Abstract
Background & Aims:
Gastrointestinal symptoms are prevalent extrapulmonary systemic manifestations of Chronic Obstructive Pulmonary Disease (COPD), but have been rarely studied. We dissected the perturbations in intestinal function in human patients with COPD using comprehensive metabolic and physiological approaches.
Methods:
In this observational study, small intestinal membrane integrity and active carrier-mediated glucose transport were quantified by sugar permeability test in 21 clinically stable patients with moderate to severe COPD (mean FEV1, 41.2 (3.2) % predicted) and 16 healthy control subjects. Protein digestion and absorption was analyzed using stable tracer kinetic methods. Plasma acetate, propionate, and butyrate concentrations were measured as markers of intestinal microbial metabolism.
Results:
Compared with healthy controls, non carrier-mediated permeability was higher (0.062 (95% CI [0.046, 0.078]) vs. 0.037 (95% CI [0.029, 0.045]), P=0.009) and active glucose transport lower in COPD (31.4 (95% CI [23.4, 39.4])% vs. 48.0 (95% CI [37.8, 58.3])%, P=0.010). Protein digestion and absorption was lower in COPD (0.647 (95% CI [0.588, 0.705] vs. 0.823 (95% CI [0.737, 0.909]), P=0007), and impairment greater in patients with dyspnea (P=0.038), exacerbations in preceding year (P=0.052), and reduced transcutaneous oxygen saturation (P=0.051), and was associated with reduced physical activity score (P=0.016) and lower quality of life (P=0.0007). Plasma acetate concentration was reduced in COPD (41.54 (95% CI [35.17, 47.91]) vs. 80.44 (95% CI [54.59, 106.30]) μmol/L, P=0.001) suggesting perturbed intestinal microbial metabolism.
Conclusions:
We conclude that intestinal dysfunction is present in COPD, worsens with increasing disease severity, and is associated with reduced quality of life.
Keywords: comorbidity, gut dysfunction, protein digestion and absorption, stable tracer kinetics, oral sugar tests, short-chain fatty acids
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is considered a systemic disease that adversely impacts extrapulmonary organs, such as the intestine. COPD patients are more often hospitalized for gastrointestinal diseases than non-COPD patients (1) and have a higher prevalence of gastrointestinal diseases and symptoms impacting their psychological well-being (2, 3). In a recent cohort study in patients with COPD, 85% suffered from at least one gastrointestinal symptom within the last week (4). Reduced pulmonary gas exchange may evoke splanchnic hypoxia with resultant impaired intestinal barrier function (5). Mechanisms protecting the intestine from ischemia-induced injury in COPD may be insufficient so that hypoxia during daily activities can enhance intestinal permeability and cause enterocyte damage in these patients (5). Consistently, gastrointestinal integrity was found to be reduced in COPD patients hospitalized for acute exacerbation with hypoxemic respiratory failure (6). While it has been proposed that enterocyte damage might reduce macronutrient digestion and absorption in COPD (5), studies investigating small intestinal digestion and absorption in COPD are lacking. In addition to the hypoxemic intestinal mucosal perturbations in COPD, intestinal microbial composition is possibly also altered contributing to changes in intestinal metabolites (7).
Short-chain fatty acid (SCFA) metabolism gained recent interest in other wasting diseases due to its link with lung and muscle health, as well as wellbeing (7, 8). Acetate, propionate, and butyrate are the most prevalent SCFAs produced primarily through bacterial fiber metabolism in the colon. Previous studies found alterations in the intestinal microbiota composition towards less SCFA producing bacteria in patients with the chronic pulmonary diseases cystic fibrosis and asthma, but alterations in the intestinal microbial metabolites in COPD have not been reported (7).
We developed a comprehensive panel of methods to measure intestinal disturbances in COPD patients and healthy controls from the Metabolism of Disease with Isotope Tracers (MEDIT) trial (9). The MEDIT trial is a controlled trial in healthy and diseased subjects in which comprehensive metabolic characterization is performed using stable tracer methodology. We assessed small intestinal membrane integrity by sugar permeability test using lactulose, rhamnose, and 3-O-methyl-glucose (10). Protein digestion and absorption of a complete high protein meal was measured using the novel, orally provided, uniformly labelled spirulina and labelled free phenylalanine (PHE) method (10–12). Since changes in protein digestion affect the availability of undigested protein in the colon and may shift the bacterial metabolism towards a lower fiber fermentation, we measured plasma concentrations of the SCFAs acetate, butyrate and propionate. We hypothesized that small intestinal digestion and absorption is perturbed in patients with moderate to severe COPD resulting in lower plasma SCFA concentrations due to alterations in intestinal SCFA metabolism.
Materials and Methods
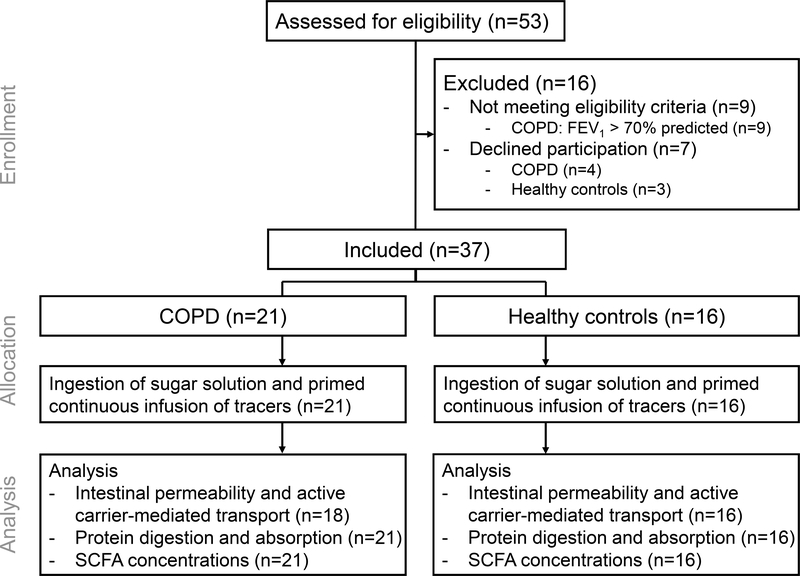
We recruited 21 clinically stable patients with moderate to very severe COPD (13) without evidence of COPD exacerbation 4 weeks prior to the study day, and 16 healthy controls (Figure 1).
Figure 1:
Consolidated Standards of Reporting Trials flow diagram. COPD: Chronic Obstructive Pulmonary Disease. FEV1: forced expiratory volume in 1 second.
Exclusion criteria were malignancy, metabolic, renal or hepatic disease, recent surgery, presence of fever in the last 3 days, intake of oral corticosteroids in the last 4 weeks and of protein containing supplements 5 days preceding the study day, which were assessed by interview and medical records provided by the treating physicians. This study was conducted in accordance with the amended Declaration of Helsinki. All subjects provided a written informed consent for inclusion before they participated in the study and the study was approved by the Institutional Review Board of Texas A&M University and registered on ClinicalTrials.gov (NCT01787682).
Disease Characteristics and Clinical History
During a screening visit, forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were quantified (14). Transcutaneous oxygen saturation was measured by pulse oximetry. We assessed gastrointestinal disorders, medication intake, long-term oxygen therapy, smoking status, exacerbations of COPD and hospitalization rates for COPD exacerbations in preceding year by patient interviews and review of medical records. The presence and severity of comorbidities were determined by the Charlson comorbidity index score (15). All COPD patients completed the modified British Medical Research Council (mMRC) questionnaire to measure dyspnea severity (16). The influence of COPD on quality of life was assessed with the St. George’s Respiratory Questionnaire adapted for COPD (17), for which a total score with the range from 0 (no impairment) to 100 (bad condition) was calculated.
Body Composition and Functional Outcome
Body weight and height were measured to calculate body mass index (14). Total fat mass, fat-free mass (FFM), and appendicular skeletal muscle mass were assessed by dual-energy X-ray absorptiometry and standardized for height as previously reported by us (9). Habitual dietary intake was assessed by 24-hour dietary recall (9), and physical activity using the Physical Activity Scale for the Elderly questionnaire (18).
Panel of Intestinal Function Measurements
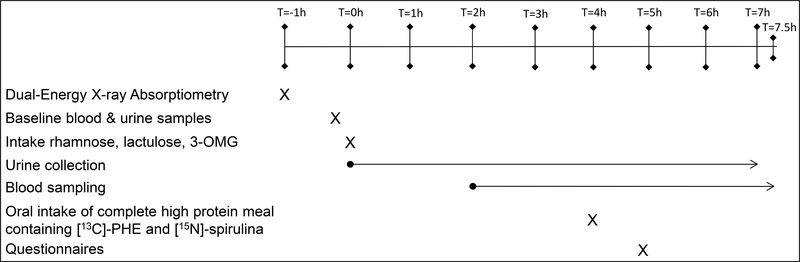
After enrolment following screening, studies were performed in the postabsorptive state (only water was allowed after 12 AM of the study day) (Figure 2).
Figure 2:
Overview of study design. 3-OMG: 3-O-methyl-glucose. PHE: phenylalanine.
Small intestinal membrane integrity was measured by urinary recovery of orally ingested inert sugars, i.e. sugars that are not metabolized in the human body and excreted in the urine. After collection of baseline urine samples to detect background lactulose, rhamnose, and 3-Omethyl-glucose (3-OMG) concentrations, subjects ingested 5 g lactulose (Wockhardt USA, Parsippany, USA), 0.5 g rhamnose, and 70 mg 3-OMG (both Sigma-Aldrich, St. Louis, USA) diluted in 70 mL water (t=0h). Urine was collected over 7 hours. Since lactulose enters the body by a paracellular route when intestinal barrier is lost, and rhamnose by non carrier-mediated transcellular transport, urinary lactulose/rhamnose ratio is an indicator of the small intestinal permeability and, thus, small intestinal barrier function (10). The urinary recovery of 3-OMG [%] is an indicator for active carrier-mediated glucose transport and, thus, for intestinal absorption capacity (10).
Protein digestion and absorption were measured using tracer kinetics as described by us (11). We inserted a catheter into a superficial vein of the lower arm and the hand was placed into a thermostatically controlled hot box (internal temperature: 50°C) for arterialized-venous blood sampling (19).
Baseline blood samples were drawn for determination of natural PHE enrichments. At t=4h participants drank a complete high protein liquid meal, the commercially available BOOST® High Protein supplement (237 mL, 15 g protein (milk protein concentrate, soy protein isolate, calcium and sodium caseinates), 6 g fat, 33 g carbohydrates) (Nestlé S.A., Vevey, Switzerland), enriched with 9.319 (1.996) μmol/kg FFM free L-[1-13C]-PHE and 1.47 g uniformly labelled 15N-Spirulina containing 5.030 (1.077) μmol/kg FFM L-[1-15N]-PHE. All stable tracers were purchased from Cambridge Isotope Laboratories (Woburn, MA, USA). Arterialized-venous blood was sampled 10 times from t=4h to t=7.5h.
Concentrations of the SCFAs acetate, propionate, and butyrate as well as high-sensitivity C-reactive protein (hsCRP), a marker of systemic inflammation, were determined in baseline blood samples.
Biochemical Analyses
We determined PHE tracer enrichments batch-wise (9). In brief, blood was collected in lithium-heparinized tubes (SARSTEDT, Nümbrecht), stored on ice to prevent enzymatic reactions and plasma separated by centrifugation (4°C, 8000 x g, 5 min). Plasma was deproteinized with trichloroacetic acid 0.1 vol of 33% (w/w) and stored at −80°C for analysis with liquid chromatography-tandem mass spectrometry (microLC 200, Eksigent, part of AB Sciex, Union City, USA).
Urine was aliquoted and stored at −80°C and lactulose, rhamnose, and 3-OMG concentrations were analyzed by high-pressure liquid mass spectrometry at the Maastricht University Medical Centre, The Netherlands (20).
To measure SCFA concentrations, 20 μL plasma and 20 μL internal standard containing known concentrations of [2,2,2-2H5]-acetate, [2,2,3,3,3-2H5]-propionate, and [2,2,3,3,4,4,4-2H7]-butyrate in PBS, were derivatized with 80 μL 100 mM pentafluorobenzyl bromide in acetone and incubated for 1 h at 65 °C. Excess pentafluorobenzyl was extracted with hordenine and captured in sulfuric acid in water. SCFAs were extracted in 1000 μL hexane. Samples (0.4 μL) were analyzed on a SCION 436-GC gas chromatograph (Bruker, Billerica, USA) equipped with a 15 m x 0.25 mm, 0.2 μm SP-2330 column (Supelco, Inc.Bellefonte, PA). Helium was used as carrier gas at a rate of 2 mL/min. The inlet was set at 200°C for 0.2 min, ramped to 230°C at 50°C/min, and ramped back to 200 °C at 200 °C/min. Splitless mode was held for 0.2 min, set to 1:100 for 20 s, and to 1:10 for the remaining duration. The initial oven temperature of 45°C was held for 0.35 min, ramped to 71°C at 175°C/min and held for 0.07 min, ramped to 124°C at 15.6°C/min, and ramped to 220°C at 200°C/min where temperature was maintained for 1.68 min.
High-sensitivity C-reactive protein (hsCRP), an inflammation marker, was measured with a particle enhanced immuno-turbidimetric assay (cobas c111, Roche Diagnostics, Mannheim, Germany).
Calculations
The urinary recovery of lactulose, rhamnose, and 3-OMG was corrected for the median of baseline (background) sugar concentrations and expressed as a percentage of oral intake. Small intestinal non carrier-mediated permeability was measured as the lactulose/rhamnose ratio and active carrier-mediated transport as the urinary 3-OMG recovery rate [%].
Protein digestion and absorption was quantified by calculating the ratio of the whole-body rate of appearance of [1-15N]-PHE from 15N-spirulina to the orally provided free [1-13C]-PHE, which does not require digestion before absorption. Spirulina degradation ratio was measured by subsequently dividing the plasma [1-15N]-PHE to [1-13C]-PHE ratio by the ratio of [1-15N]-PHE to [1-13C]-PHE in the protein meal (10–12).
Statistical Analysis
Our primary outcome was the difference of protein digestion and absorption between COPD and healthy participants. Secondary outcomes were the differences in small intestinal barrier function, active carrier-mediated transport, and SCFA concentrations between the two groups, as well as the association of all intestinal function parameters with disease characteristics. Variables are expressed as mean [95% CI range]. If data failed the normality or equal variance test, they were log-transformed where appropriate. We tested differences in general, COPD-related, and intestinal function parameters with unpaired Student’s t-test or one-way analysis of variance. We corrected for multiple comparisons by controlling the false discovery rate (21) using the two-stage step-up method of Benjamini, Krieger and Yekutieli (22) providing the false discovery rate-adjusted P value as q value. For exploratory analysis, we examined whether reduced protein digestion and absorption, active carrier-mediated transport, SCFA concentrations and/or an increased small intestinal permeability exist particularly in COPD patients with more severe disease characteristics (as reflected by long-term oxygen usage, severity of dyspnea, presence of exacerbation, exacerbation induced hospitalization in the past year) and in those with a history of smoking and presence of gastrointestinal symptoms. Small intestinal barrier function, active carrier-mediated transport, protein digestion and absorption, as well as SCFA concentrations were correlated with physical activity score, quality of life, lung function parameters, transcutaneous oxygen saturation, hsCRP concentration, pack-years of smoking, and habitual protein intake in COPD. Correlation analysis was performed with Pearson correlation coefficient. Based on the results, we built a best-fit multiple linear regression model for protein digestion and absorption in the COPD group. The significance level was chosen as α<0.05. Data analyses were performed using GraphPad Prism (version 8.2.1) (GraphPad Software Inc, San Diego, USA).
Results
General Characteristics
In this observational study, we studied 21 COPD patients and 16 healthy controls with comparable gender distribution, and range in age and body mass index (Table 1). COPD patients suffered from low-grade inflammation as noted by elevated hsCRP. Lean body mass, a measure of skeletal muscle mass, was preserved even though COPD patients were less physically active. Consistent with the clinical disease, lung function parameters and transcutaneous oxygen saturation were reduced in COPD patients and history of smoking was higher than in controls. All COPD patients received bronchodilator treatment; 12 patients used long-term oxygen therapy continuously, at night and/or as needed.
Table 1.
Characteristics of the study population
| Healthy (n=16) | COPD (n=21) | P value | |
|---|---|---|---|
| General characteristics | |||
| Age [years] | 65.1 (1.9) | 68.0 (2.2) | 0.338 |
| Gender [n male/female] | 8/8 | 10/11 | 0.744 |
| Body mass index [kg/m] | 27.1 (0.8) | 28.8 (1.4) | 0.486 |
| Fat-free mass index [kg/m] | 18.0 (0.7) | 18.0 (0.6) | 0.992 |
| Fat mass index [kg/m] | 8.47 (0.7) | 9.73 (0.9) | 0.321 |
| Appendicular skeletal muscle index [kg/m] | 7.20 (0.4) | 6.98 (0.3) | 0.666 |
| Habitual protein intake [g/kg FFM/day] | 1.62 (0.1) | 1.67 (0.2) | 0.764 |
| Habitual fiber intake [g/day] | 25.3 (2.0) | 17.3 (2.3) | 0.006 |
| Disease related parameters | |||
| FEV1 [L] | 2.83 (0.2) | 1.12 (0.1) | <0.0001 |
| FEV1 [% of predicted] | 98.1 (3.7) | 41.2 (3.2) | <0.0001 |
| FVC [L] | 3.51 (0.9) | 2.22 (0.1) | <0.0001 |
| FVC [% of predicted] | 92.2 (3.0) | 62.0 (3.2) | <0.0001 |
| FEV1/FVC [ratio] | 0.95 (0.04) | 0.50 (0.03) | <0.0001 |
| DLCO (n=11) [%] | 41.6 (4.0) | ||
| GOLD stage [n II/III/IV] | 6/9/6 | ||
| mMRC dyspnea scale [n grade 1/2/3/4] | 5/6/8/2 | ||
| Exacerbations in past year [n 0/1/2/3/4/5] | 8/5/6/0/1/1 | ||
| Oxygen therapy [n yes/no] | 0/16 | 12/9 | 0.0002 |
| St. George’s respiratory questionnaire [score] | 56.6 (4.1) | ||
| Never smoked/former smoker/active smokers [n] | 12/4/0 | 4/10/7 | 0.005 |
| Pack-years smoked [n] | 2.2 (1.2) | 35.0 (6.2) | 0.0001 |
| Oxygen saturation [%] | 97.3 (0.4) | 93.0 (0.8) | <0.0001 |
| Charlson comorbidity index [score] | 0.31 (0.2) | 1.81 (0.2) | <0.0001 |
| Physical Activity Scale for the Elderly [score] | 163 (21) | 111 (13) | 0.040 |
| High-sensitivity C-reactive protein [mg/L] | 1.72 (0.4) | 4.26 (1.0) | 0.029 |
Values are mean (SEM). COPD: Chronic Obstructive Pulmonary Disease. DLCO: diffusion capacity for carbon monoxide. FEV1: forced expiratory volume in 1 second. FFM: fat-free mass. FVC: forced vital capacity. GOLD: Global Initiative for Chronic Obstructive Lung Disease. mMRC: modified British Medical Research Council.
Intestinal Function Tests
Small Intestinal Barrier Function and Active Carrier-Mediated Transport
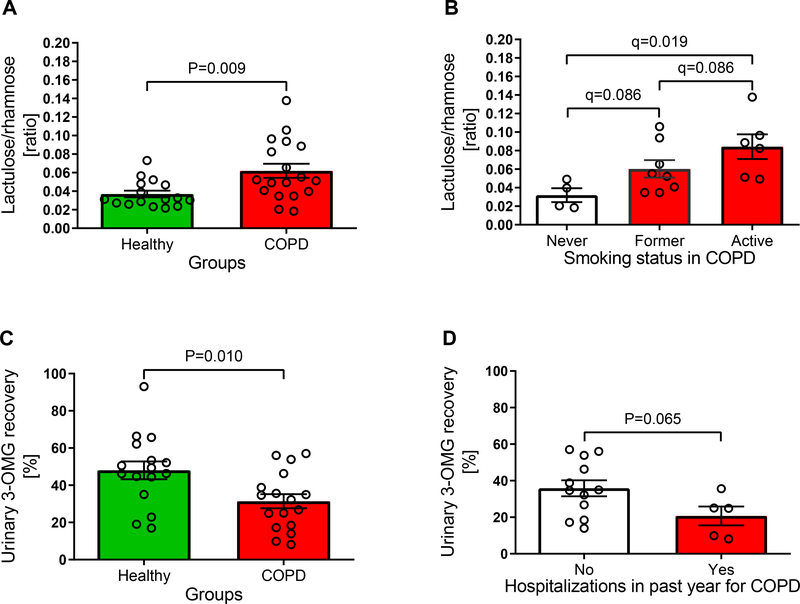
The urinary lactulose/rhamnose ratio was higher (P=0.009) in COPD patients demonstrating an elevated small intestinal non carrier-mediated permeability (0.062 [0.046, 0.078] vs. 0.037 [0.029, 0.045]) (Figure 3A). Stratification of the COPD group for exploratory analysis showed that small intestinal permeability was increased (q=0.019) in active smokers compared to patients who have never smoked (0.084 [0.050, 0.119] vs. 0.032 [0.008, 0.056]) (Figure 3B), and tended to be higher (q=0.086) than in former smokers (0.084 [0.050, 0.119] vs. 0.060 [0.038, 0.083]) and higher (q=0.086) in former smokers compared to patients who have never smoked (0.060 [0.038, 0.083] vs. 0.032 [0.008, 0.056]). Lactulose/rhamnose ratios were similar when stratifying the COPD group for disease characteristics (oxygen usage (P=0.967), exacerbations in the past year (P=0.961), exacerbation induced hospitalization in the past year (P=0.765), severity of dyspnea (P=0.769)), and presence of gastrointestinal symptoms (P=0.585) or when correlating lactulose/rhamnose ratio with physical activity score (P=0.423), quality of life (P=0.498), lung function parameters (FEV1 [% of predicted]: P=0.709; FVC [% of predicted]: P=0.856), transcutaneous oxygen saturation (P=0.280), hsCRP concentration (P=0.261), smoked pack-years (P=0.104), and habitual protein intake (P=0.953) (data not shown).
Figure 3:
Urinary lactulose/rhamnose ratio, a measure of small intestinal permeability, (A; nHealthy=16; nCOPD=18) was higher in Chronic Obstructive Pulmonary Disease (COPD) patients than in healthy controls. Among COPD patients, intestinal permeability was higher in active smokers than in participants who never smoked (B; nnever smoked=4; nformer smoker=8; nactive smoker=6). Urinary 3-O-methyl-glucose (3-OMG) recovery rate, a marker for active carrier-mediated glucose transport, was lower in COPD patients (C: nHealthy=16; nCOPD=17), and decreased to a greater extent in patients who were hospitalized due to an exacerbation in the previous year (D; nno=12; nyes=5). Data are presented as means (SEM).
Urinary 3-OMG recovery rate was decreased (P=0.010) in COPD patients implying a malfunction of the active carrier-mediated transport in enterocytes (31.4 [23.4, 39.4]% vs. 48.0 [37.8, 58.3]%) (Figure 3C). The reduction of active transport tended (P=0.065) to be more pronounced in patients who were hospitalized for an exacerbation in the previous year (YES: 20.7 [6.4, 35.0]% vs. NO: 35.9 [26.2, 45.5]%) (Figure 3D). Urinary 3-OMG recovery rate was similar in COPD patients stratified for disease characteristics (oxygen usage (P=0.630), exacerbations in the past year (P=0.531), severity of dyspnea (P=0.929)), smoking status (never vs. former smoker: q=0.788; never vs. active smoker: q=0.420; former vs. active smoker: q=0.420), and presence of gastrointestinal symptoms (P=0.452). No correlations were found between 3-OMG recovery rate and physical activity score (P=0.833), quality of life (P=0.859), lung function parameters (FEV1 [% of predicted]: P=0.664; FVC [% of predicted]: P=0.673), transcutaneous oxygen saturation (P=0.441), hsCRP concentration (P=0.222), smoked pack-years (P=0.588), and habitual protein intake (P=0.399) (data not shown).
Protein Digestion and Absorption
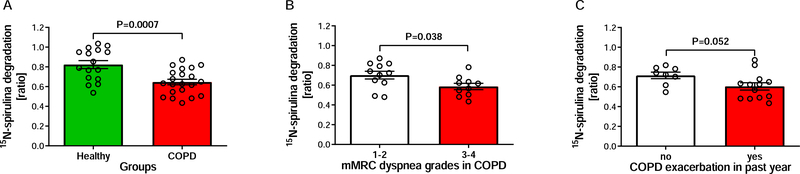
The 15N-spirulina degradation ratio was decreased (P=0.0007) in COPD patients compared to controls (0.647 [0.588, 0.705] vs. 0.823 [0.737, 0.909]) (Figure 4A). Stratification of the COPD group for exploratory analysis indicated that COPD patients classifying their dyspnea severity as mMRC grades 3 and 4 had a lower (P=0.038) protein degradation ratio than patients with mMRC grades 1 and 2 (0.587 [0.514, 0.660] vs. 0.701 [0.614, 0.789]) (Figure 4B). Moreover, COPD patients with at least one exacerbation in the past year tended to have a lower (P=0.052) protein degradation ratio than patients without exacerbations (0.604 [0.524, 0.685] vs. 0.715 [0.638, 0.793]) (Figure 4C).
Figure 4:
15N-spirulina degradation ratio, an indicator of protein digestion and absorption, was lower in Chronic Obstructive Pulmonary Disease (COPD) patients compared to healthy controls (A; nHealthy=16; nCOPD=21). Protein digestion and absorption was reduced to a greater extent in patients with higher dyspnea grades (B; n1–2=11; n3–4=10) and presence of exacerbation(s) in the past year (C; nno=8; nyes=13). Data are presented as means (SEM). mMRC: modified British Medical Research Council Questionnaire.
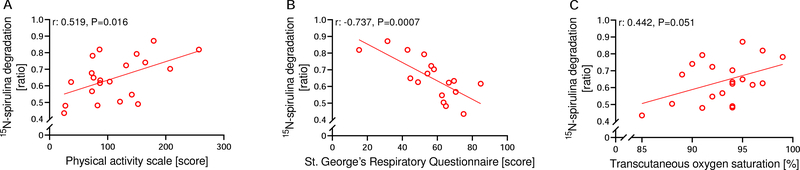
In COPD patients, a higher protein degradation ratio was associated with a higher physical activity score (r:0.519 [0.112, 0.777]; P=0.016) (Figure 5A) and quality of life (r:−0.737 [−0.899, 0.397]; P=0.0007) (Figure 5B), and tended to be associated with a higher transcutaneous oxygen saturation (r:0.442 [−0.0002, 0.740]; P=0.051) (Figure 5C).
Figure 5:
Correlations between 15N-spirulina degradation ratio, a marker of protein digestion and absorption, and different clinical parameters related to Chronic Obstructive Pulmonary Disease (COPD). Patients with a higher 15N-spirulina degradation ratio reported a higher physical activity level (A; n=21), a higher quality of life (B; n=17), and tended to have a higher transcutaneous oxygen saturation (C; n=20).
In COPD, transcutaneous oxygen saturation and self-reported physical activity level explained 52.4% of the variation in 15N-spirulina degradation ratio (Table 2).
Table 2.
Multiple Linear Regression model with 15N-spirulina degradation ratio, a marker of protein digestion and absorption, in Chronic Obstructive Pulmonary Disease patients
| Coefficients | SE | t | P value | |
|---|---|---|---|---|
| Intercept | −0.8024 [−1.885, 0.280] | 0.513 | −1.56 | 0.136 |
| Transcutaneous oxygen saturation [%] | 0.0143 [0.002, 0.026] | 0.0058 | 2.48 | 0.024 |
| Physical Activity Scale for Elderly [score] | 0.0011 [0.0001, 0.002] | 0.0005 | 2.31 | 0.034 |
R2 (weighted 1/Y2) = 0.524; P = 0.0008
No differences in 15N-spirulina degradation ratio were found when stratifying the COPD group for other disease characteristics (oxygen usage (P=0.215), hospitalization in the past year (P=0.610)), smoking status (never vs. former smoker: q=0.862; never vs. active smoker: q=0.109; former vs. active smoker: q=0.109), and presence of gastrointestinal symptoms (P=0.218). Protein degradation ratio did not correlate with lung function parameters (FEV1 [% of predicted]: P=0.505; FVC [% of predicted]: P=0.270), hsCRP concentration (P=0.898), smoked pack-years (P=0.259), and habitual protein intake (P=0.104) (data not shown).
Plasma short-chain fatty acid concentrations
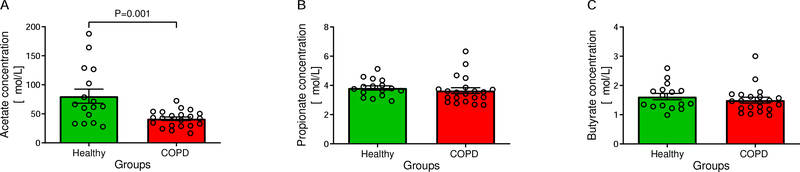
Plasma acetate concentration was decreased (P=0.001) in COPD compared to controls (41.54 [35.17, 47.91] vs. 80,44 [54.59, 106.30] μmol/L) (Figure 6A) while propionate (3.64 [3.22, 4.07] vs. 3.82 [3.51, 4.14] μmol/L; P=0.340) (Figure 6B) and butyrate (1.50 [1.29, 1.71] vs. 1.62 [1.39, 1.85] μmol/L; P=0.361) (Figure 6C) concentrations were comparable between the groups.
Figure 6:
Comparison of plasma short-chain fatty acid concentrations, a measure of fiber fermentation by intestinal microbiota. Acetate was lower in Chronic Obstructive Pulmonary Disease (COPD) patients (A), while propionate (B) and butyrate (C) concentrations were similar between groups (All: nHealthy=16; nCOPD=21). Data are presented as means (SEM).
Acetate concentrations in COPD did not correlate with habitual fiber intake (P=0.282), protein degradation ratio (P=0.365), physical activity score (P=0.491), quality of life (P=0.955), lung function parameters (FEV1 [% of predicted]: P=0.441; FVC [% of predicted]: P=0.966), transcutaneous oxygen saturation (P=0.400), hsCRP concentration (P=0.366), or smoked pack-years (P=0.912). Acetate concentrations were similar when stratifying the COPD group based on disease characteristics (oxygen usage (P=0.803), exacerbations in the past year (P=0.102), hospitalization in the past year (P=0.464), severity of dyspnea (P=0.431)), smoking status (never vs. former smoker: q=0.615; never vs. active smoker: q=0.615; former vs. active smoker: q=0.615), or presence of gastrointestinal symptoms (P=0.710) (data not shown).
Discussion
Using a comprehensive approach to study alterations in intestinal function in clinically stable patients with moderate to severe COPD, we showed increased small intestinal non carrier-mediated permeability and impaired active-carrier mediated transport and protein digestion and absorption. Intestinal dysfunction in COPD was associated with more severe disease characteristics and reduced quality of life.
In our study, the lactulose/rhamnose ratio was increased in COPD with a reduced rhamnose recovery rate while the lactulose recovery rate was similar to that in healthy controls. This observation suggests that COPD patients had an increased intestinal permeability and decreased functional absorptive area. Our observations of increased small intestinal permeability are similar to previous reports in COPD patients at rest, during activities of daily living, and during hospitalization due to an acute COPD exacerbation (5, 6). Increased small and large intestinal permeability and a reduced active carrier-mediated transport have also been reported in chronic heart failure patients, who, like those with COPD, are susceptible to intestinal ischemia and edema (23). Impaired alveolar gas exchange and a low cardiac output can cause hypoxia in metabolically active tissues, such as the intestinal epithelium, resulting in intestinal dysfunction in COPD and chronic heart failure, respectively (5, 24). Furthermore, patients with severe COPD can develop intestinal edema through right-ventricular dysfunction caused by pulmonary hypertension (cor pulmonale) which might impair their absorptive capacity (25). However, no differences in D-xylose or zinc absorption were observed between COPD patients with and without cor pulmonale in a pilot study (25)
Under physiological conditions, the intestinal epithelium at villus tips is hypoxic due to the countercurrent oxygen exchange in villi (26). Even though the intestine has adaptive mechanisms against ischemia-induced damage (27), these mechanisms may be overcome in COPD with maladaptive responses (5). In a mouse model of COPD, 8-weeks of cigarette smoke exposure resulted in the stabilization of hypoxia-inducible factor-2α in colon tissue, increased expression of angiogenic factors in the colon and ileum and an increased intestinal vascularization without improving mucosal tissue and villi hypoxia (28). Smoking can worsen intestinal damage by inhibiting cell renewal and inducing apoptosis in the intestinal epithelium (29). These data are consistent with our studies in human subjects with COPD that show progressively greater intestinal permeability in active smokers than that in former smokers and in patients who never smoked.
Increased intestinal permeability may enhance bacterial translocation and endotoxin release causing an inflammatory immune activation (23). Although our COPD patients were characterized by a low-grade inflammation indicated by elevated hsCRP, hsCRP concentration did not correlate with small intestinal permeability or other intestinal function parameters. This is consistent with reports in critically ill patients in whom hsCRP was not as reliable a predictor of sepsis as procalcitonin (30).
We applied a novel dual-tracer method to measure protein digestion and absorption in COPD. The spirulina degradation ratio in the healthy controls of the present study was similar to those reported by us and others (11, 31). The impairment of protein digestion and absorption in COPD patients was half of that previously reported in patients with cystic fibrosis who did not use pancreatic enzyme during meal intake (11) and similar to protein digestion and absorption in patients with advanced cancer undergoing chemotherapy (10). We also observed that protein digestion and absorption was impaired to a greater extent in patients with more severe disease characteristics (based on higher dyspnea, lower transcutaneous oxygen saturation and disease history (higher frequency of exacerbation(s) in the past year)). Additionally, our COPD patients with more severe intestinal dysfunction reported a lower physical activity level and rated their quality of life lower. This is consistent with previous reports that the presence of gastrointestinal symptoms in COPD patients is associated with a reduced physiological well-being (3). Proteins are digested in the intestinal lumen and can be absorbed by enterocytes as amino acids and small peptides which undergo further digestion in the enterocytes before entering the bloodstream as amino acids. If transport of small peptides from the intestinal lumen into enterocytes is impaired in COPD, peptides escape further digestion into free amino acids in the enterocytes and do not enter the systemic circulation. Thus, reduced spirulina degradation ratio in COPD patients reflects impaired luminal/enterocyte protein digestion and small peptide absorption.
In addition to impaired protein digestion and absorption, COPD patients had lower plasma acetate concentration. Acetate is a major metabolite of fiber fermentation by the intestinal microbiota. Lower plasma acetate concentration in our patients with COPD may be due to the reduced fiber intake with reduced acetate production by intestinal bacteria. However, acetate concentration within COPD patients did not correlate with their daily fiber intake and plasma propionate and butyrate concentrations were not altered in COPD. A low protein digestion and absorption may shift the metabolism of the intestinal microbiota from fiber fermentation towards amino acid degradation (32, 33). However, a lower protein degradation ratio did not correlate with a lower acetate concentration in our COPD group. Our novel observations suggest the need for future studies to investigate SCFA production rates (34), the impact of fiber supplementation on SCFA concentrations, and possible alterations in the intestinal microbiota composition in COPD (7).
A limitation of this study is that we assessed protein digestion and absorption with spirulina, which is not a common protein in the daily diet. However, spirulina is an easily digestible protein (31) and labelling with stable isotopes has been standardized. Since we did not directly measure the absorption of small peptides by enterocytes, the contribution of lower luminal protein digestion capacity, impaired small peptide uptake and reduced peptide digestion in enterocytes to lower spirulina degradation ratio cannot be quantified directly.
Our data on perturbations in intestinal digestion, absorption and intestinal permeability provide a mechanistic basis for lower responsiveness to nutritional support as observed in COPD subgroups (35) and lay the foundation for novel nutritional interventions including those that modulate intestinal permeability (36). Whether there are causal relationships between intestinal function and COPD related disease characteristics (gut-lung axis (7)), and physical activity and muscle health (gut-muscle axis (8)) need to be investigated.
Conclusions
Intestinal dysfunction in clinically stable COPD patients is particularly present in those with more severe disease characteristics and is related to reduced quality of life. Intestinal dysfunction is therefore an important systemic feature in COPD, but the specific clinical implications warrants further investigation.
Acknowledgements
We thank the COPD and healthy control subjects for their willingness to participate in this research study and all CTRAL staff who have made this work possible.
Funding
N.E.P.D. and M.P.K.J.E. were supported in part by National Institutes of Health [grant number: P30ES023512]; and George Abramson Donation. N.E.P.D., S.D., and M.P.K.J.E. were supported in part by National Institutes of Health [grant number: R56HL141744]. S.D. was supported in part by National Institutes of Health [grant numbers: R21AA022742; RO1DK113196; RO1GM119174; P50AA024333; UO1AA021890, UO1AA026975; UO1DK061732]; and the Mikati Foundation Grant.
Abbreviations
- COPD
Chronic Obstructive Pulmonary Disease
- FEV1
forced expiratory volume in 1 s
- FFM
fat-free mass
- FVC
forced vital capacity
- hsCRP
high-sensitivity C-reactive protein
- MEDIT
Metabolism of Disease with Isotope Tracers
- mMRC
modified British Medical Research Council
- PHE
phenylalanine
- SCFA
short-chain fatty acid
- 3-OMG
3-O-methyl-glucose
Footnotes
Competing interests
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nielsen HM, Rodsgaard PA, Weinreich UM. Chronic obstructive pulmonary disease as comorbidity in patients admitted to a university hospital: a cross-sectional study. The clinical respiratory journal. 2014;8(3):274–80. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Brandt L, Granath F, Lofdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung. 2008;186(3):167–72. [DOI] [PubMed] [Google Scholar]
- 3.Niklasson A, Strid H, Simren M, Engstrom CP, Bjornsson E. Prevalence of gastrointestinal symptoms in patients with chronic obstructive pulmonary disease. European journal of gastroenterology & hepatology. 2008;20(4):335–41. [DOI] [PubMed] [Google Scholar]
- 4.Rutten EP, Spruit MA, Franssen FM, Buurman WA, Wouters EF, Lenaerts K. GI symptoms in patients with COPD. Chest. 2014;145(6):1437–8. [DOI] [PubMed] [Google Scholar]
- 5.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145(2):245–52. [DOI] [PubMed] [Google Scholar]
- 6.Sprooten RTM, Lenaerts K, Braeken DCW, Grimbergen I, Rutten EP, Wouters EFM, et al. Increased Small Intestinal Permeability during Severe Acute Exacerbations of COPD. Respiration. 2018;95(5):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan A, Frazer ZA, Hansbro PM, Yang IA. COPD and the gut-lung axis: the therapeutic potential of fibre. Journal of thoracic disease. 2019;11(Suppl 17):S2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ticinesi A, Tana C, Nouvenne A. The intestinal microbiome and its relevance for functionality in older persons. Current opinion in clinical nutrition and metabolic care. 2019;22(1):4–12. [DOI] [PubMed] [Google Scholar]
- 9.Deutz NEP, Thaden JJ, Ten Have GAM, Walker DK, Engelen M. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metabolism. 2018;78:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meij BS, Deutz NEP, Rodriguez RE, Engelen M. Early Signs of Impaired Gut Function Affect Daily Functioning in Patients With Advanced Cancer Undergoing Chemotherapy. JPEN Journal of parenteral and enteral nutrition. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Engelen MP, Com G, Anderson PJ, Deutz NE. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clinical nutrition (Edinburgh, Scotland). 2014;33(6):1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur A, ten Have GA, Hritzo B, Deutz NE, Olsen C, Moroni M. Morphological and functional impairment in the gut in a partial body irradiation minipig model of GI-ARS. International Journal of Radiation Biology. 2020;96(1):112–28. [DOI] [PubMed] [Google Scholar]
- 13.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. American journal of respiratory and critical care medicine. 2017;195(5):557–82. [DOI] [PubMed] [Google Scholar]
- 14.Jonker R, Deutz NEP, Schols A, Veley EA, Harrykissoon R, Zachria AJ, et al. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clinical nutrition (Edinburgh, Scotland). 2019;38(4):1684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 16.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–6. [DOI] [PubMed] [Google Scholar]
- 17.Meguro M, Barley EA, Spencer S, Jones PW. Development and Validation of an Improved, COPD-Specific Version of the St. George Respiratory Questionnaire. Chest. 2007;132(2):456–63. [DOI] [PubMed] [Google Scholar]
- 18.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. [DOI] [PubMed] [Google Scholar]
- 19.Jonker R, Deutz NEP, Ligthart-Melis GC, Zachria AJ, Veley EA, Harrykissoon R, et al. Preserved anabolic threshold and capacity as estimated by a novel stable tracer approach suggests no anabolic resistance or increased requirements in weight stable COPD patients. Clinical nutrition (Edinburgh, Scotland). 2019;38(4):1833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Wijck K, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Novel analytical approach to a multi-sugar whole gut permeability assay. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2011;879(26):2794–801. [DOI] [PubMed] [Google Scholar]
- 21.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–7. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. [Google Scholar]
- 23.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157(1):80–5. [DOI] [PubMed] [Google Scholar]
- 24.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. Journal of the American College of Cardiology. 2014;64(11):1092–102. [DOI] [PubMed] [Google Scholar]
- 25.Andersen SK, Hardis ALS, Tupper OD, Soja AMB, Nilsson B, Ulrik CS, et al. Small intestinal absorption in patients with chronic obstructive pulmonary disease complicated by cor pulmonale - A pilot study. Clinical nutrition ESPEN. 2018;24:90–4. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd AP, Kiel JW. A model of countercurrent shunting of oxygen in the intestinal villus. Am J Physiol. 1992;262(4 Pt 2):H1136–42. [DOI] [PubMed] [Google Scholar]
- 27.Goggins BJ, Chaney C, Radford-Smith GL, Horvat JC, Keely S. Hypoxia and Integrin-Mediated Epithelial Restitution during Mucosal Inflammation. Frontiers in immunology. 2013;4:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fricker M, Goggins BJ, Mateer S, Jones B, Kim RY, Gellatly SL, et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI insight. 2018;3(3):e94040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu WK, et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review). Int J Mol Med. 2014;34(2):372–80. [DOI] [PubMed] [Google Scholar]
- 30.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Critical care medicine. 2003;31(6):1737–41. [DOI] [PubMed] [Google Scholar]
- 31.Devi S, Varkey A, Sheshshayee MS, Preston T, Kurpad AV. Measurement of protein digestibility in humans by a dual-tracer method. The American journal of clinical nutrition. 2018;107(6):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaumont M, Portune KJ, Steuer N, Lan A, Cerrudo V, Audebert M, et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. The American journal of clinical nutrition. 2017;106(4):1005–19. [DOI] [PubMed] [Google Scholar]
- 33.Blachier F, Beaumont M, Portune KJ, Steuer N, Lan A, Audebert M, et al. High-protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clinical Nutrition. 2019;38(3):1012–22. [DOI] [PubMed] [Google Scholar]
- 34.Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, et al. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients. 2015;7(11):8916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakhdar R, Rabinovich RA. Can muscle protein metabolism be specifically targeted by nutritional support and exercise training in chronic obstructive pulmonary disease? Journal of thoracic disease. 2018;10(Suppl 12):S1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]