Abstract
Background
There are limited data on the unique CVD, non-CVD, and mortality risks of primary prevention individuals with very high coronary artery calcium (CAC≥1000), especially in comparison to rates observed in secondary prevention populations.
Methods
Our study population consisted of 6814 ethnically diverse individuals age 45-84 years, free of known CVD from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective, observational, community-based cohort. Mean follow-up time was 13.6±4.4 years. Hazard ratios of CAC≥1000 were compared to both CAC=0 and CAC 400-999 for CVD, non-CVD, and mortality outcomes using Cox proportional hazards regression adjusted for age, sex, and traditional risk factors. Using a sex-adjusted logarithmic model, we calculated event rates in MESA as a function of CAC, and compared to those observed in the placebo group of stable secondary prevention patients in the FOURIER clinical trial.
Results
Compared to CAC 400–999, those with CAC≥1000 (N=257) had greater mean number of coronary vessels with CAC (3.4±0.5), greater total area of CAC (586.5±275.2 mm2), similar CAC density, and more extensive extra-coronary calcification. After full-adjustment, CAC≥1000 demonstrated a 4.71-(3.63–6.11), 7.57-(5.50–10.42), 4.86-(3.32–7.11), and 1.94-fold (1.57–2.41) increased risk for all CVD events, all CHD events, hard CHD events, and all-cause mortality, respectively, compared to CAC=0 and a 1.65-(1.25–2.16), 1.66-(1.22–2.25), 1.51-(1.03–2.23), and 1.34-fold (1.05–1.71) increased risk compared to CAC 400–999. With increasing CAC, hazard ratios increased for all event types, with no apparent upper CAC threshold. CAC≥1000 was associated with a 1.95-(1.57–2.41) and 1.43-fold (1.12–1.83) increased risk for a first non-CVD event compared to CAC=0 and CAC 400–999, respectively. CAC=1000 corresponded to an annualized 3-point MACE rate of 3.4 per 100 person-years, similar to that of the total FOURIER population (3.3), and higher than lower risk FOURIER subgroups.
Conclusions
Individuals with very high CAC (≥1000) are a unique population at substantially higher risk for CVD events, non-CVD outcomes, and mortality than those with lower CAC, and similar 3-point MACE rates as a stable treated secondary prevention population. Future guidelines should consider a less distinct stratification algorithm between primary vs. secondary prevention patients in guiding aggressive preventive pharmacotherapy.
Keywords: coronary artery calcium, CVD risk, non-CVD risk, mortality, risk scoring, cardiovascular imaging, high risk, primary prevention, secondary prevention
INTRODUCTION:
Coronary artery calcium (CAC), a crude measure of atherosclerotic burden, has evolved into a clinical decision aid in the past decade. Increased CAC scores are strongly associated with higher risk of cause-specific cardiovascular mortality and all-cause mortality in both younger and older adults.1–3 Higher CAC scores have also been linked to non-CVD related outcomes including dementia and cancer.4–8 Consistent with its usefulness as a predictor of CVD and non-CVD related outcomes, the CAC score has been shown to significantly improve upon traditional risk factors and risk scores, such as the MESA CHD Risk Score and the Pooled Cohort Equations (PCE), with meaningful reclassification of patients particularly in the borderline to intermediate risk range.9–13 One recent study in an asymptomatic primary prevention population found that combining CAC with the PCE resulted in significantly better risk discrimination for future CVD death, improving the concordance or c-statistic in middle aged adults from 0.71 to 0.75.14
CAC scores of > 300 or > 400 are generally considered to represent the highest risk group in clinical practice.15 However, there is increasing interest in the unique group of individuals with very high CAC scores. A recent clinical study investigated persons with CAC scores ≥ 1000 and found secondary prevention-level CVD mortality rates in this population; however, the authors used a retrospective clinical database of referred patients and were only able to explore long-term cause-specific and all-cause mortality.3 In addition, this study was not able to investigate non-CVD outcomes, yet CAC is known to be a marker of not only atherosclerosis but also biologic age and chronic disease.5,16–20 More data on the unique group of individuals with CAC ≥ 1000 are needed, especially with regard to: 1) non-CVD outcomes; and 2) CVD outcomes, including whether such patients truly experience secondary prevention-level risk.
The Multi-Ethnic Study of Atherosclerosis (MESA) is an NHLBI-sponsored community-based prospective cohort study of diverse adults free of known CVD with long-term follow-up for non-fatal CVD and physician-adjudicated non-CVD endpoints.21,22 We sought to use MESA to expand on the few previous studies exploring very high CAC scores (CAC ≥ 1000), investigating for the first time both CVD and non-CVD event rates in apparently healthy individuals. We also sought to conduct a more formal comparison of CVD event rates in CAC ≥ 1000 individuals to recent trials in stable secondary prevention (such as FOURIER), including establishing the CAC score cut-offs that correspond to the event rates found in these trials.
METHODS:
The study dataset is made available through the MESA website or can be obtained via BIOLINCC. Qualified researchers trained in human subject confidentiality protocols may also request to access the dataset from the Collaborative Health Studies Coordinating Center, University of Washington, at chsccweb@u.washington.edu. The corresponding author can provide the analyses that support the findings of this study on reasonable request.
Study design and study population
This analysis includes 6814 ethnically diverse participants (age 45-84 years) from the Multi-Ethnic Study of Atherosclerosis (MESA), which is a prospective, observational cohort of participants recruited from the general population from 6 U.S. cities: Baltimore, MD; Chicago, IL; Los Angeles, CA; New York, NY; St. Paul, MN; and Winston-Salem, NC. All participants were free of any baseline CVD, were not receiving active cancer treatment, and underwent baseline CAC scans at time of recruitment. Details on the data collection and design of MESA have been published elsewhere.21 Briefly, MESA is an ongoing study that collected baseline data from July 2000 to September 2002, with follow-up until December 2017 used for this analysis. Institutional Review Board approval was obtained from each field center and all study participants provided informed consent.
Computed tomography (CT) data
Three study sites used cardiac-gated electron-beam CT scans (EBCT), while the other three sites used multi-detector CT scans (MDCT). Each participant was scanned twice at baseline exam, with mean Agatston score used for analysis.23 All scans were phantom-adjusted and read by 2 trained CT image analysts at a central MESA CT reading center, with high reproducibility and comparability between EBCT versus MDCT scanning.24–26 Detailed information on CT scan methods and interpretation has been previously described.24
CAC area and density were derived from total Agatston and volume scores which were provided in the original MESA dataset. The methods for this derivation are described in a prior paper.27 Data on the total number of vessels with CAC (0-4) was available in 6543 participants (96%), aortic root calcium in 6812 participants (>99%), thoracic aortic calcium scores in 6812 participants (>99%), aortic valve calcium scores in 6812 participants (>99%), and mitral valve calcium scores in 6814 participants (100%). Data on CAC density and area could be calculated in 6543 participants (96%).
Measurement and definition of baseline characteristics and risk factors
Participants had baseline anthropometric measurements, vital signs, lifestyle characteristics, risk factors, and laboratory data collected at the time of their initial exam. Missing data is summarized in Supplemental Table I. Obesity was defined as BMI ≥ 30 kg/m2. Hypertension was defined as SBP ≥ 130 mmHg or DBP ≥ 80 mmHg. Diabetes was defined as a fasting blood glucose level ≥ 126 mg/dl or prior diagnosis of diabetes (treated or untreated). Cigarette smoking was defined as having smoked cigarettes in the past 30 days. Family history of myocardial infarction (MI) included parents, siblings, and children regardless of age. Metabolic syndrome was defined per NCEP ATP III criteria.28 Ten-year ASCVD risk scores were calculated using Pooled Cohort Equations scoring method described in the 2013 ACC/AHA guideline on the assessment of cardiovascular risk.29
Outcome ascertainment and follow-up
Telephone interviews and chart reviews were performed for all participants at 9- to 12-month intervals to collect information about any hospital admissions, outpatient diagnoses or procedures, and deaths. Self-reported diagnoses were verified using medical records, death certificates, and next-of-kin interviews for the case of out-of-hospital deaths. Follow-up time was measured from baseline examination to first occurrence of specified outcome, loss to follow-up, death, or December 31, 2017. Mean follow-up time was 13.6 ± 4.4 years (median 15.7 years, first quartile to third quartile 12.2 – 16.5 years).
CVD Events and All-Cause Mortality
Our CVD outcomes of interest included all CVD events, defined as MI, resuscitated cardiac arrest, stroke, adjudicated angina, and cardiovascular death (secondary to stroke, coronary heart disease, other atherosclerotic death, or other CVD death); hard CVD events, defined as MI, resuscitated cardiac arrest, stroke, and cardiovascular death (secondary to stroke, coronary heart disease, other atherosclerotic death, or other CVD death); all CHD events, defined as MI, resuscitated cardiac arrest, adjudicated angina, and coronary death; hard CHD events, defined as MI, resuscitated cardiac arrest, and coronary death; and all-cause mortality. Presence of angina was defined as definite or probable angina, both of which were distinct from myocardial infarction diagnoses. Definite angina required objective evidence of obstructive coronary artery disease or reversible myocardial ischemia, and probable angina was followed by revascularization. The final adjudication of each endpoint was done by the MESA Morbidity and Mortality Committee.30 The number of all CVD events, hard CVD events, all CHD events, hard CHD events, and all-cause mortality in each CAC group is summarized in Supplemental Table II.
Non-CVD Events
Other outcomes of interest included non-CVD events, which were extracted from inpatient records using ICD-9 and ICD-10 codes and were analyzed as non-competing events. For the purposes of this analysis, we included cancer, chronic kidney disease (CKD) or other indicators of end stage renal disease (ESRD), pneumonia, chronic obstructive pulmonary disease (COPD), deep vein thrombosis (DVT) / pulmonary embolism (PE), dementia, and hip fracture as non-CVD events.5 An aggregate non-CVD event variable was created from these individual events defined as time to any first non-CVD event. Supplemental Table III summarizes a breakdown of cancer type by CAC group. A full list of codes used can be found in Supplemental Table IV. The number of each specific non-CVD event type as well as aggregate non-CVD events in each CAC group is summarized in Supplemental Table V.
Statistical methods
CAC scores were categorized as CAC 0, CAC 1-399, CAC 400-999, and CAC ≥ 1000. Baseline characteristics were stratified by CAC groups, reporting number (percentage) and means (SD) as appropriate.
Hazard ratios were calculated using unadjusted and multivariable-adjusted Cox regression models to assess the relative hazards of CAC groups for CVD events, all-cause mortality, and non-CVD events compared to a reference group of CAC score 0. For the purposes of specific comparison, the same models were also used to compare the CAC ≥ 1000 group to a reference group of CAC 400–999. We chose to include two models: Model 1 (unadjusted model) and Model 2 (fully-adjusted for covariates). The covariates we used for the fully-adjusted model were age, sex, race/ethnicity, obesity, hypertension, total cholesterol, HDL-C, triglycerides, smoking status, diabetes, family history of MI, anti-hypertensive medications, and cholesterol medications at baseline.
In a multivariable model adjusted on the same covariates listed above, cubic splines with knots placed at CAC=100 and CAC=1000 were used to study the dose response relationship between CAC score and event outcomes (CVD, CHD, non-CVD, and all-cause mortality) in order to graphically examine risks around the CAC=1000 threshold.
CAC Equivalent Risk Model
To study the CAC scores in primary prevention individuals associated with equivalent CVD event risk as secondary prevention patients in clinical trials, we compared event rates in MESA to those observed in the placebo group of FOURIER.31,32 The rationale for choosing the secondary prevention population of FOURIER was that it is a modern study of individuals with stable CVD. Specifically, the population consists of patients who had a median time of >3 years since their last atherothrombotic event, and have been stable in the interim, versus other recent trials in which patients had acute coronary syndrome in the past 1-12 months.33 We chose a MESA population with clinical indication for CAC scoring under new 2019 ACC/AHA Primary Prevention of Cardiovascular Disease Guideline34 (i.e. ASCVD risk ≥ 7.5%). This produced a population with nearly identical age and sex distribution as FOURIER.
First, we used a logarithmic model to graph sex-adjusted annualized hard CVD event rate (per 100 person-years) as a function of total CAC score in MESA, using similar methods to a previous study in the literature.35 To facilitate comparison with common endpoints in clinical trials, we defined hard CVD events in this model as the familiar 3-point MACE outcome, i.e. myocardial infarction, stroke, and cardiovascular death. In this model, cardiovascular death was defined as death due to any atherosclerotic disease, stroke, or cardiovascular disease. This slightly expanded definition of cardiovascular death compared to the standard MESA definition of hard CVD death (which includes only death secondary to CHD or stroke), was used to mirror the definition used in FOURIER.
Annual placebo-group event rates were then extracted from publications summarizing the FOURIER population. Additional attention was placed on so-called low risk groups in FOURIER as defined by Sabatine et al. These were: (1) no multivessel disease, (2) only 1 prior MI, and (3) no high risk features (i.e. recent MI < 2 years ago, >1 prior MI, or multivessel disease).31,32 The FOURIER low risk subgroups with percentage of FOURIER placebo arm they represent, 3-year Kaplan-Meier rate as reported by FOURIER, and annualized event rate is summarized in Supplemental Table VI.
Finally, using the equation derived from our CAC-CVD event rate logarithmic model in MESA, we calculated the CAC score in MESA that gives the same event rate as observed in FOURIER. Equivalency with the total FOURIER population and the low-risk subgroups was then expressed graphically.
A two-sided p-value < 0.05 was considered statistically significant. All analyses were performed using Stata/SE 14.0 (Stata Corporation LP, College Station, TX, USA).
RESULTS:
Baseline characteristics
Participants with CAC ≥1000 comprised 3.8% (257 participants) of the total MESA study population. These CAC ≥ 1000 participants were older (mean 71.7 ± 7.5 years), more likely to be men (80.9%), non-Hispanic Whites (52.9%), and had a higher mean estimated 10-year ASCVD risk using the PCE (27.5 ± 14.0%) than those with lower CAC (Table 1). Compared to those with lower CAC, a higher percentage of CAC ≥ 1000 participants were also taking anti-hypertensive medication (54.1%), cholesterol medication (31.9%), had positive family history of MI (57.4%), and had albuminuria (20.6%).
Table 1 -.
Baseline characteristics according to CAC score group
| Demographic Characteristics | CAC 0 (n = 3416) | CAC 1-399 (n = 2721) | CAC 400-999 (n = 420) | CAC ≥ 1000 (n = 257) |
|---|---|---|---|---|
| Age, yr, mean (SD) | 58.0 (9.1) | 65.3 (9.6) | 69.8 (7.9) | 71.7 (7.5) |
| Sex (male), % | 36.6% | 54.7% | 63.6% | 80.9% |
| Race/Ethnicity | ||||
| White | 33.0% | 42.0% | 51.2% | 52.9% |
| Chinese | 11.7% | 12.9% | 9.0% | 5.4% |
| Black | 31.4% | 24.6% | 22.1% | 23.0% |
| Hispanic | 23.9% | 20.4% | 17.6% | 18.7% |
| Diabetes*, % | 9.3% | 14.3% | 22.9% | 22.7% |
| Systolic blood pressure, mmHg, mean (SD) | 122.4 (20.5) | 129.8 (21.5) | 135.2 (22.3) | 134.3 (20.9) |
| Diastolic blood pressure, mmHg, mean (SD) | 71.3 (10.3) | 72.3 (10.2) | 73.9 (10.4) | 72.8 (10.4) |
| Anti-hypertensive medication, % | 26.3% | 37.8% | 48.3% | 54.1% |
| Statin medication*, % | 9.6% | 18.5% | 24.3% | 30.0% |
| LDL-cholesterol*, mg/dL, mean (SD) | 116.0 (30.7) | 119.0 (32.1) | 118.9 (32.9) | 110.8 (31.4) |
| HDL-cholesterol*, mg/dL, mean (SD) | 52.5 (15.0) | 49.6 (14.4) | 49.3 (14.9) | 47.7 (14.1) |
| Triglycerides*, mg/dL, mean (SD) | 126.8 (84.9) | 136.1 (93.8) | 139.0 (89.6) | 135.8 (80.1) |
| Total cholesterol*, mg/dL, mean (SD) | 193.7 (35.0) | 195.3 (36.2) | 195.7 (36.9) | 185.9 (37.4) |
| Cholesterol medication, % | 10.7% | 20.1% | 25.7% | 31.9% |
| Cigarette smoking*, % | 13.3% | 13.0% | 11.7% | 12.9% |
| Family history of myocardial infarction*, % | 37.2% | 46.5% | 55.5% | 57.4% |
| Body mass index, kg/m2, mean (SD) | 28.3 (5.7) | 28.3 (5.3) | 28.7 (5.2) | 28.3 (4.8) |
| Metabolic syndrome*, % | 30.8% | 40.2% | 45.1% | 47.1% |
| ASCVD Risk Score*, % score, mean (SD) | 8.5% (9.5%) | 17.1% (13.9%) | 24.5% (15.2%) | 27.5% (14.0%) |
| Low (< 5%) | 49.6% | 16.9% | 3.6% | 2.0% |
| Borderline (5% to < 7.5%) | 12.2% | 11.9% | 6.2% | 2.4% |
| Intermediate (≥ 7.5% to < 20%) | 27.3% | 39.1% | 34.4% | 28.7% |
| High (≥ 20%) | 10.9% | 32.1% | 55.8% | 66.9% |
| Creatinine*, mg/dL, mean (SD) | 0.9 (0.2) | 1.0 (0.3) | 1.0 (0.3) | 1.1 (0.6) |
| eGFR (MDRD)*, mL/min/m2, mean (SD) | 83.4 (16.7) | 79.5 (20.1) | 77.9 (18.1) | 75.6 (20.0) |
| Albuminuria* (≥30 mg/g), % | 7.0% | 10.7% | 16.6% | 20.6% |
| CRP*, mg/L, mean (SD) | 3.8 (5.5) | 3.8 (6.1) | 4.0 (7.0) | 3.5 (7.1) |
Data only available in subset of study population in "Demographic Characteristics". Detailed data on number of participants in each subgroup can be found in Supplemental Table I
The proportion of men increased with increasing CAC score. There were 36.6%, 54.7%, 63.6% and 80.9% men in those with CAC=0, 1-399, 400-999 and ≥1000, respectively. The percentage of participants in the high risk group for 10-year ASCVD risk (≥ 20%) also increased with higher CAC scores, with 10.9% in the CAC = 0 group, 32.1% in the CAC 1-399 group, 55.8% in the CAC 400-999 group, and 66.9% in the CAC ≥ 1000 group.
Several traditional CVD risk factors, such as diabetes, LDL-C, HDL-C, triglycerides, cigarette smoking, family history of MI, BMI, and metabolic syndrome were similar between the CAC ≥ 1000 group and the CAC 400-999 group. The distribution of specific risk factors can be found in Table 1.
Imaging characteristics
Table 2 details the imaging characteristics across CAC score groups, including extra-coronary calcification. In those with CAC ≥ 1000, the mean number of coronary vessels with CAC was 3.4 ± 0.5, where 53.3% had 3-vessel CAC and 44.7% had 4-vessel CAC. Compared to those with CAC 400-999, a greater number of CAC ≥ 1000 participants had ARC (80.1% vs. 67.9%), TAC (77.3% vs. 61.4%), AVC (47.3% vs. 32.7%), and MVC (37.0% vs. 18.1%).
Table 2 -.
Imaging characteristics according to CAC score group
| Imaging Characteristics 1 | CAC 0 (n = 3282) | CAC 1-399 (n = 2618) | CAC 400-999 (n = 397) | CAC ≥ 1000 (n = 246) |
|---|---|---|---|---|
| # Vessels with CAC, mean (SD) | 0 (0) | 1.9 (0.9) | 3.2 (0.6) | 3.4 (0.5) |
| 0, % | 100.0% | 0.0% | 0.0% | 0.0% |
| 1, % | 0.0% | 41.0% | 0.8% | 0.0% |
| 2, % | 0.0% | 30.0% | 8.3% | 2.0% |
| 3, % | 0.0% | 23.6% | 57.9% | 53.3% |
| 4, % | 0.0% | 5.4% | 33.0% | 44.7% |
| Imaging Characteristics 2 | CAC 0 (n = 3416) | CAC 1-399 (n = 2721) | CAC 400-999 (n = 420) | CAC ≥ 1000 (n = 255) |
| TAC, % | 11.9% | 38.4% | 61.4% | 77.3% |
| TAC 1-399, % | 9.2% | 23.0% | 25.5% | 28.6% |
| TAC 400-999, % | 1.8% | 7.9% | 13.6% | 18.8% |
| TAC ≥1000, % | 0.9% | 7.5% | 22.4% | 29.8% |
| Imaging Characteristics 3 | CAC 0 (n = 3416) | CAC 1-399 (n = 2721) | CAC 400-999 (n = 419) | CAC ≥ 1000 (n = 256) |
| AVC, % | 4.9% | 17.9% | 32.7% | 47.3% |
| AVC 1-399, % | 4.5% | 16.4% | 29.4% | 37.5% |
| AVC 400-999, % | 0.3% | 0.8% | 2.6% | 6.3% |
| AVC ≥1000, % | 0.1% | 0.8% | 0.7% | 3.5% |
| Imaging Characteristics 4 | CAC 0 (n = 3416) | CAC 1-399 (n = 2721) | CAC 400-999 (n = 420) | CAC ≥ 1000 (n = 257) |
| MVC, % | 4.3% | 12.0% | 18.1% | 37.0% |
| MVC 1-399, % | 3.5% | 9.6% | 12.9% | 26.5% |
| MVC 400-999, % | 0.4% | 1.1% | 1.7% | 5.1% |
| MVC ≥1000, % | 0.4% | 1.3% | 3.6% | 5.5% |
| Imaging Characteristics 5 | CAC 0 (n = 3415) | CAC 1-399 (n = 2721) | CAC 400-999 (n = 420) | CAC ≥ 1000 (n = 256) |
| ARC, % | 16.4% | 45.8% | 67.9% | 80.1% |
| ARC 1-399, % | 15.9% | 40.6% | 52.1% | 55.5% |
| ARC 400-999, % | 0.4% | 3.9% | 13.1% | 16.4% |
| ARC ≥1000, % | 0.1% | 1.4% | 2.6% | 8.2% |
| Imaging Characteristics 6 | CAC 0 (n = 3282) | CAC 1-399 (n = 2618) | CAC 400-999 (n = 397) | CAC ≥ 1000 (n = 246) |
| Estimated Total Area, mm2, mean (SD) | 0 (0) | 32.7 (34.1) | 198.1 (56.3) | 586.5 (275.2) |
| Average CAC Density, mean (SD) | 0 | 2.6 (0.7) | 3.1 (0.4) | 3.2 (0.3) |
The specific distribution of extra-coronary calcium by CAC score group is shown in Table 2. While the CAC ≥ 1000 group had a similar average CAC density to the CAC 400-999 group (3.2 ± 0.3 vs. 3.1 ± 0.4), this extensive CAC group had a substantially greater total area of CAC than that of the CAC 400-999 group (586.5 ± 275.2 mm2 vs. 198.1 ± 56.3 mm2).
Multivariable-adjusted hazard ratios
CVD Events
After adjusting for traditional cardiovascular risk factors (listed in Table 3), those with CAC ≥ 1000 had a 4.71- (95% CI: 3.63 – 6.11), 3.18- (95% CI: 2.31 – 4.36), 7.57- (95% CI: 5.50 – 10.42), 4.86- (95% CI: 3.32 – 7.11), and 1.94-fold (95% CI: 1.57 – 2.41) increased risk for all CVD events, hard CVD events, all CHD events, hard CHD events, and all-cause mortality, respectively, compared to those with CAC = 0 (Table 3).
Table 3 -.
Hazard Ratios for CVD events by CAC score group
| CAC Score | CVD Event Type and All-cause Mortality, HR (95% CI) | ||||
|---|---|---|---|---|---|
| All CVD | Hard CVD | All CHD | Hard CHD | All-cause mortality | |
| CAC 0 as reference group | |||||
| Model 1, unadjusted HRs | |||||
| 0 | REF | REF | REF | REF | REF |
| 1-399 | 3.31 (2.84 - 3.85) | 3.00 (2.52 - 3.57) | 4.18 (3.41 - 5.11) | 3.70 (2.94 - 4.65) | 2.41 (2.15 - 2.71) |
| 400-999 | 6.88 (5.59 - 8.48) | 5.68 (4.45 - 7.24) | 9.76 (7.55 - 12.62) | 7.69 (5.68 - 10.41) | 4.23 (3.56 - 5.02) |
| ≥ 1000 | 10.95 (8.76 - 13.68) | 7.78 (5.92 - 10.21) | 15.53 (11.83 - 20.37) | 11.72 (8.48 - 16.21) | 6.65 (5.53 - 7.99) |
| Model 2, fully adjusted * HRs | |||||
| 0 | REF | REF | REF | REF | REF |
| 1-399 | 2.06 (1.74 - 2.43) | 1.80 (1.49 - 2.19) | 2.77 (2.22 - 3.45) | 2.24 (1.74 - 2.88) | 1.20 (1.05 - 1.37) |
| 400-999 | 3.12 (2.46 - 3.97) | 2.51 (1.90 - 3.32) | 4.92 (3.66 - 6.61) | 3.42 (2.41 - 4.85) | 1.48 (1.22 - 1.80) |
| ≥ 1000 | 4.71 (3.63 - 6.11) | 3.18 (2.31 - 4.36) | 7.57 (5.50 - 10.42) | 4.86 (3.32 - 7.11) | 1.94 (1.57 - 2.41) |
| CAC 400-999 as reference group | |||||
| Model 1, unadjusted HRs | |||||
| 400-999 | REF | REF | REF | REF | REF |
| ≥ 1000 | 1.57 (1.23 - 2.00) | 1.36 (1.00 - 1.84) | 1.57 (1.19 - 2.08) | 1.51 (1.07 - 2.14) | 1.57 (1.27 - 1.95) |
| Model 2, fully adjusted * HRs | |||||
| 400-999 | REF | REF | REF | REF | REF |
| ≥ 1000 | 1.65 (1.25 - 2.16) | 1.33 (0.94 - 1.86) | 1.66 (1.22 - 2.25) | 1.51 (1.03 - 2.23) | 1.34 (1.05 - 1.71) |
Adjusted on age, sex, race/ethnicity, obesity, hypertension, total cholesterol, HDL-cholesterol, triglycerides, smoking, diabetes, family history of MI, anti-hypertensive medications, cholesterol medications
In the same fully-adjusted model compared to a reference group of CAC 400-999, participants with CAC ≥ 1000 had a 1.65- (95% CI: 1.25 – 2.16), 1.33- (95% CI: 0.94 – 1.86), 1.66- (95% CI: 1.22 – 2.25), 1.51- (95% CI: 1.03 – 2.23), and 1.34-fold (95% CI: 1.05 – 1.71) increased risk for all CVD events, hard CVD events, all CHD events, hard CHD events, and all-cause mortality, respectively (Table 3).
Non-CVD Events
The risk distribution of non-CVD events by specific event type is shown in Table 4 and stratified by CAC score group. When adjusting for traditional cardiovascular risk factors, those with CAC ≥ 1000 had almost double the risk (HR 1.95, 95% CI: 1.57 – 2.41) for a first non-CVD event compared to participants in the CAC = 0 group. Specific events driving this association included cancer, CKD, pneumonia, COPD, dementia, and hip fracture, in which those with CAC ≥ 1000 had significantly increased risk for developing these outcomes vs. those with CAC = 0 (Table 4). Compared to those with CAC 400–999 in the same multivariable-adjusted model, CAC ≥ 1000 participants had a 1.43-fold (95% CI: 1.12 – 1.83) increased risk for a first non-CVD event (Table 4).
Table 4 -.
Hazard Ratios for Non-CVD events by CAC score group
| CAC Score | Non-CVD Event Type and Aggregate Non-CVD, HR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer | CKD | Pneumonia | COPD | DVT/PE | Dementia | Hip fracture | Aggregate Non-CVD | |
| CAC 0 as reference group | ||||||||
| Model 1, unadjusted HRs | ||||||||
| 0 | REF | REF | REF | REF | REF | REF | REF | REF |
| 1-399 | 1.76 (1.53 - 2.02) | 2.17 (1.85 - 2.54) | 1.85 (1.51 - 2.26) | 2.23 (1.72 - 2.90) | 1.63 (1.25 - 2.12) | 2.99 (2.36 - 3.80) | 4.03 (2.33 - 6.99) | 2.11 (1.90 - 2.35) |
| 400-999 | 2.68 (2.13 - 3.37) | 3.61 (2.81 - 4.64) | 3.64 (2.70 - 4.91) | 3.66 (2.43 - 5.49) | 1.68 (1.01 - 2.82) | 5.96 (4.27 - 8.33) | 5.86 (2.68 - 12.79) | 3.41 (2.88 - 4.05) |
| ≥ 1000 | 4.51 (3.55 - 5.74) | 6.64 (5.12 - 8.62) | 5.89 (4.28 - 8.10) | 6.02 (3.92 - 9.26) | 3.10 (1.86- 5.19) | 9.09 (6.38 - 12.96) | 9.65 (4.29 - 21.67) | 5.68 (4.73 - 6.83) |
| Model 2, fully adjusted* HRs | ||||||||
| 0 | REF | REF | REF | REF | REF | REF | REF | REF |
| 1-399 | 1.11 (0.95 - 1.29) | 1.12 (0.94 - 1.33) | 1.11 (0.88 - 1.39) | 1.29 (0.96 - 1.72) | 1.03 (0.77 - 1.39) | 1.38 (1.06 - 1.79) | 1.84 (1.00 - 3.38) | 1.21 (1.07 - 1.36) |
| 400-999 | 1.34 (1.04 - 1.73) | 1.19 (0.90 - 1.57) | 1.60 (1.14 - 2.24) | 1.69 (1.07 - 2.66) | 0.75 (0.42 - 1.34) | 1.72 (1.18 - 2.51) | 2.03 (0.86 - 4.79) | 1.41 (1.17 - 1.71) |
| ≥ 1000 | 1.80 (1.36 - 2.40) | 2.01 (1.49 - 2.70) | 2.22 (1.53 - 3.22) | 2.16 (1.32 - 3.54) | 1.49 (0.83 - 2.67) | 2.15 (1.41 - 3.26) | 3.35 (1.33 - 8.44) | 1.95 (1.57 - 2.41) |
| CAC 400-999 as reference group | ||||||||
| Model 1, unadjusted HRs | ||||||||
| 400-999 | REF | REF | REF | REF | REF | REF | REF | REF |
| ≥ 1000 | 1.68 (1.25 - 2.26) | 1.82 (1.33 - 2.50) | 1.60 (1.10 - 2.35) | 1.61 (0.96 - 2.71) | 1.79 (0.91 - 3.50) | 1.57 (1.05 - 2.34) | 1.68 (0.68 - 4.13) | 1.66 (1.33 - 2.07) |
| Model 2, fully adjusted* HRs | ||||||||
| 400-999 | REF | REF | REF | REF | REF | REF | REF | REF |
| ≥ 1000 | 1.35 (0.97 - 1.88) | 1.70 (1.19 - 2.43) | 1.33 (0.88 - 2.02) | 1.32 (0.75 - 2.34) | 1.68 (0.78 - 3.64) | 1.25 (0.79 - 1.99) | 2.45 (0.91 - 6.58) | 1.43 (1.12 - 1.83) |
Adjusted on age, sex, race/ethnicity, obesity, hypertension, total cholesterol, HDL-cholesterol, triglycerides, smoking, diabetes, family history of MI, anti-hypertensive medications, cholesterol medications
Figure 1 depicts the association between CAC score and multivariable-adjusted risk of all CVD events, all CHD events, non-CVD events, and all-cause mortality. With rising CAC, the hazard ratios increased for all types of events, including all-cause mortality. While the slope of increase for hazard ratios becomes slightly less steep above a CAC score of 1000 for CVD events, CHD events, and non-CVD events, it still continues to increase with no clear upper CAC threshold. Supplemental Figure I uses the same model to depict the curves for hard CVD events (equivalent to PCE definition of ASCVD) and hard CHD events.
Figure 1. Multivariable-adjusted hazard ratios and 95% CI for CVD events, CHD events, non-CVD events, and all-cause mortality as a function of CAC score.
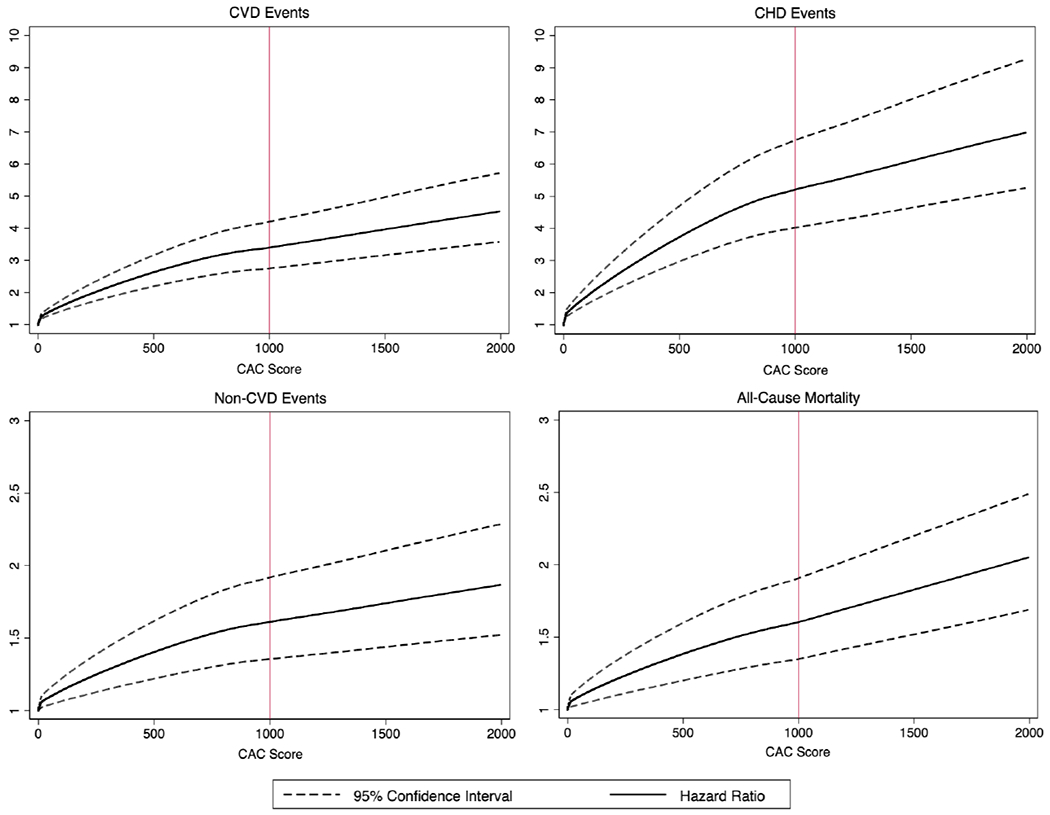
Cubic splines were used in the multivariable model with knots placed at CAC=100 and CAC=1000. Hazard ratios were adjusted for age, sex, race/ethnicity, obesity, hypertension, total cholesterol, HDL-cholesterol, triglycerides, smoking, diabetes, family history of myocardial infarction, anti-hypertensive medications, cholesterol medications.
Multivariable-adjusted logarithmic model of annualized event rates
A sex-adjusted logarithmic model graphing annualized 3-point MACE event rates as a function of CAC score is shown in Figure 2. In this logarithmic model (R2 = 0.93), the annualized 3-point MACE rate rises with a steep slope below a CAC score of approximately 200 and begins to level off above that CAC score, though still increasing. A CAC score of 1000 corresponded to an annualized 3-point MACE rate of 3.4 per 100 person-years. The annualized 3-point MACE rate of those in the FOURIER trial (3.3 per 100 person-years) corresponded to a CAC score of approximately 900 in our model.
Figure 2. Annualized 3-point MACE rate (per 100 person-years) as a function of CAC score.
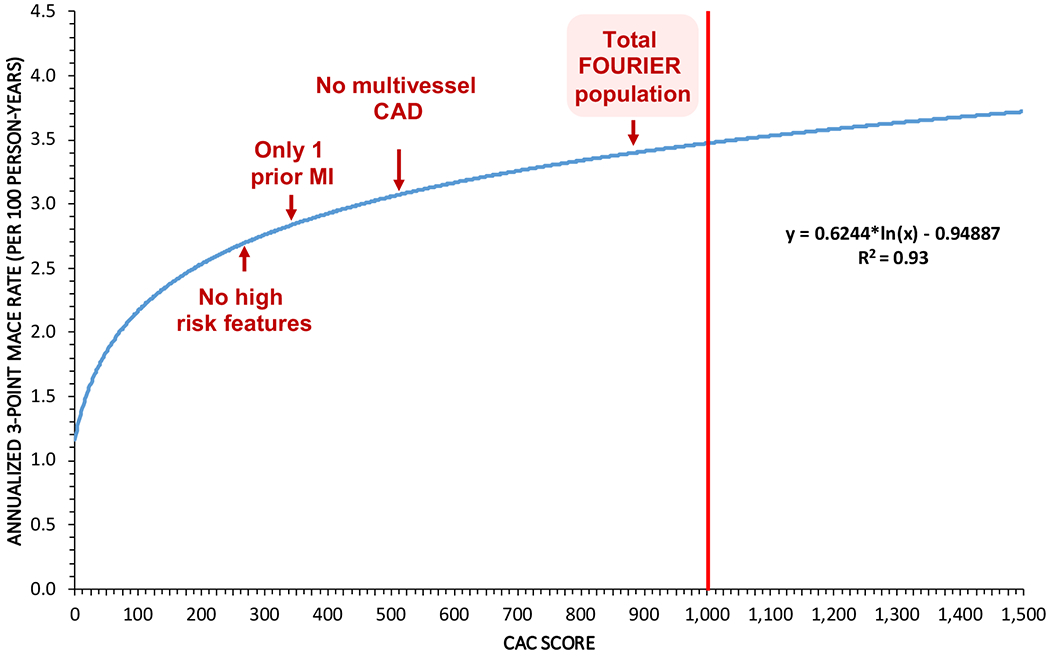
A logarithmic model was used. The annualized 3-point MACE rate (per 100 person-years) of the total FOURIER population along with low-risk subgroups of the FOURIER population are indicated on the graph with their corresponding equivalent CAC scores. For the total FOURIER population, the annualized 3-point MACE rate was 3.3 with equivalent CAC score of 902. The annualized 3-point MACE rate for following low-risk subgroups of FOURIER – no multivessel disease, only one prior MI, and no high risk features – and the corresponding equivalent CAC score for each were: 3.0 with CAC = 529; 2.7 with CAC = 364; and 2.6 with CAC = 294, respectively.
In the low risk subpopulations of FOURIER, for example those with no multivessel disease, only 1 prior MI, and no high risk features (i.e. recent MI < 2 years ago, >1 prior MI, or multivessel disease), the annualized 3-point MACE event rate corresponded to a CAC score of approximately 550, 350, and 300, respectively. The CAC-equivalency to low risk subgroups in FOURIER are shown in Figure 2.
DISCUSSION:
Our study describes in detail those with very high CAC scores (≥ 1000) in the most diverse and generalizable population of these individuals that currently exists in the literature. We demonstrate that those with CAC ≥ 1000 are primarily older, White, and male with a much more extensive pattern of coronary and extra-coronary calcification compared to those with lower CAC. These individuals also have a substantially greater total area of CAC, while remaining at a similar average CAC density compared to lower CAC score groups. Moreover, we show that CAC scores above 1000 are associated with a markedly greater risk for CVD, CHD, and non-CVD events, in addition to all-cause mortality than those with lower CAC.
Importantly, these CAC ≥ 1000 individuals are at an almost 2-times increased risk for all CVD and all CHD events and an almost 1.5-times increased risk for non-CVD events compared to those with CAC 400-999. With higher CAC scores, risk for CVD events, CHD events, non-CVD events, and all-cause mortality seem to increase without a notable upper CAC threshold. Furthermore, this distinct group of primary prevention individuals with CAC ≥ 1000 taken from the US general population has a similar 3-point MACE event rate as stable treated secondary prevention patients from the placebo arm in the FOURIER trial (3.4 vs. 3.3 per 100 person-years).
In the prior limited body of literature on those with CAC ≥ 1000, authors have investigated either only mortality events or only CHD events in their respective study cohorts and with less extensive descriptive characteristics on these individuals.3,36,37 For example, while a prior study by Shaw et al showed higher risk for all-cause mortality in asymptomatic CAC ≥ 1000 patients, the study did not focus on this population of CAC ≥ 1000 individuals and thus provided limited descriptive characteristics on this group.38 In a different study that explored CAC ≥ 1000 patients, the authors only investigated all-cause mortality and found decreased survival with increasing CAC scores, and no upper CAC threshold for mortality risk.37 In addition, the only reference categories used were those with either CAC 1-1000 or CAC 0.
In terms of non-CVD events, there is no existing literature that explores those with CAC ≥ 1000. Although Handy et al in a prior study found an association among multiple types of non-CVD events with increased CAC scores in the MESA population, the highest CAC group investigated in that paper was CAC > 400.5 In our study, we found that those with CAC ≥ 1000 had almost double the risk for a first non-CVD event compared to those with CAC = 0 and almost 1.5-fold increased risk compared to those with CAC 400-999. These findings are supported by the idea that CAC is a marker of not only atherosclerosis, but also biologic age and subclinical organ injury.16–20 While CAC may not have a direct causal relationship with non-CVD outcomes, it reflects an individual’s vulnerability to risk factors and can help predict the risk of developing future chronic disease, such as cancer and CKD, in addition to its usefulness as a risk predictor for CVD events.5,16,19,20
Most notably, in a previous 2011 study by Coylewright et al which used the MESA population to investigate CHD events and individual coronary endpoints such as MI and angina, the authors found that very high CAC ≥ 1000 was not associated with hard CHD endpoints (such as CHD death, MI, and resuscitated cardiac arrest) compared to those with CAC 400-999, although these individuals did have a higher risk for angina.36 Though these findings are in contrary to results from our analysis, they were seen as consistent with the idea that individuals with more dense CAC could have a similar risk profile to those with lower CAC scores, or even benefit from a protective effect, given that CAC density has been shown to be inversely associated with CHD and CVD risk at any level of CAC volume.27 Indeed, some have postulated that those with extensive CAC have increased calcification density rather than more plaque burden, perhaps even lessening an individual’s risk for ASCVD events.39–44 Because of this notion, those with extensive CAC were not necessarily interpreted as such a distinctly high risk population in the past.
Conversely, we show that those with extensive CAC (≥ 1000) are a unique population of individuals who are at substantially higher risk than those with CAC 400-999 for CVD, CHD, and non-CVD events, in addition to all-cause mortality. In addition, these high risk individuals are also distinct in their burden of extra-coronary calcium, with higher ARC, AVC, MVC, and TAC than those with lower CAC, similar to results found from the CAC Consortium.3 Interestingly, we show that these CAC ≥ 1000 individuals have significantly increased CAC area than those with CAC 400-999, yet almost identical CAC density.
Although our results may seem contradictory to Coylewright’s previous study on the same population of MESA participants, this could be explained by the fact that our analysis now utilizes almost 10 years longer follow-up data than the prior study.36 It is likely that this previous MESA study was underpowered due to shorter follow-up time for event endpoints, versus our study which has a median of 15.7 years follow-up. For example, at the time of the study by Coylewright et al, there were only 45 CHD events in the CAC ≥ 1000 group and 53 events in the CAC 400-999 group,36 compared with our study which has 90 CHD events in the CAC ≥ 1000 group and 109 events in the CAC 400-999 group. A tipping point analysis (Supplemental Table VII, Supplemental Figure II) performed on the MESA dataset shows that the p-value for logrank only becomes significant for CHD events after a follow-up time between 6-7 years, whereas Coylewright’s study had in-between 5-6 years of follow-up time.
When examining the extra-coronary calcification specifically in the CAC ≥ 1000 group, a univariate analysis (Supplemental Figure III) shows a trend that more extra-coronary calcification adds graded risk for all-cause mortality. However, a multivariate analysis (Supplemental Table VIII), when adjusted for age, sex, and race/ethnicity, does not show a consistent independent risk between extra-coronary calcification and risk for CVD events or all-cause mortality. Given the small sample size (n = 257) for the CAC ≥ 1000 group, we believe that the multivariate analysis is underpowered to show additive risk of extra-coronary calcification in this group. An interesting finding to note is that in the univariate analysis (Supplemental Figure III), once there is at least one site of extra-coronary calcification, the risk becomes above 2 per person-years, and above 5 per person-years for all CVD events. The outlier, with considerably lower event rates, is zero sites of extra-coronary calcification. Those with CAC ≥ 1000 who have no extra-coronary calcification may represent a unique phenotype in need of further study.
With a growing body of literature on the importance of CAC as a tool in CVD and non-CVD risk assessment, major guidelines have now incorporated the use of CAC in risk stratification.45–48 For example, the 2019 ACC/AHA Primary Prevention of CVD Guideline recommends statin use in select adults with CAC above 100 or above the 75th percentile for age/sex/race; this is an update from the 2013 ACC/AHA guidelines which state that CAC ≥ 300 could inform decision-making in starting statin therapy in those with “unclear” risk.15,34 For these primary prevention individuals, the guidelines recommend either non-pharmacologic or statin therapy depending on their risk level.34,49 More intense LDL-C lowering with the addition of non-statin drugs like ezetimibe and PCSK9 inhibitors are not indicated in this group, except in rare instances such as Familial Hypercholesterolemia in which guidelines state that these drugs may be considered as add-on treatment to maximally tolerated statin therapy.49
On the other hand, for secondary prevention patients, the 2018 AHA/ACC Cholesterol Guideline recommends more intensive LDL-C lowering with the addition of non-statin therapy (i.e. ezetimibe and PCSK9 inhibitors) in these individuals if they fall in the “high risk category” or “very high risk category”, with category criteria described in the guidelines.49,50 However, we demonstrate that primary prevention individuals with extensive CAC (≥ 1000) actually had a similar annualized rate for hard CVD events (i.e. 3-point MACE) as that of stable treated high-risk secondary prevention patients, such as those in FOURIER.31,32 Our results argue for a less distinct risk stratification algorithm between primary vs. secondary prevention patients, as we show that their risk for CVD events could overlap, or be even higher in certain primary prevention populations.
These findings are in agreement with a prior study utilizing the CAC Consortium population of primary prevention patients, showing equivalent CVD mortality event rates in their study population to secondary prevention patients from FOURIER. The authors found a CVD mortality annualized event rate of 0.80%/year in their study population vs. 0.77%/year in FOURIER.3 Our paper expands on this previous study in many ways, notably by also comparing event rates of those with CAC ≥ 1000 to lower risk groups as defined by Sabatine et al.31 In addition, the CAC Consortium population of CAC ≥ 1000 individuals were almost 90% White in ethnicity, while in our study, Black and Hispanic individuals make up almost half of those with CAC ≥ 1000, with a similar ethnicity distribution across all other CAC score groups.3 Furthermore, MESA enrolled participants from the general population, allowing for more generalizability than the CAC Consortium study, which enrolled patients who were referred for CAC scans.22 Studies with referred patients likely have a greater number of individuals with atypical symptoms or with higher pretest probability for CVD events. While more generalizable, our estimates in MESA likely underestimate events in the clinical population, which would potentially make our results more impactful in clinical practice.
In conclusion, these results establish a need for future guidelines to recognize the risk continuum according to atherosclerotic burden; that is, that some asymptomatic primary prevention patients have the same or higher CVD risk as traditional secondary prevention patients (with a previous event) and thus should have the opportunity to receive the same aggressive treatment regimen, with the addition of ezetimibe or PCSK9 inhibitors to maximally tolerated statin therapy. Though some may pose the argument that aggressive prevention may be too late, we believe that those with CAC ≥ 1000 are certainly high-risk but not of sufficiently high absolute risk that aggressive prevention could not provide considerable benefit. For example, in our CAC ≥ 1000 subset, one-year survival was 98.4%, three-year survival was 92.6%, and five-year survival was 87.5%. Even at 10-years, the survival rate was 65.9%, and by the end of our 17.5 years of follow-up time, the survival was still 38.4%. This provides a long latency for prevention, for example for statins or other pharmacologic agents to modulate plaque and prevent events. Therefore, we feel that aggressive prevention would still be very beneficial for those with CAC ≥ 1000, despite their high-risk status. We expect future guidelines to incorporate specific recommendations regarding this high-risk group. The implications from our findings also open the door for future studies to consider recruiting this high-risk primary prevention group into secondary prevention clinical trials.
Study Limitations
Our study has several limitations. First, our primary study population of CAC ≥ 1000 participants were still relatively few in number (n = 257) and one of our main comparative groups (CAC 400-999) had only 420 participants. The relatively small sample size may cause our results to be underpowered in detecting significant differences between event rates for certain outcomes of interest, such as the less common non-CVD endpoints. Because of the relatively small sample size, the limited number of events could influence the CAC threshold in terms of designating CAC = 1000 as a cut-point for the distinct high-risk group. However, CAC = 1000 is a well-established, extensively studied cut-point.
Another limitation in our study is that we did not account for change in therapy after baseline. However, prior studies in MESA showed limited impact of knowledge of CAC score on downstream behavior, and in the limited instances of imbalanced initiation of aspirin, antihypertensive therapy, or statins, this would bias our results towards the null, increasing the impact of our results for those with CAC ≥ 1000. A fourth limitation is the choice of a logarithmic model to graph CVD event rates as a function of CAC score, which might underestimate event rates at extremely high values. In our spline curves (Figure 1), the graph of hazard ratios for CVD events continued to increase past CAC scores of 1000 with no apparent risk plateau, whereas our logarithmic model graph (Figure 2) by its nature plateaus earlier. Thus, our CAC scores producing stable secondary prevention level risk may be somewhat conservative. Finally, non-CVD diagnoses in MESA were obtained using inpatient hospitalization ICD coding, which would overlook mild cases that were exclusively managed in the outpatient setting. This could potentially underestimate our observed associations between CAC score and certain non-CVD outcomes (i.e. dementia or pneumonia).
Supplementary Material
Clinical Perspectives.
What is new?
Individuals with CAC ≥ 1000 constitute a unique population at substantially higher risk for CVD events, non-CVD outcomes, and mortality than those with lower CAC.
Those with CAC ≥ 1000 had a much more extensive pattern of extra-coronary calcification than those with lower CAC, along with significantly increased CAC area yet almost identical CAC density compared to those with CAC 400–999.
CAC = 1000 corresponded to an annualized 3-point MACE rate of 3.4 per 100 person-years, similar to that of the FOURIER stable treated secondary prevention population (3.3) and higher than that of lower risk FOURIER subgroups.
What are the clinical implications?
There is a risk continuum according to atherosclerotic burden – some asymptomatic primary prevention patients, such as those with CAC ≥ 1000, have the same or higher CVD risk as traditional secondary prevention patients.
Aggressive prevention with pharmacologic agents such as statins, ezetimibe, and PCSK9 inhibitors will be important for these high-risk primary prevention patients
Future guidelines should incorporate recommendations on this unique group of CAC ≥ 1000 individuals.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding:
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). This work was funded in part by an investigator-initiated grant from Amgen.
Disclosures:
Dr. John Page is an employee of Amgen.
Grants - NIH, FDA, AHA, Amgen, Aetna Foundation
Honoraria - Amgen, Sanofi, Regeneron, Novartis, Novo Nordisk, Bayer, Akcea, 89Bio, Zogenix, Tricida, Gilead
NON-STANDARD ABBREVIATIONS AND ACRONYMS:
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARC
aortic root calcium
- ASCVD
atherosclerotic cardiovascular disease
- AVC
aortic valve calcium
- BMI
body mass index
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- CRP
c-reactive protein
- CT
computed tomography
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DVT
deep vein thrombosis
- EBCT
electron-beam computed tomography
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- FOURIER
Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk
- HDL
high-density lipoprotein
- ICD
International Statistical Classification of Diseases and Related Health Problems
- LDL
low-density lipoprotein
- MACE
major adverse cardiovascular events
- MDCT
multi-detector computed tomography
- MDRD
modification of diet in renal disease
- MESA
multi-ethnic study of atherosclerosis
- MI
myocardial infarction
- MVC
mitral valve calcium
- NCEP ATP III
National Cholesterol Education Program Adult Treatment Panel III
- NHLBI
National Heart, Lung, and Blood Institute
- PCE
pooled cohort equations
- PE
pulmonary embolism
- SBP
systolic blood pressure
- TAC
thoracic artery calcium
References:
- 1.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. [DOI] [PubMed] [Google Scholar]
- 2.Miedema MD, Dardari ZA, Nasir K, Blankstein R, Knickelbine T, Oberembt S, Shaw L, Rumberger J, Michos ED, Rozanski A, et al. Association of Coronary Artery Calcium With Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw Open. 2019;2:e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng AW, Mirbolouk M, Orimoloye OA, Osei AD, Dardari Z, Dzaye O, Budoff MJ, Shaw L, Miedema MD, Rumberger J, et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1,000: Results From the CAC Consortium. J Am Coll Cardiol Img. 2020;13:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaruvongvanich V, Wirunsawanya K, Sanguankeo A, Upala S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: A systematic review and meta-analysis. Dig Liver Dis. 2016;48:1410–1417. [DOI] [PubMed] [Google Scholar]
- 5.Handy CE, Desai CS, Dardari ZA, Al-Mallah MH, Miedema MD, Ouyang P, Budoff MJ, Blumenthal RS, Nasir K, Blaha MJ. The Association of Coronary Artery Calcium With Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai CS, Ning H, Kang J, Folsom AR, Polak JF, Sibley CT, Tracy R, Lloyd-Jones DM. Competing cardiovascular outcomes associated with subclinical atherosclerosis (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2013;111:1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W-T, Huang J-H, Hsieh M-H, Chen Y-J. Extremely high coronary artery calcium score is associated with a high cancer incidence. International Journal of Cardiology. 2012;155:474–475. [DOI] [PubMed] [Google Scholar]
- 8.Dzaye O, Al Rifai M, Dardari Z, Shaw LJ, Al-Mallah MH, Handy Marshall C, Rozanski A, Mortensen MB, Duebgen M, Matsushita K, et al. Coronary Artery Calcium as a Synergistic Tool for the Age- and Sex-Specific Risk of Cardiovascular and Cancer Mortality: The Coronary Artery Calcium Consortium. J Am Heart Assoc. 2020;9:e015306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaha MJ, Yeboah J, Al Rifai M, Liu KJ, Kronmal R, Greenland P. The Legacy of MESA – Providing Evidence for Subclinical Cardiovascular Disease in Risk Assessment. Glob Heart. 2016;11:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, et al. Ten-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the Multi-Ethnic Study of Atherosclerosis with Validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol. 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht HS. Coronary Artery Calcium Scanning: Past, Present, and Future. JACC: Cardiovascular Imaging. 2015;8:579–596. [DOI] [PubMed] [Google Scholar]
- 12.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera A, Budoff MJ, O’Donnell CJ, Ayers CA, Locke J, de Lemos JA, Massaro JM, McClelland RL, Taylor A, Levine BD. Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM) Coronary Calcium Atherosclerotic Cardiovascular Disease Risk Calculator. Circulation. 2018;138:1819–1827. [DOI] [PubMed] [Google Scholar]
- 14.Blaha MJ, Whelton SP, Al Rifai M, Dardari Z, Shaw LJ, Al-Mallah MH, Matsushita K, Rozanski A, Rumberger JA, Berman DS, et al. Comparing Risk Scores in the Prediction of Coronary and Cardiovascular Deaths: Coronary Artery Calcium Consortium. JACC Cardiovasc Imaging. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone Neil J, Robinson Jennifer G, Lichtenstein Alice H, Bairey Merz C. Noel Blum Conrad B., Eckel Robert H., Goldberg Anne C., Gordon David, Levy Daniel, Lloyd-Jones Donald M., et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 16.Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V. Biological Versus Chronological Aging: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:919–930. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Raggi P, Berman DS, Callister TQ. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188:112–119. [DOI] [PubMed] [Google Scholar]
- 18.Yano Y, O’Donnell CJ, Kuller L, Kavousi M, Erbel R, Ning H, D’Agostino R, Newman AB, Nasir K, Hofman A, et al. Association of Coronary Artery Calcium Score vs Age With Cardiovascular Risk in Older Adults. JAMA Cardiol. 2017;2:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson AJ, Raggi P, Wolf M, Gold AM, Chertow GM, Roe MT. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic Transl Sci. 2020;5:398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary Calcification in Patients with Chronic Kidney Disease and Coronary Artery Disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 22.Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al-Mallah MH, Matsushita K, Rumberger JA, Berman DS, Budoff MJ, et al. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 24.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 25.Nelson JC, Kronmal RA, Carr JJ, McNitt-Gray MF, Wong ND, Loria CM, Goldin JG, Williams OD, Detrano R. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235:403–414. [DOI] [PubMed] [Google Scholar]
- 26.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236:477–484. [DOI] [PubMed] [Google Scholar]
- 27.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular Events. JAMA. 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundy Scott M, Brewer H . Bryan, Cleeman James I., Smith Sidney C., Lenfant Claude. Definition of Metabolic Syndrome. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 29.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- 30.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, et al. Coronary Artery Calcification Compared with Carotid Intima-Media Thickness in Prediction of Cardiovascular Disease Incidence: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatine Marc S, De Ferrari Gaetano M, Giugliano Robert P, Huber Kurt, Lewis Basil S., Ferreira Jorge, Kuder Julia F., Murphy Sabina A., Wiviott Stephen D., Kurtz Christopher E., et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease. Circulation. 2018;138:756–766. [DOI] [PubMed] [Google Scholar]
- 32.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. New England Journal of Medicine. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 34.Arnett Donna K, Blumenthal Roger S, Albert Michelle A, Buroker Andrew B, Goldberger Zachary D, Hahn Ellen J, Himmelfarb Cheryl Dennison, Khera Amit, Lloyd-Jones Donald, McEvoy J William, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uddin SMI, Mirbolouk M, Kianoush S, Orimoloye OA, Dardari Z, Whelton SP, Miedema MD, Nasir K, Rumberger JA, Shaw LJ, et al. Role of Coronary Artery Calcium for Stratifying Cardiovascular Risk in Adults With Hypertension. Hypertension. 2019;73:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coylewright M, Rice K, Budoff MJ, Blumenthal RS, Greenland P, Kronmal R, Barr RG, Burke GL, Tracy R, Post WS. Differentiation of severe coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2011;219:616–622. [DOI] [PubMed] [Google Scholar]
- 37.Patel J, Blaha MJ, McEvoy JW, Qadir S, Tota-Maharaj R, Shaw LJ, Rumberger JA, Callister TQ, Berman DS, Min JK, et al. All-cause mortality in asymptomatic persons with extensive Agatston scores above 1000. Journal of Cardiovascular Computed Tomography. 2014;8:26–32. [DOI] [PubMed] [Google Scholar]
- 38.Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N, Quyyumi A, Budoff MJ, Callister TQ, Min JK. Long-Term Prognosis After Coronary Artery Calcification Testing in Asymptomatic Patients. Annals of Internal Medicine. 2015;163:14–21. [DOI] [PubMed] [Google Scholar]
- 39.Criqui MH, Knox JB, Denenberg JO, Forbang NI, McClelland RL, Novotny TE, Sandfort V, Waalen J, Blaha MJ, Allison MA. Coronary Artery Calcium Volume and Density: Potential Interactions and Overall Predictive Value: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2017;10:845–854. [DOI] [PubMed] [Google Scholar]
- 40.Forbang NI, Michos ED, McClelland RL, Remigio-Baker RA, Allison MA, Sandfort V, Ix JH, Thomas I, Rifkin DE, Criqui MH. Greater Volume But Not Higher Density of Abdominal Aortic Calcium Is Associated With Increased Cardiovascular Disease Risk: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Imaging. 2016;9:e005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bittencourt Márcio Sommer. The Denser the Merrier? Circulation: Cardiovascular Imaging. 2016;9:e005685. [DOI] [PubMed] [Google Scholar]
- 42.Aengevaeren VL, Mosterd A, Sharma S, Prakken NHJ, Möhlenkamp S, Thompson PD, Velthuis BK, Eijsvogels TMH. Exercise and Coronary Atherosclerosis: Observations, Explanations, Relevance, and Clinical Management. Circulation. 2020;141:1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Criqui MH, Forbang NI, Thomas IC. The Importance of Coronary Artery Calcium Density. JAMA Cardiol. 2020;5:290–291. [DOI] [PubMed] [Google Scholar]
- 44.Rosendael AR van, Narula J, Lin FY, Hoogen IJ van den, Gianni U, Alawamlh OAH, Dunham PC, Peña JM, Lee S-E, Andreini D, et al. Association of High-Density Calcified 1K Plaque With Risk of Acute Coronary Syndrome. JAMA Cardiol. 2020;5:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, Blankstein R, Narula J, Rumberger J, Shaw LJ. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. Journal of Cardiovascular Computed Tomography. 2017;11:157–168. [DOI] [PubMed] [Google Scholar]
- 46.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol. 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahabadi AA, Möhlenkamp S, Lehmann N, Kälsch H, Dykun I, Pundt N, Moebus S, Jöckel K-H, Erbel R. CAC Score Improves Coronary and CV Risk Assessment Above Statin Indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC: Cardiovascular Imaging. 2017;10:143–153. [DOI] [PubMed] [Google Scholar]
- 48.Amit Khera, Philip Greenland. Coronary Artery Calcium. Circulation. 2018;137:680–683. [DOI] [PubMed] [Google Scholar]
- 49.Grundy Scott M, Stone Neil J, Bailey Alison L, Beam Craig, Birtcher Kim K., Blumenthal Roger S., Braun Lynne T., de Ferranti, Faiella-Tommasino Joseph, Forman Daniel E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virani Salim S, Smith Sidney C, Stone Neil J, Grundy Scott M Secondary Prevention for Atherosclerotic Cardiovascular Disease. Circulation. 2020;141:1121–1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


