Abstract
Harmful algal blooms (HABs) are diverse phenomena involving multiple species and classes of algae that occupy a broad range of habitats from lakes to oceans and produce a multiplicity of toxins or bioactive compounds that impact many different resources. Here, a review of the status of this complex array of marine HAB problems in the U.S. is presented, providing historical information and trends as well as future perspectives. The study relies on thirty years (1990–2019) of data in HAEDAT - the IOC-ICES-PICES Harmful Algal Event database, but also includes many other reports. At a qualitative level, the U.S. national HAB problem is far more extensive than was the case decades ago, with more toxic species and toxins to monitor, as well as a larger range of impacted resources and areas affected. Quantitatively, no significant trend is seen for paralytic shellfish toxin (PST) events over the study interval, though there is clear evidence of the expansion of the problem into new regions and the emergence of a species that produces PSTs in Florida – Pyrodinium bahamense. Amnesic shellfish toxin (AST) events have significantly increased in the U.S., with an overall pattern of frequent outbreaks on the West Coast, emerging, recurring outbreaks on the East Coast, and sporadic incidents in the Gulf of Mexico. Despite the long historical record of neurotoxic shellfish toxin (NST) events, no significant trend is observed over the past 30 years. The recent emergence of diarrhetic shellfish toxins (DSTs) in the U.S. began along the Gulf Coast in 2008 and expanded to the West and East Coasts, though no significant trend through time is seen since then. Ciguatoxin (CTX) events caused by Gambierdiscus dinoflagellates have long impacted tropical and subtropical locations the U.S., but due to a lack of monitoring programs as well as under-reporting of illnesses, data on these events are not available for time series analysis. Geographic expansion of Gambierdiscus into temperate and non-endemic areas (e.g., northern Gulf of Mexico) is apparent, and, fostered by ocean warming. HAB-related marine wildlife morbidity and mortality events appear to be increasing, with statistically significant increasing trends observed in marine mammal poisonings caused by ASTs along the coast of California and NSTs in Florida. Since their first occurrence in 1985 in New York, brown tides resulting from high-density blooms of Aureococcus have spread south to Delaware, Maryland, and Virginia, while those caused by Aureoumbra have spread from the Gulf Coast to the east coast of Florida. Blooms of Margalefidinium polykrikoides occurred in four locations in the U.S. from 1921–2001 but have appeared in more than 15 U.S. estuaries since then, with ocean warming implicated as a causative factor. Numerous blooms of toxic cyanobacteria have been documented in all 50 U.S. states and the transport of cyanotoxins from freshwater systems into marine coastal waters is a recently identified and potentially significant threat to public and ecosystem health.
Taken together, there is a significant increasing trend in all HAB events in HAEDAT over the 30-year study interval. Part of this observed HAB expansion simply reflects a better realization of the true or historic scale of the problem, long obscured by inadequate monitoring. Other contributing factors include the dispersion of species to new areas, the discovery of new HAB poisoning syndromes or impacts, and the stimulatory effects of human activities like nutrient pollution, aquaculture expansion, and ocean warming, among others. One result of this multifaceted expansion is that many regions of the U.S. now face a daunting diversity of species and toxins, representing a significant and growing challenge to resource managers and public health officials in terms of toxins, regions, and time intervals to monitor, and necessitating new approaches to monitoring and management. Mobilization of funding and resources for research, monitoring and management of HABs requires accurate information on the scale and nature of the national problem. HAEDAT and other databases can be of great value in this regard but efforts are needed to expand and sustain the collection of data regionally and nationally.
Keywords: HAB, harmful algal bloom, red tide, eutrophication, time series, HAEDAT
1. Introduction
Harmful algal blooms (HABs) are a significant problem in U.S. coastal waters, dating back hundreds of years when explorers and early west-coast settlers were poisoned from what we now know were toxins in the seafood they consumed (e.g., Fortuine 1975). The species that cause HABs in the U.S. are diverse, as are the habitats in which they occur. Impacts are equally diverse and include multiple human poisoning syndromes associated with the consumption of molluscan and crustacean shellfish, fish, and other marine animals that have accumulated algal toxins. Human exposures to aerosolized or water-borne toxins, and dermatological contact with HABs can also have negative impacts. Other effects include mortalities of fish and wildlife, ecosystem disruption, hypoxia and anoxia from high biomass blooms, and noxious impacts associated with the accumulation and decay of massive micro- and macroalgal blooms.
HABs affect economic and health sectors that include commercial and recreational fisheries, aquaculture, coastal tourism, and wildlife and human health. Potentially impacted resources include commercially harvested fish and shellfish with a value of $5.6 billion in 2018, and aquaculture production valued at $1.5 billion in 2017 (NMFS 2018). Of all species of fish and shellfish harvested, shellfish are most severely impacted by HABs due to the retention of toxins in their tissues. The delay of the Dungeness crab season due to a massive domoic acid event on the U.S. West Coast in 2015 caused an estimated $48.3 million in direct economic impacts and resulted in an appropriation by Congress of $25 million in disaster aid. The direct economic impacts of a 2005 A. catenella bloom on commercial fisheries, in terms of lost gross revenues, were $2.4 million in Maine and $16–18 million in Massachusetts (Jin et al., 2008). Economic impacts on the recreational razor clam fishery that brings tourists to remote regions of the Washington State coast range from a loss of 3 to 339 full-time job equivalents and from $110,000 to $10.6 million in coastal income (Huppert and Trainer, 2014). HABs can also contribute to the stranding of numerous marine mammals, impacting ecosystem health (e.g., McCabe et al., 2019; Flewelling et al., 2005).
HAB phenomena in the U.S. are one component of the global problem currently being assessed through the GLOBALHAB Status Report (Hallegraeff et al. this volume). As countries and regions throughout the world assess the current status and future trends of HABs using common datasets and approaches, a comprehensive view of the nature and global impact of these phenomena will emerge. Here, a review of the status of marine HAB problems in the U.S. is provided using historical information and trend analysis. Brief summaries of the key drivers for bloom dynamics are presented as well. This compilation of information is of value not only to assist in current management strategies for fisheries, tourism, human health and other impacted sectors, but it will also provide the baseline against which future changes can be assessed, particularly those associated with climate change, nutrient pollution, and other global trends.
2. Methods
One challenge with a study of the trends of HAB events through time is that the datasets available at the local, state, and national levels are highly variable in terms of years of coverage, parameters measured, and the nature of bloom events that are recorded. Although these records are valuable and are used to some extent herein, this study relies predominantly on HAEDAT - the IOC-ICES-PICES1Harmful Algal Event database (http://haedat.iode.org/). HAEDAT was originally established by the ICES-IOC Working Group on Harmful Algal Bloom Dynamics in the 1990s to compile bloom data from ICES countries bordering the North Atlantic. In 2000, PICES began adding bloom records from the Pacific region (though U.S. West Coast HAB events have been recorded in HAEDAT since 1989). The database now contains 880 bloom events from the U.S.
All reports entered into HAEDAT must meet a strict definition of a ‗harmful algal event’. Specifically, the bloom must be associated with a negative impact or management action. These could include: 1) toxin accumulation in seafood above levels considered safe for human consumption; 2) water discoloration, scum or foam sufficient to cause ecosystem damage or a socio-economic impact; 3) any event where humans, animals or other organisms are negatively affected by the bloom; or 4) precautionary closures of harvesting areas based on predefined thresholds of toxic phytoplankton cells in the water. Note that a single HAEDAT event can represent dozens of individual toxin measurements that exceed regulatory levels when a bloom extends along a coast and lasts weeks or months.
A small number of freshwater cyanobacterial events have been entered in the HAEDAT dataset for the U.S., but the vast majority are for events that affect estuarine or coastal marine waters. Even those records are limited in coverage, so this review only uses peer-reviewed literature to discuss cyanoHAB problems in coastal waters. It does not directly address cyanoHABs and other blooms in freshwater systems. A similar situation pertains to records of CTXs and macroalgal blooms, both of which are currently not included in the U.S. HAEDAT records.
The U.S. National Office for Harmful Algal Blooms coordinates the collection of annual bloom data through a survey form submitted by multiple scientists and managers spanning 29 coastal regions defined for the U.S. and its territories (Fig. 1). Event information includes: the nature of the event, what was affected, the associated syndrome, species involved in the transmission, whether the report is the outcome of a monitoring program, date, location and zone, contact information, environmental conditions, and toxin assay information. HAEDAT records have evolved through time and in some instances, particularly in older records, not all information is available. All data are evaluated for accuracy and clarity prior to being entered into the database. Recently, a major effort was made to review all of the U.S. HAEDAT records to identify and remove those that did not meet the above criteria or were redundant.
Fig. 1.
U.S. map showing HAEDAT zones. Some locations discussed in the text are also indicated.
To assess changes over time in the geographical extent of bloom events, for each of the toxin families associated with a human poisoning syndrome (PSTs, ASTs, DSTs, and NSTs), a linear logistic regression model of the proportion of monitoring zones in each year that experienced at least one event was fit, with time as the regressor. All analyses were performed using the glm function from the R statistics package (version 4.0.2). The function also provides an observed significance level (or p value) for the fitted model. This approach was also used for the combination of all HAB toxins and impacts described herein to provide an overall national HAB trend.
This analysis was run for 30 years for PSTs and NSTs, and for shorter intervals for ASTs and DSTs, starting with the first year of a documented event for that toxin (1991 and 2008, respectively). It was assumed that in all years during these periods, all HAEDAT zones were monitored for the effects of these toxins. This means either phytoplankton observations or shellfish flesh testing, or management oversight were used to decide whether a specific toxin was or was not a risk within that zone. States do not monitor for all HAB toxins – each must conduct a risk assessment and then monitor for the HAB species and toxins that might occur at dangerous levels with a given zone. With the statistical approach taken here, lack of monitoring for a given toxin is not a problem if management oversight for that zone generally ensures that there were unlikely to be significant events from that toxin.
3. U.S. HABs
3.1. Poisoning syndromes
The impacts from HABs in the U.S. are diverse, with the most significant associated with the accumulation of algal toxins in shellfish, leading to poisoning of humans and animals that consume or ingest contaminated seafood. The resulting human poisoning syndromes linked to consumption of shellfish have been given the names paralytic, diarrhetic, neurotoxic, amnesic, and azaspiracid shellfish poisoning (PSP, DSP, NSP, ASP, AZP) to describe primary symptoms or the toxins involved. Except for ASP, all are caused by biotoxins synthesized by dinoflagellates; the ASP toxin, domoic acid, is produced predominantly by diatoms within the genus Pseudo-nitzschia. This review will refer to the toxins (e.g., PSTs, DSTs, NSTs, ASTs and AZTs) rather than the syndromes because the HAEDAT records are of toxin concentrations above regulatory concentrations3 and not human poisonings. A sixth human illness, ciguatera poisoning (CP) is caused by toxins produced by benthic dinoflagellates (Gambierdiscus and Fukuyoa spp.) that live on substrates or surfaces in many coral reef communities. Ciguatoxins (CTX) are transferred through the food web from herbivorous reef fishes to larger, carnivorous finfish. Additional CP vectors include giant clams and other invertebrates. Unfortunately, HAEDAT records for CTXs do not exist for the U.S. and its territories. This reflects a lack of routine monitoring of ciguatera toxins in fish, which is in turn attributed to the lack of commercially available toxin standards and analytical approaches for toxin detection that are feasible and affordable. Human poisonings do occur in the U.S. and its territories from CTXs (see e.g., Friedman et al., 2017; Chinain et al., 2020), but the true nature of the problem is hindered by misdiagnosis and under-reporting of CP cases to health authorities. Collection of data from hospitals, clinics, and medical professionals would be a significant undertaking far beyond the current effort to compile U.S. HAB event data into HAEDAT. These issues are reviewed in greater detail by Chinain et al. (2020).
AZTs, though a problem in other parts of the world (e.g., EC, 2002), are not yet a serious concern in the U.S. and thus are not addressed here in any detail. In 2002, the European Commission established a regulation for the maximum levels of AZTs, defined as the combination of AZA-1, 2, 3 in shellfish, at 160 μg kg−1 (EC, 2002). Although Azadinium poporum has been identified in the northern Gulf of Mexico (Luo et al., 2016), no AZT HAEDAT events have been reported in the U.S., except on the East Coast in Washington State where a new toxin, named AZA-59, was identified and characterized in A. poporum from Puget Sound in 2016, with a potency approximately 3-fold lower than AZA-1 (Kim et al., 2017). Based on deployment of passive solid-phase resin samplers throughout Puget Sound during 2014–2015 and detection of measurable but low amounts of AZA-59 in shellfish in 2016 and 2017, the current risk of human exposure to AZA in Washington State is low (Kim et al., 2017). Two azaspiracids, AZA-1 and AZA-2, were more recently detected for the first time on the East Coast, in Chesapeake Bay and in Virginia coastal bays (Onofrio et al., in press). While AZTs showed broad spatiotemporal distribution in these estuarine and coastal waters during a study in 2017–2018, toxin amounts detected on solid-phase resin samplers were uniformly low, suggesting a minimal threat to seafood safety.
3.2. Other impacts
Another type of HAB impact occurs when marine plants and animals are killed by algal species that release toxins and other bioactive compounds into the water. HABs also cause mortalities of fish and wildlife (e.g., seabirds, whales, dolphins, and other marine animals), typically as a result of the transfer of toxins through the food web or when aquatic toxins are ingested or transferred across gills (see e.g., Shumway, 1990; Landsberg, 2002). Physical damage of fish gills has been caused by phytoplankton with spiny processes, e.g. Chaetoceros spp. (Horner et al., 1997).
Several HAB species are highlighted herein even though they do not fall under the poisoning syndromes; they are significant in terms of areas affected and nature of impacts. Some of these cause plant and animal mortalities and are sufficiently important nationally to be described separately. They include the brown tide species Aureococcus anophagefferens and Aureoumbra lagunensis, as well as the dinoflagellates Margalefidinium polykrikoides and Karenia mikimotoi and the raphidophyte Heterosigma akashiwo. Some of these species produce poorly defined bioactive compounds, but for most, impacts derive from the high biomass that some blooms achieve. These may cause reduced light penetration in the water column and subsequent decreased densities of seagrass beds that can have dramatic impacts on coastal ecosystems, as these serve as nurseries for the young of commercially important fish and shellfish. Additionally, when large blooms of non-toxic species die, bacterial breakdown of the biomass can lead to reduced oxygen levels and subsequent mortalities of plants and animals in the affected area. High biomass blooms are sometimes linked to excessive nutrient inputs but can also occur in pristine waters.
Macroalgae (seaweeds) also cause significant problems. Over the past several decades, blooms of macroalgae have been increasing along many of the world’s coastlines (e.g., Lapointe and Bedford, 2007; Keesing et al., 2011; Wang et al., 2019). These have a broad range of ecological effects and can affect human health as well through the production of H2S as the biomass washed onto shorelines decays. Macroalgal blooms can be particularly harmful to coral reefs where under high nutrient conditions, opportunistic macroalgal species out-compete, overgrow, and replace the coral. A significant and developing problem with macroalgae involves the transport of huge floating masses of seaweeds and deposition of that material on beaches. In the U.S., problems with Sargassum are becoming serious in the Caribbean territories as well as along the coasts of Florida (Wang et al., 2019). These events are not covered here since they have not been recorded in HAEDAT. Plans are underway, however, to include these phenomena in the database going forward.
A discussion is also included on toxic cyanobacterial blooms that can affect estuarine and nearshore coastal waters.
3.3. U.S. HAB species
All potentially toxic or harmful marine microalgal HAB taxa observed in the U.S. are listed in Table 1, along with an indication of where in the country they occur. Many salinity-tolerant freshwater phytoplankton species are impacting marine coastal waters (see 4.6.6), but Table 1 is restricted to marine HAB species. Among the major genera, the diatom Pseudo-nitzschia, dinoflagellates Alexandrium, Dinophysis, Gonyaulax, and Karenia, and the raphidophyte Heterosigma are present along the East, Gulf, and West Coasts. The dinoflagellates Amphidinium, Karlodinium, Pfiesteria, Phalachroma, Pheopolykrikos, Prorocentrum, Pseudopfiesteria, and Pyrodinium, the pelagophyte Aureoumbra, and raphidophytes Chattonella and Fibrocapsa occur along the East and Gulf Coasts. Toxic dinoflagellates in the genera Gambierdiscus and Fukuyoa occur in Florida, the U.S. Virgin Islands, Puerto Rico, Hawai’i, Guam, and other Pacific Island territories. More details on the species responsible for the U.S. HAB poisoning syndromes and impacts are given in the sections below.
Table 1.
Harmful and potentially toxic marine HAB species observed along the East, Gulf, and West Coasts of the United States. E=East, G=Gulf, W=West
| Diatoms | Dinophysis acuminata | E,G,W | Ostreopsis siamensis | G | |
| Chaetoceros concavicornis | E,G,W | Dinophysis acuta | E,G,W | Pfiesteria piscicida | E,G |
| Chaetoceros convolutus | E,G,W | Dinophysis caudata | E,G | Phalacroma mitra | G |
| Halamphora coffeaeformis | E,G,W | Dinophysis fortii | E,G,W | Phalacroma rotundatum | E,G,W |
| Pseudo-nitzschia australis | G,W | Dinophysis mitra | G | Pheopolykrikos hartmannii | E,G |
| Pseudo-nitzschia calliantha | E,G,W | Dinophysis norvegica | E,G,W | Prorocentrum belizeanum | G |
| Pseudo-nitzschia cuspidata | E,G,W | Dinophysis ovum | E,G | Prorocentrum borbonicum | G |
| Pseudo-nitzschia delicatissima | E,G,W | Dinophysis sacculus | E,G | Prorocentrum cordatum | G |
| Pseudo-nitzschia fraudulenta | G,W | Dinophysis tripos | E,G,W | Prorocentrum emarginatum | G |
| Pseudo-nitzschia galaxiae | G | Fukuyoa ruetzleri | G,E | Prorocentrum faustiae | G |
| Pseudo-nitzschia multiseries | E,G,W | Fukuyoa yasumotoi | G | Prorocentrum hoffmannianum | G |
| Pseudo-nitzschia multistriata | G | Gambierdiscus belizeanus | G | Prorocentrum lima | E,G,W |
| Pseudo-nitzschia pseudodelicatissima | E,G,W | Gambierdiscus caribaeus | G | Prorocentrum maculosum | G |
| Pseudo-nitzschia pungens | E,G,W | Gambierdiscus carolinianus | E | Prorocentrum rhathymum | G |
| Pseudo-nitzschia seriata | E,G,W | Gambierdiscus carpenteri | E | Protoceratium reticulatum | G,W |
| Pseudo-nitzschia subpacifica | E,W | Gambierdiscus excentricus | G | Pseudopfiesteria shumwayae | E,G |
| Pseudo-nitzschia turgidula | E,G | Gambierdiscus silvae | G,E | Pyrodinium bahamense | G,W,E |
| Thalassiosira spp. | E,G,W | Gonyaulax spinifera | E,G,W | Takayama cladochroma | G |
| Dinoflagellates | Gymnodinium catenatum | G | Takayama pulchella | E | |
| Akashiwo sanguinea | G,W | Heterocapsa circularisquama | G | Takayama tasmanica | E |
| Alexandrium andersonii | G | Karenia bicuneiformis | G | Pelagophytes | |
| Alexandrium balechii | G | Karenia brevis | E,G | Aureococcus anophagefferens | E |
| Alexandrium catenella | E,G,W | Karenia concordia | G | Aureoumbra lagunensis | E,G |
| Alexandrium minutum | G | Karenia cristata | G | Prymnesiophytes | |
| Alexandrium monilatum | E,G | Karenia digitata | G | Phaeocystis pouchetii | E,W |
| Alexandrium ostenfeldii | E,G,W | Karenia mikimotoi | G,W | Prymnesium parvum | E,W |
| Alexandrium peruvianum | E | Karenia papilionacea | E,G | Raphidophytes | |
| Alexandrium pseudogonyaulax | G | Karenia selliformis | G | Chattonella antiqua | G |
| Alexandrium tamiyavanichii | G | Karenia umbella | G | Chattonella marina | G |
| Amphidinium carterae | E,G,W | Karlodinium armiger | G | Chattonella subsalsa | E,G |
| Amphidinium gibbosum | G | Karlodinium corsicum | G | Chrysochromulina spp. | E,W |
| Amphidinium klebsii | E | Karlodinium veneficum | E,G | Fibrocapsa japonica | E,G |
| Amphidinium operculatum | E | Lingulodinium polyedrum | G,W | Heterosigma akashiwo | E,G,W |
| Azadinium poporum | G,W | Luciella masanensis | E | Olisthodiscus | E |
| Blixaea quinquecorne | G | Margalefidinium polykrikoides | E,W | Pseudochattonella verruculosa | E,W |
| Cochlodinium fulvescens | W | Ostreopsis lenticularis | G | Vicicitus globosus | G |
| Coolia santacroce | G | Ostreopsis mascarenensis | G | ||
| Coolia tropicalis | G | Ostreopsis ovata | G |
4. U.S. HAB history and trends
4.1. PSTs
4.1.1. Background.
Paralytic shellfish poisoning (PSP) is caused by the saxitoxin family of biotoxins, hereafter termed paralytic shellfish toxins (PSTs). Taxa known to produce PSTs in the U.S. are dinoflagellate species in the genera Alexandrium and Pyrodinium. The species Alexandrium catenella (historically called Gonyaulax excavata, G. tamarensis, A. fundyense, A. tamarense Group I, and A. tamarense North American Strain) is the most widespread, responsible for outbreaks along the U.S. East and West Coasts, including Alaskan coastal waters. PSTs have also been reported in A. peruvianum in Rhode Island (Borkman et al., 2012) though outbreaks are limited in scale and toxin levels are low. Isolates of Alexandrium ostenfeldii from the Gulf of Maine produce spirolide toxins, but not PSTs (Gribble et al., 2005), while Pyrodinium bahamense causes PST incidents on both coasts of Florida (Landsberg et al., 2006; Lopez et al., 2019). Several other Alexandrium species that can produce saxitoxins are also present in U.S. waters and are included in Table 1, but they have not been linked to PST events.
4.1.2. History of PSTs from Alexandrium catenella.
On the East Coast of North America, PSP (which we can now reliably attribute to A. catenella) was reported in northeastern Canada over 100 years ago (Ganong, 1889), but in the New England region of the U.S., PSTs were first documented in 1958 in far-eastern sections of Maine near the Canadian border (Hurst, 1975; Shumway et al., 1988). In 1972, a massive, visible red tide of A. catenella stretched from southern Maine through New Hampshire and into Massachusetts, causing PST-related shellfish toxicity in southern areas for the first time. Virtually every year since the 1972 outbreak, Maine, New Hampshire, and Massachusetts have experienced PST outbreaks, a direct result of Alexandrium cysts being retained in bottom sediments of southern New England following their introduction by the massive bloom (Anderson and Wall, 1978; Anderson et al., 2014). Low levels of toxicity had occasionally been reported in Maine west of Penobscot Bay prior to 1972 (Shumway et al., 1988), but present-day outbreaks in that area are far more numerous and involve much higher levels of toxicity, consistent with a dispersal event.
Further to the south, PSTs and A. catenella cells and cysts were first documented in small embayments in Connecticut and Long Island in the early 1980s (Anderson et al., 1982; Schrey et al., 1984). Despite the presence of cysts at some sites in Connecticut, there have been very few closures due to PSTs in that state, the most recent being in 2003. On Long Island, New York, the first PST outbreak and shellfish bed closure occurred in 2006 when blooms first became significant issues in Northport Harbor (Hattenrath et al., 2010). Since then, PST-induced shellfish bed closures have occurred at more than six different locations across Long Island, with closures becoming annual events. Cysts and motile cells of A. catenella have been reported as far south as New Jersey (Cohn et al., 1988), but no PSTs have been reported in that region.
Recent history is thus suggestive of a gradual expansion of the PST problem from north to south in the northeastern U.S., in part due to major species dispersal events like the 1972 bloom, but also small-scale dispersal events, as may have occurred on Long Island. Some of this apparent expansion can also be attributed to the detection of indigenous, low-toxicity populations that had long existed in southern waters (Anderson, 1997).
On the U.S. West Coast, the oldest putative PST incident associated with A. catenella occurred in Alaska in 1799 when approximately 100 crew members of a fur-trading ship died after eating blue mussels in an area near Sitka, now appropriately called Peril Strait (Khlebnikov, 1837, Fortuine, 1975). In fact, saxitoxins were originally extracted from their namesake butter clams (Saxidomus giganteus) harvested from this Peril Strait region (Lewitus et al., 2012). Elsewhere on the West Coast, human poisonings from PSTs were apparently common among European settlers in California (Meyer et al., 1928), but the first recorded incident occurred in 1903 when twelve people became ill and five died after eating mussels (Sommer and Meyer, 1937). PSTs were finally recognized as a serious health risk in that state in 1927 when a major outbreak near San Francisco led to more than 100 illnesses and multiple deaths (Price et al., 1991). This led to the establishment of a PST shellfish monitoring program, the first in the U.S.
Three fatalities due to PSP were recorded in Washington State in 1942 (Quayle, 1969) resulting in seasonal closures for all shellfish harvest, except razor clams, on the outer coast. That state has also instituted a routine shellfish monitoring program for HAB toxins, as have Oregon and Alaska, though the latter focuses on commercial harvesting sites while keeping the remainder of the massive coastline permanently closed. Tribal entities now have their own monitoring and testing programs, run through SEATOR – Southeastern Alaskan Tribal Ocean Research.
The PST problem along much of the U.S. West Coast thus has a long history. Monitoring has been somewhat limited, however, due to seasonal, coastwide closures of shellfish harvest areas in several states. There is evidence for regional expansions in severity and geographic extent in Oregon and Washington, as indicated by the increase in harvesting closures along the Oregon coast from 1990 to the 2000s (Lewitus et al., 2012), and the increase in shellfish closures in Puget Sound WA that paralleled the gradual incursion of A. catenella into the Sound since monitoring first began in the 1940s and 1950s (Trainer et al., 2003).
PSTs have also long been a problem in Alaska, particularly along much of the Gulf of Alaska coast from the British Columbia border in the southeast to the Aleutian Islands (RaLonde et al., 2001; Vandersea et al., 2018). Between 1973 and 2012, over 200 cases of PSP and several deaths from consuming PST-contaminated shellfish were reported in Alaska (Gessner, 1996, with updates from AK Epidemiology bulletins). Because of the huge coastline, lack of comprehensive toxicity testing and underreporting of illnesses, the historical and geographical extent of PST and the number of people affected by PSP in Alaska have been grossly underestimated (Gessner and Middaugh, 1995; Gessner and McLaughlin, 2008; Trainer et al., 2014). The historical record of PSTs in Alaska from 1991–2012 shows several measurements of PSTs in shellfish in southeast Alaska at >5000 μg 100g−1, more than 50x the regulatory level. A record of >30,000 μg 100g−1 was measured in mussels from Ketchikan in May 2011, when several people became ill with PSP (Trainer et al, 2014). The PST problem stretches north into the Bering Sea and beyond. Recent research studies have documented huge blooms of A. catenella just north of the Bering Strait and in the Chukchi Sea areas of the Alaskan Arctic (Natsuike et al., 2017; D. M. Anderson, unpub. data.) There are records of A catenella (as Goniaulax tamarensis) in far northern Alaska near Pt. Barrow more than 60 years ago (e.g., Bursa, 1963), but it is not known if the distribution, frequency, and magnitude of blooms of this species have expanded due to the rapid warming of waters in that region, though this seems likely given the recent changes in bottom and surface water temperatures that are now supportive of germination and growth (Danielson et al., 2020; D. M. Anderson, unpub. data).
4.1.3. History of PSTs from Pyrodinium bahamense
Pyrodinium bahamense is a well-known PST producer in the tropical Indo-Pacific region (e.g., MacLean, 1989; Azanza and Taylor, 2001), but reports of toxic strains of this species in the Atlantic Ocean are relatively new (Landsberg et al., 2006; Philips et al., 2006). Until the early 2000s, it had been assumed that the Atlantic strain of P. bahamense (distinguished as P. bahamense bahamense from P. bahamense compressum) did not produce PSTs (Steidinger et al., 1980; Azanza and Taylor, 2001) until an extensive bloom in the Indian River Lagoon on the east coast of Florida coincided with pufferfish toxicity events in 2002 (Philips et al., 2004). Landsberg et al. (2006) later reported saxitoxin bioaccumulation in pufferfish from the same area, concurrent with P. bahamense blooms. Toxic P. bahamense blooms also occur regularly in Tampa Bay and Charlotte Harbor on Florida’s west coast (FWC HAB Monitoring Database, Lopez et al., 2019), with cells also present more sporadically in estuaries in the northern panhandle of Florida, as well as in Florida Bay and the Florida Keys (FWC HAB Monitoring Database; Accoroni et al., 2020). Puffer fish have tested positive for PTXs in all of these areas (Abbott et al., 2009).
To distinguish PST (saxitoxin) exposures in humans from consumption of toxic puffer fish, any public health incidents involving puffer fish rather than shellfish, are referred to as saxitoxin puffer fish poisonings (SPFPs) (Landsberg et al., 2006). Due to extensive monitoring of P. bahamense and the management of PSTs in shellfish in Florida, to date there have been no documented human cases of PSP since this link was discovered. Prior to this, 28 cases of SPFP in humans occurred from pufferfish harvested from January 2002 to May 2004, with further potential incidents managed by an indefinite ban on pufferfish harvesting implemented in June 2004 (Landsberg et al., 2006). If toxicity above the regulatory limit is detected, shellfish beds in areas managed by the state are closed (e.g., in the Indian River Lagoon in 2009). Note that cases of SPFP are not distinguished from shellfish-related PST events in HAEDAT.
The documented range of P. bahamense in the U.S. is currently restricted to the Florida coasts and Puerto Rico (Philips et al., 2004). Although known to be present in the Indian River Lagoon in the 1990s, Pyrodinium bahamense was not associated with toxicity as noted above. It is possible, but unverified, that PSTs were present in the Indian River Lagoon in the 1960s but were not linked to any poisoning events. As part of a study into the closely-related PST sodium-channel blocker, tetrodotoxin (TTX), puffer fish research conducted in the early 1960s by Lalone et al. (1963) demonstrated by mouse bioassay that muscle from the southern puffer fish, Sphoeroides nephelus, was the most lethal to mice compared to other tissues (skin, gonad, liver). Researchers assumed (without other methods of analytical verification) that the toxicity was from TTX even though TTX is not typically accumulated in the muscle of puffer fish in the Indian River Lagoon. Recent work by Deeds et al. (2008) and Abbott et al. (2009) determined that high concentrations of PSTs are found there (up to 20,106 μg STX eq. 100g−1 tissue).
The anecdotal data from the 1960s might hint of earlier PST presence in the Indian River Lagoon potentially originating from P. bahamense, but there was no indication of toxin levels reaching the high levels observed in puffer fish after the early 2000s. Theories for the apparent appearance of P. bahamense toxicity relate to an increase of bloom biomass from environmental triggers, high nutrient loadings, and increased water residency time (see below) rather than from the introduction or dispersal of a toxic strain, improved public health surveillance, or improved analytical methods. While there has been interest in conducting P. bahamense cyst analyses for retrospective toxin testing in sediment profiles, this has not yet been possible.
4.1.4. PST trends
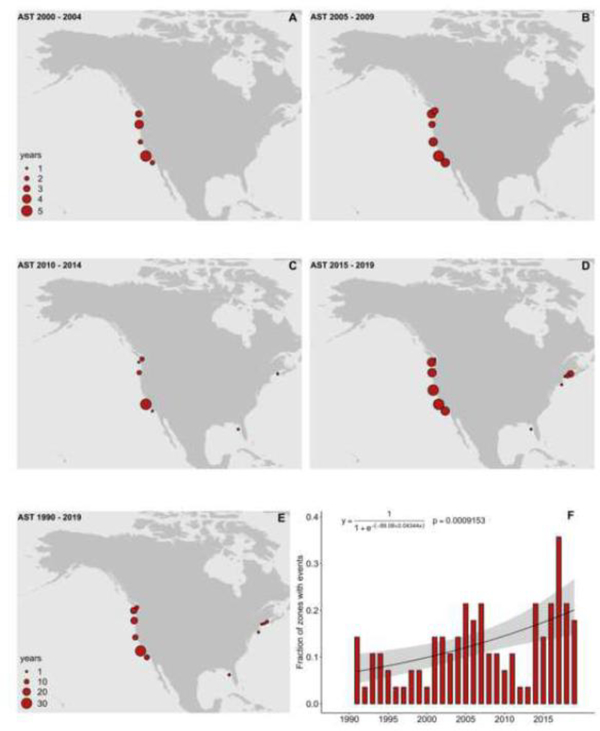
PST event data for the U.S. in HAEDAT covering the last thirty years (1990–2019) are presented in Fig. 2. Panels A-D show the frequency of documented PST events in five-year increments beginning in 2000, panel E shows the pattern of events over a 30-year interval, and panel F shows a 30-year time series of the fraction of the 28 U.S. HAEDAT zones that had at least one PST event each year. The five-year maps are provided for only the last 20 years because of space and clarity considerations.
Fig. 2.
The frequency of PST events (defined as at least one closure in a defined region or HAEDAT zone in a given year) in the U.S. derived from HAEDAT. Data include events linked to either A. catenella or P. bahamense. A-D, five-year frequencies, with the size of the circle denoting the number of events during that interval; E, PST frequencies (events per year) for the entire U.S. over the 30-year study interval (1990 – 2019); F, time series of observed (bars) and modeled (line) proportions of monitoring zones with at least one event. Also reported is the fitted linear logistic model and its non-significant p value (p>.05).
It is evident from Fig. 2 that PST outbreaks over the last 20 years are annually recurrent on both coasts of the U.S. In the east, PSTs are a problem from Long Island north to the U.S. border with Canada. A clear biogeographic boundary of A. catenella exists near New York and New Jersey, as PST events have not been documented further to the south except for Florida where the causative species is Pyrodinium bahamense. On the West Coast, PSTs are recorded from Alaska to California, with the number of events each year decreasing from north to south.
One conclusion from the HAEDAT PST time series maps is the distribution of Alexandrium-related PST events in New England and along the coasts of California, Oregon, and Washington has not changed significantly over the last 20 years. Some of the expansions noted above in the Gulf of Maine, Long Island, and Puget Sound WA are not evident in this figure, either because the events occurred before the study interval or nearby PST events obscure the newer ones at the scale of these maps. In other parts of the country, expansions are evident. One is in Florida, where toxic P. bahamense blooms first appeared on the East Coast in the 2002 – 2007 interval (Fig. 2A and B), and then on the West Coast between 2008 and 2012. The lack of a HAEDAT record for the Florida west coast in the most recent interval (Fig. 2D) is because the P. bahamense blooms that occur are in an area where shellfish beds are closed for other reasons, or shellfish beds are outside areas with known blooms (Lopez et al., 2019) and thus no PST testing is occurring.
There is also a suggestion of an expansion in Alaska, toward the Bering Strait and northern waters, but the data are too sparse to say anything conclusive at this stage. Alaska is one of several states where commercially harvested and farmed products are routinely tested, but most recreationally harvested shellfish are not. Many PST events undoubtedly go unnoticed under this type of management program. Section 4.1.6 discusses PST and animal mortalities, with many new reports of PSTs in wildlife, but again, no time series data are available to reveal trends.
Between 2 and 14 PST events have been observed nationwide each year over the 30-year study interval, with 334 total events and a mean of 11.1 per year. No significant trend in the fraction of U.S. HAEDAT zones is evident for PST events over the last 30 years (p> .05), though there is a hint of multi-year cycling, with the lowest number of events around the years 1999, 2006, and 2010 (Fig. 2F). Note that there is also no significant trend in PST events if all those caused by P. bahamense are removed from the analysis. On the other hand, if only PST events caused by P. bahamense are examined, a significant increasing trend would be evident given the recent emergence of toxicity from this organism over the past 20 years and the increasing regulatory scrutiny this has engendered.
4.1.5. Other time series for PSTs
In the context of decadal trends or patterns, one of the longest and most comprehensive HAB toxicity time series in the U.S. is the monitoring program for PSTs conducted by the State of Maine, dating back to 1978. Using that dataset, a HAB Index was developed (Anderson et al., 2014) based on three measures of PST toxicity for each year (the percent of monitoring stations showing detectable toxicity over the year, the cumulative amount of toxin per station measured in all shellfish samples during that year, and the duration of measurable toxicity). These metrics were combined into an index that provides a single measure of annual toxin severity. When viewed from 1978 to 2012, the Index reveals at least two, and perhaps three multi-year intervals or eras, each with different patterns and levels of toxicity (Fig. 3). Due to budget constraints, Maine dramatically reduced the number of monitoring stations in 2012, so it is not possible to calculate the Index to the present day. A new Index is currently being formulated that will use a smaller set of sampling stations to allow the Index time series to be sustained into the future.
Fig. 3.
The HAB Index for western Maine. This metric combines multiple measures of PST toxicity to provide a single value that is indicative of overall severity for each year. Modified from Anderson et al., 2014.
4.1.6. PST Impacts in marine fish and wildlife
Thus far wildlife mortalities from PSTs in the U.S. involving shellfish (Shumway, 1990, Landsberg, 2002) and higher vertebrates have only been associated with Alexandrium catenella. In HAEDAT, PSTs were first reported to affect marine mammals, after the mortality of 14 endangered humpback whales (Megaptera novaeangliae) in November 1987 in Cape Cod Bay, Massachusetts. The whales were lethally exposed to PSTs after feeding on toxic mackerel, Scomber scombrus that had migrated south from the St. Lawrence River, Canada. This was the first documented case demonstrating the vectoring of PSTs to higher vertebrates via the food chain (Geraci et al., 1989). Earlier the same year, a mortality of 60 sea otters, Enhydra lutris, from PSTs in Alaska was suspected, but unproven (De Gange and Vacca, 1989). Concentrations of up to 9.9 ng PSTs g−1 were recorded in bottlenose dolphin carcasses from the Indian River Lagoon over a 10-year period, indicating that P. bahamense is a potential mortality risk factor (Fire et al., 2020).
Mortality events of birds involving PSTs originating from A. catenella and vectored through fish have occasionally been documented. In 1978, mortalities of common terns, Sterna hirundo, in Massachusetts (Nisbet, 1983) and of Kittlitz’s murrelet, Brachyramphus brevirostris, in the last decade in Alaska, were caused by PSTs (Shearn-Boschler et al., 2014). Noteworthy because of other wildlife HAB toxicity events in Alaska (see ASTs), PSTs were also detected coincident with die offs of common murres, Uria aalge in the Gulf of Alaska from 2015–2016 (concurrent with ASTs) and in mortalities in the East Bering Sea of tufted puffins, Fratercula cirrhata, from 2016–2017, but lethal HAB toxicity was unproven (Jones et al., 2019, Van Hemert et al., 2020). The mortality of at least 13 endangered shortnose sturgeon, Acipenser brevirostrum, in Sagadahoc Bay, Maine, in the summer of 2009 occurred during a high biomass A. catenella bloom. Extremely high concentrations of PSTs were detected in local shellfish. PST-like activity was found in sturgeon tissues and stomach contents containing amethyst gem clams, Gemma gemma, were confirmed to contain PSTs (Fire et al., 2012).
4.1.7. Key drivers
One key factor underlying both the biogeography and bloom dynamics of Alexandrium catenella and Pyrodinium bahamense is that both species include a resting cyst stage in their life histories (Azanza et al., 2018). Cyst germination provides the inoculum for blooms, and the transformation back to the resting state can remove substantial numbers of cells from the bloom population and act as a major factor in bloom termination. The timing of cyst germination (excystment) and the ultimate formation of new cysts (encystment) is regulated by both internal and external factors, and leads to highly episodic or seasonal outbreaks (Fischer et al., 2018; Lopez et al., 2019). Cysts are also important for population dispersal and can even be sources of toxin to shellfish and other benthic animals.
Once in the water column, the development of blooms of these species is driven by a range of environmental factors, the most important of which include temperature, nutrients, and water column stratification. Both PST-producing species migrate vertically in the water column and can form dense, near-surface aggregations during daylight hours and subsurface layers at night where higher nutrients are generally available. Salinity can play an important role in the blooms of both species through stratification and perhaps the provision of nutrients, humic substances and other growth enhancers from terrestrial sources. For example, blooms of A. catenella are closely associated with a buoyant coastal current formed by river outflow into the Gulf of Maine, providing a transport pathway to deliver cells to southern regions, while also providing a stratified layer that favors dinoflagellate growth and accumulation over non-motile diatoms (Franks and Anderson, 1992).
While the occurrence of HABs is controlled by multiple biological, chemical and physical processes, temperature is a central organizing factor determining the potential for HABs to occur (Smayda and Reynolds, 2001) and may have contributed to an expansion or continuance of A. catenella blooms in some regions of the U.S. As with many phytoplankton species, A. catenella has maximal laboratory growth rates at temperatures higher than the annual maximum in some ecosystems within which it blooms. Ocean warming thus has the potential to promote blooms of A. catenella in the U.S., as shown by Gobler et al. (2017) who used models to indicate that ocean warming from 1982–2017 significantly increased the potential growth rate and duration of the bloom season from Cape Cod through Canada and the Gulf of Maine and within regions of southern Alaska and Puget Sound. Similarly, Moore et al. (2008; 2011) predicted a significant expansion of the bloom season in Puget Sound with the progressive warming predicted to occur this century, leading to longer blooms and higher toxicity. These two forecasts, however, focused solely on the growth of the motile, vegetative cells in the A. catenella life cycle. Brosnahan et al. (2020) recently explored the impacts of global warming on the germination dynamics of the cysts of this species and argued that cyst accumulation zones may persist longer in more seasonally-variable, shallow inshore habitats than in deep offshore ones, promoting HABs that are more localized and commence earlier. This work also suggests that A. catenella will be more resilient to future warming in habitats with high temperature seasonality (i.e., shallow embayments and estuaries). These types of climate-related forecasts are further complicated by the encystment process, as shown by Ralston et al. (2014) who demonstrated that warm winters did lead to earlier bloom development for A. catenella (as predicted by Gobler et al. (2017) and Moore et al. (2008)), but that blooms also ended earlier due to encystment driven by factors other than temperature. There was no expansion of the temperature window for rapid growth.
Pyrodinium bahamense var. bahamense is able to form blooms under low-salinity conditions (i.e., salinities from 13 to 35) that are found in many lagoons and estuaries in Florida (Phlips et al., 2006), but is usually restricted to the warmer months from April to October. The most intense P. bahamense blooms coincided with higher than normal rainfall from 2002–2006 (Phlips et al., 2015). Pyrodinium bahamense is hypothesized to have high nutrient requirements (Phlips et al., 2006) and blooms during elevated nutrient loading associated with high precipitation events that contribute to lower salinities (Phlips et al., 2015). In the Indian River Lagoon, one of the primary driving factors for blooms is the extended mean water residence period (Steward et al., 2005; Phlips et al., 2015), providing time for the accumulation of biomass, fueled by increased anthropogenic nutrient loading during elevated rainfall. However, in Tampa Bay, environmental factors only explain a small percentage of the variability in P. bahamense abundance (Corcoran et al., 2017) and life cycle dynamics play a primary role in seasonal patterns and recurrence of blooms (Lopez et al., 2019). Despite significant reductions in Tampa Bay N-loadings and recovery of other Bay segments, prolonged blooms of P. bahamense each summer continue to pose challenges for Old Tampa Bay (Sherwood et al., 2016). Blooms of P. bahamense in Tampa Bay occur primarily in the northern segment, Old Tampa Bay, which has high abundance of resting cysts in the sediments (Karlen and Campbell, 2012) and long water residence times (Meyers et al., 2017). Changes in top-down zooplankton pressure may also favor blooms of P. bahamense (Badylak and Phlips, 2008).
With increasingly likely variability in the frequency of hurricanes and precipitation events, it is possible that P. bahamense blooms will change in frequency or longevity in various areas of Florida. The potential for an increase in temperature due to global warming may also provide optimal conditions for the range expansion of this species. As pointed out by Brosnahan et al. (2020) the bloom occurrence of P. bahamense extends beyond the known resting cyst distribution in the sediments, suggesting that an expansion may occur. However, this may be contingent upon the effects of temperature on dormancy and quiescence of the cysts (Lopez et al., 2019).
4.2. ASTs
4.2.1. Background
Amnesic shellfish poisoning is caused by domoic acid (DA) and its isomers, hereafter termed amnesic shellfish toxins (ASTs). In the U.S., taxa known to produce ASTs are multiple diatom species in the genera Pseudo-nitzschia (Table 1). Of the more than 50 Pseudo-nitzschia species identified, over 25 are known to produce ASTs at varying concentrations (Bates et al., 2018), dependent upon the species and the environmental conditions to which they are exposed (reviewed in Lelong et al., 2012; Trainer et al., 2012; Bates et al., 2018). Domoic acid has several means of entering the food web - through filter feeding molluscan shellfish, suspension-feeding finfish, and zooplankton. Shellfish closures to protect human health occur when DA concentrations exceed 20 ppm in most shellfish except Dungeness crab, for which the regulatory closure level is 30 ppm in viscera. The most highly toxic species of Pseudo-nitzschia on the U.S. West Coast are P. australis and P. multiseries. On the East Coast, the species responsible for toxic episodes in the Gulf of Maine first reported in 2016 is P. australis and in the Gulf of Mexico the P. pseudodelicatissima species complex (including P. cuspidata) has been associated with AST closures of shellfish harvest starting in 2013 (Bates et al., 2018).
4.2.2. AST history
Because of the relatively recent discovery of domoic acid as a HAB toxin (Bates et al., 1989), the 1990–2019 HAEDAT records show the complete story of the initial documented DA-related shellfish closures on the U.S. West Coast in 1991 and the more recent emergence of DA in the Gulf of Mexico and U.S. East Coast starting in 2014 and 2016, respectively. Soon after the first DA poisoning event was reported in 1987 in eastern Canada (Bates et al., 1989; Todd 1993), DA poisoning caused the illness and death of seabirds in Monterey Bay, California in 1991 (Work et al., 1993). Anchovies and sardines that had fed on the DA-producing P. australis (Buck et al., 1992; Fritz et al., 1992) were the vectors of toxin transfer to seabirds. Later that same year, DA was measured in razor clams (Siliqua patula) and Dungeness crabs on the Washington coast (Wekell et al., 1994). In May-June 1998, the first confirmed death of a marine mammal was recognized to be associated with the trophic transfer of DA from sardines and anchovies to sea lions (Gulland et al., 2000; Lefebvre et al., 1999; Scholin et al., 2000). ASTs continue to cause shellfish closures and marine mammal and bird deaths on the U.S. West Coast to the present day. A massive, coastwide bloom in 2015 has been linked to a warm water anomaly in the Pacific Ocean and resulted in record concentrations of DA in seawater, shellfish and planktivorous fish and numerous marine mammal and bird deaths (McCabe et al., 2016; McKibben et al., 2017).
In contrast to the recurring events observed on the U.S. West Coast, the first AST closures in the Gulf of Mexico and the U.S. East Coast occurred recently, in 2013 and 2016, respectively. Toxic Pseudo-nitzschia species and particulate DA were present in the Gulf of Maine prior to the first shellfish closure there in 2016, which coincided with the first observation of P. australis in that region after many years of monitoring (Fernandes et al., 2016; Bates et al., 2018). Since 2016, repeated AST closures associated with P. australis blooms have been observed. In the Gulf of Mexico, the first shellfish closures were imposed in April 2013 after eastern oysters (Crassostrea virginica) tested above the AST regulatory level (Bates et al., 2018). Since then, two additional closures have been warranted (2014, 2017); all three Gulf of Mexico closures occurred in the same embayment in northwest Florida (Bates et al., 2018).
4.2.3. AST trends in shellfish
HAEDAT records document an increasing trend of AST events in the U.S. (Fig. 4), with a hint of multi-year cycling. Initial events were only on the West Coast, occurring nearly every year from 2000–2019. Gulf of Mexico events first appeared in 2013 and thereafter occurred sporadically, compared to Gulf of Maine outbreaks which began in 2016 and recurred each year since. The overall pattern is one of relatively frequent outbreaks on the U.S. West Coast and far fewer on the East Coast and the Gulf of Mexico. This is most evident in Fig. 4E which shows the 30-year history, highlighting ASTs as a predominantly West Coast phenomenon, with emerging problems in the east. Figure 4F shows the fraction of HAEDAT zones with at least one AST event per year through time, documenting an increasing and significant trend in AST incidence (p< .05). There were 91 total AST events in the U.S. over the 30-year study interval, with a mean of 3.0 per year.
Fig. 4.
The frequency of AST events in the U.S. derived from HAEDAT. A-D, five-year frequencies, with the size of the circle denoting the number of events during that interval; E, AST frequencies (events per year) for the entire U.S. (1991 – 2019); F, time series of observed (bars) and modeled (line) proportions of HAEDAT monitoring zones with at least one event. Also reported is the fitted linear logistic model and its significant p value (p<.05).
The increasing national trend is in part a reflection of growing management awareness of this new HAB toxin and its discovery in locations where it likely had occurred before but was undetected. Since the original discovery of DA in Canadian shellfish in 1987 (Bates et al., 1989), some U.S. states have been slow to set up comprehensive monitoring programs for this toxin, as qualitative risk assessments indicate that the likelihood of significant events is low. In some cases, shellfish flesh testing is not implemented until phytoplankton sampling indicates that Pseudo-nitzschia spp. cell abundance reaches certain thresholds. Interestingly, the recent AST events in the Gulf of Maine have been linked to P. australis, a species that had not been observed in decades of monitoring by U.S. and Canadian workers in that region (Bates et al., 2018). This therefore seems to be a clear example of the expansion of a HAB problem to a new region. Current thinking is that the species was introduced to the Gulf of Maine in 2016 with water masses from the Scotian shelf (Clark et al., 2019). It is not known whether recurrent events in that region since 2016 reflect the persistence of the species within the Gulf, or are due to additional water mass intrusions and introduction events. The link between these events and a warming climate is also unknown and is currently under investigation.
4.2.4. Other time series for ASTs
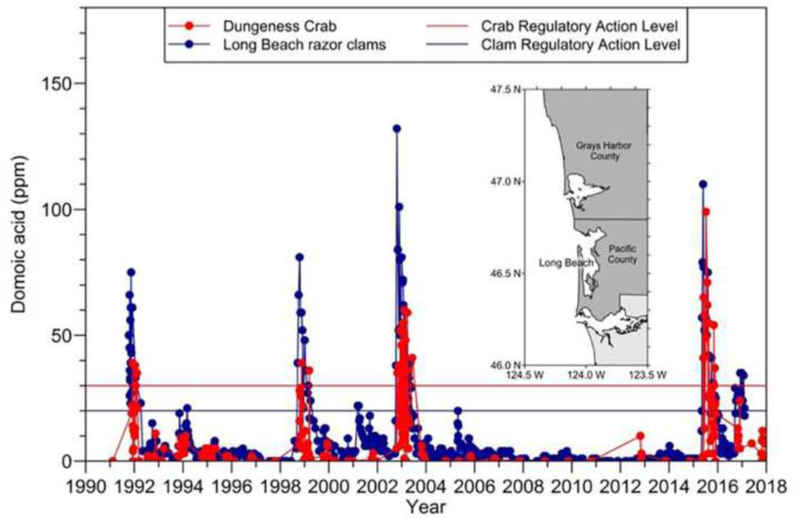
State shellfish safety programs such as those led by the Washington State Department of Health, the Oregon Department of Fish and Wildlife and California Department of Public Health Services have provided useful shellfish time series data that help pinpoint trends and areas of concern. For example, DA time series data from both Dungeness crab and razor clams collected near Long Beach, Washington show the close linkage of toxin in these two species (Fig. 5). In almost every case, when razor clam DA increases, so does crab DA, illustrating the importance of clams as crab food. Other important data sets that provide insights into environmental drivers of DA events include the Olympic Region HAB (ORHAB; e.g. Trainer and Suddleson 2005) phytoplankton monitoring data, Monitoring Oregon’s Coast for Harmful Algae (MOCHA; e.g. McKibben et al., 2015) and the California Department of Public Health Marine Biotoxin Monitoring Program monthly report (Smith et al., 2018; https://www.cdph.ca.gov/Programs/CEH/DRSEM/Pages/EMB/Shellfish/Marine-Biotoxin-Monitoring-Reports.aspx)
Fig. 5.
Time series of AST in Dungeness crab (red) and razor clams (blue) near Long Beach, WA. Monthly razor clam DA at Long Beach provides a 1–2 week early warning of Dungeness crab DA. The crab and clam regulatory limits are shown with red and blue horizontal lines respectively. Source: Washington State Department of Health.
4.2.5. AST impacts in marine wildlife
Animal mortalities from DA exposure through the food web are common, with this syndrome being termed domoic acid poisoning or DAP. During DAP events in seabirds and marine mammals, the toxin moves rapidly through food webs by accumulating in filter-feeding invertebrates and planktivorous fish during blooms, and is then consumed by marine mammals and birds resulting in serious neurologic illness and death (Scholin et al., 2000; Work et al., 1993). Toxin concentrations detected in bivalve and fish vector species are high enough to cause documented illness and mortality in marine mammal and seabird predators, but unlike some other shellfish toxins, there are no reports of acute excitotoxic health impacts or die-offs due to DAP in fish or bivalve vectors. Domoic acid is an excitotoxic agonist for glutamate, a primary neurotransmitter in the central nervous system (CNS) (Berman and Murray, 1997). As such, it exerts its toxicity through over-excitation of neurons in the CNS of mammals and birds, which may explain the lack of obvious neurotoxic impact in bivalves that lack this system. Planktivorous fish have been shown to have glutamate receptors in the CNS that are susceptible to DA over-excitation in laboratory studies (Lefebvre et al., 2001). However, oral gavage studies and field observations provide strong evidence that fish are more tolerant of oral consumption of DA than birds and mammals, and are not likely impacted acutely in the wild under the ecologically-relevant bloom conditions observed to date (Lefebvre et al., 2001, 2007, 2012).
DAP in seabirds and marine mammals via consumption of DA-contaminated planktivorous fish has been well documented on the U.S. West Coast (Fritz et al., 1992; Work et al., 1993; Lefebvre et al., 1999; Scholin et al., 2000). The highest incidence of DAP occurs along the coast of California. The first documented DAP event in the U.S. occurred in 1991 in Monterey Bay, California involving large numbers of brown pelicans (Pelicanus occidentalis) and Brandt’s cormorants (Phalacrocorax penicillatus) that had consumed DA-contaminated Northern anchovies (Engraulis mordax) (Fritz et al., 1992). Behavioral excitotoxicity observed in DA-exposed birds consisted of head weaving, scratching and vomiting (Work et al., 1993). In 1998, a highly toxic Pseudo-nitzschia bloom in central California was linked to reduced interannual survival of the marbled murrelet (Brachyramphus marmoratus) (Peery et al., 2006). This highly toxic bloom also caused the first documented DAP event observed in a marine mammal species. Starting in May of 1998, hundreds of California sea lions began stranding on beaches exhibiting signs of DAP such as scratching, disorientation, ataxia, and seizures as a result of consuming highly toxic anchovies (Lefebvre et al., 1999; Gulland, 2000; Scholin et al., 2000; Silvagni et al., 2005). Since this event, California sea lions have been experiencing DAP events on an almost yearly basis (Bejarano et al., 2008a). As a result of the regular occurrence, the highly visible neurotoxic symptoms, and the high numbers of animals affected, California sea lions have become a model system for studying mechanisms of DA toxicity.
In the absence of overt excitotoxic signs like seizures, it is difficult to diagnose DAP in stranded and dead marine mammals. As such, it is likely that more animals are impacted than have been reported. The toxin has been detected in many stranded marine mammal species along the entire U.S. West Coast (Landsberg et al., 2014) as well as in pygmy and dwarf sperm whales from southeastern and mid-Atlantic U.S. waters (Fire et al., 2009). Mortality events associated with DA have been reported in California sea lions (Zalophus californianus), Harbor seals (Phoca vitulina), Pacific harbor seal (P. vitulina richardii), Northern fur seals (Callorhinus ursinus), Southern sea otters (Enhydra lutris nereis), Long-beaked common dolphins (Delphinus capensis), Short-beaked common dolphins (D. delphis), Bottlenose dolphins (Tursiops truncatus), Risso’s dolphins (Grampus griseus), Harbor porpoise (Phocoena phocoena), Dall’s porpoise (Phocoenoides dalli), Minke whales (Balaenoptera acutorostrata), humpback whales (Megaptera novaeangliae), Cuvier’s beaked whales (Ziphius cavirostris), and gray whales (Eschrichtius robustus) (Heyning, 2003; Kreuder et al., 2003, 2005; Torres de la Riva et al., 2009; Lefebvre et al. 2010; Fire and Van Dolah 2012; Fire et al. 2010).
Anomalously warm ocean conditions have been linked to increased toxicity, geographic range, and duration of DA-producing algal blooms on the U.S. West Coast, resulting in exceptionally high DA contamination in vector species and sea lion mortality rates (McCabe et al., 2016). As waters warm, Pseudo-nitzschia blooms may expand and/or increase in toxicity, posing even greater risks to seabirds and marine mammals in new geographic regions. A recent long-term study of the presence of algal toxins in 13 marine mammal species from Arctic and subarctic regions revealed potential exposure risks in all species tested (Fig. 6). Although it could not be determined if these animals had high enough toxin doses to cause DAP, the data did confirm that DA was present in Arctic and subarctic food webs at levels high enough to be detected, regardless of feeding strategies, thereby posing increasing exposure risks as future ocean conditions warm (Lefebvre et al., 2016; McCabe et al., 2016).
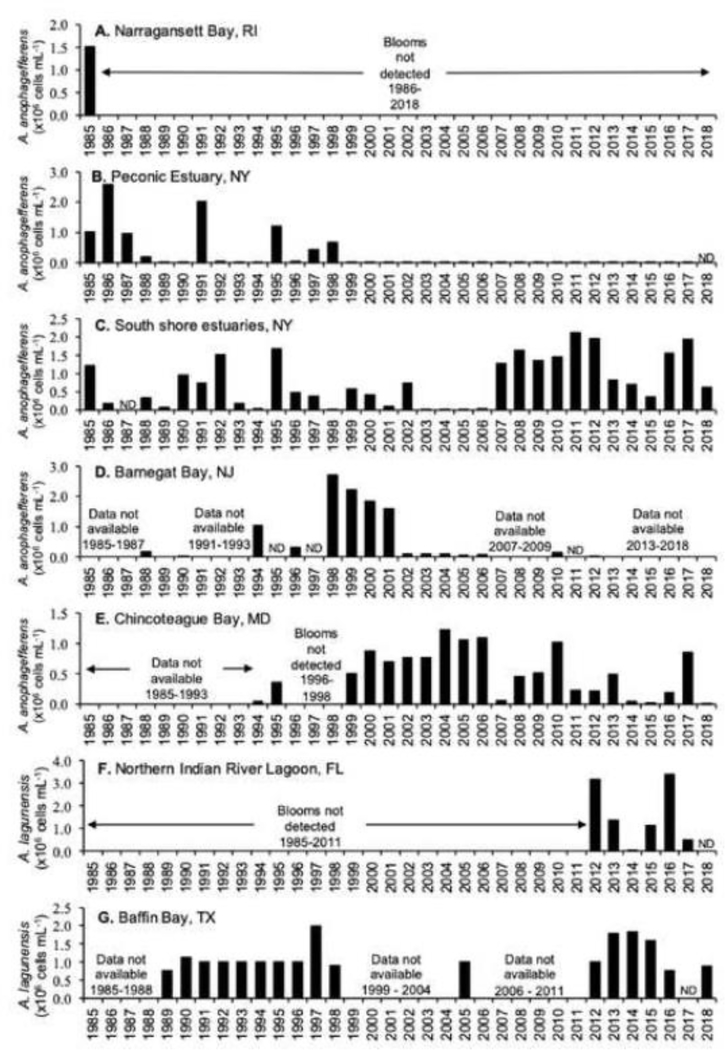
Fig. 6.
Locations where algal toxins were detected in stranded (s) and harvested (h) marine mammals between 2004 and 2013. Red images represent species positive for domoic acid (DA) and purple images represent species positive for saxitoxin (STX). Many species contained both toxins confirming co-exposure. The 13 species that were sampled are listed on the side of the figure in gray (Source: Lefebvre et al., 2016).
4.2.6. AST-related marine mammal mortality trends
There were no reported marine mammal poisonings attributed to DA until 1998 in Central California (Scholin et al., 2000), but since then, DAP has impacted marine mammals almost every year, marking a definite increase in DAP-related mortality after 1998 along the California Coast (Fig. 7; Bejarano et al., 2008a; Bargu et al., 2010; McCabe et al., 2016). As a result of this common occurrence and well-defined symptomology, retrospective analyses of veterinarian records at The Marine Mammal Center in Sausalito, California were performed to identify DAP events prior to its initial discovery in 1998. Records revealed that at least one animal per year since 1990 has been impacted by DAP. The number of cases began increasing in 1998 (Fig. 7). Dozens to hundreds of sea lions have been diagnosed with DAP or DAP-related mortality yearly along the California coast since 1998, with higher-than-average numbers recorded in 2015 during the widespread coastal bloom that year. In fact, the first sea lion documented to exhibit DA-related excitotoxic seizures north of California also occurred in 2015 in Long Beach, Washington, raising concerns of a northward expansion of intense DA-producing blooms (McCabe et al., 2016). Although confirmed DA toxicosis events in wildlife have not been documented on the East Coast nor in the Gulf of Mexico, there is an emerging trend for DA detection in stranded marine mammals from those areas (Fire et al., 2009, 2011).
Fig. 7.
Increasing trend in cases of California sea lions diagnosed with domoic acid poisoning as recorded at the Marine Mammal Center in Sausalito CA. Dotted line shows the significant regression (p < .05).
4.2.7. Key drivers
Past field studies have shown that toxigenic Pseudo-nitzschia blooms often occur when macronutrients are not limiting; there is often no significant correlation between DA and ambient concentrations of macronutrients in situ (Trainer et al., 2009). However, toxin-producing Pseudo-nitzschia species have a unique capability of surviving extreme ocean conditions, including high temperatures and low macronutrients (e.g., Trainer et al., 2019). Certain Pseudo-nitzschia species appear to exhibit special strategies for survival under nutrient or trace metal stress. Some may have the ability to acquire strongly complexed iron, even when available at very low concentrations, using a high-affinity iron acquisition system that requires copper and the production of DA (Wells et al., 2005). In addition, the high affinity of at least one Pseudo-nitzschia species, P. australis, for nitrate and ammonium (Cochlan et al., 2008) provides it with a competitive advantage for acquiring nitrogen (N) under N-depleted conditions, but especially following a N surge during upwelling when its maximal rate of nitrate uptake exceeds those of virtually all the other phytoplankton species commonly found in upwelling systems (Kudela et al., 2010). Recent genetic studies have shed light on the interplay of several genes involved in trace metal and vitamin acquisition on the ability of Pseudo-nitzschia species to cope with nutrient limitation (summarized in Bates et al., 2018). Toxic species often produce DA in culture under silicate limitation (e.g., Lelong et al., 2012), and field data suggests that interannual variability in the ratio of nitrogen to silicate may be important for DA production as well (Clark et al., 2019; Ryan et al., 2016). However, there is no consensus on any universal drivers for toxin production; in fact, geographical and species differences likely exist. However, long-term records are providing some clues. An analysis of the historical record of large-scale toxigenic Pseudo-nitzschia blooms has demonstrated a potential link to periods of anomalously warm ocean conditions such as El Niño, positive phases of the Pacific Decadal Oscillation (PDO), or record-setting marine heatwaves (McCabe et al., 2016; McKibben et al., 2017).
4.3. DSTs
4.3.1. Background
In the U.S., diarrhetic shellfish poisoning (DSP) is a relatively new threat to human health with only three confirmed illnesses in 2011 (Trainer et al., 2013). The lipophilic phycotoxins associated with this syndrome are okadaic acid (OA), dinophysistoxins-1 (DTX1) and −2 (DTX2) and their derivatives, hereafter referred to as diarrhetic shellfish toxins (DSTs). Eight toxigenic species of the genus Dinophysis are present in U.S. waters (Table 1) and initial culturing studies have demonstrated that toxin profiles can vary at both the Dinophysis species and strain designation (Fux et al., 2011, Wolny et al., 2020). While a cross-regional comparison is still needed in the U.S., general trends can be identified amongst strains in culture thus far: East Coast D. acuminata contains both OA and DTX1 (Fux et al., 2011; Wolny et al., 2020); Gulf Coast D. ovum contains only OA (Fux et al., 2011), and only dihydro-DTX1 is present in D. norvegica from the Northeast Coast (Deeds et al., 2020). Less prominent are DST-producers Prorocentrum lima, an epiphytic/epibenthic dinoflagellate (Morton et al., 1999), and two species of Phalacroma (P. rotundatum, P. mitra), previously of the genus Dinophysis (Table 1). Yessotoxins and pectenotoxins are not regulated in U.S. seafood and so are excluded from this analysis
4.3.2. DST history
The earliest reports of DSTs in the U.S. date back over thirty years, to the mid 1980s, and first appeared along the East Coast. Multiple species of toxigenic Dinophysis and P. lima have been identified along this coastline and blooms can temporally overlap (Hargraves and Maranda, 2002). As such, the causative species of a DST event has not always been clear. In Narragansett Bay, Rhode Island, elevated cell concentrations of D. acuminata and a mixed assemblage of other toxigenic Dinophysis spp. coincided with the detection of DSTs in edible shellfish meat from 1984 – 1985 (Maranda and Shimizu 1987). Toxin levels, however, did not exceed FDA guidance levels of 16 μg okadaic acid eq. 100g−1, thus shellfish and harvesting closures were not imposed. The first suspected human illness in the U.S., due to DSTs, parallels this timeline with reports of gastroenteritis after the consumption of bivalves from New York (Freudenthal and Jijina 1985) and Maine (Maranda and Shimizu 1987) in the mid-1980s. The causative organism(s) could not be confirmed. New reports of DST did not occur for another decade, until 1998, when cases of gastroenteritis surfaced and DSTs were detected in low concentrations in shellfish collected off the coast of Maine (Morton et al., 1999). The causative organism was suspected to be P. lima due to the detection of DTX1 in epiphyte samples, a history of P. lima and associated DSP outbreaks in adjacent Canadian waters (Cembella 1989), and the presence of empty P. lima thecae in blue mussel digestive tracts. Potential DST-producers D. norvegica and D. acuminata, however, were also present, but plankton samples lacked DSTs as determined via multiple detection methods (Morton et al., 1999). Later field studies demonstrated that while P. lima abundance was correlated with DSTs in the epibiotic community, accumulation in shellfish was minimal (Maranda et al., 2007a,b), again suggesting low risk for DSP outbreaks and causative species along the coast of Maine. In 2002, a bloom of D. acuminata was detected in the East Coast estuary of Chesapeake Bay, Maryland (Tango et al., 2004). The elevated abundance led to a precautionary harvesting closure based on cell abundance that was lifted when DST levels in shellfish were confirmed to have been well below federal guidelines.
The events described between 1984–2002 are suggestive of a history of DST in the U.S., but reports were sporadic and DSP illnesses were not confirmed, nor was identification of causative organisms conclusive. DSTs in shellfish were not detected above FDA regulatory guidelines during this period so reports were not entered into the HAEDAT database. In 2008, however, toxigenic D. ovum was observed in the Gulf of Mexico (Texas) (Campbell et al., 2010, Deeds et al., 2010). Toxin levels in the meat of the eastern oyster (Crassostrea virginica) exceeded the FDA regulatory guidance level, so harvesting for the species was prohibited for a month as product was recalled. To many, this notable event on the Texas Gulf Coast marked the emergence of DSP as a HAB concern in the U.S. This was the first DST entry into HAEDAT.
The first conclusive DSP illness in the U.S. was then documented in Sequim Bay, Washington, in 2011 with a bloom of D. acuminata and the sickening of three people who consumed blue mussels (Mytilus edulis) (Trainer et al., 2013). In a follow-up study in 2012, DSTs were also found above FDA guidance levels in the varnish clam (Nuttalia obscurata), California mussel (Mytilus californianus), Pacific oyster (Crassostrea gigas), and manila clam (Venerupis philippinarum), demonstrating that several seafood products were susceptible to contamination. This DSP event and subsequent study made it apparent that DSTs were a concern for the West Coast. On the same coastline, DSTs were detected in the California mussel above FDA guidance levels in Monterey Bay, California during a field study from 2013–2016 (Shultz et al., 2019).
While the East Coast had the earliest reports of DSTs in the U.S., the region did not experience a substantial event until 2011–2012 on Long Island, New York, when D. acuminata cells exceeded 2 × 106 cells L−1 and DSTs in shellfish far surpassed FDA guidance levels (Hattenrath-Lehmann et al., 2013). Later, in 2015 and 2018, DST events were recorded for the first time off the coast of New England (Fig. 8D), with elevated concentrations of Dinophysis spp. and DSTs above FDA guidelines in shellfish collected from Massachusetts and Maine, respectively. DSTs have also been found above FDA guidance levels between 2010–2016 in shellfish from the mid-Atlantic states of Delaware and Maryland where small embayments had Dinophysis spp. concentrations of 104 to 106 cells L−1 (Wolny et al., 2020). No closures resulted, however, as elevated DSTs were detected in areas already closed to harvesting or detected in noncommercial shellfish species. Together, this recent history indicates the threat of DSP now exists along all coasts of the U.S., with toxic shellfish detected in Texas, Washington, California, New York, Massachusetts, Maine, Delaware, and Maryland.
Fig. 8.
The frequency of DST events in the U.S. derived from HAEDAT. A-D, five-year frequencies, with the size of the circle denoting the number of events during that interval; E, DST frequencies (events per year) for 2008 – 2019; F, time series of observed (bars) and modeled (line) proportions of HAEDAT monitoring zones with at least one event. Also reported is the fitted linear logistic model and its non-significant p value (p>.05).
4.3.3. DST trends
Since the first documented DST event in 2008 (Fig. 8B), at least one event has been recorded in the U.S. every year, except 2009 (Fig. 8F). This is in stark contrast to the two decades prior, where reports were sparse and less intense (i.e., lower concentrations of Dinophysis spp. cells recorded and lower, unactionable levels of DSTs measured in edible shellfish meat). The 2008 DST event on the Gulf Coast and the subsequent events on the West and East Coasts (Fig. 8C,D), also more clearly identified the causative organisms, linking Dinophysis spp., and not P. lima, to a higher risk of DSP illness in the U.S. The entries in HAEDAT typically do not identify the species of Dinophysis, but when specified, the causative species has been D. acuminata or D. ovum, part of the D. acuminata-complex: a group of Dinophysis species currently undistinguishable using light microscopy (Reguera et al., 2014) or molecular methods (Raho et al., 2008). The importance of P. lima and related species in DST prevalence in the U.S. is still an unknown. The small dinoflagellate is considered benthic and often epiphytic, and therefore, would likely be excluded from typical monitoring programs that enumerate cells in surface water samples.
The number of HAEDAT zones impacted by DSTs shows no significant trend with time since 2008 (p > .05; Fig. 8F). Since 2008, 34 events were recorded, with an average of 2.83 events per year. It is evident from Figure 8E that while DSTs are a problem on all three U.S. coastlines, the West Coast is the region with the most occurrences.
When comparing HAEDAT entries to the literature, it becomes apparent that multiple DST events were not included in the database despite the data meeting the criteria of “biotoxin accumulation in seafood above levels considered safe for human consumption” (e.g., Hattenrath-Lehmann et al., 2013; Trainer et al., 2013; Shultz et al., 2019; Wolny et al. 2020), suggesting an underreporting of these occurrences in the U.S. These DST events were likely excluded because a closure was not imposed in association with the elevated toxin levels in seafood. This may be because areas affected were already closed for other contaminants or toxins, or Dinophysis enumeration and DST quantification were not yet incorporated into shellfish monitoring programs. Toxins may also have been quantified in shellfish well after the DST event as part of a scientific study instead of a shellfish monitoring program.
Monitoring for Dinophysis and DSTs has been progressively incorporated into state shellfish monitoring programs over the last decade, as has been the case for AST over the last three decades. Regional thresholds have recently been defined to trigger enhanced phytoplankton and shellfish monitoring or precautionary harvesting closures based on the concentration of toxic phytoplankton cells in the water, i.e., 2 – 10 Dinophysis mL−1. Even with these improvements, however, DSTs may continue to be underreported, as harmful Dinophysis blooms typically do not reach cell concentrations capable of causing water discoloration, Dinophysis spp. may form subsurface layers evading detection (Velo-Suarez et al., 2008), and DSP symptoms can be confused with gastroenteritis in humans. To date, there are no unique behaviors or pathology in aquatic animals attributed to DSTs and so these types of entries have not yet been included in HAEDAT.
4.3.4. DST impacts in marine wildlife
DSTs have not solely been attributable to wildlife mortality events, but okadaic acid has been detected concurrently with other HAB toxins. Following a large-scale bottlenose dolphin mortality that occurred off the coast of Texas in the winter-spring of 2008, at least three dolphins were determined to be concurrently exposed to ASTs and DSTs (Fire et al., 2011). Although still unproven for possible effects on wildlife, it should be recognized that okadaic acid is a tumor promoter (Fujiki and Suganuma, 1993). Possible links with wildlife neoplasia and okadaic acid exposure via trophic transfer from benthic Prorocentrum have been investigated but potential connections remain equivocal (Landsberg et al., 1999, 2014).
4.3.5. Key drivers
Not enough information is available in the U.S. HAEDAT database yet to make conclusions regarding the drivers of DST events or to predict trends with climate change or eutrophication. The lack of DST events along the southern portions of the East or West Coasts (Fig. 8) may provide some clues as to the parameters that constrict blooms. Outside of HAEDAT, drivers are being explored regionally with emphasis being placed on predator-prey dynamics (Hattenrath-Lehman et al., 2013, Harred and Campbell 2014), nitrogenous nutrients (Hattenrath and Gobler, 2015), and basic environmental parameters (Shultz et al., 2019). Models linking changes in surface temperatures to the growth rates of D. acuminata indicate that ocean warming from 1982–2017 has significantly increased the potential growth rate and duration of blooms in the northeast U.S. and Puget Sound (Gobler et al., 2017), both regions that have newly experienced these HABs during the past decade. More field studies are needed to determine drivers of DST events across species and regions including warm water anomalies, the Pacific Decadal Oscillation, El Niño, and the 2015 marine heatwave in the Pacific (McCabe et al., 2016; McKibben et al., 2017).
4.4. NSTs and Karenia brevis in the Gulf of Mexico
4.4.1. Background
Ingestion of neurotoxic shellfish toxins (NSTs, also known as brevetoxins), can cause Neurotoxic Shellfish Poisoning (NSP), and NSTs can cause other negative human health impacts when inhaled in aerosolized form (Backer et al., 2003; Kirkpatrick et al., 2006; Fleming et al., 2011). In the U.S., the vast majority of NST events are Gulf of Mexico blooms of the dinoflagellate Karenia brevis (historically known as Gymnodinium brevis, Gymnodinium breve, and Ptychodiscus brevis). Karenia brevis produces NSTs constitutively although to varying degrees (Baden and Tomas, 1988; Lekan and Tomas, 2010; Corcoran et al., 2014). Blooms of K. brevis with associated NSTs cause recurring, frequent impacts and are observed nearly every year in Florida and more sporadically in Texas. Events rarely occur in northern Gulf of Mexico states (Alabama, Mississippi, and Louisiana) where they are linked to western advection of northwest Florida blooms (Dortch et al., 1998; Maier Brown et al., 2006; Soto et al., 2018). Blooms from southwest Florida – the area most frequently impacted by NSTs – are sometimes transported to Florida’s Atlantic coast (Weisberg et al., 2019) and may become entrained in the Gulf Stream. This impacts other U.S. Atlantic states, including North Carolina where one event was documented (Tester et al., 1988), and Delaware where there was a putative event (Delaware Department of Natural Resources and Environmental Control, DNREC, http://www.dnrec.delaware.gov/Pages/RedTideINformation.aspx. Other observations of NST-producing species in the U.S. include Karenia papilionacea found in the Gulf of Mexico, Florida Straits, and Delaware (Coyne et al., 2015), and a flagellate, Chloromorum toxicum, identified in Delaware and formerly reported as Chattonella cf. verruculosa (Bourdelais et al., 2002; Giner et al., 2008). Recent phylogenetic analysis indicates that this species instead falls within a new class of heterokonts that requires further taxonomic assessment (Medlin and Desdevises, 2018). All other U.S. NST events have been attributed to K. brevis. Other Karenia spp. occur in the Gulf of Mexico and are generally more broadly distributed but have been shown to produce different toxins not considered to be NSTs (reviewed in Villac et al., 2020).
4.4.2. History of NSTs
Even with routine monitoring in place, impacts such as wildlife mortality, respiratory irritation, and water discoloration can provide some of the first indications of an NST event. Historical reports of these impacts suggest that K. brevis may have occurred prior to European settlement. Spanish explorers and early settlers provided some of the first accounts of recurring fish kills along the coasts of what are now eastern Mexico and Texas, yet most of these were not likely related to HABs (Gunte, 195; Magaña 2003), with only ~7% of fish kills in coastal Texas attributed to red tide from 1951–2006 (Thronson and Quigg 2008). Severe and widespread fish kills in Mexico (reviewed by Magaña, 2003) were noted in 1648 (with a foul odor), in 1792, and throughout the 1800s (1853, 1861, 1865, 1871, and 1875). Fish mortalities were first associated with respiratory impacts (a “dry cough”) in 1875. In southwest Florida, from the greater Tampa Bay region to the Florida Keys, extensive wildlife mortalities were first noted in the 1800s and early 1900s, and during this time, other impacts typically associated with NSTs began to be more consistently observed (Ingersoll, 1882; Feinstein et al., 1955; Rounsefell and Nelson 1966). Ingersoll (1882) described a series of unusual severe wildlife mortality events in southwest Florida in 1844, 1854, 1878, and 1880 and also recurring, less severe events (i.e., between 1854 and 1878). During the severe 1880 event, which followed a hurricane, a peculiar smell was associated with water discoloration, benthic fauna mortalities preceding pelagic species, and “spoiled” oysters in the greater Tampa Bay region (Ingersoll, 1882). In the early 1900s, further reports of wildlife mortalities and water discoloration also noted a “suffocating” or “acrid” gas (Taylor, 1917; Woodcock, 1948; Feinstein et al., 1955), likely aerosolized NSTs. In 1948, following the particularly severe 1946–1947 bloom event in southwest Florida, K. brevis was first identified and associated with these phenomena (Gunter et al., 1947; Davis, 1948).
The first documented NST event in Texas was in 1935 with widespread fish mortalities and “irritating gas” that extended to Mexico, and this and another event in 1948 were subsequently attributed to K. brevis based on similarities to Florida events (Lund, 1936; Gunter 1952). In between those events, less severe but recurring localized impacts were reported in the Galveston Bay area (Connell and Cross 1950). Fish and mammal mortalities linked to K. brevis blooms were reported again in Florida from 1953–1955 (Landsberg 2002) and a severe fish mortality event occurred in Mexico and southern Texas in 1955 (reviewed by Buskey, 1996). These and subsequent events motivated more intensive monitoring by Gulf of Mexico states, demonstrating that in areas where blooms occur regularly, i.e., Texas and Florida, periodic severe events are interspersed with less severe events (Magaña et al., 2003; Walsh et al., 2006). Southwest Florida is the only U.S. area impacted nearly annually by blooms (defined as ≥100,000 K. brevis cells L−1), lasting months to years that result in shellfish harvest closures, marine mammal, turtle, bird, and/or fish mortalities, negative economic impacts, and human respiratory irritation and other symptoms associated with NST exposure (Rounsefell and Nelson, 1966; Steidinger, 2009; Diaz et al., 2019; see 4.4.5). Blooms of K. brevis are also known to occur regularly in northwest Florida (on average every 3 years), and less frequently on Florida’s east coast (with 10 events from 1972–2020; Florida Wildlife Commission HAB Monitoring Database, http://myfwc.com/research/redtide/monitoring/database).
Monitoring for K. brevis and brevetoxins in designated shellfish harvest areas by U.S. states around the Gulf of Mexico has been successful in that reports of human illnesses due to NSP are uncommon. Rare NSP outbreaks in southwest Florida (e.g., 1995, 1996, 2001, 2005, 2006) have been associated with unapproved recreational harvest from shellfish harvest areas during severe, prolonged blooms (Watkins et al., 2008; Florida Department of Health, 2011, http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/gsi-neurotoxic-shellfish.pdf). The 2006 outbreak was the second largest event on record in the U.S., with clusters representing at least 20 documented NSP cases related primarily to clam consumption, resulting in emergency room visits (Watkins et al., 2008). Regional scale advection of blooms in some years can cause the geographic extent to span multiple states and U.S. regions (Tester et al., 1988; Soto et al., 2018; Novoveská and Robertson, 2019). The first K. brevis bloom in the northern Gulf of Mexico that was associated with NSTs (in oysters) occurred in 1996 (Dortch et al., 1998; Maier Brown et al., 2006) when a northwest Florida bloom was advected west following a tropical storm, resulting in NST-related shellfish harvest closures as far west as Louisiana. Blooms have been noted infrequently since then in the northern Gulf (Soto et al., 2018). Western advection of northwest Florida blooms to Alabama and/or Mississippi has occurred at least six times (1996, 2000, 2005, 2007, 2015, and 2018) with varied impacts (Dortch et al., 1998; Maier Brown et al., 2006; Soto et al., 2018; Alabama Department of Public Health). The first and only reported observation of K. brevis in North Carolina occurred in 1987–1988 when a southwest Florida bloom was advected east around the southern tip of the state and transported north via the Gulf Stream. A shoreward intrusion brought K. brevis cells into North Carolina coastal and estuarine waters (Tester et al., 1988). Human health impacts included reports of skin, eye, and respiratory irritation, as well as 48 documented cases of NSP, the largest recorded outbreak in the U.S., resulting in shellfish harvest area closures that lasted several months. The only other Atlantic observation outside Florida was in 2007, when K. brevis was putatively observed at low concentrations in Delaware, coincident with anecdotal reports of respiratory irritation (DNREC, http://www.dnrec.delaware.gov/Pages/RedTideINformation.aspx). While K. brevis appears to be endemic to the Gulf of Mexico, several other Karenia spp., including the NST-producing species K. papilionacea, occur in and outside the Gulf, but have yet to cause issues in the U.S. (Villac et al., 2020 and references therein).
Karenia papilionacea, initially described as K. brevis, was recognized as a separate species with differing morphotypes (i.e., “butterfly” and K. brevis-like) in 2004 (Haywood et al., 2004). This species has been documented in the North and South Pacific, North Atlantic, and Indian Oceans, as well as the eastern and western Gulf of Mexico, Florida Straits, and the Mediterranean Sea (summarized in Villac et al., 2020). Production of PbTx-2, one of the major forms of NSTs produced by K. brevis, was recently confirmed in strains from New Zealand and Delaware, but the levels produced by K. papilionacea strains tested were orders of magnitude less than K. brevis, and not all strains may be toxic (McNabb et al., 2006; Corcoran et al., 2014 Fowler et al., 2015). Production of NSTs by K. papilionacea has rarely been confirmed in nature, largely because this species occurs with other Karenia species that can produce NSTs (McNabb et al., 2006; Fowler et al., 2015). In the U.S., the recurring presence of low levels of K. papilionacea in Delaware during late summer suggests that there may be a local or regional population there (Coyne et al., 2015).
Like K. papilionacea, K. concordia was initially described as Karenia cf. brevis (Chang and Ryan, 2004; Haywood et al., 2004) but also has features similar to K. mikimotoii (Chang and Ryan, 2004). This species has been observed only in New Zealand. In 2002, a bloom in the Hauraki Gulf was associated with widespread fish mortalities (Chang et al., 2008) and in 2006, K. concordia was confirmed to produce multiple NSTs with PbTx-2 as the dominant form (Chang et al., 2006). Chang et al. (2006) suggested that the K. brevis-like cells observed in 1993 (when reported human health issues included respiratory distress and an NSP outbreak) were actually K. concordia, however, multiple Karenia spp. were present during both events and this could not be further confirmed.
The only other producer of NSTs to date is a flagellate first described as Chattonella cf. verruculosa, then proposed as the novel species Chloromorum toxicum, and now more recently placed in a novel class of heterokonts most closely related to Raphidophyceae (Medlin and Desdevises, 2018). This flagellate occurred widely in inland Delaware bays in 2000 and was associated with extensive fish mortalities (Bourdelais et al., 2002, Giner et al., 2008). Pigment and sterol analysis were unique but most similar to Raphidophyceae (Giner et al., 2008). Given the absence of type material and new phylogenetic analysis, Medlin and Desdevises (2018) suggest taxonomic reclassification at the class level is necessary which precludes assignment of genus and species nomenclature. Analysis of field and culture material indicated the presence of multiple NSTs, but further confirmation has not been conducted nor have subsequent events been reported.
4.4.3. NST trends
The HAEDAT dataset is focused on K. brevis—the only species known to cause recurring NST events in the U.S.—to provide further insight into bloom variability over the last 30 years (1990–2019; Fig. 9). HAEDAT also records aerosolized toxin impacts, but all of these were associated with NST event reports; aerosols are not discussed separately here to avoid double counting. This dataset primarily shows NST events across the four regions where repeated impacts have been described: the western Gulf of Mexico (Texas), the northern Gulf (Louisiana, Mississippi, and Alabama), Florida’s Gulf Coast, and Florida’s Atlantic Coast. The frequency of documented NST events is remarkably similar across these regions over time. This is shown in five-year increments from 2000–2019 (Fig. 9), capturing near annual occurrences in southwest Florida, frequent blooms in Texas, and rare events in the northern Gulf of Mexico and along Florida’s Atlantic Coast. A total of 58 NST events are recorded over 30 years, with a mean of 1.9. No significant trend over time is evident (p> .05; Fig. 9F). Although it appears that there might be a slight increase in the number of events over time, a greater than four-fold increase in monitoring effort occurred in Florida alone from 1990 to the present, and the possibility that some early events were missed or were not as comprehensively characterized cannot be excluded.
Fig. 9.
The frequency of NST events in the US derived from HAEDAT. A-D, five-year frequencies, with the size of the circle denoting the number of events during that interval; E, DST frequencies (events per year) for the entire US over the 30 years study interval (1990–2019); F, time series of observed (bars) and modeled (line) proportions of HAEDAT monitoring zones with at least one event. Also reported is the fitted linear logistic model and its non-significant p value (p>.05)
Reports of sporadic impacts from the northern Gulf of Mexico (Soto et al., 2018) should be similarly considered. Nevertheless, NST events occurred nearly annually with the exception of five years (1990, 1991, 1993, 1997, and 2010), of which the absence of a 2010 southwest Florida bloom was attributed to anomalous physical forcing that year (Weisberg et al., 2014). In HAEDAT records, the longest-lasting events—up to 30 months—occurred in southwest Florida (1994–1997, 2002–2004, 2004–2006, and 2017–2019), with a hint of multi-year cycling. Across the 30-year series, the greatest fraction of impacted zones occurred in 1996 and 2018, both of which are years with severe Florida blooms (Fig. 9). Integrating other metrics such as duration, spatial extent, and cell abundance could help further resolve spatiotemporal trends in bloom severity (i.e., Tominack et al., 2020).
4.4.4. Other NST time series
States along the Gulf Coast, including Florida, Alabama, Mississippi, Louisiana and Texas, conduct routine and event-driven sampling to inform management of shellfish harvest areas and testing for NSTs, and maintain time series data and information about bloom severity. Florida and Texas especially began to monitor more consistently for K. brevis in the mid-1900s with sampling intensity increasing over time (Steidinger, 2009; Tominack et al., 2020). Most of these K. brevis cell count data are available online ( NOAA National Centers for Environmental Information, 2014, https://data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.nodc:0120767), which is updated regularly, and is incorporated into other tools for tracking and predicting K. brevis blooms and their impacts. These include satellite chlorophyll imagery, oceanic and atmospheric models for short-term forecasting of bloom transport and/or respiratory irritation impacts, wildlife mortality investigations (state and federal), and public health reporting systems (Abbott et al., 2009; Stumpf et al., 2009; review by Hu et al., 2015). States around the Gulf of Mexico provide K. brevis abundance data that feeds into a centralized respiratory irritation forecast produced by NOAA (Stumpf et al., 2009; Kavanaugh et al., 2016). Because of the impacts related to these blooms, there are also other records of fish, marine mammal, and other wildlife mortalities maintained by state and federal agencies.
4.4.5. NST impacts in marine fish and wildlife
The most devastating bloom-forming species in the U.S. with respect to diverse effects on wildlife and ecosystems is K. brevis whose blooms produce NSTs that directly affect an extensive range of marine and estuarine organisms, including marine mammals, birds, sea turtles, fish (osteichthyes and chondrichthyes), and invertebrates (Quick and Henderson, 1974; O’Shea et al., 1991, Kreuder et al., 2002; Landsberg et al., 2009, 2014; Flewelling et al., 2010; Twiner et al., 2012;, van Deventer et al., 2012; Fauquier et al., 2013a, b). Even terrestrial organisms can be impacted, such as dogs or coyotes that have ingested beached toxic fish (Castle et al., 2013) or animals exposed to aerosolized NSTs or toxic rainwater (e.g., green tree frogs, spotted ground squirrels; Buttke et al., 2018). Aquatic organisms can be directly exposed to NSTs by ingestion of toxic cells or prey, through absorption of NSTs from the water through the gills, by drinking, or when cells lyse, releasing toxin on the gill filaments (Landsberg, 2002). NSTs are vectored to higher trophic levels through the food chain that can include filter-feeding phytoplankton, zooplankton, fish, and shellfish, through to piscivores or vectored by benthic organisms such as seagrass with toxic epiphytes (Tester et al., 2000; Landsberg, 2002; Flewelling et al., 2005; Fire et al., 2008; Landsberg et al., 2009).
Fish kills associated with K. brevis red tides in the Gulf of Mexico have been documented since 1844 and observed routinely during almost annual red tides in the Gulf of Mexico usually lasting for months (Ingersoll, 1882; Landsberg, 2002; Magaña et al., 2003; Steidinger, 2009). The first dramatic widescale event documenting fish and invertebrate mortalities occurred along the west coast of Florida from November 1946-August 1947 (Gunter et al., 1947, 1948). It is only in prolonged bloom periods lasting for months to over one year (e.g. 2005) where noticeable short-term declines have been noted in finfish communities (Gannon et al., 2009; Flaherty and Landsberg, 2011; Walters et al., 2013), but these data are not reflected in HAEDAT. An almost total recruitment failure of bay scallops, Argopecten irradians, was documented during an unusual K. brevis bloom that impacted North Carolina coastal waters in 1987 (Summerson and Peterson, 1990).
Mortalities of other invertebrates such as shrimp, sponges, sea urchins, and stone crabs often occur during K. brevis blooms (Steidinger et al., 1973; Landsberg, 2002; Gravinese et al., 2018), with documented effects on larval molluscs (Leverone et al., 2006). Sustained persistent blooms can cause ecosystem-wide effects, as cascading toxins and dead bloom biomass influence water quality, leading to hypoxic and anoxic events and increased biological oxygen demand, that together with an increase in hydrogen sulfide from degrading blooms and decomposing dead animals, can cause benthic mortalities. During intense and prolonged K. brevis blooms in 1971 and 2005, entire benthic communities over thousands of km2 in the eastern Gulf of Mexico were effectively wiped out (Smith, 1975; Landsberg et al., 2009; Dupont et al., 2010). Many of these events have not been adequately reported in HAEDAT.
4.4.6. NST-related marine animal mortality trends
Other examples that may show a mortality trend are from K. brevis red tides killing endangered manatees, bottlenose dolphins, and sea turtles in Florida (Fig. 10). Impacts of K. brevis blooms to manatees were first documented in 1963, and the first mass mortality event was reported in 1982. Since 1996, and especially over the last two decades, mortalities of manatees have steadily increased but with some periodicity. This may be attributable in part to an increased population size, but it is also associated with increased frequency and longevity of blooms in coastal areas that manatee frequent. Extensive brevetoxicosis-associated mortalities affecting ~ 1000 manatees occurred in 2002, 2003, 2005–2006, 2007–2008, 2009, 2011-Feb 2012, Oct 2012-May 2013, 2015-April 2016, Oct 2016-May 2017, and Dec 2017-Jan 2019 (Landsberg et al., 2009; Fire et al., 2015; FWC 2020). Bottlenose dolphin mortalities have also been associated with brevetoxicosis (often temporally concurrent with manatee mortality events but not spatially) with ~ 500 hundred deaths reported since 1999 in the Gulf of Mexico, and mass die-offs occurring in 1999–2000, 2004, 2005–2006 (two events in SW and NW FL), and Oct 2007- Jan 2008 (east coast FL) (Van Dolah, 2003; Twiner et al., 2012, Fire et al., 2015). Suspected mortality events of bottlenose dolphins from brevetoxicosis also occurred in 1991 in Florida and 1996 in Mississippi (concurrent with K. brevis blooms) and NSTs were detected at low concentrations in other mortality events with undetermined causes of death in Texas in 2008 and the central Gulf of Mexico from 2010–2014 (Litz et al., 2015).
Fig. 10.
NST-related mortality in manatees from 1990–2019. Dotted line shows the significant regression (p < .05). (Data from FWC, 2020)
For all the documented sea turtle strandings from all causes in the state of Florida from 1986–2013, Foley et al. (2019) reported some 2000 dead endangered loggerheads (Caretta caretta), green turtles (Chelonia mydas), Kemp’s ridleys (Lepidochelys kempii), hawksbills (Eretmochelys imbricata) and leatherbacks (Dermochelys coriacea) from central west Florida (the high frequency zone with combined 100 months of red tide during this time period) as being directly attributable to brevetoxicosis. Statewide during the same years, some 2,900 turtles were killed by red tide. In 2005 (a red tide lasting all year), over a quarter of the turtle strandings statewide were attributed to brevetoxicosis (Foley et al., 2019).
4.4.7. Key drivers
Although much progress has been made in recognizing some drivers associated with recurring K. brevis blooms, the onset, duration, and severity of blooms and NST events are complex and vary from event to event (Vargo, 2009). Laboratory and field studies have been essential in revealing insights into K. brevis bloom dynamics. Unlike many HABs, a resting stage has not yet been described for K. brevis and the life cycle has not been fully resolved. A thin-walled resting cyst in the closely related species Karenia mikimotoi was described by Liu et al. (2020) as being similar to those described for other athecate dinoflagellates. Periodic observations of spherical K. brevis cells have been associated with salinity stress (e.g., Novoveská and Robertson, 2019; Tilney et al., 2019). Even if a resting cyst is found for K. brevis, vegetative cells occur throughout the Gulf of Mexico year-round (Geesey and Tester, 1993; Steidinger, 2009; Weisberg et al., 2019) and theoretically either or both could contribute to bloom initiation.
The low growth rates that K. brevis exhibits in culture suggests that other factors are important in the formation of high biomass blooms (Steidinger, 2009; Vargo, 2009). Ecological flexibility within the Gulf of Mexico seems to be key. Field and laboratory studies show that K. brevis can tolerate a wide range of salinity, temperature, and light conditions (Maier Brown et al., 2006; Vargo, 2009; Soto et al., 2018; Novoveská and Robertson, 2019), has the ability utilize a broad array of nitrogen and phosphorus sources (Lenes et al., 2010; Heil et al., 2014; Richardson and Corcoran, 2015), produces allelopathic chemicals (Kubanek et al., 2005), inhibits grazers (Waggett et al., 2012), and undergoes diel vertical migration (Schaeffer et al., 2009).
Physical forcing has been identified as a key driver of bloom initiation and severity, and thus observed interannual variability. Both Texas and Florida K. brevis blooms typically initiate offshore in late summer or fall; regional hurricane and tropical storm activity typically peak during the same window (i.e., August-October). These stochastic events have impacts on blooms that are difficult to predict and have contributed to localized intensification, expansion to new regions, and dissipation. Blooms of the nitrogen-fixing cyanobacterium Trichodesmium often precede and coincide with K. brevis, and associated nitrogen fixation as well as bloom decomposition potentially act as substantive nutrient sources. Karenia brevis can utilize an array of nutrient sources including those of anthropogenic origins (Heil et al., 2014).
Florida blooms are hypothesized to initiate on the West Florida Shelf, 18–74 km offshore, spanning from north of Tampa Bay to Sanibel (e.g., Steidinger, 1975; Weisberg et al., 2019). Interactions of the Loop Current with the West Florida Shelf are important for inoculating the inner shelf with deeper, nutrient-rich, oceanic water. Interannual variability in the location, duration, and intensity of Loop Current/slope interactions is important for K. brevis bloom severity in that blooms appear to require moderate upwelling but may be inhibited by strong upwelling events, as was observed in 2010 (Liu et al., 2016; Weisberg et al., 2014). Shoreward transport occurs via the bottom Ekman layer under upwelling favorable conditions typical of late summer/fall (Liu et al., 2016; Weisberg et al., 2009). Wind-driven forcing near shore coupled with the vertical swimming behavior of K. brevis can help transport cells to the surface and/or cause cells to aggregate at density fronts (Stumpf et al., 2008). Blooms in Florida typically endure for months to years, are patchy in space and time, and severity varies widely across events (Walsh et al., 2006; Liu et al., 2016).
Initiation in Texas is suspected to occur >15km offshore (Villareal et al., 2001), however, modeling studies point to a distant rather than local source (Hetland and Campbell, 2007; Stumpf et al., 2008; Thyng et al., 2013). Blooms occur regularly along the Mexican coast of the Gulf of Mexico during late summer/fall as well and often coincide with Texas events (Magaña et al., 2003; Núñez-Vázquez et al., 2016). Northeast transport along the shelf from Mexico to Louisiana typically occurs during upwelling-favorable conditions, which start in spring and summer. During fall, the switch to downwelling-favorable winds begins in the north and translates back down the coast, resulting in a reversal to the alongshore flow. Under strong downwelling conditions, a severe bloom is unlikely to develop along the Texas coast (Henrichs et al., 2015; Thyng et al., 2013); blooms are instead prevalent during years when alongshore, southwestern transport is weak. Shoreward Ekman transport—in tandem with vertical migration of K. brevis—helps create a convergence zone that concentrates cells near the coast (Hetland and Campbell, 2007; Thyng et al., 2013). A summary of HAB events in Mexico’s Yucatan coast along the Bay of Campeche from 1996–2014 indicated that NST impacts occurred in 14 of those 18 years, and the Mexican state which borders Texas (Tamaulipas) had the most NST impacts relative to the rest of the Mexican Gulf states (Núñez-Vázquez et al., 2016); fewer events occurred in Texas during this same time (Fig. 6). The timing of many Mexico events relative to the seasonal transition to downwelling favorable conditions along the coast raise the possibility that advection along the U.S.-Mexico coast and/or localized processes are important drivers of blooms there. While genetically distinct populations of K. brevis have been identified over time and sometimes between the eastern and western Gulf of Mexico during years when blooms occur in both areas, there is no consistent indication that Florida and Texas populations are genetically distinct (Henrichs et al., 2013). Weisberg and Liu (2017) and more recently, Yang et al. (2020) highlight how intrusions of the Loop Current into the Gulf of Mexico may reach Texas, potentially providing one possible means of connectivity across these regions.
In the context of climate change, Errera et al. (2014) examined cultures under variable pCO2 and temperature scenarios and did not find a response between pCO2 or temperature and NST content but did find a response between pCO2 and growth rate. Subsequently, Bercel and Kranz (2019) examined K. brevis growth in cultures at a constant temperature under future pCO2 conditions and observed no change in growth, carbon or nitrogen cellular composition, or photosynthetic rates. NST content and pCO2 were positively correlated, however. Both papers ultimately came to same conclusion – K. brevis blooms are likely to persist and NSTs might increase with a warmer, more acidic ocean, so toxin severity could increase. However, circulation changes and more storms in the region, also to be expected with climate change, make it difficult to assess whether conditions will support worsening blooms in existing areas, or whether the distribution of areas experiencing severe blooms will shift or expand.
4.5. CTX and CP
4.5.1. Background
Ciguatoxins (CTX) are a complex group of potent neurotoxins that can accumulate to dangerous levels in coral reef fish and invertebrates, and cause Ciguatera Poisoning (CP) in human consumers of seafood contaminated with these toxins. CTXs are produced by tropical dinoflagellate species in the genera Gambierdiscus and Fukuyoa, which are benthic and epiphytic, living on the surfaces of a variety of macrophytes as well as detritus on dead coral (Parsons et al., 2012; Cruz-Rivera and Villareal, 2006). CTXs enter the coral reef food web via grazing by herbivorous fishes and invertebrates, and can be bioaccumulated and concentrated in fish at higher trophic levels. Toxin levels are frequently highest in carnivorous reef fishes, many of which are targeted by commercial and recreational fisheries, although a wide variety of species have the potential to be ciguatoxic. The CTXs associated with ciguatera are chemically diverse compounds composed of a ladder shape polyether structure, and differences in the number of ether rings and backbone structure are regionally specific (reviewed by Soliño and Costa, 2018). Of the more than 30 CTX congeners identified thus far, the main Pacific CTX (P-CTX-1) is most potent, producing clinical symptoms in humans at levels >0.08 ng P-CTX-1 g−1 fish flesh (Lehane and Lewis, 2000; Pasinszki et al., 2020). The main Caribbean CTX (C-CTX-1) is approximately ten-fold less potent than P-CTX-1. The U.S. FDA has proposed advisory levels of 0.10 ppb C-CTX-1 equivalent toxicity in fish from the tropical Atlantic, Gulf of Mexico and Caribbean, and 0.01 ppb P-CTX-1 equivalent toxicity in fish from Pacific regions (U.S. FDA, 2019). In addition to CTXs, some species of Gambierdiscus and Fukuyoa have been shown to produce other polyether compounds such as maitotoxins (MTXs), gambierol, gambieric acids, and gambierone, but their contribution to CP is not established (Soliño and Costa, 2020).
Taxonomic studies have identified two Fukuyoa species and at least 18 species within the Gambierdiscus genus, many of which co-occur and differ significantly in toxicity. At least ten of these species have been found in tropical and subtropical regions of the U.S., including Hawai’i, the Gulf of Mexico, the Florida Keys, Puerto Rico, and the U.S. Virgin Islands (Table 1). Not all species are globally distributed, and this includes the most toxic species identified thus far (Chinain et al., 1999, 2010; Longo et al., 2019; Robertson et al., 2018). For example, G. polynesiensis has been reported in the Pacific (including Hawai’i) but not in the Caribbean, while G. silvae and G. excentricus have been found at locations in the Caribbean and Gulf of Mexico, but not in the Pacific or Indian Oceans. Several species – G. carpenteri, G. caribaeus, G. carolinianus– are widely distributed, and have been found at locations in both the Pacific Ocean and Caribbean Sea. Details regarding the current geographic distribution of Gambierdiscus species are reviewed by Chinain et al. (2020).
4.5.2. History of CTX and CP
CTXs have been found in fish from tropical or subtropical areas globally, and are endemic to coral reef ecosystems between 35° north and 35° south latitude. In the U.S., CP caused by CTX afflicts hundreds to thousands of people annually (Friedman et al., 2017), and outbreaks are most severe in Florida (Radke et al., 2015), the U.S. Virgin Islands (Radke et al., 2013), Puerto Rico (Azziz-Baumgartner et al., 2012), and Hawai’i, as well as U.S. affiliated territories (Copeland et al., 2014; Skinner et al., 2011). There have also been sporadic reports of CTX and CP from temperate waters of the U.S., and in 1987 ten cases of CP were reported from North Carolina during an outbreak involving several fish species, including barracuda (Pottier et al., 2001). CTXs are known to occur in the northwestern Gulf of Mexico, and have been found in fish caught near oil production platforms, which historically have not been regarded as risky areas for CP (Villareal et al., 2007). In addition to detecting CTX in fish, this study also found Gambierdiscus dinoflagellates in samples collected from the fouling community present on the platforms and from algae collected at these sites. In 2008, CP outbreaks were linked to grouper and amberjack species caught near the Flower Garden Banks National Marine Sanctuary in the northern Gulf, prompting the U.S. FDA to issue guidance identifying several species of concern (e.g., hogfish, grouper, snapper, amberjack) for which ciguatera is a “reasonably likely hazard” when captured near the Sanctuary.
Of all the poisoning syndromes associated with HAB toxins, CP caused by CTX is the most prevalent globally, and is estimated to afflict tens of thousands of people each year, with many cases unreported. Unlike many other HAB poisoning syndromes, CP is not associated with large scale blooms of the causative organism, and monitoring programs to test fish for CTXs have been hindered by complex toxin chemistry and involvement of multiple toxins, as well as the lack of commercially available toxin standards and affordable and practicable methods for toxin detection. In the absence of monitoring programs, prevention of CP largely relies on avoidance of high-risk carnivorous fish species most commonly associated with outbreaks (e.g., barracuda, snapper, grouper) as well as local ecological knowledge regarding the distribution of toxic fish, which can function as an informal type of “closure,” in which high risk locations are avoided.
4.5.3. CTX and CP trends
Although CP has been endemic to certain regions of the U.S. for decades, there are no sustained monitoring programs for Gambierdiscus or CTX, and HAEDAT datasets do not exist to establish time series trends. In the absence of routine monitoring, prevalence and trends for CTXs and CP can only be derived from state health department records, emergency room reports, and other local systems used in CP surveillance. Obtaining complete data on CP is difficult and is an undertaking far beyond this current effort.
The scientific literature includes several efforts to examine CP incidence at local or regional levels in an effort to establish time series trends, and specifically to test hypotheses regarding climate-driven warming on CP incidence and extent. Based on these studies, CP and CTXs appear to be increasing in some locations where it is endemic, but declining in others. For example, Radke et al. (2013) found a possible decline in CP incidence on St. Thomas, USVI over a 30-year period (1980–2010) based on a review of emergency room reports and household surveys, despite the steady increase in sea surface temperatures (SSTs) during that same period. Similarly, CP incidence was estimated over a ten-year period in Florida based on an analysis of reports to the Florida Department of Health (FDOH), combined with a survey of recreational fisherman (Radke et al. 2015). This study also found that CP may have declined over the ten-year period. The study also determined, however, that the majority of possible cases of CP are never reported to the FDOH, illustrating the potential biases in current surveillance systems and the need for data-driven estimates of underreporting to more accurately determine prevalence and impact of CTXs and CP (Radke et al., 2015).
There is recent evidence of geographic range expansion of Gambierdiscus and CP into higher latitudes in U.S. waters, associated with climate-driven warming. Over the past two decades CP and CTXs were reported for the first time in the northern Gulf of Mexico, and ephemeral populations of Gambierdiscus have also been documented in North Carolina. Prolonged periods of elevated water temperatures at these locations and in northern Florida are hypothesized to result in increased Gambierdiscus and Fukuyoa cell densities, and in turn, increased CTX flux.
In addition to shifts in CTX range, CP events throughout the U.S. are likely to increase due to expanding seafood trade (supplying retail markets or restaurants), and importation of ciguatoxic fish to temperate and land-locked locations for local consumption. CP outbreaks have already been reported from multiple non-endemic locations such as Illinois, Maryland, Massachusetts, New York, North Carolina, Vermont, District of Columbia, Texas, Kansas, and California (Vogt, 1985; Juranovic, 1989; Barton et al., 1995; Graber et al., 2013).
4.5.4. Key drivers
Key drivers of CTX events and CP risk are not fully understood, but involve a combination of: the species composition of Gambierdiscus and Fukuyoa populations, particularly the presence of highly toxic species, and the environmental conditions that promote CTX production and/or foster growth of toxigenic strains and species. Additionally, patterns of toxicity and bioaccumulation are affected by CTX uptake by herbivorous fishes and invertebrates, and trophic biotransformation and transfer in coral reef food webs.
Temperature is a key factor controlling CTX occurrence, and laboratory experiments investigating the influence of temperature on growth of various Gambierdiscus species have shown that most species exhibit maximum growth rates between 24–30°C, but that growth responses varied by species (Kibler et al., 2012; Xu et al., 2016). Thus, higher temperatures may favor growth of more thermally tolerant species (e.g., G. belizeanus and G. caribaeus) in ciguatera endemic regions, and promote establishment of species shown to be less tolerant (e.g., G. silvae) in subtropical and temperate regions. Warm surface temperatures may also shift the Gambierdiscus and Fukuyoa populations to deeper waters.
Natural and anthropogenic damage to reef ecosystems have also been implicated in CTX events, such as public or military construction activities involving dredging, explosives, dumping of materials; coral bleaching, shipwrecks, and hurricanes (Randall, 1958; Ruff, 1989; Hales, 1999; Lehane and Lewis, 2000). As the areal distribution and abundance of Gambierdiscus and Fukuyoa dinoflagellates is largely a function of algal cover, it was hypothesized that disturbances create free space on dead coral that is colonized by filamentous algae, enabling the proliferation of epiphytic dinoflagellates (Randall, 1958; Yasumoto, 1977; Yasumoto, 1980; Kohler and Kohler, 1992).
Few studies have examined these linkages directly, however. In the U.S., Gingold et al. (2014) examined the association between CP incidence and warming SSTs as well as tropical storm frequency in the Caribbean Sea, based on calls made to U.S. National Poison Control Centers over a ten-year time frame. These studies found that monthly CP calls to poison control centers in the continental U.S. were associated with both tropical storm frequency and peak SST in the Caribbean basin, with SST increases having a stronger association with CP compared with the effect of storms. Additional data on cases with confirmed disease, perhaps focused on high incidence areas, are needed to explore these findings further, and to pinpoint the proper lag time between weather and SST disturbance and CP.
4.6. Other HAB species
4.6.1. Brown tides
4.6.1.1. Background
Brown tides are caused by the pelagophytes Aureococcus anophagefferens Hargraves et Sieburth and Aureoumbra lagunensis DeYoe et Stockwell. Although Aureococcus and Aureoumbra are genetically distinct (DeYoe et al., 1995), they both are small (4–5 μm for Aureoumbra and 2–3 μm for Aureococcus), spherical, non-motile cells (DeYoe et al., 1997; Gobler and Sunda, 2012). Neither alga had been described prior to the onset of HABs in the U.S. caused by each species and both were originally assigned to the class Chrysophyceae (Sieburth et al., 1988; Buskey and Stockwell, 1993), although later examination led to their formal placement in a new class, Pelagophyceae (DeYoe et al., 1997).
Brown tides are considered ecosystem disruptive, as they are harmless to humans but are damaging to marine life and habitats. Brown tides are high biomass blooms with concentrations exceeding 106 ml−1, which result in increased light attenuation and the destruction of seagrass meadows (Zostera marina; Cosper et al., 1987; Onuf, 1996; Gobler et al., 2013). Both pelagophytes produce extracellular polysaccharides which adversely impact larval, juvenile and adult hard clams (M. mercenaria; e.g., Bricelj et al., 2001; Newell et al., 2009), larval and adult bay scallops (A. irradians; Bricelj and Kuenstner, 1989; Gallager et al., 1989), adult and juvenile blue mussels (Mytilus edulis; Bricelj and Kuenstner, 1989; Bricelj et al., 2001), and dwarf surf clams Mulinia lateralis (Montagna et al., 1993). Brown tides are also harmful to micro- and mesozooplankton (e.g., Buskey and Stockwell, 1993; Lonsdale et al., 1996; Smith et al., 2008). Aureococcus has been shown to negatively affect bivalves at cell densities > 3.5 × 104 cells ml−1 (Bricelj et al., 2001).
4.6.1.2. History of brown tides
The first A. anophagefferens blooms in the U.S. occurred simultaneously in the summer of 1985 in Narragansett Bay, Rhode Island, Great South Bay, and the Peconic Estuary on Long Island, New York, and putatively in Barnegat Bay, New Jersey (Fig. 11; Sieburth et al., 1988; Cosper et al., 1989; Olsen, 1989). During the 1990s, blooms expanded south along the U.S. East Coast into bays in New Jersey (Fig. 11; Barnegat Bay; Gastrich et al., 2004), Delaware (Fig. 11; Little Assawoman Bay; Popels et al., 2003), Maryland, and Virginia (Fig 10; Chincoteague Bay; Trice et al., 2004; Glibert et al., 2007; Mulholland et al., 2009a). Low abundances of A. anophagefferens cells have been observed along the entire eastern seaboard of the U.S. from Maine to Florida (Anderson et al., 1993; Popels et al., 2003).
Fig. 11.
Yearly maximum concentrations of Aureococcus anophagefferens in: A. Narrangansett Bay (Rhode Island, RI), B. Peconic Estuary (New York, NY) C. the south shore estuaries (New York, NY), D. Barnegat Bay (New Jersey, NJ), and E. Chincoteague Bay (Maryland, MD), and Aureoumbra lagunensis in F. eastern Florida estuaries (FL) and G. Baffin Bay (Texas, TX) from 1985 – 2018. ND indicates no detection of brown tide alga. ‗Data not available’ means no data was collected / no data exists. In cases where there is no bar and no ND or ‘data not available’, the densities were detectable but low. Data sources for: Rhode Island (Anderson et al., 1993; Sieburth et al., 1988), all New York estuaries (Suffolk County Department of Health Services, 1985–2018; C.J. Gobler, unpublished), New Jersey (Anderson et al., 1993; Bricelj et al., 2017; Mahoney et al., 2003; M.D. Gastrich, unpublished), Maryland (Maryland Department Nuzzi et al., 1996;of Natural Resources, 2009–2018; Trice et al., 2004), Florida (St. Johns River Management District, 2012–2018), and Texas (Buskey et al., 1997, 1999, 2001; Villareal et al., 2004; Wetz et al., 2017; M. Wetz, unpublished).
The first A. lagunensis brown tide bloom began in shallow lagoons in and around the Laguna Madre and Baffin Bay, Texas in January 1990 and subsequently persisted for nearly eight years, making it the longest continuous harmful algal bloom (HAB) event on record (Fig. 11; Buskey et al., 1997, 2001). Surveys across the Gulf of Mexico have detected low densities of A. lagunensis through the entire Laguna Madre south into Mexico as well as in southern Florida, specifically Florida Bay, but did not detect cells in Mississippi or along the northeast Texas coast (Villareal et al., 2004).
4.6.1.3. Brown tide time series
Since the onset of brown tides caused by A. anophagefferens, they have vanished from some ecosystems but have been annual occurrences in other locales. In Rhode Island’s Narragansett Bay, the brown tide of 1985 never returned (Fig. 11A; Smayda, 2008). In New York, brown tides occurred in the Peconic Estuary from 1985–1998 but have not occurred since (up to 104 ml−1; Fig. 11B; Nuzzi and Waters, 2004). In contrast, Aureococcus form dense blooms annually within the south shore lagoons of Long Island, with the intensity and frequency from 2007–2018 surpassing the blooms from 1985–2005 (Fig. 11C; Great South, Moriches, Shinnecock, and Quantuck Bays; Gobler et al., 2011). In New Jersey, monitoring has been inconsistent (Fig. 11D) but dense blooms (> 105 cells mL−1) were recorded from 1998–2001 (Gastrich et al., 2004). Sporadic monitoring since then has revealed more modest densities (104–105 cells mL−1; Bricelj et al., 2017). In Maryland, dense blooms have been occurring near annually in Chincoteague Bay since the mid-1990s (Fig. 11E). Brown tides were more intense and consistent in Maryland during the decade of 1999–2008 than the decade since, during which there have been only two events with densities exceeding 106 cells mL−1 (Fig. 11E). This system represents the southern extent of A. anophagefferens blooms, perhaps partly due to warmer temperatures and lower salinities of lagoons south of this locale.
The first ever brown tide caused by A. lagunensis persisted from late 1989 through the fall of 1997 and then recurred during the summer and fall of 1998 in Laguna Madre and Baffin Bay, Texas (Buskey et al., 1997, 2001). Reporting of blooms was sporadic from 1999–2010, with at least one bloom occurring during that period (Fig. 11G; Wetz et al., 2017). Since 2012, the Wetz laboratory (Texas A&M University) has surveyed Baffin Bay annually and has reported dense blooms (105–106 cells mL−1, Fig. 11G) annually. Over the past decade, the geographic extent of A. lagunensis has greatly expanded, with blooms in the northern Indian River Lagoon and Mosquito Lagoon on the east coast of Florida starting in 2012 (Gobler et al., 2013). This was the furthest east these blooms had ever been observed and the densest brown tides ever recorded, with cell concentrations exceeding 3 × 106 cells mL−1 in 2012 and again in 2016 (Fig. 11F). Interestingly, in the year after the expansion to eastern Florida, A. lagunensis blooms were noted in Cuba for the first time (Koch et al., 2014a).
4.6.1.4. Key drivers
Although harmful algal blooms in coastal waters have been commonly attributed to nutrient loading, the role of nutrients in the occurrence of brown tides appears to be more complex than a simple excess inorganic nutrient stimulation of blooms (Sunda et al., 2006; Gobler and Sunda, 2012). Multiple lines of evidence demonstrate that A. anophagefferens is at a competitive disadvantage when the concentrations of inorganic nitrogen are elevated and instead blooms form when inorganic nutrient levels are low (Cosper et al., 1989; LaRoche et al., 1997; Keller and Rice, 1989; Gobler and Sanudo-Wilhelmy, 2001a; Gobler et al., 2002, 2004a,b; Taylor et al., 2006) due to its superior ability to utilize organic forms of C, N, and P (Berg et al., 2002; Mulholland et al., 2002, 2009a; Gobler et al., 2004, 2011; Kang et al., 2017). Similar conclusions have been drawn regarding A. lagunensis (Buskey et al., 1997; Muhlstein and Villareal, 2007; Sunda and Hardison, 2007, 2010) although there has been some evidence that elevated ammonium levels might favor this alga (Buskey et al., 1997; DeYoe et al., 2007; Kang et al., 2015).
Regarding physical drivers, brown tides occur in estuaries, mainly lagoons, with long residence times (weeks-months; Gobler and Sunda, 2012; Cira and Wetz, 2019) that permit the accumulation of high algal biomass (106 cells mL−1) and low light conditions (Gobler and Sunda, 2012; Cira and Wetz, 2019). Both brown tide species achieve near-maximum growth rates at low light levels (50 μmol photons m−2 s−1, ~ 2% of noon solar irradiance; MacIntyre et al., 2004; Sunda and Hardison, 2010) which are common during dense blooms (Gobler and Sunda, 2012). Within the sub-tropical estuaries of Texas and Florida, A. lagunensis blooms have been associated with hypersaline conditions (40 – 90 PSU; Buskey et al., 1998; Gobler et al., 2013) that may inhibit algal grazers and competing algal species (Buskey et al., 1998; Gobler et al., 2013; Cira and Wetz, 2019). As warming accelerates during this century, hypersalinity is likely to be a more frequent occurrence in sub-tropical and tropical estuaries with extended residence times, favoring the occurrence of A. lagunensis blooms. This may have contributed to the expansion of this species to Florida and Cuba during the past decade (Gobler et al., 2013; Koch et al., 2014). Alternatively, warming may restrict the distribution and intensity of A. anophagefferens blooms which have a significantly lower upper temperature threshold compared to A. langenensis (24°C v 32°C; Gobler and Sunda, 2012). These warmer temperatures may be driving A. anophagefferens blooms to occur and end earlier in ecosystems that commonly exceed this temperature, including those in New York, New Jersey, and Maryland.
Zooplankton grazing rates on brown tides are typically low due to unpalatability, toxicity, and/or physical interference with grazing, contributing to the initiation and persistence of blooms (Buskey et al., 1997; Gobler et al., 2002; Sunda et al., 2006; Smayda, 2008). Similarly, brown tides tend to inhibit filter feeding by bivalves in the shallow systems they occur (Bricelj and Kuenstner, 1989; Buskey et al., 1997; Bricelj et al., 2001; Harke et al., 2011) and an absence of benthic feeding may also enhance blooms (Cerrato et al., 2004). Grazing communities can evolve resistance to harmful algae over time via natural selection processes (Hairston et al., 2002), and the selective impact of blooms on more sensitive phenotypes within populations (Caron et al., 2004) could eventually lead to the establishment of grazer communities that are better adapted to co-exist with and consume A. anophagefferens. Recent studies have shown that in some New York estuaries a shift occurred in which zooplankton communities that formerly consumed Aureococcus at low rates during massive blooms have shifted to communities that actively graze Aureococcus at rates similar to those for other algal species (Deonarine et al., 2006). Similarly, although Aureococcus inhibits grazing by mollusks, the filter feeding slipper limpet, Crepidula fornicata, robustly grazes Aureococcus (Harke et al., 2011). This limpet is now present at high abundance in estuaries that no longer experience brown tide blooms (e.g., the Peconic Estuary; Harke et al., 2011). Thus, the recent abatement or reduced magnitude of brown tides in some estuaries (Fig. 11) may, at least partly, reflect a shift in the grazing communities toward populations more capable of feeding on A. anophagefferens. Finally, allelopathic interactions with other phytoplankton may facilitate the persistence of brown tide blooms, as both A. anophagefferens and A. langenensis have recently been shown to significantly reduce the cell densities and growth rates of multiple species of co-occurring phytoplankton (Kang and Gobler, 2018).
4.6.2. Margalefidinium/Cochlodinium
Dinoflagellates of the genus Cochlodinium were first identified in 1895 by Schütt (1895) and two species, C. polykrikoides and C. fulvescens, have been forming harmful algal blooms on the U.S. East Coast and West Coast, respectively, for several decades. These HAB species were recently renamed M. polykrikoides and M. fulvescens by Gomez et al. (2017) due to ribosomal DNA sequence differences from the type-species, Cochlodinium strangulatum. Blooms of M. polykrikoides have caused >$100M in fisheries losses in Korea, the Gulf of Oman, and Canada (Kim, 1998; Whyte et al., 2001; Richlen et al., 2008) and have caused fish and wild and aquacultured shellfish kills in the U.S. (Gobler et al., 2008; Kudela and Gobler, 2012; Griffith et al., 2019a). Blooms of M. polykrikoides are macroscopically and microscopically distinctive but are also highly ephemeral and horizontally and vertically heterogenous (Koch et al., 2014b). Consequentially, consistent long-term monitoring records of these HABs in the U.S. are rare, and as such, the assessment here covers published occurrences of blooms that can be interpreted as reliable due to the visually distinctive nature of these HABs, commonly referred to as ‗rust tides’.
4.6.2.1. History and trends
The first reported blooms of M. polykrikoides were on the U.S. East Coast in Barnegat Bay, New Jersey (Silva, 1967), the York River, Virginia (Ho and Zubkoff, 1979), and Greenwich Cove, Rhode Island (Tomas and Smayda, 2008) in the 1960s, 1970s, and 1980s, respectively. Since these reports, there has been a significant expansion of blooms reported along the U.S. East Coast. On Long Island, New York, M. polykrikoides has formed dense blooms in the Peconic Estuary and Shinnecock Bay annually since 2004 through 2020 (Gobler et al., 2008; Tang and Gobler 2010; C. Gobler pers. obs.) and has recently (since 2011) expanded across Long Island into Great South Bay and Long Island Sound (since 2015; C. Gobler, pers. obs.). There were no reports of this alga prior to 2004 on Long Island despite robust HAB monitoring programs established decades prior (e.g. SCDHS, 1976-2020). To the north of this region, blooms have been observed this century in Point Judith Pond and Narragansett Bay, Rhode Island (Hargraves and Maranda, 2002). Within Buzzards Bay (Massachusetts), M. polykrikoides blooms were not observed until 2005, but now are observed annually (Rheuban et al., 2016). Since 2010, blooms of M. polykrikoides have emerged within both Martha’s Vineyard and Nantucket Sound (Massachusetts; Leavitt et al., 2010). To the south of this region, blooms of M. polykrikoides had been sporadic events in the York River, Maryland, (Ho and Zubkoff, 1979) but have recently become regular, annual events within multiple tributaries of Chesapeake Bay in Maryland and Virginia (Marshall et al., 2005; Marshall, 2009; Mulholland et al., 2009b; Morse et al., 2011, 2013). Blooms of M. polykrikoides have also been newly reported in the southeast U.S. specifically within the Skidaway Estuary (Georgia, U.S.A; Verity, 2010) and the Indian River Lagoon (Florida; Philips et al., 2011). On the West Coast of North America, Margalefidinium spp. cells had been observed in southern California coastal waters during the twentieth century (i.e., La Jolla Bay, California; Kofoid and Swezy,1921: Holmes et al., 1967). Since that time, Curtiss et al. (2008) and Kudela et al. (2008) reported annual M. fulvescens blooms in Monterey Bay (California), with recurrent sightings across central and Southern California since 2004. While many of these studies have been strictly observational, investigations in Virginia, New York, and Massachusetts have confirmed the ability of M. polykrikoides to cause mass kills of fish and shellfish (Gobler et al., 2008; Mulholland et al., 2009b; Levitt et al., 2010; Li et al., 2012 Griffith et al., 2019a).
4.6.2.2. Key drivers
Given the striking visual attributes of M. polykrikoides blooms (large, dense, surface patches of rust-colored water), the occurrence of these events in multiple new regions on the U.S. East Coast during the past two decades seems likely to be a real phenomenon rather than a function of improved methods of detection. Several potential drivers for these trends have emerged. First, it has been discovered during the past decade that M. polykrikoides populations on the U.S. East Coast produce cysts in culture (Tang and Gobler, 2012) and cysts beds have been identified within estuaries that experience recurrent blooms (Hattenrath-Lehmann et al., 2016) suggesting anthropogenic and natural transport of cysts could facilitate the spread of this HAB (Smayda, 2007). Nitrogen loading has been associated with the intensification of M. polykrikoides blooms in New York and Virginia (Gobler et al., 2012; Morse et al., 2011, 2013). Finally, the warming of surface waters since 1982 has yielded significant increases in potential growth rates of M. polykrikoides and bloom season duration in coastal zones from Chesapeake Bay through Cape Cod, areas where blooms have become newly established and/or intensified this century (Fig. 12; Griffith et al., 2019b).
Fig. 12.
Change in the duration of the bloom season (days per year) from 1982 to 2016 for M. polykrikoides (American/Malaysian ribotype) along the US East Coast. Stippling (dots) indicates regions where trends were statistically significant (p<.05; Mann–Kendall test). Further analytical detail appears in Griffith et al., 2019b.
4.6.3. Heterosigma
4.6.3.1. Background
The motile raphidophyte Heterosigma akashiwo (=H. carterae) is known for its dense surface blooms, often leading to water discoloration. In the U.S., it occurs in the Gulf of Mexico and on both the East and West Coasts, though it is considered harmful predominantly on the West Coast due to its effects on fish farms. Reports of wild salmon and marine aquacultured fish mortalities due to blooms of this species have been reported primarily in Washington State inland waterways (Hershberger et al., 1997; Horner et al., 1997). Farmed fish are particularly vulnerable when winds or currents move blooms into penned areas where the fish are unable to escape. Consequently, H. akashiwo has caused the death of salmonids held in net pens in Puget Sound since at least 1976 (Rensel, 2007; Rensel et al., 1989). These HABs were considered a serious risk to site development for new net-pen facilities, particularly given that as few as 500 cells/L can cause fish deaths when cells are expressing toxicity (Horner, 1998). The salmon aquaculture industry in Washington State has suffered economic losses of ~ $2 to 6 million per episode due to H. akashiwo blooms. Recent problems with net pen collapse have led to the prohibition of aquacultured salmon operations in Puget Sound as of 2017, thus records of mortalities due to H. akashiwo are no longer available. The most complete timelines of H. akashiwo occurrence and associated aquacultured fish mortalities are recorded in nearby Canadian inland waterways (see Table 2 in Rensel et al., 2010).
4.6.3.2. Key drivers
Heterosigma akashiwo is considered a mixotroph as it can supplement its normal phototrophic physiology and nutrition through the ingestion of bacteria and other particles (Jeong, 2011). It also has a cyst stage that allows it to persist in a region when conditions are not suitable for growth (Imai and Itakura, 1999). In the Puget Sound region of the U.S., H. akashiwo blooms have been more frequently associated with specific times of year (early July and mid-September), warm water temperatures (>15 °C) and higher than normal riverine discharge from snowmelt, resulting in reduced salinity and a stable mixed layer in the coastal zone (Taylor and Haigh, 1993; Rensel, 1995; Connell and Jacobs, 1997; Li and Smayda, 2000; Anderson et al., 2001). In north Puget Sound, blooms are believed to initiate in the southern Strait of Georgia and U.S. waters north of San Juan Island in association with the brackish plume of the Fraser River in Canada (Rensel, 1995, 2007). The Fraser River, which empties into the southernmost portion of the Strait of Georgia, is the largest river contributing freshwater (Mackas and Harrison, 1997) to the Strait of Georgia, Puget Sound and Strait of Juan de Fuca region. Its effects on these marine waters and circulation therefore are pronounced, as H. akashiwo blooms in North Puget Sound and the Strait of Georgia have been strongly associated with its larger than normal discharge (Rensel, 2007).
4.6.4. Karenia mikimotoi
Blooms of Karenia mikimotoi have been observed on both the U.S. East and West Coasts. This species is globally distributed and is not known to produce toxins that impact human health or wildlife. It has, however, been associated with widespread fish and benthic mortality events. Although K. mikimotoi occurs regularly in the Gulf of Mexico, blooms or related impacts have not been identified in those waters (Villac et al., 2020). A high-density bloom occurred in Alaska in 2013 that caused water discoloration and fish kills, but was not detected in preceding or following years and the source is not known (Vandersea et al., 2020). This species also bloomed in the Gulf of Maine in 2017 at levels of 107 cells/L, and has been observed there in subsequent years. Recently, a resting stage was identified for K. mikimotoi (Liu et al., 2020), and thus the observation of recurring blooms in the Gulf of Maine could be a result of cyst germination giving rise to localized populations, though advection from another region can’t be ruled out. Genetic differentiation has been observed among geographically distinct populations, which could be a useful diagnostic tool to better understand potential linkages with source populations, especially in areas with emerging events.
4.6.5. Effects of other marine HABs on fish and wildlife
From the 1930s onwards, sporadic kills affecting diverse estuarine and marine fish and invertebrates in the Gulf of Mexico (Texas) and in the Indian River Lagoon, east coast Florida were associated with Alexandrium monilatum (subsequently determined to produce goniodomin, but its role in kills was unknown) (Gunter, 1942; Howell, 1953; Wardle et al., 1975, Landsberg, 2002). Since 2007, this species has bloomed almost annually in the Chesapeake Bay (Wolny et al., 2020) and in that same year was associated with a mortality of veined rapa whelk, Rapana venosa, presumptively attributed to exposure to goniodomin causing respiratory paralysis (Harding et al., 2009). Takayama pulchella was associated with fish and invertebrate kills in the Indian River Lagoon in 1996 (Steidinger et al., 1998) and in 2004. A large shellfish kill in Maine in 1988 was linked to Gymnodinium aureolum, and attributed to a combination of low dissolved oxygen, possible toxicity, and production of mucus (Shumway, 1990; Heinig and Campbell, 1992). Lingulodinium polyedra (producing yessotoxins) had previously been associated with fish and invertebrate kills in California (Torrey, 1902), and a fish kill in 2003 in the same state was reported to HAEDAT, although a direct role for the toxins is unproven. Extensive study on Karlodinium veneficum toxicity along the U.S. Atlantic eastern seaboard has shown multiple strains producing polyketide karlotoxins that are cytotoxic, hemolytic, and ichthyotoxic (Deeds et al., 2004, Van Wagoner et al., 2008; Bachvaroff et al., 2009; Place et al., 2012). Karlotoxins bind to 4-desmethyl sterols (e.g., cholesterol) on cell bilayer membranes causing pore formation in epithelial and chloride cells in fish gills leading to respiratory dysfunction and consequent acute mortalities, as well as effects on shellfish larvae (Deeds and Place, 2006; Deeds et al., 2006). As Karlodinium is a small unarmored dinoflagellate similar to Pfiesteria, multiple fish kills attributed to the latter from 1991–1996 in North Carolina (Burkholder and Marshall, 2012) are listed in HAEDAT as Karlodinium. HAEDAT has eight fish mortality events attributed to Karlodinium, but some fish kills that were reported in the 1990s as Pfiesteria are not in the database. Karlodinium has been reported killing diverse species of estuarine fish in mid-Atlantic coastal waters: Chesapeake Bay rivers and tributaries, aquaculture facilities in Maryland, the Neuse and Pamlico Rivers, North Carolina, and in brackish retention ponds in South Carolina (Deeds et al., 2002, 2006; Kempton et al., 2002; Goshorn et al., 2004), with increasing blooms of K. veneficum and sporadic fish kills since the mid-2000s in the Chesapeake Bay (Wolny et al., 2020). Not all events are in HAEDAT.
Periodically, other HAB species are occasionally associated with a fish kill. In some cases, toxicity is suspected but not known or kills are attributed to poor water quality because most algal species at high biomass have the potential to be associated with low dissolved oxygen. Examples of diverse fish kills in HAEDAT are from the dinoflagellates Prorocentrum micans causing a fish kill in New Jersey in 1968, P. minimum and fish kills in Louisiana in 2001 and the Potomac River, Chesapeake Bay in June 2016, Katodinium rotundatum fish and shellfish kills in New Jersey in 1993 and 2000, and Levanderina fissa associated with fish and crab die-off in Colonial Beach, Virginia in 2019.
Diatom blooms, mostly in the Pacific Northwest, have been attributed to fish kills, both natural and in aquaculture net pens. Usually intense blooms of Chaetoceros, Skeletonema, and Rhizosolenia with morphological structures such as spines and setae can clog fish gills, causing mechanical irritation, and puncturing of the surface gill lamellae can lead to secondary bacterial infections. Fish trapped in the confines of net pens can succumb fairly quickly if the pens are not moved (Landsberg, 2002). A mixed bloom of Ceratium spp., Thalassiosira, and Nitzschia sp. in Kodiak Island, Alaska in September 2000 was reported to HAEDAT and associated with mortalities of flounder, dolly varden, and sea lance. Fish kills involving a mixed algal bloom of Skeletonema costatum, Thalassiosira gravida, T. nordenskioeldii, Cerataulina pelagica, Rhizosolenia delicatula, Olisthodiscus luteus, Katodinium rotundatum, Prorocentrum spp., and Eutreptia lanowii in Raritan and Sandy Hook bays, New Jersey in late May-June and July 1995 and reported to HAEDAT, were attributed to associated low dissolved oxygen.
Haptophytes, primarily P. parvum, are the biggest issue in brackish waters in Texas, with occasional reports in other southern states. Only four events are documented in the HAEDAT database, with three in Texas (2002, 2003, 2011), and one in Vero Beach, Florida (2004), but additional widespread reports have been documented since the first associated fish kill in 1985 in the Pecos River, Texas (James and De La Cruz, 1989; Roelke et al., 2016). Multiple toxins or bioactive compounds characterized as prymnesins and polyunsaturated fatty acids have been implicated in Prymnesium “ichthyotoxic” properties. As a low salinity tolerant species, the spread of P. parvum blooms in inland, south-central U.S. was attributed to drought years and increased salinities of various rivers and lakes (Roelke et al., 2016) with enhanced risks from water management activities projected to boost the formation of coastal blooms (Lundgren et al., 2015).
In the 1970s-1980s, raphidophytes, principally Heterosigma akashiwo were associated with fish kills, primarily of aquaculture fish (salmonids) in the Pacific Northwest. A kill was also associated with H. akashiwo in 1994 in Puget Sound (see 4.6.3.1). There are several reports in HAEDAT of Chattonella-associated fish kills in Virginia (C. subsalsa, 2010), New Jersey (Chattonella spp., 2017) and one in Core Sound, North Carolina in 2001. A closely-related organism (formerly reported as Chattonella cf. verruculosa) was involved in ten fish mortality events, killing over a million fish, primarily menhaden, in Rehoboth Bay, Delaware in 2000 (Bourdelais et al., 2002).
In 1984 (Harper and Guillen, 1989), 1994, 2003, and 2007 (HAEDAT), blooms of the dinoflagellate Akashiwo sanguinea (formerly Gymnodinium sanguineum) were occasionally associated with fish kills in the Gulf of Mexico and attributed to consequential poor water quality. In 2007, during a large-scale bloom, some 14 seabird species were killed in Monterey Bay, California, demonstrating for the first time that at high biomass, A. sanguinea could produce vast quantities of proteinaceous foam. The biosurfactant foam coated the birds’ feathers and affected their natural water repellency and insulation. Being unable to maintain body temperature and forage, seabirds such as Pacific loons, Gavia pacifica, red-throated loons, G. stellata, Clark’s grebes, Aechmophorus clarkii, and western grebes, A. occidentalis, became hypothermic and emaciated. Of 550 birds stranded alive, almost half were northern fulmar, Fulmarus glacialis (Jessup et al., 2009). In the fall of 2009, another unusual A. sanguinea bloom further north along the U.S. Pacific Northwest coast (Du et al., 2011; White et al., 2014), affected several hundred loons, Gavia immer, common murres, Uria aalge, Pacific loons, red-throated loons, surf scoters, Melanitta perspicillata, and western and Clark’s grebes causing a widescale dieoff (Phillips et al., 2011).
4.6.6. Cyanobacteria
While harmful cyanobacterial blooms (CHABs) have been reported in the scientific literature for more than 130 years (Francis, 1878), in recent decades, the incidence, intensity, and impacts of these blooms has increased in freshwater and marine systems (Hudnell, 2008; Paerl and Huisman, 2008; O’Neill et al., 2012). CHABs most commonly occur in water bodies that experience accelerated nutrient loading and can be promoted by elevated temperatures (O’Neill et al., 2012). Every state in the U.S. experiences CHABs, and while these blooms can be harmful by causing hypoxia, turbidity, and general ecosystem disruption, they also represent a serious human and animal health threat via their synthesis of hepatotoxins (microcystin, nodularin, cylindrospermopsin) and neurotoxins (anatoxin-a, saxitoxin; Paerl et al., 2001).
Beyond the effects of CHABs and their toxins within freshwater bodies across the U.S., there is now clear evidence that these toxins can become persistent contaminants when advected into estuarine and coastal ecosystems. Many CHAB events occur in close proximity to the land-sea interface on all coasts of the U.S. Miller et al. (2010) provided the first evidence of microcystins from land contaminating a marine ecosystem - specifically Monterey Bay, California. In this case, microcystins from rivers and lakes were flushed into Monterey Bay and accumulated in bivalves that were subsequently consumed by sea otters that died due to acute liver necrosis. Beyond identifying a novel source of marine mammal mortality, this study also revealed a striking potential human health threat, namely the bioaccumulation of microcystin in filter-feeding bivalves (as well as in secondary consumers including crabs and snails). Miller et al. (2010) demonstrated that bivalves can concentrate microcystin from the water column more than 100-fold and that it takes weeks for contaminated bivalves to depurate themselves of that toxin, emphasizing the likely widespread nature of this scenario.
While this initial study identified a novel mechanism by which marine bivalves and marine mammals are exposed to harmful freshwater biotoxins, this finding was not necessarily a surprise. Microcystis, the most common CHAB in the U.S., is tolerant of brackish waters with most strains persisting at salinities between 10 and 15 (Preece et al., 2017), meaning that Microcystis cells can be expected to survive in brackish environments where many bivalves such as oysters thrive. Furthermore, senescent or dead intact cells are still toxic. This has been obvious in places like the Saint Lucie River Estuary where Florida has declared states of emergency in 2016 and 2018 when massive Microcystis blooms originating from Lake Okeechobee were flushed into estuarine regions with salinities up to 30 (Kramer et al., 2018). In addition, microcystins can be stable and persistent in saltwater and freshwater habitats (Harke et al., 2016). Moreover, while microcystin can be degraded by physical processes (Tsuji et al., 1994) or microbially (Rohrlack and Hyenstrand, 2007), 90% of microcystin is typically found within cells where the toxin is significantly more stable (Harke et al., 2016). Finally, the primary exposure route of HAB toxins to humans is via bivalves, yielding well-known syndromes such as PSP, ASP, etc. (Shumway, 1990). While the poisoning of humans from shellfish contaminated with microcystin has yet to be documented (hepatotoxic shellfish poisoning; HSP), evidence that has emerged since the Miller et al. (2010) study suggests such occurrences are likely common, but unreported.
Studies since Miller et al. (2010) have both affirmed the findings of that initial study and have demonstrated that filter-feeding bivalves on U.S. West and East Coasts regularly accumulate high levels of microcystin. Gibble and Kudela (2014) demonstrated that microcystins in and around Monterey Bay were widespread and found at moderate levels throughout the year and throughout the region. Across all of San Francisco Bay, microcystins were found to be chronically present in SPATT (solid phase absorptive toxin trackers) samplers over a five-year period and were also detected in mussel samples from sites across the entire bay, during all months (Peacock et al., 2018). One quarter of the mussels tested in this study exceeded the California Office of Environmental Health and Hazard Assessment guideline level to protect human health (10 μg kg−1; Peacock et al., 2018). Further north, in Puget Sound, Preece et al., (2015) reported that mussels contained 2–15 μg microcystin kg−1. Bukaveckas et al. (2018) recently reported on the accumulation of microcystin in shellfish in the oligohaline region of the James River, Virginia (>50 km from freshwater) to levels exceeding 100 μg microcystin kg tissue−1 or an order of magnitude beyond the California guidance value. Similarly, Garcia et al. (2010) reported on blue crabs within the Barataria Estuary system (Louisiana) with > 100 μg microcystin kg−1 in muscle and up to 820 μg microcystin kg tissue−1 in the hepatopancreas. These observations demonstrate that the accumulation of microcystins and other CHAB toxins in shellfish is a phenomenon not isolated to California but rather represents an emerging human health threat across the U.S. that is poorly understood. Given the commonality of CHABs in water bodies that discharge into marine environments, the transport and accumulation of CHAB toxins in marine food webs may be a common occurrence.
5. Conclusions
The foregoing emphasizes that U.S. HABs are diverse phenomena involving multiple species and classes of algae that occupy a range of habitats and produce numerous toxins or bioactive compounds that impact a wide range of resources. This enormous diversity greatly complicates efforts to identify trends in bloom incidence, as some toxins (e.g., PSTs, NSTs) have been monitored on a routine basis for many years while others (e.g., ASTs, DSTs) have only emerged as threats in recent times, so monitoring efforts and entries in HAEDAT are less consistent and have a shorter history. The U.S. is also a large country with three major oceanic coastlines having very different plankton communities, hydrography, and habitats. Furthermore, many HAB species that do not threaten public health but that still cause harm (e.g., M. polykrikoides, H. akashiwo, K. mikimotoi) are not monitored on a routine basis, so events can be missed and are only entered into databases like HAEDAT on a sporadic basis. This is equally true for records of wildlife mortalities which also tend to be reported sporadically.
5.1. Trends for individual toxin families or HAB species
In the search for national trends and given these caveats, it is perhaps most meaningful to examine toxin families and individual HAB species and classes separately and to look for trends within each, as has been the focus throughout this review. The findings for these categories can be summarized as follows.
PSTs.
PSTs are the most widespread of all HAB poisoning syndrome toxins in the U.S., affecting large areas of the East and West Coasts as well as small parts of Florida with annually recurrent blooms. Statistically, there is no significant national trend in PST incidence over the last 30 years, though there has been some geographic expansion of toxicity, such as with the emergence of P. bahamense in Florida and of A. catenella on Long Island, in Puget Sound, and in northern areas of Alaska, with major expansion events occurring in the western Gulf of Maine and in Puget Sound before the HAEDAT time series began. PSTs currently represent a sustained and persistent annual threat to U.S. coastlines in terms of the number of events, with individual blooms and years varying dramatically in scale and impact. At this time, the only suggestion of a link between changing PST distributions or dynamics in the U.S. and climate change is the apparent expansion of toxicity in the Bering and Chukchi Seas, though the latter is based on research cruises and is not yet evident in HAEDAT because of the lack of routine PST monitoring in that remote region. Theoretically, warming temperatures may lead to increases in the timing and severity of PST events in that region going forward, while the balance between localized estuarine blooms and those in deeper coastal waters may shift in other areas of the U.S.
ASTs.
ASTs are a relatively recent HAB problem in the US that can impact both humans and marine wildlife. The number and magnitude of AST events has been on the rise in the U.S. since the first reports in California in 1991 though there is strong evidence that the causative organisms were present on that coast decades before the discovery of domoic acid as the ASP toxin. The first events in the Gulf of Mexico were recorded in 2014 and in the Gulf of Maine in 2016, the latter representing the apparent dispersal of highly toxic P. australis into this region for the first time. The national pattern is one of relatively frequent outbreaks on the US West Coast and emerging, recurring outbreaks on the East Coast, with sporadic incidents in the Gulf. The ability of P. australis, and perhaps other Pseudo-nitzschia species to survive in warmer, nutrient-depleted waters suggests that ASTs will likely be a persistent, recurrent problem in the future, warmer ocean.
DSTs.
HAEDAT entries for DST events in the U.S. begin in 2008, with no significant trend through time since then. The records provide evidence that the causative organism, Dinophysis spp. is now established on all three coasts. Comparison with the literature suggests an underreporting of DST events in HAEDAT and it is unclear if this problem will continue into the future, though reporting should improve as Dinophysis spp. enumeration and DST quantification are further incorporated into state and tribal monitoring programs. DST-related illnesses may remain inconspicuous, however, due to overlapping symptoms with other human diseases and unknown symptoms in wildlife. Not enough information is available to make conclusions regarding the drivers of DST events or predict trends with climate change or eutrophication. Field investigations into the drivers of DST events along all three U.S. coastlines are needed.
NSTs.
Like PSTs, there is a long historical record of NST events yet no significant trend was observed over the past 30 years. Instead, remarkably similar patterns were found in areas where events have recurred. The lack of a clear trend is at least partly due to sporadic severe events—which can last for multiple years—that are interspersed with less severe events. Incidents are documented nearly annually in the eastern Gulf of Mexico and can span international borders in the western Gulf. Ocean circulation appears to be a major driver of the recurring K. brevis blooms in the Gulf of Mexico and is important for bloom severity on event to interannual time scales (Stumpf et al., 2008). Culture and field studies that highlight the broad ecology of K. brevis suggest that this species may continue to thrive under future ocean conditions, however, changes in ocean circulation and storm frequency and intensity are likely to have impacts on bloom dynamics and distribution.
CTXs.
Due to a lack of monitoring programs for Gambierdiscus and CTXs as well as under-reporting of cases, data on these events are not available to establish time series trends. Epidemiological data have indicated possible declines at some locations (e.g., Florida, USVI), but these studies also identified significant underreporting and potential biases in current surveillance systems. Additional data-driven estimates of underreporting are needed to more accurately determine prevalence and impact of CTXs. Geographic expansion of Gambierdiscus spp. into temperate and non-endemic areas (e.g., northern Gulf of Mexico) appears to be occurring, fostered by ocean warming, and human health impacts from CTXs are expected to increase as this range expansion continues.
Wildlife toxicoses.
HAB-related marine wildlife morbidity and mortality events appear to be growing in number, with statistically significant increasing trends observed in marine mammal poisonings caused by domoic acid (ASTs) along the coast of California and brevetoxins (NSTs) in Florida. Sustained mortalities of other animals continue in parallel with the incidence of HAB events nationally, though events are not systematically recorded and reported. Some emerging HAB toxins (e.g., DSTs) will likely have fish and wildlife and ecosystem impacts, but more data and research on exposure effects are needed.
Brown tides.
Brown tides caused by A. anophagefferens have ceased to occur in some estuaries in the Northeast U.S. (Rhode Island), have continued in other estuaries (New York, New Jersey), and have expanded into the mid-Atlantic region (Maryland, Virginia). Brown tides caused by A. lagunensis have regularly recurred in Texas since 1990 and have expanded into lagoons along the east coast of Florida this decade. Brown tides caused by Aureoumbra are often coincident with hypersaline events that are caused by warming and droughts in sub-tropical lagoons of Texas and Florida which may be more common today and in the future.
Margalefidinium.
While blooms of this dinoflagellate were noted in the literature in four U.S. locations during the twentieth century, there has been a significant expansion in the reported occurrences and recurrence of these events in the recent past, now including > 15 previously unimpacted estuaries. The unique macroscopic and microscopic nature of these blooms suggest these are truly new occurrences in new locations. Eutrophication, ocean warming, and cyst-facilitated dispersal may all have contributed to this trend.
Heterosigma.
Heterosigma akashiwo occurs in the Gulf of Mexico and on both the East and West coasts, though it is considered harmful predominantly on the West Coast due to its effects on farmed fish. Lack of routine monitoring and changes in the scale of fish aquaculture operations obscure possible trends in national bloom frequency or magnitude.
Karenia mikimotoi.
This species occurs in many parts of the U.S., from Alaska to Maine, including the Gulf of Mexico. Lack of routine monitoring prevents the identification of national or regional trends, nor is there sufficient information on ecophysiology to suggest outcomes in a warming ocean.
Cyanobacteria.
Toxic cyanobacterial blooms are widespread across the U.S., often identified in multiple locations in all 50 states. During the past decade, a new threat associated with cyanobacteria has been identified, specifically the ability of freshwater blooms and toxins to be transported to coastal ecosystems where they can accumulate in shellfish and subsequently intoxicate marine mammals or other shellfish consumers. These events are not yet recorded in HAEDAT, so trends cannot be identified. Given the strong link between CHAB events and anthropogenic nutrient increases and warm temperatures, however, the scale of this recently discovered HAB impact has likely expanded in recent years, and is likely to maintain that trend as global warming and global change continue.
5.2. All HABs combined
An effort was also made to identify national trends for all HAB types by combining HAEDAT records for PSTs, ASTs, DSTs, NSTs, marine mammal mortalities, and other HAB events into a single time series (“other” includes Heterosigma, Aureococcus, Aureoumbra, Chattonella, Gymnodinium, Margalefidinium, Prymnesium, and Pyrodinium). Results are presented in two forms. Fig. 13A shows the number of events through time, and for statistical purposes and trend analysis, Fig. 13B shows the proportion of the U.S. HAEDAT zones that experience one or more of these types of HAB events each year. Both panels reveal similar patterns, with major interannual variability in events. In some years, the number of marine HAB events is 10 or less nationally, while in other years it is 30+.
Fig. 13.
Time series of all HAEDAT-recorded marine HAB events in the U.S., including PSTs, ASTs, DSTs, NSTs, marine mammal mortalities, and others (defined above). A) Number of events; B) Observed (bars) and modeled (line) proportions of U.S. HAEDAT zones with at least one HAB event. Also reported is the fitted linear logistic model and its significant p value (p<.05).
Importantly, panel 13B shows a statistically significant increasing trend in the proportion of U.S. HAEDAT zones impacted by HAB events through time (p< .05). The U.S. HAB problem has therefore expanded during the study interval; HABs are affecting more regions with more toxins and impacts than was the case decades ago. Part of that expansion simply reflects a better realization of the true or historic scale of the problem, long obscured by inadequate or inconsistent monitoring. For example, two “new” toxins (DSTs and ASTs) emerged within the last several decades, leading to a progressively expanding national monitoring effort and thus to more findings of events in recent years. Prior to the discovery of those toxins in the U.S., there were no records of them and thus no events to tabulate. The absence of event data in the early years of the study does not, however, mean that there were no events. Indeed, the report by Bargu et al. (2012) demonstrating erratic and aggressive behavior of birds in California in 1961 is strong evidence that ASTs were present and causing harm nearly 30 years before domoic acid was documented as a toxin. Likewise, DST events probably did occur prior to 2008, but were not detected for reasons listed in section 4.3.3.
Also contributing to the overall trend in HAB incidence are events caused by the natural- or human-assisted dispersion of HAB species to new regions and the stimulatory effects of factors such as nutrient pollution, aquaculture expansion, and climate change, among others. There are multiple examples of species dispersion, such as the expansion of A. catenella and PSTs into southern New England, throughout Long Island, and within Puget Sound – all likely due to natural dispersion and subsequent colonization of the species via its encysted stage. Another example would be the identification of P. australis in the Gulf of Maine after decades of negative monitoring results for that species (Bates et al., 2019). Others would be the emergence of ciguatera dinoflagellates in the northern Gulf of Mexico or range expansions of non-toxic HABs like Aureoumbra and Margalefidinium along the U.S. East Coast in recent years.
In terms of nutrient pollution-driven expansion, Anderson et al. (2008) examined U.S. HABs on a regional basis and concluded that most large-scale HABs along open coasts appear to be unrelated or weakly related to anthropogenic nutrient sources, whereas nutrient pollution is a much larger, and in some cases, dominant factor in HAB species success in certain estuaries, embayments, and sounds. The emerging cyanobacterial problem in the freshwater-to-marine continuum (e.g., Paerl et al., 2018) is one example of nutrient pollution-driven enhancement of HAB incidence. Another might be the severity of K. brevis blooms along the coast of Florida, though this warrants further examination to understand which of the many potential nutrient sources are utilized in the development and maintenance of massive but also spatiotemporally patchy blooms (Vargo et al., 2008, Heil et al. 2014).
The role of climate or global warming-driven expansion is equally uneven, with some regions (e.g., the Alaskan Arctic and the northern Gulf of Mexico) showing signs of temperature increases that are supportive of HAB species expansion into or within the region, whereas such inferences are not yet as evident in other regions. Environmental influences on HABs are complex and not all species will become more problematic as the climate warms (Hallegraeff 2010; Wells et al., 2015). For example, some species will thrive more than others as waters warm, while others may shift their geographic ranges (Kibler et al., 2015; Gobler et al., 2017). Recent extreme weather events, such as the 2015 marine heatwave on the U.S. West Coast and the subsequent widespread Pseudo-nitzschia bloom (McCabe et al., 2016) or the warm winter of 2012 on the East Coast and its effect on Alexandrium blooms (Ralston et al., 2014) have provided insights into potential future responses by HABs to climate change, summarized by Wells et al. (2015).
Note that the statistical approach using the proportion of zones with HAB events will reveal changes in event frequency and geographic range of HAB species, but says nothing about changes in the severity or duration of those events. This type of information is available in HAEDAT and other data sources and could be examined for trends in subsequent studies, as in the HAB Index approach of Anderson et al., (2014).
One result of the multifaceted expansion documented here is that many regions of the U.S. face a daunting diversity of species and toxins compared to the past. This multiplicity presents a significant and growing challenge to resource managers and public health officials in terms of toxins, regions, and time intervals to monitor, necessitating new approaches to monitoring and management. Many programs have evolved to include both phytoplankton monitoring and flesh testing for HAB toxins, the former often used as a screening method to identify locations and times to implement the more time consuming and expensive targeted toxin analysis. The development of powerful sensors capable of detecting and quantifying HAB cells and toxins in situ is a rapidly developing area that shows promise to expand the frequency, extent, and efficiency of monitoring.
Mobilization of funding and resources for research, monitoring and management of HABs requires accurate information on the scale and nature of the national problem. As shown here, HAEDAT and other databases can be of great value in this regard, but efforts are needed to expand and sustain the collection of HAB data nationally and regionally.
Highlights.
The first effort to quantitatively analyze trends in U.S. marine HAB events
Some HABs (PSTs and NSTs) show no trend over 30 years; DSTs show no trend over 12 years
Others (AST, CTXs, Margalefidinium, brown tides, cyanotoxins) increased in frequency and/or geographic extent
A statistically significant increasing trend is seen for all U.S. HAB events combined
Part of the expansion is a realization of the true scale of the problem, long obscured by inadequate monitoring
Increases also linked to species dispersion and stimulation from nutrient pollution, aquaculture, and ocean warming
Acknowledgements.
The many datasets referenced in this paper required the efforts of a host of individuals. We gratefully acknowledge the following for help with the reporting of event records: J. Kleindinst, V. Uva, E. Bresnan, K.Kanwit, D. Couture, A. Sirois, B. Lewis, A. Hamilton Vailea, C. Nash, M. Hickey, T. O’Neil, E. Van Gulick, K. DeRosia-Banick, A. Dragan, G. Wickfors, D. Borkman, A. Griffith, T. Hattenrath-Lehmann, Y. Kang, D. Greenfield, W. Hastback, R. Schuster, P. Petty, T.Egerton, K. Reece, K. Coyne, A. Place, P. Tester, J. Wolny, C. Wazniak,, B. Webb, D. Sheehan, N. Rabalais, C. Lemaire, C. Blankenship, A. Nunez, V. Zubkousky-White, F. Gulland, T. Norris, A. Manderson, J. Borchert, D. Ayres, A. Coyne, C. Bursch, B. Wright, D. Hondolero, R. Masui, K. Lanphier, N. Card, D. Whalen, E. Fachon, G. Di Cecco, M. Tengler, G. Langlois, R. Horner, J. Borchert, and F. Cox. We acknowledge R. Horner, Z. Forster, C. DeSouza, C. Villac, C. Holland, N. Hall, K. Steidinger, and R. Kudela for assistance with species information in Table 1. We also thank C. Anacreon for manuscript assistance, C. Young for data compilation, and M. Wetz and L. Hall for sharing TX and FL Aureoumbra data, C. Lopez for Pyrodinium data, and two anonymous reviewers for their insightful comments. Support for DMA, MLR, and DMK was provided through the Woods Hole Center for Oceans and Human Health (National Science Foundation grant OCE-1840381 and National Institutes of Health grants NIEHS-1P01-ES028938–01) and the U.S. National Office for Harmful Algal Blooms with funding from NOAA’s National Centers for Coastal Ocean Science (NCCOS) through the Cooperative Institute for the North Atlantic Region (CINAR) (NA14OAR4320158, NA19OAR4320074). Funding for KAL and DMA was provided by the National Oceanic and Atmospheric Administration National Centers for Coastal Ocean Science Competitive Research Program under award NA20NOS4780195 to the Woods Hole Oceanographic Institution and NOAA’s Northwest Fisheries Science Center. We also acknowledge support for A.H. from the National Oceanic and Atmospheric Administration [NOAA] Office of Ocean and Coastal Resource Management Award NA19NOS4780183, C.J.G from NOAA-MERHAB (NA19NOS4780186) and (NA16NOS4780189) for VLT Support was also received for JLS, CJG, and VLT from NOAA-NCCOS-ECOHAB under awards NA17NOS4780184 and NA19NOS4780182. This is ECOHAB publication number ECO972.
Footnotes
Disclaimer: These data and related items of information have not been formally disseminated by NOAA, and do not represent any agency determination, view, or policy.
Declaration of Interest Statement
Authors have no competing interest to declare. The data will not be published elsewhere in the same form in any language, including electronically without the written consent of the copyright holder if accepted.
IOC – International Oceanographic Commission; ICES – International Council for Exploration of the Seas; PICES -North Pacific Marine Science Organization
States along the Gulf Coast, including Florida, Alabama, Mississippi, Louisiana and Texas, conduct monitoring for NSTs following federal public health safety policies set by the National Shellfish Sanitation Program (NSSP), which recently changed from using a cell threshold (5000 Karenia brevis cells L−1) or a toxin threshold in shellfish measured using an approved method (20 MU/100 grams or 0.8 mg brevetoxin-2 equivalents kg−1 of shellfish tissue) to using only the toxin threshold. Many older HAEDAT NST events thus
States along the Gulf Coast, including Florida, Alabama, Mississippi, Louisiana and Texas, conduct monitoring for NSTs following federal public health safety policies set by the National Shellfish Sanitation Program (NSSP), which recently changed from using a cell threshold (5000 Karenia brevis cells L−1) or a toxin threshold in shellfish measured using an approved method (20 MU/100 grams or 0.8 mg brevetoxin-2 equivalents kg−1 of shellfish tissue) to using only the toxin threshold. Many older HAEDAT NST events thus reflect cell count criteria, not direct toxin measurements.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6 References
- Abbott GM, Landsberg JH, Reich AR, Steidinger KA, Ketchen S, Blackmore C, 2009. Resource guide for public health response to harmful algal blooms in Florida: Based on recommendations of the Florida Harmful Algal Bloom Task Force Public Health Technical Panel, Fish and Wildlife Research Institute Technical Report. Florida Fish and Wildlife Conservation Commission, Saint Petersburg, pp. 1–132. [Google Scholar]
- Abbott JP, Flewelling LJ, Landsberg JH, 2009. Saxitoxin monitoring in three species of Florida puffer fish. Harmful Algae 8, 343–348. [Google Scholar]
- Accoroni S, Totti C, Romagnoli T, Giulietti S, Glibert PM, 2020. Distribution and potential toxicity of benthic harmful dinoflagellates in waters of Florida Bay and the Florida Keys. Mar. Environ. Res. 155, 104891. [DOI] [PubMed] [Google Scholar]
- Amzil Z, Fresnel J, Le Gal D, Billard C, 2001. Domoic acid accumulation in French shellfish in relation to toxic species of Pseudo-nitzschia multiseries and P. pseudodelicatissima. Toxicon 39, 1245–1251. [DOI] [PubMed] [Google Scholar]
- Anderson DM, 1997. Bloom dynamics of toxic Alexandrium species in the northeastern United States. Limnol. Oceanogr. 42, 1009–1022. [Google Scholar]
- Anderson DM, Wall D, 1978. Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms. J. Phycol. 14(2), 224–234. [Google Scholar]
- Anderson DM, Kulis DM, Orphanos JA, Ceurvels AR, 1982. Distribution of the toxic dinoflagellate Gonyaulax tamarensis in the southern New England region. Estuar. Coast. Shelf Sci. 14(4), 447–458. [Google Scholar]
- Anderson DM, Keafer BA, Kulis DM, Waters RM, Nuzzi R, 1993. An immunofluorescent survey of the brown tide chrysophyte Aureococcus anophagefferens along the northeast coast of the United States. J. Plankton Res. 15, 563–580. [Google Scholar]
- Anderson DM, Keafer BA, Geyer WR, Signell RP, Loder TC, 2005. Toxic Alexandrium blooms in the western Gulf of Maine: The plume advection hypothesis revisited. Limnol. Oceanogr. 50(1), 328–345. [Google Scholar]
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ Jr, Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B, 2014. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep Sea Res. Part II: Top. Stud. Oceanogr. 103, 6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azanza RV, Taylor FJR., 2001. Are Pyrodinium blooms in the southeast Asian region recurring and spreading? A view at the end of the millennium. Ambio 30, 356–364. [PubMed] [Google Scholar]
- Azanza RV, Brosnahan ML, Anderson DM, Hense I, Montresor M, 2018. The role of life cycle characteristics in harmful algal bloom dynamics. In: Glibert PM, Berdalet E, Burford M, Pitcher G, Zhou M (Eds.), Global Ecology and Oceanography of Harmful Algal Blooms (GEOHAB), Ecological Studies 232. Springer, Cham, Switzerland, pp. 133–161. [Google Scholar]
- Azziz-Baumgartner E, Luber G, Conklin L, Tosteson TR, Granade HR, Dickey RW, Backer LC, 2012. Assessing the incidence of ciguatera fish poisoning with two surveys conducted in Culebra, Puerto Rico, during 2005 and 2006. Environ. Health Perspect. 120 (4) 526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvaroff TR, Adolf JE, Place AR, 2009. Strain variation in Karlodinium veneficum (Dinophyceae): toxin profiles, pigments, and growth characteristics. J. Phycol. 45, 138–153. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng Y-S, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DC, 2003. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2(1), 19–28. [Google Scholar]
- Baden DC, Tomas CR, 1988. Variations in major toxin composition for six clones of Ptychodiscus brevis. Toxicon 26(10), 961–963. [DOI] [PubMed] [Google Scholar]
- Badylak S, Phlips EJ, 2008. Spatial and temporal distributions of zooplankton in Tampa Bay, Florida, including observations during a HAB event. J. Plankton Res. 30, 449–465. [Google Scholar]
- Bargu S, Powell CL, Coale SL, Busman M, Doucette GJ, Silver MW, 2002. Krill: a potential vector for domoic acid in marine food webs. Mar. Ecol. Prog. Ser. 237, 209–216. [Google Scholar]
- Bargu S, Powell CL, Wang Z, Doucette GJ, Silver MW, 2008. Note on the occurrence of Pseudo-nitzschia australis and domoic acid in squid from Monterey Bay, CA (USA). Harmful Algae 7, 45–51. [Google Scholar]
- Bargu S, Silver M, Goldstein T, Roberts K, Gulland F, 2010. Complexity of domoic acid-related sea lion strandings in Monterey Bay, California: Foraging patterns, climate events, and toxic blooms. Marine Ecology Progress Series, 418, 213–222. [Google Scholar]
- Bargu S, Silver MW, Ohman MD, Benitez-Nelson CR, Garrison DL, 2012. Mystery behind Hitchcock’s birds. Nature Geosci. 5(1), 2–3. [Google Scholar]
- Barton ED, Tanner P, Turchen SG, Tunget CL, Manoguerra A, Clark RF, 1995. Ciguatera fish poisoning. A southern California epidemic. Western J. Med. 163 (1), 31. [PMC free article] [PubMed] [Google Scholar]
- Bates SS, Bird CJ, de Freitas ASW, Foxall R, Gilgan M, Hanic LA, Johnson GR, McCulloch AW, Odense P, Pocklington R, Quilliam MA, Sim PG, Smith JC, Subba Rao DV, Todd ECD, Walter JA, Wright JLC, 1989. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish Aquat. Sci. 46, 1203–1215. [Google Scholar]
- Bates SS, Hubbard KA, Lundholm N, Montressor M, Leaw CP 2018. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 79, 3–43. [DOI] [PubMed] [Google Scholar]
- Bean LL, McGowan JD, Hurst JW Jr, 2005. Annual variations of paralytic shellfish poisoning in Maine, U.S.A 1997–2001. Deep Sea Res. Part II: Top. Stud. in Oceanogr, 52(19–21), 2834–2842. [Google Scholar]
- Bejarano AC, Gulland FM, Goldstein T, St Leger J, Hunter M, Schwacke LH, Vandolah FM, Rowles TK, 2008. Demographics and spatio-temporal signature of the biotoxin domoic acid in California sea lion (Zalophus californianus) stranding records. Mar. Mammal Sci. 24(4), 899–912. [Google Scholar]
- Bercel TL Kranz SA, 2019. Insights into carbon acquisition and photosynthesis in Karenia brevis under a range of CO2 concentrations. Prog. Oceanogr. 172, 65–76. [Google Scholar]
- Berg GM, Repeta DJ, LaRoche J, 2002. Dissolved organic nitrogen hydrolysis rates in axenic cultures of Aureococcus anophagefferens (Pelagophyceae): comparison with heterotrophic bacteria. Appl. Environ. Microbiol. 68, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman FW, Murray T, 1997. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J. Neurochem. 69, 693–703. [DOI] [PubMed] [Google Scholar]
- Blanco J, Livramento F, Rangel IM, 2010. Amnesic shellfish poisoning (ASP) toxins in plankton and molluscs from Luanda Bay, Angola. Toxicon 55, 541–546. [DOI] [PubMed] [Google Scholar]
- Bogan YM, Kennedy DJ, Harkin AL, Gillespie J, Vause BJ, Beukers-Stewart BD, Hess P, Slater JW, 2007. Variation in domoic acid concentration in king scallop (Pecten maximus) from fishing grounds around the Isle of Man. Harmful Algae 6: 81–92. [Google Scholar]
- Borkman DG, Smayda TJ, Tomas CR, York R, Strangman W, Wright JLC, 2012. Toxic Alexandrium peruvianum (Balech and de Mendiola) Balech and Tangen in Narragansett Bay, Rhode Island (USA). Harmful Algae 19, 92–100. [Google Scholar]
- Bourdelais AJ, Tomas CR, Naar J, Kubanek J, Baden DG, 2002. New fish-killing alga in coastal Delaware produces neurotoxins. Environ. Health Perspect. 110, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricelj VM, Carpenter EJ (Eds.), Novel phytoplankton blooms: Causes and impacts of recurrent brown tides and other unusual blooms. Springer, Berlin, pp. 189–212. [Google Scholar]
- Bricelj VM, Kuenstner SH, 1989. Effects of the “brown tide” on the feeding physiology and growth of bay scallops and mussels. In: Cosper EM, Bricelj VM, Carpenter EJ (Eds.), Novel phytoplankton blooms: Causes and impacts of recurrent brown tides and other unusual blooms. Springer, Berlin, pp. 491–509. [Google Scholar]
- Bricelj VM, MacQuarrie SP, Schaffner RA, 2001. Differential effects of Aureococcus anophagefferens isolates (“brown tide”) in unialgal and mixed suspensions on bivalve feeding. Mar. Biol. 139, 605–615. [Google Scholar]
- Bricelj VM, Kraeuter JN, Flimlin G 2017. Status and trends of hard clam, Mercenaria mercenaria, populations in a coastal lagoon ecosystem, Barnegat Bay-Little Egg Harbor, New Jersey. J. Coast. Res. 78., 205–253. [Google Scholar]
- Brosnahan ML, Fischer AD, Lopez CB, Moore SK, Anderson DM, 2020. Cyst-forming dinoflagellates in a warming climate. Harmful Algae, 91, 101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KR, Uttalcooke L, Pilskaln CH, Roelke DL, Villac MC, Fryxell GA, Cifuentes L, Chavez FP, 1992. Autecology of the diatom Pseudo-nitzschia australis, a domoic acid producer, from Monterey Bay, California. Mar. Ecol. Prog. Ser. 84, 293–302. [Google Scholar]
- Bukaveckas PA, Franklin R, Tassone S, Trache B, Egerton T 2018. Cyanobacteria and cyanotoxins at the river-estuarine transition. Harmful Algae, 76, 11–21. [DOI] [PubMed] [Google Scholar]
- Burkholder JM, Marshall HG, 2012. Toxigenic Pfiesteria species—Updates on biology, ecology, toxins, and impacts. Harmful Algae 14, 196–230. [Google Scholar]
- Bursa A, 1963. Phytoplankton in coastal waters of the Arctic Ocean at Point Barrow, Alaska. Arctic, 16(4), 239–262. [Google Scholar]
- Buskey EJ, 1996. Current status and historical trends of brown tide and red tide phytoplankton blooms in the Corpus Christi Bay National Estuary Program study area. Corpus Christi Bay National Estuary Program, pp. 1–65. [Google Scholar]
- Buskey EJ, Stockwell DA, 1993. Effects of a persistent “brown tide” on zooplankton populations in the Laguna Madre of south Texas. In: Smayda TJ, Shimizu Y (Eds.), Toxic Phytoplankton Blooms in the Sea. Elsevier, Amsterdam, pp. 659–666. [Google Scholar]
- Buskey EJ, Wysor B, Hyatt C, 1998. The role of hypersalinity in the persistence of the Texas ‘brown tide’ in the Laguna Madre. J. Plankton Res. 20, 1553–1565. [Google Scholar]
- Buskey EJ, Villareal TA, Lopez-Barreiro T, 1999. Reconstructing the initiation of the Texas brown tide bloom of Aureoumbra lagunensis from archived samples using an immunofluorescence assay. Plankton Biology and Ecology 46(2), 159–161. [Google Scholar]
- Buskey EJ, Liu HB, Collumb C, Bersano JGF, 2001. The decline and recovery of a persistent Texas brown tide algal bloom in the Laguna Madre (Texas, USA). Estuaries 24, 337–346. [Google Scholar]
- Buttke DE, Walker A, Huang I-S, Flewelling L, Lankton J, Ballmann AE, Clapp T, Lindsay J, Zimba PV, 2018. Green tree frog (Hyla cinerea) and ground squirrel (Xerospermophilus spilosoma) mortality attributed to inland brevetoxin transport at Padre Island National Seashore, Texas, U.S.A, 2015. J. Wildl. Dis. 54, 142–146. [DOI] [PubMed] [Google Scholar]
- Campbell DA, Kelly MS, Busman M, Bolch CJ, Wiggins E, Moeller PDR, Morton SL, Hess P, Shumway SE, 2001. Amnesic shellfish poisoning in the king scallop, Pecten maximus, from the west coast of Scotland. J. Shellfish Res. 20, 75–84. [Google Scholar]
- Campbell L, Olson RJ, Sosik HM, Abraham A, Henrichs DW, Hyatt CJ, Buskey EJ, 2010. First harmful Dinophysis (Dinophyceae, Dinophysiales) bloom in the US is revealed by automated imaging flow cytometry. J. Phycol. 46(1), 66–75. [Google Scholar]
- Castle KT, Flewelling LJ, Bryan J, Kramer A, Lindsay J, Nevada C, Stablein W, Wong D, Landsberg JH, 2013. Coyote (Canis latrans) and domestic dog (Canis familiaris) mortality and morbidity due to a Karenia brevis red tide in the Gulf of Mexico. J Wild Dis 49, 955–964. [DOI] [PubMed] [Google Scholar]
- Cembella A, 1989. Occurrence of okadaic acid, a major diarrheic shellfish toxin, in natural populations of Dinophysis sp. from the eastern coast of North America. J. Appl. Phycol. 1, 307–310. [Google Scholar]
- Cerrato RM, Caron DA, Lonsdale DJ, Rose JM, Schaffner RA, 2004. An experimental approach to examine the effect of the hard clam Mercenaria mercenaria on the development of blooms of the brown tide alga, Aureococcus anophagefferens. Mar. Ecol. Prog. Ser. 281, 93–108. [Google Scholar]
- Chang FH, Ryan KG, 2004. Karenia concordia sp. nov. (Gymnodiniales, Dinophyceae), a new nonthecate dinoflagellate isolated from the New Zealand northeast coast during the 2002 harmful algal bloom events. Phycologia 43(5), 552–562. [Google Scholar]
- Chang FH, Bourdelais AJ, Baden DG, Gall M, Hulston D, Webb V, 2006. Karenia concordia (Dinophyceae) as a brevetoxin-producer and comparison with two closely related species K. brevisulcata and K. mikimotoi, 12th International Conference on Harmful Algae, Copenhagen, Denmark, 51–52. [Google Scholar]
- Chang FH, Uddstrom MJ, Pinkerton MH, Richardson KM, 2008. Characterising the 2002 toxic Karenia concordia (Dinophyceae) outbreak and its development using satellite imagery on the north-eastern coast of New Zealand. Harmful Algae 7(4), 532–544. [Google Scholar]
- Chinain M, Faust MA, Pauillac S, 1999. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 35 (6), 1282–1296. [Google Scholar]
- Chinain M, Darius HT, Ung A, Cruchet P, Wang Z, Ponton D, Laurent D, Pauillac S, 2010. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 56 (5), 739–750. [DOI] [PubMed] [Google Scholar]
- Chinain M, Gatti CMI, Darius HT, Quod JP, Tester PA, 2020. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae, 101873. [DOI] [PubMed] [Google Scholar]
- Cira EK, Wetz MS 2019. Spatial-temporal distribution of Aureoumbra lagunensis (“brown tide”) in Baffin Bay, Texas. Harmful Algae 89, 101669. [DOI] [PubMed] [Google Scholar]
- Clark S, Hubbard KA, Anderson DM, McGillicuddy DJ Jr, Ralston DK, Townsend DW, 2019. Pseudo-nitzschia bloom dynamics in the Gulf of Maine: 2012–2016. Harmful Algae 88, 101656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochlan WP, Herndon J, Kudela RM, 2008. Inorganic and organic nitrogen uptake by the toxigenic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 8, 111–118. [Google Scholar]
- Cohn MS, Olsen P, Mahoney JPB, Feerst E, 1988. Occurrence of the dinoflagellate Gonyaulax tamarensis, in New Jersey. Bull. New Jersey Acad. Sci. 33, 45–49 [Google Scholar]
- Connell CH, Cross JB, 1950. Mass mortality of fish associated with the protozoan Gonyaulax in the Gulf of Mexico. Science 112(2909), 359–363. [DOI] [PubMed] [Google Scholar]
- Connell L, Jacobs M, 1997. Anatomy of a Bloom: Heterosigma akashiwo in Puget Sound 1997. Puget Sound Res. 98, 830–834. [Google Scholar]
- Copeland NK, Palmer WR, Bienfang PK, 2014. Ciguatera fish poisoning in Hawai’i and the Pacific. Hawai’i journal of medicine & public health : A Journal of Asia Pacific Medicine & Public Health 73 (11 Suppl 2), 24–27. [PMC free article] [PubMed] [Google Scholar]
- Corcoran AA, Richardson B, Flewelling LJ, 2014. Effects of nutrient-limiting supply ratios on toxin content of Karenia brevis grown in continuous culture. Harmful Algae 39, 334–341. [Google Scholar]
- Corcoran AA, Wolny J, Leone E, Ivey J, Murasko S 2017. Drivers of phytoplankton dynamics in Old Tampa Bay, FL (USA), a subestuary lagging in ecosystem recovery. Estuar. Coast. Shelf Sci. 185, 130–140. [Google Scholar]
- Cosper EM, Dennison WC, Carpenter EJ, Bricelj VM, Mitchell JG, Kuenstner SH, Colflesh DC, Dewey M, 1987. Recurrent and persistent “brown tide” blooms perturb coastal marine ecosystem. Estuaries 10, 284–290. [Google Scholar]
- Cosper EM, Dennison W, Milligan A, 1989. An examination of environmental factors important to initiating and sustaining “brown tide” blooms. In: Cosper EM, Bricelj VM, Carpenter EJ (Eds.), Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tides and Other Unusual Blooms. Springer, Berlin, pp. 317–340. [Google Scholar]
- Costa PR, Rodrigues SM, Botelho MJ, Sampayo MA, 2003. A potential vector of domoic acid: the swimming crab Polybius henslowii Leach (Decapoda-Brachyura). Toxicon 42, 135–141. [DOI] [PubMed] [Google Scholar]
- Costa PR, Garrido S 2004. Domoic acid accumulation in the sardine Sardina pilchardus and its relationship to Pseudo-nitzschia diatom ingestion. Mar. Ecol. Prog. Ser. 284, 261–268. [Google Scholar]
- Costa PR, Rosa R, Sampayo MAM, 2004. Tissue distribution of the amnesic shellfish toxin, domoic acid, in Octopus vulgaris from the Portuguese coast. Mar. Biol. 144, 971–976. [Google Scholar]
- Costa PR, Rosa R, Duarte-Silva A, Brotas V, Sampayo MA, 2005a. Accumulation, transformation and tissue distribution of domoic acid, the amnesic shellfish poisoning toxin, in the common cuttlefish, Sepia officinalis. Aquat. Toxicol. 74, 82–91. [DOI] [PubMed] [Google Scholar]
- Costa PR, Rosa R, Pereira J, Sampayo MAM, 2005b. Detection of domoic acid, the amnesic shellfish toxin, in the digestive gland of Eledone cirrhosa and E. moschata (Cephalopoda, Octopoda) from the Portuguese coast. Aquat. Living Resour. 18, 395–400. [Google Scholar]
- Coyne KJ, Whereat E, Muns F, Wang Y, Farrell J, Tomas C, Rouse D, 2015. Recurrent blooms of Karenia papilionacea in Delaware coastal waters, Eighth Symposium on Harmful Algae in the U.S., Long Beach, California, p. 114. [Google Scholar]
- Cruz-Rivera E, Villareal TA, 2006. Macroalgal palatability and the flux of ciguatera toxins through marine food webs. Harmful Algae, 5, 497–525. [Google Scholar]
- Curtiss CC, Langlois GW, Busse LB, Mazzillo F, Silver MW, 2008. The emergence of Cochlodinium along the California Coast (U.S.A). Harmful Algae 7, 337–346. [Google Scholar]
- Danielson SL, Ahkinga O, Ashjian C, Basyuk E, Cooper LW, Eisner L, Farley E, Iken KB, Grebmeier JM, Juranek L, Khen G, 2020. Manifestation and consequences of warming and altered heat fluxes over the Bering and Chukchi Sea continental shelves. Deep-Sea Res. Part II: Top. Stud. Oceanogr. 177, 104781. [Google Scholar]
- Davis CC, 1948. Gymnodinium brevis sp. nov., a cause of discolored water and animal mortality in the Gulf of Mexico. Bot. Gaz. 109(3), 358–360. [Google Scholar]
- Deeds JR, Place AR, 2006. Sterol specific membrane interactions with the toxins from Karlodinium micrum (Dinophyceae) – A strategy for self protection. Afr. J. Mar. Sci. 28, 421–425. [Google Scholar]
- Deeds JR, Terlizzi DE, Adolf JE, Stoecker DK, Place AR, 2002. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae) - a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae 1, 169–189. [Google Scholar]
- Deeds JR, Reimschuessel R, Place AR, 2006. Histopathological effects in fish exposed to the toxins from Karlodinium micrum. J. Aquat. Anim. Health 18, 136–148. [Google Scholar]
- Deeds JR, White KD, Etheridge SM, Landsberg JH, 2008. Concentrations of saxitoxin and tetrodotoxin in three species of puffers from the Indian River Lagoon, Florida, the location for multiple cases of saxitoxin puffer poisoning from 2002 to 2004. Trans. Am. Fish. Soc. 137, 1317–1326. [Google Scholar]
- Deeds JR, Wiles K, Heideman GB, White KD, Abraham A, 2010. First U.S. report of shellfish harvesting closures due to confirmed okadaic acid in Texas Gulf coast oysters. Toxicon, 55, 1138–1146. [DOI] [PubMed] [Google Scholar]
- Deeds JR, Stutts WL, Celiz MD, MacLeod J, Hamilton AE, Lewis BJ, Miller DW, Kanwit K, Smith JL, Kulis DM, McCarron P, 2020. Dihydrodinophysistoxin-1 produced by Dinophysis norvegica in the Gulf of Maine, USA and its accumulation in shellfish. Toxins, 12(9), 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGange AR, Vacca MM, 1989. Sea otter mortality at Kodiak Island, Alaska, during summer 1987. J. Mamm. 70, 836–838. [Google Scholar]
- Del Rio R, Bargu S, Baltz D, Fire S, Peterson G, Wang Z, 2010. Gulf menhaden (Brevoortia patronus): A potential vector of domoic acid in coastal Louisiana food webs. Harmful Algae 10, 19–29. [Google Scholar]
- DeYoe HR, Chan AM, Suttle CA, 1995. Phylogeny of Aureococcus anophagefferens and a morphologically similar bloom-forming alga from Texas as determined by 18S ribosomal RNA sequence analysis. J. Phycol. 31, 413–418. [Google Scholar]
- DeYoe HR, Stockwell DA, Bidigare RR, Latasa M, Johnson PW, Hargraves PE, Suttle CA, 1997. Description and characterization of the algal species Aureoumbra lagunensis gen. et sp. nov. and referral of Aureoumbra and Aureococcus to the Pelagophyceae. J. Phycol. 33, 1042–1048. [Google Scholar]
- DeYoe HR, Buskey EJ, Jochem FJ, 2007. Physiological responses of Aureococcus lagunensis and Synechococcus sp. to nitrogen addition in a mesocosm study. Harmful Algae 6, 48–55. [Google Scholar]
- Dickey RW, Plakas. SM, 2010. Ciguatera: A public health perspective. Toxicon 56 (2), 123–136. [DOI] [PubMed] [Google Scholar]
- Dortch Q, Moncreiff CA, Mendenhall WM, Parsons ML, Franks JS, Hemphill KW, 1998. Spread of Gymnodinium breve into the northern Gulf of Mexico, In: Reguera B, Blanco J, Fernandez ML, Wyatt T (Eds.). Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO 1998, Santiago de Compostela, Spain, pp. 143–144. [Google Scholar]
- Du X, Peterson W, McCulloch A, Liu G, 2011. An unusual bloom of the dinoflagellate Akashiwo sanguinea off the central Oregon, U.S.A, coast in autumn 2009. Harmful Algae 10, 784–793. [Google Scholar]
- Dupont JM, Hallock P, Jaap WC, 2010. Ecological impacts of the 2005 red tide on artificial reef epibenthic macroinvertebrate and fish communities in the eastern Gulf of Mexico. Mar. Ecol. Prog. Ser. 415, 189–200. [Google Scholar]
- EC. 2002. Commission Decision of 15 March 2002. Laying down rules for the implementation of Council Directive 9¼92/EEC as regards the maximum levels and the methods of analysis of certain marine biotoxins in bivalve molluscs, echinoderms, tunicates and marine gastropods. Off. J. Eur. Communities. (2002./225/EC) L 75, 62–63. [Google Scholar]
- Errera RM, Yvon-Lewis S, Kessler JD, Campbell L, 2014. Reponses of the dinoflagellate Karenia brevis to climate change: pCO2 and sea surface temperatures. Harmful Algae, 37, 110–116. [Google Scholar]
- Fauquier DA, Flewelling LJ, Maucher JM, Keller M, Kinsel MJ, Johnson CK, Henry M, Gannon JG, Ramsdell JS, Landsberg JH, 2013a. Brevetoxicosis in sea birds naturally exposed to Karenia brevis blooms along the central west coast of Florida. J. Wildl. Dis. 49, 246–260. [DOI] [PubMed] [Google Scholar]
- Fauquier DA, Flewelling LJ, Maucher JM, Manire CA, Socha V, Kinsel MJ, Stacy BA, Henry M, Gannon JG, Ramsdell JS, Landsberg JH, 2013b. Brevetoxin in blood, biological fluids, and tissues of sea turtles naturally exposed to Karenia brevis blooms in central west Florida. J. Zoo Wildl. Med. 44, 364–375. [DOI] [PubMed] [Google Scholar]
- Feinstein A, Ceurvels AR, Hutton RF, Snoek E, 1955. Red tide outbreaks off the Florida west coast, In: Smith FGW (Ed.). The Marine Laboratory, University of Miami, Florida State Board of Conservation, Coral Gables, Florida, pp. 1–39. [Google Scholar]
- Ferdin ME, Kvitek RG, Bretz CK, Powell CL, Doucette GJ, Lefebvre KA, Coale S, Silver MW, 2002. Emerita analoga (Stimpson)-possible new indicator species for the phycotoxin domoic acid in California coastal waters. Toxicon. 40, 1259–1265. [DOI] [PubMed] [Google Scholar]
- Fernandes LF, Hubbard KA, Richlen ML, Smith J, Bates SS, Ehrman J, Léger C, Mafra LL Jr, Kulis D, Quilliam M and Libera K, 2014. Diversity and toxicity of the diatom Pseudo-nitzschia Peragallo in the Gulf of Maine, Northwestern Atlantic Ocean. Deep Sea Res. Part II: Top. Stud. Oceanogr, 103, 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire SE, Silver MW, 2005. Domoic acid in the Santa Cruz wharf fishery. Calif. Fish Game 91, 179–192. [Google Scholar]
- Fire SE, Van Dolah FM, 2012. Marine biotoxins: Emergence of harmful algal blooms as health threats to marine wildlife. In: Aguirre AA, Ostfeld RS, Daszak P (Eds.), New Directions in Conservation Medicine: Applied Cases of Ecological Health. Oxford University Press, NY, pp. 374–389. [Google Scholar]
- Fire SE, Flewelling LJ, Naar J, Twiner MJ, Henry MS, Pierce RH, Gannon DP, Wang Z, Davidson L, Wells RS, 2008. Prevalence of brevetoxins in prey fish of bottlenose dolphins in Sarasota Bay, Florida. Mar. Ecol. Prog. Ser. 368, 283–294. [Google Scholar]
- Fire SE, Wang Z, Leighfield TA, Morton SL, McFee WE, McLellan WA, Litaker RW, Tester PA, Hohn AA, Lovewell G, Harms C, Rotstein DS, Barco SG, Costidis A, Sheppard B, Bossart GD, Stolen M, Durden WN, Van Dolah FM, 2009. Domoic acid exposure in pygmy and dwarf sperm whales (Kogia spp.) from southeastern and mid-Atlantic U.S. waters. Harmful Algae 8, 658–664. [Google Scholar]
- Fire SE, Wang Z, Berman M, Langlois GW, Morton SL, Sekula-Wood E, Benitez-Nelson CR, 2010. Trophic transfer of the harmful algal toxin domoic acid as a cause of death in a minke whale (Balaenoptera acutorostrata) stranding in southern California. Aquat. Mamm. 36, 342–350. [Google Scholar]
- Fire SE, Wang ZH, Byrd M, Whitehead HR, Paternoster J, Morton SL, 2011. Co-occurrence of multiple classes of harmful algal toxins in bottlenose dolphins (Tursiops truncatus) stranding during an unusual mortality event in Texas, USA. Harmful Algae 10, 330–336. [Google Scholar]
- Fire SE, Pruden J, Couture D, Wang Z, Dechraoui Bottein MY, Haynes BL, Knott T, Bouchard D, Lichtenwalner A, Wippelhauser G, 2012. Saxitoxin exposure in an endangered fish: association of a shortnose sturgeon mortality event with a harmful algal bloom. Mar. Ecol. Prog. Ser. 460, 145–153. [Google Scholar]
- Fire SE, Flewelling LJ, Stolen M, Durden WN, de Wit M, Spellman AC, Wang Z, 2015. Brevetoxin-associated mass mortality event of bottlenose dolphins and manatees along the east coast of Florida, USA. Mar. Ecol. Prog. Ser. 526, 241–251. [Google Scholar]
- Fire SE, Browning JA, Durden WN, Stolen MK, 2020. Comparison of during-bloom and inter-bloom brevetoxin and saxitoxin concentrations in Indian River Lagoon bottlenose dolphins, 2002–2011. Aquat. Toxicol. 218, 105371. [DOI] [PubMed] [Google Scholar]
- Fischer AD, Brosnahan ML, Anderson DM, 2018. Quantitative response of Alexandrium catenella cyst dormancy to cold exposure. Protist, 169(5), 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KE, Landsberg JH, 2011. Effects of a persistent red tide (Karenia brevis) bloom on community structure and species-specific relative abundance of nekton in a Gulf of Mexico estuary. Estuaries and Coasts 34, 417–439. [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Walsh CJ, Nierenberg K, Clark J, Reich A, Hollenbeck J, Benson J, Cheng YS, Naar J, Pierce R, Bourdelais AJ, Abraham WM, Kirkpatrick G, Zaias J, Wanner A, Mendes E, Shalat S, Hoagland P, Stephan W, Bean J, Watkins S, Clark T, Byrne M, Baden DG, 2011. Review of Florida red tide and human health effects. Harmful Algae 10(2), 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling LJ, Naar JP, Abbott JP, Baden DG, Barros NB, Bossart GD, Bottein MY, Hammond DG, Haubold EM, Heil CA, Henry MS, Jacocks HM, Leighfield TA, Pierce RH, Pitchford TD, Rommel SA, Scott PS, Steidinger KA, Truby EW, Van Dolah FM, Landsberg JH, 2005. Red tides and marine mammal mortalities. Nature 435, 755–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling LJ, Adams DH, Naar JP, Atwood KA, Granholm AA, O’Dea SN, Landsberg JH, 2010. Brevetoxins in sharks and rays (Chondrichthyes, Elasmobranchii) from Florida coastal waters. Mar. Biol. 157, 1937–1953. [Google Scholar]
- Florida Department of Health (FDOH), 2011. Neurotoxic Shellfish Poisoning, Guide to Surveillance and Investigation. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/gsi-neurotoxic-shellfish.pdf [accessed 10/2020]
- Florida Fish and Wildlife Conservation Commission (FWC). 2020. Red tide manatee mortalities. https://myfwc.com/research/manatee/rescue-mortality-response/statistics/mortality/red-tide/
- Foley A, Stacy BA, Schueller P, Flewelling LJ, Schroeder B, Minch K, Fauquier DA, Foote JJ, Manire C, Atwood KE, Granholm AA, Landsberg JH, 2019. Assessing Karenia brevis red tide as a mortality factor of sea turtles in Florida. Dis. Aquat. Org. 132, 109–124. [DOI] [PubMed] [Google Scholar]
- Fortuine R, 1975. Paralytic shellfish poisoning in the north Pacific: two historic accounts and implications for today. Alaska Med. 17, 71–76. [PubMed] [Google Scholar]
- Fowler N, Tomas C, Baden D, Campbell L, Bourdelais A, 2015. Chemical analysis of Karenia papilionacea. Toxicon 101, 85–91. [DOI] [PubMed] [Google Scholar]
- Francis G, 1878. Poisonous Australian Lake. Nature 18, 11–12. [Google Scholar]
- Francis G, 1878. Poisonous Australian Lake. Nature 18, 11–12. [Google Scholar]
- Franks PJS, Anderson DM, 1992. Alongshore transport of a toxic phytoplankton bloom in a buoyancy current: Alexandrium tamarense in the Gulf of Maine. Mar. Biol, 112(1), 153–164. [Google Scholar]
- Freudenthal AR, Jijina J, 1985. Shellfish poisoning episodes involving or coincidental with dinoflagellates. In: 3. International Conference on Toxic Dinoflagellates, St. Andrews, New Brunswick (Canada), 8–12 Jun 1985. Elsevier. [Google Scholar]
- Friedman MA, Fernandez M, Backer LC, Dickey RW, Bernstein J, Schrank K, Kibler S, Stephan W, Gribble MO, Bienfang P, 2017. An updated review of ciguatera fish poisoning: clinical, epidemiological, environmental, and public health management. Mar. Drugs 15, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L, Quilliam MA, Wright JLC, Beale AM, Work TM, 1992. An outbreak of domoic acid poisoning attributed to the pennate diatom Pseudonitzschia australis. J. Phycol. 28, 439–442. [Google Scholar]
- Fujiki H, Suganuma M, 1993. Tumor promotion by inhibitors of protein phosphatases 1 and 2A: the okadaic acid class of compounds. Adv. Cancer Res. 61, 143–194. [DOI] [PubMed] [Google Scholar]
- Fux E, Smith JL, Tong M, Guzman L, Anderson DM, 2011. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon, 57, 275–287. [DOI] [PubMed] [Google Scholar]
- Gallager SM, Stoecker DK, Bricelj VM, 1989. Effects of the brown tide algae on growth, feeding physiology and locomotory behavior of scallop larvae (Argopecten irradians).. In: Cosper EM, Bricelj VM, Carpenter EJ (Eds.), Novel phytoplankton blooms: Causes and impacts of recurrent brown tides and other unusual blooms. Springer, Berlin, pp. 511–542. [Google Scholar]
- Gannon DP, McCabe EJB, Camilleri SA, Gannon JG, Brueggen MK, Barleycorn AA, Palubok VI, Kirkpatrick GJ, Wells RS, 2009. Effects of Karenia brevis harmful algal blooms on nearshore fish communities in southwest Florida. Mar. Ecol. Prog. Ser. 378, 171–186. [Google Scholar]
- Ganong WF, 1889. The economic mollusca of Acadia. Bull. Natur. Hist. Soc. N.B. 8, l16 p. [Google Scholar]
- Garcia AC, Bargu S, Dash P, Rabalais NN, Sutor M, Morrison W, Walker ND, 2010. Evaluating the potential risk of microcystins to blue crab (Callinectes sapidus) fisheries and human health in a eutrophic estuary. Harmful Algae 9, 134–143. [Google Scholar]
- Gastrich MD, Bell JL, Gobler CJ, Anderson OR, Wilhelm SW, 2004. Viruses as potential regulators of regional brown tide blooms caused by the alga, Aureococcus anophagefferens: a comparison of bloom years 1999–2000 and 2002. Estuaries 27, 112–119. [Google Scholar]
- Geesey M, Tester PA, 1993. Gymnodinium breve: ubiquitous in Gulf of Mexico waters? In: Smayda TJ, Shimizu Y (Eds.), Toxic Phytoplankton Blooms in the Sea. Elsevier, New York, pp. 251–255. [Google Scholar]
- Geraci JR, Anderson DM, Timperi RJ, St. Aubin DJ, Early GA, Prescott JH, Mayo CA, 1989. Humpback whales (Megaptera novaeangliae) fatally poisoned by dinoflagellate toxin. Can. J. Fish. Aquat. Sci. 46, 1895–1898. [Google Scholar]
- Gessner BD 1996. Epidemiology of paralytic shellfish poisoning outbreaks in Alaska. Alaska Mar. Res. 8, 16–17. [Google Scholar]
- Gessner BD, Middaugh JP, 1995. Paralytic shellfish poisoning in Alaska: A 20-year retrospective analysis. Amer. J. Epidemiol, 141, 766–770. [DOI] [PubMed] [Google Scholar]
- Gessner BD, McLaughlin JB, 2008. Epidemiologic impact of toxic episodes: neurotoxic toxins. In: Botana LM, editor. Seafood and Freshwater Toxins: pharmacology, physiology, and detection, 2nd edition. CRC Press. Boca Raton, pp. 77–104. [Google Scholar]
- Gibble CM, Kudela RM, 2014. Detection of persistent microcystin toxins at the land–sea interface in Monterey Bay, California. Harmful Algae, 39, 146–153. [Google Scholar]
- Gibble CM, Peacock MB, Kudela RM, 2016. Evidence of freshwater algal toxins in marine shellfish: Implications for human and aquatic health. Harmful Algae 59, 59–66. [DOI] [PubMed] [Google Scholar]
- Giner J-L, Zhao H, Tomas C, 2008. Sterols and fatty acids of three harmful algae previously assigned as Chattonella. Phytochemistry 69(11), 2167–2171. [DOI] [PubMed] [Google Scholar]
- Gingold DB, Strickland MJ, Hess JJ, 2014. Ciguatera fish poisoning and climate change: analysis of National Poison Center data in the United States, 2001–2011. Environ. Health Perspect. 122 (6), 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glibert PM, Wazniak CE, Hall MR, Sturgis B, 2007. Seasonal and interannual trends in nitrogen and brown tide in Maryland’s coastal bays. Ecol. Appl. 17, S79–S87. [Google Scholar]
- Gobler CJ, Sañudo-Wilhelmy SA, 2001. Effects of organic carbon, organic nitrogen, inorganic nutrients, and iron additions on the growth of phytoplankton and bacteria during a brown tide bloom. Mar. Ecol. Prog. Ser. 209, 19–34. [Google Scholar]
- Gobler CJ, Sunda WG, 2012. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae, 14, 36–45. [Google Scholar]
- Gobler CJ, Renaghan MJ, Buck NJ, 2002. Impacts of nutrients and grazing mortality on the abundance of Aureococcus anophagefferens during a New York brown tide bloom. Limnol. Oceanogr. 47, 129–141. [Google Scholar]
- Gobler CJ, Boneillo GE, Debenham C, Caron DA, 2004. Nutrient limitation, organic matter cycling, and plankton dynamics during an Aureococcus anophagefferens bloom in Great South Bay, NY. Aquat. Microb. Ecol. 35, 31–43. [Google Scholar]
- Gobler CJ, Berry DL, Anderson OR, Burson A, Koch F, Rodgers BS, Moore LK, Goleski JA, Allam B, Bowser P, Tang YZ, Nuzzi R, 2008. Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. Harmful Algae 7, 293–307. [Google Scholar]
- Gobler CJ, Berry DL, Dyhrman ST, Wilhelm SW, Salamov A, Lobanov AV, Zhang Y, Collier JL, Wurch LL, Kustka AB, Dill BD, Shah M, VerBerkmoes NC, Kuo A, Terry A, Pangilinan J, Lindquist EA, Lucas S, Paulsen IT, Hattenrath-Lehmann TK, Talmage SC, Walker EA, Koch F, Burson AM, Marcoval MA, Tang YZ, Lecleir GR, Coyne KJ, Berg GM, Bertrand EM, Saito MA, Gladyshev VN, Grigoriev IV, 2011. Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proc. Natl. Acad. Sci. USA 108, 4352–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobler CJ, Burson A, Koch F, Tang Y, Mulholland MR, 2012. The role of nitrogenous nutrients in the occurrence of harmful algal blooms caused by Cochlodinium polykrikoides in New York estuaries (USA). Harmful Algae, 17, 64–74. [Google Scholar]
- Gobler CJ, Koch F, Kang Y, Berry DL, Tang YZ, Lasi M, Walters L, Hall L, Miller JD, 2013. Expansion of harmful brown tides caused by the pelagophyte, Aureoumbra lagunensis DeYoe et Stockwell, to the US east coast. Harmful Algae, 27, 29–41. [Google Scholar]
- Gobler CJ, Doherty OM, Hattenrath-Lehmann TK, Griffith AW, Kang Y, Litaker RW, 2017. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. 114, 4975–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez F, Richlen ML, Anderson DM 2017. Molecular characterization and morphology of Cochlodinium strangulatum, the type species of Cochlodinium, and Margalefidinium gen. nov. for C. polykrikoides and allied species (Gymnodiniales, Dinophyceae). Harmful Algae 63, 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshorn D, Deeds J, Tango P, Poukish C, Place A, McGinty M, Butler W, Luckett C, Magnien R, 2004. Occurrence of Karlodinium micrum and its association with fish kills in Maryland estuaries, In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA (Eds.), Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, FL, U.S.A, pp. 361–363. [Google Scholar]
- Graber N, Stavinsky F, Hoffman R, Button J, Clark N, Martin S, Robertson A, Hustedt J, 2013. Ciguatera fish poisoning - New York City, 2010–2011. MMWR. Morbidity and Mortality Weekly Report 62 (4), 61. [PMC free article] [PubMed] [Google Scholar]
- Gribble KE, Keafer BA, Quilliam MA, Cembella AD, Kulis DM, Manahan A, Anderson DM, 2005. Distribution and toxicity of Alexandrium ostenfeldii (Dinophyceae) in the Gulf of Maine, USA. Deep Sea Res. Part II: Top. Stud. Oceanogr, 52(19–21), 2745–2763. [Google Scholar]
- Griffith AW, Shumway SE, Gobler CJ, 2019a. Differential mortality of North Atlantic bivalve molluscs during harmful algal blooms caused by the dinoflagellate, Cochlodinium (aka Margalefidinium) polykrikoides. Estuaries Coast 42, 190–203. [Google Scholar]
- Griffith AW, Doherty OM, Gobler CJ 2019b. Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms. Proc. R. Soc. B 286, 20190340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland F, 2000. Domoic acid toxicity in California sea lions (Zalophus californianus) stranded along the central California coast, May-October 1998. Report to the National Marine Fisheries Service Working group on Unusual Marine Mammal Mortality Events. NOAA Technical Memorandum NMFS-OPR-17, 45 pp. [Google Scholar]
- Gunter G, 1942. Recurrent summer fish mortalities on the Texas coast. Amer. Midl. Nat. 28, 631. [Google Scholar]
- Gunter G, 1952. The importance of catastrophic mass mortalities for marine fisheries along the Texas coast. J Wildl Manag 16(1), 63–69. [Google Scholar]
- Gunter G, Smith FGW, Williams RH, 1947. Mass mortality of marine animals on the lower west coast of Florida, November 1946 - January 1947. Science 105, 256–257. [DOI] [PubMed] [Google Scholar]
- Gunter G, Williams RH, Davis CC, Smith FGW, 1948. Catastrophic mass mortality of marine animals and coincident phytoplankton bloom on the west coast of Florida, November 1946 to August 1947. Ecol. Monogr. 18, 309–324. [Google Scholar]
- Hairston NG, Holtmeier CL, Lampert W, Weider LJ, Post DM, Fisher JM, Caceres CE, Gaedke U, 2002. Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution 55, 2203–2214. [DOI] [PubMed] [Google Scholar]
- Hales S, Weinstein P, Woodward A, 1999. Ciguatera (fish poisoning), El Nino, and Pacific sea surface temperatures. Ecosyst. Health 5, 20–25. [Google Scholar]
- Hallegraeff GM, 2010. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge 1. J. Phycol. 46(2), 220–235. [Google Scholar]
- Hallegraeff G.M. A. Zingone, and Enevoldsen H (eds). Global Harmful Algal Bloom Status Reporting. HARALG. Vol.100. [DOI] [PubMed] [Google Scholar]
- Harding JM, Mann R, Moeller P, Hsia MS, 2009. Mortality of the veined rapa whelk, Rapana venosa, in relation to a bloom of Alexandrium monilatum in the York River, United States. J. Shellfish Res. 28, 363–367. [Google Scholar]
- Hargraves PE, Maranda L, 2002. Potentially toxic or harmful microalgae from the northeast coast. Northeastern Naturalist 9, 81–120. [Google Scholar]
- Harke MJ, Gobler CJ, Shumway SE, 2011. Suspension feeding by the Atlantic slipper limpet (Crepidula fornicata) and the northern quahog (Mercenaria mercenaria) in the presence of cultured and wild populations of the harmful brown tide alga, Aureococcus anophagefferens. Harmful Algae 10, 503–511. [Google Scholar]
- Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW, 2016. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54, 4–20. [DOI] [PubMed] [Google Scholar]
- Harper DEJ, Guillen G, 1989. Occurrence of a dinoflagellate bloom associated with an influx of low salinity water at Galveston, Texas, and coincident mortalities of demersal fish and benthic invertebrates. Contrib. Mar. Sci. 31, 147–161. [Google Scholar]
- Harred LB, Campbell L, 2014. Predicting harmful algal blooms: a case study with Dinophysis ovum in the Gulf of Mexico. J. Plankton Research, 36, 1434–1445. [Google Scholar]
- Hattenrath TK, Anderson DM, Gobler CJ, 2010. The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) estuary. Harmful Algae 9, 402–412. [Google Scholar]
- Hattenrath-Lehmann T, Gobler CJ, 2015. The contribution of inorganic and organic nutrients to the growth of a North American isolate of the mixotrophic dinoflagellate, Dinophysis acuminata. Limnol. Oceanogr, 60(5), 1588–1603. [Google Scholar]
- Hattenrath-Lehmann TK, Marcoval MA, Berry DL, Fire S, Wang Z, Morton SL, Gobler CJ, 2013. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae, 26, 33–44. [Google Scholar]
- Hattenrath-Lehmann TK, Marcoval MA, Mittlesdorf H, Goleski JA, Wang Z, Haynes B, Morton SL, Gobler CJ, 2015. Nitrogenous nutrients promote the growth and toxicity of Dinophysis acuminata during estuarine bloom events. PloS One, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattenrath-Lehmann TK, Zhen Y, Wallace RB, Tang YZ, Gobler CJ, 2016. Mapping the distribution of cysts from the toxic dinoflagellate Cochlodinium polykrikoides in bloom-prone estuaries by a novel fluorescence in situ hybridization assay. Appl. Environ. Microbiol. 82, 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattenrath-Lehmann TK, Lusty MW, Wallace RB, Haynes B, Wang Z, Broadwater M, Chytalo K, 2018. Evaluation of rapid, early warning approaches to track shellfish toxins associated with Dinophysis and Alexandrium blooms. Mar. Drugs, 16, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood AJ, Steidinger KA, Truby EW, Bergquist PR, Bergquist PL, Adamson J, Mackenzie L, 2004. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J Phycol 40(1), 165–179. [Google Scholar]
- Heil CA, Dixon LK, Hall E, Garrett M, Lenes JM, O’Neil JM, Walsh BM, Bronk DA, Killberg-Thoreson L, Hitchcock GL, Meyer KA, Mulholland MR, Procise L, Kirkpatrick GJ, Walsh JJ, Weisberg RW, 2014. Blooms of Karenia brevis (Davis) G. Hansen & Ø. Moestrup on the West Florida Shelf: Nutrient sources and potential management strategies based on a multi-year regional study. Harmful Algae 38 (C), 127–140. [Google Scholar]
- Heinig C, Campbell D, 1992. The environmental context of a Gyrodinium aureolum bloom and shellfish kill in Maquoit Bay, Maine, September 1988. J. Shellfish Res. 11, 111–122. [Google Scholar]
- Henrichs DW, Renshaw MA, Gold JR, Campbell L, 2013. Genetic diversity among clonal isolates of Karenia brevis as measured with microsatellite markers. Harmful Algae 21–22, 30–36. [Google Scholar]
- Henrichs DW, Hetland RD, Campbell L, 2015. Identifying bloom origins of the toxic dinoflagellate Karenia brevis in the western Gulf of Mexico using a spatially explicit individual-based model. Ecol Model. 313, 251–258. [Google Scholar]
- Hershberger PK, Rensel JE, Postel JR, Taub FB, 1997. Heterosigma bloom and associated fish kill. Harmful Algae News 16, 1–4. [Google Scholar]
- Hetland RD, Campbell L, 2007. Convergent blooms of Karenia brevis along the Texas coast. Geophys Res Lett 34(19), 5. [Google Scholar]
- Heyning JE, 2003. Final report on the multi-species marine mammal unusual mortality event along the southern California coast 2002. National Marine Fisheries Service Technical Memorandum; 59 pp. [Google Scholar]
- Ho MS, Zubkoff PL, 1979. The effects of a Cochlodinium heterolobatum bloom on the survival and calcium uptake by larvae of the American oyster, Crassostrea virginica. In: Taylor F, Seliger H (Eds.), Toxic Dinoflagellate Blooms. Elsevier, NY, pp. 409–412. [Google Scholar]
- Holmes R, William P, Eppley R, 1967. Red water in La Jolla Bay, 1964–1966. Limnol. Oceanogr. 12, 503–512. [Google Scholar]
- Horner RA 1998. Harmful Algal Blooms in Puget Sound: General Perspective. 4th Puget Sound Research Conference, Seattle, WA, March 12-13, 1998. [Google Scholar]
- Horner RA, Postel JR, 1993. Toxic diatoms in western Washington waters (U.S. West Coast). Hydrobiologia 269, 197–205. [Google Scholar]
- Horner RA, Garrison DL, Plumley FG, 1997. Harmful algal blooms and red tide problems on the U.S. West Coast. Limnol. Oceanogr. 42, 1076–1088. [Google Scholar]
- Howell JF, 1953. Gonyaulax monilata, sp.nov., the causative dinoflagellate of a red tide on the east coast of Florida in August-September, 1951. Trans. Am. Microsc. Soc. 72, 153–156. [Google Scholar]
- Hu C, Murch B, Corcoran AA, Zheng L, Barnes BB, Weisberg RH, Atwood K, Lenes JM, 2015. Developing a smart semantic web with linked data and models for near-real-time monitoring of red tides in the Eastern Gulf of Mexico. IEEE Systems J. 10(3), 1282–1290. [Google Scholar]
- Hudnell HK, Dortch Q, Zenick H, 2008. An overview of the interagency, international symposium on cyanobacterial harmful algal blooms (ISOC-HAB): advancing the scientific understanding of freshwater harmful algal blooms, Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer, pp. 1–16. [DOI] [PubMed] [Google Scholar]
- Hurst Jr., J. W., 1975. History of paralytic shellfish poisoning on the Maine coast. In: Toxic Dinoflagellate Blooms (LoCicero VR, ed.). Proceedings of the International Conference (lst) Massachusetts Science and Technology Foundation, 525–528. [Google Scholar]
- Imai I, Itakura S, 1999. Importance of cysts in the population dynamics of the red tide flagellate Heterosigma akashiwo (Raphidophyceae). Mar. Biol. 133(4), 755–762. [Google Scholar]
- Ingersoll E, 1882. On the fish mortality in the Gulf of Mexico. Proc. U. S. Natl. Mus. 4, 74–80. [Google Scholar]
- James KJ, Gillman M, Amandi MF, Lopez-Rivera A, Puente PF, Lehane M, Mitrovic S, Furey A, 2005. Amnesic shellfish poisoning toxins in bivalve molluscs in Ireland. Toxicon 46, 852–858. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, 2011. Mixotrophy in red tide algae raphidophytes 1. J. Eukaryot. Microbiol. 58 (3), 215–222. [DOI] [PubMed] [Google Scholar]
- Jessup DA, Miller MA, Ryan JP, Nevins HM, Kerkering HA, Mekebri A, Crane DB, Johnson TA, Kudela RM, 2009. Mass stranding of marine birds caused by a surfactant-producing red tide. PloS One 4, e4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Divine LM, Renner H, Knowles S, Lefebvre KA, Burgess HK, Wright C, Parrish JK, 2019. Unusual mortality of Tufted puffins (Fratercula cirrhata) in the eastern Bering Sea. PloS One 14 (5) e0216532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranovic LR, 1989. Determination of the toxic/mutagenic potential of toxins associated with ciguatera dinoflagellates. Master of Science Thesis, University of Arizona, Tuscon, AZ. [Google Scholar]
- Kang Y, Gobler CJ, 2018. The brown tide algae, Aureococcus anophagefferens and Aureoumbra lagunensis (Pelagophyceae), allelopathically inhibit the growth of competing microalgae during harmful algal blooms. Limnol. Oceanogr. 63, 985–1003. [Google Scholar]
- Kang Y, Koch F, Gobler CJ, 2015. The interactive roles of nutrient loading and zooplankton grazing in facilitating the expansion of harmful algal blooms caused by the pelagophyte, Aureoumbra lagunensis, to the Indian River Lagoon, FL, USA. Harmful Algae, 49, 162–173. [Google Scholar]
- Kang Y, Kudela RM, Gobler CJ, 2017. Quantifying nitrogen assimilation rates of individual phytoplankton species and plankton groups during harmful algal blooms via sorting flow cytometry. Limnol. Oceanogr. Methods, 15(8), 706–721. [Google Scholar]
- Karlen D, Campbell K, 2012. The distribution of Pyrodinium bahamense cysts in Old Tampa Bay sediments. Tampa Bay Estuary Program Technical Report #07–12. Environmental Protection Commission of Hillsborough County, Tampa, Florida, 41 pp. [Google Scholar]
- Kavanaugh KE, Derner K, Fisher KM, Davis EEP, 2013. Assessment of the Eastern Gulf of Mexico Harmful Algal Bloom Operational Forecast System (GOMX HAB-OFS): a comparative analysis of forecast skill and utilization from May 1, 2008 to April 30, 2014, NOAA Technical Report NOS CO-OPS 066. [Google Scholar]
- Keesing JK, Liu D, Fearns P, Garcia R, 2011. Inter-and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar. Pollut. Bull. (6), 1169–1182. [DOI] [PubMed] [Google Scholar]
- Keller AA, Rice RL, 1989. Effects of nutrient enrichment on natural populations of the brown tide phytoplankton Aureococcus anophagefferens (Chrysophyceae). J. Phycol. 25, 636–646. [Google Scholar]
- Kempton JW, Lewitus AJ, Deeds JR, Law JM, Place AR, 2002. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae 1, 233–241. [Google Scholar]
- Khlebnikov KT 1837. BARANOV chief manager of the Russian colonies in America. Kingston, Ontario: Limestone Press. [1973 English translation of the Russian original]. 319 pp. [Google Scholar]
- Kibler SR, Litaker RW, Holland WC, Vandersea MW, Tester PA, 2012. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae 19, 1–14. [Google Scholar]
- Kibler SR, Tester PA, Kunkel KE, Moore SK and Litaker RW, 2015. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 316, 194–210. [Google Scholar]
- Kim HG, 1998. Harmful algal blooms in Korean coastal waters focused on three fish killing dinoflagellates. In: Lee H, Lee S, Lee C (Eds.), Harmful Algal Blooms in Korea and China. National Fisheries Research and Development Institute, Pusan, Korea, pp. 1–20. [Google Scholar]
- Kim J-H, Tillmann U, Adams NG, Krock B, Stutts WL, Deeds JR, Han M-S, Trainer VL 2017. Identification of Azadinium species and a new azaspiracid from Azadinium poporum in Puget Sound, Washington State, U.S.A. Harmful Algae. 68, 152–167. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG, 2006. Environmental exposures to Florida red tides: effects on emergency room respiratory diagnoses admissions. Harmful Algae 5(5), 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Kang Y, Villareal TA, Anderson DM, Gobler CJ 2014a. A novel immunofluorescence flow cytometry technique detects the expansion of brown tides caused by Aureoumbra lagunensis to the Caribbean Sea. App. Environ. Microbiol. 80, 4947–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Burson A, Tang YZ, Collier JL, Fisher NS, Sanudo-Wilhelmy S, Gobler CJ, 2014b. Alteration of plankton communities and biogeochemical cycles by harmful Cochlodinium polykrikoides (Dinophyceae) blooms. Harmful Algae 33, 41–54. [Google Scholar]
- Kofoid CA, Swezy O, 1921. The free living unarmoured Dinoflagellata. Berkeley: Memoirs of the University of California. [Google Scholar]
- Kramer BJ, Davis TW, Meyer KA, Rosen BH, Goleski JA, Dick GJ, Oh G, Gobler CJ, 2018. Nitrogen limitation, toxin synthesis potential, and toxicity of cyanobacterial populations in Lake Okeechobee and the St. Lucie River Estuary, Florida, during the 2016 state of emergency. PloS One 13: e0196278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuder C, Mazet JA, Bossart GD, Carpenter TE, Holyoak M, Elie MS, Wright SD, 2002. Clinicopathologic features of suspected brevetoxicosis in double-crested cormorants (Phalacrocorax auritus) along the Florida Gulf Coast. J. Zoo Wildl. Med. 33, 8–15. [DOI] [PubMed] [Google Scholar]
- Kreuder C, Miller MA, Jessup DA, Lowenstine LJ, Harris MD, Ames JA, Carpenter TE, Conrad PA, Mazet JA, 2003. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. J. Wildl. Dis. 39, 495–509. [DOI] [PubMed] [Google Scholar]
- Kreuder C, Miller MA, Lowenstine LJ, Conrad PA, Carpenter TE, Jessup DA and Mazet JA, 2005. Evaluation of cardiac lesions and risk factors associated with myocarditis and dilated cardiomyopathy in southern sea otters (Enhydra lutris nereis). Am. J. Vet. Res. 66, 289–299. [DOI] [PubMed] [Google Scholar]
- Kubanek J, Hicks MK, Naar J, Villareal TA, 2005. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol. Oceanogr. 50(3), 883–895. [Google Scholar]
- Kudela RM, Gobler CJ, 2012. Harmful dinoflagellate blooms caused by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14, 71–86. [Google Scholar]
- Kudela R, Ryan J, Blakely M, Lane J, Peterson T, 2008. Linking the physiology and ecology of Cochlodinium to better understand harmful algal bloom events: A comparative approach. Harmful Algae 7, 278–292. [Google Scholar]
- Kudela RM, Seeyave S, Cochlan WP, 2010. The role of nutrients in regulation and promotion of harmful algal blooms in upwelling systems. Prog. Oceanogr. 85, 122–135. [Google Scholar]
- Kvitek RG, Goldberg JD, Smith GJ, Doucette GJ, Silver MW, 2008. Domoic acid contamination within eight representative species from the benthic food web of Monterey Bay, California, USA Mar. Ecol. Prog. Ser. 367, 35–47. [Google Scholar]
- Lalone RC, DeVillez ED, Larson E, 1963. An assay of the toxicity of the Atlantic puffer fish, Spheroides maculatus. Toxicon 1, 159–164. [Google Scholar]
- Landsberg JH, 2002. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 10, 113–390. [Google Scholar]
- Landsberg JH, Balazs GH, Steidinger KA, Baden DG, Work TM, Russell DJ, 1999. The potential role of natural tumor promoters in marine turtle fibropapillomatosis. J. Aquat. Anim. Health 11, 199–210. [Google Scholar]
- Landsberg JH, Hall S, Johannessen JN, White KD, Conrad SM, Abbott JP, Flewelling LJ, Richardson RW, Dickey RW, Jester EL, Etheridge SM, Deeds JR, Van Dolah FM, Leighfield TA, Zou Y, Beaudry CG, Benner RA, Rogers PL, Scott PS, Kawabata K, Wolny JL, Steidinger KA, 2006. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 114, 1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg JH, Flewelling LJ, Naar J, 2009. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: decadal advancements. Harmful Algae 8, 598–607. [Google Scholar]
- Landsberg JH, Lefebvre K, Flewelling L, 2014. Effects of toxic microalgae on marine organisms, In: Rossini GP (Ed.), Toxins and Biologically Active Compounds from Microalgae. CRC Press, Boca Raton, Florida, pp. 379–449. [Google Scholar]
- Lapointe BE, 1997. Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limn. Oceanogr. 42, 1119–1131. [Google Scholar]
- Lapointe BE and Bedford BJ, 2007. Drift rhodophyte blooms emerge in Lee County, Florida, U.S.A: evidence of escalating coastal eutrophication. Harmful Algae, 6(3), 421–437. [Google Scholar]
- LaRoche J, Nuzzi R, Waters R, Wyman K, Falkowski PG, Wallace DWR, 1997. Brown tide blooms in Long Island’s coastal waters linked to interannual variability in groundwater flow. Glob. Change Biol. 3, 397–410. [Google Scholar]
- Leavitt D, Karney R, Surier A, 2010. Biology of the Bay Scallop. Northeastern Regional Aquaculture Center 213, 1–8. [Google Scholar]
- Lee C, 1980. Fish poisoning with particular reference to ciguatera. J. Trop. Med. Hyg. 83, 93–97. [PubMed] [Google Scholar]
- Lefebvre KA, Powell CL, Busman M, Doucette CJ, Moeller PDR, Sliver JB, Miller PE, Hughes MP, Singaram S, Silver MW, Tjeerdema RS, 1999. Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Nat. Toxins 7, 85–92. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Dovel SL, Silver MW, 2001. Tissue distribution and neurotoxic effects of domoic acid in a prominent vector species, the northern anchovy. Mar. Biol. 138(4), 693–700. [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, Silver MW, 2002a. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon 40, 971–977. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Silver MW, Coale SL, Tjeerdema TS, 2002b. Domoic acid in planktivorous fish in relation to toxic Pseudo-nitzschia cell densities. Mar. Biol. 140, 625–631. [Google Scholar]
- Lefebvre KA, Noren DP, Schultz IR, Bogard SM, Wilson J, Eberhart BL, 2007. Uptake, tissue distribution and excretion of domoic acid after oral exposure in coho salmon (Oncorhynchus kisutch). Aquat. Toxicol. 81(3), 266–274. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Robertson A, Frame ER, Colegrove KM, Nance S, Baugh KA, Wiedenhoft H, Gulland FMD, 2010. Clinical signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of toxin detection methods. Harmful Algae 9, 374–383. [Google Scholar]
- Lefebvre KA, Frame ER, Kendrick PS, 2012. Domoic acid and fish behavior: A review. Harmful Algae 13, 126–130. [Google Scholar]
- Lefebvre KA, Quakenbush L, Frame E, Burek Huntington K, Sheffield G, Stimmelmayr R, Bryan A, Kendrick P, Ziel H, Dickerson B and Gill V, 2016. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful Algae 55, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane L, Lewis R, 2000. Ciguatera: recent advances but the risk remains. Int. J. Food Microbiol. 61, 91–125. [DOI] [PubMed] [Google Scholar]
- Lekan DK, Tomas CR, 2010. The brevetoxin and brevenal composition of three Karenia brevis clones at different salinities and nutrient conditions. Harmful Algae 9(1), 39–47. [Google Scholar]
- Lelong A, Hégaret H, Soudant P, Bates SS, 2012a. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: revisiting previous paradigms. Phycologia 51, 168–216. [Google Scholar]
- Lenes JM, Heil CA, 2010. A historical analysis of the potential nutrient supply from the N2 fixing marine cyanobacterium Trichodesmium spp. to Karenia brevis blooms in the eastern Gulf of Mexico. J Plankton Res 32(10), 1421–1431. [Google Scholar]
- Leverone JR, Blake NJ, Pierce RH, Shumway SE, 2006. Effects of the dinoflagellate Karenia brevis on larval development in three species of bivalve mollusc from Florida. Toxicon 48, 75–84. [DOI] [PubMed] [Google Scholar]
- Lewitus AJ, Horner RA, Caron DA, Garcia-Mendoza E, Hickey BM, Hunter MD, Huppert DD, Kudela RM, Langlois GW, Largier JL, Lessard EJ, RaLonde R, Rensel JEJ, Strutton PG, Trainer VL, Tweddle JF, 2012. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 19, 133–159. [Google Scholar]
- Li Y, Meseck SL, Dixon MS, Rivara K, Wikfors GH 2012. Temporal variability in phytoplankton removal by a commercial, suspended eastern oyster nursery and effects on local plankton dynamics. J Shellfish Res 31, 1077–1089. [Google Scholar]
- Litz JA, Baran MA, Bowen-Stevens SR, Carmichael RH, Colegrove KM, Garrison LP, Fire SE, Fougeres EM, Hardy R, Holmes S, Jones W, Mase-Guthrie BE, Odell DK, Rosel PE, Saliki JT, Shannon DK, Shippee SF, Smith SM, Stratton EM, Tumlin MC, Whitehead HR, Worthy GAJ, Rowles TK, 2014. Review of historical unusual mortality events (UMEs) in the Gulf of Mexico (1990–2009): providing context for the multi-year northern Gulf of Mexico cetacean UME declared in 2010. Dis. Aquat. Org. 112, 161–175. [DOI] [PubMed] [Google Scholar]
- Liu H, Laws EA, Villareal TA, Buskey EJ, 2001. Nutrient-limited growth of Aureoumbra lagunensis (Pelagophyceae), with implications for its capability to outgrow other phytoplankton species in phosphate-limited environments. J. Phycol. 37, 500–508. [Google Scholar]
- Liu Y, Hu Z, Deng Y, Tang YZ, 2020. Evidence for production of sexual resting cysts by the toxic dinoflagellate Karenia mikimotoi in clonal cultures and marine sediments. J. Phycol. 56(1), 121–134. [DOI] [PubMed] [Google Scholar]
- Longo S, Sibat M, Viallon J, Darius HT, Hess P, Chinain M, 2019. Intraspecific variability in the toxin production and toxin profiles of in vitro cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia. Toxins 11 (12), 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale DJ, Cosper EM, Kim W-S, Doall MH, Divadeenam A, Jonasdottir SH, 1996. Food web interactions in the plankton of Long Island bays, with preliminary observations on brown tide effects. Mar. Ecol. Prog. Ser. 134, 247–263. [Google Scholar]
- Lopez CB, Smith CG, Marot ME, Karlen DJ, Corcoran AA, 2015. The role of seedbeds in Pyrodinium bahamense bloom dynamics in Tampa Bay. Navigating Changing Tides, Basis 6: Proceedings of the Sixth Tampa Bay Area Scientific Information Symposium, 28–30th September 2015, St. Petersburg, Florida, 78. [Google Scholar]
- Lopez CB, Karim A, Murasko S, Marot M, Smith CG, Corcoran AA, 2019. Temperature mediates secondary dormancy in resting cysts of Pyrodinium bahamense (Dinophyceae). J. Phycol, 55(4), 924–935. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivera A, Pinto M, Insinilla A, Suarez Isla B, Uribe E, Alvarez G, Lehane M, Furey A, James KJ, 2009. The occurrence of domoic acid linked to a toxic diatom bloom in a new potential vector: the tunicate Pyura chilensis (piure). Toxicon 54, 754–762. [DOI] [PubMed] [Google Scholar]
- Lund EJ, 1936. Some facts relating to the occurrences of dead and dying fish on the Texas coast during June, July, and August, 1935, Annual Report of the Texas Game, Fish and Oyster Commission, 1934–35. Texas Game, Fish and Oyster Commission, pp. 47–50. [Google Scholar]
- Lundgren VM, Roelke DL, Brooks BW, Granéli E, Davis SL, Baty T, Scott WC, 2015. Prymnesium parvum invasion success into coastal bays of the Gulf of Mexico: Galveston Bay case study. Harmful Algae 43, 31–45. [Google Scholar]
- Luo Z, Krock B, Mertens KN, Price AM, Turner RE, Rabalais NN, Gu H, 2016. Morphology, molecular phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the Gulf of Mexico. Harmful Algae 55, 56–65. [DOI] [PubMed] [Google Scholar]
- MacIntyre HL, Lomas MW, Cornwell J, Suggett DJ, Gobler CJ, Koch EW, Kana TM, 2004. Mediation of benthic-pelagic coupling by microphytobenthos: an energy- and material-based model for initiation of blooms of Aureococcus anophagefferens. Harmful Algae 3, 403–437. [Google Scholar]
- Maclean JL, 1989. Indo-Pacific red tides, 1985–1988. Marine Pollution Bulletin. 20, 304. [Google Scholar]
- Magaña HA, Contreras C, Villareal TA, 2003. A historical assessment of Karenia brevis in the western Gulf of Mexico. Harmful Algae 2, 163–171. [Google Scholar]
- Mahoney JB, Jeffress D, Zeitlin C, Olsen PS, Grebe H, Brooks J 2003. Distribution of the brown tide picoplankter Aureococcus anophagefferens in western New York Bight coastal waters. Northeast Fisheries Science Center Reference Document 03–12. U. S. Department of Commerce, Woods Hole, MA. 23pp. [Google Scholar]
- Maier-Brown AF, Dortch Q, Van Dolah FM, Leighfield TA, Morrison W, Thessen AE, Steidinger KA, Richardson B, Moncreiff CA, Pennock JR, 2006. Effect of salinity on the distribution, growth, and toxicity of Karenia spp. Harmful Algae 5(2), 199–212. [Google Scholar]
- Maranda L, Shimizu Y, 1987. Diarrhetic shellfish poisoning in Narragansett Bay. Estuaries 10, 298–302. [Google Scholar]
- Maranda L, Corwin S, Hargraves PE, 2007a. Prorocentrum lima (Dinophyceae) in northeastern USA coastal waters: I. Abundance and distribution. Harmful Algae 6, 623–631. [Google Scholar]
- Maranda L, Corwin S, Dover S, Morton SL, 2007b. Prorocentrum lima (Dinophyceae) in northeastern USA coastal waters : II: Toxin load in the epibiota and in shellfish. Harmful Algae 6, 632–641. [Google Scholar]
- Martin JL, Haya K, Burridge LE, Wildish DJ, 1990. Nitzschia pseudodelicatissima –a source of domoic acid in the Bay of Fundy, eastern Canada. Mar. Ecol. Prog. Ser. 67, 177–182. [Google Scholar]
- Marshall HG, 2009. Phytoplankton of the York River. J. Coastal Res. 57, 59–65. [Google Scholar]
- Marshall HG, Egerton TA, Burchardt L, Cerbin S, Kokocinski M, 2005. Long term monitoring results of harmful algal populations in Chesapeake Bay and its major tributaries in Virginia, U.S.A. Oceanol. Hydrobiol. Stud. 34, Supp. 3, 35–41. [Google Scholar]
- Maryland Department of Natural Resources (MD DNR). 2009. –2018. Maryland Department of Natural Resources water quality monitoring for 2009–2018. [Google Scholar]
- Mazet JA, 2005. Evaluation of cardiac lesions and risk factors associated with myocarditis and dilated cardiomyopathy in southern sea otters (Enhydra lutris nereis). Am. J. Vet. Res. 66, 289–299. [DOI] [PubMed] [Google Scholar]
- McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, Gulland FMD, Thomson RE, Cochlan WP, Trainer VL, 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res, Lett. 43, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinness KL, Fryxell GA, McEachran JD, 1995. Pseudonitzschia species found in digestive tracts of northern anchovies (Engraulis mordax). Can. J. Zool. 73, 642–647. [Google Scholar]
- McKibben SM, Watkins-Brandt KS, Wood AM, Hunter M, Forster Z, Hopkins A, Du X, Eberhart BT, Peterson WT, White A 2015. Monitoring Oregon Coastal Harmful Algae: Observations and implications of a harmful algal bloom-monitoring project. Harmful Algae 50, 32–44. [Google Scholar]
- McKibben SM, Peterson W, Wood AM, Trainer VL, Hunter M, White AE 2017. Climatic regulation of the neurotoxin domoic acid. Proceedings of the National Academy of Sciences of the United States of America 114, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb P, Rhodes L, Adamson J, Holland P, 2006. Brevetoxin—an elusive toxin in New Zealand waters. Afr. J. Mar. Sci. 28(2), 375–377. [Google Scholar]
- Medlin LK, Desdevies Y, 2018. Sequence analysis confirms a new algal class. Vie et Milieu 68, 151–155. [Google Scholar]
- Meyer KF, Sommer H, Schoenholz P, 1928. Mussel poisoning. J. Prevent. Med. 2, 365–394. [Google Scholar]
- Meyers SD, Moss AJ, Luther ME, 2017. Changes in residence time due to large-scale infrastructure in a coastal plain estuary. J. Coastal Res. 33, 815–828. [Google Scholar]
- Miller MA, Kudela RM, Mekebri A, Crane D, Oates SC, Tinker MT, Staedler M, Miller WA, Toy-Choutka S, Dominik C, Hardin D, Langlois G, Murray M, Ward K, Jessup DA, 2010. Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to sea otters. PLoS One 5, e12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna PA, Stockwell DA, Kalke RD, 1993. Dwarf surf clam Mulinea lateralis (Say, 1822) populations and feeding during the Texas brown tide. J. Shellfish Res. 12, 833–842. [Google Scholar]
- Moore SK, Trainer VL, Mantua NJ,Parker MS, Laws EA, Fleming LE, Backer LC, 2008. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 7, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SK, Mantua NJ, Salathé EP Jr, 2011. Past trends and future scenarios for environmental conditions favoring the accumulation of paralytic shellfish toxins in Puget Sound shellfish. Harmful Algae 10(5), 521–529. [Google Scholar]
- Morse RE, Shen J, Blanco-Garcia JL, Hunley WS, Fentress S, Wiggins M, Mulholland MR, 2011. Environmental and physical controls on the formation and transport of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in the lower Chesapeake Bay and its tributaries. Estuaries Coasts 34, 1006–1025. [Google Scholar]
- Morse RE, Mulholland MR, Hunley WS, Fentress S, Wiggins M, Blanco-Garcia JL, 2013. Controls on the initiation and development of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in lower Chesapeake Bay and its tributaries. Harmful Algae, 28, 71–82. [Google Scholar]
- Morton SL, Leighfield TA, Haynes BL, Petitpain DL, Busman MA, Moeller PD, Bean L, Mcgowan J, Hurst JW, Van Dolah FM, 1999. Evidence of diarrhetic shellfish poisoning along the coast of Maine. J. Shell. Res. 18, 681–686. [Google Scholar]
- Muhlstein HI, Villareal TA, 2007. Organic and inorganic nutrient effects on growth rate-irradiance relationships in the Texas brown-tide alga Aureoumbra lagunensis (Pelagophyceae). J. Phycol. 43, 1223–1226. [Google Scholar]
- Mulholland MR, Gobler CJ, Lee C, 2002. Peptide hydrolysis, amino acid oxidation, and nitrogen uptake in communities seasonally dominated by Aureococcus anophagefferens. Limnol. Oceanogr. 47, 1094–1108. [Google Scholar]
- Mulholland MR, Boneillo GE, Bernhardt PW, Minor EC, 2009a. Comparison of nutrient and microbial dynamics over a seasonal cycle in a mid-Atlantic coastal lagoon prone to Aureococcus anophagefferens (brown tide) blooms. Estuaries Coasts 32, 1176–1194. [Google Scholar]
- Mulholland MR, Morse RE, Boneillo GE, Bernhardt PW, Filippino KC, Procise LA, Blanco-Garcia JL, Marshall HG, Egerton TA, Hunley WS, Moore KA, Berry DL, Gobler CJ, 2009b. Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries Coasts 32, 734–747. [Google Scholar]
- Murphy E, Steidinger K, Roberts B, Williams J, Jolley J Jr, 1975. An explanation for the Florida east coast Gymnodinium breve red tide of November 1972. Limnol. Oceanogr. 20(3), 481–486. [Google Scholar]
- Natsuike M, Matsuno K, Hirawake T, Yamaguchi A, Nishino S, Imai I, 2017. Possible spreading of toxic Alexandrium tamarense blooms on the Chukchi Sea shelf with the inflow of Pacific summer water due to climatic warming. Harmful Algae 61, 80–86. [Google Scholar]
- Newell RIE, Tettelbach ST, Gobler CJ, Kimmel DG, 2009. Relationships between reproduction in suspension-feeding hard clams, Mercenaria mercenaria, and phytoplankton community structure. Mar. Ecol. Prog. Ser. 387, 179–196. [Google Scholar]
- Nisbet ICT, 1983. Paralytic shellfish poisoning; effects on breeding terns. The Condor 85, 338–345. [Google Scholar]
- NOAA National Centers for Environmental Information. 2014. Physical and biological data collected along the Texas, Louisiana, Mississippi, Alabama, and Florida Gulf coasts in the Gulf of Mexico as part of the Harmful Algal Blooms Observing System from 1953–08-19 to 2020–03-09 (NCEI Accession 0120767). NOAA National Centers for Environmental Information. https://accession.nodc.noaa.gov/0120767 [accessed 10/2020]. [Google Scholar]
- Novoveská L, Robertson A, 2019. Brevetoxin-producing spherical cells present in Karenia brevis bloom: evidence of morphological plasticity? J. Mar. Sci. Eng. 7(2), 24. [Google Scholar]
- NSSP, 2017. National Shellfish Sanitation Program, Guide for the control of molluscan shellfish: 2017 revision, U.S. Food and Drug Administration and Interstate Shellfish Sanitation Conference, Washington, D.C., p. 478. [Google Scholar]
- Núñez-Vázquez E, Ramírez-Camarena C, Poot-Delgado C, Almazán-Becerril A, Aké-Castillo J, Pérez-Morales A, Hernández-Sandoval F, Fernández-Herrera L, Heredia-Tapia A, Band-Schmidt C, Bustillos-Guzman J, Lopez-Cortes DJ, Dominguez-Solis G, Ley-Martinez TC, Cauich-Sanchez YR, Barra Gonzalez LA, 2016. Toxinas marinas en el Golfo de México: orígenes e impactos, In: García Mendoza E, Quijano Scheggia SI, Olivos Ortiz A, Núñez-Vázquez EJ (Eds.), Florecimientos algales nocivos en México. CICESE, Ensenada, Mexico, pp. 308–321. [Google Scholar]
- Nuzzi R, Olsen PS, Mahoney JB, Zodi G, 1996. The first Aureococcus anophagefferens brown tide in New Jersey. Harmful Algae News 15, 8–9. [Google Scholar]
- Nuzzi R, Waters RA, 2004. Long-term perspective on the dynamics of brown tide blooms in Long Island coastal bays. Harmful Algae 3, 279–293. [Google Scholar]
- O’Neil JM, Davis TW, Burford MA, Gobler CJ, 2012. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 14, 313–334. [Google Scholar]
- Olsen PS, 1989. Development and distribution of a brown-water algal bloom in Barnegat Bay, New Jersey with perspective on resources and other red tides in the region. In: Cosper EM, Bricelj VM, Carpenter EJ (Eds.), Novel phytoplankton blooms: Causes and impacts of recurrent brown tides and other unusual blooms. Springer, Berlin, pp. 189–212. [Google Scholar]
- Onofrio MD, Egerton T, Reece K, Pease SKD, Sanderson MP, Jones W, Yeargan E, Roach A, DeMent C, Wood A, Reay WG, Place A, Smith JL (in press) Spatiotemporal distribution of phycotoxins and their co-occurrence within nearshore waters. Harmful Algae [DOI] [PubMed] [Google Scholar]
- Onuf CP, 1996. Seagrass responses to long-term light reduction by brown tide in upper Laguna Madre, Texas: distribution and biomass patterns. Mar. Ecol. Prog. Ser. 138, 219–231. [Google Scholar]
- O’Shea TJ, Bonde RK, Buergelt CD, Odell DK, 1991. An epizootic of Florida manatees associated with a dinoflagellate bloom. Mar. Mamm. Sci. 7, 165–179. [Google Scholar]
- Paerl HW, Huisman J, 2008. Blooms like it hot. Science 320, 57–58. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Fulton RS, Moisander PH, Dyble J, 2001. Harmful freshwater algal blooms with an emphasis on cyanobacteria. The Scientific World 1, 76–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW, Otten TG, Kudela R, 2018. Mitigating the expansion of harmful algal blooms across the freshwater-to-marine continuum. Environ. Sci. Technol. 52, 5519–5529. [DOI] [PubMed] [Google Scholar]
- Parsons ML, Aligizaki K, Bottein MYD, Fraga S, Morton SL, Penna A, Rhodes L, 2012. Gambierdiscus and Ostreopsis: reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 14, 107–129. [Google Scholar]
- Pastorok RA, Bilyard GR, 1985. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 21, 175–189. [Google Scholar]
- Peacock MB, Gibble CM, Senn DB, Cloern JE, Kudela RM, 2018. Blurred lines: Multiple freshwater and marine algal toxins at the land-sea interface of San Francisco Bay, California. Harmful Algae 73, 138–147. [DOI] [PubMed] [Google Scholar]
- Peery ZM, Beissinger SR, Burkett E, Newman SH, 2006. Local survival of marbled murrelets in central California: roles of oceanographic processes, sex, and radiotagging. J. Wildl. Manage. 70, 78–88. [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd ECD, Remis RS, 1990. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med, 322(25), 1775–1780. [DOI] [PubMed] [Google Scholar]
- Phillips EM, Zamon JE, Nevins HM, Gibble CM, Duerr RS, Kerr LH, 2011. Summary of birds killed by a harmful algal bloom along the south Washington and north Oregon coasts during October 2009. Northwest. Nat. 92, 120–126. [Google Scholar]
- Phlips EJ, Badylak S, Youn S, Kelley K, 2004. The occurrence of potentially toxic dinoflagellates and diatoms in a subtropical lagoon, the Indian River Lagoon, Florida, USA. Harmful Algae 3, 39–49. [Google Scholar]
- Phlips EJ, Badylak S, Bledsoe E, Cichra M, 2006. Factors affecting the distribution of Pyrodinium bahamense var. bahamense in coastal waters of Florida Mar. Ecol. Prog. Ser. 322, 99–115. [Google Scholar]
- Phlips EJ, Badylak S, Christman M, Wolny J, Brame J, Garland J, Hall L, Hart J, Landsberg J, Lasi M, Lockwood J, Paperno R, Scheidt D, Staples A, Steidinger K, 2011. Scales of temporal and spatial variability in the distribution of harmful algae species in the Indian River Lagoon, Florida, USA. Harmful Algae 10, 277–290. [Google Scholar]
- Phlips EJ, Badylak S, Lasi MA, Chamberlain R, Green WC, Hall LM, Hart JA, Lockwood JC, Miller JD, Morris LJ, Steward JS, 2015. From red tides to green and brown tides: Bloom dynamics in a restricted subtropical lagoon under shifting climatic conditions. Estuaries Coasts 38, 886–904. [Google Scholar]
- Phlips EJ, Badylak S, Nelson NG, Havens KE, 2020. Hurricanes, El Niño and harmful algal blooms in two sub-tropical Florida estuaries: Direct and indirect impacts. Scientific Reports 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place AR, Bowers HA, Bachvaroff TR, Adolf JE, Deeds JR, Sheng J, 2012. Karlodinium veneficum - The little dinoflagellate with a big bite. Harmful Algae 14, 179–195. [Google Scholar]
- Popels LC, Coyne KJ, Forbes R, Pustizzi F, Gobler CJ, Cary SC, Hutchins DA, 2003. The use of quantitative polymerase chain reaction for the detection and enumeration of the harmful alga Aureococcus anophagefferens in environmental samples along the United States east coast. Limnol. Oceanogr. Methods 1, 92–102. [Google Scholar]
- Pottier I, Vernoux JP, Lewis RJ, 2001. Ciguatera fish poisoning in the Caribbean islands and Western Atlantic. In: Reviews of Environmental Contamination and Toxicology. Springer, New York, NY, pp. 99–141. [DOI] [PubMed] [Google Scholar]
- Price DW, Kizer KW, Hangsen KH, 1991. California’s paralytic shellfish poisoning prevention program 1927–1989. J. Shellfish Res. 10, 119–145. [Google Scholar]
- Radke EG, Grattan LM, Cook RL, Smith TB, Anderson DM, Morris JG Jr, 2013. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. J. Trop. Med. Hyg. 88 (5), 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raho NS, Pizarro G, Escalera L, Reguera B, Marìn I, 2008. Morphology, toxin composition and molecular analysis of Dinophysis ovum Schütt, a dinoflagellate of the Dinophysis acuminata complex. Harmful Algae 7, 839–848. [Google Scholar]
- RaLonde R (Ed.)., 2001. Harmful algal blooms on the North American West Coast. University of Alaska Sea Grant, AK-SG-01–05, Fairbanks. [Google Scholar]
- Ralston DK, Keafer BA, Brosnahan ML, Anderson DM, 2014. Temperature dependence of an estuarine harmful algal bloom: Resolving interannual variability in bloom dynamics using a degree-day approach. Limnol. Oceanogr. 59(4), 1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall JE, 1958. A review of ciguatera, tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci. Gulf Caribb. 8, 236–267. [Google Scholar]
- Reguera B, Riobû P, Rodrìguez F, Dìaz PA, Pizarro G, Paz B, Franco JM, Blanco J, 2014. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 12, 394–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheuban JE, Williamson S, Costa JE, Glover DM, Jakuba RW, McCorkle DC, Neill C, Williams T, Doney SC, 2016. Spatial and temporal trends in summertime climate and water quality indicators in the coastal embayments of Buzzards Bay, Massachusetts. Biogeosci. 13, 253–265. [Google Scholar]
- Richardson B, Corcoran AA, 2015. Use of dissolved inorganic and organic phosphorus by axenic and nonaxenic clones of Karenia brevis and Karenia mikimotoi. Harmful Algae 48, 30–36. [DOI] [PubMed] [Google Scholar]
- Richlen ML, Morton SL, Jamali EA, Rajan A, Anderson DM, 2010. The catastrophic 2008–2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9, 163–172. [Google Scholar]
- Robertson A, Richlen ML, Erdner D, Smith TB, Anderson DM, Liefer J, Xu Y, McCarron P, Miles C, Parsons M, 2018. Toxicity, chemistry, and implications of Gamberdiscus silvae: A ciguatoxin superbug in the Greater Caribbean Region. International Conference on Harmful Algae (ICHA), 21–26 October, Nantes, France. [Google Scholar]
- Roelke DL, Barkoh A, Brooks BW, Grover JP, Hambright KD, LaClaire II JW, Moeller PDR, Patino R, 2016. A chronicle of a killer alga in the west: ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 764, 29–50. [Google Scholar]
- Rohrlack T, Hyenstrand P 2007. Fate of intracellular microcystins in the cyanobacterium Microcystis aeruginosa (Chroococcales, Cyanophyceae). Phycologia, 46, 277–283. [Google Scholar]
- Rounsefell GA, Nelson WR, 1966. Red-tide research summarized to 1964 including an annotated bibliography, Special Scientific Report - Fisheries. United States Department of the Interior, Fish and Wildlife Service, Bureau of Commercial Fisheries, Washington, DC, pp. 1–85. [Google Scholar]
- Ruff TA, 1989. Ciguatera in the Pacific: a link with military activities. The Lancet, 201–204. [DOI] [PubMed] [Google Scholar]
- Ryan JP, Kudela RM, Birch JM, Blum M, Bowers HA, Chavez FP, Doucette GJ, Hayashi K, Marin R, Mikulski CM, Pennington JT, Scholin CA, Smith GJ, Woods A, Zhang Y, 2017. Causality of an extreme harmful algal bloom in Monterey Bay, California, during the 2014–2016 northeast Pacific warm anomaly. Geophys. Res. Lett. 44, 5571–5579. [Google Scholar]
- Quayle DB, 1969. Paralytic shellfish poisoning in British Columbia. Bull. Fish. Res. Bd. Can. 168, 1–68. [Google Scholar]
- Quick JA Jr., Henderson GE, 1974. Effects of Gymnodinium breve red tides on fishes and birds: a preliminary report on behavior, anatomy, hematology, and histopathology, In: Amborski RL, Hood MA, Miller RR (Eds.), Proceedings of the Gulf Coast Regional Symposium on Diseases of Aquatic Animals. Louisiana Sea Grant: Louisiana State University, pp. 85–113. [Google Scholar]
- SCDHS, 1976-2020. Suffolk County Department of Health Services annual report on water quality conditions in Suffolk County, NY, USA. [Google Scholar]
- SCDHS. 1985-2018. Suffolk County Department of Health Services water quality monitoring for 1985–2018.
- Schaeffer BA, Kamykowski D, McKay L, Milligan EJ, 2009. Diel vertical migration thresholds of Karenia brevis (Dinophyceae). Harmful Algae 8(5), 692–698. [DOI] [PubMed] [Google Scholar]
- Scheuer PJ, Takahashi W, Tsutsumi J, Yoshida T, 1967. Ciguatoxin: Isolation and chemical nature. Science 155, 1267–1268. [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R 3rd, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM, 2000. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403 (6765), 80–84. [DOI] [PubMed] [Google Scholar]
- Schrey SE, Carpenter EJ, Anderson DM, 1984. The abundance and distribution of the toxic dinoflagellate, Gonyaulax tamarensis, in Long Island estuaries. Estuaries 7, 472–477. [Google Scholar]
- Schütt F, 1895. Peridineen der Plankton-Expedition. Ergebn. Plankton--Expedition der Humboldt-Stiftung 4, M, a, A, 1–170, 127 pps. [Google Scholar]
- Shearn-Bochsler V, Lance EW, Corcoran R, Piatt J, Bodenstein B, Frame E, Lawonn J, 2014. Fatal paralytic shellfish poisoning in Kittlitz’s Murrelet (Brachyramphus brevirostris) nestlings, Alaska, U.S.A. J. Wildl. Dis. 50, 933–937. [DOI] [PubMed] [Google Scholar]
- Sherwood ET, Greening H, Garcia L, Kaufman K, Janicki T, Pribble R, Cunningham B, Peene S, Fitzpatrick J, Dixon K, Wessel M 2016. Development of an integrated ecosystem model to determine effectiveness of potential watershed management projects on improving Old Tampa Bay. In: Stringer CE, Krauss KW, Latimer JS (Eds.), Headwaters to Estuaries: Advances in Watershed Science and Management - Proceedings of the Fifth Interagency Conference on Research in the Watersheds. March 2–5, 2015, North Charleston, South Carolina. e-General Technical Report SRS-211. U.S. Department of Agriculture Forest Service, Southern Research Station, Asheville, NC, pp. 156–163. [Google Scholar]
- Shultz D, Campbell L, Kudela RM, 2019. Trends in Dinophysis abundance and diarrhetic shellfish toxin levels in California mussels (Mytilus californianus) from Monterey Bay, California. Harmful Algae, 88. 101641. [DOI] [PubMed] [Google Scholar]
- Shumway SE, 1990. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 21, 65–104. [Google Scholar]
- Shumway SE, Sherman-Caswell S, Hurst JW, 1988. Paralytic shellfish poisoning in Maine: Monitoring a monster. J. Shellfish Res. 7, 643–652. [Google Scholar]
- Sieburth JM, Johnson PW, Hargraves PE, 1988. Ultrastructure and ecology of Aureococcus anophagefferens gen. et sp. nov. (Chrysophyceae): the dominant picoplankter during a bloom in Narragansett Bay, Rhode Island, summer 1985. J. Phycol. 24, 416–425. [Google Scholar]
- Sierra-Beltrán AP, Palafox-Uribe M, Grajales-Montiel J, Cruz-Villacorta A, Ochoa JL, 1997. Sea bird mortality at Cabo San Lucas, Mexico: Evidence that toxic diatom blooms are spreading. Toxicon 35, 447–453. [DOI] [PubMed] [Google Scholar]
- Silva ES, 1967. Cochlodinium heterolobactum n. sp.: Structure and some cytophysiological aspects. J. Protozoo. 14, 745–754. [Google Scholar]
- Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, Gulland F, 2005. Pathology of domoic acid toxicity in California sea lions (Zalophus californianus). Vet. Pathol. 42, 184–191. [DOI] [PubMed] [Google Scholar]
- Skinner MP, Brewer TD, Johnstone R, Fleming LE, Lewis RJ, 2011, Ciguatera fish poisoning in the Pacific Islands (1998 to 2008). PLoS Negl. Trop. Dis. 5(12), e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smayda TJ, 2007. Reflections on the ballast water dispersal--harmful algal bloom paradigm. Harmful Algae 6, 601–622. [Google Scholar]
- Smayda TJ, 2008. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae 8, 140–151. [Google Scholar]
- Smayda TJ, Reynolds CS, 2001. Community assembly in marine phytoplankton: application of recent models to harmful dinoflagellate blooms. J. Plankton Res. 23, 447–461. [Google Scholar]
- Smith GB, 1975. The 1971 red tide and its impact on certain reef communities in the mid-eastern Gulf of Mexico. Environ Lett 9, 141–152. [DOI] [PubMed] [Google Scholar]
- Smith J, Connell P, Evans RH, Gellene AG, Howard MD, Jones BH, Kaveggia S, Palmer L, Schnetzer A, Seegers BN, Seubert EL, 2018. A decade and a half of Pseudo-nitzschia spp. and domoic acid along the coast of southern California. Harmful Algae 79, 87–104. [DOI] [PubMed] [Google Scholar]
- Smith JK, Lonsdale DJ, Gobler CJ, Caron DA, 2008. Feeding behavior and development of Acartia tonsa nauplii on the brown tide alga Aureococcus anophagefferens. J. Plankton Res. 30, 937–950. [Google Scholar]
- Soliño L, Costa PR, 2020. Global impact of ciguatoxins and ciguatera fish poisoning on fish, fisheries and consumers. Environ. Res. 182, 109111. [DOI] [PubMed] [Google Scholar]
- Sommer H, Meyer KF, 1937. Paralytic shellfish poisoning. Arch. Path. 24, 560–598. [Google Scholar]
- Soto IM, Cambazoglu MK, Boyette AD, Broussard K, Sheehan D, Howden SD, Shiller AM, Dzwonkowski B, Hode L, Fitzpatrick PJ, Arnone RA, Mickle PF, Cressman K, 2018. Advection of Karenia brevis blooms from the Florida Panhandle towards Mississippi coastal waters. Harmful Algae 72, 46–64. [DOI] [PubMed] [Google Scholar]
- Steidinger KA, 1975. Basic factors influencing red tides. In: LoCicero VR (ed.). Toxic Dinoflagellate Blooms. Proceedings of the International conference (lst) Massachusetts Science and Technology Foundation, Wakefield, MA: pp. 153–162. [Google Scholar]
- Steidinger KA, 2009. Historical perspective on Karenia brevis red tide research in the Gulf of Mexico. Harmful Algae 8, 549–561. [Google Scholar]
- Steidinger KA, Burklew MA, Ingle RM, 1973. The effects of Gymnodinium breve toxin on estuarine animals. In: Martin DF, Padilla GM (Eds.), Marine Pharmacognosy: Action of Marine Toxins at the Cellular Level. Academic Press, New York (NY), pp. 179–202. [Google Scholar]
- Steidinger KA, Tester LS, Taylor FJR, 1980. A redescription of Pyrodinium bahamense var. compressa (Böhm) stat. nov. from Pacific red tides. Phycologia 19, 329–337. [Google Scholar]
- Steidinger KA, Landsberg JH, Truby EW, Roberts BS, 1998. First report of Gymnodinium pulchellum (Dinophyceae) in North America and associated fish kills in the Indian River, Florida. J. Phycol. 34, 431–437. [Google Scholar]
- Steward JS, Virnstein RW, Morris LJ, Lowe EF, 2005. Setting seagrass depth, coverage, and light targets for the Indian River Lagoon system, Florida. Estuaries 28, 923–935. [Google Scholar]
- St. Johns River Water Management District (SJRWMD). 2012-2018. St. Johns River Water Management District water quality monitoring for 2012–2018.
- Stumpf RP, Litaker RW, Lanerolle L, Tester P, 2008. Hydrodynamic accumulation of Karenia off the west coast of Florida. Cont Shelf Res 28(1), 189–213. [Google Scholar]
- Stumpf RP, Tomlinson MC, Calkins JA, Kirkpatrick B, Fisher K, Nierenberg K, Currier R, Wynne TT, 2009. Skill assessment for an operational algal bloom forecast system. J Mar Syst 76(1–2), 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerson HC, Peterson CH, 1990. Recruitment failure of the bay scallop, Argopecten irradians concentricus, during the first red tide, Ptychodiscus brevis, outbreaks recorded in North Carolina. Estuaries 13, 322–331. [Google Scholar]
- Sunda WG, Hardison DR, 2007. Ammonium uptake and growth limitation in marine phytoplankton. Limnol. Oceanogr. 52, 2496–2506. [Google Scholar]
- Sunda WG, Hardison DR, 2010. Evolutionary tradeoffs among nutrient acquisition, cell size, and grazing defense in marine phytoplankton promote ecosystem stability. Mar. Ecol. Prog. Ser. 401, 63–76. [Google Scholar]
- Sunda WG, Graneli E, Gobler CJ, 2006. Positive feedback and the development and persistence of ecosystem disruptive algal blooms. J. Phycol. 42, 963–974. [Google Scholar]
- Tang YZ, Gobler CJ, 2010. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 406, 19–31. [Google Scholar]
- Tang YZ, Gobler CJ, 2012. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 20, 71–80. [Google Scholar]
- Tango P, Butler W, Wazniak C, 2004. Assessment of harmful algae bloom species in the Maryland Coastal Bays. In: Wazniak C, Hall M (Eds.), Maryland’s Coastal Bays Ecosystem Health Assessment 2004. Maryland Department of Natural Resources, Annapolis, MD. [Google Scholar]
- Taylor GT, Gobler CJ, Sañudo-Wilhelmy SA, 2006. Speciation and concentrations of dissolved nitrogen as determinants of brown tide Aureococcus anophagefferens initiation. Mar. Ecol. Prog. Ser. 312, 67–83. [Google Scholar]
- Taylor HF, 1917. Mortality of fishes on the west coast of Florida, Department of Commerce, Bureau of Fisheries, pp. 5–24. [Google Scholar]
- Tester PA, Stumpf RP, Fowler PK, 1988. Red tide, the first occurrence in North Carolina waters: an overview, OCEANS ‘88. A Partnership of Marine Interests. Proceedings. IEEE, Baltimore, MD, USA, pp. 808–811. [Google Scholar]
- Tester PA, Stumpf RP, Vukovich FM, Fowler PK, Turner JT, 1991. An expatriate red tide bloom: transport, distribution, and persistence. Limnol Oceanogr 36(5), 1053–1061. [Google Scholar]
- Tester PA, Turner JT, Shea D, 2000. Vectorial transport of toxins from the dinoflagellate Gymnodinium breve through copepods to fish. J. Plankton Res. 22, 47–61. [Google Scholar]
- Tester PA, Feldman RL, Nau AW, Kibler SR, Litaker RW, 2010. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 56 (5), 698–710. [DOI] [PubMed] [Google Scholar]
- Thronson A, Quigg A, 2008. Fifty-five years of fish kills in coastal Texas. Estuar Coasts 31, 802–813. [Google Scholar]
- Thyng KM, Hetland RD, Ogle MT, Zhang X, Chen F, Campbell L, 2013. Origins of Karenia brevis harmful algal blooms along the Texas coast. Limnol. Oceanogr. Fluids Environ 3, 269–278. [Google Scholar]
- Tilney CL, Shankar S, Hubbard KA, Corcoran AA, 2019. Is Karenia brevis really a low-light-adapted species? Harmful Algae 90, 101709. [DOI] [PubMed] [Google Scholar]
- Todd ECD, 1993. Amnesic shellfish poisoning - a review. J. Food Prot. 56, 69–83. [DOI] [PubMed] [Google Scholar]
- Tomas C, Ono C, Yoshimatsu S, Gobel J, 2004. Chattonella verruculosa and related species from Japan, Europe (North Sea) and U. S. coastal waters: case of mistaken identity?, In: Steidinger KA, Landsberg JH, Tomas C, Vargo GA (Eds.), Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, pp. 425–427 [Google Scholar]
- Tomas CR and Smayda TJ, 2008. Red tide blooms of Cochlodinium polykrikoides in a coastal cove. Harmful Algae 7, 308–317. [Google Scholar]
- Tominack SA, Coffey KZ, Yoskowitz D, Sutton G, Wetz MS, 2020. An assessment of trends in the frequency and duration of Karenia brevis red tide blooms on the South Texas coast (western Gulf of Mexico). PLoS One, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres De la Riva G, Johnson CK, Gulland FM, Langlois GW, Heyning JE, Rowles TK, Mazet JA, 2009. Association of an unusual marine mammal mortality event with Pseudo-nitzschia spp. blooms along the southern California coastline. J. Wildl. Dis. 45, 109–121. [DOI] [PubMed] [Google Scholar]
- Torrey HB, 1902. An unusual occurrence of Dinoflagellata on the California coast. Amer. Nat. 36, 187–192. [Google Scholar]
- Trainer VL, Eberhart BTL, Wekell JC, Adams NG, Hanson L, Cox F, Dowell J, 2003. Paralytic shellfish toxins in Puget Sound, Washington state. J. Shellfish Res. 22, 213–224. [Google Scholar]
- Trainer VL, Suddleson M, 2005. Monitoring approaches for early warning of domoic acid events in Washington state. Oceanogr. 18, 228–237. [Google Scholar]
- Trainer VL, Wells ML, Cochlan WP, Trick CG, Bill BD, Baugh KA, Beall BF, Herndon J, Lundholm N, 2009. An ecological study of a massive toxigenic bloom of Pseudo-nitzschia cuspidata off the Washington State coast. Limnol. Oceanogr. 54, 1461–1474. [Google Scholar]
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, Trick CG, 2012. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 14, 271–300. [Google Scholar]
- Trainer VL, Moore L, Bill BD, Adams NG, Da Silva DAM, Eberhart BTL, Harrington N, Borchert J, 2013. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Mar. Drugs 11, 1815–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainer VL, Sullivan K, Eberhart BTL, Paternoster J, Shuler A, Hignutt E Jr., Kiser J, Eckert G, Morton SL, 2014. The impacts of paralytic shellfish poisoning on the Alaska shellfish industry and the implementation of an early warning system. J. Shellfish Res. 33, 531–539. [Google Scholar]
- Trice TM, Glibert PM, Lea C, Van Heukelem L, 2004. HPLC pigment records provide evidence of past blooms of Aureococcus anophagefferens in the Coastal Bays of Maryland and Virginia, U.S.A. Harmful Algae 3, 295–304. [Google Scholar]
- Tsuji K, Naito S, Kondo F, Ishikawa N, Watanabe MF, Suzuki M, Harada KI, 1994. Stability of microcystins from cyanobacteria: effect of light on decomposition and isomerization. Environ. Sci. Technol. 28, 173–177. [DOI] [PubMed] [Google Scholar]
- Twiner MJ, Flewelling LJ, Fire SE, Bowen-Stevens SR, Gaydos JK, Johnson CK, Landsberg JH, Leighfield TA, Mase-Guthrie B, Schwacke L, Van Dolah FM, Wang Z, Rowles TK, 2012. Comparative analysis of three brevetoxin-associated bottlenose dolphin (Tursiops truncatus) mortality events in the Florida Panhandle region (U.S.A). PloS One 7, e42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA (United States Food and Drug Administration), 2019. Fish and Fishery Products Hazards and Controls Guidance, 4th ed.
- Van Dolah FM, Doucette GJ, Gulland FMD, Rowles TL, Bossart GD, 2003. Impacts of algal toxins on marine mammals, In: Vos JG, Bossart GD, Fournier M, O’Shea TJ (Eds.), Toxicology of Marine Mammals. Taylor and Francis, New York, pp. 247–269. [Google Scholar]
- Van Hemert C, Schoen SK, Litaker RW, Smith MM, Arimitsu ML, Piatt JF, Holland WC, Hardison DR, Pearce JM, 2020. Algal toxins in Alaskan seabirds: Evaluating the role of saxitoxin and domoic acid in a large-scale die-off of Common Murres. Harmful Algae 92, 101730. [DOI] [PubMed] [Google Scholar]
- Van Wagoner RM, Deeds JR, Satake M, Ribeiro AA, Place AR, Wright JL, 2008. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetrahedron Lett. 49, 6457–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandersea M, Tester P, Holderied K, Hondolero D, Kibler S, Powell K, Baird S, Doroff A, Dugan D, Meredith A, Tomlinson M, 2020. An extraordinary Karenia mikimotoi “ beer tide” in Kachemak Bay Alaska. Harmful Algae 92, 101706. [DOI] [PubMed] [Google Scholar]
- Vargo GA, 2009. A brief summary of the physiology and ecology of Karenia brevis Davis (G. Hansen and Moestrup comb. nov.) red tides on the West Florida Shelf and of hypotheses posed for their initiation, growth, maintenance, and termination. Harmful Algae 8(4), 573–584. [Google Scholar]
- Vargo GA, Heil CA, Fanning KA, Dixon LK, Neely MB, Lester K, Ault D, Murasko S, Havens J, Walsh J and Bell S, 2008. Nutrient availability in support of Karenia brevis blooms on the central West Florida Shelf: what keeps Karenia blooming? Cont. Shelf Res. 28 (1), 73–98. [Google Scholar]
- Velo-Suarez L, Gonzalez-Gil S, Gentien P, Lunven M, Bechemin C, Fernand L, Raine R, Reguera B, 2008. Thin layers of Pseudo-nitzschia spp. and the fate of Dinophysis acuminata during an upwelling -- downwelling cycle in a Galician Ria. Limnol. Oceanogr. 53, 1816–1834. [Google Scholar]
- Verity PG, 2010. Expansion of potentially harmful algal taxa in a Georgia Estuary (U.S.A). Harmful Algae 9, 144–152. [Google Scholar]
- Vigilant VL,Silver MW, 2007. Domoic acid in benthic flatfish on the continental shelf of Monterey Bay, California, USA. Mar. Biol. 151, 2053–2062. [Google Scholar]
- Villac MC, Hoeglund A, Tilney C, Garrett M, Lopez C, Hubbard K, Steidinger KA, 2020. Ecophysiology and bloom dynamics of Karenia with emphasis on Karenia brevis in Florida waters, In: Rao DVS (Ed.), Dinoflagellates Classification, Evolution, Physiology and Ecological Significance. Nova Science Publishers, New York, pp. 261–301. [Google Scholar]
- Villareal TA, Brainard MA, McEachron LW, 2001. Gymnodinium breve (Dinophyceae) in the western Gulf of Mexico: resident versus advected populations as a seed stock for blooms, In: Hallegraeff G, Blackburn SI, Bolch CJ, Lewis RJ (Eds.), Harmful Algal Blooms 2000. Intergovernmental Oceanographic Commission of UNESCO; Paris, pp. 153–156. [Google Scholar]
- Villareal TA, Chirichella T, Buskey EJ, 2004. Regional distribution of the Texas brown tide (Aureoumbra lagunensis) in the Gulf of Mexico. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA (Eds.), Harmful Algae. 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, FL, pp. 374–376. [Google Scholar]
- Villareal TA, Hanson S, Qualia S, Jester ELE, Granade HR, Dickey RW, 2007. Petroleum production platforms as sites for the expansion of ciguatera in the northwestern Gulf of Mexico. Harmful Algae 6, 253–259. [Google Scholar]
- Waggett RJ, Hardison DR, Tester PA, 2012. Toxicity and nutritional inadequacy of Karenia brevis: synergistic mechanisms disrupt top-down grazer control. Mar. Ecol. Prog. Ser. 444, 15–30. [Google Scholar]
- Walsh JJ, Jolliff JK, Darrow B, Lenes J, Milroy SP, Remsen AW, Dieterle D, Carder K, Chen F, Vargo GA, Weisberg RH, Fanning K, Muller-Karger FE, Shinn E, Steidinger K, Heil CA, Tomas CR, Prospero JM, Lee T, Kirkpatrick GJ, Whitledge TE, Stockwell D, Villareal TA, Jochens A, Bontempi P, 2006. Red tides in the Gulf of Mexico: where, when, and why? J. Geophys. Res. 111(C11003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters S, Lowerre-Barbieri S, Bickford J, Tustison J, Landsberg JH, 2013. Effects of Karenia brevis red tide on the spatial distribution of spawning aggregations of sand seatrout Cynoscion arenarius in Tampa Bay, Florida. Mar. Ecol. Prog. Ser. 479, 191–202. [Google Scholar]
- Wang M, Hu C, Barnes BB, Mitchum G, Lapointe B and Montoya JP, 2019. The great Atlantic Sargassum belt. Science, 365(6448), 83–87. [DOI] [PubMed] [Google Scholar]
- Wardle WJ, Ray SM, Aldrich AS, 1975. Mortality of marine organisms associated with offshore summer blooms of the toxic dinoflagellate Gonyaulax monilata Howell at Galveston, Texas, In: LoCicero VR (Ed.), Proceedings of the First International Conference on Toxic Dinoflagellate Blooms. Massachusetts Science and Technology Foundation, Wakefield, Massachusetts, pp. 257–263. [Google Scholar]
- Watkins SM, Reich A, Fleming LE, Hammond RD, 2008. Neurotoxic shellfish poisoning. Mar. Drugs 6(3), 431–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg RH, Liu Y, 2017. On the loop current penetration into the Gulf of Mexico. J Geophys. Res. C. Oceans 122(12), 9679–9694. [Google Scholar]
- Weisberg RH, Barth A, Alvera-Azcárate, Zheng L, 2009. A coordinated coastal ocean observing and modeling system for the West Florida Continental Shelf. Harmful Algae 8(4), 585–597. [Google Scholar]
- Weisberg RH, Zheng L, Liu Y, Lembke C, Lenes JM, Walsh JJ, 2014. Why no red tide was observed on the West Florida Continental Shelf in 2010. Harmful Algae 38, 119–126. [Google Scholar]
- Weisberg RH, Liu Y, Lembke C, Hu C, Hubbard K, Garrett M, 2019. The coastal ocean circulation influence on the 2018 West Florida Shelf K. brevis red tide bloom. J. Geophys. Res. C. Oceans 124(4), 2501–2512. [Google Scholar]
- Wekell JC, Gauglitz EJ Jr., Barnett HJ, Hatfield CJ, Simons D, Ayres D, 1994. Occurrence of domoic acid in Washington state razor clams (Siliqua patula) during 1991–1993. Nat. Toxins 2, 197–205. [DOI] [PubMed] [Google Scholar]
- Wells M,L, Trick CG, Cochlan WP, Hughes MP, Trainer VL, 2005, Domoic acid: The synergy of iron, copper, and the toxicity of diatoms, Limnol. Oceanogr, 50 (6) 1908–1917. [Google Scholar]
- Wells ML, Trainer VL, Smayda TJ, Karlson BSO, Trick CG, Kudela RM, Ishikawa A, Bernard S, Wulff A, Anderson DM, Cochlan WP 2015. Harmful algal blooms and climate change: learning from the past and present to forecast the future. Harmful Algae 49, 68–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells ML, Karlson B, Wulff A, Kudela R, Trick C, Asnaghi V, Berdalet E, Cochlan W, Davidson K, De Rijcke M, Dutkiewicz S, Hallegraeff G, Flynn KJ, Paerl H, Legrand C, Silke J, Suikkanen S, Thompson P, Trainer VL,. 2019. Future HAB science: directions and challenges in a changing climate. Harmful Algae 91, 101632. [DOI] [PubMed] [Google Scholar]
- Wetz MS, Cira EK, Sterba-Boatwright B, Montagna PA, Palmer TA, Hayes KC, 2017. Exceptionally high organic nitrogen concentrations in a semi-arid South Texas estuary susceptible to brown tide blooms. Estuar. Coast. Shelf Sci. 188, 27–37. [Google Scholar]
- White AE, Watkins-Brandt KS, McKibben SM, Wood AM, Hunter M, Forster Z, Du X, Peterson WT, 2014. Large-scale bloom of Akashiwo sanguinea in the Northern California current system in 2009. Harmful Algae 37, 38–46. [Google Scholar]
- Whyte JNC, Haigh N, Ginther NG, Keddy LJ, 2001. First record of blooms of Cochlodinium sp. (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 40, 298–304. [Google Scholar]
- Wolny JL, Tomlinson MC, Schollaert Uz S, Egerton TA, McKay JR, Meredith A, Reece KS, Scott GP, Stumpf RP, 2020. Current and future remote sensing of harmful algal blooms in the Chesapeake Bay to support the shellfish industry. Front. Mar. Sci. 7. 337 [Google Scholar]
- Woodcock AH, 1948. Note concerning human respiratory irritation associated with high concentrations of plankton and mass mortality of marine organisms. J. Mar. Res. 7(1) 56–62. [Google Scholar]
- Work TM, Barr B, Beale AM, Fritz L, Quilliam MA, and Wright JLC, 1993. Epidemiology of domoic acid poisoning in brown pelicans (Pelecanus occidentalis) and Brandt’s cormorants (Phalacrocorax penicillatus) in California. J. Zoo Wildlife Med. 24(1) 54–62. [Google Scholar]
- Vogt RL, 1986. Ciguatera fish poisoning-Vermont. Morbidity and Mortality Weekly Report 35, 263–264. [PubMed] [Google Scholar]
- Xu Y, Richlen ML, Liefer JD, Robertson A, Kulis D, Smith TB, Parsons ML, Anderson DM, 2016. Influence of environmental variables on Gambierdiscus spp. (Dinophyceae) growth and distribution. PLoS One 11(4), e0153197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Weisberg RH, Liu Y, Liang XS, 2020. Instabilities and multiscale interactions underlying the loop current eddy shedding in the Gulf of Mexico. J. Phys. Oceanogr. 50(5), 1289–1317. [Google Scholar]
- Yasumoto T, Nakajima I, Bagnis RA, Adachi R, 1977. Finding of a dinoflagellate as a likely culprit of ciguatera. Bull. Jpn. Soc. Sci. Fish 46, 1397–1404. [Google Scholar]