Abstract
Background
Up-regulated interleukin 6 (IL-6) signaling, immune system activation, and pronociceptive autoantibodies are characteristic of complex regional pain syndrome (CRPS). IL-6 is known to promote B cell differentiation, thus we hypothesized that IL-6 signaling plays a crucial role in the development of adaptive immune responses and nociceptive sensitization in a murine tibia fracture model of CRPS.
Methods
Mice deficient in IL-6 expression (IL-6−/−) or B cell deficient (muMT) underwent tibia fracture and 3 weeks of cast immobilization or sham injury. The deposition of IgM in fractured limbs was followed using Western blotting, and passive serum transfer to muMT fracture mice was used to detect nociception-supporting autoantibodies. Lymph nodes were assessed for hypertrophy, IL-6 expression was measured using qPCR and ELISA, and germinal center formation was evaluated using FACS and immunohistochemistry. The therapeutic effects of exogenous neutralizing anti-IL-6 antibodies were also evaluated in the CRPS fracture model.
Results
Functional IL-6 signaling was required for the post fracture development of nociceptive sensitization, vascular changes, and IgM immune complex deposition in the skin of injured limbs. Passive transfer of sera from wild-type, but not IL-6−/− fracture mice into muMT fracture mice caused enhanced allodynia and postural unweighting. IL-6−/− fracture mice displayed reduced popliteal lymphadenopathy after fracture. Germinal center responses were detected in the popliteal lymph nodes of wild-type, but not in IL-6−/− fracture mice. We observed that IL-6 expression was dramatically enhanced in popliteal lymph node tissue after fracture. Conversely, administration of anti-IL-6 antibodies reduced nociceptive and vascular changes after fracture and inhibited lymphadenopathy.
Conclusions
Collectively, these data support the hypothesis that IL-6 signaling in the fracture limb of mice is required for germinal center formation, IgM autoantibody production and nociceptive sensitization. Anti-IL-6 therapies might, therefore, reduce pain after limb fracture or in the setting of CRPS.
Keywords: Fracture, Complex regional pain syndrome, Pain, Autoimmunity, Germinal center, Interleukin 6
1. Introduction
Pain is a nearly ubiquitous experience following many types of traumatic injuries including surgeries, soft tissue strains, fractures, and others. While pain resolution generally parallels the healing of damaged tissues, the chronification of pain after trauma is now recognized to be a major public health issue and is included as a diagnostic category in the revamped ICD-11 classification of diseases (Treede et al., 2015). Chronic pain after surgery affects 10–80% of surgical patients (Kehlet et al., 2006), and unresolved postsurgical and posttraumatic pain is a major factor leading to pain clinic referrals (Barke et al., 2018; Crombie et al., 1998). Limb injuries and joint surgeries are particularly closely associated with lingering pain as approximately 20% or more of those with trauma to the hands as well as fractures of the ankle or wrist report pain and disability for a year or more after their injuries (Friesgaard et al., 2016; Richards et al., 2011). Although the etiology of chronic posttraumatic pain is enigmatic and may be heterogeneous, aberrant immune system function, dysregulated autonomic signaling, neuropeptide release, glial activation and neuroplastic changes in several regions of the brain have all been suggested as causes or contributors (Clark et al., 2018; Marinus et al., 2011). A more complete knowledge of these mechanisms may facilitate the design of interventions intended to disrupt these maladaptive changes and prevent the chronification of pain.
One specific type of chronic pain generally beginning with limb trauma or surgery and persisting long after the resolution of underlying tissue derangement is complex regional pain syndrome (CRPS). Recent observations directly implicate the activation of the adaptive immune system and presence of pain-supporting autoantibodies in patients with this condition. These observations include the passive transfer of human CRPS IgG to rats with hindpaw incisions leading to very delayed resolution of nociceptive sensitization and direct activation of afferent neurons (Cuhadar et al., 2019; Helyes et al., 2019). Other experiments from our group show that the transfer of IgM from human CRPS patients or IgM from tibia fracture/casted wild-type mice reconstitutes the pain phenotype in congenitally B lymphocyte deficient muMT fracture mice (Guo et al., 2017; Guo et al., 2020). The specificity of the autoantibodies involved is incompletely defined, but we demonstrated interactions with keratin and other proteins in skin and spinal cord (Guo et al., 2020; Tajerian et al., 2017). Very recently we showed that regional lymph node activation and autoantibody formation take place in mice after tibia fracture with casting, including the formation of germinal centers within 3 weeks of injuries (Li et al., 2020), although the signals supporting lymph node activation after fracture remain unknown.
The inflammatory cytokine IL-6 may be a key pain chronification immune modulator as patients exhibit increased skin IL-6 levels a month after hand surgery with cast immobilization, and tibia fracture followed by cast immobilization in rodents causes regional IL-6 elevations lasting for months (Birklein et al., 2018; Pepper et al., 2013). Likewise, studies of cytokines in the affected skin of patients with CRPS have shown IL-6 to be robustly elevated for months after the inciting injury, relative to levels observed in the contralateral limb (Birklein et al., 2014). Interleukin-6, originally labeled B-cell stimulatory factor 2 (BSF-2), is a well-known modulator of B cell maturation and immunoglobulin production (Chen-Kiang, 1995). With this in mind we designed a set of experiments to address the hypothesis that IL-6 is required for lymph node activation after limb fracture and the production of pain-supporting autoantibodies.
2. Materials and methods
2.1. Animals
All animal experiment protocols were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the National Institutes of Health guide for the care and use of Laboratory animals. Male C57BL/6J mice, B cell deficient mice (muMT), and IL-6−/− deficient mice (B6.129S2-IL-6tm1Kopf/J) were obtained from The Jackson Laboratory (Sacramento, CA, USA) at 8–12 weeks of age. Experiments were done after a 7–10-day acclimation period. The animals were housed 4 per cage under pathogen-free conditions with soft bedding and were given food and water ad libitum, with a 12:12 light:dark cycle. The animals were fed Teklad lab rodent diet 2018 (Teklad Diets; Harlan Laboratories, Indianapolis, IN, USA), which contains 1.0% calcium, 0.7% phosphorus, and 1.5 IU/g vitamin D3. Data collection was conducted blind to group assignment.
2.2. Tibia fracture surgery
The mouse fracture model was used (Birklein et al., 2018). Briefly, under isoflurane anesthesia, a hemostat was used to make a closed fracture of the right tibia just distal to the middle of the tibia. The hind limb was then wrapped in casting tape (Delta-Lite; BSN Medical, Hamburg, Germany) so the hip, knee, and ankle were all fixed. The cast extended from the metatarsals of the hind paw up to a spica formed around the abdomen. A window was left open over the dorsal paw and ankle to prevent constriction if post-fracture edema developed. After fracture and casting, the mice were given, subcutaneously, 2 days of buprenorphine (0.05 mg/kg) and baytril (5 mg/kg), as well as 1.0 mL of normal saline to maintain hydration. At 3 weeks after surgery, the mice were anesthetized with isoflurane to facilitate cast removal. All mice had union at the fracture site, by manual inspection.
2.3. Hind paw nociceptive testing
Allodynia - To measure mechanical allodynia in the mice, an up-down von Frey testing paradigm was used as we previously described (Guo et al., 2017). Briefly, mice were placed on wire mesh platforms in clear cylindrical plastic enclosures 10 cm in diameter and 40 cm in height, and after 15 minutes of acclimation, von Frey fibers of sequentially increasing stiffness were applied against the hind paw plantar skin at approximately midsole, taking care to avoid the tori pads, and pressed upward to cause a slight bend in the fiber and left in place for 5 seconds. Withdrawal of or licking the hind paw after fiber application was scored as a response. When no response was obtained, the next stiffest fiber in the series was applied to the same paw; if a response was obtained, a less stiff fiber was applied. Testing proceeded in this manner until 4 fibers had been applied. Estimation of the mechanical withdrawal threshold by data-fitting algorithm permitted the use of parametric statistics for analysis. Hind paw mechanical nociceptive thresholds were analyzed as the difference between the fractured side (right hind paw) and the contralateral unfractured side (left hind paw).
Hind paw unweighting - An incapacitance device (IITC Inc. Life Science, Woodland Hills, CA, USA) was used to measure hind paw unweighting. The mice were manually held in a vertical position over the apparatus with the hind paws resting on separate metal scale plates, and the entire weight of the mouse was supported on the hind paws. The duration of each measurement was 6 seconds, and 6 consecutive measurements were taken at 10-second intervals. All 6 readings were then averaged (Guo et al., 2017). Hind paw weight-bearing data were analyzed as a ratio between twice the right hind paw weight and the sum of right and left hind paw values ([2R/(R+L)]×100%).
2.4. Hind paw volume testing
A laser sensor technique was used to determine the dorsal-ventral thickness of the hind paw, as we have previously described (Guo et al., 2017). The measurement sensor device (Limab, Göteborg, Sweden) used in these experiments has a measurement range of 200mm, with a 0.01-mm resolution. Hind paw volume data were analyzed as the difference between the fractured side and the contralateral unfractured side.
2.5. Hind paw temperature testing
The temperature of the hind paw was measured using a fine-wire thermocouple (Omega Engineering Inc, Stamford, CT, USA) applied to the paw skin, as previously described (Guo et al., 2017). The investigator held the wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hind paw: the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hind paw, the same site was immediately tested in the contralateral hind paw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The 6 measurements for each hind paw were averaged for the mean temperature. Hind paw temperature data were analyzed as the difference between the fractured side and the contralateral unfractured side.
2.6. Passive transfer experiments in the fracture mouse CRPS model
The passive transfer of serum was performed as we described previously (Guo et al., 2017; Li et al., 2020). To prepare serum for passive transfer, blood was collected by transcardial puncture in isoflurane anesthetized wild-type 3-week post-fracture and IL-6−/− post-fracture mice. The blood was left undisturbed at room temperature for 60 min to allow clotting, then the blood samples were centrifuged at 1,500g for 20 min at 4°C, and the serum supernatants were aliquoted and frozen at −80°C.
Serum recipient muMT mice underwent tibia fracture and casting 3 weeks before use with casts removed one day before serum injection. These mice were injected with serum (500 μl/mouse, i.p.) originating from the various experimental groups. Nociceptive behavioral testing was performed at 1, 7, 14, and 21 days after injection.
2.7. Popliteal lymph node dissection and size measurement
This experiment looked at size of the popliteal lymph node with fracture in both wild-type and IL-6−/− mice (Li et al., 2020). Mice underwent a right distal tibia fracture and were casted for 3 weeks Non-fracture intact mice served as control. The popliteal lymph node is embedded in adipose tissue of the popliteal fossa and is spherical. To harvest these structures, mice were euthanized by carbon dioxide asphyxiation followed by cervical dislocation 3 or 7 weeks after fracture. The hair surrounding the foot-draining popliteal lymph nodes was clipped, the skin over the node was incised, and the subcutaneous tissue, fat, and fascia were carefully dissected under a low-power microscope. The lymph node size (diameter) was measured in millimeters (mm) using a caliper, with the average diameter for each lymph node defined as [(short-axis diameter + long-axis diameter)/2].
2.8. RNA extraction and real-time polymerase chain reaction
Animals were euthanized by carbon dioxide asphyxiation at 3 weeks after fracture. The popliteal lymph nodes were harvested and immediately snap-frozen on dry ice and stored at −80°C before RNA isolation. Total RNA was extracted using the GeneAll Hybrid-R kit (GeneAll Biotechnology, Seoul, South Korea), dissolved in RNase-free water, and the purity and concentration were determined spectrophotometrically. For real-time quantification of IL-6 RNA, validated primer sets from Qiagen, QuantiTect Primer Assays were used, together with the recommended QuantiTect SYBR Green one-step RT-PCR kit (Qiagen, Hilden, Germany). RNA was diluted in RNase free water in order to obtain the same input template concentration (0.5 ng/μl) for each reaction. The manufacturer’s protocol for PCR from Applied Biosysytems was followed. Briefly, reactions were prepared using QIAGEN OneStep RT-PCR kit, and each reaction contains 25 μl of 2x QuantiTect SYBR Green
RT-PCR Master Mix, 5 μl of 10x QuantiTect Primer Assay, 0.5 μl of QuantiTect RT Mix, 10 μl of Template RNA (8ng), and 9.5 μl of RNase-free water for a total volume of 50 μl. The ABI 7900HT real-time device was programmed as follows: reverse transcription at 50 C for 30 min, initial polymerase activation step at 95 C for 15 min followed by 3-step amplification cycles (denaturation at 94 C for 15 s, annealing at 55 C for 20 s and elongation at 72 C for 20 s) for 40 cycles. The fluorescence intensity reflecting the amount of double-stranded formed PCR-product was read in real-time at the end of each elongation step. All samples were run in duplicate together with appropriate non-template controls. The coefficient of variation was <2% for all replicates. The data from real-time polymerase chain reaction experiments were analyzed by the comparative cycle threshold method.
2.9. Protein extraction and enzyme-linked immunosorbent assay
The popliteal lymph nodes were collected from animals euthanized 3 weeks after fracture, and the tissue was minced into fine pieces in ice-cold phosphate-buffered saline, pH 7.4, containing a cocktail of protease inhibitors (Roche Applied Science, USA) and followed by homogenization using a TissurLyser LT (Qiagen, Hiden, Germany). Homogenates were centrifuged at 12,000g for 15 min at 4°C, and supernatant fractions were frozen at −80°C until required for enzyme-linked immunosorbent assay performance. An aliquot was subjected to protein assay (Bio-Rad Laboratories Inc, USA) to normalize mediator levels. Interleukin IL-6 protein levels were determined using mouse IL-6 ELISA kits (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Absorbance of standards and samples was determined spectrophotometrically at 450 nm using a microplate reader (Bio-Rad Laboratories Inc., USA). Results were plotted against the linear portion of the standard curve, and the protein concentration of each sample was expressed as pg/mg protein of sample.
2.10. Western blot analysis
This experiment examined IgM deposition in hindlimb after fracture in both wild-type fracture and IL-6−/− fracture mice. As we described previously (Guo et al., 2017), mouse hind paw skin was harvested and stored at −80°C. Tissues were homogenized in ice-cold Tris buffer with 0.7% (v/v) β-mercaptoethanol and 10% glycerol. Lysates were centrifuged at 13,000g for 15 minutes at 4°C, and the protein concentration of the supernatant was measured by protein assay reagent (Bio-Rad). Equal amounts of protein (50 μg) were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The blots were blocked overnight with 5% normal serum in Tris-buffered saline with 0.5% Tween-20, and incubated with primary antibodies against immunoglobulin M (IgM) or β-actin (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 hour on a rocking platform at room temperature. After 3 washes, the blots were incubated with secondary antibody for 1 hour at room temperature. The membrane was then washed again, and proteins were detected using ECL chemiluminescence reagent (GE Healthcare, Pittsburgh, PA, USA). The band intensities were quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA), IgM/Actin band intensity ratio was calculated to demonstrate the changes in skin IgM levels after fracture.
2.11. Fluorescence-activated cell sorting (FACS)
This experiment investigated whether IL-6 signaling is required for the germinal center response in the secondary lymphoid tissues after fracture. Mice from control non-fracture, wildtype fracture, IL-6−/− fracture were euthanized and the popliteal lymph nodes were immediately collected. and stored in 4 C Hanks balanced salt solution (HBSS; Gibco, Life Technologies, Grand Island, NY, USA). FACS analysis was performed as we previously described (Li et al., 2020). In brief, the nodes were passed through a 50-μm filter with HBSS. Cells were pelleted by centrifugation at 400g for 5 minutes, and re-suspended in fresh HBSS. Cells were diluted and transferred to fresh 5-mL tubes for staining. Prepared samples were kept on ice and in the dark during the staining procedure. About 1.25×106 cells were incubated with LIVE/DEAD Aqua (Invitrogen), washed, and incubated with unconjugated anti-CD16/CD32 (FcγRII/III) mAb to block Fc-receptors. Cells were then stained on ice for 20 min with a “cocktail” of fluorochrome-conjugated antibodies. After washing, 0.1–0.3 × 106 cells were analyzed on an Aria flow cytometer (BD Bioscience). Data were analyzed with FlowJo (TreeStar).
Fluorochrome-conjugated antibodies include: anti-CD38-Alexa488 (clone 90, Biolegend), anti-CD43-PE (clone S11, Biolegend), anti-CD5-PE-Cy5 (clone 53–7.3, Biolegend), anti-CD19-PE-Cy5.5 (clone ID3, Invitrogen, cat#35-0193-82), anti-IgG1-PE-Cy7(clone RMG1-1, Biolegend), anti-IgM-APC (clone RMM1, Biolegend), anti-IgD-APC-Cy7(clone 11-26c.2a, Biolegend), anti-CD95-Qdot605 (clone SA367H8, Biolegend), anti-CD11b-PB (clone M1/70, Biolegend), anti-Gr-1-PB(clone RB6-8C5, Biolegend), anti-TCRαβ-PB(clone H57, Invitrogen), anti-CD11c-PB (clone N418, Biolegend), anti-CD3ε-PB (clone 145-2C11, Biolegend), anti-F4/80-PB (clone BM8, Biolegend). Dead, myeloid, and T cells were gated out and live CD19 positive B cells were further characterized (live myeloid− CD3− CD19+) to reveal CD95 and CD38 surface expression. Germinal center B cells were defined as CD19+ CD38− CD95+.
2.12. Tissue processing and immunofluorescence confocal microscopy
Confocal microscopy was performed to detect if IL-6 signaling mediates germinal center formation after fracture. Mice were euthanized and immediately perfused with 4% paraformaldehyde (PFA) in PBS, pH 7.4, via the ascending aorta; the popliteal lymph nodes were removed and post-fixed in 4% PFA for 4 hours, and then the tissues were treated with 10%, 20%, and 30% sucrose in PBS at 4 °C for 30 min respectively followed by 30% sucrose at 4 C overnight. The tissue was embedded in optimum cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA), and 6-μm thick slices were made using a cryostat, mounted onto Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA, USA), and stored at −80 C.
Frozen sections were permeabilized and blocked with “immunomix” staining solution (i.e., 0.3% Triton X-100, 0.2% bovine serum albumin, and 0.1% sodium azide in 1X PBS) containing 5% normal donkey serum and followed by incubated with rat anti-mouse CD16/CD32 (clone 2.4G2, mouse BD Fc block) before primary antibody incubation. Sections were incubated in primary antibody cocktail, i.e., FITC conjugated rat anti-mouse T- and B-cell activation antigen (Clone GL7), 1:100 (BD Pharmingen, USA), Alexa Flour 647 anti-mouse CD279 (PD-1, clone 29F.1A12), 1:100 (BioLegend), and PE rat anti-mouse IgD (clone 11-26c), 1:100 (eBioscience™, USA) diluted in imunomix containing 1% donkey serum at 4 °C overnight. After washing in PBS, the sections were counterstained with DAPI (diluted 1:3000; Thermo Scientific, Waltham, MA, USA) to locate nuclei. After 3 washes, the sections were mounted with anti-fade mounting medium (Invitrogen, Life Technologies). Images were visualized and captured using a confocal microscope (Zeiss LSM710, Carl Zeiss, Jena, Germany). Control experiments included incubation of slices in primary and secondary antibody-free solutions, both of which led to low-intensity nonspecific staining patterns in preliminary experiments (data not shown).
2.13. Chronic administration of anti-mouse IL-6 treatments in the fracture mouse CRPS model
Mice were subjected to repeated administration of neutralizing anti-IL-6 or isotype control IgG while casted after fracture. Mice underwent tibia fracture and were casted for 3 weeks. The mice received twice per week intraperitoneal (i.p.) injections of 400 ug InVivoMab antibody (BioXCell, Lebanon, NH) with the first dose immediately following fracture and the last one at the time of cast removal. Hind paw nociceptive testing and assessment of warmth and edema were performed prior to fracture and 3 weeks after fracture at 1 day following cast removal. Then the hindpaw skin and spinal cord were collected for IgM analysis by ELISA and the popliteal lymph nodes were dissected to measure hypertrophy.
2.14. Statistical analysis
Datasets involving 3 or more independent groups were analyzed using 1-way analysis of variance followed by Sidak’s multiple comparisons test (Figure 2, 4, 5, 7, 8, 9, and 10), others were analyzed using repeated measures 2-way analysis of variance with Sidak’s correction for multiple comparisons (Figure 1 and 3). All data are presented as the mean ± SEM, and differences are considered significant at a P value <0.05 (Prism 6, GraphPad Software, La Jolla, CA, USA).
Figure 2.
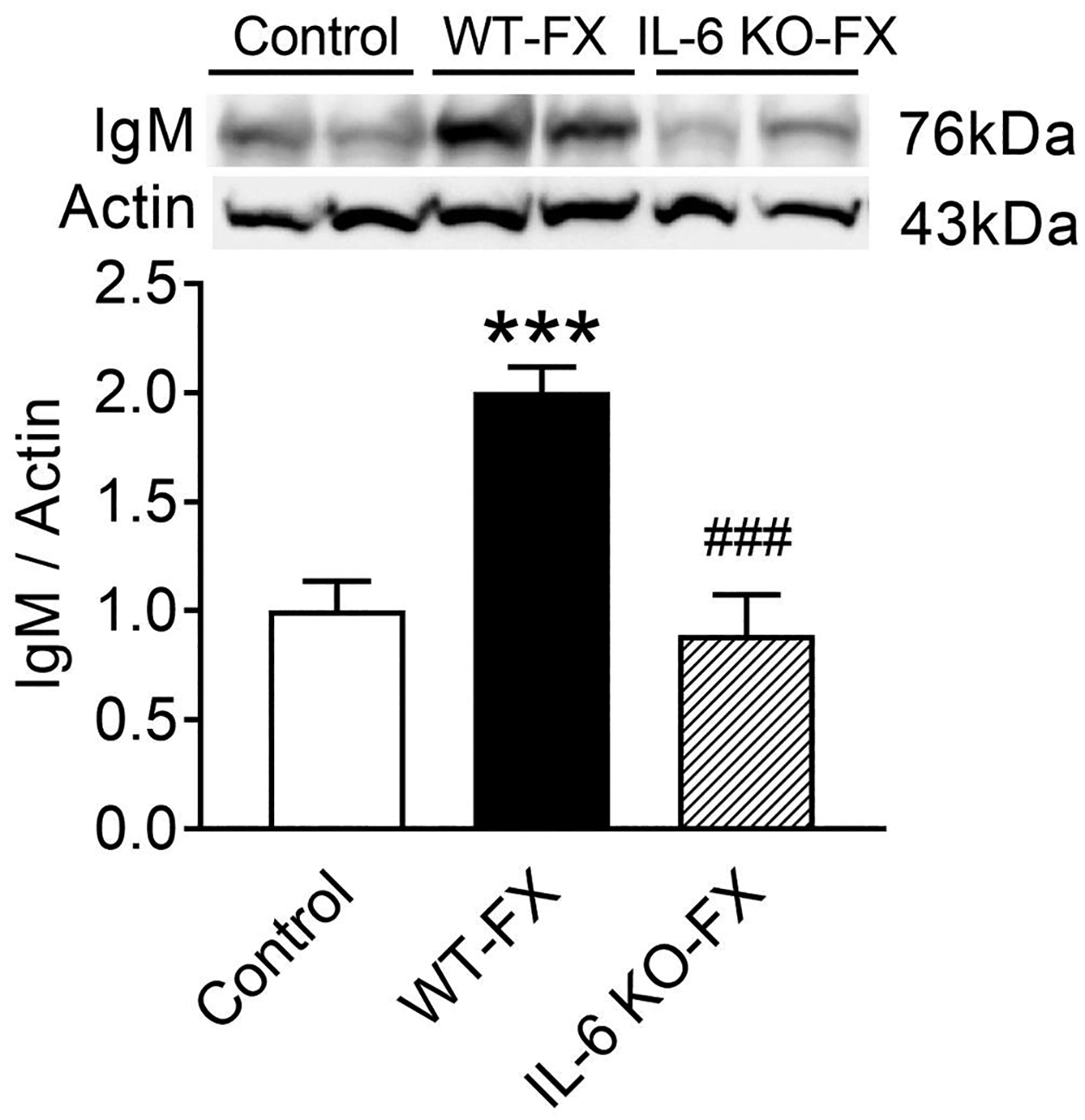
Western blot analysis of immunoglobulin M (IgM) protein deposition in hind paw skin tissue after tibial fracture and cast immobilization. Compared with control non-fracture wild-type (Control) mice, IgM protein levels were greatly increased in the ipsilateral hindpaw skin of WT fracture (WT-FX) mice, but the hindpaw skin IgM levels in IL-6 deficient mice did not increase after fracture (IL-6 KO-FX). Data were analyzed using a one-way analysis of variance (ANOVA) with a Holm-Sidak’s correction test for post hoc contrasts. Data are expressed means ± SEM, n = 6 per cohort analyzed in duplicate. ***P < 0.001 vs. control, ### p<0.001 compared to WT-FX.
Figure 4.

Hypertrophy of the popliteal lymph node at 3 and 7 weeks (wk3, wk7) after fracture (FX) in wild-type (WT) and IL-6 deficient (IL-6 KO) mice. Post-FX popliteal lymphadenopathy was observed at 3 and 7 weeks after FX with maximum hypertrophy occurring at 3 weeks after fracture in WT mice, compared to non-fractured wild-type controls (Control). In contrast, in the IL-6 KO-FX mice popliteal lymph node hypertrophy was significantly reduced at 3 weeks, relative to WT-FX mice, and normalized at 7 weeks after fracture, compared to controls. Data were analyzed using a one-way analysis of variance with a Holm-Sidak’s test for post hoc contrasts, error bars indicate SEM, n = 10 per cohort. ***P < 0.001 vs. Control, ##P < 0.01 and ###P < 0.001 vs. WT-FX wk3, $P < 0.05 vs IL-6 KO-FX wk3.
Figure 5.

FACS analysis of germinal center (GC) B cells in popliteal lymph node tissue at 3 weeks after fracture. At 3 weeks post-fracture there was a dramatic increase in GC B cells the popliteal lymph nodes of wild-type fracture mice (WT-FX), whereas IL-6 deficient fracture mice (IL-6 KO-FX) demonstrated only a minimal increase in GC B cell numbers at 3 weeks post-fracture. (A) FACS plots showing GC B cells in popliteal lymph nodes of control, wild-type fracture mice (WT-FX), and IL-6 knockout fracture mice (IL-6 KO-FX) mice. Live T and myeloid cells-negative, CD19-positive cells (myeloid−CD3−CD19+) are gated to reveal CD95 and CD38 surface expression. GC B cells are defined as (CD19+CD38−CD95+). (B) Absolute numbers of GC B cells in popliteal lymph nodes of control mice, or mice of WT-FX, IL-6KO-FX at 3 weeks after fracture are shown. Data were analyzed using a one-way analysis of variance with a Holm-Sidak’s correction test for post hoc contrasts, error bars indicate SEM. **P < 0.01 for WT-FX (n = 8) vs. Control (n = 8), ## P < 0.01 for IL-6 KO-FX (n = 8) vs. FX-WT (n = 8).
Figure 7.

Post-fracture IL-6 mRNA and protein expression in the ipsilateral popliteal lymph node. Levels of IL-6 mRNA and protein expression were detected using quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assays (ELISA). At 3 weeks after fracture in wild-type mice the IL-6 mRNA (A) and protein (B) levels were greatly increased in the node ipsilateral to the fracture (FX-Ipsi), but no changes detected in the contralateral (FX-Contra), compared to non-fractured controls (Control). Data were analyzed using a one-way analysis of variance with a Holm-Sidak’s correction test for post hoc contrasts, error bars indicate SD, **p < 0.01, ***p < 0.001 for FX-Ipsi (n = 8 in A, n=10 in B) vs Control values (n=8), #P < 0.05, and ##P < 0.01 for FX-Contra (n=8 in A, n = 4 in B) vs. FX-Ipsi (n = 8 in A, n=10 in B).
Figure 8.

Changes in size of the popliteal lymph node after fracture in anti-IL-6 and control IgG treated wild-type mice. Wild-type 3-week fracture (FX) mice injected with control IgG developed hypertrophic popliteal lymph nodes in the ipsilateral, but not the contralateral hindlimbs. Chronic administration of anti-IL-6 (400ug i.p. twice a week for 3 weeks) reduced ipsilateral lymph node hypertrophy at 3 weeks after fracture, compared to control IgG injections. The average diameter of popliteal lymph nodes in FX+anti-IL-6 mice was 23.5 % smaller at 3 weeks after fracture (1.7 ± 0.11 mm in FX+anti-IL-6, n = 8 vs 1.3 ± 0.17 mm in FX+Control IgG groups, n = 8). Data were analyzed using a one-way analysis of variance with a Holm-Sidak’s correction test for post hoc contrasts, error bars indicate SD, ***p < 0.001 vs contralateral, and ##P < 0.01 for FX+anti-IL-6 vs FX+IgG. FX: fracture.
Figure 9.

Western blot analysis of immunoglobulin M (IgM) protein deposition in hind paw skin tissue after tibial fracture and cast immobilization in mice treated with anti-IL-6 IgG, or control IgG. Compared with control non-fracture wild-type (Control) mice, IgM protein levels were greatly increased in the ipsilateral hindpaw skin of WT fracture (WT-FX) mice and separate fracture mice treated with control IgG. However, the hindpaw skin IgM levels in fracture mice treated with anti-IL-6 IgG. Data were analyzed using a one-way analysis of variance (ANOVA) with a Holm-Sidak’s correction test for post hoc contrasts. Data are expressed means ± SEM, n = 4 per cohort analyzed in duplicate. ***P < 0.001 vs. control, ### p<0.001 compared to WT-FX.
Figure 10.

Chronic administration of anti-IL-6 significantly reduced nociceptive and vascular changes after fracture. Two groups of wild-type mice were fractured/casted and subjected to chronic intraperitoneal administration of either 400 ug of anti-IL-6 (n=8) or control IgG (n=8) twice a week for 3 weeks. Hind paw nociceptive testing and assessment of warmth and edema were performed prior to fracture (baseline; BL) and 3 weeks after fracture (1 day after cast removal; 3wk). At 3 weeks after fracture the control IgG treated mice exhibited hindpaw allodynia (A), unweighting (B), edema (C), and warmth (D) similar to that observed in untreated wild-type fracture mice (Fig. 1). Anti-IL-6 treatments reduced hindpaw allodynia (A), unweighting (B), and warmth (D). No significant changes in hindpaw edema were observed after anti-IL-6 treatment, although there was a nonsignificant reduction in edema (C). Data were analyzed using a one-way analysis of variance with a Holm-Sidak’s correction test for post hoc contrasts, error bars indicate SEM, **p < 0.01, ***p < 0.001 for FX+IgG 3wk vs FX+IgG BL or FX+anti-IL-6 3wk vs FX+anti-IL-6 BL, #P < 0.05, ##P < 0.01, and ###P < 0.001 for FX+anti-IL-6 3wk vs FX+IgG 3wk. FX: fracture, BL: baseline, 3wk: 3 weeks.
Figure 1.

Post-fracture nociceptive and vascular changes in wild-type (WT) and interleukin (IL)-6 deficient (IL-6 KO) mice. Both WT and IL-6 KO mice underwent distal tibia fracture with hind limb casting for 3 weeks, then the cast was removed and behavioral testing performed prior to fracture (baseline) and at 3, and 7 weeks after fracture. A repeated measures 2-way analysis of variance was used to test the effects of IL-6-deficiency on the dependent variables, with a Holm-Sidak’s correction. WT fracture mice developed mechanical allodynia (A), unweighting (B), warmth (C), and edema (D), but IL-6 KO fracture mice exhibited a reduction in von Frey mechanical allodynia (A; 3 and 7 weeks), unweighting (B; 3, and 7 weeks), warmth (C; 3 week), and edema (D; 3 week). *P<0.05, **P<0.01, and ***P <0.001 for WT fracture (n = 10) vs IL-6 KO fracture (n = 10). ### p<0.001 compared to Baseline (n=10). Data are expressed as mean values ± SEM.
Figure 3.

Intraperitoneal injection of sera from wild-type fracture mice (WT/FX) had pronociceptive effects in B cell deficient muMT fracture mice (muMT/FX), while injection of sera from IL-6 deficient FX mice (IL-6 KO/FX) had no effect. When serum from 7-week post-fracture WT mice was injected (500 μl, i.p.) into 7-week post-FX muMT mice, the mice gradually developed increased hindpaw von Frey allodynia (A) and unweighting (B) over the ensuing week, and consistent with the half-life of immunoglobulin, these pronociceptive effects resolved by 2 weeks post-injection. The pronociceptive effects of the WT/FX serum were restricted to the FX limb and not observed in nonfractured mice (data not shown). Injecting serum from IL-6 KO 7-week FX mice did not alter hindpaw von Frey allodynia (A) and unweighting (B) in the muMT/FX mice. A 2-way repeated measures analysis of variance was performed followed by a Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. N = 8 per cohort. #P < 0.05, ##P < 0.01, ###P < 0.001 for WT/FX serum vs. IL-6 KO/FX serum.
3. Results
3.1. Reduction of CRPS-like changes in IL-6−/− tibia fracture mice
Interleukin IL-6, formerly known as B cell stimulatory factor, is strongly involved in supporting adaptive immunity including the differentiation of B cells into antibody-producing plasma cells (Hunter and Jones, 2015). We first hypothesized that IL-6 support is required for nociceptive sensitization after limb trauma. To examine this hypothesis, we employed the mouse fracture/cast CRPS model.
As showed in Figure 1, wildtype mice developed hindpaw allodynia, unweighting, warmth, and edema at 3-, and 7-weeks post-fracture, but the IL-6−/− mice had significantly less hindlimb allodynia and unweighting than WT fracture mice across this time period. Similarly, IL-6 deficient mice also failed to develop significant hindpaw warmth or edema. These findings suggest that IL-6 signaling supports nociceptive and vascular changes observed in the CRPS mouse model.
3.2. IL-6 signaling contributes to IgM deposition in hindlimb skin after fracture
The above observations and the essential role of B cell activation and autoantibody formation in post-fracture sensitization prompted us to ask whether IL-6 signaling was required for IgM immune complex deposition in hindpaw skin tissue after limb fracture. Western immunoblots were therefore undertaken skin from wild-type non-fracture controls, wild-type fractured, and IL-6−/− fractured mice. As showed in Figure 2, compared to non-fracture control mice, the 7-week post fracture WT mice exhibited two-fold IgM increases in skin (Fig. 2), while IL-6−/− mice had attenuated levels of IgM in the hindlimb after fracture. This result suggests that IL-6 signaling promotes post-fracture IgM deposition in the injured hindlimb.
3.3. Sera from IL-6−/− fracture mice have reduced pronociceptive effects when injected into B-cell deficient fracture mice
Based on the behavioral and biochemical data, passive serum transfer experiments were designed to test the hypothesis that intact IL-6 signaling is required to produce pronociceptive autoantibodies after fracture and casting in mice. Both wild-type and IL-6−/− mice were fractured and casted for 3 weeks. One day after cast removal the sera were collected for injection into 3-week post-fracture muMT mice. Nociceptive behavioral tests were performed before and at 1, 7, 14, and 21 days after the injection. Consistent with our previous finding (Guo et al., 2017), the fracture muMT mice that received sera from fracture wild-type mice, gradually developed increased hindpaw von Frey allodynia (Figure 3A) and unweighting (Figure 3B) over the ensuing week. Allodynia was the more sensitive measure, although the time courses of changes for the two measures were compatible. Consistent with the half-life of IgM, these pronociceptive effects resolved by 2 weeks post-injection. However, fracture muMT mice receiving fracture sera from IL-6−/− mice did not exhibit hindpaw nociceptive or postural changes. Analysis of data from non-fractured limbs showed no sensitization due to passive transfer (data not shown) consistent with our previous results (Guo et al., 2017). These results indicate that a fracture-induced component of fracture mouse serum, likely IgM, has pronociceptive effects, and IL-6 signaling is crucial for the development of these pronociceptive serum effects.
3.4. IL-6 deficient fracture mice have reduced popliteal lymphadenopathy after fracture
We recently observed that limb fracture mice exhibit lymph node hypertrophy (Li et al., 2020) consistent with other’s observations in laboratory animals and humans after fracture (Szczesny et al., 2004). Based on these studies we determined whether IL-6 signaling plays any role in lymphadenopathy after fracture. Mice underwent a right distal tibia fracture and cast immobilization for 3 weeks, then the casts were removed. As shown in Figure 4, tibia fracture in wild-type mice was accompanied by significant enlargement of popliteal lymph nodes at 3 and 7 weeks after fracture, but lymph node hypertrophy was less significant in IL-6 deficient fracture mice. The average diameter of popliteal lymph nodes in wild-type mice was 194% and 163% greater at 3 and 7 weeks after fracture respectively (1.9 ± 0.27 mm in 3-week post-fracture n = 10 vs 0.98 ± 0.1 mm in control groups, n = 10; 1.60 ± 0.20 in 7-week post-fracture n = 10 vs 0.98 ± 0.1 mm in control groups, n = 10) in wild-type fracture mice, but only 143% and 122% greater in IL-6−/− mice at 3 and 7 weeks after fracture respectively (1.4 ± 0.13 mm in 3-week post-fracture n = 10 vs 0.98 ± 0.1 mm in control groups, n = 10; 1.2 ± 0.24 in 7-week post-fracture n = 10 vs 0.98 ± 0.1 mm in control groups, n = 10). These findings suggest that IL-6 signaling contributed to post-fracture popliteal lymphadenopathy.
3.5. IL-6 deficiency blocked post-fracture germinal center B cell differentiation
Germinal centers are sites formed within peripheral lymphoid organs such as lymph nodes in which B cells proliferate and differentiate. These B cells form antibody-secreting plasma cells and memory B cells, thus playing an important role in adaptive immunity. Using fluorescence-activated cell sorting (FACS) analysis, we recently observed that germinal center responses gradually develop in the ipsilateral hypertrophic popliteal lymph nodes of fracture mice, but not in other lymphoid tissues such as the contralateral popliteal lymph nodes or spleen (Li et al., 2020). In the current study we analyzed post-fracture germinal center B cell differentiation using FACS analysis of cell suspensions from lymph node tissues. As showed in Figure 5, germinal center B cells (CD38− CD95+) are rare in popliteal lymph nodes from uninjured control mice. Contrariwise, at 3 weeks after fracture there was a dramatic 53-fold increase in germinal center B cells in the ipsilateral popliteal lymph node and only a slight increase in germinal center B cells were observed in nodes from fractured IL-6−/− mice. Collectively, these data indicate that IL-6 signaling is critical for germinal center response in the regional lymph nodes following the limb fracture.
3.6. Immunohistochemical analysis demonstrates that post-fracture germinal center formation is impaired in IL-6−/− fracture mice
This study used immunohistochemical techniques to further examine IL-6 signaling in the regulation of lymph node germinal center response 3 weeks after fracture, the time point of maximal germinal center formation per FACS analysis (Li et al., 2020). Figure 6 illustrates that both germinal center cell types, i.e., the T follicular helper (Tfh) cells defined as cells expressing high levels of the programmed cell death-1 marker (PD-1) and the germinal center B cells defined as expressing the GL7 marker, were induced in lymph nodes from fracture mice. These popliteal lymph node germinal centers were surrounded by immature B cells defined by their expression of IgD. Only rare germinal center cell B cells or Tfh cells were identified in the popliteal lymph nodes of control animals (Figure 6). However, germinal center B cell and Tfh cells are barely detected in the ipsilateral popliteal lymph nodes from IL-6−/− mice 3 weeks after fracture (Figure 6). These data support the FACS results and demonstrate that IL-6 signaling is required for fracture-induced germinal center formation.
Figure 6.

Representative fluorescent photomicrographs of immunostaining for DAPI (counterstain to show DNA content and nuclei, Blue) GL7 (a murine germinal center (GC) B-cell marker, green), IgD1 (immature B-cell marker, yellow), and PD1 (T follicular helper cell (Tfh) marker, red) in the popliteal lymph node at 3 weeks post-fracture (FX). Top panels are from a popliteal lymph node collected at 3 weeks after fracture in a wild-type mouse (WT-FX) on the side contralateral to fracture, middle panels are from the ipsilateral popliteal lymph node a 3 weeks after fracture, and the bottom panels are from an IL-6 deficient fracture mouse (IL-6 KO-FX) ipsilateral popliteal lymph node collected 3 weeks after fracture. Triple labelling demonstrates activated B-cell (GC B-cell) and Tfh cells surrounded by immature B cells in the ipsilateral popliteal lymph node at 3 weeks after fracture. The GC B-cells are intimately with Tfh cells. However, GC B-cell and Tfh cells are barely detected in the ipsilateral popliteal lymph node of IL-6 deficient fracture mice. This image demonstrates that IL-6 signaling is required for fracture induced germinal center formation. Scale bar = 50 μm.
3.7. Tibial fracture enhances IL-6 mRNA and protein expression in popliteal lymph node tissue
In this study, two sets of experiments were used to measure IL-6 expression in popliteal lymph node tissue after fracture. First, we evaluated IL-6 mRNA levels using qPCR. This experiment revealed that IL-6 mRNA levels in the fracture popliteal lymph nodes were significant higher (2.2-fold) at 3 weeks after fracture as compared to non-fracture control mice (Figure 7A). Second, we looked at IL-6 protein levels using ELISA. Figure 7B shows a 4-fold elevation in levels of IL-6 protein in ipsilateral popliteal lymph node tissue vs non-fracture controls. Furthermore, there were no significant changes in IL-6 mRNA or protein levels in the contralateral popliteal lymph nodes. These data indicate that tibial fracture induced up-regulated IL-6 expression only in injured limb draining lymph nodes.
3.8. Chronic administration of anti-IL-6 reduces lymph node hypertrophy and nociceptive changes after fracture
The IL-6 deficient fracture mouse results prompted us to look at the potential therapeutic efficacy of IL-6 neutralization after fracture. Two groups of mice were fractured and subjected to intraperitoneal administration of either 400 ug anti-IL-6 or isotype control IgG twice a week for 3 weeks. Hind paw nociceptive testing and assessment of warmth and edema were performed at 3 weeks after fracture (1 day after cast removal). The hindpaw skin was collected for IgM analysis and the popliteal lymph nodes were dissected to measure hypertrophy.
As shown in Figure 8, intraperitoneal administration of the anti-IL-6 antibody partially reduced lymph hypertrophy. The average diameter of popliteal lymph nodes in FX+anti-IL-6 mice was smaller at 3 weeks after fracture. The data in Figure 9 demonstrate that repeated administration of anti-IL-6 suppressed the deposition of IgM in hindpaw skin after fracture. Finally, as shown in Figure 10, after tibia fracture and 3 weeks cast immobilization, the control IgG antibody-treated mice developed hind paw mechanical allodynia, unweighting, warmth, and edema. However, anti-IL-6 treatment significantly reduced both nociceptive and vascular changes suggesting that neutralizing IL-6 antibodies can inhibit the development of CRPS-like changes after fracture.
4. Discussion
The resolution of pain after trauma and surgery is highly variable with some patients experiencing pain long after healing of the underlying injuries potentially leading to excessive health care costs, loss of employment, and prolonged suffering (Kehlet et al., 2006). Of the many mechanisms suggested to play a role in the development and maintenance of chronic post-traumatic pain, an increasing body of experimental and clinical data suggests that injury-induced autoimmune responses contribute to pain chronification. In this series of studies, we advance the understanding of post-traumatic autoimmunity in a well-characterized tibia fracture chronic pain model (Birklein et al., 2018), utilizing IL-6 deficient fracture mice. The IL-6 −/− fracture mice, relative to wild-type mice, exhibited; 1) diminished pain-related behaviors, 2) reduced popliteal lymphadenopathy, 3) failure to develop post-fracture germinal center B cell responses or increased IgM immune complex deposition in the injured limb, and 4) absence of pronociceptive serum effects. Furthermore, we observed the up-regulation of IL-6 in popliteal lymph nodes ipsilateral but not contralateral to tibial fracture. Clinically it has been observed that anti-IL-6 therapy reduces autoantibody titers in autoimmune diseases such as in rheumatoid arthritis (Noguchi et al., 2020). Thus, while the pleiotropic cytokine IL-6 has long been associated with acute pain, perhaps by directly activating local nociceptive neurons (Zhou et al., 2016), we now provide evidence that trauma-related IL-6 production may support longer term pronociceptive autoimmune responses. A schematic diagram of past observations regarding IL-6 and chronic pain after fracture and our present findings is provided as Figure 11.
Figure 11.

Diagram the dual actions of IL-6 in supporting chronic pain after fracture. Fracture and other forms of trauma lead to the generation of IL-6 in tissues surrounding the injury and in regional lymph nodes. This cytokine can act directly on nociceptive neurons to support spontaneous and evoked pain. Germinal centers are also formed in response to IL-6 production in lymph nodes, and pain-supporting autoantibodies are formed. Agents that sequester IL-6 or ones that prevent the formation of antibody secreting cells block these pain-supporting pathways.
Autoimmunity has long been associated with certain painful conditions including rheumatic diseases, e.g. rheumatoid arthritis and ankylosing spondylitis, as well as neurological conditions, e.g. Gillian-Bare Syndrome and rare paraneoplastic neuropathies (Zis et al., 2017). More recently, however, information has accumulated suggesting that chronic pain after injuries might also have an autoimmune etiology. For example, genetic studies have reported that specific human leukocyte antigen (HLA) alleles are associated with CRPS, specifically HLA-DQ8 and HLA-B62 (van Rooijen et al., 2012). These and other HLA antigens are associated with autoimmune diseases of many types (Djilali-Saiah et al., 2006). Additional studies using antibodies purified from CRPS patient sera showed reactivity with autonomic neurons and specific cell surface receptors such as the alpha-1 and beta-2 adrenergic receptors (Dubuis et al., 2014); autonomic dysregulation is commonly noted in CRPS (Knudsen et al., 2019). The task of autoantibody discovery in CRPS was also approached in hypothesis-free fashion using liquid chromatography-mass spectrometry (LC-MS). In these experiments, IgM class autoantibodies against structural and cytoskeletal proteins such as keratin as well as nuclear proteins such as histones were identified by our group in the sera of mice weeks after tibia fracture and the sera of CRPS patients (Guo et al., 2020; Tajerian et al., 2017). Unclear to this point has been whether trauma exposes antigens in tissue that normally have some degree of immune privilege, or whether “neo” antigens are expressed in the injured limb after fracture thus stimulating an autoimmune response.
The results of experiments in which either the serum or purified IgG and IgM antibodies were administered to recipient laboratory animals such as done in the present studies have been informative. For example, the administration of IgG purified from well-characterized patients with CRPS prolonged allodynia caused by a small hindpaw incision for weeks (Cuhadar et al., 2019). In the same set of experiments, it was shown that CRPS IgG interacted with afferent neurons to sensitize those fibers to stimulation. Recently we observed that systemic administration of serum or purified IgM but not IgG from fractured wild-type mice induced nociceptive sensitization and unweighting in the healed fracture limb of muMT lacking immunoglobulin (Guo et al., 2017). Furthermore, we observed pro-nociceptive effects of early phase CRPS patient IgM but not IgG when passively transferred to tibia fractured mice (Guo et al., 2020). Thus the IgM-mediated effects might be more important for symptoms manifest during the first several months to one year after the onset of CRPS. Interestingly, it required approximately 3 weeks for pronociceptive IgM autoantibodies to develop in the wild-type fracture mice, paralleling the time course for germinal center formation in the ipsilateral popliteal lymph node and the deposition of IgM immune complexes in the fracture limb skin(Li et al., 2020). On the other hand, maneuvers that blocked T follicular helper cell signaling and germinal center formation, such as the administration of the calcineurin inhibitor tacrolimus (FK506), blocked pain-related autoantibody production (Li et al., 2020). In the current study we identify IL-6 to be necessary for the activation of the regional lymph nodes and the production of pain-related autoantibodies; we found no pronociceptive effects after serum transfer from IL-6−/− fracture mice and observed no significant deposition of IgM in the fracture limb skin of these mice.
The pro-inflammatory cytokine under study, IL-6, is very strongly associated with trauma. It is rapidly generated in injured tissues, and an elevated abundance of the cytokine can be measured for days in samples from surgical wound drains, the skin surrounding wounds and plasma (Di Vita et al., 2006). Many patients with CRPS show elevated levels of IL-6 in skin ipsilateral compared with contralateral for 30 months or more after diagnosis (Munnikes et al., 2005). One recent study conducted by our group linked elevated skin levels of IL-6 five weeks after hand surgery to the likelihood of experiencing pain in the surgical hand a month later (Pepper et al., 2013). Cytokines including IL-6 can support pain by acting on nociceptive neurons directly (Zhou et al., 2016). However, in its capacity as a mediator between the innate and adaptive systems of immunity, IL-6 supports the maturation of B cells and antibody production (Chen-Kiang, 1995) as well as supporting the induction of antigen-specific T cells in models of experimental autoimmune encephalomyelitis (Serada et al., 2008). Germinal center size in lymph nodes is diminished in IL-6 deficient mice (Kopf et al., 1998), and the follicular dendritic cell is one of the cell types supplying lymph nodes with IL-6 (Wu et al., 2009). Lymphocytes in the germinal centers were noted not to be major producers of IL-6 in previous studies (Kopf et al., 1998; Wu et al., 2009). Interestingly autoreactive B cells may themselves produce much of the IL-6 necessary for T follicular helper cell activation and germinal center formation (Arkatkar et al., 2017).
We documented strong increases in IL-6 mRNA and protein in popliteal lymph nodes ipsilateral but not contralateral to tibial fractures in the present experiments. Conversely, mice engineered to produce excess IL-6 display hypergammaglobulinemia, lymphadenopathy and extensive plasma cell infiltration of lymph nodes, spleen, liver, and lung (Brandt et al., 1990). Thus, our observations of diminished lymph node size, reduced germinal center formation and failure to develop pain-related autoantibodies after limb trauma in IL-6−/− mice, along with enhanced IL-6 production after fracture, are in line with the known immunological functions of IL-6.
The strategy of reducing IL-6 signaling may be a viable one for those suffering from chronic post-traumatic pain. There exist FDA-approved anti-IL-6 or anti-IL-6 receptor biologic drugs, e.g. tocilizumab, sarilumab, and siltuximab, that are used clinically to fight rheumatologic diseases or Castleman’s disease, a condition characterized by lymph node enlargement (Choy et al., 2020). While a reduction in pain-related autoantibody formation would be predicted based on the present studies, it is also the case that reducing IL-6 signaling could reduce pain by lessening the sensitization of nociceptors. For example, we observed that the local injection of a small molecule IL-6 inhibitor reduced hindpaw sensitization within minutes of administration in the same tibia fracture model used for these studies (Shi et al., 2018). IL-6-mediated effects may result from direct sensitization of nociceptive neurons or through the enhanced expression of TRPA1 channels (Malsch et al., 2014). Additional mechanisms for IL-6 induced sensitization include the enhancement of transcription and translation of several other genes including Janus-activated kinase/signal transducer activator of transcription (JAK/STAT), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) (Zhou et al., 2016). It should be borne in mind, however, that prolonged anti-IL-6 treatment may predispose patients to infection as has been demonstrated in long-term registry and surveillance studies (Narazaki and Kishimoto, 2018).
While our data are consistent with and expand the understanding of the roles of IL-6 in post-traumatic chronic pain, there are limitations to the work. For example, we studied a single sex of mice. While the laboratory has previously characterized sex differences in the tibia fracture model, including post-fracture changes in cutaneous and spinal IL-6 expression (Tajerian et al., 2015), we have not specifically addressed sex-related roles in the function of IL-6. We have observed, however, that the development of pain-related adaptive immune changes is delayed but robust in female versus male mice after fracture (Guo et al., 2019). Also, while we did use both nociceptive and functional endpoints in these studies, we did not study memory or changes related to affect in the fracture model mice, both of which are important consequences of chronic pain (Tajerian et al., 2014). Nevertheless, further study of the roles of IL-6 after limb trauma and surgery and the pursuit of anti-IL-6 therapies for those with slowly resolving post-traumatic pain should be considered.
Highlights.
Limb fracture in mice caused pain sensitization, regional lymph node hypertrophy and germinal center formation.
The passive transfer of serum from fractured wild-type to fractured B-cell deficient mice recapitulation the pain phenotype in the recipient mice.
Genetic deletion of IL-6 or the administration of anti-IL-6 antibodies after limb fracture prevented the pain, immunological and passive transfer effects.
Funding:
This work was supported by the National Institutes of Health R01NS117340 (JDC), R01NS094438 (WSK, JDC), R01AI128839 (LAH); the Department of Defense CP190028 (JDC); the Department of Veterans Affairs I01RX001475 (JDC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: The authors have no disclosures relevant to this work.
References
- Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, Rawlings DJ, Jackson SW, 2017. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 214, 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barke A, Korwisi B, Casser HR, Fors EA, Geber C, Schug SA, Stubhaug A, Ushida T, Wetterling T, Rief W, Treede RD, 2018. Pilot field testing of the chronic pain classification for ICD-11: the results of ecological coding. BMC Public Health 18, 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birklein F, Drummond PD, Li W, Schlereth T, Albrecht N, Finch PM, Dawson LF, Clark JD, Kingery WS, 2014. Activation of cutaneous immune responses in complex regional pain syndrome. J Pain 15, 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birklein F, Ibrahim A, Schlereth T, Kingery WS, 2018. The Rodent Tibia Fracture Model: A Critical Review and Comparison With the Complex Regional Pain Syndrome Literature. J Pain 19, 1102 e1101–1102 e1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW, 1990. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest 86, 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S, 1995. Regulation of terminal differentiation of human B-cells by IL-6. Curr Top Microbiol Immunol 194, 189–198. [DOI] [PubMed] [Google Scholar]
- Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T, 2020. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol 16, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Tawfik VL, Tajerian M, Kingery WS, 2018. Autoinflammatory and autoimmune contributions to complex regional pain syndrome. Mol Pain 14, 1744806918799127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie IK, Davies HT, Macrae WA, 1998. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain 76, 167–171. [PubMed] [Google Scholar]
- Cuhadar U, Gentry C, Vastani N, Sensi S, Bevan S, Goebel A, Andersson DA, 2019. Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain 160, 2855–2865. [DOI] [PubMed] [Google Scholar]
- Di Vita G, Patti R, D’Agostino P, Caruso G, Arcara M, Buscemi S, Bonventre S, Ferlazzo V, Arcoleo F, Cillari E, 2006. Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair. Wound Repair Regen 14, 259–264. [DOI] [PubMed] [Google Scholar]
- Djilali-Saiah I, Fakhfakh A, Louafi H, Caillat-Zucman S, Debray D, Alvarez F, 2006. HLA class II influences humoral autoimmunity in patients with type 2 autoimmune hepatitis. J Hepatol 45, 844–850. [DOI] [PubMed] [Google Scholar]
- Dubuis E, Thompson V, Leite MI, Blaes F, Maihofner C, Greensmith D, Vincent A, Shenker N, Kuttikat A, Leuwer M, Goebel A, 2014. Longstanding complex regional pain syndrome is associated with activating autoantibodies against alpha-1a adrenoceptors. Pain 155, 2408–2417. [DOI] [PubMed] [Google Scholar]
- Friesgaard KD, Gromov K, Knudsen LF, Brix M, Troelsen A, Nikolajsen L, 2016. Persistent pain is common 1 year after ankle and wrist fracture surgery: a register-based questionnaire study. Br J Anaesth 116, 655–661. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS, 2017. Passive transfer autoimmunity in a mouse model of complex regional pain syndrome. Pain 158, 2410–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS, 2019. Sex differences in the temporal development of pronociceptive immune responses in the tibia fracture mouse model. Pain 160, 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TZ, Wei T, Tajerian M, Clark JD, Birklein F, Goebel A, Li WW, Sahbaie P, Escolano FL, Herrnberger M, Kramer HH, Kingery WS, 2020. Complex regional pain syndrome patient immunoglobulin M has pronociceptive effects in the skin and spinal cord of tibia fracture mice. Pain 161, 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Tekus V, Szentes N, Pohoczky K, Botz B, Kiss T, Kemeny A, Kornyei Z, Toth K, Lenart N, Abraham H, Pinteaux E, Francis S, Sensi S, Denes A, Goebel A, 2019. Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proc Natl Acad Sci U S A 116, 13067–13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Jones SA, 2015. IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ, 2006. Persistent postsurgical pain: risk factors and prevention. Lancet 367, 1618–1625. [DOI] [PubMed] [Google Scholar]
- Knudsen LF, Terkelsen AJ, Drummond PD, Birklein F, 2019. Complex regional pain syndrome: a focus on the autonomic nervous system. Clin Auton Res 29, 457–467. [DOI] [PubMed] [Google Scholar]
- Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH, 1998. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med 188, 1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Yang Y, Shi XY, Guo TZ, Guang Q, Kingery WS, Herzenberg LA, Clark JD, 2020. Germinal center formation, immunoglobulin production and hindlimb nociceptive sensitization after tibia fracture. Brain Behav Immun 88, 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsch P, Andratsch M, Vogl C, Link AS, Alzheimer C, Brierley SM, Hughes PA, Kress M, 2014. Deletion of interleukin-6 signal transducer gp130 in small sensory neurons attenuates mechanonociception and down-regulates TRPA1 expression. J Neurosci 34, 9845–9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS, van Hilten JJ, 2011. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol 10, 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ, 2005. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators Inflamm 2005, 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narazaki M, Kishimoto T, 2018. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, Yasuda S, Hisada R, Kato M, Oku K, Bohgaki T, Suzuki M, Matsumoto Y, Atsumi T, 2020. Anti-cyclic citrullinated peptide antibody titers decrease in rheumatoid arthritis patients treated with tocilizumab: A pilot study. Mod Rheumatol 30, 276–281. [DOI] [PubMed] [Google Scholar]
- Pepper A, Li W, Kingery WS, Angst MS, Curtin CM, Clark JD, 2013. Changes resembling complex regional pain syndrome following surgery and immobilization. J Pain 14, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Garvert DW, McDade E, Carlson E, Curtin C, 2011. Chronic psychological and functional sequelae after emergent hand surgery. J Hand Surg Am 36, 1663–1668. [DOI] [PubMed] [Google Scholar]
- Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, Yoshida H, Nishikawa T, Terabe F, Ohkawara T, Takahashi T, Ripley B, Kimura A, Kishimoto T, Naka T, 2008. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 105, 9041–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Guo TZ, Li W, Sahbaie P, Rice KC, Sulima A, Clark JD, Kingery WS, 2018. Exercise Reverses Nociceptive Sensitization, Upregulated Neuropeptide Signaling, Inflammatory Changes, Anxiety, and Memory Impairment in a Mouse Tibia Fracture Model. Anesthesiology 129, 557–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny G, Olszewski WL, Zaleska M, 2004. Limb lymph node response to bone fracture. Lymphat Res Biol 2, 155–164. [DOI] [PubMed] [Google Scholar]
- Tajerian M, Hung V, Khan H, Lahey LJ, Sun Y, Birklein F, Kramer HH, Robinson WH, Kingery WS, Clark JD, 2017. Identification of KRT16 as a target of an autoantibody response in complex regional pain syndrome. Exp Neurol 287, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Hsu V, Kingery W, Huang TT, Becerra L, Clark JD, 2014. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology 121, 852–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajerian M, Sahbaie P, Sun Y, Leu D, Yang HY, Li W, Huang TT, Kingery W, David Clark J, 2015. Sex differences in a Murine Model of Complex Regional Pain Syndrome. Neurobiol Learn Mem 123, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JW, Wang SJ, 2015. A classification of chronic pain for ICD-11. Pain 156, 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen DE, Roelen DL, Verduijn W, Haasnoot GW, Huygen FJ, Perez RS, Claas FH, Marinus J, van Hilten JJ, van den Maagdenberg AM, 2012. Genetic HLA associations in complex regional pain syndrome with and without dystonia. J Pain 13, 784–789. [DOI] [PubMed] [Google Scholar]
- Wu Y, El Shikh ME, El Sayed RM, Best AM, Szakal AK, Tew JG, 2009. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int Immunol 21, 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK, 2016. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 13, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis P, Paladini A, Piroli A, McHugh PC, Varrassi G, Hadjivassiliou M, 2017. Pain as a First Manifestation of Paraneoplastic Neuropathies: A Systematic Review and Meta-Analysis. Pain Ther 6, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]


