Summary:
Ticks are hematophagous arthropods with unique molecular mechanisms for digesting host blood meal while acting as vectors for various pathogens of public health significance. The tick’s pharmacologically active saliva plays a fundamental role in modulating the host’s immune system for several days to weeks, depending on the tick species. The vector tick has also developed sophisticated molecular mechanisms to serve as a competent vector for pathogens, including the spotted fever group rickettsiae. Evidence is still inadequate concerning tick–rickettsiae–host interactions and saliva-assisted transmission of the pathogen to the mammalian host. Rickettsia parkeri, of the spotted fever group rickettsia, can cause a milder version of Rocky Mountain spotted fever known as American Boutonneuse fever. The Gulf Coast tick (Amblyomma maculatum) often transmits this pathogenic rickettsia in the United States. This review discusses the knowledge gap concerning tick–rickettsiae–host interactions by highlighting the spotted fever group rickettsia and the Am. maculatum model system. Filling this knowledge gap will provide a better understanding of the tick–rickettsiae–host interactions in disease causation, which will be crucial for developing effective methods for preventing tick-borne diseases.
Keywords: Ticks, Hematophagy, Rickettsiae, Tick-borne disease, antioxidants
1. Introduction
Arthropod vector ticks harbor a diverse range of viral, bacterial, and protozoan agents and transmit them to their mammalian host, making them a major public health threat (Estrada-Peña and de la Fuente, 2014; Mansfield et al., 2017; Parola et al., 2013; Wikel, 2013, 2018a). According to the Centers for Disease Control and Prevention (CDC), a total of 491,671 cases of tick-borne diseases (~76.5% of all vector-borne diseases) were reported in the United States and territories from 2004–2016 (CDC, 2019). Lyme disease is the most common vector-borne disease in the United States, and a recently released estimate based on medical insurance records suggests that approximately 476,000 Americans are annually diagnosed and treated for this disease. Even more tick-borne diseases (TBDs) are recorded by the CDC (CDC, 2019). The total number of reported rickettsial cases jumped from 495 infections in 2000 to 6,248 infections in 2017 (Biggs et al., 2016). As exposure to ticks will likely increase, due to expanding deer and rodent populations and global climate change, an increasing number of people will be at risk of contracting rickettsiosis that is vectored by tick species.
Various tick species can transmit >20 emerging and resurgent agents, all capable of causing significant diseases, including alpha-gal syndrome (Crispell et al., 2019), in humans and animals, including livestock, pets, and wildlife. Rickettsial pathogens cause life-threatening human infections and are significant causes of morbidity globally. Arthropod vectors, such as ticks, fleas, lice, and chiggers, transmit the intracellular bacteria that cause these diseases. Rickettsial diseases have been responsible for the loss of millions of lives throughout history (Sahni et al., 2013). An estimated one billion people worldwide are at risk of rickettsial diseases, which are caused by an obligate intracellular Gram-negative bacterium (Walker and Ismail 2008; Parola et al., 2005), and this enhanced risk is partly due to rapid global travel and the high-volume international livestock trade. Previously, strict quarantine and surveillance protocols contained the spread of tick-borne diseases. However, the high frequency and ease of global movement constitute a risk for the transportation of ticks and tick-borne diseases that may have previously been isolated to one region. Migratory birds also provide a means of tick dispersal over thousands of miles (Mukherjee et al., 2013; Budachetri et al., 2017). Intriguingly, Rickettsia parkeri, an emerging spotted fever group rickettsia, causes a disease of public health significance that is characterized by fever, headache, malaise, myalgia, arthralgia, the presence of a maculopapular rash, and multiple eschars (Walker and Ismail, 2008; Parola et al., 2005; Paddock et al., 2004; Whitman et al., 2007; Paddock et al., 2008). In the United States, a six-fold increase in clinical cases of spotted fever group (SFG) rickettsiosis has been reported since 2005 (Walker and Ismail 2008; Groseclose et al., 2004; Adams et al., 2015). The increase in R. parkeri rickettsiosis clinical cases is mainly due to the underreporting and serological conservation of R. rickettsii, the agent that causes Rocky Mountain spotted fever (Paddock et al., 2009; 2008).
R. parkeri is maintained within the Gulf Coast tick Amblyomma maculatum populations through both transstadial (between life-stage molts) and transovarial transmission (TOT, deposition into eggs of the developing next generation; Budachetri et al., 2014). R. parkeri infection of tick salivary glands and ovaries is an essential stage of the bacterial life cycle. There is a dearth of information about the interplay between the tick vector Am. maculatum and intracellular pathogenic bacterium R. parkeri. The first sialotranscriptome of Am. maculatum opened up a new avenue of research by enabling the development of molecular tools to investigate the functional role of tick genes in hematophagy and vector competence using an Am. maculatum–R. parkeri model (Karim et al., 2011; Villarreal et al., 2013). The interactions between the tick vector and rickettsia bacteria are an understudied research area, and Am. maculatum together with R. parkeri offers a unique model system for understanding pathogen infection and saliva-assisted transmission of the emerging SFG of rickettsia and their tick vectors (Socolovschi et al., 2009).
2. Tick hematophagy
To survive, ticks must maintain homeostasis (a stable equilibrium maintained by physiological processes) and obtain a disproportionately large blood meal of up to 100 times their unfed weight (Fig. 1). The Gulf Coast tick, Amblyomma maculatum, has recently gained increased attention due to its now established role as a competent vector for Rickettsia parkeri, which causes an emerging rickettsial disease of public health significance (Paddock and Goddard 2015). This hard tick species is distributed across several regions of Central and South American countries bordering the Gulf of Mexico and the Caribbean Sea, with a range larger than both Amblyomma americanum and Ixodes scapularis (Estrada-Pena et al., 2005). Bird migration and animal movement have likely contributed to the vast distribution of this tick across the United States (Mukherjee et al., 2014). It is a known vector of Rickettsia parkeri and other bacterial species, including Candidatus Rickettsia andeanae (Noden et al., 2020).
Figure 1.
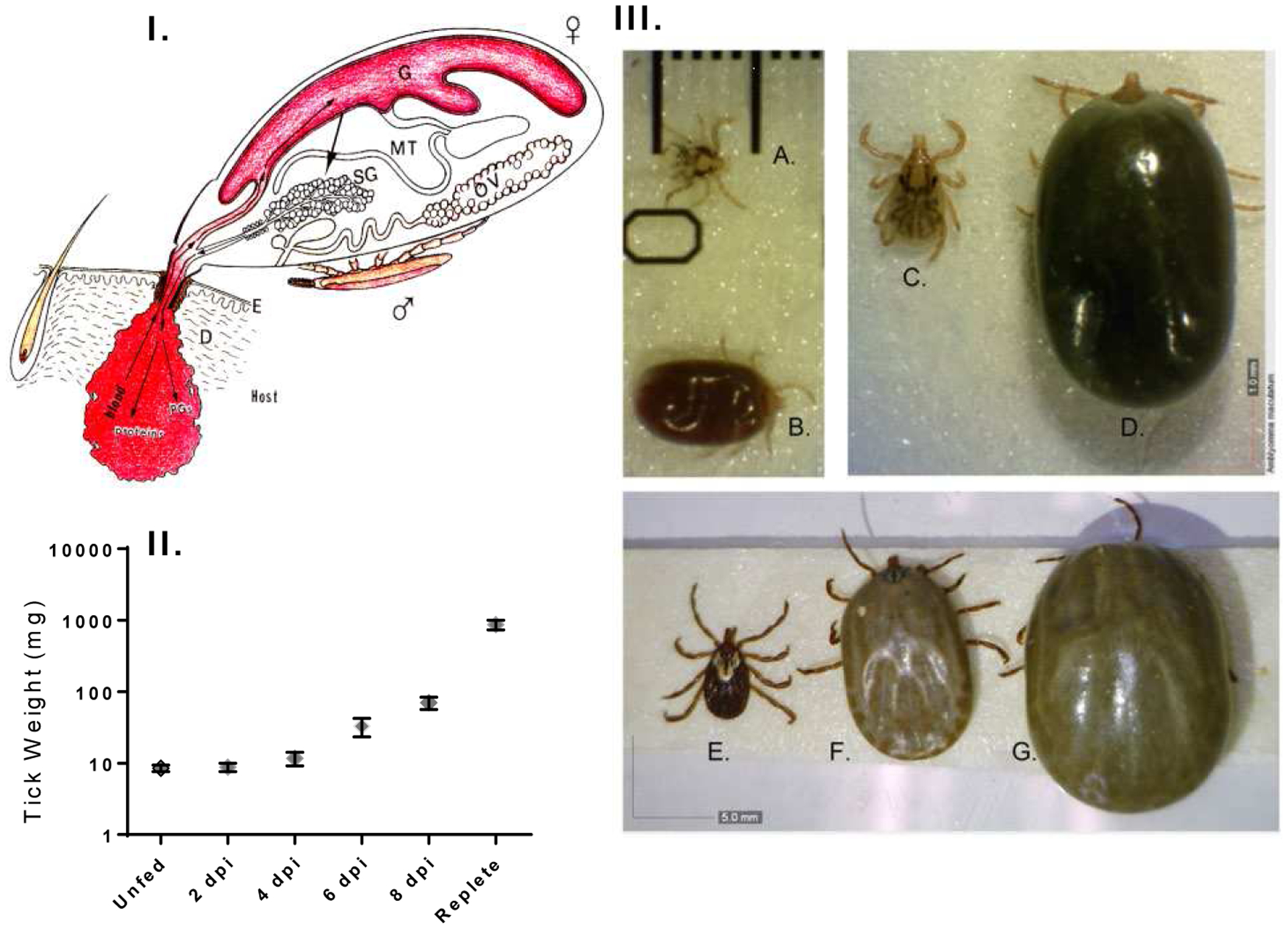
The tick feeding process involves multiple host-response inhibitors secreted from the tick salivary glands, and bacteria at the tick–host interface take advantage during transmission to enter susceptible hosts. A representation of tick feeding and tick mating on the host (I), depicting the preparation of the blood pool inside the host skin dermis, which is rapidly engulfed later. The tick weight gain by an Amblyomma maculatum female (II) represents two distinct stages of feeding: slow, until day six, and rapid, after 8–12 days (10 female ticks were weighed at each stage). Larval (A, B) and nymphal (C, D) ticks at the unfed and fully engorged stages and adult female ticks (E, F, G) at the unfed, slow-feeding, and engorged stages. Scale: 1 mm (A, B and C, D); 5 mm (E, F, G).
In hard ticks, the blood-feeding process occurs once in each post-embryonic life stage, whereas soft ticks feed multiple times on the host. The tick feeding on the host can be divided into the stages of attachment, slow feeding, fast feeding (24–48 h before detachment), repletion, and disengagement from the host (Fig. 1). Successful tick feeding requires a repertoire of pharmacologically active proteins and compounds to evade host hemostasis, inducing blood coagulation, platelet aggregation, and vasoconstriction, which is the reason for investigating tick factors for vaccine development (Francischetti et al., 2009; Karasuyama et al., 2020). An updated inventory of the secreted proteins in tick saliva (sialome/secretome) suggested complex antigenic variation and a sialome switch at different time points in the host for the successful feeding (Karim et al., 2021, 2011; Karim and Ribeiro, 2015; Ribeiro and Mans, 2020).
Tick saliva is instrumental in the biological success of tick vectors, including blood-feeding success, and the secretion of saliva proteins is regulated by a conserved exocytotic machinery composed of soluble N-ethyl sensitive factor attachment protein receptors (SNAREs, Karim et al., 2002). These SNARE genes were silenced using RNA interference to demonstrate their role in saliva secretion and tick feeding (Browning and Karim, 2013; Villarreal et al., 2013). The study of tick exosomes, which are sources of potent host-response inhibitors, provided new therapeutic components that potentially play a role in wound healing (Zhou et al., 2020). Modulation of the tick secretome at the tick–host interface helps infectious agent transmission to the host (Kazimírová and Štibrániová, 2013; Kotál et al., 2015; Wikel, 2018b). The exosomes responsible for saliva secretion have received more attention lately, although more study is needed for the characterization of exosomes in preparation of the tick feeding site, maintenance of the blood pool for a prolonged period, successful healing of the wound site, and detachment from the host. Exosomes and their accumulated “secretome” cargo determine the integrity of the tick–host interface in such a way that host responses are evaded and tick pathogens traffic into the host via saliva.
3. Tick-borne rickettsiosis
Rickettsial diseases are caused by infection with obligate intracellular Gram-negative alphaproteobacteria transmitted by arthropod vectors and may affect an estimated one billion people worldwide (Parola et al., 2013). Tick-borne rickettsial diseases are caused by two groups of intracellular bacteria belonging to the order Rickettsiales and including (a) bacteria belonging to spotted fever group (SFG) of the genus Rickettsia within the family Rickettsiaceae and (b) bacteria within the family Anaplasmataceae, including several genera, such as Anaplasma and Ehrlichia (Dumler et al., 2001). Traditionally, rickettsial agents have been divided into three groups based on immunological cross-reactivity and vector species in the SFG. Most of these are tick-associated, although the typhus group is associated with body lice (R. prowazekii) or fleas (R. typhi), as is the scrub typhus group, Orientia tsutsugamushi (Renvoise et al., 2009). There were few known rickettsial infections before 1984, and from 1984–2004 many rickettsial pathogens were identified with the utilization of cell culture and molecular techniques (Tomassone et al., 2018). The discovery of increased numbers of rickettsial agents removed the old concept that only one tick-borne rickettsiosis is prevalent within a geographical area. These rickettsial agents include bacteria for which no species have been identified, and typical rickettsiosis has been found to be caused by additional rickettsial species (Renvoise et al., 2009). Further genomic studies revealed the genomic reduction of Rickettsia due to a highly selective intracellular lifestyle (Diop et al., 2018). Several Rickettsia species have been discovered, and many are recently known to be a causative agent of human disease.
There are several important rickettsial pathogens and diseases prevalent in North America, such as R. parkeri (maculatum disease; Paddock et al., 2008), R. rickettsii (Rocky Mountain spotted fever; Ricketts, 1991; Sahni et al., 2019), SPECIES (rickettsia pox; Reeves et al., 2007), Candidatus R. philippi (eschar-associated illness; Shapiro et al., 2010), R. prowazekii (a zoonosis spread to humans from infected flying squirrels in the United States; Chapman et al., 2009, p. 201), R. typhi (typhus; Blanton and Walker, 2017), and R. africae (associated with traveling in sub-Saharan Africa; Raoult et al., 2001). Most of the rickettsial diseases are grouped by severity of illness and contain overlapping clinical manifestations. Several Rickettsia pathogens, such as R. rickettsii, R. prowazekii, R. conorii, and R. typhi, can cause life-threatening diseases. The fatality rate of Rocky Mountain spotted fever prior to the age of antibiotics was 20–25% but has now been reduced to 3–4% (Dumler and Walker, 2005; Walker, 1989). Due to several virulent strains of R. rickettsii in Latin America, the current fatality rate is as high as 30–40% (Sahni et al., 2019). During the first few days of human rickettsioses, the symptoms progress from chills, fever, headaches, myalgia, nausea, and vomiting to the appearance of rashes on the body within 3–5 days, which can gradually progress to a severe condition and cause respiratory failure, kidney injury, hypotensive shock, hemorrhagic lesions, jaundice, and even coma. But rickettsioses due to R. parkeri and R. africae are non-life-threatening, may develop epidermal and dermal necrosis (eschar) at the tick feeding site, and may cause headache, fever, myalgias, and draining lymphadenopathy (Paddock et al., 2008; Raoult et al., 2001). Another tick pathogen, R. amblyommatis, is carried by almost 50% of Amblyomma americanum ticks, which are known to be the most prevalent human biting ticks in the southeastern and south-central regions of the USA, are spreading steadily north, and have caused subclinical infections in several patients, who asymptomatically developed anti-SFG antibodies (Sahni et al., 2019; Walker and Ismail, 2008).
Several cases of R. parkeri infection have been found within the USA (Paddock and Goddard, 2015). It is likely that some of the 13,500 uncharacterized cases of SFG rickettsioses that were reported in the United States during the period 2008–2012 were caused by R. parkeri (Drexler et al., 2016). In other countries, such as Uruguay, Argentina, and parts of Brazil, R. parkeri is known as the most important pathogen for spotted fever rickettsiosis (Nava et al., 2008; Saito et al., 2019; Venzal et al., 2005; Weck et al., 2017). The most common zoonotic bacteria reported in Africa are the SFG rickettsiae, mainly represented by R. africae, R. aeschlimannii, R. conorii, and R. massiliae (Macaluso et al., 2003; Parola et al., 2005). Point-of-care diagnostic tools and molecular surveillance studies of tick vectors should help in detection of new and emerging tick-borne rickettsiae.
4. Pathogenic and endosymbiotic rickettsiae
It was suggested in a recent rickettsiae review that each member of the SFG should be considered a potential pathogen (Parola et al., 2013). The ability of endosymbiotic rickettsiae to invade tick host cells has been lost during evolution, and this characteristic differentiates them from pathogenic rickettsiae. R. peacockii, an endosymbiont rickettsia in the Dermacentor andersoni tick, has lost the ability to enter hemocytes and salivary gland tissues, which establishes its endosymbiotic nature and prevents its infecting vertebrates (Baldridge et al., 2004; Novakova and Smajs, 2018). The line between pathogenic bacteria and endosymbionts is not well defined, as there are several virulent strains of pathogenic rickettsiae, such as the R. rickettsii strain, which lives inside ticks and can be transmitted transovarially (Ellison et al., 2008). The pathogenic and endosymbiotic nature of rickettsiae may have evolved through different scenarios. First, a loss of pathogenicity, for example, by R. peacockii, a strictly endosymbiotic Rickettsia that is closely related to the severely pathogenic R. rickettsii. Studies have shown various deletions and mutations in the genome of R. peacockii by transposon recombination that eliminated its pathogenic ability (Felsheim et al., 2009; Gillespie et al., 2012). Rickettsia buchneri (an endosymbiont) and Rickettsia monacensis (a pathogen) also define a similar situation (Kurtti et al., 2016). Second, gain of pathogenicity, since the repeated occurrences of horizontal transfer in rickettsia may have led to novel bacterial phenotypes, as in Coxiella burnetii, which infects vertebrate cells, causes Q fever, and originated from Coxiella-like endosymbionts (CLEs; Duron et al., 2017).
5. Animal models and rickettsiosis
Rickettsial infection begins with inoculation of the host skin by a tick bite. Initially, the target cells of infection are macrophages and/or dendritic cells; next, the rickettsiae spread into the regional lymph nodes via lymphatic vessels, as observed in the case of R. sibirica mongolitimonae infection, which cause lymphangitis (Fournier et al., 2005). Rickettsiae bacteria then spread throughout the body hematogenously, mainly infecting endothelial cells and, to a lesser extent, macrophages, skin, the gastrointestinal tract, lungs, kidneys, heart, brain, liver, and other organs. As a result of rickettsial infection of the endothelium, several cell-signaling cascades are activated to secrete several host innate immune signaling molecules, including cytokines and chemokines.
The increasing number of cases of these SFG rickettsia (SFGR) infections brings urgent attention to understanding the mechanism of disease development and immune responses to SFGR infections. Several animal models have been developed, but all have ultimately been found incomplete due to drawbacks in the potential approaches related to the host immune system or a lack of tick transmission and pathogenesis of these diseases. Tick-transmission mouse models (C3H/HeN) for R. parkeri were established and provided evidence that tick transmission significantly increases the bacterial load inside mouse organs compared with intravenous injection (Saito et al., 2019). Furthermore, a rat model was successfully used for A. maculatum for an R. parkeri transmission study, and traditionally used guinea pigs have been similarly proposed to be useful models for R. parkeri tick-transmission studies (Stokes et al., 2020; Suwanbongkot et al., 2019). The consensus tick transmission model for R. parkeri will depend on the ease of infesting ticks, a sufficient pathogen load in animal organs, and accurate representation of human disease. The standard animal transmission model of spotted fever rickettsiosis is needed to perform many tick-transmission-blocking experiments. The Macaluso laboratory further studied the tick transmission of R. parkeri in primates, representing one more step forward, and demonstrated that the tick bite plays a critical role in infection (Banajee et al., 2015). Of all animal models, tick transmission in primates best represents the natural mode of pathogen infection in humans and was significantly better than the injection method.
6. Vector biology of rickettsial diseases
The ability of ticks to harbor and be colonized by infectious agents in their gut tissues during a blood meal, on first infection or as a reservoir of infection, is determined by tick–pathogen interactions. The various pathogens are vectored by different tick species, which is the outcome of these interactions. The vector biology of rickettsial pathogens is not well studied or understood. The human body louse, a vector for R. prowazekii, is known to be an unsuccessful host, as 100% of the infected lice are killed by the rickettsiae (Sahni et al., 2019). The most pathogenic rickettsia, R. rickettsia, is found in <0.1% of its vector tick, Dermacentor variabilis (in the United States), while less pathogenic rickettsia, such as R. africae and R. amblyommatis, have high colonization rates in their respective tick vectors.
Several studies on the salivary secretions of Ixodes scapularis, which transmits several diseases, such as anaplasmosis, Lyme borreliosis, babesiosis, Powassan virus encephalitis, and Ehrlichia muris eauclairensis, have revealed salivary anticoagulants that maintain blood flow during feeding, immunomodulators to suppress the host immune system, and pain suppressors for the tick to go unnoticed by the host (Sahni et al., 2019). There are very few studies of the salivary secretions of ticks vectoring rickettsioses, but similar phenomena most likely take place, as mentioned above, in the case of the well-studied Ixodes scapularis tick (Sahni et al., 2019). There is a gap in knowledge of the vector biology of Dermacentor, Amblyomma, and Rhipicephalus ticks, including several significant phenomena, such as reactivation of rickettsial virulence in unfed ticks that do not cause disease but are reactivated to virulence during tick feeding.
7. Tick–pathogen interactions
It is well established that during blood feeding, the tick bite makes a feeding lesion and suppresses host hemostatic, immune, and inflammatory responses for successful feeding, while pathogens manipulate tick and host molecular processes to facilitate successful infection, multiplication, and transmission (Fig. 1). Vector competence is defined as the ability to acquire, maintain, and transmit pathogens; it is a multifactorial process that involves multiple genes and multiple gene networks in multiple organs. Vector colonization is defined as the acquisition, survival, multiplication, and trafficking of the pathogen in a tick vector. Understanding the functional consequence of pathogen colonization within the tick vector and transmission to the host are fundamental to the development of new paradigms based on the targeting of tick proteins to control ticks and tick-transmitted pathogens. Simultaneously, both the tick vector and the mammalian host react against tick infestation and pathogen infection by activating different mechanisms. Intracellular pathogenic bacteria facilitate infection, multiplication, and transmission by suppressing the host response. To suppress the host response, pathogenic bacteria have developed several molecular mechanisms, including manipulation of the immune response, inhibition of cell apoptosis, remodeling of the cytoskeleton, and control of host cell epigenetics (de la Fuente et al., 2016). Genomic reprogramming of ticks with infection or the ability of the pathogen to evade the tick’s potent innate immunity by differentially expressing their genome still results in infected ticks. In the case of Rickettsia rickettsii, the prevalence of human pathogens in ticks is always very low, which suggests that ticks might be continually clearing these infections (Niebylski et al., 1999). However, the bacterium itself modulates its genome depending on the environment, whether in the tick or in animals, as in the case of Ehrlichia (Kuriakose et al., 2011). While in ticks or mammals, the intracellular bacterium Ehrlichia differentially regulates its outer membrane proteins, especially Omp1B or P30–10, which are expressed at significantly higher levels in ticks than in mammalian hosts (Felek et al., 2003; Unver et al., 2002). The entry-triggering protein (EtpE) of Ehrlichia, known as the key to entry into host cells, is highly expressed in infected ticks and inhibits the host cellular redox response from NADPH oxidase (Budachetri et al., 2020; Teymournejad et al., 2017). The Lyme disease agent Borrelia expresses OspA and OspB during tick colonization, whereas spirochetes express OspC during mouse infection (Tilly et al., 2016). In the case of Rickettsia risticii, the genes upregulated by temperature are different than those upregulated by a blood meal (Galletti et al., 2016).
Rickettsia generally invades host cells by binding their outer-surface cell antigens (sca0, also known as rompA, and sca5, also known as rOmpB) to the outer surface of cellular receptors and is then internalized by receptor-mediated endocytosis via clathrin-coated vesicles (Chan et al., 2009). A similar sca5-mediated invasion mechanism used by rickettsiae against vertebrates is also used to invade tick cells (Thepparit et al., 2010). Upon invasion, rickettsiae lyse these inclusions and escape into the cytosol, where they replicate and hijack the host cell actin cytoskeleton and attach themselves to the actin tails (Sonenshine and Macaluso, 2017). The actin protein complex Arp2/3 is essential for the internalization of several known SFG rickettsiae (Petchampai et al., 2014; Sonenshine and Macaluso, 2017). Rickettsia express RickA in vertebrate host cells, which in turn promotes activation of the host cell actin complex and enables these bacteria to be propelled throughout host cells and into cell protrusions, which mediate cell-to-cell infection, and that is how infection can be spread throughout the surrounding tissues (Jeng et al., 2004; Kumar et al., 2004; Sonenshine and Macaluso, 2017). Actin bridges are required for Rickettsia rickettsii infection, while R. parkeri and other SFGRs are spread by manipulating the intercellular tension and mechano-transduction between host cells (Lamason et al., 2016).
In tick salivary glands, A. phagocytophilum facilitates its own infection by inhibiting the intrinsic apoptosis pathway (through porin downregulation), while tick cells promote tick survival by activating the extrinsic apoptosis pathway to limit A. phagocytophilum infection (de la Fuente et al., 2016; Narasimhan and Fikrig, 2015; Neelakanta et al., 2010; Busby et al., 2012; Hajdušek et al., 2013; Gulia-Nuss et al., 2016; Sonenshine and Macaluso, 2017; Hajdušek et al., 2013). Studies of field-collected A. maculatum composition showed the presence of two bacterial symbionts, Francisella-like endosymbiont (FLE) and Candidatus Midichloria mitochondrii endosymbiont (CMM). Several genetically diverse FLEs were reported in Dermacentor variabilis and D. andersoni (Dergousoff and Chilton, 2012; Liu et al., 2016). Francisella is a Gram-negative coccobacilli, a gamma proteobacterium widely recognized because of Francisella tularensis, causing a fatal disease known to infect more than 100 mammalian species (Gerhart et al., 2016). It was hypothesized that pathogenic Francisella tularensis is transformed into symbiotic FLE in ticks (Gerhart et al., 2016). In most microbiome studies, FLE are prevalent in ticks and perhaps replace the pathogenic R. parkeri and favor Candidatus R. andeanae, another rickettsial symbiont within the tick vector (Paddock et al., 2015). In ticks, a new symbiont, Candidatus Midichloria mitochondrii (CMM), with a unique localization to tick cell mitochondria or cytoplasm, was discovered previously (Sassera et al., 2006). The authors of phylogenetic and statistical studies of 16S rRNA sequences of “Midichloria and like organisms” proposed a novel family “Candidatus Midichloriaceae” within the order Rickettsiales (Montagna et al., 2013). In our study, we observed the mutualistic relationship between A. maculatum-transmitted R. parkeri with CMM (Budachetri et al., 2018a). Recently ten different genera of maternally inherited bacteria have been described in ticks (Noda et al., 1997; Nováková and Šmajs, 2018; Perlman et al., 2006; Zhong et al., 2007, p. 200). Among them, the most prevalent bacterial genera found in ticks are Coxiella-LE (60.5%) and Rickettsia (55.6%), which have also been identified in more tick species than in any other genera (Duron et al., 2017). Rickettsia was also found to aggregate specifically (nonrandomly) with Midichloria. This type of endosymbiotic association suggests a need to synthesize all the components of certain essential pathways, such as vitamin B, for tick fitness (Duron et al., 2017). Collaboration of FLE and Rickettsia is a more efficient alternative for synthesizing B7 and B9 vitamins than relying on CLE (Duron et al., 2017; Hunter et al., 2015).
Transovarial transmission of more than one rickettsial species from the SFG have not been proven, but the coexistence of R. bellii with SFG rickettsiae has already been described (Blanc et al., 2007), and the presence of R. bellii in D. andersoni ticks prevents the infection of Anaplasma marginale (Gall et al., 2016). Symbiosis with vitamin-provisioning rickettsiae is essential and warrants in-depth studies on the nutrition and reproductive fitness of tick species.
8. Tick–rickettsia interactions: redox reactions and selenoproteins
Upon tick attachment, the vertebrate host’s immune system activates phagocytes, such as neutrophils, monocytes, macrophages, and eosinophils, to prevent invasion of foreign microorganisms by producing ample amounts of superoxide ions—one of the components responsible for high oxidative stress in ticks. Ticks have a significantly elevated level of anti-oxidant capacity, suggested by their tolerance for oxidizing agents, such as 20 mM paraquat and up to 7% H2O2, which is significantly higher than in animal/human cells (Kumar et al., 2016). The ability of ticks to offset starvation and blood-feeding-related stress suggests that they have a proactive antioxidant system. Tick saliva promotes feeding and pathogen transmission by modulating the host immune and inflammatory responses. Tick saliva composition, as revealed by our sialotranscriptome (from the Greek “sialo” for saliva), indicates the presence of over 5,000 putative secreted peptides containing representatives of dozens of protein families (Karim et al., 2011; Karim and Ribeiro 2015; Karim et al., 2021).
In A. maculatum, superoxide dismutase (SOD) and catalase, together with a battery of selenogenes, quench radicals or break down peroxides during blood feeding and pathogen colonization (Table 1). The tick is a unique model with which to study redox biology, as the feeding of ticks on blood generates toxic levels of reactive oxygen species (ROS) that could damage lipids, proteins, and DNA, thus promoting mutation, cellular dysfunction, and cell death. To successfully feed and survive, ticks must somehow prevent these detrimental effects and promote the beneficial aspects of ROS, which suggests that there are precise regulatory strategies for maintaining appropriate ROS levels, both within the tick and possibly at the tick–host interface. Our studies have shown an adaptive coevolutionary process that has enabled tick-borne pathogen (TBP) survival by manipulating an antioxidant defense system associated with selenium (Se), including a full set of selenoproteins and other antioxidants (Karim et al., 2011; Karim and Ribeiro 2015; Adamson et al., 2013; Budachetri et al., 2017, 2017a, 2018; Budachetri and Karim 2015; Crispell et al., 2016; Adamson et al., 2014; Kumar et al., 2019). The generation of ROS is among the first lines of host defense against invading microbes (Hoffman 2003; Ha et al., 2005). Selenoproteins exhibit diverse biological functions, such as detoxification of peroxides, regeneration of reduced thioredoxin, and reduction of oxidized methionine residues by oxidation of the selenium (Se−) active site (Grommer et al, 2005; Reeves and Hoffman 2009). In this review, we focused on unique antioxidant genes playing significant roles in oxidative stress management in ticks with blood meal and R. parkeri infection.
Table 1:
How selenoproteins respond during hematophagy and pathogen infection. All functional roles of selenoproteins were studied in Amblyomma maculatum, except for SelK, which was studied in both A. maculatum/Rp and I.sca/Bb. MG, midgut; SG, salivary glands; dpa, days post attachment.
| Tick antioxidant | GenBank Acc # | Gene expression (Rickettsiaparkeri infection) | Gene expression (blood meal) | Impact of knockdown (kd) | References |
|---|---|---|---|---|---|
| SOD1 (Cu/Zn SOD) | JO844140 | Increased in both MG and SG. | MG: gradually reduced with dpa. SG: increased initially and later remained similar to that of unfed. |
Rp significantly reduced in MG and SG. | (Crispell et al., 2016) |
| SOD3 (MnSOD) | JO843979 | No impact | MG: remained similar with dpa. SG: increased before fast feeding and then decreased to level similar to unfed. |
N/A | (Crispell et al., 2016) |
| Cat | JO843741 | Increased in SG but not in MG. | MG: gradually reduced with dpa. SG: remained constant. |
Reduced in load in MG, SG, and eggs. | (Budachetri et al., 2017; Kumar et al., 2016) |
| GST | Increased only in SG. | (Budachetri et al., 2018a) | |||
| SEF | KC989559 | Increased | MG: gradually reduced SG: remained constant. |
MG: pathogen load reduced. SG: increased. |
(Adamson et al., 2013) |
| SBP2 | MF115980 | Increased in both tissues. | Reduced and then restored in gut, while gradually reduced in SG with dpa. | Reduced transovarial transmission. No impact on MG or SG. |
(Budachetri et al., 2017) |
| SEPHS2 | Increased in tissues. | Increased strongly in gut tissues, while gradually reduced in SG with dpa. | N/A | (Budachetri et al., 2017) | |
| SelK* | JO843326 | Increased in Rp- and B. burgdorferi-infected MG and SG. | Increased with blood meal in both tissues. | Reduced load of Bb. | (Adamson et al., 2014; Kumar et al., 2019) |
| TrxR | JO843723 | Induced in SG only. | Expression remained constant in SG, while MG reduced before fast-feeding step. | Total bacterial load reduced in MG and SG. Not done with rp. | (Budachetri & Karim, 2015) |
| SelP | MF115978 | Increased in tissues. | Increased strongly in gut tissues, and gradually reduced in SG. | No effect in tick gut or salivary gland tissue Rp load. | (Budachetri et al., 2017) |
| SelM | Increased in SG and OV. No impact on MG. | Increased in both tissues. | Reduced only in SG. | (Adamson et al., 2014) | |
| SelO | KC989561 | Increased in MG and SG. | Remained similar in MG, gradually reduced in SG. | Reduced load in MG and SG. | ((Budachetri et al., 2018a) |
| SelS | JO842687 | Increased in MG and SG. | Constant in MG, while spiked with blood in SG. | Reduced load in SG only. | (Budachetri et al., 2018a) |
| SelT | KC989562 | No change | (Budachetri et al., 2018a) | ||
| SelX | JO845128 | Induced only in gut. | (Budachetri et al., 2018a) |
Selenoproteins are selenium-containing proteins known for redox function and centered on the selenium atom in selenocysteine (U), encoded by UGA (Opal codon) (Allmang and Krol, 2006). The alternate coding of UGA to selenocysteine (known as the 21st amino acid) occurs in all three domains of life, with a mechanism involving specific synthesis machinery, including Sec-tRNAsec, the SECIS element in the 3’-UTR of selenoprotein mRNAs; SECIS-binding protein; selenocysteine-specific elongation factors; and selenophosphate synthetase, supplying selenium (Allmang and Krol, 2006). There are 25 known human selenoproteins, and the best studied are glutathione peroxidases, thioredoxin reductases, and iodothyronine deiodinases (Labunskyy et al., 2014). Selenoproteins are known for their antioxidant properties, and selenium plays a central role in redox reactions. Nature has chosen selenium over sulfur, because of Se’s rate and redox advantages. Se is a better nucleophile and reacts with greater ease to ROS, and the Se–O bond is readily reduced and resists permanent oxidation (Reich and Hondal, 2016). The silencing of selenocysteine elongation factor (eEFsec) in R. parkeri-infected Am. maculatum salivary glands suggested epigenetic control of tick gene expression (Adamson et al., 2013) and has provided further support for the idea that tick-borne pathogens modulate the system to promote their survival and transmission to the vertebrate host. However, the question of how these selenoproteins contribute to the survival of R. parkeri within the tick vector has yet to be answered. We utilized a reverse genetic approach (RNAi) to silence tick genes and estimate the silencing impact on the level of R. parkeri colonization inside tick organs. In Table 1 we summarize the work related to selenoproteins and their roles in pathogen infection within the tick vector. Most of the work was focused on A. maculatum and R. parkeri, although we started expanding our work to Ixodes scapularis and Borrelia burgdorferi interactions as well. We utilized an RNAi approach to specifically silence tick selenogenes and certain non-selenogenes (SOD and catalase) and observed the impact on tick blood feeding, fecundity, and bacterial load (for total bacteria, R. parkeri, or symbionts) in each experiment (Table 1).
Homoeostasis between ROS generation and antioxidants is of vital significance to the survival of the pathogen within the arthropod. A study in Drosophila revealed that the ingestion of a bacterial pathogen within a catalase-knockout fly led to the death of the fly because of uncontrolled oxidative stress (Ha et al., 2005). The role of SOD in R. parkeri colonization within A. maculatum strongly indicates the impact of antioxidants on tick–pathogen interactions (Crispell et al., 2016). The silencing of SOD (Cu/Zn-SOD and Mn-SOD) reduces the R. parkeri load, with an elevated level of oxidative stress due to less complete quenching of hydroxide radicals produced with infection or by the natural KREBs cycle in mitochondria (Crispell et al., 2016). Catalase, which significantly reduces hydrogen peroxides or other organic peroxides, is a significant component of the tick antioxidant system and, upon silencing, not only interferes with reduced rickettsial load but negatively affects tick fecundity and transovarial transmission of R. parkeri (Budachetri, 2017; Kumar et al., 2016).
Recent work has demonstrated the role of antioxidants, including selenoproteins, in R. parkeri colonization in tick tissues (Budachetri et al., 2018a). The functional role of selenoproteins in tick hematophagy and vector competence is in larval or nymph ticks, but the available literature supports their importance in tick homeostasis, obligate hematophagy, and vector competence and for protecting the tick microbiome from high oxidative stress (Adamson et al., 2014, 2013; Budachetri et al., 2018b; Budachetri and Karim, 2015; Kumar et al., 2016). While information regarding the impact of antioxidants on vector–pathogen interactions is limited, several studies have suggested the role of antioxidants as redox switches in pathogen colonization (Crispell et al., 2016; Stolf et al., 2011; Walczak et al., 2012). Apart from redox reactions, two selenoproteins, SelO and SelS, are especially known for endoplasmic reticulum (ER) stress mitigation, which is required for the successful dissemination of R. parkeri, and this bacterium mitigates ER stress by manipulating expression of the selenogenes selS and selM (Adamson et al., 2013). However, its functional role is unknown. Recent studies involving the tick’s selenocysteine elongation factor (eEFSec) demonstrate the involvement of selenoproteins in gene regulation as well as their putative role in tick vectorial competence (Adamson et al., 2013).
9. Conclusions and future research
The Amblyomma maculatum and Rickettsia parkeri model is a unique system with which to study tick–pathogen–host interactions. The acquisition, infection, survival, and proliferation of R. parkeri inside tick organs (such as midgut, hemocytes, salivary glands, and ovarian tissues), including the ability to transmit to eggs, needs to be explored in the context of vector competence. The reduced genome and intracellular lifestyle of R. parkeri make it an appropriate bacterium with which to study the innate cellular immune response and how it avoids phagolysosome digestion. A survey of field-collected ticks showed a 20–30% R. parkeri infection rate, which suggests an active role for the tick’s innate immune system defense. The rickettsia inside tick organs modulates the tick genome in such a way that the bacterium avoids elevated ROS by manipulating the tick’s robust antioxidant machinery, which is comprised of a battery of selenoproteins and various other non-selenoproteins (SOD, catalase, glutathione reductase).
The microbiome composition of ticks is comprised of pathogenic and non-pathogenic microbes, which interact within the tick vector synergistically, like CMM and R. parkeri, or competitively, like CMM and FLE. In-depth insight into the endosymbiont and its interaction with pathogenic microbes is needed to decipher the molecular mechanism existing during the colonization of the tick vector by pathogenic microbes. The blood-feeding success of Ixodid ticks on the host skin for several days offers pathogenic bacteria inside the tick sufficient time to transmit to the host. Interestingly, most infectious agents, such as the Lyme disease-causing bacterium (Borrelia sp.), Ehrlichia, or Anaplasma, are transmitted within 2–3 days of the arrival of ticks on the host. The early tick feeding behavior is important, and transmission-blocking strategies should focus on highly upregulated proteins, protein families, or pathways during early time points.
Funding:
National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103476; USDA NIFA (2017-67016-26864 & 2017-67017-26171); Pakistan-U.S. Science and Technology cooperation Program (Phase 7) (2000008306).
Footnotes
Data Availability Statement:
Data availability is not applicable to this article as no new data generated or analyzed in this study
Conflict of Interest Statement:
The authors explicitly declare that they have no conflict of interest to disclose.
Bibliography
- Adams D, Fullerton K, Jajosky R, Sharp P, Onweh D, Schley A, Anderson W, Faulkner A, Kugeler K. (2015). Summary of notifiable infectious diseases and conditions - United States, 2013. M.M.W.R. Morb. Mortal. Wkly. Rep 62: 1–122. [DOI] [PubMed] [Google Scholar]
- Adamson S, Browning R, Singh P, Nobles S, Villarreal A, Karim S, 2014. Transcriptional activation of antioxidants may compensate for selenoprotein deficiencies in Amblyomma maculatum (Acari: Ixodidae) injected with selK- or selM-dsRNA. Insect Mol. Biol 23, 497–510. 10.1111/imb.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson SW, Browning RE, Budachetri K, Ribeiro JMC, Karim S, 2013. Knockdown of Selenocysteine-Specific Elongation Factor in Amblyomma maculatum Alters the Pathogen Burden of Rickettsia parkeri with Epigenetic Control by the Sin3 Histone Deacetylase Corepressor Complex. PloS One 8, e82012. 10.1371/journal.pone.0082012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C, Krol A, 2006. Selenoprotein synthesis: U.G.A. does not end the story. Biochimie 88, 1561–1571. 10.1016/j.biochi.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Baldridge GD, Burkhardt NY, Simser JA, Kurtti TJ, Munderloh UG, 2004. Sequence and expression analysis of the ompA gene of Rickettsia peacockii, an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni. Appl Env. Microbiol 70, 6628–6636. https://doi.org/70/11/6628 [pii] 10.1128/AEM.70.11.6628-6636.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banajee KH, Embers ME, Langohr IM, Doyle LA, Hasenkampf NR, Macaluso KR, 2015. Amblyomma maculatum Feeding Augments Rickettsia parkeri Infection in a Rhesus Macaque Model: A Pilot Study. PloS One 10, e0135175. 10.1371/journal.pone.0135175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Folk SM, Kato CY, Lash RR, Levin ML, Massung RF, Nadelman RB, Nicholson WL, Paddock CD, Pritt BS, Traeger MS, 2016. Diagnosis and Management of Tick-borne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis — United States. M.M.W.R. Recomm. Rep 65, 1–44. 10.15585/mmwr.rr6502a1 [DOI] [PubMed] [Google Scholar]
- Blanc G, Ogata H, Robert C, Audic S, Claverie J-M, Raoult D, 2007. Lateral gene transfer between obligate intracellular bacteria: Evidence from the Rickettsia massiliae genome. Genome Res. 17, 1657–1664. 10.1101/gr.6742107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton LS, Walker DH, 2017. Flea-Borne Rickettsioses and Rickettsiae. Am. J. Trop. Med. Hyg 96, 53–56. 10.4269/ajtmh.16-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning R, Karim S, 2013. R.N.A. interference-mediated depletion of N-ethylmaleimide Sensitive Fusion Protein and Synaptosomal Associated Protein of 25 kDa results in the inhibition of blood feeding of the Gulf Coast tick, Amblyomma maculatum. Insect Mol Biol. 10.1111/imb.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, 2017. Study of Rickettsia parkeri Colonization and Proliferation in the Tick Host Amblyomma maculatum (Acari: Ixodidae). Dissertations. [Google Scholar]
- Budachetri K, Browning RE, Adamson SW, Dowd SE, Chao C, Ching W, Karim S, 2014. An insight into the microbiome of the Amblyomma maculatum (Acari: Ixodidae). J. Med. Entomol 51. 10.1603/ME12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri Khemraj, Crispell G, Karim S, 2017. Amblyomma maculatum S.E.C.I.S. binding protein 2 and putative selenoprotein P are indispensable for pathogen replication and tick fecundity. Insect Biochem. Mol. Biol 88, 37–47. 10.1016/j.ibmb.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Karim S, 2015. An insight into the functional role of thioredoxin reductase, a selenoprotein, in maintaining normal native microbiota in the Gulf Coast tick (Amblyomma maculatum). Insect Mol. Biol 24, 570–581. 10.1111/imb.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Kumar D, Crispell G, Beck C, Dasch G, Karim S, 2018a. The tick endosymbiont Candidatus Midichloria mitochondrii and selenoproteins are essential for the growth of Rickettsia parkeri in the Gulf Coast tick vector. Microbiome 6, 141. 10.1186/s40168-018-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Williams J, Mukherjee N, Sellers M, Moore F, Karim S. (2017). The microbiome of neotropical ticks parasitizing on passerine migratory birds. Ticks and Tick-borne Diseases 8(1): 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Kumar D, Karim S, 2017. Catalase is a determinant of the colonization and transovarial transmission of Rickettsia parkeri in the Gulf Coast tick Amblyomma maculatum. Insect Mol. Biol 26. 10.1111/IMB.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Teymournejad O, Lin M, Yan Q, Mestres-Villanueva M, Brock GN, Rikihisa Y, 2020. An Entry-Triggering Protein of Ehrlichia Is a New Vaccine Candidate against Tick-Borne Human Monocytic Ehrlichiosis. mBio 11. 10.1128/mBio.00895-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby AT, Ayllón N, Kocan KM, Blouin EF, de la Fuente G, Galindo RC, Villar M, de la Fuente J, 2012. Expression of heat shock proteins and subolesin affects stress responses, Anaplasma phagocytophilum infection and questing behaviour in the tick, Ixodes scapularis. Med. Vet. Entomol 26, 92–102. 10.1111/j.1365-2915.2011.00973.x [DOI] [PubMed] [Google Scholar]
- C.D.C., 2019. Tick-borne Diseases of the U.S.: A Reference for H.C. Providers | C.D.C. [W.W.W. Document]. Cent. Dis. Control Prev URL https://www.cdc.gov/ticks/tickbornediseases/index.html (accessed 12.24.20).
- Chan YGY, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ, 2009. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell. Microbiol 11, 629–644. 10.1111/j.1462-5822.2008.01279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AS, Swerdlow DL, Dato VM, Anderson AD, Moodie CE, Marriott C, Amman B, Hennessey M, Fox P, Green DB, Pegg E, Nicholson WL, Eremeeva ME, Dasch GA, 2009. Cluster of Sylvatic Epidemic Typhus Cases Associated with Flying Squirrels, 2004–2006. Emerg. Infect. Dis 15, 1005–1011. 10.3201/eid1507.081305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K, Klyachko O, Grindle N, Civitello D, Oleske D, Fuqua C, 2008. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol Ecol 17, 4371–4381. [DOI] [PubMed] [Google Scholar]
- Crispell G, Commins SP, Archer-Hartmann SA, Choudhary S, Dharmarajan G, Azadi P, Karim S. (2019). Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Frontiers in Immunology doi: 10.3389/fimmu.2019.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispell G, Budachetri K, Karim S, 2016. Rickettsia parkeri colonization in Amblyomma maculatum: The role of superoxide dismutases. Parasit. Vectors 9. 10.1186/s13071-016-1579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Waterhouse RM, Sonenshine DE, Roe RM, Ribeiro JM, Sattelle DB, Hill CA, 2016. Tick Genome Assembled: New Opportunities for Research on Tick-Host-Pathogen Interactions. Front. Cell. Infect. Microbiol 6, 103. 10.3389/fcimb.2016.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergousoff SJ, Chilton NB, 2012. Association of different genetic types of Francisella-like organisms with the rocky mountain wood tick (Dermacentor andersoni) and the American dog tick (Dermacentor variabilis) in localities near their northern distributional limits. Appl. Environ. Microbiol 78, 965–71. 10.1128/AEM.05762-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop A, Raoult D, Fournier P-E, 2018. Rickettsial genomics and the paradigm of genome reduction associated with increased virulence. Microbes Infect. 20, 401–409. 10.1016/j.micinf.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G, 2009. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 5, e1000423. 10.1371/journal.ppat.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler NA, Dahlgren FS, Heitman KN, Massung RF, Paddock CD, Behravesh CB, 2016. National Surveillance of Spotted Fever Group Rickettsioses in the United States,. Am. J. Trop. Med. Hyg 94, 26–34. 10.4269/ajtmh.15-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR, 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combi. Int J Syst Evol Microbiol 51, 2145–2165. 10.1099/00207713-51-6-2145 [DOI] [PubMed] [Google Scholar]
- Dumler JS, Walker DH, 2005. Rocky Mountain spotted fever--changing ecology and persisting virulence. N. Engl. J. Med 353, 551–553. 10.1056/NEJMp058138 [DOI] [PubMed] [Google Scholar]
- Duron O, Binetruy F, Noël V, Cremaschi J, McCoy KD, Arnathau C, Plantard O, Goolsby J, Pérez de León AA, Heylen DJA, Van Oosten AR, Gottlieb Y, Baneth G, Guglielmone AA, Estrada-Peña A, Opara MN, Zenner L, Vavre F, Chevillon C, 2017. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol 26, 2905–2921. 10.1111/mec.14094 [DOI] [PubMed] [Google Scholar]
- Ellison DW, Clark TR, Sturdevant DE, Virtaneva K, Porcella SF, Hackstadt T, 2008. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infect. Immun 76, 542–550. 10.1128/IAI.00952-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, de la Fuente J, 2014. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 108, 104–128. 10.1016/j.antiviral.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Estrada-Pena A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA, Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA, 2005. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsumNeumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol 60, 99–112. 10.1007/s11230-004-1382-9 [DOI] [PubMed] [Google Scholar]
- Felek S, Greene R, Rikihisa Y, 2003. Transcriptional analysis of p30 major outer membrane protein genes of Ehrlichia canis in naturally infected ticks and sequence analysis of p30–10 of E canis from diverse geographic regions. J. Clin. Microbiol 41, 886–888. 10.1128/jcm.41.2.886-888.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsheim RF, Kurtti TJ, Munderloh UG, 2009. Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent rickettsia rickettsii: identification of virulence factors. PloS One 4, e8361. 10.1371/journal.pone.0008361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Gouriet F, Brouqui P, Lucht F, Raoult D, 2005. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: seven new cases and review of the literature. Clin Infect Dis 40, 1435–1444. https://doi.org/CID35284 [pii] 10.1086/429625 [DOI] [PubMed] [Google Scholar]
- Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC, 2009. The role of saliva in tick feeding. Front. Biosci. Landmark Ed 14, 2051–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CA, Reif KE, Scoles GA, Mason KL, Mousel M, Noh SM, Brayton KA, 2016. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. I.S.M.E. J 10, 1846–1855. 10.1038/ismej.2015.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti MFBM, Fujita A, Rosa RD, Martins LA, Soares HS, Labruna MB, Daffre S, Fogaça AC, 2016. Virulence genes of Rickettsia rickettsii are differentially modulated by either temperature upshift or blood-feeding in tick midgut and salivary glands. Parasit. Vectors 9, 331. 10.1186/s13071-016-1581-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart JG, Moses AS, Raghavan R, 2016. A Francisella-like endosymbiont in the Gulf Coast tick evolved from a mammalian pathogen. Sci. Rep 6, 33670. 10.1038/srep33670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Joardar V, Williams KP, Driscoll T, Hostetler JB, Nordberg E, Shukla M, Walenz B, Hill CA, Nene VM, Azad AF, Sobral BW, Caler E, 2012. A Rickettsia genome overrun by mobile genetic elements provides insight into the acquisition of genes characteristic of an obligate intracellular lifestyle. J Bacteriol 194, 376–394. https://doi.org/JB.06244-11 [pii] 10.1128/JB.06244-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromer S, Eubel JK, Lee BL, Jacob J. 2005. Human selenoproteins at a glance. Cell Mol Life Sci 62(21):2414–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose SL, Brathwaite WS, Hall PA, Connor FJ, Sharp P, Anderson WJ, Fagan RF, Aponte JJ, Jones GF, Nitschke DA., et al. (2004). Summary of notifiable diseases–United States, 2002. M.M.W.R. Morb. Mortal. Wkly. Rep 51: 1–84. [PubMed] [Google Scholar]
- Gulia-Nuss M, Nuss AB, Meyer JM, Sonenshine DE, Roe RM, Waterhouse RM, Sattelle DB, de la Fuente J, Ribeiro JM, Megy K, Thimmapuram J, Miller JR, Walenz BP, Koren S, Hostetler JB, Thiagarajan M, Joardar VS, Hannick LI, Bidwell S, Hammond MP, Young S, Zeng Q, Abrudan JL, Almeida FC, Ayllón N, Bhide K, Bissinger BW, Bonzon-Kulichenko E, Buckingham SD, Caffrey DR, Caimano MJ, Croset V, Driscoll T, Gilbert D, Gillespie JJ, Giraldo-Calderón GI, Grabowski JM, Jiang D, Khalil SMS, Kim D, Kocan KM, Koči J, Kuhn RJ, Kurtti TJ, Lees K, Lang EG, Kennedy RC, Kwon H, Perera R, Qi Y, Radolf JD, Sakamoto JM, Sánchez-Gracia A, Severo MS, Silverman N, Šimo L, Tojo M, Tornador C, Van Zee JP, Vázquez J, Vieira FG, Villar M, Wespiser AR, Yang Y, Zhu J, Arensburger P, Pietrantonio PV, Barker SC, Shao R, Zdobnov EM, Hauser F, Grimmelikhuijzen CJP, Park Y, Rozas J, Benton R, Pedra JHF, Nelson DR, Unger MF, Tubio JMC, Tu Z, Robertson HM, Shumway M, Sutton G, Wortman JR, Lawson D, Wikel SK, Nene VM, Fraser CM, Collins FH, Birren B, Nelson KE, Caler E, Hill CA, 2016. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun 7, 10507. 10.1038/ncomms10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha E-M, Oh C-T, Ryu J-H, Bae Y-S, Kang S-W, Jang I-H, Brey PT, Lee W-J, 2005. An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell 8, 125–32. 10.1016/j.devcel.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Hajdušek O, Síma R, Ayllón N, Jalovecká M, Perner J, de la Fuente J, Kopáček P, 2013. Interaction of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol 3, 26. 10.3389/fcimb.2013.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JS (2003). The immune responses of Drosophila. Nature 426:33–38. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Torkelson JL, Bodnar J, Mortazavi B, Laurent T, Deason J, Thephavongsa K, Zhong J, 2015. The Rickettsia Endosymbiont of Ixodes pacificus Contains All the Genes of De Novo Folate Biosynthesis. PLOS ONE 10, e0144552. 10.1371/journal.pone.0144552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng RL, Goley ED, D’Alessio JA, Chaga OY, Svitkina TM, Borisy GG, Heinzen RA, Welch MD, 2004. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell. Microbiol 6, 761–769. 10.1111/j.1462-5822.2004.00402.x [DOI] [PubMed] [Google Scholar]
- Karasuyama H, Miyake K, Yoshikawa S, 2020. Immunobiology of Acquired Resistance to Ticks. Front. Immunol 11, 601504. 10.3389/fimmu.2020.601504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Kumar D, Adamson S, Ennen JR, Qualls CP, Ribeiro JMC, 2020. The sialotranscriptome of the gopher-tortoise tick, Amblyomma tuberculatum. Ticks Tick-Borne Dis. 12, 101560. 10.1016/j.ttbdis.2020.101560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Ribeiro JMC, 2015. An Insight into the Sialome of the Lone Star Tick, Amblyomma americanum, with a Glimpse on Its Time Dependent Gene Expression. PloS One 10, e0131292. 10.1371/journal.pone.0131292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JMC, 2011. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One 6, e28525. 10.1371/journal.pone.0028525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Essenberg RC, Dillwith JW, Tucker JS, Bowman AS, Sauer JR. (2002). Identification of SNARE and cell trafficking regulatory proteins in the salivary glands of the lone star tick, Amblyomma americanum (L). Insect Biochemistry and Molecular Biology 32:1711–1721. [DOI] [PubMed] [Google Scholar]
- Kazimírová M, Štibrániová I, 2013. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol 3. 10.3389/fcimb.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotál J, Langhansová H, Lieskovská J, Andersen JF, Francischetti IMB, Chavakis T, Kopecký J, Pedra JHF, Kotsyfakis M, Chmelař J, 2015. Modulation of host immunity by tick saliva. J. Proteomics 128, 58–68. 10.1016/j.jprot.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Budachetri K, Meyers VCC, Karim S, 2016. Assessment of tick antioxidant responses to exogenous oxidative stressors and insight into the role of catalase in the reproductive fitness of the Gulf Coast tick, Amblyomma maculatum. Insect Mol. Biol 25, 283–94. 10.1111/imb.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Gupta L, Han YS, Barillas-Mury C, 2004. Inducible peroxidases mediate nitration of anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J. Biol. Chem 279, 53475–53482. 10.1074/jbc.M409905200 [DOI] [PubMed] [Google Scholar]
- Kuriakose JA, Miyashiro S, Luo T, Zhu B, McBride JW, 2011. Ehrlichia chaffeensis transcriptome in mammalian and arthropod hosts reveals differential gene expression and post transcriptional regulation. PLoS One 6, e24136. 10.1371/journal.pone.0024136PONE-D-11-09798 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti TJ, Burkhardt NY, Heu CC, Munderloh UG, 2016. Fluorescent Protein Expressing Rickettsia buchneri and Rickettsia peacockii for Tracking Symbiont-Tick Cell Interactions. Vet. Sci 3. 10.3390/vetsci3040034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labunskyy VM, Hatfield DL, Gladyshev VN, 2014. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev 94, 739–77. 10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamason RL, Bastounis E, Kafai NM, Serrano R, Del Álamo JC, Theriot JA, Welch MD, 2016. Rickettsia Sca4 Reduces Vinculin-Mediated Intercellular Tension to Promote Spread. Cell 167, 670–683.e10. 10.1016/j.cell.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-N, Yu Z-J, Liu L-M, Li N-X, Wang R-R, Zhang C-M, Liu J-Z, 2016. Identification, Distribution and Population Dynamics of Francisella-like Endosymbiont in Haemaphysalis doenitzi (Acari: Ixodidae). Sci. Rep 6, 35178. 10.1038/srep35178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Davis J, Alam U et al. , (2003). Spotted fever group rickettsiae in ticks from the Masai Mera region of Kenya. Am. J. Trop. Med. Hyg 68:551–553. [DOI] [PubMed] [Google Scholar]
- Mansfield KL, Jizhou L, Phipps LP, Johnson N, 2017. Emerging Tick-Borne Viruses in the Twenty-First Century. Front. Cell. Infect. Microbiol 7, 298. 10.3389/fcimb.2017.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna M, Sassera D, Epis S, Bazzocchi C, Vannini C, Lo N, Sacchi L, Fukatsu T, Petroni G, Bandi C, 2013. “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alphaproteobacteria. Appl. Environ. Microbiol 79, 3241–8. 10.1128/AEM.03971-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N, Beati L, Sellers M, Burton L, Adamson S, Robbins RG, Moore F, Karim S, 2014. Importation of exotic ticks and tick-borne spotted fever group rickettsiae into the United States by migrating songbirds. Ticks Tick Borne Dis 5, 127–134. 10.1016/j.ttbdis.2013.09.009S1877-959X(13)00100-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Fikrig E, 2015. Tick microbiome: the force within. Trends Parasitol 31, 315–323. 10.1016/j.pt.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava S, Elshenawy Y, Eremeeva ME, Sumner JW, Mastropaolo M, Paddock CD, 2008. Rickettsia parkeri in Argentina. Emerg. Infect. Dis 14, 1894–1897. 10.3201/eid1412.080860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakanta G, Sultana H, Fish D, Anderson JF, Fikrig E, 2010. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Invest 120, 3179–3190. 10.1172/JCI42868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niang EHA, Bassene H, Fenollar F, Mediannikov O, 2018. Biological Control of Mosquito-Borne Diseases: The Potential of Wolbachia-Based Interventions in an IVM Framework. J. Trop. Med 2018, 1470459. 10.1155/2018/1470459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Schwan TG, 1999. Lethal effect of rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl Env. Microbiol 65, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Munderloh UG, Kurtti TJ, 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Env. Microbiol 63, 3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden BH, Roselli MA, Loss SR, 2020. Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Group Ticks - Volume 26, Number 2—February 2020 - Emerging Infectious Diseases journal - C.D.C 26. 10.3201/eid2602.190664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nováková M, Šmajs D, 2018. Rickettsial Endosymbionts of Ticks. Ticks Tick-Borne Pathog. 10.5772/intechopen.80767 [DOI] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR et al. , (2004). Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States, Clin. Infect. Dis 38:805–811. [DOI] [PubMed] [Google Scholar]
- Paddock CC, Denison AM, Dryden MW et al. , 2015. High prevalence of ‘Candidatus Rickettsia andeanae’ and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculutum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks and Tick-Borne Diseases 6, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME, 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 47, 1188–1196. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Goddard J, 2015. The Evolving Medical and Veterinary Importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol 52, 230–252. [DOI] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Raoult D. (2005). Tick-borne rickettsioses around the world: emerging disease challenging old concepts. Clin Microbiol Reviews 18, 719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier P-E, Raoult D, 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev 26, 657–702. 10.1128/CMR.00032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SJ, Hunter MS, Zchori-Fein E, 2006. The emerging diversity of Rickettsia. Proc. R. Soc. B Biol. Sci 273, 2097–2106. 10.1098/rspb.2006.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchampai N, Sunyakumthorn P, Guillotte ML, Verhoeve VI, Banajee KH, Kearney MT, Macaluso KR, 2014. Novel identification of Dermacentor variabilis Arp2/3 complex and its role in rickettsial infection of the arthropod vector. PloS One 9, e93768. 10.1371/journal.pone.0093768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MA and Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66: 2457–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, de Pina JJ, Caruso G, Jones N, Laferl H, Rosenblatt JE, Marrie TJ, 2001. Rickettsia africae, a Tick-Borne Pathogen in Travelers to Sub-Saharan Africa. N. Engl. J. Med 344, 1504–1510. 10.1056/NEJM200105173442003 [DOI] [PubMed] [Google Scholar]
- Reich HJ, Hondal RJ, 2016. Why Nature Chose Selenium. A.C.S. Chem. Biol 11, 821–41. 10.1021/acschembio.6b00031 [DOI] [PubMed] [Google Scholar]
- Reeves WK, Loftis AD, Szumlas DE, Abbassy MM, Helmy IM, Hanafi HA, Dasch GA Rickettsial Pathogens in the Tropical Rat Mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian Rats (Rattus spp.) Exp. Appl. Acarol 2007;41:101–107. doi: 10.1007/s10493-006-9040-3. [DOI] [PubMed] [Google Scholar]
- Renvoise A, Mediannikov O, Raoult D, 2009. Old and new tick-borne rickettsioses. Int. Health 1, 17–25. 10.1016/j.inhe.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, Mans BJ, 2020. TickSialoFam (TSFam): A Database That Helps to Classify Tick Salivary Proteins, a Review on Tick Salivary Protein Function and Evolution, With Considerations on the Tick Sialome Switching Phenomenon. Front. Cell. Infect. Microbiol 10, 374. 10.3389/fcimb.2020.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts HT, 1991. Some aspects of Rocky Mountain spotted fever as shown by recent investigations. 1909. Rev. Infect. Dis 13, 1227–1240. 10.1093/clinids/13.6.1227 [DOI] [PubMed] [Google Scholar]
- Sahni SK, Narra HP, Sahni A, Walker DH. (2013). Recent molecular insights into rickettsial pathogenesis and immunity. Future Microbiol. 8:1265–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni A, Fang R, Sahni SK, Walker DH, 2019. Pathogenesis of Rickettsial Diseases: Pathogenic and Immune Mechanisms of an Endotheliotropic Infection. Annu. Rev. Pathol. Mech. Dis 14, 127–152. 10.1146/annurev-pathmechdis-012418-012800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito TB, Bechelli J, Smalley C, Karim S, Walker DH, 2019. Vector Tick Transmission Model of Spotted Fever Rickettsiosis. Am. J. Pathol 189, 115–123. 10.1016/j.ajpath.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassera D, Beninati T, Bandi C, Bouman EAP, Sacchi L, Fabbi M, Lo N, 2006. “Candidatus Midichloria mitochondrii”, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int J Syst Evol Microbiol 56, 2535–2540. 10.1099/ijs.0.64386-0 [DOI] [PubMed] [Google Scholar]
- Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME, 2010. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 50, 541–548. 10.1086/649926 [DOI] [PubMed] [Google Scholar]
- Socolovschi C, Mediannikov O, Raoult D, Parola P, 2009. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Vet. Res 40. 10.1051/vetres/2009017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE, Macaluso KR, 2017. Microbial Invasion vs. Tick Immune Regulation. Front. Cell. Infect. Microbiol 7. 10.3389/fcimb.2017.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JV, Walker DH, Varela-Stokes AS, 2020. The guinea pig model for tick-borne spotted fever rickettsioses: A second look. Ticks Tick-Borne Dis. 11, 101538. 10.1016/j.ttbdis.2020.101538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolf BS, Smyrnias I, Lopes LR, Vendramin A, Goto H, Laurindo FRM, Shah AM, Santos CXC, 2011. Protein Disulfide Isomerase and Host-Pathogen Interaction. Sci. World J 11, 1749–1761. 10.1100/2011/289182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanbongkot C, Langohr IM, Harris EK, Dittmar W, Christofferson RC, Macaluso KR, 2019. Spotted Fever Group Rickettsia Infection and Transmission Dynamics in Amblyomma maculatum. Infect. Immun 87. 10.1128/IAI.00804-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Ferreira Á, Ashburner M, 2008. The Bacterial Symbiont Wolbachia Induces Resistance to R.N.A. Viral Infections in Drosophila melanogaster. PLoS Biol. 6, e1000002. 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teymournejad O, Lin M, Rikihisa Y, 2017. Ehrlichia chaffeensis and Its Invasin EtpE Block Reactive Oxygen Species Generation by Macrophages in a DNase X-Dependent Manner. mBio 8. 10.1128/mBio.01551-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepparit C, Bourchookarn A, Petchampai N, Barker SA, Macaluso KR, 2010. Interaction of Rickettsia felis with histone H2B facilitates the infection of a tick cell line. Microbiology 156, 2855–2863. 10.1099/mic.0.041400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Bestor A, Rosa PA, 2016. Functional Equivalence of OspA and OspB, but Not OspC, in Tick Colonization by Borrelia burgdorferi. Infect. Immun 84, 1565–1573. 10.1128/IAI.00063-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassone L, Portillo A, Nováková M, de Sousa R, Oteo JA, 2018. Neglected aspects of tick-borne rickettsioses. Parasit. Vectors 11, 263. 10.1186/s13071-018-2856-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver A, Rikihisa Y, Stich RW, Ohashi N, Felek S, 2002. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun 10.1128/IAI.70.8.4701-4704.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venzal JM, Félix ML, Olmos A, Mangold AJ, Guglielmone AA, 2005. A collection of ticks (Ixodidae) from wild birds in Uruguay. Exp. Appl. Acarol 36, 325–31. 10.1007/s10493-005-8433-z [DOI] [PubMed] [Google Scholar]
- Villareal MV, Mingala CN, Rivera WL, 2013. Molecular characterization of Trypanosoma evansi isolates from water buffaloes (Bubalus bubalis) in the Philippines. Acta Parasitol 58, 6–12. 10.2478/s11686-013-0110-5 [DOI] [PubMed] [Google Scholar]
- Villarreal AM, Adamson SW, Browning RE, Budachetri K, Sajid MS, Karim S, 2013. Molecular characterization and functional significance of the Vti family of SNARE proteins in tick salivary glands. Insect Biochem. Mol. Biol 43, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CP, Bernardi KM, Tsai B, 2012. Endoplasmic Reticulum-Dependent Redox Reactions Control Endoplasmic Reticulum-Associated Degradation and Pathogen Entry. Antioxid. Redox Signal 16, 809–818. 10.1089/ars.2011.4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, 1989. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin. Microbiol. Rev 2, 227–240. 10.1128/cmr.2.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, Ismail N, 2008. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol 6, 375–386. https://doi.org/nrmicro1866 [pii] 10.1038/nrmicro1866 [DOI] [PubMed] [Google Scholar]
- Weck B, Dall’Agnol B, Souza U, Webster A, Stenzel B, Klafke G, Martins JR, Reck J, 2017. Rickettsia parkeri in Amblyomma dubitatum ticks in a spotted fever focus from the Brazilian Pampa. Acta Trop. 171, 182–185. 10.1016/j.actatropica.2017.03.028 [DOI] [PubMed] [Google Scholar]
- Werren JH, Windsor DM, 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc Biol Sci 267, 1277–1285. 10.1098/rspb.2000.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel S, 2013. Ticks and tick-borne pathogens at the cutaneous interface: Host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front. Microbiol 4. 10.3389/fmicb.2013.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, 2018a. Ticks and Tick-Borne Infections: Complex Ecology, Agents, and Host Interactions. Vet. Sci 5. 10.3390/vetsci5020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, 2018b. Tick-host-pathogen systems immunobiology: an interactive trio. Front. Biosci. Landmark Ed 23, 265–283. 10.2741/4590 [DOI] [PubMed] [Google Scholar]
- Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek PJ, Jiang J, Byers DK, Sanders JW. (2007). Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis 13: 334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Jasinskas A, Barbour AG, 2007. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS One 2, e405. 10.1371/journal.pone.0000405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Tahir F, Wang JC-Y, Woodson M, Sherman MB, Karim S, Neelakanta G, Sultana H, 2020. Discovery of Exosomes From Tick Saliva and Salivary Glands Reveals Therapeutic Roles for CXCL12 and IL-8 in Wound Healing at the Tick-Human Skin Interface. Front. Cell Dev. Biol 8, 554. 10.3389/fcell.2020.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]


