Abstract
The glycosylation of the fragment crystallizable (Fc) region of immunoglobulins (Ig) is critical for the modulation of antibody effects on inflammation. Moreover, antibody glycosylation may induce pathologic modifications and ultimately contribute to the development of autoimmune diseases. Thanks to progress in the analysis of glycosylation, more data are available on IgG and its subclass structures in the context of autoimmune diseases. In this review, we focused on the impact of Ig glycosylation in autoimmunity, describing how it modulates the immune response and how glycome profiles can be used as biomarkers of disease activity. The analysis of antibody glycosylation demonstrated specific features in human autoimmune and chronic inflammatory conditions, including rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease and autoimmune liver diseases, among others. Within the same disease, different patterns are associated with disease severity and treatment options. Future research may increase the information available on the distinct glycome profiles and expand their potential role as biomarkers and as targets for treatment, ultimately favoring an individualized approach.
Keywords: Glycosylation, Antibody, Autoimmune Diseases, Inflammation, Fc fragment
1. Introduction
The appearance of autoantibodies and the loss of humoral tolerance are a hallmark of autoimmune diseases [1, 2]. Autoantibodies may contribute to autoimmunity through the activation of type I and type II FcRs, as well as through the activation of the complement system [2–5]. However, the presence of autoantibodies has been demonstrated before the onset of symptoms, and their titer does not correlate with disease activity with few exceptions [3, 6]. Therefore, other factors are involved in the final immune response aside from autoantibody specificity. [7, 8]. Antibodies mediate a wide range of immune responses including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP). The effects and strength of these responses are dependent on the binding affinity and specificity of the antibody fragment crystallizable (Fc) domain for different receptors, such as Fc receptors (FcRs) and complement protein C1q. The interaction between antibodies and the receptors plays an important role in autoimmune diseases.
Glycosylation is a post-translational modification that enhances the conformational diversity of antibodies, affecting their interactions with receptors. During the glycosylation process, glycans are added to the antibody protein through two types of molecular linkages: on asparagine residues (N-glycans) or on serine/threonine residues (O-glycans) [9]. Human IgG can be divided in four subclasses and 36 possible glycoforms, which allow up to 144 functional states, resulting in more complex and precise regulation of antibody effects [10]. Glycosylation of the Fc region is critical for modulating the inflammatory functions of IgG [11–14]. Altered antibody glycosylation has been demonstrated in patients with rheumatoid arthritis (RA) before the onset of arthritis, in parallel with increasing systemic inflammation [6]. Antibody glycosylation may be considered as a “switch” that converts antibodies from protective to autoreactive, ultimately resulting in autoimmune diseases. The ability to manipulate antibody activity makes glycosylation a promising target for the treatment of autoimmune diseases. A commonly used treatment for various autoimmune/inflammatory diseases is the administration of high-dose intravenous immunoglobulins (IVIg), in which specific glycosylation patterns promote the anti-inflammatory activity [15]. In a similar fashion, the administration of engineered enzymes able to attach either galactose or sialic acid to existing antibodies is effective in attenuating inflammation in an arthritogenic K/BxN mice model, whereas the removal of IVIg terminal sialic acid residues abrogated their anti-inflammatory activity [16, 17]. In this review, we aim to provide a comprehensive overview and discuss (i) the impact of N-glycosylation on antibody (mainly IgG) stability and activity and (ii) the association between glycosylation changes and the severity of autoimmune diseases.
2. Impact of antibody N-glycosylation.
Antibody N-glycosylation has an impact on numerous pathways, including antibody half-life, antibody-dependent cell cytotoxicity (ADCC), complement (C3a or C5a)-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and other receptor-mediated immunoregulation (Figure 1).
Figure 1.
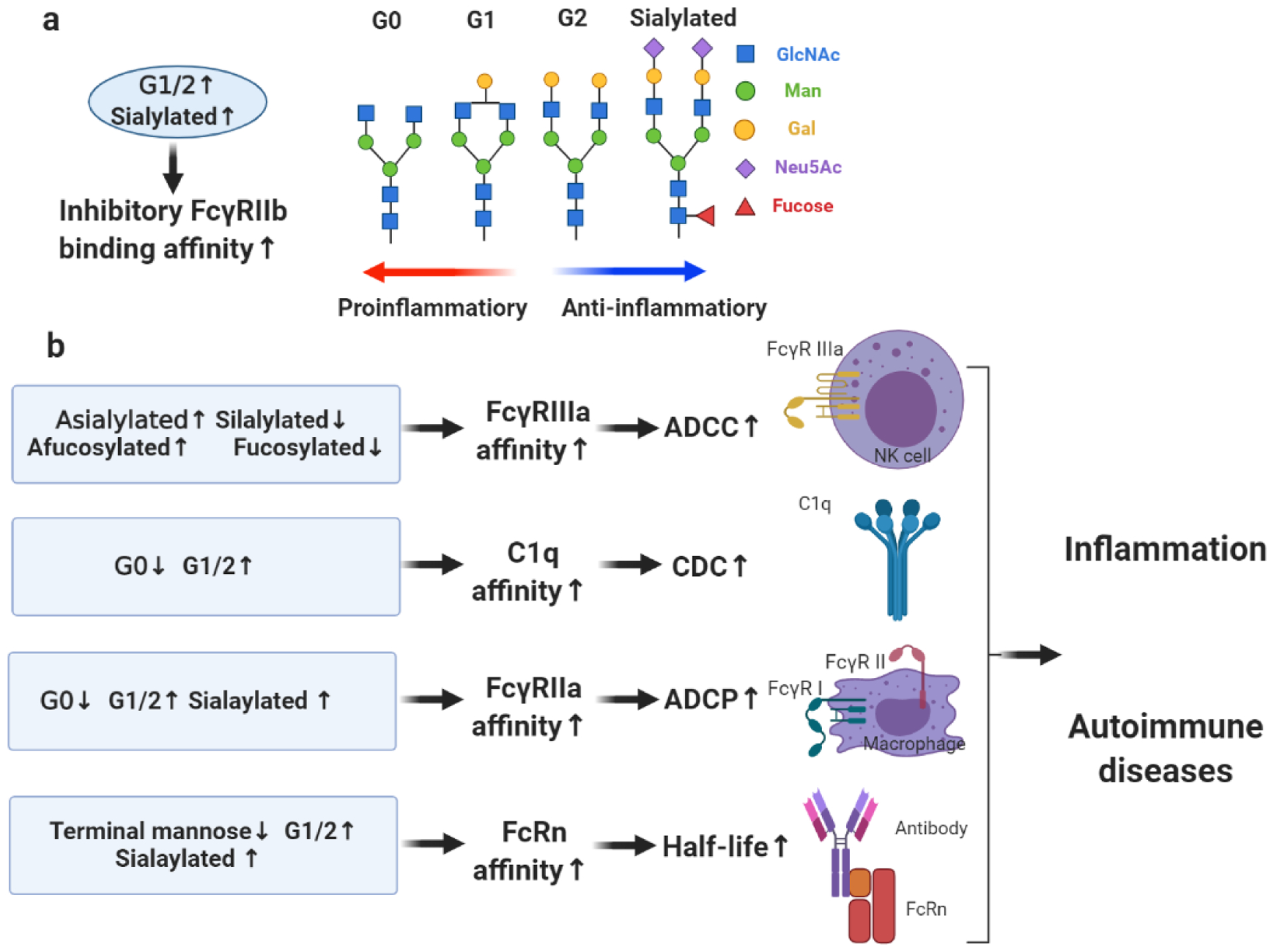
Immunoregulation pathways of antibody glycosylation in autoimmune diseases. a) increased galactosylation or sialylation of antibodies promotes binding affinity to inhibitory receptor FcγRIIb. Galactosylated, sialylated or fucosylated glycans promote the anti-inflammatory functions of antibody. b) Increased sialylation or fucosylation of antibody promote binding affinity to activating receptor FcγRIIIa, which mediates ADCC; Increased galactosylation of antibodies promotes binding affinity to C1q, which mediates CDC; Increased galactosylation or sialylation of antibody promotes binding affinity to FcγRIIa, which mediates ADCP; Decreased terminal mannose, increased galactosylation or sialylation of antibody promotes binding affinity to FcRn, which determines antibody half-life. Antibody effector functions play a role in initiation, development, and pathology of autoimmune diseases. G0, agalactosylated N-linked glycans; G1/2, singly galactosylated or digalactosylated N-linked glycans; G1, singly galactosylated N-linked glycans; G2, digalactosylated N-linked glycans.
2.1. Antibody half-life
Antibody half-life for IgA and IgM is typically less than one week and sometimes less than 1 day [18] while IgG has a half-life of up to 3 weeks due to being recycled by FcRn receptors [19, 20]. This is due to the glycans attached to antibodies, which affect their conformational stability and their binding to their receptors [21]. Addition of N-glycans to the antigen-binding fragment (Fab) through rational engineering enhances the conformational stability of monoclonal antibodies (mAbs), thus increasing their half-life [22].
One of the known glycosylation patterns resulting in antibody half-life modification is N-glycosylation with terminal mannose (G0). In humans, therapeutic IgG mAbs containing high-mannose glycans in the Fc region are cleared more rapidly than antibodies glycosylated with other glycans [23]. In a mouse model, mAbs with complex glycosylation had a longer half-life compared with those with high-mannose glycosylation in vivo [24]. Furthermore, mAbs with G0 oligosaccharides have a slightly faster clearance rate than mAbs with other structures in the variable domain [25]. These studies suggest that antibodies with terminal mannose exhibit faster clearance and shorter half-life. However, one former study on BALB/c mice described a significantly longer half-life of G0 IgG than the fully glycosylated variants [26]. Therefore, the impact of mannose on half-life of antibody needs to be further clarified.
In addition to mannose, terminal sialic acid residues have an effect on antibody half-life. A recent study based on temperature-controlled nanoelectrospray showed that the α2,6-sialylated mAb: FcγRIIIa complex exhibited higher thermal stability when measured in the temperature gradient from 20 to 50°C [27]. Furthermore, sialic acid engineering of mAbs demonstrated an 11%~35% increase in half-life of mAbs when compared with mAbs without sialic acid [28]. Previous studies reported an increased serum persistence and longer half-life of IgG variants combining high Fc sialylation and FcRn+ Fc mutations in mice models [29]. These findings suggest that antibodies glycosylated with sialic acid have a longer half-life than non-sialylated antibodies.
2.2. Antibody-dependent cellular cytotoxicity (ADCC)
Glycosylation of antigen-specific antibodies may promote the death of normal cells via the modulation of ADCC. Ig glycosylation influences ADCC by affecting the binding to the FcγRIIIa receptors, which mediate proinflammatory effects [30]. A recent study demonstrated that the glycosylation removal abolished the ability of anti-RhD antibodies to induce human natural killer (NK) cell degranulation [31]. A specific glycosylation pattern affecting the binding between antibodies and FcγRIIIa receptors is core fucosylation. Human IgG1 Fc with core α1-6 fucosylation exhibits a lower affinity to FcγRIIIa than IgG1 Fc without core α1-6 fucosylation [32, 33] . In contrast core fucosylation of IgG1 increases the binding affinity to FcγRIIIa up to 17-fold [34, 35]. The improved binding of afucosylated antibodies to FcγRIIIa likely mediates an enhancement of ADCC [36]. Several studies have demonstrated that the lack of fucosylation on IgG1 plays a critical role in enhancing ADCC [33, 37–39]. Glycoengineered afucosylated anti-CD20 IgG induced significantly greater ADCC, NK cell activation, FcγRIIIa binding, and in vivo IgG-mediated cellular depletion, compared with core-fucosylated IgG [40, 41]. ADCC activity of human anti-CD20 IgG4 isotype antibody had a positively linear correlation with IgG4 afucosylation, both in vivo and in vitro [42]. The impact of antibody fucosylation on ADCC is evident in pharmacology studies and in the pathophysiology of some autoimmune diseases. For example, the removal of fucose from pertuzumab increased its ADCC activity [28]. In the hemolytic disease of the fetus and newborn (HDFN), the level of anti-RhD fucosylation negatively correlated with the binding of FcγRIIIa and FcγRIIIa-mediated ADCC [43]. Moreover, ADCC is strongly mediated by human low fucosylated anti-RhD mAbs [44].
Sialylation is an additional modification of antibodies associated with modulation of ADCC. Similar to fucosylation, attachment of terminal sialic acid residues to antibodies reduces ADCC activity, due to lower binding affinity to FcγRIIIa [36, 45]. In the presence of core fucosylation, sialylation of antibodies led to decreased ADCC [40]. Moreover, the removal of sialic acid residues increased ADCC activity of pertuzumab, similarly to defucosylation [28]. Removing sialic acids from IgG increased ADCC activity in an in vitro model of thyroiditis [46]. In addition, engineered aglycosylated IgG Fc variants increased their binding ability to FcγRIIIa and enhanced ADCC [47].
Different studies showed contradictory results on the effect of galactosylation of antibodies on ADCC. One suggested that hyper-galactosylation of IgG1 increased the binding to FcγRIIIa, resulting in enhanced NK cell-mediated ADCC [34]. However, another study reported no significant variation in the affinity of human IgG isotypes for FcγRIIIa and no increased ADCC [48]. On the other hand, galactosylation of a fucosylated Fc region resulted in increased FcγRIIIa affinity when compared with non-galactosylated variants [27, 32, 49]. This could indicate that galactosylation may alter the impact of other glycoforms on FcγRIIIa affinity and ADCC. Furthermore, reduced or enhanced ADCC was demonstrated depending on addition of a galactose to the a-3 or a-6 mannose arm, which provides a potential explanation for the contradictory impact of galactose[50]. Finally, a 10-fold increase in ADCC resulted from high bisecting N-acetyl glucosamine (GlcNAc) of mAbs [51]. Further research is certainly needed on the mechanistic effect of galactosylation.
2.3. Complement-dependent cytotoxicity (CDC)
Antibody glycosylation influences CDC activities by affecting the binding of antibody to the complement component 1q (C1q) complex. Agalactosylated IgG have decreased binding affinity to C1q, while IgG1 with terminal galactosylation bind with high affinity to C1q, which promotes CDC [30, 34, 52]. Degalactosylation of remodeled therapeutic mAbs reduces CDC [51]. There is conflicting evidence regarding the effect of fucose on CDC. One study suggested that fucosylation increases the CDC activity of antibodies [53], but in another study, afucosylated rituximab induced lower CDC than normal rituximab [41].
Sialic acid is another residue with unclear effects on CDC; a recent study in thyroiditis demonstrated that the removal of sialic acids from IgG N-glycans decreased CDC activity [46]. In addition, afucosylated rituximab reduced CDC, compared to normal rituximab [41]. These findings are consistent with the observation that IgG1 with terminal sialylation have an increased binding affinity to C1q [36]. However, the removal of sialic acid has been shown to increase CDC activity of pertuzumab [28]. Moreover, Fc sialylation of human monoclonal IgG1 impaired its efficacy to induce CDC [54]. This could suggest that other factors are potentially involved in the interaction between sialylated Ig and the C1q complex.
2.4. Antibody-dependent cellular phagocytosis (ADCP)
A study based on therapeutic mAbs demonstrated that the galactosylation of antibodies had a positively linear correlation with FcγRIIa binding, which mediates the ADCP activity [55]. Another study demonstrated that galactosylated mAbs were associated with increased ADCP, whereas agalactosylated antibodies had a reduced ADCP. Furthermore, sialylated antibodies were significantly associated with enhanced ADCP activity, regardless of galactosylation content [50].
2.5. Other receptor-mediated antibody effector functions
The Fc domain mediates effector activities by selectively binding to FcRs; the binding depends on the Fc conformational state determined by the Fc-associated N-linked glycans [4]. Differences in antibody activity are determined by variation in IgG’s affinity for activating and inhibitory IgG FcRs [56]. Therefore, Fc-associated N-linked glycans play a major role in antibody effector functions.
2.5.1. Type I FcRs-mediated antibody effector functions.
Type I FcRs include the activating receptors FcγRI, FcγRIIa, FcγRIIc, FcγRIIIa, and FcγRIIIb and the inhibitory receptor FcγRIIb. Among these receptors, FcγRIIIa and FcγRIIb play an important role in the antibody effector function and FcγRIIIa mediates ADCC, as discussed in previous sections. In the relationship between antibody glycosylation and binding to FcγRIIb, recent data indicate that the anti-inflammatory activities of IgG are dependent on sialylation of the IgG Fc fragment [57]. Sialylated IgG Fc reduces inflammation by stimulating the up-regulation of the inhibitory receptor FcγRIIB and the production and secretion of interleukin (IL)-33 [58]. In mouse models of autoimmune diseases, sialylation played a key role in IVIG-mediated anti-inflammatory activity, which depends on the inhibitory FcγRIIB [59, 60]. In other studies, however, sialylated IgG inhibited maturation of dendritic cells and proliferation of B cells in an FcγRIIb independent manner [61, 62]. It has also been observed that sialylated IVIG is able to suppress the activity of autoantibodies, independently of the human FcγR genotype [63]. Therefore, the complete mechanism through which sialylation enhances Ig anti-inflammatory properties remains unclear.
Increased galactosylation of IgG1 is another pattern known to mediate anti-inflammatory activities, by promoting the association between FcγRIIB and Dectin-1 [64]. In murine models of autoimmune hemolytic anemia galactosylation of IgG1 is critical for modulating FcγRIIB-mediated inhibition of disease activity [65]. According to a binding analysis, human IgG1 Fc with core α1-6 fucosylation has a lower binding affinity to FcγRIIb than IgG1 Fc without core α1-6 fucosylation [32]. In addition, we should note that the glycoprofile of FcγRs also has an impact on their interaction with the Fc domain of IgG1 [66].
2.5.2. Type II FcRs-mediated antibody effector function.
Type II FcRs are represented by the family of C-type lectin receptors (CLRs) and we will here discuss the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and CD23. SIGN-R1, the murine ortholog of DC-SIGN, is a lectin receptor expressed on macrophages in the splenic marginal zone and is strongly involved in the interaction with glycosylated antibodies. The anti-inflammatory activity of sialylated IVIG is dependent on innate Th2 response via binding to SIGN-R1 or its human orthologue, DC-SIGN [15, 67]. This finding was demonstrated in multiple autoimmune disease models, where blockade of SIGN-R1 using either SIGN-R1 blocking antibodies or SIGN-R1 deficient mice resulted in the abrogation of the therapeutic immunomodulatory activity of IVIG [17, 59]. Moreover, inducing DC-SIGN expression in SIGN-R1-deficient mice restored IVIG anti-inflammatory activities in a mice model of arthritis [68]. On the contrary, the binding of IgG Fc to DC-SIGN does not play a role in IVIG effect [69] but further data is needed to confirm these results.
CD23 is a CLR involved in glycosylated antibody binding and, based on solid-phase binding competition assays, glycoprotein blotting experiments, and glycan array analysis, the lectin-like domains of cow and mouse CD23 are able to bind mannose, GlcNAc, glucose, and fucose as well as glycoproteins bearing these sugars in nonreducing terminal positions [70]. Additional data have shown that non-N-glycosylated CD23 has higher affinity to IgE than with glycosylated CD23 [71].
2.5.3. Other binding receptors-mediated antibody effector function
Glycosylation also mediates the binding of antibodies to receptors other than type I and type II FcRs, such as dendritic cell immunoreceptor (DCIR), Dectin-1, CD22, Siglec-H and mannose-binding lectin (MBL). The inhibition of IVIg binding to DCIR blocks the internalization of sialylated IVIG into dendritic cells (DCs), as well as the induction of tolerogenic DCs and Treg cells, identifying DCIR as a specific receptor for sialic acid-enriched-IVIG on DCs, mediating a variety of downstream responses [72]. Dectin-1 was also recently identified by a combination of biophysical experiments as a receptor for the core fucose on IgG antibodies [73]. Furthermore, sialic acid-positive but not sialic acid-negative IVIG promotes B-cell apoptosis through its interaction with CD22, indicating the ability of CD22 to discriminate the presence of sialic acid residues [74]. Similar observations were made for sialic acid-binding immunoglobulin-like lectin H (Siglec-H) [75]. The role of MBL in interacting with glycosylated antibodies, however, is unclear. Pro-inflammatory activity increased after binding of agalactosylated IgG to MBL [76]. Nevertheless, a later study showed that the effects of IgG-G0 are unimpaired in MBL-deficient mice, suggesting no significant role of MBL in immune response modulation [52]. This finding is consistent with several other studies where no significant correlation was found between MBL levels and IgG galactosylation and between MBL polymorphisms and RA disease activity [77, 78].
3. Methods for protein glycosylation analysis
The analysis of cell surface glycans may contribute to understand the function of protein glycosylation, glycan-related biological processes, and eventually disease diagnosis and treatment. Conventional methods for glycomic analysis are by lectin microarrays, high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), mass spectrometry (MS), and their combinations. New methods based on electrochemistry and barcoding technology have also been successfully used for multiple analysis of glycans on intact cell surface. In this section, these methods will be compared based on their procedures and applications (Table 1).
Table 1.
Available methods for the analysis of glycosylation.
| Method | Advantage | Shortcoming | |
|---|---|---|---|
| Soluble protein | Glycan specific lectin [79, 80] | Rapid, efficient, quantitative, low-infrastructure-requiring. | Only apply for few samples, no glycan structure information; no site-specific analysis. |
| Lectin microarray [81–85] | Rapid, efficient, and low-infrastructure-requiring; simple procedure, apply to large sample. | No quantitative, no glycan structures information, no site-specific analysis. | |
| Mass spectrometry (MS)-related methods [86–88] | Quantitative, provide structural information apply to complex glycan features of large sample; site-specific analysis. | Long procedure, complex, professional requirements, high-infrastructure-requiring. | |
| High-performance liquid chromatography (HPLC)-related methods [89, 90] | Separate different glycans components, quantitative, combine with MS to get structural information. | Long procedures complex, professional requirements, high-infrastructure-requiring. | |
| In situ cell surface protein | Barcoding technology and electrochemistry for glycan analysis of in situ cell surface proteins [91–94] | Allow analysis of glycans on cell surface, quantitative, useful for complex glycan features of large samples. | Long procedure, complex, high-infrastructure-requiring, no glycan structure information, no site-specific analysis. |
3.1. Lectin and lectin microarrays for glycosylation analysis
Lectin has traditionally been used to analyze the glycome for its specificities in binding to monosaccharides [79]. Glycan-specific lectins, including fucose-binding lectin, galactose-binding lectin, sialic-binding lectin, and mannose-binding lectin, have been developed and utilized in the analysis of these glycans. Once lectin-labeled glycoproteins are formed, traditional techniques can be used to detect lectin and characterize glycome composition. Techniques based on lectin including flow cytometry, histochemical staining, western blot analysis, and ELISA provide rapid, efficient, quantitative, and low-infrastructure-requiring methods to detect glycans on glycoproteins [80]. However, these methods do not apply to complex glycans of large samples and do not offer detailed information on glycan structure. Thus lectin microarray for glycomic analysis was developed [81–84], which detects the target glycoproteins through direct labeling with fluorescent reagents or by overlaying a fluorescent-labeled lectin antibody. Lectin microarray is not quantitative, and it also cannot offer information on glycan structures. Unlike mass spectrometry (MS), application of lectin microarray is more efficient for comparative purposes of complex glycan features of large samples, since it does not require the release of glycans or additional derivatizations [85].
3.2. Mass spectrometry-related methods for glycosylation analysis
Mass spectrometry (MS) has several advantages that make it ideal for glycomic and glycoproteomic analysis. First, the technique is quantitative. Second, it can provide structural information of glycans on small amounts of material. Third, different types of mass analysis can be combined to obtain more precise structural information on the glycan of interest. For example, quadrupole time-of-flight mass spectrometry (QTOF-MS) can recognize peptides and glycopeptides of different Ig subclasses and quantitate glycans and site-specific glycosylation of antibodies [86]. Nevertheless, compared with lectin, MS involves more complex procedures and 5 steps are required before acquiring quantitative and structural information. First, N-or O-glycans need to be released from glycoproteins or glycopeptides for mass analysis. Second, derivatization of glycans is necessary in order to improve stability, separation, ionization efficiency, and quantitation accuracy for glycomic analysis. After derivatization, complex glycan structures formed by monosaccharides need to be separated to obtain their accurate mass. This can be achieved by several separation methods, including hydrophilic interaction chromatography (HILIC), liquid chromatography, capillary electrophoresis, and ion mobility. In addition, imaging mass spectrometry (IMS) is required for detailed structural identification. Finally, bioinformatics tools are utilized to perform glycomics and glycoproteomics analysis. In summary, MS provides more detailed structural information than lectin analysis, but requires more complex procedures and equipment to obtain the result [87, 88].
3.3. High-performance liquid chromatography (HPLC)
High-performance liquid chromatography (HPLC) is a technique that isolates the different components of a sample in a pressurized liquid solvent depending on the slightly different interactions between each component and the adsorbent material in column. Glycosylation analysis by HPLC generally involves these following steps: enrichment, releasing glycans, derivatization of glycans, and separation and quantification using HPLC paired with a fluorescence or combined with other methods [89]. The techniques used for releasing glycans are similar to those used in MS. During the derivatization step, a fluorescent tag such as 2-aminobenzamide is often used to detect the glycans. Other fluorescent reagents include aniline, 2-aminoacridone, and procainamide. Lectin and other glycan affinity chromatography are commonly used as the adsorbent material in the separation step. After separation, the individual components can be used for further investigation of their structural information and quantification by combining with other methods such as MS. Glycan differences between different groups of samples can be obtained by the detection of fluorescence or other methods [90]. In summary, HPLC is a useful method to separate different glycans components and can be used in combination with MS to acquire detailed structural information on the glycans. However, similar to MS, HPLC requires complex procedures and equipment.
3.4. Barcoding technology and electrochemistry for glycan analysis of intact cells
New methods based on barcoding technology and electrochemistry have recently been successfully developed for the analysis of glycans on intact cell surfaces [91, 92]. These methods are similar to lectin microarrays for glycomic analysis. First, the glycans on cell surfaces are labeled with lectin probes conjugated with fluorescein, biotin, and horseradish peroxidase (HRP). Other techniques such as chemical covalent recognition and metabolic labeling are also used for glycan labeling and recognition [93]. Next, electrochemical analysis is used to detect the electrochemical signal of lectin probes labeled with HRP or quantum dots for its high sensitivity and easy and fast operation in live cells [94]. In addition, DNA-encoded lectins are also a great option to label the glycans on intact cells. DNA is an ideal barcode because of the great variety of sequences that can be recognized by advanced DNA detection techniques. Quantification of the labeled glycans can then be achieved by capturing the cleaved DNA barcodes using DNA detection methods such as DNA microarray [91]. These new methods allow the analysis of glycans on intact cells. Nevertheless, as methods of indirect detection, they cannot be used to determine complete glycan structures as MS can.
4. Immunoglobulin Glycosylation in Autoimmune Diseases
4.1. Rheumatoid Arthritis
In the case of RA [95, 96], specific glycosylation patterns generally found in patients include lower galactosylation, lower sialylation, and higher fucosylation levels of IgG compared with healthy controls [97–99]. Abnormal N-glycosylation of serum IgG in RA patients was reported for the first time in 1985 [100]. Patients with RA exhibit decreased IgG galactosylation (G1 or G2) or increased IgG agalactosylation (G0) [101–105]. A recent prospective study suggested that people with low IgG galactosylation have higher risk for RA development than people with high IgG galactosylation [106]. Hypogalactosylated and hyposialylated IgG glycans have also been reported in patients with juvenile idiopathic arthritis [107, 108]. Agalactosylation seems especially relevant in autoantibody activity of IgG rheumatoid factors in RA patients [105]. Rheumatoid factor is an autoantibody targeting the Fc region of autologous IgG and is a biomarker of RA. Rheumatoid factors exhibiting high affinity for agalactosyl IgG have been identified in RA patients [109]. The levels of IgG sialylation and galactosylation are lower in rheumatoid factor-positive patients than in seronegative RA patients, while the IgG sialylation level of seronegative patients are similar to that of healthy controls [105].
In addition to low galactosylation, also serum IgG sialylation levels are lower in RA compared to healthy controls [103]. Reduced sialylation of IgG was also found in a mouse models of arthritis [110]. Furthermore, an increase in IgG fucosylation was found in RA patients compared to healthy controls [103]. Taken together, the evidence demonstrates a special glycosylation pattern of IgG in patients with RA and could represent a potential biomarkers for the diagnosis of subsets of rheumatoid arthritis [111] (Table 2).
Table 2.
Aberrant antibody glycosylation observed in autoimmune diseases.
| Glycosylation | RA | SLE | CD | UC | AAV | PBC | PSC |
|---|---|---|---|---|---|---|---|
| Agalactosylation | IgG↑ [104, 105, 107, 108] | IgG↑ [119, 120] | IgG↑ [124, 126] | IgG↑ [124, 126] | IgG↑ [133, 134] | IgG1 and IgG2 ↑ [86] | IgG1 ↑ [86] |
| Galactosylation | IgG↓ [97–99, 102, 103, 105, 106] | IgG ↓ [121] | IgG↓ [124, 125, 127] | IgG↓ [124, 125, 127] | IgG↓ [134] | IgG1↓ [86] | IgG↓ [86, 142] |
| Asialylation | IgG↑ [108] | IgA1/2↑ [86] | |||||
| Sialylation | IgG↓ [97, 99, 105, 110] | IgG ↓ [121] | IgG↓ [125, 127] | IgG↓ [125] | IgG↓ [134, 135] | IgG1↓ [142] | |
| Afucosylation | IgM J chain↑ [86] | IgG2↑ [86] | |||||
| Fucosylation | IgG↑ [97, 103] | IgG ↑[122] ↓[121] | IgG ↑ [125] | IgG↓ [125] | IgM J chain↓ [86] | IgG4↑ [142] | |
| Bisection | IgG↑ [121] | IgG1 and IgG2/3 ↑; IgG4↓ [125] | IgG↓ [125] | IgG1↓ [135] | IgA1/2↑ [86] | IgG4↑ [142] | |
| Diantennary | IgA↑ [86] | IgG2/3 ↑[142] |
The anti-citrullinated protein antibodies (ACPA) are more specific than the rheumatoid factors for RA [2]. The Fc glycosylation patterns of ACPA-IgG1 and total IgG1 were studied in patients with arthralgia who later developed arthritis who had no difference in Fc galactosylation, sialylation, and bisecting at baseline; a decrease in ACPA-IgG1 Fc galactosylation and sialylation was revealed in RA patients at the onset of arthritis. In addition, ACPA-IgG1 also displayed higher levels of core fucosylation compared with total IgG1, either before and at the onset of arthritis [6, 112]. The decrease in galactosylation occurred around 3 months prior to the onset of RA and was paralleled by an increase in systemic inflammation [6]. A recent study also showed that significantly less sialylation and galactosylation of IgG and ACPA were present in asymptomatic ACPA positive subjects who subsequently developed RA within 12 months, compared with individuals who remained healthy throughout the 12 months period [113]. These findings support the hypothesis that changes in ACPA glycosylation parallel the onset of RA. The synovial fluid (SF) of patients with RA has also been investigated. Aberrant Fc-linked glycan patterns of ACPA were predominant in SF compared with serum [112]. Higher agalactosylation and lower sialylation of ACPA IgG1, but not total IgG1, were detected in SF of RA patients [112]. Moreover, higher agalactosylation of ACPA IgG1 was found in rheumatoid factor positive compared with seronegative RA [112] (Table 3).
Table 3.
Aberrant auto-antibody glycosylation observed in autoimmune diseases
| Glycosylation | RA | AAV | FNAIT | |
|---|---|---|---|---|
| Glycosylation of autoantibody Fc region | Agalactosylation | ACPA IgG1↑ [112] | ANCA IgG1↑ [135] | |
| Galactosylation | ANCA IgG1↓ [135] | HPA-1a specific IgG1↑ [138] | ||
| Sialylation | ACPA IgG1↓ [112] | ANCA IgG1↓ [135] | HPA-1a specific IgG1↑ [138] | |
| Fucosylation | ACPA IgG1↑ [6, 112] | HPA-1a specific IgG1↓ [35, 138, 139] | ||
| Bisection | ANCA IgG1↓ [135] |
As described above, the development of RA is paralleled by changes in Ig glycosylation. Therefore, certain Ig glycosylation patterns may serve as a marker of disease activity and treatment efficacy in RA patients. Low galactosylation of IgG may indicate high disease activity. The galactosylation levels of IgG negatively correlated with RA disease activity [101]. A study based on ELISA-plate analysis of serum IgG of RA patients demonstrated that IgG agalactosylation was positively associated with C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), indicating increased disease severity [114]. A positive correlation between IgG G0/G1 and markers of inflammation such as CRP and ESR was also found in JIA [107]. In RF positive RA patients, the decrease in IgG galactosylation and sialylation correlated with the increase in RF activity [105]. This trend was confirmed by a recent study where increased IgG G0/G1 was observed to parallel an increase of CRP in RA patients [115]. Furthermore, agalactosylated IgG were shown to enhance pathogenicity of collagen induced arthritis (CIA) in animal models [116]. These findings suggest a strong correlation between IgG galactosylation and RA disease activity, which makes IgG galactosylation levels a promising candidate for disease activity biomarker. The well documented association between IgG galactosylation and RA activity was also analysed in pregnant RA patients. Most pregnant women with RA have low disease activity during pregnancy. One study found that IgG1 and IgG2 galactosylation increased during pregnancy and decreased in the postpartum period, while GlcNAc was not influenced by pregnancy status [102]. Moreover, increased total and Fc galactosylation, but not Fab galactosylation, were associated with lower disease activity in RA patients during pregnancy [117].
In addition to galactosylation, IgG sialylation correlates with RA activity through a lower IgG sialylation compared with patients with inactive RA, ACPA positive inactive RA, and healthy controls [113]. Furthermore, the genetic block of sialylation in activated B cells promotes joint inflammation in CIA model [110]. Therefore, low IgG sialylation may also serve as an indicator of high RA disease activity. Monitoring disease activity and the efficacy of treatment is crucial in clinical practice and, as glycosylation patterns of Ig may serve as an indicator of disease activity, changes in Ig glycosylation may reflect the effectiveness of a specific drug or serve to monitor treatment. The use of methotrexate (MTX) in RA is associated with increased IgG galactosylation and sialylation levels, but no changes in IgG fucosylation level [103]. A recent prospective study also demonstrated that the low abundance of galactosylated glycans in untreated patients with early RA can be restored by MTX treatment [118]. Moreover, IgG galactosylation is increased in RA patients during a combined treatment with MTX and infliximab (a chimeric anti-TNF-alpha antibody) [114]. Therefore, an increase in IgG galactosylation can potentially be used to indicate treatment efficacy.
In summary, increased agalactosylation, decreased galactosylation, and decreased sialylation of IgG are signs associated with severe disease activity in RA patients. After treatment, decreased IgG agalactosylation and increased galactosylation and sialylation can be observed. Since changes in Ig glycosylation parallels the development of RA, the detection and analysis of Ig glycosylation patterns may be a useful tool for monitoring disease activity (Table 4).
Table 4.
Evidence of the correlation between antibody glycosylation and disease activity in autoimmune diseases.
| RA | SLE | CD | UC | AAV | FNAIT | PBC | PSC | |
|---|---|---|---|---|---|---|---|---|
| More severe clinical and biochemical (CRP, ESR, ALP, TB) manifestations | IgG agalactosylation↑ [114] ; IgG G0/G1↑ [107, 115]; | IgG galactosylation↓; IgG sialylation ↓; IgG bisection↑ [121] | IgG agalactosylation↑ [126] | Fucosylation of HPA-1a specific IgG1↓ [35]; Galactosylation of HPA-1a specific IgG1↑ [139]; | IgG1 agalactosylation↑; Branching IgG2 ↑;[86] | IgG1 agalactosylation↑; Branching IgG2 ↑;[86] | ||
| Severe pathological change | IgG agalactosylation↑ [116] | IgG2/3 agalatosylation↑; IgG2/3 digalactosylation↓; IgG2/3 sialylation ↓[125]; IgG1 agalactosylation↑; IgG1 G0F/G2F ↑[125, 128]; | IgG agalatosylation↑; IgG digalactosylation↓; IgG G0F/G2F ↑[125, 128]; | Galacosylation of IgG autoantibodies↑ [140]; Fucosylation of HPA-1a specific IgG1↓ [35] | Branching IgG1 and IgG2↑; IgG2 agalactosylation ↑;[86] | Branching IgG1 and IgG2↑; IgG2 agalactosylation ↑;[86] | ||
| Active disease status | IgG galactosylation↓ [105] ; IgG sialylation ↓[105, 113] | IgG fucosylation↑[122] | IgG agalactosylation↑; IgG G0F/G2F ↑[128, 131]; | IgG G0F/G2F ↑[128] | IgG1 Sialylation of PR3-ANCA and total IgG↓; IgGl agalactosylation↑; IgGl galactosylation↓ [134, 136]; IgG bisection↓ [135]; | IgG agalactosylation↑; IgG galactosylation ↓;[86] | ||
| Difficulty in treatment and management (need for surgery or relapse) | IgG2/3 agalatosylation↑; IgG2/3 digalactosylation↓; IgG2/3 sialylation ↓[125] | IgG agalatosylation↑; IgG digalactosylation↓; IgG sialylation ↓[125] | IgG1 galactosylation↓; IgG1 sialylation ↓[137]; |
B2, stricturing Crohn’s disease; B3, internal penetrating Crohn’s disease; CRP, C-reactive protein; ALB, serum albumin; BVAS, Birmingham vasculitis activity scores; G0/G1, agalactosylated N-linked glycans to singly galactosylated N-linked glycans ratio; G0F/G2F, fucosylated agalactosylated N-linked glycans to fucosylated digalactosylated N-linked glycans ratio.
4.2. Systemic Lupus Erythematosus (SLE)
As early as 1992, aberrant glycosylation of Ig, such as increased IgG agalactosylation, was reported in SLE [119, 120]. Recently, analyses based on ultra-performance liquid chromatography showed that decreased galactosylation, decreased sialylation, decreased core fucosylation, and increased bisecting N-acetylglucosamine were observed in serum IgG of SLE patients compared with healthy controls [121]. However, another study observed increased serum IgG fucosylation in SLE patients [122]. Low IgG galactosylation was also reported in lupus mice models [123]. Alpha-mannosidase II is an enzyme that modulates the trimming of mannose residues from hybrid N-linked oligosaccharides. A α-mannosidase II-deficient mice presented a systemic autoimmune disease similar to human SLE [40], suggesting a potential pathogenic role of mannose in SLE.
Decreased galactosylation, decreased sialylation, and increased bisecting glycan were positively associated with serum antinuclear antibody (ANA) and SLE organ involvement [121]. Another study demonstrated that increased serum IgG fucosylation was associated with high SLE disease activity and low serum C3 levels [122]. These associations make IgG glycosylation patterns a promising biomarker for SLE disease activity.
4.3. Inflammatory Bowel Disease (IBD)
Similar to autoimmune diseases, patients with IBD including Crohn’s disease (CD) and ulcerative colitis (UC) exhibit increased IgG agalactosylation (G0) and decreased IgG monogalactosylation (G1) and digalactosylation (G2) [124–127]. In addition, an increase in the agalactosylated and fucosylated IgG to digalactosylation and fucosylated IgG ratio (G0F/G2F) was observed in CD and UC patients [128]. In a large retrospective analysis of plasma samples, increased IgG1 and IgG2/3 core fucosylation was identified in patients with CD, but decreased IgG2/3 core fucosylation was reported in patients with UC [125]. Fut8 is an enzyme that transfers fucose to N-linked glycopeptides. Fut8 knockout mice showed less severe colitis in chemical and T cell-transfer induced IBD mouse models [129], further suggesting a role of Ig fucosylation in IBD. Sialylation is another Ig glycosylation pattern with potential involvement in IBD. Decreased IgG sialylation was observed in both CD and UC patients [125, 127]. Sialic acid acetylesterase (SIAE), an enzyme that modifies sialic acid and might act as a mediator of autoimmune disease pathogenesis, has been reported to be defective in IBD patients and shown to be associated with their susceptibility to IBD [130]. A lower level of evidence is available on the bisecting of antibody, especial for IBD and one recent study showed that IgG4 bisection was low for both CD and UC patients compared with healthy controls. On the other hand, IgG1 and IgG2/3 bisection was increased in patients with CD and decreased in patients with UC compared to healthy controls [125].
Antibody glycosylation was significantly associated with IBD disease activity. For CD patients, increased IgG2/3 agalactosylation, decreased IgG2/3 digalactosylation, or decreased IgG2/3 sialylation have been associated with disease worsening, thus increasing their risk for surgery. In addition, increased IgG1 agalactosylation or increased agalactosylated and fucosylated IgG to digalactosylation and fucosylated IgG ratio (G0F/G2F) correlated with more extensive CD [125, 128]. This correlation was further demonstrated by significantly higher IgG agalactosylation and G0F/G2F observed in patients with active CD when compared with patients in remission [128, 131]. Moreover, increased agalactosylation was associated with increased CRP and decreased serum albumin in CD [126]. In UC, an increase in IgG agalactosylation(G0), a decrease in IgG digalactosylation (G2), or a decrease in IgG sialylation were positively associated with their need for surgery [125]. Meanwhile, increased IgG G0, decreased IgG G2, or increased G0F/G2F were associated with more extensive UC (pancolitis) [125, 128]. Furthermore, G0F/G2F was significantly higher in active UC patients than in patients in remission[128]. Alterations in antibody glycosylation change according to the treatment for IBD. An increase in IgG galactosylation and IgG1 and IgG4 sialylation was detected in CD and UC patients treated with azathioprine/6-mercaptopurine, when compared with those treated with anti-TNFα [125]. In CD patients, increased IgG galactosylation was observed in response to anti-TNFα therapy [132]. A decrease in total IgG agalactosylation was also observed in UC patients after treatment with mesalazine, compared with those treated with steroids [125]. Furthermore, CD patients treated with infliximab exhibited a significant decrease in IgG agalactosylation [131]. In summary, increased IgG agalactosylation, decreased IgG galactosylation, and decreased IgG sialylation are commonly observed in CD and UC patients. High level IgG agalactosylation or low-level IgG galactosylation are associated with more severe CD and UC. A decrease in IgG agalactosylation can be observed in response to most treatments. These findings suggest that changes in IgG glycosylation patterns, especially fluctuations in IgG galactosylation levels, may serve as a tool to monitor IBD disease activity and assess treatment efficacy.
4.4. ANCA-associated vasculitis (AAV)
The group of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitidies (AAV) includes microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Vasculitis-associated ANCA target proteinase-3 (PR3-ANCA) and myeloperoxidase (MPO-ANCA). Several studies focusing on antibody glycosylation in patients with AAV have been conducted, and some specific glycosylation patterns have been observed. The IgG agalactosylation levels were significantly higher in PR3-ANCA positive or MPO-ANCA positive AAV patients compared with healthy controls. On the contrary, the IgG galactosylation levels were significantly lower in AAV patients compared with healthy controls [133, 134]. Similarly to galactosylation, the sialylation levels of PR3- or MPO-ANCA IgG from AAV patients were lower than those from healthy controls [134]. One study focusing on GPA demonstrated a decrease in total IgG1 and IgG2 galactosylation, sialylation, and bisection in patients with GPA compared with healthy controls. Moreover, in GPA patients, the anti-PR3 specific IgG1 ANCA exhibited lower galactosylation, sialylation, and bisection than total IgG1, while the anti-PR3 specific IgG1 ANCA agalactosylation was higher than total IgG1 agalactosylation [135].
The antibody glycosylation patterns seem to change with disease activity variation in AAV. The sialylation levels of PR3-ANCA and total IgG were significantly lower in patients with active GPA than in those with weakly active or inactive GPA. A negative correlation was found between sialylation levels and GPA disease activity measured by Birmingham Vasculitis Activity Score (BVAS) [136]. Similarly, bisection of PR3-specific IgG1 was negatively associated with disease activity measured by BVAS [135]. IgG1 agalactosylation was significantly higher and IgG1 galactosylation was significantly lower in patients with active AAV compared with patients in remission and with healthy controls, while no significant difference was observed in the latter two groups [134]. However, one study showed that galactosylation and sialylation of anti-PR3 ANCA Fc positively correlated with the levels of inflammatory cytokines and that galactosylation of anti-PR3 ANCA was positively associated with the time to remission [135].
Relapse after treatment is commonly seen in AAV and specific changes in the ANCA glycosylation patterns have been demonstrated before AAV relapse, which may provide valuable information for predicting the course of the disease. Patients with PR3-ANCA with low total IgG1 galactosylation or sialylation seem more prone to relapse after an increase of ANCA levels. Moreover, a decrease in total IgG1 galactosylation, sialylation, and bisection, as well as an increase in IgG1 core fucosylation were observed during the period from ANCA increase to relapse, while these changes were not found in non-relapsing patients. On the other hand, both relapsing and non-relapsing patients exhibited a decrease in PR3-ANCA IgG1 galactosylation, sialylation, and core fucosylation. An increase in PR3-ANCA IgG1 bisection was only observed in non-relapsing patients [137]. The described differences in glycosylation patterns could be monitored in AAV patients to predict potential relapses.
4.5. Immune thrombocytopenia purpura (ITP) and fetal and neonatal alloimmune thrombocytopenia (FNAIT)
ITP is caused by IgG autoantibodies directed against platelets. FNAIT is a an alloimmune disorder determined by maternal antibodies directed against fetal and neonatal platelet alloantigens. Anti–human platelet antigen (HPA)-1a antibodies are the major autoantibodies involved in FNAIT. Based on mass spectrometry, increased galactosylation, increased sialylation, and decreased core fucosylation were observed in HPA-1a specific IgG1 compared with total IgG1 in patients with FNAIT [35, 138, 139]. Moreover, FNAIT patients experience a decrease in HPA-1a specific IgG1 fucosylation levels after their first pregnancy, while no other changes in glycosylation patterns were found during and after pregnancy and in subsequent pregnancies [139].
The impact of antibody glycosylation on disease severity of ITP or FNAIT has been reported in several studies. Deglycosylation of autoantibodies from ITP patients seems able to reduce platelet phagocytosis activity in vitro and to impair their ability of destroying platelets in vivo [140]. In patients with FNAIT, IgG with low levels of Fc fucosylation exhibited enhanced platelet phagocytosis activity, by binding to FcγRIII on polymorphonuclear cells and monocytes. Moreover, decreased fucosylation and increased galactosylation of anti-HPA-1a showed positive correlation with lower platelet counts and higher risk of bleeding in FNAIT patients [35, 139]. These findings suggest an association between ITP and FNAIT disease activity and antibody glycosylation, which may serve as an indicator of the severity of these diseases.
4.6. Autoimmune liver disease
Autoimmune cholestatic liver diseases include primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) [141]. PBC is characterized by the presence of highly specific antimitochondrial antibodies (AMA) and progressive destruction of intrahepatic small bile ducts [3]. Primary sclerosing cholangitis (PSC) is a cholestatic autoimmune liver disease characterized by fibrotic structures and dilatations of large intrahepatic and extrahepatic bile ducts [141]. Recently, we performed a study to elucidate site-specific glycomic profiling of Ig from patients with autoimmune cholestatic liver diseases using triple quadruple (QqQ) mass spectroscopy with subsequent multiple reaction monitoring. Increased IgG1 agalactosylation and decreased IgG1 galactosylation were identified in both PBC and PSC patients. IgG2 afucosylation levels were higher in PSC patients compared with PBC patients and healthy controls. Decreased IgG 3/4 glycosylation was observed in patients with PBC. Increased IgA 1/2 bisecting glycoforms, decreased IgA diantennary glycoforms, and increased IgA 1/2 asialylation were observed in PBC patients. Furthermore, decreased glycosylation was identified in all N-glycan sites of IgM in patients with PBC. Higher afucosylation and lower fucosylation levels were also identified in the IgM J chain of PBC patients [86]. The decrease in IgG galactosylation observed in PSC patients was consistent with the findings of another study, where increased IgG4 fucosylation, increased IgG4 bisection, increased IgG2/3 diantennary glycoforms, and decreased IgG1 sialylation were detected in patients with PSC [142].
The distinct branching and composition patterns of Ig glycoforms were closely associated with disease severity, disease duration, stage, and liver cirrhosis in PBC and PSC. IgG1 agalactosylation was positively correlated with aspartate aminotransferase and alkaline phosphatase in both groups, while IgG2 agalactosylation was positively associated with different stages of PBC. Branching IgG2 was directly correlated with total bilirubin and disease duration and inversely correlated with platelet count in PSC and PBC patients. Furthermore, branching levels of IgG1 and IgG2 were higher in PSC and PBC patients with cirrhosis compared with patients without cirrhosis. Most distinctive patterns of antibody glycoforms were associated with Mayo Risk Score, which provides prognostic information in PSC [86]. These findings highlights the potential role of lg glycoforms in monitoring disease progression, as biomarkers and surrogate endpoints in clinical trials on autoimmune cholestatic liver disease.
5. The impact of antibody glycosylation on the management of autoimmune diseases
As described in previous sections dedicated to specific conditions, the glycosylation of antibodies likely mediates their pro- or anti-inflammatory activities. For example, sialylated IgG antibodies have anti-inflammatory effector functions. The transfer of sialylated IgG autoantibodies was able to reduce the development of the disease in murine models of arthritis and lupus nephritis [143]. Therefore, modification of the glycosylation pattern of antibodies may be a potential target for the treatment of autoimmune diseases. At present, several glycosylation related therapies are being investigated in the treatment of autoimmune disease patients or in animal models. These include glycoengineered IVIg and monoclonal antibody, glycosylase (e.g. EndoS, B4GalTI)-based methods, nutritional substrate for glycosaminoglycan synthesis (e.g. GlcNAc), and chemical glycosylation (e.g. phytoestrogens).
IVIg are commonly used in the treatment of autoimmune diseases for their anti-inflammatory properties. IVIG with enriched Fc-domain sialylation suppressed joint inflammation, while the removal of terminal sialic acid residues from IVIG abrogated their anti-inflammatory activity in ITP models [15, 17]. Moreover, deglycosylated IVIG were unable to mediate anti-inflammatory activity in a model of RA [144]. Similarly, deglycosylated monoclonal antibody Ter119 lost its ability to ameliorate murine ITP [145]. However, many studies showed contradictory results [146–148]. The role of glycosylation in IVIG anti-inflammatory activity remains unclear and thus requires further investigation. Promising results were observed with endoglycosidase (EndoS), an enzyme able to deglycosylate the IgG Fc domain. EndoS reduced the proinflammatory properties of immune complexes purified from SLE patients and arthritis mice models [149, 150]. Furthermore, EndoS-treated antibodies and serum showed reduced ability to induce arthritis or lupus-like disease in RA and SLE mice models [151, 152]. In models of AAV, EndoS-treated ANCA IgG also exhibited reduced pathogenicity by showing attenuated ANCA-mediated neutrophil activation [153]. Similarly to the use of EndoS, attaching a galactose or a sialic acid to antibodies using solubilized glycosyltransferases was able to attenuate the antibody pathogenicity in vivo [16]. Mice defective in β-1,4-galactosyltransferase showed resistance to the development of chemically induced colitis by increasing the production of IL-10 [154]. A phase 2 trial demonstrated that the administration of GlcNAc greatly improved histology, disease activity, and time before surgery in patients with IBD [155]. In a colitis mouse model, treatment with GlcNAc reduced the severity of the disease and the disease progression via inhibiting T cell-mediated immune response in the intestinal mucosa [156]. Finally, phytoestrogens were able to protect mice from collagen induced arthritis by increasing sialylation and galactosylation of IgG, leading to amelioration of inflammation [157].
6. Conclusions
The analysis of antibody glycosylation demonstrated specific and, in some cases, shared characteristics in human autoimmune diseases and, within the same disease, different patterns associated with severity and treatment. Future research may increase the information available on the distinct glycome profiles and expand their potential role as biomarkers and as targets for treatment. This could allow a better patient assessment, a more precise prognostic stratification and a personalized management.
Highlights.
N-Glycosylation of immunoglobulin (Ig) regulates structure, stability and activity.
Ig glycosylation is altered in autoimmune and chronic inflammatory conditions.
Recent advances in glycosylation analysis provide unique and deep structural insights into Ig glycosylation.
The ability to manipulate antibody activity makes glycosylation a promising target for the treatment of autoimmune diseases.
Funding supports:
Weici Zhang is supported by AASLD Pinnacle Research Award in Liver Disease, AASLD Gupta Family Pilot Awards in PSC Research and American Liver Foundation PBC Fund for the Cure Award. M. Eric Gershwin is supported by NIH 1R01DK123262-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Selmi C, Bowlus CL, Gershwin ME and Coppel RL, Primary biliary cirrhosis, Lancet 377 (2011) 1600–9. [DOI] [PubMed] [Google Scholar]
- [2].Ge C, Tong D, Liang B, Lonnblom E, Schneider N, Hagert C, Viljanen J, Ayoglu B, Stawikowska R, Nilsson P, Fields GB, Skogh T, Kastbom A, Kihlberg J, Burkhardt H, Dobritzsch D and Holmdahl R, Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage, JCI Insight 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hirschfield GM and Gershwin ME, The immunobiology and pathophysiology of primary biliary cirrhosis, Annu Rev Pathol 8 (2013) 303–30. [DOI] [PubMed] [Google Scholar]
- [4].Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM and Ravetch JV, Type I and type II Fc receptors regulate innate and adaptive immunity, Nat Immunol 15 (2014) 707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schmidt RE and Gessner JE, Fc receptors and their interaction with complement in autoimmunity, Immunology Letters 100 (2005) 56–67. [DOI] [PubMed] [Google Scholar]
- [6].Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Huizinga TW, Wuhrer M, van Schaardenburg D, Toes RE and Scherer HU, Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis, Ann Rheum Dis 74 (2015) 234–41. [DOI] [PubMed] [Google Scholar]
- [7].Rantapaa-Dahlqvist S, Diagnostic and prognostic significance of autoantibodies in early rheumatoid arthritis, Scand J Rheumatol 34 (2005) 83–96. [DOI] [PubMed] [Google Scholar]
- [8].Seeling M, Bruckner C and Nimmerjahn F, Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity?, Nat Rev Rheumatol 13 (2017) 621–630. [DOI] [PubMed] [Google Scholar]
- [9].Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH and Prestegard JH, Essentials of Glycobiology [internet], (2015). [PubMed]
- [10].Pučić M, Knežević A, Vidič J, Adamczyk B, Novokmet M, Polašek O, Gornik O, Šupraha-Goreta S, Wormald MR, Redžić IJM and Proteomics C, High throughput isolation and glycosylation analysis of IgG–variability and heritability of the IgG glycome in three isolated human populations, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goulabchand R, Vincent T, Batteux F, Eliaou JF and Guilpain P, Impact of autoantibody glycosylation in autoimmune diseases, Autoimmun Rev 13 (2014) 742–50. [DOI] [PubMed] [Google Scholar]
- [12].Arnold JN, Wormald MR, Sim RB, Rudd PM and Dwek RA, The impact of glycosylation on the biological function and structure of human immunoglobulins, Annu Rev Immunol 25 (2007) 21–50. [DOI] [PubMed] [Google Scholar]
- [13].Schwab I and Nimmerjahn F, Intravenous immunoglobulin therapy: how does IgG modulate the immune system?, Nat Rev Immunol 13 (2013) 176–89. [DOI] [PubMed] [Google Scholar]
- [14].Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR and Lebrilla CB, Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review, Journal of Autoimmunity 57 (2015) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Anthony RM, Kobayashi T, Wermeling F and Ravetch JV, Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway, Nature 475 (2011) 110–U133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pagan JD, Kitaoka M and Anthony RM, Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease, Cell 172 (2018) 564–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schwab I, Biburger M, Kronke G, Schett G and Nimmerjahn F, IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1, Eur J Immunol 42 (2012) 826–30. [DOI] [PubMed] [Google Scholar]
- [18].Langereis JD, Jacobs JFM, de Jonge MI and van Deuren M, Plasma therapy leads to an increase in functional IgA and IgM concentration in the blood and saliva of a patient with X-linked agammaglobulinemia, J Transl Med 17 (2019) 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anderson CL, Chaudhury C, Kim J, Bronson CL, Wani MA and Mohanty S, Perspective-- FcRn transports albumin: relevance to immunology and medicine, Trends Immunol 27 (2006) 343–8. [DOI] [PubMed] [Google Scholar]
- [20].Akilesh S, Christianson GJ, Roopenian DC and Shaw AS, Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism, J Immunol 179 (2007) 4580–8. [DOI] [PubMed] [Google Scholar]
- [21].Wang Z, Zhu J and Lu H, Antibody glycosylation: impact on antibody drug characteristics and quality control, Appl Microbiol Biotechnol 104 (2020) 1905–1914. [DOI] [PubMed] [Google Scholar]
- [22].Reslan M, Sifniotis V, Cruz E, Sumer-Bayraktar Z, Cordwell SP and Kayser V, Enhancing the stability of adalimumab by engineering additional glycosylation motifs, Int J Biol Macromol 158 (2020) 189–196. [DOI] [PubMed] [Google Scholar]
- [23].Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV and Flynn GC, High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans, Glycobiology 21 (2011) 949–59. [DOI] [PubMed] [Google Scholar]
- [24].Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, Shitara K and Satoh M, Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types, Glycobiology 17 (2007) 104–18. [DOI] [PubMed] [Google Scholar]
- [25].Huang L, Biolsi S, Bales KR and Kuchibhotla U, Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization, Anal Biochem 349 (2006) 197–207. [DOI] [PubMed] [Google Scholar]
- [26].Newkirk MM, Novick J, Stevenson MM, Fournier MJ and Apostolakos P, Differential clearance of glycoforms of IgG in normal and autoimmune-prone mice, Clin Exp Immunol 106 (1996) 259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hajduk J, Brunner C, Malik S, Bangerter J, Schneider G, Thomann M, Reusch D and Zenobi R, Interaction analysis of glycoengineered antibodies with CD16a: a native mass spectrometry approach, MAbs 12 (2020) 1736975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luo C, Chen S, Xu N, Wang C, Sai WB, Zhao W, Li YC, Hu XJ, Tian H, Gao XD and Yao WB, Glycoengineering of pertuzumab and its impact on the pharmacokinetic/pharmacodynamic properties, Sci Rep 7 (2017) 46347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bas M, Terrier A, Jacque E, Dehenne A, Pochet-Beghin V, Beghin C, Dezetter AS, Dupont G, Engrand A, Beaufils B, Mondon P, Fournier N, de Romeuf C, Jorieux S, Fontayne A, Mars LT and Monnet C, Fc Sialylation Prolongs Serum Half-Life of Therapeutic Antibodies, J Immunol 202 (2019) 1582–1594. [DOI] [PubMed] [Google Scholar]
- [30].Raju TS, Terminal sugars of Fc glycans influence antibody effector functions of IgGs, Curr Opin Immunol 20 (2008) 471–8. [DOI] [PubMed] [Google Scholar]
- [31].Elias S, Kol I, Kahlon S, Amore R, Zeibak M, Mevorach D, Elchalal U, Zelig O and Mandelboim O, Anti-RhD antibody therapy modulates human natural killer cell function, Haematologica (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Subedi GP and Barb AW, The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor, MAbs 8 (2016) 1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH and Presta LG, Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity, J Biol Chem 277 (2002) 26733–40. [DOI] [PubMed] [Google Scholar]
- [34].Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, Lissenberg-Thunnissen SN, Visser R, Brouwer M, Mok JY, Matlung H, van den Berg TK, van Esch WJE, Kuijpers TW, Wouters D, Rispens T, Wuhrer M and Vidarsson G, Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities, Front Immunol 8 (2017) 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kapur R, Kustiawan I, Vestrheim A, Koeleman CA, Visser R, Einarsdottir HK, Porcelijn L, Jackson D, Kumpel B, Deelder AM, Blank D, Skogen B, Killie MK, Michaelsen TE, de Haas M, Rispens T, van der Schoot CE, Wuhrer M and Vidarsson G, A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy, Blood 123 (2014) 471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wada R, Matsui M and Kawasaki N, Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms, MAbs 11 (2019) 350–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N and Shitara K, The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity, J Biol Chem 278 (2003) 3466–73. [DOI] [PubMed] [Google Scholar]
- [38].Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M and Kato K, Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans, Genes Cells 16 (2011) 1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W and Taniguchi N, The alpha1–6-fucosyltransferase gene and its biological significance, Biochim Biophys Acta 1473 (1999) 9–20. [DOI] [PubMed] [Google Scholar]
- [40].Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV and Wang LX, Modulating IgG effector function by Fc glycan engineering, Proc Natl Acad Sci U S A 114 (2017) 3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gasdaska JR, Sherwood S, Regan JT and Dickey LF, An afucosylated anti-CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity and B-cell depletion and lower complement-dependent cytotoxicity than rituximab, Mol Immunol 50 (2012) 134–41. [DOI] [PubMed] [Google Scholar]
- [42].Gong Q, Hazen M, Marshall B, Crowell SR, Ou Q, Wong AW, Phung W, Vernes JM, Meng YG, Tejada M, Andersen D and Kelley RF, Increased in vivo effector function of human IgG4 isotype antibodies through afucosylation, MAbs 8 (2016) 1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kapur R, Della Valle L, Sonneveld M, Hipgrave Ederveen A, Visser R, Ligthart P, de Haas M, Wuhrer M, van der Schoot CE and Vidarsson G, Low anti-RhD IgG-Fc-fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn, Br J Haematol 166 (2014) 936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Siberil S, de Romeuf C, Bihoreau N, Fernandez N, Meterreau JL, Regenman A, Nony E, Gaucher C, Glacet A, Jorieux S, Klein P, Hogarth MP, Fridman WH, Bourel D, Beliard R and Teillaud JL, Selection of a human anti-RhD monoclonal antibody for therapeutic use: impact of IgG glycosylation on activating and inhibitory Fc gamma R functions, Clin Immunol 118 (2006) 170–9. [DOI] [PubMed] [Google Scholar]
- [45].Scallon BJ, Tam SH, McCarthy SG, Cai AN and Raju TS, Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality, Mol Immunol 44 (2007) 1524–34. [DOI] [PubMed] [Google Scholar]
- [46].Zabczynska M, Polak K, Kozlowska K, Sokolowski G and Pochec E, The Contribution of IgG Glycosylation to Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Complement-Dependent Cytotoxicity (CDC) in Hashimoto’s Thyroiditis: An in Vitro Model of Thyroid Autoimmunity, Biomolecules 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jo M, Kwon HS, Lee KH, Lee JC and Jung ST, Engineered aglycosylated full-length IgG Fc variants exhibiting improved FcgammaRIIIa binding and tumor cell clearance, MAbs 10 (2018) 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Peschke B, Keller CW, Weber P, Quast I and Lunemann JD, Fc-Galactosylation of Human Immunoglobulin Gamma Isotypes Improves C1q Binding and Enhances Complement-Dependent Cytotoxicity, Front Immunol 8 (2017) 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K and Kato K, Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy, Biochim Biophys Acta 1760 (2006) 693–700. [DOI] [PubMed] [Google Scholar]
- [50].Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S and Alter G, Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function, AIDS 28 (2014) 2523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hodoniczky J, Zheng YZ and James DC, Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro, Biotechnol Prog 21 (2005) 1644–52. [DOI] [PubMed] [Google Scholar]
- [52].Nimmerjahn F, Anthony RM and Ravetch JV, Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity, Proc Natl Acad Sci U S A 104 (2007) 8433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Natsume A, In M, Takamura H, Nakagawa T, Shimizu Y, Kitajima K, Wakitani M, Ohta S, Satoh M, Shitara K and Niwa R, Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities, Cancer Res 68 (2008) 3863–72. [DOI] [PubMed] [Google Scholar]
- [54].Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang LX, Munz C, Nimmerjahn F, Dalakas MC and Lunemann JD, Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity, J Clin Invest 125 (2015) 4160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kuhns S, Shu J, Xiang C, Guzman R, Zhang Q, Bretzlaff W, Miscalichi N, Kalenian K and Joubert M, Differential influence on antibody dependent cellular phagocytosis by different glycoforms on therapeutic Monoclonal antibodies, J Biotechnol 317 (2020) 5–15. [DOI] [PubMed] [Google Scholar]
- [56].Nimmerjahn F and Ravetch JV, Divergent immunoglobulin g subclass activity through selective Fc receptor binding, Science 310 (2005) 1510–2. [DOI] [PubMed] [Google Scholar]
- [57].Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC and Ravetch JV, Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc, Science 320 (2008) 373–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fiebiger BM, Maamary J, Pincetic A and Ravetch JV, Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs, Proc Natl Acad Sci U S A 112 (2015) E2385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schwab I, Mihai S, Seeling M, Kasperkiewicz M, Ludwig RJ and Nimmerjahn F, Broad requirement for terminal sialic acid residues and FcgammaRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo, Eur J Immunol 44 (2014) 1444–53. [DOI] [PubMed] [Google Scholar]
- [60].Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H, Garofalo K, Meccariello R, Meador JW 3rd, Rutitzky L, Schultes BC, Ling L, Avery W, Nimmerjahn F, Manning AM, Kaundinya GV and Bosques CJ, Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity, Proc Natl Acad Sci U S A 112 (2015) E1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, Schommartz T, Petzold D, Bitterling J, Schoen AL, Stoehr AD, Vu Van D, Darcan-Nikolaisen Y, Blanchard V, Schmudde I, Laumonnier Y, Strover HA, Hegazy AN, Eiglmeier S, Schoen CT, Mertes MM, Loddenkemper C, Lohning M, Konig P, Petersen A, Luger EO, Collin M, Kohl J, Hutloff A, Hamelmann E, Berger M, Wardemann H and Ehlers M, Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs, J Allergy Clin Immunol 129 (2012) 1647–55 e13. [DOI] [PubMed] [Google Scholar]
- [62].Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, Schommartz T, Mertes MM, Schoen CT, Tiburzy B, Herrmann A, Kohl J, Manz RA, Madaio MP, Berger M, Wardemann H and Ehlers M, T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies, J Clin Invest 123 (2013) 3788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schwab I, Lux A and Nimmerjahn F, Pathways Responsible for Human Autoantibody and Therapeutic Intravenous IgG Activity in Humanized Mice, Cell Rep 13 (2015) 610–620. [DOI] [PubMed] [Google Scholar]
- [64].Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Kohl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M and Kohl J, Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1, Nat Med 18 (2012) 1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yamada K, Ito K, Furukawa J, Nakata J, Alvarez M, Verbeek JS, Shinohara Y and Izui S, Galactosylation of IgG1 modulates FcgammaRIIB-mediated inhibition of murine autoimmune hemolytic anemia, J Autoimmun 47 (2013) 104–10. [DOI] [PubMed] [Google Scholar]
- [66].Cambay F, Forest-Nault C, Dumoulin L, Seguin A, Henry O, Durocher Y and De Crescenzo G, Glycosylation of Fcgamma receptors influences their interaction with various IgG1 glycoforms, Mol Immunol 121 (2020) 144–158. [DOI] [PubMed] [Google Scholar]
- [67].Zhang G, Massaad CA, Gao T, Pillai L, Bogdanova N, Ghauri S and Sheikh KA, Sialylated intravenous immunoglobulin suppress anti-ganglioside antibody mediated nerve injury, Exp Neurol 282 (2016) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Anthony RM, Wermeling F, Karlsson MC and Ravetch JV, Identification of a receptor required for the anti-inflammatory activity of IVIG, Proc Natl Acad Sci U S A 105 (2008) 19571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yu X, Vasiljevic S, Mitchell DA, Crispin M and Scanlan CN, Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain, J Mol Biol 425 (2013) 1253–8. [DOI] [PubMed] [Google Scholar]
- [70].Jegouzo SAF, Feinberg H, Morrison AG, Holder A, May A, Huang ZY, Jiang LH, Lasanajak Y, Smith DF, Werling D, Drickamer K, Weis WI and Taylor ME, CD23 is a glycan-binding receptor in some mammalian species, Journal of Biological Chemistry 294 (2019) 14845–14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Selb R, Eckl-Dorna J, Twaroch TE, Lupinek C, Teufelberger A, Hofer G, Focke-Tejkl M, Gepp B, Linhart B, Breiteneder H, Ellinger A, Keller W, Roux KH, Valenta R and Niederberger V, Critical and direct involvement of the CD23 stalk region in IgE binding, Journal of Allergy and Clinical Immunology 139 (2017) 281–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, Mourad W, Piccirillo CA and Mazer BD, Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells, J Allergy Clin Immunol 133 (2014) 853–63 e5. [DOI] [PubMed] [Google Scholar]
- [73].Manabe Y, Marchetti R, Takakura Y, Nagasaki M, Nihei W, Takebe T, Tanaka K, Kabayama K, Chiodo F, Hanashima S, Kamada Y, Miyoshi E, Dulal HP, Yamaguchi Y, Adachi Y, Ohno N, Tanaka H, Silipo A, Fukase K and Molinaro A, The Core Fucose on an IgG Antibody is an Endogenous Ligand of Dectin-1, Angew Chem Int Ed Engl 58 (2019) 18697–18702. [DOI] [PubMed] [Google Scholar]
- [74].Seite JF, Cornec D, Renaudineau Y, Youinou P, Mageed RA and Hillion S, IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes, Blood 116 (2010) 1698–704. [DOI] [PubMed] [Google Scholar]
- [75].Handa-Narumi M, Yoshimura T, Konishi H, Fukata Y, Manabe Y, Tanaka K, Bao GM, Kiyama H, Fukase K and Ikenaka K, Branched Sialylated N-glycans Are Accumulated in Brain Synaptosomes and Interact with Siglec-H, Cell Struct Funct 43 (2018) 141–152. [DOI] [PubMed] [Google Scholar]
- [76].Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA and Sim RB, Glycosylation Changes of Igg Associated with Rheumatoid-Arthritis Can Activate Complement Via the Mannose-Binding Protein (Vol 1, Pg 237, 1995), Nature Medicine 1 (1995) 599–599. [DOI] [PubMed] [Google Scholar]
- [77].van de Geijn FE, de Man YA, Wuhrer M, Willemsen SP, Deelder AM, Hazes JM and Dolhain RJ, Mannose-binding lectin does not explain the course and outcome of pregnancy in rheumatoid arthritis, Arthritis Res Ther 13 (2011) R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].van de Geijn FE, Hazes JM, Geleijns K, Emonts M, Jacobs BC, Dufour-van den Goorbergh BC and Dolhain RJ, Mannose-binding lectin polymorphisms are not associated with rheumatoid arthritis--confirmation in two large cohorts, Rheumatology (Oxford) 47 (2008) 1168–71. [DOI] [PubMed] [Google Scholar]
- [79].Wu AM, Sugii SJ and Herp A, A guide for carbohydrate specificities of lectins, Adv Exp Med Biol 228 (1988) 819–47. [DOI] [PubMed] [Google Scholar]
- [80].Srinivasan K, Roy S, Washburn N, Sipsey SF, Meccariello R, Meador JW 3rd, Ling LE, Manning AM and Kaundinya GV, A Quantitative Microtiter Assay for Sialylated Glycoform Analyses Using Lectin Complexes, J Biomol Screen 20 (2015) 768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, Kochhar S, Sigrist H and Sprenger N, Glycoprofiling with micro-arrays of glycoconjugates and lectins, Glycobiology 15 (2005) 31–41. [DOI] [PubMed] [Google Scholar]
- [82].Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M and Hirabayashi J, Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling, Nat Methods 2 (2005) 851–6. [DOI] [PubMed] [Google Scholar]
- [83].Zheng T, Peelen D and Smith LM, Lectin arrays for profiling cell surface carbohydrate expression, J Am Chem Soc 127 (2005) 9982–3. [DOI] [PubMed] [Google Scholar]
- [84].Pilobello KT, Krishnamoorthy L, Slawek D and Mahal LK, Development of a lectin microarray for the rapid analysis of protein glycopatterns, Chembiochem 6 (2005) 985–9. [DOI] [PubMed] [Google Scholar]
- [85].Hirabayashi J, Yamada M, Kuno A and Tateno H, Lectin microarrays: concept, principle and applications, Chem Soc Rev 42 (2013) 4443–58. [DOI] [PubMed] [Google Scholar]
- [86].Zhou X, Kailemia MJ, Sun Y, Shuai Z, Yang GX, Dhaliwal S, Cristoferi L, Leung PSC, Invernizzi P, Bowlus CL, Lebrilla CB, Ansari AA, Ridgway WM, Zhang W and Gershwin ME, Glycomic analysis of antibody indicates distinctive glycosylation profile in patients with autoimmune cholangitis, J Autoimmun (2020) 102503. [DOI] [PubMed] [Google Scholar]
- [87].Ruhaak LR, Xu G, Li Q, Goonatilleke E and Lebrilla CB, Mass Spectrometry Approaches to Glycomic and Glycoproteomic Analyses, Chem Rev 118 (2018) 7886–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Banazadeh A, Veillon L, Wooding KM, Zabet-Moghaddam M and Mechref Y, Recent advances in mass spectrometric analysis of glycoproteins, Electrophoresis 38 (2017) 162–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Toth E, Ozohanics O, Bobaly B, Gomory A, Jeko A, Drahos L and Vekey K, HPLC enrichment/isolation of proteins for post-translational modification studies from complex mixtures, J Pharm Biomed Anal 98 (2014) 393–400. [DOI] [PubMed] [Google Scholar]
- [90].Knezevic A, Bones J, Kracun SK, Gornik O, Rudd PM and Lauc G, High throughput plasma N-glycome profiling using multiplexed labelling and UPLC with fluorescence detection, Analyst 136 (2011) 4670–3. [DOI] [PubMed] [Google Scholar]
- [91].Chen Y, Ding L, Liu T and Ju H, Arrayed profiling of multiple glycans on whole living cell surfaces, Anal Chem 85 (2013) 11153–8. [DOI] [PubMed] [Google Scholar]
- [92].Chen Y, Ding L, Xu J, Song W, Yang M, Hu J and Ju H, Micro-competition system for Raman quantification of multiple glycans on intact cell surface, Chem Sci 6 (2015) 3769–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen Y, Ding L and Ju H, In Situ Cellular Glycan Analysis, Acc Chem Res 51 (2018) 890–899. [DOI] [PubMed] [Google Scholar]
- [94].Cheng W, Ding L, Ding S, Yin Y and Ju H, A simple electrochemical cytosensor array for dynamic analysis of carcinoma cell surface glycans, Angew Chem Int Ed Engl 48 (2009) 6465–8. [DOI] [PubMed] [Google Scholar]
- [95].Gibofsky A, Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis, Am J Manag Care 18 (2012) S295–302. [PubMed] [Google Scholar]
- [96].Scott DL, Wolfe F and Huizinga TW, Rheumatoid arthritis, Lancet 376 (2010) 1094–108. [DOI] [PubMed] [Google Scholar]
- [97].Su Z, Xie Q, Wang Y and Li Y, Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS, Int J Mol Sci 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sun D, Hu F, Gao H, Song Z, Xie W, Wang P, Shi L, Wang K, Li Y, Huang C and Li Z, Distribution of abnormal IgG glycosylation patterns from rheumatoid arthritis and osteoarthritis patients by MALDI-TOF-MS(n), Analyst 144 (2019) 2042–2051. [DOI] [PubMed] [Google Scholar]
- [99].Huang C, Liu Y, Wu H, Sun D and Li Y, Characterization of IgG glycosylation in rheumatoid arthritis patients by MALDI-TOF-MS(n) and capillary electrophoresis, Anal Bioanal Chem 409 (2017) 3731–3739. [DOI] [PubMed] [Google Scholar]
- [100].Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K and et al. , Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG, Nature 316 (1985) 452–7. [DOI] [PubMed] [Google Scholar]
- [101].Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM and Rademacher TW, Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity, Lancet 1 (1988) 966–9. [DOI] [PubMed] [Google Scholar]
- [102].van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM and Dolhain RJ, Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study, Arthritis Res Ther 11 (2009) R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gindzienska-Sieskiewicz E, Radziejewska I, Domyslawska I, Klimiuk PA, Sulik A, Rojewska J, Gabryel-Porowska H and Sierakowski S, Changes of glycosylation of IgG in rheumatoid arthritis patients treated with methotrexate, Adv Med Sci 61 (2016) 193–197. [DOI] [PubMed] [Google Scholar]
- [104].Soltys AJ, Hay FC, Bond A, Axford JS, Jones MG, Randen I, Thompson KM and Natvig JB, The binding of synovial tissue-derived human monoclonal immunoglobulin M rheumatoid factor to immunoglobulin G preparations of differing galactose content, Scand J Immunol 40 (1994) 135–43. [DOI] [PubMed] [Google Scholar]
- [105].Matsumoto A, Shikata K, Takeuchi F, Kojima N and Mizuochi T, Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation, J Biochem 128 (2000) 621–8. [DOI] [PubMed] [Google Scholar]
- [106].Gudelj I, Salo PP, Trbojevic-Akmacic I, Albers M, Primorac D, Perola M and Lauc G, Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10years of follow-up, Biochim Biophys Acta Mol Basis Dis 1864 (2018) 2034–2039. [DOI] [PubMed] [Google Scholar]
- [107].Ercan A, Barnes MG, Hazen M, Tory H, Henderson L, Dedeoglu F, Fuhlbrigge RC, Grom A, Holm IA, Kellogg M, Kim S, Adamczyk B, Rudd PM, Son MB, Sundel RP, Foell D, Glass DN, Thompson SD and Nigrovic PA, Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation, Arthritis Rheum 64 (2012) 3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cheng HD, Stockmann H, Adamczyk B, McManus CA, Ercan A, Holm IA, Rudd PM, Ackerman ME and Nigrovic PA, High-throughput characterization of the functional impact of IgG Fc glycan aberrancy in juvenile idiopathic arthritis, Glycobiology 27 (2017) 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Imafuku Y, Yoshida H and Yamada Y, Reactivity of agalactosyl IgG with rheumatoid factor, Clin Chim Acta 334 (2003) 217–23. [DOI] [PubMed] [Google Scholar]
- [110].Ohmi Y, Ise W, Harazono A, Takakura D, Fukuyama H, Baba Y, Narazaki M, Shoda H, Takahashi N, Ohkawa Y, Ji S, Sugiyama F, Fujio K, Kumanogoh A, Yamamoto K, Kawasaki N, Kurosaki T, Takahashi Y and Furukawa K, Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis, Nat Commun 7 (2016) 11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang JR, Gao WN, Grimm R, Jiang S, Liang Y, Ye H, Li ZG, Yau LF, Huang H, Liu J, Jiang M, Meng Q, Tong TT, Huang HH, Lee S, Zeng X, Liu L and Jiang ZH, A method to identify trace sulfated IgG N-glycans as biomarkers for rheumatoid arthritis, Nat Commun 8 (2017) 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, Haupl T, Burmester GR, Deelder AM, Huizinga TW, Wuhrer M and Toes RE, Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid, Arthritis Rheum 62 (2010) 1620–9. [DOI] [PubMed] [Google Scholar]
- [113].Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S, Haugg B, Hueber AJ, Daum P, Heidkamp GF, Ge C, Bohm S, Lux A, Schuh W, Magorivska I, Nandakumar KS, Lonnblom E, Becker C, Dudziak D, Wuhrer M, Rombouts Y, Koeleman CA, Toes R, Winkler TH, Holmdahl R, Herrmann M, Bluml S, Nimmerjahn F, Schett G and Kronke G, Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease, Nat Immunol 18 (2017) 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pasek M, Duk M, Podbielska M, Sokolik R, Szechinski J, Lisowska E and Krotkiewski H, Galactosylation of IgG from rheumatoid arthritis (RA) patients--changes during therapy, Glycoconj J 23 (2006) 463–71. [DOI] [PubMed] [Google Scholar]
- [115].Schwedler C, Haupl T, Kalus U, Blanchard V, Burmester GR, Poddubnyy D and Hoppe B, Hypogalactosylation of immunoglobulin G in rheumatoid arthritis: relationship to HLA-DRB1 shared epitope, anticitrullinated protein antibodies, rheumatoid factor, and correlation with inflammatory activity, Arthritis Res Ther 20 (2018) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rademacher TW, Williams P and Dwek RA, Agalactosyl glycoforms of IgG autoantibodies are pathogenic, Proc Natl Acad Sci U S A 91 (1994) 6123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bondt A, Wuhrer M, Kuijper TM, Hazes JM and Dolhain RJ, Fab glycosylation of immunoglobulin G does not associate with improvement of rheumatoid arthritis during pregnancy, Arthritis Res Ther 18 (2016) 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lundstrom SL, Hensvold AH, Rutishauser D, Klareskog L, Ytterberg AJ, Zubarev RA and Catrina AI, IgG Fc galactosylation predicts response to methotrexate in early rheumatoid arthritis, Arthritis Res Ther 19 (2017) 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tomana M, Schrohenloher RE, Reveille JD, Arnett FC and Koopman WJ, Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases, Rheumatol Int 12 (1992) 191–4. [DOI] [PubMed] [Google Scholar]
- [120].Pilkington C, Yeung E, Isenberg D, Lefvert AK and Rook GA, Agalactosyl IgG and antibody specificity in rheumatoid arthritis, tuberculosis, systemic lupus erythematosus and myasthenia gravis, Autoimmunity 22 (1995) 107–11. [DOI] [PubMed] [Google Scholar]
- [121].Vuckovic F, Kristic J, Gudelj I, Teruel M, Keser T, Pezer M, Pucic-Bakovic M, Stambuk J, Trbojevic-Akmacic I, Barrios C, Pavic T, Menni C, Wang Y, Zhou Y, Cui L, Song H, Zeng Q, Guo X, Pons-Estel BA, McKeigue P, Leslie Patrick A, Gornik O, Spector TD, Harjacek M, Alarcon-Riquelme M, Molokhia M, Wang W and Lauc G, Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome, Arthritis Rheumatol 67 (2015) 2978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sjowall C, Zapf J, von Lohneysen S, Magorivska I, Biermann M, Janko C, Winkler S, Bilyy R, Schett G, Herrmann M and Munoz LE, Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus, Lupus 24 (2015) 569–81. [DOI] [PubMed] [Google Scholar]
- [123].Mizuochi T, Hamako J, Nose M and Titani K, Structural changes in the oligosaccharide chains of IgG in autoimmune MRL/Mp-lpr/lpr mice, J Immunol 145 (1990) 1794–8. [PubMed] [Google Scholar]
- [124].Tomana M, Schrohenloher RE, Koopman WJ, Alarcon GS and Paul WA, Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases, Arthritis Rheum 31 (1988) 333–8. [DOI] [PubMed] [Google Scholar]
- [125].Simurina M, de Haan N, Vuckovic F, Kennedy NA, Stambuk J, Falck D, Trbojevic-Akmacic I, Clerc F, Razdorov G, Khon A, Latiano A, D’Inca R, Danese S, Targan S, Landers C, Dubinsky M, C. Inflammatory Bowel Disease Biomarkers, McGovern DPB, Annese V, Wuhrer M and Lauc G, Glycosylation of Immunoglobulin G Associates With Clinical Features of Inflammatory Bowel Diseases, Gastroenterology 154 (2018) 1320–1333 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Dube R, Rook GA, Steele J, Brealey R, Dwek R, Rademacher T and Lennard-Jones J, Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein, Gut 31 (1990) 431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Trbojevic Akmacic I, Ventham NT, Theodoratou E, Vuckovic F, Kennedy NA, Kristic J, Nimmo ER, Kalla R, Drummond H, Stambuk J, Dunlop MG, Novokmet M, Aulchenko Y, Gornik O, Campbell H, Pucic Bakovic M, Satsangi J, Lauc G and Consortium I-B, Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome, Inflamm Bowel Dis 21 (2015) 1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shinzaki S, Iijima H, Nakagawa T, Egawa S, Nakajima S, Ishii S, Irie T, Kakiuchi Y, Nishida T, Yasumaru M, Kanto T, Tsujii M, Tsuji S, Mizushima T, Yoshihara H, Kondo A, Miyoshi E and Hayashi N, IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease, Am J Gastroenterol 103 (2008) 1173–81. [DOI] [PubMed] [Google Scholar]
- [129].Fujii H, Shinzaki S, Iijima H, Wakamatsu K, Iwamoto C, Sobajima T, Kuwahara R, Hiyama S, Hayashi Y, Takamatsu S, Uozumi N, Kamada Y, Tsujii M, Taniguchi N, Takehara T and Miyoshi E, Core Fucosylation on T Cells, Required for Activation of T-Cell Receptor Signaling and Induction of Colitis in Mice, Is Increased in Patients With Inflammatory Bowel Disease, Gastroenterology 150 (2016) 1620–1632. [DOI] [PubMed] [Google Scholar]
- [130].Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, Liu H, Bell DW, Driscoll DR, Diederichs S, Haider K, Netravali I, Le S, Elia R, Dow E, Lee A, Freudenberg J, De Jager PL, Chretien Y, Varki A, MacDonald ME, Gillis T, Behrens TW, Bloch D, Collier D, Korzenik J, Podolsky DK, Hafler D, Murali M, Sands B, Stone JH, Gregersen PK and Pillai S, Functionally defective germline variants of sialic acid acetylesterase in autoimmunity, Nature 466 (2010) 243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Shinzaki S, Kuroki E, Iijima H, Tatsunaka N, Ishii M, Fujii H, Kamada Y, Kobayashi T, Shibukawa N, Inoue T, Tsujii M, Takeishi S, Mizushima T, Ogata A, Naka T, Plevy SE, Takehara T and Miyoshi E, Lectin-based immunoassay for aberrant IgG glycosylation as the biomarker for Crohn’s disease, Inflamm Bowel Dis 19 (2013) 321–31. [DOI] [PubMed] [Google Scholar]
- [132].Varadi C, Hollo Z, Poliska S, Nagy L, Szekanecz Z, Vancsa A, Palatka K and Guttman A, Combination of IgG N-glycomics and corresponding transcriptomics data to identify anti-TNF-alpha treatment responders in inflammatory diseases, Electrophoresis 36 (2015) 1330–5. [DOI] [PubMed] [Google Scholar]
- [133].Holland M, Takada K, Okumoto T, Takahashi N, Kato K, Adu D, Ben-Smith A, Harper L, Savage CO and Jefferis R, Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis, Clin Exp Immunol 129 (2002) 183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Lardinois OM, Deterding LJ, Hess JJ, Poulton CJ, Henderson CD, Jennette JC, Nachman PH and Falk RJ, Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity, PLoS One 14 (2019) e0213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wuhrer M, Stavenhagen K, Koeleman CA, Selman MH, Harper L, Jacobs BC, Savage CO, Jefferis R, Deelder AM and Morgan M, Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation, J Proteome Res 14 (2015) 1657–65. [DOI] [PubMed] [Google Scholar]
- [136].Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, Chereau C, Pagnoux C, Michalski JC, Guillevin L, Weill B, Batteux F and Guilpain P, Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s), Arthritis Rheum 63 (2011) 2105–15. [DOI] [PubMed] [Google Scholar]
- [137].Kemna MJ, Plomp R, van Paassen P, Koeleman CAM, Jansen BC, Damoiseaux J, Cohen Tervaert JW and Wuhrer M, Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis, EBioMedicine 17 (2017) 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wuhrer M, Porcelijn L, Kapur R, Koeleman CA, Deelder A, de Haas M and Vidarsson G, Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens, J Proteome Res 8 (2009) 450–6. [DOI] [PubMed] [Google Scholar]
- [139].Sonneveld ME, Natunen S, Sainio S, Koeleman CA, Holst S, Dekkers G, Koelewijn J, Partanen J, van der Schoot CE, Wuhrer M and Vidarsson G, Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia, Br J Haematol 174 (2016) 310–20. [DOI] [PubMed] [Google Scholar]
- [140].Bakchoul T, Walek K, Krautwurst A, Rummel M, Bein G, Santoso S and Sachs UJ, Glycosylation of autoantibodies: insights into the mechanisms of immune thrombocytopenia, Thromb Haemost 110 (2013) 1259–66. [DOI] [PubMed] [Google Scholar]
- [141].Li Y, Tang R, Leung PSC, Gershwin ME and Ma X, Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases, Autoimmun Rev 16 (2017) 885–896. [DOI] [PubMed] [Google Scholar]
- [142].Culver EL, van de Bovenkamp FS, Derksen NIL, Koers J, Cargill T, Barnes E, de Neef LA, Koeleman CAM, Aalberse RC, Wuhrer M and Rispens T, Unique patterns of glycosylation in immunoglobulin subclass G4-related disease and primary sclerosing cholangitis, J Gastroenterol Hepatol 34 (2019) 1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bartsch YC, Rahmoller J, Mertes MMM, Eiglmeier S, Lorenz FKM, Stoehr AD, Braumann D, Lorenz AK, Winkler A, Lilienthal GM, Petry J, Hobusch J, Steinhaus M, Hess C, Holecska V, Schoen CT, Oefner CM, Leliavski A, Blanchard V and Ehlers M, Sialylated Autoantigen-Reactive IgG Antibodies Attenuate Disease Development in Autoimmune Mouse Models of Lupus Nephritis and Rheumatoid Arthritis, Front Immunol 9 (2018) 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kaneko Y, Nimmerjahn F and Ravetch JV, Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation, Science 313 (2006) 670–3. [DOI] [PubMed] [Google Scholar]
- [145].Yu X, Menard M, Seabright G, Crispin M and Lazarus AH, A monoclonal antibody with anti-D-like activity in murine immune thrombocytopenia requires Fc domain function for immune thrombocytopenia ameliorative effects, Transfusion 55 (2015) 1501–11. [DOI] [PubMed] [Google Scholar]
- [146].Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, Maraskovsky E, Zuercher AW, Neschadim A, Leontyev D, McKenzie BS and Kasermann F, Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils, J Immunol 192 (2014) 5031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Guhr T, Bloem J, Derksen NI, Wuhrer M, Koenderman AH, Aalberse RC and Rispens T, Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia, PLoS One 6 (2011) e21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Leontyev D, Katsman Y, Ma XZ, Miescher S, Kasermann F and Branch DR, Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin, Transfusion 52 (2012) 1799–805. [DOI] [PubMed] [Google Scholar]
- [149].Nandakumar KS, Collin M, Happonen KE, Lundstrom SL, Croxford AM, Xu B, Zubarev RA, Rowley MJ, Blom AM, Kjellman C and Holmdahl R, Streptococcal Endo-beta-N-Acetylglucosaminidase Suppresses Antibody-Mediated Inflammation In Vivo, Front Immunol 9 (2018) 1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lood C, Allhorn M, Lood R, Gullstrand B, Olin AI, Ronnblom L, Truedsson L, Collin M and Bengtsson AA, IgG glycan hydrolysis by endoglycosidase S diminishes the proinflammatory properties of immune complexes from patients with systemic lupus erythematosus: a possible new treatment?, Arthritis Rheum 64 (2012) 2698–706. [DOI] [PubMed] [Google Scholar]
- [151].Nandakumar KS, Collin M, Olsen A, Nimmerjahn F, Blom AM, Ravetch JV and Holmdahl R, Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis, Eur J Immunol 37 (2007) 2973–82. [DOI] [PubMed] [Google Scholar]
- [152].Albert H, Collin M, Dudziak D, Ravetch JV and Nimmerjahn F, In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner, Proc Natl Acad Sci U S A 105 (2008) 15005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].van Timmeren MM, van der Veen BS, Stegeman CA, Petersen AH, Hellmark T, Collin M and Heeringa P, IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis, J Am Soc Nephrol 21 (2010) 1103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shinzaki S, Iijima H, Fujii H, Kuroki E, Tatsunaka N, Inoue T, Nakajima S, Egawa S, Kanto T, Tsujii M, Morii E, Takeishi S, Asano M, Takehara T, Hayashi N and Miyoshi E, Altered oligosaccharide structures reduce colitis induction in mice defective in beta-1,4-galactosyltransferase, Gastroenterology 142 (2012) 1172–82. [DOI] [PubMed] [Google Scholar]
- [155].Salvatore S, Heuschkel R, Tomlin S, Davies SE, Edwards S, Walker-Smith JA, French I and Murch SH, A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease, Aliment Pharmacol Ther 14 (2000) 1567–79. [DOI] [PubMed] [Google Scholar]
- [156].Dias AM, Correia A, Pereira MS, Almeida CR, Alves I, Pinto V, Catarino TA, Mendes N, Leander M, Oliva-Teles MT, Maia L, Delerue-Matos C, Taniguchi N, Lima M, Pedroto I, Marcos-Pinto R, Lago P, Reis CA, Vilanova M and Pinho SS, Metabolic control of T cell immune response through glycans in inflammatory bowel disease, Proc Natl Acad Sci U S A 115 (2018) E4651–E4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Du N, Song L, Li Y, Wang T, Fang Q, Ou J and Nandakumar KS, Phytoestrogens protect joints in collagen induced arthritis by increasing IgG glycosylation and reducing osteoclast activation, Int Immunopharmacol 83 (2020) 106387. [DOI] [PubMed] [Google Scholar]


