Abstract
Objective
The aim of this systematic review was to assess the evidence from randomized controlled trials (RCTs) and cohort studies for the effectiveness of digital interventions designed to enhance adherence to physical activity (PA) for people with inflammatory arthritis and describe the intervention content using established coding criteria.
Methods
Six electronic databases were searched for published and unpublished studies. Independent data extraction and quality assessment (Cochrane risk of bias II or ROBINS-I) were conducted by two reviewers. The primary outcome was self-reported adherence to PA post-intervention. Secondary outcomes included self-reported adherence to PA at other time points, level of PA or engagement with intervention at any follow-up time point. Intervention content was assessed using the Consensus on Exercise Reporting Template and the Behaviour Change Techniques Taxonomy version 1.
Results
From 11 136 citations, four moderate risk of bias studies (three RCTs and one cohort study) including 1160 participants with RA or JIA were identified. Owing to heterogeneity of outcomes, a narrative synthesis was conducted. Only one RCT reported a small between-group difference in adherence to PA [mean difference (95% CI) −0.46 (−0.82, −0.09)] in favour of the intervention. There were no between-group differences in any secondary outcomes. Interventions included between 3 and 11 behaviour change techniques but provided minimal information on exercise prescription.
Conclusion
There is currently limited moderate-quality evidence available to provide confident evaluation of the effect of web-based and mobile health interventions on adherence to PA or level of PA post-intervention in people with inflammatory arthritis.
Keywords: systematic review, inflammatory arthritis, digital health interventions, adherence, physical activity, exercise
Key messages
Digital interventions to support adherence to physical activity in people with inflammatory arthritis seem promising.
There is insufficient evidence to evaluate the effect of digital interventions on physical activity confidently.
Future studies need to report intervention content in line with standardized reporting guidelines.
Introduction
Physical activity (PA) is a key management strategy for people with inflammatory arthritis (rheumatoid arthritis (RA), psoriatic arthritis (PsA), axial spondyloarthritis, juvenile inflammatory arthritis (JIA)) [1, 2]. Guidance suggests that people with inflammatory arthritis should complete ≥150 min of moderate-intensity PA or equivalent per week and strengthening and flexibility exercises twice a week [1–3].
The importance of PA was reinforced by the World Health Organization during the current COVID-19 pandemic [4], but adherence to PA in people with inflammatory arthritis tends to be low [5–7]. There are complex and distinctive barriers that hamper participation in PA and make the adoption and adherence to new health behaviours challenging, without appropriate support [8–10]. However, restricted resources and increasing demand mean that access to face-to-face health-care interventions to support the uptake and maintenance of PA is limited [11], and this is exacerbated by social distancing requirements owing to the COVID-19 pandemic. To address this need, there has been a rapid reconfiguration of services and adoption and scaling up of remote patient care, including new ways to increase and support adherence to PA [12–14].
Interventions that use digital technologies [e.g. mobile applications (apps), websites and wearable devices] that can be delivered across a range of telecommunication devices (e.g. smartphones or tablets) offer a potential solution to support people with inflammatory arthritis in adhering to PA recommendations [15, 16].
However, changing PA behaviour can be complex, and theory- and evidence-based principles are recommended when designing and implementing interventions [17–20]. Previous reviews of the evidence for the effectiveness of digital interventions to support PA in people with inflammatory arthritis highlight the limited number and low methodological quality of studies and poor integration or reporting of evidence-based intervention content [21–23]. One narrative synthesis of four randomized controlled trials (RCTs; 492 participants with inflammatory arthritis) identified no evidence of effect of interactive digital interventions on objectively measured PA and reported limited evidence of effect on self-reported PA [22]. However, only evidence from RCTs was included, potentially missing evidence of effect from other study designs. Another systematic review including six studies (567 participants with RA) found limited evidence for the effectiveness for web-based rehabilitation interventions on self-management, health information and/or PA [21]. This review focused only on people with RA, which limited the generalizability of the findings to the inflammatory arthritis population, and did not report the theoretical underpinning or assess intervention content for the inclusion of behavioural change techniques (BCTs). Neither of the previous reviews assessed the content of the exercise prescriptions against established guidelines.
Consequently, although digital interventions appear promising, the effectiveness of interventions to support PA in people with inflammatory arthritis is unclear. Thus, an up-to-date review is required that also includes the evaluation of PA prescription and BCTs using standardized approaches, such as the Consensus on Exercise Reporting Template (CERT) [24] and Behaviour Change Techniques Taxonomy (BCTTv1) [20]. This will help health-care professionals to identify the specific characteristics or active ingredients associated with effectiveness in interventions [25, 26].
Aims and objectives
In this study, we aimed to (a) systematically identify and quality appraise the evidence from studies evaluating the effectiveness of digital interventions (web-based and mobile apps) on PA for people with inflammatory arthritis conditions; (b) identify and describe the content of PA interventions using standardized reporting formats; and (c) identify whether behavioural theory has been applied to underpin development of the intervention.
Methods
This review was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [27], the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [28] (see Supplementary Data S1, available at Rheumatology Advances in Practice online) and Synthesis Without Meta-Analysis (SWiM) [29] reporting guidelines. The protocol was registered prospectively on the international prospective register of systematic reviews, PROSPERO: CRD35019129341.
Eligibility criteria
Types of studies
We included RCTs, quasi-experimental trials, prospective cohort studies, retrospective cohort analyses and before–after trials that reported baseline and follow-up measurements of adherence to PA or PA levels in at least two groups.
Types of participants
Participants with inflammatory arthritis diagnosed according to established criteria were included (i.e. adults ≥18 years old with RA, PsA or axial spondylarthritis or children with JIA) [30–33]. Data from studies evaluating several rheumatic populations were included if data from different clinical populations were reported separately.
Types of interventions
All types of clinician-guided or self-directed digital interventions were included. We defined digital interventions as interventions delivered via the Internet (static or interactive websites, or web-based apps), personal computers, social media or smartphones (mobile websites or smartphone apps) [34].
Types of comparisons
The study comparison groups comprised interventions not including digital technologies, usual/standard care, information only or waiting list comparisons.
Types of outcomes
The primary outcome of interest was self-reported measure of adherence to PA at the end of the intervention. Outcomes could be reported as exercise diaries, questionnaires and self-reported data uploaded to an app (i.e. user recording exercise completion data). Secondary outcomes included self-reported measures of adherence to PA at any other follow-up time point, levels of PA by any validated measure (e.g. monitoring device, i.e. step-count, accelerometer) or engagement (i.e. usage of the intervention, reported via number of times the participant logged in, minutes active on webpage) at the end of the intervention or any other follow-up time point, if available.
Search strategy
Search terms included MeSH, keyword and wild-card terms located in the title or abstract that reflected disease type, intervention (e.g. web-based, mobile app) and outcome (e.g. self-reported activity) (full search strategy is shown in Supplementary Table S1, available at Rheumatology Advances in Practice online). Studies were retrieved by: (a) searching electronic databases [MEDLINE, CINAHL, PsychINFO, EMBASE, PEDro, Cochrane Central Register of Controlled Trials (CENTRAL), Opengrey]; (b) cross-referencing from retrieved studies, previous relevant systematic reviews and meta-analyses; and (c) soliciting studies from experts in the field and authors who have published studies of web-based and mobile app interventions. Databases were searched from January 2005 to June 2019. The final search was completed on 28 June 2019.
Study selection
The search results were exported to an online platform designed to facilitate systematic and transparent management of reviews, Covidence (http://www.covidence.org). After de-duplication, all retrieved titles and abstracts were examined by two independent reviewers against eligibility criteria. Conflicts were resolved by consensus. A third reviewer acted as arbiter if necessary. Reviewers were not masked to the name(s) of the study author(s), institution(s) or publication source. Authors were contacted when full-text manuscripts were not available or when additional information was needed.
Data extraction
Coding and data extraction from the full text of eligible studies were conducted by two trained reviewers independently (bespoke data extraction tool available on request). Conflicts were resolved by discussion and a third reviewer acted as an arbitrator if necessary, Information was extracted regarding:
Study characteristics (i.e. author, year of publication, study design, sample size).
Characteristics of populations (i.e. number of patients included in each study, type of inflammatory arthritis condition).
Intervention details (i.e. type of intervention: Web-based, mobile type, duration of intervention).
Outcomes of interest (i.e. self-reported measure of adherence, levels of adherence and engagement, e.g. number of logins/access to webpages) at each assessment time point.
The content of the intervention. This included:
Theoretical underpinning of interventions (i.e. authors explicitly stated/reported use of theory in manuscript; yes/no).
Coding to identify the presence of BCTs [20]. The BCTTv1, presents 93 discrete BCTs that are ‘observable, replicable and irreducible component of an intervention designed to alter or redirect causal processes that regulate behavior’ (yes/no).
Completeness of reporting of the exercise prescriptions [Consensus of Exercise Reporting Template (CERT)] [24]. The CERT consists of 16 items across 7 domains applied to evaluate the reporting of exercise interventions (yes/no/not applicable).
Risk of bias
Full texts were assessed for risk of bias by two independent reviewers. The risk of bias tool 2, developed by the Cochrane collaboration, was used to assess RCTs [27]. This tool comprises five domains (randomization process, deviations from intended interventions, missing data, measurement of the outcome and selection of the reported results) and is classified as having either the presence or potential presence of a source of bias (Yes), no risk of bias (No) or some concerns.
The Risk Of Bias in Non-randomized Studies – of Interventions (ROBINS-I) tool was applied to non-RCTs [35]. This tool includes seven domains (bias attributable to cofounding, bias in selection of participants into the study, bias in classification of interventions, bias attributable to deviations from intended interventions, bias attributable to missing data, bias in measurement of outcomes and bias in selection of the reported results). Risk of bias was evaluated as being low risk, moderate risk, serious risk or critical risk. Any disagreements were resolved though discussions with a third reviewer.
Data analysis
We grouped studies according to the type of study design (RCT or cohort), mode of intervention delivery (via intervention website, Internet webpage) and population. Standardized mean differences (SMDs) for within- and between-group differences on the primary outcome (self-reported adherence to PA) for all RCTs were calculated using Cochrane Review Manager (RevMan v.5.41) [36]. For SMDs, effect sizes were interpreted as follows: 0.20 = small effect, 0.50 = moderate effect and 0.80 = large effect [37]. Studies with multiple interventions were grouped together and combined as recommended in the Cochrane handbook for systematic reviews of interventions [27]. In studies that reported the median and interquartile range, we calculated the mean (s.d.) as recommended in the Cochrane handbook for systematic reviews of interventions [27], reporting the conversion in line with published recommendations [38–40]. We assessed clinical heterogeneity by inspecting the types of participants, interventions and outcomes of each study. Owing to the clinical and methodological heterogeneity, results could not be combined reliably to complete a meta- analysis. Therefore, a narrative synthesis without meta-analysis (SWiM) of all studies (RCTs and cohort studies) was conducted [29]. Intervention engagement data (e.g. usage data) were reported as the mean (s.d.) for the number of times participants logged into the intervention.
Results
Articles identified
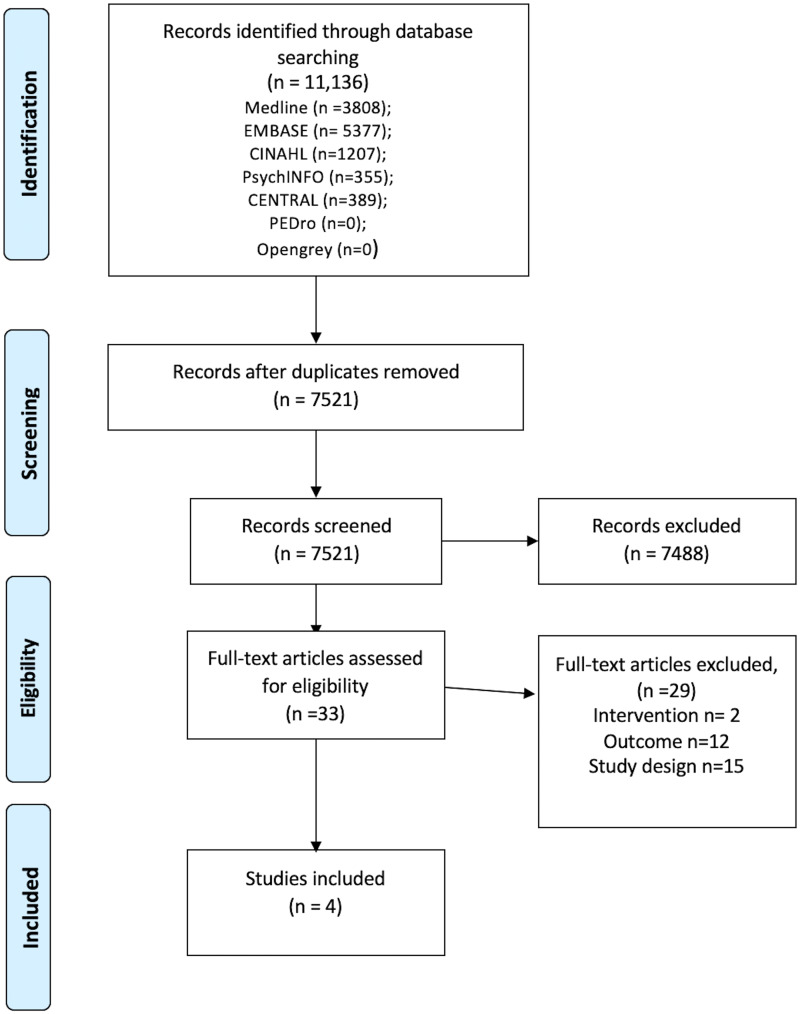
Fig. 1 presents a flow diagram of study selection. We identified 11 136 studies. After de-duplication (n = 3615), 7521 titles and abstracts were screened for eligibility and 33 manuscripts progressed to full review. Four publications reporting three trials and one cohort study were included. No unpublished studies were included.
Fig. 1.
Flow diagram illustrating study selection
General characteristics of included studies and populations
The characteristics of included studies are presented in Table 1. Included studies were published between 2008 and 2016. The RCTs were completed in Switzerland [40], The Netherlands [41] and the USA [42], and the cohort study was conducted in Canada [43].
Table 1.
Characteristics of included studies
| Author (year) country | Study design | Population | Sample size | Age [years, mean (s.d.)], % male | Intervention group (n) and description intervention | Comparator group (n) and description of intervention | Intervention duration | Length of follow-up | Measure of adherence to PA | Measure of levels of PA | Theory, BCTs (n) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Allam et al. (2015) [40] Switzerland |
RCT | RA | 157 |
53.46 (9.96) 54% |
Information (n = 30) Information and social support (n = 29) Gaming (n = 28) Social support and gaming (n = 28) Combined groups (n = 115) |
Information on RA management delivered via the Web page Social support provided via online forum Participants’ actions and contributions to the platform were reviewed by collective time points Social support provided via online forum plus participants’ actions and contributions to the platform were reviewed by collective time points |
Control group (n = 40) | No access to website | 8 weeks | 2 months (post-intervention) | Self-report completion of exercise (min/week) | N/A |
No theory reported Information: Information about health consequences, credible source, problem solving (n = 3) Information and social support: Information about health consequences, credible source, problem solving, social support (practical); social support (emotional) (n = 5) Gaming: Information about health consequences, credible source, problem solving, non-specific reward, social comparison (n = 6) Social support and gaming: Information about health consequences, credible source, problem solving, social support (practical); social support (emotional) non-specific reward, social comparison (n = 7) |
|
Armbrust et al. (2017) [41] The Netherlands |
RCT | JIA | 49 |
10.2 (9–10.8) 43% |
Intervention group (n = 28) | Web-based cognitive behavioural programme to improve physical activity. Combination of Internet-based and individual instruction, supplemented with four group sessions | Standard care (n = 21) | Standard care, not restricted in any activities | 14 weeks |
14 weeks (post-intervention) 12 months |
Self-report 7 day activity dairy, moderate to vigorous physical activity duration (min/week) |
Accelerometer readings over 7 days Moderate to vigorous physical activity duration (min/week) |
Pender’s Health Protection Model [45] Goal setting (behaviour), information about health consequences, problem solving, social support (emotional), instructions on how to perform the behaviour, action planning, commitment, reward (material), feedback on behaviour, goal setting (outcome), prompts and cues, problem solving (n = 11) |
|
Lorig et al. (2008) [42] USA |
RCT | RA | 855 |
52.2 (12.2) 9.5% |
Intervention group (n = 425) | The internet‐based arthritis self‐management program (ASMP): interactive, web-based instruction; web-based bulletin board discussion; individually tailored tools, such as exercise logs, medication diaries and an exercise programme | Usual care (n = 441) | No access to ASMP | 6 weeks |
6 months 12 months |
Self-reported aerobic exercise (min/week) | N/A |
Self-efficacy theory [46] Goal setting, action planning, demonstration of the behaviour, reduce negative emotions, self-talk, social support (unspecified). Feedback on behaviour, problem solving, verbal persuasion, prompts and cues (n = 10) Control group: reward (material reward) (n = 1) |
|
Brosseau et al. (2014) [43] Canada |
Cohort | RA | N/A |
56.4 (12.5) 19% |
Intervention group (n = 99) | Online educational programme, focusing on self-management in RA, delivered via Facebook | N/A | N/A | 2 weeks |
2 weeks (post-intervention) 3 months |
Self-reported intention to complete hand-strengthening exercises (%) | N/A |
Knowledge to action cycle [47, 48] Credible source; demonstration of the behaviour; instruction on how to perform behaviour (n = 3) |
BCT: behaviour change technique; N/A: not applicable; PA: physical activity; RCT: randomized controlled trial.
The review included a total of 1160 participants (1061 participants in three RCTs, and 99 participants in one cohort study). One RCT included adults with RA [40], one trial included people with RA, OA and FM, but the results were reported separately for each condition at baseline, 6 and 12 month follow-up [42], and one trial included children with JIA [41]. No studies included people with PsA or AS. The cohort study included adults with RA and OA, and the results were presented separately for each condition at baseline and 3 month follow-up [43]. Sample size ranged from 49 [41] to 855 participants [42], and the mean age of participants ranged from 10.6 [41] to 56.4 years [43].
Self-reported adherence to PA was measured in the three RCTs. Two RCTs [40, 42] used the Exercise Behaviours Scale [44], and another trial used an activity diary post-intervention [41]. One trial measured self-reported PA immediately after delivery of the intervention [40], and a further trial assessed self-reported PA after intervention delivery and at 12 month follow-up [41]. One trial did not assess self-reported adherence to PA post-intervention but assessed it at 6 months and 12 months post-intervention [42].
One trial assessed moderate to vigorous physical activity post-intervention and at 12 month follow-up using an accelerometer during a 7 day period [41]. The cohort study assessed the percentage of RA participants’ intention to apply exercises 2 weeks post-intervention and assessed the percentage of participants that applied the exercises at 3 month follow-up [43].
Characteristics of intervention and comparators
All studies investigated interventions delivered via the Internet, and no studies evaluated interventions delivered via mobile applications (Table 1). The duration of the intervention ranged from 2 [43] to 14 weeks [41]. Interventions included in one RCT [42] and one cohort study [43] were delivered remotely. One RCT evaluated an intervention that included access to an online support forum as part of the intervention [40], and another RCT investigated a digital intervention for JIA participants that was supplemented with four group sessions [41]. Comparison groups consisted of usual care with no access to the web-based interventions in two trials [40, 42], and in another trial the control group received standard care and were not restricted in any activities [41].
Risk of bias
Fig. 2 summarizes the sources of risk of bias for included RCT studies. There were three moderate risk of bias RCTs [40, 41, 42]. Table 2 provides details for the one moderate risk bias cohort study [43]. Specifically, the measurement bias, classification bias and intended intervention bias were unclear owing to the lack of reporting of relevant details. All RCTs reported appropriate methods for randomization. Given that it is not possible to blind participants to the nature of the intervention, risk of bias from this source was universally high, and this domain was excluded from the rating of overall study quality. An appropriate analysis for estimating the effect of assignment (intention-to-treat analysis) was performed in two trials [40, 42]. A high dropout rate (72–78%) was present in one RCT, contributing to the missing data bias, and this study also did not report the blinding of the assessor [42]. The pre-planned analysis was not published and described in detail before the start of all three trials. Thus, the main sources of bias include deviations from intended intervention for two trials [40, 41]; missing outcome data and measurement of outcome for one trial [42]; and selection of reported bias for all three trials [40, 41, 42].
Fig. 2.
Risk of bias for included randomized controlled trials
Table 2.
ROBINS-I risk of bias for included cohort studies
| Author (year) | ROBINS-I risk of bias domains |
||||||
|---|---|---|---|---|---|---|---|
| Bias attributable to cofounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias attributable to deviations from intended outcomes | Bias attributable to missing data | Bias in measurement of outcomes | Bias in selection of related result | |
| Brosseau et al. (2014) [43] | Moderate risk | Low risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk |
Effect of adherence to physical activity
Table 3 displays the between- and within-group differences for our primary outcome (adherence to PA post-intervention) and secondary outcome (PA level) for included studies. There was a small between-group difference in adherence to PA at the end of the intervention in one trial with moderate risk of bias [−0.46 (95% CI −0.82, −0.09)] where four interventions were combined and compared with a single comparator group, favouring the intervention [40]. However, there were no differences in individual intervention groups compared with the comparison group in this trial. There was a substantial baseline difference between the control group and intervention groups [40].
Table 3.
Baseline, post-intervention, between- and within-group differences for primary and secondary outcomes
| Author (year) | Description of intervention | Measures | Assessment time point | Baseline |
Follow-up
|
Between-group difference [SMD (95% CI)] | Within-group difference [SMD (95% CI)] | ||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group [n, mean (s.d.)] | Control group [n, mean (s.d.)] | Intervention group [n, mean (s.d.)] | Control group [n, mean (s.d.)] | ||||||
| Allam et al. (2015) [40] | Information |
Adherence to PA: Self-reported completion of exercise (min/week) |
2 months |
30 30.67 (23.13) |
40 50.5 (22.03) |
29 33.36 (26.55) |
40 44.12 (29.69) |
−0.45 (−0.93, 0.04) |
−0.12 (−0.63, 0.39) |
| Allam et al. (2015) [40] | Information and social support |
Adherence to PA: Self-reported completion of exercise (min/week) |
2 months |
29 32.41 (31.92) |
40 50.5 (22.03) |
29 28.10 (29.68) |
40 44.12 (29.69) |
−0.64 (−1.13, −0.15) |
0.14 (−0.38, 0.65) |
| Allam et al. (2015) [40] | Gaming |
Adherence to PA: Self-reported completion of exercise (min/week) |
2 months |
28 33.57 (32.59) |
40 50.5 (22.03) |
28 33.30 (32.43) |
40 44.12 (29.69) |
−0.41 (−0.90, 0.08) |
0.01 (−0.52, 0.53) |
| Allam et al. (2015) [40] | Social support and gaming |
Adherence to PA: Self-reported completion of exercise (min/week) |
2 months |
28 26.96 (27.10) |
40 50.5 (22.03) |
28 28.21 (27.39) |
40 44.12 (29.69) |
−0.66 (−1.16, −0.17) |
−0.05 (−0.57, 0.48) |
| Allam et al. (2015) [40] | Intervention groups combined |
Adherence to PA: Self-reported completion of exercise (min/week) |
2 months |
115 30.91 (28.60) |
40 50.5 (22.03) |
114 30.8 (31.4) |
40 44.12 (29.69) |
−0.46 (−0.82, −0.09) |
0.00 (−0.26, 0.26) |
| Lorig et al. (2008) [42] | The internet‐based arthritis self‐management program (ASMP) |
Adherence to PA: Self-reported aerobic exercise (min/week) |
6 months 12 months |
441 99.1 (104) 347 91.1 (104) |
425 88.5 (100) 375 88.5 (100) |
310 103 (102.7) 307 111.5 (109) |
331 90.5 (93.9) 344 91.0 (92.8) |
−0.13 (−0.29, 0.02) 20.50 (4.85, 36.15) |
−0.04 (−0.19, 0.10) −0.12 (−0.27, 0.04) |
| Armbrust et al. (2017) [41] | Web-based cognitive behavioural program |
Adherence to PA: Self-reported 7 day activity dairy, moderate to vigorous physical activity (min/week) PA level: Accelerometer, moderate to vigorous physical activity (min/week) |
14 weeks 12 months 14 weeks 12 months |
28 58.73 (38.04) 22 58.89 (40.34) 28 44.04 (18.44) 21 43.92 (19.64) |
21 70.9 (25.60) N/A 21 41.24 (20.43) N/A |
28 82.8 (51.24) 22 93.85 (73.31) 28 41.52 (18.44) 21 43.67 (18.76) |
21 77.6 (65.35) N/A 21 51.71 (21.94) N/A |
0.09 −0.00, 0.65 N/A 0.01 −0.55, 0.26 N/A |
−0.53 −1.06, 0.01 −34.96 (−69.93, 0.01) 0.13 (−0.39, 0.66) 0.01 (−0.59, 0.62) |
| Brosseau et al. (2014) [43] | Self-management in RA delivered via Facebook |
Adherence to PA: Self-reported intention to complete hand-strengthening exercises (%) and follow through on using techniques (%) |
2 weeks (intention) 3 months (follow through) |
Percentage of self-reported intention to complete strengthening exercises for the hand 74% | N/A | Percentage that followed through on intention to use strengthening exercises for the hand 78% | N/A | N/A | N/A |
N/A: not applicable; PA: physical activity; SMD: standard mean difference.
Likewise, there were no between-group differences in self-reported adherence to PA in two other trials with moderate risk of bias post-intervention [41], at 6 month follow-up [42] or at 12 month follow-up [41, 42].
For all three trials with moderate risk of bias, no within-group differences were identified [40, 41, 42]. The cohort study with moderate risk of bias found that 74% of RA participants had the intention to use a specific self-management technique relating to strengthening exercises for the hand 2 weeks after accessing the online Facebook page [43]. At 3 months post-intervention, 78% of participants followed through on completing the hand-strengthening exercises [43].
Effect on physical activity levels
Only one trial measured PA level [41]. Post-intervention, there were no within- or between-group differences in objectively measured PA levels post-intervention in one trial with moderate risk of bias [41]. Only participants randomized to the intervention group were followed up at 12 months, and no within-group difference in objectively measured PA levels was identified [41].
Engagement
Two trials reported data on intervention engagement (usage data) [41, 42]. In one trial with moderate risk of bias, 24 of 355 participants randomized to the ASMP intervention group did not engage with the web-based intervention (11 participants dropped out from the study before being assigned to a group session, and 13 participants did not log in after being assigned) [42]. A total of 409 participants logged in 31.6 (24.5) [mean (s.d.)] times (range 1–220) over 6 weeks, and 25 of 355 participants generated between 400 and 600 posts on the bulletin board [42].
In another trial, all participants randomized to the intervention group logged in to the intervention 53.7 (93.1) [mean (s.d.)] times [41]. Participants within the gaming group and the social support plus gaming group accessed the website a mean (s.d.) of 66.8 (112.4) times, whereas participants in the information group and the information plus social support group accessed the website less frequently [mean (s.d.) of 26.2 (27.1) times] [41].
Theoretical underpinning of intervention and behaviour change techniques
Three studies explicitly reported a theory or model to underpin the design of the interventions [41–43]. One trial [41] was based on Pender’s Health Protection Model [45], and another trial [42] was underpinned by self-efficacy theory [46]. The included cohort study [43] was underpinned by the knowledge to action cycle [47, 48].
BCTs were identified in all interventions and ranged from 3 [43] to 11 BCTs [41] (Table 1). There was no single BCT applied across all four studies. The most commonly included BCTs in three interventions included problem solving [40, 41, 42] and prompts and cues [41, 42]. Most studies shared a minimum of two BCTs; these included information about health consequences [40, 41], credible source [40, 43], in addition to goal setting and action planning [41, 42]. Only one trial [42] applied one BCT (material reward) to the control group.
Consensus on exercise reporting template (CERT)
None of the RCTs [40, 41, 42] provided sufficient details to be coded against the CERT [24].
Only the included cohort study [43] provided details on the non-exercise components (item 10) and information regarding the setting in which the exercises were to be performed (item 12), resulting in a total CERT score of 2/16 (Supplementary Table S2 and Supplementary Data S2, available at Rheumatology Advances in Practice online).
Discussion
This systematic review shows that there is currently limited, moderate-quality evidence available to enable confident evaluation of the effect of web-based and mobile health interventions on adherence to PA post-intervention in people with RA and JIA. Only one of three trials found a small improvement in adherence to PA post-intervention. Likewise, there is insufficient evidence of the effects of digital interventions on PA level at any time point, because few studies evaluated this outcome. No studies evaluated the effect of PA mobile applications designed for people with inflammatory arthritis, and, surprisingly, no studies evaluated the impact of web-based interventions in people with PsA or AS.
Our findings broadly concur with the findings from previous reviews of web-based interventions in people with inflammatory arthritis [22] and in people with RA [21]. One narrative synthesis identified no trials that reported any significant between-group differences in objectively measured PA, and only one trial with low risk of bias found a significant between-group difference in self-reported vigorous but not moderate PA in people with inflammatory arthritis [22]. Another systematic review including six studies also found limited evidence for the effectiveness of web-based rehabilitation interventions on self-management, health information and/or PA in people with RA [21].
Our review updates and extends these findings by including a broad range of inflammatory arthritis populations, study designs and evaluation of the intervention content. Despite the fast-paced development of digital interventions, broad review eligibility criteria for inflammatory arthritis and updated, comprehensive searches our review included only four studies and confirmed that there is a paucity of high-quality evidence evaluating web-based and mobile health interventions on PA adherence or activity level in people with inflammatory arthritis. However, the COVID-19 pandemic has accelerated the adoption and evaluation of digital interventions; therefore, more evidence might become available [49].
Surprisingly, our review did not include any studies that investigated mobile applications to support adherence to PA. Mobile apps that aim to support adherence to PA are available for people with inflammatory arthritis conditions [50–54], although app content and quality are heterogeneous, and integrated measures to assess PA are often not evidence based [23]. A recent review identified one high-quality mobile app that was designed for people with RA, although its effectiveness has not been established [23].
To our knowledge, our review is one of the first reviews also to synthesize findings on user engagement with digital interventions. Although no within- or between-group differences were detected in our included studies, in one RCT the intervention groups with access to social support had a greater number of logins to the web-based intervention [40]. This suggests that interventions that include a component of social support might enhance participation and interaction with the intervention itself [55]. For example, in a self-management PA mobile Internet service co-designed by people with RA, the inclusion of an interactive forum also enhanced participant engagement with the intervention [56].
Although three studies investigated interventions that were underpinned by theory, the trial that showed a small effect on adherence to PA was not underpinned by theory [40]. The application of theory is strongly advocated to underpin an intervention, because theory provides guidance on what should be the targeted focus of an intervention (i.e. behavioural determinant) and guidance on how to target the specified behavioural determinants (e.g. what constructs would be most effective, or application of BCTs) [57]. However, our findings suggest that identifying the most appropriate theory that effectively targets determinants that influence adherence to PA is challenging, and no included studies described how theory was applied during the development of the digital intervention investigated [58].
All interventions in the studies included in our review incorporated between 3 [40] and 11 BCTs [41]. The most commonly reported BCT was problem solving, which was included in all three RCTs [40, 41, 42]. In this review, the intervention with the small effect included a total of seven BCTs [40]. Although there is limited evidence with regard to the optimal number of BCTs and dosage to support adherence to PA [59, 60], a recent review suggested that interventions with fewer than seven BCTs are most effective at enhancing adherence to prescribed exercise in individuals with musculoskeletal conditions [26].
We evaluated the explicit reporting of PA intervention content using a standardized tool, (CERT) for the first time. Only one cohort study [43] provided some limited details of the intervention PA content. This was disappointing and was predominantly because the descriptions of interventions did not provide specific details of intervention delivery, exercise dosage or adaptations.
It is crucial that future digital interventions include evidence-based PA prescriptions that are aligned to public health guidance and the EULAR [2] for people with inflammatory arthritis recommendations (i.e. ≥150 min of moderate PA/week and twice weekly strengthening and flexibility exercises) [3] Exercise formats and dosages should be described accurately and the options for tailoring, progression and regression of exercises highlighted such that they can be replicated safely [3, 61]. Interventions should also optimize an individual’s capacity, motivation and opportunity to adhere to PA. Evidence-informed BCTs, such as goal setting, instruction and demonstration of appropriate PA, strategies to facilitate regular practice and social support should be incorporated.
This review has a number of methodological considerations. Our review included a comprehensive search strategy, and broad eligibility criteria were applied, thus extending and updating previous reviews [21, 22]. A rigorous assessment of risk of bias of studies [27, 35] was completed, and intervention content was described via a recognized taxonomy [20] and exercise reporting template [24].
However, the findings of this review are compromised by the paucity and quality of the included studies (e.g. measures of PA adherence were often self-reported). The only study that reported an intervention effect had a substantial between-group difference at baseline, and this might have masked the true impact of the intervention [40]. Our review included a wide range of inflammatory arthritis populations to try to estimate the effect of digital interventions on PA. However, this resulted in a substantial age heterogeneity in the population of participants in our included studies. This might have influenced our interpretation of the effect of digital interventions on PA, because children and young people might find hand-held mobile devices easier to use and be more inclined to engage with digital interventions than other generations. Children and adults might also have different life commitments that could impact on adherence to PA. However, because there is only limited research on the effect of digital interventions on PA in people with inflammatory arthritis, we were unable to explore this aspect within our review. Although we reported some data on intervention engagement, this review did not consider whether participants were involved in the development of the web-based interventions or extracted data on the participant experience and acceptability of the interventions.
Conclusion
Our review findings indicate that there is limited evidence evaluating the effect of digital interventions on adherence to PA in people with inflammatory arthritis. The available evidence suggests that there is likely to be no effect of digital interventions on adherence to PA post-intervention or at other follow-up time points. Consequently, clinicians do not have an evidence base to help them select digital interventions to support PA. Future trials need to ensure that the content of web-based and mobile health interventions are reported in line with standardized reporting guidelines [62], report the specific exercise prescriptions [24] and apply validated measures for PA adherence [e.g. Exercise Adherence Reporting Scale (EARS)] [63, 64] and objective measures for PA level (e.g. accelerometers).
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
All data are incorporated into the article and its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
Supplementary Material
References
- 1. Van der Heijde D, Ramiro S, Landewé R. et al. 2016 update of the ASAS‐EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 2. Osthoff AK, Niedermann K, Braun J. et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 201877:1251–60. [DOI] [PubMed] [Google Scholar]
- 3. Bull FC, Al-Ansari SS, Biddle S. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. 2020. #HealthyAtHome - Physical activity. https://www.who.int/news-room/campaigns/connecting-the-world-to-combat-coronavirus/healthyathome/healthyathome---physical-activity
- 5. Pedersen BK, Saltin B.. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25(S3):1–72. [DOI] [PubMed] [Google Scholar]
- 6. O'Dwyer T, Rafferty T, O'Shea F, Gissane C, Wilson F.. Physical activity guidelines: is the message getting through to adults with rheumatic conditions? Rheumatology 2014;53:1812–7. [DOI] [PubMed] [Google Scholar]
- 7. Bell K, Hendry G, Steultjen M.. Physical activity and sedentary behaviour in people with inflammatory joint disease: a cross sectional study. Arthritis Care Res 2020. doi: 10.1002/acr.24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meade LB, Bearne LM, Godfrey EL.. “It’s important to buy in to the new lifestyle”: barriers and facilitators of exercise adherence in a population with persistent musculoskeletal pain. Disabil Rehabil 2021;43:468–78. [DOI] [PubMed] [Google Scholar]
- 9. Manning VL, Hurley MV, Scott DL, Bearne LM.. Are patients meeting the updated physical activity guidelines? Physical activity participation, recommendation, and preferences among inner-city adults with rheumatic diseases. J Clin Rheumatol 2012;18:399–404. [DOI] [PubMed] [Google Scholar]
- 10. Baxter S, Smith C, Treharne G, Stebbings S, Hale L.. What are the perceived barriers, facilitators and attitudes to exercise for women with rheumatoid arthritis? A qualitative study. Disabil Rehabil Int Multidiscip J 2016;38:773–80. [DOI] [PubMed] [Google Scholar]
- 11. Ndosi M, Ferguson R, Backhouse MR. et al. National variation in the composition of rheumatology multidisciplinary teams: a cross-sectional study. Rheumatol Int 2017;37:1453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torous J, Myrick KJ, Rauseo-Ricupero N, Firth J.. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Mental Health 2020;7:e18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirby T. Rheumatologists rapidly adjust patient care during COVID-19 pandemic. Lancet Rheumatol 2020;2:e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinto AJ, Dunstan DW, Owen N, Bonfá E, Gualano B.. Combating physical inactivity during the COVID-19 pandemic. Nat Rev Rheumatol 2020;16:347–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Berg MH, de Boer IG, le Cessie S, Breedveld FC, Vlieland TP.. Most people with rheumatoid arthritis undertake leisure-time physical activity and exercise in the Netherlands: an observational study. Aust J Physiother 2007;53:113–8. [DOI] [PubMed] [Google Scholar]
- 16. Becker S, Miron-Shatz T, Schumacher N. et al. mHealth 2.0: experiences, possibilities, and perspectives. JMIR Mhealth Uhealth 2014;2:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carey R, Jenkins E, Williams P. et al. A taxonomy of modes of delivery of behaviour change interventions: development and evaluation. Eur Health Psychol 2017;19. [Google Scholar]
- 18. Moore GF, Audrey S, Barker M. et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Craig P, Dieppe P, Macintyre S. et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michie S, Richardson M, Johnston M. et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. [DOI] [PubMed] [Google Scholar]
- 21. Srikesavan C, Bryer C, Ali U, Williamson E.. Web-based rehabilitation interventions for people with rheumatoid arthritis: a systematic review. J Telemed Telecare 2019;25:263–75. [DOI] [PubMed] [Google Scholar]
- 22. Griffiths AJ, White CM, Thain PK, Bearne LM.. The effect of interactive digital interventions on physical activity in people with inflammatory arthritis: a systematic review. Rheumatol Int 2018;38:1623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bearne LM, Sekhon M, Grainger R. et al. Smartphone apps targeting physical activity in people with rheumatoid arthritis: systematic quality appraisal and content analysis. JMIR mHealth uHealth 2020;8:e18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slade SC, Dionne CE, Underwood M, Buchbinder R.. Consensus on Exercise Reporting Template (CERT): explanation and elaboration statement. Br J Sports Med 2016;50:1428–37. [DOI] [PubMed] [Google Scholar]
- 25. Michie S, Prestwich A.. Are interventions theory-based? Development of a theory coding scheme. Health Psychol 2010;29:1–8. [DOI] [PubMed] [Google Scholar]
- 26. Meade LB, Bearne LM, Sweeney LH, Alageel SH, Godfrey EL.. Behaviour change techniques associated with adherence to prescribed exercise in patients with persistent musculoskeletal pain: systematic review. Br J Health Psychol 2019;24:10–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JP, Thomas J, Chandler J. et al. , eds. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2019. [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell M, McKenzie JE, Sowden A. et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:I6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 31. Rudwaleit MV, van der Heijde D, Landewé R. et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 32. Taylor W, Gladman D, Helliwell P, et al. ; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 33. Martini A, Ravelli A, Avcin T, et al. ; Pediatric Rheumatology International Trials Organization (PRINTO). Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol 2019;46:190–7. [DOI] [PubMed] [Google Scholar]
- 34. Yardley L, Spring BJ, Riper H. et al. Understanding and promoting effective engagement with digital behavior change interventions. Am J Prev Med 2016;51:833–42. [DOI] [PubMed] [Google Scholar]
- 35. Sterne JA, Hernán MA, Reeves BC. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cochrane Training. Review Manager (RevMan). https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman
- 37. Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 38. Shi J, Luo D, Weng H. et al. Optimally estimating the sample standard deviation from the five‐number summary. Res Synth Methods 2020;11:641–54. [DOI] [PubMed] [Google Scholar]
- 39. Luo D, Wan X, Liu J, Tong T.. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–805. [DOI] [PubMed] [Google Scholar]
- 40. Allam A, Kostova Z, Nakamoto K, Schulz PJ.. The effect of social support features and gamification on a Web-based intervention for rheumatoid arthritis patients: randomized controlled trial. J Med Intern Res 2015;17:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Armbrust W, Bos GJ, Wulffraat NM. et al. Internet program for physical activity and exercise capacity in children with juvenile idiopathic arthritis: a multicenter randomized controlled trial. Arthritis Care Res 2017;69:1040–9. [DOI] [PubMed] [Google Scholar]
- 42. Lorig KR, Ritter PL, Laurent DD, Plant K.. The internet‐based arthritis self‐management program: A one‐year randomized trial for patients with arthritis or fibromyalgia. Arthritis Care Res 2008;59:1009–17. [DOI] [PubMed] [Google Scholar]
- 43. Brosseau L, Wells GA, Brooks S. et al. People getting a grip on arthritis II: an innovative strategy to implement clinical practice guidelines for rheumatoid arthritis and osteoarthritis patients through Facebook. Health Educ J 2014;73:109–25. [Google Scholar]
- 44. Lorig K, Stewart A, Ritter P. et al. Outcome measures for health education and other health care interventions. London: SAGE, 1996. [Google Scholar]
- 45. Srof BJ, Velsor-Friedrich B.. Health promotion in adolescents: a review of Pender’s health promotion model. Nurs Sci Q 2006;19:366–73. [DOI] [PubMed] [Google Scholar]
- 46. Bandura A, Freeman WH, Lightsey R.. Self-efficacy: the exercise of control. New York: WH Freeman, 1997. [Google Scholar]
- 47. Graham ID, Logan J, Harrison MB. et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof 2006;26:13–24. [DOI] [PubMed] [Google Scholar]
- 48. Straus S, Tetroe J, Graham ID, eds. Knowledge translation in health care: moving from evidence to practice. West Sussex: John Wiley & Sons, 2013. [Google Scholar]
- 49. Budd J, Miller BS, Manning EM. et al. Digital technologies in the public-health response to COVID-19. Nat Med 2020;26:1183–0. [DOI] [PubMed] [Google Scholar]
- 50. Najm A, Gossec L, Weill C. et al. Mobile health apps for self-management of rheumatic and musculoskeletal diseases: systematic literature review. JMIR Mhealth Uhealth 2019;7:e14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luo D, Wang P, Lu F. et al. Mobile apps for individuals with rheumatoid arthritis: a systematic review. J Clin Rheumatol 2019;25:133–41. [DOI] [PubMed] [Google Scholar]
- 52. Knitza J, Tascilar K, Messner E-M. et al. German mobile apps in rheumatology: review and analysis using the Mobile Application Rating Scale (MARS). JMIR Mhealth Uhealth 2019;7:e14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kwan YH, Ong WJ, Xiong M. et al. Evaluation of mobile apps targeted at patients with spondyloarthritis for disease monitoring: systematic app search. JMIR mHealth and uHealth 2019;7:e14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grainger R, Townsley H, White B, Langlotz T, Taylor WJ.. Apps for people with rheumatoid arthritis to monitor their disease activity: a review of apps for best practice and quality. JMIR Mhealth Uhealth 2017;5:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perski O, Blandford A, West R, Michie S.. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med 2017;7:254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Revenäs Å, Opava CH, Ahlén H. et al. Mobile internet service for self-management of physical activity in people with rheumatoid arthritis: evaluation of a test version. RMD Open 2016;2:e000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Michie S, Johnston M, Francis J, Hardeman W, Eccles M.. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660–80. [Google Scholar]
- 58. Brand R, Cheval B.. Theories to explain exercise motivation and physical inactivity: ways of expanding our current theoretical perspective. Front Psychol 2019;10:1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Michie S, Abraham C, Whittington C, McAteer J, Gupta S.. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690–701. [DOI] [PubMed] [Google Scholar]
- 60. Bishop FL, Fenge-Davies AL, Kirby S, Geraghty AW.. Context effects and behaviour change techniques in randomised trials: a systematic review using the example of trials to increase adherence to physical activity in musculoskeletal pain. Psychol Health 2015;30:104–21. [DOI] [PubMed] [Google Scholar]
- 61. Boniface G, Gandhi V, Norris M. et al. A systematic review exploring the evidence reported to underpin exercise dose in clinical trials of rheumatoid arthritis. Rheumatology 2020;59:3147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoffmann TC, Glasziou PP, Boutron I. et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 63. Meade LB, Bearne LM, Godfrey EL.. Comprehension and face validity of the Exercise Adherence Rating Scale in patients with persistent musculoskeletal pain. Musculoskelet Care 2018;16:409–12. [DOI] [PubMed] [Google Scholar]
- 64. Newman-Beinart NA, Norton S, Dowling D. et al. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: the Exercise Adherence Rating Scale (EARS). Physiotherapy 2017;103:180–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.