Abstract
Early life adversity has been linked to poor health, including obesity. Understanding the role of unhealthy food intake, may elucidate the importance of self-soothing behaviors in explaining the association between early life adversity and poor health in adulthood. The purpose of this study was to assess the association between early life adversity and dietary quality in a sample of adults from the Lifestyle Influences of Family Environment study. Early life adversity, demographic, and dietary data were obtained for 145 participants using formal interviews and two days of interviewer-administered 24-hour recalls. Dietary quality was measured using the 2015 Healthy Eating Index (HEI) scoring algorithm to compute total and component scores. The association between early life adversity and dietary quality was assessed through linear regression and in models adjusted for age and sex. The mean ± SD HEI score for all participants was 54.6 ± 12.8. Individuals with early life adversity had a 4.51 lower overall HEI score when compared to those without early life adversity, 95% CI (0.35, 8.68). After adjusting for age and sex, early life adversity was associated with a 4.6 lower HEI score, 95% CI (0.45, 8.73). HEI component scores indicated that individuals with early life adversity were significantly more likely to have lower whole grain (0.7 versus 2.4) and total dairy (4.3 versus 6.1) scores compared to those without early life adversity. ELA was associated with lower measures of dietary quality. Results warrant future research on dietary and behavioral factors that underly the association between early life adversity and poor health outcomes.
Keywords: Early life adversity, Childhood adversity, Dietary Quality, Healthy Eating Index, Adverse health outcomes
1. Introduction
Early life adversity (ELA) such as parental loss and maltreatment has been linked to manifold lasting negative effects on health and well-being (Felitti et al., 1998; Merrick & Guinn, 2018; Metzler et al., 2017; National Center for Injury Prevention [NCIJ], 2021). In the United States (U.S.), up to 45% of children have experienced at least one form of ELA (Sacks & Murphey, 2018). ELA is associated with adverse health outcomes like obesity (Alvarez et al., 2007; Li et al., 2015; Mutlu et al., 2016; Richardson et al., 2013; Sokol et al., 2018, 2019), the metabolic syndrome (Delpierre et al., 2016; Farr et al., 2015; Suglia et al., 2018), and eating disorders (Imperatori et al., 2016; Nusslock & Miller, 2016); and these adverse health outcomes are among the costliest in U.S. healthcare expenditures (Hruby & Hu, 2015). Identifying modifiable mechanisms that underpin the burden of adverse health outcomes becomes increasingly integral in assuaging U.S. healthcare financial costs.
The relationship between ELA and unhealthy dietary behaviors has been examined in previous research (Abajobir et al., 2017; Gavrieli et al., 2015; Hemmingson, 2019; Hinchliff et al., 2016; Isohookana et al., 2016; Jackson & Vaugn, 2019; Marques et al., 2019; Miller & Lumeng, 2018; Non et al., 2018; Russell et al., 2019; Windle et al., 2018). Individuals with ELA are more likely to have lower consumption of natural foods and higher consumption of processed foods (Marques et al., 2019), to engage in “obesogenic” food intake (Abajobir et al., 19; Hemmingson, 2019; Jackson & Vaughn, 2019), to choose ‘feel good’ foods over whole foods (28), and to engage in risky dieting behavior (Hinchliff et el., 2016; Isohookana et al., 2016). For example, one cross-sectional study found that individuals who engaged in binge eating and endorsed “food addiction” (chronic and severe cravings for certain foods to achieve pleasure or to gain relief), also reported significantly higher body mass index (BMI) and increased severity of childhood trauma (Imperatori et al., 2016). Indeed, unhealthy eating behaviors among survivors of ELA (Russell et al., 2019) may serve as a self-soothing or “self-medicating” mechanism to manage negative affect and memories (Hemmingson, 2018; Nusslock & Miller, 2016).
Although the relationship between consumption of unhealthy foods and ELA has been evaluated, most studies have used binary measures of dietary quality, collected dietary data at a single time point, and/or focused on high-fat or obesogenic food consumption. In fact, only one study used comprehensive profiles of dietary composition, but did not use the most updated 2015–2020 version of the Healthy Eating Index (HEI; Gavrieli et al., 2015). Thus, in the current study, we examined dietary intake in a sample of adults with and without a history of ELA. Using 24-hour dietary recalls and the 2015-2020 version of the HEI, we aimed to provide a broader assessment of dietary intake to better understand adult dietary patterns and quality related to ELA. To the authors’ knowledge, this was the first study to employ 24-hour dietary recalls and the HEI to examine the relationship between dietary intake and ELA. Knowledge of dietary mechanisms in adulthood will inform the development of novel interventions, which may ultimately serve to ameliorate the established burden of adverse health outcomes in populations affected by ELA.
2. Methods
2.1. Subjects
This study used data from a sub-set of the Lifestyle Influences of Family Environment study (LIFE). Between 2014-2019, individuals were recruited through newspaper and internet advertisements. The inclusion criteria were: 1) individuals aged 18-40 years, 2) individuals without acute or chronic health conditions who were not taking daily medication other than hormonal contraceptives, 3) individuals who were not currently breastfeeding or pregnant. The ELA group was composed of individuals with a childhood history of maltreatment, with or without a history of childhood parental loss (detailed description provided in the ELA measure). Individuals without ELA had no childhood abuse, neglect, or parental loss, or lifetime history of major psychiatric disorder. The LIFE study was approved by the Butler Hospital Institutional Review Board.
The analytical sample was comprised of 145 participants, ELA (n = 71) and non-ELA (n = 74). One participant was excluded due to missing dietary data. Demographic, clinical, and dietary information was collected across three visits through structured interviews and self-administered questionnaires.
2.2. Measures and Methodology
2.2.1. Sociodemographics
Sociodemographic variables including sex, race, ethnicity, education, income, and marital status were assessed categorically. Age was assessed as a continuous variable. Due to the presence of students in the sample and the high collinearity of education and income variables, highest completed level of education was considered as a proxy for socioeconomic position (SEP) and was assessed categorically.
2.2.2. Early life adversity
The Childhood Experience of Care and Abuse (CECA; Bifulco et al., 1994) is a semi-structured interview that assesses childhood parental loss and the following types of maltreatment: physical abuse, sexual abuse, parental neglect, and psychological abuse and antipathy. Inter-rater reliability ranges from .82 to 1.0, and construct validity has been shown in two separate samples of adult sisters, with 84-94% agreement for between-sister reports of the presence or absence of maltreatment (Bifulco et al., 1994, 1997). Interviews and scoring were conducted by a trained interviewer, and scores were independently reviewed by a second trained interviewer/scorer, with unclear cases discussed in a group format with consensus scoring. The ELA group in this study met the criteria for at least moderate severity of at least one type of maltreatment occurring prior to age 19. Parental loss was defined as death or desertion of a parent for at least 12 months prior to the age 11 and during which time the child rarely had contact with the parent (less than monthly). Additional parental separations that lasted for fewer than 12 months or occurred after age 10 were also assessed and termed “Other Parental Separation”. The group without ELA did not have any form of maltreatment or parental loss, except for one individual who experienced intimate partner abuse. Table 1 provides detailed information regarding ELA and non-ELA grouping.
Table 1.
Childhood Experience of Care and Abuse, Early Life Adversity (ELA) and non-ELA grouping
| Adversity (moderate or marked) | ELA, n (%) | Non-ELA, n (%) |
|---|---|---|
| Antipathy | 65 (88) | 0 (0) |
| Neglect | 59 (80) | 0 (0) |
| Physical abuse | 50 (68) | 0 (0) |
| Sexual abuse | 44 (60) | 1 (1) |
| Psychological abuse | 42 (57) | 1 (1) |
| Parental death, desertion, or other separation | 64 (87) | 3 (4) |
| ≥ 2 Adversity types | 74 (100) | 1 (1) |
| ≥ 3 Adversity types | 67 (91) | 0 (0) |
| ≥ 4 Adversity types | 57 (77) | 0 (0) |
2.2.3. Dietary Assessment
Dietary intake data were collected using the Automated Self-Administered 24-hour (ASA24) Dietary Assessment Tool, versions 2014 and 2016, developed by the National Cancer Institute (National Cancer Institute [NCI], 2017, 2019). The tool was administered by an interviewer during participants’ second and third study visits. At the time of visit 2, participants were asked to fast the night before starting at 8:00 PM for another aspect of the study. Eighty-six percent (n = 125) of participants reported two days of dietary intake data (visits 2 and 3) and 14% (n = 20) reported one day of dietary intake data (visit 2 or 3).
2.2.4. Healthy Eating Index
The Healthy Eating Index (HEI), which is defined by the 2015-2020 Dietary Guidelines for Americans (DGA), was calculated as a measure of dietary quality (Kirkpatrick et al., 2018; NCI, 2019; Reedy et al., 2018; U.S. Department of Agriculture [USDA], 2015). The overall HEI score, ranging from 0 to 100, indicates to what extent an individual’s overall diet adheres to guidelines across 13 major dietary components. Higher overall and component HEI scores indicate dietary quality that more closely matches the DGA.
The HEI components are derived from various food groups and nutrients that are most associated with health outcomes according to the DGA (USDA, 2015). The 13 components are comprised of adequacy components as well as moderation components. The former category consists of 9 dietary items, which the DGA encourages for consumption, including total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and fatty acids. The latter category consists of 4 dietary items, which the DGA suggest are best consumed in moderation, including refined grains, sodium, added sugars, and saturated fats (NCI, 2017, 2019; USDA, 2015). The most current HEI integrates additional food and nutrient items associated with health outcomes such as sodium and alcohol intake, by counting these calories as “empty” calories and increasing total caloric intake without any added nutrients (Kirkpatrick et al., 2018; Reedy et al., 2018; USDA, 2015). We examined both moderation and adequacy component HEI scores along with total HEI scores in this study.
2.3. Statistical Analysis
Computation of HEI scores was conducted using SAS® software version 9.4 and publicly available code (SAS Institute, 2019). For all other analyses, the statistical software R, version 3.6 was used (R Core Team, 2013). Descriptive statistics for sociodemographic variables were assessed for the overall sample and stratified by exposure group. For categorical variables, demographic differences across exposure groups were assessed using Fisher’s Exact Tests and Chi-squared tests. For normally distributed continuous variables, differences were assessed using two-sample t-tests. For non-normal continuous variables, Kruskall-Wallis rank sum tests were used. Differences were considered significant if they were below a two-tailed significance level of p < 0.05.
Overall and component HEI scores were calculated using the HEI scoring algorithm (NCI, 2019). Since there were no differences in HEI scores comparing those with or without two days of dietary data (p < 0.05), HEI scores were also used for individuals with a single day of dietary data (14%). Since HEI component scores were highly skewed with scores tending either towards the minimum or maximum values, Kruskall-Wallis rank sum tests were employed to assess, and radar plots were used to visualize, effects for these variables.
Multiple regression was used to assess differences in overall HEI scores between groups and the influence of potential confounders, age, and sex. The selection of covariates was informed by published data on confounding factors (Hiza et al., 2013; Kirkpatrick et al., 2018; Reedy et al., 2018). Established confounders were treated as candidate covariates for a final model. Covariates for the first candidate model were race, ethnicity, age, sex, and highest completed level of education. Only covariates with a predetermined p-value of < 0.2 were included in the final model. The predetermined p-value of < 0.2 was chosen to better account for confounding. The utility of this value has been shown in previous studies (Vittinghoff et al., 2011). Goodness-of-fit tests were used to assess model fit and efficacy. To further assess the model fit, regression coefficient standard errors and confidence intervals were re-estimated using the non-parametric bootstrap (Fox, 2002).
Sensitivity Analysis
Although most individuals who comprised the group without exposure to ELA did not have any form of maltreatment or parental loss, one individual in this group reported intimate partner abuse. Given the inclusion of this individual within the group without exposure to ELA, a sensitivity analysis was conducted to assess the influence of this observation. The analyses described above were replicated in a sample excluding this individual.
3. Results
3.1. Sociodemographic variables
Table 2 provides details about sociodemographic variables. The mean age was 27.7 (SD = 6.0; range = 18-40) years. Most of the sample was female (n = 107; 74%) and identified as non-Hispanic and/or White (n = 103; 71%). In the main analyses and sensitivity analyses, individuals with ELA were significantly more likely to report a lower completed level of education and a lower income compared to the non-ELA group. There were no other sociodemographic differences between the ELA and non-ELA groups.
Table 2.
| Total (n = 145) |
Early life
adversity (n = 74) |
No early life
adversity (n = 71) |
|
|---|---|---|---|
| Age, y ± SD | 27.6 ± 6.0 | 28.3 ± 5.9 | 26.9 ± 6.0 |
| Males | 38 (26) | 16 (22) | 22 (31) |
| Race | |||
| American Indian/Alaska Native | 3 (2) | 3 (4) | 0 (0) |
| Asian | 9 (6) | 3 (4) | 6 (9) |
| Black/African American | 12 (8) | 9 (12) | 3 (4) |
| More than one race | 13 (9) | 9 (12) | 4 (6) |
| Unknown | 5 (3) | 4 (5) | 1 (1) |
| White | 103 (71) | 46 (62) | 57 (80) |
| Ethnicity, non-Hispanic/Latino | 118 (81) | 56 (76) | 62 (88) |
| White + non-Hispanic/Latino | 89 (62) | 39 (53) | 50 (70) |
| Education* | |||
| College graduate | 80 (55) | 32 (43) | 48 (68) |
| High school or less | 15 (10) | 9 (12) | 6 (9) |
| Partial college | 50 (34) | 33 (45) | 17 (24) |
| Total Income* | |||
| $0-$24,999 | 33 (26) | 19 (30) | 14 (22) |
| $25,000-$49,999 | 41 (32) | 29 (45) | 12 (19) |
| $50,000-$74,999 | 19 (15) | 7 (11) | 12 (19) |
| $75,000+ | 35 (27) | 9 (14) | 26 (41) |
| Marital status | |||
| Divorced | 4 (3) | 4 (5) | 0 (0) |
| Married/Cohabitation | 24 (17) | 11 (15) | 13 (18) |
| Never married | 113 (78) | 56 (76) | 57 (80) |
| Separated | 4 (3) | 3 (4) | 1 (1) |
| BMI, kg/m2* | 27.2 ± 5.7 | 28.2 ± 5.7 | 26.1 ± 5.5 |
Data are given as mean ± SD or n (%).
* indicates statistical significance at p < 0.05.
3.2. Dietary Outcomes
The entire sample had a mean overall HEI score of 54.6 (SD = 12.8; range = 22.3-84.6), which is only slightly lower than the average overall HEI score of 59 for Americans (USDA, 2020). Figure 1 displays the overall HEI scores based on the two ELA groups. A crude regression model indicated that having a history of ELA was associated with a 4.5 lower average overall HEI score (95% (0.35, 8.68), p < 0.05). Covariates for the first candidate adjusted model were race, ethnicity, age, sex, and highest completed level of education. The covariates with p values less than 0.2 were age and sex. After adjusting for age and sex, having a history of ELA was associated with a 4.6 lower average overall HEI score (95% (0.45, 8.73), p < 0.05). Bootstrap estimated confidence intervals agreed closely with the adjusted regression model. Table 3 provides details about multivariate analyses. Sensitivity analyses produced similar results, although ELA-specific differences in overall HEI scores were larger in both crude and adjusted models. In sensitivity analyses, crude models demonstrated that ELA was associated with 4.8 lower average overall HEI scores (95% (0.60, 8.93, p < 0.05). Adjusted models indicated that ELA was associated with 4.9 lower average overall HEI scores (95% (0.75, 9.04), p < 0.05).
Figure 1.
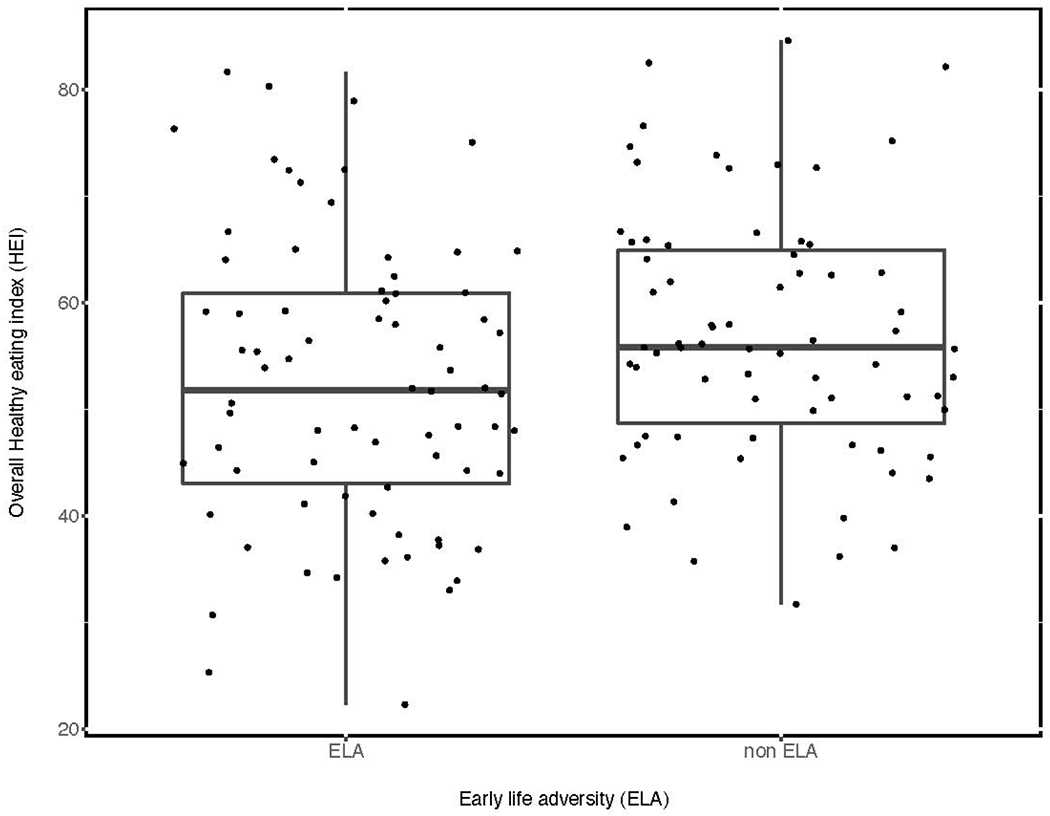
Overall Healthy Eating Index scores by early life adversity status.
Table 3.
Multiple regression coefficients (95% CI) and bootstrap estimated CIs of overall Healthy Eating Index scores1
| Candidate adjusted | ||||
|---|---|---|---|---|
| Crude model | model | Final adjusted model | Bootstrap | |
| Early life adversity | −04.51 (−8.68, −0.35)* | −4.84 (−9.43, −0.26)* | 4.59 (−8.73, −0.45)* | −8.58, −0.50 |
| Age, y | −0.36 (−0.74, 0.01) | −0.29 (−0.64, −0.05) | −0.62, 0.03 | |
| Male | −4.15 (−9.20, 0.90) | −5.28 (−9.98, −0.57) * | −9.95, −0.71 | |
| High school or less | −0.41 (−8.06, 7.24) | |||
| Partial college | 2.44 (−2.49, 7.37) | |||
| American Indian/Alaska | ||||
| Native | −5.67 (−20.59, 9.25) | |||
| Asian | 2.45 (−6.35, 11.27) | |||
| Black/African American | −1.11 (−8.99, 6.77) | |||
| More than one race | 0.74 (−6.80, 8.28) |
* indicates statistical significance at p < 0.05.
Table 4 provides details about mean HEI component scores. In both the main analyses and sensitivity analyses, Individuals with ELA were more likely to have lower whole grain (0.7 versus 2.4, p < 0.05) and lower total dairy (4.3 versus 6.1, p < 0.05) component scores compared to those without ELA. No other component scores differed significantly between the two groups. Figure 2 displays the median component scores for the two groups.
Table 4.
| Total (n = 145) | Early life adversity (n = 74) | No early life adversity (n = 71) | |
|---|---|---|---|
| Calories ± SD | 3586 ± 1401 | 3530 ± 1451 | 3644 ±1355 |
| Total HEI score ± SD* | 54.6 ± 12.8 | 52.4 ± 13.5 | 56.9 ± 11.8 |
| Total vegetables | 3.8 (2.5, 5.0) | 3.6 (2.3, 5.0) | 3.9 (2.6, 5.0) |
| Greens and beans | 3.4 (0.0, 5.0) | 3.3 (0.0, 5.0) | 3.6 (0.3, 5.0) |
| Total fruits | 2.3 (0.2, 4.7) | 2.3 (0.2, 4.9) | 1.9 (0.2, 4.3) |
| Whole fruits | 2.5 (0.1, 5.0) | 1.7 (0.0, 5.0) | 3.1 (0.4, 5.0) |
| Whole grains* | 1.4 (0.0, 4.4) | 0.7 (0.0, 2.9) | 2.4 (0.4, 5.7) |
| Refined grains | 7.3 (4.2, 9.8) | 7.1 (3.1, 9.5) | 7.6 (4.9, 10.0) |
| Total dairy* | 4.9 (2.8, 8.1) | 4.3 (2.4, 6.9) | 6.2 (3.3, 9.0) |
| Total protein foods | 5.0 (4.3, 5.0) | 5.0 (4.6, 5.0) | 5.0 (3.8, 5.0) |
| Seafood plant protein | 4.3 (1.1, 5.0) | 5.0 (0.6, 5.0) | 4.3 (1.9, 5.0) |
| Fatty acids | 4.1 (2.1, 8.2) | 4.0 (2.3, 8.0) | 4.1 (1.8, 8.0) |
| Sodium | 3.3 (0.0, 5.8) | 2.5 (0.0, 6.0) | 3.4 (0.0, 5.5) |
| Saturated fats | 5.7 (1.9, 7.8) | 5.4 (1.7, 7.9) | 5.8 (2.2, 7.7) |
| Added sugars | 8.6 (6.3, 10.0) | 8.2 (6.3, 10.0) | 9.2 (7.3, 10.0) |
Data are given as median and interquartile range (IQR) to account for skewedness.
Greater Healthy Eating Index scores indicate dietary intake that more closely aligns with the Dietary Guidelines for Americans.
* indicates statistical significance at p < 0.05.
Figure 2.
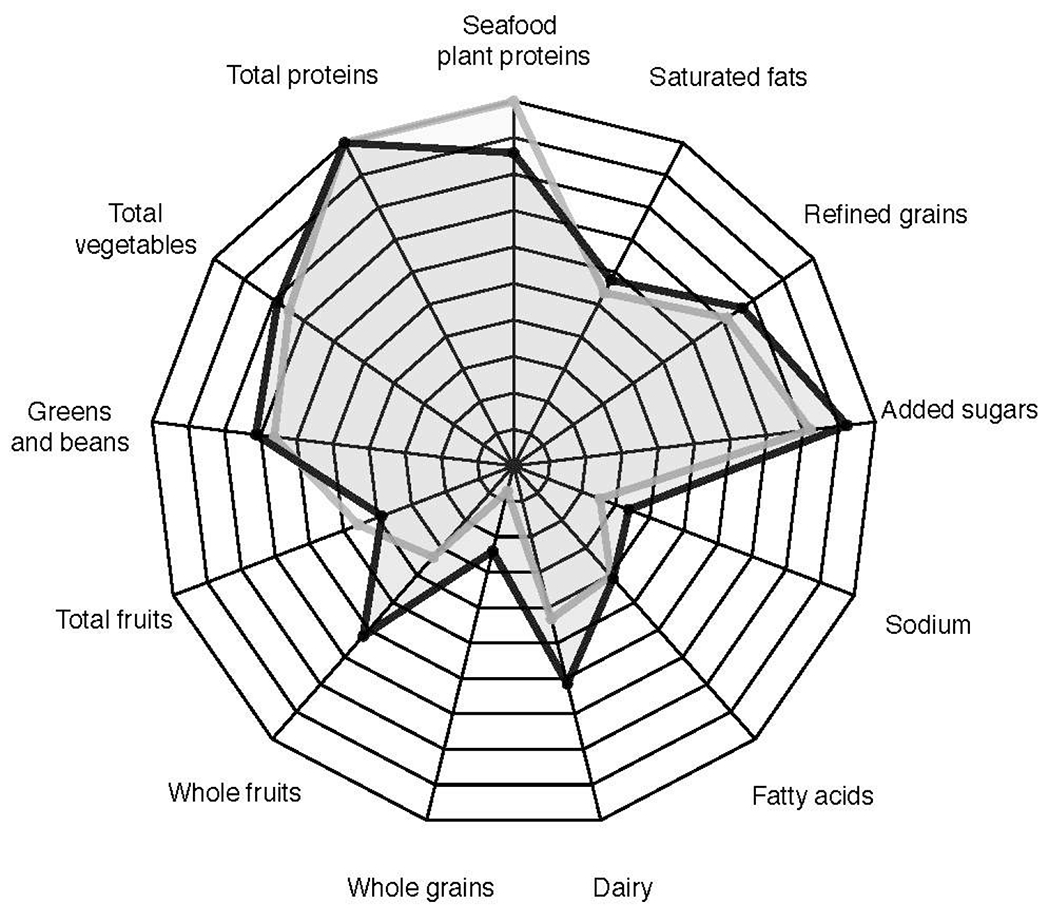
Component Healthy Eating Index scores by early life adversity status.
Early life adversity: gray line
No early life adversity: black line
4. Discussion
The objective of this study was to ascertain whether there are differences in dietary intake between individuals with and without ELA. To our knowledge, this was the first study to employ 24-hour dietary recalls and the most updated 2015-2020 version of HEI to assess dietary intake in relation to ELA. Thus, the current study uniquely contributes to the existing literature that has categorically assessed high fat and obesogenic food intake (Abajobir et al., 2017; Hemmingson, 2018; Jackson & Vaughn, 2019) and shown an increased propensity for unhealthy foods and an increased dietary intake of processed foods among individuals with ELA (Marques et al., 2019; Russell et al., 2019). Although results from this study did not find an association between ELA and high fat or obesogenic dietary intake, findings quantitatively demonstrated differences in dietary components among adults with and without ELA. Such differences may indicate possible mechanisms that underpin the association between ELA and adverse health outcomes.
Our findings elucidate a meaningful association between overall dietary quality and ELA and provide insight regarding dietary patterns (Kirkpatrick et al., 2018). Kirkpatrick and colleagues (2018) recommend that the appropriate interpretation of HEI scores include total scores to evaluate overall dietary quality and component scores to examine quality of foods consumed. In the current study, differences in HEI component scores suggest that patterns of lower intake of whole grains and dairy may drive the association between dietary intake and ELA. Additionally, these two dietary factors worked in conjunction with the other 11 HEI component scores to comprise overall HEI scores that were statistically significantly different between the ELA and non-ELA groups. Although both the ELA and non-ELA groups had low dietary quality (i.e., overall HEI scores in the range of 0-59), our results indicate an inverse relationship between ELA and overall dietary quality that is meaningful and meets the criterion of an effect size of 0.5 (Kirkpatrick et al., 2018). Our results are consistent with the one prior study that examined ELA with another version of the HEI (Gavrieli et al., 2015). Moreover, overall dietary quality among individuals with ELA appears to be driven by various dietary factors versus a single or small number of unhealthy dietary habits, which fits with use of HEI as an overall assessment of diet quality. Also, the difference in HEI between groups found in this our study match meaningful differences found in other studies with morbidity and mortality outcomes. A recent study has illustrated that 5–6-point differences in HEI scores are associated with better cardiovascular outcomes (Brauer et al., 2021). Similarly, greater HEI scores are linked to reduced risk of all-cause mortality, cardiovascular disease, cancer, and type 2 diabetes mellitus (Schwingshakl & Hoffmann, 2015), all of which are linked to ELA (Felitti et al., 1998).
Differences in component HEI scores between ELA groups may not have emerged due to Type 2 error, or our modest sample size. To further elucidate the relationship between dietary quality and ELA, future studies should include 24-hour dietary recalls for at least two days, to allow a comparison to our study sample as well as account for the degree of within-person or day-to-day variations. Indeed, the use of 24-hour recall data for the calculation of HEI is preferred over the use of food frequency questionnaire data (Kirkpatrick et al., 2018). However, the day-to-day variability of intake is different across different types of foods, which may result in differences in validity between HEI component scores. Moreover, dietary recall measures are subject to recall and reporting biases, which may differ between a variety of food types and populations (Kirkpatrick et al., 2018). Given marked associations between ELA and food insecurity (Chilton et al., 2015), eating disorders (Imperatori et al., 2016), depressed mood, and risky dieting (Hinchliff et al., 2016), day-to-day variability may be increased in populations with histories of early life adversity. Although increased day-to-day variability among individuals with ELA may be expected, overall HEI scores did not differ significantly between individuals with a single 24-hour recall and individuals with 2 24-hour recalls in our sample.
The design of this study and its retrospective appraisal of ELA allowed us to investigate how dietary behaviors may differ years after ELA has occurred. We also investigated how dietary practices differ across two otherwise comparable groups, thereby reducing the potential for omitted variable bias. By employing HEI scoring methodology, this study found a difference in dietary practices among individuals with and without substantial early life adversity within the context of the evidence-based 2015-2020 National Dietary Guidelines for Americans (USDA, 2015).
The combined use of 24-hour recalls and HEI scoring allowed us to assess how closely the dietary composition of each group matched evidence-based dietary guidelines. This underscores the utility of the HEI scoring methodology in showing detailed dietary differences between individuals with and without ELA. The focus of past studies on high fat (Abajobir et al., 2017), obesogenic food consumption (Jackson & Vaughn, 2019), and binary measures of dietary quality (Marques et al., 2019, Russell et al., 2019) along with the use of dietary measurements collected at a single time point limited the capacity of past studies to obtain dietary measures that were representative of long-term dietary patterns. Through repeated 24-h recall measurements in adulthood and HEI scoring methodology, this study was able to partially address this limitation. Results from this study also suggest the utility of the HEI as a measure to track the improvement or deterioration of diet in survivors of ELA. This knowledge may serve to inform future studies seeking to attenuate the harmful effects of ELA as they pertain to dietary composition.
The unique contributions of this study should be considered in light of some limitations. First, given the retrospective appraisal of exposure to ELA, a causal link between ELA and lower dietary quality cannot be definitively established from this study alone. Second, it is not possible to extrapolate the findings of this study to other populations because of the inclusion and exclusion criteria and how ELA was defined. Third, while 24-hour dietary recall is a standard method, shortcomings of 24-hour recall data are well known (Kirkpatrick et al., 2018; NCI, 2019; Reedy et al., 2018; USDA, 2015). Since 24-hour recalls are a snapshot of usual dietary intake, two 24-hour recalls may not adequately describe the variation present in an individual’s usual diet, especially variation in micronutrients and specific foods (Reedy et al., 2018). Finally, the modest sample size of this study was a limitation. In particular, a larger sample size may have enhanced our ability to detect statistically significant differences in added sugars and refined grain HEI component scores. Given ELA-specific differences in these components and marked associations between obesogenic food intake and ELA (Jackson & Vaughn, 2019), it is clear that added sugars and refined grains may be important contributors to unhealthy food intake among individuals with ELA. Nevertheless, this study was unable to detect statistically significant differences in these components.
Despite these limitations, our study used an in-depth, intensive interview of early stress, and recruited individuals with a significant history of early stress compared with those with no such early stress or lifetime trauma. Future research investigating the association between ELA and dietary quality would benefit from longitudinal designs, repeated 24-hour recalls over more than 2 days, and comprehensive measures of ELA. Moreover, technological advancements may facilitate data acquisition in this domain, through methods such as ecological momentary assessment of dietary intake.
Our results describe the association between ELA and overall dietary quality. However, dietary quality is not adequately explained by ELA alone. There are other drivers of dietary quality, such as limited access to healthy foods, cultural or ethnic differences (Mackenbach et al., 2019), SEP (Chilton et al., 2015; Ghosh-Dastidar et al., 2014; Mackenbach et al., 2019; Non et al., 2020), and social support (Non et al., 2020). Although these other factors may themselves be driven by a history of ELA (Felitti et al., 1998; Metzler et al., 2017; NCIJ, 2019), research describing the direction of the mechanisms by which this occurs is scarce. Future research using longitudinal data that employs repeated measurements would facilitate the identification of the direction of the relationships between ELA and health outcomes. Furthermore, research that examines mediational factors influencing the relationship between ELA and adverse health outcomes is essential to understand the nuance of these relationships.
Given the established association between proximity and cost of healthy foods and obesity (Ghosh-Dastidar et al., 2014; Mackenbach et al., 2019), future studies might utilize geographical measures and childhood socioeconomic position to evaluate access to healthy foods, which could contribute to setting life-long eating habits. Measures of emotional eating and resilience may also shed light on psychosocial factors that moderate the association between ELA and dietary intake. Furthermore, increased efforts to include more historically disadvantaged populations are needed in all fields of biomedical research. Such inclusion may unravel the heterogeneity in the relationship between a history of ELA and diverse patterns of dietary quality, adverse health outcomes, and mental health outcomes across populations with diverse risk profiles (Chilton et al., 2015; Hiza et al., 2013; Mackenbach et al., 2019; Office of Disease Prevention and Health Promotion, 2019).
In summary, findings from this study provide insight to the dietary patterns that underlie the relationship between overall dietary quality and history of ELA by quantitatively demonstrating dietary differences in adults with and without ELA.
Acknowledgements & Author Contributions
ART designed and oversaw the research of the original study; APG provided essential materials (data necessary for research); FDM performed statistical analyses with guidance from PMR and AS; FDM and KJM wrote paper; and all authors have responsibility for final content and read, edited, and approved the final manuscript. The authors report no potential conflicts of interest.
Sources of Support
The work was supported by the National Institutes of Mental Health [R01MH101107] and the Rockefeller University Heilbrunn Family Center for Research Nursing through the generosity of the Heilbrunn Family.
Abbreviations
- ASA24
Automated Self-Administered 24-hr Recall
- BMI
Body Mass Index
- CECA
Childhood Experience of Care and Abuse
- CI
Confidence Interval
- ELA
Early life adversity
- HEI
Healthy Eating Index
- LIFE
Lifestyle Influences of Family Environment study
- NCI
National Cancer Institute
- NCIP
National Center for Injury Prevention
- SEP
socioeconomic position
- U.S.
United States
- DGA
Dietary Guidelines for Americans
- USDA
U.S. Department of Agriculture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Center for Health Promotion and Health Equity Research
Ethics Statement
As a secondary data analysis, this study obtained exempt status from the University of Rhode (#00000599). The original study was approved by the Butler Hospital IRB, and voluntary written informed consent was obtained.
References
- Abajobir AA, Kisely S, Williams G, Strathearn L, & Najman JM (2017). Childhood maltreatment and high dietary fat intake behaviors in adulthood: A birth cohort study. Child Abuse & Neglect, 72, 147–153. 10.1016/j.chiabu.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Alvarez J, Pavao J, Baumrind N, & Kimerling R (2007). The Relationship Between Child Abuse and Adult Obesity Among California Women. American Journal of Preventive Medicine, 33(1), 28–33. 10.1016/j.amepre.2007.02.036 [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Lillie A, & Jarvis J (1997). Memories of Childhood Neglect and Abuse: Corroboration in a Series of Sisters. Journal of Child Psychology and Psychiatry, 38(3), 365–374. 10.1111/j.1469-7610.1997.tb01520.x [DOI] [PubMed] [Google Scholar]
- Brauer P, Royall D, & Rodrigues A (2021). Use of the Healthy Eating Index in Intervention Studies for Cardiometabolic Risk Conditions: A Systematic Review. Advances in Nutrition. 10.1093/advances/nmaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Craig TKJ, Harris TO, Handley RV, & Harvey AL (2007). Development of a retrospective interview measure of parental maltreatment using the Childhood Experience of Care and Abuse (CECA) instrument — A life-course study of adult chronic depression — 1. Journal of Affective Disorders, 103(1–3), 205–215. 10.1016/jjad.2007.05.022 [DOI] [PubMed] [Google Scholar]
- Chilton M, Knowles M, Rabinowich J, & Arnold KT (2015). The relationship between childhood adversity and food insecurity: ‘It’s like a bird nesting in your head.’ Public Health Nutrition, 18(14), 2643–2653. 10.1017/s1368980014003036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre C, Fantin R, Barboza-Solis C, Lepage B, Darnaudéry M, & Kelly-Irving M (2016). The early life nutritional environment and early life stress as potential pathways towards the metabolic syndrome in mid-life? A lifecourse analysis using the 1958 British Birth cohort. BMC Public Health, 16(1). 10.1186/s12889-016-3484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Ko B-J, Joung KE, Zaichenko L, Usher N, Tsoukas M, … Mantzoros CS (2015). Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutrition, Metabolism and Cardiovascular Diseases, 25(5), 479–488. 10.1016/j.numecd.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Fox J (2002). Bootstrapping Regression Models: Appendix to An R and S-PLUS Companion to Applied Regression. In An R and S-PLUS Companion to Applied Regression. Thousand Oaks CA, Sage. [Google Scholar]
- Gavrieli A, Farr OM, Davis CR, Crowell JA, & Mantzoros CS (2015). Early life adversity and/or posttraumatic stress disorder severity are associated with poor diet quality, including consumption of trans fatty acids, and fewer hours of resting or sleeping in a US middle-aged population: A cross-sectional and prospective study. Metabolism, 64(11), 1597–1610. 10.1016/j.metabol.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Dastidar B, Cohen D, Hunter G, Zenk SN, Huang C, Beckman R, & Dubowitz T (2014). Distance to Store, Food Prices, and Obesity in Urban Food Deserts. American Journal of Preventive Medicine, 47(5), 587–595. 10.1016/j.amepre.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsson E (2018). Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Current Obesity Reports, 7(2), 204–209. 10.1007/s13679-018-0310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliff GLM, Kelly AB, Chan GCK, Patton GC, & Williams J (2016). Risky dieting amongst adolescent girls: Associations with family relationship problems and depressed mood. Eating Behaviors, 22, 222–224. 10.1016/j.eatbeh.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Hiza HAB, Casavale KO, Guenther PM, & Davis CA (2013). Diet Quality of Americans Differs by Age, Sex, Race/Ethnicity, Income, and Education Level. Journal of the Academy of Nutrition and Dietetics, 113(2), 297–306. 10.1016/j.jand.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Hruby A, & Hu FB (2014). The Epidemiology of Obesity: A Big Picture. PharmacoEconomics, 33(7), 673–689. 10.1007/s40273-014-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatori C, Innamorati M, Lamis DA, Farina B, Pompili M, Contardi A, & Fabbricatore M (2016). Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse & Neglect, 58, 180–190. 10.1016/j.chiabu.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Isohookana R, Marttunen M, Hakko H, Riipinen P, & Riala K (2016). The impact of adverse childhood experiences on obesity and unhealthy weight control behaviors among adolescents. Comprehensive Psychiatry, 71, 17–24. 10.1016/j.comppsych.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Jackson DB, & Vaughn MG (2019). Obesogenic food consumption among young children: the role of maltreatment. Public Health Nutrition, 22(10), 1840–1849. 10.1017/s1368980019000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick SI, Reedy J, Krebs-Smith SM, Pannucci TRE, Subar AF, Wilson MM, … Tooze JA (2018). Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. Journal of the Academy of Nutrition and Dietetics, 118(9), 1603–1621. 10.1016/j.jand.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chassan RA, Bruer EH, Gower BA, & Shelton RC (2015). Childhood maltreatment increases the risk for visceral obesity. Obesity (Silver Spring, Md.), 23(8), 1625–1632. 10.1002/oby.21143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach JD, Dijkstra SC, Beulens JW, Seidell JC, Snijder MB, Stronks K, … Nicolaou M (2019). Socioeconomic and ethnic differences in the relation between dietary costs and dietary quality: the HELIUS study. Nutrition Journal, 18(1). 10.1186/s12937-019-0445-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques ES, Leite TH, de Oliveira AG, Cunha DB, Verly Júnior E, & Azeredo CM (2019). Association Between Family Physical Violence Victimization and Food Consumption Among Brazilian Adolescents. Journal of Interpersonal Violence, 088626051988466. 10.1177/0886260519884668 [DOI] [PubMed] [Google Scholar]
- Merrick MT, & Guinn AS (2018). Child Abuse and Neglect: Breaking the Intergenerational Link. American Journal of Public Health, 108(9), 1117–1118. 10.2105/ajph.2018.304636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler M, Merrick MT, Klevens J, Ports KA, & Ford DC (2017). Adverse childhood experiences and life opportunities: Shifting the narrative. Children and Youth Services Review, 72, 141–149. 10.1016/j.childyouth.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, & Lumeng JC (2018). Pathways of Association from Stress to Obesity in Early Childhood. Obesity, 26(7), 1117–1124. 10.1002/oby.22155 [DOI] [PubMed] [Google Scholar]
- Mutlu H, Bilgiç V, Erten S, Aras Ş, & Tayfur M (2016). Evaluation of the Relationship between Childhood Traumas and Adulthood Obesity Development. Ecology of Food and Nutrition, 55(4), 390–401. 10.1080/03670244.2016.1198791 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2017, August 29). Overview & Background of The Healthy Eating Index. Overview & Background of Healthy Eating Index (HEI) | EGRP/DCCPS/NCI/NIH. https://epi.grants.cancer.gov/hei/.
- National Cancer Institute. (2019, May). HEI Scoring Algorithm. HEI Scoring Algorithm | EGRP/DCCPS/NCI/NIH. https://epi.grants.cancer.gov/hei/hei-scoring-method.html.
- National Center for Injury Prevention. (2021, January 28). Preventing Multiple Forms of Violence: A Strategic Vision for Connecting the Dots. Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/about/connectingthedots.html. [Google Scholar]
- Non AL, Román JC, Clausing ES, Gilman SE, Loucks EB, Buka SL, … Kubzansky LD (2020). Optimism and Social Support Predict Healthier Adult Behaviors Despite Socially Disadvantaged Childhoods. International Journal of Behavioral Medicine, 27(2), 200–212. 10.1007/s12529-020-09849-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non AL, Román JC, Gross CL, Gilman SE, Loucks EB, Buka SL, & Kubzansky LD (2016). Early childhood social disadvantage is associated with poor health behaviours in adulthood. Annals of Human Biology, 43(2), 144–153. 10.3109/03014460.2015.1136357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biological Psychiatry, 80(1), 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion. (2019). Healthy People - Healthy People 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/healthy_people/hp2020.html. [Google Scholar]
- R Foundation for Statistical Computing. (2013). R Core Team. R: A language and environment for statistical computing. Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TRE, Wilson MM, … Tooze (2018). Evaluation of the Healthy Eating Index-2015. Journal of the Academy of Nutrition and Dietetics, 118(9), 1622–1633. 10.1016/j.jand.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AS, Dietz WH, & Gordon-Larsen P (2013). The association between childhood sexual and physical abuse with incident adult severe obesity across 13 years of the National Longitudinal Study of Adolescent Health. Pediatric Obesity, 9(5), 351–361. 10.1111/j.2047-6310.2013.00196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Hughes K, & Bellis MA (2016). Impact of childhood experience and adult well-being on eating preferences and behaviours. BMJ Open, 6(1). 10.1136/bmjopen-2015-007770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc Copyright ©. (2019). The computation of HEI for this paper was generated using SAS software, Version 9.4 for Windows. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. [Google Scholar]
- Sacks V, & Murphey D (2018). The prevalence of adverse childhood experiences, nationally, by state, and by race or ethnicity. Child Trends. https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity.
- Schwingshackl L, & Hoffmann G (2015). Diet Quality as Assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Journal of the Academy of Nutrition and Dietetics, 115(5). 10.1016/j.jand.2014.12.009 [DOI] [PubMed] [Google Scholar]
- Sokol RL, Ennett ST, Shanahan ME, Gottfredson NC, Poti JM, Halpern CT, & Fisher EB (2019). Maltreatment experience in childhood and average excess body mass from adolescence to young adulthood. Child Abuse & Neglect, 96, 104070. 10.1016/j.chiabu.2019.104070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RL, Gottfredson NC, Shanahan ME, & Halpern CT (2018). Relationship between child maltreatment and adolescent body mass index trajectories. Children and Youth Services Review, 93, 196–202. 10.1016/j.childyouth.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, … Zachariah JP (2018). Childhood and Adolescent Adversity and Cardiometabolic Outcomes: A Scientific Statement From the American Heart Association. Circulation, 137(5). 10.1161/cir.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. (2015, December). 2015-2020 Dietary Guidelines for Americans. USDA. https://www.fns.usda.gov/2015-2020-dietary-guidelines-americans. [Google Scholar]
- U.S. Department of Agriculture: Food and Nutrition Service. (2020). Healthy Eating Index (HEI). USDA. https://www.fns.usda.gov/resource/healthy-eating-index-hei. [Google Scholar]
- Vittinghoff E, C MCE, Glidden DV, & Shiboski SC (2011). 3 — Linear and Non-Linear Regression Methods in Epidemiology and Biostatistics. In Essential Statistical Methods for Medical Statistics (pp. 66–103). North-Holland, Elsevier B.V. [Google Scholar]
- Windle M, Haardörfer R, Getachew B, Shah J, Payne J, Pillai D, & Berg CJ (2018). A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. Journal of American College Health, 66(4), 246–251. 10.1080/07448481.2018.1431892 [DOI] [PMC free article] [PubMed] [Google Scholar]


