Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly achieved global pandemic status. The pandemic created huge demand for relevant medical and personal protective equipment (PPE) and put unprecedented pressure on the healthcare system within a very short span of time. Moreover, the supply chain system faced extreme disruption as a result of the frequent and severe lockdowns across the globe. In such a situation, additive manufacturing (AM) becomes a supplementary manufacturing process to meet the explosive demands and to ease the health disaster worldwide. Providing the extensive design customization, a rapid manufacturing route, eliminating lengthy assembly lines and ensuring low manufacturing lead times, the AM route could plug the immediate supply chain gap, whilst mass production routes restarted again. The AM community joined the fight against COVID-19 by producing components for medical equipment such as ventilators, nasopharyngeal swabs and PPE such as face masks and face shields. The aim of this article is to systematically summarize and to critically analyze all major efforts put forward by the AM industry, academics, researchers, users, and individuals. A step-by-step account is given summarizing all major additively manufactured products that were designed, invented, used, and produced during the pandemic in addition to highlighting some of the potential challenges. Such a review will become a historical document for the future as well as a stimulus for the next generation AM community.
Keywords: COVID-19, Additive manufacturing, 3D printing, Personal protective equipment (PPE), Pandemic
1. Introduction
Additive manufacturing (AM), also known as 3D printing, is an advanced manufacturing process [1]. In contrast to conventional manufacturing processes, AM fabricates objects by adding materials as required which eliminates the necessity of subtracting materials (by means of machining, milling, carving, etc.) to obtain desired shapes [2]. In the AM processes, precise geometric shapes are directly printed from a computer-aided-design (CAD) model via slicing software. The technology offers a simplified way of fabricating complex geometries in a single step with a lower manufacturing cost. Making prototypes and improving the parts by a trial and error process is more straightforward and typically faster using AM technology [3], [4]. Parts can be fabricated in a single step removing the need for assembly in some cases, thus reducing post-processing and lead times. Thanks to these attractive manufacturing advantages, AM is extensively utilised in medical, aerospace, and automotive industries [5], [6], [7], [8], to mention a few. The advantage of part customisation is utilised highly in medical applications, in which parts can be customised to the individual patient's data [9], [10]. Providing rapid manufacturing facilities, reduced or zero expensive assembly line requirements, low manufacturing lead times and easier and cheaper customized manufacturing-AM is undoubtedly a key way to minimize supply chain bottlenecks in the time of sudden and extensive demands.
Coronavirus disease 2019 (widely known as the COVID-19), which was first recognized in December 2019 in the Wuhan city of China, had spread so fast that within a very short time it was classified as a global pandemic [11]. From the very outset of the COVID-19 pandemic, healthcare and personal protective equipment (PPE) suppliers began to struggle to meet the acute demands of specific items such as face masks, face shields, test kits, ventilators, etc. [12], [136]. The existing production capacities could not cope with the unexpected and acute demands. Moreover, global supply chains were disrupted as a result of reduced employees and lockdown in many areas of the world, making the situation even more critical [13]. In most cases, the shortage of required testing kits to detect and isolate infected patients and the large backlog in the PPE supply chain to protect individuals has been considered as a major contributing factor in the fast spread of the disease. For instance, the nasopharyngeal (NP) swab is one of the most essential kits in detecting COVID-19 infected patients. Until the COVID-19 outbreak, there were only two major suppliers of the specialized NP swabs required to collect test samples [14]. Due to the shortage of NP swabs, a number of countries could not perform the required number of tests to detect and isolate infected patients [15]. Another important device required for treating critically-ill patients is the ventilator that supports breathing. Ranney et al. [16] studied the extreme shortages and challenges of providing this life saving (in some cases) equipment experienced by the hospitals at the beginning of the outbreak.
Ensuring proper supply of the necessary equipment within a very short span of time was one of the most pressing challenges worldwide to combat the disease from the beginning. However, regular and established manufacturers were unable to ramp up their production capacities overnight. Furthermore, the equipment is often manufactured in countries with lower labour costs which can introduce geographic constraints of extended lead times of weeks to months in the system. The AM technology in such an emergency situation enabled localised rapid manufacturing of alternative PPE and other equipment, easing the burden on traditional manufacturing routes and removing the bottlenecks of the supply chain [49]. The 3D printing technology is also attractive considering the environmental issues surrounding PPE during the pandemic. Waste management of the PPE is an issue of significant concern that has already been reported to spark a surge in the ocean pollution [50]. 3D printed PPEs can reduce waste through near-net shape production [51], [52].The majority of the 3D printed COVID-19 items being made from thermoplastic polymers are recyclable adding an additional environmental advantage [53], [54], [55].
The deployment of 3D printers in the most COVID-19 affected regions in the time of need greatly improved the gap between demand and supply [56], [57]. Almost all 3D printing conglomerates and hubs put unprecedented amount of efforts into collaboration with each other to produce necessary components of medical equipment and PPE. Being flexible in design and manufacturing, AM facilities could instantly start producing parts, which had never been produced using such AM technology before. Hundreds of corporations over the world in collaboration with hospitals, academia, and research institutes deployed their 3D printers to combat the challenges. For instance, Formlabs, a 3D printing giant, could produce about 112,500 NP swabs per day using Stereolithography (SLA) additive manufacturing machines in their network [58]. Volkswagen, one of the largest car makers in the world, deployed their 125 industrial 3D printers in making ventilator parts [59]. Fast Radius, an industrial AM parts producer, produced 10,000 face shields per day [60]. Even individual engineers, designers, students, doctors, and charity organizations used 3D printers to produce urgently required parts for the outbreak. Common items related to the COVID-19 pandemic, that were manufactured using 3D printing technology, include ventilator components, face masks, face shields, NP swabs, hands-free door openers, quarantine homes, and drone parts. Polymer based 3D printing technologies were mainly used in producing these parts and equipment [61].
The responses and collaborations by the 3D printing corporations and associations to combat the COVID-19 outbreak are unprecedented and admirable. However, to the best of the authors’ knowledge any article containing all major collaborations and attempts emerging from the 3D printing community to face the COVID-19 challenges has not yet been published. Although few published articles were found in the open literature, their content and scopes of discussion were very limited. For instance, Vafea et al. [56] studied the most frequently used emergent technologies used to face the COVID-19 challenges, and briefly mentioned the significant innovation of 3D printing in rapidly designing and manufacturing medical equipment. Combined contributions of the 3D printing technology to the COVID-19 supply chain shortage were separately studied by Tino et al. [17] and Larraneta et al. [49]. Amin et al. [61] summarized various approaches for making face shields by means of 3D printing technologies including required materials, workflow, time, and costing. Furthermore, Amin et al. [61] and Swennen et al. [62] studied technical and medical aspects of the 3D printed face shield and face mask, respectively. A quantitative and comparative study on the 3D printed face shields and face masks was carried out by Novak and Loy [63]. Cox and Koepsell [15] worked on making NP swabs using 3D printing and published their experience on the detailed manufacturing process, advantages, and cautions required.
Very recently, Patel and Gohil [137] studied the role of additive manufacturing in COVID-19 scenario from Indian landscape. Therein, they clearly demonstrated that government agencies, individuals, corporations and universities have been working together to quickly develop various 3D-printed products especially when established supply chains are under distress in India. Nazir et al. [138] observe that 3D printing together with smart Computer Aided Design (CAD) show promise to overcome the disruption caused by the lockdown of classical manufacturing units specially for medical and testing equipment, and protective gears. Furthermore, Malik et al. [139] studied how the ventilator production in the face of COVID-19 can be ramped-up using human-robot teams. They particularly explored advantages of both the ease of integration and maintaining social distancing. Another article on the 3D printed NP swabs was published by Tay et al. [64] focusing on the design, development, and validation for the effective medical application. Faryami and Harris [65] as well as Ayyildiz et al. [66] separately worked on the manufacturing, testing, and capability assessment of different 3D printed ventilation parts. Clifton et al. [67] studied and analyzed the potential consideration and caution required in using 3D printed PPE. Erickson et al. [57] introduced a novel 3D printing technique to produce helmet modifications for PPE. Francois et al. [68] studied contact-free devices made by 3D printing for the COVID-19 pandemic. Other minor contributions of the AM technology to meet the COVID-19 challenges are also found in the literature [69], [70], [71], [140], [141], [142], [143], [144], [145], [146], [147]. However, all aforementioned articles are based on discrete efforts and focused on specific items.
Hence, there is a growing need to study all major efforts from the AM community in a single article that assist in facing the COVID-19 challenges. Such a study will undoubtedly help individuals, corporations, designers, developers, hospitals, and health workers to combat the pandemic in efficient ways to get rid of the challenges they have been facing in their day-to-day roles. This will also be a good resource for the AM community to reflect on their efforts and motivate and inspire the next innovations in the 3D printing arena to take the technology to the next level. This review article is dedicated to surveying most of the efforts of the global AM community to tackle the pandemic challenge in one place. The primary and essential COVID-19 items that were produced in large volumes such as face shields, face masks, ventilator parts, and NP swabs are discussed herein. However, some minor items that may not be considered as primary but played a significant role in facing the pandemic challenges and produced in comparatively lower volumes; such as hands-free door openers, unmanned aerial vehicle (drone) parts used for the pandemic time, 3D printed quarantine booths and homes, hand sanitizer holders, etc., are omitted here due to space limitations. Corporations, research institutes, hospitals, and associations working together in producing specific items have been discussed in the respective section. The materials and processes, or devices used by the parties during the manufacturing have also been listed for each respective item in addition to mentioning the state of the open access of the technology and design. Finally, the technical and legal challenges experienced by the parties in deploying the AM technologies are discussed along with the potential limitations and opportunities in facing the COVID-19 global challenges.
2. Additive manufacturing techniques used during the COVID-19 pandemic
Most of the parts manufactured by the 3D printing technology to meet the COVID-19 challenges were made of polymeric materials. Major AM techniques to print polymeric parts have been listed in Fig. 1 . However, the technological discussion of the article is limited to the polymeric AM technologies that have been mainly used in making the medical parts as well as the PPE during the pandemic crisis. The main processing mechanisms of different technologies rely on photopolymerization, laser fusion, and thermal melting. Based on the AM techniques, polymer materials can be used in different forms such as powder, filament, liquid, and sheet. However, extra caution needs to be taken in selecting the materials for the medical applications. Biocompatibility, non-toxicity, and disinfection procedures are some important criteria that are considered during selecting the polymeric materials for producing the medical parts and PPE applications.
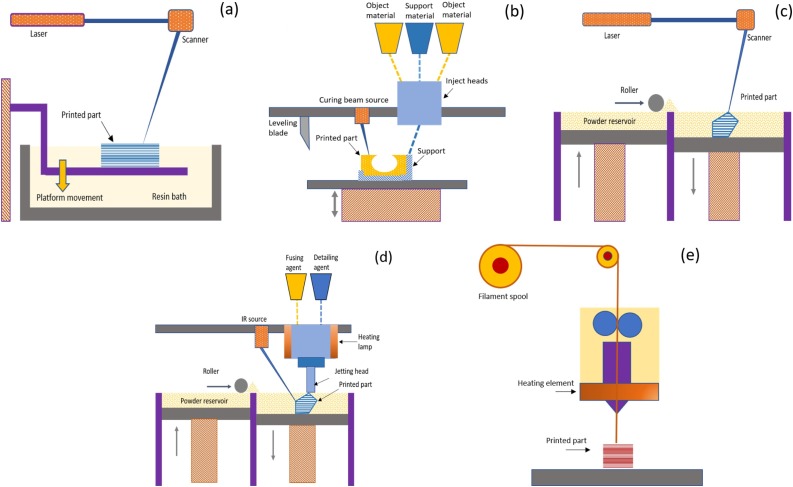
Fig. 1.
Basic steps of the five major AM techniques widely used in making the 3D printed products during the COVID-19 pandemic: (a) SLA: Stereolithography Apparatus, (b) MultiJet (or PolyJet) Printing, (c) SLS: Selective Laser Sintering, (d) MJF: Multi Jet Fusion, (e) FDM/FFF: Fused Deposition Modelling/Fused Filament Fabrication.
Stereolithography apparatus (SLA): SLA is the most commonly used vat photopolymerization process. The vat photopolymerization is a generic class of polymerization that uses a ultra-violet (UV) light source to cure the resin from a vat of polymer. A schematic diagram of the SLA process is shown in Fig. 1a. The basic principle of the technique is that a UV-light source is used to selectively polymerize (cure) a photosensitive polymer resin layer by layer which ultimately builds the desired 3D part [18], [19], [20]. The UV source scans through a resin bath according to the control commands from the AM slicing software. The built part is supported by a structure, which holds pattern in the liquid resin bath, and prevents the newly formed layer from moving out of the position from the already built portion. Once a layer is scanned and cured, the platform or the support structure moves downward or upward by several micrometers (μm, based on the resolution of a printer) to make another layer of liquid resin available to be cured [22], [23], [7], [8], [21].
MultiJet (or PolyJet) printing: This AM technique is also based on the photopolymerization principle which follows the inject printing method to build a 3D part. A schematic diagram of the technique is shown in Fig. 1b. The printing head is consisting of a couple of minute apertures that jets the multiple photocurable build materials and support materials at a single run [100], [25], [26]. The jetted materials are cured upon simultaneous application of a UV light. Since several build materials are jetted from multiple apertures, materials with various colors and properties can be used to easily build parts possessing different colors and properties at different geometric (spatial) locations [27], [28], [29], [30], [31], [32].
Selective laser sintering (SLS): SLS is one of the most common 3D printing techniques that belongs to the powder bed fusion family of the AM classification. In this technique, laser energy is used to selectively fuse and sinter pre-polymers (mainly thermoplastics) layer by layer to ultimately form a 3D part. A schematic diagram of the SLS technique is shown in Fig. 1c. At first, a thin layer of the dispersed polymer particles is formed on top of the build platform. Before applying laser energy, the particles are preheated close to the melting temperature and above the recrystallization temperature which significantly reduces the laser energy required to sinter the selected portion [36], [37]. The preheating is also advantageous to avoid the thermal gradient that may cause thermal stresses in the part resulting in distortion [38], [39]. At present, mostly used polymer particles for the SLS technique are Polyamides (PA 12 or Nylon 12); although poly(acrylonitrile/butadiene/styrene) (ABS), polystyrene (PS), polycarbonate (PC), high-density polyethylene, polyether ether ketone (PEEK) have been developed in the powder form to be used in the SLS applications [40].
Multi jet fusion (MJF): MJF is another type of powder bed fusion 3D printing technology that is similar to the SLS technique in many aspects. In both techniques, initial polymeric particles (usually nylon) are dispersed as the powder bed form that is selectively defused to build the part under the application of a thermal energy [41], [42], [43]. In both techniques the powder bed is preheated to the near-sintering temperature to eliminate thermal stresses. The main difference lies on the heat source used to defuse the polymers. In the SLS technique, CO2 laser source is directly used to selectively scan and defuse the polymeric powders. Whereas, in the MJF technique, an ink (as a fusing agent) is first dispensed on the powder to promote the infrared light (IR) absorptivity and a detailing agent is simultaneously printed near the edge of the part to inhibit sintering in these areas. Then a high-power IR energy source is applied to scan over the building platform to only fuse the inked areas which bonds the polymer powder together [41], [42], see Fig. 1d.
Fused deposition modeling (FDM): FDM, also known as the fused filament fabrication (FFF), uses preform thermoplastic materials in the filament form to print 3D parts [100], [27], [44]. A schematic of the FDM process is shown in Fig. 1e. The polymer filament is passed through a heating zone, in which the filament is melted, and the melted polymer is then deposited on the building space through the printing head or nozzle. Instead of one printing head, sometimes printer with multiple heads is used to defuse any temporary support structure required during printing the complex overhanging parts. Multi-head printer is also useful to build part with different local mechanical and aesthetic properties since multiple polymer melts can be easily defused simultaneously at the different x − y plane locations. In some senses, FDM is an updated version of the conventional extrusion or injection molding except that the technique does not require any mold to provide the part with the expected shape. FDM and SLS are similar in the aspect that both use polymer preform as the initial materials [44], [45], [46], [47], [48] (Fig. 2 ).
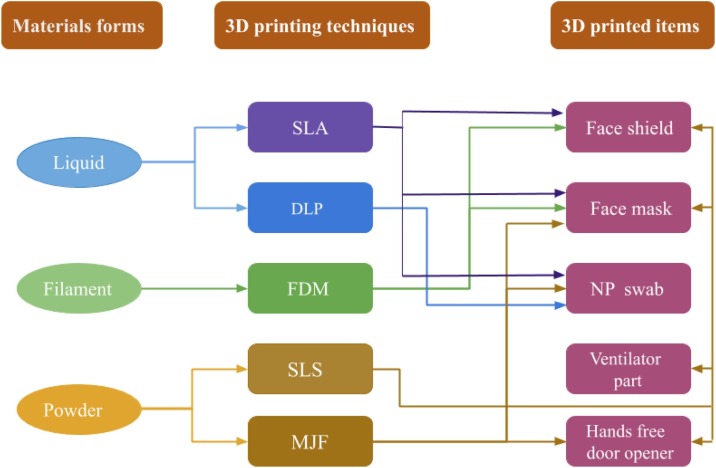
Fig. 2.
A flowchart showing the interrelations among various materials, AM techniques, and major 3D printed products to face the COVID-19 pandemic challenges. SLA: Stereolithography, DLP: Digital Light Processing, FDM: Fused Deposition Modelling, SLS: Selective Laser Sintering, MJF: Multi Jet Fusion. For a detailed description of all major AM techniques used in polymeric materials can be found in Stansbury et al. [100].
3. Additively manufactured products for the COVID-19
In this section, additively manufactured items produced meeting the COVID-19 challenges have been discussed, surveyed in terms of the manufacturing processes, materials used along with reference to the parties/manufacturers working to produce the respective item(s). These items include face shields, face masks, ventilator parts, and NP swabs. All these efforts and collaborations to produce the major COVID-19 products have been briefly discussed in the following sections.
3.1. Face shield
As droplets of Coronavirus can be spread via the mouth, nose and eyes, frontline healthcare workers are the most susceptible to the virus. National Occupational Safety and Health Institute (NIOSH, USA) and CDC (Centers for Disease Control and Prevention) have guidelines for healthcare providers who directly come into contact with respiratory virus infected patients. For them, CDC accredited respirator protecting equipment are recommended. Face shield designs may vary, however, most consist of a frame (i.e., headband), an elastic retainer, and a clear visor. Some include 3D printed brackets (stiffeners) on the clear visor at the bottom and 3D printed fasteners to replace the elastic. A wide range of companies around the world in close collaboration with universities, medical research centres, and hospitals have been at the forefront in manufacturing different parts of this assembly using 3D printing technologies to meet the demands during the COVID-19 pandemic. Some companies have been producing all three parts of a face shield and then assembling them, while many of them have been manufacturing only a particular item and assembling with other parts from different sources. Note that a few of the companies released detailed designs of their 3D printed products so that other interested persons or institutes were able to print face shields easily [87] (Fig. 3 ).
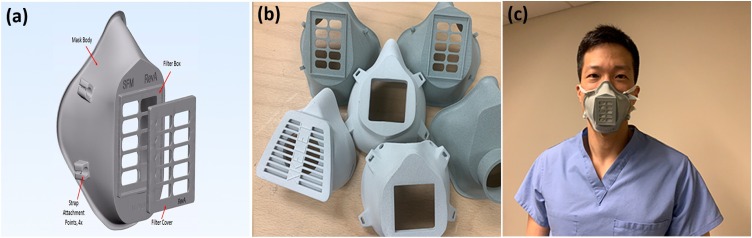
Fig. 3.
Face shield, an essential PPE in the pandemic. (a) An FDM-based printer creates frames of face shields, (b) a face shield with various components, e.g., frame/headband, bracket, and visor, (c) 3D printed face shield from (b) with other PPE worn by an Intensive Care Consultant (Photo credit: Swansea University/UK, University of California San Francisco/USA).
Flashforge is one of the global 3D printers’ suppliers that has been manufacturing face shields to support essential service workers all over Indonesia with the help of its local dealer, Evolusi 3D [72]. For this, Flashforge mainly uses PLA (Polylactic Acid) and Pro filaments. Airwolf3D, a California-based 3D printing machine and accessories distributor, also responded to the pandemic by releasing its design file for face shields in the public domain. Moreover, they released detailed guidance to use ABS (Acrylonitrile Butadiene Styrene) for printing the bracket and assemble with commercially available plastic shields and elastic straps. Similarly in Europe, in order to ensure sufficient supplies of medical equipment, BCN3D (a 3D printing company in Barcelona) came forward to manufacture face shields for hospitals and health workers in Spain [73]. They designed, prototyped, and printed face shields utilizing the FDM technique. Moreover, they used independent dual extrusion (IDEX) system in which Polyethylene Terephthalate Glycol (PETG) was the main filament. The company also shared the technology in the public domain. Another major 3D printer manufacturer, Stratasys, has been active in manufacturing face shields. They even released all design files in the public domain. Moreover, the company formed a COVID-19 coalition team with Minnesota Dunwoody College of Technology and organized 100 teams around the world. For instance, they additively manufactured and distributed 100,000 face shields to different hospitals within one month (April 2020) [86].
During the pandemic, hospitals across the UK experienced a large and urgent demand for face shields. Photocentric, an UK-based 3D printer manufacturer, invented a novel form of 3D printing that could rapidly create polymeric objects in a large scale. With a visible light curing technology and a photopolymer resin, Photocentric's LC Magna printers started manufacturing thousands of shield parts daily. After the mass production capability, the company made an official agreement with the UK government to provide 7.6 million 3D printed face shields for healthcare providers [74]. New York was one of the hardest pandemic hit cities in the world. Therein, 3D printer reseller iMakr established a new dedicated facility with one hundred printers to print face shields with a view to support healthcare providers [75]. The company additively manufactured and distributed thousands of face shields in a critical time when a large number of healthcare activists were protesting in demand of PPE. Note that iMakr helped hospitals to meet the demand of PPE using their own networks. Similarly, Guy's and St Thomas in London, with the help of local academics, started printing face shields using two hundred printers given by iMakr in London [75].
California-based 3D printer manufacturer Nexa3D responded similarly to the PPE shortage. The company launched a facility producing 3D printed face shields at a large scale with two different designs [76]. 3D Systems, another major global player in the additive manufacturer and distributor supply chain, made their own face shield design and released it as open access with detailed assembly instructions [77]. Moreover, they published guidance to sterilize their reusable PPE products. Materials biocompatibility and strength data were also made available to users so that they can print according to the product requirements. Czech Republic-based additive manufacturing company, Prusa3D, also came forward to help healthcare workers. Their Czech health ministry-approved open access design file of the headband (a part of the face shield) was downloaded 250,000 times as of April 2020. They printed and distributed 200,000 face shields in their own facility as of May, 2020 [78]. One of the co-authors of this contribution used this design and modified it. Furthermore, they set up a re-purposed manufacturing unit at Swansea University to manufacture face shields [79]. Companies like Tessy and Ricoh 3D utilized additively manufacturing techniques along with the traditional injection molding process to increase their production capability. With the help of 3D-printed modified designs for the injection molding, they had the capacity to produce forty thousand face shields per week. A summary of all major efforts in producing 3D printed face shield parts is presented in Table 1 . Note that the production capacity mentioned in the table is mainly related to the respective company's number of 3D printers involved in the manufacturing process.
Table 1.
A summary of major efforts for producing face shields using various AM techniques with a wide range of materials.
| Material type | Manufacturing process/device | Production capacity | Manufacturer | Open source | Reference |
|---|---|---|---|---|---|
| Premium PLA Profilament | Flashforge Guider II Printer | 45/day | Evolusi3D | Yes | [72] |
| PETG | FFD 3D Printer | 140/day | BCN3D | Yes | [73] |
| Photopolymer resin | LC Magna Printer | 500,000/day | Photocentric | No | [74] |
| PLA/PETG | SLS/FDM | 500/day | iMakr | No | [75] |
| Nexa3D developed material | SLA | 150/day | Nexa3D | No | [76] |
| Medical grade nylon | ProX SLS 6100 Printer | 100/day | 3D Systems | Yes | [77] |
| PETG | Prusa i3 Printer | 10,000/day | Prusa3D | Yes | [78] |
| PLA/PETG | Budmen 3D Printer | 30,000/day | Budmen | Yes | [80] |
| Azul3D developed material | High-Area Rapid Printing (HARP) | 1000/day | Azul3D | No | [81] |
| DSM Somos resin | SLA/SLS/DLS | 700/day | Paragon Rapid Tech. | Yes | [82] |
| PLA/PETG | SLA/SLS | 150/day | I-Form | Yes | [84] |
| PETG/PC | HP MJ Fusion Printer | 10,000/day | Fast Radius | No | [60] |
| ABS-42 Filament | Omni500 LITE | 150/day | Omni3D | No | [83] |
| ABS 3D-FC | SLS/SLA | 300/day | Ford Motor | No | [84] |
| PLA/PETG | KUKA Robotic Printer | 1000/day | Caracol-AM | No | [85] |
| Medical grade nylon | SLS | 700/day | 3DPRINTUK | No | [84] |
| ABS | FDM | 700/day | Stratasys | Yes | [86] |
| DPR 10/UMA 90 | DLS | NA | Carbon3D | Yes | [87] |
| NA | NA | 15,000/day | Nissan | No | [83] |
3.2. Face mask
A device covering a person's nose and mouth regardless of the filtration efficiency is considered as a face mask according to FDA (Food and Drug Administration) [89]. When a mask can protect its user against fluids and particulate materials and serves as a physical barrier between the nose, mouth, and the surroundings, it is called a surgical mask. The N95 respirator represents one of the most essential PPE used to protect a user from airborne contaminants and fluids. It can filter at least 95% of airborne particles. FDA has distinguished definition and standards for all kind of face masks to avoid potential health hazards [89]. Note that N95 or any surgical respirator requires a fit test for the healthcare providers that ensures an adequate sealing to the face. Such a fit test is not mandatory in wearing a normal face mask, which is loose-fitting and provides only protection against droplets, including large respiratory particles. The CDC and World Health Organization (WHO) both have recommended people to use mask or cloth face covering to minimize the community transmission of the Coronavirus [90].
The shortage of face masks was so acute that FDA issued Emergency Use Authorization (EUA) to mitigate the scarcity of PPE [89]. AM researchers and industries came forward to print different kinds of face masks as per their competence. Czech Technical University (CTU) developed their own face mask prototype which can be printed using HP's MultiJet Fusion (MJF) 3D printers [91]. Collaborating with other Czech companies, they improved designs to such a scale that the RP95-M mask passed the standard of providing the highest FFP3 level of protection against micro-organisms including the Coronavirus infection. Having the CE certification pledging conformance to European Union (EU) standard safety requirements, CTU modified the design for a mass production of the mask by injection moulding with 3D printed moulds. Coordinated by CARDAM company, the mask has been manufactured ten thousands per day [88].
3D printer manufacturer Roboze realized the predicament of healthcare workers as a result of the respiratory N95 mask shortage during the pandemic. They designed and provided N95 mask manufacturers with a 3D printed mold to accelerate mass production, in which a high temperature resistant thermoplastic was used. Their design file was released in the public domain [92]. Similarly, Maker Mask, a nonprofit worldwide digital platform, designed the first US National Institute of Health (NIH)-approved 3D printable respiratory mask and shared their design in the public domain with detailed instructions [93]. The company, using their networks, organized more than ten thousand makers including 179 universities and 214 hospitals. Moreover, they additively manufactured 100,000 respiratory masks as of June 2020. The cost of a 3D printed mask was only 3 dollars which can be reused by only changing the filter and can save the usage of N95 respiratory masks, see Fig. 4a and b.
Fig. 4.
Surgical face mask is a common PPE in usual time that becomes scarce during the pandemic. (a) Various components of the main structure of a 3D printed face mask, (b) AM creates ample opportunities and customizations in the design of face masks, (c) a perfectly fit 3D printed face mask (Photo credit: National Institute of Health/NIH).
3D systems was also open to share their face mask design and released a detailed print instruction [104] along with the guidance to sterilize their reusable PPE product. Anyone can utilize this publicly available file to 3D print their face mask and use during standard PPE shortage [77]. A number of companies modified other available suitable products to provide protection against Coronavirus infection during the respirator shortage. Masks On, a nonprofit initiative, collaborating with academics, industries, and hospitals adopted a full face snorkel mask to use as PPE in the absence of standard respirators [105]. They 3D printed an adapter replacing the tube to make an airtight seal over the snorkel holes and attached a medical grade filter available in the hospitals [106]. With an user manual and configuration of different filters of the mask, Masks On delivered twenty thousand emergency snorkel masks to different medical centers till June 2020 [107].
Stanford University research lab came up with a similar idea of converting the scuba mask to a reusable PPE face mask that turned finally into the Pneumask project. With the help of AM companies globally, they 3D printed an adapter and assembled with an off-the-shelf scuba mask and a medical grade filter [96]. Such masks can be used as emergency respiratory masks in the absence of standard respirators. Besides fulfilling unprecedented demand of PPE items during the public health crisis, these efforts are saving money and the environment because they can replace single-use PPE [50]. Most healthcare providers had to wear face masks for a long time to protect themselves against the infection while giving care to patients. Farsoon Technologies printed facial mask adjusters to fit all types of facial masks to improve comfort by utilising an open source design [103]. A summary of all major AM community initiatives in producing face masks is shown in Table 2 .
Table 2.
A detailed account of the key initiatives for producing face masks using various AM techniques during the pandemic.
| Material type | Manufacturing process/device | Production capacity | Manufacturer | Open source | Reference |
|---|---|---|---|---|---|
| PA 12 | HP MJF 540 Printer | 10,000/day | CIIRU CTU &CARDAM | Yes | [88] |
| PEEK | Argo 500 Printer | Prototype Mold | Roboze | Yes | [92] |
| PLA | FDM/SLA | 1500/day | Maker Mask | Yes | [93] |
| Biocompatible Nylon | SLS | Design File | 3D Systems | Yes | [94] |
| PLA/ABS | SLS/SLA | 1000/day | MaskOn | No | [95] |
| PLA/ABS | SLS/SLA | 500/day | Pneumask | No | [96] |
| PLA/ABS | SLS/SLA | 50/day | Nexteer Automotive | No | [97] |
| TPU/PETG | FDM | 35/day | Filament Innovations | Yes | [98] |
| PLA | FDM | NA | Barrow Innovation Center | Yes | [99] |
| Formlabs Draft Resin | SLA | 50/day | Formlabs | No | [58] |
| PA Nylon 12 | HP's MJF | NA | ProtoCAM | Yes | [101] |
| TPU74D | High Speed Extrusion | 700/day | Essentium | Yes | [102] |
| PA 12 | HP's MJF | 400/day | Ferrovial | No | [84] |
| Farsoon FS3300PA | Polymer Laser Sintering | 1000/day | Farsoon Technologies | Yes | [103] |
3.3. Ventilator parts
Ventilators are mechanical devices that provide breathing support to patients. During inhalation difficulties, a ventilator helps the patient by blowing oxygen into the lungs. An oxygen flow regulator, input and output pipes and an endotracheal tube are the integral parts of a mechanical ventilator [115]. As COVID-19 created severe respiratory problems to many individuals, there was increased demand for a wide range of ventilation therapies for patients around the world [116]. 2–4% affected patients required ventilator supports [117]. Hospitals struggled to obtain enough ventilators to treat thousands of seriously ill patients as manufacturers failed to meet the abrupt and unprecedented need for this life saving equipment [16], [70]. The shortage of ventilator parts was so acute that in some cases, doctors had to take emergency decisions regarding which patients would receive ventilator support, and have a better chance of survival [118]. FDA issued Emergency Use Authorization (EUA) for ventilators as their standard ventilators became insufficient amid the pandemic [119].
To the best of the authors’ knowledge, Italian company Isinnova responded at the very beginning of the pandemic outbreak with the idea of 3D printed ventilator valves. Following an acute crisis of respiratory valves in local hospitals, they designed a prototype and formed a team with a 3D printing company Lonati. They printed a respiratory valve, which is necessary to connect respiratory tubing with the ventilator and ensure flow in the right direction for oxygen input and outgoing, see Fig. 5a. Isinnova made the design available in the public domain so that anyone can print the valve in times of emergency. They also provided detailed guidelines on the 3D printing of the ventilator valve, named ‘Charlotte’, to convert a snorkeling mask into an oxygen mask that minimized the ventilator shortage and helped in saving lives [108].
Fig. 5.
COVID-19, a respiratory-related disease, creates enormous demands for ventilators. Ventilators and their various components are some of the most essential and life-saving medical equipment. (a) An additively manufactured ventilator valve perfectly replicates an original valve made by traditional manufacturing techniques, (b) 3D printing technology efficiently creates ample options by manufacturing T-connector, Y-connector, etc. for using a single ventilator for multiple patients (Photo credit: Isinnova, VESper).
Airwolf3D, a 3D printing company in the USA, dedicated its facility to print emergency respiratory valves and other parts [109]. Another Italian 3D printing company, CRP technology, used the open source Charlotte ventilator valve designed by Isinnova and provided printed valves to hospitals using their own filaments. Such a valve helps hospitals to use available snorkeling masks instead of oxygen masks during the acute shortage in COVID-19 [110]. Furthermore, 3D printer manufacturer Roboze implemented a practical but creative idea of using a T-connector to use one ventilator device for two patients in the case of emergency, see Fig. 5b. They designed, printed, and distributed T-connectors to local hospitals along with the Charlotte ventilator valve using Isinnova's open source design [92].
Italian 3D printing company Weerg also helped hospitals in mitigating the ventilator crisis. Using Charlotte's design, they printed valves and distributed to local hospitals [111]. Prisma Health collaborated with Clemson University and University of South Carolina that resulted in the design and development of a Y-connector which facilitated the use of one ventilator device for two patients at the same time. Having EUA from FDA, the ventilator valve (known as VESper) has been produced and distributed in a large scale with the help of industries [112]. Utilizing Neyman and Irvin's concept of ventilator sharing published in 2006 [120], a group of doctors from the University of Texas Health Science Center published an open source design for ventilator splitter [121]. 3D Systems, a global company in the additive manufacturing sector, used this open source design and printed T-connectors and adaptors. For this, the company used medical grade nylon to 3D print the T-connectors. They also printed and distributed flow limiters which effectively regulate ventilating pressures and flow [77].
Materialise, a Belgium-based 3D printing company, took multiple approaches to solve the ventilator crisis. They developed an efficient way to provide Positive End Expiratory Pressure (PEEP) to COVID-19 patients, shown to be beneficial in some types of respiratory failure. The company designed and 3D printed a connector which can hold a non-invasive mask, a filter, and a PEEP valve. Materialise named this 3D printed and adjustable PEEP ventilation as Materialise Passive NIP (Non-invasive PEEP). The device can deliver oxygen by creating a high positive pressure without the use of a ventilator [113]. Beside this, the company also developed a 3D printed ventilator valve that can optimize ventilating pressure and flow while using a single ventilator for multiple patients. They further improved the design of Isinnova's ventilator valve to make it stronger and fit to convert a scuba mask into an oxygen mask for the ventilator support. They made the design file as an open source [122]. Sports car manufacturer Ferrari developed and distributed a respirator valve [114]. A detailed account of all major efforts in producing 3D printed ventilator parts is summarised in Table 3 .
Table 3.
Some main AM initiatives to meet the challenges of COVID-19 by manufacturing ventilator parts with a wide range of materials.
| Material type | Manufacturing process/device | Production capacity | Manufacturer | Open source | Reference |
|---|---|---|---|---|---|
| PLA | SLS | 100/day | Isinnova, Lonati | Yes | [108] |
| ABS, PC, TPU | AXIOM 20 | NA | Airwolf3D | No | [109] |
| Windform P1 | High Speed Sintering | NA | CRP Technology | Yes | [110] |
| NA | SLS | FFF | Roboze | No | [92] |
| PA 12 Nylon | HP's MJF 5210 Printer | 500/day | Weerg | Yes | [111] |
| NA | NA | NA | Prisma Health | No | [112] |
| Medical Grade Nylon | SLS | NA | 3D Systems | No | [77] |
| NA | NA | NA | Materialise | No | [113] |
| ABS | FDM | NA | Ferrari | No | [114] |
3.4. Nasopharyngeal swab
Nasopharyngeal (NP) swab is a flexible stick with a bristle at the end. Such sticks are used to collect the COVID-19 testing sample from patients’ nose. This is a compulsory kit required for the initial diagnostic test of a COVID-19 symptomatic person. However, as a result of the exploding demand during the COVID-19 outbreak, medical services encountered an unprecedented and high shortage of the kit. In most cases, the lack of NP swabs supply was the only barrier in testing a symptomatic person [14]. The testing kit is quite different from that of the known standard Q-tips [130]. NP swabs need to be skinny and long enough to reach the upper part of the throat through the nose, see Fig. 6a. According to CDC, a swab needs to be made of a synthetic fiber and cannot contain calcium alginate which can kill Coronavirus [130]. However, until the COVID-19 outbreak, two companies (Puritan Medical Products, USA and Copan Diagnostics Inc., Italy) were the top suppliers of such specialized NP swabs for the entire world [14].
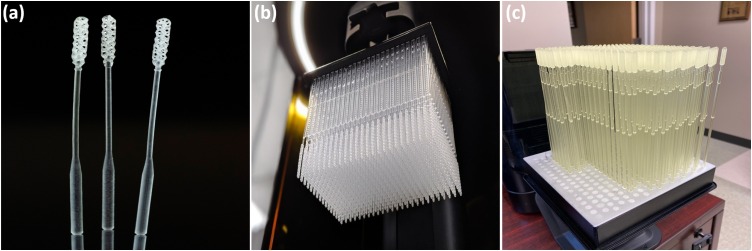
Fig. 6.
Additively manufactured nasopharyngeal swab, an essential ingredient in the fight against the COVID-19: (a) a closeup of three NP sticks that depict the intricate geometries of the instrument, (b) a batch of the NP swabs printed by Carbon3D's DLS technology, (c) Formlabs’ manufactured a variety of NP swabs with the SLA technique of vat polymerization (Photo credit: Carbon3D, Formlabs).
In such a pressing situation, several 3D printing companies throughout the world in collaboration with academia, medical research centers, and hospitals came to the frontline to produce NP swabs. BIDMC (Beth Israel Deaconess Medical Center), a Boston-based biomedical laboratory, has performed multi-step preclinical evaluations on 160 different swab designs and 48 materials from 24 companies for the purpose of mass production using different AM techniques [131]. While there were only few types of NP swabs available earlier, FDA approved several variants of them during the outbreak. These are easier to manufacture within a short period of time [58]. In addition, different consortia including academia, medical centres, and commercial enterprises were formed to facilitate and expedite the production of NP swabs. One such consortium was formed that included the Harvard Medical School, BIDMC, and six different ISO13485 verified 3D printing companies. Based on medical requests, the consortium is able to produce up to 4 million NP swabs per week following the FDA registered design. Furthermore, a number of companies have approached to collaborate among themselves for the mass production of NP swabs with the help of several research laboratories and hospitals. Formlabs, a Boston-based 3D printing company, collaborated with three leading USA hospitals-USF Health, Northwell Health, and Tampa General Hospital. The collaboration was to design, develop and test NP swabs to be printed using Formlabs’ 3D printers [58]. They could produce 300 swabs at a time with a single printer resulting up to 150,000 swabs per day. Validation and rapid clinical tests proved that 3D printed swabs performed as well as or even better than the traditionally manufactured NP swabs [58]. The company is open to share the technology to scale up the production nationwide to meet the critical challenges. An array of 3D printed NP swabs using Formlabs printers is seen in Fig. 6c.
In a similar effort, EnvisionTEC (Michigan-based company) collaborated with Harvard Microbiology Lab for design and mass production of NP swabs [123]. Initial printing and testing was done at Nilson Laboratories, USA. After ten phases of mechanical testing, two absorption tests; a biological test and a chemical test, it was confirmed that materials utilized in the printing process are chemically safe. One of the crucial elements is the flexibility of the NP swabs as they can be bent up to 180 degree without any fracture and can collect enough virus particles from the nasal passage [131]. With the help of BIDMC, EnvisionTEC obtained IRB (Institutional Review Board) approval for its mass production. BIDMC confirmed that EnvisionTEC's swab performed same as or even better than other test swabs. Especially the NP swabs, being sterilized by steam at temperature of 270°F and 27 Pa in an autoclave, continued to perform the same. The company made the technology open with FDA registered and listed companies. However, the protocol developed needs to use EnvisionTEC's One cDLM printer with a PCA 2000 curing unit.
Forecast 3D (a California-based company), in collaboration with Abiogenix, Fathom, and Hewlett-Packard (HP), produced more than 100,000 pieces of NP swabs in one day [124]. Abiogenix selected a spiral design for the swabs considering patients’ sensitivity, comfort, and ability to collect sufficient viral fluid and breakpoint reliability. In a similar effort, Hospital virtual Valdecilla (HvV) (Spain) has designed and developed their own swabs to fulfill the increasing demand in the area [125]. The hospital has provided complete open access for the full manufacturing process, testing, and quality control. Structo, a Singapore-based 3D printing startup, is collaborating with the healthcare professionals to deliver about one million NP swabs [129]. Also, a number of 3D printer hubs, that usually deal with the additive manufacturing of soft polymers such as Origin and Carbon3D, have been making NP swabs in response to the pandemic in addition to their regular business works. The motivation for such initiative was to serve the community in the challenging time in addition to making revenues since their regular business had slowed down. For example, Origin, which generally focuses on selling 3D printers to material manufacturers, has deployed most of their 3D printers to produce NP swabs [127]. Carbon3D, a Silicon Valley-based company which generally develops 3D printing technology of soft materials, has dedicated all of their 3D printers to combat the pandemic challenges [128]. The company collaborated with Resolution Medical, an FDA registered medical device manufacturer, to produce the NP swabs. The NP swabs printed in the Resolution Medical using Carbon3D printing facilities exhibit a conformal lattice design and provide a flexible geometry to enhance the functionality and comfort. A study from Stanford University revealed that Carbon's 3D printed lattice swabs showed lower false negative rate than the traditional flocked swabs [84]. A bundle of 3D printed NP swabs using Carbon3D DLS technology is illustrated in Fig. 6b. Some more initiatives noted around the world are summarised in Table 4 .
Table 4.
Some key efforts that AM community put forward in producing the nasopharyngeal swabs.
| Material type | Manufacturing process/device | Production capacity | Manufacturer | Open source | Reference |
|---|---|---|---|---|---|
| Surgical Guide resin | Formlabs SLA Printer | 112,000/day | Formlabs | Yes | [58] |
| Soft C-29C resin | Envision One cDLM Printer | 500,000/day | EnvisionTEC | Yes | [123] |
| Company developed material | HP's MJF Printer | 100,000/day | Forecast 3D and Abigenix | No | [124] |
| 3D Systems biocompatible material | Figure 4 Printer | 3000/day | 3D Systems | No | [77] |
| Surgical Guide resin | Form 2 SLA Printer | 324/print | Hospital virtual Valdecilla | Yes | [125] |
| Nexa3D material | NXE 400 | 75,000/day | Nexa3D | No | [126] |
| Company developed material | Origin One Printer | 190,000/day | Origin and Stratasys | No | [127] |
| Carbon3D resin | Carbon3D M2 Printer | NA | Carbon3D | No | [128] |
| Structo developed material | Structo MSLA Printer | 15,000/day | Structo | No | [129] |
| Nylon base and Rayon | Markforged Industrial Series Printer | 10,000/day | Markforged | No | [84] |
4. Challenges
The extensive efforts that the additive manufacturing community has made to meet the extreme pandemic demands, have sometimes met with hurdles and potential challenges in different phases from the design to the final production stage. The challenges can be described in terms of design, manufacturing, legal issues, and critical approval system. Most of the parts or products required were never produced before using 3D printing technologies. The first time production of some critical components faced design challenges. In addition, since most of the 3D printed items are being used in direct human contact, there were additional safety challenges. For instance, nasopharyngeal (NP) swabs or ventilator parts are extremely sensitive products related to the human breathing systems. Hence, ultra-caution regarding various health and safety regulations had to be considered during various phases of all 3D printed items. A great challenge experienced by the 3D printing designers is to design the face masks ensuring perfect fit for the end users. For example, America Makes, an USA-based umbrella organization for the AM community, called for the design challenge ‘Fit to Face’. The idea is to involve designers and manufacturers nationwide to rapidly innovate the 3D printing designs for the face mask that will ensure proper fit and better seal around the face area. The organization worked with the Department of Veterans Affairs to address the challenges. Another similar design challenge was organized by Challenge America and the Veterans Health Administration (VHA) [133].
Note that only few materials are suitable for the 3D printing of biomedical applications. Moreover, detailed data of relevant raw materials that are to be used for the medical applications were not available in the time of need. Without explicit data on the material properties, restrictions have usually been enforced on their selection to be used for the specific application. Furthermore, available printing processes sometimes also have been made limited because of the compulsory design considerations related to the health and safety regulations. Therefore, obeying all relevant constraints for materials and process techniques, it was not easy but challenging tasks for the designers and manufacturers to finalize the materials and processes to be used for a mass production. In particular, extra caution must be taken in selecting materials for some items used in direct human contact such as face masks, NP swabs, ventilator parts etc. For example, to produce the stopgap mask, NIH recommended nylon as the only material considering its biocompatibility and disinfection aspects. Moreover, the recommended printing method for the stopgap masks were MJF and SLS. Some manufacturers found using PLA in FDM technology is convenient in precisely printing the stopgap mask. However, PLA was not recommended considering the disinfection difficulties. Some medical devices or parts with complex geometries need to combine with electronics or mumps during the services at hospitals. Such devices, that require maintaining same specifications, dimensions, performance, are difficult to replicate in the 3D printing process [132].
Another major challenge that the AM community has been experiencing during the incredible journey is the copyright and legal issues in producing the items in mass volumes. While the European Association for Additive Manufacturing (CECIMO) reached out to all major AM corporations to contribute to the pandemic challenges, they also expressed concerns regarding copyright issues. For instance, due to the supply of 3D printed oxygen valves during the emergency crisis in the one of the worst hit areas in Italy, the CEO of Isinnova has faced IP (Intellectual Properties) infringement lawsuits issues. Another challenge arises as a result of the disparity between developing and developed countries. Although 3D printing technologies have widely been used in developed countries to solve the supply chain crisis, the technology is still rarely used in developing and underdeveloped countries. This is mainly because of the considerable expense of the printing devices along with the fact that the technology is not familiar in most of these countries. Therefore, only few countries could utilize the advantages of the AM technology in combating the COVID-19 pandemic challenges. However, the gap between developing and developed countries for the case of AM is shrinking in which India is an example. In a recent report, Patel and Gohil [137] studied the role of additive manufacturing in medical application COVID-19 scenario for an Indian perspective.
There are also regulatory requirements and liability risks for anyone working to produce medical or associated PPE for the pandemic – be they a large corporation, community/maker group or solo ‘hobbyist’. Of course, these requirements vary between countries. In order to illustrate some of the issues faced when manufacturing PPE using AM techniques, a real case study will be used, which describes the setting up of a re-purposed AM site at Swansea University (SU) by one of the co-authors to produce 3D printed face shields [79]. This group initially produced a few hundred face shields based on the open-source Prusa design [78]. However, when it became clear that any such face shields produced constitute PPE, and must be certified. The design was altered in line with certification requirements, and feedback from users, and with consideration for the mass production taken into account. The British Standards Institute (BSI) produced the following guidance: Regulatory status of equipment being used to help prevent Coronavirus (COVID-19) [134], [135] which clarified the above certification requirement for any PPE released into the market (even if provided at zero cost). The SU team's face shield design was certified and is one of the first 3D printed designs to have received CE certification under the COVID-19 reduced PPE requirements. The question remains as to how many tens-to-thousands of other designs have been released to healthcare workers, or other frontline services from maker communities, or other organisations without the required certification. The challenge of achieving certification cannot be underestimated. It takes experienced individuals and a considerable time-effort, to negotiate what can be a grey area, in addition to the £ 5000 cost which is likely to be prohibitive to smaller maker communities. The discussion of regulations does raise a genuine concern over the quality and safety of any uncertified products present; e.g. if products such as face shields are produced in an unsafe way, or not according to the required legal standards, then more harms than good could very well occur. For example, if one of the ‘makers’ – unknowingly – had COVID-19, and then coughed over the products they were making and did not isolate the products for at least 72 h, they could well pass COVID-19 to the people they are looking to help. This example is specifically related to the EU standards (BS EN harmonised) [135].
5. Conclusion
The COVID-19 period has demonstrated the power of localised manufacturing and the entrepreneurial nature of people on a scale from the individual ‘maker’ with a 3D printer in their shed, through to networks of makers who have organised themselves as re-purposed manufacturers, and to universities utilising the skills of their staff and students and resources, and onto the more typical larger organisations who provide products in what were ‘normal’ times. There has been a great effort and collaboration to address the pandemic and the acute need for PPE and medical equipment that could not be fulfilled by existing manufacturing routes, due to increased lead times, lack of government planning and procurement of these equipment, and associated factors. As from the experience of the authors, it can be very difficult to obtain clarification on what is required for specific categories of products, when it takes time for large organisations such as FDA, CDC, BSI to catch up with the rapid pace of individuals and groups manufacturing PPE and equipment. There is a debate to be had about the risk-benefit analysis of individuals or maker networks producing 3D printed PPE and medical equipment for situations where there is not other alternative: is it better for a healthcare worker to potentially wear a face shield that has a non-compliant elastic band for a strap instead of an elastic band of a minimum 10 mm width, and potentially be safeguarded from a patient? Or is it better to have nothing? Further questions which need investigating are how standards agencies or bodies provide more cost-effective support for maker-networks or individuals to gain certification advice early on, which they can afford. In addition perhaps there could be ‘pop up’ certification centres/ advice centres from government bodies which provide quick advice/help/testing for rapidly produced PPE and medical equipment, which can then be checked prior to use, with the caveat it is only for situations where there are no alternative certified products available. Copyright and legal responsibilities are other aspects that have to have considered before the mass production. Also, there were challenges in the rapid design process being most of the parts first produce in the AM techniques. However, corporations and agencies spontaneously came out to find the solutions to meet the urgent situation requirements. Several 3D printing companies have committed to share the complete manufacturing technology in public to be used by any corporation or individual interested in contributing by producing parts for the outbreak.
Declaration of interests
None.
References
- 1.Gebhardt A. Carl Hanser Verlag GmbH Co. KG; Munich: 2011. Understanding additive manufacturing: rapid prototyping, rapid tooling, rapid manufacturing. [Google Scholar]
- 2.Attaran M. The rise of 3-D printing: the advantages of additive manufacturing over traditional manufacturing. Bus Horizons. 2017;60:677–688. [Google Scholar]
- 3.Kruth J.P., Leu M.C., Nakagawa T. Progress in additive manufacturing and rapid prototyping. CIRP Ann Manuf Technol. 1998;47:525–540. [Google Scholar]
- 4.Campbell I., Bourell D., Gibson I. Additive manufacturing: rapid prototyping comes of age. Rapid Prototyp J. 2012;18:255–258. [Google Scholar]
- 5.Rauch E., Unterhofer M., Dallasega P. Industry sector analysis for the application of additive manufacturing in smart and distributed manufacturing systems. Manuf Lett. 2018;15:126–131. [Google Scholar]
- 6.Stavropoulos P., Foteinopoulos P., Papacharalampopoulos A., Bikas H. Addressing the challenges for the industrial application of additive manufacturing: towards a hybrid solution. Int J Lightweight Mater Manuf. 2018;1:157–168. [Google Scholar]
- 7.Hossain M., Liao Z. 3D printed elastomeric polyurethane: viscoelastic experimental characterisations and constitutive modelling with nonlinear viscosity functions. Int J Non-Linear Mech. 2020;126:103546. [Google Scholar]
- 8.Hossain M., Liao Z. An additively manufactured silicone polymer: thermo-viscoelastic experimental study and computational modelling. Addit Manuf. 2020;35:101395. [Google Scholar]
- 9.Javaid M., Haleem A. Additive manufacturing applications in medical cases: a literature based review. Alexandria J Med. 2018;54:411–422. [Google Scholar]
- 10.Ventola C.L. Medical applications for 3D printing: current and projected uses. Pharm Ther. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organisation. WHO Timeline – COVID-19. https://www.who.int/news-room/detail/27-04-2020-who-timeline-covid-19 [accessed 13.06.20].
- 12.Diaz D, Sands G, Alesci C. Cost of protective equipment rises amid competition and surge in demand. https://edition.cnn.com/2020/04/16/politics/ppe-price-costs-rising-economy-personal-protective-equipment/index.html [accessed 13.06.20].
- 13.Volkin S. How has COVID-19 impacted supply chains around the world? John Hopkins University. https://hub.jhu.edu/2020/04/06/goker-aydin-global-supply-chain/ [accessed 22.06.20].
- 14.NPR Organisation. Testing swabs run in short supply as makers try to speed up production. https://www.npr.org/sections/health-shots/2020/03/18/817801222/testing-swabs-run-in-short-supply-as-makers-try-to-speed-up-production [accessed 05.06.20].
- 15.Cox J.L., Koepsell S.A. 3D-printing to address COVID-19 testing supply shortages. Lab Med. 2020;51:e45–e46. doi: 10.1093/labmed/lmaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages – the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 17.Tino R., Moore R., Antoline S., Ravi P., Wake N., Ionita C.N. COVID-19 and the role of 3D printing in medicine. 3D Print Med. 2020;6(11) doi: 10.1186/s41205-020-00064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neckers D.C. Stereolithography: an introduction. Chemtech. 1990;20:615–619. [Google Scholar]
- 19.Kaplan H. Stereolithography – a marriage of technologies. Photon Spectra. 1990;24:74. [Google Scholar]
- 20.Zakeri S., Vippola M., Levaenen E. A comprehensive review of the photopolymerization of ceramic resins used in stereolithography. Addit Manuf. 2020;35:101177. [Google Scholar]
- 21.Mu Q., Wang L., Dunn C.K., Kuang X., Duan F., Zhang Z. Digital light processing 3D printing of conductive complex structures. Addit Manuf. 2017;18:74–83. [Google Scholar]
- 22.Borrello J., Nasser P., Iatridis J.C., Costa K.D. 3D printing a mechanically-tunable acrylate resin on a commercial DLP-SLA printer. Addit Manuf. 2018;23:374–380. doi: 10.1016/j.addma.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumbleston J.R., Shirvanyants D., Ermoshkin N., Janusziewicz R., Johnson A.R., Kelly D. Continuous liquid interface production of 3D objects. Science. 2015;347:6228. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 25.Liu W., Song H., Huang C. Maximizing mechanical properties and minimizing support material of PolyJet fabricated 3D lattice structures. Addit Manuf. 2020;35:101257. [Google Scholar]
- 26.Singh R. Process capability study of polyjet printing for plastic components. J Mech Sci Technol. 2011;25:1011–1015. [Google Scholar]
- 27.Tanikella N.G., Wittbrodt B., Pearce J.M. Tensile strength of commercial polymer materials for fused filament fabrication 3D printing. Addit Manuf. 2017;15:40–47. [Google Scholar]
- 28.Sugavaneswaran M., Arumaikkannu G. Analytical and experimental investigation on elastic modulus of reinforced additive manufactured structure. Mater Des. 2015;66:29–36. [Google Scholar]
- 29.Larimore Z., Jensen S., Parsons P., Good B., Smith K., Mirotznik M. Use of space-filling curves for additive manufacturing of three dimensionally varying graded dielectric structures using fused deposition modeling. Addit Manuf. 2017;15:48–56. [Google Scholar]
- 30.Cooperstein I., Layani M., Magdassi S. 3D printing of porous structures by UV-curable O/W emulsion for fabrication of conductive objects. J Mater Chem C. 2015;3:2040–2044. [Google Scholar]
- 31.Fahad M., Dickens P., Gilbert M. Novel polymeric support materials for jetting based additive manufacturing processes. Rapid Prototyp J. 2013;19:230–239. [Google Scholar]
- 32.Hofmann M. 3D printing gets a boost and opportunities with polymer materials. ACS Macro Lett. 2014;3:382–386. doi: 10.1021/mz4006556. [DOI] [PubMed] [Google Scholar]
- 36.Mokrane A., Boutaous M., Xin S. Process of selective laser sintering of polymer powders: modeling, simulation, and validation. Comptes Rendus Mecanique. 2018;346:1087–1103. [Google Scholar]
- 37.Kandis M., Bergman T.L. A simulation-based correlation of the density and thermal conductivity of objects produced by laser sintering of polymer powders. J Manuf Sci Eng. 2000;122:439–444. [Google Scholar]
- 38.Ahmadi Dastjerdi A., Movahhedy M.R., Akbari J. Optimization of process parameters for reducing warpage in selected laser sintering of polymer parts. Addit Manuf. 2017;18:285–294. [Google Scholar]
- 39.Minetola P., Calignano F., Galati M. Comparing geometric tolerance capabilities of additive manufacturing systems for polymers. Addit Manuf. 2020;32:101103. [Google Scholar]
- 40.Schmid M., Amado A., Wegener K. Polymer powders for selective laser sintering (SLS) AIP Conf Proc. 2015;1664:160009. [Google Scholar]
- 41.Habib F.N., Iovenitti P., Masood S.H., Nikzad M. Fabrication of polymeric lattice structures for optimum energy absorption using Multi Jet Fusion technology. Mater Des. 2018;155:86–98. [Google Scholar]
- 42.Xu Z., Wang Y., Wu D., Ananth K.P., Bai J. The process and performance comparison of polyamide 12 manufactured by multi jet fusion and selective laser sintering. J Manuf Process. 2019;47:419–426. [Google Scholar]
- 43.O’Connor H.J., Dickson A.N., Dowling D.P. Evaluation of the mechanical performance of polymer parts fabricated using a production scale multi jet fusion printing process. Addit Manuf. 2018;22:381–387. [Google Scholar]
- 44.Rocha C.R., Perez A.R.T., Roberson D.A., Shemelya C.M., MacDonald E., Wicker R.B. Novel ABS-based binary and ternary polymer blends for material extrusion 3D printing. J Mater Res. 2014;29:1859–1866. [Google Scholar]
- 45.Hunt E.J., Zhang C., Anzalone N., Pearce J.M. Polymer recycling codes for distributed manufacturing with 3-D printers. Resour Conserv Recycl. 2015;97:24–30. [Google Scholar]
- 46.Compton B.G., Lewis J.A. 3D-printing of lightweight cellular composites. Adv Mater. 2014;26(34):5930–5935. doi: 10.1002/adma.201401804. [DOI] [PubMed] [Google Scholar]
- 47.Shaffer S., Yang K., Vargas J., Di Prima M.A., Voit W. On reducing anisotropy in 3D printed polymers via ionizing radiation. Polymer. 2014;55:5969–5979. [Google Scholar]
- 48.Korpela J., Kokkari A., Korhonen H., Malin M., Naerhi T., Seppaelea J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J Biomed Mater Res B Appl Biomater. 2013;101B:610–619. doi: 10.1002/jbm.b.32863. [DOI] [PubMed] [Google Scholar]
- 49.Larraneta E., Dominguez-Robles J., Lamprou D.A. Additive manufacturing can assist in the fight against COVID-19 and other pandemics and impact on the global supply chain. 3D Print Addit Manuf. 2020;7:100–103. doi: 10.1089/3dp.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Guardian. More masks than jellyfish: coronavirus waste ends up in ocean. https://www.theguardian.com/environment/2020/jun/08/more-masks-than-jellyfish-coronavirus-waste-ends-up-in-ocean [accessed 03.08.20].
- 51.Huang S.H., Liu P., Mokasdar A., Hou L. Additive manufacturing and its societal impact: a literature review. Int J Adv Manuf Technol. 2013;67:1191–1203. [Google Scholar]
- 52.Sreenivasan R., Goel A., Bourell D.L. Sustainability issues in laser-based additive manufacturing. Phys Proc. 2010;5:81–90. [Google Scholar]
- 53.Cruz Sanchez F.A., Boudaoud H., Hoppe S., Camargo M. Polymer recycling in an open-source additive manufacturing context: mechanical issues. Addit Manuf. 2017;17:87–105. [Google Scholar]
- 54.Shi Q., Yu K., Kuang X., Mu X., Dunn C.K., Dunn M.L. Recyclable 3D printing of vitrimer epoxy. Mater Horizons. 2017;4:598–607. [Google Scholar]
- 55.Bernardo C.A., Cunha A.M., Oliveira M.J. The recycling of thermoplastics: prediction of the properties of mixtures of virgin and reprocessed polyolefins. Polymer Eng Sci. 1996;36:511–519. [Google Scholar]
- 56.Vafea M.T., Atalla E., Georgakas J., Shehadeh F., Mylona E.K., Kalligeros M. Emerging technologies for use in the study, diagnosis, and treatment of patients with COVID-19. Cell Mol Bioeng. 2020 doi: 10.1007/s12195-020-00629-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erickson M.M., Richardson E.S., Hernandez N.M., Bobbert D.W., Gall K., Fearis P. Helmet modification to PPE with 3D printing during the COVID-19 pandemic at Duke University Medical Center: a novel technique. J Arthroplasty. 2020;35:S23–S27. doi: 10.1016/j.arth.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Formlabs. 3D Printed COVID-19 Test Swabs. https://formlabs.com/covid-19-response/covid-test-swabs/ [accessed 01.06.20].
- 59.Volkswagen AG. From making cars to ventilators. https://www.volkswagenag.com/en/news/stories/2020/04/from-making-cars-to-ventilators.html [accessed 18.06.20].
- 60.Fast Radius. Request reusable medical face shield kits. https://pages.fastradius.com/medical-face-shields-covid-19.html [accessed 24.06.20].
- 61.Amin D., Nguyen N., Roser S.M., Abramowicz S. 3D printing of face shields during COVID-19 pandemic: a technical note. J Oral Maxillofac Surg. 2020 doi: 10.1016/j.joms.2020.04.040. S0278239120304468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swennen G.R.J., Pottel L., Haers P.E. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020;49:673–677. doi: 10.1016/j.ijom.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novak J.I., Loy J. A quantitative analysis of 3D printed face shields and masks during COVID-19. Emerald Open Res. 2020;2:42. doi: 10.35241/emeraldopenres.13815.1. [DOI] [Google Scholar]
- 64.Tay J.K., Cross G.B., Lee C.K., Yan B., Loh J., Lim Z.Y. Design and clinical validation of a 3D-printed nasopharyngeal swab for COVID-19 testing. MedRxiv. 2020 doi: 10.1101/2020.06.18.20134791. [DOI] [Google Scholar]
- 65.Faryami A., Harris C.A. Open source 3D printed Ventilation Device. BioRxiv. 2020 doi: 10.1101/2020.05.21.108043. [DOI] [Google Scholar]
- 66.Ayyildiz S., Dursun A.M., Yildirim V., Ince M.E., Guelcelik M.A., Erdoel C. 3D-printed splitter for use of a single ventilator on multiple patients during COVID-19. 3D Print Addit Manuf. 2020 doi: 10.1089/3dp.2020.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clifton W., Damon A., Martin A.K. Considerations and cautions for three-dimensional-printed personal protective equipment in the COVID-19 crisis. 3D Print Addit Manuf. 2020;7:97–99. doi: 10.1089/3dp.2020.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francois P.M., Bonnet X., Kosior J., Adam J., Khonsari R.H. 3D-printed contact-free devices designed and dispatched against the COVID-19 pandemic: the 3D COVID initiative. J Stomatol Oral Maxillofac Surg. 2020 doi: 10.1016/j.jormas.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishack S., Lipner S.R. Applications of 3D printing technology to address COVID-19-related supply shortages. Am J Med. 2020;133:771–773. doi: 10.1016/j.amjmed.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuniga J.M., Cortes A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev Med Dev. 2020;17:477–481. doi: 10.1080/17434440.2020.1756771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vordos N., Gkika D.A., Maliaris G., Tilkeridis K., Antoniou A., Bandekas D.V. How social media and 3D printing tackles the PPE shortage during Covid-19 pandemic. MedRxiv. 2020 doi: 10.1101/2020.04.27.20081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evolusi 3D. EVOLUSI 3D Fight COVID-19. https://www.evolusi3d.com/evolusi-3d-fight-covid-19.html [accessed 21.06.20].
- 73.BCN3D Technology. 3D printing at BCN3D to change the face of the global Covid-19 pandemic. https://www.bcn3d.com/here-to-help-3d-printing-to-change-the-covid-19-pandemic/ [accessed 21.06.20].
- 74.Photocentric 3D printing. Printing Parts for Covid 19-Photocentric 3D Printing, Face Shields. https://photocentricgroup.com/printing-parts-for-covid-19/ [accessed 22.06.20].
- 75.iMakr. iMakr, 3D Printers, 3D Scanners, Services online. https://www.imakr.com/ [accessed 24.06.20].
- 76.Nexa 3D. 3D Printing Leader Nexa3D Launches Service to Deliver NexaShield PPE to Frontline Healthcare and Other Mission Critical Workers. https://nexa3d.com/3d-printing-leader-nexa3d-launches-service-to-deliver-nexashield-ppe-to-frontline-healthcare-and-other-mission-critical-workers/ [accessed 23.06.20].
- 77.3D Systems. COVID-19 Call to Action. https://www.3dsystems.com/covid-19-response [accessed 23.06.20].
- 78.Prusa 3D. 3D printed face shields for medics and professionals. https://www.prusa3d.com/covid19/ [accessed 24.06.20].
- 79.Swansea University. Face shields designed by Swansea University team get CE mark for safety for NHS to use. https://www.swansea.ac.uk/press-office/news-events/news/2020/06/face-shields-designed-by-university-team-get-ce-mark-for-safety-for-nhs-to-use.php [accessed 10.08.20].
- 80.Budmen Industries. 3D Face Shield-Producer FAQ. https://budmen.com/face-shield/faq/ [accessed 24.06.20].
- 81.Azul 3D. Azul 3D Protects Healthcare Workers Facing COVID-19 Crisis-Printing 1,000 Face Shields Per Day Per Printer. https://www.businesswire.com/news/home/20200330005666/en/Azul-3D-Protects-Healthcare-Workers-Facing-COVID-19 [accessed 24.06.20].
- 82.Paragon Rapid Technologies. https://paragon-rt.com/ [accessed 24.06.20].
- 83.3D Printing Industry. 3D Printing Community responds to COVID-19 and Coronavirus resources. https://3dprintingindustry.com/news/3d-printing-community-responds-to-covid-19-and-coronavirus-resources-169143/ [accessed 20.06.20].
- 84.Griffiths L., O’Connor D. Sam Davies. The latest 3D printing efforts against Covid-19. TCT Mag. 2020 [Google Scholar]
- 85.KUKA AG. KUKA Robots Print Protective Equipment in Northern Italy. https://www.kuka.com/en-us/press/news/2020/05/kuka-robots-print-protective-equipment-in-northern-italy [accessed 24.06.20].
- 86.Stratasys. Stratasys Responds to COVID-19 with 3D Printed Face Shields & Testing Swabs. https://www.stratasys.com/covid-19 [accessed 22.06.20].
- 87.Carbon3D. DLS Face Shield Assembly and Care Instructions. https://www.carbon3d.com/dls-face-shield-instructions/ [accessed 24.06.20].
- 88.Novakova A. Mass production of original Czech mask with the highest degree of protection has been launched. More than thirty Czech companies helped with preparations. https://www.ciirc.cvut.cz/seriova-vyroba-ceske-masky-s-nejvyssim-stupnem-ochrany-byla-zahajena-do-priprav-se-zapojilo-vice-nez-tricet-ceskych-spolecnosti/ [accessed 27.06.20].
- 89.FDA. Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency (Revised). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health [accessed 21.06.20].
- 90.WHO. Coronavirus disease (COVID-19) advice for the public: when and how to use masks. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks [accessed 05.08.20].
- 91.Czech Technical University. CIIRC CTU Develops Own Prototype of CIIRC RP95 Respirator/Half Mask. https://www.ciirc.cvut.cz/covid/ [accessed 27.06.20].
- 92.Roboze. COVID-19, the value of collaboration and sharing. https://www.roboze.com/en/news/covid-19-the-value-of-collaboration-and-sharing.html [accessed 28.06.20].
- 93.Maker Mask. News Releases. https://www.makermask.com/news-releases.html [accessed 28.06.20].
- 94.NIH. Stopgap Surgical Face Mask (SFM), NIH 3D Print Exchange. https://3dprint.nih.gov/discover/3dpx-013429 [accessed 28.06.20].
- 95.Maskson. FAQ, MasksOn.Org. https://maskson.org/faq/ [accessed 29.06.20].
- 96.Amenabar T. Stanford has made a reusable mask from scuba gear-and it's shipping it to the front lines of the pandemic, The Washington Post. https://www.washingtonpost.com/lifestyle/2020/04/29/stanford-mask-ppe-scuba-pneumask/ [accessed 30.06.20].
- 97.Nexeer. Nexteer Supports Fight Against COVID-19 with Medical Mask & Face Shield Components Production. https://www.nexteer.com/release/nexteer-supports-fight-against-covid-19-with-medical-mask-face-shield-components-production/ [accessed 30.06.20].
- 98.NIH. 3d Mask-SLUHN and Filament Innovations, NIH 3D Print Exchange. https://3dprint.nih.gov/discover/3dpx-013321 [accessed 30.06.20].
- 99.Barrow Innovation Center. 3D Printed N95 Replacement Mask. https://www.barrowneuro.org/get-to-know-barrow/barrow-innovation-center-2/3d-printed-n95-mask/ [accessed 30.06.20].
- 100.Stansbury J.W., Idacavage M.J. 3D printing with polymers: challenges among expanding options and opportunities. Dent Mater. 2016;32:54–64. doi: 10.1016/j.dental.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 101.ProtoCAM. Additive Manufacturing News, ProtoCAM. https://www.protocam.com/learningcenter/news/3d-masks-covid-19/ [accessed 30.07.20].
- 102.EssentiumWRX. https://essentiumwrx.com/about/ [accessed 30.06.20].
- 103.Farsoon. Farsoon Technologies-Open for Industry. http://en.farsoon.com/yl_detail/productId=98.html [accessed 30.06.20].
- 104.3D Systems. Stopgap Face Mask (SFM)-Instructions for Use. https://www.3dsystems.com/sites/default/files/2020-05/Stopgap.
- 105.NPR.ORG. Snorkel Kits Help Doctors Get Through PPE Shortage. https://www.npr.org/2020/04/23/842195578/snorkel-kits-help-doctors-get-through-ppe-shortage [accessed 29.06.20].
- 106.MasksOn: A COVID-19 Story of Collaboration, MasksOn.Org. https://maskson.org/blog/maskson-a-covid-19-story-of-collaboration/ [accessed 29.06.20].
- 107.MasksOn. User Guide, MasksOn.Org. https://maskson.org/info/ [accessed 29.06.20].
- 108.Isinnova. Easy-Covid19 ENG. https://www.isinnova.it/easy-covid19-eng/ [accessed 26.07.20].
- 109.Wolf E. AIRWOLF3D Offering Emergency Additive Manufacturing Services. https://airwolf3d.com/2020/03/17/airwolf3d-offering-emergency-additive-manufacturing-services/ [accessed 26.07.20].
- 110.CRP Technology. CRP Technology on the front line in the fight against Covid-19. https://www.crptechnology.com/front-line-fight-against-covid19-mask-3d-printing/ [accessed 26.07.20].
- 111.Editors DE. Weerg 3D Prints Valves for Emergency Respiratory Masks. https://www.digitalengineering247.com/article/weerg-3d-prints-valves-for-emergency-respiratory-masks [accessed 26.07.20].
- 112.Prisma Health. Prisma Health introduces VESper. https://www.prismahealth.org/vesper/ [accessed 26.07.20].
- 113.Materialise. 3D-Printed Non-Invasive PEEP Masks Aim to Alleviate Ventilator Shortage. https://www.materialise.com/en/node/7056 [accessed 27.07.20].
- 114.Ferrari Corporate. Ferrari continues its efforts to fight the Covid-19 pandemic. https://corporate.ferrari.com/en/ferrari-continues-its-efforts-fight-covid-19-pandemic [accessed 27.07.20].
- 115.Dondorp A.M., Hayat M., Aryal D., Beane A., Schultz M.J. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102(6):1191–1197. doi: 10.4269/ajtmh.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meng L., Qiu H., Wan L., Ai Y., Xue Z., Guo Q. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anaesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance. https://www.who.int/publications/i/item/clinical-management-of-covid-19.
- 118.Banker S. COVID-19 And 3D Printing. https://www.forbes.com/sites/stevebanker/2020/04/13/covid-19-and-3d-printing/ [accessed 25.07.20].
- 119.FDA. Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-ventilators-and-accessories-and-other-respiratory-devices-during-coronavirus [accessed 25.07.20].
- 120.Neyman G., Irvin C.B. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006;13:1246–1249. doi: 10.1197/j.aem.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ventsplitter. A Free 3D Printable Ventilator Circuit Splitter. http://ventsplitter.org [accessed 27.07.20].
- 122.Materialise. Fast and Reliable: MedTech Solutions amid the COVID-19 Pandemic. https://www.materialise.com/en/node/7046 [accessed 27.07.20].
- 123.EnvisionTEC. EnvisionTEC to 3D Print Mass Quantities of Nasopharyngeal Swabs for COVID-19 Testing Based on Successful Clinical Trial Medical. https://envisiontec.com/envisiontec-to-3d-print-mass-quantities-of-nasopharyngeal-swabs-for-covid-19-testing-based-on-successful-clinical-trial/ [accessed 02.06.20].
- 124.FORECAST 3D. Forecast 3D now producing nasopharyngeal swabs for COVID-19 test kits. https://www.gknpm.com/en/news-and-media/news-releases/2020/forecast-3d-now-producing-nasopharyngeal-swabs-for-covid-19-test-kits/ [accessed 06.06.20].
- 125.Hospital Virtual Valdecilla. Recogida de muestras: Hisopos. https://www.hvvaldecilla.es/proyectos/recogidas-de-muestras-hisopos/ [accessed 04.06.20].
- 126.Nexa 3D. 3D Printing Leader Nexa3D Launches Service to Deliver NexaShield PPE to Frontline Healthcare and Other Mission Critical Workers. https://nexa3d.com/3d-printing-leader-nexa3d-launches-service-to-deliver-nexashield-ppe-to-frontline-healthcare-and-other-mission-critical-workers/ [accessed 04.06.20].
- 127.Origin 3D-BIDMC. Origin 3D-Printed COVID-19 Test Swabs Clinical Trial and Validation Completed with Beth Israel Deaconess Medical Center. https://www.origin.io/news/origin-3d-printed-covid-19-test-swabs-clinical-trial-and-validation-completed-with-beth-israel-deaconess-medical-center/ [accessed 04.06.20].
- 128.Carbon3D. Carbon COVID-19 Response. https://www.carbon3d.com/covid19/ [accessed 24.07.20].
- 129.Structo 3D. Structo starts production of millions of sterilized nasopharyngeal testing swabs. https://www.structo3d.com/blogs/press-releases/singapore-based-3d-printing-startup-structo-starts-production-of-millions-of-pre-sterilized-nasopharyngeal-testing-swabs [accessed 24.07.20].
- 130.Centers for Disease Control and Prevention (CDC). Information for Laboratories about Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html.
- 131.Callahan C.J., Lee R., Zulauf K., Tamburello L., Smith K.P., Previtera J. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. MedRxiv. 2020 doi: 10.1101/2020.04.14.20065094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.FDA. FAQs on 3D Printing of Medical Devices, Accessories, Components, and Parts During the COVID-19 Pandemic. https://www.fda.gov/medical-devices/3d-printing-medical-devices/faqs-3d-printing-medical-devices-accessories-components-and-parts-during-covid-19-pandemic [accessed 30.06.20].
- 133.COVID-19 Makers Challenge. https://www.covid19makerchallenge.com/ [accessed 29.06.20].
- 134.UK Govt. Regulatory status of equipment being used to help prevent coronavirus COVID-19. https://www.gov.uk/guidance/regulatory-status-of-equipment-being-used-to-help-prevent-coronavirus-covid-19 [accessed 10.08.20].
- 135.European Union (EU). Coronavirus response in relation to personal protective equipment. https://ec.europa.eu/growth/sectors/mechanical-engineering/personal-protective-equipment_en [accessed 10.08.20].
- 136.Choong Y.Y.C., Tan H.W., Patel D.C., Choong W.T.N., Chen C.H., Low H.Y. The global rise of 3D printing during the COVID-19 pandemic. Nat Rev Mater. 2020;5:637–639. doi: 10.1038/s41578-020-00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel P., Gohil P. Role of additive manufacturing in medical application COVID-19 scenario: India case study. J Manuf Syst. 2020 doi: 10.1016/j.jmsy.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nazir A., Azhar A., Nazir U., Liu Y.F., Qureshi W.S., Chen J.E. The rise of 3D Printing entangled with smart computer aided design during COVID-19 era. J Manuf Syst. 2020 doi: 10.1016/j.jmsy.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malik A.A., Masood T., Kousar R. Reconfiguring and ramping-up ventilator production in the face of COVID-19: can robots help? J Manuf Syst. 2020 doi: 10.1016/j.jmsy.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar S., Khanna V., Singh B.P., Patil R.K., Mehrotra D. Purview of 3D printing in medical applications during COVID-19. Health Policy Technol. 2020 doi: 10.1016/j.hlpt.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oladapo B.I., Ismail S.O., Afolalu T.D., Olawade D.B., Zahedi M. Review on 3D printing: fight against COVID-19. Mater Chem Phys. 2021;258:123943. doi: 10.1016/j.matchemphys.2020.123943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manoj A., Bhuyan M., Banik S.R., Sankar M.R. 3D Printing of Nasopharyngeal Swabs for COVID-19 diagnose: past and current trends. Mater Today Proc. 2020 doi: 10.1016/j.matpr.2020.11.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ballard D.H. Quantitative fit tested N95 respirator-alternatives generated with CT imaging and 3D Printing: a response to potential shortages during the COVID-19 pandemic. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arjunan A., Zahid S., Baroutaji A., Robinson J. 3D printed auxetic nasopharyngeal swabs for COVID-19 sample collection. J Mech Behav Biomed Mater. 2020 doi: 10.1016/j.jmbbm.2020.104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shpichka A., Bikmulina P., Peshkova M., Kosheleva N., Zurina I., Zahmatkesh E. Engineering a model to study viral infections: bioprinting, microfluidics, and organoids to defeat coronavirus disease 2019 (COVID-19) Int J Bioprint. 2020;6(4):302. doi: 10.18063/ijb.v6i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Celik H.K., Kose O., Ulmeanu M.E., Rennie A.E.W., Abram T.N., Akinci I. Design and additive manufacturing of medical face shield for healthcare workers battling coronavirus (COVID-19) Int J Bioprint. 2020;6(4):286. doi: 10.18063/ijb.v6i4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bishop E.G., Leigh S.J. Using large-scale additive manufacturing as a bridge manufacturing process in response to shortages in personal protective equipment during the COVID-19 outbreak. Int J Bioprint. 2020;6(4):281. doi: 10.18063/ijb.v6i4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]