Abstract
STUDY QUESTION
What is willingness, preference and decision-making about planning for the possibility of needing multiple cycles of IVF/ICSI treatment among patients consulting for a first or repeat stimulated IVF/ICSI cycle?
SUMMARY ANSWER
The majority of patients seem to value the opportunity to plan for multiple cycles of treatment while acknowledging both possible challenges and benefits of doing so and decisions that might need to be made in advance.
WHAT IS KNOWN ALREADY
Patients have strong intentions to do treatment to achieve pregnancy and approximately 48–54% continue treatment when confronted with a failed cycle, undergoing at least three complete cycles of treatment. However, there is inconsistency between this apparent willingness to do multiple cycles of treatment and the way treatment is currently planned on a cycle-by-cycle basis with patients.
STUDY DESIGN, SIZE, DURATION
The study was of cross-sectional design, comprising a mixed-methods English online survey posted between November 2019 and March 2020. Eligibility criteria were being a patient who had had a consultation to start a stimulated cycle of IVF/ICSI for the first time or for a repeat stimulated cycle after an unsuccessful cycle in the eight weeks prior to survey completion. Individuals were also required to be aged 18 or older (upper age limit of 42 years for women) and able to respond in English. In total 881 clicked on the survey link, 118 did not consent, 41 were excluded after data screening, 57 did not meet the inclusion criteria, 331 started the survey but did not complete it, 28 had missing data on critical variables (e.g., age) and 306 completed the survey (40.1% completion, 57 men, 249 women).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants were allocated to either the willing or unwilling to plan for multiple cycles of treatment group based on their responses to three variables: willingness to plan for three complete cycles, whether they would choose to have another cycle of IVF and whether they would continue treatment after an unsuccessful cycle. Quantitative questions gathered data on preferences towards planning for multiple cycles (i.e., attitudes, subjective norms and perceived behavioural control), challenges, benefits of planning for multiple cycles, decisional conflict experienced and treatment decisions involved in planning for multiple cycles. Demographic, fertility and fertility treatment information were also collected. Qualitative questions gathered textual data on other perceived benefits and challenges of planning for multiple cycles and solutions to the challenges. Descriptive and inferential statistics were used on quantitative data. Thematic analysis (inductive coding) was performed on the textual data.
MAIN RESULTS AND THE ROLE OF CHANCE
Overall, 73.2% (n = 224) of participants had had a consultation to start a first cycle of IVF/ICSI. Participants were on average 33 years of age and had been trying to conceive for three years. A total of 63.07% (n = 193) were university educated. A total of 56% (n = 172) of participants were willing to plan for multiple cycles of IVF/ICSI in advance of treatment. Repeated measures ANOVA, t-tests and chi-square analysis showed the willing group to be significantly more likely to have been in a relationship for longer (p<.05), have higher education (p<.05) and be resident in the United Kingdom (p<.05). The willing group had positive attitudes towards planning for multiple cycles (p<.001) and stronger agreement with subjective norms (p<.001), perceived behavioural control (p<.001), benefits of planning for multiple cycles (p<.01) and felt able and attached more importance to making treatment decisions in advance of treatment (p<.05). Data saturation was achieved for the thematic analysis of textual data which revealed a total of four other challenges (e.g., less decisional freedom) and six other benefits (e.g., having a realistic view of treatment) to planning for multiple cycles. Qualitative analysis also revealed that most patients could anticipate and provide solutions for the nine challenges of planning for multiple cycles (e.g., using flexible working for the negative effect of treatment on work).
LIMITATIONS, REASONS FOR CAUTION
Limitations included the outcome measure being willingness to plan for multiple cycles rather than actual multi-cycle planning behaviour. The unwilling group represented a heterogeneous group with possibly unknown motivational coherence (e.g., definitely against planning, ambivalent about planning). Other limitations included the cross-sectional nature of the survey and the recruitment source.
WIDER IMPLICATIONS OF THE FINDINGS
Treatment consultations about undergoing fertility treatment could re-frame treatment to be a multi-cycle process in line with patient’s willingness, preference and decision-making. This multi-cycle approach could empower patients and clinicians to discuss treatment expectations realistically and formulate fully informed treatment plans that take account of the high likelihood of cycle failure in addition to the treatment decisions that may need to be made during treatment when a cycle fails. This multi-cycle approach could help us support patients in adhering to their treatment plans even when faced with challenges, and help ascertain the level of treatment engagement possible to achieve parenthood goals.
STUDY FUNDING/COMPETING INTEREST(S)
This project is funded by an Investigator-Sponsor Non-interventional Study from Merck Serono Ltd (MS200059_0010). Professor Boivin reports personal fees from Merck KGaA, Darmstadt, Germany, Merck AB an affiliate of Merck KGaA, Darmstadt Germany, Theramex, Ferring Pharmaceuticals A/S, grant from Merck Serono Ltd, outside the submitted work and that she is co-developer of Fertility Quality of Life (FertiQoL) and MediEmo app. Dr. Gameiro reports consultancy fees from Ferring Pharmaceuticals A/S, Access Fertility and SONA-Pharm LLC, and grants from Merck Serono Ltd. Dr. Harrison declares no conflicts of interest.
TRIAL REGISTRATION NUMBER
n/a
Keywords: willingness and intentions / implementation intentions / multiple cycles / IVF/ICSI treatment planning / preferences and decision-making
Introduction
Infertility affects approximately one in six heterosexual couples in the United Kingdom (Hardway & Younas, 2018; Oakley et al., 2008). A total of 563,244 IVF and ICSI cycles were carried out in Europe in 2016 (Wyns et al., 2020). In the United Kingdom, the number of complete IVF cycles recommended is three (National Institute for Health and Care Excellence, NICE, 2013). Past research shows that patients have strong intentions to do as much treatment as needed to achieve pregnancy and approximately 50% undergo at least three cycles of treatment (Gameiro et al., 2013; McLernon, 2016). There is, however, inconsistency between this apparent willingness to do multiple cycles of treatment and the way treatment is currently presented and discussed on a cycle-by-cycle basis with patients (Kupka et al., 2016). The present study investigated patients’ willingness to plan for having up to three complete cycles of IVF/ICSI (hereafter, multiple cycles) in advance of treatment. Results will inform whether planning for multiple cycles can become a viable option at clinics and what such treatment planning should entail.
NICE recommends that patients should be offered up to three complete cycles of IVF/ICSI. A complete cycle refers to all embryo transfers (including frozen) resulting from one episode of ovarian stimulation. The reason for this recommendation is that although most women typically see success rates of 20–35% per cycle, the likelihood of getting pregnant decreases with each successive round, while the cost increases. The cumulative effect of three complete cycles increases the chances of a successful pregnancy up to 45–53% for women under the age of 40 (NICE, 2013). Consequently, according to the NICE guidelines, undergoing three complete cycles of IVF is the most cost and clinically effective option. Funding practices from other countries also support a multi-cycle approach to treatment, subsidising three (e.g., the Netherlands, Germany, Spain), or more (e.g., France, Australia, Belgium) complete cycles. Notwithstanding this, some countries support less (e.g., New Zealand, the United States, Switzerland; Berg Brigham et al., 2013; Fertility Associates, 2020). The NICE recommendations, funding practices in other countries that support three or more complete cycles and the general evidences for cumulative success rates imply that IVF treatment should be planned on a multi-cycle rather than a single-cycle basis.
Patients starting treatment generally have strong intentions to achieve their goal of parenthood. Most patients seem to implement these intentions into actual behaviour. Overall, audit studies suggest that between 48% (McLernon, 2016) and 54% (Gameiro et al., 2013) of people who experience a failed cycle (1st or 2nd) decide to continue, but this number can be higher (i.e., 66%) with funding (Gameiro et al., 2013). Despite this apparent willingness, fertility treatment tends to be planned with patients cycle by cycle. By planned, we mean the presentation of information (e.g., chance of pregnancy, likely number of cycles, procedures) and subsequent discussions concerning the intended course of treatment. Kupka et al. (2016) and McLernon (2016) have both highlighted that planning is typically guided by the livebirth rate of a single fresh cycle rather than cumulative live birth rates. The shift in worldwide routine clinical practice to cryopreservation of surplus embryos and subsequent frozen embryo transfers (Chambers et al., 2017; HFEA, 2020; McLernon, 2016) needs to be reflected in planning consultations and highlights a need to better understand patient’s willingness to plan for multiple complete cycles of treatment.
Previous research demonstrates treatment engagement to be accompanied by a gradual weakening of intentions. Gameiro et al. (2013) reported two out of ten patients to discontinue treatment earlier than expected with more patients discontinuing treatment after the second (24.7%) than first failed cycle (18.2%; Gameiro et al., 2013). Discontinuation has been found to occur even when there is a high likelihood of success and sufficient financial resources to cover the cost of treatment (e.g., subsidised treatment as per Brandes et al., 2009). Treatment discontinuation has been attributed to treatment intentions being compromised by the challenges faced during treatment (e.g., emotional and physical burden of treatment) and/or the experience of an unsuccessful treatment cycle (Domar, 2004; Domar et al., 2010; Domar et al., 2018; Gameiro et al., 2012; Smeenk et al., 2004), which cause patients to experience a negative motivational state, discouraging their re-engagement with treatment (Akyuz & Sever, 2009; Bailey et al., 2017; Gameiro et al., 2020; Peddie et al., 2005; Rauprich et al., 2011). Gameiro et al. (2020) suggest that pre-planning for the challenges of treatment (e.g., what to do if treatment fails) could help rebuild hope after treatment failure and decrease the chances of treatment postponement and ultimate discontinuation. Understanding the factors that may impact willingness to plan for multiple cycles is therefore important to ensure that patient treatment expectations and plans are fully informed and aligned to parenthood goals from the moment patients start treatment.
The Theory of Planned Behaviour (TPB; Ajzen 1991) and the Health Belief Model (HBM; Rosenstock, 1974) are useful for understanding behaviour in health contexts. These theories highlight the importance of supporting patients to translate willingness into action (i.e., actual behaviours) even when challenges are encountered. According to Gollwitzer (1999), this translation requires ‘if-then’ planning to identify in advance the challenges people may face and consideration of possible solutions for each challenge. Planning in this way (implementation intentions) is effective in a range of situations to shield intentions from unwanted influences, bolster intentions and translate intentions into behaviour (Gollwitzer & Sheeran, 2006).
We do not yet know whether patients could consider at the start of treatment the many decisions that planning for multiple cycles of treatment might imply. Planning for multiple cycles would encourage clinicians and patients to consider, from the start of treatment, the potential need for multiple cycles and thereby reasons for this need (e.g., poor response to medication). That then could additionally imply that some treatment protocol decisions might also need to be considered in advance. For example, if people are informed that 5–24% of IVF patients have a too-low response to ovarian stimulation that can lead to cycle cancellation (Kuang et al., 2014) then they might also need to be informed that in such cases people have to decide whether to increase medication dose in their next cycle. Previous research suggests it would be better to make decisions such as these in advance of treatment rather than during periods of stress and tension after treatment cancellation (Boivin, 2000) or an unsuccessful cycle (Gameiro et al., 2020; Mesquita et al., 2018). However, we do not know whether patients would be willing to consider such decisions in advance, or how conflicted they would be about such advanced decision-making.
The aim of the present study was to gain understanding of willingness to plan for the possibility of having multiple cycles of treatment. The specific objectives were to learn:
Whether patients were willing to plan for multiple cycles of treatment
The antecedents of willingness to plan for multiple cycles as relates to psychological theory, namely attitudes, subjective norms and perceived behavioural control
The challenges and benefits of planning for multiple cycles according to theory and as generated by patients, and their possible solutions according to patients
The decisions and nature of decision-making context of planning for multiple cycles: the ability and importance of making treatment decisions in advance of treatment, decisional conflict
Materials and methods
Participants
Participants were eligible if they had a consultation to start a stimulated cycle of IVF/ICSI for the first time or for a repeat cycle after an unsuccessful cycle within the eight weeks prior to completing the survey, were aged 18 or older (upper age limit of 42 years for women), and able to respond in English. The upper age limit for women was applied because it is the upper age limit for NHS-funded fertility treatment (HFEA, 2019). Patients were excluded if they had been advised to stop IVF/ICSI, had already had more than two complete cycles or if their most recent consultation (i.e., within the previous eight weeks) was for a frozen embryo transfer. Participants were also excluded if they had undergone IVF/ICSI for pre-implementation genetic diagnosis because of a genetic disorder, fertility preservation, surrogacy or were using donated gametes (egg or sperm).
A priori power calculation computed for logistic regression to identify determinants that can differentiate people who were willing/unwilling to plan for multiple cycles estimated a total sample size of N = 231 was required to detect medium (0.5) effect size (alpha=.05, power=.80).
Materials
The Treatment Planning and Continuation Study (TPCS) was a mixed format (i.e., quantitative-qualitative), online survey created using Qualtrics (Qualtrics, Provo Utah, USA). Questions were centred on willingness, preferences and decision-making about planning for multiple cycles of IVF/ICSI if recommended by their doctor. Survey wording was adapted to be appropriate to men and women who had/had not had any previous fertility treatment. The TPCS took approximately 30–45 minutes to complete. Only questions relevant to analyses presented in this paper are described (see Supplementary Table SI). The complete survey is available via the Open Science Framework (https://osf.io/yevkz/).
Demographic, fertility and fertility treatment characteristics
Participants stated their gender, age, number of years cohabiting/married, whether they had paid employment, at least a university level of education, their income and whether they had had trouble paying bills or buying household items in the past 12 months.
Participants indicated whether they had previously given birth, had any adopted or step children, how long they had been trying to conceive, their fertility diagnosis, if their treatment was funded, whether their recent consultation was for a first or repeat cycle, and how many previous complete cycles they had had if applicable.
Willingness to plan for multiple cycles
Participants were assigned to willingness groups based on their responses to three separate intention questions. Participants indicated whether they would 1) be willing to plan for having up to three complete cycles of IVF/ICSI at the start of treatment, 2) probably agree to repeat a standard cycle of IVF/ICSI and 3) opt to continue with treatment (if the doctor thought they still had a chance of pregnancy) in the event of an unsuccessful cycle (all responses were no/yes). Respondents who answered yes to all three questions were assigned to the willing group (WG) whereas those who did not answer yes to all three or who were missing on one or two of the items were assigned to the unwilling group (UWG).
Antecedents of willingness
Three items measured attitudes towards planning for multiple cycles. Six items measured subjective norms and one item measured perceived behavioural control. For attitudes and subjective norms, mean scores were computed with higher scores indicating more agreement.
Challenges and benefits: quantitative
Nine items were used to measure the challenges of planning for multiple cycles and 11 items were used to measure the possible benefits.
Challenges and benefits: qualitative
An open-ended text box, with no word limit, asked patients to describe in their own words any other benefits or challenges they saw in planning for multiple cycles.
Solutions to challenges: qualitative
A series of nine open-ended text boxes, with no word limit, asked patients to describe possible solutions to overcoming the nine challenges of planning for multiple cycles.
Decision-making context
Seven items from the Treatment Decisional Conflict Scale (O’Connor, 1995) measured decisional conflict in relation to whether participants were willing to plan for multiple cycles. Participants were categorised according to whether they met the decisional conflict score (i.e., ≥ 25) threshold (no/yes).
Participants indicated whether they could make ten treatment decisions if they were provided with the relevant information in advance of treatment (no/yes). The total number of participants responding yes to each treatment decision was calculated. Participants were additionally asked to rate how important it would be to make these decisions in advance of treatment. A composite ‘advanced decision-value’ score was computed from the cross-product of advanced decision-making ability X advanced decision-making importance. Higher scores (range 1–5) indicated higher value placed on planning decisions in advance.
Procedure
The School of Psychology, Cardiff University, provided study ethical review and approval (EC.19.10.08.5715). The TPCS was uploaded to Qualtrics and distributed via social media adverts (e.g., Facebook and Instagram) and online advertising from collaborating fertility charities/advocates worldwide (e.g., Fertility Network UK, RESOLVE). Participants were offered either a £10 Amazon voucher or were entered into a prize draw to win one of eight £25 Amazon vouchers in exchange for their participation. The survey was live from November 4th 2019 to March 12th 2020. Upon clicking the survey link, participants were presented with an information sheet outlining the study and data collection procedures. Participants were additionally informed that their participation was voluntary and they were free to withdraw from the study at any time. A consent form was provided after the information sheet. Once participants had consented to take part, they were presented with the survey. There was no time limit to survey completion, but interrupted surveys had to be completed within one week of last input. At the end of the survey, participants were thanked for the participation and provided with more information about the survey and research project.
Data analysis
Descriptive statistics were used to profile the sample on demographic, fertility and fertility treatment characteristics. Multiple items measuring the same construct were used to create composite variables and internal consistency (see Supplementary Table SI) was assessed using Cronbach alpha coefficient (α; Schmitt, 1996). Chi-square (χ2) or t-tests (t) were used for group (i.e., willingness) comparisons, as appropriate to units of measurement. The probability value of .05 was considered significant.
A series of univariate analyses were used to identify variables that could discriminate willingness groups. Mixed factorial ANOVA were used to examine the main effect of group (between-subject factor) on antecedents of willingness (i.e., attitudes, subjective norms, perceived behavioural control), challenges, benefits and importance of treatment decisions (within-subject factors, when multiple scales measuring same constructs). Significant Group × Measure interactions were explored using simple comparison analysis, with Bonferroni adjustment for alpha inflation. Independent t-tests were used to examine group differences on single variables of decisional conflict, perceived behavioural control and weighted treatment decisions.
Multivariate logistic regression analysis was used to examine the associations between the significant TPB antecedents of intentions (attitudes, subjective norms, perceived behavioural control) and willingness (dependent measure, UWG coded 0). Statistics were standardised beta coefficients (β), SE, odd ratios (exp(B)) and CI (± 95% CI).
Thematic analysis was carried out on textual data according to the method of Braun and Clarke (2006) with first steps being familiarisation with data, inductive coding (attaching meaningful labels to textual data segments) and reviewing coding with colleagues. Coding was carried out until no new codes were identified (i.e., data saturation reached). CH was coder. Authors came together multiple times for peer debriefing, to reflect, discuss, and review the codes generated. Textual data analysis was presented as a summary accompanied by illustrative verbatim quotations. Quotations are referenced by participant number (P), gender (Male, Female) and age (in years).
Results
Results are presented according to study objectives.
Recruitment outcome
In total, 881 clicked the survey link, 118 did not consent, 41 were excluded after data was screened for duplicate responses, responses with more than 50% of missing data and questionnaire response time of less than 10 minutes because this was regarded as low quality or invalid data (Buchanan & Scofield., 2018). A total of 57 participants did not meet the inclusion criteria, 331 started the survey but did not complete it, 28 had missing data on critical variables (i.e., age, relationship status, time trying, number of previous complete treatment cycles) which were used to validate eligibility. The final sample (N = 306) consisted of 57 men (19%) and 249 women (81%) who had had a consultation for a first (n = 224, 73%) or repeat cycle (n = 82, 27%).
Are patients willing to plan for multiple cycles of IVF/ICSI, and what is associated with willingness?
Table I shows the pattern of responses to the three classification questions for the total sample and according to whether respondents had had any previous treatment. A total of 134 (44%) individuals were categorised into the UWG and 172 (56%) were categorised into the WG. No significant differences were found between those having a consult for a first or repeat cycle.
Table I.
Descriptive statistics for responses to the three classification questions for the total sample and according to whether or not the respondent had any previous treatment.
| Total (N = 306) | First Cycle (n = 224) | Repeat Cycle (n = 82) | Test statistic (χ2) | |
|---|---|---|---|---|
| Willing to plan for multiple cycles % yes (n) | 86.15 (249) | 87.38 (187) | 82.67 (62) | 1.04 |
| Repeat a standard cycle of treatment % yes (n) | 84.11 (254) | 84.55 (186) | 82.93 (68) | .12 |
| Continue treatment % yes (n) | 76.32 (232) | 77.93 (173) | 71.95 (59) | 1.18 |
| Willingness (i.e., yes to all three classification questions) | 56.21 (172) | 58.48 (131) | 50.00 (41) | 1.76 |
Note: percentages may vary due to missing cases.
Table II shows the demographic, fertility and treatment characteristics of the sample overall and according to willingness group. Compared to the UWG, the WG were significantly older (t(304)=2.05, p<.05), had been in a relationship for longer (t(304)=2.20, p<.05), had higher education (χ2(1, 305)=3.90, p<.05) and were more likely to be from the United Kingdom (χ2(1, 306)=5.10, p<.05).
Table IV.
The nine challenges (number of participants who left a textual response) of planning for multiple cycles of IVF/ICSI and the coded reported solutions.
| Challenges | Solutions |
|---|---|
| Ability to afford multiple cycles (n = 155) | Saving in advance, using savings, getting into debt (e.g., loans, credit cards, borrowing from family and friends, re-mortgaging), taking another job or extra work, minimise non-essential spending, research to know the true costs of treatment. |
| Negative physical effect of treatment (n = 143) | Take time off work, preparing mentally, practicing self-care |
| Burden of treatment on relationship (n = 140) | Open, honest and/or frequent communication with partner, plan to spend time with partner, engage in fun activities, support and consideration for each other, counselling. |
| Stressful interactions with staff (n = 118) | Research which clinic to use, choose the right clinic, increase communication with staff, communicate concerns with staff/clinic, knowing complaints procedure, accept that it is occurring and continue treatment, |
| Negative effect of treatment on work/daily activities (n = 141) | Plan in advance, take time off, arrange work around treatment, use flexible working, work from home, seek support from employer, communicate with work colleagues/boss about what treatment will involve, quit job if incompatible with treatment. |
| Coping with the possibility that the cycle is unsuccessful (n = 139) | Counselling, support from family and friends, remain positive, hopeful and optimistic about treatment success, being realistic about treatment success from the start, get advice from individuals who have previous experience. |
| Negative emotional effect of treatment (n = 129) | Counselling, seeking support from family, friends, partner and support group, remain positive, hopeful and optimistic about treatment success, learn from others, take one thing at a time. |
| Not knowing how body would react (n = 116) | Research effects of treatment, know possible side effects, prepare body to be in optimal condition, take treatment one step at a time. |
| Not knowing chances of pregnancy with another cycle (n = 122) | Not knowing success is part of the journey, general acceptance that it might not work, ask doctors and research success rates, being realistic from start of treatment, have treatment cut off point. |
The antecedents of willingness to plan for multiple cycles of IVF/ICSI
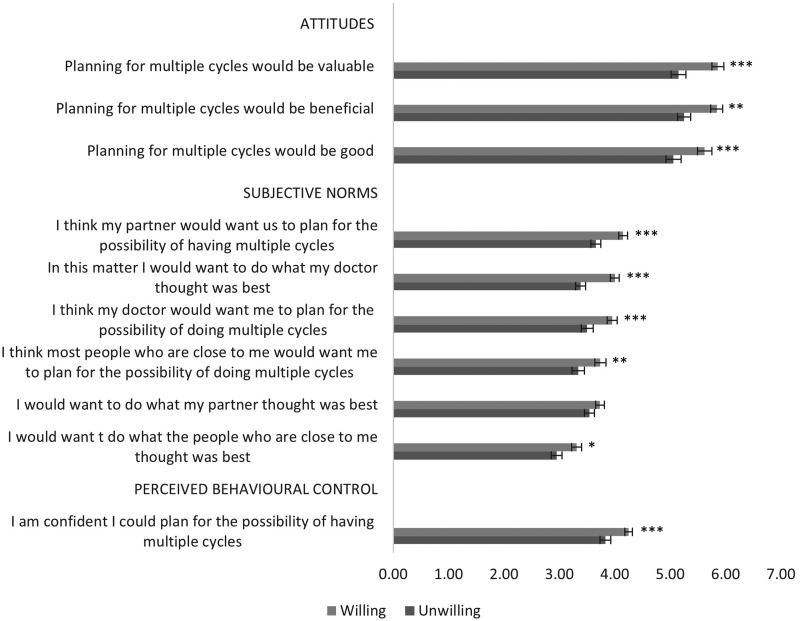
Figure 1 shows data for TPB constructs attitudes, subjective norms and behavioural control.
Figure 1.
Mean attitudes towards planning for multiple cycles of treatment and agreement with subjective norms and perceived behavioural control according to willingness group. Higher scores indicate more positive attitudes (scale 1–7) and agreement (scale 1–5). Error bars indicate 95% confidence interval around the mean. *p<.05, **p<.01, ***p<.001 for significant group differences.
A 2 × 3 ANOVA with attitudes as the within subjects variable showed a main effect of willingness group (F(1, 294)=17.66, p<.000), a main effect of type of attitude (F(2, 296)=3.61, p<.05) with a non-significant interaction between attitude and willingness (F(2, 296)=.55, p=.58). Figure 1 shows that both groups had favourable attitudes, but the WG had overall significantly more favourable attitudes in seeing planning as more beneficial, good and of value than UWG (Mdiff=.619, p<.000).
A 2 × 7 ANOVA, with subjective norms as the within subjects factor, showed a significant main effect of willingness group (F(1, 301)=23.23, p<.000), a significant main effect of subjective norm (F(5, 301)=23.79, p<.000) with a non-significant interaction between subjective norm and willingness (F(5, 301)=1.59, p=.16). Compared to the UWG, the WG had higher subjective norms for all items except for wanting to do what their partner thought was best (see Figure 1).
For perceived behavioural control, an independent t-test showed that the WG had significantly higher mean (M) scores (M = 4.25, SD=.94) compared to the UWG (t(303)=3.63, p<.000) as shown in Figure 1.
Table III shows the logistic regression to examine the independent associations of attitudes, subjective norms and perceived behavioural control with willingness to plan for multiple cycles. The model was statistically significant (χ2(3) =28.67, p<.001) explaining 12.1% (Nagelkerke R square) of the variance in willingness. When all variables were in the equations, attitudes and subjective norms remained positively and significantly associated with willingness.
Table II.
Descriptive statistics for demographic, fertility, and fertility treatment characteristics for the total sample, and according to willingness to plan for multiple cycles.
| Total (N = 306) | WG (n = 172) | UWG (n = 134) | Test statistic ( χ2/t) | |
|---|---|---|---|---|
| Demographic characteristic | ||||
| Gender % (n) | .00 | |||
| Male | 18.62 (57) | 18.60 (32) | 18.66 (25) | |
| Female | 81.37 (249) | 81.40 (140) | 81.34 (109) | |
| Age in years M(SD) | 32.57 (4.18) | 33.00 (4.29) | 32.02 (4.00) | 2.05* |
| > 39 years % (n) | 6.12 (19) | 9.70 (13) | 3.29 (6) | |
| Relationship length in years M(SD) | 6.21 (3.89) | 6.634 (4.14) | 5.66 (3.48) | 2.20* |
| At least university level % (n) | 63.07 (193) | 68.02 (117) | 57.46 (77) | 3.90* |
| In paid employment % (n) | 96.08 (294) | 97.67 (168) | 95.52 (128) | 1.95 |
| Income % (n) | 3.89 | |||
| Lower | 11.76 (36) | 8.72 (15) | 15.67 (21) | |
| The same | 61.43 (188) | 62.21 (107) | 60.45 (81) | |
| Higher | 26.80 (82) | 29.07 (50) | 23.88 (32) | |
| Problem paying bills % (n) | 15.36 (47) | 15.12 (26) | 15.67 (21) | .01 |
| Problem buying things needed in household % (n) | 10.78 (33) | 10.47 (18) | 11.19 (15) | .04 |
| Country of residence % (n) | 5.10* | |||
| United Kingdom | 79.08 (242) | 83.7 (144) | 73.1 (98) | |
| Other1 | 20.92 (64) | 16.27 (28) | 26.86 (36) | |
| Fertility and treatment characteristics | ||||
| Has given birth/fathered a child % (n) | 22.6 (69) | 21.51 (37) | 23.88 (32) | .24 |
| Has adopted a child/children % (n) | 5.23 (16) | 4.07 (7) | 6.72 (9) | 1.14 |
| Has Stepchild/children % (n) | 4.90 (15) | 4.07 (7) | 5.97 (8) | .68 |
| Time trying to conceive in years M(SD) | 3.05 (2.33) | 3.09 (2.31) | 3.00 (2.36) | .36 |
| Fertility diagnosis % (n) | 1.96 | |||
| Problem with woman | 26.80 (82) | 27.33 (47) | 26.12 (35) | |
| Problem with the man | 31.37 (96) | 30.81 (53) | 32.10 (43) | |
| Problem with both partners | 21.90 (67) | 21.51 (37) | 22.39 (30) | |
| Problem is unexplained | 17.97 (55) | 17.44 (30) | 18.66 (25) | |
| Not been given a diagnosis | 1.96 (6) | 2.91 (5) | .74 (1) | |
| IVF/ICSI funded % (n) | 4.16 | |||
| All the costs are covered | 31.37 (96) | 36.05 (62) | 25.37 (34) | |
| Some of the cost are covered | 34.64 (106) | 33.13 (57) | 36.57 (49) | |
| None of the costs will be covered | 28.76 (88) | 26.16 (45) | 32.09 (43) | |
| Unsure | 5.23 (16) | 4.65 (8) | 5.97 (8) | |
| Cycle % (n) | 1.76 | |||
| First cycle | 73.20 (224) | 76.16 (131) | 69.40 (93) | |
| Repeat cycle | 26.80 (82) | 23.84 (41) | 30.60 (41) | |
| 1 previous cycle | 75.61 (62) | 73.17 (30) | 78.05 (32) | .27 |
| 2 previous cycles | 24.39 (20) | 26.83 (11) | 21.95 (9) | |
Note: WG = willing group, UWG = unwilling group. M=mean, χ2=chi-square test, t= t-test.
Other countries were USA (12.7%, n = 39), Canada (4.6%, n = 14), Australia (.3%, n = 1), New Zealand (.3%, n = 1), France (.3%, n = 1), Italy (.3%, n = 1), Spain (.3%, n = 1), Norway (.7%, n = 2), Qatar (.3%, n = 1), India (.3%, n = 1).
p<.05,
p<.01,
p<.001.
Agreement with the challenges and benefits of planning for multiple cycles of IVF/ICSI
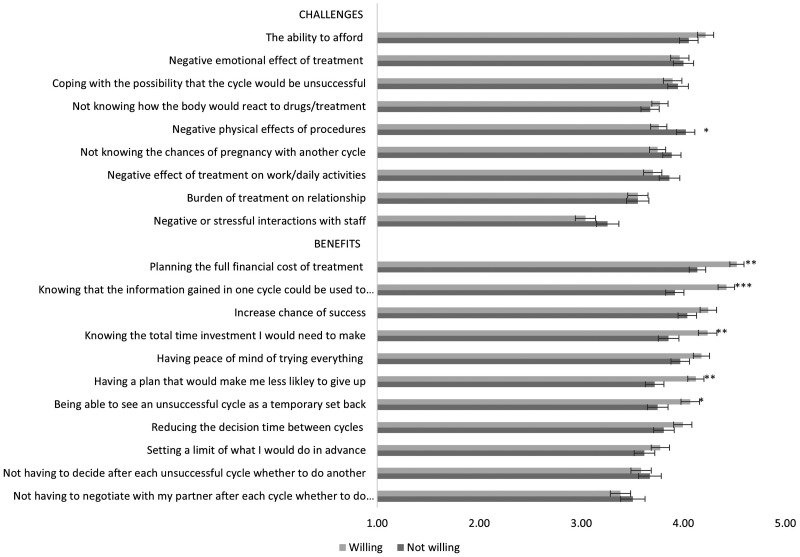
Figure 2 shows mean agreement with the nine challenges and 11 benefits of planning for multiple cycles according to willingness group. A 2 × 9 ANOVA with challenges as the within-subject factor showed that the main effect of willingness group (F(1, 300)=.70, p=.41) and interaction (F(8, 300)=1.68, p=.10) were not significant. The main effect of challenges was significant (F(8, 300)=27.20, p<.000) with some challenges (e.g., being able to afford multiple cycles) being significantly endorsed more than others (e.g., stressful interactions with staff) as shown in Figure 2.
Figure 2.
Mean level of agreement with challenges and benefits of planning for multiple cycles according to willingness group. Higher scores indicate more agreement (Scale 1–5). Error bars indicate 95% confidence interval around the mean. *p<.05, **p<.01, ***p<.001 for significant group differences.
A 2 × 11 ANOVA, with benefits as the within subjects factor, showed a main effect of willingness group (F(1, 293)=8.35, p<.01), a main effect of benefit (F(10, 295)=23.01, p<.000) and a significant interaction between willingness group and benefit (F(10, 295)=3.33, p<.000). The main effect of group showed that compared to the UWG, the WG more strongly endorsed all benefits. The main effect of benefit showed all bar three items (e.g., negotiating with partner) were rated higher than the mean for all participants across all benefits (M = 3.95, SD=.70). For the interactions, simple comparisons showed that the WG were significantly more likely to endorse all bar two of the benefits compared to the UWG (see Figure 2).
Participant-generated challenges and benefits of planning for multiple cycles of IVF/ICSI
A total of 106 participants generated other challenges and benefits. Thematic analysis revealed four additional challenges and eight additional benefits coded from these responses.
New challenges included the possibility that planning for multiple cycles could mean having less interaction with clinical staff during treatment [‘I would worry that it just means fewer conversations and explanations in between treatments with the clinical staff’ P1, Female, 37], difficulty because of invested hope in current cycle success [‘the idea of it not working and having to do multiple cycles is so overwhelming’ P4, Female, 36] and that planning in advance could result in a lack of decisional freedom, for example, ‘not being able to re-evaluate after each treatment’ (P53, Female, 32), or having the ‘liberty of stopping or withdrawing from the process’ (P54, Female, 36) taken away. Additionally, participants perceived planning for the unknown a challenge in itself due to lack of treatment experience to inform planning ‘before having undertaken one [cycle] and without knowing the effect it will have on you’ (P131, Female, 38).
New benefits largely centred on managing expectations and making the treatment journey practically, physically and psychologically easier. For example, patients reported that planning for three cycles would give them a realistic view of treatment ‘as a process rather than an instant win’ (P138, Female, 29) and would be beneficial because it would encourage them to consider the ‘bigger picture’ and think about treatment beyond the initial cycle [‘It may make you realise it is unlikely that IVF is going to work successfully on your first cycle so may help your emotional wellbeing when it isn't successful’ P63, Female, 38; ‘It makes the failure of an initial IVF cycle less devastating as you have mentally prepared yourself for it taking 3 cycles’ P94, Female, 31, ‘may not work initially and that multiple rounds is normal for a live birth’ P174, Female, 33]. Other benefits included planning facilitating the treatment process by encouraging organisational skills in addition to health management [‘planning in advance would give me a better chance of controlling and keeping control of my diabetes’ P83, Female, 28]. Planning was also perceived to reduce ‘the stress and anxiety of the unknown’ (P101, Female, 25) and ‘calm tension between partners’ (P100, Female, 32) because, for example, the ‘decision-making along the way’ (P44, Female, 33) would become less pressured [‘It takes the pressure and unknown out of the process’ P273, Female, 33]. Planning was also reported to facilitate a sense of control which ‘might feel empowering’ (P56, Female, 31). Other benefits included reducing self-blame that can accompany fertility treatment [‘three cycles won’t let women or man blame themselves’, P74, Female, 29], providing a goal and sense of hope to patients [‘It gives you a goal and hope if your first cycle isn't successful’ P86, Female, 29], and facilitating discussions with social networks about treatment [‘clarity with family & friends over our plans’ P65, Female, 35].
Reported solutions to the challenges of planning for multiple cycles
Participants were asked to report how they might plan to overcome the nine challenges of planning for multiple cycles presented in the survey. Table IV shows the nine challenges and the coded solutions from the participant’s textual responses. For most challenges, multiple solutions were provided. However, for some challenges (i.e., negative physical, emotional and relational effect of treatment, not knowing how the body will react to treatment and stressful interactions with staff), a proportion of participants reported that they were unable to provide a solution in advance. This was because they lacked previous treatment experience and did not know what to expect and how to prepare (e.g., ‘Impossible to know before and what it is going to be like to be able to prepare!’ P138, Female, 29).
Table III.
Logistic regression analysis for the antecedents of willingness to plan for multiple cycles (N = 306).
| β | S.E | Exp(B) | 95% C.I.for Exp(B) Lower Upper |
||
|---|---|---|---|---|---|
| Attitudes | .35 | .14 | 1.42* | 1.08 | 1.86 |
| Subjective norms | .46 | .18 | 1.59* | 1.11 | 2.27 |
| Perceived behavioural control | .14 | .14 | 1.15 | .87 | 1.51 |
Note: β=Standardised beta coefficient, Exp(B)= odd ratios.
p<.05,
p<.01,
p<.001.
Decision-making context: is planning in advance a conflicted decision for patients to make?
A 2 × 2 chi-square examining the percent of those meeting the decisional conflict score threshold (i.e., ≥25) according to group showed no significant difference between UWG and WG (χ2(306) = 25.82, p =.18).
Decision-making context: the ability and importance of making treatment planning decisions in advance of treatment
The treatment decisions and percentage of patients who indicated that they could make treatment decisions in advance of treatment are presented in Table V. Significance tests showed that WG were significantly more likely to be able to decide to put back more than one embryo, add an extra technique with a good quality study associated with it and change lifestyle than UWG. However, UWG were significantly more likely to be able to decide in advance about using donated eggs than WG.
Table V.
Percentage (n) of patients who could decide in advance about treatment decisions, mean (SD) importance of making the decisions and weighted rating for each treatment decision for WG (n = 172) and UWG (n = 134).
| Make treatment decision % (n) |
Test statistic χ2a | Importance M(SD)b |
Weighted rating M(SD)c |
Test statistic td | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Decision to… | Total | WG | UWG | Total | WG | UWG | Total | WG | UWG | ||
| …Use less medication | 42.48 (130) | 43.60 (75) | 41.04 (55) | .20 | 3.12 (1.11) | 3.10 (1.09) | 3.14 (1.13) | 1.38 (1.79) | 1.40 (1.76) | 1.36 (1.83) | .21 |
| …Use more medication | 69.08 (210) | 72.09 (124) | 64.18 (86) | 2.16 | 3.32 (1.22) | 3.49 (1.22) | 3.09 (1.20)** | 2.37 (1.90) | 2.63 (1.92) | 2.04 (1.82) | 2.73** |
| …Use ICSI instead of IVF | 69.28 (212) | 72.67 (125) | 64.92 (87) | 2.13 | 3.76 (1.16) | 3.95 (1.12) | 3.50 (1.17)*** | 2.76 (2.07) | 3.02 (2.07) | 2.43 (2.02) | 2.47* |
| …Put back more than one embryo in the womb | 73.86 (226) | 78.49 (135) | 67.91 (91) | 4.37* | 3.65 (1.25) | 3.77 (1.26) | 3.49 (1.22)* | 2.78 (1.96) | 3.03 (1.93) | 2.46 (1.95) | 2.53* |
| …Add on an extra technique that did not have evidence | 42.16 (129) | 43.02 (74) | 41.04 (55) | .12 | 3.13 (1.24) | 3.20 (1.23) | 3.03 (1.25) | 1.35 (1.78) | 1.41 (1.83) | 1.26 (1.72) | .74 |
| …Add on an extra technique that had little/mixed evidence | 41.97 (128) | 45.35 (78) | 37.31 (50) | 1.85 | 3.15 (1.23) | 3.26 (1.25) | 3.00 (1.19) | 1.47 (1.90) | 1.62 (1.96) | 1.28 (1.81) | 1.51 |
| …Add on an extra technique that had good evidence | 71.80 (219) | 80.23 (138) | 60.44 (81) | 15.22*** | 3.55 (1.20) | 3.65 (1.20) | 3.42 (1.21) | 2.64 (1.95) | 3.02 (1.85) | 2.16 (1.97) | 2.92*** |
| …Use donated eggs | 16.34 (50) | 12.20 (21) | 21.64 (29) | 4.90* | 3.05 (1.43) | 3.18 (1.41) | 2.88 (1.44) | .57 (1.39) | .45 (1.29) | .72 (.149) | 1.69 |
| …Use donated sperm | 22.70 (69) | 20.93 (36) | 24.63 (33) | .51 | 3.11 (1.44) | 3.17 (1.45) | 3.02 (1.42) | .77 (1.56) | .70 (1.51) | .86 (1.62) | .88 |
| …Change your lifestyle | 79.54 (241) | 83.72 (144) | 72.39 (97) | 6.36* | 3.97 (1.15) | 3.88 (1.12) | 3.66 (1.18) | 3.20 (1.88) | 3.46 (1.73) | 2.86 (2.01) | 2.82** |
Note. WG = willing group, UWG = unwilling group, M=mean.
χ2 Chi-square for difference between willing and unwilling for the treatment decisions that could be made in advance of treatment.
Importance rating is a 5-point likert scale ranging from 1= ‘not at all important’ to 5= ‘extremely important’.
Weighted rating =percent could make treatment decision x importance rating.
t= t-test.for difference between wiling and unwilling for weighted rating.
p<.05,
p<.01,
p<.001.
Table IV also shows the mean importance of making the treatment decisions in advance of treatment according to willingness group. A 2 × 9 repeated measures ANOVA, with importance of decision as the within subjects factor, showed a main effect of willingness group (F(1, 299)=6.50, p<.05) and importance (F(9, 301)=24.90, p<.000) with a non-significant interaction between importance and willingness (F(9, 301)=1.54, p=.13).
For the weighted scores, the WG rated all treatment decisions higher than the UWG. Specifically, they were more likely to rate the decisions using more medication, ICSI instead of IVF, putting back more than one embryo, adding an extra with good evidence and changing lifestyle significantly higher than the UWG. Overall, changing lifestyle had the highest rated score, using donated eggs had the lowest and the biggest difference between the WG and UWG was for adding a technique that had good evidence.
Discussion
The central finding from this study is that the majority of patients seemed to value the opportunity to plan for the possibility of having three complete cycles of treatment (i.e., multiple cycles) while acknowledging both possible challenges and benefits of doing so, and the decisions that might need to be made in advance. The findings broadly support the idea that developing a planning approach to treatment that integrates the full treatment process is compatible with patient’s mental model of treatment. Re-framing planning consultations so that fertility treatment is considered a multi-cycle process could empower patients and clinicians to discuss treatment expectations realistically and formulate fully informed treatment plans that are aligned with the value patients attribute to having children. These plans could then be less affected by transitory or reactive negative motivational states. This study was a cross-sectional examination of willingness to plan for multiple cycles. Future research should assess the acceptability and feasibility of reframing consultations and replicate the current research prospectively to examine actual multi-cycle planning behaviour in fertility clinics.
There was good evidence that a majority of patients (56%) were unambiguously willing to plan for the possibility of having multiple cycles of IVF/ICSI in advance of treatment. Participants had a positive attitude to planning for multiple cycles, felt they had positive backing of significant others (family, doctor), were able to envisage undergoing multiple cycles, felt able to make most of the decisions that such planning would necessarily entail (without conflict) and placed importance on making these decisions in advance of treatment. Further willingness was informed; people endorsed and generated, in their own words, the challenges and benefits of a multi-cycle process. Findings are consistent with previous research showing that patients consider the cumulative live birth rate to be more important than single cycles (Malizia et al., 2013) because it empowers them (and their) clinicians to make strategic decisions about care over a period of time (Maheshwari et al., 2015). However, this level of willingness seems at odds with the medical field, which plans treatment using single-cycle success rates (McLernon, 2016). Discrepancy could be capturing as yet unmeasured patient self-directed learning and understanding of assisted reproductive technologies, efforts of stakeholders that strive to provide a realistic view of fertility treatment (e.g., patient support groups, regulators), the influence of online fertility communities (e.g., social media platforms), and even a shift towards using cumulative live births and complete cycles as measures in outcome studies. Regardless of specific determinants, these findings imply that planning treatment on a multi-cycle basis would be welcomed among patients starting IVF/ICSI (or repeating after unsuccessful treatment).
One could claim that the unwilling group was sizable too, indicating that for many patients (44%) planning for multiple cycles was, in fact, not acceptable. We agree with this possible interpretation but wish to qualify it to some extent in saying that rather than being opposed to planning this group may have been more ambivalent, conflicted or conditional about a multi-cycle approach. Only 2% (n = 6) of participants in the UWG indicated no to all three willingness classification questions, with the remaining saying yes to two (n = 96, 31.4%) or one (n = 27, 8.8%). Further, some unwillingness may represent conditional willingness in that patients may be willing to plan for multi-cycles, choose to continue after failure but not choose to repeat the same type of cycle. As such, these unwilling patients may represent a sub-group that could carry on with treatment if provided with a suitable alternative (e.g., different protocol or treatment). We could not study this group in detail due to small numbers (e.g., 23/306 or 7.5% of sample). While we agree this sub-group could express some form of willingness, we would argue that the conditional element to their willingness also shows ambivalence about their multi-cycle intentions since they would carry on only if an alternative to the standard IVF was provided. Results from sensitivity analysis excluding these participants from the ‘unwilling’ group (data not reported) indicate that the findings remain valid. Alternatively, the UWG may represent people who face challenges at different points in the behavioural continuum because the three questions referred to different aspects of willingness. Specifically, one question concerned willingness to plan for multiple cycles pre-treatment (i.e., intention formation) while two concerned the choice patients would make if their cycle was unsuccessful (i.e., implementation of intention). Multi-cycle planning consultations would therefore need to target possible sources of ambivalence as well as different components of the intention-behaviour motivational complex (intention formation, implementation). If implementation is an issue, planning consults may need to promote if-then planning to encourage patients to adhere to their treatment plans when faced with treatment ambivalence or challenges, and preferences for alternative treatment options (Gollwitzer, 1999). We did not disaggregate these in the present study due to small sample size but identifying and profiling sub-groups of unwilling patients could be a direction for future research focused more on detailed aspects of willingness. Indeed, such targeted research could help us be more precise in our understanding of why patients do not uptake or continue with treatment. Considering the discussion about the sub-group of participants who rejected a standard cycle but could carry on with treatment if provided with a suitable alternative, we recommend removing the word ‘standard’ when asking patients if they agree to ‘repeat a cycle of IVF/ICSI’. This would allow disaggregation of whether and how patients continue with treatment.
Further support for ambivalence is shown in that the UWG was very similar to the WG in many respects, for example, demographic characteristics (though younger life stage and less educated), having generally positive views (but lower intensity) towards treatment (e.g., attitude, backing from significant others, behavioural control) and being able to make decisions (with less certainty) but all variables seemed somewhat weaker in intensity compared to the WG. We can only speculate from study findings what might be the source of this ambivalence or weakening of treatment intentions because we did not examine moderators. One possibility might be about the valuing of challenges and benefits, or perceived ability to overcome the challenges of undergoing treatment, since here we saw some challenges differentially rated between groups or with significant interactions. The most highly rated challenges concerned affordability, emotional upheaval and loss of control, stressors that have been previously identified as significant determinants of treatment drop out (Gameiro et al., 2012). For benefits, gaining control and the ability to stay positive during treatment were highly rated among the WG compared to UWG. These results reinforce those from previous research that show that having a sense of control in relation to fertility (Lopes et al., 2013) and maintaining hope are key to building up one’s strength to continue with treatment after an unsuccessful cycle (Bailey et al., 2017; Mesquita et al., 2018). Results also highlight that multi-cycle planning consultations should address issues of controllability, maintaining hope in the face of adversity in addition to emotional distress. Different members of fertility staff (e.g., nurses, counsellors, psychologists) could therefore support patients (Gameiro et al., 2015) plan for the possible need of multiple cycles, though all could have different roles in doing so. Counsellors could, for instance, work towards helping patients (particularly those more distressed or psychologically vulnerable) have a sense of control over their treatment, make value based efficacious treatment plans and prevent premature treatment discontinuation (Boivin et al., 2012). The solutions patients spontaneously provided to the challenges of planning multiple cycles of treatment should be harnessed by health care professionals, including counsellors, for patient support (see supplementary Table SII) and the development of effective if-then planning to help patients overcome any challenges or ambivalence experienced, implement intentions (Gollwitzer 1999) and engage in multiple cycles of treatment.
Patients, overall, seemed to find advance decision-making acceptable and important. This was especially so for the WG, which might imply these patients to be more advanced in their decision deliberation than the UWG. Results also show patients’ tendency to be risk averse. Patients find it easier to decide about those options that jeopardise less their chance of success (e.g., putting back more than one embryo, changing lifestyle, add-ons with good evidence) than those that do so more (e.g., add-ons without any evidence, less medication). However, most patients were unable to make advance decisions about the use of donated gametes, which could be indicative of caution for decisions involving significant shift in perspective about parenthood goals. The use of donated eggs may not be considered desirable when starting treatment but its possibility could become more acceptable when it becomes a necessity to the prospect of achieving parenthood, as often is the case with adoption (Daniluk & Tench, 2007). Alternatively, inability to decide about donated gametes could simply reflect personal treatment limits (i.e., rejection of third party reproduction). These are important considerations when planning for multiple cycles of treatment and ascertaining the level of treatment engagement patients are prepared to have to achieve their parenthood goals.
In line with behavioural theories, willingness to plan for multiple cycles of treatment was found to be underpinned by positive attitudes towards planning and the perception that important others, especially partners and clinicians, endorse the multi-cycle approach to planning (Ajzen, 1991). There appears to be a desire from patients to adhere to treatment plans developed with their doctors. The combination of personal attitudes and doctor norms could suggest that patients’ view on multiple cycles should be seen as a collaborative endeavour. There is precedence for such a suggestion, for example, opting for single rather than double embryo transfer is enhanced if doctor’s attitude is also for single embryo transfer (De Lacy et al., 2007). Collaboratively planning for multiple cycles could additionally minimise decisional ambivalence during the treatment journey by moving many decisional points away from the stressful periods of treatment (e.g., moment of cycle cancellation or unsuccessful cycle) that have been shown to deplete treatment motivation and discourage couples from carrying on with treatment (Akyuz & Sever, 2009; Gameiro et al., 2020, Mesquita et al., 2018). However, multi-cycle collaborative plans would need to be managed carefully to be respectful of ethics (Klock, 2015). Clinicians would need to ensure that patients are not over-sold or persuaded into consenting to pay for treatment or add-ons when success is unlikely (Harper et al., 2017) or when these options are not aligned to patient values. Being mindful of ethics, a multi-cycle treatment plan could bolster individual and couple treatment intentions after treatment failure, reduce time to next treatment cycle and discontinuation rates, which could ultimately increase the likelihood of achieving parenthood. By mapping all treatment from the start, multiple cycle planning has the benefit of helping patients feel sure they have done all they could to realise their parenthood goals (see perceived benefits). This can facilitate post-treatment transitions when it is unsuccessful, and moving-on options (e.g., self-guided support to come to terms with an unfulfilled childwish, https://www.myjourney.pt) can be made available to patients.
Strengths and limitations
By definition, the UWG represented a heterogeneous group with possibly unknown motivational coherence. Our operational definition of ‘willing’ required a yes to all willingness questions, with remaining participants assigned to the ‘unwilling’ group. The latter group thus consisted of people that answered no to all, no or yes to some or failed to reply to some of the classification questions (participants missing on all were omitted). The cross-sectional nature of the study and the outcome measure being willingness to plan rather than actual behaviour is problematic because we know the association between intentions and behaviour can be weak (Gollwitzer, 1999). Furthermore, cross-sectional data makes it difficult to ascertain cause and effect. Thus while social pressure from significant others could cause people to be more willing, it may also be that those more willing could elicit more support for their plans from their network. Prospective replication of the current study is needed to confirm applicability of the results found to actual multi-cycle planning behaviour, examine the validity and sensitivity of our definition of willingness to plan for multiple cycles throughout the treatment continuum, and determine direction of causality. Note however, that the size of the willing group (56%) using our classification method produced continuation intentions consistent with actual behaviour as observed in previous research (i.e., Gameiro et al., 2013; McLernon et al., 2016). Nonetheless, considering the discussion focused on the sub-group of participants who rejected a standard cycle but could carry on with treatment if provided with a suitable alternative, we acknowledge that the use of the qualifier ‘standard’ may be misleading and recommend its removal when asking patients if they ‘agree to repeat a cycle of IVF/ICSI’. Asking patients who had previously engaged in at least one previous cycle of IVF/ICSI to consider their preferences and decision-making retrospectively was needed to understand multi-cycle perspectives for those repeating treatment but could imply recall bias on some questions and/or amplify our definition of willingness because some patients had previous treatment experience (i.e., had implemented their intentions). Nevertheless, the responses to the three classification questions and therefore willingness was similar for repeat and first time users. Another limitation could be the single recruitment source (i.e., social media), which could have biased the sample towards those more keenly interested in treatment issues. Although the use of incentives would have arguably increased the likelihood of those less keen on participating there is a need for replication of the study with other populations of fertility patients recruited directly from clinics without participation incentives. Finally, the study was advertised worldwide but only 20% of the sample were non-UK residents. Consequently, there was an insufficient number of non-UK residents to examine country differences and the possible influence variances in health care contexts and the provision of treatment (e.g., costs of treatment) could have had on willingness to plan for multiple cycles. Future research should examine the possible country differences that may exist in willingness to plan for multiple cycles.
Conclusion
Results highlight the acceptability to patients of reframing treatment to be a multi-cycle process, which could empower patients (and clinicians) to work collaboratively to formulate fully informed treatment plans that account for the high likelihood of cycle failure. This approach would be in line with patient preferences and could ultimately facilitate treatment being delivered according to those preferences and not eroded through treatment challenges. Future research should prioritise investigating the acceptability and feasibility of re-framing consultations toward a multi-cycle approach and investigate best ways to elicit preferences at the start of treatment, and as they change along the treatment journey.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
Thank you to Fertility Network UK, Fertility Europe, Fertility Matters Canada, RESOLVE, Fertility New Zealand, Think Fertility, Fair Treatment for the Women of Wales and all the fertility clinics and social influencers for helping to distribute the survey.
Authors’ roles
C.H., S.G. and J.B. contributed to the conception and design of the study, the acquisition of data and the analysis and interpretation of data. They drafted all versions of the article and approved the final version for publication.
Funding
This research was financially supported by Merck Serono Ltd, an affiliate of Merck KGaA, Darmstadt, Germany. Merck KGaA, Darmstadt, Germany, reviewed the manuscript for medical accuracy only before journal submission. The Authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors.
Conflict of interest
Professor Boivin reports personal fees from Merck KGaA, Darmstadt, Germany, Merck AB an affiliate of Merck KGaA, Darmstadt Germany, Theramex, Ferring Pharmaceuticals A/S, grant from Merck Serono Ltd, outside the submitted work and that she is co-developer of Fertility Quality of Life (FertiQoL) and MediEmo app. Dr. Gameiro reports consultancy fees from Ferring Pharmaceuticals A/S, Access Fertility and SONA-Pharm LLC, and grants from Merck Serono Ltd. Dr. Harrison declares no conflicts of interest.
References
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50:179–211. [Google Scholar]
- Akyuz A, Sever N.. Reasons for infertile couples to discontinue in vitro fertilization (IVF) treatment. J Reprod Infant Psychol 2009;27:258–268. [Google Scholar]
- Bailey A, Ellis-Caird H, Croft C.. Living through unsuccessful conception attempts: a grounded theory of resilience among women undergoing fertility treatment. Journal of Reproductive and Infant Psychology 2017;35:324–333. [DOI] [PubMed] [Google Scholar]
- Berg Brigham K, Cadier B, Chevreul K.. The diversity of regulation and public financing of IVF in Europe and its impact on utilization. Human Reproduction 2013;28:666–675. [DOI] [PubMed] [Google Scholar]
- Boivin J, Domar A, Shapiro D, Wischmann T, Fauser B, Verhaak C.. Tackling burden in ART: an integrated approach for medical staff. Human Reproduction 2012;27:941–950. [DOI] [PubMed] [Google Scholar]
- Brandes M, van der Steen JOM, Bokdam SB, Hamilton CJCM, de Bruin JP, Nelen WLDM, Kremer JAM.. When and why do subfertile couples discontinue their fertility care? A longitudinal cohort study in a secondary care subfertility population. Hum Reprod 2009;24:3127–3134. [DOI] [PubMed] [Google Scholar]
- Braun V, Clarke V.. Using thematic analysis in psychology. Qualitative Research in Psychology 2006;3:77–101. [Google Scholar]
- Buchanan EM, Scofield JE.. Methods to detect low quality data and its implication for psychological research. Behav Res 2018;50:2586–2596. [DOI] [PubMed] [Google Scholar]
- Chambers GM, Paul RC, Harris K, Fitzgerald O, Boothroyd CV, Rombauts L, Chapman MG, Jorm L.. Assisted reproductive technology in Australia and New Zealand: cumulative live birth rates as measures of success. Med J Aust 2017;207:114–118. [DOI] [PubMed] [Google Scholar]
- Calhaz-Jorge C, de Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V, European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Hum Reprod 2016;31:1638–1652.27496943 [Google Scholar]
- Daniluk JC, Tench E.. Long‐term adjustment of infertile couples following unsuccessful medical intervention. Journal of Counselling & Development 2007;85:89–100. [Google Scholar]
- De Lacey S, Davies M, Homan G, Briggs N, Norman RJ.. Factors and perceptions that influence women's decisions to have a single embryo transferred. Reproductive Biomedicine Online 2007;15:526–531. [DOI] [PubMed] [Google Scholar]
- Domar A. Impact of psychological factors on dropout rates in insured infertility patients. Fertil. Steril 2004;81:271–273. [DOI] [PubMed] [Google Scholar]
- Domar A, Smith K, Conboy L, Iannone M, Alper M.. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil. Steril 2010;94:1457–1459. [DOI] [PubMed] [Google Scholar]
- Domar AD, Rooney K, Hacker MR, Sakkas D, Dodge LE.. Burden of care is the primary reason why insured women terminate in vitro fertilization treatment. Fertility and Sterility 2018;109:1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertility Associates ( 2020. ). Public funding and eligibility. https://www.fertilityassociates.co.nz/treatment-costs-and-payment-options/public-funding-and-eligibility/.
- Gameiro S, Boivin J, Peronace L, Verhaak CM.. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Human Reproduction Update 2012;18:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro S, Verhaak CM, Kremer JAM, Boivin J.. Why we should talk about compliance with assisted reproductive technologies (ART): a systematic review and meta-analysis of ART compliance rates. Human Reproduction Update 2013;19:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro S, Boivin J, Dancet E, Klerk C, Emery M, Lewis-Jones C, Thorn P, Van den Broeck U, Venetis C, Verhaak C, Wischmann. et al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction—a guide for fertility staff. Hum Reprod 2015;30:2476–2485. [DOI] [PubMed] [Google Scholar]
- Gameiro S, Mesquita da Silva S, Gordon U, Baccino G, Boivin J. (2020). In-depth analysis of what influences whether patients’ commit to achieve parenthood (parenthood-engagement) and undergo fertility treatment (treatment-engagement) before and after a treatment cycle. European Society of Human Reproduction and Embryology (ESHRE). France, July 2020.
- Gliozheni O, Hambartsoumian E, Strohmer H, Petrovskaya E, Tishkevich O, Bogaerts K, Wyns C, Balic D, Antonova I, Pelekanos M, The European IVF-monitoring Consortium (EIM)‡ for the European Society of Human Reproduction and Embryology (ESHRE) et al. ART in Europe, 2016: results generated from European registries by ESHRE. Human Reproduction Open 2020; 10.1093/hropen/hoaa032. [DOI] [Google Scholar]
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. American Psychologist 1999;54:493–503. [Google Scholar]
- Gollwitzer PM, Sheeran P.. Implementation intentions and goal achievement: A meta‐analysis of effects and processes. Advances in Experimental Social Psychology 2006;38:69–119. [Google Scholar]
- Hardway M, Younas K.. Female infertility. InnovAiT 2018;11:201–204. [Google Scholar]
- Harper J, Jackson E, Sermon K, Aitken RJ, Harbottle S, Mocanu E, Hardarson T, Mathur R, Viville S, Vail A. et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Human Reproduction 2017;32:485–491. [DOI] [PubMed] [Google Scholar]
- Human Fertilisation and Embryology Authority (HFEA). Commissioning guidance for fertility treatment Human Fertilisation and Embryology Authority. London., UK: 2019. [Google Scholar]
- Human Fertilisation and Embryology Authority (HFEA) Fertility Treatment 2018: trends and Figures. London, UK: Human Fertilisation and Embryology Authority. [Google Scholar]
- Klock SC. When treatment appears futile: the role of the mental health professional and 552 end-of-treatment counseling. Fertil Steril 2015;104:267–270. [DOI] [PubMed] [Google Scholar]
- Mesquita da Silva S, Place JM, Boivin J, Gameiro S.. Failure after fertility treatment: Regulation strategies when facing a blocked parenthood goal. Human Fertility 2020;23:179–185. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z.. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reproductive Biomedicine Online 2014;29:684–691. [DOI] [PubMed] [Google Scholar]
- Lopes V, Canavarro MC, Verhaak CM, Boivin J, Gameiro S.. Are patients at risk for psychological maladjustment during fertility treatment less willing to comply with treatment? Results from the Portuguese validation of the SCREENIVF. Human Reproduction 2014;29:293–302. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, McLernon D, Bhattacharya S.. Cumulative live birth rate: time for a consensus? Hum Reprod 2015;30:2703–2707. [DOI] [PubMed] [Google Scholar]
- Malizia BA, Dodge LE, Penzias AS, Hacker MR.. The cumulative probability of liveborn multiples after in vitro fertilization: a cohort study of more than 10,000 women. Fertil Steril 2013;99:393–399. [DOI] [PubMed] [Google Scholar]
- McLernon DJ. Predicting the chances of live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113873 women. BMJ 2016;355: doi: 10.1136/bmj.i5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita da Silva S, Place JM, Boivin J, Gameiro S.. Failure after fertility treatment: regulation strategies when facing a blocked parenthood goal. Hum Fertil 2018;1–6. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence Fertility Problems: assessment and 554 Treatment. UK: NICE Clinical Guideline CG156, 2013. [PubMed] [Google Scholar]
- Oakley L, Doyle P, Maconochie N.. Lifetime prevalence of infertility and infertility treatment in the UK: results from a population-based survey of reproduction. Hum Reprod 2007;23:447–450. [DOI] [PubMed] [Google Scholar]
- Ockhuijsen H, Hoogen V, Eijkemans M, Macklon N, Boivin J.. Clarifying the benefits of the positive reappraisal coping intervention for women waiting for the outcome of IVF. Human Reproduction 2014;29:2712–2718. [DOI] [PubMed] [Google Scholar]
- O'Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- Peddie VL, van Teijlingen E, Bhattacharya S.. A qualitative study of women's decision-making at the end of IVF treatment. Human Reproduction 2005;20:1944–1951. [DOI] [PubMed] [Google Scholar]
- Rauprich O, Berns E, Vollmann J.. Information provision and decision-making in assisted reproduction treatment: results from a survey in Germany. Human Reproduction 2011;26:2382–2391. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM. The health belief model and preventive health behavior. Health Education Monographs 1974;2:354–386. [Google Scholar]
- Schmitt N. Uses and abuses of coefficient apha. Psychological Assessment 1996;8:350–353. [Google Scholar]
- Smeenk J, Verhaak C, Stolwijk A, Kremer J, Braat D.. Reasons for dropout in an in vitro fertilization/intracytoplasmic sperm injection program. Fertil. Steril 2004;81:262–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.