Abstract
Tissue-resident stem cells (SCs) are critical players in the maintenance of tissue homeostasis. SCs reside in complex and uniquely anatomically organized microenvironments (SC niches), that carefully control SC lineage outputs depending on localized tissue needs. Upon environmental perturbations and tissue-stressors, SCs respond and restore the tissue to homeostasis, as well as protect it from secondary assaults. Critical to this function are two key processes, SC lineage plasticity and SC memory. In this review, we delineate the multi-factorial determinants, and key principles underlining these two remarkable SC behaviors. Understanding lineage plasticity and SC memory will be critical not only to design new regenerative therapies, but also to determine how these processes are altered in a multitude of pathologies such as cancer and chronic tissue damage.
Introduction
Each tissue-specific adult stem cell (SC) is responsible for maintaining its organ’s structure and function by replacing dying cells and balancing proliferation with differentiation. Tissue SCs reside in specialized environments called niches, where they are connected to supporting cells, protected from harmful stimuli, and regulated by appropriate activating signals. During steady-state (homeostasis), some SCs, such as those of the epidermis, give rise to only one specific cell-fate, while others such as in the hair follicle (HF), intestine or hematopoietic system give rise to multiple lineages. Temporally, SC renewal can be continuous (such as in the epidermis, intestine and lung airways), very slow (in muscle, and sweat glands), or in bursts of regenerative activity (as seen by HFSCs and lactating mammary glands)1.
While SCs show autonomy in their functions, they act in concert with, and are dependent on, their neighboring environment to convey information about the ever-changing needs and demands of the tissue (Fig 1A)2,3. These interactions must be finely tuned4,5. When SC activity is excessive, tissue overgrowth and cancer can arise. In contrast, decreases in SC activity, can result in defective tissue regeneration and barrier dysfunction. The niche, therefore, must be poised to convey ever-changing physical, metabolic, inflammatory and wound-induced changes to SCs, regulating their behavior accordingly. As such, it is not surprising to find that SC niches include a multitude of diverse components, including mesenchymal cells, immune cells, peripheral neurons, vascular and lymphatic vessels, and a specialized extracellular matrix (ECM), all of which can intimately influence SC behavior3,6–8. In addition to these, SCs are influenced by neighboring progeny, directly responding to stimulatory or inhibitory signals that they produce9. Hence, fate determination of tissue-resident SCs depends on the complex integration between inductive environmental cues, and their intrinsic abilities to respond appropriately to them (Fig 1B).
Figure 1: SC niche microenvironment.
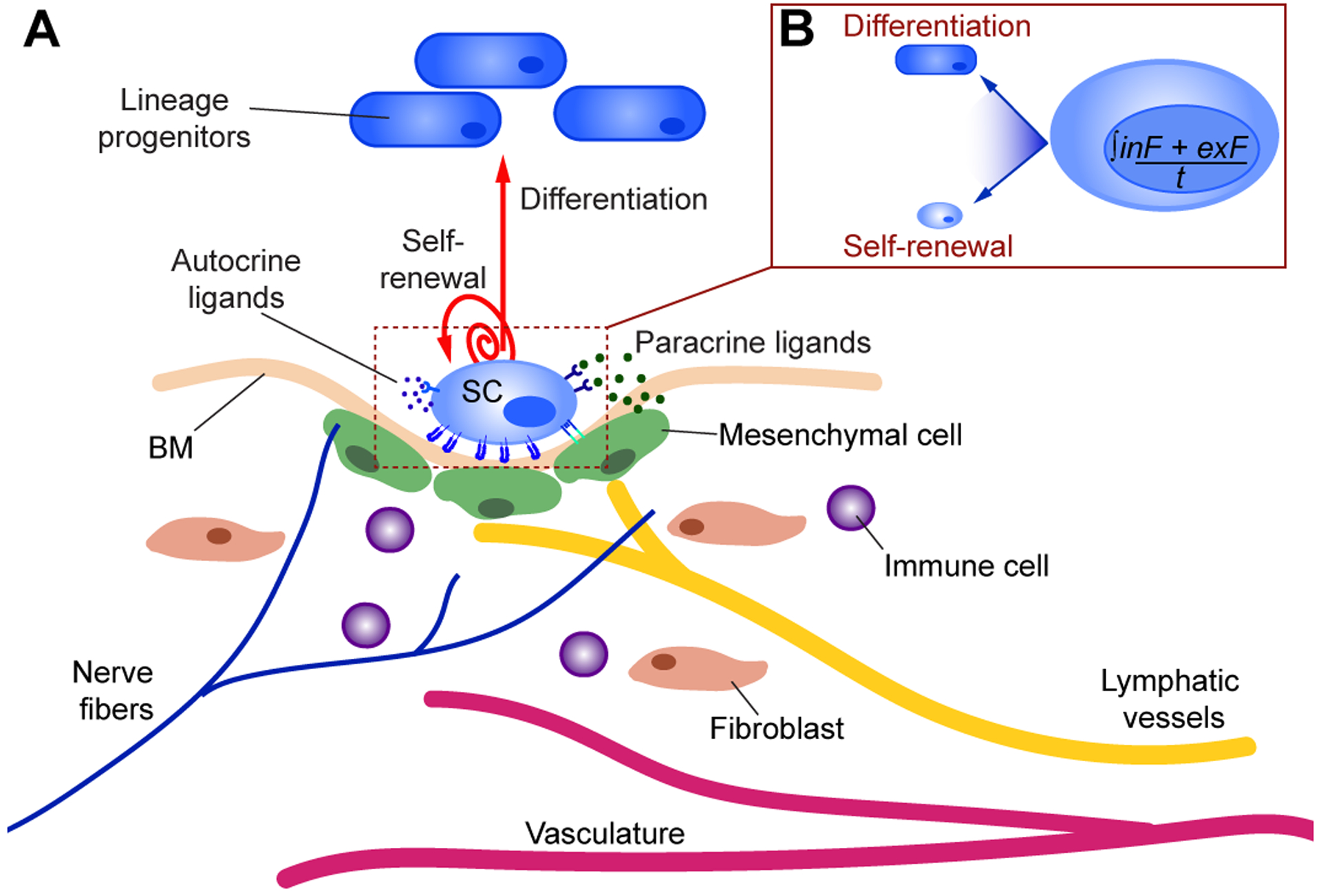
A) The SC niche involves a multitude of tissue specific cellular components (blood vessels, neurons, fibroblasts, immune cells), secreted factors (ex: growth factors, Wnts, BMPs, chemokines), and mechanical forces in part influenced by the extracellular matrix [basement membrane (BM)]. At homeostasis, the SC niche provides structural and trophic support, topographical information, and physical cues (soluble paracrine or autocrine signals, as well as mechanical stimuli), which influence SC functions and fate over time. While in some tissues SCs are continually active (such as in the epidermis, intestine, and lungs), in other tissues SCs are predominantly quiescent (as in muscle, and sweat glands), or undergo bursts of regenerative activity (as seen by HFSCs and lactating mammary glands). Local interactions are bi-directional, such that SCs not only receive signals from their surrounding niche cells but also transmit signals to their neighbouring cells. Moreover, increasing studies show that SCs can also directly shape their local niches, resulting in a unique localized micro-environment. B) SC behavior can therefore be summarized by the integration of intrinsic (inF) and extrinsic factors (exF) over time (t). How these complex and dynamic pathways are combined to result in the required SC fate-decision (differentiation or self-renewal) at any moment in time is currently an area of active investigation.
A major challenge in SC biology is discerning how SCs respond and adapt once their local niche has been perturbed. How do SCs replace neighboring cells following tissue damage? How do they adapt to a local dynamic environment? And, do they retain information of previous stressors to better guide cell-fate decisions at later times? In this review, we focus on two key processes: SC lineage plasticity, a state that SCs enter when their normal niche signals have been altered, and SC memory, a process whereby long-lasting SC activity remains altered even after initial insult resolution. While the molecular mechanisms behind these cell behaviours are still incompletely understood, preliminary work suggests that unique alterations in chromatin dynamics play a central role. Dissecting the molecular and cellular networks that regulate SC functions will be central to our understanding of regeneration and host protection.
Stem Cell Lineage Plasticity
Adult SCs maintain homeostasis and tissue integrity by undergoing local proliferation and differentiation. However, in response to environmental changes and tissue stressors, particularly wounding, adult SCs enter a state of plasticity, where the lineage choices afforded to SCs become more flexible. This is in part mediated by loss of homeostatic canonical transcription factors, and ensuing changes in chromatin accessibility and co-expression of transcription factors specific for other SC lineages. These changes empower SCs with a greater capacity to repair tissue damage (Fig 2A)10. Early evidence of SC plasticity emerged from serial transplantation and/or engraftments following cell culture. No longer under the controls exerted by their niche, these transplanted SCs generated a wider range of cellular lineages than they normally do within their physiological constraints10,11. This can be exemplified by skin sweat gland SCs, which when transplanted into different local environments (either mammary fat pads or skin), can either form milk producing glandular structures, or epidermal tissue12. How SCs respond to injuries varies dramatically depending not only on the particular SC niche, but also its proximity to the wound edge. Thus, lineage plasticity has been studied either using cell ablation techniques, or tracking cells during tissue injury and repair.
Figure 2: Local perturbations or environmental stressors alter SC behaviors.
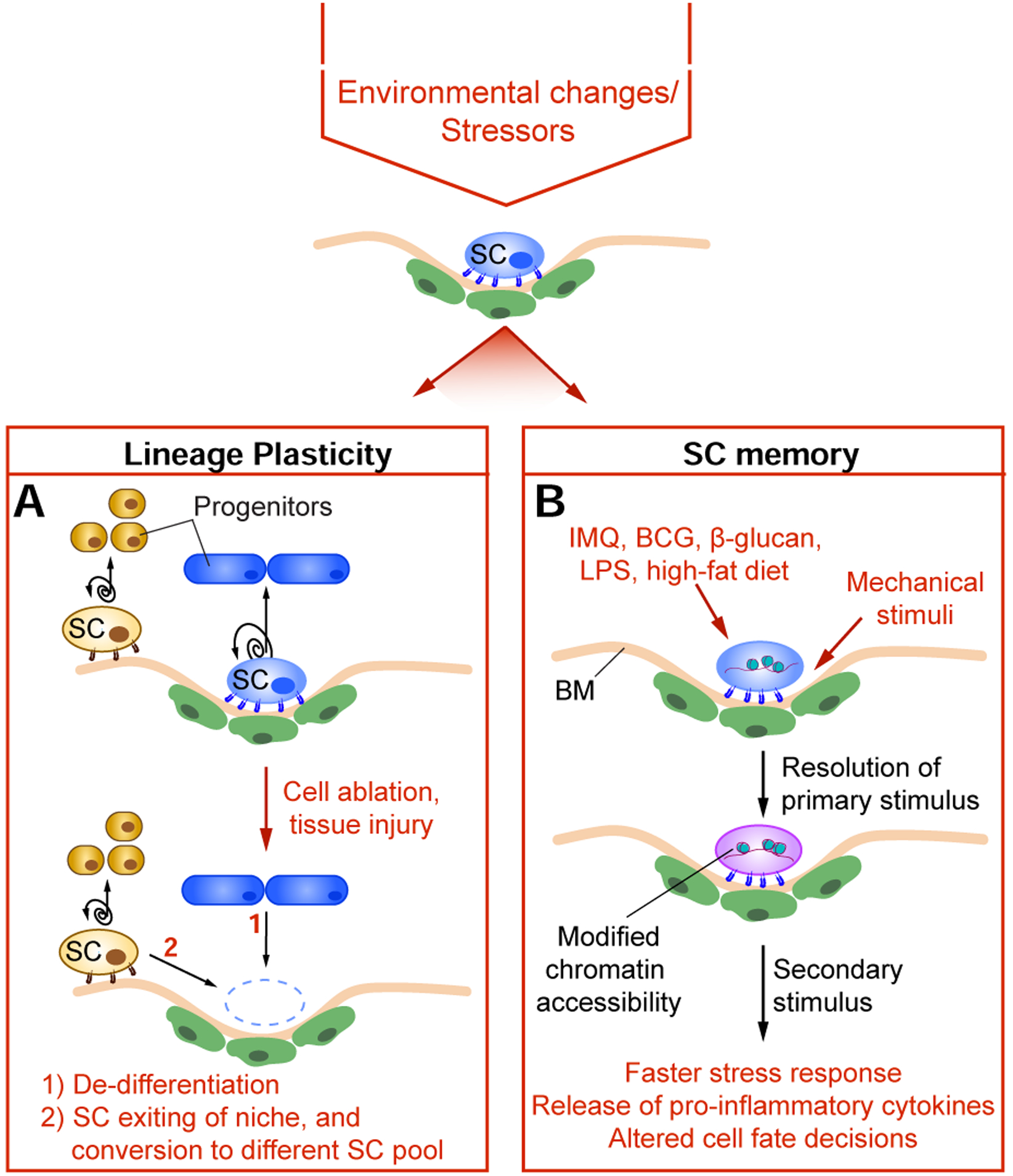
Environmental stimuli can alter stem cell (SC) function in a multitude of ways, with two key processes being lineage plasticity and SC memory. A) Under homeostatic conditions, each SC compartment is maintained by corresponding resident SCs. Following environmental perturbations, localized cell ablation or tissue injury, SCs become more plastic (i.e. their lineage choices become broadened and more flexible) and can migrate out of their niche, allowing for increased capacity to repair local tissue damage. Furthermore, differentiated progenitor cells can similarly undergo de-differentiation. Lineage plasticity is a key process in optimizing expeditious healing, where prolonged unresolved wounds would otherwise have detrimental consequences to the host. B) Exposure to environmental stimuli and stressors (such as IMQ (Imiquimod), BCG (Bacillus-Calmette-Guérin), β-glucan, LPS (Lipopolysaccharide), and high-fat diet) can have a long-lasting impact on SC function following resolution of the initial insult and its associated pathology. Adult SCs also intimately interact with the basement membrane (BM) to maintain their potency, with mechanical stimuli having broad effects on SC memory and function. SC memory results in long-term cellular alterations, often at the chromatin level. Upon re-exposures to environmental stressors, SCs exhibit altered behaviors, such as faster stress response (such as re-epithelization), elevated production of inflammatory cytokines (such as IFNγ and TNFα), and altered cell fate decisions (such as increased myelopoiesis in the case of HSCs, or osteogenic differentiation in the case of MSCs).
SC plasticity following cell ablation
Cell ablation studies via a multitude of experimental approaches have shown that although nearby SCs can undergo self-renewal to replenish SC vacancy, committed progeny can also de-differentiate to assume the behavior of their SC parents. This has been demonstrated in the HFSC niche, an anatomical region known as the ‘bulge’. Laser ablation and diphtheria toxin-targeted genetic approaches have shown that in response to HFSC vacancies within the bulge, nearby SCs or differentiated progeny above and below the bulge are able to repopulate the niche13–15. Lineage plasticity has also been observed for basal Keratin-5+ SCs of the lung and their secretory progeny. In culture, secretory cells can dedifferentiate into basal SCs when basal cells are absent; however, when directly in contact with neighboring basal SCs, the dedifferentiation process is inhibited16. Similarly, following injury in the intestinal epithelium, Bmi1+ cells just above the crypt SC niche and secretory committed Dl11+ cells can both revert back to a SC-like state and contribute to tissue repair17–20. However, apoptosis of Lgr5+-intestinal SCs (ISCs) by irradiation or cytotoxic damage results in severe crypt loss and deterioration of crypt-villus architecture, implying that ISCs are crucial for robust long-term intestinal regeneration21. These data suggest that lineage plasticity is in part context dependent, supporting the notion that SC fate is finely regulated by local environmental cues.
SC plasticity in tissue injury and repair
While cellular ablation provides a localized and precise tool to remove SCs resulting in repaired niche vacancies, healthy SCs must similarly be able to survive tissue damage and inflammatory insults, restoring local homeostasis. Intriguingly, during wound healing, tissue regeneration often engages signaling networks and programs that are active during embryonic development, a time when the SC precursors naturally have greater fate options4. As tissues develop and SC niches mature, SC fate options become constrained by the local niche. However, upon tissue injury, SCs are either directly damaged or are recruited to the wound bed. In either case, the resulting new environment drives in part the silencing of homeostatic transcription factors while inducing new ones. The outcome are changes in chromatin dynamics that enable SCs to survive their new location by entering a state of plasticity.
Following tissue injury, key stages of healing occur broadly defined as hemostasis, inflammation, proliferation and tissue remodeling22. Although these key steps of wound repair are well characterized, the complexities of how individual SCs behave at the clonal level are just beginning to be uncovered. In a wound response, the nearest SCs will be stimulated, even if they have to undergo fate switching23,24. Wound-induced SC plasticity has been well characterized in the skin, where HF neogenesis occurs following deep tissue wounds25,26. Furthermore, following deep tissue injury, inter-follicular epithelial (IFE) SCs maintain their expression of transcription factor Klf5, but now exhibit open chromatin regions enriched for HFSC transcription factor Sox923. SC plasticity was also seen in Gata6+ isthmus-residing SCs following tissue damage. These cells, which normally give rise to the skin sebaceous ducts, undergo migration towards the injured barrier, and revert from a differentiated to IFE SC fate27. Finally, in response to shallow wounds, HFSCs exhibit open chromatin regions enriched for Klf5, while maintaining their expression of Sox923. The plastic Klf5+ and Sox9+ “lineage infidelity” state allows HFSCs to undergo a fate switch and regenerate the epidermis, while enabling IFE SCs to induce de novo HF morphogenesis. Interestingly, this dual lineage state appears to be transient during wound repair, but it persists in skin cancer23, perhaps a reflection of the permanently altered niche and chromatin profile of tumorigenic SCs. Analogous to the situation in skin, cells in the intestinal epithelium that are committed to terminal differentiation can also revert back to a progenitor-like state and contribute to tissue repair following injury. Indeed, following intestinal injury, committed precursors of Paneth and enteroendocrine lineages enter a state of plasticity enabling them to generate all major ISC lineage cell types28.
Lineage infidelity in SCs is not limited to wounding and tumorigenesis. Infection-induced inflammation can trigger SC plasticity in some remarkable ways. In the intestine, distinct cytokines are known to impact lineage differentiation and stem cell renewal. For instance, during gut infection, the pathogen specific cytokine milieu produced by activated T-helper cells drives the expansion and cell fate decision of transient amplifying ISC progeny29. Mice infected with Salmonella resulted in high levels of Th1-cytokines (IFNγ) and consequent increase in Paneth cell numbers. In contrast, mice infected with an intestinal worm (Heligmosomoides polygyrus) had increased Th2-cytokines (IL13) levels and expansion of Tuft cells. Similarly, a cytokine circuitry was also recently shown in the context of helminths infections which were shown to alter the metabolic state of the nearby intestinal epithelial tissue. The increased succinate in the tissue stimulates resident innate-like immune (ILC2s) cells to produce IL-13, which skews ISCs cell fate towards Tuft cell generation. In turn, Tuft cells secrete IL-25, a proliferative stimulus for ILC2s30. Lastly, in a process that is reminiscent of that described for skin SCs in a wound-induced state4, ISCs exposed to parasitic infections upregulate fetal transcriptional programs to repair tissue damage31.
As SC behavior is significantly altered during induction and maintenance of inflammatory states, the field awaits elucidation of the feedback mechanisms required to halt cellular plasticity, initiate healing, and resolve inflammation. Insights emerging from studies of the hematopoietic system and muscle suggest the involvement of regulatory T (Treg) cells and type-2 macrophages32–34, but a conceptual framework is still needed to understand how SCs and immune players efficiently collaborate. Lastly, a recent study by Song et al. shows that both dietary and microbial factors can influence the steady state status of bile acid metabolites in the gut, which in turn is required to maintain intestinal RORγ+ Tregs which reduce susceptibility to inflammatory colitis35. These glimpses point to a future need to probe deeper also into the metabolic milieu surrounding SC niches and interrogate the changes that occur in different stress states.
Stem Cell Memory
Exposure to environmental stimuli and stressors can have long-lasting impacts on SC function after the initial insult has ended (Fig 2B). Triggers of SC memory can be either biochemical or biophysical in nature, with biochemical stimuli broadly divisible as either immunogenic stressors (resulting in trained immunity) or metabolic. This SC memory results from cellular modifications (often at the chromatin level) that are retained long-term and alter subsequent SC behaviors.
Biochemical stimuli
A multitude of reports have now shown that immunogenic stressors can have long-lasting impacts on cellular function, resulting in trained immunity (a term proposed to describe long-term functional reprogramming after an insult, which leads to an altered response towards a secondary challenge)36. Initially described in innate immune cells, data suggest that changes in chromatin organization, DNA methylation, and transcription of long non-coding RNAs are key molecular players in maintaining trained immunity36.
In addition to innate immune cells (of which some are relatively short-lived), there is now clear evidence that long lived, tissue resident stromal and SCs compartments can also exhibit inflammatory memory. This was elegantly demonstrated in the skin, where following resolution of acute skin inflammation with an IL-17-inducing stimulus [imiquimod (IMQ)], the accessibility of key stress response genes was altered in epithelial SCs, such that these “poised” genomic regions enable faster re-epithelization following wounding37. Importantly, these chromatin domains harboring inflammation sensing activity were found to remain accessible up to 6 months post primary challenge. While the molecular details of this inflammatory memory remain to be unfolded, IL-1β was an essential component of SC memory recall response37.
In addition to the skin, SC memory has also been found in mesenchymal stromal SCs (MSCs) and in human lung epithelial explants. Albeit on a shorter-time scale, LPS (Lipopolysaccharide) or TNF stimulated adipose-derived MSCs showed increased ability to produce pro-inflammatory cytokines upon secondary challenges38. Increased cytokine production may be critical to tailor the phenotype and temporal dynamics of local immune responses. Although preliminary, inflammatory SC memory may also exist in humans, as following ex vivo culturing of lung epithelial explants, chromatin dynamics elicited by an IL4/IL13-stimulating allergic response persisted for weeks even with regular media changes. These findings exemplify the intrinsic nature of this memory39. As SCs express receptors for a multitude of inflammatory receptors40,41, future work will be needed to decipher the quality, longevity, and functional consequences of SC memory to different types stimuli.
Recent work in HSCs has revealed another fascinating facet of SC memory which results in altered SC fate determination upon secondary re-activation. Intravenous Bacillus-Calmette-Guérin (BCG) administration not only markedly alters the transcriptional and chromatin landscape of HSCs and multipotent progenitors (MPPs), but also, results in enhanced expression of several key cytokines (IFNγ, TNFα, IL-1β) and increased myelopoiesis upon secondary infection42. In a similar study, β-glucan (a sepsis-inducing agent and key inducer of IL-1β) skewed the fate of HSCs towards myeloid lineages at the expense of lymphoid precursors43. Recently, de Laval et al. also showed that following a primary inflammatory LPS insult, novel open chromatin regions (OCRs) persist long-term in HSCs, with accessible myeloid enhancers enriched for C/EBPβ targets44. Interestingly, C/EBPβ deletion erased the long-term inscription of LPS-induced epigenetic marks hinting to a direct mechanism for SC memory. These studies clearly highlight the physiological importance of HSC memory, which can result in improved host survival following exposure to a multitude of pathogens42,44. Furthermore, they also demonstrate how inflammatory memory retained in long-lived SCs, can result in lineage alteration that influence a short-lived progeny (as exemplified by HSCs and their monocytic progenitors).
Recent data have extended the concept of epigenetic memory to metabolic encounters45–47. Thus, a “Western” diet rich in fats exacerbates the IL-1β epigenetic skewing of HSCs along the myeloid lineage44, again, invoking a role for IL-1β and suggesting tantalizing parallels in the mechanisms controlling inflammatory memory in adult SCs7. Additionally in Drosophila, dietary cholesterol influences new intestinal cell differentiation in an Hr96-dependent manner by altering the level and duration of Notch signaling48. Even after removal from a high-cholesterol diet, intestinal SCs maintain enhanced generation of enteroendocrine cells. While more studies will need to be done in mammalian systems, the intersection between metabolic and inflammatory “priming” on SC memory is of great potential interest. Indeed, exposure to immunological stimuli (such as β-glucan) appear not only to trigger trained immunity, but also metabolically alter HSCs progenitors towards aerobic glycolysis43.
Biophysical stimuli
Similar to extrinsic biochemical factors, mechanical cues resulting from externally applied forces have broad effects on SC function, fate and memory. Adult SCs require physical interactions with the ECM to maintain their potency, cell fate, and tissue organization49,50. In fact, in vitro differentiation of MSCs derived from adipose or bone marrow tissue is in part regulated by the elasticity of the matrix composition51,52. Stiff matrix cultures result in MSC spreading, nuclear translocation of transcriptional co-activators YAP-TAZ (Yes-associated protein, and transcriptional coactivator with PDZ-binding domain), and differentiation to osteogenesis. In contrast, MSCs cultured on soft matrices result in cell rounding, cytoplasmic localization of YAP/TAZ and adipogenesis. Importantly, YAP-TAZ appear to act as intracellular mechanical rheostats, mediating the effects of mechanical dosing on SC fate by persistent localization in the nucleus53. Retention of mechanical history in MSCs is further regulated by levels of microRNA-21 promoting a MSC fibrogenic program54. Preliminary data also suggest that histone acetylation and chromatin organization may adapt rapidly to matrix stiffness, a process that can be reversible or irreversible depending on duration and composition of matrix exposure55. Such findings suggest a potential mechanism by which these cells may be able to retain a mechanical memory of their prior environments. Thus far, SC memory from mechanical stimuli has only been observed in osteogenic differentiation, and further work will be needed to determine the extent to which this might be a widespread feature of tissue SCs. Additionally, more work will be necessary to understand the underlying mechanisms of this intriguing mechanical memory and its physiological in vivo occurrence.
Conclusions and perspectives
The multitude of experimental approaches ranging from mouse genetics, intravital imaging and high throughput “omics” have greatly advanced our understanding of adult SCs. It has become clear that the SC niche and microenvironment can alter SC behavior not only in the short, but also long term. Although more work will need to be done to mechanistically understand these processes, it appears that chromatin modifications and transcriptional changes play a central role in modulating SC functions. As the complexities of SCs and their local interactions continue to unfold, so too will come a better understanding of the underlying biological principles that govern SC behavior, as well as ways in which we may be able to harness the potential of SC plasticity for regenerative medicine.
Acknowledgments
We are grateful to the many friends and colleagues in the stem cell, wound-repair and immunology fields whose work inspired us to write this review, specifically we thank KAU Gonzales, CJ Cowley, A Bonny, and J Novak for helpful comments. Given the reference constraints and the focus on recent findings, we regret that we could not cite all the worthy papers on this topic. However, we have tried to present a balanced view of the field, attempting to clarify areas of seeming discrepancies and present a collective view of where the field stands and the exciting areas of research that await us all. A.G. is a recipient of a Damon Runyon Postdoctoral Fellowship. E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the NIH (R01-AR050452, R01-AR31737 and R01-AR27883 E.F.), NYSTEM (C32585GG) and the Starr Foundation (AWD00000251).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Given the reference constraints and the focus on recent findings, we regret that we could not cite all the worthy papers on this topic. However, we have tried to present a balanced view of the field, attempting to clarify areas of seeming discrepancies and present a collective view of where the field stands and the exciting areas of research that await us all. The authors have not direct conflicts of interest.
References
- 1.Gonzales KAU & Fuchs E Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev Cell 43, 387–401, doi: 10.1016/j.devcel.2017.10.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L & Clevers H Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545, doi: 10.1126/science.1180794 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu YC, Li L & Fuchs E Emerging interactions between skin stem cells and their niches. Nat Med 20, 847–856, doi: 10.1038/nm.3643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Y & Fuchs E Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet 19, 311–325, doi: 10.1038/nrg.2018.9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Key review on SC function during homeostasis, in wound healing, and in cancer with a focus on lineage plasticity.
- 5.Asada N et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223, doi: 10.1038/ncb3475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gur-Cohen S et al. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225, doi: 10.1126/science.aay4509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated the role of SC-lymphatic vessels interactions to mediate effective skin regeneration.
- 7.Naik S, Larsen SB, Cowley CJ & Fuchs E Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 175, 908–920, doi: 10.1016/j.cell.2018.08.071 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins BA, Fontecilla NM, Lu CP, Fuchs E & Lumpkin EA The cellular basis of mechanosensory Merkel-cell innervation during development. Elife 8, doi: 10.7554/eLife.42633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesa KR et al. Homeostatic Epidermal Stem Cell Self-Renewal Is Driven by Local Differentiation. Cell Stem Cell 23, 677–686 e674, doi: 10.1016/j.stem.2018.09.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Elucidates the role for epidermal SC progenitors in driving self-renewal of neighboring SCs.
- 10.Wagers AJ & Weissman IL Plasticity of adult stem cells. Cell 116, 639–648, doi: 10.1016/s0092-8674(04)00208-9 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Blanpain C & Fuchs E Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281, doi: 10.1126/science.1242281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu CP et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150, 136–150, doi: 10.1016/j.cell.2012.04.045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rompolas P, Mesa KR & Greco V Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518, doi: 10.1038/nature12602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Kizawa K, Hamada K & Cotsarelis G Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72, 548–557, doi: 10.1111/j.1432-0436.2004.07209008.x (2004). [DOI] [PubMed] [Google Scholar]
- 15.Hoeck JD et al. Stem cell plasticity enables hair regeneration following Lgr5(+) cell loss. Nat Cell Biol 19, 666–676, doi: 10.1038/ncb3535 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Tata PR et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503, 218–223, doi: 10.1038/nature12777 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA & Henning SJ Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 297, G461–470, doi: 10.1152/ajpgi.90446.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian H et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259, doi: 10.1038/nature10408 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Es JH et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14, 1099–1104, doi: 10.1038/ncb2581 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetteh PW et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell 18, 203–213, doi: 10.1016/j.stem.2016.01.001 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Metcalfe C, Kljavin NM, Ybarra R & de Sauvage FJ Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14, 149–159, doi: 10.1016/j.stem.2013.11.008 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Dekoninck S & Blanpain C Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol 21, 18–24, doi: 10.1038/s41556-018-0237-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent review covering a multitude of SCs actvities during wound healing.
- 23.Ge Y et al. Stem cell lineage infidelity drives wound repair and cancer. Cell 169, 636–650 e614, doi: 10.1016/j.cell.2017.03.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page ME, Lombard P, Ng F, Gottgens B & Jensen KB The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482, doi: 10.1016/j.stem.2013.07.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim CH et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat Commun 9, 4903, doi: 10.1038/s41467-018-07142-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320, doi: 10.1038/nature05766 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Donati G et al. Wounding induces dedifferentiation of epidermal Gata6(+) cells and acquisition of stem cell properties. Nat Cell Biol 19, 603–613, doi: 10.1038/ncb3532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buczacki SJ et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69, doi: 10.1038/nature11965 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Biton M et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 175, 1307–1320 e1322, doi: 10.1016/j.cell.2018.10.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Determines the role played by Th cytokines in intestinal SC renewal through the use of organoid co-culturing systems and in vivo genetic manipulations
- 30.Schneider C et al. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 174, 271–284 e214, doi: 10.1016/j.cell.2018.05.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Elucidates an elegant cytokine circuit in the gut involving Tuft Cell-ILC2-ISCs
- 31.Nusse YM et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 559, 109–113, doi: 10.1038/s41586-018-0257-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Following parasitic infections, ISCs in culture can revert back to fetal-like epithelium by forming spheroids and activating fetal-like transcriptional programs.
- 32.Burzyn D et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295, doi: 10.1016/j.cell.2013.10.054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arpaia N et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 162, 1078–1089, doi: 10.1016/j.cell.2015.08.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krzyszczyk P, Schloss R, Palmer A & Berthiaume F The Role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9, 419, doi: 10.3389/fphys.2018.00419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song X et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415, doi: 10.1038/s41586-019-1865-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netea MG et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 20, 375–388, doi: 10.1038/s41577-020-0285-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Key review on functions and mechanisms of trained immunity in a multitude of different cell types and tissues.
- 37.Naik S et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480, doi: 10.1038/nature24271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu GY et al. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol Immunol 13, 369–378, doi: 10.1038/cmi.2015.11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ordovas-Montanes J et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 560, 649–654, doi: 10.1038/s41586-018-0449-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Estiblishes a role for allergic inflammatory memory in human epithelial lung SCs via ex vivo culturing
- 40.Pevsner-Fischer M et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109, 1422–1432, doi: 10.1182/blood-2006-06-028704 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Nagai Y et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812, doi: 10.1016/j.immuni.2006.04.008 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufmann E et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172, 176–190 e119, doi: 10.1016/j.cell.2017.12.031 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Mitroulis I et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172, 147–161 e112, doi: 10.1016/j.cell.2017.11.034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Kaufmann et al., and Mitroulis et al., provides elegant examples of trained immunity in HSCs, showing that SC memory can not only influence local tissues but also play a key role in altering circulating short-lived progeny.
- 44.de Laval B et al. C/EBPbeta-Dependent Epigenetic Memory Induces Trained Immunity in Hematopoietic Stem Cells. Cell Stem Cell 26, 657–674 e658, doi: 10.1016/j.stem.2020.01.017 (2020). [DOI] [PubMed] [Google Scholar]; * Suggests a key role for C/EBPbeta in maintaining an inflammatory memory in HSCs following LPS administration.
- 45.Christ A et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 172, 162–175 e114, doi: 10.1016/j.cell.2017.12.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng SC et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684, doi: 10.1126/science.1250684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novakovic B et al. beta-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 167, 1354–1368 e1314, doi: 10.1016/j.cell.2016.09.034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obniski R, Sieber M & Spradling AC Dietary Lipids Modulate Notch Signaling and Influence Adult Intestinal Development and Metabolism in Drosophila. Dev Cell 47, 98–111 e115, doi: 10.1016/j.devcel.2018.08.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a key example on how dietary lipids can directly modulate notch signaling and ISC fate in Drosophila.
- 49.Vining KH & Mooney DJ Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18, 728–742, doi: 10.1038/nrm.2017.108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shyer AE et al. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 357, 811–815, doi: 10.1126/science.aai7868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huebsch N et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9, 518–526, doi: 10.1038/nmat2732 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689, doi: 10.1016/j.cell.2006.06.044 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Yang C, Tibbitt MW, Basta L & Anseth KS Mechanical memory and dosing influence stem cell fate. Nat Mater 13, 645–652, doi: 10.1038/nmat3889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li CX et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16, 379–389, doi: 10.1038/nmat4780 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Killaars AR et al. Extended Exposure to Stiff Microenvironments Leads to Persistent Chromatin Remodeling in Human Mesenchymal Stem Cells. Adv Sci (Weinh) 6, 1801483, doi: 10.1002/advs.201801483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


