Abstract
The hippocampus is a key limbic region involved in higher-order cognitive processes including learning and memory. Although both typical and atypical functional connectivity patterns of the hippocampus have been well-studied in adults, the developmental trajectory of hippocampal connectivity during infancy and how it relates to later working memory performance remains to be elucidated. Here we used resting state fMRI (rsfMRI) during natural sleep to examine the longitudinal development of hippocampal functional connectivity using a large cohort (N=202) of infants at 3 weeks (neonate), 1 year, and 2 years of age. Next, we used multivariate modeling to investigate the relationship between both cross-sectional and longitudinal growth in hippocampal connectivity and 4-year working memory outcome. Results showed robust local functional connectivity of the hippocampus in neonates with nearby limbic and subcortical regions, with dramatic maturation and increasing connectivity with key default mode network (DMN) regions resulting in adult-like topology of the hippocampal functional connectivity by the end of the first year. This pattern was stabilized and further consolidated by 2 years of age. Importantly, cross-sectional and longitudinal measures of hippocampal connectivity in the first year predicted subsequent behavioral measures of working memory at 4 years of age. Taken together, our findings provide insight into the development of hippocampal functional circuits underlying working memory during this early critical period.
Keywords: Connectivity, Development, Infant, Hippocampus, Working Memory
1. Introduction
The hippocampus is a critical limbic region that facilitates learning and memory throughout development (Tamnes et al., 2014; Alberini and Travaglia, 2017). Along with other limbic regions, the hippocampus develops early on (Insausti et al., 2010), with the number and density of synapses reaching adult-like levels within the first 6 postnatal months (Seress and Ábrahám, 2008). Infancy is also a period of dramatic structural growth; development of myelination along major axonal bundles connecting distant parts of the brain likely enable the emergence and establishment of extensive hippocampal functional circuits during the first years of life (Benes et al., 1994; Gao et al., 2009a; Huang et al., 2015; Van Den Heuvel et al., 2015). However, to the best of our knowledge, no study has characterized the longitudinal growth trajectory of hippocampal functional connectivity during this early critical period.
Early infancy is a period defined by dramatic growth in functional architecture (Gao et al., 2015a; Gilmore et al., 2018). Resting-state networks, detectable perinatally (Fransson et al., 2009; Gao et al., 2009b; Fransson et al., 2011; Thomason et al., 2013), are plastic, modifiable, and demonstrate nonlinear, patterned, and network-specific growth trajectories during the first two years of life (Gao et al., 2011, 2017). In neurotypical infants, primary sensory networks (e.g., sensorimotor, auditory) are the first to resemble adult-like patterns whereas higher-order networks such as the default mode network (Raichle et al., 2001), dorsal attention network (Fox et al., 2006), and executive control network (Seeley et al., 2007) develop more gradually over the first years of life (Smyser et al., 2011; Gao et al., 2015a). Furthermore, a pattern featuring long-range synchronization and local differentiation has been consistently observed during typical development (Fair et al., 2009; Gao et al., 2009b; Supekar et al., 2009; Uddin et al., 2010). Importantly, the early development of functional networks has been shown to index later behavioral outcomes (Alcauter et al., 2014; Salzwedel et al., 2019a; Chen et al., 2020; Gao et al., 2020), underscoring the behavioral significance of early functional brain development. Collectively, a growing body of work suggests that different resting-state networks show unique critical periods, developmental trajectories, and behavioral implications. Functional connectivity between the hippocampus and a widespread network of cortical and subcortical regions is critical for processes underlying learning and memory and can be detected shortly after birth (Gao et al., 2011; Alcauter et al., 2014; Bajic et al., 2016). Although the developmental profile of hippocampal functional connectivity has been characterized in children and adolescents (Zhong et al., 2014; Blankenship et al., 2017), the development of hippocampal functional connectivity during the first years of life remains poorly understood.
In contrast to primary systems such as sensorimotor and visual functions, complex external environmental input is crucial for shaping hippocampus-supported learning and memory behaviors for better adaptation to the individualized environment (Maguire et al., 2000; Brunson et al., 2003; Gogtay et al., 2006; Lavenex and Banta Lavenex, 2013; Gómez and Edgin, 2016). Importantly, early development of learning and memory functions during infancy likely lays the foundation for later prolonged development of different cognitive functions (Gogtay et al., 2006). Among these, working memory refers to the ability to retain and manipulate information over a short period of time, which is critical for learning and supports many other cognitive capabilities (Baddeley, 2003; D’Esposito and Postle, 2015). In adults, working memory functions have traditionally been associated with frontoparietal areas (Cabeza and Nyberg, 2000), but a growing body of work indicates that the hippocampus may also play a key role in working memory (Olson et al., 2006; Jeneson and Squire, 2011).
The structural and functional architecture of the hippocampus during development has been demonstrated to be related to later working memory outcome. For example, several studies have shown that lower preterm infant hippocampal volumes are associated with later working memory/cognitive deficits (Beauchamp et al., 2008; Nosarti and Froudist-Walsh, 2016; Strahle et al., 2019), indicating the importance of early hippocampal development for later working memory performance. The capacity for working memory increases throughout childhood (Gonthier et al., 2019) and early measures of working memory skills are predictive of later outcome. Indeed, working memory skills assessed as early as 2–4 years of age predict school readiness at 6 years of age (Fitzpatrick and Pagani, 2012). Furthermore, preschoolers (2- to 5-years-old) with attention-deficit/hyperactivity disorder (ADHD) already show deficits in working memory compared with typically developing controls (Mahone and Hoffman, 2007), highlighting the importance of investigating the relationship between early resting state connectivity and later working memory performance to provide insight into potential mechanisms underlying working memory well before the onset of overt behavioral symptoms. Prior studies in children have specifically linked the development of hippocampal functional circuits to working memory performance; longitudinal growth of hippocampal-neocortical functional circuits is related to individual gains in working memory-based problem solving in 7- to 9-year-old children (Qin et al., 2014). However, to our knowledge, there have been no studies on how hippocampal functional connectivity in infancy may relate to later working memory outcome. As such, the relationship between early synchronization of hippocampal functional circuits and later working memory outcome remains to be elucidated.
In this study, we used resting-state functional magnetic resonance imaging (rsfMRI) to delineate the development of hippocampal functional connectivity patterns during the first two years of life using a large (N=202) longitudinal sample of infants at 3 weeks (neonate), 1 year, and 2 years of age. Next, we characterized the behavioral implications of early hippocampal functional connectivity using working memory scores measured at 4 years of age in a subsample of our infants. We expected to find local connectivity in neonates followed by dramatic synchronization during the first year of life (Gao et al., 2009b), especially with default mode network regions as reported in adults (Andrews-Hanna et al., 2010). We also hypothesized that functional connectivity growth during the first year of life would significantly correlate with 4-year working memory scores. Given previous reports of environmental influences such as socioeconomic status on the structure and function of the hippocampus (Farah, 2017; Hanson et al., 2011; Yu et al., 2018), we further explored whether maternal education might be related to early hippocampal connectivity in a supplemental analysis. To our knowledge, this is the first study to examine the longitudinal development of hippocampal connectivity during the first two years of life and how it may relate to later behavioral outcome.
2. Materials and Methods
2.1. Participants
Typically developing infant participants were part of the University of North Carolina Early Brain Development Study, characterizing early childhood brain and behavior development (Gao et al., 2017; Gilmore et al., 2018). We retrospectively identified 202 subjects with at least one successful rsfMRI scan during the first two years of life. Participant characteristics are listed in Table 1. Exclusion criteria included gestational age at birth <37 weeks, maternal mental disorder status, and any neonatal illness requiring more than a 24-hour stay at a neonatal intensive care unit. These criteria were established before data analysis. Study protocols were approved by the University of North Carolina at Chapel Hill and Cedars-Sinai Institutional Review Board.
Table 1.
Subject Demographics
| Subjects (N=202) | |
|---|---|
| Sex (Female/Male) | 106/96 |
| Race (White/Non-white) | 137/65 |
| Mean (SD) | |
| Birth Weight (pounds) | 7.05 (1.10) |
| Gestational Age at Birth (days) | 273.86 (9.41) |
| Gestational Age at Scan (days) | |
| Neonates (N=143) | 297.28 (11.47) |
| 1-Year-Olds (N=96) | 657.98 (20.77) |
| 2-Year-Olds (N=68) | 1021.44 (26.17) |
| Maternal Education (years) | 15.77 (3.24) |
| BRIEF-P Working Memory Score (4 years) | 52.23 (10.13)a |
| S-B Working Memory Score (4 years) | 113.07 (10.20)b |
N=111,
N=97
2.2. Imaging Acquisition
Longitudinal rsfMRI data were acquired from the cohort of typically developing infants (N=202) at 3 weeks (neonates), 1 year, and 2 years of age. The distribution of available datasets for functional connectivity analyses is shown in Figure 1. As expected, there was attrition across longitudinal time points, but there was no differential loss due to follow-up by sex (p = .62) or race (p = .11). Subjects were fed, swaddled, and fitted with ear protection prior to imaging. All subjects were in a natural sleep state during the imaging session. All MRI data were collected on a Siemens 3T Allegra (circular polarization head coil; neonates: N=121, 1-year-olds: N=88, 2-year-olds: N=61) or Tim Trio scanner (32-channel head coil; neonates: N=22, 1-year-olds: N=8, 2-year-olds: N=7). Scanner was included as a covariate of no-interest in all subsequent analyses. Functional images were acquired using a T2*-weighted echo planar imaging (EPI) sequence: TR=2000ms, TE=32ms, 33 slices, voxel size=4mm2, 150 volumes. Structural images were acquired using a three-dimensional magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence: TR=1820ms, TE=4.38ms, inversion time=1100ms, voxel size=1mm2. The acquisition parameters were identical for both scanners.
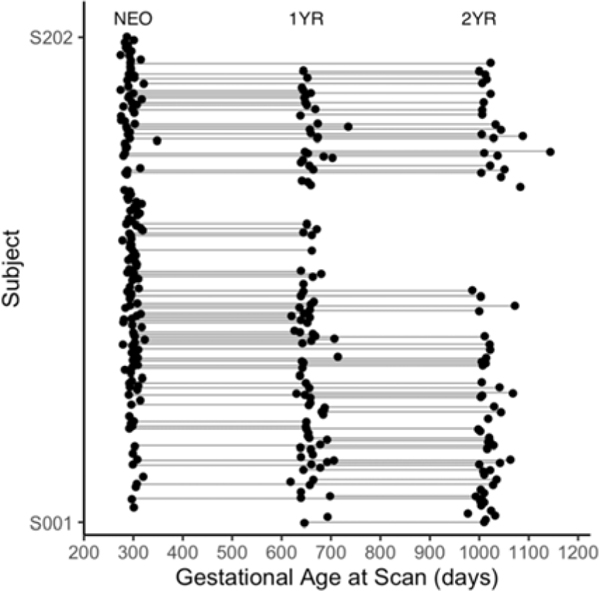
Figure 1. Data Distribution.
The distribution of gestational age at scan for all included infant subjects (N=202 totaling 307 datasets) whose image quality passed the quality control procedures is shown. Each dot represents a successful rsfMRI scan, and dots along each line represent all the available longitudinal scans for a given subject. Neonates (NEO): N=143; 1-year-olds (1YR): N=96; 2-year-olds (2YR): N=68; NEO and 1YR: N=53; 1YR and 2YR: N=44; NEO and 2YR: N=33; NEO, 1YR, and 2YR: N=25.
2.3. Working Memory Measures at 4 Years of Age
Developmental assessments examining the development of working memory were conducted at 4 years of age (Table 1), with a subset of infants providing measurements at this time point (Table S2). The Behavior Rating Inventory of Executive Function – Preschool Version (BRIEF-P; Gioia et al., 2003) was completed by parents to assess their child’s executive function behaviors in five main domains including inhibition, ability to shift, emotional control, working memory, and ability to plan/organize. Here we focused on the domain standard working memory subscale, which assesses a more generalized ability of working memory across multiple situations (i.e., assesses the application of skills in everyday situations). Parents are asked to rate how often each of the behaviors listed has been a problem during the past six months (rating options include: “Never,” “Sometimes,” “Often”). Items on the working memory subscale include behaviors such as: ”When given two things to do, remembers only the first or last;” “Has trouble concentrating on games, puzzles, or play activities;” “Unable to finish describing an event, person, or story.” Higher BRIEF-P working memory scores indicate worse working memory capability and a score of 65 or higher is indicative of more clinically significant difficulties with the index; 26 subjects had working memory scores falling in this range (see Figure 2A for full distribution of scores).
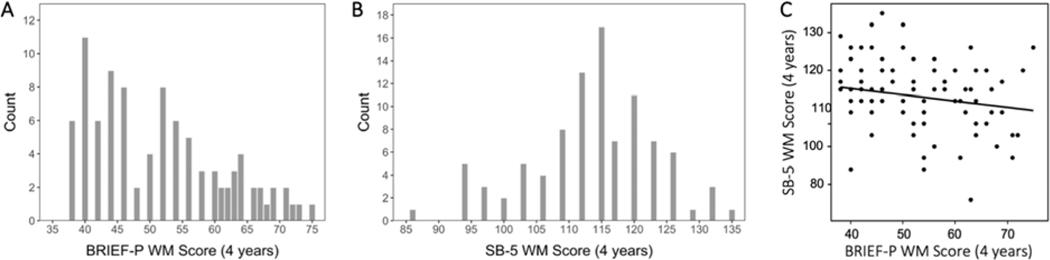
Figure 2. Distribution and correlation of 4-year working memory (WM) scores.
Higher BRIEF-P WM scores indicate worse working memory capability; a score of 65 or higher is indicative of more clinically significant difficulties with the index (A). Higher SB-5 scores indicate better working memory capability (B). In our sample of infants, the two measures were negatively correlated (r = −.18, p = .02).
The Stanford-Binet Intelligence Scales, Fifth Edition (SB-5; Roid, 2003) was also administered at 4 years of age. This is a task-based assessment administered individually in a structured setting to assess intelligence across the lifespan, specifically focusing on five domains including fluid reasoning, knowledge, quantitative reasoning, visual-spatial processing, and working memory. The working memory subscale provides a measure of performance (i.e., assesses underlying skills) on two distinct working memory tasks, one verbal and one nonverbal. Tasks evaluated during the verbal portion of the assessment include memory for sentences (e.g., “Drink milk;” “The circus came to town”) and recall of last word in a sequence (e.g., “fast, bark;” “hot, green, soft, wet”). Tasks evaluated during the nonverbal portion of the assessment include delayed response (e.g., “Car under middle cup”) and a block span task where individuals are asked to copy a block tapping pattern. In the current study, the working memory standard score was used whereby a higher score indicates better working memory capability (see Figure 2B for full distribution of scores).
It is important to note that the two measures utilized in this study employ different modes of assessment: the BRIEF-P is parent-report whereas the SB-5 is task-based. Parent-report (i.e., rating scales) and task-based assessments represent different aspects of cognitive and behavioral function; both provide important and nonredundant information about an individuals’ efficiency and success in achieving goals (McAuley et al., 2010; Toplak et al., 2013; Ten Eycke and Dewey, 2016). A growing body of work has begun to examine the association between these two types of measures; in particular, the association between ratings on the BRIEF and task-based measures of executive function (including working memory) are weak (r = .18; Toplak et al., 2013). Indeed, we observed this in our sample as well, whereby the correlation between the BRIEF-P and SB-5 (Figure 2C) is statistically significant albeit weak (r = −.18, p = .02). Given that higher BRIEF-P scores indicate worse working memory capability whereas higher SB-5 scores indicate better working memory capability, a negative correlation is expected.
2.4. fMRI Data Preprocessing
Functional imaging data were preprocessed using FMRIB’s Software Library (FSL; Smith et al., 2004) and Analysis of Functional Neuroimages (AFNI; Cox J.S., 1996). Preprocessing included discarding the first 10 volumes, slice-timing correction, rigid-body motion correction, bandpass filtering (0.01–0.08 Hz), and nuisance signal regression. To further reduce noise and other confounds, 24 motion-related parameters (six motion correction parameters, their derivatives, and their quadratic terms) were included as nuisance regressors in addition to the following measures: mean white matter time series, mean cerebrospinal fluid time series, and mean global time series, as well as the temporal derivatives and quadratic terms of these regressors (Power et al., 2014). All nuisance signals were band-pass filtered (0.01–0.08 Hz) before regression to match the frequency of the blood oxygenation level dependent (BOLD) signal. Data scrubbing was performed as an added motion correction step in addition to the standard rigid-body motion correction procedures; volumes with global signal changes > 0.5% and/or frame-wise displacements (FD) > 0.3 mm were removed (“scrubbed”) from the data; one volume immediately preceding and two volumes following the scrubbed volume were also removed (Power et al., 2012). Subjects with fewer than 90 volumes remaining after scrubbing were excluded from the study. The number of volumes removed and residual framewise displacement (rFD) were compared cross-sectionally to ensure that there were no differences in motion; rFD was included as a covariate of no-interest in all subsequent analyses. The data were spatially smoothed with a Gaussian kernel of 6mm full width at half maximum (FWHM) and truncated to 90 volumes. The University of North Carolina (UNC) infant brain templates were used for co-registration (Shi et al., 2011). Specifically, spatial normalization to an infant brain template was achieved using a two-step approach (as recommended for pediatric datasets to improve accuracy and correct for changes in brain size; Cusack et al., 2018; Pfeifer et al., 2018): 1) subject-specific nonlinear plus age-specific nonlinear warping (both using Advanced Normalization Tools; ANTs; Avants et al., 2008) to the age-specific template, and 2) between-age-group nonlinear transformations (ANTs) to the 2-year template (Shi et al., 2011), which served as the final target for spatial normalization. Nonlinear warping using ANTs has been shown to be particularly effective for registering pediatric data (Sanchez et al., 2012) and each subject was visually checked to ensure good registration between time points. Functional connectivity analyses were conducted in age-specific space (i.e., in the standard neonate template space for neonates, the standard 1-year template space for 1-year-olds, and the standard 2-year template space for 2-year-olds) and the resulting measures were subsequently aligned to the 2-year template space (Shi et al., 2011) for cross-sectional and longitudinal functional connectivity analyses.
2.5. fMRI Data Analysis
2.5.1. Effect of Age
To examine whole-brain functional connectivity of the hippocampus, average residual time series were extracted from anatomical regions-of-interest (ROIs) for left and right hippocampus, as derived from an infant brain atlas (Shi et al., 2011). For each ROI, the time series extracted from processed residuals in standard space was correlated with that of every other voxel in the brain, and the resulting correlation measures were normalized using Fisher’s Z transformation and analyzed at the regional (i.e., voxelwise) level. Specifically, t tests and linear mixed-effect (LME) models in MATLAB (R2019a) were used to quantify cross-sectional (neonates, 1-year-olds, and 2-year-olds) and longitudinal (neonates to 1-year-olds, 1-year-olds to 2-year-olds, and neonates to 2-year-olds) effects, respectively. Given prior work showing log-linear growth trends of functional connectivity during the first 2 years of life (Alcauter et al., 2015; Gao et al., 2015b; Pendl et al., 2017; Salzwedel et al., 2019b), log transformation of age was used in the LME modeling. The LME models included random intercept and slope terms, with the effect estimate associated with gestational age at scan being the principle variable of interest. Other participant characteristics were included as covariates of no interest in the LME models including scanner, sex, gestational age at birth, birthweight, and rFD. Significance was defined using a clustering approach (AFNI’s 3dClustSim). We used conservative settings (Eklund et al., 2016; Cox et al., 2017a, 2017b) to achieve the desired correction rate of α = .05. Specifically, we imposed a voxelwise cutoff of p < .001 and generated smoothness estimates from the preprocessed data using the mixed-model autocorrelation function. Cluster sizes (bisided, nearest neighbor = 1) were established for each subsample (Table S1).
2.5.2. Brain-Behavior Analyses
Voxelwise linear regression was used to investigate whether infant hippocampal functional connectivity would predict later working memory scores at 4 years of age. Both crosssectional (neonate, 1-year-olds, and 2-year-olds) and longitudinal (neonates to 1-year-olds, 1-year-olds to 2-year-olds, and neonates to 2-year-olds) effects were examined. In the longitudinal prediction analyses, the difference between the connectivity from each time point was calculated as an individual growth measurement. In the linear regression model, the cross-sectional and longitudinal measures were used to predict 4-year BRIEF-P WM and SB-5 WM scores. For both cross-sectional and longitudinal analyses, covariates of no interested included scanner, sex, gestational age at birth, birthweight, and rFD. Significance thresholding was defined by a voxelwise cutoff of p < .001 and cluster-corrected at α = .05 using cluster sizes (bisided, nearest neighbor = 1) established for each subsample (Table S1). The number of data points for each model (i.e., number of infants who contributed both imaging data at each time point and behavioral data at 4 years of age) are listed in Table S2.
2.5.3. Relationship between Functional Connectivity and Maternal Education
Supplementary analyses investigating the cross-sectional and longitudinal association between infant hippocampal functional connectivity and maternal education were conducted using voxelwise linear regression and LME models. Significance thresholding was defined by a voxelwise cutoff of p < .001 and cluster-corrected at α = .05. However, as no results survived correction at this stringent threshold, results from this exploratory analysis are reported at a more lenient threshold (voxelwise cutoff of p < .05 and cluster-corrected at α = .05) in the Supplementary Information.
3. Results
3.1. Hippocampal Functional Connectivity During the First 2 Years of Life
Connectivity maps generated from left and right hippocampus seeds in neonates, 1-year-olds, and 2-year-olds are shown in Figure 3A with corresponding longitudinal effects (log(Age); neonates to 1-year-olds, 1-year-olds to 2-year-olds, and neonates to 2-year-olds) presented in Figure 3B. Across all three time points, similar functional connectivity patterns were observed between left (Table S4) and right hippocampus (Table S5). In neonates, whole-brain connectivity maps demonstrated robust local connectivity with adjacent limbic regions (parahippocampal gyrus, amygdala, insula), temporal areas (temporal pole, middle temporal gyrus), and subcortical regions (thalamus, caudate, putamen) (Figure 3A; see also Supplementary Information Figure S1A for axial slices).
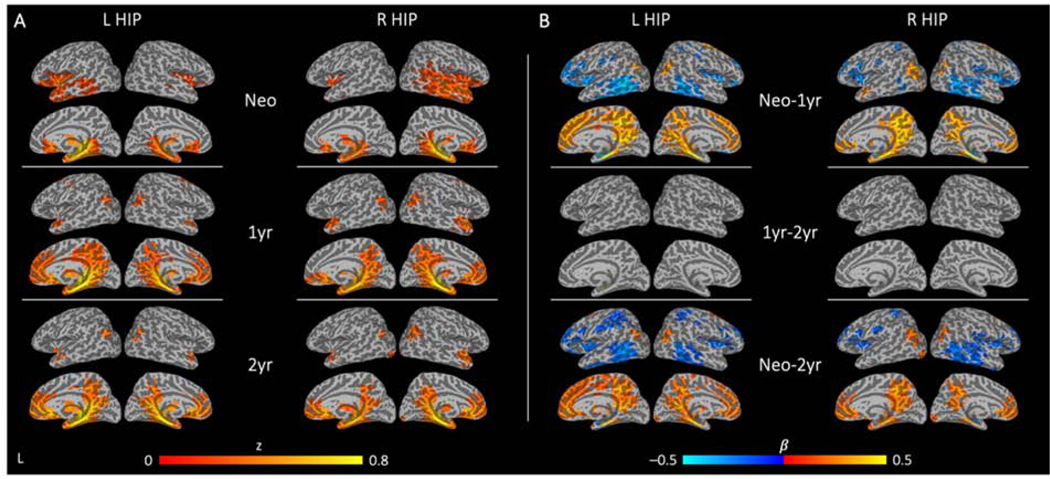
Figure 3. Regional functional connectivity and corresponding longitudinal changes for the left and right hippocampus.
Cross-sectional patterns of significant hippocampal functional connectivity are detected at all three time points (A). Patterns of significant changes in hippocampal connectivity over time show dramatic growth from neonate to 1 year, with minimal differences from 1 to 2 years of age (B; warm colors indicate increases in the strength of connectivity and cool colors indicate decreases in the strength of connectivity between the twotime points). See Supplemental Tables S4–S7 for full breakdown of significant clusters (abbreviations denoted in Table S3). (L HIP: left hippocampus; R HIP: right hippocampus; Neo: neonate; 1yr: 1-year-old; 2yr: 2-year-old; Neo-1yr: neonate to 1-year-old; 1yr-2yr: 1-year-old to 2-years-old; Neo-2yr: neonate to 2-years-old)
During the first year (i.e., neonates to 1-year-olds), there was significant growth of hippocampal functional connectivity with most default mode network (DMN) regions including the medial prefrontal cortex, middle/posterior cingulate cortex, and inferior parietal lobule areas, resulting in adult-like functional connectivity of the hippocampus with a constellation of DMN core areas (Figure 3A). Connections between the hippocampus and subcortical areas persisted while connectivity with orbitofrontal cortex and inferior temporal regions decreased during this period (Figure 3B, top row; Tables S6–7). Qualitatively, 1- and 2-year hippocampal connectivity patterns were similar, although there appeared to be some retraction of hippocampal connectivity with dorsal DMN regions (e.g., the dorsal medial prefrontal/parietal cortex), a trend that is consistent with the connectivity pattern observed in adults whereby the hippocampus is more strongly associated with the ventral part of the DMN (Greicius et al., 2004; Vincent et al., 2006; Andrews-Hanna et al., 2010). However, this trend was not statistically significant and the longitudinal LME model only showed minimal increase of connectivity between the left hippocampus and a small parahippocampal gyrus cluster from 1 to 2 years of age (Figure 3B, middle row; Figure S1B; Table S6). Consistent with these observations, growth from neonates to 2-year-olds primarily reflected first year growth of hippocampal connectivity (Figure 3B, bottom row; Figure S3B; Tables S6–7).
3.2. Hippocampal Functional Connectivity During Infancy is Associated with 4-Year Behavioral Outcome
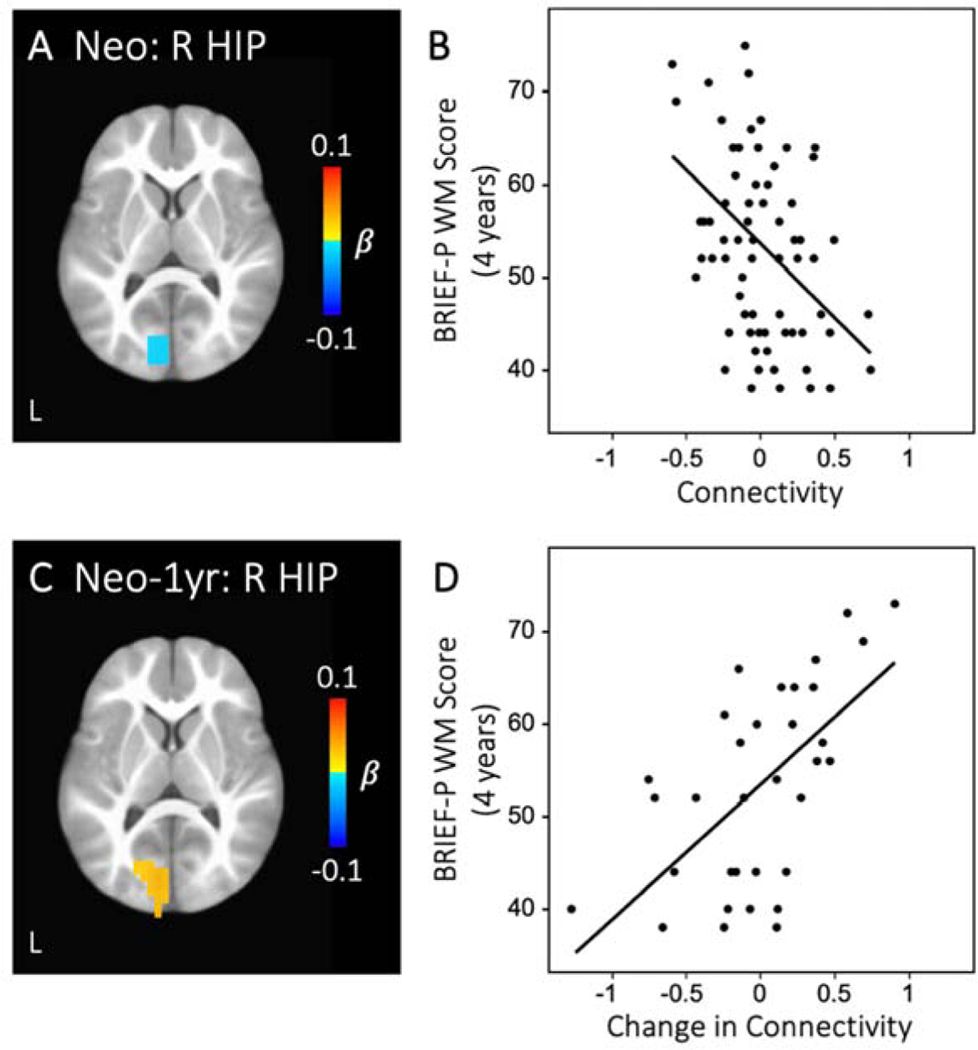
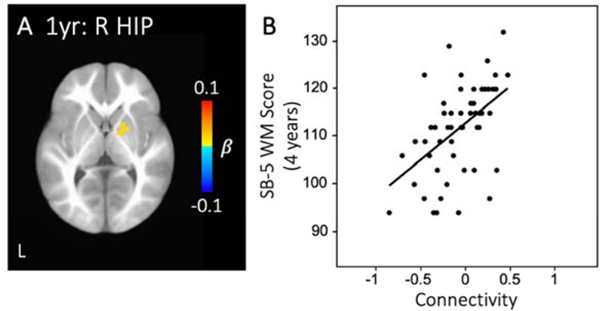
In the brain-behavioral analysis, hippocampal functional connectivity with a visual cortex region revealed that both cross-sectional values and longitudinal growth were significantly related to 4-year BRIEF-P working memory scores. Specifically, greater connectivity between right hippocampus and a left visual cortex cluster at the neonate time point was associated with better (i.e., lower) BRIEF-P working memory scores at 4 years (Figure 4A–B, Table S9). Interestingly, infants who showed decreasing connectivity between right hippocampus and this same visual cortex region during the first year (i.e., neonate to 1-year-old) had better BRIEF-P working memory scores at 4 years of age (Figure 4C–D, Table S9). In addition to this visual cluster, infants who displayed greater intrahemispheric connectivity between right hippocampus and putamen at 1 year of age had better (i.e., higher) SB-5 working memory scores at 4 years (Figure 5, Table S9).
Figure 4. Hippocampal connectivity predicts 4-year BRIEF-P working memory score.
Greater neonatal connectivity between right hippocampus and left visual cortex was related to better (i.e., lower) BRIEF-P WM scores at 4 years of age (A-B). Longitudinal growth in connectivity between these regions was also associated with BRIEF-P WM outcome (C-D). See Supplemental Table S9 for full breakdown of significant clusters (abbreviations denoted in Table S3). (R HIP: right hippocampus; Neo: neonate; Neo-1yr: neonate to 1-year-old; WM: working memory)
Figure 5. Hippocampal connectivity predicts 4-year SB-5 working memory score.
Greater connectivity between right hippocampus and right putamen at 1 year was related to better (i.e., higher) SB-5 WM scores at 4 years of age (A-B). See Supplemental Table S9 for full breakdown of significant clusters (abbreviations denoted in Table S3). (R HIP: right hippocampus; 1yr: 1-year-old; WM: working memory)
4. Discussion
In this study, we examined the development of hippocampal functional connectivity during the first two years of life. Early on, neonates exhibited robust positive connectivity with nearby limbic and subcortical areas whereas long-range connectivity with key DMN regions emerged during the first year and further consolidated through the second year. Both cross-sectional and longitudinal growth of hippocampal connectivity during the first year indexed 4-year working memory scores. These findings support the developmental significance of hippocampal functional connectivity during the first year of life. Overall, our findings provide a better understanding of the developing networks underlying learning and memory in typical development and lay the groundwork for identifying brain-based biomarkers for atypical development of working memory processes seen in many neurodevelopmental disorders.
The hippocampus develops early in life (Insausti et al., 2010), with the number and density of synapses in most regions reaching levels similar to those in adults within the first 6 postnatal months (Seress and Ábrahám, 2008). Thus, it is not surprising to observe significant functional connectivity of the hippocampus with adjacent limbic and subcortical areas in neonates (Blum et al., 2015), including the parahippocampal gyrus, amygdala, caudate, putamen, and thalamus. These are key regions associated with emotional appraisal and salience detection (Seeley et al., 2007; Lindquist et al., 2015; Salzwedel et al., 2019b). Indeed, the hippocampus plays a major role in the detection of salient environmental events (Sridharan et al., 2008), which supports selective attention and is crucial for the development of working memory (Plebanek and Sloutsky, 2019). Our findings indicate that synchronization of these regions is present shortly after birth and may help support early development of these processes.
Consistent with prior work demonstrating that long-range connections, which are less developed during the prenatal period, primarily emerge and strengthen during the first two years of life (Gao et al., 2015b, 2017; Emerson et al., 2016; Cao et al., 2017), we observed dramatic synchronization of hippocampal functional connectivity with key DMN regions resulting in adult-like topology by the end of the first year, which stabilized and further consolidated by 2 years of age. This is in line with prior research demonstrating that long-range connectivity increases while short-range connectivity decreases during typical development (Fair et al., 2009; Gao et al., 2009b; Supekar et al., 2009; Uddin et al., 2010). Indeed, short-range connectivity between the hippocampus and brain regions that were anatomically nearby (e.g., inferior temporal regions) decreased over time whereas long-range connectivity between the hippocampus and regions that were anatomically more distant (e.g., DMN regions such as anterior and posterior cingulate gyri) increased over time. The observed dramatic synchronization with DMN regions is also highly consistent with the adult hippocampal connectivity pattern (Buckner et al., 2008; Gao et al., 2009b) and previous reports of DMN growth during the first year (Gao et al., 2015b). Interestingly, the qualitative trend of decreasing connectivity between the hippocampus and dorsal DMN regions is also in line with connectivity patterns observed in adults whereby the hippocampus is more associated with the ventral part of the DMN (Andrews-Hanna et al., 2010; Wylie et al., 2014; Staffaroni et al., 2018). However, this trend was not statistically significant, suggesting that the functional specialization process between the hippocampus and dorsal DMN may require additional experienced-based pruning later in life.
Development of hippocampal functional connectivity was significantly related to 4-year working memory outcomes. Specifically, neonates showing greater connectivity between right hippocampus and a left visual cortex cluster were reported by parents (i.e., BRIEF-P) to have better working memory at 4 years of age. Basic sensory networks including the primary visual network develop early on in infancy; in fact, fetal fMRI studies have demonstrated that intrinsic functional connectivity of the occipital lobe is already detectable prenatally (Thomason et al., 2013; Jakab et al., 2014). After birth, visual areas dramatically enhance their connectivity during the first 3 months postnatally and are followed by protracted development of higher-order networks including attention and DMN regions across the first 6 months (Gao et al., 2015a, 2015b). In addition to being one of the earlier regions to mature during development, the visual cortex also provides crucial sensory input to the hippocampus (Tsanov and Manahan-Vaughan, 2009). Our results indicate that this might be especially critical for working memory development during early infancy.
In line with prior work in older children showing that improvement of working memory performance is associated with attenuated connections between occipital regions and DMN (Zhong et al., 2014), we observed a shift in the brain-behavior relationship whereby decreasing connectivity between right hippocampus and a similar left visual cortex cluster during the first year was related to better parent-reported working memory scores at 4 years of age. This may reflect underlying pruning processes that emerge towards the end of the first year and mediate experience-dependent development (Huttenlocher, 1984; Toga et al., 2006). Indeed, the visual cortex experiences a period of rapid synapse production that ends at around 8 months of age, followed by a longer period of synapse elimination (i.e., pruning) which extends past 3 years of age (Huttenlocher et al., 1982). Thus, while positive connectivity between the hippocampus and visual cortex may be critical early on in infancy, synaptic pruning and accompanying regression in functional connectivity that occur towards the end of the first year may also be crucial for the development of neural networks underlying working memory. Consistent with a previous study showing that changes in hippocampal connectivity were related to individual gains in memory-based problem solving in neurotypical children (Qin et al., 2014), our findings further highlight that developmental changes in connectivity may provide more dynamic insight into the underlying neural processes for working memory. Overall, the finding that cross-sectional/longitudinal functional connectivity between the hippocampus and a visual cluster indexes 4-year working memory performance measure by parent report (i.e., BRIEF-P) indicates that early visual input to/interactions with the hippocampus might play a critical role in the application of working memory skills in everyday situations, as measured by BRIEF-P.
In addition to the relationship between the hippocampus and visual cortex, connectivity between the right hippocampus and putamen at 1 year of age was related to later working memory skills assessed by a task-based measure (i.e., SB-5). The putamen is involved the integration of behavior and plays a major role in recruiting frontal motor areas for higher cognitive processes such as working memory (Yin and Knowlton, 2006; Chang et al., 2007). Therefore, this finding likely reflects the importance of early hippocampus-putamen functional interactions that support the cognitive basis of task-based verbal/non-verbal working memory development, as measured by SB-5. Previous studies have demonstrated that the putamen is activated during performance of working memory tasks in children (Ciesielski et al., 2006) and adults (Pessoa et al., 2002; Koelsch et al., 2009). Furthermore, children with ADHD show disrupted connectivity with the putamen as well as with the DMN (Cao et al., 2009) in addition to reduced hippocampal volumes (Boedhoe et al., 2020). Here, we found that greater connectivity between right hippocampus and right putamen at 1 year was related to better task-based working memory performance at 4 years of age. This suggests that greater hippocampal connectivity with striatal areas supports the development of networks underlying working memory and may provide a possible mechanism for impaired working memory capacity seen in neurodevelopmental disorders such as ADHD (Darki and Klingberg, 2015). In this study, functional connectivity growth patterns for left and right hippocampus were largely similar, but the detected brain-behavior relationships with 4-year working memory outcomes were lateralized to the right hippocampus. This is consistent with prior findings demonstrating that the right hippocampus is strongly implicated in spatial processing, which is critical for early working memory development (Burgess et al., 2002; Kühn and Gallinat, 2014).
It is worth noting that the two assessments utilized in the present study measure different aspects of behaviors associated with working memory. The primary difference rests in the mode of assessment: the BRIEF-P is parent-report whereas the SB-5 is task-based. The BRIEF-P assesses the application of working memory skills in everyday situations as evaluated by the parents (Gioia et al., 2003), and the SB-5 provides a measure of the underlying cognitive skills associated with performance on two distinct working memory tasks (verbal and nonverbal; Roid, 2003). Although the BRIEF-P is more commonly utilized as a measure for assessing executive function (including working memory), it can be influenced by the parent’s interpretation of their child’s behavior and their perspective on how they are interpreting the questions (e.g., whether they are comparing their child’s ability to what they have observed in other children), as well as parent-child relationship quality (Soto et al., 2020). By contrast, the SB-5, which is more broadly used to assess intelligence and general cognition, is structured to specifically tap into the cognitive basis of working memory (Roid, 2003). Since parent-report and task-based assessments represent different aspects of cognitive and behavioral function (i.e., assess different aspects of the same underlying construct), both provide important and nonredundant information about an individuals’ ability and success in achieving goals (McAuley et al., 2010; Toplak et al., 2013; Ten Eycke and Dewey, 2016). As such, our findings of two distinct clusters associated with the two measures may indicate that two sets of hippocampal connections are related to two different aspects of working memory development, as measured by BRIEF-P and SB-5, respectively. Furthermore, impairment on ratings of executive function (e.g., parent-report on BRIEF-P) does not translate to impairment on task-based measures of executive function (e.g., SB-5; Biederman et al., 2008). Although 26 children in the present study were rated by their parents as having BRIEF-P scores indicative of elevated clinically significant difficulties on the working memory subscale, this does not necessarily translate to or affect the interpretation of their task-based performance on the SB-5.
Several limitations warrant further discussion. All infants in this study were scanned during natural sleep. A recent study found that different sleep stages may be a potential confound in rsfMRI (Mitra et al., 2017), but overt monitoring of sleep stage using electrophysiological methods has proven to be difficult to accomplish in this population (Raschle et al., 2012). In addition, recent work using rapid acquisition fMRI in neonates has demonstrated that higher frequency signals (> 0.2 Hz) are of interest and may carry meaningful signal that can provide a more nuanced portrait of developmental changes in functional brain connectivity (Smith-Collins et al., 2015). In order to remain consistent and ensure comparability with prior studies using the same dataset with identical imaging parameters (i.e., with a relatively long TR = 2000 ms; Alcauter et al., 2014, 2015; Gao et al., 2015b, 2015a, 2019; Emerson et al., 2016; Salzwedel et al., 2019b), we chose to implement the same temporal bandpass filtering range to examine fluctuations at frequencies between 0.01–0.8 Hz. Future studies with large sample sizes employing rapid acquisition fMRI methods (e.g., developing human connectome project, dHCP; Ciarrusta et al., 2020) will provide an opportunity to examine higher frequency signals in this population. While prior research has linked SES and maternal education to long-term changes in the connectivity, volume, and microstructure of hippocampal regions in later childhood (Hackman and Farah, 2009; Hanson et al., 2011; Barch et al., 2016; Farah, 2017; Yu et al., 2018), we did not detect significant (i.e., p < .001) maternal education effects on early hippocampal functional connectivity development in our exploratory analyses (marginally significant at p < .05; see Supplemental Information). This may be related to the minimal variance in maternal education within our sample (Figure S3) with an average of 15 years of education (Table 1). Future work with a widespread distribution of maternal education is needed to better investigate the potential impact of SES on the early development of hippocampal networks. In addition to SES, previous studies have shown that risk factors such as prenatal drug exposure (Grewen et al., 2015; Salzwedel et al., 2015; Geng et al., 2018; Morie et al., 2019) and maternal stress/depression (Sheridan et al., 2013; Rotem-Kohavi et al., 2019; Scheinost et al., 2020) can profoundly influence hippocampal structure and function. In the present study, although maternal mental illness was part of the exclusionary criteria, we were unable to exclude based on paternal mental illness status since that information was not collected. A small but growing body of work is beginning to demonstrate that paternal psychiatric disorders also impact infant development (Aktar et al., 2019); as such, future work should take this into account when defining exclusionary criteria in infant samples. Future studies of different environmental risk factors and related behavioral implications are needed to provide better insight into atypical developmental trajectories in these populations and to better inform early interventions.
To our knowledge, this is the first study to characterize the longitudinal development of hippocampal functional connectivity during the first two years of life. Our findings highlight the dramatic postnatal functional synchronization between the hippocampus and the DMN, resulting in an adult-like hippocampal network by the end of the first year, with further consolidation through the second year of life. Importantly, both cross-sectional and longitudinal growth of hippocampal functional connectivity during the first year indexed 4-year working memory outcomes, underscoring the critical importance of early growth of hippocampal functional circuits for later behavioral outcomes
Supplementary Material
Acknowledgements
The authors thank the families who generously gave their time to participate in this study.
Funding
This work was supported by the National Institutes of Health (R34DA050255, R01DA042988, R01DA043678, R21NS088975, R21DA043171, and R03DA036645 to W.G.; R01MH064065 and R01HD05300 to J.H.G.) and by Cedars–Sinai Precision Medicine Initiative Award and institutional support (to W.G.).
Footnotes
Other Statements:
We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
Due to confidentiality reasons, data used in this manuscript can be requested and shared after University of North Carolina-Chapel Hill and Cedars-Sinai Medical Center IRB review and necessary data sharing agreement being established.
The codes used for the analysis and generation of the data presented in this paper are publicly available through this link: https://osf.io/8fmx4/?view_only=b743b43671504125bc56595e45843069
No part of the study procedures and analyses was pre-registered prior to the research being conducted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aktar E, Qu J, Lawrence PJ, Tollenaar MS, Elzinga BM, Bögels SM (2019) Fetal and infant outcomes in the offspring of parents with perinatal mental disorders: Earliest influences. Front Psychiatry 10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Travaglia A (2017) Infantile amnesia: A critical period of learning to learn and remember. J Neurosci 37:5783–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Keith Smith J, Gilmore JH, Gao W (2015) Consistent anterior-posterior segregation of the insula during the first 2 years of life. Cereb Cortex 25:1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Keith Smith J, Short SJ, Goldman BD, Steven Reznick J, Gilmore JH, Gao W (2014) Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci 34:9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010) Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 65:550–562 Available at: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2003) Working memory: Looking back and looking forward. Nat Rev Neurosci 4:829–839. [DOI] [PubMed] [Google Scholar]
- Bajic D, Craig MM, Borsook D, Becerra L (2016) Probing intrinsic resting-state networks in the infant rat brain. Front Behav Neurosci 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, Tillman R, Luby J (2016) Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am J Psychiatry 173:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ (2008) Preterm infant hippocampal volumes correlate with later working memory deficits. Brain 131:2986–2994. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P (1994) Myelination of a Key Relay Zone in the Hippocampal Formation Occurs in the Human Brain during Childhood, Adolescence, and Adulthood. Arch Gen Psychiatry 51:477–484. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Black S, Faneuil A, Doyle AE, Seidman LJ, Faraone S V. (2008) Discordance between psychometric testing and questionnaire-based definitions of executive function deficits in individuals with ADHD. J Atten Disord 12:92–102. [DOI] [PubMed] [Google Scholar]
- Blankenship SL, Redcay E, Dougherty LR, Riggins T (2017) Development of hippocampal functional connectivity during childhood. Hum Brain Mapp 38:182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Habeck C, Steffener J (2015) Dominant As We Age. 5:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe PSW et al. (2020) Subcortical Brain Volume, Regional Cortical Thickness, and Cortical Surface Area Across Disorders: Findings From the ENIGMA ADHD, ASD, and OCD Working Groups. Am J Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Chen YC, Avishai-Eliner S, Baram TZ (2003) Stress and the developing hippocampus. Mol Neurobiol 27:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000) Neural bases of learning and memory: functional neuroimaging. Curr Opin Neurol 13:415–421. [DOI] [PubMed] [Google Scholar]
- Cao M, Huang H, He Y (2017) Developmental Connectomics from Infancy through Early Childhood. Trends Neurosci 40:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y (2009) Abnormal restingstate functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res 1303:195–206 Available at: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V (2007) Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage 34:1253–1269 Available at: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu S, Salzwedel A, Stephens R, Cornea E, Goldman BD, Gilmore JH, Gao W (2020) The subgrouping structure of newborns with heterogeneous brain-behavior relationships. Cereb Cortex 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrusta J, Dimitrova R, Batalle D, O’Muircheartaigh J, Cordero-Grande L, Price A, Hughes E, Kangas J, Perry E, Javed A, Demilew J, Hajnal J, Edwards AD, Murphy D, Arichi T, McAlonan G (2020) Emerging functional connectivity differences in newborn infants vulnerable to autism spectrum disorders. Transl Psychiatry 10 Available at: 10.1038/s41398-020-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski KT, Lesnik PG, Savoy RL, Grant EP, Ahlfors SP (2006) Developmental neural networks in children performing a Categorical N-Back Task. Neuroimage 33:980–990. [DOI] [PubMed] [Google Scholar]
- Cox JS H RW (1996) AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173 Available at: https://ac-els-cdncom.ezp-prod1.hul.harvard.edu/S0010480996900142/1-s2.0-S0010480996900142main.pdf?_tid=c22bae7a-b8f5-4a8b-9dac-91c1ed841d53&acdnat=1549393398_e37181b8933a2ac88c2d7dc0eab14413. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017a) FMRI clustering and falsepositive rates. Proc Natl Acad Sci U S A 114:E3370–E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017b) FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack R, McCuaig O, Linke AC (2018) Methodological challenges in the comparison of infant fMRI across age groups. Dev Cogn Neurosci 33:194–205 Available at: 10.1016/j.dcn.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR (2015) The Cognitive Neuroscience of Working Memory. Annu Rev Psychol 66:115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F, Klingberg T (2015) The role of fronto-parietal and fronto-striatal networks in the development of working memory: A longitudinal study. Cereb Cortex 25:1587–1595. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Gao W, Lin W (2016) Longitudinal study of the emerging functional connectivity asymmetry of primary language regions during infancy. J Neurosci 36:10883–10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE (2009) Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ (2017) The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron 96:56–71 Available at: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick C, Pagani LS (2012) Toddler working memory skills predict kindergarten school readiness. Intelligence 40:205–212 Available at: 10.1016/j.intell.2011.11.007. [DOI] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Åden U, Blennow M, Lagercrantz H (2011) The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex 21:145–154. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Engström M, Hallberg B, Mosskin M, Åden U, Lagercrantz H, Blennow M (2009) Spontaneous brain activity in the newborn brain during natural sleep-an fMRI study in infants born at full term. Pediatr Res 66:301–305. [DOI] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W (2015a) Functional network development during the first year: Relative sequence and socioeconomic correlations. Cereb Cortex 25:2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W (2015b) Development of human brain cortical network architecture during infancy. Brain Struct Funct 220:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Chen Y, Cornea E, Goldman BD, Gilmore JH (2020) Neonatal brain connectivity outliers identify over forty percent of IQ outliers at 4 years of age. Brain Behav:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, Lin W (2011) Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH (2009a) Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol 30:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Grewen K, Gilmore JH (2017) Functional connectivity of the infant human brain: Plastic and modifiable. Neuroscientist 23:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Salzwedel AP, Carlson AL, Xia K, Azcarate-Peril MA, Styner MA, Thompson AL, Geng X, Goldman BD, Gilmore JH, Knickmeyer RC (2019) Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl) 236:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W (2009b) Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A 106:6790–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F, Salmeron BJ, Ross TJ, Black MM, Riggins T (2018) Long-term effects of prenatal drug exposure on the neural correlates of memory at encoding and retrieval. Neurotoxicol Teratol 65:70–77 Available at: 10.1016/j.ntt.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W (2018) Imaging structural and functional brain development in early childhood. Nat Rev Neurosci 19:123–137 Available at: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G, Espy K, Isquith P (2003) BRIEF-P: Behavior Rating Inventory of Executive Function—Preschool Version: Professional Manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM (2006) Dynamic Mapping of Normal Human Hippocampal Development. Hippocampus 1031:664–672. [DOI] [PubMed] [Google Scholar]
- Gómez RL, Edgin JO (2016) The extended trajectory of hippocampal development: implications for early memory development and disorder. Dev Cogn Neurosci 18:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonthier C, Zira M, Colé P, Blaye A (2019) Evidencing the developmental shift from reactive to proactive control in early childhood and its relationship to working memory. J Exp Child Psychol 177:1–16 Available at: 10.1016/j.jecp.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Salzwedel AP, Gao W (2015) Functional connectivity disruption in neonates with prenatal marijuana exposure. Front Hum Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ (2009) Socioeconomic status and the developing brain. Trends Cogn Sci 13:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD (2011) Association between income and the hippocampus. PLoS One 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, Rollins N, Gong G, Cheng H, Peng Y, Dong Q, He Y (2015) Development of human brain structural networks through infancy and childhood. Cereb Cortex 25:1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1984) Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic 88:488–496. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H (1982) Synaptogenesis in human visual cortex — evidence for synapse elimination during normal development. Neurosci Lett 33:247–252. [DOI] [PubMed] [Google Scholar]
- Insausti R, Cebada-Sánchez S, Marcos P (2010) Postnatal development of the human hippocampal formation. Adv Anat Embryol Cell Biol 206:1–86. [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schöpf V, Langs G (2014) Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci 8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Squire LR (2011) and Medial Temporal Lobe Function. Learn Mem 19:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Schulze K, Sammler D, Fritz T, Müller K, Gruber O (2009) Functional architecture of verbal and tonal working memory: An fMRI study. Hum Brain Mapp 30:859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J (2014) Segregating cognitive functions within hippocampal formation: A quantitative meta-analysis on spatial navigation and episodic memory. Hum Brain Mapp 35:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P (2013) Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav Brain Res 254:8–21 Available at: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Kober H, Bliss-Moreau E, Barrett LF, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2015) The brain basis of emotion: A meta-analytic review. Behav Brain Sci 35:121–143 Available at: https://www.cambridge.org/core/product/identifier/S0140525X11000446/type/journal_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Frith CD (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A 97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Hoffman J (2007) Behavior ratings of executive function among preschoolers with ADHD. Clin Neuropsychol 21:569–586. [DOI] [PubMed] [Google Scholar]
- McAuley T, Chen S, Goos L, Schachar R, Crosbie J (2010) Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc 16:495–505. [DOI] [PubMed] [Google Scholar]
- Mitra A et al. (2017) Resting-state fMRI in sleeping infants more closely resembles adult sleep than adult wakefulness. PLoS One 12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, Crowley MJ, Mayes LC, Potenza MN (2019) Prenatal drug exposure from infancy through emerging adulthood: Results from neuroimaging. Drug Alcohol Depend 198:39–53 Available at: 10.1016/j.drugalcdep.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Froudist-Walsh S (2016) Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Dev Med Child Neurol 58:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M (2006) Working memory for conjunctions relies on the medial temporal lobe. J Neurosci 26:4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendl SL, Salzwedel AP, Goldman BD, Barrett LF, Lin W, Gilmore JH, Gao W (2017) Emergence of a hierarchical brain during infancy reflected by stepwise functional connectivity. Hum Brain Mapp 38:2666–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L (2002) Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron 35:975–987. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB, Byrne ML, Mills KL (2018) Modeling Developmental Change: Contemporary Approaches to Key Methodological Challenges in Developmental Neuroimaging. Dev Cogn Neurosci 33:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebanek DJ, Sloutsky VM (2019) Selective attention, filtering, and the development of working memory. Dev Sci 22:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154 Available at: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320– 341 Available at: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Cho S, Chen T, Rosenberg-Lee M, Geary DC, Menon V (2014) Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat Neurosci 17:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, Gaab N (2012) Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Ann N Y Acad Sci 1252:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH (2003) Stanford-Binet Intelligence Scales (5th ed.). In. Ithasca, IL: Riverside Publishing. [Google Scholar]
- Rotem-Kohavi N, Williams LJ, Muller AM, Abdi H, Virji-Babul N, Bjornson BH, Brain U, Werker JF, Grunau RE, Miller SP, Oberlander TF (2019) Hub distribution of the brain functional networks of newborns prenatally exposed to maternal depression and SSRI antidepressants. Depress Anxiety 36:753–765 Available at: 10.1002/da.22906. [DOI] [PubMed] [Google Scholar]
- Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W (2015) Prenatal drug exposure affects neonatal brain functional connectivity. J Neurosci 35:5860–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Stephens RL, Goldman BD, Lin W, Gilmore JH, Gao W (2019a) Development of Amygdala Functional Connectivity During Infancy and Its Relationship With 4-Year Behavioral Outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging 4:62–71 Available at: 10.1016/j.bpsc.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Stephens RL, Goldman BD, Lin W, Gilmore JH, Gao W (2019b) Development of Amygdala Functional Connectivity During Infancy and Its Relationship With 4-Year Behavioral Outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging 4:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CE, Richards JE, Almli CR (2012) Age-specific MRI templates for pediatric neuroimaging. Dev Neuropsychol 37:379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Spann MN, McDonough L, Peterson BS, Monk C (2020) Associations between different dimensions of prenatal distress, neonatal hippocampal connectivity, and infant memory. Neuropsychopharmacology Available at: 10.1038/s41386-0200677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L, Ábrahám H (2008) Pre- and postnatal morphological development of the human hippocampal formation. In: Handbook of Developmental Cognitive Neuroscience, 2nd Editio. (Nelson CA, Luciano M, eds), pp 187–209. Cambridge, Massachusetts: The MIT Press. [Google Scholar]
- Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA (2013) What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev Sci 16:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D (2011) Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Collins APR, Luyt K, Heep A, Kauppinen RA (2015) High frequency functional brain networks in neonates revealed by rapid acquisition resting state fMRI. Hum Brain Mapp 36:2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:208–219. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ (2011) Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain. Neuroimage 56:1437–1452 Available at: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto EF, Kofler MJ, Ph D, Singh LJ, Ph D, Wells EL, Ed M, Irwin LN, Groves NB, Miller CE (2020) Executive functioning rating scales: Ecologically valid or construct invalid? Neuropsychology 34:605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, Cobigo Y, Mumford PS, Walters S, Saloner R, Karydas A, Coppola G, Rosen HJ, Miller BL, Seeley WW, Kramer JH (2018) The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J Neurosci 38:2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle JM, Triplett RL, Alexopoulos D, Smyser TA, Rogers CE, Limbrick DD, Smyser CD (2019) Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. NeuroImage Clin 22:101787 Available at: 10.1016/j.nicl.2019.101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V (2009) Development of large-scale functional brain networks in children. PLoS Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Engvig A, Grydeland H, Krogsrud SK, Østby Y, Holland D, Dale AM, Fjell AM (2014) Regional hippocampal volumes and development predict learning and memory. Dev Neurosci 36:161–174. [DOI] [PubMed] [Google Scholar]
- Ten Eycke KD, Dewey D (2016) Parent-report and performance-based measures of executive function assess different constructs. Child Neuropsychol 22:889–906. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, Yeo L, Mody S, Hernandez-Andrade E, Hassan SS, Studholme C, Jeong JW, Romero R (2013) Crosshemispheric functional connectivity in the human fetal brain. Sci Transl Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER (2006) Mapping brain maturation. Trends Neurosci 29:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, West RF, Stanovich KE (2013) Practitioner Review: Do performance-based measures and ratings of executive function assess the same construct? J Child Psychol Psychiatry Allied Discip 54:131–143. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D (2009) Visual cortex plasticity evokes excitatory alterations in the hippocampus. Front Integr Neurosci 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V (2010) Typical and atypical development of functional human brain networks: Insights from resting-state fMRI. Front Syst Neurosci 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Kersbergen KJ, De Reus MA, Keunen K, Kahn RS, Groenendaal F, De Vries LS, Benders MJNL (2015) The neonatal connectome during preterm brain development. Cereb Cortex 25:3000–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL (2006) Coherent Spontaneous Activity Identifies a Hippocampal-Parietal Memory Network. J Neurophysiol 96:3517–3531. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Rojas DC, Ross RG, Hunter SK, Maharajh K, Cornier MA, Tregellas JR (2014) Reduced brain resting-state network specificity in infants compared with adults. Neuropsychiatr Dis Treat 10:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat Rev Neurosci 7:464–476. [DOI] [PubMed] [Google Scholar]
- Yu Q, Daugherty AM, Anderson DM, Nishimura M, Brush D, Hardwick A, Lacey W, Raz S, Ofen N (2018) Socioeconomic status and hippocampal volume in children and young adults. Dev Sci 21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Rifkin-Graboi A, Ta AT, Yap KL, Chuang KH, Meaney MJ, Qiu A (2014) Functional networks in parallel with cortical development associate with executive functions in children. Cereb Cortex 24:1937–1947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.