Abstract
Hematoma size after intracerebral hemorrhage (ICH) significantly affects patient outcome. However, our knowledge of endogenous mechanisms that underlie hematoma clearance and the potential role of the anti-inflammatory cytokine interleukin-10 (IL-10) is limited. Using organotypic hippocampal slice cultures and a collagenase-induced ICH mouse model, we investigated the role of microglial IL-10 in phagocytosis ex vivo and hematoma clearance in vivo. In slice culture, exposure to hemoglobin induced IL-10 expression in microglia and enhanced phagocytosis that depended on IL-10–regulated expression of CD36. Following ICH, IL-10–deficient mice had more severe neuroinflammation, brain edema, iron deposition, and neurologic deficits associated with delayed hematoma clearance. Intranasal administration of recombinant IL-10 accelerated hematoma clearance and improved neurologic function. Additionally, IL-10–deficient mice had weakened in vivo phagocytic ability owing to decreased expression of microglial CD36. Moreover, loss of IL-10 significantly increased monocyte-derived macrophage infiltration and enhanced brain inflammation in vivo. These results indicate that IL-10 regulates microglial phagocytosis and monocyte-derived macrophage infiltration after ICH and that CD36 is a key phagocytosis effector regulated by IL-10. Leveraging the innate immune response to ICH by augmenting IL-10 signaling may provide a useful strategy for accelerating hematoma clearance and improving neurologic outcome in clinical translation studies.
Keywords: Intracerebral hemorrhage, IL-10, Microglia, Macrophage, Hematoma clearance
1. Introduction
Spontaneous intracerebral hemorrhage (ICH) is a significant cause of mortality and morbidity throughout the world. ICH occurs when a weakened vessel ruptures and bleeds into the surrounding brain. The blood accumulates and compresses the surrounding brain tissue, causing tissue damage and neuronal death (Li et al., 2017a, 2017c). Minimally invasive catheter drainage of the hematoma after repeated injections of tissue plasminogen activator into the hematoma failed to show improved one-year outcome (Hanley et al., 2019), although residual hematoma volume < 15 mL was associated with good outcome (Awad et al., 2019). Thus, leveraging endogenous mechanisms of red blood cell (RBC) phagocytosis may assist in bringing hematoma volume below this threshold.
Activated microglia and infiltrating immune cells such as monocyte-derived macrophages protect the surrounding cells by removing toxic elements (blood, dying neurons, and myelin debris) (Fu et al., 2015; Lan et al., 2017a, 2017b). In animals, insufficient RBC elimination by phagocytes prolongs damage and worsens outcomes (Yao and Tsirka 2012). Conversely, overactive microglia/macrophages (MMΦ) can also cause secondary damage by releasing proinflammatory cytokines and chemokines (Lan et al., 2017a, 2017b). Classically activated macrophages increase proinflammatory cytokines and upregulate reactive oxygen and nitrogen species, whereas alternatively activated macrophages secrete anti-inflammatory cytokines and growth factors, promote wound repair and remodeling, and may accelerate efferocytosis (Miron et al., 2013; Lan et al., 2017a, 2017b). Although microglia and macrophages share similarities, it is still debated whether microglia have two different activation states (Ransohoff 2016). Classically activated MMΦ appear as early as 6 h after ICH but later transition to an alternatively activated phenotype (Chang et al., 2017; Lan et al., 2017a, 2017b). However, how these activation states are regulated in the context of ICH is incompletely understood.
MMΦ secrete the anti-inflammatory cytokine interleukin-10 (IL-10), and IL-10 has been shown to enhance phagocytosis by monocytes (David and Kroner, 2011). In permanent ischemic stroke, IL-10 deficiency was associated with a greater inflammatory response but no delay in lesion resolution (Pérez-de Puig et al., 2013). Although increased IL-10 plasma levels have been reported in patients after ICH (Dziedzic et al., 2002; Wang et al., 2016), its precise role and mechanisms of action have not been fully investigated in ICH animal models.
CD36 belongs to a class B scavenger receptor family and is a key receptor that mediates the phagocytosis response of microglia, macrophages, and monocytes (Fadok et al., 1998, Fang et al., 2014, Woo et al., 2016). It also promotes hematoma resolution after ICH (Fang et al., 2014; Zhao et al., 2015a). In vitro studies showed that signaling via CD36 up-regulates IL-10 production in macrophages stimulated with oxidized low-density lipoprotein (Rios et al., 2013), and that silencing CD36 expression blunts IL-10 production in macrophages stimulated with lipopolysaccharide (Zhao et al., 2014). Further, in primary microglial cultures, IL-10 increases CD36 expression and enhances RBC phagocytosis (Fang et al., 2014). Thus, the complex interrelationships among IL-10, CD36, and phagocytosis in the clearance of hematoma are unclear. The goals of this study were to better understand the role of IL-10 in regulating MMΦ-mediated hematoma clearance and inflammation and to identify the major downstream target molecule of IL-10 after ICH. We utilized the collagenase and blood injection models of ICH in mice, as well as organotypic hippocampal slice cultures (OHSCs), which avoid recruitment of blood-borne immune cells. The insights gained from this study will advance our knowledge of innate immune regulation after ICH and will be essential for advancing future clinical and translational studies targeting endogenous hematoma clearance.
2. Methods
2.1. Animals
All procedures on mice were approved by the Johns Hopkins University Animal Care and Use Committee and followed NIH guidelines for care of animals. Two-month-old C57BL/6 male mice were obtained from Charles River Laboratories (Germantown, MD). Six-week-old male CX3CR1GFP/+ mice (on C57BL/6 background), which express enhanced GFP in monocytes, dendritic cells, NK cells, and microglia under control of the endogenous Cx3cr1 locus (Jung et al., 2000), were obtained from Dr. Jonathan Bromberg (University of Maryland, Baltimore, MD) and were used to visualize microglia. Six-week-old male GFP-UBC mice (on C57BL/6 background), which express ubiquitin C promoter-derived GFP in all cell types (Derecki et al., 2012) were obtained from Dr. Roger Johns (Johns Hopkins University, Baltimore, MD) and were used to visualize RBCs. Two-week-old male IL-10−/− (B6.129P2-Il10tm1Cgn/J) mice (Kühn et al., 1993) obtained from Dr. Xuhang Li (Johns Hopkins University, Baltimore, MD) were used to investigate the role of IL-10 in the phagocytic function of microglia and macrophages. Two-week-old male CD36−/− (B6.129S1-Cd36tm1Mfe/J) mice (Pascual et al., 2017) obtained from Dr. Alan Scott (Johns Hopkins University, Baltimore, MD) were used to explore whether IL-10 regulates CD36 and thereby promotes phagocytosis.
2.2. ICH models
Mice were anesthetized by 1–3% isoflurane inhalation and ventilated with oxygen-enriched air (20%:80%). In one model, the left striatum of mice was injected with 0.5 μL of 0.075 U collagenase VII-S (Sigma, St. Louis, MO) at 0.1 μL/minute. Injections were administered at 0.8 mm anterior and 2.1 mm lateral of the bregma, and 3.1 mm in depth, as previously described (Li et al., 2017a, 2017c). To confirm the role of IL-10 on ICH outcomes, we used a second ICH model in which 8 μL of autologous whole arterial blood was injected into the left striatum at a rate of 0.5 μL/minute (Li et al., 2017b). Blood removed from the tail artery was infused in two time blocks (2 μL followed by a 5-minute pause and then 6 μL followed by a 10-minute pause) (Li et al., 2017b). The craniotomy was sealed with Super Glue (Loctite, Westlake, OH). Rectal temperature was maintained at 37.0 ± 0.5 °C throughout the experimental and recovery periods. Sham-operated mice received the same treatment, including needle insertion. Animals that died or were euthanized within 24 h of surgery were excluded from the sample size; otherwise all animals proceeded into the final analysis. All efforts were made to minimize the number of animals used and ensure minimal suffering. Body weight, rectal temperature, and survival rates of mice were recorded before and after surgery until the experimental endpoint (Supplemental Fig. 6C, D). All treatment strategies followed ARRIVE and RIGOR guidelines (Lapchak et al., 2013), including randomization and concealment of treatment and genetic mouse line for analysis of neurologic outcome, volumetric morphometry, and immunopositive cell counts.
2.3. Organotypic hippocampal and striatal slice cultures
OHSCs or striatal slice cultures were cultured as previously described (Fujimoto et al., 2006; Li et al., 2017a, 2017c). Briefly, C57BL/6 or CX3CR1GFP/+ mouse pups (P7-P9) were rapidly perfused with cold PBS, decapitated, and the hippocampus placed in ice-cold Hanks’ balanced salt solution (HBSS, with Ca2+ and Mg2+; Life Technologies, Frederick, MD) supplemented with 25 mM HEPES (Life Technologies). A McIlwain tissue chopper was used to cut 350-μm-thick sections that were immediately plated on a hydrophilic PTFE cell culture insert (pore size: 0.4 μm; Millicell-CM, Millipore, Darmstadt, Germany) in 50% DMEM (Life Technologies), 25% heat-inactivated horse serum (Invitrogen, Grand Island, NY), 25% HBSS, 35 mM glucose (Sigma), and 25 mM HEPES. The sections were incubated at 37 °C in a 5% CO2 atmosphere. After 1 day in vitro, the medium was changed to 70% DMEM and 5% heat-inactivated horse serum. Medium was changed every 2–3 days. After 10–14 days of culture in vitro, brain slices were maturing and thinning. Slices were placed in serum-free medium (75% DMEM) 24 h before use. To perform immunostaining, the slices on the membrane were cut from culture insert and incubated with antibodies in a 24-well-plate. Immunostained slices were transferred onto microscope slides and covered with coverslips. Images were taken under fluorescence microscope and analyzed with Image J software blindly.
2.4. Phagocytosis assay
Fluorescence-conjugated latex beads (Life Technologies, P35364 and A37309) or aged red blood cells (RBCs) obtained from GFP-UBC mice were incubated with OHSCs or cells for 4–6 h. Then, samples were fixed with 4% PFA and immunostained. Alternatively, cells were seeded in 96-well plates and beads were added into treated cells. Real-time fluorescence intensity of beads in the cells was measured with a plate reader.
RBCs were obtained from male GFP-UBC mice and aged as described previously (Kroner et al., 2014). Briefly, blood was collected in EDTA-coated tubes, washed with PBS, centrifuged, and resuspended in HEPES buffer containing calcium (2.5 mM; Sigma) and Ca2+ ionophore (0.5 μM; Sigma). RBCs were incubated at 30 °C overnight, washed twice in PBS, and incubated with treated OHSCs.
For in vivo experiments, aged RBCs (1 × 106) in 1 μL PBS were injected into left striatum of WT or IL-10−/− mice at 1 μL/minute. Sham animals were injected with 1 μL of PBS. Injections were administered at 0.8 mm anterior and 2.1 mm lateral of the bregma, and 3.1 mm in depth. The craniotomy was sealed with Super Glue (Loctite, Westlake, OH). Rectal temperature was maintained at 37.0 ± 0.5 °C throughout the experimental and recovery periods.
2.5. Hematoma size and hemoglobin content analysis
Mice were euthanized by deep anesthesia and perfused with cold PBS. Coronal brain sections (1-mm each) were freshly cut on a mouse brain matrix (Chang et al., 2015). All coronal fresh sections were scanned. Hematoma volume in cubic millimeters was analyzed with Image J software and calculated as the summation of the hematoma areas multiplied by the interslice distance (1 mm) (Chang et al., 2017).
The hemoglobin content of brains was quantified with the Hemoglobin Assay Kit (Sigma, MAK115–1KT) at different time points after collagenase injection (Li et al., 2017a, 2017b). Briefly, mice were anesthetized and then transcardially perfused with PBS. The injured hemisphere was collected in a tube with 500 μL of ice-cold PBS. The tissue was homogenized for 1 min and then centrifuged at 16000g for 15 min. Fifty microliters of supernatant was transferred to flat-bottom, 96-well plates. After a 5-minute incubation with reaction reagent, we measured the absorbance at 400 nm. Hemoglobin content was calculated by a standard curve.
2.6. Neurologic function evaluations
An experimenter blinded to treatment group evaluated mice for neurologic function at various times after ICH. In the neurologic deficit scoring system, mice were evaluated for body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling. Each test was graded from 0 to 4, establishing a maximum deficit score of 24 (Li et al., 2017a, 2017c). Forelimb placing was assessed with a vibrissae-elicited forelimb placing test. The mouse was placed on the edge of a tabletop, and the vibrissae on one side were brushed. Intact animals placed the contralateral forelimb quickly on the tabletop. Placing was quantified as the percentage of successful responses in 10 trials (Li et al., 2017a, 2017c). For the hind limb placing test, the mouse was placed on the edge of a tabletop and the contralateral hind limb was pulled down. The test was scored as follows: immediate pullback of limb = 0; delayed pullback = 1; inability to pull back = 2. Placing was quantified in 10 successful trials; trials were excluded when the animal attempted to turn around or walk away (Li et al., 2017a, 2017c). Results are shown as a total score for each mouse.
2.7. Brain water content measurement
To assess global cerebral edema, we measured brain water content in different treatment groups as previously reported (Wu et al., 2011). Briefly, on day 3 or 5 after ICH, mice were euthanized by deep anesthesia. The brains were removed and divided into ipsilateral cortex, ipsilateral striatum, contralateral cortex, contralateral striatum, and cerebellum (an internal control). Samples were immediately weighed on an analytical balance to record the wet weight, and then dried at 100 °C for 24 h to obtain the dry weight. Brain water content was expressed as (wet weight – dry weight)/wet weight of brain tissue × 100% (Zhao et al., 2015b).
2.8. Bone marrow chimeras
To specifically study the role of microglial CD36 after ICH, we made BM chimeras as previously described (Peake et al., 2015). Briefly, 8-week-old male WT and CD36−/− mice were injected intraperitoneally with 20 mg/kg busulfan (Sigma-Aldrich) for 4 days and then reconstituted with BM (1 × 106 cells in 200 μL PBS) from a WT or CD36−/− donor. BM was allowed to engraft 8–10 weeks prior to ICH. Percent chimerism was measured at 8 weeks after BM engraftment by flow cytometry. Successful chimerism was defined as the recipient mouse having at least 80% of the donor’s BM cells in its peripheral blood. When establishing the method, we used flow cytometry to check the chimerism rate in WT and CX3CR1GFP/+ mice (Supplemental Fig. 6E, F).
2.9. Flow cytometry
Mouse brain dissociation and FAC sorting were followed our previous publication (Li et al., 2018). Generally, mice (8–10 weeks old, male) were injected with collagenase or aged RBCs. Three- or five- days later, they were anesthetized and perfused with 50 mL ice-cold PBS. Brains were then removed and 4-mm coronal sections that included the major hemorrhagic territory were collected in HBSS. Single cells were dissociated from tissue with a gentle MACS Dissociator and Neural Tissue Dissociation kit (Cat. No. 130–092–628, Miltenyi Biotec, San Diego, CA). Myelin was removed by using anti-myelin immunoglobulin-conjugated magnetic microbeads (Cat. No. 130–096–733, Miltenyi Biotec). After a 10-minute blocking step, single cells were stained for 30 min on ice for BV421-CD11b (Cat. No. 562605, BD Biosciences), APC-CD45 (Cat. No. 103112, Biolegend, San Diego, CA), FITC-CD11b (Cat. No. 130–081–201, Miltenyi Biotec), PE-CD45 (Cat. No. 130–102–596, BD Biosciences), BV421-CD86 (Cat. No. 564198, BD Biosciences), and/or APC-CD206 (Cat. No. 141708, Biolegend) for flow cytometry. Gated microglia and macrophage populations were back-gated to double check the size of the gated cells, and lymphocytes were ruled out. PI staining was used to distinguish dead cells. Cells were sorted by a MoFlo cytometer (Beckman Coulter). For the fluorescence-minus-one (FMO) controls, samples were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA).
2.10. Cell cultures
The human iPSC line iPS (IMR90)-1 (WiCell Research Institute) was infected by lentiviruses harboring doxycycline-inducible neurogenin 2. After doxycycline treatment for 6 days, neuronal precursors (3 × 105 cells/cm2) were seeded on dishes pre-coated with poly-D-lysine (1 μg/mL) and laminin (1 μg/mL; Sigma) in neuron culture medium (Neurobasal medium [Life Technologies] with B27 supplement [Life Technologies], brain-derived neurotrophic factor [20 ng/mL; PeproTech, Rocky Hill, NJ], glial cell line-derived neurotrophic factor [20 ng/mL; PeproTech], L-ascorbic acid [0.2 mM; Sigma], dibutyryl cAMP [0.5 mM; Sigma], and γ-secretase inhibitor DAPT [10 μM; Stemgent, Cambridge, MA]) (Sagal et al., 2014). Neurons were matured for 20 days before Hb treatment. Medium was changed every 3 days.
Mouse primary corticostriatal neurons were prepared from embryos at gestational day 15.5 and cultured in serum-free conditions as described previously (Zhang et al., 2017). Briefly, the tissue was dissociated in Neurobasal medium (containing B27 supplement) and digested with papain (Sigma) to obtain single cells. The cells were seeded onto poly-D-lysine–coated plates at a density of 5 × 104 cells/cm2. They were maintained in Neurobasal medium and used at 7 days in vitro. The corticostriatal cultures contained mostly neurons and < 2% glia cells, as determined by immunofluorescence staining.
Mouse primary microglia and astrocytes were prepared from pups at postnatal day 0–1 and cultured as described previously (Lan et al., 2017a, 2017b). Briefly, the tissue was digested into single cells with papain and then dissociated in DMEM/F12 medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS; Life Technologies) and 1% penicillin/streptomycin. Cells were maintained in the DMEM/F12 medium for 14 days in vitro. Finally, they were shaken at 200 rpm at 37 °C for 4 h to separate microglia and astrocytes. Medium was changed every 3 days.
Mouse bone marrow-derived macrophages were prepared from 6- to 8-week-old male mice and cultured as described previously (Weischenfeldt and Porse, 2008). Briefly, bone marrow cells from the femurs of mice were flushed out with MEM (Life Technologies). These cells were cultured overnight in MEM medium supplemented with 10% FBS and 1% penicillin/streptomycin. Non-adherent cells were collected and RBCs were lysed in RBC lysis buffer (Sigma). Cells were cultured in the described medium supplemented with murine M−CSF (20 ng/μL; PeproTech) for 7 days at 37 °C in a 5% CO2 atmosphere. Medium was changed every 3 days.
2.11. PI staining
OHSCs were incubated with 5 μg/mL PI (Sigma) for 30 min and photographed under a fluorescence microscope (Nikon TE 2000-E) at 200-millisecond exposure time (P0). The structure of the slices was examined under brightfield. After treatment, the slices were incubated with PI for 30 min, and images were captured (P16). Slices were then incubated for another 24 h to reach maximum death (Pmax). Percentage cell death was expressed as the number of pixels in the region of interest above a threshold in the PI fluorescent image divided by the total number of pixels in the region of interest (Li et al., 2017a, 2017c). The fluorescence intensity was measured with Image J software and the PI+ cells were calculated as (P16 – P0)/(Pmax – P0) × 100%.
2.12. LDH activity assay
Activity was measured by using an LDH activity assay kit (Sigma) (Li et al., 2017a, 2017c). Briefly, medium from treated slices or cells was collected and centrifuged. Then the samples were incubated with substrate and assay buffer in a clear 96-well plate. LDH in the samples reduced NAD to NADH, which was specifically detected at 450 nm with a plate reader (Spectramax M2 microplate reader; Molecular Devices LLC, Sunnyvale, CA).
2.13. ROS measurement
OHSCs were incubated with 63 μM HEt (Invitrogen) for 30 min (Li et al., 2017a, 2017c) before images were captured under a fluorescence microscope (Nikon Eclipse 90i). An investigator blinded to treatment measured fluorescence intensity using Image J software.
2.14. Iron deposition
We used Turnbull’s blue staining to detect ferrous (Fe2+) iron in tissues. Briefly, fixed OHSCs were incubated in 10% ammonium sulfide solution (Sigma) for 90 min, followed by 20% potassium-ferricyanide (Sigma) with 1% hydrochloric acid for 15 min and methanol with 0.01 M NaN3 (Sigma) and 0.3% H2O2 (Sigma) for 60 min (Kroner et al., 2014). Sections were washed between incubations with PBS.
To detect ferric iron (Fe3+) in tissues, we used 3,3′-diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA)-enhanced Perls’ staining as previously described (Li et al., 2017b) with slight modification. Briefly, fixed OHSCs or sections of brain tissue were washed with PBS and incubated in freshly prepared Perls’ solution (5% potassium ferrocyanide [Sigma]/10% hydrochloric acid) for 1 h, followed by five PBS washes. After DAB incubation for 3 min and hematoxylin (Sigma) counterstaining, iron deposition was digitized and analyzed with Image J software.
2.15. FluoroJade B (FJB) staining
FJB or FJC was used to identify degenerating neurons as previously described (Li et al., 2017a, 2017c). Treated OHSCs or brain sections were observed and photographed under a fluorescence microscope at an excitation wavelength of 450–490 nm.
2.16. Immunostaining
Treated OHSCs were cut from the inserts, fixed in 4% paraformaldehyde (PFA) for 2 h, and then blocked in 10% goat serum (Invitrogen) for 1 h at room temperature. Next, the slices were incubated overnight at 4 °C with one of the following primary antibodies: anti-CD11b (1:200, Cat. No. MAB1387Z, Millipore), anti-Arg1 (1:100; Cat. No. 610708, BD Biosciences, Franklin Lakes, NJ), anti-IL-10 (1:200), anti-NeuN (1:500; Cat. No. 12943S, Cell Signaling, Danvers, MA), or anti-GFAP (1:500; Cat. No. 13–0300, Life Technologies). After the sections were washed three times, they were incubated for 2 h in Alexa Fluor 488-conjugated and/or Alexa Fluor 594-conjugated secondary antibody (1:500; Cat. Nos. A11001 and A11006, Invitrogen) at room temperature. After being stained with DAPI, the slices were mounted. All slices were observed and photographed under a fluorescence microscope (Nikon Eclipse 90i).
Mouse primary microglia or bone marrow-derived macrophages were fixed in 4% PFA for 30 min and then blocked in 10% goat serum for 1 h at room temperature. Next, the cells were incubated with anti-IL-10 (1:1000) or F4/80 (1:500; Cat. No. MCA497, AbD Serotec, Bangkok, Thailand) primary antibody overnight at 4 °C. For the phospho-STAT3 (pSTAT3) staining, primary microglia were incubated with the primary antibodies of anti-phospho-STAT3 (1:200; Cat. No. 9145 T, Cell Signaling Technology) and anti-CD11b (1:200, Cat. No. MAB1387Z, Millipore). After the cells were washed, they were incubated for 2 h in Alexa Fluor 594-conjugated or Alexa Fluor 488-conjugated secondary antibody at room temperature. After being stained with DAPI, the slices were mounted. All slices were observed and photographed under a fluorescence microscope (Nikon Eclipse 90i).
For in vivo experiments, brain sections (15 μm) were incubated overnight at 4 °C with primary antibodies including anti-Iba-1 (1:1000; Cat. No. 019–19741, Wako Chemicals, Richmond, VA), anti-Tmem119 (1:100; Cat. No. ab209064, Abcam), anti-P2y12 (1:100; Cat. No. PA5–77671, ThermoFisher), anti-iNOS (1:50; Cat. No. 610329, BD Biosciences), anti-CD206 (1:50; Cat. No. ab8918, Abcam), anti-IL-10 (1:500) or anti-CD36 (1:500; Cat. No. sc-9154, Santa Cruz Biotechnology, Dallas, TX). Then the sections were incubated with secondary antibodies (1:1000; Alexa Fluor 488 and/or Alexa Fluor 594) for 1 h at room temperature. All sections were photographed under a fluorescence microscope (Nikon Eclipse TE2000-E or Leica DM6). We acquired at least 9 locations in perihematoma region (Fig. 7A) per mouse (3 fields (upper, left, and right) per section × 3 sections (bregma 1.70 mm, 0.74 mm, and −0.46 mm) per mouse) for quantifications. The mean value of the 9 fields was calculated and used for statistics. Positive stained cells were counted manually and blindly.
2.17. Real-time reverse transcription PCR (RT-PCR) and phagocytosis PCR array
Total mRNA was extracted from brain tissues, OHSCs, cultured cells, or flow cytometry-sorted cells by QIAzol Lysis Reagent (miRNeasy Mini Kit; QIAGEN, Gaithersburg, MD). The SuperScript VILO cDNA Synthesis Kit (Invitrogen) was used to transcribe 200 to 1000 ng of RNA from each sample into cDNA. Real-time PCR was performed on an ABI 7500 Fast real-time PCR System (Applied Biosystems, Foster City, CA) in TaqMan Universal PCR master mixture II with uracil N-glycosylase (Applied Biosystems). The following TaqMan Gene Expression Assay Mixes (Applied Biosystems) were used: CD11b (Mm00434455_m1), CD16 (Mm00438882_m1), CD32 (Mm004388875_m1), CD86 (Mm00444543_m1), iNOS (Mm00440502_m1), TNF-α (Mm00443258_m1), IL-6 (Mm00446190_m1), IL-1β (Mm00434228_m1), CD163 (Mm00474091_m1), Arg1 (Mm00475988_m1), YM-1 (Mm00657889_m1), IL-4 (Mm00445259_m1), IL-10 (Mm00439614_m1), CD206 (Mm00485148_m1), TGF-β (Mm01227699_m1), Tlr3 (Mm01207404_m1), Myd88 (Mm00440338_m1), CD36 (Mm01135198_m1), Angpt2 (Mm00545822_m1), CCL2 (Mm00441242_m1), CCL11 (Mm00441238_m1), and CXCL10 (Mm00445235_m1). The endogenous control was GAPDH (Mm99999915_g1). The cycle time values of candidate genes were normalized to GAPDH in the same sample. The expression levels of mRNA were then presented as fold change versus control as described (Li et al., 2014).
To detect phagocytosis-related genes and signaling pathways in treated OHSCs, we performed an RT2 Profiler™ phagocytosis PCR array (Cat. No. PAMM-173ZC, QIAGEN) according to the manufacturer’s instructions. Briefly, total mRNA was extracted with Trizol reagent (Life Technologies) as previously described (Li et al. 2011). One microgram of RNA from each sample was transcribed into cDNA with the RT2 First Strand Kit (Cat. No. 330401, QIAGEN). Then, the cDNA was mixed with the RT SYBR green/ROX PCR master mix (Cat. No. 330522, QIAGEN) and added into 96-well plates for real-time PCR. Results were analyzed by PCR array data analysis software provided on the QIAGEN website.
2.18. Cytokine/chemokine array
Conditioned medium was collected from Hb-treated OHSCs. Cytokines and chemokines were detected with a proteome profiler mouse cytokine array kit (R&D Systems; ARY006) according to the manufacturer’s instructions. Briefly, sample was mixed with a cocktail of biotinylated detection antibodies and then incubated overnight at 4 °C with the array membrane, which is spotted with antibodies to specific proteins. After being washed, the membrane was incubated with streptavidin-HRP, and bound proteins were visualized with chemiluminescent detection reagents. The intensity of the dots was quantified with Image J software.
For in vivo experiments, 4-mm sections of ipsilateral hemisphere tissue that included hematoma core and perihematomal regions were collected from WT and IL-10−/− mice at 3 days after ICH or from the corresponding brain areas of sham mice. Cytokines and chemokines were detected with a proteome profiler mouse XL cytokine array (R&D Systems; ARY028) according to the manufacturer’s instructions. Briefly, brain tissue was homogenized and protein was extracted as previously described (Li et al., 2017a, 2017c). Protein was incubated with the membrane and visualized by chemiluminescent detection reagents as described earlier in this section. Dot intensity was quantified with Image J software.
2.19. ELISA
ELISAs (R&D Systems) were used to measure concentrations of cytokines IL-1β (MLB00C), IL-6 (M6000B), TNF-α (MTA00B), IL-10 (M1000B), IL-4 (M4000B), and TGF-β (MB200) in treated OHSC medium, cell culture medium, or brain tissue, as described previously (Han et al., 2019).
2.20. Western blot analysis
OHSCs were homogenized in ice-cold protein extraction reagent (T-PER Reagent; Pierce, Appleton, WI) with a complete mini protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany). Total protein was quantified by BCA protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 and probed with primary antibodies against p38 MAPK (1:1000; Cat. No. 8690p, Cell Signaling), Phospho-p38 MAPK (1:500; Cat. No. 4511p, Cell Signaling), NeuN (1:500; Cat. No. MAB377, Millipore), GFAP (1:500; Cat. No. ab7260, Abcam), Iba-1 (1:500), CD36 (1:500), or β-actin (1:5000; Cat. No. sc-47778, Santa Cruz Biotechnology) at 4 °C overnight. The membranes were washed and incubated with horseradish peroxidase-linked anti-rabbit or anti-mouse secondary antibodies (Santa Cruz Biotechnology; 1:1000) for 1 h.
2.21. Transwell migration assay
A transwell assay was used to evaluate the migration ability of bone marrow-derived macrophages as previously reported (Li et al., 2011, 2014) with slight changes. Briefly, Hb-treated mouse primary astrocytes which are the main cell population that secretes chemokines (Xie and Yang, 2015; Parajuli et al., 2015) or medium containing 10% FBS was pipetted onto the bottom of 6-well plates. Treated bone marrow-derived macrophages were seeded on top of the Transwell chambers (Cat. No. 3422, Corning, Corning, NY). After 24 h of incubation at 37 °C, migrated cells were stained with a Diff-Quik stain set (Cat. No. B4132–1A, Fisher Scientific, Hampton, NH). We acquired at least 9 locations per sample for quantifications.
2.22. Drug administration
For OHSC experiments, we added 20 μM Hb (Sigma) (Li et al., 2017a, 2017c), 0.2 mM FeCl2 (Sigma) (Li et al., 2017a, 2017c), 0.5 ng/mL mouse recombinant IL-10 protein (PeproTech), IL-10 neutralizing antibodies (0–1 μg/mL; Cat. No. MAB417, R&D Systems, Minneapolis, MN) (Lybeck et al., 2009), or CD36 neutralizing antibodies (2 μg/mL; Cat. No. ab23680, Abcam, Cambridge, UK) for 16 h. For pStat3 measurement, primary microglia were incubated with 5 ng/mL mouse recombinant IL-10 for 1 h (Braun et al., 2013) and then stimulated with 20 μM Hb for 30 or 60 min. We added empty liposomes (FormuMax Scientific, Sunnyvale, CA) or clodronate liposomes (75 μg/mL; FormuMax Scientific) for 14 days to deplete microglia.
For human iPSC-derived neurons, mouse primary neurons, mouse primary astrocytes, or mouse primary microglia, we added 20 μM Hb, 25 ng/mL lipopolysaccharides (Sigma), or vehicle for 16 h. For mouse bone marrow-derived macrophages, 10 ng/mL mouse recombinant IL-10 protein (Wang and Rice 2006), 20 ng/mL mouse recombinant IFN-γ protein (Zajac et al., 2013), or vehicle was added to the medium without M−CSF for 48 h.
For in vivo experiments, we administered mouse recombinant IL-10 protein (0.1 μg/mouse in 5 μL PBS/nostril) (Wang et al., 2014) or vehicle (PBS) intranasally to WT, IL-10−/−, or CD36−/− mice beginning at 2 h after ICH and then once daily until sacrifice.
2.23. Study design
A power analysis based on our previous studies (Wu et al., 2011, Li et al., 2017a) and pilot data indicated that 8 mice/group would provide at least 80% power for detecting a 20% decrease in lesion volume at α = 0.05 (2-sided). To account for potential animal death, we used 10 mice/group. Animals that had a neurologic deficit score higher than 20 at 24 h post-surgery were euthanized under deep anesthesia. Animals that died or were euthanized were excluded from the sample size. Outlying data points were defined with statistical software assuming a normal distribution (threshold was set as 2.0-fold SD from the mean) and were excluded from the data set. Three or more independent experiments were performed for all ex vivo and in vitro experiments unless indicated. Animals, slice cultures, and cell cultures for each group were randomized with the website www.randomization.com (Li et al., 2017a, 2017c). Treatment, data collection, and data analyses were blinded by using different investigators or by masking sample labels. All analyses were carried out with GraphPad Software (GraphPad Prism 5.0; GraphPad Software, Inc., La Jolla, CA).
2.24. Statistics
Data are presented as mean ± SD with dot plots or bar graphs. We made two-group comparisons with a two-tailed Student’s t-test followed by Welch’s correction. We evaluated group comparisons of all behavioral tests by Kruskal-Wallis analysis of ranks followed by Dunn’s multiple comparison post hoc test. In anatomical and biochemical studies, one-way or two-way ANOVA was used for comparisons among multiple groups. Bonferroni or Dunn’s post hoc analysis was used to determine where those differences occurred. We evaluated survival data using a log-rank (Gehan-Breslow-Wilcoxon Test) test. For comparison of macrophage phagocytosis, we used repeated measurements with the Bonferroni correction for multiple timepoint comparisons between the two groups. The criterion for statistical significance was p < 0.05.
3. Results
3.1. Hemoglobin (Hb) induces neuronal cell death and activates microglia ex vivo
We first implemented an ex vivo model with Hb exposure to OHSCs in which we previously characterized the dose- and time-dependent cell death, as assessed by propidium iodide (PI) staining (Li et al., 2017a, 2017c). In OHSCs derived from C57BL/6 wild-type (WT) mouse pups, exposure to Hb at 20 μM for 16 h caused cell death in the dentate gyrus and hippocampal CA1 and CA3 regions comparable to that of exposure to Fe2+, which was used as a positive control (Supplemental Fig. 1A). This dose and duration of Hb exposure was previously shown to increase reactive oxygen species (ROS) (Li et al., 2017a, 2017c). Here, we detected Hb-induced ferrous iron deposition at 16 h of incubation with Turnbull’s blue staining (Supplemental Fig. 1B, upper row) and ferric iron deposition with Perl’s staining at 3 days (Supplemental Fig. 1B, bottom row); the latter is thought to represent separation of ferrous iron from ferritin and subsequent oxidation by ROS. Fluoro-Jade B (FJB) staining suggested preferential cell death of neurons (Supplemental Fig. 1C). To visualize microglia, we used slices derived from CX3CR1GFP/+ mouse pups in which green fluorescent protein (GFP) is under the control of the Cx3cr1 locus. Hb exposure did not induce microglial cell death, as we observed no PI+/GFP+ colocalization (Supplemental Fig. 1D). However, Hb incubation resulted in a significant increase in microglial cell body size (Supplemental Fig. 1E). This result suggests that, under the conditions used here, Hb exposure induces microglial activation rather than microglial cell death.
3.2. Hb exposure induces overexpression of both proinflammatory and anti-inflammatory microglial markers ex vivo
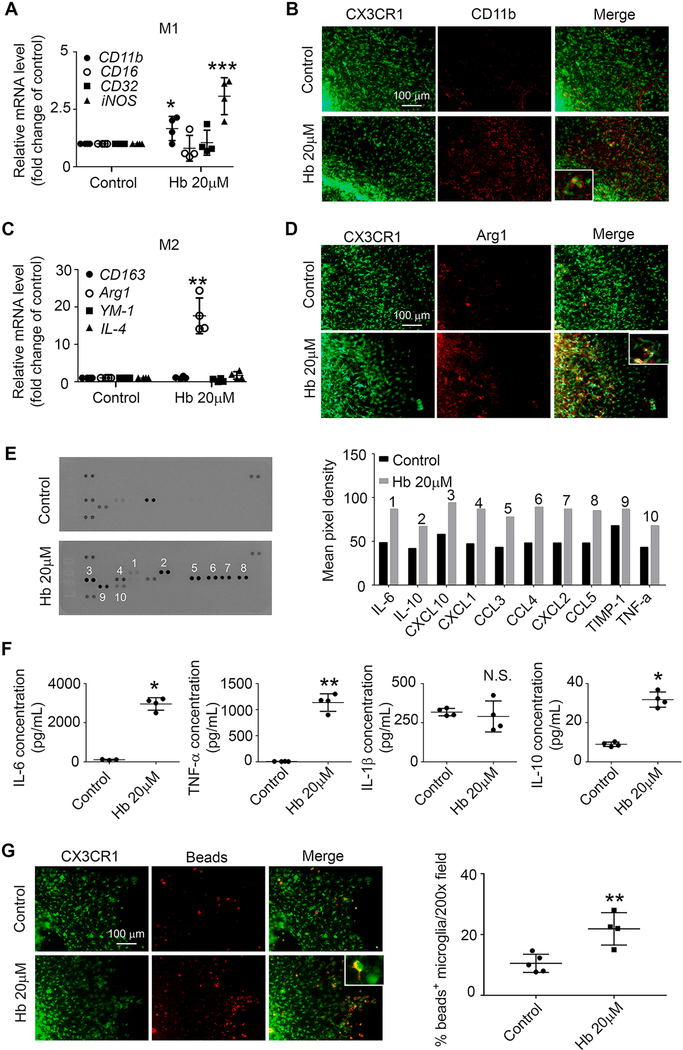
To determine if exposure to Hb polarizes microglia to a classical or alternatively activated state in OHSCs, we extracted mRNA from Hb- or vehicle-treated slices and assessed gene expression with real-time PCR. Hb incubation significantly increased gene expression of CD11b, iNOS, and Arg1 (Fig. 1A, C). Immunostaining revealed that overexpressed CD11b and Arg1 colocalized with GFP+ cells (Fig. 1B, D).
Fig. 1.
Hemoglobin (Hb) induces overexpression of both proinflammatory and anti-inflammatory microglial markers in OHSCs. OHSCs prepared from CX3CR1GFP/+ pups were treated with 20 μM Hb or vehicle for 16 h. (A, C) mRNA was extracted from the OHSCs, and real-time RT-PCR was performed. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Control. n = 4. (B, D) Slices were fixed and immunostained with CD11b or Arg1 antibodies. Insets are high-power images showing colocalization of CX3CR1+/CD11b+ or CX3CR1+/Arg1+ cells. (E) Culture medium was collected and concentrated for use in a cytokine/chemokine array assay. Quantification of mean pixel density is shown; n = 1. (F) Culture medium was collected and concentrated for use in an ELISA. *p < 0.05, **p < 0.01 vs. Control; N.S., not significant. n = 3–4. (G) OHSCs were incubated with fluorescence-conjugated latex beads (4-μm diameter) for 4 h and fixed before imaging. Representative images from the CA1 region and quantification of bead+/GFP+ cells are shown. Insets are high-power images showing microglia with engulfed beads. **p < 0.01 vs. Control. n = 4–5. A, C: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test; F, G: Student’s t-test followed by Welch’s correction. All results (except E) are from at least three independent experiments. Scale bars: (B), (D) and (G) 100 μm.
To investigate cytokine and chemokine dynamics after Hb incubation, we applied medium from Hb- and vehicle-treated OHSCs to membranes with pre-spotted antibodies. Hb exposure for 16 h resulted in increased secretion of inflammatory cytokines and chemokines including pro-inflammatory IL-6, TNF-α, and CCL3 and anti-inflammatory IL-10 (Fig. 1E). The increased secretion of IL-6, TNF-α, and IL-10 was confirmed by ELISA (Fig. 1F). Importantly, Hb treatment significantly enhanced microglial phagocytosis of fluorescence-conjugated latex beads (Fig. 1G).
3.3. Hb treatment enhances microglial phagocytosis by regulating IL-10 ex vivo
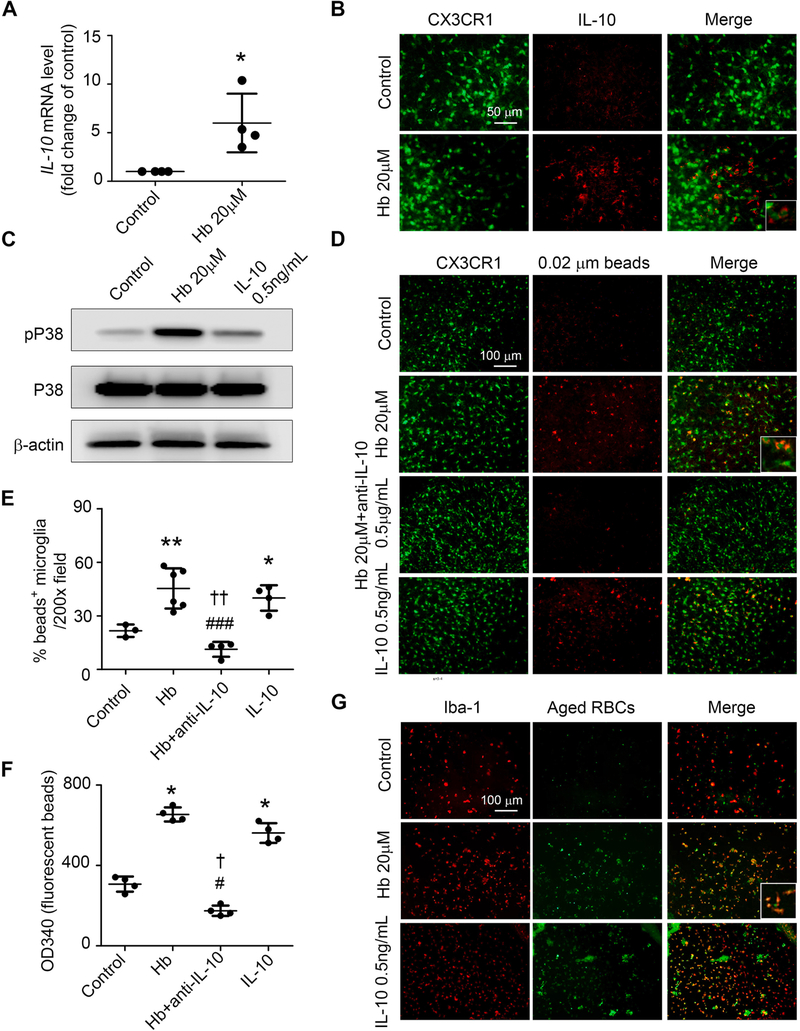
IL-10 polarizes peripheral macrophages to a protective phenotype associated with increased phagocytosis (Bian et al., 2016). To investigate whether IL-10 is regulated directly by Hb, we extracted mRNA from Hb- or vehicle-treated OHSCs and assessed gene expression with real-time PCR. Incubation with Hb significantly increased IL-10 mRNA and protein expression (Fig. 2A, B). Most IL-10+ staining co-localized with GFP+ cells in OHSCs obtained from CX3CR1GFP/+ pups (Fig. 2B). IL-10 can be regulated through the phosphorylated P38 (pP38) signaling pathway (Foey et al., 1998). Therefore, we next incubated slices with Hb, recombinant IL-10 protein (0.5 ng/mL), or vehicle. Western blots showed that Hb incubation increased pP38 without changing total P38 expression. Consistent with P38 acting upstream from IL-10 incubation did not alter pP38 or P38 protein expression level (Fig. 2C).
Fig. 2.
Hemoglobin (Hb) induces microglial phagocytosis by regulating IL-10 in OHSCs. OHSCs prepared from CX3CR1GFP/+ (A-F) or C57BL/6 (G) pups were treated as indicated for 16 h. (A) mRNA was extracted from the OHSCs, and real-time RT-PCR was carried out with IL-10 primers. *p < 0.05 vs. Control. n = 4. (B) Slices were fixed and immunostained with IL-10 antibodies. (C) OHSCs were collected and homogenized for Western blotting. β-actin served as a loading control. (D-F) OHSCs were incubated with fluorescence-conjugated latex beads (0.02-μm diameter) for 4 h and fixed before imaging. Representative images from the CA1 region (D) and quantification of bead+/GFP+ cells (E) are shown. Insets are high-power images showing microglia with engulfed beads. Tissue from another experimental group was collected and lysed to detect fluorescence intensity on a plate reader (F). *p < 0.05, **p < 0.01 vs. Control; #p < 0.05, ###p < 0.001 vs. Hb; †p < 0.05, ††p < 0.01 vs. IL-10. n = 3–6. (G) OHSCs were incubated for 4 h with aged RBCs prepared from GFP-UBC mice, fixed, and immunostained with Iba-1 antibodies. Representative images from the CA1 region are shown. Insets are high-power images showing colocalization of GFP-RBCs and microglia. A: Student’s t-test followed by Welch’s correction; E, F: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test. Results are from at least three independent experiments. Scale bars: (B) 50 μm; (D) and (G) 100 μm.
To determine if Hb exposure results in altered microglial phagocytosis directly by regulating IL-10 production, we first established that 0.5 μg/mL of an IL-10 neutralizing antibody was sufficient to neutralize the IL-10 production by Hb-treated OHSCs (Supplemental Fig. 1F). Next, we incubated OHSCs pups with Hb, Hb plus IL-10 neutralizing antibodies, recombinant IL-10 protein, or vehicle for 16 h and assessed CX3CR1GFP/+ microglial phagocytosis of non-opsonized, fluorescence-conjugated 0.02 μm latex beads. Incubation with Hb only or IL-10 only increased microglial phagocytosis significantly compared to that of the control group when quantified by the percentage of phagocytic microglia or by fluorescence intensity (Fig. 2D, F). The Hb enhancement of phagocytosis was completely reversed by co-incubation with IL-10 neutralizing antibodies (Fig. 2D, F). Furthermore, both Hb and IL-10 treatment enhanced microglial engulfment of larger, 4-μm beads (Supplemental Fig. 1G) and aged red blood cells (RBC), thereby inferring that uptake is not the result of simple diffusion (Fig. 2G). Moreover, results in striatal slice cultures confirmed these main results observed in OHSCs (Supplemental Fig. 1H–J).
3.4. Microglia are the primary secretors of IL-10 in response to Hb ex vivo
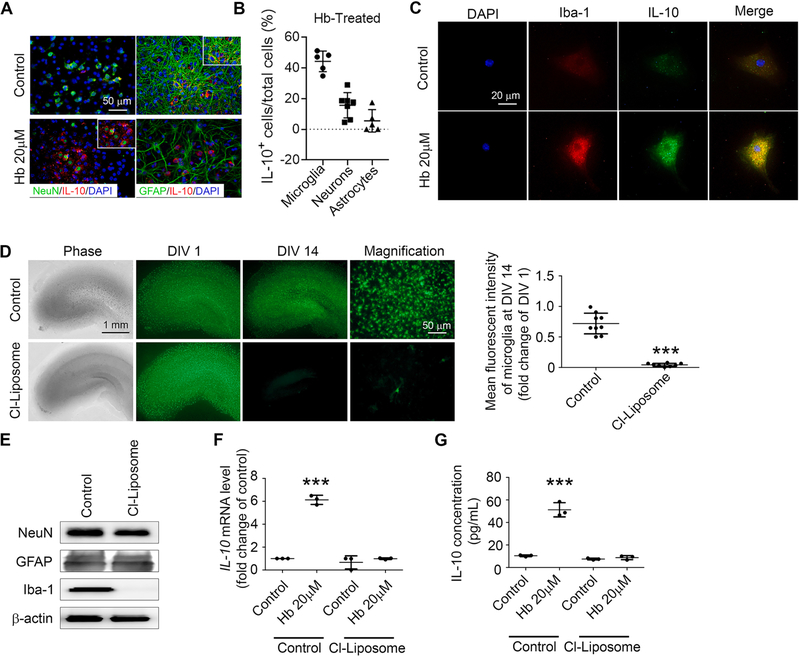
Neurons (Sharma et al., 2011), astrocytes (Lobo-Silva et al., 2016), and microglia (Koscsó et al., 2012) all have been reported to produce IL-10. To determine which cell population expresses IL-10 in response to Hb exposure, we immunostained Hb-treated OHSCs and found that microglia (Fig. 2B and 3B) and the majority of neurons (Fig. 3A, B), but not astrocytes (Fig. 3A, B), stained positive for IL-10. However in cell cultures, Hb did not increase IL-10 secretion in human induced pluripotent stem cell (iPSC)-derived neurons (Supplemental Fig. 1K) or in mouse primary neurons (Supplemental Fig. 1L); nor did it change the secretion of IL-10 in mouse primary astrocytes (Supplemental Fig. 1M). In mouse primary microglial cells, in contrast, Hb did increase IL-10 secretion (Supplemental Fig. 1N), mRNA expression (Supplemental Fig. 1O), and protein expression (Fig. 3C). We also used clodronate-containing liposomes to selectively deplete microglia from OHSCs (Fig. 3D), as evident by the loss of Iba-1 with no change in NeuN or GFAP expression (Fig. 3E). After microglia depletion, Hb exposure failed to upregulate IL-10 mRNA (Fig. 3F) or protein (Fig. 3G). Collectively, these ex vivo data implicate microglia as the main source of IL-10 evoked by Hb and that IL-10 is required for evoked phagocytosis.
Fig. 3.
Hemoglobin (Hb) upregulates microglial IL-10 in OHSCs. (A) OHSCs prepared from C57BL/6 pups were treated as indicated for 16 h. Slices were fixed and immunostained with IL-10 and NeuN or GFAP antibodies. Nuclei were stained with DAPI. Representative images from the CA1 region are shown. Insets are high-power images showing colocalization of IL-10+/NeuN+ cells but not colocalization of IL-10+ and GFAP+ cells. (B) Quantifications of percentage of IL-10+ microglia/neurons/astrocytes in Hb-treated OHSCs are shown. n = 5–7. (C) Mouse primary microglia were treated as indicated for 16 h, fixed, and immunostained with IL-10 antibodies. Nuclei were stained with DAPI. (D-G) OHSCs prepared from CX3CR1GFP/+ pups were treated with control-liposome (Control) (Pichler et al., 2013) or clodronate-liposome (Cl-Liposome) for 14 days. DIV, days in vitro. (D) Representative images from the slices and mean fluorescence intensity are shown. ***p < 0.001 vs. Control. n = 8. (E) Slices were collected and homogenized for Western blotting. β-actin served as a loading control. (F, G) OHSCs were treated with control-liposome (Pichler et al., 2013) or Cl-Liposome for 14 days and further incubated with either Hb or vehicle for 16 h. (F) Culture medium was collected and concentrated for use in an ELISA. ***p < 0.001 vs. Control. n = 3. (G) mRNA was extracted from the OHSCs, and real-time RT-PCR was carried out with IL-10 primers. GAPDH was used as an internal control. ***p < 0.001 vs. Control. n = 3. D: Student’s t-test followed by Welch’s correction; F, G: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test. Results are from at least three independent experiments. Scale bars: (A) and right panels for (D) 50 μm; (C) 20 μm; left panels for (D) 1 mm.
3.5. IL-10–deficient mice have delayed hematoma clearance and more severe acute outcomes after ICH
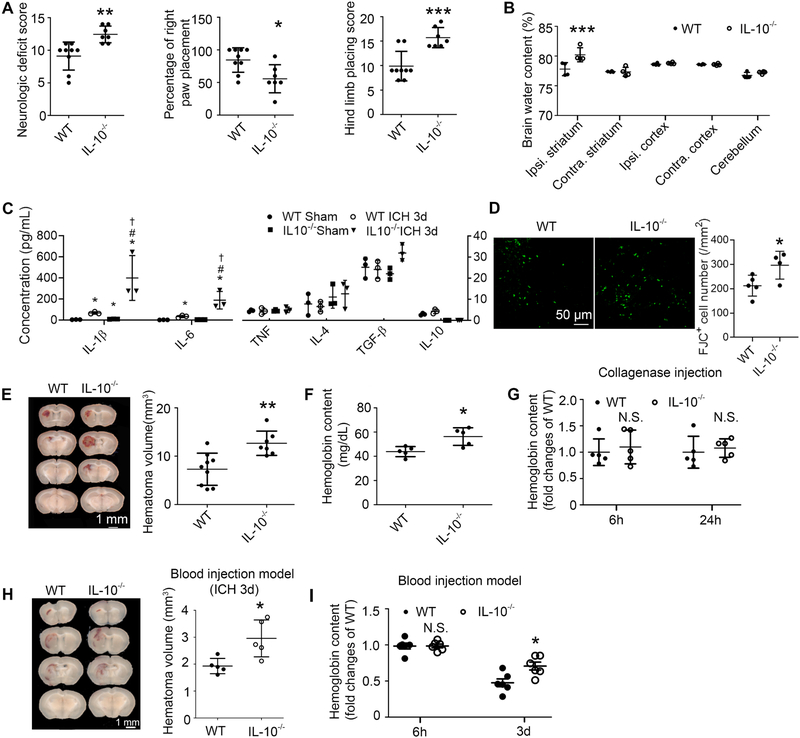
To determine whether data obtained in slice cultures can be replicated in vivo, we performed collagenase-induced ICH in IL-10−/− mice and in wild-type (WT) mice. At 5 days post-ICH, IL-10−/− mice had significantly greater neurologic deficit, right forelimb muscle weakness, and hind limb muscle weakness than did WT mice (Fig. 4A). Sham-operated WT and IL-10−/− mice displayed no behavior differences (data not shown). Brain edema peaks 3 days after ICH in the collagenase mouse model; however, we found no significant difference in the ipsilateral striatum between WT and IL-10−/− ICH mice (Supplemental Fig. 2A). However, at day 5 the percentage of ipsilateral striatal water content was elevated in IL-10−/− mice (Fig. 4B). ELISAs showed that at 3 days post-ICH, IL-10−/− mice displayed greater increases in brain IL-1β and IL-6 than did WT mice. No significant differences were seen among groups for TNF-α, IL-4, TGF-β, and IL-10 (Fig. 4C). Furthermore, at 5 days post-ICH, IL-10−/− mice had markedly increased number of FJC+ cells in the perihematomal region than did WT mice (Fig. 4D).
Fig. 4. IL-10 deficiency aggravates acute ICH outcomes and delays hematoma clearance in vivo.
(A-G) Male C57BL/6 (WT) or IL-10−/− mice underwent collagenase injection or sham procedure. (A) Neurologic deficit score, right front paw placement, and hind limb placing scores preference tested at day 5. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT. n = 7–9. (B) Ipsilateral (Ipsi.) and contralateral (Contra.) regional water content measured at day 5. Cerebellum served as an internal control. ***p < 0.001 vs. WT Ipsi. striatum. n = 4. (C) ELISA-based cytokine measurements from homogenates of 4-mm slices from hematoma core and perihematoma region at 3-day post-surgery. *p < 0.05 vs. corresponding WT Sham; #p < 0.05 vs. WT ICH 3d, †p < 0.05 vs. IL-10−/− Sham. n = 3–4. (D) Mice were sacrificed at day 5 post-ICH and brain sections were stained with Fluoro-Jade C (FJC). Representative images from perihematomal region and quantification of FJC+ cells are shown. *p < 0.05 vs. WT. n = 4–5. (E) Representative images from fresh coronal sections harvested at day 5 and quantified hematoma volume. **p < 0.01 vs. WT. n = 7–9. (F, G) Hemoglobin content measured at hour 6, hour 24 (G) and day 5 (F) post-ICH in injured hemisphere after PBS perfusion. *p < 0.05 vs. WT. n = 5. (H, I) Male WT or IL-10−/− mice (8–10 weeks old) underwent injection with 8 μL of autologous whole arterial blood. (H) Mice were sacrificed at day 3 post-ICH. Left: Representative images of fresh brain coronal sections; Right: Quantification of hematoma volume. *p < 0.05 vs. WT. n = 5. (I) Mice were perfused with PBS at 6 h or 3 days post-ICH. Tissue from the injured hemisphere was homogenized for measuring hemoglobin content. N.S., not significant; *p < 0.05 vs. corresponding WT. n = 6. Results are presented as scatter plots (mean ± SD). A, D, E, F, H: Student’s t-test followed by Welch’s correction. B, G, I: Two-way ANOVA followed by Bonferroni post-hoc test. C: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test. Results are from at least three independent experiments. Scale bars: (D) 50 μm; (E) and (H) 1 mm.
Because IL-10−/− ICH mice exhibited significantly prolonged neurologic deficiency, brain edema, more severe inflammation and greater number of degenerative neurons, we evaluated whether this was associated slower disposition of the hematoma. At day 5, IL-10−/− ICH mice had significantly larger hematoma volume (Fig. 4E) and tissue hemoglobin content (Fig. 4F) than did WT mice despite no difference in hemoglobin content at 6 or 24 h (Fig. 4G). For further confirmation, we injected autologous arterial blood into WT and IL-10−/− mice. Again, IL-10−/− mice exhibited slower hematoma and hemoglobin clearance (Fig. 4H, I). Moreover, IL-10−/− mice had greater iron deposition after collagenase injection (Supplemental Fig. 2B, C).
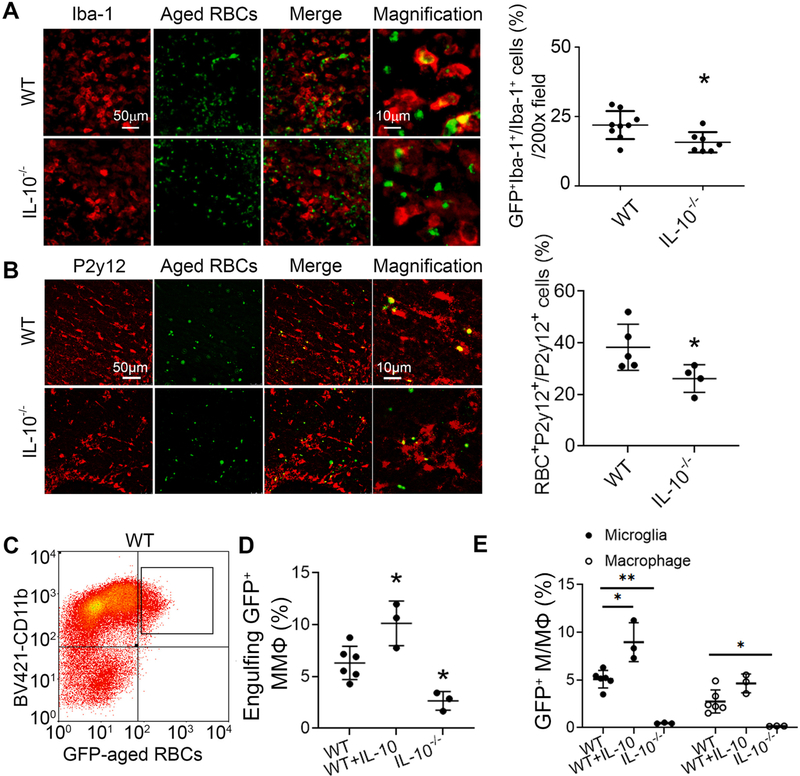
To further investigate the delayed hematoma clearance, we injected aged RBCs prepared from GFP-UBC mice into striatum. At 5 days, Iba-1–positive microglia/macrophages were visible with phagocytosed RBCs (Fig. 5A). IL-10−/− ICH mice had significantly fewer aged RBC phagocytes than did the WT group. We further confirmed this result using a microglial specific marker P2y12 (Fig. 5B). In addition, we administered mouse recombinant IL-10 protein to RBC-injected WT mice (WT + IL-10). We further dissociated cells from ipsilateral brain tissue of injured WT, WT + IL-10 and IL-10−/− mice, and then performed flow cytometry using GFP to identify aged RBCs, BV421-CD11b to identify microglia and macrophages, and APC-CD45 to separate microglia from monocyte-derived macrophages (Fig. 5C and Supplemental Fig. 3A). Compared with WT ICH animals, administration of recombinant IL-10 increased and IL-10 deficiency decreased the phagocytic population significantly, as assessed by the percentage of GFP+CD11b+/CD11b+ cells (Fig. 5D). Moreover, the majority of phagocytic cells were microglia (CD11b+CD45int) and the minority were macrophages (CD11b+CD45high) in all groups (microglia vs. macrophage cell number in 50,000 live cells, WT: 894 ± 328.9 vs. 467 ± 183.5; WT + IL-10: 1213 ± 106.2 vs. 666 ± 248.47; IL-10−/− : 351 ± 40.4 vs. 141 ± 33.8), indicating that deficiency of IL-10 may affect both cell populations concurrently (Fig. 5E). Notably, administered IL-10 enhanced the microglial phagocytosis ability specificity (Fig. 5E). In addition, we sorted GFP+CD11b+CD45intPI− and GFP+CD11b+CD45high PI− cells to confirm the cell type by morphology and cell body diameters (Supplemental Fig. 3B). Additionally, we obtained aged RBCs from WT mice, stained them with PKH26 fluorescent cell membrane labeling dye and injected them into WT and IL-10−/− mouse striatum to confirm our results with flow cytometry (Supplemental Fig. 3C, D). Although fewer CD11b+ phagocytes were detected in this experiment, the results again showed that IL-10 deficiency decreased phagocytic population compared with that in WT mouse.
Fig. 5. IL-10 deficiency decreases engulfing red blood cells (RBCs) phagocytes after ICH in vivo.
(A, B) Male C57BL/6 (WT) or IL-10−/− mice (8–10 weeks old) were injected with aged RBCs. Brain slices obtained at day 5 were stained with Iba-1 (A) or P2y12 (B) antibodies. Representative images from the perihematomal region and the percentage of Iba-1+/GFP+ and P2y12+/GFP+ cells are shown. *p < 0.05 vs. WT. n = 4–9. (C-E) WT, IL-10−/−, or WT that were administered IL-10 (WT + IL-10) mice were injected with aged RBCs. Mouse brain was perfused with PBS and dissociated into single cells. Cells were stained with different antibodies for flow cytometry. (C) Representative flow plot shows the CD11b+ GFP+ population after gating the PI− cells (population inside the solid line). Graphs show the percentage of GFP+CD11b+ cells (D) and percentage of phagocytes with engulfed RBCs (E). D: *p < 0.05, **p < 0.01 from corresponding WT group. n = 3–6. Results are presented as scatter plots (mean ± SD). A, B: Student’s t-test followed by Welch’s correction. D: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test E: Two-way ANOVA followed by Bonferroni post-hoc tests. Results are from at least three independent experiments. Scale bars: (A) and (B) left panels 50 μm; (A) and (B) right panels 10 μm. MMФ: microglia and macrophages; M/MФ: microglia/macrophages. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. IL-10 deficiency increases macrophage infiltration and polarizes phagocytes to proinflammatory phenotype in vivo
To investigate the effects of IL-10 deficiency on the activation of microglia and macrophages, we measured the percentage of microglia and macrophages with flow cytometry. Gating strategy and proper controls are shown in Supplemental Fig. 4. Staining with FITC-CD11b and PE-CD45 showed that the percentage of cells in WT and IL-10−/− sham groups that were CD11b+ increased significantly from approximately 15% to around 30% at 3 and 5 days post-ICH in both substrains (Fig. 6A, B). As expected, sham mice had few CD11b+CD45high cells (Fig. 6A, C). However, at 3 days post-ICH, IL-10−/− mice had a significantly lower percentage of CD11b+CD45int cells and higher percentage of CD11b+CD45high cells than did WT mice (Fig. 6A, C).
Fig. 6. IL-10 deficiency increases monocyte-derived macrophage infiltration and changes its surface marker expression in vivo.
Male C57BL/6 (WT) and IL-10−/− mice underwent collagenase injection or sham procedure. Brains were perfused with PBS and dissociated into single cells that were stained with antibodies for flow cytometry. (A) Representative flow plots show the CD11b+CD45+ population after gating the PI− cells (population inside the solid line). Ma, macrophage; Mi, microglia. (B) Percentages of CD11b+ cells. *p < 0.05 vs. corresponding Sham; N.S., not significant; n = 3–4. (C) Percentages of CD45highCD11b+/CD11b+ cells (macrophage) and CD45intCD11b+/CD11b+ cells (microglia). *p < 0.05, **p < 0.01 vs. corresponding WT group; n = 3–4. (D-I) Cells were stained with different antibodies for flow cytometry. The absolute cell counts of CD86+CD11b+ (E) and CD206+CD11b+ (G) were quantified based on the total events. The percentages of CD86+CD11b+ (D, H) and CD206+CD11b+ (F, I) cells were quantified. Representative flow plots show CD45+CD86+ (D) and CD45+CD206+ (F) populations after gating the CD11b+PI− cells. (E, G): *p < 0.05 vs. corresponding Sham; N.S., not significant. (H, I): *p < 0.05 vs. corresponding WT. n = 3–4. Results are presented as scatter plots (mean ± SD). B, E, G: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test; C, H, I: Two-way ANOVA followed by Bonferroni post-hoc tests. All results are from at least three independent experiments. M/MФ: microglia/macrophages.
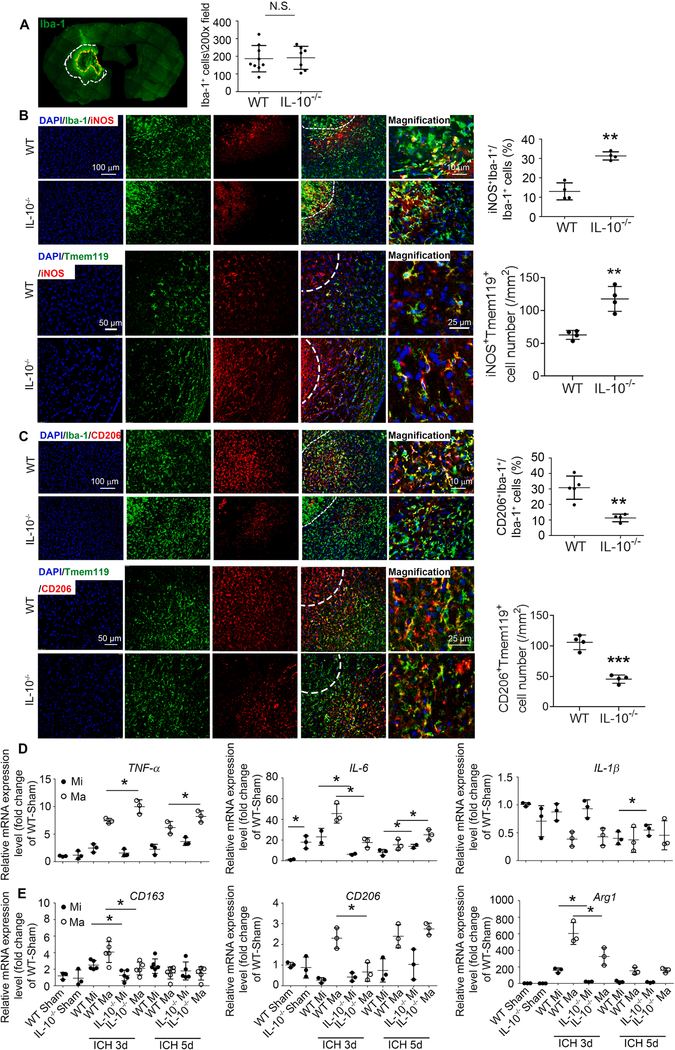
Additionally, we stained cells with surface markers BV421-CD86 or APC-CD206. The cell number of CD11b+ cells with these markers increased after ICH but without differences between WT and IL-10−/− mice (Fig. 6D–G). However, selective macrophage staining with PE-CD45 revealed an increased population of CD86+ macrophages (Fig. 6H) and decreased population of CD206+ macrophages in IL-10−/− mice at 3 days post-ICH (Fig. 6I). To determine whether IL-10 deficiency changes surface marker expression in vivo, we performed immunostaining to target the perihematoma region in the ICH brain (Fig. 7A). When compared with WT ICH mice, IL-10−/− ICH mice had no difference in number of Iba-1+ cells in perihematoma regions but a significantly greater percentage of iNOS+Iba-1+/ Iba-1+ cells and a reduced percentage of CD206+Iba-1+/ Iba-1+ cells at 5 days (Fig. 7B, C). Similar results were observed using a microglial specific marker Tmem119 (Fig. 7B, C).
Fig. 7. IL-10 deficiency changes microglia and monocyte-derived macrophage activation status in vivo.
Male C57BL/6 (WT) and IL-10−/− mice (8–10 weeks old) underwent collagenase injection or sham procedure. (A-C) Mice were sacrificed at day 5 post-ICH. Brain slices were stained with different antibodies. DAPI indicates nuclei. (A) Yellow dash line indicates hematoma core, and the area between white dash line and yellow dash line indicates perihematoma area. Quantification of the numbers of Iba-1+ cells is shown. N.S., not significant; n = 7–9. (B, C) Representative images from the perihematomal region and quantification of the percentages/numbers iNOS+Iba-1+, CD206+Iba-1+, iNOS+Tmem119+, and CD206+Tmem119+ cells are shown. **p < 0.01, ***p < 0.001 vs. WT. n = 4. (D, E) Brains were perfused with PBS and dissociated into single cells that were stained with antibodies for flow cytometry. CD11b+ cells in sham mice and CD45highCD11b+ (macrophage) and CD45intCD11b+ (microglia) cells in ICH mice were sorted after gating the PI− cells. mRNA was extracted from sorted cells, and real-time RT-PCR was carried out with different primers. *p < 0.05 vs. corresponding WT group. n = 3–5. Results are presented as scatter plots (mean ± SD). A-C: Student’s t-test followed by Welch’s correction. D, E: Two-way ANOVA followed by Bonferroni post-hoc tests. All results are from at least three independent experiments. Scale bars: (B) and (C) left panels 100 μm for Iba-1 staining and 50 μm for Tmem119 staining; (B) and (C) right panels 10 μm for Iba-1 staining and 25 μm for Tmem119 staining. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We further sorted microglia and macrophages from ICH groups based on the expression of CD45 (the entire CD11b+ cell population was sorted in sham groups owing to the small number of macrophages). We evaluated proinflammatory status with extracted mRNA measurements of CD16, CD86, CD32, TNF-α, IL-6, IL-1β, and iNOS. Compared with levels in WT mice, IL-10 deficiency increased TNF-α expression on macrophages at 3 and 5 days post-ICH, increased IL-6 expression on both microglia and macrophages (MMΦ) at 5 days post-ICH (Fig. 7D), decreased CD32 on MMΦ at 3 days post-ICH and decreased expression of iNOS on MMΦ at 5 days post-ICH (Supplemental Fig. 3E). No significant changes were detected in CD16, CD86, or IL-1β post-ICH in either mouse substrain (Fig. 7D and Supplemental Fig. 3E). We evaluated anti-inflammatory status with CD163, CD206, Arg1, YM-1, and TGF-β. Compared with levels in WT mice, IL-10 deficiency significantly decreased expression of CD163 and Arg1 on MMΦ at 3 days post-ICH, decreased expression of CD206 on macrophages at 3 days post-ICH (Fig. 7E), increased YM-1 expression on macrophages at 5 days post-ICH, and increased TGF-β expression on macrophages at 3 days post-ICH (Supplemental Fig. 3E).
3.7. IL-10 deficiency upregulates monocyte-derived macrophage-related cytokines/chemokines in vivo and recombinant IL-10 enhances macrophage phagocytosis in vitro
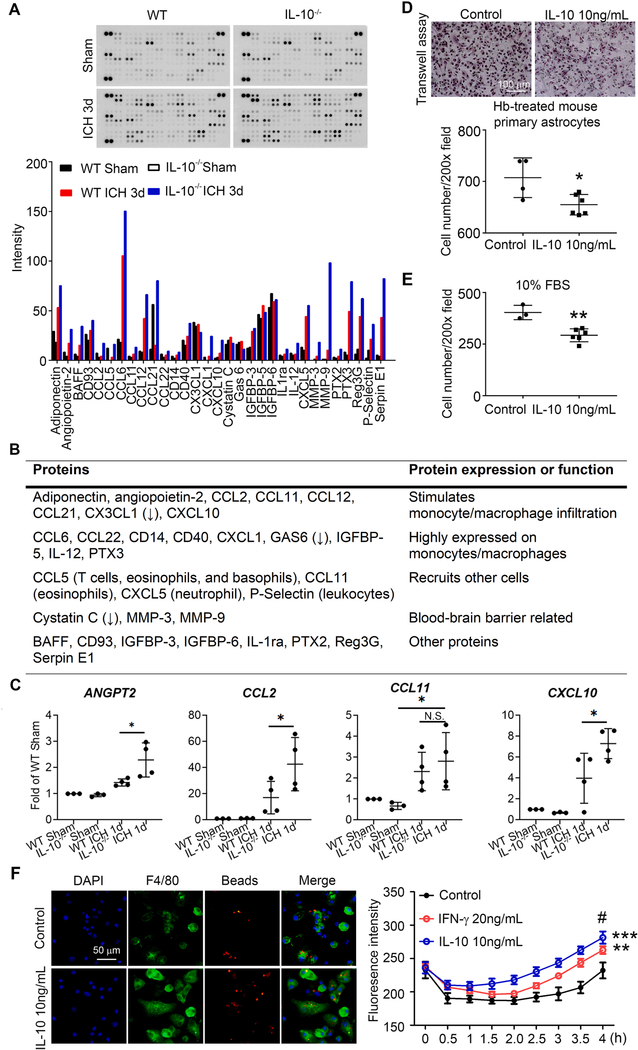
To investigate which chemokines were affected, we performed a cytokine array using homogenized brain tissue from WT and IL-10−/− mice before and after ICH. Three days after ICH, IL-10−/− mice had greater expression of monocyte-derived macrophage-related factors (adiponectin, angiopoietin-2, CCL2, CCL11, CCL12, CCL21, and CXCL10) and other blood cell (T cells, eosinophils, basophils, neutrophils, and leukocytes) infiltration-related factors (CCL5, CCL11, CXCL5, and P-Selectin) (Fig. 8A, B). Cytokines/chemokines that are highly expressed by monocytes/macrophages were also increased in IL-10−/− ICH mouse brain (CCL6, CCL22, CD14, CD40, CXCL1, IGFBP-5, IL-12, and Pentraxin 3; Fig. 8A, B). We further confirmed expression of chemokines that stimulate monocyte/macrophage infiltration with extracted mRNA measurements of ANGPT2, CCL2, CCL11, and CXCL10. Compared with levels in WT mice, IL-10 deficiency increased ANGPT2, CCL2, and CXCL10 expression at 1 day post-ICH (Fig. 8C).
Fig. 8. IL-10 deficiency upregulates macrophage-infiltration–related cytokines in vivo and enhances macrophage phagocytosis in vitro.
(A-C) Male C57BL/6 (WT) and IL-10−/− mice (8–10 weeks old) underwent collagenase injection or sham procedure. Four-millimeter tissue slices were collected from the hematoma core and perihematomal region of 3 day post-ICH and sham animals. Tissue was homogenized for cytokine/chemokine array. (A) Representative images and quantification of mean pixel density are shown. n = 1. (B) Functions of proteins identified by cytokine/chemokine assay are listed in table. (C) mRNA was extracted at 1 day after ICH, and real-time RT-PCR was carried out with different primers. *p < 0.05 vs. corresponding WT or Sham group; N.S., not significant. n = 4. (D-F) Bone marrow-derived macrophages from C57BL/6 mice were cultured. Cells were treated as indicated for 48 h. Migration of macrophages was assessed by Transwell assay in Transwell chambers plated with hemoglobin (Hb)-treated astrocytes (D) or medium containing 10% FBS (E). Representative images are shown and number of migrating macrophages was quantified. *p < 0.05, **p < 0.01 vs. control. n = 3–6. (F) Cells were incubated with fluorescence conjugated latex beads (4 μm in diameter) for 4 h. Fluorescence intensity was measured every 30 min in a plate reader. Fluorescence intensity from beads engulfed by macrophages was quantified. Another set of cells was incubated with beads for 4 h, fixed, and immunostained with F4/80. Representative images are shown. **p < 0.01, ***p < 0.001 vs. control; #p < 0.05 vs. IFN-γ. Results are presented as bar graph, line chart (mean ± SD), or scatter plots (mean ± SD). C: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test. D, E: Student’s t-test followed by Welch’s correction. F: Repeated measurement followed by Tukey’s multiple comparison. Results are from at least three independent experiments except for A. Scale bars: (D) 100 μm; (F) 50 μm.
To confirm that IL-10 affects macrophage migration and phagocytosis, we cultured bone marrow (BM)-derived macrophages from 6 to 8–week-old WT mice. Pretreatment with recombinant IL-10 significantly decreased migration of macrophages toward Hb-treated mouse primary astrocytes (Fig. 8D) or 10% FBS (Fig. 8E) in a Transwell assay. IL-10 incubation also increased BM-derived macrophage phagocytosis of latex beads at a level comparable to the IFN-γ positive control (Fig. 8F).
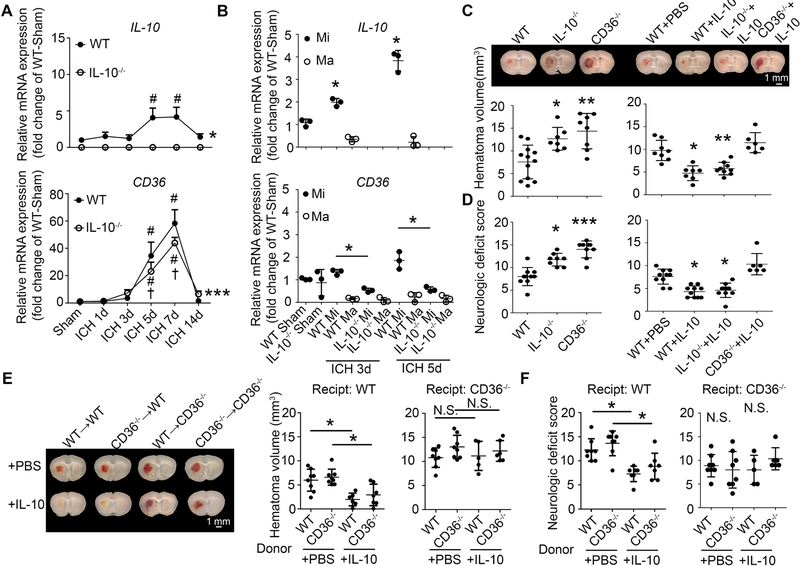
3.8. Decreased microglial CD36 expression causes delayed hematoma clearance in IL-10−/− mice
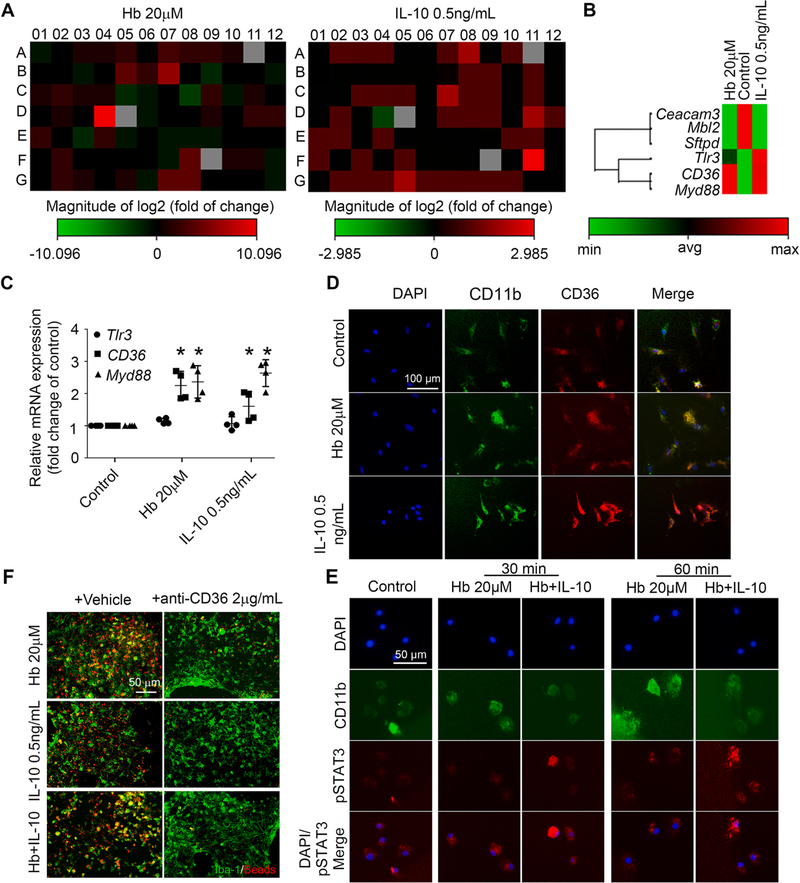
To determine which phagocytosis-related factors were upregulated by Hb-induced IL-10 production, we performed phagocytosis PCR arrays using Hb-, IL-10-, or vehicle-treated OHSCs (Supplemental Fig. 5A). We found that treatment with Hb or IL-10 upregulated CD36, an important receptor involved in microglial/macrophage phagocytosis (Fig. 9A, B). Consistent with this result, Hb and IL-10 upregulated CD36 mRNA and protein expression in OHSCs (Supplemental Fig. 5B–D) and primary microglia (Fig. 9C, D). It is known that IL-10 activates STAT3 and promotes CD36 gene transcription (Hutchins et al., 2013; Sp et al., 2018). To further confirm activation of IL-10/STAT3/CD36 axis in microglia after Hb treatment, we examined the STAT3 phosphorylation (pSTAT3) in primary microglia. We found that IL-10 promoted pSTAT3 levels and accumulation in the nucleus in Hb-treated primary microglia (Fig. 9E). Moreover, enhancement of phagocytosis by Hb, IL-10, and Hb plus IL-10 was substantially reversed by adding CD36 neutralizing antibodies (Fig. 9F).
Fig. 9. IL-10 and CD36 are required for microglial phagocytosis activity in vitro.
(A, B) OHSCs prepared from C57BL/6 pups were treated as indicated for 16 h, mRNA was extracted, and real-time RT-PCR was carried out with a phagocytosis PCR array. (A) Heat map of gene changes compared with control group. (B) Hemoglobin (Hb)- and IL-10–co-regulated gene sets. (C, D) Mouse primary microglia were treated as indicated for 16 h. (C) mRNA was extracted from the mouse primary microglia, and real-time RT-PCR was carried out with different primers. *p < 0.05 vs. corresponding Control; n = 3–4. (D) Cells were fixed and immunostained with CD11b and CD36 antibodies. Nuclei were stained with DAPI. Representative images are shown. (E) Mouse primary microglia were treated as indicated. Cells were fixed and immunostained with CD11b and pSTAT3 antibodies. Nuclei were stained with DAPI. Representative images are shown. (F) OHSCs were treated as indicated for 16 h and then incubated with fluorescence-conjugated latex beads (4-μm diameter) for 4 h, fixed, and immunostained with Iba-1 antibodies. Representative images are shown. C: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test. All results are from at least three independent experiments except for A and B. Scale bars: (D) 100 μm; (E) and (F) 50 μm.
To determine whether decreased expression of CD36 could account for delayed hematoma clearance in IL-10−/− mice, we analyzed mRNA from WT and IL10−/− mice after collagenase-induced ICH. IL-10 mRNA increased as early as 1 day, peaked at 5 days, and decreased at 14 days post-ICH (Fig. 10A); CD36 mRNA level peaked at 7 days but was lower in IL-10−/− ICH mice (Fig. 10A). Additionally, we performed real-time PCR on CD11b+ sorted microglia and monocyte-derived macrophages from sham and ICH mice (3 and 5 days). Consistent with previous results, mRNA expression of CD36 peaked on day 5 and was significantly decreased in IL-10−/− mice (Fig. 10B). Interestingly, most of the CD36 was expressed on microglia, not on monocyte-derived macrophages (Fig. 10B). Similarly, most IL-10 was expressed by microglia rather than monocyte-derived macrophages (Fig. 10B). Moreover, CD36 was expressed mainly on Iba-1+ cells with microglial morphology around the hematoma core (Supplemental Fig. 5E). This result was further confirmed by using Tmem119 antibodies (Supplemental Fig. 5F). IL-10 deficiency markedly decreased CD36 expression (Supplemental Fig. 5E, F).
Fig. 10. IL-10−/− mice exhibit delayed hematoma clearance due to decreased microglial CD36 expression.
(A, B) Male C57BL/6 (WT) and IL-10−/− mice underwent collagenase injection or sham procedure. Brains were perfused with PBS, and 4-millimeter tissue slices were collected from the hematoma core and perihematoma region of injured and sham animals. (A) Tissues were homogenized, mRNA was extracted, and real-time RT-PCR was performed. *p < 0.05, ***p < 0.001 vs. WT; #p < 0.05 vs. corresponding Sham; †p < 0.05 vs. corresponding WT; n = 3–4. (B) Tissue was dissociated into single cells that were stained with antibodies for flow cytometry. CD11b+ cells in sham animals and CD45highCD11b+ cells (macrophage) and CD45intCD11b+ cells (microglia) in injured animals were sorted after gating the PI− cells. mRNA was extracted from sorted cells, and real-time RT-PCR was performed and normalized by the WT sham microglia expression for each run. *p < 0.05 vs. Sham (upper panel), or *p < 0.05 vs. corresponding WT group (lower panel). n = 3. (C, D) At 2 h after collagenase injection, male WT, IL-10−/−, and CD36−/− mice received recombinant IL-10 protein or PBS via intranasal administration. (C) Hematoma volume was measured on fresh brain coronal sections and representative images are shown. *p < 0.05, **p < 0.01 vs. WT or WT + PBS. n = 6–12. (D) Neurologic deficit score was assessed at day 5. *p < 0.05, ***p < 0.001 vs. WT or WT + PBS. n = 6–10. (E, F) WT and CD36−/− mice received transplanted bone marrow as indicated. After recovery, mice underwent collagenase injection followed 2 h later by intranasal administration of recombinant IL-10 protein or PBS. (E) Hematoma volume was measured on fresh brain coronal sections, and representative images are shown. *p < 0.05 vs. WT + PBS; N.S., not significant. n = 5–8. (F) Neurologic deficit score was assessed at day 5. *p < 0.05 vs. WT or WT + PBS. n = 6–10. A: Two-way ANOVA followed by Bonferroni post-hoc test; B-F: One-way ANOVA followed by Dunn’s multiple comparison post-hoc test; Results are from at least three independent experiments. Scale bars: (C) and (E) 1 mm.
To confirm the role of CD36 in hematoma clearance, we injected collagenase into CD36−/− and WT mice. Although hemoglobin content was similar at 24 h post-ICH (Supplemental Fig. 6B), hemoglobin content and hematoma volume were significantly higher in the CD36−/− group than in the WT group at 5 days after ICH (Supplemental Fig. 6A, B).
Intraventricular injection of IL-10 can decrease hematoma volume (Chang et al., 2017). Here, we used intranasal administration to enhance clinical relevance and determine whether it regulates CD36. At 5 days post-ICH, IL-10–deficient and CD36-deficient mice had worse neurologic deficit scores and motor function than did WT mice. Daily intranasal IL-10 administration starting at 2 h after collagenase injection significantly corrected the neurologic deficits in WT and IL-10−/− groups but not in the CD36−/− group (Fig. 10D). Consistent with behavioral changes, IL-10−/− and CD36−/− mice had larger hematomas than did WT mice. IL-10 administration accelerated hematoma clearance in WT and IL-10−/− mice but did not alter hematoma size in CD36−/− mice (Fig. 10C).
To further confirm that microglial CD36 is the main effector of hematoma clearance, we made bone marrow (BM) chimeras using WT or CD36−/− mice as recipient or donor mice. As busulfan depletes only peripheral myeloid cells and microglia remain of host origin (Peake et al., 2015), this BM transplantation experiment allows us to compare the function of CD36 on microglia and monocyte-derived macrophages after ICH. At 5 days after ICH, no significant difference in hematoma volume was detected in chimeras when BM was transplanted from WT to WT mice vs. CD36−/− to WT mice (Fig. 10E). Although hematoma volumes were larger in CD36−/− recipient mice, no difference was detected between chimeras when BM was transplanted from WT to CD36−/− mice or from CD36−/− to CD36−/− mice (Fig. 10E). In addition, when we administered recombinant IL-10 protein or PBS intranasally to chimeras, IL-10 administration significantly decreased hematoma volume in WT recipient mice, but not in CD36−/− recipient mice (Fig. 10E). IL-10 administration corrected neurologic deficits in WT recipient mice, but not in CD36−/− recipient mice at 5 days post-ICH (Fig. 10F). These data indicate that microglial CD36, but not CD36 on monocyte-derived macrophages, could be the main downstream effector in response to IL-10 treatment.
4. Discussion
IL-10 is a classic anti-inflammatory cytokine that induces macrophage alternative activation (Zhu et al., 2019). Its overexpression improves ischemic stroke outcomes (de Bilbao et al., 2009) and protects against spirochetal brain infection (Londoño et al., 2008); however, blocking IL-10 benefits mice in an Alzheimer’s disease model by promoting β-amyloid clearance (Guillot-Sestier et al., 2015). Thus, the role of IL-10 in acute and chronic brain diseases is unsettled, and little is known about the role of IL-10 in regulating phagocytosis and hematoma clearance after ICH. In this study, we demonstrate that IL-10–deficient mice have greater inflammation, slower phagocytosis of RBCs associated with lower expression of CD36, and worse outcome from ICH, whereas intranasal administration of IL-10 accelerated hematoma clearance and neurologic recovery as long as CD36 expression was intact.
Using an ex vivo mouse OHSC model, which preserves cytoarchitecture and mirrors the ultra-early stage of ICH before recruitment of blood-derived immune cells, we found that RBC metabolite hemoglobin induced IL-10 overexpression and secretion. In the ICH mouse brain, IL-10 mRNA was upregulated in the perihematoma region at 1 day, peaked at 7 days, and was nearly back to baseline at 14 days. Taylor et al. (Taylor et al., 2017) did not detect overexpression of IL-10 in the brain tissue in the blood-injection mouse model, but the expression might have been too low to detect (10–100 pg/mL) (Zhu et al., 2019). We and others have shown in animal studies (Gao et al., 2014; Zhu et al., 2019) that IL-10 mRNA and protein level starts increasing as early as 1.5 h after collagenase-induced ICH and peaks at 7 days. In ICH patients, plasma IL-10 can increase by day 1 (Dziedzic et al., 2002) and peak at day 3 to levels that negatively correlate with severity of cerebral edema (Wang et al., 2016). Although plasma IL-10 level was associated with rebleeding in ICH patients (Wang et al., 2011), we did not observe increased bleeding with intranasal administration of recombinant IL-10.
All of this evidence indicates that IL-10 expression is increased after ICH in plasma and brain tissue and that increasing IL-10 could be beneficial for ICH outcomes. Indeed, IL-10−/− mice had more severe brain edema and iron overload, more severe neurologic behavior dysfunction, and weaker front and hind limb muscle force. Furthermore, IL-10–deficient mice had more severe inflammation after ICH than did WT mice, as evidenced by increased expression of IL-1β and IL-6. Interestingly, brain inflammation was detected in IL-10−/− sham animals. It has been reported that IL-10−/− mice have uncontrolled immune responses and that they spontaneously develop gut inflammation and chronic enterocolitis after weaning (Kuhn et al., 1993; Keubler et al., 2015). To the best of our knowledge, we are the first group to report inflammatory markers in the IL-10−/− sham brain. Loss of IL-10 may eliminate the immunosuppressive role of microglia (Iyer and Cheng, 2012).
Major functions of mononuclear cells include the phagocytosis of dead cells and the removal of cellular debris (Kettenmann et al., 2013) and the timely removal of RBCs from the extravascular spaces (de Back et al., 2014). However, the specific function of microglia and monocyte-derived macrophages in hematoma clearance after ICH is not fully understood. The fact that open surgery trials (Mendelow et al., 2013) and thrombolytic irrigation trials (Hanley et al., 2019) of hematoma evacuation have not shown clear positive results emphasizes the need to investigate the endogenous mechanisms of hematoma removal. We found that Hb effectively stimulated microglia and macrophages engulfment of latex beads in vitro; both microglia and macrophages engulfed RBCs in vivo, but the majority of phagocytes were microglia due, in part, to the greater abundance of microglia. Unexpectedly, only a small percentage of RBCs (approximately 5%) were engulfed by phagocytes on days 3 and 5. It is possible that phagocytosed and digested RBCs in the phagolysosomes are undetectable by immunostaining and flow cytometry at these time points. Whether hematoma clearance depends on mechanisms (e.g., RBC lysis) other than MMΦ phagocytosis and over longer time intervals needs further investigation.
Using multiple different methods, we confirmed that IL-10−/− mice had delayed hematoma clearance compared with that in WT mice. One possibility is that IL-10 deficiency inhibits phagocytosis by MMΦ in vitro and in vivo. Although IL-10−/− mice recruited more monocyte-derived macrophages to the lesion by secreting monocyte/macrophage-related chemokines, the infiltrating IL-10−/− monocytes-derived macrophages were not able to rectify the attenuated phagocytic function. We also found that those infiltrating monocyte-derived macrophages expressed higher levels of proinflammatory cytokine markers, which may further increase the inflammatory response after ICH. Some evidence indicates that infiltrating macrophages are capable of suppressing microglia phagocytic activity via actions of prostaglandin E2 on microglia EP2 receptors (Greenhalgh et al., 2018).
A PCR array revealed that one of the downstream targets of IL-10 signaling is CD36, a class B scavenger receptor that plays an important role in recognition of oxidized lipids and as part of the receptor system that promotes phagocytosis (Fadok et al., 1998; Woo et al., 2016). CD36 has been identified as a key regulator of hematoma clearance after ICH (Fang et al., 2014; Zhao et al., 2015a). In our study, we confirmed that CD36-deficient mice had delayed hematoma clearance and that IL-10-mediated signaling acted as a master regulator of CD36 in vitro and in vivo. In previous studies of ICH, CD36 expression was shown to be regulated by nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) (Zhao et al., 2015a), PPARγ (Zhao et al., 2007) and TLR4 (Fang et al., 2014). IL-10 also upregulates CD36 expression in microglial cell cultures (Fang et al., 2014). However, the in vivo relationship between IL-10 and CD36 after ICH has not yet been fully elucidated. Studies have shown that CD36 regulates the expression of IL-10 in macrophages in contact with oxidized low-density lipoprotein, which mimics atherosclerosis development (Rios et al., 2013), and that macrophage IL-10 production is partially dependent on CD36 in a model of phagocytic apoptotic cells (Chung et al., 2007; Ferracini et al., 2013). These findings raise the question of whether the regulatory relationship between IL-10 and CD36 is disease-dependent or cell type-dependent. We found that IL-10 was expressed and upregulated mainly in microglia, and that CD36 was primarily upregulated in microglia as well. Importantly, we demonstrated that microglia are the main source of IL-10 and CD36 in mouse brain after ICH.
Microglia may have different responses than infiltrating monocyte-derived macrophages to the same stimulation (ICH in this case). Indeed, although gene expression changes in microglia and monocyte-derived macrophage populations exhibited similar trends from day 3 to 5 post-ICH, the changes were more profound in monocyte-derived macrophages in most cases. Consistent with our findings, Hickman et al. (Hickman et al., 2013) showed that gene expression levels differed dramatically between the two cell populations in a study of aging. Likewise, in ischemic stroke, microglia produced relatively higher levels of reactive oxygen species and TNF-α, whereas monocytes produced mainly IL-1β (Ritzel et al., 2015; Zarruk et al., 2018). On the other hand, transcriptome analysis of infiltrating monocyte-derived macrophages acquire some of the distinctive markers of microglia in the setting chronic inflammation associated with experimental autoimmune encephalomyelitis (Grassivaro et al., 2020), thereby emphasizing the role of two-way communication between microglia and infiltrating macrophages. Furthermore, infiltrating macrophages can downregulate microglial phagocytosis (Greenhalgh et al., 2018), which could then alter the kinetics of RBC engulfment by microglia after ICH.
Our study had some limitations. First, we used global IL-10–deficient mice. As we found that neurons also express IL-10, a conditional microglial-specific IL-10–depleted mouse strain should be used in future studies to confirm the role of microglial IL-10 in phagocytosis and inflammation. Second, we focused only on acute ICH outcomes in this study, and long-term effects will require further research. Additionally, we did not investigate the signaling pathway by which IL-10 regulates CD36. Third, although slice cultures provide the advantage of deciphering the effects of hemoglobin on microglia, it also eliminates potentially important interactions between blood-borne immune cells and microglia whereby monocyte-derived macrophages and microglia behave as an integrated functional unit (Grassivaro et al., 2020). Fourth, CD36 effects in immature brain after stroke differ from mature brain (Woo et al., 2012). Although our slice cultures were allowed 10–14 days to mature and thin, we cannot exclude incomplete maturation of inflammatory signaling pathways in the slice cultures. Finally, collagenase/autologous blood injection could also introduce peripheral monocytes to the mouse brain. Monocytes/macrophages that were analyzed and sorted from ipsilateral mouse brain by flow cytometry may include recruited peripheral monocytes/macrophages, and also a portion that was introduced to the brain during ICH modeling.
In conclusion, we found that Hb/ICH induced microglial IL-10 expression and enhanced microglial phagocytosis by regulating the IL-10–CD36 axis. Mice lacking IL-10 had worse outcomes after ICH, including worse neurologic deficits, greater inflammation, and delayed hematoma clearance. Importantly, intranasal administration of recombinant IL-10 significantly improved hematoma clearance and functional outcomes. These novel findings fill an important gap in knowledge regarding the role of IL-10/CD36 signaling in MMΦ phagocytic function after ICH and provide a vital foundation to develop cytokine–based immunotherapy for treating ICH.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (R01NS078026, R01NS102583, and R01NS105894 to J. Wang and R.C. Koehler), the American Heart Association (Grant-in-Aid 17GRNT33660766 to J. Wang; Scientist Development Grant 16SDG30980031 to XH; Career Development Award 19CDA34660065 to XL; Postdoctoral Fellowship Awards, 16POST29640010 to QL, 17POST33660191 to XL, and 18POST33970007 to J. Wan), and a Stimulating and Advancing ACCM Research (StAAR) Award from the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University.
We thank Dr. Jonathan Bromberg from University of Maryland for the gift of CX3CR1GFP/+ mice; Dr. Roger Johns from Johns Hopkins University for the gift of GFP-UBC mice; Dr. Xuhang Li from Johns Hopkins University for the gift of IL-10−/− mice; Dr. Alan Scott from Johns Hopkins University for the gift of CD36−/− mice; Dr. Mingyao Ying from Johns Hopkins University for the gift of human iPSC-derived neurons; and Dr. Hao Zhang from Flow Cytometry Cell Sorting Core Facility at Bloomberg School of Public Health, Johns Hopkins University, for doing FACS sorting. This facility was supported by 1S10OD016315–01,1S10RR13777001 and in part by CFAR: 5P30AI094189–04. We thank the Anesthesiology and Critical Care Medicine Flow Cytometry Core at Johns Hopkins School of Medicine for doing flow cytometry FMO control. We thank Claire Levine, MS, ELS, for assistance with manuscript preparation.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2021.02.001.
References
- Awad IA, Polster SP, Carrion-Penagos J, Thompson RE, Cao Y, Stadnik A, Money PL, Fam MD, Koskimaki J, Girard R, Lane K, McBee N, Ziai W, Hao Y, Dodd R, Carlson AP, Camarata PJ, Caron JL, Harrigan MR, Gregson BA, Mendelow AD, Zuccarello M, Hanley DF and M. I. T. Investigators (2019). Surgical Performance Determines Functional Outcome Benefit in the Minimally Invasive Surgery Plus Recombinant Tissue Plasminogen Activator for Intracerebral Hemorrhage Evacuation (MISTIE) Procedure. Neurosurgery 84(6): 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z, Shi L, Guo YL, Lv Z, Tang C, Niu S, Tremblay A, Venkataramani M, Culpepper C, Li L, Zhou Z, Mansour A, Zhang Y, Gewirtz A, Kidder K, Zen K, Liu Y, 2016. Cd47-Sirpalpha interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc. Natl. Acad. Sci. U.S.A 113 (37), E5434–E5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DA, Fribourg M, Sealfon SC, 2013. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J. Biol. Chem 288 (5), 2986–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-F, Cai L.i., Wang J, 2015. Translational intracerebral hemorrhage: a need for transparent descriptions of fresh tissue sampling and preclinical model quality. Transl Stroke Res. 6 (5), 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Wan J, Li Q, Renfroe SC, Heller NM, Wang J, 2017. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol. Dis 103, 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X, 2007. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27 (6), 952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A, 2011. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci 12 (7), 388–399. [DOI] [PubMed] [Google Scholar]
- de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R, 2014. Of macrophages and red blood cells; a complex love story. Front. Physiol 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, Giannakopoulos P, 2009. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J. Neurochem 110 (1), 12–22. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SBG, Guyenet PG, Kipnis J, 2012. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484 (7392), 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic T, Bartus S, Klimkowicz A, Motyl M, Slowik A, Szczudlik A, 2002. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke 33 (9), 2334–2335. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Warner ML, Bratton DL, Henson PM, 1998. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J. Immunol 161 (11), 6250–6257. [PubMed] [Google Scholar]
- Fang H, Chen J, Lin S, Wang P, Wang Y, Xiong X, Yang Q, 2014. CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling. J. Immunol 192 (12), 5984–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracini M, Rios FJ, Pecenin M, Jancar S, 2013. Clearance of apoptotic cells by macrophages induces regulatory phenotype and involves stimulation of CD36 and platelet-activating factor receptor. Mediators Inflamm. 2013, 950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM, 1998. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol 160 (2), 920–928. [PubMed] [Google Scholar]
- Fu Y, Liu Q, Anrather J, Shi F-D, 2015. Immune interventions in stroke. Nat. Rev. Neurol 11 (9), 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Kume T, Akaike A, 2006. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol. Dis 22 (1), 130–142. [DOI] [PubMed] [Google Scholar]
- Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ, Huang WL, ZhuGe WS, Zhang YL, Fu B, Jin KL, ZhuGe QC, 2014. Transplanted neural stem cells modulate regulatory T, gammadelta T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int. J. Mol. Sci 15 (3), 4431–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassivaro F, Menon R, Acquaviva M, Ottoboni L, Ruffini F, Bergamaschi A, Muzio L, Farina C, Martino G, 2020. Convergence between Microglia and Peripheral Macrophages Phenotype during Development and Neuroinflammation. J. Neurosci 40 (4), 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, Zarruk JG, Healy LM, Baskar Jesudasan SJ, Jhelum P, Salmon CK, Formanek A, Russo MV, Antel JP, McGavern DB, McColl BW, David S, 2018. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 16 (10), e2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier M-V, Doty K, Gate D, Rodriguez J, Leung B, Rezai-Zadeh K, Town T, 2015. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85 (3), 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Li Q, Lan X, EL-Mufti L, Ren H, Wang J, 2019. Microglial Depletion with Clodronate Liposomes Increases Proinflammatory Cytokine Levels, Induces Astrocyte Activation, and Damages Blood Vessel Integrity. Mol. Neurobiol 56 (9), 6184–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, Mayo SW, Bistran-Hall AJ, Gandhi D, Mould WA, Ullman N, Ali H, Carhuapoma JR, Kase CS, Lees KR, Dawson J, Wilson A, Betz JF, Sugar EA, Hao Y.i., Avadhani R, Caron J-L, Harrigan MR, Carlson AP, Bulters D, LeDoux D, Huang J, Cobb C, Gupta G, Kitagawa R, Chicoine MR, Patel H, Dodd R, Camarata PJ, Wolfe S, Stadnik A, Money PL, Mitchell P, Sarabia R, Harnof S, Barzo P, Unterberg A, Teitelbaum JS, Wang W, Anderson CS, Mendelow AD, Gregson B, Janis S, Vespa P, Ziai W, Zuccarello M, Awad IA, Abdul-Rahim A, Abou-Hamden A, Abraham M, Ahmed A, Alba CA, Aldrich EF, Altschul D, Amin-Hanjani S, Anderson D, Ansari S, Antezana D, Ardelt A, Arikan F, Baguena M, Baker A, Barrer SJ, Becker KJ, Bergman T, Boström A, Braun J, Brindley P, Broaddus WC, Brown R, Buki A, Cao B, Cao Y, Carrion-Penagos J, Chalela J, Chang T, Chorro IM, Chowdhry S, Corral L, Csiba L, Davies J, Díaz AT, Derdeyn CP, Diringer M, Dlugash R, Ecker R, Economas T, Enriquez P, Ezer E, Fan Y, Feng H, Franz D, Freeman WD, Fusco M, Galicich W, Gelea ML, Goldstein J, Gonzalez AC, Grabarits C, Greenberg S, Gress D, Gu E, Hall C, Hernandez FM, Hoesch R, Hoh BL, Houser J, Hu R, Huang Y.i., Hussain MA, Insinga S, Jadhav A, Jaffe J, Jahromi BS, Jallo J, James M, James RF, Jankowitz B, Jeon E, Jichici D, Jonczak K, Jonker B, Karlen N, Keric N, Kerz T, Knopman J, Koenig C, Krishnamurthy S, Kumar A, Kureshi I, Laidlaw J, Lakhanpal A, Latorre JG, Leifer D, Leiphart J, Lenington S, Li Y, Lopez G, Lovick D, Lumenta C, Luo J, Maas MB, MacDonald J, MacKenzie L, Madan V, Majkowski R, Major O, Malhorta R, Malkoff M, Mangat H, Maswadeh A, Matouk C, McArthur K, McCaul S, Medow J, Mezey G, Mighty J, Miller D, Mohan KK, Muir K, Muñoz L, Nakaji P, Nee A, Nekoovaght-Tak S, Nyquist P, O’Kane R, Okasha M, O’Kelly C, Ostapkovich N, Pandey A, Parry-Jones A, Perla KR, Pollack A, Polster S, Pouratian N, Quinn T, Rajajee V, Reddy K, Rehman M, Reimer R, Rincon F, Rybinnik I, Sanchez B, Sansing L, Schneck M, Schuerer L, Schul D, Schweitzer J, Seder DB, Seyfried D, Sheth K, Spiotta A, Stechison M, Szabo K, Tamayo G, Tanczos K, Taussky P, Terry J, Testai F, Thomas K, Thompson CB, Thompson G, Torner JC, Tran H, Tucker K, Ungar L, Varelas P, Vargas NM, Vatter H, Venkatasubramanian C, Vermillion K, Vollmer D, Wang Y, Wang Y, Wen J, Whitworth LT, Willis B, Wrencher M, Wright SE, Xu Y, Yanase L, Yi X, Yu Z, Zomorodi A, 2019. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 393 (10175), 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang L-C, Means TK, El Khoury J, 2013. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci 16 (12), 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins AP, Diez D, Miranda-Saavedra D, 2013. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct. Genomics 12 (6), 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Cheng G, 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol 32 (1), 23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR, 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol 20 (11), 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A, 2013. Microglia: new roles for the synaptic stripper. Neuron 77 (1), 10–18. [DOI] [PubMed] [Google Scholar]
- Keubler LM, Buettner M, Häger C, Bleich A, 2015. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm. Bowel Dis. 21 (8), 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscsó B, Csóka B, Selmeczy Z, Himer L, Pacher P, Virág L, Haskó G, 2012. Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. J. Immunol 188 (1), 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh A, Zarruk J, Passos dos Santos R, Gaestel M, David S, 2014. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83 (5), 1098–1116. [DOI] [PubMed] [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W, 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75 (2), 263–274. [DOI] [PubMed] [Google Scholar]
- Lan X, Han X, Li Q, Wang J, 2017a. (−)-Epicatechin, a Natural Flavonoid Compound, Protects Astrocytes Against Hemoglobin Toxicity via Nrf2 and AP-1 Signaling Pathways. Mol. Neurobiol. 54 (10), 7898–7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Han X, Li Q, Yang Q-W, Wang J, 2017b. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol 13 (7), 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Zhang JH, Noble-Haeusslein LJ, 2013. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl. Stroke Res. 4 (3), 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J, 2017a. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2 (7), e90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wan J, Lan X, Han X, Wang Z, Wang J, 2017b. Neuroprotection of brain-permeable iron chelator VK-28 against intracerebral hemorrhage in mice. J. Cereb. Blood Flow Metab. 37 (9), 3110–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Han X, Lan X, Hong X, Li Q, Gao Y, Luo T, Yang Q, Koehler RC, Zhai Y, Zhou J, Wang J, 2017c. Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol. Dis 108, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lan X, Han X, Wang J, 2018. Expression of Tmem119/Sall1 and Ccr2/CD69 in FACS-Sorted Microglia- and Monocyte/Macrophage-Enriched Cell Populations After Intracerebral Hemorrhage. Front. Cell. Neurosci 12, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Shi L, Gui B, Yu W, Wang J, Zhang D.i., Han X, Yao Z, Shang Y, 2011. Binding of the JmjC demethylase JARID1B to LSD1/NuRD suppresses angiogenesis and metastasis in breast cancer cells by repressing chemokine CCL14. Cancer Res. 71 (21), 6899–6908. [DOI] [PubMed] [Google Scholar]
- Li Q, Wijesekera O, Salas SJ, Wang JY, Zhu M, Aprhys C, Chaichana KL, Chesler DA, Zhang H, Smith CL, Guerrero-Cazares H, Levchenko A, Quinones-Hinojosa A, 2014. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin. Cancer Res. 20 (9), 2375–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M, 2016. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm 13 (1), 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño D, Carvajal J, Arguelles-Grande C, Marques A, Cadavid D, 2008. Interleukin 10 protects the brain microcirculation from spirochetal injury. J. Neuropathol. Exp. Neurol 67 (10), 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybeck KR, Storset AK, Olsen I, 2009. Neutralization of interleukin-10 from CD14(+) monocytes enhances gamma interferon production in peripheral blood mononuclear cells from Mycobacterium avium subsp. paratuberculosis-infected goats. Clin. Vaccine Immunol. 16 (7), 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, Investigators, S.I., 2013. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 382 (9890), 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C,, 2013. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci 16 (9), 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli B, Horiuchi H, Mizuno T, Takeuchi H, Suzumura A, 2015. CCL11 Enhances Excitotoxic Neuronal Death by Producing Reactive Oxygen Species in Microglia. Glia 63 (12), 2274–2284. [DOI] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini C-O, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA, 2017. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541 (7635), 41–45. [DOI] [PubMed] [Google Scholar]
- Peake K, Manning J, Lewis CA, Barr C, Rossi F, Krieger C, 2015. Busulfan as a myelosuppressive agent for generating stable high-level bone marrow chimerism in mice. J. Vis. Exp 98, e52553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-de Puig I, Miró F, Salas-Perdomo A, Bonfill-Teixidor E, Ferrer-Ferrer M, Márquez-Kisinousky L, Planas AM, 2013. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J. Cereb. Blood Flow Metab. 33 (12), 1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler I, Del Greco MF, Gogele M, Lill CM, Bertram L, Do CB, Eriksson N, Foroud T, Myers RH, Consortium PG, Nalls M, Keller MF, International C Parkinson’s Disease Genomics, C. Wellcome Trust Case Control, B. Benyamin JB Whitfield C Genetics of Iron Status, Pramstaller PP, Hicks AA, Thompson JR and Minelli C (2013). “Serum iron levels and the risk of Parkinson disease: a Mendelian randomization study.” PLoS Med. 10(6): e1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, 2016. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci 19 (8), 987–991. [DOI] [PubMed] [Google Scholar]
- Rios FJ, Ferracini M, Pecenin M, Koga MM, Wang Y, Ketelhuth DF, Jancar S, 2013. Uptake of oxLDL and IL-10 production by macrophages requires PAFR and CD36 recruitment into the same lipid rafts. PLoS ONE 8 (10), e76893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD, 2015. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflamm 12, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagal J, Zhan X, Xu J, Tilghman J, Karuppagounder SS, Chen L, Dawson VL, Dawson TM, Laterra J, Ying M, 2014. Proneural transcription factor Atoh1 drives highly efficient differentiation of human pluripotent stem cells into dopaminergic neurons. Stem Cells Transl. Med. 3 (8), 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI, 2011. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 1373, 189–194. [DOI] [PubMed] [Google Scholar]
- Sp N, Kang DY, Kim DH, Park JH, Lee HG, Kim HJ, Darvin P, Park YM, Yang YM, 2018. Nobiletin Inhibits CD36-Dependent Tumor Angiogenesis, Migration, Invasion, and Sphere Formation Through the Cd36/Stat3/Nf-Kappab Signaling Axis. Nutrients 10 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RA, Chang CF, Goods BA, Hammond MD, Mac Grory B, Ai Y, Steinschneider AF, Renfroe SC, Askenase MH, McCullough LD, Kasner SE, Mullen MT, Hafler DA, Love JC, Sansing LH, 2017. TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Invest 127 (1), 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K-W, Cho C-L, Chen H-J, Liang C-L, Liliang P-C, Tsai Y-D, Wang H-K, Lu K, 2011. Molecular biomarker of inflammatory response is associated with rebleeding in spontaneous intracerebral hemorrhage. Eur. Neurol 66 (6), 322–327. [DOI] [PubMed] [Google Scholar]
- Wang SB, Deng YQ, Ren J, Xiao BK, Liu Z, Tao ZZ, 2014. Exogenous interleukin-10 alleviates allergic inflammation but inhibits local interleukin-10 expression in a mouse allergic rhinitis model. BMC Immunol. 15, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Zhang YG, Li AL, Long ZH, Wang D, Li XX, Xia JH, Luo SY, Shan YH, 2016. Expressions of serum inflammatory cytokines and their relationship with cerebral edema in patients with acute basal ganglia hemorrhage. Eur. Rev. Med. Pharmacol. Sci 20 (13), 2868–2871. [PubMed] [Google Scholar]
- Wang Y, Rice AP, 2006. Interleukin-10 inhibits HIV-1 LTR-directed gene expression in human macrophages through the induction of cyclin T1 proteolysis. Virology 352 (2), 485–492. [DOI] [PubMed] [Google Scholar]
- Weischenfeldt J and Porse B (2008). “Bone Marrow-Derived Macrophages (BMM): Isolation and Applications.” CSH Protoc 2008: pdb prot5080. [DOI] [PubMed] [Google Scholar]
- Woo M-S, Wang X, Faustino JV, Derugin N, Wendland MF, Zhou P, Iadecola C, Vexler ZS, 2012. Genetic deletion of CD36 enhances injury after acute neonatal stroke. Ann. Neurol 72 (6), 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M-S, Yang J, Beltran C, Cho S, 2016. Cell Surface CD36 Protein in Monocyte/Macrophage Contributes to Phagocytosis during the Resolution Phase of Ischemic Stroke in Mice. J. Biol. Chem 291 (45), 23654–23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J, Wang J, 2011. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 31 (5), 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LK, Yang SH, 2015. Interaction of astrocytes and T cells in physiological and pathological conditions. Brain Res. 1623, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Tsirka SE, 2012. The CCL2-CCR2 system affects the progression and clearance of intracerebral hemorrhage. Glia 60 (6), 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, Deryugina EI, 2013. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 122 (25), 4054–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk JG, Greenhalgh AD, David S, 2018. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp. Neurol 301 (Pt B), 120–132. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li X, Kwansa H, Kim YT, Yi L, Hong G, Andrabi SA, Dawson VL, Dawson TM, Koehler RC, Yang Z-J, 2017. Augmentation of poly(ADP-ribose) polymerase-dependent neuronal cell death by acidosis. J. Cereb. Blood Flow Metab. 37 (6), 1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun G, Ting S-M, Song S, Zhang J, Edwards NJ, Aronowski J, 2015a. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem 133 (1), 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J, 2007. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann. Neurol 61 (4), 352–362. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q, Hou Z, Maruyama T, Zhang J, Wang J, 2015b. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav. Immun. 46, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiong Z, Lechner EJ, Klenotic PA, Hamburg BJ, Hulver M, Khare A, Oriss T, Mangalmurti N, Chan Y, Zhang Y, Ross MA, Stolz DB, Rosengart MR, Pilewski J, Ray P, Ray A, Silverstein RL, Lee JS, 2014. Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 7 (2), 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, Che F, Chen X, Ren H, Hong M, Wang J, 2019. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol 101610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.