Abstract
Background: Cervical anastomotic leakage (CAL) is one of the most common complications that occur minimally invasive esophagectomy (MIE). It is associated with high postoperative mortality. Some risk factors still remained controversial and so accurate prediction of risk groups for CAL remained very difficult. This study aimed to identify the risk factors of CAL after McKeown MIE to predict the accuracy of the technique as early as possible.
Material and Methods: A total of 129 patients with esophageal cancer who underwent McKeown MIE at the Department of Thoracic Surgery, the Fourth Hospital of Hebei Medical University, between January 2018 and June 2019 were retrospectively reviewed. Multivariate logistic regression analysis was used to identify the risk factors for CAL and receiver operating characteristic (ROC) curve analysis was used to predict the accuracy for each quantitative data variable and determine the cutoff value.
Results: There were statistically significant differences between Group CAL and Group NCAL in FEV1 (p = 0.031), neoadjuvant chemotherapy (p = 0.001), intraoperative minimum PaCO2 (p = 0.002), and hospital stays (p <0.001). In multivariate logistic regression, FEV1 (OR = 0.440, p = 0.047), neoadjuvant chemotherapy (OR = 4.425, p = 0.003), and intraoperative minimum PaCO2 (OR = 1.14, p <0.001) were identified to be three risk factors of CAL. The ROC curve analysis showed that FEV1 <2.18L (p = 0.029) and intraoperative minimum PaCO2 >45.5 mmHg (p = 0.002) demonstrated good accuracy.
Conclusion: FEV1, neoadjuvant chemotherapy, and intraoperative minimum PaCO2 in arterial blood gas (ABG) were considered as risk factors of CAL after McKeown MIE for esophageal cancer. Preoperative FEV1 <2.18L and intraoperative minimum PaCO2 >45.5 mmHg in ABG showed good accuracy in predicting risk factors for CAL.
Keywords: cervical anastomotic leakage, risk factors, minimally invasive esophagectomy, esophageal cancer
Introduction
Esophageal cancer ranks sixth among all cancer-related deaths worldwide.1,2) Esophagectomy is still considered as the primary curative treatment for esophageal cancer.1–4) With the continuous development of minimally invasive technology, the approach of minimally invasive esophagectomy (MIE) has been widely used.3) According to several previous studies, MIE is associated with lower in-hospital mortality, morbidity of postoperative complications, and postoperative hospital stays than open esophagectomy.3,5) However, cervical anastomotic leakage (CAL) is one of the most common complications that occur after MIE.2,4,6,7) Among these, anastomotic tissue hypoperfusion is regarded as the major risk factor of anastomotic leakage.2,4,8,9) Despite several advances in surgical treatment and perioperative care in recent years, the incidence of CAL after esophagectomy still remained high, ranging from 8 to 35%.2,10–15) CAL after esophagectomy is associated with high postoperative mortality.2,4)
Previous studies have identified various risk factors for CAL, such as neoadjuvant chemotherapy,4,15,16) chronic obstructive pulmonary disease (COPD),2) smoker at diagnosis,6) pneumonia,17,18) calcification of arteries,9,19) diabetes mellitus,2,6,20) body mass index (BMI),1) and arrhythmia.2,6,19,21) Some risk factors still remained controversial and so accurate prediction of risk groups for CAL remained very difficult. This study aimed to identify the risk factors of CAL after McKeown MIE via preoperative, intraoperative, and postoperative first day data and also offered the cutoff values. This subsequently assists in predicting the accuracy of the technique as early as possible and in decision-making regarding the surgical treatment and perioperative care.
Materials and Methods
A total of 129 patients with esophageal cancer who underwent McKeown MIE at the Department of Thoracic Surgery, the Fourth Hospital of Hebei Medical University, between January 2018 and June 2019 were retrospectively reviewed.
This study was approved by the Institutional Ethics Board of the Fourth Hospital of Hebei Medical University and an exemption from informed consent was obtained. This study was conducted according to the approved guidelines.
The inclusion criteria were as follows: (1) pathological confirmation of esophageal malignancy; (2) patients who underwent McKeown esophagectomy with left cervical anastomosis; and (3) patients with complete clinical data. Patients with incomplete clinical data were excluded, such as the lung function examination cannot be completed due to poor coordination of patients.
The information regarding gender, age, BMI, FEV1, FEV1/FVC, diabetes mellitus, hypertension, coronary artery disease, smoker at diagnosis, neoadjuvant therapy, tumor location, duration of operation, the count of red blood cell, hemoglobin, blood platelet, alanine transaminase, albumin, D-dimer, histology, venous thrombosis, arrhythmia, TNM stages, hospital stays and intraoperative maximum, minimum, mean values of pH, PaO2, PaCO2 in arterial blood gas (ABG) were collected as potential risk factors of CAL after McKeown MIE.
TNM stages of the patients were determined according to the eighth edition of the Union for International Cancer Control (UICC) staging system. Venous thrombosis contained deep vein thrombus and intermuscular vein thrombosis. Arrhythmia contained atrial fibrillation, tachycardia, and ventricular fibrillation.
Surgical procedures
All 129 patients who received thoracoscopic-laparoscopic McKeown MIE with left cervical anastomosis were included. First, the esophagus was separated by performing right video-assisted thoracoscopic surgery. After that, laparoscopic gastric resection and cutting off of the esophageal cervical region were performed simultaneously. Finally, the gastric tube reconstruction and cervical anastomosis of esophagus and gastric tube were performed. Left gastric vessels, common hepatic artery, and short gastric vessels were divided and right gastroepiploic artery was remained. A slender conduit (a width of 3–5 cm) was reconstructed and an end-to-side esophagogastric anastomosis was performed in the left neck by circular stapled anastomotic technique. Straight line stapler was used to remove the tubular gastric remnant at 2 cm from the anastomosis. The anastomotic stoma was interrupted suture, and suspended at the neck. Single lumen intubation and continuous CO2 insufflation were performed during the thoracic phase to obtain a better surgical vision. ABG was detected per hour during the operation and if a patient had low oxygen saturation or abnormal blood gas analysis, more blood gas analysis should be added. Nasal feeding tube or jejunostomy tube was used for nutritional support after esophagectomy.
Diagnosis of CAL
The diagnosis of CAL was determined according to the imageological examinations, which include the upper gastrointestinal radiography and computed tomography (CT) scan with oral contrast medium. All patients underwent imageological examination. Of the 129 patients, 104 underwent imageological examinations on day 7 after esophagectomy, and 21 patients were found to be diagnosed with CAL. Eight of the 129 patients who underwent imageological examinations before day 7 after esophagectomy had flow of pus, saliva, or digestive juice from the site of cervical anastomosis, and all patients were diagnosed with CAL. In all, 17 of the 129 patients were unable to undergo imageological examinations before day 7 after esophagectomy because they were transferred to intensive care unit ward due to respiratory failure, and imageological examinations were performed after their return to the thoracic surgery ward. Two of these patients were diagnosed with CAL.
Statistical analysis
SPSS program (version 22.0; SPSS Inc., Chicago, IL, USA) was used to analyze the differences in the data. P values of <0.05 were defined as statistically significant. Quantitative data between Group CAL and Group NCAL was tested by Student's t-test or Mann–Whitney U-test, as appropriate. Qualitative data were tested by Chi-square test or its continuous correction. Fisher’s exact test was used if any expected value was less than 1. Multivariate logistic regression analysis was used to identify the risk factors of CAL and the factors with p <0.05 in univariate analysis entered into multivariate model. The adjusted odds ratios (ORs), 95% confidence intervals (CIs) and P values of respective risk factors were shown. Finally, the receiver operating characteristic (ROC) curve analysis was used to predict the accuracy of quantitative data and determine the cutoff value.
Results
McKeown MIE was successfully performed in 129 patients, with an average age of 64.05 ± 7.71 years. Four patients who were converted to thoracotomy and one patient who was converted to laparotomy were excluded from the study. Two patients who were unable to cooperate the lung function examination were also excluded. Of the 129 patients, 85 were males and 44 were females. The number of patients with diabetes mellitus, hypertension, coronary artery disease, smoke at diagnosis, neoadjuvant therapy, venous thrombosis, and arrhythmia was 11 (8.5%), 52 (40.3%), 2 (1.6%), 63 (48.8%), 29 (22.5%), 24 (18.6%), and 34 (26.4%), respectively. The histology of most of the patients was confirmed to be squamous cell carcinoma, which accounted for 119 (92.2%) patients, while other histologies including adenocarcinoma, large cell carcinoma, small cell carcinoma, and mixed types accounted for 10 (7.8%). Intraoperative ABG showed maximum pH (7.36 ± 0.06), minimum pH (7.23 ± 0.06), mean pH (7.29 ± 0.05), maximum PaCO2 (60.22 ± 10.93 mmHg), minimum PaCO2 (45.65 ± 6.89 mmHg), mean PaCO2 (49.99 ± 7.17 mmHg), maximum PaO2 (393.64 ± 87.61 mmHg), minimum PaO2 (126.15 ± 74.51 mmHg), and mean PaO2 (262.94 ± 78.25 mmHg). The average hospital stay was 23.42 (± 14.01) days.
The patients were divided into two groups: Group CAL (patients with postoperative complications of CAL) and Group NCAL (patients without postoperative complications of CAL). Complications were observed in 31 (24.0%) patients in Group CAL. Comparison of patient characteristics between the two groups is presented in Table 1. No statistical differences were found in the BMI (p = 0.429), FEV1/FVC (p = 0.090), arrhythmia (p = 0.186), and TNM stages (p = 0.063) in both the groups. FEV1 in Group CAL (2.31 ± 0.55L) was significantly smaller than that in Group NCAL (2.58 ± 0.62L) (p = 0.031). There was a statistically significant difference in neoadjuvant chemotherapy (p = 0.001). In addition, statistically significant difference was also found in intraoperative minimum PaCO2 in ABG between the two groups (p = 0.002). The complications associated with CAL have significantly prolonged the hospital stay from 19.42 ± 11.42 days to 35.94 ± 14.59 days (p <0.001).
Table 1. Characteristics of patients with esophageal cancer according to CAL.
| Variable | Total | CAL (n = 31) | NCAL (n = 98) | t/z | p value |
|---|---|---|---|---|---|
| Preoperative | |||||
| Age (years) | 64.05 ± 7.71 | 64.77 ± 6.69 | 63.82 ± 8.02 | 0.602 | 0.549a |
| Gender | 1.251 | 0.263b | |||
| Male | 85 (65.9%) | 23 (74.2%) | 62 (63.3%) | ||
| Female | 44 (34.1%) | 8 (25.9%) | 36 (36.7%) | ||
| BMI (kg/m2) | 23.25 ± 2.94 | 23.70 ± 3.02 | 23.10 ± 2.92 | 0.791 | 0.429c |
| FEV1 (L) | 2.52 ± 0.61 | 2.31 ± 0.55 | 2.58 ± 0.62 | 2.177 | 0.031a |
| FEV1/FVC (%) | 75.75 ± 9.66 | 72.88 ± 10.36 | 76.66 ± 9.29 | 1.695 | 0.090c |
| Diabetes mellitus | 0.069 | 0.792b | |||
| Yes | 11 (8.5%) | 3 (9.7%) | 8 (8.2%) | ||
| No | 118 (91.5%) | 28 (90.3%) | 90 (91.8%) | ||
| Hypertension | 2.157 | 0.142b | |||
| Yes | 52 (40.3%) | 9 (29.0%) | 43 (43.9%) | ||
| No | 77 (59.7%) | 22 (71.0%) | 55 (56.1%) | ||
| Coronary heart disease | 0.424d | ||||
| Yes | 2 (1.6%) | 1 (3.2%) | 1 (1.0%) | ||
| No | 127 (98.4%) | 30 (96.8%) | 97 (99.0%) | ||
| Smoker at diagnosis | 1.390 | 0.238b | |||
| Yes | 63 (48.8%) | 18 (58.1%) | 45 (45.9%) | ||
| No | 66 (51.2%) | 13 (41.9%) | 53 (54.1%) | ||
| Neoadjuvant chemotherapy | 12.045 | 0.001b | |||
| Yes | 29 (22.5%) | 14 (45.2%) | 15 (15.3%) | ||
| No | 100 (77.5%) | 17 (54.8%) | 83 (84.7%) | ||
| Tumor location | 0.273 | 0.873b | |||
| Upper | 17 (13.2%) | 4 (12.9%) | 13 (13.3%) | ||
| Middle | 67 (51.9%) | 15 (48.4%) | 52 (53.1%) | ||
| Lower | 45 (34.9%) | 12 (38.7%) | 33 (33.7%) | ||
| Intraoperative | |||||
| Duration of operation (minutes) | 290.40 ± 46.99 | 298.35 ± 46.69 | 287.89 ± 47.04 | 1.182 | 0.237c |
| Maximum pH in ABG | 7.36 ± 0.06 | 7.34 ± 0.07 | 7.37 ± 0.06 | 1.386 | 0.166c |
| Minimum pH in ABG | 7.23 ± 0.06 | 7.22 ± 0.06 | 7.23 ± 0.06 | 1.127 | 0.260c |
| Mean pH in ABG | 7.29 ± 0.05 | 7.28 ± 0.06 | 7.30 ± 0.05 | 1.546 | 0.125a |
| Maximum PaCO2 in ABG (mmHg) | 60.22 ± 10.93 | 61.52 ± 10.85 | 59.82 ± 10.98 | 1.123 | 0.262c |
| Minimum PaCO2 in ABG (mmHg) | 45.65 ± 6.89 | 44.58 ± 7.34 | 39.41 ± 6.28 | 3.128 | 0.002c |
| Mean PaCO2 in ABG (mmHg) | 49.99 ± 7.17 | 52.10 ± 8.17 | 49.33 ± 6.73 | 1.657 | 0.098c |
| Maximum PaO2 in ABG (mmHg) | 393.64 ± 87.61 | 402.10 ± 86.02 | 390.96 ± 88.73 | 0.626 | 0.532c |
| Minimum PaO2 in ABG (mmHg) | 126.15 ± 74.51 | 120.87 ± 63.25 | 127.82 ± 77.95 | 0.196 | 0.845c |
| Mean PaO2 in ABG (mmHg) | 262.94 ± 78.25 | 266.5 ± 71.7 | 261.8 ± 80.51 | 0.551 | 0.581c |
| Postoperative first day | |||||
| Red blood cell (×1012/L) | 3.88 ± 0.58 | 3.94 ± 0.46 | 3.87 ± 0.61 | 0.565 | 0.573a |
| Hemoglobin (g/L) | 121.58 ± 17.04 | 121.35 ± 15.45 | 121.66 ± 17.59 | 0.807 | 0.931a |
| Blood platelet (109/L) | 200.26 ± 60.26 | 195.26 ± 56.82 | 201.84 ± 61.51 | 0.240 | 0.810c |
| Alanine transaminase (U/L) | 40.87 ± 32.6 | 45.94 ± 51.52 | 39.27 ± 23.98 | 0.466 | 0.641c |
| Albumin (g/L) | 30.32 ± 3.58 | 32.11 ± 3.71 | 32.39 ± 3.91 | 0.355 | 0.723a |
| Postoperative | |||||
| D-dimer (mg/L) | 30.23 ± 2.30 | 3.22 ± 1.96 | 3.24 ± 2.41 | 0.309 | 0.758c |
| Venous thrombosis | 0.015 | 0.902b | |||
| Yes | 24 (18.6%) | 6 (19.4%) | 18 (18.4%) | ||
| No | 105 (81.4%) | 25 (80.6%) | 80 (81.6%) | ||
| Arrhythmia | 1.751 | 0.186b | |||
| Yes | 34 (26.4%) | 11 (35.5%) | 23 (23.5%) | ||
| No | 95 (73.6%) | 20 (64.5%) | 75 (76.5%) | ||
| Histology | 0.006 | 0.940e | |||
| Squamous cell carcinoma | 119 (92.2%) | 28 (90.3%) | 91 (92.9%) | ||
| Others | 10 (7.8%) | 3 (9.7%) | 7 (7.1%) | ||
| TNM stages | 7.299 | 0.063b | |||
| I | 5 (27.1%) | 3 (9.7%) | 32 (32.7%) | ||
| II | 36 (27.9%) | 10 (32.3%) | 26 (26.5%) | ||
| III | 40 (31.0%) | 11 (35.5%) | 29 (29.6%) | ||
| IVA | 18 (14.0%) | 7 (22.6%) | 11 (11.2%) | ||
| Hospital stays (days) | 23.42 ± 14.01 | 35.94 ± 14.59 | 19.42 ± 11.42 | 6.606 | <0.01c |
aIndependent t-test,
bChi-square test,
cMann–Whitney U-test,
dFisher exact test,
eContinuous correction Chi-square test.
ABG: arterial blood gas; BMI: body mass index; CAL: cervical anastomotic leakage
Univariate analysis showed significant differences between the two groups in FEV1 (p = 0.034), neoadjuvant chemotherapy (p = 0.001), and intraoperative minimum PaCO2 in ABG (p = 0.001). These factors were selected for multiple logistic regression analysis. In multivariate logistic regression (Table 2), FEV1 (OR = 0.440, 95% CI = 0.196–0.987, p = 0.047), neoadjuvant chemotherapy (OR = 4.425, 95% CI = 1.639–11.948, p = 0.003), and intraoperative minimum PaCO2 in ABG (OR = 1.14, 95% CI = 1.052–1.236, p <0.001) were identified as the three risk factors for CAL.
Table 2. Result of multiple logistic regression analysis in FEV1, neoadjuvant chemotherapy, intraoperative minimum PaCO2 in ABG.
| Variable | Adjusted OR | 95% CI | p value |
|---|---|---|---|
| FEV1 | 0.440 | 0.196–0.987 | 0.047 |
| Neoadjuvant chemotherapy | 4.425 | 1.639–11.948 | 0.003 |
| Intraoperative minimum PaCO2 in ABG | 1.14 | 1.052–1.236 | 0.001 |
ABG: arterial blood gas; CI: confidence interval; OR: odds ratio
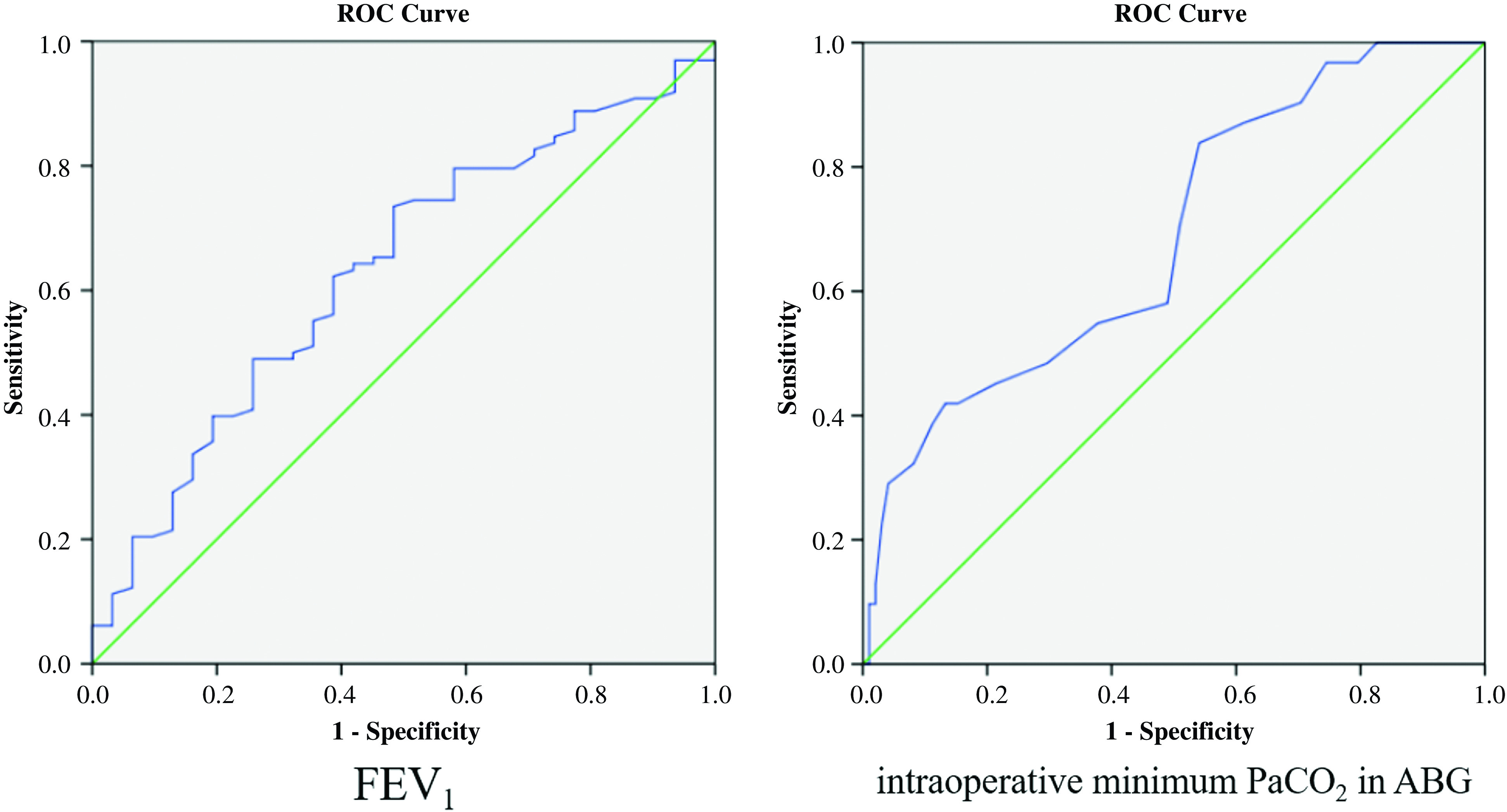
The ROC curve (Table 3) (Fig. 1) showed that two factors demonstrated good accuracy in predicting the risk factors for CAL, which included the FEV1 (area under the curve: 0.630, p = 0.029) and the intraoperative minimum PaCO2 in ABG (area under the curve: 0.686, p = 0.002). The cutoff value was defined as the value with maximum Youden index, representing the best compromise between the sensitivity and specificity. The cutoff values were 2.18L and 45.5mmHg in FEV1 and intraoperative minimum PaCO2 in ABG, respectively (Table 3).
Table 3. Sensitivity, specificity, AUC, and cutoff of predictors.
| Variable | Sensitivity | Specificity | AUC | Cutoff | p value |
|---|---|---|---|---|---|
| FEV1 | 73.5% | 51.6% | 0.630 | 2.18 | 0.029 |
| Intraoperative Minimum PaCO2 in ABG | 41.9% | 86.7% | 0.686 | 45.5 | 0.002 |
ABG: arterial blood gas; AUC: area under the curve
Fig. 1. The receiver operating characteristic (ROC) curve of FEV1 and intraoperative minimum PaCO2 in ABG. ABG: arterial blood gas.
Discussion
McKeown MIE is the main procedure of MIE that has been widely used in patients with esophageal cancer.5,11,19) The main anastomotic method of esophagectomy included intrathoracic anastomosis and cervical anastomosis and left CAL has been the main complication of McKeown MIE. It provided a wider range of lymphadenectomy, especially the bilateral para-laryngeal recurrent nerve lymph nodes.3) However, this surgical method also had some disadvantages, such as long duration of operation and high incidence of CAL.2–4,6,7) The therapy of CAL included continuous drainage, enhanced nutrition support and anti-infection. Even though the treatment is known, four patients had mediastinal infection and two patients died due to this procedure. CAL increased the length of hospital stay and increased the postoperative mortality.2,4) Hence, it is imperative to predict the risk factors for CAL early and accurately after undergoing McKeown MIE.
In this study, the preoperative FEV1 <2.18L and intraoperative minimum PaCO2 >45.5mmHg in ABG were identified as risk factors for CAL after undergoing McKeown MIE procedure. For patients whose FEV1 lower than 2.18L, preoperative preparation strategies could be adjusted to further improve patients' lung function and reduce the risk of CAL. The intraoperative minimum value of PaCO2 could help predict the risk groups for CAL early. For patients whose intraoperative minimum PaCO2 higher than 45.5 mmHg, postoperative diagnosis and treatment strategies can be adjusted, such as delay the time of oral feeding or giving more nutrition intake to reduce the risk of CAL. Previous studies have shown that anastomotic tissue hypoperfusion led to postoperative anastomotic leakage and insufficient oxygen supply caused tissue hypoperfusion.2,4,8,9) Adequate tissue oxygen supply is considered as a prerequisite for successful anastomotic healing, and this has been established previously.8) Both lower preoperative FEV1 and higher intraoperative PaCO2 decrease anastomotic tissue hypoperfusion, which cause insufficient perfusion of anastomotic tissue and eventually lead to CAL. Recently, a meta-analysis revealed that CAL was associated with hemodynamic instability caused by atrial fibrillation.21) Only one study involved intraoperative blood gas analysis.17) Another factor that caused changes in the homeostatic environment, which was a postoperative high serum lactate level,4) was found to be risk factors for CAL. More importantly, no relationship between intraoperative pH in ABG and CAL was found. The factors that led to decreased lung function,22) including the COPD,2) smoker at diagnosis,6) and pneumonia,17,18) were identified as risk factors for CAL. This study only focused on CAL and McKeown MIE, while other previous studies included the above discussed intrathoracic anastomotic leakage, open esophagectomy, and other surgical methods of MIE and the definitions of CAL were not exactly the same in all the studies. These factors might cause differences in the results of these studies. Severe tissue hypoperfusion also led to tissue necrosis, subsequently causing subcutaneous infection of the neck, and increasing the probability of anastomotic leakage. Vasopressors are used to treat tissue hypoperfusion caused by hypotension, but it has been proved that the application of vasopressor does not reduce the incidence of CAL, and appeared to increase the risk by three-fold.23) Perioperative use of inotropes increased cardiac output, but did not reduce the risk of CAL.24) Intraoperative artificial pneumothorax and single lumen intubation increase the value of PaCO2. But artificial pneumothorax and single lumen intubation were applied among all patients during the MIE. The continuously high value of intraoperative minimum PaCO2 indicates poor lung function, which suggests that CO2 continues to accumulate in the body. The conclusion of a previous study showed that postoperative high serum lactate level increased the probability of anastomotic leakage4) which is similar to this study. Intraoperative artificial pneumothorax and single lumen intubation only increased the value of PaCO2, not decreased it, so it had no influence on the value of intraoperative minimum PaCO2. On the contrary, the value of intraoperative mean and maximum PaCO2 were greatly affected. This study did not find the value of intraoperative mean and maximum PaCO2 contribute to CAL. The value of PaO2 and tissue oxygenation decreased because of intraoperative artificial pneumothorax and single lumen intubation. According to recent studies,25–27) low tissue oxygenation during or after operation was found to be one of the factors of CAL. Although the reduction of PaO2 is able to decrease the tissue oxygenation, no study has shown an association between PaO2 reduction and CAL. In this study, for patients who cannot keep SPO2 above 90 during the operation received high flow oxygen supply. In this case, this study was not found the maximum, minimum and mean PaO2 were associated with CAL. In these recent studies, the value of tissue oxygenation has been evaluated by optical fiber spectroscopy25,26) and Indocyanine dye.27) There is no study describing how PaO2 reflects the tissue oxygenation.
In the study of Morita, esophagectomy was performed in 310 patients and the 27.9% of patients with preoperative neoadjuvant chemotherapy had CAL. But only 16.5% of patients without preoperative neoadjuvant chemotherapy had CAL, and the results concluded close association of neoadjuvant chemotherapy with CAL.16) The same result was found in the study of Ip.4) In our study, the results of multivariate logistic regression confirmed neoadjuvant chemotherapy as one of the risk factors. Neoadjuvant chemotherapy affected the overall nutritional status, especially immune function,18) increasing the risk of postoperative infection and finally affecting the anastomotic healing negatively. However, some studies showed contrast results. Preoperative neoadjuvant chemotherapy had more survival benefits,28,29) and showed no correlation with CAL.2,13,14) The differences in indications of neoadjuvant chemotherapy, choice of chemotherapeutics, and methods of operation caused differences in the results. Furthermore, patients receiving neoadjuvant chemotherapy also demonstrated more postoperative complications and greater impact on cardiopulmonary function,14,30) which, in turn, reduced tissue perfusion and increased the risk of poor anastomotic healing.
More risk factors for CAL after esophagectomy have been identified, which included diabetes mellitus,2,6,20) BMI,1) arrhythmia,2,6,19,21) and smoker at diagnosis.6) However, these factors were not identified in this study as no uniform conditions were followed. Arrhythmia contained atrial fibrillation, tachycardia, and ventricular fibrillation. Other studies have only confirmed atrial fibrillation as a risk factor of CAL.21) During the perioperative period, blood glucose was measured four times a day in all patients, and blood glucose was controlled within the normal range, so the relationship between diabetes mellitus and CAL was not found. Only one study included 82 patients and mentioned the relationship between pH and anastomotic leakage.17) The small sample size may lead to the difference from this study. Different surgical methods and locations of anastomosis resulted in variations in the results. Few studies have focused on one surgical approach, and included a large sample size as well. Tiny CAL and its influence on the results were ignored because of different definitions of CAL. Moreover, a considerable discrepancy between Asians and Europeans is observed,19) and so it is hard to study them together.
The incidence of postoperative CAL in this study accounted for 24.0% of all patients, and was higher than those shown in some recent studies.2,12–14) This is because the definition of CAL is kept in a very loose position and contained all the tiny CAL, which could be proved by any imageological evidence. Moreover, a recent multicenter randomized study reported that the incidence of CAL was 22%–30%, and the incidence of CAL in this study has fallen within this range.10) Hospital stays for patients without CAL is 23.42 ± 14.01 days. It is longer than some studies2,6) and similar to one study.4) Patients without CAL began oral feeding on the 7th day after MIE, with a gradual transition from a liquid to a semi-liquid diet. The food intake is increased by 500 ml per day. If there are no other symptoms after reaching 3000 ml food intake, the patient can be discharged from the hospital, which takes about 7 days. Thus, a patient can recover and being discharged from the hospital in about 14 days. Patients without CAL also include the ones with serious lung diseases or digestive tract diseases, and their hospital stays seemed to be longer.
For those patients with high risk of CAL, it is important to take corresponding measures actively. First, patients with preoperative FEV1 <2.18L are necessary to accept the treatment to improve the lung function before esophagectomy. Next, during esophagectomy, the surgical procedure is improved to reduce the incidence of pneumonia. Moreover, after esophagectomy, pneumonia is treated by aggressive anti-infective therapy, and high nutrition support is also considered. However, to what extent is preoperative pulmonary function controlled, how to improve surgical procedure and how to choose postoperative antibiotics are still unclear, and requires confirmation by other studies.
However, this study had several limitations that require consideration. First, the sample size in this study was small. Second, this study was limited by single-center retrospective trial. Third, this study focused on a single surgical approach, McKeown MIE with left cervical anastomosis, and it might no longer be applicable when the surgical approach or anastomotic location was changed. Therefore, according to these limitations, the results should be validated in a larger sample size and multicenter studies.
Conclusion
This study included 129 patients who received McKeown MIE with cervical anastomosis and the incidence of CAL was 24.0%. FEV1, neoadjuvant chemotherapy, and intraoperative minimum PaCO2 in ABG were considered as risk factors of CAL after McKeown MIE for esophageal cancer. Preoperative FEV1 <2.18L and intraoperative minimum PaCO2 >45.5mmHg in ABG showed good accuracy in predicting risk factors for CAL.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Ethics Board of the Fourth Hospital of Hebei Medical University and an exemption from informed consent was obtained.
Acknowledgments
We thank all the patients for participating in this study and all the authors for their contribution. This research was supported by “the construction of early diagnosis and treatment platform for esophageal cancer”, funded under the Fourth Hospital of Hebei Medical University “Double first-class” talent construction project.
This research was also supported by “the correlations between the expression of long noncoding RNA (SPRY4-lT1) and the lymph node metastasis in esophageal squamous cell carcinoma”, funded under the specific project of the national cancer center of China on the scientific research of oncology, grant number No.NCC2017A24.
Authors’ Contributions
Gao WD and Tian ZQ conceived and designed the study. Gao WD, Wang MB, Su P, and Zhang F acquired and sorted out the data. Fan Zhang and Chao Huang contributed to analysis of data and production of tables and figure. Gao WD drafted the manuscript. All authors have read and given the final approval of the study.
Disclosure Statement
The authors declare no conflict of interest in the study.
References
- 1). Wang P, Li Y, Sun H, et al. Predictive value of body mass index for short-term outcomes of patients with esophageal cancer after esophagectomy: a meta-analysis. Ann Surg Oncol 2019; 26: 2090– 103. [DOI] [PubMed] [Google Scholar]
- 2). Gooszen JAH, Goense L, Gisbertz SS, et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg 2018; 105: 552– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012; 379: 1887– 92. [DOI] [PubMed] [Google Scholar]
- 4). Ip B, Ng KT, Packer S, et al. High serum lactate as an adjunct in the early prediction of anastomotic leak following oesophagectomy. Int J Surg 2017; 46: 7– 10. [DOI] [PubMed] [Google Scholar]
- 5). Gottlieb-Vedi E, Kauppila JH, Malietzis G, et al. Long-term survival in esophageal cancer after minimally invasive compared to open esophagectomy: a systematic review and meta-analysis. Ann Surg 2019; 270: 1005– 17. [DOI] [PubMed] [Google Scholar]
- 6). Liu YJ, Fan J, He HH, et al. Anastomotic leakage after intrathoracic versus cervical oesophagogastric anastomosis for oesophageal carcinoma in Chinese population: a retrospective cohort study. BMJ Open 2018; 8: e021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011; 28: 29– 35. [DOI] [PubMed] [Google Scholar]
- 8). Van Daele E, Van Nieuwenhove Y, Ceelen W, et al. Assessment of graft perfusion and oxygenation for improved outcome in esophageal cancer surgery: protocol for a single-center prospective observational study. Medicine (Baltimore) 2018; 97: e12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Goense L, van Rossum PSN, Weijs TJ, et al. Aortic calcification increases the risk of anastomotic leakage after ivor-lewis esophagectomy. Ann Thorac Surg 2016; 102: 247– 52. [DOI] [PubMed] [Google Scholar]
- 10). van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074– 84. [DOI] [PubMed] [Google Scholar]
- 11). Zhai C, Liu Y, Li W, et al. A comparison of short-term outcomes between Ivor-Lewis and McKeown minimally invasive esophagectomy. J Thorac Dis 2015; 7: 2352– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Okamura A, Watanabe M, Imamura Y, et al. Preoperative glycosylated hemoglobin levels predict anastomotic leak after esophagectomy with cervical esophagogastric anastomosis. World J Surg 2017; 41: 200– 7. [DOI] [PubMed] [Google Scholar]
- 13). Huang J, Zhou Y, Wang C, et al. Logistic regression analysis of the risk factors of anastomotic fistula after radical resection of esophageal-cardiac cancer. Thorac Cancer 2017; 8: 666– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Gronnier C, Tréchot B, Duhamel A, et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 2014; 260: 764– 70; discussion 770-1. [DOI] [PubMed] [Google Scholar]
- 15). Merritt RE, Whyte RI, D'Arcy NT, et al. Morbidity and mortality after esophagectomy following neoadjuvant chemoradiation. Ann Thorac Surg 2011; 92: 2034– 40. [DOI] [PubMed] [Google Scholar]
- 16). Morita M, Masuda T, Okada S, et al. Preoperative chemoradiotherapy for esophageal cancer: factors associated with clinical response and postoperative complications. Anticancer Res 2009; 29: 2555– 62. [PubMed] [Google Scholar]
- 17). Goense L, van Rossum PS, Tromp M, et al. Intraoperative and postoperative risk factors for anastomotic leakage and pneumonia after esophagectomy for cancer. Dis Esophagus 2017; 30: 1– 10. [DOI] [PubMed] [Google Scholar]
- 18). Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg 2016; 132: 549– 55. [DOI] [PubMed] [Google Scholar]
- 19). Zhao L, Zhao G, Li J, et al. Calcification of arteries supplying the gastric tube increases the risk of anastomotic leakage after esophagectomy with cervical anastomosis. J Thorac Dis 2016; 8: 3551– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Li SJ, Wang ZQ, Li YJ, et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2017; 30: 1– 12. [DOI] [PubMed] [Google Scholar]
- 21). Schizas D, Kosmopoulos M, Giannopoulos S, et al. Meta-analysis of risk factors and complications associated with atrial fibrillation after oesophagectomy. Br J Surg 2019; 106: 534– 47. [DOI] [PubMed] [Google Scholar]
- 22). Goense L, Meziani J, Bülbül M, et al. Pulmonary diffusion capacity predicts major complications after esophagectomy for patients with esophageal cancer. Dis Esophagus 2019; 32: doy082. [DOI] [PubMed] [Google Scholar]
- 23). Zakrison T, Nascimento BA, Tremblay LN, et al. Perioperative vasopressors are associated with an increased risk of gastrointestinal anastomotic leakage. World J Surg 2007; 31: 1627– 34. [DOI] [PubMed] [Google Scholar]
- 24). Choudhuri AH, Uppal R, Kumar M. Influence of non-surgical risk factors on anastomotic leakage after major gastrointestinal surgery: Audit from a tertiary care teaching institute. Int J Crit Illn Inj Sci 2013; 3: 246– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Gareau DS, Truffer F, Perry KA, et al. Optical fiber probe spectroscopy for laparoscopic monitoring of tissue oxygenation during esophagectomies. J Biomed Opt 2010; 15: 061712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Pham TH, Perry KA, Enestvedt CK, et al. Decreased conduit perfusion measured by spectroscopy is associated with anastomotic complications. Ann Thorac Surg 2011; 91: 380– 5. [DOI] [PubMed] [Google Scholar]
- 27). Campbell C, Reames MK, Robinson M, et al. Conduit vascular evaluation is associated with reduction in anastomotic leak after esophagectomy. J Gastrointest Surg 2015; 19: 806– 12. [DOI] [PubMed] [Google Scholar]
- 28). Mariette C, Piessen G, Briez N, et al. Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol 2011; 12: 296– 305. [DOI] [PubMed] [Google Scholar]
- 29). Dewberry LC, Wingrove LJ, Marsh MD, et al. Pilot prehabilitation program for patients with esophageal cancer during neoadjuvant therapy and surgery. J Surg Res 2019; 235: 66– 72. [DOI] [PubMed] [Google Scholar]
- 30). Sathornviriyapong S, Matsuda A, Miyashita M, et al. Impact of neoadjuvant chemoradiation on short-term outcomes for esophageal squamous cell carcinoma patients: a meta-analysis. Ann Surg Oncol 2016; 23: 3632– 40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


