Abstract
Introduction
Although multiple home blood pressure variability (HBPV) indices have been proposed, the superiority of one over another is not clear in treated hypertensives.
Aim
We evaluated the correlation between different indices of HBPV and hypertension-mediated organ damage (HMOD) in this population and determined predictors of greater HBPV.
Methods
We included adult treated hypertensives who performed an HBP monitoring (duplicate sitting BP readings in the morning, afternoon, and evening for 4 days, Omron HEM-705CP-II), laboratory measurements, transthoracic echocardiogram and carotid-femoral pulse wave velocity. We selected HBPV indices from three different calculation approaches: coefficient of variation (CoV), difference between maximum and minimum BP (MMD), and morning BP increase (MI), and evaluated their correlation with left ventricular mass index, relative wall thickness (RWT), ejection fraction, arterial stiffness and estimated glomerular filtration rate through a correlation matrix. For those variability indices significantly associated with HMOD, we constructed multiple linear regression models to determine independent predictors of HBPV.
Results
We included 204 patients, mean age 67.2 (± 13.8) years, 64% female. CoV and MMD for systolic BP showed the greatest correlation with HMOD. Factors independently associated both with CoV and MMD were: older age (b = 0.07; 95% CI 0.04–0.07; p < 0.001 and b = 0.4; 95% CI 0.2–0.5; p < 0.001, respectively), history of stroke (b = 3.6; 95% CI 0.9–6.4; p = 0.01 and b = 25.7; 95% CI 10.1–41.2; p = 0.001, respectively), and body mass index [b = − 0.1; 95% CI − 0.2 to (− 0.02); p = 0.01 and b = − 0.5; 95% CI − 0.9 to (− 0.1); p = 0.01, respectively].
Conclusion
CoV and MMD showed the greatest association with HMOD in treated hypertensives. Older age, history of stroke and lower body mass index were easy-to-detect predictors.
Keywords: Home blood pressure, Self-measurement, Hypertension-mediated organ damage, Variability
Introduction
Blood pressure (BP) is a continuous variable that constantly fluctuates in response to an interplay between environmental, physical and emotional factors [1]. The changes in BP induced by these factors trigger regulatory mechanisms that seek to maintain the so-called BP homeostasis. Despite the fact that, for practical reasons, the mean of several BP recordings is used as the main parameter to characterize a hypertensive patient, as in any complex system [2], the implications of BP may not be captured simply through an average. Furthermore, growing evidence suggests that BP variability imposes additional stress on the cardiovascular system, showing an association with hypertension-mediated organ damage and cardiovascular events regardless of the mean value [3–5].
On the other hand, home blood pressure monitoring (HBPM), a standardized technique for measuring BP outside the office, is widely available, inexpensive and well tolerated by patients and [6], especially in the context of mandatory isolation imposed by the COVID-19 pandemic, it has been positioned as an invaluable tool for the evaluation and monitoring of hypertensive patients. The variability of BP measured through HBPM (HBPV) has also shown an independent role in the cardiovascular damage induced by hypertension. However, it is important to note that there are multiple and varied indices of variability, some easily calculable and others more difficult to determine [7]. Such indices probably represent different components of these variable BP patterns, and the superiority of one over the other is not clear. Nor is it clear what characteristics of the patients or their treatment should be more closely considered in patients with probable BP hypervariability. Moreover, most studies evaluating the relationship between HBPV and hypertension-mediated organ damage have focused on untreated hypertensive individuals or other populations, such as patients with diabetes or chronic kidney disease [7]. Data on HBPV regarding treated hypertensive patients are scarce. Therefore, we aimed to evaluate the correlation between different indices of HBPV and hypertension-mediated organ damage in treated hypertensives and to determine predictors of greater HBPV that could be easily detected in the office.
Methods
Study Population
This was a cross-sectional study that included adult hypertensive patients under treatment in the Hypertension Section of the Hospital Italiano de Buenos Aires, who performed an HBPM prescribed by their treating physician in order to assess hypertension control status. As part of a routine evaluation of the patients, laboratory measurements, transthoracic echocardiogram and carotid-femoral pulse wave velocity measurements were performed in order to detect subclinical hypertension-mediated organ damage. The design of the study complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1964 and Declaration of Tokyo, 1975, as revised in 2008). The recruitment period lasted from July 2014 to December 2016. The study protocol was approved by the local ethics committee [Comité de Ética de Protocolos de Investigación (CEPI)] and all patients who accepted to participate gave informed consent.
Blood Pressure Measurements
Office Blood Pressure
Patients underwent three consecutive BP measurements, with 1-min intervals in between, after resting for 5 min. For this purpose, a physician used a validated oscillometric OMRON® HEM-7200 device (Omron, Tokyo, Japan) during consultation in the office, with an appropriate cuff size according to the patient’s arm circumference, and remained present during the whole process.
Home Blood Pressure
After receiving appropriate training, patients returned home with a validated Omron HEM-705CP-II® device (Omron, Tokyo, Japan) and registered duplicate sitting BP readings (1 min apart) in the nondominant arm, during fixed hours in the morning (8–12 a.m.), afternoon (14–18 p.m.) and evening (20–24 p.m.), for four days [8]. Patients were instructed to measure home BP after a 5-min rest, with legs uncrossed, their back supported, and refraining from talking. Morning readings were taken before breakfast and drug intake. The average of BP readings stored in the devices’ memory (not self-reported measurements) was used for analysis and, according to current recommendations, first day measurements were discarded [9, 10]. Subjects with less than 16 measurements were excluded from analysis.
Blood Pressure Variability Indices
Among different categories of HBPV indices, we selected one which assesses dispersion, one which assesses instability, and one which measures a specific pattern of variability, respectively [1]: the coefficient of variation, which is the standard deviation of all home BP measurements divided by the mean BP obtained during HBPM and multiplied by 100; the difference between the maximum BP value and the minimum BP value obtained during HBPM; and the morning BP increase, which is obtained by subtracting the average of all evening BP values from all morning BP values during HBPM. All indices were calculated both for systolic and diastolic BP. The formulas used for the calculations are detailed in Table 1.
Table 1.
Formulas used for the calculation of blood pressure variability indices
| Coefficient of variation for SBP (CoVs) | SBP standard deviation/mean home SBP] * 100 |
| Coefficient of variation for DBP (CoVd) | DBP standard deviation/mean home DBP] * 100 |
| Difference between maximum and minimum SBP value (MMDs) | Maximum SBP-minimum SBP |
| Difference between maximum and minimum DBP value (MMDd) | Maximum DBP-minimum DBP |
| Morning SBP increase | Morning SBP-evening SBP |
| Morning DBP increase | Morning DBP-evening DBP |
BP blood pressure, DBP diastolic blood pressure, SBP systolic blood pressure
Hypertension-Mediated Organ Damage Assessment
We recorded demographic and laboratory variables, calculating estimated glomerular filtration rate (eGFR) through the Modification of Diet in Renal Disease Study (MDRD) equation: eGFR (mL/min/1.73 m2) = 175 × (Scr) − 1.154 × (Age) − 0.203 × (0.742 if female). We measured left ventricular mass index (LVMI, g/m2) through Devereux’s modified formula [11], relative wall thickness and ejection fraction using transthoracic echocardiography (Philips iE33®; Phillips Healthcare, Andover, Massachusetts), as well as carotid-femoral pulse wave velocity (PWV, m/s) with the patient in supine position after a 5-min rest using either Sphygmocor® (AtCor Medical, Sydney, Australia) or Aortic® (Exxer, Buenos Aires, Argentina) devices. The latter was subjected to prior validation against the former, with an excellent degree of accordance [12].
Other Variables
Subjects were interrogated and their medical records were reviewed to gather data regarding risk factors (diabetes, smoking status) and history of cardiovascular disease (coronary heart disease, cerebrovascular disease). The number and type of antihypertensive drugs were also registered.
Statistical Analysis
Results are reported as mean ± standard deviation for continuous variables, and percentages for categorical variables. We evaluated the relationship of each of the HBPV indices, with the following markers of hypertension-mediated organ damage: left ventricular mass index, relative wall thickness (RWT), ejection fraction, arterial stiffness (measured by PWV) and estimated glomerular filtration rate, through a correlation matrix.
For the variability indices that showed a significant association with hypertension-mediated organ damage, we constructed multiple linear regression models with the purpose of establishing the independent association with HBPV of different characteristics detected in bivariate analyses. Therefore, the b coefficients (with their 95% confidence intervals) were estimated, which determine how much HBPV increases (or decreases) for each increase of one unit in each of the independent variables (if they are continuous variables) or by comparing the group with a certain characteristic versus the group without the characteristic (in the case of dichotomous variables). Observational units were independent. Other assumptions for linear regression were corroborated through visual inspection of residuals plotted against fitted values, two-way scatter plots between the dependent and each of the independent variables, and p–p plots. The Shapiro–Wilk test was also used to test the residuals’ normality assumption and White’s general test for heteroskedasticity, for the homoscedasticity assumption.
A two-sided p value of 0.05 was considered statistically significant.
Results
We included 216 patients in the study. Among them, 12 subjects did not reach the established minimum of 16 measurements on HBPM and were excluded. Therefore, 204 patients were finally included in the analysis. Mean age was 67.2 (± 13.8) years, and 64% patients were female. Additionally, they were medicated with a mean of 2.1 (± 1.2) drugs per patient, and mean BP was 140.9 (± 17.1)/78 (± 9.7) and 134.1 (± 13.9)/73.8 (± 8.8) mmHg, for office and home respectively (Table 2 and Fig. 1).
Table 2.
Characteristics of the study population
| n | 204 |
| Age, years (SD) | 67.2 (13.8) |
| Female sex (%) | 64 |
| Diabetes (%) | 8.7 |
| Current smokers (%) | 5 |
| History of ischemic heart disease (%) | 3.2 |
| History of cerebrovascular diseasea (%) | 3.4 |
| BMI, kg/m2 (SD) | 28.9 (5.4) |
| Office SBP, mmHg (SD) | 140.9 (17.1) |
| Office DBP, mmHg (SD) | 78 (9.7) |
| Home SBP, mmHg (SD)b | 134.1 (13.9) |
| Home DBP, mmHg (SD)b | 73.8 (8.8) |
| Number of antihypertensive drugs (SD) | 2.1 (1.2) |
| FPG, mg/dL (SD) | 101.4 (17.8) |
| eGFR, mL/min/1.73 m2 (SD) | 81.7 (20.3) |
| SCr, mg/dL (SD) | 0.88 (0.24) |
| Total cholesterol, mg/dL (SD) | 187.5 (34.2) |
| LVMI, g/m2 (SD) | 95 (22.1) |
| Ejection fraction, % (SD) | 57 (3.7) |
| Relative wall thickness (SD) | 0.44 (0.06) |
| Carotid-femoral pulse wave velocity, m/s (SD) | 9.3 (2.4) |
BMI body mass index, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, LVMI left ventricular mass index, m/s meters per second, SBP systolic blood pressure, Scr serum creatinine, SD standard deviation
aStroke or transient ischemic attack.
bAverage of all BP measurements from the 4-day monitoring.
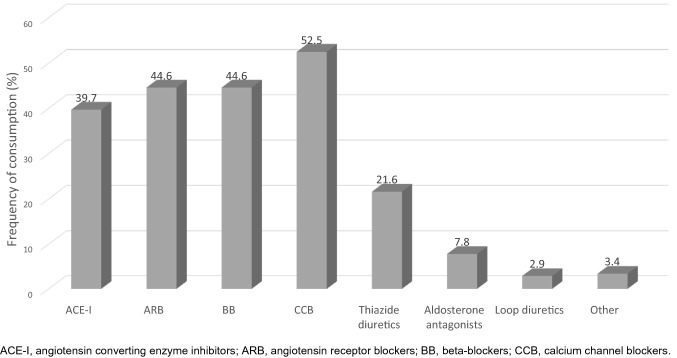
Fig. 1.
Antihypertensive drug consumption among the study population
Regarding home BP variability indices, the coefficient of variation (CoV) was 8.5 (2.6)/8.1 (2.5)% for systolic and diastolic BP, respectively; the difference between maximum and minimum BP during HBPM (MMD) was 43 (± 15.2)/22.4 (± 7.4) mmHg for systolic and diastolic BP, respectively; and morning BP increase (MI) was 0.5 (± 12.2)/1.3 (± 5.9) mmHg for systolic and diastolic BP, respectively.
Of all the indices, the coefficient of variation for systolic BP (CoVs) and the difference between maximum and minimum systolic BP (MMDs) were the ones that showed a greater association with hypertension-mediated organ damage: in both cases, they were significantly associated with RWT, arterial stiffness and glomerular filtration rate: r = 0.16, p = 0.03; r = 0.21, p = 0.004; and r = − 0.23, p = 0.001, respectively, for CoVs and r = 0.19, p = 0.009; 0.24, p = 0.001; and r = − 0.26, p < 0.001, respectively, for MMDs (Table 3).
Table 3.
Correlation coefficients among home blood pressure variability indices and target organ damage markers
| LVMI | Ejection fraction | RWT | cfPWV | eGFR | |
|---|---|---|---|---|---|
| CoVs |
0.01 p = ns |
− 0.05 p = ns |
0.16 p = 0.03 |
0.21 p = 0.004 |
− 0.23 p = 0.001 |
| CoVd |
− 0.01 p = ns |
− 0.06 p = ns |
0.15 p = 0.04 |
0.16 p = 0.03 |
− 0.13 p = ns |
| MMDs |
0.09 p = ns |
− 0.04 p = ns |
0.19 p = 0.009 |
0.24 p < 0.001 |
− 0.26 p < 0.001 |
| MMDd |
0.05 p = ns |
− 0.07 p = ns |
0.16 p = 0.02 |
0.05 p = ns |
− 0.03 p = ns |
| Morning SBP increase |
− 0.01 p = ns |
− 0.1 p = ns |
− 0.01 p = ns |
0.08 p = ns |
0.07 p = ns |
| Morning DBP increase |
− 0.02 p = ns |
− 0.07 p = ns |
− 0.01 p = ns |
0.08 p = ns |
0.07 p = ns |
cfPWV carotid-femoral pulse wave velocity, CoVd coefficient of variation for diastolic blood pressure, CoVs coefficient of variation for systolic blood pressure, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, ns non significant, MMDd difference between maximum and minimum diastolic BP, MMDs difference between maximum and minimum systolic BP, ns non significant, RWT relative wall thickness, SBP systolic blood pressure
In multiple linear regression analyses, the factors independently associated with CoVs were: older age, female gender, history of stroke, blood glucose level (positive associations), and body mass index (negative association). For the MMDs, the independent predictors were: older age, history of stroke, office systolic BP level (positive associations), and body mass index (negative association) (Table 4).
Table 4.
Multiple lineal regression analyses: predictors of the coefficient of variation for systolic blood pressure (panel A) and of the difference between maximum and minimum systolic blood pressure (panel B)
|
Panel A. Dependent variable: coefficient of variation for systolic BP R2 = 0.34 |
Panel B. Dependent variable: difference between maximum and minimum systolic BP R2 = 0.35 |
|||||
|---|---|---|---|---|---|---|
| b | 95% CI | p value | b | 95% CI | p value | |
| Female gender | 1.1 | 0.4 to 1.8 | 0.003 | 3.9 | − 0.1 to 7.9 | ns |
| Age | 0.07 | 0.04 to 0.07 | < 0.001 | 0.4 | 0.2 to 0.5 | < 0.001 |
| Diabetes | − 0.23 | − 1.5 to 1.1 | ns | − 0.3 | − 7.8 to 7.2 | ns |
| Current smoker | − 0.01 | − 1.5 to 1.5 | ns | − 1.7 | − 10.4 to 6.9 | ns |
| Ischemic heart disease | − 0.6 | − 2.4 to 1.2 | ns | − 2.3 | − 12.7 to 8.2 | ns |
| Stroke | 3.6 | 0.9 to 6.4 | 0.01 | 25.7 | 10.1 to 41.2 | 0.001 |
| Number of antihypertensive drugs | − 0.3 | − 0.6 to 0 | ns | − 0.9 | − 2.6 to 0.8 | ns |
| FPG | 0.02 | 0.01 to 0.05 | 0.02 | 0.1 | − 0.03 to 0.2 | ns |
| Total cholesterol | 0.003 | 0 to 0.01 | ns | 0.03 | − 0.02 to 0.1 | ns |
| Office SBP | 0.01 | − 0.01 to 0.03 | ns | 0.2 | 0.04 to 0.3 | 0.01 |
| Office DBP | 0 | − 0.04 to 0.04 | ns | − 0.05 | − 0.3 to 0.2 | ns |
| BMI | − 0.1 | − 0.2 to (− 0.02) | 0.01 | − 0.5 | − 0.9 to (− 0.1) | 0.01 |
95% CI 95% confidence interval, BMI body mass index, BP blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, ns non significant, SBP systolic blood pressure
Finally, regarding antihypertensive treatment, the use of aldosterone antagonists was associated with a lower variability in home BP, by either of the two evaluated indices: b = − 1.7 [95% CI – 3 to (− 0.4)], p = 0.01, for CoVs, and b = − 10.8 [95% CI − 18.5 to (− 3.1)], p = 0.01, for MMDs (Table 5).
Table 5.
Association between antihypertensive treatment and home blood pressure variability
| Drug class | Home BP variability index | |||||
|---|---|---|---|---|---|---|
| CoVs | MMDs | |||||
| b | 95% CI | p value | b | 95% CI | p value | |
| ACE-I | − 0.3 | − 1 to 0.5 | ns | − 2.9 | − 7.2 to 1.4 | ns |
| ARB | 0.4 | − 0.4 to 1.1 | ns | 3.2 | − 1 to 7.4 | ns |
| BB | 0.02 | − 0.7 to 0.8 | ns | 2.1 | − 2.1 to 6.3 | ns |
| CCB | − 0.7 | − 1.4 to 0.1 | ns | − 0.8 | − 5.1 to 3.7 | ns |
| Loop diuretics | 1.6 | − 0.6 to 3.7 | ns | 6.4 | − 6.1 to 18.8 | ns |
| Thiazide diuretics | − 0.4 | − 1.3 to 0.4 | ns | − 2.2 | − 7.3 to 2.9 | ns |
| Aldosterone antagonists | − 1.7 | − 3 to (− 0.4) | 0.01 | − 10.8 | − 18.5 to (− 3.1) | 0.01 |
| Other | − 0.3 | − 2.3 to 1.7 | ns | 1.3 | − 10.2 to 12.9 | ns |
ACE-I angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, BB beta-blockers, BP blood pressure, CCB calcium channel blockers, CoVs coefficient of variation for systolic blood pressure, ns non significant, MMDs difference between maximum and minimum systolic blood pressure
Discussion
In our study, we found that HBPV, particularly assessed through the coefficient of variation for systolic blood pressure and the difference between maximum and minimum systolic BP, is associated with hypertension-mediated organ damage. Several characteristics that are easily detected in the office, such as age, history of stroke and BMI, are predictors of this variability.
Several studies have shown significant associations between HBPV and cardiac markers, such as LVMI [13]; renal markers, such as eGFR and proteinuria [14–16]; and large artery stiffness markers, such as PWV [17, 18]. For instance, a study conducted by Ishiyama et al. in subjects with at least one cardiovascular risk factor found that arterial stiffness amplified the association between cardiac overload and home BP variability, measured through the standard deviation, the coefficient of variation, and the average real variability [18]. Of note, these studies included different populations from ours, mostly patients with diabetes, chronic kidney disease or untreated hypertension. Our results show that the relationship between HBPV and hypertension-mediated organ damage holds in treated hypertensives. The link between HBPV and hypertension-mediated organ damage might be reflecting the influences of increased sympathetic drive and reduced arterial and cardiopulmonary reflexes [19]. It must be noted, however, that there is wide variety among studies regarding the HBPV indices used to assess the association with hypertension-mediated organ damage or events: some studies used easy-to-calculate indices, such as the standard deviation, the coefficient of variation, the morning-evening difference or the difference between maximum and minimum BP, while others used rather cumbersome indices, such as average real variability (ARV) and variability independent of the mean (VIM). In that sense, a study conducted by Juhanoja et al. [19] from the International Database of Home Blood Pressure in Relation to Cardiovascular Outcome (IDHOCO) evaluated four HBPV indices: standard deviation, coefficient of variation, VIM and ARV. Although all of them were predictors of all-cause mortality, cardiovascular mortality, cardiovascular events and stroke events, only the easily calculated in clinical practice coefficient of variation was additionally associated with cardiac events. Moreover, adding systolic/diastolic coefficient of variation in Cox regression models that included the conventional cardiovascular risk factors significantly increased the discrimination of the models for cardiovascular events. Of note, this was one of the HBPV indices detected in our study to be more consistently associated with hypertension-mediated organ damage.
Beyond the association between HBPV and hypertension-mediated organ damage, it is important to investigate this increase in variability given its relationship with cardiovascular events [19] and with other hypervariability markers which are also predictors of morbidity and mortality, such as orthostatic hypotension [20]. As shown in this and other studies, screening for HBPV can be carried out through HBPM, a simple, low-cost, widely available, and better tolerated technique than ambulatory blood pressure monitoring [21]. In that line, a study conducted by Andreadis et al. has shown that an alternative to HBPM, such as automated office blood pressure recordings (AOBP), appears to be at least equally reliable to 24-h monitoring in the evaluation of morning BP peak—a type of BP variability—in order to predict cardiovascular events [22]. In the current COVID 19 pandemic context, it must be noted that HBPM has also been an invaluable tool for the remote evaluation of patients with hypertension during social isolation [23].
Several characteristics have been described to be related to a higher HBPV. In accordance to our findings, one of the largest studies assessing real world HBPV in over 56,000 individuals found that the coefficient of variation showed the strongest associations with female gender and older age [24]. These two variables have also been shown to be the most consistent predictors of HBPV across several previous studies [7]. Regarding BMI, both a study conducted by Ishikura et al. in 1933 treated hypertensive patients [25] and another by Schutte et al. conducted in a general population sample of 2944 individuals [26], found a negative association between BMI and HBPV, which is in accordance with our findings. Moreover, consistent with our results, the latter study also showed an association between mean systolic BP and HBPV, determined through the MMD.
A history of stroke was another variable independently associated with HBPV in our study, both measured through CoVs and MMDs. In line with these results, the Finn Home study conducted in a general population, also found an independent association between HBPV and previous cardiovascular disease, including stroke [27].
Regarding diabetes, some studies have related it to a higher HBPV [27, 28]. In our study, this variable did not reach statistical significance. However, fasting plasma glucose did show a significant positive association with HBPV, a finding that was also observed in the aforementioned study by Schutte et al. [26].
The effects of antihypertensive treatment on HBPV have been assessed in several studies. Imai et al. [29] and Asayama et al. [30] found that HBPV is higher in treated versus untreated hypertensives. The number of antihypertensive drugs has also been related to a higher HBPV [28], although we could not find this association in our study. Considering different drug classes, beta-blockers and renin angiotensin system inhibitors have been related to a higher HBPV [25, 26], whereas calcium channel blockers [31] and alpha blockers were found to be associated with a lower HBPV [17], the former being the drug class most consistently related with a lower HBPV across studies [32–34]. We were not able to find other studies in humans that confirm our findings of a lower HBPV in patients taking aldosterone antagonists. In fact, in a study conducted in patients with heart failure and reduced ejection fraction, the relationship between the coefficient of variation for systolic BP and cardiovascular mortality or heart failure hospitalization was not modified by eplerenone (p value for interaction = 0.48 [35]. On the other hand, eplerenone prevented BPV-induced aggravation of hypertensive cardiac remodeling in a rat model of a combination of hypertension and high BPV, created by performing bilateral sinoaortic denervation in spontaneously hypertensive rats [36]. This finding provides biological plausibility to a possible protective role for aldosterone antagonists regarding HBPV-related damage, which warrants further investigation in humans. On the other hand, the observation that antialdosterone agents resulted to be associated with lower HBPV than other drugs might be explained by the fact these patients were those who received more than 2–3 drugs, and the combination of them could have had a major impact on BP variability.
Our results must be interpreted within the context of their potential limitations and strengths: first, the cross-sectional nature of the study precludes the establishment of a causal role between HBPV and hypertension-mediated organ damage ; second, due to the small number of subjects included, some factors known to be associated to HBPV, such as diabetes and number of antihypertensive drugs taken, might have been underestimated; third, our study is representative of Argentine middle-class medicated hypertensives, mainly from European descent, and our results may not be generalizable to other populations; fourth, the time in which the subjects took their antihypertensive medication was not controlled, which may have overestimated the variability in BP measurements. Among the strengths of our study, BP measurements were performed with the same validated oscillometric device and following the same protocol in all study subjects; the HBPM protocol used is in accordance with the optimal schedule for assessing HBPV based on the Finn-Home study, which suggested that home BP should be measured for at least 3 days when assessing HBPV, and that increasing the number of measurement days from 3 to 7 resulted in only marginal improvement in prognostic accuracy [37]; home BP data used for the analysis were extracted directly from the device's memory, precluding a misreporting of BP readings which has been found to reach 35% in some studies when patients used a logbook [38]. Finally, the fact that HBPM instead of ambulatory blood pressure monitoring (ABPM) was used to evaluate BP variability is of special importance in the COVID-19 pandemic, particularly in elderly subjects in whom strict isolation is required at the same time that proper BP evaluation is still needed. In this context, HBPM, a method that has been shown to be superior to office BP measurement and comparable to ABPM for many every day practice indications, becomes very useful to monitor patients without leaving their homes.
In conclusion, our results suggest that HBPV is related to different markers of hypertension-mediated organ damage in treated hypertensives, CoVs and MMDs being the indices that showed the greater association. Certain clinical parameters easily obtainable in the office, such as age, gender, BMI, history of stroke and office systolic BP may help to raise the suspicion of increased HBPV. More research is needed to determine the optimal way to manage individuals with this phenotype.
Declarations
Funding
The authors did not receive support from any organization for the submitted work.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Availability of data and material/code availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JB: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, revising the article critically for important intellectual content and final approval of the submitted version. RM: acquisition of data, interpretation of data, revising the article critically for important intellectual content, and final approval of the submitted version. LA: acquisition of data, interpretation of data, revising the article critically for important intellectual content, and final approval of the submitted version.
Ethics approval
The design of the study complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1964 and Declaration of Tokyo, 1975, as revised in 2008). The study protocol was approved by the local ethics committee (Comité de Ética de Protocolos de Investigación [CEPI]).
Consent to participate
Subjects gave informed consent.
Consent for publication
Not applicable.
References
- 1.Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich). 2018;20(7):1133–1137. doi: 10.1111/jch.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23(1):1–11. doi: 10.1016/S0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57(2):160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 5.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52(6):1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 6.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi: 10.1097/HJH.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 7.Stergiou GS, Ntineri A, Kollias A, Ohkubo T, Imai Y, Parati G. Blood pressure variability assessed by home measurements: a systematic review. Hypertens Res. 2014;37(6):565–572. doi: 10.1038/hr.2014.2. [DOI] [PubMed] [Google Scholar]
- 8.Barochiner J, Cuffaro PE, Aparicio LS, Elizondo CM, Giunta DH, Rada MA, et al. Reproducibility and reliability of a 4-day HBPM protocol with and without first day measurements. Rev Fac Cien Med Univ Nac Cordoba. 2011;68(4):149–153. [PubMed] [Google Scholar]
- 9.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36(12):2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 10.Villar R, Sánchez RA, Boggia J, Peñaherrera E, Lopez J, Barroso WS, et al. Recommendations for home blood pressure monitoring in Latin American countries: a Latin American Society of Hypertension position paper. J Clin Hypertens (Greenwich). 2020;22(4):544–554. doi: 10.1111/jch.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Morales MS, Cuffaro PE, Barochiner J, Rada M, Alfie J, Aparicio L, et al. Validation of a new piezo-electronic device for non-invasive measurement of arterial pulse wave velocity according to the artery society guidelines. Artery Res Elsevier. 2015;10:32–37. doi: 10.1016/j.artres.2015.03.001. [DOI] [Google Scholar]
- 13.Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K. Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension. 2011;57:1087–1093. doi: 10.1161/HYPERTENSIONAHA.111.171645. [DOI] [PubMed] [Google Scholar]
- 14.Okada T, Matsumoto H, Nagaoka Y, Nakao T. Association of home blood pressure variability with progression of chronic kidney disease. Blood Press Monit. 2012;17:1–7. doi: 10.1097/MBP.0b013e32834f7125. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura M, Kato Y, Tanaka T, Todo R, Tone A, Yamada K, et al. Significance of estimating the glomerular filtration rate for the management of hypertension in type 2 diabetes with microalbuminuria. Hypertens Res. 2013;36:705–710. doi: 10.1038/hr.2013.22. [DOI] [PubMed] [Google Scholar]
- 16.Ushigome E, Fukui M, Hamaguchi M, Senmaru T, Sakabe K, Tanaka M, et al. The coefficient variation of home blood pressure is a novel factor associated with macroalbuminuria in type 2 diabetes mellitus. Hypertens Res. 2011;34:1271–1275. doi: 10.1038/hr.2011.128. [DOI] [PubMed] [Google Scholar]
- 17.Fukui M, Ushigome E, Tanaka M, Hamaguchi M, Tanaka T, Atsuta H, et al. Home blood pressure variability on one occasion is a novel factor associated with arterial stiffness in patients with type 2 diabetes. Hypertens Res. 2013;36:219–225. doi: 10.1038/hr.2012.177. [DOI] [PubMed] [Google Scholar]
- 18.Ishiyama Y, Hoshide S, Kanegae H, Kario K. Increased arterial stiffness amplifies the association between home blood pressure variability and cardiac overload: the J-HOP study. Hypertension. 2020;75(6):1600–1606. doi: 10.1161/HYPERTENSIONAHA.119.14246. [DOI] [PubMed] [Google Scholar]
- 19.Juhanoja EP, Niiranen TJ, Johansson JK, Puukka PJ, Thijs L, Asayama K, International Database on Home Blood Pressure in Relation to Cardiovascular Outcome (IDHOCO) Investigators et al. Outcome-driven thresholds for increased home blood pressure variability. Hypertension. 2017;69(4):599–607. doi: 10.1161/HYPERTENSIONAHA.116.08603. [DOI] [PubMed] [Google Scholar]
- 20.Tabara Y, Matsumoto T, Murase K, Setoh K, Kawaguchi T, Nagashima S, Nagahama study group et al. Day-to-day home blood pressure variability and orthostatic hypotension: the Nagahama Study. Am J Hypertens. 2018;31(12):1278–1285. doi: 10.1093/ajh/hpy131. [DOI] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 22.Andreadis EA, Geladari CV, Angelopoulos ET, Kolyvas GN, Papademetriou V. Morning surge and peak morning ambulatory blood pressure versus automated office blood pressure in predicting cardiovascular disease. High Blood Press Cardiovasc Prev. 2019;26(3):209–215. doi: 10.1007/s40292-019-00315-7. [DOI] [PubMed] [Google Scholar]
- 23.Kario K, Morisawa Y, Sukonthasarn A, Turana Y, Chia YC, Park S, Hypertension Cardiovascular Outcome Prevention, Evidence in Asia (HOPE Asia) Network et al. COVID-19 and hypertension-evidence and practical management: Guidance from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2020 doi: 10.1111/jch.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KI, Nikzad N, Quer G, Wineinger NE, Vegreville M, Normand A, et al. Real world home blood pressure variability in over 56,000 individuals with nearly 17 million measurements. Am J Hypertens. 2018;31(5):566–573. doi: 10.1093/ajh/hpx221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikura K, Obara T, Kato T, Kikuya M, Shibamiya T, Shinki T, J-HOME-Morning Study Group et al. Associations between day-by-day variability in blood pressure measured at home and antihypertensive drugs: the J-HOME-Morning study. Clin Exp Hypertens. 2012;34(4):297–304. doi: 10.3109/10641963.2012.681087. [DOI] [PubMed] [Google Scholar]
- 26.Schutte R, Thijs L, Liu YP, Asayama K, Jin Y, Odili A, et al. Within-subject blood pressure level-not variability-predicts fatal and nonfatal outcomes in a general population. Hypertension. 2012;60(5):1138–1147. doi: 10.1161/HYPERTENSIONAHA.112.202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Factors affecting the variability of home-measured blood pressure and heart rate: the Finn-home study. J Hypertens. 2010;28(9):1836–1845. doi: 10.1097/HJH.0b013e32833b6c8a. [DOI] [PubMed] [Google Scholar]
- 28.Okada T, Nakao T, Matsumoto H, Nagaoka Y, Tomaru R, Iwasawa H, et al. Day-by-day variability of home blood pressure in patients with chronic kidney disease. Nihon Jinzo Gakkai Shi. 2008;50:588–596. [PubMed] [Google Scholar]
- 29.Imai Y, Nishiyama A, Sekino M, Aihara A, Kikuya M, Ohkubo T, et al. Characteristics of blood pressure measured at home in the morning and in the evening: the Ohasama study. J Hypertens. 1999;17:889–898. doi: 10.1097/00004872-199917070-00004. [DOI] [PubMed] [Google Scholar]
- 30.Asayama K, Kikuya M, Schutte R, Thijs L, Hosaka M, Satoh M, et al. Home blood pressure variability as cardiovascular risk factor in the population of Ohasama. Hypertension. 2013;61:61–69. doi: 10.1161/HYPERTENSIONAHA.111.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui Y, O’Rourke MF, Hoshide S, Ishikawa J, Shimada K, Kario K. Combined effect of angiotensin II receptor blocker and either a calcium channel blocker or diuretic on day-by-day variability of home blood pressure: the Japan Combined Treatment With Olmesartan and a Calcium-Channel Blocker Versus Olmesartan and Diuretics Randomized Efficacy Study. Hypertension. 2012;59:1132–1138. doi: 10.1161/HYPERTENSIONAHA.111.189217. [DOI] [PubMed] [Google Scholar]
- 32.Webb AJ, Wilson M, Lovett N, Paul N, Fischer U, Rothwell PM. Response of day-to-day home blood pressure variability by antihypertensive drug class after transient ischemic attack or nondisabling stroke. Stroke. 2014;45(10):2967–2973. doi: 10.1161/STROKEAHA.114.005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nozato S, Yamamoto K, Nozato Y, Takeda M, Hongyo K, Takeya M, et al. Comparison between L-type and N/L-type calcium channel blockers in the regulation of home blood-pressure variability in elderly hypertensive patients. Hypertens Res. 2018;41(4):290–298. doi: 10.1038/s41440-018-0018-4. [DOI] [PubMed] [Google Scholar]
- 34.Xu M, Wu Y, Wang H, Xu X, Zhao S, Zhang M, et al. Effects of lercanidipine hydrochloride versus felodipine sustained-release on day-to-day home blood pressure variability. Curr Med Res Opin. 2016;32(sup2):43–52. doi: 10.1080/03007995.2016.1220932. [DOI] [PubMed] [Google Scholar]
- 35.Monzo L, Ferreira JP, Abreu P, Szumski A, Böhm M, McMurray JJV, et al. Visit-to-visit blood pressure variation and outcomes in heart failure with reduced ejection fraction: findings from the eplerenone in patients with systolic heart failure and mild symptoms trial. J Hypertens. 2020;38(3):420–425. doi: 10.1097/HJH.0000000000002275. [DOI] [PubMed] [Google Scholar]
- 36.Yasuoka S, Kai H, Kajimoto H, Kudo H, Takayama N, Anegawa T, et al. Blood pressure variability activates cardiac mineralocorticoid receptor and induces cardiac remodeling in hypertensive rats. Circ J. 2013;77(6):1474–1481. doi: 10.1253/circj.CJ-12-1253. [DOI] [PubMed] [Google Scholar]
- 37.Juhanoja EP, Johansson JK, Puukka PJ, Jula AM, Niiranen TJ. Optimal schedule for assessing home BP variability: the Finn-Home Study. Am J Hypertens. 2018;31(6):715–725. doi: 10.1093/ajh/hpy030. [DOI] [PubMed] [Google Scholar]
- 38.Mengden T, Hernandez Medina RM, Beltran B, Alvarez E, Kraft K, Vetter H. Reliability of reporting self-measured blood pressure values by hypertensive patients. Am J Hypertens. 1998;11:1413–1417. doi: 10.1016/S0895-7061(98)00241-6. [DOI] [PubMed] [Google Scholar]