Abstract
Background:
Stress precipitates depression and may do so in part by increasing susceptibility to inflammation-induced depressive symptoms. However, this has not been examined among individuals facing a major life stressor. Accordingly, the present study tested the moderating role of stress on the longitudinal association between inflammation and depressive symptoms among women with breast cancer.
Methods:
Women recently diagnosed with early-stage breast cancer (N = 187) were enrolled before starting adjuvant/neoadjuvant treatment. Blood draws and self-reported depressive symptoms were collected pre-treatment, post-treatment, and at 6, 12, and 18-month post-treatment follow ups. C-reactive protein (CRP) was used to index inflammation. Measures of psychological stress, including cancer-related stress, general stress perceptions, and childhood stress, were administered pre-treatment.
Results:
Stress moderated the association between CRP and depressive symptoms, such that higher levels of CRP were associated with elevated depressive symptoms only among women who reported high cancer-related stress (β = .080, p = .002) and perceived stress (β = .053, p = .044); childhood stress effects were non-significant. Moreover, elevated CRP was associated with increased odds of exhibiting clinically significant depressive symptoms (OR = 1.64, p < .001) among women who reported high cancer-related stress. Results were independent of age, BMI, race and cancer-related covariates.
Conclusions:
Stress was found to heighten sensitivity to inflammation-associated depressive symptoms over a 2-year period, with notably stronger effects for subjective stress responses to a concurrent life event. Individuals who are most distressed following a major life event may exhibit the greatest risk for inflammation-induced depression.
Keywords: Depression, Inflammation, Breast Cancer, Stress, CRP
1. Introduction
Stress precipitates depression, such that major life events (Hammen, 2003; Kendler et al., 1999) and daily stressors (Melchior et al., 2007) significantly increase a person’s risk for depression. Indeed, some estimate that 80% of depression cases are preceded by a major life event (Mazure, 1998). The striking association between stress and depression may be partially attributed to inflammation (Dantzer et al., 2008; Miller et al., 2009; Miller and Raison, 2016; Raison et al., 2006). Stress is well known to elicit increases in inflammatory activity (Segerstrom and Miller, 2004; Steptoe et al., 2007; Herbert and Cohen, 1993), and inflammation can elicit behavioral alterations that include depressive symptoms (Dantzer et al., 2008). Notably, experimentally induced inflammation leads to increased depressed mood (DellaGioia and Hannestad, 2010) and alterations in depression-related neural processes (Eisenberger et al., 2010; Felger, 2018). Similarly, inflammation predicts the development of depression in observational studies (Colasanto et al., 2020; Gimeno et al., 2009; Zalli et al., 2016), and inflammatory markers are reliably elevated in depressed populations relative to healthy controls (Osimo et al., 2020). Stress may therefore precipitate depression in part by upregulating inflammation (Slavich and Irwin, 2014).
In addition to its role in eliciting inflammation and depression, stress may also influence susceptibility to inflammation-related depression. The association between inflammation and depressive symptoms exhibits notable heterogeneity, such that only 25–35% of depression cases clearly exhibit higher inflammation than healthy controls (Raison, 2020; Raison et al., 2013; Raison and Miller, 2011). Similarly, only 45% of individuals treated with interferon alpha (which elicits inflammatory activity) develop symptoms consistent with major depressive disorder (Musselman et al., 2001). Accordingly, a subset of individuals may be particularly susceptible to depression when inflammation rises (Raison and Miller, 2011). Stress has been shown to increase vulnerability to inflammation-induced depressive symptoms in preclinical models (Cunningham, 2013; Dudek et al., 2020; Foti Cuzzola et al., 2013; Frank et al., 2020; Menard et al., 2017; Sántha et al., 2016), and initial clinical studies also support stress as a moderator of the association between inflammation and depression. For example, healthy young adults who reported higher levels of perceived stress (Irwin et al., 2019) and early life adversity (Kuhlman et al., 2020) exhibit a larger increase in depressive symptoms following acute inflammatory challenge.
Despite promising evidence that stress may both trigger inflammation and modulate its association with depression, the moderating role of stress has yet to be examined in the context of a major life event. Accordingly, the present study tested stress as a moderator of the association between inflammation and depressive symptoms among women undergoing breast cancer diagnosis, treatment and recovery. Examining stress moderation effects in this population is ideal because cancer diagnosis is a major life event that is known to increase risk for depression (Linden et al., 2012; Mitchell et al., 2011). In addition, applying this program of research to a breast cancer context is important (Bower, 2007; Bower et al., 2011; Irwin et al., 2013) because depressed cancer survivors exhibit reduced quality of life (Bower et al., 2011), treatment adherence (DiMatteo et al., 2002), health care utilization (Goldstein et al., 2012), and survival (Pinquart and Duberstein, 2010). The study sample comprises women recently diagnosed with early stage breast cancer who were followed for approximately 2 years with repeated assessments of inflammation and depressive symptoms. CRP was used to index systemic inflammation given that it is reliably elevated in depressed samples (Osimo et al., 2020), longitudinally associated with depressive symptoms (Valkanova et al., 2013), and indicates risk for de novo major depressive disorder (Pasco et al., 2010). In accordance with current guidelines (Crosswell and Lockwood, 2020; Epel et al., 2018), we utilized several stress measures including event-based (i.e., cancer-related stress), global (i.e., general stress perceptions), and early life (i.e., childhood stress) measures. Based on research showing that stress increases depressive symptoms following inflammatory challenge (Irwin et al., 2019; Kuhlman et al., 2020), we hypothesized that women who reported higher levels of stress at baseline would exhibit greater depressive symptoms and higher odds of clinically significant depressive symptoms at times when CRP was elevated. Given the focus on stress responses to a concurrent life event, primary analyses focused on baseline stress assessments to capture post-diagnosis stress responses. However, repeated assessments were available for cancer-related stress and general stress perceptions, and were used to examine if stress moderation effects detected at baseline extended to subsequent assessments.
2. Material and Method
2.1. Participants
Patients were recruited from oncology practices in Los Angeles to participate in a longitudinal, observational study of cancer-related fatigue (RISE study) between January 2013 and July 2015 (Bower et al., 2019). Women were eligible if they were 1) newly diagnosed with early stage (0 – IIIA) breast cancer, 2) had yet to begin (neo)adjuvant treatment with radiation, chemotherapy, or endocrine therapy, and 3) were proficient in English. All participants provided written consent and the UCLA Institutional Review Board pre-approved all procedures. Prior publications using the RISE study data examined risk factors for cancer-related fatigue (Bower et al., 2019, in press), associations between hypothalamic-pituitary-adrenocortical axis functioning and depressive symptoms (Kuhlman et al., 2017b), pathways linking childhood maltreatment to depressive symptoms (Kuhlman et al., 2017a), associations between childhood maltreatment and monocyte gene expression (Bower et al., 2020).
2.2. Procedure
Two-hundred and seventy women completed baseline assessments after breast cancer diagnosis but before starting (neo)adjuvant treatment. Most women enrolled within 60 days of diagnosis (78.4%), and had completed surgery (lumpectomy or mastectomy) at the time of enrollment (91%); the remaining 9% were enrolled before neoadjuvant therapy and thus had not undergone surgery at baseline. Women who received radiation and/or chemotherapy (N = 207) had the opportunity to complete a post-treatment assessment scheduled 2–4 weeks after the end of adjuvant treatment. Next, all women were assessed at 6-, 12-, and 18-month follow-ups. Study retention was high, with at least 90% of participants completing each of the follow-up assessments (see Bower et al., in press for CONSORT diagram and additional information). At each study assessment, participants completed online questionnaires and provided blood samples for immune assessment. The current study included participants who provided at least two blood samples (N = 187), which was required for analyses of within-person effects (described below).
2.3. Measures
Depressive symptoms, cancer-related stress, and general stress perceptions were measured at each study assessment, whereas childhood stress was only measured during the baseline assessment. All psychological measures were administered via online questionnaires using Qualtrics.
2.3.1. Depressive Symptoms
The primary outcome of the present study—depressive symptoms—was assessed using the Center for Epidemiologic Studies Depression scale (CES-D), a valid and reliable measure that includes affective, cognitive, and somatic symptoms of depression (Radloff, 1977). Participants were presented with 20 statements and asked to rate how often they experienced each statement over the past week using a 0 to 3 scale. Scores were summed; higher CES-D scores indicate more depressive symptoms. In addition to sum scores, binary variables indicating the presence of clinically significant depressive symptoms were created using the traditional clinical cutoff of ≥ 16 for the CES-D (Lewinsohn et al., 1997) as well as a cutoff of ≥ 20, given that a recent meta-analysis (Vilagut et al., 2016) found that a ≥ 20 threshold yielded a better balance of sensitivity and specificity then the traditional ≥ 16 clinical cutoff.
2.3.2. Stress Measures
2.3.2.1. Cancer-related Stress
Cancer-related stress over the past week was measured using the intrusions subscale of the Impact of Event Scale (IES), a measure of subjective distress in response to a specific traumatic event (Horowitz et al., 1979), including breast cancer (Dupont et al., 2014). Participants were presented with 7 statements (e.g., I thought about it when I didn’t mean to), and asked to indicate “how frequently these comments were true for you during the past seven days with regard to your breast cancer” on a 0 to 5 scale. Scores on this scale were averaged; higher IES scores indicate more frequent cancer-related intrusive thoughts.
2.3.2.2. General Stress Perceptions
General stress perceptions over the past month was measured using the Perceived Stress Scale (PSS), one of the most commonly used instruments to assess subjective stress (Cohen et al., 1983). Participants rated the frequency with which they experienced 10 statements pertaining to feelings of stress over the last month (e.g., How often have you found that you could not cope with all the things you had to do?) on a 0 to 4 scale. Scores were summed; higher PSS scores indicate greater general stress perceptions.
2.3.2.3. Childhood Stress
Childhood stress was measured using a shortened version of the 28-item Childhood Trauma Questionnaire (CTQ) that omitted the 3-item minimization/denial subscale (Bernstein et al., 1994). The CTQ assesses physical, emotional, and sexual abuse during childhood as well as physical and emotional neglect. Participants were presented with statements pertaining to traumatic childhood experiences and asked to rate how true each statement was for them on a 5-point scale. A total CTQ score was computed, consistent with past work (Bevilacqua et al., 2012). Higher CTQ scores indicate greater childhood stress.
2.3.3. C-reactive Protein
Blood samples were collected through venipuncture by a licensed phlebotomist on up to 5 occasions throughout the study period. Blood draws were scheduled to coincide with clinic visits, when possible, and typically took place before noon. Blood samples were transported on dry ice to the Inflammatory Biology Core Laboratory at the Cousins Center for Psychoneuroimmunology (UCLA, CA), where they were centrifuged for acquisition of plasma and stored at – 80°C until assayed. Human Quantikine ELISA assays (R&D Systems, Minneapolis, MN) were used to quantify circulating plasma levels of C-reactive protein (CRP); all samples were assayed in duplicate and averaged. The CRP assay lower limit was 0.2 mg/L. Inter-assay and intra-assay coefficient of variation were both low (4.6% and 1.8%, respectively).
2.3.4. Demographic, Medical, and Cancer-related Variables
Age, body mass index (BMI), race, cancer stage, surgery type, as well as receipt of chemotherapy, radiation therapy, and endocrine therapy were included as covariates, given their potential association with depressive symptoms and/or inflammation (Nolen-Hoeksema and Ahrens, 2002; Rexrode et al., 2003; Sun et al., 2019). Height and weight were measured by clinic staff. Age and race were self-reported as part of baseline demographic questionnaires. Type of surgery received (lumpectomy, mastectomy), cancer stage, as well as receipt of chemotherapy, radiation therapy, and endocrine therapy were determined from medical records.
2.4. Analytic Plan
Robust mixed linear models were fitted to the present data using the robustlmm (Koller, 2016) R package in R 4.0.2 (R Core Team, 2019). Sampling occasions (level-1) were nested within individuals (level-2) to yield 2-level models. Individual-level intercepts were modeled as random effects. CRP values were natural log transformed before analysis to normalize level-1 residuals. Cluster-level centering was used to isolate within-person variability in repeated CRP measurement (Enders and Tofighi, 2007). Person-centered CRP was computed by subtracting individuals’ average CRP values across sampling occasion from CRP values corresponding to each sampling occasion. Accordingly, positive person-centered scores indicate that an individual exhibited higher values than (their own) average across time. Person-centered CRP was entered as a level 1 fixed and random effect, consistent with guidelines for testing cross-level interactions (Heisig and Schaeffer, 2019).
In primary analyses, baseline levels of cancer-related intrusive thoughts, general stress perceptions, and childhood stress were examined as moderators of the within-person association between CRP and depressive symptoms. Moderation tests were carried out by including these variables (in separate models) as a level-2 fixed effect interacting with person-centered CRP slopes. For cancer-related intrusive thoughts and general stress perceptions, tests of moderation were repeated while entering these variables as level 1 variables (because repeated assessments were available) to examine if moderation effects were evident across assessments. Significant interactions were followed-up by tests of simple slopes contrasting low (−1 SD), moderate (mean), and high (+1 SD) levels of the moderator variable. Plots were created using sjPlot (Lüdecke, 2018). Consistent with extant guidelines (Lorah, 2018), effect size estimates (reported as β) were calculated by standardizing outcome variables and continuous predictors.
Primary analyses were repeated while predicting binary variables (derived from the ≥ 16 and 20 cutoffs) indicating the presence of clinically significant depressive symptoms (i.e., 1 = clinically significant depressive symptoms; 0 = not clinically significant depressive symptoms). The presence of clinically significant depressive symptoms was modeled using generalized linear mixed effect model (with a binomial link function) via the lme4 R package (Bates et al., 2015). These models were otherwise identical to models used to test primary hypotheses for continuous CES-D scores.
All covariate measures (i.e., age, BMI, race, surgery, cancer stage, and receipt of chemotherapy, radiation therapy, and endocrine therapy) were entered as level-2 fixed effects. Age and BMI were mean centered. Race (i.e., White v. Black v. Asian v. Other) and cancer stage were dummy coded and centered on “White” and “stage 0 or 1.” Surgery was dichotomized (i.e., lumpectomy v. mastectomy) and centered on “lumpectomy.” Receipt of chemotherapy, radiation and endocrine therapy were centered on the most frequently endorsed value (i.e., “did not receive chemotherapy”, “received radiation therapy” and “received endocrine therapy”).
3. Results
3.1. Descriptive Analyses
As shown on Table 1, study participants were primarily middle aged (M = 55.5, SD = 11.2), white (75%), married (65%), had a college degree (71%), and an annual household income over $100K (54%). Most women presented with stage 0 or 1 breast cancer (60%) and underwent lumpectomy (61%), radiation treatment (72%) and endocrine therapy (63%), and did not receive chemotherapy (60%).
Table 1.
Demographics and Clinical Characteristics of the Sample.
| Variable | N | % | Mean | (SD) |
|---|---|---|---|---|
| Age (years) | 187 | 55.55 | (11.21) | |
| Education | ||||
| High School Diploma or less | 8 | 4.28% | ||
| Some College | 46 | 24.60% | ||
| College Degree | 72 | 38.50% | ||
| Post-Graduate Degree | 61 | 32.62% | ||
| Race | ||||
| White/Caucasian | 140 | 74.87% | ||
| Black/African American | 8 | 4.28% | ||
| Asian | 20 | 10.70% | ||
| Other | 19 | 10.16% | ||
| Married | ||||
| Married or living as married | 121 | 64.71% | ||
| Divorced, separated, widowed, or never married | 66 | 35.29% | ||
| Income | ||||
| < $60,000 | 47 | 25.41% | ||
| $60,000 - $100,000 | 38 | 20.54% | ||
| > $100,000 | 100 | 54.05% | ||
| Breast Cancer Stage | ||||
| 0 or 1 | 112 | 59.89% | ||
| 2 or 3 | 75 | 40.11% | ||
| Surgery Type | ||||
| Lumpectomy | 114 | 60.96% | ||
| Mastectomy | 73 | 39.04% | ||
| Received chemotherapy | 74 | 39.57% | ||
| Received radiation therapy | 135 | 72.19% | ||
| Received endocrine therapy | 118 | 63.10% |
As shown on Table 2, baseline CES-D levels were elevated relative to community-residing older adults (Lewinsohn et al., 1997) but below the clinical threshold of 16, and tended to decrease over time. Across all assessments, 28.5% of CES-D scores were ≥ 16, and 19.7% of CES-D scores were ≥ 20. Levels of CRP were also somewhat elevated at baseline (> 3 mg/L) with a tendency to remain elevated post-treatment and decrease thereafter.
Table 2.
Descriptive Statistics for Depressive symptoms, CRP and Stress Measures
| Variable | N | % | α | Mean | (SD) |
|---|---|---|---|---|---|
| Criterion: | |||||
| Depressive Symptoms (CES-D) | |||||
| Pre-treatment | 187 | .908 | 12.95 | (10.25) | |
| Post-treatment | 144 | .917 | 10.70 | (9.91) | |
| 6 Month Follow-up | 178 | .924 | 12.06 | (10.80) | |
| 12 Month Follow-up | 176 | .911 | 11.52 | (9.76) | |
| 18 Month Follow-up | 172 | .916 | 11.14 | (10.00) | |
| Clinically Significant Depressive Symptoms (CES-D ≥ 16) | |||||
| Pre-treatment | 63 | 33.69 % | |||
| Post-treatment | 38 | 26.39 % | |||
| 6 Month Follow-up | 54 | 30.34 % | |||
| 12 Month Follow-up | 44 | 25.00 % | |||
| 18 Month Follow-up | 45 | 26.16 % | |||
| Clinically Significant Depressive Symptoms (CES-D ≥ 20) | |||||
| Pre-treatment | 42 | 22.46 % | |||
| Post-treatment | 26 | 18.06 % | |||
| 6 Month Follow-up | 38 | 21.35 % | |||
| 12 Month Follow-up | 34 | 19.32 % | |||
| 18 Month Follow-up | 29 | 16.86 % | |||
| Predictor: | |||||
| CRP (mg/L) | |||||
| Pre-treatment | 185 | 3.44 | (5.25) | ||
| Post-treatment | 144 | 3.74 | (7.85) | ||
| 6 Month Follow-up | 168 | 2.41 | (3.76) | ||
| 12 Month Follow-up | 159 | 2.30 | (3.60) | ||
| 18 Month Follow-up | 159 | 2.56 | (4.45) | ||
| Stress Moderators: | |||||
| Cancer-related Intrusive Thoughts (IES) | |||||
| Pre-treatment | 187 | .898 | 1.78 | (1.35) | |
| Post-treatment | 144 | .909 | 1.12 | (1.18) | |
| 6 Month Follow-up | 177 | .903 | 1.21 | (1.18) | |
| 12 Month Follow-up | 176 | .884 | 1.15 | (1.11) | |
| 18 Month Follow-up | 172 | .892 | 1.08 | (1.09) | |
| General stress perceptions (PSS) | |||||
| Pre-treatment | 187 | .876 | 15.50 | (6.63) | |
| Post-treatment | 144 | .909 | 13.16 | (6.98) | |
| 6 Month Follow-up | 178 | .925 | 14.87 | (7.68) | |
| 12 Month Follow-up | 176 | .912 | 14.52 | (7.04) | |
| 18 Month Follow-up | 172 | .907 | 14.46 | (7.25) | |
| Childhood stress (CTQ) | 184 | .928 | 37.24 | (14.13) |
CES-D = Center for Epidemiological Studies – Depression; CRP = C-reactive Protein; IES = Impact of Event Scale; PSS = Perceived Stress Scale; CTQ = Childhood Trauma Questionnaire.
Higher baseline stress was generally associated with both elevated depressive symptoms and inflammation across time. Women who reported more intrusive thoughts about cancer, greater general stress perceptions and higher childhood stress exhibited higher depressive symptoms over the course of the study (b = 1.90, SE = .42, β = .23, t(172) = 4.6, p < .001, b = .81, SE = .07, β = .54, t(172) = 12.0, p < .001, and b = .17, SE = .04, β = .21, t(172) = 4.0, p < .001, respectively). Similarly, perceived stress and childhood stress were positively associated with CRP levels across sampling occasions (b = 0.02, SE = .012, β = .098, t(176) = 1.7, p = .091, and b = .013, SE = .006, β = .13, t(174) = 2.3, p = .019, respectively). However, CRP was not associated with cancer-related intrusive thoughts (p = .46). General stress perceptions were significantly correlated with cancer-related intrusive thoughts (r = .51, p < .001) and childhood stress (r = .30, p < .001), but cancer-related intrusive thoughts and childhood stress were not significantly correlated (r = .11, p = .10).
3.2. Association between CRP and depressive symptoms as moderated by stress measures
Analyses were first conducted to test the association between CRP and depressive symptoms. There was no evidence that person-centered CRP was associated with CES-D scores (b = 0.22, SE = .29, β = .013, t(69) = 0.75, p = .45). Excluding covariate measures did not influence this result.
Next, we tested our primary hypothesis that stress would moderate the association between CRP and depressive symptoms. Indeed, we found that baseline intrusive thoughts about cancer moderated the association between person-centered CRP and depressive symptoms (b = .744, SE = .219, β = .059, t(70) = 3.40, p = .001), such that CRP was positively associated with depressive symptoms only among women who reported high (+1 SD) levels of intrusive thoughts about cancer (b = 1.284, SE = .407, β = .080, t(96) = 3.16, p = .002) (Figure 1a). By contrast, the association between person-centered CRP and depressive symptoms was non-significant for women who reported low (−1 SD) or moderate (mean) intrusive thoughts about cancer (b = −.618, SE = .400, β = −.038, t(52) = 1.54, p = .13, and b =.333, SE = .291, β = .021, t(69) = 1.15, p = .25, respectively). Moderation tests examining cancer-related intrusive thoughts as a level 1 moderator revealed a comparable pattern of results (p < .001), suggesting that more intrusive thoughts were associated with a stronger coupling of CRP and depressive symptoms at any given assessment.
Figure 1.
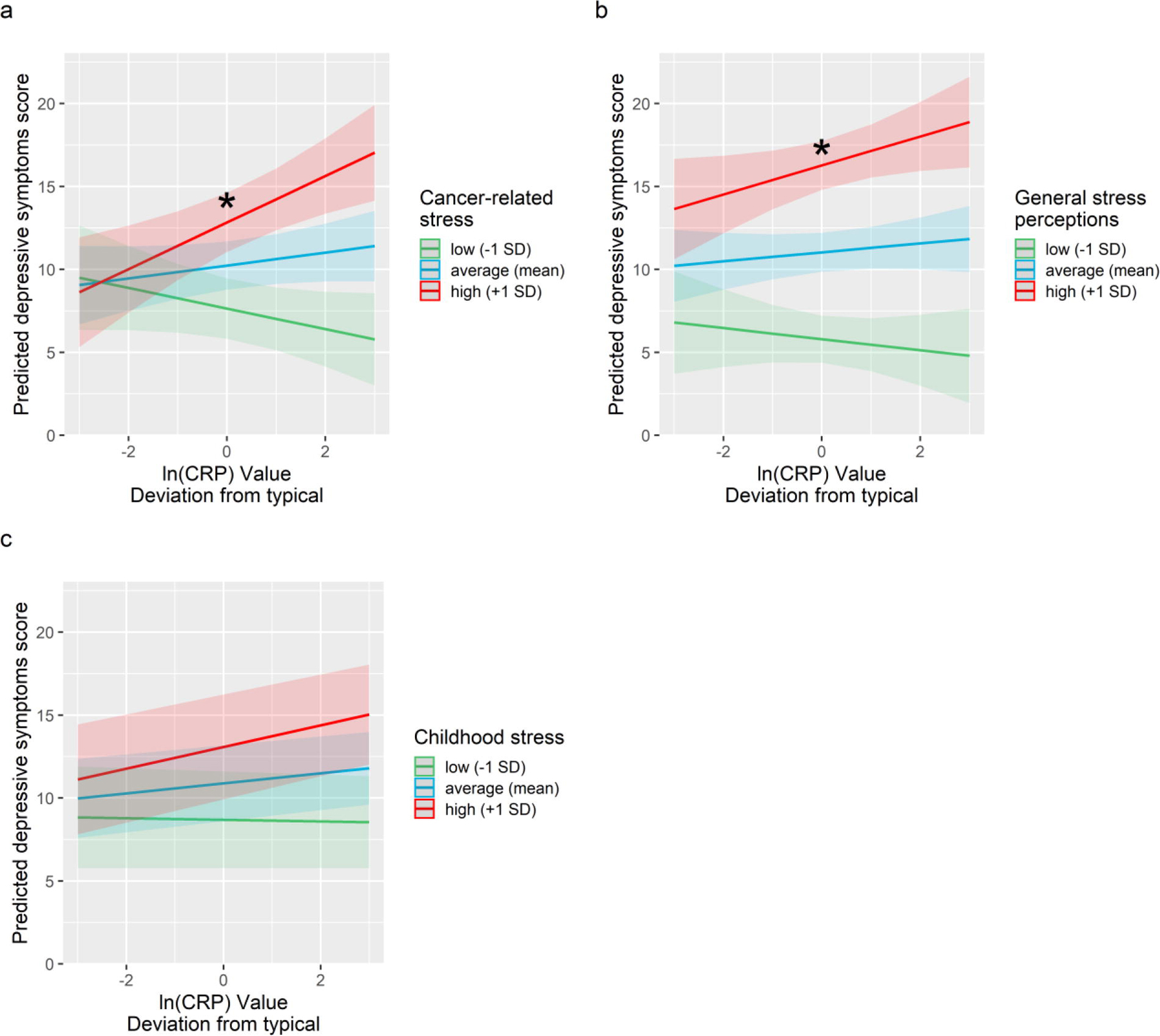
Predicted depressive symptoms as a function of person-centered CRP and cancer-related stress (panel a), general stress perceptions (panel b), and childhood stress (panel c). Elevated CRP was associated with increased depressive symptoms only among women who reported high cancer related stress and high general stress perceptions. Predicted depressive symptom scores were adjusted for age, BMI, race, surgery, cancer stage and receipt of chemotherapy, radiation therapy and endocrine therapy. Shaded areas depict confidence intervals of simple slopes. The asterisk symbols (*) index statistically significant simple slopes (p < .05).
Similarly, general stress perceptions at the time of study enrollment marginally moderated the association between person-centered CRP and depressive symptoms (b = 0.092, SE = .048 β = .039, t(97) = 1.89, p = .062), such that women with high (+1 SD) reported general stress perceptions showed higher depressive symptoms at assessments when their CRP levels were elevated (b = .864, SE = .419, β = .054, t(82) = 2.06, p = .042), whereas women with low (−1 SD) or moderate (mean) reported general stress perceptions showed no association between CRP and depressive symptoms (b = −.389, SE = .465, β = −.024, t(81) = .84, p = .41, and b = .238, SE = .293, β = .015, t(67) = .81, p = .42, respectively). Moderation tests examining general stress perceptions as a level 1 moderator were non-significant (p = .32), suggesting that greater stress perceptions were associated with a marginally stronger coupling of CRP and depressive symptoms only at baseline.
Childhood stress did not moderate the association between person-centered CRP and depressive symptoms (all ps > .23). Excluding covariate measures or controlling for baseline CRP levels did not influence tests of moderation for any stress measure.
3.3. Association between CRP and clinically-significant depression as moderated by stress measures
Tests of primary hypotheses were repeated using binary variables indicative of clinically significant depressive symptoms. Using the ≥ 20 CES-D cutoff revealed a significant interaction of cancer-related intrusive thoughts by person-centered CRP predicting clinically significant depressive symptoms (OR = 1.46, z = 2.78, p = .005). As shown on Figure 2, higher levels of CRP increased the odds of displaying clinically significant depressive symptoms only among women who reported high (+1 SD) cancer-related intrusive thoughts (OR = 1.64, p < .001). By contrast, person-centered CRP did not predict the odds of displaying clinically significant depressive symptoms for women who reported moderate (mean) or low (−1 SD) cancer-related intrusive thoughts (all ps > .10). Using the traditional ≥ 16 CES-D cutoff, the interaction of baseline cancer-related intrusive thoughts by person-centered CRP was non-significant (p = .16), but tests of simple slopes indicated a similar pattern of results where higher CRP levels were associated with greater odds of displaying clinically significant depressive symptoms only among women who reported high (+1 SD) cancer-related intrusive thoughts (OR = 1.52, p = .041). Moderation tests examining cancer-related intrusive thoughts as a level 1 moderator revealed a comparable pattern of results with significant moderation for both clinical cutoffs (all ps < .005), suggesting that moderation effects observed for intrusive thoughts were not limited to the baseline assessment.
Figure 2.
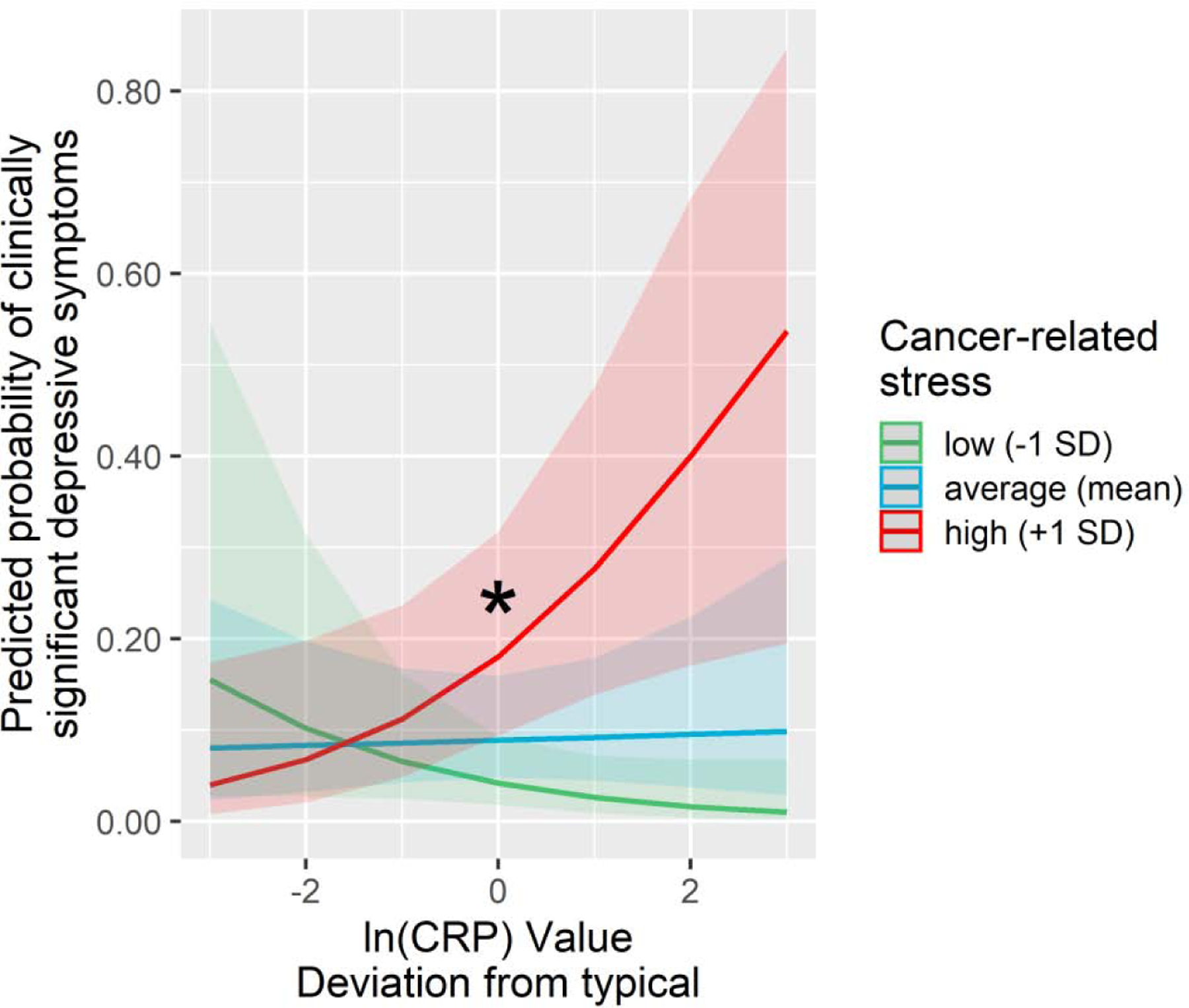
Predicted probability of clinically significant depressive symptoms (CES-D scores ≥ 20) as a function of person-centered CRP and cancer-related stress. Elevated CRP was associated with increased probability of exhibiting clinically significant depressive symptoms only among women who reported high cancer-related stress. Models were adjusted for age, BMI, race, surgery, cancer stage and receipt of chemotherapy, radiation therapy and endocrine therapy. Shaded areas depict confidence intervals of simple slopes. The asterisk symbols (*) index statistically significant simple slopes (p < .05).
Consistent with tests of continuous CES-D scores, the presence of clinically significant depressive symptoms (as indexed by the ≥ 20 or the ≥ 16 clinical cutoffs) was not predicted by the interaction of person-centered CRP and general stress perceptions, or the interaction of person-centered CRP and childhood stress (all ps > .21). Moderation tests examining general stress perceptions as a level 1 moderator were non-significant (all ps > .62). Excluding covariate measures or controlling for baseline CRP levels did not influence results of analyses predicting the presence of clinically significant depressive symptoms.
4. Discussion
Inflammation is reliably linked with depression, but this association appears to be driven by a subset of depressed individuals (Raison, 2020; Raison et al., 2013; Raison and Miller, 2011). Accordingly, there is a growing need to identify individuals who are most susceptible to depressive symptoms when inflammation is elevated. Stress may increase susceptibility to inflammation-induced depression (Irwin et al., 2019; Kuhlman et al., 2020), but no studies to date have examined this relationship in the context of an intense, real-life stressor such as cancer diagnosis and treatment. Accordingly, the present study tested the moderating role of three key components of stress - cancer-related stress, general stress perceptions and childhood stress - on the association between inflammation and depressive symptoms over a 2-year period in women with breast cancer. Consistent with hypotheses, the association between CRP and depressive symptoms was moderated by stress, such that women who reported higher levels of cancer-related stress were more likely to report higher depressive symptoms when their CRP levels were elevated. A similar pattern of findings was noted for general stress perceptions, though this association was evident only at the pre-treatment assessment. In contrast, childhood stress did not moderate the association between CRP and depressive symptoms. Finally, elevated CRP predicted the odds of exhibiting clinically significant depressive symptoms only among women who reported high cancer-related intrusive thoughts.
There is growing interest in identifying individuals who are susceptible to inflammation-associated depression (Raison, 2020; Raison et al., 2013; Raison and Miller, 2011). In rodents, experimental stressors are shown to increase blood brain barrier permeability (Dudek et al., 2020; Foti Cuzzola et al., 2013; Menard et al., 2017; Sántha et al., 2016), promote myeloid cells migration to the brain (Wohleb et al., 2016), and heighten inflammatory responses of microglia (Cunningham, 2013; Frank et al., 2020). On this basis, stressors are hypothesized to heighten the effect of peripheral inflammation on the brain, thereby rendering individuals more susceptible to depressive symptoms when peripheral inflammation is upregulated. Indeed, greater perceived stress is associated with a larger rise in depressive symptoms following endotoxin administration in healthy adults (Irwin et al., 2019). The present study extends this body of work to individuals experiencing a major life stressor, demonstrating that women who experienced higher levels of stress following breast cancer diagnosis were most vulnerable to inflammation-associated depressive symptoms over a 2-year period.
The present study included multiple stress measures to evaluate event-specific and general stress perceptions as well as childhood stress experiences. Results best supported a moderating effect of cancer-related stress, as measured by the frequency of intrusive thoughts about cancer over the past week. Even sub-clinical elevations in CRP (i.e., 2.72 nmol/L) were associated with a 64% increase in the odds of exhibiting clinically significant depressive symptoms for women who reported high cancer-related intrusive thoughts at baseline. Of note, this levels of intrusive thoughts is comparable with patients seeking psychotherapy following a major life event (Horowitz et al., 1979). A similar pattern of results was observed for general stress perceptions over the past month, but results were marginally significant, and limited to the baseline assessment. In contrast, childhood stress did not moderate the association between inflammation and depression, although almost half of study participants reported some type of maltreatment. More distal stress exposure may be less relevant in this sample of middle-aged and older women confronting the immediate threat of cancer diagnosis and treatment. Overall, findings suggest that event-specific stress and general stress perceptions immediately following a major life event best index vulnerability to inflammation-associated depressive symptoms. From a clinical perspective, monitoring stress levels at the time of stress exposure could serve to identify individuals most vulnerable to behavioral symptoms and subsequent disruptions in quality of life (Dupont et al., 2014).
Strengths of the present study included a relatively large sample and repeated assessments of CRP and depressive symptoms over a 2-year period among women confronting a major life stressor. This design provides an ideal opportunity to examine how “naturalistic” variation in inflammation would be associated with depressive symptoms in stressed individuals. Furthermore, the present sample exhibited a wide range of depressive symptom levels of which approximately 1/5 exceeded the clinical threshold, suggesting that results are applicable to populations exhibiting increased depression prevalence. Finally, the present study included varied stress measures, which allowed for a more granular test of stress effects. Nevertheless, the presented data were observational, and causality may not be inferred. Furthermore, observed effect sizes were small, and the moderating effect of baseline general stress perceptions was only marginally significant. Finally, results may be specific to the experience of cancer diagnosis/treatment, and future work should evaluate if findings generalize to other life events.
5. Conclusions
The present study sought to examine the moderating role of stress on susceptibility to inflammation-associated depressive symptoms among women with breast cancer followed over approximately 2 years. Results revealed that elevated CRP was associated with increased depressive symptoms only among women who reported higher levels of cancer-related stress at any given time and general stress perceptions shortly after diagnosis. Moreover, elevated CRP also predicted the odds of exhibiting clinically significant depressive symptoms among women who reported high cancer-related stress. There is a growing need to identify for whom elevated inflammation will lead to depression, and the present study demonstrates that heightened subjective stress following a major life event increases susceptible to inflammation-associated depressive symptoms over a 2-year period. Monitoring stress levels at the onset of a major life event (e.g., following cancer diagnosis) could therefore serve to identify individuals at risk for inflammation-induced depression such that they may be targeted by preventive measures.
Highlights:
Stressed cancer survivors showed greater coupling of depression and inflammation
Stress heightens sensitivity to inflammation-associated depression over 2 years
Effects were strongest for measures of stress responses to a concurrent life event
Acknowledgment
This work was supported by the National Cancer Institute R01 CA160427 and by the Breast Cancer Research Foundation. C.M.C. was supported by the National Cancer Institute (P30 CA016042). Preparation of this article was made possible by the National Institute of Mental Health awarded to A.W.M (T32MH015750), and K.R.K. (K08MH112773).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement
The authors reported no biomedical financial interests or potential conflicts of interest.
6. References
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw 67. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch M-A, 2012. Interaction Between FKBP5 and Childhood Trauma and Risk of Aggressive Behavior. Arch. Gen. Psychiatry 69, 62–70. 10.1001/archgenpsychiatry.2011.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, 2007. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain. Behav. Immun 21, 863–871. 10.1016/j.bbi.2007.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Asher A, Garet D, Petersen L, Ganz PA, Irwin MR, Cole SW, Hurvitz SA, Crespi CM, 2019. Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125, 633–641. 10.1002/cncr.31827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Cole SW, Garet D, Petersen L, Asher A, Hurwitz SA, Crespi CM, in press. Do all cancer patients experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer [DOI] [PMC free article] [PubMed]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW, 2011. Inflammation and Behavioral Symptoms After Breast Cancer Treatment: Do Fatigue, Depression, and Sleep Disturbance Share a Common Underlying Mechanism? J. Clin. Oncol 29, 3517–3522. 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Kuhlman KR, Ganz PA, Irwin MR, Crespi CM, Cole SW, 2020. Childhood maltreatment and monocyte gene expression among women with breast cancer. Brain. Behav. Immun 88, 396–402. 10.1016/j.bbi.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A Global Measure of Perceived Stress. J. Health Soc. Behav 24, 385–396. [PubMed] [Google Scholar]
- Colasanto M, Madigan S, Korczak DJ, 2020. Depression and inflammation among children and adolescents: A meta-analysis. J. Affect. Disord 277, 940–948. 10.1016/j.jad.2020.09.025 [DOI] [PubMed] [Google Scholar]
- Crosswell AD, Lockwood KG, 2020. Best practices for stress measurement: How to measure psychological stress in health research. Health Psychol. Open 7, 2055102920933072. 10.1177/2055102920933072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, 2013. Microglia and neurodegeneration: The role of systemic inflammation. Glia 61, 71–90. 10.1002/glia.22350 [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J, 2010. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci. Biobehav. Rev 34, 130–143. 10.1016/j.neubiorev.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW, 2002. Patient adherence and medical treatment outcomes: a meta-analysis. Med. Care 40, 794–811. 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, Ferrer Perez C, Golden SA, Tamminga C, Turecki G, Mechawar N, Russo SJ, Menard C, 2020. Molecular adaptations of the blood–brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci 117, 3326–3336. 10.1073/pnas.1914655117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont A, Bower JE, Stanton AL, Ganz PA, 2014. Cancer-Related Intrusive Thoughts Predict Behavioral Symptoms Following Breast Cancer Treatment. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc 33, 155–163. 10.1037/a0031131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-Induced Anhedonia: Endotoxin Reduces Ventral Striatum Responses to Reward. Biol. Psychiatry, Positron Emission Tomography Imaging of Dopamine and Opiate Receptors in Addiction 68, 748–754. 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Tofighi D, 2007. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol. Methods 12, 121–138. 10.1037/1082-989X.12.2.121 [DOI] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB, 2018. More than a feeling: A unified view of stress measurement for population science. Front. Neuroendocrinol 49, 146–169. 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, 2018. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr. Neuropharmacol 16, 533–558. 10.2174/1570159X15666171123201142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti Cuzzola V, Galuppo M, Iori R, De Nicola GR, Cassata G, Giacoppo S, Bramanti P, Mazzon E, 2013. Beneficial effects of (RS)-glucoraphanin on the tight junction dysfunction in a mouse model of restraint stress. Life Sci 93, 288–305. 10.1016/j.lfs.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Watkins LR, Maier SF, 2020. Acute stress induces chronic neuroinflammatory, microglial and behavioral priming: A role for potentiated NLRP3 inflammasome activation. Brain. Behav. Immun S0889159120308369. 10.1016/j.bbi.2020.05.063 [DOI] [PMC free article] [PubMed]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GDO, Rumley A, Marmot MG, Ferrie JE, 2009. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med 39, 413–423. 10.1017/S0033291708003723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D, Bennett BK, Webber K, Boyle F, de Souza PL, Wilcken NRC, Scott EM, Toppler R, Murie P, O’Malley L, McCourt J, Friedlander M, Hickie IB, Lloyd AR, 2012. Cancer-Related Fatigue in Women With Breast Cancer: Outcomes of a 5-Year Prospective Cohort Study. J. Clin. Oncol 30, 1805–1812. 10.1200/JCO.2011.34.6148 [DOI] [PubMed] [Google Scholar]
- Hammen C, 2003. Interpersonal stress and depression in women. J. Affect. Disord., Women and Depression 74, 49–57. 10.1016/S0165-0327(02)00430-5 [DOI] [PubMed] [Google Scholar]
- Heisig JP, Schaeffer M, 2019. Why You Should Always Include a Random Slope for the Lower-Level Variable Involved in a Cross-Level Interaction. Eur. Sociol. Rev 35, 258–279. 10.1093/esr/jcy053 [DOI] [Google Scholar]
- Herbert TB, & Cohen S, 1993. Stress and immunity in humans: a meta-analytic review. Psychosom. Med 55, 364–379. 10.1097/00006842-199307000-00004 [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W, 1979. Impact of Event Scale: A Measure of Subjective Stress: Psychosom. Med 41, 209–218. 10.1097/00006842-197905000-00004 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole S, Olmstead R, Breen EC, Cho JJ, Moieni M, Eisenberger NI, 2019. Moderators for depressed mood and systemic and transcriptional inflammatory responses: a randomized controlled trial of endotoxin. Neuropsychopharmacology 44, 635–641. 10.1038/s41386-018-0259-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead RE, Ganz PA, Haque R, 2013. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain. Behav. Immun., Advances in Cancer and Brain, Behavior, and Immunity: A Decade of Progress 30, S58–S67. 10.1016/j.bbi.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal Relationship Between Stressful Life Events and the Onset of Major Depression. Am. J. Psychiatry 156, 837–841. 10.1176/ajp.156.6.837 [DOI] [PubMed] [Google Scholar]
- Koller M, 2016. robustlmm: An R Package for Robust Estimation of Linear Mixed-Effects Models. J. Stat. Softw 75, 1–24. 10.18637/jss.v075.i06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Boyle CC, Irwin MR, Ganz PA, Crespi CM, Asher A, Petersen L, Bower JE, 2017a. Childhood maltreatment, psychological resources, and depressive symptoms in women with breast cancer. Child Abuse Negl 72, 360–369. 10.1016/j.chiabu.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Irwin MR, Ganz PA, Crespi CM, Petersen L, Asher A, Bower JE, 2017b. Cortisol Awakening Response as a Prospective Risk Factor for Depressive Symptoms in Women After Treatment for Breast Cancer: Psychosom. Med 79, 763–769. 10.1097/PSY.0000000000000499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Haydon MD, Dooley L, Boyle CC, Bower JE, 2020. Early life stress sensitizes individuals to the psychological correlates of mild fluctuations in inflammation. Dev. Psychobiol 62, 400–408. 10.1002/dev.21908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB, 1997. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 12, 277–287. 10.1037//0882-7974.12.2.277 [DOI] [PubMed] [Google Scholar]
- Linden W, Vodermaier A, MacKenzie R, Greig D, 2012. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord 141, 343–351. 10.1016/j.jad.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Lorah J, 2018. Effect size measures for multilevel models: definition, interpretation, and TIMSS example. Large-Scale Assess. Educ 6, 8. 10.1186/s40536-018-0061-2 [DOI] [Google Scholar]
- Lüdecke D, 2018. sjPlot: Data visualization for statistics in social science
- Mazure CM, 1998. Life Stressors as Risk Factors in Depression. Clin. Psychol. Sci. Pract 5, 291–313. 10.1111/j.1468-2850.1998.tb00151.x [DOI] [Google Scholar]
- Melchior M, Caspi A, Milne BJ, Danese A, Poulton R, Moffitt TE, 2007. Work stress precipitates depression and anxiety in young, working women and men. Psychol. Med 37, 1119–1129. 10.1017/S0033291707000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonté B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad Z, Tang CY, Merad M, Russo SJ, 2017. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci 20, 1752–1760. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 65, 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N, 2011. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 12, 160–174. 10.1016/S1470-2045(11)70002-X [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH, 2001. Paroxetine for the Prevention of Depression Induced by High-Dose Interferon Alfa. N. Engl. J. Med 344, 961–966. 10.1056/NEJM200103293441303 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Ahrens C, 2002. Age differences and similarities in the correlates of depressive symptoms. Psychol. Aging 17, 116–124. 10.1037/0882-7974.17.1.116 [DOI] [PubMed] [Google Scholar]
- Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD, 2020. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain. Behav. Immun 87, 901–909. 10.1016/j.bbi.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, Schneider HG, Leonard BE, Berk M, 2010. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry J. Ment. Sci 197, 372–377. 10.1192/bjp.bp.109.076430 [DOI] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR, 2010. Depression and cancer mortality: a meta-analysis. Psychol. Med 40, 1797–1810. 10.1017/S0033291709992285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Radloff LS, 1977. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raison CL, 2020. Microglial Activation and Response to Anti-inflammatory Treatment in Major Depressive Disorder: Another Piece in the Inflammation–Mood Disorders Puzzle. Biol. Psychiatry 88, 594–596. 10.1016/j.biopsych.2020.08.003 [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27, 24–31. 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH, 2011. Is Depression an Inflammatory Disorder? Curr. Psychiatry Rep 13, 467–475. 10.1007/s11920-011-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry 70, 31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM, 2003. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann. Epidemiol 13, 674–682. 10.1016/S1047-2797(03)00053-X [DOI] [PubMed] [Google Scholar]
- Sántha P, Veszelka S, Hoyk Z, Mészáros M, Walter FR, Tóth AE, Kiss L, Kincses A, Oláh Z, Seprényi G, Rákhely G, Dér A, Pákáski M, Kálmán J, Kittel Á, Deli MA, 2016. Restraint Stress-Induced Morphological Changes at the Blood-Brain Barrier in Adult Rats. Front. Mol. Neurosci 8. 10.3389/fnmol.2015.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE, 2004. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull 130, 601–630. 10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin M, 2014. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull 140, 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y, 2007. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain. Behav. Immun 21, 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Sun L, Yan J, Wang L, 2019. Postoperative depression in female patients with breast cancer surgery: an analysis of risk factors and assessment of. Int. J. Clin. Exp. Med 12, 9. [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord 150, 736–744. 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Vilagut G, Forero CG, Barbaglia G, Alonso J, 2016. Screening for Depression in the General Population with the Center for Epidemiologic Studies Depression (CES-D): A Systematic Review with Meta-Analysis. PLOS ONE 11, e0155431. 10.1371/journal.pone.0155431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, Duman RS, 2016. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci 17, 497–511. 10.1038/nrn.2016.69 [DOI] [PubMed] [Google Scholar]
- Zalli A, Jovanova O, Hoogendijk WJG, Tiemeier H, Carvalho LA, 2016. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology (Berl.) 233, 1669–1678. 10.1007/s00213-015-3919-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


