Abstract
The bacterial pathogen Borrelia burgdorferi is the causative agent of Lyme disease and is transmitted to humans through an Ixodes tick vector. B. burgdorferi is able to survive in both mammalian and tick hosts through careful modulation of its gene expression. This allows B. burgdorferi to adapt to the environmental and nutritional changes that occur when it is transmitted between the two hosts. Distinct interactions between the spirochete and its host occur at every step of the enzootic cycle and dictate the ability of the spirochete to survive until the next stage of the cycle. Studying the interface between B. burgdorferi, the Ixodes tick vector, and the natural mammalian reservoirs has been made significantly more feasible through the complete genome sequences of the organisms and the advent of high throughput screening technologies. Ultimately, a thorough investigation of the interplay between the two domains (and two phyla within one domain) are necessary in order to completely understand how the pathogen is transmitted.
1. Introduction
Arthropod borne diseases are becoming increasingly prevalent across the globe, and an understanding of the pathogen-arthropod interface can be an important tool in control of these diseases. Ixodes ticks carry many pathogens of human importance, including Anaplasma, Babesia, Borrelia, tick-borne encephalitis virus and Powassan virus. The most researched of these pathogens is the spirochete Borrelia burgdorferi, the causative agent of Lyme disease and the most common vector-borne disease in the United States (1–3). There are three important components to maintenance of the B. burgdorferi life cycle: the spirochete, the invertebrate tick vector, and the vertebrate host (Figure 1).
Figure 1: The enzootic cycle of Borrelia burgdorferi.
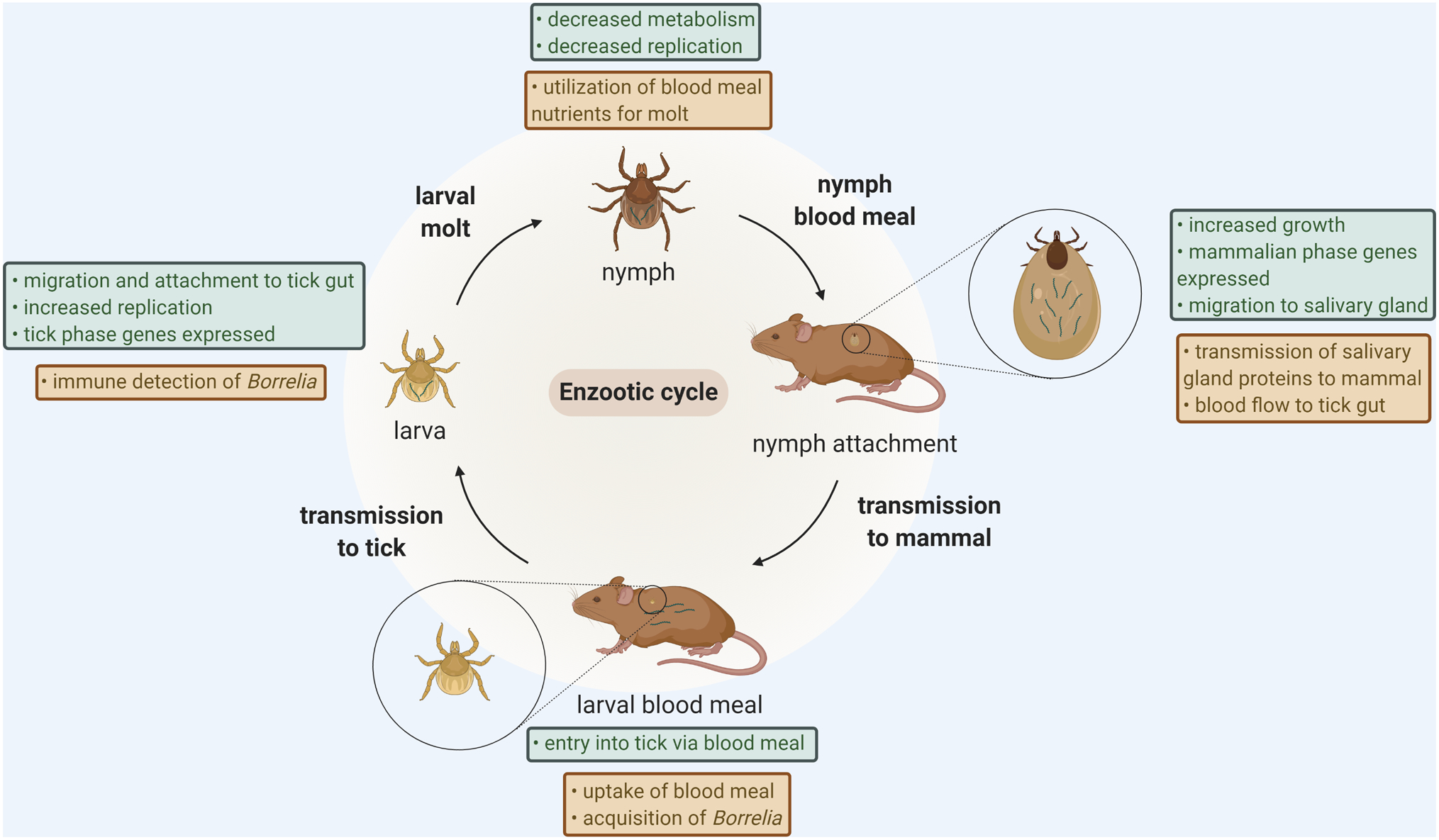
Throughout the enzootic cycle, B. burgdorferi must adapt to new challenges within the tick. Upon entering the tick, B. burgdorferi must rapidly adapt to changes in environment and upregulate tick-phase genes, while at the same time evade detection by the tick immune system. The influx of blood into the tick gut during the nymphal blood meal promotes the migration of B. burgdorferi to the salivary glands and eventual entry into the host, where it must again adjust to environmental changes. B. burgdorferi associated changes are indicated in green boxes, changes in the tick are indicated in brown boxes. Created with BioRender.com.
Ixodes ticks are hematophagous and take one blood meal during the larval, nymph and adult stage of their lifecycle. B. burgdorferi is not transmitted from adult ticks to eggs, so larval ticks must acquire the spirochetes from infected animals, such as birds and mice, with the first blood meal. The spirochetes will remain within the tick after feeding and molting into the nymphal stage. A B. burgdorferi infected nymph will feed on another reservoir host and transmit the spirochetes, continuing the enzootic cycle. After another molt into the adult stage, the adult tick will typically feed on larger animals (such as deer) that may not be competent reservoir hosts for B. burgdorferi but are critical to tick mating. Ticks in the nymphal stage are primarily responsible for transmission of B. burgdorferi to humans, but humans are not important in the enzootic cycle and are considered dead-end hosts (1).
The tick salivary glands and midgut play important roles in the colonization and transmission of B. burgdorferi. During acquisition from an infected host that occurs with the tick blood meal, B. burgdorferi enters the tick with blood, and interacts with tick commensal microbes and tick proteins to establish infection and persistence within the vector. Ticks, in response to the bloodmeal and the presence of the pathogen, will modify expression of certain genes. B. burgdorferi then has to respond to the vast environmental changes of moving from a vertebrate host to an invertebrate host. These include but are not limited to differences in host immunity, nutrient availability, and temperature. For example, after a blood meal, hard ticks such as Ixodes will not feed again for many months. To adapt, the organism needs to slow its growth rate, evade tick immunity and survive a wide range of temperatures. Then, when the tick takes its next blood meal, spirochetes must identify the signals of a new feeding and initiate the changes required for successful migration to the salivary glands in preparation for transmission into a new mammalian host (1,4–8). In this review, we will provide an overview of the enzootic cycle, focusing on the interplay between the mammalian host, the Ixodes tick vector, and B. burgdorferi, at each step.
2. Tick acquisition and initial Borrelia adaptations
During the first stage of the enzootic cycle, B. burgdorferi is transmitted into ticks during the larval blood meal. This change in environment requires B. burgdorferi to rapidly adapt from the mammal to the tick in order to survive. B. burgdorferi must sequester nutrients from the blood meal and evade elimination by components of the tick immune system (Figure 1).
2.1. Borrelia transmission into ticks
Ixodes ticks use a feeding apparatus, made of a ventral barbed hypostome, which acts as an anchor and two chelicerae, lined by rows of dentricles, to saw into the host skin (9). In the case of I. scapularis but not I. ricinus, the tick anchors the mouthparts to the skin via a cement-like material that is created by the salivary glands (10,11). The blood meal lasts for several days, so they have had to develop many strategies to remain attached to the host without triggering key host immune defenses and allowing for adequate blood flow. To accomplish this, tick saliva contains immunosuppressant, vasodilator and anticoagulant molecules that are beneficial to feeding and are utilized by pathogens such as B. burgdorferi to assist with transmission (12–14). These will be discussed in detail later in the review. When spirochetes enter the midgut of the larval tick, they utilize the nutrients from the blood-meal and rapid replication occurs (15). Very little is known about spirochete nutrient utilization and metabolism in larval ticks, but nutrient utilization during the nymphal blood meal is better characterized and will be discussed in later sections.
B. burgdorferi’s biphasic life cycle requires the spirochete to adapt to the changes in environmental conditions from the vertebrate phase to the arthropod phase, including shifts in pH, temperature, immune defenses, and nutrient availability. B. burgdorferi combats these changing conditions by activating transcriptional regulators important in controlling expression of genes that are involved in attachment to the tick and evading tick immunity (4). A two-component system, consisting of Histidine kinase 1 (Hk1) and response regulatory protein 1 (Rrp1), have been studied in the tick phase and appear to work concurrently during both the larval and nymphal life stages to regulate expression of genes required for survival within the tick. There are still large gaps of knowledge surrounding the regulation and activation of Rrp1 and Hk1 (16–20). Hk1 signaling seems to be induced by stimuli during the process of larval and nymphal feeding but it is unclear if these signals are derived from the vertebrate host at the site of the tick bite or from the tick midgut (21).
It is known that Hk1/Rrp1 play a critical role in promoting Borrelia survival within the gut and regulating production of the nucleotide second messenger cyclic-dimeric-GMP (c-di-GMP) (17). Hk1 binding of free amino acids or their derivatives (18) results in a signaling cascade culminating in the phosphorylation of Rrp1 (reviewed in (4)). Rrp1 phosphorylation catalyzes the synthesis of c-di-GMP, which in turn modulates the expression of genes required for survival within ticks including those important for chemotaxis, nucleotide and carbohydrate metabolism (17,20,22). Rrp1-deficient Borrelia strains, which are unable to synthesize c-di-GMP, are unable to survive within the tick vector (19), underscoring the importance of both the Hk1/Rrp1 two-component system and c-di-GMP on Borrelia survival. Indeed, levels of c-di-GMP help regulate other functions of Borrelia survival, including motility and virulence (23,24).
2.2. Tick immune system, detection of Borrelia, and Borrelia evasion
Ticks take in relatively large blood meals and are therefore vulnerable to the many invading pathogenic species that may be present in the blood. Arthropods defend themselves through many immune signaling pathways like Janus kinase-signaling transducer activator of transcription (JAK-STAT), immune deficiency (IMD), and Toll signaling, but our knowledge of these immune mechanisms in humans and mice cannot necessarily be applied to tick immunity, as ticks are phylogenetically distinct (25,26). I. scapularis encodes 33 genes that potentially belong to the Toll pathways, but the roles of these genes and Toll pathways are yet to be elucidated (27). The I. scapularis IMD pathway, though significantly different than the traditional insect IMD pathway, produces antimicrobial responses to B. burgdorferi and Anaplasma phagocytophilum to reduce colonization of these pathogens. During A. phagocytophilum infection, this pathway is trigged by infection-derived lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) and 1-palmitoyl-2-oleoyldiacylglycerol (PODAG), however it is not known whether infection-derived lipids are the trigger for this pathway in B. burgdorferi infection (28).
A crucial component of arthropod physiology, the peritrophic membrane (PM) also acts as part of the tick immune defenses. The PM allows metabolites and other materials to be transported between the gut lumen and surrounding tissues, and also acts to prevent abrasive particles from getting through the gut epithelium (29,30). During early stages of feeding, the PM can be detected in Ixodes tick midguts, and can act as an immune barrier to pathogens (30,31). Some Babesia microti parasites, also transmitted by Ixodes ticks, develop an organelle called the arrowhead that passes through the PM and allows parasites to enter the epithelial cells (32). Pathogens that inhabit other arthropods such as Aedes aegypti mosquitoes and sand flies have been known to secrete chitinases that break down the PM barrier and allow for movement through the gut epithelium (33,34). B. burgdorferi does not appear to have homologs of this protein. Proteome analyses of PM isolated from fed ticks indicated that there were few unique proteins and the predominant protein was homologous to arthropod chitin deacytlase. Knockdown of this predominant protein, I. scapularis CDA-like protein (IsCDA), did not prevent formation of the PM or persistence of B. burgdorferi (30). However, passive transfer of antibodies against this protein did increase persistence of B. burgdorferi in ticks without impacting the total bacterial population in the gut, perhaps demonstrating a role for the PM in selectively limiting the levels of B. burgdorferi in the gut. It is hypothesized from the amino acid sequence that perhaps IsCDA, while containing a conserved enzymatic domain, provides mechanical strength for the PM rather than acting as an active enzyme (30). Yang et al. recently discovered that a PM-associated protein, Peritrophic Membrane Chitin Binding Protein (PM_CBP), likely plays a role in the structure and organization of the PM (35). Interference of PM_CBP expression resulted in decreased thickness and increased permeability of the PM, as well as delayed tick feeding (35).
With the blood meal, ticks ingest immune molecules from the vertebrate host that are released in response to the feeding ticks. Vertebrate cytokines and chemokines may impact the tick’s own innate immune signaling processes. Smith et al. found that vertebrate cytokine IFNγ taken up in the blood meal induces I. scapularis Rho-like GTPase (IGTPase) in a STAT-dependent fashion, which then regulates expression of a borreliacidal peptide, domesticated amidase effector 2 (Dae2) (Figure 2). Knockdown of IGTPase in nymphal ticks caused an increase in spirochete burden (36). Dae2, which is expressed during the unfed nymphal and unfed adult stages of I. scapularis ticks, is thought to act on the cell wall of B. burgdorferi to regulate levels of the spirochete after the tick has acquired the bacteria. It does not, however, limit the amount of bacteria taken in by the tick at the blood, as knockdown in nymphal ticks immediately after engorgement did not result in lower spirochete burden (37).
Figure 2: Interactions between B. burgdorferi and the Ixodes tick vector.
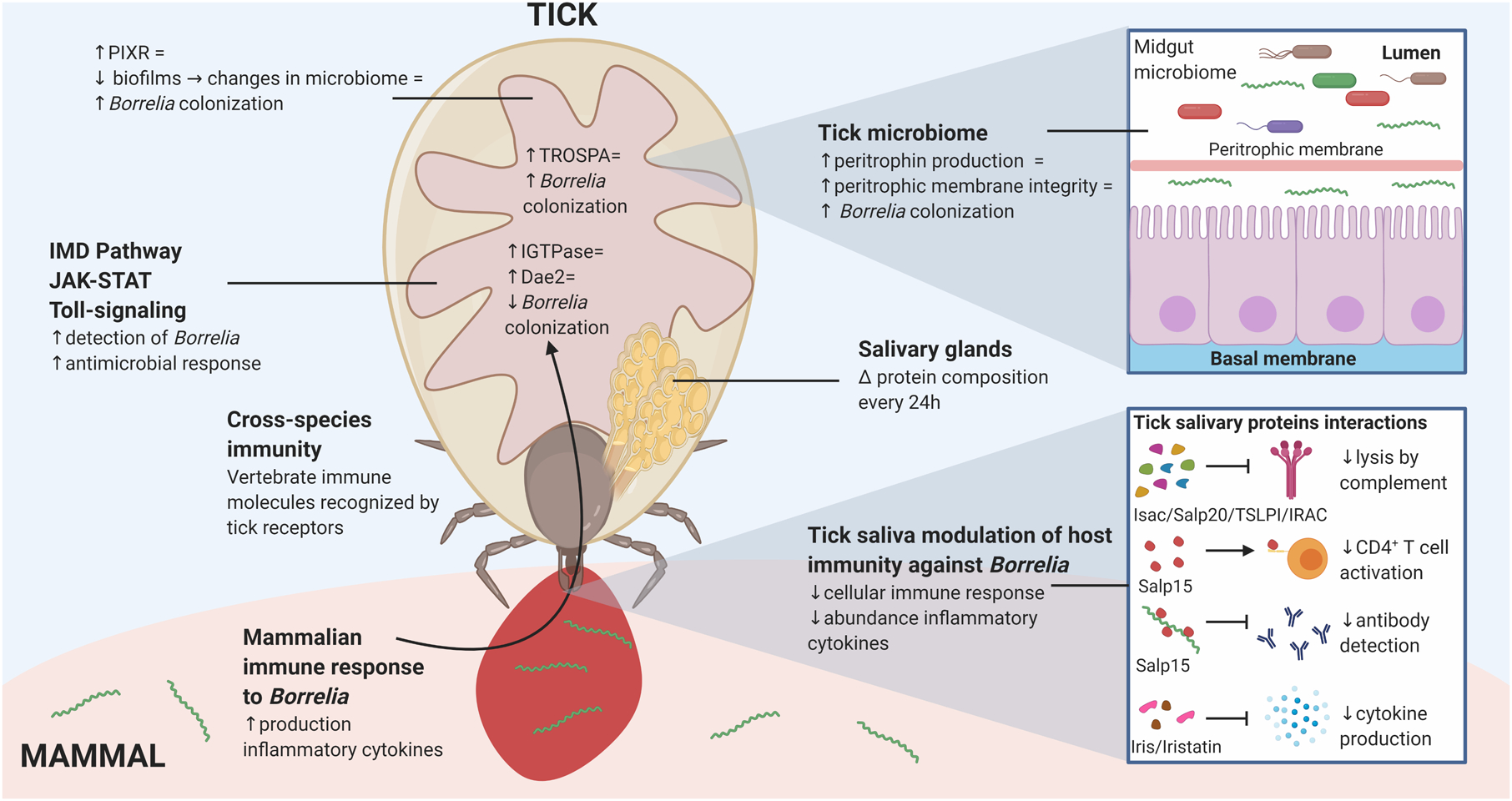
Interactions between B. burgdorferi and tick proteins occur at multiple locations within the tick. The tick midgut provides an opportunity to influence B. burgdorferi colonization through the peritrophic membrane, tick gut microbiome, and other tick gut proteins, including TROSPA and PIXR. Tick salivary gland proteins are able to impair the mammalian immune response and aid B. burgdorferi survival within the mammal. Some of these interactions include Isac, Salp20, TSLPI and IRAC prevention of complement mediated lysis of B. burgdorferi, Salp15 binding to CD4+ T cells and inhibiting their activation, while simultaneously binding to B. burgdorferi and preventing antibody mediated detection of the spirochete, and Iris and Iristatin mediated reduction of proinflammatory cytokine production. The equal sign denotes “leads to.” Created with BioRender.com.
3. Borrelia interactions with the tick gut
Following uptake from the blood meal, B. burgdorferi establishes residence within the tick gut. Within this environment, the spirochete interacts with other bacteria within the gut microbiome and tick gut proteins in order to remain viable.
3.1. Tick gut microbiome
The tick gut microbiome may also influence pathogen success and colonization, and manipulation of the microbiome may allow for interruption of this colonization. B. burgdorferi may be particularly susceptible to this, as it does not possess the genetic mechanisms for direct interactions with other bacteria, demonstrated by the finding that increases in bacterial genera like Pseudomonas, Bacillus, and Enterobacteriaceae are associated with decreases in B. burgdorferi in the tick midgut (38). However, studies on hard ticks such as I. scapularis have observed differing results in terms of the tick microbiome, with some studies finding few bacterial genera in the tick gut and other studies observing 20 or more. The geographical location of field collected ticks, the life stage of the tick, the tick species, and time from molting and feeding may also play a role in the inconsistencies reported in tick microbiome research, as well as a lack of agreement as to whether the tick microbiome includes environmental microbes and symbionts (39,40).
Recent evidence has leaned towards lack of a stable microbiome. Ross et al. found through visualization of bacteria and direct measurements of bacterial burden, that I. scapularis ticks do not have a stable midgut microbiome (38). A possible explanation for the discrepancies is that many of the initial studies were done by 16s rRNA sequencing and that low biomass has been associated with misinterpretation of sequencing data as well as susceptibility to contamination. Pooling samples when studying the tick microbiome may lead to more consistent results in the future (38,41,42). In a recent study supporting the results from Ross et al., Guizzo et al. found that the hard ticks I. ricinus and Rhipicephalus microplus ticks also have relatively small midgut bacterial communities compared to other blood sucking arthropods, even after the blood meal. Interestingly, the ovaries of both species had much larger microbial communities (43). However, despite a relatively small and inconsistent taxonomic composition, Estrada-Pena et al. found that I. scapularis midgut microbiomes are functionally redundant. This redundancy implies that the microbiomes have a core functionality that can be accomplished by many taxonomic combinations, and this allows the functional tick microbiome to be quite resistant to perturbation (39).
Narasimhan et al. found that the microbiome of the tick may play a key role in the tick’s immune defenses against pathogens attempting to colonize the gut (44). They suggest that the expression of STAT, a key component in the JAK/STAT signaling pathway, is modulated by the tick gut microbiome, and that this may facilitate the expression of a core glycoprotein of the tick PM, peritrophin. Increased expression of peritrophin would therefore strengthen the integrity of the PM barrier and they theorize that this may protect the spirochetes from the harsh environment of the gut lumen (44) (Figure 2). Indeed, it has been recently demonstrated that administration of anti-PM_CBP antibodies resulted in impaired spirochete survival in the tick gut (35), again suggesting a potentially critical role of PM integrity on Borrelia survival. This research presents a novel and atypical role for the PM, as it is typically thought to be a physical immune barrier that prevents pathogens from breaking through the epithelial layer (29).
Different microorganisms have also been found to manipulate the tick microbiome to facilitate colonization and persistence. A protein upregulated in ticks infected with B. burgdorferi and induced by feeding is Protein of I. scapularis with a Reeler domain (PIXR). Knockdown of PIXR via RNAi or ticks fed on mice immunized with recombinant PIXR caused a decrease in B. burgdorferi colonization in larval and nymphal ticks, and it is hypothesized that PIXR plays a role in inhibiting gram-positive bacterial biofilm formation in the tick gut. This is similar to the role of IAFGP, a protein induced by Anaplasma phagocytophilum, which inhibits biofilm formation by binding to the peptidoglycan in the cell walls of gram-positive bacteria (45). Though it is not clear how the induction of PIXR benefits B. burgdorferi, it may be elimination of the physical biofilm barrier that allows for easier colonization, or it may be a reduction in some detrimental immune responses provoked by biofilm production and changes in the tick microbiome (46).
3.2. Gut protein-Borrelia interactions
The B. burgdorferi outer surface proteins OspA and OspB have been shown to be critical for survival within and colonization of the tick gut (47,48). Of the two, much more is known about the importance of OspA for survival within the tick, and the importance of OspA antibodies in preventing binding of B. burgdorferi to the tick gut, as this has been the focus of vaccination efforts (49). OspA is selectively expressed when B. burgdorferi enters larval ticks, and this allows for attachment and colonization in the gut. During tick feeding, some spirochetes will stop producing OspA, which is thought to allow the spirochetes to detach from the tick gut and travel to the salivary glands for transmission to the host. It is also thought that some adherence to the tick gut occurs via OspA-OspA interactions and that the downregulation of OspA during migration to the salivary glands reduces these interactions and prevents clumping of the spirochetes during transmission to the vertebrate (50). Some spirochetes will continue to produce OspA but these spirochetes disappear during the establishment of mammalian infection (51–53). It is unclear if it is because the spirochetes eventually downregulate OspA or if those that do not are cleared by the host immune system. There is an abundance of research demonstrating that OspA elicits immunity against B. burgdorferi and OspA was the antigen for the only approved human vaccine for Lyme disease (54). C3H mice administered recombinant OspA have been shown to be protected against multiple strains of B. burgdorferi (54–56). Additionally, antibodies to OspA bind to the spirochetes in the tick gut during feeding, so mice administered OspA antibodies are also protected from challenge with B. burgdorferi (57,58).
The related protein, OspB, has also been shown to play a role in tick colonization by the spirochete. Non-borreliacidal antibodies to OspB have been shown to inhibit the interaction between the spirochetes and the tick gut, and some of this inhibition may be due to antibody binding to multiple epitopes, causing steric hinderance (59). It is possible that, due to the genetic and structural similarities of the two outer surface proteins, and the colocalization of these proteins on the surface of the spirochete, antibodies to OspB may impact the binding of OspA to the tick gut (59,60). Neelakanta et al. demonstrated in their data that OspB facilitates the survival of spirochetes in the tick, as spirochetes deficient in OspB were not able to colonize, resulting in low numbers of spirochetes in the tick gut despite entering the ticks from infected mice at the same rate as their wild type counterparts (47). The mutant spirochetes that were able to persist may have been able to utilize OspA to adhere to the tick gut, as OspA was not affected in the OspB mutants (47).
At the same time as B. burgdorferi is highly expressing OspA, I. scapularis is highly expressing the OspA specific ligand, Tick Receptor for OspA (TROSPA). TROSPA expression does appear to be influenced by the presence of spirochetes within the tick, as ticks that do not harbor any B. burgodorferi express less TROSPA. This upregulation within the tick would be beneficial to the spirochetes, as the OspA-TROSPA interaction increases spirochete colonization in the tick gut (Figure 2). After tick feeding and engorgement, expression of TROSPA decreases within the tick, in parallel to decreased expression of B. burgdorferi OspA (61). Another protein found to impact B. burgdorferi colonization in the tick gut is I. scapularis protein disulfide isomerase A3 (IsPDIA3). Knockdown of this protein in nymphs resulted in decreased B. burgdorferi colonization. Furthermore, larvae fed on mice that were given anti-recombinant IsPDIA3 sera had decreased B. burgdorferi colonization. When IsPDIA3 was knocked down in ticks already infected with B. burgdorferi, however, it did not impact the spirochete burden, demonstrating that IsPDIA3 is important for colonization but not viability or persistence of B. burgdorferi (62).
4. Borrelia survival between blood meals
While the blood meal ushers in a large wave of nutrients, spirochetes need to survive within the tick for months after the blood meal nutrients have been depleted until the following blood meal has been taken. During this time, there are many changes in B. burgdorferi gene expression, and the spirochete has to modify its utilization of carbon sources in order to survive within the tick until the next blood meal (Figure 1).
Studies have identified several Borrelia genes required for persistence within the tick after the blood meal nutrients have waned, although a complete picture of which genes are critical for survival between blood meals remains unknown. BB0690, a Dps protein, homologs of which have been shown to protect other bacteria against DNA damage and oxidative stress (63,64), is highly expressed in ticks and is required for Borrelia persistence, as Dps-deficient spirochetes are unable to survive for prolonged periods in unfed ticks (65). While BB0690 does not bind DNA or protect against oxidative stress in vitro, it does bind to iron and copper, which in turn helps to protect the spirochete against peroxide stress (65,66). Additionally, the B. burgdorferi DnaK suppressor protein (DksA) helps to mediate the response to starvation through downregulating DNA replication machinery proteins, flagellar components, and ribosomal proteins (67,68). The surface lipoprotein of unknown function, bptA, has been shown to be a critical regulator of Borrelia virulence and persistence within the tick although the mechanism by which it acts is still unknown (69).
Rrp1, the diguanylate cyclase that synthesizes c-di-GMP, is required to control expression of glycerol transport and metabolism in B. burgdorferi (19,70). During the initial part of the tick phase of the enzootic cycle, the influx of mammalian blood into the tick gut allows for the utilization of glucose as a carbon source by B. burgdorferi. However, in the period of time after the larval blood meal and prior to the nymphal blood meal, glucose and other nutrients provided by the mammalian blood are depleted quickly and B. burgdorferi switches its primary carbon source to glycerol, as well as chitobiose and maltose for glycolysis, in order to survive within the tick (20,71–76). Glycerol is readily available in the tick midgut, as it is produced by ticks to serve as “antifreeze” during the winter. Spirochetes that are deficient in either Rrp1 (16,19) or genes encoding the glycerol metabolism operon (71) are unable to survive in ticks. Glycerol utilization and metabolism in B. burgdorferi is regulated by the stringent response and RelBbu (BB0198) (77,78). RelBbu is a homolog of RelA and SpoT (79), enzymes that control the levels of two nucleotide signaling molecules, termed alarmones: guanosine pentaphosphate (pppGpp) and guanosine tetraphosphate (ppGpp). Together, these molecules modulate transcription, translation, and numerous other cellular activities. In B. burgdorferi, RelBbu is responsible for (p)ppGpp synthesis (77,80,81) and is critical for spirochete survival within the tick in between blood meals, partially due to its regulation of the glycerol metabolism pathway (77). It is possible that the importance of RelBbu in spirochete survival is not limited to regulation of glycerol metabolism genes, as RNA sequencing and microarray analysis has revealed a number of genes that are differentially regulated by RelBbu during nutrient stress (77,78). Through identifying specific pathways regulated by RelBbu, we can further understand how B. burgdorferi survives in the tick between blood meals.
5. Borrelia migration from the midgut to the salivary glands to a new host
While Borrelia reside in the tick midgut between blood meals, the onset of the blood meal prompts the spirochetes to migrate out of the midgut and into the salivary glands in preparation for transmission into the mammalian host (82). The influx of blood into the tick ushers in further changes to the midgut environment, including temperature and pH, inducing changes to Borrelia gene expression necessary for adapting to the new environment (5,83–86). While acquiring the blood meal, ticks remain attached to their host for several days. The tick salivary proteins expressed throughout the feeding period evolve to enhance successful transmission of Borrelia to the vertebrate host (14).
5.1. Nutrient uptake during the nymphal blood meal
The onset of the nymphal blood meal ends the period of nutritional deprivation for the spirochete. Immediately following the nymphal blood meal, spirochete numbers in the midgut remain low but increase as the feeding continues in response to the influx of nutrients (15,87,88). Hoxmeier et al. used metabolomics to dissect the interaction between I. scapularis ticks and B. burgdorferi during the blood meal, as B. burgdorferi does not possess the pathways for synthesis of nucleotides, amino acids, fatty acids, or enzyme cofactors and therefore it competes with the tick for these nutrients (89). Elucidating these interactions and identifying these metabolites could be important for controlling spirochete growth and persistence within the tick vector.
B. burgdorferi lacks the enzymes for the classical pathway for purine salvage, including hypoxanthine-guanine phophoribosyltransferase (hpt), adenylosuccinate synthase (purA), and adenylosuccinate lysase (purB). It also does not contain the genes that encode the enzymes for de novo synthesis of purines so it sequesters deoxynucleotides and purine bases, the most common purine being hypoxanthine, from the tick blood meal through novel purine salvage pathways (90–92). Similarly, B. burgdorferi obtains amino acids from the blood meal, but very little is known about how the spirochetes sequester them from the tick (93). Peptides are a source of amino acids for many bacteria, and this is likely the case for B. burgdorferi, as it possesses a peptide transport system similar to oligopeptide permease (Opp) transporters. The genome encodes five peptide-binding proteins, all of which are capable of working with the transporter to facilitate peptide transport (94). The peptide binding proteins show different binding specificity and patterns of expression suggesting that they may play specific roles in particular parts of the lifecycle (94–97). Ablation of this Opp system in vitro results in spirochetes that are morphologically abnormal and unable to replicate, further highlighting the critical role of this system for spirochete survival (95). Immediately after the nymphal blood meal, B. burgdorferi switches its carbon source to glucose, the most prevalent carbohydrate source in mammalian blood and one of the limited amounts of carbohydrates B. burgdorferi can utilize for energy (19,71,98,99). This utilization of glucose is supported by the presence of multiple phosphotranferase-type glucose transporter genes in the B. burgdorferi genome (4,72,73,100). B. burgdorferi is also dependent on the blood meal in the tick for fatty acids and cholesterol as it cannot synthesize them, elongate fatty acid chains or oxidize exogenous fatty acids on its own (101–103). The spirochete’s outer membrane is composed of cholesterol and fatty acids and free cholesterol appears to be crucial to the interactions with the tick through lipid rafts (104).
5.2. Changes in Borrelia and tick gene expression occurring during the blood meal(s)
As previously discussed, Rrp1-Hk1 mediates the initial transition of B. burgdorferi to the tick host during the blood meal. The environment during the blood meal is harsh, with activation of reactive oxygen and nitrogen species as well as activation of the tick immune system (7,27,28,105–107). By studying a transposon library of Borrelia mutants (108), Phelan et al. were able to identify 46 genes that were essential to Borrelia survival in ticks after the blood meal (109). Most of these genes were of unknown function, and likely have a variety of functions critical for Borrelia survival and migration out of the gut. However, several of the genes appeared to be involved in protection against reactive oxygen species. BB0017, a protein known to protect B. burgdorferi against reactive oxygen species, is required for tick survival through control of expression of other genes (109).
After establishing infection in the tick midgut, the next major event in the lifecycle is the nymphal blood meal. Spirochete gene expression changes in response to nymphal blood uptake are critical for B. burgdorferi survival and transmission to a new host. During uptake of the nymphal blood meal, the Hk2/Rrp2 two-component system becomes active, although the specific signal that leads to this activation is unknown. Phosphorylation of Rrp2 by Hk2 results in Rrp2 activation (110–112). In turn, Rrp2 acts as a transcriptional activator for RpoN, which controls expression of RpoS, both of which are alternate sigma factors (112–117). While many components of this pathway have yet to be elucidated, the Rrp2-RpoN-RpoS pathway is essential for successful spirochete transmission into mice. Genes in this regulon, including ospc (important in early mammalian infection) and decorin binding protein A (dbpa, important in binding to mammalian extracellular matrix proteins), are involved in the tick-mammal transmission cycle (116,118–120).
Spirochete motility is also critical for survival in the tick following the blood meal (88). Spirochetes deficient in flaB (121), the periplasmic flagellar filament, or cheY3 (122), a chemotaxis response regulator, survive in ticks to a lower extent than wild-type Borrelia. While it is unknown when during the tick phase of the enzootic cycle these genes are required for survival, it is possible that motility is upregulated during feeding when the spirochetes need to migrate from the midgut into the salivary glands. In addition to motility, other genes have been documented to change expression in feeding ticks or are important for spirochete transmission from ticks to mammals, including bba52 (123), bba03 (124), bba07 (125), and bba66 (126). In addition to identifying Borrelia proteins that are critical for spirochete transmission from ticks, researchers have also begun to identify tick proteins that impact spirochete transmission. For example, the tick gut protein ISDLP (I. scapularis dystroglycan-like protein) is upregulated during tick feeding and is important for B. burgdorferi transmission (127). In order to understand the specific impact of each of these proteins on spirochete survival and successful transmission from tick to mammal, the specific functions and interacting partners of these B. burgdorferi and tick proteins need to be elucidated.
Interactions between spirochete proteins and tick proteins are critically important to both establishment of the spirochete in the midgut and migration out of the midgut and into the salivary glands. While the interaction between OspA and TROSPA is critical for spirochete maintenance in the midgut, downregulation of OspA and TROSPA during the blood meal help promote spirochete egress from the midgut (50,61). To identify additional interacting proteins, Narasimhan et al. screened a yeast display library of tick gut proteins against total Borrelia membrane extracts (128). From this screen, the tick gut protein Ixofin3D was found to help congregate spirochetes into clusters in the midgut epithelium, which was required for transmission to the salivary glands and egress from the midgut (128). While the Borrelia protein that specifically interacts with Ixofin3D is unknown, other Borrelia genes have been shown to be important for interacting with tick proteins and inducing migration to the salivary glands. Interactions between the Borrelia surface protein BBE31, which is upregulated during nymph feeding, and the secreted gut protein TRE31 results in spirochete migration through the hemolymph to the salivary glands (129).
5.3. Salivary gland composition
Upon egress from the tick midgut, Borrelia enter the salivary glands prior to transmission into the vertebrate host. The tick salivary gland is a complex environment, composed of several hundred known proteins (130). While many of these proteins are critical for Borrelia transmission into the mammalian host (see “Immunomodulatory effects of tick saliva”), there have been recent studies that have emphasized the diversity of the salivary gland proteins at different stages of feeding.
Acquiring a blood meal from a mammal is a lengthy process, with the tick remaining attached to the mammal for a few days to up to one week (14). During this time, the proteins secreted from the salivary glands change rapidly. Using proteomics, researchers have determined that ticks secrete functionally similar but distinct proteins every 24 hours in order to evade detection by the mammalian immune system (131). Other groups have used sequencing technologies, including cDNA libraries (130,132,133), phage display (134), and next generation sequencing (135,136), to characterize salivary gland proteins and have found that the complexity and diversity observed in tick salivary gland proteins is likely due to the long host attachment time Ixodes ticks require in order to acquire a blood meal. Many of the proteins identified in these studies have known functions, including proteases, cell adhesion proteins, cytoskeletal proteins, antimicrobial proteins, and others (132). However, a substantial portion of the salivary gland proteins are of unknown function. Determining whether these proteins interact with Borrelia surface proteins or mammalian proteins can help identify their role and function in the tick salivary gland.
In addition to differential expression over the course of feeding, it has become evident that proteins in the salivary gland, including lipocalins, Kunitz-domain containing proteins, and metalloproteases have molt stage-specific expression, suggesting that the interactions between the mammal and the tick are likely to differ between stages (136). Likewise, tick salivary protein expression differs depending on the mammalian host, as the tick transcriptome and proteome composition is distinct when fed on mice or guinea pigs (137). These adaptions by the tick that are dependent on the molt stage or mammalian host help to ensure adequate blood meal uptake and evasion of mammalian immune detection.
5.4. Immunomodulatory effects of tick saliva
Proteins in tick saliva have immunomodulatory properties that impact multiple aspects of mammalian host defense. While their primary purpose is to ensure that the tick can feed to repletion, B. burgdorferi take advantage of some of these salivary proteins or their functions to facilitate movement from the tick to the mammalian host (138–140).
One salivary gland protein, Salp15, is particularly influential in impacting the mammalian CD4+ T cell response to Borrelia. Salp15 has been documented to bind to the CD4 co-receptor on CD4+ T cells, preventing T cell receptor signaling through the inhibition of Lck and Zap70 phosphorylation (138,141) (Figure 2). Together, this results in a decrease in IL-2 production, a cytokine critical for T cell expansion (138). The impact on the CD4+ T cell response is not limited to Salp15, as other unknown tick salivary gland proteins have been shown to bind IL-2 directly, preventing T cell proliferation (142). Other salivary proteins, such as Iris and Iristatin, inhibit production of several proinflammatory cytokines, including IL-6, TNFα, and IFNγ (143,144). Tick saliva has also been documented to impact more upstream pathways, specifically impairment of dendritic cell maturation, antigen presentation, and cytokine production (139,145,146), all of which can inhibit the downstream CD4+ T cell response.
The impact of tick saliva on the mammalian host response is not limited to T cells. In addition to its influence in inhibiting cytokine production, Salp15 has also been documented to bind to OspC on Borrelia, protecting the bacterium from antibody mediated killing in mice (147). Antibody targeting of Salp15 resulted in enhanced phagocytosis of Borrelia and targeting of OspC antibodies to the Borrelia surface (148). Other salivary gland proteins, such as the B cell inhibitory protein (BIP), inhibit B cell proliferation and activation (140,149). Salp15 and other salivary gland proteins, including Isac, Salp20, TSLPI and IRAC, have also been reported to interfere with the complement cascade, preventing both neutrophil phagocytosis and complement-induced lysis of Borrelia (150–154) (Figure 2). Furthermore, tick saliva can reduce integrin expression on neutrophils, reducing chemotaxis and inhibiting neutrophil mediated killing of Borrelia (155,156). Through inhibition of multiple aspects of the mammalian immune response, tick salivary proteins help ensure adequate transmission of Borrelia from the tick to the mammal and completion of the enzootic cycle.
6. Utilizing insights from the tick-pathogen interface
Repeat exposure to tick salivary proteins can induce tick immunity in hosts such as rabbits and guinea pigs, but not in the natural Peromyscus leucopus reservoir (157). Tick immunity can result in a decrease in tick feeding and longer time to engorgement, among other detriments to tick health. Immunity to tick proteins can be driven by several immune components, including eosinophils, basophils, mast cells, and antibodies against the salivary proteins (158,159). Interestingly, although repeat tick bites do not appear to cause tick immunity in mice, when infected ticks are fed on mice that had been repeatedly infested with ticks, the immune responses against tick proteins did reduce transmission of B. burgdorferi (160). Furthering this, when serum from rabbits with tick immunity was passively transferred to mice, B. burgdorferi transmission was reduced upon challenge with infected ticks, demonstrating the importance of tick salivary proteins to the transmission of B. burgdorferi (161). This effect can also be seen in humans, as individuals living in endemic Lyme disease areas who have been repeatedly exposed to tick bites have lower rates of Lyme disease (162). However, it is unclear if this is driven entirely by immunity to tick saliva, or if the reaction to the tick bite simply allows for a more rapid identification and removal of the tick.
Interfering with tick salivary proteins that impede mammalian immunity against Borrelia is an attractive option to combatting long-term Borrelia infections in mammals. There have been numerous studies that have utilized gene silencing technologies in ticks to determine the effects of salivary proteins on Borrelia transmission and survival within the tick, and are promising targets for therapeutics aimed to decrease Borrelia transmission. RNA-interference (RNAi) mediated silencing of the salivary protein tick histamine release factor (tHRF), which binds to basophils and stimulates histamine release, has been shown to reduce B. burgdorferi burden in mice and reduce tick feeding efficiency (163). Similarly, silencing of Isac reduced tick weight and Borrelia burdens in ticks (164). RNAi silencing of the salivary protein sialostatin L, which has a predominately anti-inflammatory role in mammals through targeted inhibition of several proteases (165), reduced tick weight and survival following attachment (166).
Furthermore, vaccinating mice and other mammals against tick salivary proteins is a potential way to combat Borrelia transmission from ticks to mammals and vice versa. There have been several promising studies indicating that vaccination against tick salivary proteins could be a successful avenue to developing an effective anti-tick vaccine. Vaccination of mice with recombinant Salp15 has been shown to protect mice from B. burgdorferi colonization (148), evidence that tick salivary proteins can be immunogenic in mammals. Similarly, mice vaccinated with recombinant TSLPI reduced B. burgdorferi burdens, while RNAi silencing of TLSPI in ticks reduced Borrelia survival in ticks following the blood meal (151). Oral vaccination of mice using recombinant vaccinia virus expressing the tick gene subolesin resulted in significantly lower tick infestation on vaccinated mice and substantially reduced uptake of B. burgdorferi by ticks that fed to repletion (167). Larval ticks fed on mice administered antiserum against the secreted salivary gland protein IsPDIA3 showed a reduction in B. burgdorferi colonization both in the fed larvae and once the ticks had molted into nymphs (62), suggesting that reducing acquisition by ticks from mice could further limit the spread of the spirochete from ticks to other mammals. These vaccination studies are not limited to efficacy in mice – vaccination of tick sialostatin L2 in guinea pigs lead to decreased feeding ability of ticks, including longer feeding time, increased tick rejection rate, and enhanced inflammation (168). Although guinea pigs differ from the natural mouse hosts, these studies are encouraging when thinking about potential tick vaccination strategies.
Proteomic studies have also aided in the discovery of potential salivary protein vaccine candidates. Comparing the tick salivary proteome at 24 hours and 66 hours post-attachment, Narasimhan et al. discovered that salivary gland proteins expressed in the first 24 hours following attachment are sufficient to induce mammalian directed anti-tick immunity and can prevent tick feeding and Borrelia transmission (161). Yeast display libraries have been used to identify salivary gland proteins that impair mammalian immune function (169). These data have been useful to further immunization studies, as vaccination of rabbits with several newly discovered tick salivary proteins led to a reduction in nymph feeding (169). Together, these studies suggest that targeted interference with tick salivary proteins, whether through RNAi silencing or vaccination with recombinant protein, is a promising method for an anti-tick vaccine that can ultimately reduce Borrelia transmission from ticks to mammals.
While vaccinating humans against one or several of these tick proteins is a potential preventative approach, it does not address the geographic spread of the disease or the increasing prevalence of infection among ticks. Reservoir targeted vaccine approaches targeting B. burgdorferi infection in ticks and wild mice have the potential to greatly reduce the prevalence of disease. Vaccination of wild P. leucopus mice could help reduce total spirochete burdens in the wild, thereby lessening the chances of human B. burgdorferi infection. Success for this approach was shown in a field study in southern Connecticut, where mice were hand captured and injected with OspA. This resulted in a decreased prevalence of infected ticks in the subsequent season (170). Another method of reservoir targeted vaccines involves oral vaccination of animals. The benefit of oral vaccination is that delivery of an anti-tick or anti-B. burgdorferi vaccine could occur through placed baits. Successful oral strategies of vaccination have utilized recombinant B. burgdorferi proteins or viral vectors encoding either spirochete or tick genes (171–174). Targeting tick antigens in a reservoir targeted vaccine approach also has added benefits of reducing transmission rates of other tick borne pathogens, including B. microti and A. phagocytophilum (167).
7. Conclusions and Perspectives
The stages of the enzootic cycle result in several distinct environments to which B. burgdorferi must adapt in order to survive (Figure 1). Spirochete gene regulation is a critical component of this adaptation, as it is necessary for preventing elimination by host defenses and altering metabolism according to nutrient availability. Entry into the tick via a blood meal from a mammal requires B. burgdorferi to change its gene expression from evading mammalian host detection to evading the tick immune pathways. The nutrient availability from the larval blood meal wanes quickly, and B. burgdorferi must alter its carbon source in order to survive until the nymphal blood meal. Upon tick uptake of the nymphal blood meal, B. burgdorferi must again change anatomical locations within the tick by migrating from the gut to the salivary glands, which contain an entirely new set of defenses that the spirochete must overcome in order to be successfully transmitted into the mammalian host. Ultimately, when B. burgdorferi returns to the mammal, it must alter its gene expression yet again in order to evade mammalian immune detection. Studying the interface between B. burgdorferi and its tick and mammalian hosts is now more accessible than in the past, as the genomes of all three species have been sequenced and are readily available (101,175,176). High throughput screens that utilize sequencing technologies have emerged as critical methods to identify points of intersection and interaction between the spirochete and its hosts. There still remains much to be known about specific protein-protein interactions and signaling pathways between the hosts and B. burgdorferi, and how these interactions change according to tick molt stage and the different mammalian species. While there is no approved vaccine to prevent transmission of the spirochete from ticks to mammals, unique perspectives on the enzootic cycle and interactions between B. burgdorferi and the Ixodes tick vector can help identify potential targets for blocking mammal to tick transmission, tick to mammal transmission, or spirochete survival in the tick vector. It is our hope that this review provides adequate context for future research designed to identify interactions between I. scapularis and B. burgdorferi.
Acknowledgements
This work was supported by the National Institutes of Health through grants R21AI146841 and R01AI131656. We apologize to those colleagues whose work we could not cite or discuss due to space limitations.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to report.
References
- 1.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: Understanding the dual-host lifestyle of Lyme disease spirochaetes. Vol. 10, Nature Reviews Microbiology. 2012. p. 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. 2008;13:6938–46. [DOI] [PubMed] [Google Scholar]
- 3.Mead PS. Epidemiology of Lyme Disease. Infect Dis Clin North Am. 2015;29(2):187–210. [DOI] [PubMed] [Google Scholar]
- 4.Caimano MJ, Drecktrah D, Kung F, Samuels DS. Interaction of the Lyme disease spirochete with its tick vector. Vol. 18, Cellular Microbiology. Blackwell Publishing Ltd; 2016. p. 919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, et al. Profiling of Temperature-Induced Changes in Borrelia burgdorferi Gene Expression by Using Whole Genome Arrays. Infect Immun. 2003;71(4):1689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuels DS, Samuels LRN. Gene Regulation During the Enzootic Cycle of the Lyme Disease Spirochete. For Immunopathol Dis Therap. 2016;7(3–4):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurokawa C, Lynn GE, Pedra JHF, Pal U, Narasimhan S, Fikrig E. Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol. 2020;18:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw DK, Tate AT, Schneider DS, Levashina EA, Kagan JC, Pal U, et al. Vector Immunity and Evolutionary Ecology: The Harmonious Dissonance. Trends Immunol. 2018;39(11):862–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter D, Matuschka FR, Spielman A, Mahadevan L. How ticks get under your skin: insertion mechanics of the feeding apparatus of Ixodes ricinus ticks. Proc Biol Sci. 2013;280(1773):20131758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nosek J, Rajcani J, Kozuch O. Reaction of the host to the tick bite III. The bite of viruliferous Ixodes ricinus female. Zentralbl Bakteriol Orig A. 1978;242(2):141–7. [PubMed] [Google Scholar]
- 11.Kemp DH Binnington KC S BF. Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions and the host response. In: Physiology of ticks. Oxford, UK: Pergamon Press; 1982. p. 119–143. [Google Scholar]
- 12.Kazimírová M, Štibrániová I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol. 2013;4(AUG):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wikel S. Ticks and tick-borne pathogens at the cutaneous interface: Host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol. 2013;4(NOV):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci. 2009;14(6):2051–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piesman J, Oliver JR, Sinsky RJ. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg. 1990;42(4):352–7. [DOI] [PubMed] [Google Scholar]
- 16.Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol. 2011;81(1):219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, et al. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun. 2011;79(8):3117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer WJ, Luthra A, Zhu G, Radolf JD, Malkowski MG, Caimano MJ. Structural characterization and modeling of the Borrelia burgdorferi hybrid histidine kinase Hk1 periplasmic sensor: A system for sensing small molecules associated with tick feeding. J Struct Biol. 2015;192(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, et al. Cyclic di-gmp is essential for the survival of the lyme disease spirochete in ticks. PLoS Pathog. 2011;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, et al. Study of the Response Regulator Rrp1 Reveals Its Regulatory Role in Chitobiose Utilization and Virulence of Borrelia burgdorferi. Infect Immun. 2013;81(5):1775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam R, Saier Jr. MH. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57(2):320–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers EA, Terekhova D, Zhang H, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009;71(February):1551–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR, Motaleb MA. Analysis of the HD-GYP domain cyclic dimeric gmp phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi. Infect Immun. 2011;79(8):3273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. Analysis of the Borrelia burgdorferi cyclic-di-GMP-binding protein PlzA reveals a role in motility and virulence. Infect Immun. 2011;79(5):1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21(11):2568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6(9):946–53. [DOI] [PubMed] [Google Scholar]
- 27.Smith AA, Pal U. Immunity-related genes in Ixodes scapularis-perspectives from genome information. Front Cell Infect Microbiol. 2014;4(AUG):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw DK, Wang X, Brown LJ, Chávez ASO, Reif KE, Smith AA, et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat Commun. 2017;8(14401):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. [DOI] [PubMed] [Google Scholar]
- 30.Kariu T, Smith A, Yang X, Pal U. A chitin deacetylase-like protein is a predominant constituent of tick peritrophic membrane that influences the persistence of Lyme disease pathogens within the vector. PLoS One. 2013;8(10):e78376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Z, Gern L, Aeschlimann A. The peritrophic membrane of Ixodes ricinus. Parasitol Res. 1991;77(7):635–41. [DOI] [PubMed] [Google Scholar]
- 32.Rudzinska MA, Spielman A, Lewengrub S, Piesman J, Karakashian S. Penetration of the peritrophic membrane of the tick by Babesia microti. Cell Tissue Res. 1982;221(3):471–81. [DOI] [PubMed] [Google Scholar]
- 33.Langer RC, Vinetz JM. Plasmodium ookinete-secreted chitinase and parasite penetration of the mosquito peritrophic matrix. Trends Parasitol. 2001;17(6):269–72. [DOI] [PubMed] [Google Scholar]
- 34.Schlein Y, Jacobson RL, Shlomai J. Chitinase secreted by Leishmania functions in the sandfly vector. Proc Biol Sci. 1991;245(1313):121–6. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Koči J, Smith AA, Zhuang X, Sharma K, Dutta S, et al. A novel tick protein supports integrity of gut peritrophic matrix impacting existence of gut microbiome and Lyme disease pathogens. Cell Microbiol. 2020;(September):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AA, Navasa N, Yang X, Wilder CN, Buyuktanir O, Marques A, et al. Cross-Species Interferon Signaling Boosts Microbicidal Activity within the Tick Vector. Cell Host Microbe. 2016;20:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou S, Daugherty MD, Peterson SB, Biboy J, Yang Y, Jutras BL, et al. Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature. 2015;518(7537):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross BD, Hayes B, Radey MC, Lee X, Josek T, Bjork J, et al. Ixodes scapularis does not harbor a stable midgut microbiome. ISME J. 2018;12(11):2596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estrada-Pena A, Cabezas-Cruz A, Obregon D. Resistance of Tick Gut Microbiome to Anti-Tick Vaccines, Pathogen Infection and Antimicrobial Peptides. Pathogens. 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart PE, Bloom ME. Sharing the Ride : Ixodes scapularis Symbionts and Their Interactions. Front Cell Infect Microbiol. 2020;10(April):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glassing A, Dowd SE, Galandiuk S, Davis B, Chiodini RJ. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 2016;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D, Hofstaedter CE, Zhao C, Mattei L, Tanes C, Clarke E, et al. Optimizing methods and dodging pitfalls in microbiome research. Microbiome. 2017;5(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guizzo MG, Neupane S, Kucera M, Perner J, Frantova H, da Silva Vaz I, et al. Poor Unstable Midgut Microbiome of Hard Ticks Contrasts With Abundant and Stable Monospecific Microbiome in Ovaries. Front Cell Infect Microbiol. 2020;10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe. 2014;15(1):58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc Natl Acad Sci U S A. 2017;114(5):E781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narasimhan S, Schuijt TJ, Abraham NM, Rajeevan N, Coumou J, Graham M, et al. Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat Commun. 2017;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neelakanta G, Li X, Pal U, Liu X, Beck DS, DePonte K, et al. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 2007;3(3):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199(5):641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal U, Montgomery RR, Lusitani D, Voet P, Weynants V, Malawista SE, et al. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J Immunol. 2001;166(12):7398–403. [DOI] [PubMed] [Google Scholar]
- 50.Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, et al. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest. 2000;106(4):561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the lyme disease- associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38(1):382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnishi J, Piesman J, De Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A. 2001;98(2):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rathinavelu S, De Silva AM. Purification and characterization of Borrelia burgdorferi from feeding nymphal ticks (ixodes scapularis). Infect Immun. 2001;69(6):3536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, et al. Vaccination against Lyme disease with recombinant Borrelia burgorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339(4):209–15. [DOI] [PubMed] [Google Scholar]
- 55.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the lyme disease agent by immunizing with recombinant OspA. Science (80- ). 1990;250(4980):553–6. [DOI] [PubMed] [Google Scholar]
- 56.Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339(4):216–22. [DOI] [PubMed] [Google Scholar]
- 57.De Silva AM, Telford SR, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183(1):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Silva AM, Fish D, Burkot TR, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun. 1997;65(8):3146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fikrig E, Pal U, Chen M, Anderson JF, Flavell RA. OspB antibody prevents Borrelia burgdorferi colonization of Ixodes scapularis. Infect Immun. 2004;72(3):1755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Escudero R, Halluska ML, Backenson PB, Coleman JL, Benach JL. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect Immun. 1997;65(5):1908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, DeSilva AM, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119(4):457–68. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y, Rosen C, Arora G, Booth CJ, Murfin KE, Cerny J, et al. An Ixodes scapularis protein disulfide isomerase contributes to Borrelia burgdorferi colonization of the vector. Infect Immun. 2020;(September). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA- binding protein Dps. J Bacteriol. 1997;179(16):5188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor σ(B) in Bacillus subtilis. J Bacteriol. 1997;179(23):7251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Pal U, Ramamoorthi N, Liu X, Desrosiers DC, Eggers CH, et al. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol. 2007;63(3):694–710. [DOI] [PubMed] [Google Scholar]
- 66.Wang P, Lutton A, Olesik J, Vali H, Li X. A novel iron- and copper-binding protein in the Lyme disease spirochaete. Mol Microbiol. 2012;86(6):1441–51. [DOI] [PubMed] [Google Scholar]
- 67.Boyle WK, Groshong AM, Drecktrah D, Boylan JA, Gherardini FC, Blevins JS, et al. DksA controls the response of the lyme disease spirochete borrelia burgdorferi to starvation. J Bacteriol. 2019;201(4):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mason C, Thompson C, Ouyang Z. DksA plays an essential role in regulating the virulence of Borrelia burgdorferi. Mol Microbiol. 2020;114(1):172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Revel AT, Blevins JS, Almazán C, Neil L, Kocan KM, De La Fuente J, et al. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc Natl Acad Sci U S A. 2005;102(19):6972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. Cyclic di-GMP Modulates Gene Expression in Lyme Disease Spirochetes at the Tick-Mammal Interface to Promote Spirochete Survival During the Blood Meal and Tick-to-Mammal Transmission. Infect Immun. 2015;83(8):3043–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troy EB, Lin T, Gao L, Lazinski DW, Lundt M, Camilli A, et al. Global Tn-seq analysis of carbohydrate utilization and vertebrate infectivity of Borrelia burgdorferi. Mol Microbiol. 2016;101(6):1003–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corona A, Schwartz I. Borrelia burgdorferi: Carbon Metabolism and the Tick-Mammal Enzootic Cycle. Microbiol Spectr. 2015;3(3):MBP-0011–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tilly K, Grimm D, Bueschel DM, Krum JG, Rosa P. Infectious Cycle Analysis of a Borrelia burgdorferi Mutant Defective in Transport of Chitobiose, a Tick Cuticle Component. Vector-Borne Zoonotic Dis. 2004;4(2):159–68. [DOI] [PubMed] [Google Scholar]
- 75.Rhodes RG, Coy W, Nelson DR. Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS. BMC Microbiol. 2009;9(108):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoon-Hanks LL, Morton EA, Lybecker MC, Battisti JM, Scott Samuels D, Drecktrah D. Borrelia burgdorferi malQ mutants utilize disaccharides and traverse the enzootic cycle. FEMS Immunol Med Microbiol. 2012;66:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. The Borrelia burgdorferi RelA/SpoT Homolog and Stringent Response Regulate Survival in the Tick Vector and Global Gene Expression during Starvation. PLoS Pathog. 2015;11(9):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bugrysheva JV, Pappas CJ, Terekhova DA, Iyer R, Godfrey HP, Schwartz I, et al. Characterization of the RelBbu regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One. 2015;10(2):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittenhuber G Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J Mol Microbiol Biotechnol. 2001;3(4):585–600. [PubMed] [Google Scholar]
- 80.Bugrysheva J, Dobrikova EY, Sartakova ML, Caimano MJ, Daniels TJ, Radolf JD, et al. Characterization of the stringent response and relBbu expression in Borrelia burgdorferi. J Bacteriol. 2003;185(3):957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bugrysheva JV, Bryksin AV, Godfrey HP, Cabello FC. Borrelia burgdorferi rel is responsible for generation of guanosine-3′-diphosphate-5′-triphosphate and growth control. Infect Immun. 2005;73(8):4972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53(4):397–404. [DOI] [PubMed] [Google Scholar]
- 83.Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67(7):3181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramamoorthy R, Scholl-Meeker D. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect Immun. 2001;69(4):2739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, et al. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37(6):1470–9. [DOI] [PubMed] [Google Scholar]
- 86.Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun. 2004;72(9):5419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piesman J, Schneider BS, Zeidner NS. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J Clin Microbiol. 2001;39(11):4145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119(12):3652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoxmeier JC, Fleshman AC, Broeckling CD, Prenni JE, Dolan MC, Gage KL, et al. Metabolomics of the tick-Borrelia interaction during the nymphal tick blood meal. Sci Rep. 2017;7(February):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jain S, Sutchu S, Rosa PA, Byram R, Jewett MW. Borrelia burgdorferi harbors a transport system essential for purine salvage and mammalian infection. Infect Immun. 2012;80(9):3086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jewett MW, Lawrence KA, Bestor A, Byram R, Gherardini F, Rosa PA. GuaA and GuaB are essential for Borrelia burgdorferi survival in the tick-mouse infection cycle. J Bacteriol. 2009;191(20):6231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pettersson J, Schrumpf ME, Raffel SJ, Porcella SF, Guyard C, Lawrence K, et al. Purine salvage pathways among Borrelia species. Infect Immun. 2007;75(8):3877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tilly K, Rosa PA, Stewart PE. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22(2):217–34, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medrano MS, Ding Y, Wang XG, Lu P, Coburn J, Hu LT. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. J Bacteriol. 2007;189(7):2653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Groshong AM, Dey A, Bezsonova I, Caimano MJ, Radolf JD. Peptide uptake is essential for Borrelia burgdorferi viability and involves structural and regulatory complexity of its oligopeptide transporter. MBio. 2017;8(6):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang XG, Lin B, Kidder JM, Telford S, Hu LT. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J Bacteriol. 2002;184(22):6198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang XG, Kidder JM, Scagliotti JP, Klempner MS, Noring R, Hu LT. Analysis of Differences in the Functional Properties of the Substrate Binding Proteins of the Borrelia burgdorferi Oligopeptide Permease (opp) Operon. J Bacteriol. 2004;186(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Young DS, Harris EK, Cotlove E. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. IV. Results of a study designed to eliminate long-term analytic deviations. Clin Chem. 1971;17(5):403–10. [PubMed] [Google Scholar]
- 99.Khajanchi BK, Odeh E, Gao L, Jacobs MB, Philipp MT, Lin T, et al. Phosphoenolpyruvate Phosphotransferase System Components Modulate Gene Transcription and Virulence of Borrelia burgdorferi. Infect Immun. 2015;84(3):754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.von Lackum K, Stevenson B. Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol Lett. 2005;243(1):173–9. [DOI] [PubMed] [Google Scholar]
- 101.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390(6660):580–6. [DOI] [PubMed] [Google Scholar]
- 102.Livermore BP, Bey RF, Johnson RC. Lipid metabolism of Borrelia hermsi. Infect Immun. 1978;20(1):215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Laar TA, Lin YH, Miller CL, Karna SLR, Chambers JP, Seshu J. Effect of Levels of Acetate on the Mevalonate Pathway of Borrelia burgdorferi. PLoS One. 2012;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crowley JT, Toledo AM, LaRocca TJ, Coleman JL, London E, Benach JL. Lipid exchange between Borrelia burgdorferi and host cells. PLoS Pathog. 2013;9(1):e1003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourret TJ, Boylan JA, Lawrence KA, Gherardini FC. Nitrosative damage to free and zinc-bound cysteine thiols underlies nitric oxide toxicity in wild-type Borrelia burgdorferi. Mol Microbiol. 2011;81(1):259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bourret TJ, Lawrence KA, Shaw JA, Lin T, Norris SJ, Gherardini FC. The Nucleotide Excision Repair Pathway Protects Borrelia burgdorferi from Nitrosative Stress in Ixodes scapularis Ticks. Front Microbiol. 2016;7(SEP):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramsey ME, Hyde JA, Medina-Perez DN, Lin T, Gao L, Lundt ME, et al. A high-throughput genetic screen identifies previously uncharacterized Borrelia burgdorferi genes important for resistance against reactive oxygen and nitrogen species. PLoS Pathog. 2017;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, et al. Analysis of an Ordered, Comprehensive STM Mutant Library in Infectious Borrelia burgdorferi: Insights into the Genes Required for Mouse Infectivity. PLoS One. 2012;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phelan JP, Kern A, Ramsey ME, Lundt ME, Sharma B, Lin T, et al. Genome-wide screen identifies novel genes required for Borrelia burgdorferi survival in its Ixodes tick vector. PLoS Pathog. 2019;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65(5):1193–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mulay VB, Caimano MJ, Iyer R, Dunham-Ems S, Liveris D, Petzke MM, et al. Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod- and mammalian host-specific signals. J Bacteriol. 2009;191(8):2783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100(19):11001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blevins JS, Xu H, He M, Norgard MV, Reitzer L, Yang XF. Rrp2, a sigma54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J Bacteriol. 2009;191(8):2902–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. 2008;76(9):3844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiol. 2008;154(Pt 9):2641–58. [DOI] [PubMed] [Google Scholar]
- 116.Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV, et al. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN – RpoS regulatory pathway. Proc Natl Acad Sci. 2001;98(22):12724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caimano MJ, Groshong AM, Belperron A, Mao J, Hawley KL, Luthra A, et al. The RpoS Gatekeeper in Borrelia burgdorferi: An Invariant Regulatory Scheme that Promotes Spirochete Persistence in Reservoir Hosts and Niche Diversity. Front Microbiol. 2019;10(AUG). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun. 2004;72(11):6433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, et al. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102(14):5162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, et al. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 2012;12(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW, et al. Motility is crucial for the infectious life cycle of borrelia burgdorferi. Infect Immun. 2013;81(6):2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Novak EA, Sekar P, Xu H, Moon KH, Manne A, Wooten RM, et al. The Borrelia burgdorferi CheY3 response regulator is essential for chemotaxis and completion of its natural infection cycle. Cell Microbiol. 2016;18(12):1782–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis. 2010;201(7):1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bestor A, Rego ROM, Tilly K, Rosa PA. Competitive advantage of Borrelia burgdorferi with outer surface protein BBA03 during tick-mediated infection of the mammalian host. Infect Immun. 2012;80(10):3501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu H, He M, He JJ, Yang XF. Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infect Immun. 2010;78(7):2910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patton TG, Brandt KS, Nolder C, Clifton DR, Carroll JA, Gilmore RD. Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse infection by tick transmission. Infect Immun. 2013;81(7):2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coumou J, Narasimhan S, Trentelman JJ, Wagemakers A, Koetsveld J, Ersoz JI, et al. Ixodes scapularis dystroglycan-like protein promotes Borrelia burgdorferi migration from the gut. J Mol Med. 2016;94(3):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Narasimhan S, Coumou J, Schuijt TJ, Boder E, Hovius JW, Fikrig E. A Tick Gut Protein with Fibronectin III Domains Aids Borrelia burgdorferi Congregation to the Gut during Transmission. PLoS Pathog. 2014;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L, Zhang Y, Adusumilli S, Liu L, Narasimhan S, Dai J, et al. Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph. PLoS Pathog. 2011;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perner J, Kropáčková S, Kopáček P, Ribeiro JMC. Sialome diversity of ticks revealed by RNAseq of single tick salivary glands. PLoS Negl Trop Dis. 2018;12(4):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim TK, Tirloni L, Pinto AFM, Moresco J, Yates JR, da Silva Vaz I, et al. Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS Negl Trop Dis. 2016;10(1):1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ribeiro JMC, Alarcon-Chaidez F, Ivo IM, Mans BJ, Mather TN, Valenzuela JG, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36(2):111–29. [DOI] [PubMed] [Google Scholar]
- 133.Valenzuela JG, Francischetti IMB, Pham VM, Garfield MK, Mather TN, Ribeiro JMC. Exploring the sialome of the tick Ixodes scapularis. J Exp Biol. 2002;205(18):2843–64. [DOI] [PubMed] [Google Scholar]
- 134.Lewis LA, Radulović ŽM, Kim TK, Porter LM, Mulenga A. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks Tick Borne Dis. 2015;6(3):424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schwarz A, Tenzer S, Hackenberg M, Erhart J, Gerhold-Ay A, Mazur J, et al. A systems level analysis reveals transcriptomic and proteomic complexity in Ixodes ricinus midgut and salivary glands during early attachment and feeding. Mol Cell Proteomics. 2014;13(10):2725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kotsyfakis M, Schwarz A, Erhart J, Ribeiro JMC. Tissue- and time-dependent transcription in Ixodes ricinus salivary glands and midguts when blood feeding on the vertebrate host. Sci Rep. 2015;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Narasimhan S, Booth CJ, DePonte K, Wu MJ, Liang X, Mohanty S, et al. Host-specific expression of Ixodes scapularis salivary genes. Ticks Tick Borne Dis. 2019;10(2):386–97. [DOI] [PubMed] [Google Scholar]
- 138.Anguita J, Ramamoorthi N, Hovius JWR, Das S, Thomas V, Persinski R, et al. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity. 2002;16(6):849–59. [DOI] [PubMed] [Google Scholar]
- 139.Hovius JWR, De Jong MAWP, Dunnen J Den, Litjens M, Fikrig E, Van Der Poll T, et al. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog. 2008;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hannier S, Liversidge J, Sternberg JM, Bowman AS. Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: A potential role in enhanced Borrelia burgdoferi transmission. Immunology. 2004;113(3):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garg R, Juncadella IJ, Ramamoorthi N, Ashish, Ananthanarayanan SK, Thomas V, et al. Cutting Edge: CD4 Is the Receptor for the Tick Saliva Immunosuppressor, Salp15. J Immunol. 2006;177(10):6579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gillespie RD, Dolan MC, Piesman J, Titus RG. Identification of an IL-2 Binding Protein in the Saliva of the Lyme Disease Vector Tick, Ixodes scapularis. J Immunol. 2001;166(7):4319–26. [DOI] [PubMed] [Google Scholar]
- 143.Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, et al. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem. 2002;277(12):10083–9. [DOI] [PubMed] [Google Scholar]
- 144.Kotál J, Stergiou N, Buša M, Chlastáková A, Beránková Z, Řezáčová P, et al. The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin. Cell Mol Life Sci. 2019;76(10):2003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Skallová A, Iezzi G, Ampenberger F, Kopf M, Kopecký J. Tick Saliva Inhibits Dendritic Cell Migration, Maturation, and Function while Promoting Development of Th2 Responses. J Immunol. 2008;180(9):6186–92. [DOI] [PubMed] [Google Scholar]
- 146.Sá-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, et al. Prostaglandin E2 Is a Major Inhibitor of Dendritic Cell Maturation and Function in Ixodes scapularis Saliva. J Immunol. 2007;179(3):1497–505. [DOI] [PubMed] [Google Scholar]
- 147.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436(7050):573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, et al. Antibodies against a Tick Protein, Salp15, Protect Mice from the Lyme Disease Agent. Cell Host Microbe. 2009;6(5):482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hannier S, Liversidge J, Sternberg JM, Bowman AS. Ixodes ricinus tick salivary gland extract inhibits IL-10 secretion and CD69 expression by mitogen-stimulated murine splenocytes and induces hyporesponsiveness in B lymphocytes. Parasite Immunol. 2003;25(1):27–37. [DOI] [PubMed] [Google Scholar]
- 150.Schuijt TJ, Hovius JWR, Van Burgel ND, Ramamoorthi N, Fikrig E, Van Dam AP. The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect Immun. 2008;76(7):2888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the Lyme disease agent. Cell Host Microbe. 2011;10(2):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valenzuela JG, Charlab R, Mather TN, Ribeiro JMC. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275(25):18717–23. [DOI] [PubMed] [Google Scholar]
- 153.Tyson K, Elkins C, Patterson H, Fikrig E, De Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol. 2007;16(4):469–79. [DOI] [PubMed] [Google Scholar]
- 154.Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 2007;9(2):247–50. [DOI] [PubMed] [Google Scholar]
- 155.Montgomery RR, Lusitani D, De Boisfleury Chevance A, Malawista SE. Tick Saliva Reduces Adherence and Area of Human Neutrophils. Infect Immun. 2004;72(5):2989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo X, Booth CJ, Paley MA, Wang X, DePonte K, Fikrig E, et al. Inhibition of neutrophil function by two tick salivary proteins. Infect Immun. 2009;77(6):2320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Donahue JG, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987;36(1):92–6. [DOI] [PubMed] [Google Scholar]
- 158.Brossard M, Fivaz V. Ixodes ricinus L.: mast cells, basophils and eosinophils in the sequence of cellular events in the skin of infested or re-infested rabbits. Parasitology. 1982;85 (Pt 3):583–92. [DOI] [PubMed] [Google Scholar]
- 159.Brossard M, Girardin P. Passive transfer of resistance in rabbits infested with adult Ixodes ricinus L: humoral factors influence feeding and egg laying. Experientia. 1979;35(10):1395–7. [DOI] [PubMed] [Google Scholar]
- 160.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65(1):335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Narasimhan S, DePonte K, Marcantonio N, Liang X, Royce TE, Nelson KF, et al. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One. 2007;2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burke G, Wikel SK, Spielman A, Telford SR, McKay K, Krause PJ, et al. Hypersensitivity to ticks and Lyme disease risk. Emerg Infect Dis. 2005;11(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the Lyme disease agent. PLoS Pathog. 2010;6(11):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Soares CAG, Lima CMR, Dolan MC, Piesman J, Beard CB, Zeidner NS. Capillary feeding of specific dsRNA induces silencing of the isac gene in nymphal Ixodes scapularis ticks. Insect Mol Biol. 2005;14(4):443–52. [DOI] [PubMed] [Google Scholar]
- 165.Kotsyfakis M, Sá-Nunes A, Francischetti IMB, Mather TN, Andersen JF, Ribeiro JMC. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281(36):26298–307. [DOI] [PubMed] [Google Scholar]
- 166.Kotsyfakis M, Karim S, Andersen JF, Mather TN, Ribeiro JMC. Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J Biol Chem. 2007;282(40):29256–63. [DOI] [PubMed] [Google Scholar]
- 167.Bensaci M, Bhattacharya D, Clark R, Hu LT. Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of Borrelia burgdorferi. Vaccine. 2012;30(42):6040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IMB, Mather TN, et al. Cutting Edge: Immunity against a “Silent” Salivary Antigen of the Lyme Vector Ixodes scapularis Impairs Its Ability to Feed. J Immunol. 2008;181(8):5209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schuijt TJ, Narasimhan S, Daffre S, DePonte K, Hovius JWR, van’t Veer C, et al. Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display. PLoS One. 2011;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: Vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci. 2004;101(52):18159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scheckelhoff MR, Telford SR, Hu LT. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine. 2006;24(11):1949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bhattacharya D, Bensaci M, Luker KE, Luker G, Wisdom S, Telford SR, et al. Development of a baited oral vaccine for use in reservoir-targeted strategies against Lyme disease. Vaccine. 2011;29(44):7818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gomes-Solecki MJC, Brisson DR, Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine. 2006;24:4440–9. [DOI] [PubMed] [Google Scholar]
- 174.Richer LM, Aroso M, Contente-Cuomo T, Ivanova L, Gomes-Solecki M. Reservoir Targeted Vaccine for Lyme Borreliosis Induces a Yearlong, Neutralizing Antibody Response to OspA in White-Footed Mice. Clin Vaccine Immunol. 2011;18(11):1809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gulia-Nuss M, Nuss AB, Meyer JM, Sonenshine DE, Roe RM, Waterhouse RM, et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat Commun. 2016;7(May 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Long AD, Baldwin-Brown J, Tao Y, Cook VJ, Balderrama-Gutierrez G, Corbett-Detig R, et al. The genome of Peromyscus leucopus, natural host for Lyme disease and other emerging infections. Sci Adv. 2019;5(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


