Abstract
Synthetic mRNAs are an appealing platform with multiple biomedical applications ranging from protein replacement therapy to vaccination. In comparison with conventional mRNA, synthetic self-amplifying mRNAs (sa-mRNAs) are gaining interest because of their higher and longer-lasting expression. However, sa-mRNAs also elicit an innate immune response, which may complicate their clinical application. Approaches to reduce the innate immunity of sa-mRNAs have not been studied in detail. Here we investigated, in vivo, the effect of several innate immune inhibitors and a novel cellulose-based mRNA purification approach on the type I interferon (IFN) response and the translation and vaccination efficacy of our formerly developed sa-mRNA vaccine against Zika virus. Among the investigated inhibitors, we found that corticosteroids and especially topical application of clobetasol at the sa-mRNA injection site was the most efficient in suppressing the type I IFN response and increasing the translation of sa-mRNA. However, clobetasol prevented formation of antibodies against sa-mRNA-encoded antigens and should therefore be avoided in a vaccination context. Residual dsRNA by-products of the in vitro transcription reaction are known inducers of immediate type I IFN responses. We additionally demonstrate a drastic reduction of these dsRNA by-products upon cellulose-based purification, reducing the innate immune response and improving sa-mRNA vaccination efficacy.
Keywords: innate immunity inhibitors, self-amplifying mRNA, type I IFN, mRNA purification, cellulose, clobetasol, Zika vaccine
Graphical abstract
The innate immunity of sa-mRNAs may complicate their clinical application. In this study, Zhong et al. investigated, in vivo, the effect of several innate immune inhibitors and a novel cellulose-based mRNA purification approach on the type I interferon response and the translation and vaccination efficacy of an sa-mRNA vaccine against Zika virus.
Introduction
Synthetic mRNAs have become an appealing therapeutic platform with multiple biomedical applications ranging from protein replacement therapy to vaccination.1,2 Compared with plasmid DNA and viral vectors, synthetic mRNAs have some important advantages. First, they do not have to cross the nuclear barrier to perform their function, making them effective in dividing and non-dividing cells.1,3 Furthermore, synthetic mRNAs allow cell-free production and have transient and more predictable expression.1,2 In recent years, synthetic self-amplifying mRNAs (sa-mRNAs) have also gained interest because of their higher and longer-lasting expression compared with non-amplifying mRNAs.4,5 Self-amplifying RNAs encode an RNA-dependent RNA polymerase (replicase) that gives them the capacity to trigger temporal amplification of their backbone. Additionally, this replicase also generates many copies of smaller “subgenomic RNA(s)” that encode the protein(s) of interest. By using synthetic sa-mRNAs, it is possible to reduce the dose and the need for repeated injections while still benefiting from the desirable features of synthetic mRNAs.
However, innate immunity triggered by sa-mRNA may complicate the clinical translation of this platform. The current in vitro production process of synthetic (sa)-mRNAs generates by-products such as short abortive transcripts and double-stranded RNA (dsRNA) species, which are recognized as non-self by Toll-like receptors (TLRs), cytoplasmic RIG-I-like receptors (RLRs), and other cellular pattern recognition receptors (PRRs).1,6,7 This triggers production of proinflammatory cytokines and type I interferons (IFNs), which are undesirable when synthetic (sa)-mRNAs are considered for protein (replacement) therapy.7 In contrast, the cytokines induced by this self-defense mechanism may serve as adjuvants and facilitate the effects of synthetic (sa)-mRNA vaccines.8 However, this view needs to be nuanced because studies demonstrated that, depending on the administration approach, type I IFN responses can also decrease the efficacy of mRNA vaccines by negatively affecting immune responses9,10 and by inducing enzymes that inhibit mRNA translation.11,12 An important breakthrough was achieved when it was demonstrated that the innate immunity of synthetic mRNAs can be reduced drastically by incorporation of modified nucleotides and by reverse-phase high-performance liquid chromatography (HPLC) purification.13, 14, 15 However, this was only demonstrated for non-amplifying mRNAs. We recently demonstrated that the innate immune response triggered by the sa-mRNA platform4,5,12 reduced the cellular and humoral responses of an sa-mRNA vaccine against Zika virus.16 Therefore, strategies that can reduce the innate immunity of sa-mRNAs may improve the efficacy of sa-mRNA vaccines and acceptance of sa-mRNA therapeutic agents in general. Tempering the innate immunity of sa-mRNA by inclusion of modified nucleosides is expected to have a negative effect on replication of sa-mRNA.4,17 Moreover, analogous to the size-dependent sensitivity of DNA to shear-mediated degradation, we can expect that the large size (>10 kb) of sa-mRNAs makes them more prone to shear forces, which complicates their purification by reverse phase HPLC purification.18 Therefore, alternative strategies to decrease the innate immunity of sa-mRNAs are needed.
In this work, we investigated the capacity of several innate immune inhibitors to temper the innate immune response and improve expression of an sa-mRNA vaccine against Zika virus (ZIKVac-sa-mRNA). The tested inhibitors (Figure 1) were mixed with the sa-mRNA vaccine or administered locally at the intradermal injection site or administered orally via drinking water. Corticosteroids strongly reduced innate immunity and especially local application of clobetasol drastically improved the in vivo translation of sa-mRNA. In an alternative approach to mitigate innate immune responses triggered by dsRNA by-products, we also purified the ZIKVac-sa-mRNA using a new cellulose-based procedure. This new purification process drastically reduced innate immunity and improved the expression and vaccination efficacy of our ZIKVac-sa-mRNA vaccine.
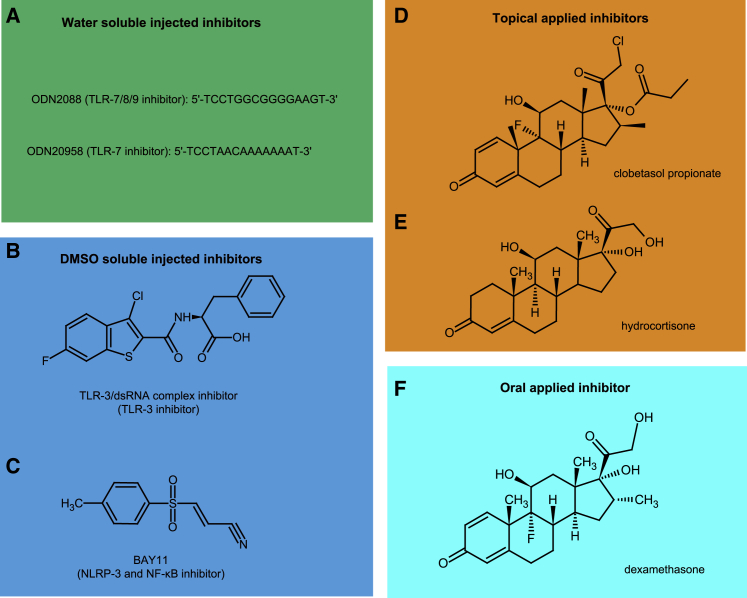
Figure 1.
Structure of the innate inhibitors used in this study
(A) The oligonucleotides ODN2088 and ODN20958 inhibit TLR7/8/9 and TLR7, respectively. (B) The chemical structure of the TLR3/dsRNA complex inhibitor (called “TLR3 inhibitor” in this paper). (C) BAY11 inhibits the intracellular NRLP3 receptor and NF-κB activation. (D–F) Clobetasol propionate (D) and hydrocortisone (E) are topical corticosteroids, and dexamethasone (F) is an oral corticosteroid. All three have broad immunosuppressive properties, including NF-κB inhibition.
Results
Tempering the innate immunogenicity of sa-mRNA with innate immune inhibitors
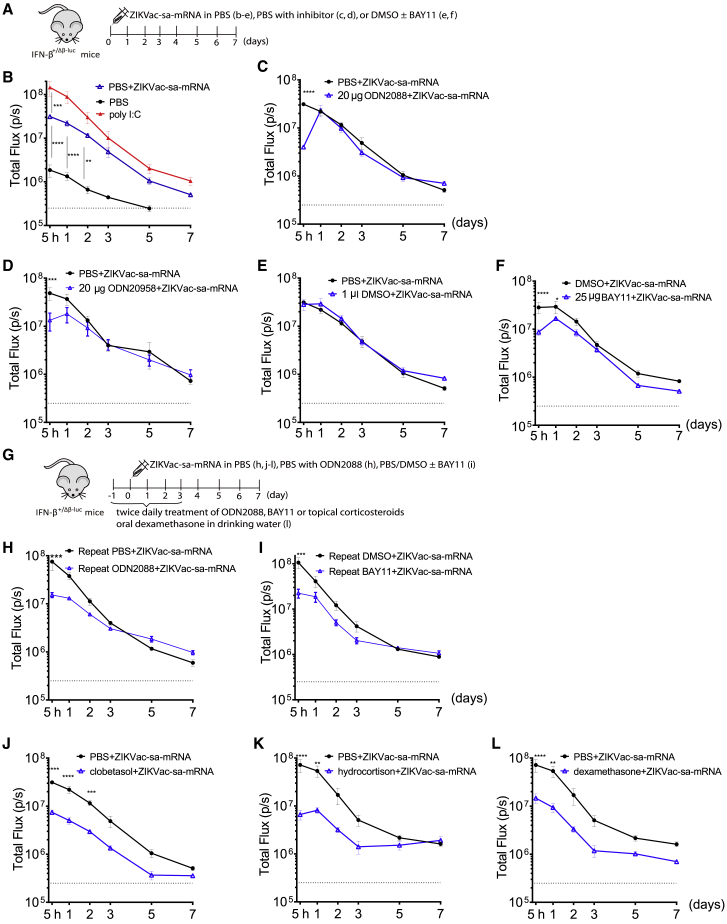
It is known that the in vivo safety and efficacy of sa-mRNA therapeutic agents are compromised by their strong activation of type I IFNs. To address this issue, we evaluated the capacity of a series of innate immune inhibitors (Figure 1) to suppress the in vivo type I IFN response elicited by ZIKVac-sa-mRNA. To this end, we co-administered innate immune inhibitors with the ZIKVac-sa-mRNA vaccine in IFN-β luciferase reporter mice (IFN-β+/Δβ-luc) (Figure 2A).19 In these transgenic mice, luciferase expression is under control of the promotor of IFN-β, a key type I IFN. In the absence of co-administered innate immune inhibitors, IFN-β reporter gene expression increased rapidly and peaked within 0–5 h after intradermal electroporation of the ZIKVac-sa-mRNA vaccine. After this peak, IFN-β reporter gene expression dropped sharply, and background IFN-β reporter gene expression was reached after about 1 week (Figure 2B). Furthermore, we confirmed that the elicited IFN-β response mainly occurred because of the ZIKVac-sa-mRNA vaccine, electroporation of solely PBS induced only a moderate type I IFN response (Figure 2B, black curve). Co-injection of the water-soluble oligonucleotide-based TLR inhibitors ODN2088 and ODN20958 (Figure 1A) with the ZIKVac-sa-mRNA vaccine significantly reduced the immediate type I IFN response (Figures 2C and 2D). However, this innate immune-tempering effect was lost after 1 day. The inhibitory effect of ODN2088, which blocks TLR7/8/9, was slightly higher than that of ODN20958, which only blocks TLR7 (Figures 2C and 2D). A lower but still significant reduction of the early IFN-β response was also achieved when the ZIKVac-sa-mRNA was co-injected with lower doses (<20 μg) of these TLR inhibitors (Figures S1A and S1B).
Figure 2.
Effect of innate immune inhibitors on the kinetics of the type I IFN response after intradermal electroporation of ZIKVac-sa-mRNA in IFN-β+/Δβ-luc reporter mice
(A) Inhibitors were mixed with the ZIKVac-sa-mRNA vaccine before administration to IFN-β+/Δβ-luc reporter mice. (B–F) The graphs represent the kinetics of the type I IFN response (y axis) after a single intradermal electroporation of the ZIKVac-sa-mRNA vaccine (1 μg in 50 μL), poly(I:C) (1 μg in 50 μL), or PBS control (B) and the capacity of the inhibitors ODN2088 (C), ODN20958 (D), and BAY11 (F) to temper type I IFN responses elicited by the ZIKVac-sa-mRNA. Because DMSO is needed to dissolve BAY11, the influence of DMSO was also studied (E). (G) The treatment schedule of repeated administration of inhibitors to IFN-β+/Δβ-luc reporter mice. (H and I) The effect of repeated ODN2088 or BAY11 administration; the injection site was injected intradermally with the inhibitors 5 h prior to ZIKVac-sa-mRNA administration. Subsequently, the ZIKVac-sa-mRNA vaccine was administered together with the inhibitors ODN2088 (H) or BAY11 (I). On the day of sa-mRNA administration, a second local injection of ODN2088 or BAY11 was given after 7 h. Local injection of the inhibitors continued twice daily until day 3. (J and K) In contrast to the other inhibitors, clobetasol propionate (J) and hydrocortisone (K) were applied topically (25 μg/1 cm2) 1 day prior to ZIKVac-sa-mRNA administration, and this was repeated twice daily until day 3. (L) Dexamethasone was administered orally in drinking water for 4 days starting on day −1. Each symbol represents the mean of four individual mice, and the error bars represent SEM. The PBS+ZIKVac-sa-mRNA blanc was repeated each time, except in (C) and (L), where we used the same blanc as in (A) and (K), respectively.
Next we evaluated the TLR-3/dsRNA complex inhibitor and BAY11-7082 (BAY11) (Figures 1B–1C). The latter inhibits the intracellular oligomerization domain (NOD)-like receptor pyrin 3 (NLRP3) and nuclear factor κB (NF-κB). DMSO was used to dissolve these inhibitors because both are water insoluble. We first confirmed that addition of a small amount of DMSO (1 μL) to our ZIKV-sa-mRNA vaccine (50 μL) did not change its IFN-β induction capacity (Figure 2E). Co-injection of ZIKV-sa-mRNA with 25 μg BAY11 significantly suppressed the IFN-β response during the first 24 h (Figure 2F), whereas 12.5 μg of BAY11 was not effective (Figures S1C). In contrast, neither of the tested TLR-3 inhibitor doses tempered the intrinsic innate immunogenicity of the ZIKVac-sa-mRNA vaccine (Figures S1D and S1E).
In a subsequent experiment, we studied whether pre-treatment and post-treatment of the injection spot with BAY11 or ODN2088 could increase and prolong their capacity to temper the innate immunogenicity of our sa-mRNA vaccine (Figure 2G). Surprisingly, pre-treatment of the injection site with BAY11 or ODN2088 and twice daily intradermal administration of these inhibitors after injection of the ZIKVac-sa-mRNA slightly, but not drastically, increased or prolonged suppression of the IFN-β response (Figures 2H and 2I). In another attempt to quell the type I IFN response, we considered topical application of the corticosteroid clobetasol and hydrocortisone as well as oral administration of dexamethasone. To that end, the injection site was pre-treated with a clobetasol or hydrocortisone ointment 12 h prior to administration of ZIKVac-sa-mRNA vaccine. Subsequently, clobetasol or hydrocortisone treatment was repeated twice daily for 3 days, starting on the day of ZIKVac-sa-mRNA injection. Dexamethasone was added to drinking water, and the mice had free access to the water for 4 days. This schedule of local corticosteroid treatment or oral application of dexamethasone drastically reduced the elicited type I IFN response (Figures 2J–2L and S1F-S1H). A significant inhibitory effect was observed up to 2 days after ZIKVac-sa-mRNA injection, and overall, a 3-fold reduction of IFN-β reporter gene expression was observed with these corticosteroids (Figure S1F-S1H).
Co-administration of multiple innate immune inhibitors
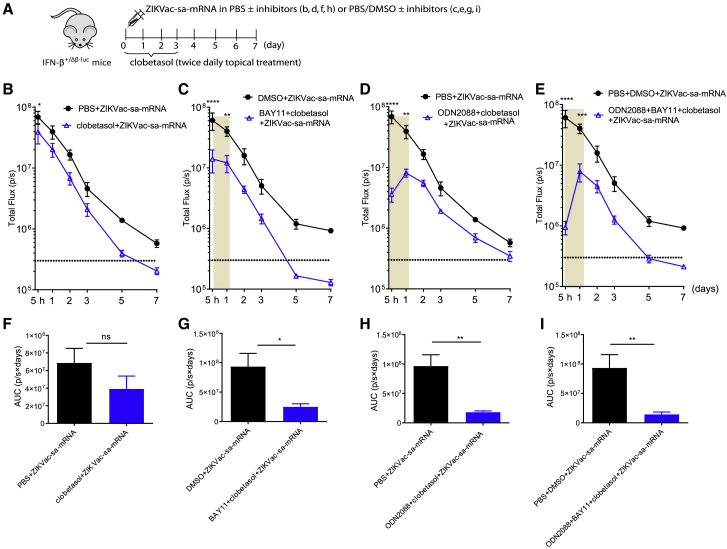
We next evaluated whether co-administration of multiple innate immune inhibitors could further decrease the type I IFN response elicited by our ZIKVac-sa-mRNA vaccine. In more detail, clobetasol was co-administered with ODN2088 or BAY11 or with both inhibitors. In these experiments, clobetasol was applied locally at the injection site twice daily for 3 days starting on the day of ZIKVac-sa-mRNA injection. The ODN2088 or BAY11 inhibitors were given as a single co-injection with the ZIKV-sa-mRNA vaccine (Figure 3A). The clobetasol pre-treatment, which was skipped in this experiment, seems to be of great importance because inhibition of the IFN-β response was much lower without clobetasol pre-treatment (Figures 2J and S1F versus 3B and 3F). A drastic reduction of the type I IFN response was observed when clobetasol was combined with BAY11 or/and ODN2088 (Figures 3C–3E and 3G–3I). Especially combining the three inhibitors (clobetasol+ODN2088+BAY11) was very successful in inhibiting the type I IFN response elicited by our sa-mRNA vaccine (Figures 3E and 3I).
Figure 3.
Effect of topical clobetasol in combination with other inhibitors on the type I IFN response kinetics after intradermal electroporation of ZIKVac-sa-mRNA in IFN-β+/Δβ-luc reporter mice
(A) Treatment schedule for the injection site. (B) ZIKVac-sa-mRNA vaccine (1 μg in 50 μL) administration was directly followed by topical application of clobetasol propionate (25 μg/1 cm2) twice per day and continued over 3 days. (C–E) Additionally, clobetasol was combined with 25 μg BAY11 (C), 20 μg ODN2088 (D), or ODN2088 and BAY11 together (E). These inhibitors were co-injected once with the ZIKVac-sa-mRNA. The areas under the curve (AUCs) in (B)–(E) are presented in (F)–(I), respectively. Each symbol or bar represents the mean of four individual mice, and the error bars represent SEM.
Influence of innate immune inhibitors on translation of sa-mRNA
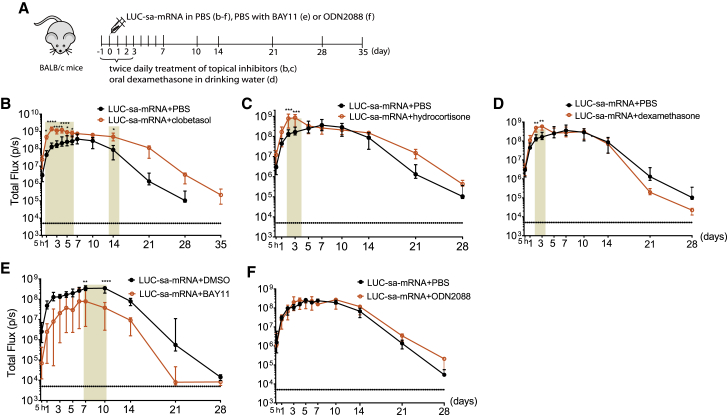
It is generally accepted that a strong innate immune response after mRNA delivery has a negative effect on its translation efficacy.9,11,12,16 Therefore, we evaluated, in BALB/c mice, the effect of all three corticosteroids (clobetasol, hydrocortisone, and dexamethasone), BAY11, and ODN2088 on the translation efficacy of sa-mRNA encoding luciferase (LUC-sa-mRNA). The LUC-sa-mRNA was again administered by intradermal electroporation. Pre-treatment and post-treatment of the LUC-sa-mRNA injection site twice daily with a clobetasol ointment for 3 days prolonged the translation with 1 week and increased the initial translation within the first 6 days (Figures 4A and 4B). This treatment regimen with clobetasol caused a 3.5-fold increase in overall translation of the LUC-sa-mRNA (Figures 4B and S2A). Topical treatment of the injection site with hydrocortisone and oral dexamethasone only improved initial in vivo translation on days 2 and 3 after sa-mRNA administration (Figure 4C, 4D, S2B, and S2C). Surprisingly, co-administration of BAY11 drastically reduced translation of our LUC-sa-mRNA (Figures 4E and S2D). This was not due to the DMSO solvent because LUC-sa-mRNA with equal amounts of PBS and DMSO was as effective as LUC-sa-mRNA with only PBS (Figures S2E). Co-injection of LUC-sa-mRNA with ODN2088 did not change the translation profile (Figure 4F).
Figure 4.
Influence of innate immune inhibitors on translation of sa-mRNA encoding LUC in BALB/c mice
(A) Time schedule of the animal experiment. (B–F) Wild-type BALB/c mice were electroporated intradermally with 1 μg of sa-mRNA encoding luciferase (LUC-sa-mRNA) in the presence of clobetasol propionate (B), hydrocortisone (C), dexamethasone (D), BAY11 (E), or ODN2088 (F). Topical treatment of the injection site with clobetasol propionate and hydrocortisone (25 μg/1 cm2) was performed 1 day prior to LUC-sa-mRNA injection and subsequently twice daily for 3 days. Dexamethasone was administered orally in drinking water for 4 days starting on day −1. BAY11 (25 μg) and ODN2088 (20 μg) were co-injected with the LUC-sa-mRNA (1 μg in 50 μl). LUC expression was determined by measuring the bioluminescence at the injection spot for 28 or 35 days. Each symbol represents the median of four individual mice, and the error bars indicate the interquartile range.
Innate immunogenicity and translation efficacy of sa-mRNA purified by cellulose chromatography
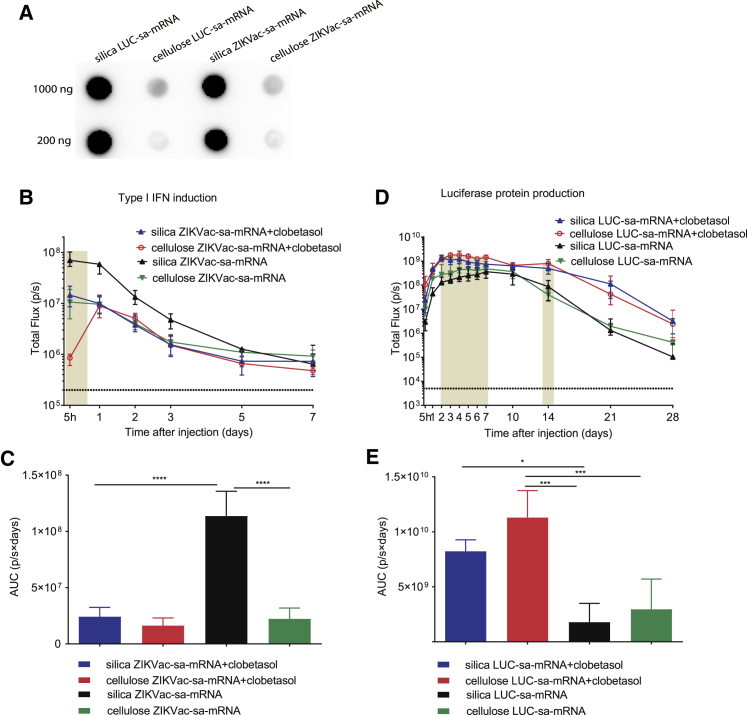
It is well known that dsRNA contaminants in synthetic (in vitro transcription [IVT]) mRNAs play an important role in activation of type I IFNs and translational inhibition.1,20 The classic purification strategies, like the silica-based columns we use routinely, do not efficiently remove dsRNAs. However, it has been shown recently that these dsRNAs can be removed from short non-amplifying synthetic mRNAs by a cellulose-based purification method.20 The applicability of this method to synthetic sa-mRNAs, which are 3 to 4 times longer than non-amplifying mRNAs, is unknown. Therefore, we investigated the capacity of this method to remove dsRNAs, decrease innate immunity, and improve the translation and vaccination efficacy of synthetic sa-mRNAs. We first demonstrated that synthetic sa-mRNAs purified with silica columns contained substantial amounts of dsRNA contaminants that could be removed efficiently by the novel cellulose-based purification method (Figure 5A). Subsequently, the effect of cellulose-based purification on the innate immunogenicity of the ZIKVac-sa-mRNA vaccine and expression of LUC-sa-mRNA was studied after intradermal electroporation in IFN-β reporter and BALB/c mice, respectively. In addition, the effect of pre- and post-treatment (twice daily for 3 days) of the injection site with clobetasol was also studied. We confirmed that the silica-purified ZIKVac-sa-mRNA vaccine elicits a strong type I IFN response that can be tempered by clobetasol (Figure 5B, blue and black curves). Interestingly, a similar reduction of the type I IFN response could be obtained when the ZIKVac-sa-mRNA vaccine was purified by the cellulose-based method (Figure 5B, green curve). Topical application of clobetasol could not further decrease the overall elicited type I IFN response of the cellulose-purified ZIKVac-sa-mRNA (Figure 5C), but clobetasol strongly reduced IFN-β reporter gene expression (approximately 10-fold) 5 h after administration of the cellulose-purified ZIKVac-sa-mRNA vaccine (Figures 5B, red curve, and 5C). Although cellulose purification of the sa-mRNA significantly reduced the elicited type I IFN response, it only slightly improved the translation efficacy of the sa-mRNA (Figure 5D, black and green curves). Nevertheless, co-administration of clobetasol significantly increased translation of the silica-purified as well as cellulose-purified LUC-sa-mRNA. The highest expression was observed in mice that received the cellulose-purified LUC-sa-mRNA together with clobetasol (Figures 5D and 5E). As also shown in Figure 4A, clobetasol seems to prolong translation of the silica- and cellulose-purified LUC-sa-mRNA (Figure 5D).
Figure 5.
The effect of cellulose purification and clobetasol on the type I IFN response and translation of sa-mRNAs
(A) dsRNA by-products in silica- and cellulose-purified sa-mRNAs (1,000 or 200 ng per dot) as analyzed by dot blotting with the J2 dsRNA-specific monoclonal antibody (mAb). (B) Type I IFN response kinetics after intradermal electroporation of silica- and cellulose-purified ZIKVac-sa-mRNA (1 μg) in IFN-β+/Δβ-luc reporter mice with or without topical clobetasol treatment of the injection site. (C) The AUCs of the curves in (B) (n = 4). (D) LUC expression kinetics after intradermal electroporation of LUC-sa-mRNA in wild-type BABL/c mice after silica- or cellulose-based purification with or without clobetasol treatment of the injection site. (E) The AUCs of the curves in (D). Each symbol or bar represents the mean of four individual mice, and the error bars represent SEM. The statistical analysis of the data shown in (B) and (D) can be found in Table S1.
Cellulose-purified sa-mRNA vaccines elicit a stronger humoral and cellular immune response
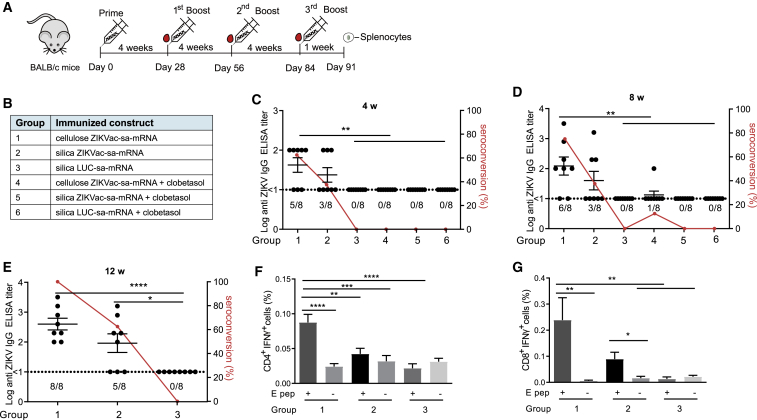
We finally investigated whether the novel cellulose-based purification method could improve the efficacy of our ZIKVac-sa-mRNA vaccine. It has been reported that inhaled and oral corticosteroids do not affect the efficacy of influenza vaccines.21,22 We were triggered by these counter-intuitive reports and decided to also investigate the effect of clobetasol pre- and post-treatment on the efficacy of our ZIKVac-sa-mRNA vaccine. Forty-eight BALB/c mice were randomized into six groups and vaccinated by intradermal electroporation of cellulose- or silica-purified ZIKVac-sa-mRNA with or without clobetasol. LUC-sa-mRNA was used as a negative control (Figures 6A and 6B). The interval between the vaccinations was 4 weeks, and the dose was 1 μg. Compared with the silica-purified vaccine, higher antibody titers and seroconversion rates were observed in mice receiving the cellulose-purified ZIKVac-sa-mRNA vaccine after the prime as well as after the booster vaccination (Figures 6C and 6D). As expected, a second vaccination further increased the antibody titers and seroconversion rates of both vaccines. Four weeks after the first booster, the mean antibody titer and seroconversion rate of the mice vaccinated with the cellulose-purified ZIKVac-sa-mRNA vaccine were 122 and 75%, whereas these values were 40 and 37.5% in mice that received the silica-purified ZIKVac-sa-mRNA vaccine (Figure 6D). Topical application of clobetasol at the injection site completely abolished the efficacy of the ZIKVac-sa-mRNA vaccine (Figures 6C and 6D). Because the humoral immune response improved after the booster vaccination, we decided to give a second booster to mice that received the ZIKVac-sa-mRNA vaccine without co-administration of clobetasol (Groups 1–3). Again, the antibody titer and seroconversion rates increased in both vaccinated groups, and the mice that received the cellulose-purified sa-mRNA vaccine developed the highest antibody titers. Moreover, the seroconversion rate in this group increased to 100%, whereas the seroconversion rate in mice immunized with the silica-purified ZIKVac-sa-mRNA vaccine was only 62.5% (Figure 6E). In addition to this strong humoral response, increased ZIKV E protein-specific CD4+ and CD8+ T cell responses were seen after the final immunization with the cellulose-purified ZIKVac-sa-mRNA vaccine. These cellular responses were significantly higher than those obtained with LUC-sa-mRNA-vaccinated mice (Figures 6F and 6G).
Figure 6.
Vaccination efficacy of silica- and cellulose-purified ZIKVac-sa-mRNA in BALB/c mice treated topically with or without clobetasol
(A and B) Experimental setup (A) and overview of the different vaccinated groups (B). Mice were electroporated intradermally with 1 μg of cellulose- or silica-purified ZIKVac-sa-mRNA vaccine or LUC-sa-mRNA control on day 0, day 28, day 56, and day 84 (Groups 1–3, without clobetasol treatment) or on day 0 and day 28 (groups 4–6, with clobetasol treatment). (C–E) Antibody titers in mice were determined by ZIKV E-protein-specific IgG ELISA 4 weeks (C), 8 weeks (D), or 12 weeks (E) after initial immunization (n = 8). The dashed lines indicate the limit of detection of the assay. The percentage of seroconverted mice is depicted by a red line (right y axis). (F and G) Antigen-specific CD4+ (F) and CD8+ (G) T cell responses in mice from groups 1–3 were assessed 1 week after the third booster by IFN-γ staining in T cells stimulated with a ZIKV E protein peptide pool (E pep +). Each bar represents the mean of eight individual mice, and the error bars represent SEM.
Discussion
Synthetic sa-mRNAs are known for their high in vivo translation efficiency.23,24 However, in vivo administration of sa-mRNAs may induce a strong type I IFN response.4,5,12 Although this can be considered advantageous when the sa-mRNA is used for vaccination purposes,12,25 several studies have demonstrated that triggering type I IFN production can negatively affect the intended adaptive immune response of intramuscularly and intradermally administered mRNA vaccines.9,12,16 Evidently, a strong type I IFN-mediated inflammatory response should also be avoided when synthetic mRNAs are considered for protein replacement therapy, gene editing, or stem cell reprogramming.26,27 In line with previous reports,8,12,16 we found that intradermal electroporation of our formerly developed ZIKVac-sa-mRNA vaccine results in very rapid upregulation of type I IFNs with maximal induction within 5 h after sa-mRNA administration (Figure 2). By-products originating from the IVT process, like dsRNA and uncapped or untailed RNAs species, contribute to this innate immune response.1 There is also concern that intracellular amplification of sa-mRNAs, which occurs through dsRNA intermediates, may strongly trigger intracellular sensors such as RIG-I and MDA5.28 However, our data do not fully support this idea because the peak in IFN-β production occurs shortly after delivery of the sa-mRNA instead of at maximal sa-mRNA replication. Moreover, we recently reported similar IFN-β induction for replication-deficient and replication-competent sa-mRNAs in mice.16 The absence of a strong innate immune response against the dsRNA intermediates that arise during sa-mRNA replication may be due to the location of these intermediates in spherules that shield them from cytoplasmic dsRNA sensors like RIG-I and MDA5.29,30
In an attempt to block the immediate type I IFN response elicited by our ZIKVac-sa-mRNA vaccine, we screened several commercial TLR and NF-κB/NLRP3 inhibitors (Figure 1). Endosomal or cell surface-associated TLRs31,32 are one type of PRRs that recognize dsRNAs and single-stranded RNAs (ssRNAs) in synthetic sa-mRNA.33,34 Co-injection of TLR7 (ODN20958) or TRL7/8/9 (ODN2088) antagonists with the ZIKVac-sa-mRNA vaccine seems to quickly block recognition of ssRNA species (Figure 2). This indicates that TLR7, which recognizes single nucleosides and short ssRNAs (oligoribonucleotides), is involved in sensing our sa-mRNA vaccine.7,35 We also hypothesize that degradation products from sa-mRNAs that did not enter the cells after in vivo electroporation are recognized by cell surface-associated TLR7. Moreover, it is also possible that TLR-mediated innate responses solely originate from sa-mRNAs that enter cells by endocytosis during the brief period between injection and electroporation. This may also explain the short and limited effect of the TLR inhibitors. Co-administration of our sa-mRNA vaccine with BAY11, which blocks nuclear translocation of NF-κB and inhibits the NLRP3 inflammasome,36,37 also significantly decreased the innate immune response, but only directly after administration. The short-lived downregulation of the type I IFN response by co-injected ODN2088, ODN20958, or BAY11 may also be due to rapid dilution of the inhibitors from the injection site. Therefore, we tested pre- and post-treatment of the injection site with these inhibitors. However, this only slightly increased and prolonged the reduction of the innate immune response (Figure 2). Pre- and post-treatment with these inhibitors should probably be performed closer to the moment of injection; e.g., 15 min (instead of 5 h) before and 1 h (instead of 12 h) after injection of the sa-mRNA vaccine. An interesting future approach would be co-encapsulation of these innate immune inhibitors with sa-mRNA into, e.g., lipid nanoparticles. Interestingly, no diminution of the type I IFN response was observed when 12.5 μg or even 25 μg of a TLR3 inhibitor was co-administered (Figure S1). This is remarkable because a dot blot assay clearly indicated that dsRNA species are present in the silica-purified sa-mRNA (Figure 5A). The massive amounts of dsRNA in our silica-purified sa-mRNA possibly completely outcompeted binding of the TLR3 antagonist to TLR3 receptors.
Besides the aforementioned specific innate immune inhibitors, we also tested oral dexamethasone, topical hydrocortisone, and clobetasol. The latter is a more potent topical corticosteroid than hydrocortisone.38,39 Topical application of hydrocortisone and clobetasol at the injection site efficiently inhibited the type I IFN response elicited by our ZIKVac-sa-mRNA vaccine. However, it is essential that the injection spot is treated with the corticosteroid prior to sa-mRNA administration (Figures 2 and 3). Moreover, additive effects were observed when topical clobetasol was combined with ODN2088 and/or BAY11 (Figure 3). Oral dexamethasone was also able to temper the innate immunity of the sa-mRNA, and its effects were similar to that of topical corticosteroids. We also investigated the effects of these inhibitors on in vivo translation of sa-mRNA. Surprisingly, only the corticosteroids improved the in vivo translation efficacy of the sa-mRNA, and the highest improvement was observed with clobetasol (Figures 4 and S2A–S2C). In more detail, a combination of pre-, co-, and post-treatment of the injection site with clobetasol prolonged expression of the LUC-encoding sa-mRNA by 1 week and significantly increased overall expression (Figures 4 and S2A). However, topical corticosteroids can also induce systemic effects; it is well known that they can pass through the skin and enter the circulation.40 Moreover, although the ointment had penetrated the skin completely before the mice woke up, we cannot exclude that topical corticosteroids were ingested by licking the treated spot. Surprisingly, ODN2088 did not improve in vivo translation, and BAY11 even decreased in vivo expression of the sa-mRNA (Figures 4 and S2D). This observation agrees with the findings of Liu et al.,41 who screened 15 different inhibitors and found that reduced IFN production was not associated with enhanced mRNA translation in cultured human foreskin fibroblast cells. Similar to our results, 7 of the tested inhibitors even reduced mRNA translation efficacy.41 In contrast, Awe et al.42 reported enhanced in vitro translation of the transcription factor OCT4 from a synthetic mRNA upon BAY11 supplementation. However, enhanced OCT4 translation was not achieved when the type I IFN decoy receptor B18R was supplemented, indicating that the observed increase in translation was independent of type I IFNs.42
As mentioned earlier, synthetic mRNAs produced by IVT contain by-products such as dsRNAs and small abortive ssRNA species that are known to strongly stimulate innate immune responses in mammalian cells.1,20 Cellulose purification has been reported to efficiently remove small by-products like dsRNA species larger than 30 bp.1,20,43 The method has been applied successfully to non-amplifying in-vitro-transcribed mRNA.20 Here we investigated whether cellulose-based purification20 could also reduce the type I IFN response and increase the in vivo translation efficacy of sa-mRNAs by removing dsRNA by-products. Immunoblotting confirmed that cellulose-mediated purification of sa-mRNA efficiently removed dsRNA species and, compared with standard silica-based purification, cellulose-purified sa-mRNA elicited a much lower type I IFN response that could be reduced further by topical clobetasol (Figure 5). However, this beneficial effect of cellulose purification was not completely reflected in the translation efficacy because significant increases in translation efficacy were only observed when the mice were treated with clobetasol (Figure 5). The untranslated regions (UTRs) in our sa-mRNA are based on the RNA genome of Venezuelan equine encephalitis virus (VEEV), which possesses structural elements in its 5′ UTR that can (partly) evade translational inhibition induced by a type I IFN response.44 Therefore, this may explain why strategies that reduce type I IFN do not drastically improve the translation efficacy of our synthetic sa-mRNA. Alternatively, it is also possible that the drop in type I IFNs induced by TLR7/8 antagonists or cellulose purification is not enough to improve the translation efficiency of the sa-mRNA. To further decrease the type I IFN response, inhibitors of other PRRs, like the RLR, can be used, and cellulose-based purification can be improved further. Indeed, the dot blot (Figure 5A) shows some remaining dsRNA species that are most likely caused by minor amounts of remaining dsRNA-contaminants (because of incomplete removal) or extensive double-stranded secondary structures in the sa-mRNA. Short uncapped dsRNAs, which are especially recognized by RIG-I,45 are probably also present in the sa-mRNA. Therefore, phosphatase treatment of the sa-mRNA prior to injection can also circumvent RIG-I-mediated detection of di/tri-phosphate 5′ ends.46
In a final experiment, we demonstrated that the cellulose-purified ZIKVac-sa-mRNA vaccine induced higher antigen-specific humoral and cellular immune responses than the silica-purified ZIKVac-sa-mRNA vaccine. This confirms that the by-products after IVT have negative effects on the efficacy of our sa-mRNA-based vaccine. These results also support our previous finding that silica-purified ZIKVac-sa-mRNA elicits stronger humoral and cellular immune responses in IFNAR1−/− mice, which show defective type I IFN signaling.16 Clobetasol treatment of the vaccination site prevented induction of a humoral immune response despite the beneficial effects of clobetasol on the IFN response and translation of the sa-mRNA (Figure 6). This is an important finding because it has been reported that inhaled and oral corticosteroids do not affect the efficacy of influenza vaccines.21,22 Moreover, these data indicate that corticosteroids can be used to prevent antibodies being raised against mRNA-encoded therapeutic proteins; e.g., clotting factors or erythropoietin.
In summary, among a handful of commercial TLR and NF-κB/NLRP3 inhibitors, topical application of clobetasol caused the strongest reduction of the innate immune response elicited after intradermal electroporation of our ZIKVac-sa-mRNA vaccine. Combining clobetasol with a TLR7 antagonist and/or a NRLP-3/NF-κB inhibitor further reduced the innate immune response. Clobetasol also increased translation of intradermally electroporated sa-mRNA. In a vaccination context, however, co-administration of clobetasol with our ZIKVac-sa-mRNA vaccine completely blocked the cellular and humoral immune response. In contrast, purification of the ZIKVac-sa-mRNA vaccine with a novel cellulose-based method tripled the antibody titers, doubled the cellular immune response, and increased the seroconversion rate from 62.5% to 100%. This improvement was associated with a drastic reduction of dsRNA by-products, which significantly decreased the type I IFN response elicited by the sa-mRNA vaccine and slightly improved expression of the sa-mRNA. It is important to note that the data in this study were achieved by intradermal electroporation of the sa-mRNA vaccine and, thus, without use of a carrier, which are known to drastically increase mRNA vaccination efficacy. In a future project, we aim to determine whether this novel purification method also improves the efficacy of sa-mRNA therapeutics or vaccines that are delivered by lipid nanoparticles.
Materials and methods
Mice
Female BALB/c mice, aged 6–8 weeks, were purchased from Janvier (France). The heterozygous BALB/c IFN-β reporter mice (IFN-β+/Δβ-luc) used in this study were from the Institute for Laboratory Animal Science, Hannover Medical School (Germany), and the breed was maintained inhouse. All mice were housed in individually ventilated cages and had free access to food and water. The mouse experiments were approved by the Ethics Committee of the Faculty of Veterinary Medicine, Ghent University (EC2019/62). During intradermal injections and bioluminescence imaging, mice were under isoflurane anesthesia (5% for induction and 2% for maintenance).
Inhibitors
The TLR3 and TLR7 inhibitors ODN2088 and ODN20958 (Miltenyi Biotec, Belgium) were used in this study. Phosphorothioate-modified ODN2088 (5′-TCCTGGCGGGGAAGT-3′) is a TLR7/8/9 antagonist, and the phosphorothioate-modified oligonucleotide ODN20958 is a TLR7 inhibitor (5′-TCCTAACAAAAAAAT-3′). The TLR3/dsRNA complex inhibitor (C18H13ClFNO3S) was bought from Merck Millipore (Belgium). The NF-κB and NLRP3 inhibitor BAY11 were from InvivoGen (Belgium). The TLR3/dsRNA complex inhibitor and BAY11 were dissolved in DMSO. Clobetasol propionate ointment (0.05%, Dermovate cream) was from GlaxoSmithKline (GSK). Hydrocortisone ointment (1% cream, Pannocort) and dexamethasone (2 mg/mL solution of Rapidexon) were obtained from a local pharmacy.
mRNA and silica purification
Sa-mRNA was synthetized by IVT as described previously.16 Briefly, the template DNA for transcription of ZIKVac-sa-mRNA was constructed by inserting the sequence of the Zika virus prM-E fusion protein of the Brazilian Rio-S1 ZIKV strain (GenBank: KU926310.1) containing a signal peptide of Japanese encephalitis virus (JEV) at the 5′ terminal end into the pTK155 plasmid using Gateway Cloning (Invitrogen). The sequence of firefly LUC was cloned into pTK155 to produce LUC-sa-mRNA. The plasmids of VEEV-based ZIKVac-sa-mRNA and LUC-sa-mRNA were transformed into competent E. coli bacteria (Invitrogen, Waltham, MA, USA) and, after 24 h, purified with the Plasmid Plus Midi kit (QIAGEN, Germany). Subsequently, linearized plasmids were obtained using I-SceI endonuclease (New England Biolabs [NEB], Ipswich, MA, USA), and the sa-mRNAs were synthetized by IVT with a MEGAscript T7 transcription kit (Thermo Fisher Scientific, Waltham, MA, USA). Next, the sa-mRNA was purified with the RNeasy Mini Kit (QIAGEN, Germany) and capped post-transcriptionally using the ScriptCap m7G capping system and a 2′-O-methyltransferase kit (CELLSCRIPT, Madison, WI, USA) to obtain cap-1. After capping, the sa-mRNA was purified again with the RNeasy Mini Kit (QIAGEN, Germany). Because a 40-nt-long poly(A) was encoded in the linearized plasmid template, poly(A) tailing was not required. The quantity and quality of the sa-mRNAs were determined with a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and sa-mRNAs were stored at −80°C.
Cellulose-based purification of sa-mRNAs
After IVT, the mRNAs (LUC-sa-mRNA and ZIKVac-sa-mRNA) were purified by LiCl precipitation and subsequently capped enzymatically as described above. Next, the capped sa-mRNAs were again precipitated with LiCl and resuspended in HEPES-ethanol buffer (10 mM HEPES [pH 7.2], 0.1 mM EDTA, 125 mM NaCl, and 16% ethanol). Subsequently, additional cellulose-based purification was performed to remove dsRNA by-products as described previously.20 Briefly, cellulose fibers (Sigma-Aldrich, Belgium) were suspended in HEPES-ethanol buffer at a concentration of 0.2 g/mL. After 10 min of vigorous mixing, 630 μL of the cellulose suspension was transferred to a microcentrifuge spin column (NucleoSpin Filters, Macherey-Nagel, Düren, Germany) and centrifuged for 1 min at 14,000 × g. The flowthrough was discarded, and 450 μL HEPES-ethanol buffer was added to the cellulose fibers, followed by vigorous shaking for 5 min. Subsequently, the spin column was centrifuged for 1 min at 14,000 × g, and the flowthrough was discarded. 100–500 μg of sa-mRNA in 450 μL HEPES-ethanol buffer was added to the cellulose in the spin column, followed by vigorous shaking for 30 min to allow association of the dsRNA by-products with the cellulose. Separation of the cellulose-associated dsRNA from the sa-mRNA occurred by centrifugation at 14,000 × g for 1 min. The collected flowthrough containing the sa-mRNA was precipitated by adding 0.1 volume of 3 M NaOAc (pH 5.5, 50 μL) and 1 volume of isopropanol and incubating this mixture for 30 min at −20°C. Next, the mRNA was pelleted by centrifugation at 4°C for 15 min at 14,000 × g, and the supernatant was discarded. The pellet was washed with 500 μL 70% pre-cooled ethanol and centrifuged at 4°C for 5 min at top speed. The supernatant was removed, and the cellulose-purified mRNA was finally dissolved in nuclease-free water.
Dot blot analysis of dsRNA by-products
LUC-sa-mRNA and ZIKVac-sa-mRNA were diluted in nuclease-free water to final concentrations of 40 and 200 ng/μL. Subsequently, 5 μL aliquots (200 or 1,000 ng sa-mRNAs per dot) were loaded onto a positively charged nylon membrane (Whatman Nytran SuPerCharge, Sigma-Aldrich) that was taped on a sheet of Whatman GB005 blotting paper. After drying, the membrane was blocked in 5% (w/v) non-fat dried milk in PBS-T buffer (0.1% (v/v) Tween-20 in PBS) for 1 h at room temperature. After three washes with PBS-T buffer, the membrane was incubated overnight at 4°C on a rolling mixer with mouse J2 anti-dsRNA murine antibody (Scicons, Budapest, Hungary) diluted 1:5,000 in PBS-T buffer containing 1% (w/v) non-fat dried milk. Next, the membranes were washed three times with PBS-T buffer and incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated donkey anti-mouse immunoglobulin G (IgG) (H+L, Jackson ImmunoResearch Laboratories, Cambridgeshire, UK) diluted 1:10,000 in PBS-T buffer containing 1% (w/v) non-fat dried milk. After washing the membranes three times with PBS-T buffer, detection of the target dsRNAs on the membrane was performed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and the ChemiDoc MP Imaging System (Bio-Rad, USA).
In vivo IFN response
IFN-β+/Δβ-luc mice were used to investigate the effect of several innate immune inhibitors on the IFN response elicited by our ZIKVac-sa-mRNA vaccine. IFN-β+/Δβ-luc mice were shaved at their flanks and injected intradermally into both flanks with 0.5 μg sa-mRNA vaccine in 25 μL PBS (without Ca2+ and Mg2+) using 29G insulin needles (VWR, the Netherlands). Electroporation, when used, was performed immediately after each sa-mRNA injection with a 2-needle array electrode containing 4 needles per row of 4 mm (AgilePulse, BTX Harvard Apparatus, Massachusetts, USA). The procedure for electroporation involved two short high-voltage pulses of 450 V with a duration of 0.05 ms and an interval of 300 ms, followed by eight long low-voltage pulses of 100 V with a duration of 10 ms and an interval of 300 ms.4,16 Innate immune inhibitors (Figure 1) were co-injected with the sa-mRNA vaccine. For certain experiments, the injection site was pre-treated 5 h before sa-mRNA administration and post-treated twice daily by intradermal injection of the innate inhibitors. The corticosteroids were not injected but applied topically as an ointment (clobetasol and hydrocortisone) at the injection site (25 μg clobetasol/cm2) or administered orally in drinking water (final concentration of dexamethasone, 5 mg/L in water). The type I IFN response was monitored by measuring the bioluminescent signal at the injection sites daily for 7 days. To that end, mice were injected subcutaneously with 200 μL D-luciferin (15 mg/mL, Gold Biotechnology, USA). Twelve minutes later, the mice were anesthetized using isoflurane, and the in vivo bioluminescence signal was recorded using an IVIS Lumina II (PerkinElmer, USA).
In vivo translation kinetics
The effect of selected innate inhibitors and the cellulose-based purification method on in vivo translation of the sa-mRNA was investigated by intradermal electroporation (see above for the protocol) of 1 μg silica- or cellulose-purified LUC-sa-mRNA in BALB/c mice in the presence or absence of the indicated innate immune inhibitors. LUC expression was monitored for 28 or 35 days by in vivo optical imaging, as described above.
Vaccination experiment
Shaved female BALB/c mice (6 weeks old) were anesthetized by inhalation of isoflurane and immunized by intradermal electroporation of 0.5 μg ZIKVac-sa-mRNA vaccine or the LUC-sa-mRNA in both flanks using the vaccination schedule depicted in Figure 6A. Silica- and cellulose-purified ZIKVac-sa-mRNA vaccines with and without clobetasol treatment of the injection site were investigated. Topical treatment with clobetasol involved treatment of the injection site 12 h prior to vaccination and a treatment twice daily until 3 days after immunization. Electroporation was performed immediately after each ZIKVac-sa-mRNA injection using the protocol described above.
Zika virus-specific antibody titers
A mouse ZIKV ELISA kit (Alpha Diagnostic International, TX, USA) was used to determine ZIKV E protein-specific antibody titers. 96-well plates pre-coated with ZIKV E protein were equilibrated for 5 min at room temperature with 300 μL of the provided wash buffer. Subsequently, 2-fold serial dilutions of the serum samples were made (starting from a 50-fold dilution), and 100 μL of these dilutions was added per well along with the calibration standards. After 1 h of incubation at room temperature, the plates were washed four times with wash solution. Next, 100 μL of anti-mouse IgG HRP conjugate working solution was added to the wells and incubated at room temperature. After 30 min, the wells were washed five times and incubated with 100 μL of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate at room temperature. Enzymatic conversion of TMB was stopped after 15 min by adding 100 μL of stop solution, and the absorbance was measured at 450 nm in an EZ 400 microplate reader (Biochrom, UK). The antibody endpoint titers were defined as the highest reciprocal dilution with an absorbance that was at least two times the background (obtained with serum from unvaccinated mice).
Zika virus-specific cellular immune response
Intracellular cytokine staining was performed to determine Zika virus-specific CD4+ and CD8+ T cell responses with flow cytometry. Splenocytes were isolated 1 week after the last booster and stimulated in 96-well plates (1 × 106 cells/well) with 2 μg/mL of overlapping 15-amino-acid-long peptides covering the ZIKV E protein (JPT, Berlin, Germany) in 1640 RPMI medium. After 1 h of stimulation at 37°C 0.3 μL of protein transport inhibitor cocktail (Brefeldin A [5.3 mM] + Monensin [1 mM], eBioscience) was added to 150 μL of stimulated splenocytes, and the samples were incubated for 5 h at 37°C. Splenocytes were then harvested, washed with cold PBS, treated with mouse BD Fc Block (BD Biosciences), and stained with anti-CD3-APC/CD4-PerCP/CD8-Alexa Fluor 488 antibodies (clones 145-2C11, RM4-5, and 53-6.7, BioLegend) for 30 min at 4°C according to the manufacturer’s instructions. Subsequently, the cells were fixed and permeabilized with fixation/permeabilization buffer (eBioscience) for 30 min at 4°C before intracellular staining with anti-IFN-γ-PE antibody (clone XMG1.2, BioLegend) for 30 min at room temperature. Finally, all samples were washed and stored at 4°C until analysis using a Cytoflex flow cytometer (Beckman Coulter). Single and live cells were gated, and 300,000 events were collected for each sample. Samples treated with cell stimulation cocktail (eBioscience) served as positive controls and unstimulated samples as negative controls.
Statistical analyses
Statistical analyses were performed with GraphPad Prism software (version 7.0, GraphPad, San Diego, CA, USA). The longitudinal experiments with different animal groups were analyzed using repeated-measures two-way ANOVA corrected for multiple comparisons (Bonferroni method). Differences between two groups were compared with Student’s t test (non-parametric Mann-Whitney U test). The data are represented as means ± SEM unless otherwise noted. A p value below 0.05 is considered statistically significant (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Acknowledgments
This work was supported by the Concerted Research Action (GOA) Fund of Ghent University (project code BOF15/GOA/013), BOF-UGent (BOF.BAS.2018.0028.01), and the Research Foundation-Flanders (FWO; project code G087516N). Z.Z. acknowledges funding from the China Scholarship Council (CSC; 201607650018).
Author contributions
Z.Z. and N.N.S. conceived experiments, participated in experimental studies, interpreted the results, and wrote the manuscript. S.M. and L.O. assisted with cellulose-based purification of sa-mRNA. F.C. and N.N.S. critically revised the manuscript. S.M., H.W., H.H., J.D.T., and J.P.P.C. helped with the in vivo experiments. S.L. provided the IFN-β+/Δβ-luc reporter mice. All authors approved the final version of the manuscript.
Declaration of interest
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.01.023.
Supplemental information
References
- 1.Zhong Z., Mc Cafferty S., Combes F., Huysmans H., De Temmerman J., Gitsels A., Vanrompay D., Portela Catani J., Sanders N.N. mRNA therapeutics deliver a hopeful message. Nano Today. 2018;23:16–39. [Google Scholar]
- 2.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andries O., De Filette M., Rejman J., De Smedt S.C., Demeester J., Van Poucke M., Peelman L., Peleman C., Lahoutte T., Sanders N.N. Comparison of the gene transfer efficiency of mRNA/GL67 and pDNA/GL67 complexes in respiratory cells. Mol. Pharm. 2012;9:2136–2145. doi: 10.1021/mp200604h. [DOI] [PubMed] [Google Scholar]
- 4.Huysmans H., Zhong Z., De Temmerman J., Mui B.L., Tam Y.K., Mc Cafferty S., Gitsels A., Vanrompay D., Sanders N.N. Expression Kinetics and Innate Immune Response after Electroporation and LNP-Mediated Delivery of a Self-Amplifying mRNA in the Skin. Mol. Ther. Nucleic Acids. 2019;17:867–878. doi: 10.1016/j.omtn.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyman B., Huysmans H., Mc Cafferty S., Combes F., Cox E., Devriendt B., Sanders N.N. Comparison of the Expression Kinetics and Immunostimulatory Activity of Replicating mRNA, Nonreplicating mRNA, and pDNA after Intradermal Electroporation in Pigs. Mol. Pharm. 2018;15:377–384. doi: 10.1021/acs.molpharmaceut.7b00722. [DOI] [PubMed] [Google Scholar]
- 6.Andries O., De Filette M., De Smedt S.C., Demeester J., Van Poucke M., Peelman L., Sanders N.N. Innate immune response and programmed cell death following carrier-mediated delivery of unmodified mRNA to respiratory cells. J. Control. Release. 2013;167:157–166. doi: 10.1016/j.jconrel.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Devoldere J., Dewitte H., De Smedt S.C., Remaut K. Evading innate immunity in nonviral mRNA delivery: don’t shoot the messenger. Drug Discov. Today. 2016;21:11–25. doi: 10.1016/j.drudis.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Edwards D.K., Jasny E., Yoon H., Horscroft N., Schanen B., Geter T., Fotin-Mleczek M., Petsch B., Wittman V. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017;15:1. doi: 10.1186/s12967-016-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Beuckelaer A., Pollard C., Van Lint S., Roose K., Van Hoecke L., Naessens T., Udhayakumar V.K., Smet M., Sanders N., Lienenklaus S. Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses. Mol. Ther. 2016;24:2012–2020. doi: 10.1038/mt.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Hoecke L., Roose K., Ballegeer M., Zhong Z., Sanders N.N., De Koker S., Saelens X., Van Lint S. The Opposing Effect of Type I IFN on the T Cell Response by Non-modified mRNA-Lipoplex Vaccines Is Determined by the Route of Administration. Mol. Ther. Nucleic Acids. 2020;22:373–381. doi: 10.1016/j.omtn.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollard C., Rejman J., De Haes W., Verrier B., Van Gulck E., Naessens T., De Smedt S., Bogaert P., Grooten J., Vanham G., De Koker S. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013;21:251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepini T., Pulichino A.M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 2017;198:4012–4024. doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Weissman D., Pardi N., Muramatsu H., Karikó K. HPLC purification of in vitro transcribed long RNA. Methods Mol. Biol. 2013;969:43–54. doi: 10.1007/978-1-62703-260-5_3. [DOI] [PubMed] [Google Scholar]
- 15.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Z., Portela Catani J.P., Mc Cafferty S., Couck L., Van Den Broeck W., Gorlé N., Vandenbroucke R.E., Devriendt B., Ulbert S., Cnops L. Immunogenicity and Protection Efficacy of a Naked Self-Replicating mRNA-Based Zika Virus Vaccine. Vaccines (Basel) 2019;7:96. doi: 10.3390/vaccines7030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauffman K.J., Mir F.F., Jhunjhunwala S., Kaczmarek J.C., Hurtado J.E., Yang J.H., Webber M.J., Kowalski P.S., Heartlein M.W., DeRosa F., Anderson D.G. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials. 2016;109:78–87. doi: 10.1016/j.biomaterials.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengsfeld C.S., Anchordoquy T.J. Shear-induced degradation of plasmid DNA. J. Pharm. Sci. 2002;91:1581–1589. doi: 10.1002/jps.10140. [DOI] [PubMed] [Google Scholar]
- 19.Lienenklaus S., Cornitescu M., Zietara N., Łyszkiewicz M., Gekara N., Jabłónska J., Edenhofer F., Rajewsky K., Bruder D., Hafner M. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J. Immunol. 2009;183:3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 20.Baiersdörfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Karikó K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue S., Shibata Y., Takabatake N., Igarashi A., Abe S., Kubota I. Influence of corticosteroid therapy on the serum antibody response to influenza vaccine in elderly patients with chronic pulmonary diseases. EXCLI J. 2013;12:760–765. [PMC free article] [PubMed] [Google Scholar]
- 22.Kubiet M.A., Gonzalez-Rothi R.J., Cottey R., Bender B.S. Serum antibody response to influenza vaccine in pulmonary patients receiving corticosteroids. Chest. 1996;110:367–370. doi: 10.1378/chest.110.2.367. [DOI] [PubMed] [Google Scholar]
- 23.Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., Wicke L., Perkovic M., Beissert T., Haas H. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018;26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geall A.J., Verma A., Otten G.R., Shaw C.A., Hekele A., Banerjee K., Cu Y., Beard C.W., Brito L.A., Krucker T. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen D.N., Mahon K.P., Chikh G., Kim P., Chung H., Vicari A.P., Love K.T., Goldberg M., Chen S., Krieg A.M. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc. Natl. Acad. Sci. USA. 2012;109:E797–E803. doi: 10.1073/pnas.1121423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theofilopoulos A.N., Baccala R., Beutler B., Kono D.H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 28.Brisse M., Ly H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019;10:1586. doi: 10.3389/fimmu.2019.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietilä M.K., Hellström K., Ahola T. Alphavirus polymerase and RNA replication. Virus Res. 2017;234:44–57. doi: 10.1016/j.virusres.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Hellström K., Kallio K., Utt A., Quirin T., Jokitalo E., Merits A., Ahola T. Partially Uncleaved Alphavirus Replicase Forms Spherule Structures in the Presence and Absence of RNA Template. J. Virol. 2017;91:e00787-17. doi: 10.1128/JVI.00787-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanno A., Tanimura N., Ishizaki M., Ohko K., Motoi Y., Onji M., Fukui R., Shimozato T., Yamamoto K., Shibata T. Targeting cell surface TLR7 for therapeutic intervention in autoimmune diseases. Nat. Commun. 2015;6:6119. doi: 10.1038/ncomms7119. [DOI] [PubMed] [Google Scholar]
- 32.Agier J., Żelechowska P., Kozłowska E., Brzezińska-Błaszczyk E. Expression of surface and intracellular Toll-like receptors by mature mast cells. Cent. Eur. J. Immunol. 2016;41:333–338. doi: 10.5114/ceji.2016.65131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moresco E.M., LaVine D., Beutler B. Toll-like receptors. Curr. Biol. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 34.Linares-Fernández S., Lacroix C., Exposito J.Y., Verrier B. Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends Mol. Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Desmet C.J., Ishii K.J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J., Zhang H., Huang Y., Wang H., Wang S., Zhao C., Liang Y., Yang N. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Int. Immunopharmacol. 2013;17:116–122. doi: 10.1016/j.intimp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Hu S., Luo Q., Cun B., Hu D., Ge S., Fan X., Chen F. The pharmacological NF-κB inhibitor BAY11-7082 induces cell apoptosis and inhibits the migration of human uveal melanoma cells. Int. J. Mol. Sci. 2012;13:15653–15667. doi: 10.3390/ijms131215653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobson C., Cornell R.C., Savin R.C. A comparison of clobetasol propionate 0.05 percent ointment and an optimized betamethasone dipropionate 0.05 percent ointment in the treatment of psoriasis. Cutis. 1986;37:213–214, 216, 218–220. [PubMed] [Google Scholar]
- 39.Allenby C.F., Main R.A., Marsden R.A., Sparkes C.G. Effect on adrenal function of topically applied clobetasol propionate (Dermovate) BMJ. 1975;4:619–621. doi: 10.1136/bmj.4.5997.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carruthers J.A., August P.J., Staughton R.C. Observations on the systemic effect of topical clobetasol propionate (Dermovate) BMJ. 1975;4:203–204. doi: 10.1136/bmj.4.5990.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Krishnan M.N., Phua K.K.L. Suppression of mRNA Nanoparticle Transfection in Human Fibroblasts by Selected Interferon Inhibiting Small Molecule Compounds. Biomolecules. 2017;7:7. doi: 10.3390/biom7030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awe J.P., Crespo A.V., Li Y., Kiledjian M., Byrne J.A. BAY11 enhances OCT4 synthetic mRNA expression in adult human skin cells. Stem Cell Res. Ther. 2013;4:15. doi: 10.1186/scrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascolo S. Messenger RNA-based vaccines. Expert Opin. Biol. Ther. 2004;4:1285–1294. doi: 10.1517/14712598.4.8.1285. [DOI] [PubMed] [Google Scholar]
- 44.Hyde J.L., Gardner C.L., Kimura T., White J.P., Liu G., Trobaugh D.W., Huang C., Tonelli M., Paessler S., Takeda K. A viral RNA structural element alters host recognition of nonself RNA. Science. 2014;343:783–787. doi: 10.1126/science.1248465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlee M., Roth A., Hornung V., Hagmann C.A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schott J.W., Morgan M., Galla M., Schambach A. Viral and Synthetic RNA Vector Technologies and Applications. Mol. Ther. 2016;24:1513–1527. doi: 10.1038/mt.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.