Abstract
Background:
Race- and sex-specific differences in heart failure (HF) risk may be related to differential burden and effect of risk factors. We estimated the population attributable fraction (PAF), which incorporates both prevalence and excess risk of HF associated with each risk factor (obesity, hypertension, diabetes, current smoking, and hyperlipidemia), in specific race-sex groups.
Methods:
A pooled cohort was created using harmonized data from six US longitudinal population-based cohorts. Baseline measurements of risk factors were used to determine prevalence. Relative risk of incident HF was assessed using a piecewise constant hazards model adjusted for age, education, other modifiable risk factors, and the competing risk of death from non-HF causes. Within each race-sex group, PAF of HF was estimated for each risk factor individually and for all risk factors simultaneously.
Results:
Of 38,028 participants, 55% were female and 22% Black. Hypertension had the highest PAF among Black men (28.3% [18.7, 36.7]) and women (25.8% [16.3, 34.2%]). In contrast, PAF associated with obesity was highest in White men (21.0% [14.6, 27.0]) and women (17.9% [12.8, 22.6]). Diabetes disproportionately contributed to HF in Black women (PAF 16.4%, 95% CI 12.7, 19.9%). The cumulative PAF of all 5 risk factors was highest in Black women (51.9% [39.3, 61.8]).
Conclusions:
The observed differences in contribution of risk factors across race-sex groups can inform tailored prevention strategies to mitigate disparities in HF burden. This novel competing risk analysis suggests that a sizeable proportion of HF risk may not be associated with modifiable risk factors.
Keywords: Primary prevention, heart failure, risk factors, race and ethnicity
Heart failure (HF) is a growing public health burden and national estimates of HF prevalence exceed 6 million Americans.1 Recent data suggest that cardiovascular mortality related to HF is increasing in all race-sex subgroups, with death rates being significantly higher in Black men and women.2 The increasing prevalence and morbidity have led to rising costs, totaling $30.7 billion in 2012 and are expected to increase to $69.8 billion by 2030.3 Despite therapeutic advances, the persistently high burden of morbidity and mortality with HF highlights the need to focus on prevention.
Targeting modifiable risk factors for HF, such as obesity, hypertension, diabetes, cigarette smoking, and hyperlipidemia, is an effective means of reducing HF burden.4–6 A better understanding of the proportion of HF cases in the population that can be attributed to each modifiable risk factor, a measure known as population attributable fraction (PAF), can help refine and prioritize public health interventions as well as target key contributors towards disparities in HF development. Specifically, PAF quantifies the potential impact of a risk factor by accounting for both the prevalence and excess risk of disease associated with the risk factor. While prior studies have evaluated the PAF of HF for certain modifiable risk factors, they have been limited by lack of generalizability in diverse populations where prevalence or risk associated with HF may vary. Furthermore, prior studies have predominantly examined short-term risk and have not utilized a competing risk framework 7–17, which accounts for deaths that are due to causes other than HF. Including the competing risk of death is essential in obtaining an accurate risk estimate for HF as each modifiable risk factor is associated with other life-limiting conditions. Thus, not accounting for the competing risk of death in time-to-event analyses results in bias and systematic overestimation of the relative risk (RR).18
Therefore, we pooled and harmonized data from six contemporary population-based cohorts in the Lifetime Risk Pooling Project (LRPP) to estimate the PAF of HF for the common modifiable risk factors using a competing risk framework, stratified by race and sex.
METHODS
Study Population
The LRPP is an individual-level pooled dataset from numerous community-based or population-based cardiovascular disease (CVD) cohorts in the United States.19 Participants were free of clinical CVD at baseline and had nearly 100% complete follow up for vital status. This large dataset provided a unique opportunity to assess PAF of HF for modifiable risk factors in each race-sex group separately. We included 38,028 Black and White participants from the following prospective cohorts: Atherosclerosis Risk in Communities Study (baseline period 1987–1989), Coronary Artery Risk Development in Young Adults Study (1985–1986), Cardiovascular Health Study (1989–1990, 1992–1993 for Black participants), Framingham Heart Study starting from Exam 12 (1972), Framingham Offspring Study (1971–1975), and the Multi-Ethnic Study of Atherosclerosis (2000–2002). These cohorts were chosen as they represent relatively contemporary longitudinal studies with available measurements of modifiable risk factors and adjudication of HF events (Supplemental Methods and Supplemental Table I). All data were de-identified, and all study protocols and procedures were approved by the Institutional Review Board at Northwestern University with a waiver for informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Risk Factor Ascertainment
We included participants with at least one baseline measurement of each modifiable risk factor: systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), smoking status, body mass index (BMI), and fasting lipid profile. Baseline demographics of age, race, sex, and education were self-reported. Height, weight, and blood pressure were measured by trained staff. Hypertension was defined as SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, or self-reported use of blood pressure medications. In a separate analysis, hypertension was defined as SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg in accordance with the 2017 ACC/AHA blood pressure guidelines.20 Obesity was defined using the BMI threshold of ≥ 30 kg/m2. Smoking status was self-reported. Diabetes was defined as FPG ≥ 126 mg/dl, self-reported physician diagnosis of diabetes, or self-reported use of diabetes medications. Hyperlipidemia was defined as total cholesterol levels ≥ 240 mg/dl, or self-reported use of lipid-lowering medications. We excluded participants of non-Black or non-White self-reported race due to small sample sizes and participants over the age of 80 years to minimize including elderly individuals with undiagnosed prevalent HF.
Outcome Ascertainment
All participants had at least 10 years of follow-up. Adjudication criteria for incident HF were pre-specified and detailed descriptions are provided in the Supplemental Table I. Vital status was obtained through linkage with National Death Index and all cardiovascular deaths were adjudicated by review of medical records and available autopsies. Deaths were then categorized as those related to HF and those not related to HF, which included both non-cardiovascular and cardiovascular causes. These other non-HF causes of death included non-cardiovascular death including but not limited to cancer, neurological disease, accidents, respiratory illness, renal disease, and infections as well as cardiovascular death not attributed to HF such as myocardial infarction or stroke.
Statistical Analysis
PAF is defined as the proportion of incident HF events attributable to a given risk factor. Specifically, PAF integrates both the prevalence and the RR to represent the proportion of incident HF events that would not have occurred if the prevalence of the risk factor were zero (e.g. eliminating hypertension).
First, we estimated the RR and the 95% confidence interval to show the strength of the association between each risk factor and HF and death not related to HF, adjusting for age, education, and other modifiable risk factors. The total follow-up period was defined as the time from baseline to date of incident HF, death or loss of follow up, which occurred first. The survival times were assumed to follow a parametric proportional hazards model with piecewise constant baseline hazard function.21 Maximum likelihood estimation with iterative method was applied to assess the parameter and their covariance estimates.22
Second, we combined the exposure or risk factor prevalence and maximum likelihood estimates to quantify the PAF using the formula and program developed by Laaksonen et. al. accounting for the competing risk of death not related to HF.22, 23 This method provides freedom with respect to setting the reference level for risk factor modification and also allows for simultaneous analysis of multiple risk factors. Assessment of joint cumulative estimates assumes that all risk factors will be modified to the reference level with the lowest risk. Thus, the joint cumulative estimates obtained quantify the expected proportional reduction in incident HF if all the risk factors of interest were simultaneously eliminated from the population. PAFs were estimated for each risk factor individually and for all risk factors cumulatively. The variance estimates of PAF was assessed using the delta method, and 95% confidence interval were estimated using a symmetrizing complementary logarithmic transformation of PAF. In secondary analysis, we also estimated the PAF in individuals presenting with multiple risk factors by performing an ordinal competing analysis. We categorized individuals into one of the following three groups based on the number of risk factors present: 1 risk factor, 2 risk factor, or ≥3 risk factors. We used a P value of less than 0.05 for a 2-sided significance test. We performed all statistical analyses using SAS V.9.4 and PAF program based on SAS macros.23
RESULTS
A total of 38,028 participants were included with 607,382 person-years of follow up; 22% were Black and 55% women. Baseline characteristics are shown in Table 1 stratified by race and sex. Hypertension was more prevalent in Black women (53%) and Black men (51%) than in White women (35%) and White men (35%). The prevalence of diabetes in Black women (15%) and Black men (16%) was 2-fold higher than that observed in White women (7%) and White men (8%). The prevalence of obesity was also markedly higher in Black women (49%) and Black men (35%) compared with White women (22%) and White men (22%). The prevalence of current smoking ranged from 22% to 30% across groups. Prevalence of hyperlipidemia ranged from 19% to 27% across groups.
Table 1.
Baseline characteristics and heart failure event rates stratified by race and sex
| Black Women | White Women | Black Men | White Men | |
|---|---|---|---|---|
| n = 4,848 | n = 14,912 | n = 3,559 | n = 12,699 | |
| Age, years | 54 ± 10 | 56 ± 11 | 54 ± 10 | 56 ± 10 |
| Education ≥ high school (%) | 75 | 83 | 73 | 83 |
| Risk Factors | ||||
| Mean Systolic Blood Pressure (SBP), mm Hg | 128 ± 22 | 122 ± 20 | 129 ± 20 | 125 ± 18 |
| Mean Diastolic Blood Pressure (DBP), mm Hg | 76 ± 11 | 71 ± 11 | 79 ± 12 | 75 ± 11 |
| Hypertension, SBP ≥140 or DBP ≥90 mm Hg, or treated (%) | 53 | 35 | 51 | 35 |
| Hypertension, SBP ≥130 or DBP ≥80 mm Hg, or treated (%) | 65 | 48 | 67 | 55 |
| Diabetes (%) | 15 | 7 | 16 | 8 |
| Mean Body Mass Index (BMI), kg/m2 | 31 ± 7 | 27 ± 5 | 29 ± 6 | 27 ± 4 |
| Obesity, BMI ≥ 30 kg/m2 (%) | 49 | 22 | 35 | 22 |
| Current Smoker (%) | 22 | 22 | 30 | 24 |
| Hyperlipidemia, total cholesterol ≥ 240 mg/dL or treated (%) | 23 | 27 | 19 | 22 |
| Mean Total Cholesterol, mg/dL | 203 ± 42 | 213 ± 4 | 198 ± 42 | 206 ± 38 |
| Unadjusted Event Rates | ||||
| Heart Failure events/1000 person-years | 9 | 7 | 10 | 8 |
| Median follow-up time, years | 15 | 18 | 15 | 17 |
| Follow-up time, total person-years | 73,290 | 267,830 | 50,002 | 216,260 |
During the study period, a total of 4,636 incident HF cases occurred over a median follow-up time ranging from 15 to 18 years across race-sex subgroups. The baseline characteristics of those who did and did not develop HF are shown in Table 2, stratified by race and sex. Within each race-sex subgroup, individuals who developed HF were older at baseline and had lower levels of education. As expected, the prevalence of each of the five modifiable risk factors was higher in those with HF compared with those without HF across all groups.
Table 2.
Baseline characteristics in participants with and without heart failure stratified by race and sex
| Black Women | White Women | Black Men | White Men | |||||
|---|---|---|---|---|---|---|---|---|
| HF | No HF | HF | No HF | HF | No HF | HF | No HF | |
| n = 635 | n = 4,213 | n = 1,808 | n = 13,104 | n = 501 | n = 3,058 | n = 1,692 | n = 11,007 | |
| Age, years | 59 ± 9 | 54 ± 10 | 64 ± 9 | 55 ± 10 | 59 ± 9 | 54 ± 10 | 62 ± 9 | 55 ± 10 |
| Education, ≥ high school (%) | 54 | 78 | 71 | 85 | 57 | 75 | 73 | 84 |
| Risk Factors | ||||||||
| Mean Systolic Blood Pressure (SBP), mm Hg | 138 ± 24 | 126 ± 21 | 133 ± 21 | 121 ± 20 | 137 ± 22 | 127 ± 19 | 133 ± 21 | 123 ± 18 |
| Mean Diastolic Blood Pressure (DBP), mm Hg | 78 ± 13 | 76 ± 11 | 71 ± 11 | 71 ± 11 | 81 ± 14 | 79 ± 11 | 75 ± 12 | 75 ± 11 |
| Hypertension, SBP ≥140 or DBP ≥90 mm Hg, or treated (%) | 75 | 50 | 59 | 31 | 73 | 47 | 57 | 32 |
| Hypertension, SBP ≥130 or DBP ≥80 mm Hg, or treated (%) | 83 | 63 | 71 | 45 | 83 | 64 | 71 | 51 |
| Diabetes (%) | 35 | 12 | 15 | 6 | 29 | 14 | 19 | 7 |
| Mean Body Mass Index (BMI), kg/m2 | 32 ± 7 | 31 ± 7 | 28 ± 6 | 26 ± 5 | 30 ± 6 | 29 ± 5 | 28 ± 5 | 27 ± 4 |
| Obesity, BMI ≥ 30 kg/m2 (%) | 58 | 48 | 33 | 20 | 40 | 34 | 29 | 21 |
| Current Smoker (%) | 26 | 21 | 24 | 22 | 34 | 29 | 25 | 24 |
| Hyperlipidemia, total cholesterol ≥ 240 mg/dL or treated (%) | 32 | 22 | 35 | 26 | 23 | 19 | 25 | 21 |
| Mean Total Cholesterol, mg/dL | 216 ± 46 | 201 ± 41 | 223 ± 42 | 211 ± 41 | 206 ± 46 | 196 ± 41 | 210 ± 40 | 205 ± 38 |
The multivariable-adjusted RRs of incident HF for each of the five modifiable risk factors as well as age and education are shown in Table 3. In Black women, diabetes (RR 2.58, 95% CI 2.17, 3.07), hypertension (RR 1.84, 95% CI 1.52, 2.23), current smoking (RR 1.74, 95% CI 1.45, 2.10), and obesity (RR 1.28, 95% CI 1.00, 1.64) were significantly associated with incident HF but hyperlipidemia was not. Similar associations were observed in Black men, although the association with obesity and incident HF did not reach statistical significance (RR 1.23, 95% CI 0.96, 1.58). In both White men and White women, diabetes, hypertension, obesity, and current smoking were also significantly associated with incident HF. Hyperlipidemia was significantly associated with incident HF in White men but not in White women. When the contemporary cutoff of ≥130/80 mm Hg was used to define hypertension, the RR of incident HF associated with hypertension was lower across all groups (Supplemental Table II).
Table 3.
Prevalence and multivariable-adjusted relative risk of incident heart failure across groups by risk factors
| Black Women | White Women | Black Men | White Men | |||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | Prevalence | RR (95% CI) | Prevalence | RR (95% CI) | Prevalence | RR (95% CI) | Prevalence | |
| Age, per year | 1.05 (1.04–1.06) | -- | 1.09 (1.09–1.10) | -- | 1.05 (1.04–1.06) | -- | 1.09 (1.09–1.10) | -- |
| Education (> high school) | 0.55 (0.45–0.66) | 0.52 | 0.65 (0.58–0.74) | 0.49 | 0.57 (0.46–0.70) | 0.51 | 0.66 (0.58–0.74) | 0.55 |
| Hypertension* | 1.84 (1.52–2.23) | 0.53 | 1.72 (1.55–1.91) | 0.35 | 2.08 (1.68–2.54) | 0.51 | 1.81 (1.63–2.01) | 0.35 |
| Diabetes | 2.58 (2.17–3.07) | 0.15 | 2.03 (1.77–2.32) | 0.07 | 2.06 (1.66–2.51) | 0.16 | 2.09 (1.84–2.37) | 0.08 |
| Obesity | 1.28 (1.00–1.64) | 0.49 | 1.87 (1.66–2.11) | 0.22 | 1.23 (0.96–1.58) | 0.35 | 1.76 (1.53–2.02) | 0.22 |
| Current Smoker | 1.74 (1.45–2.10) | 0.22 | 1.80 (1.61–2.02) | 0.22 | 1.67 (1.37–2.04) | 0.30 | 1.57 (1.40–1.76) | 0.24 |
| Hyperlipidemia | 1.04 (0.88–1.24) | 0.23 | 0.95 (0.86–1.05) | 0.27 | 1.05 (0.85–1.30) | 0.19 | 1.12 (1.00–1.25) | 0.22 |
Adjusted for age, education, all modifiable risk factors when not included as the primary exposure and competing risk of death from other causes.
Hypertension: SBP ≥140 or DBP ≥90 mm Hg, or treated
RR: relative risk; CI: confidence interval
We then evaluated the PAF for each of the five modifiable risk factors across all race-sex groups (Figure and Supplemental Table III). In Black women, the highest PAF was observed with hypertension (PAF 25.8%, 95% CI 16.3, 34.2%) and diabetes (PAF 16.4%, 95% CI 12.7, 19.9%). While the estimate for PAF for obesity in Black women was comparable to that from hypertension and diabetes, it had a wide confidence interval (PAF 13.1%, 95% CI 0, 26.8%). Current smoking made a more modest contribution (PAF 6.9%, 95% CI 3.3, 10.3%) while the contribution from hyperlipidemia was negligible in Black women. In Black men, hypertension had the highest PAF of 28.3% (95% CI 18.7, 36.7%). The PAF for obesity in Black men was comparable to that from hypertension but had a wide confidence interval (PAF 16.2%, 95% CI 2.2, 28.1%). Compared with hypertension, the contributions from diabetes (PAF 9.2%, 95% CI 5.1, 13.0%) and current smoking (PAF 6.8%, 95% CI 1.8, 11.5%) were significantly lower in Black men. The PAF for hyperlipidemia was also negligible in Black men. In White women, the highest PAF was observed with obesity (PAF 17.9%, 95% CI 12.8, 22.6%) and hypertension (PAF 17.3%, 95% CI 13.0, 21.4%). In contrast, the contributions from diabetes (PAF 4.4%, 95% CI 2.9, 5.9%) and current smoking (PAF 6.2%, 95% CI 4.1, 8.2%) were significantly lower. There was no contribution to HF burden from hyperlipidemia in White women. The highest PAF in White men was observed with obesity (PAF 21.0%, 95% CI 14.6, 27.0%) and hypertension (PAF 17.3%, 95% CI 13.2, 21.1%). In comparison, the contributions from diabetes (PAF 6.1%, 95% CI 4.4, 7.7%), current smoking (PAF 3.8%, 95% CI 1.6, 6.0%), and hyperlipidemia (PAF 2.3%, 95% CI 0, 4.6%) were significantly lower. The cumulative PAF of HF for all five modifiable risk factors ranged from 39.3% (95% CI 33.9, 44.%) in White women to 51.9% (95% CI 39.3, 61.8%) in Black women. When the more contemporary blood pressure cutoff of ≥ 130/80 for diagnosis of hypertension was used, the PAF for hypertension was higher across all groups with the largest absolute change occurring in Black women (PAF 28.2%, 95% CI 16.3, 38.5%). There were no meaningful changes to the overall results and trends from the primary analysis (Supplemental Figure I and Supplemental Table IV).
Figure. Race- and sex-specific estimates of population attributable fractions of heart failure for modifiable risk factors.
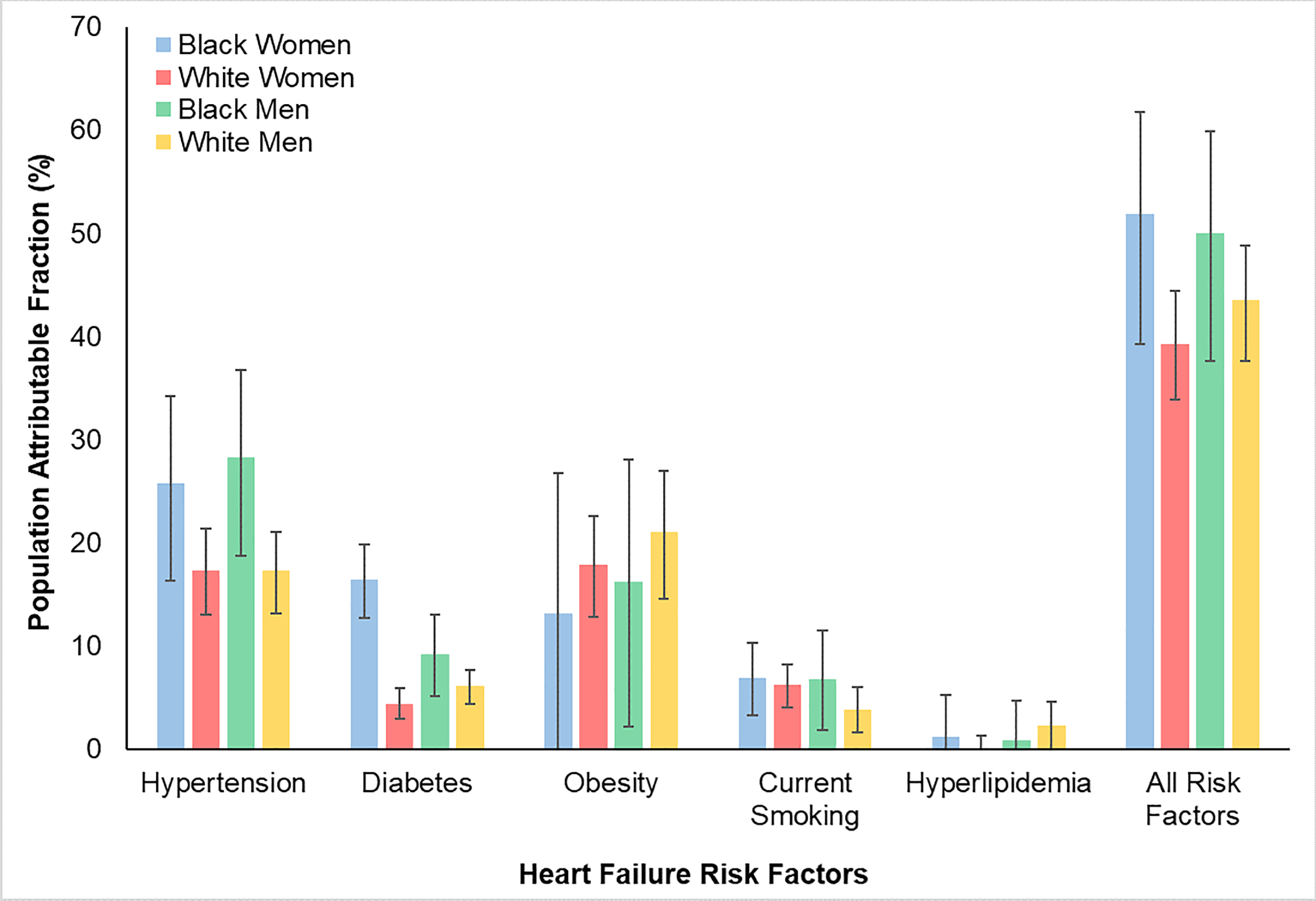
Differences in population attributable fractions for major modifiable risk factors stratified by race and sex. Hypertension, defined as SBP ≥140 or DBP ≥90 or treated, had the highest PAF for HF in Black men and women qualitatively. Obesity had the highest PAF for HF in white men and women. Diabetes disproportionately contributed to HF burden in Black women. Current smoking made a modest contribution to HF burden across all subgroups. Hyperlipidemia did not meaningfully contribute to HF burden in any of the subgroups.
Finally, we estimated PAF for an absolute count of total risk factors (1, 2, 3 or more) in each race-sex group (Supplemental Table V). Among White men, 38.3% had one risk factor, 30.0% had two risk factors, and 9.1% had three of more risk factors. Within White women, 36.0% had one risk factor, 23.0% had two risk factors, and 9.5% had three or more risk factors. In the cohort of Black men, 36.2% had one risk factor, 30.1% had two risk factors, and 16.8% had three or more risk factors. Among Black women, 32.5% had one risk factor, 31.5% had two risk factors, and 20.5% had three or more risk factors. There were no significant race-sex differences in the PAFs for 1 or 2 risk factors. However, the PAFs of HF in adults presenting with 3 or more risk factors was significantly higher in Black men (20.1%, 95% CI 15.4, 24.5%) and Black women (28.8%, 95% CI 23.5, 33.7%) compared with White men (10.3%, 95% CI 8.6, 11.9%) and White women (12.5%, 95% CI 10.6, 14.3%).
DISCUSSION
In the present study, we leveraged individual-level data harmonized from six longitudinal cohorts of well-phenotyped Black and White men and women to estimate adjusted PAFs for HF associated with five major risk factors after accounting for competing risk of non-HF death. Our large sample size, long-term follow-up, and use of the competing risk framework allowed us to provide race and sex-specific PAFs and more accurate estimates. While addressing each of these risk factors is critical in an individual patient, the race and sex-specific findings can help prioritize often limited community-level resources and target public health interventions aimed at mitigating burden of and disparities in risk of HF. Many of the prior studies have not used a competing risk framework, leading to overestimation of PAFs. Since the risk factors assessed here are strongly associated with other life-limiting comorbidities, use of a competing risk of death model is innovative and necessary to provide life course estimates of PAF with long-term follow-up, as utilized here. For example, an individual with hypertension may die from a myocardial infarction or stroke or a smoker may die from lung cancer prior to developing HF. The competing risk model estimates the marginal probability of death from another cause and thus provides a more accurate risk estimate to inform policies.
Comparison of our results to prior findings must account for the use of the competing risk model, the context of individual cohort characteristics, and the changing epidemiology of risk factors. Prior analysis in predominantly White, older cohorts such as Framingham Heart Study and Framingham Offspring Study have provided PAF estimates for hypertension ranging from 39% in men to 59% in women.7. In contrast, we estimated the PAF for hypertension to be 17% in both White men and women. This difference is likely related to the use of a competing risk model that adjusted for non-HF causes of death during follow-up.
As observed in White men and women, hypertension had the highest PAF for HF in Black men and women as well. The PAF point estimates for hypertension in Black adults were higher than White adults, although this did not meet statistical significance. Previous analysis of the Southern Community Cohort Study found the PAF for hypertension to be 41% in Black men and 32% in Black women.15 However, a competing risk framework was not used, and the findings are difficult to generalize as participants were from a low-income, underinsured population in the southeast United States. In an analysis of the Jackson Heart Study, the PAFs for HF from hypertension based on the 2017 ACC/AHA guidelines threshold of ≥130/80 mm Hg were estimated to be 30% in Black men and 15% in Black women.17 Similar to our analysis, a competing risk framework was used. When we used the ≥130/80 cutoff, we found hypertension to have a similar PAF in Black men (29%) but a higher PAF in Black women (28%). Given limited data from longitudinal population-based Black cohorts, our results add to these findings and demonstrate the importance of targeting hypertension in Black men and women.
An important finding in our study was the higher contribution of diabetes to the PAF of HF in Black women compared to prior findings in older cohorts.24 This difference likely represents the increase in the prevalence of diabetes in Black adults over the past few decades.25 In Black women, the adjusted RR of HF was significantly higher with diabetes than other modifiable risk factors. Given the rising prevalence and the greater RR, we observed that the contribution of diabetes to the PAF of HF was markedly higher in Black women than the other race-sex groups. Our results highlight the need to better understand this relationship between diabetes and HF in Black women and determine whether this group may derive greater benefit from use of sodium-glucose cotransporter 2 inhibitors as a first-line agent, as they have been shown to prevent incident HF in favor of other therapies such as glucagon-like peptide-1 receptor agonists, which have a greater benefit in prevention of atherothrombotic events.26, 27
Estimating the PAF of HF from obesity is critical given the rising prevalence and strong association with HF.1, 28 While half of the Black women in our cohort were obese, the association with HF was relatively weak. This led to an imprecise PAF estimate with a wide confidence interval. In contrast, White women had a lower prevalence of obesity but the association with HF was significantly greater in this subgroup compared with Black women. Similarly, there was a significant association between obesity and HF in White men but not in Black men. Thus, obesity along with hypertension were the leading contributors to the population burden of HF in White men and women. The PAF estimates from our more contemporary cohort are higher than those previously reported (21% vs 11% in White men, 18% vs 14% in White women).9 The difference in the RR of HF in White compared with Black adults with obesity may represent differences in pathophysiologies or greater competing risks in Black adults compared with White adults. The effect of obesity on HF in Black adults may also be largely explained through other risk factors such as diabetes and hypertension. Obesity is strongly linked to risk factors such as hypertension and diabetes and may in fact have a synergistic effect; thus, it is difficult to ascertain its true PAF on HF.
We also estimated the PAFs for smoking and hyperlipidemia. Smoking had a modest PAF across all race-sex subgroups. Hyperlipidemia did not contribute to the population burden of HF in Black adults or in White women but had a modest PAF in White men. These findings again highlight the importance of the competing risk framework as smoking and hyperlipidemia are strongly associated with atherosclerotic CVD (and malignancy for smoking); thus, individuals are likely to die from a competing event before developing HF.
Finally, our analysis examining the cumulative PAF of all five risk factors demonstrates that antecedent major risk factors contributed to a substantial proportion of risk and point estimates were higher in Black compared with White adults, although the difference did not meet statistical significance. In particular, as many patients also present with multiple risk factors at baseline, we estimated the PAFs for increasing count of risk factors and observed significantly greater PAFs for ≥3 risk factors in Black men (20.1%) and women (28.8%) than that in White men (10.3%) and women (12.5%) due to higher prevalence. However, a large proportion of risk remains unaccounted for in all race-sex groups. This may be because each risk factor has a continuous, dose-dependent impact on HF risk. While cut points have been adopted to define clinical intervention, this approach underestimates the true effect of subclinical elevations in these risk factors. A second explanation may be related to alternate pathophysiologic pathways that contribute to HF, independent of traditional risk factors, such as risk of HF related to genetic cardiomyopathy, inflammation, and amyloidosis.
This study is not without limitations. Given the observational nature of the study, residual confounding remains a limitation. We adjusted for other risk factors and the competing risk of non-HF death to mitigate this bias. We were unable to estimate PAFs for HF subtypes as data on left ventricular ejection fraction was not available in all patients. However, these major risk factors are associated with both HF with preserved ejection fraction and HF with reduced ejection fraction; thus, assessing the contribution of each risk factor to the comprehensive burden of HF was felt to be appropriate. Secular changes in risk factor prevalence over time may also reduce the generalizability of our pooled cohort as it includes data over several decades, but creation of a large longitudinal pooled cohort was necessary to generate robust long-term risk estimates by race and sex. Use of only baseline prevalence rates, which was done to maximize follow-up time, may also lead to underestimation of PAF as prevalence of risk factors such as obesity, hypertension, and diabetes have increased over time. Furthermore, baseline medications were not included in the adjustment model, which may affect the PAF estimates. Third, we were not able to evaluate differences in treatment between groups as we did not have rigorous and accurate real-time assessment of medication changes that occur in between follow-up visits. Finally, potential cohort-specific effects are possible since the different cohorts used in this pooled analysis spanned the course of decades, thus the prevalence of risk factors and association with HF could be different between cohorts. However, each cohort was separately analyzed without significant differences from the overall results. Importantly, our pooled cohort, which excluded individuals with underlying baseline CVD, allows for better understanding of the effects of different risk factors on the development of incident HF as individuals with CVD are likely to already be on therapy for secondary prevention including HF prevention.
In summary, we present an analysis of a large harmonized cohort of Black and White adults from six population-based studies describing race- and sex-specific contributions of five modifiable risk factors to development of incident HF using PAF estimates within a competing risk framework. Our results highlight that hypertension and obesity are leading contributors to the population burden of HF in all race-sex subgroups with hypertension playing a larger role in Black adults and obesity playing a larger role in White adults. Contributions of all 5 risk factors on HF burden were greater in Black adults compared with White adults. Given the increasing number of deaths from HF, widening Black-White disparities, and an aging population, PAF provides a useful metric to inform public health strategies to equitably reduce the burden of and disparities related to HF.
Supplementary Material
What is New?
We provide race- and sex-specific contributions of the five common modifiable risk factors to the development of incident HF.
We calculated PAF estimates using a competing risk framework, which provides a more accurate assessment of the HF risk independently associated with each risk factor while adjusting for the competing risk of non-HF death.
What are the Clinical Implications?
Hypertension and obesity are the leading contributors to the population burden of HF in all race-sex groups but there are important differences by race-sex group.
The contribution of diabetes to the burden of HF is disproportionately high in Black women. The PAF of HF in adults presenting with multiple risk factors is higher in Black adults compared with White adults.
These findings emphasize the need for clinicians to focus on primordial and primary prevention and inform public health policies to close the gap in HF-related disparities.
Acknowledgments:
The authors thank the investigators of all the cohort studies included in this analysis for their hard work and dedication in collecting the underlying data, and the study participants for their time and commitment.
Sources of Funding: This work was supported by grants from the National Institutes of Health (KL2TR001424, P30AG059988; P30DK092939) and the American Heart Association (AHA#19TPA34890060) to Dr. Sadiya Khan. The Lifetime Risk Pooling Project was supported in its inception by the National Institutes of Health/National Heart, Lung, and Blood Institute (R21 HL085375). Dr. Arjun Sinha is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL069771.
Abbreviations
- HF
heart failure
- PAF
population attributable fraction
- RR
relative risk
- LRPP
Lifetime Risk Pooling Project
- CVD
cardiovascular disease
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- FPG
fasting plasma glucose
- BMI
body-mass index
- CI
confidence interval
- ACC
American College of Cardiology
- AHA
American Heart Association
Footnotes
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in Cardiovascular Mortality Related to Heart Failure in the United States. J Am Coll Cardiol. 2019;73:2354–5. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633–44. [DOI] [PubMed] [Google Scholar]
- 6.Ogunmoroti O, Oni E, Michos ED, Spatz ES, Allen NB, Rana JS, Virani SS, Blankstein R, Aronis KN, Blumenthal RS, et al. Life’s Simple 7 and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6:e005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–62. [PubMed] [Google Scholar]
- 8.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 10.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. Jama. 2009;302:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Deswal A, Folsom AR, Heiss G. The potentially modifiable burden of incident heart failure due to obesity: the atherosclerosis risk in communities study. Am J Epidemiol. 2010;172:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, Rosamond WD, Heiss G. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. Journal of the American College of Cardiology. 2012;60:1640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubicki DM, Xu M, Akwo EA, Dixon D, Munoz D, Blot WJ, Wang TJ, Lipworth L, Gupta DK. Race and Sex Differences in Modifiable Risk Factors and Incident Heart Failure. JACC Heart Fail. 2020;8:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spahillari A, Talegawkar S, Correa A, Carr JJ, Terry JG, Lima J, Freedman JE, Das S, Kociol R, de Feranti S, et al. Ideal Cardiovascular Health, Cardiovascular Remodeling, and Heart Failure in Blacks: The Jackson Heart Study. Circ Heart Fail. 2017;10:e003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark D III, Colantonio LD, Min Y-I, Hall ME, Zhao H, Mentz RJ, Shimbo D, Ogedegbe G, Howard G, Levitan EB. Population-Attributable Risk for Cardiovascular Disease Associated With Hypertension in Black Adults. JAMA Cardiology. 2019;4:1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Qadir H, Fang J, Lee DS, Tu JV, Amir E, Austin PC, Anderson GM. Importance of Considering Competing Risks in Time-to-Event Analyses: Application to Stroke Risk in a Retrospective Cohort Study of Elderly Patients With Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2018;11:e004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, Garside DB, Dyer A, Lloyd-Jones DM. Data Resource Profile: The Cardiovascular Disease Lifetime Risk Pooling Project. International Journal of Epidemiology. 2015;44:1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:2199–269.29146533 [Google Scholar]
- 21.Friedman M Piecewise Exponential Models for Survival Data with Covariates. Ann Statist. 1982;10:101–13. [Google Scholar]
- 22.Laaksonen MA, Härkänen T, Knekt P, Virtala E, Oja H. Estimation of population attributable fraction (PAF) for disease occurrence in a cohort study design. Stat Med. 2010;29:860–74. [DOI] [PubMed] [Google Scholar]
- 23.Laaksonen MA, Virtala E, Knekt P, Oja H, Härkänen T. SAS Macros for Calculation of Population Attributable Fraction in a Cohort Study Design. 2011. 2011;43:25. [Google Scholar]
- 24.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–8. [DOI] [PubMed] [Google Scholar]
- 25.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 26.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9. [DOI] [PubMed] [Google Scholar]
- 27.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841–51. [DOI] [PubMed] [Google Scholar]
- 28.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd-Jones DM. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiology. 2018;3:280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci 1963;107:539–56. [DOI] [PubMed] [Google Scholar]
- 30.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, Greenlan P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 32.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida ALC, Wu CO, Gomes AS, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 34.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 36.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995;5:270–7. [DOI] [PubMed] [Google Scholar]
- 37.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


