Abstract
Phenolic compounds are parts of secondary metabolites mostly found in plant species with enormous structural diversities. They can exist as glycosides or aglycones; matrix or free-bound compounds; and comprising mostly polymerized or monomer structures. Additionally, these compounds are not universally dispensed within plants with varied stability. This has contributed to challenging extraction processes; implying that employing a single step or inappropriate extraction technique might change the recovery of phenolic components from the plant samples. Hence, it is important to select an appropriate extraction method so as to recover the targeted phenolic compounds. This is will helps to recover substantial yields from the sample matrix. Therefore, this review mainly focuses on the phenolic compounds and several methods of extraction that are used to obtaining them from plant materials. These extraction methods includes both conventional and unconventional techniques.
Keywords: Extraction, Phenolic compounds, Polyphenols, Flavonoids, Assisted extraction, Conventional techniques, Unconventional techniques, Solid-liquid extraction, Liquid-liquid extraction, Secondary metabolites
Graphical abstract
Highlights
-
•
Phenolic compounds from natural sources.
-
•
Methods of extracting phenolic compounds.
-
•
Selection of an appropriate extraction method to recover the targeted phenolic compounds from plant materials.
List of abbreviations
- CC
Column chromatography
- DCCC
Droplet counter-current chromatography
- EAE
Enzyme assisted extraction
- HHPE
High hydrostatic pressure
- LC-MS
Liquid chromatography mass spectrometry
- LLE
Liquid-liquid extraction
- MAE
Microwave assisted extraction
- MIPs
Molecularly imprinted polymers
- MSPD
Matrix solid-phase dispersion
- MW
Molecular weight
- PFE
Pressurized fluid extraction
- PLE
Pressurized liquid extraction
- SC-CO2
Supercritical CO2 extraction
- SFE
Supercritical fluid extraction
- SPE
Solid-phase extraction
- SLE
Solid-liquid extraction
1. Introduction
Compounds with more or single aromatic rings coupled to a single or more hydroxyl groups are commonly called phenolic. They are the most common secondary plant metabolites with over 8000 known structures. They range from the simple phenolic such as phenolic acids; to the complex compounds like tannins. The compounds participate in plant defence against ultra violet (UV), pathogens, and other predators. Their presence in all plant organs makes them a vital ingredient of the human diet (Balasundram et al., 2006; Shah et al., 2018).
Phenolics are found mainly in fruits, legumes, vegetables, tea, wine, coffee, and accounts for the organoleptic characteristics of plant food. Likewise, phenolic compounds are responsible for the bitterness of fruits due to their interaction with salivary glycoprotein. Phenolics can also added to the colour of many fruits and vegetables. Phenolics are known to account for the differences in the flavour and colour of different wine brands. Among the plants, phenolics are lignans, tannins, phenolics acids, stilbenes, and flavonoids.
Flavonoids are the major polyphenols in human diets. Structurally, flavonoids composed of a flavan nucleus with 15 carbon atoms arranged in 3 rings such as C6–C3–C6 labelled A, B, and C. There are six subgroups of flavonoid, these are flavones, flavanones, flavonols, flavanols, anthocyanins, and isoflavones. This grouping operates on the oxidation state of central C ring in the flavonoid structure. The differences in the structure of each subgroup are partly attributed to the pattern and degree of hydroxylation, prenylation, glycosylation, or methoxylation. The commonest flavonoids are quercetin, catechin, naringenin, cyanidin-glycoside, and daidzein (D’Archivio et al., 2007; Dai and Mumper, 2010).
There are 2 classes of phenolic, they are acids-benzoic acid derivatives (gallic acid) and cinnamic acid derivatives such as coumaric and ferulic acid. Caffeic acid is mostly found in vegetables and fruits which are mostly esterified with quinic acid. Ferulic acid is another common phenolic acid found in cereals; mostly esterified with hemicelluloses (Dai and Mumper, 2010).
Tannins are commonly subdivided into 2 groups: Hydrolysable and condensed tannins. The hydrolysable tannins contain a central glucose core in an esterified form with gallic acid. The formation of oxidative linkage between the components of these structures’ accounts for the great differences in their structures. Several oligomeric compounds with molecular weight (MW) ranging from 200 to 5000 Da are formed from various intermolecular oxidation reactions (Khanbabaee and van Ree, 2001). Regarding the condensed tannins, they are either oligomers or polymers of flavan-3-ol bonded via the interflavan carbon bond. Condensed tannins are called proanthocyanidins due to the fact that when heated in an acidic alcohol solution, they can be degraded into anthocyanidins via an acid-catalyzed oxidation process (Naumann et al., 2017).
Recently, the attention of nutritionist has only been drawn to the nutritional and health benefits of dietary polyphenols despite their wide abundance. The major point of attraction to researchers and food manufacturers are the potent antioxidant properties of polyphenols and their preventive role in various oxidative stress-related conditions (Alara et al., 2018a, Alara et al., 2018b; Manach et al., 2004). Different studies have proven the preventive role of polyphenols in cardiovascular and neurodegenerative conditions, as well as in other diseases and cancer in both in vitro and in vivo experiments (Cory et al., 2018; Forni et al., 2019; Potì et al., 2019; Vauzour et al., 2010). They have been found to influence the activity of several enzymes and cell receptors. Hence, there are other biological activities of polyphenols in addition to their antioxidant properties.
Moreover, the solubility and separation properties of polyphenols are affected by their structural differences. For instance, the structure of any compound has a significant influence on its polarity level, conjugation, and interaction with the sample matrix. High molecular weight phenolics are often insoluble because of their structural composition on their solubility. Furthermore, the stability of phenolic compounds varies due to their non-uniform distribution in plants; for instance, some phenolic compounds are stable while the remaining ones are either prone to oxidation, thermolabile or volatile. The recovery of polyphenols from source is a tedious task due to the high level of enzyme activity in most foods and plants. Hence, the selection of the extraction process must be done with utmost care to avoid the chemical alteration of the target compounds (Bohlin, 1998; Robards, 2003).
Currently, there is no generally accepted procedure for the recovery of all phenolics or those of a specific group from plant materials. Thus, there is a need to establish an optimized process for the recovery of phenolic compounds from plant materials by considering the following:
-
(a)
The type of sample and targeted compounds such as total phenolics, specific class of phenolics, a specific phenolic, and others;
-
(b)
The object of the analysis that is being used for quantitation or structural elucidation purpose;
-
(c)
Technique availability.
Owing to the complexity of most samples, their preparation method usually has a noticeable impact on result of the entire extraction processes. Some of the common sample preparation methods before extraction include drying, homogenization, filtration, and grinding. Additionally, a hydrolysis step is added in most cases to facilitate the release of compounds from the sample matrix with ease. Crude extracts are commonly prepared using solvent extraction method; however, assisted extraction methods, including those that added the use of ultrasounds, microwaves, and pressurized/supercritical fluids have been commonly used to extract phenolic compounds from plant materials (Selvamuthukumaran and Shi, 2017). Crude extracts are often a pool of different classes of compounds which are soluble in the employed solvent system. However, the unwanted phenolics and other interfering substances may need to be removed via an additional step, thereby requiring an effective cleaning method such as solid-phase extraction (SPE), droplet counter-current chromatography (DCCC), and column chromatography (CC). Moreover, new alternative methods which include the utilization of molecularly imprinted polymers (MIPs) are being developed in the extraction of targeted compounds (Sticher, 2008). Therefore, this review focuses on the various ways of extracting phenolic compounds from their sources.
2. Phenolic compounds from natural sources
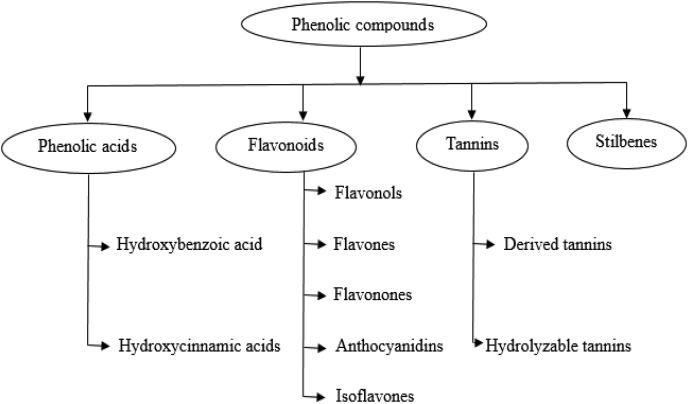
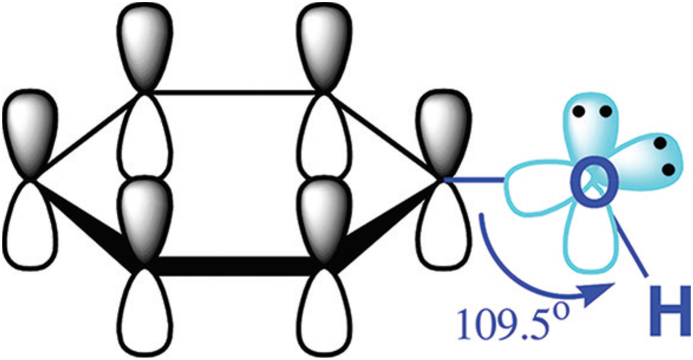
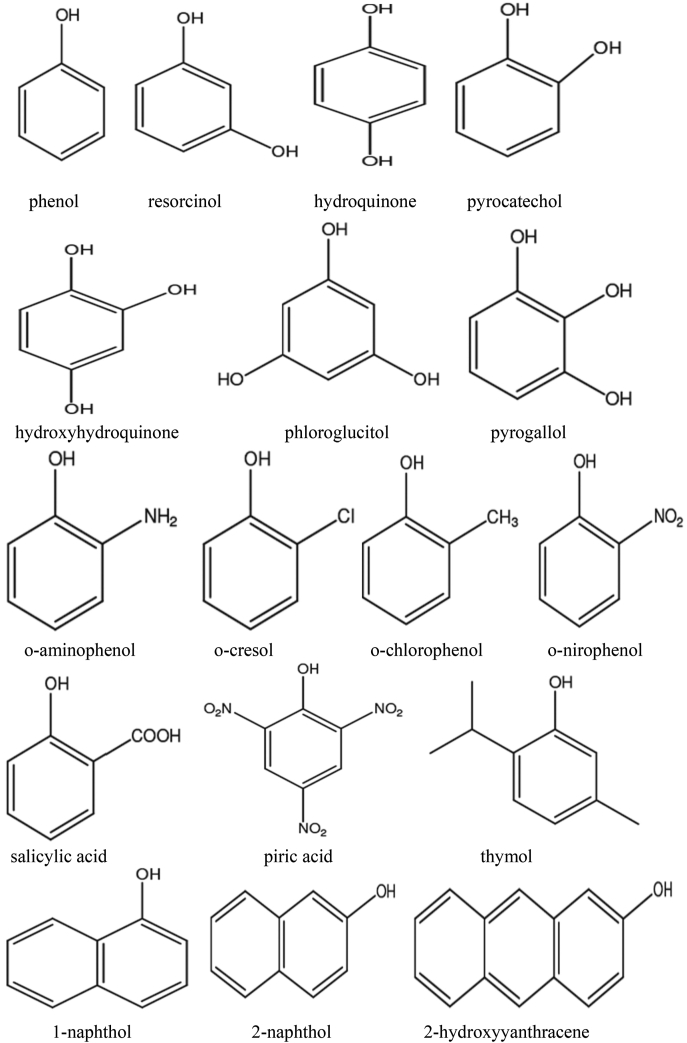
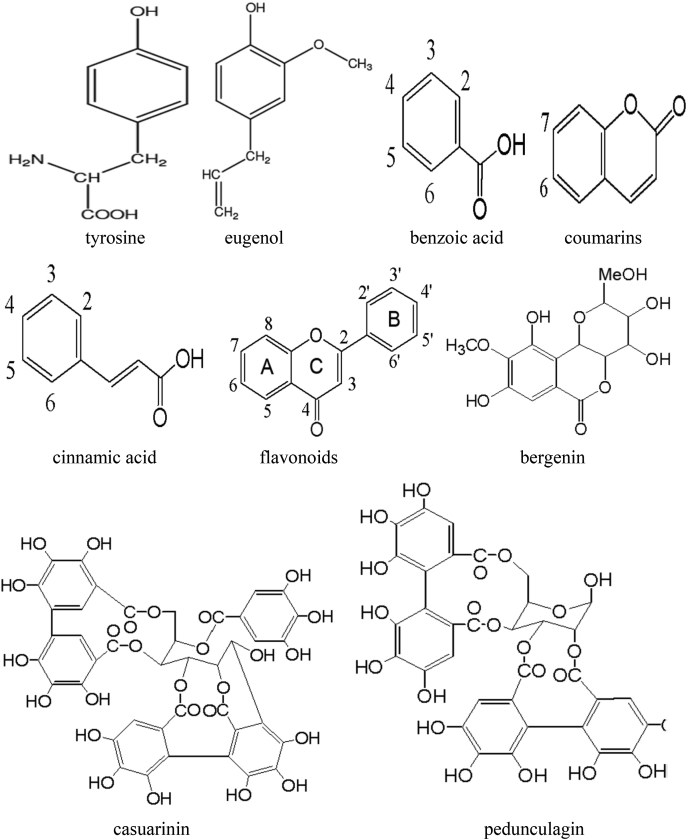
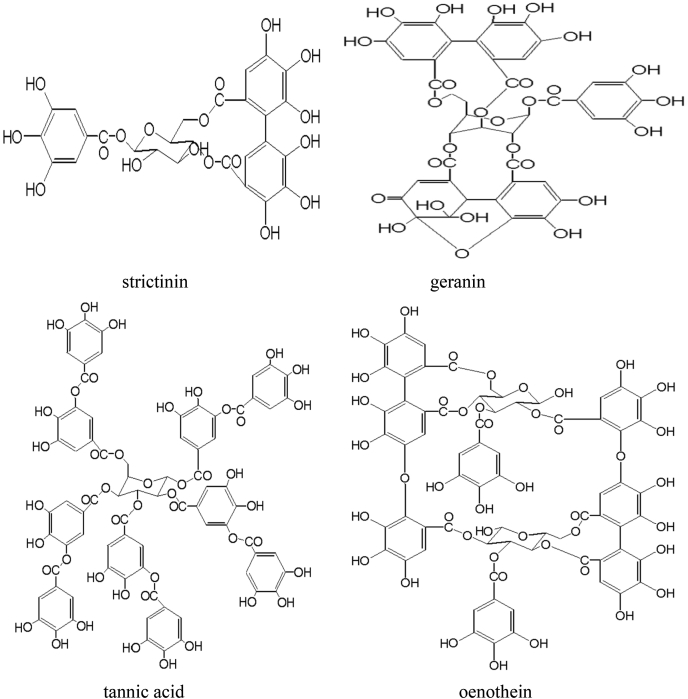
Plants can synthesize several organic compounds called secondary metabolites either during normal metabolic processes or in regarding to certain environmental conditions including wounds, temperature, UV-radiation, infection, and others (Cheynier, 2012; Tiago et al., 2017). These metabolites are grouped into different clusters in relative to the occurrence of phenol rings in their structures, and on the structures that hold the ring in place. Phenolic compounds occurred as functional derivatives such as methyl esters, esters and glycoside. They are seen inform of the conjugate with poly- and monosaccharides which joined one or more phenolic compounds (Balasundram et al., 2006). They include flavonoids, phenolic acids, simple phenols, and hydroxycinnamic acid derivatives. Phenolic compounds are categorized into different classes as shown in Fig. 1. They are toxic to micro-organisms due to the presence of several numbers of hydroxyl groups on the phenols. The actual structure of phenol and structural identification of some phenolic compounds are provided in Fig. 2, Fig. 3, respectively. The physicochemical properties of some phenolic compounds are presented in Table 1.
Fig. 1.
Main classes of phenolic compounds. These include phenolic acids, flavonoids, tannis, and stilbenes.
Fig. 2.
Structure of phenol.
Fig. 3.
Structural identification of some phenolic compounds.
Table 1.
Physicochemical properties of some phenolic compounds.
| Chemical compound (CAS number) | Form/colour | Melting point (°C) | Boiling point (°C) | Solubility in water | Molecular weight | Relative density (water = 1) | Flash point (°C) | Vapour pressure (kPa) | Relative vapour density (air = 1) | Auto ignition point (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
|
p-tert-butylphenol 98-54-4 |
98 | 237 | soluble | 150.21 | 0.908@ 80 °C | |||||
| 4-tert-butylpyrocatechol 98-29-3 |
53.5 | 285 | 166.21 | |||||||
| catechol 120-80-9 |
monoclinic tablets, prisms from toluene; colourless crystals; discolours to brown on exposure to light and moist air |
105 | 245 | very soluble | 110.11 | 1.344 | 127 cc | 3 × 104 mm Hg | 3.79 | 510 |
|
p-chloro-m-cresol 59-50-7 |
dimorphous crystals; slightly pink or white crystals |
67 | 235 | slightly soluble | 142.58 | |||||
| 2-chlorophenol 95-57-8 |
light amber liquid; colourless to yellow brown liquid |
9.3 | 174.9 | slightly soluble | 128.6 | 1.2634 | 64 cc | 0.23 | 4.4 | |
| 3-chlorophenol 108-43-0 |
needles; white crystals | 33 | 214 | slightly soluble | 128.6 | 1.268@ 25 °C | 121 cc | 0.13@44.2 °C | ||
| 4-chlorophenol 106-48-9 |
needle-like, white to straw coloured crystals; pink crystals |
43 | 220 | slightly soluble | 128.60 | 1.2238@78 °C/4 °C | 13 Pa | 4.43 | ||
| cresol, all isomers 1319-77-3 |
yellowish, colourless, pinkish liquid, or brownish-yellow |
11–35 | 191–203 | 50% soluble | 108.13 | 1.0300-1.0380@25 °C/25 °C | 43–82 | 14–32 Pa@ 25 °C | 3.72 | 559 |
| o-cresol-95-48-7 | colourless crystalline compound; white crystals/liquid |
31 | 191 | soluble | 108.10 | 1.0470 | 81 cc | 33 Pa@ 25 °C | 3.72 | 599 |
|
m-cresol 108-39-4 |
yellowish liquid or colourless |
12 | 202 | slightly soluble | 108.10 | 1.0340 | 86 cc | 20 Pa@ 25 °C | 3.72 | 588 |
|
p-cresol 106-44-5 |
prisms; crystals; white crystals; colourless; crystalline mass |
35 | 201.9 | slightly soluble | 108.13 | 1.0178 | 86 cc | 15 Pa@ 25 °C | 3.72 | 559 |
| 2,6-di-tert-butyl-p--cresol 128-37-0 |
white crystalline solid; pale yellowish crystalline powder |
70 | 265 | insoluble | 220.34 | 1.0480 | 127 cc | 7.6 | ||
| 2,6-di-tert-butylphenol 128-39-2 |
39 | 133 | 206.31 | |||||||
| 2,4-dichlorophenol 120-83-2 |
colourless crystals; hexagonal needles from benzene; white solid |
45 | 210 | slightly soluble | 163.00 | 1.383@ 60 °C/25 °C | 114 | 0.075 mm Hg@ 25 °C | 5.62 | |
| 2,5-dichlorophenol 583-78-8 |
prisms from petroleum ether and benzene | 59 | 211 @ 744 mm Hg | slightly soluble | 163.00 | 16.60 Pa @ 25 °C | 5.60 | |||
| 3,5-dichlorophenol 591-35-5 |
prisms from petroleum ether |
68 | 233 @ 757 mm Hg | slightly soluble | 163.00 | 1.10 pa @ 25 °C | 5.60 | |||
| 2,4-dimethylphenol 105-67-9 |
crystals; needles from water; colourless needles | 25.4–36 | 211.5 @ 766 mm Hg | slightly soluble | 122.16 | 0.9650 | 10 mm Hg @ 92.3 °C | |||
| dinitro-o-cresol 534-52-1 |
87 | 312 | slightly soluble | 198.13 | 1.05 × 104 mm Hg @ 25 °C | 6.80 | ||||
| hydroquinone 123-31-9 |
colourless, hexagonal prisms; white crystals; monoclinic prisms (sublimation); needles from water; prisms from methanol |
172 | 285–287 | soluble | 110.11 | 1.332 | 165 | 0.12 Pa | 3.81 | 515 |
| 2-hydroxybiphenyl 90-43-7 |
needles from petroleum ether; white, flaky crystals; pinkish crystals; colourless crystals |
59 | 286 | insoluble | 170.20 | 1.213 @ 25 °C/4 °C | 124 cc | 2.70 @ 163 °C | 530 | |
| 4-methoxyphenol 150-76-5 |
plates from water; white waxy solid |
57 | 243 | soluble | 124.14 | 1.550 | 132 cc | 421 | ||
| nonylphenol, all isomers 25,154-52-3 |
thick light yellow, straw coloured liquid |
−10 | 293–297 | insoluble | 220.39 | 0.950 | 140 cc | <0.01 | 7.59 | 370 |
| pentachlorophenol 87-86-5 |
colourless crystals (pure); flakes (crude product) or dark greyish powder; flakes or solid beads; white monoclinic, crystalline solid; needle-like crystals |
190–191 | 309–310 | slightly soluble | 266.30 | 1.978 @ 22 °C/4 °C | 0.02 Pa | 9.20 | ||
| pentachlorophenol, sodium salt 131-52-2 |
buff coloured flakes; tan or white powder | 33% @ 25 °C | 288.34 | |||||||
| phenol 108-95-2 |
colourless, white or acicular crystals, crystalline mass; colourless to light pink, interlaced or separate, needle-shaped crystal, or a light pink, crystalline mass |
43 | 181.8 | soluble | 94.11 | 1.0576 | 79 cc | 47 pa | 3.24 | 715 |
| pyrogallic acid 87-66-1 |
white crystals; orthorhombic; needles or leaflets from benzene |
133 | 309 | very soluble | 126.11 | 1.45 | 1.33 @ 168 °C | |||
| resorcinol 108-46-3 |
white needle-like crystal; needles from benzene; plates from water; pyramids and rhombic tablets |
111 | 280 | soluble | 110.11 | 1.2717 | 1 mm Hg @ 108.40 °C | 1.0739 | ||
| 2,3,4,6-tetrachlorophenol 58-90-2 |
needles from ligroin, acetic acid; brown flakes or sublimed mass; light brown mass |
70 | 150@15 mm Hg | insoluble | 231.89 | 1.83 @ 25 °C/4 °C | 1 mm Hg @ 100.00 °C | |||
| 2,3,5,6-tetrachlorophenol 935-95-5 |
leaf, from ligroin | 115 | 288 | slightly soluble | 231.89 | 1.7 | <10 Pa | 8.1 | ||
| leaf, from ligroin | light grey powder; fine white crystals |
358.58 | 150 | 0.08% | 358.58 | |||||
| 2,3,4-trichlorophenol 15,950-66-0 |
Needles or white powder | 83.5 sublimes | 197.50 | |||||||
| 2,3,5-trichlorophenol 933-78-8 |
colourless crystals | 62 | 248–249 | insoluble | 197.40 | 6.8 | ||||
| 2,3,6-trichlorophenol 933-75-5 |
needles from diluted alcohol, petroleum ether; colourless needles |
58 | 253 | slightly soluble | 197.44 | 1.5 | 78 | 6.82 | ||
| 2,4,5-trichlorophenol 95-95-4 |
needles from ligroin or alcohol; grey flakes in sublimed mass; colourless needles |
67 | 253 | slightly soluble | 197.40 | 1.678 @ 25 °C/4 °C | 2.90 Pa @ 25 °C | |||
| 2,4,6-trichlorophenol 88-06-2 |
crystals from ligroin; rhombic needles from acetic acid; yellow flakes; colourless needles |
69 | 246 | 800 mg/l @ 25° | 197.45 | 1.4901 | 133 Pa @ 76.5 °C | 6.8 |
Several studies had been performed on various sources of polyphenols including food wastes, vegetables, fruits, microalgae, algae, teas, and others (Alara and Abdurahman, 2019a, Alara and Abdurahman, 2019b; Hollman et al., 1997). Huge quantities of polyphenols have been reported in most plant-derived foods and agro-food wastes in recent years. Table 2 presents a summary of some recent studies and their result on different sources of polyphenols. Although polyphenols exist in several plant materials, however, their quantity and type are dependent on the extraction methods used, their chemical nature, the particle size, the presence of interfering compounds, and storage condition (Suwal and Marciniak, 2018). Similarly, they vary in their nature and chemical structure, ranging from simple to highly complex substances with different contents of phenolic acids, anthocyanins, phenylpropanoids, and tannins (Balasundram et al., 2006; Tsao, 2010). Besides, they could exist in complexed forms with proteins, carbohydrates, and the other insoluble high-MW phenolics (Herrero et al., 2012).
Table 2.
Extracted phenolic compounds from plant materials.
| Phenolic compounds | Examples of plant sources |
|---|---|
| Flavonoids | |
| Anthocyanins | Grape skins, red wine, grape seeds, fermented grape pomace, winery by-products, grapes, red and back-currants, strawberries, plums, raspberries, red cabbage, pomegranate |
| Chalcones | Apples |
| Flavanols | Grapes, apples, tomatoes, leeks, lettuces, curly kale, berries, onions, red grapes, beans, green and black, cider, tea, red wine and red winery by-products |
| Flavanones | Citrus juices, citrus fruits, seed wastes, orange peels |
| Flavonols | Apples, apple peels, beans, leeks, lettuce, onions, tomatoes, olive leafs, chestnut, olives and olive fermented pomaces |
| Flavones | Capsicum pepper, citrus fruits, spinach, celery, capsicum pepper |
| Isoflavones | Soy processing waste, soybeans, soymilk, soy flour |
| Stilbenes | Grape seeds, red grape fermented pomaces, grape skins, red grapes |
| Xanthones | Mango fruits and peels |
| Phenolic acids | |
| Hydroxycinnamic acids | Coffee, cherries, cereals, peaches, spinach, citrus juices and fruits, plums, tomatoes, rice flour, wheat flour, corn flour, olive mill wastewaters, potato, artichoke wastewaters and almonds |
| Hydroxybenzoic acids | Oilseeds, cereals, coffee, cowpea, wheat flour, black currant, raspberry, squash shells and seeds, blackberry |
| Tannins | |
| Condensed tannins | Pears, grapes, pears, apples, peaches, chestnut, hazelnuts |
| Hydrolyzable tannins | Pomegranates, raspberries |
Source: Alfredo (2017).
Therefore, phenolic compounds are often extracted from plant materials in a crude form, thereby demanding the modification of a given extraction method to ensure the removal of the unwanted interferences such as pigments, terpenes, fats, and wax. Solid-phase extraction methods, fractionation and purification based on acidity are usually the methods of choice for removing these interferences (Żwir-Ferenc and Biziuk, 2006), but despite the benefits of the technology improvements used for extracting polyphenol from natural origins, it must be certain that their extraction efficiency is a function of several critical parameters like solvent, the nature of the material, light, duration of extraction period, pH, temperature, material size, solvent/substrate ratio, as well as liquid-liquid or solid-liquid ratio (Herrero et al., 2012).
2.1. Flavonoids
All flavonoids have a similar structure as they consist of two aromatic rings which include A and B attached to 3C atoms to give an oxygenated heterocycle such as ring C. There are 6 subclasses of flavonoids according to the type of heterocycle involved. These include flavanones, anthocyanidins, flavonols, flavones, flavanols, and isoflavones.
2.1.1. Flavonols
These are the commonest type of flavonoids in foods, with myricetin, kaempferol and quercetin being the major representatives. They are found abundantly in leeks, broccoli, kale, and onions with the concentrations of up to 1.2 g/kg fresh wt (Herrero et al., 2012). Also, flavonols are abundant in tea and red wine in their glycosylated form with some simple sugars like glucose or rhamnose. The outer part of some fruits contains about 5–10 different flavonol glycosides (Kumar and Pandey, 2013). Observably, the concentration of flavonols in different fruits (even those from the same species) vary due to the variation in their biosynthesis in the presence of sunlight (Jeganathan et al., 2016). This is the case in leafy vegetables where the greener outer part of the leaves contains more flavonols compared to the inner pale coloured ones (José et al., 2015). Beverages such as apple and cranberry drinks are rich in flavonols (Boyer and Liu, 2004).
2.1.2. Flavones
The concentration of flavones in fruits and vegetables is less than that of flavonols. Flavones are glycosides of apigenin and luteolin with little reported sources, including sweet bell pepper, celery and parsley. They exist as glycosides of flavones in cereals such as wheat and millet; while in citrus skin, they exist as poly-methoxylated flavones including nobiletin, sinensetin, and tangeretin (Stuetz et al., 2010).
2.1.3. Isoflavones
This subclass of flavonoids exists as phytoestrogens owing to their affinity for estrogens receptors. Even though they are non-steroids, they contain OH- in positions 7 and 4’ in a configuration like estradiol. The basic difference between isoflavones and other flavonoids is the position of benzene ring B in C3. Leguminous plants such as processed soya are the essential sources of isoflavones in diets. Soybeans-sourced isoflavones contain three important molecules: glycitein, daidzein and genistein. These molecules exist majorly as malonyl or acetyl glycosides. Several isoflavonoids have been identified, soybeans were the most investigated plant source (Wang et al., 2013). The processing method is one factor that affects isoflavonoids content; for instance, fermentation can result in the generation of miso and tempeh while heating can elicit the hydrolysis of glycosides to aglycones that needs more heat.
2.1.4. Flavanones
Flavanones are the major flavonoids of citrus; they are found as hesperitin in oranges, eriodictyol in lemons and aglycones naringenin in grapefruit. They are majorly glycosylated in position 7 by a disaccharide, accounting for the bitter taste of some citrus skin. Between 40 and 140 mg flavanone glycosides can be found in one glass of orange juice (Kaur and Kaur, 2014). Being that the solid part of citrus fruit contains the highest flavanone content, the flavanone glycosides content of the whole fruit may be up to 5 times more. Besides, aromatic plants such as mint may contain flavanones.
2.1.5. Anthocyanins
These are the commonest studied flavonoids which exist as pigments in several foods, accounting for the purple, red, pink, or cyan colour of such foods. Structurally, it is a degradation product of the flavylium ion: These are heterosides of an aglycone unit. Different anthocyanins mainly vary in several aspects, including the position of these bonds, the nature and number of bonded sugars, aliphatic or the aromatic carboxylates attached to the sugar in the molecules, as well as the number of hydroxylated groups in the aglycone (Khoo et al., 2017).
The chemical characteristics of anthocyanins rely on their structures; hence, the structures of anthocyanins must be perfectly understood concerning their stability, reactivity, colour, and antioxidant activity. Anthocyanins can exist in different forms, such as aglycones, esterified with different organic acids, glycosylated with glucose at position 3, and phenolic acids. They stabilize by complexing with other flavonoids (Khoo et al., 2017). Anthocyanins are abundant in cereals, red wine and some root vegetables like onions, cabbage, beans, and others. However, they are generally seen in certain fruits such as cherries, red berries and pomegranates (Martín et al., 2017).
2.1.6. Flavanols (proanthocyanidins and catechins)
Flavanols occur either as catechins or proanthocyanidins. The A ring of the monomer undergoes several levels of hydroxylation on the 5th and 7th positions while the B ring undergoes the same on the 3′, 4′ and 5’ positions. The 3-position of the C ring is either esterified with gallic acid or contain an OH- group. Although catechins can be found in different fruits, they are majorly sourced from beverages like red wine, fruit juices, chocolate, and green tea (Arts et al., 2000). Flavanols found in food are not glycosylated as found in the other flavonoids. Proanthocyanidins or condensed tannins are varying flavonols bonded by C–C bond at either 4–6 or 4–8 such as B-type proanthocyanidins. Fruits including apples, grapes, pears, and kiwis are the main sources of condensed flavanols; they can also be sourced from tea, cocoa and others. Condensed flavanols account for the bitter taste of chocolates, astringency of beverages and fruit due to their capability to produce complexes with salivary proteins (Katz et al., 2011).
2.2. Stilbenes
Stilbenes exist in the human diet only in small amounts and mainly in the form of resveratrol which has been investigated severally for anticarcinogenic, anti-inflammatory characteristics, and cardio protective (Bahare et al., 2018). The identified sources of resveratrol are trees, few flowering plants, grapevines, and peanuts but the main dietary origins are wines, peanuts, grapes, and peanut products. In red wine, the resveratrol content contains 15 mg glycosides/L and 0.3–7 mg aglycones/L (Claudine et al., 2004).
2.3. Lignans
Lignans are produced inform of 2-phenylpropane units. They are phytoestrogens with reported estrogenic/anti-estrogenic activity. Their food sources include linseed (probably the richest source), dietary fibre, antioxidants, protein, oilseeds, vegetables, nuts, vegetables, garlic, fruits, olive oil, wine, tea, beer, and coffee in small amounts. Lignans exist in food as matairesinol or secoisolariciresinol, but in the human system as enterodiol or enterolactone. As per several studies, the benefits of lignans in regard to health depends on the exact kind of lignan (Durazzo et al., 2013).
3. Methods of extracting phenolic compounds
The updated outlines for the extraction techniques employed in determining the phenolic compounds in plant materials from conventional to unconventional methods are presented in this section. Conventional extraction methods are mostly designated by utilizing larger volume of extraction solvents and manual procedures that are mostly dependent on the investigator and labour-intensive; thus, the techniques are not ideally consistent (Alara et al., 2018a, Alara et al., 2018b). These methods which include solid-liquid extraction (SLE) or soxhlet extraction, liquid-liquid extraction (LLE) and maceration are the most utilized methods under this category. Since the conventional extraction methods suffer some drawbacks, it is however important to overcome these challenges; this brought about the use of unconventional extraction methods which have been generated with the purpose of filling the missing gaps of conventional methods; these methods include pressurized liquid extraction (PLE), subcritical water extraction (SWE), supercritical fluid extraction (SFE), microwave assisted extraction (MAE), solid phase extraction (SPE), ultrasounds assisted extraction (UAE), high hydrostatic pressure extraction (HHPE), solid-supported liquid-liquid extraction (SSLLE), matrix solid-phase dispersion (MSPD), and counter-current chromatography (CCC) (Shams et al., 2015). Some of the improved properties of unconventional techniques include automation, enhanced selectivity, higher extraction efficiency, and reduced consumption of extraction solvents (Alara et al., 2018a, Alara et al., 2018b; Azwanida, 2015).
3.1. Conventional extraction
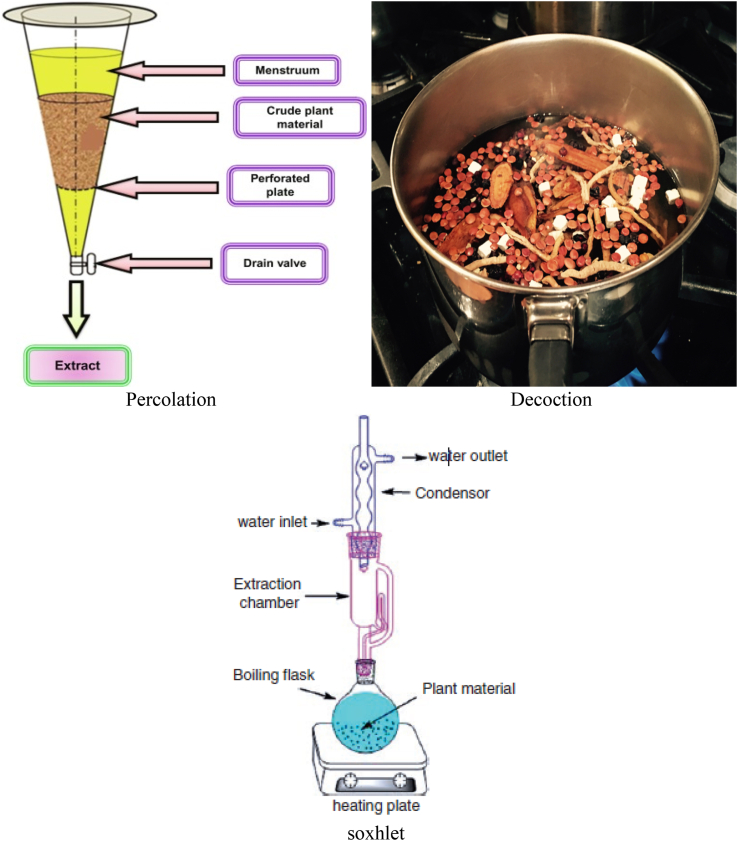
The conventional extraction techniques of phenolic compounds are maceration, decoction, percolation, infusion, digestion, serial exhaustive extraction, and soxhlet extraction (Fig. 4) (Alara et al., 2018a, Alara et al., 2018b; Kaufmann and Christen, 2002; Sticher, 2008). Currently, the maceration method is not commonly used due to the availability of other more feasible methods. Extraction by maceration is a simple process of soaking a pulverized sample in the appropriate solvent in a closed system, followed by constant or sporadic agitation at room temperature (Olejar et al., 2015; Sticher, 2008). After the extraction period, a separation process is applied to separate the solid parts from the solvent. This is usually achieved by either filtration, decantation, or clarification (Ćujić et al., 2016). Although this is an easy technique, it has the demerit of being time-consuming and requiring solvents in large volumes (Alara et al., 2018a, Alara et al., 2018b; Kaufmann and Christen, 2002; Sticher, 2008).
Fig. 4.
The pictorial representation of (a) percolation (b) decoction (c) soxhlet extraction.
Decoction technique involves boiling the plant samples for a shorter period of time or pouring boiled water over the plant samples and allow the mixture to stand for a certain duration of time. This method is mostly suitable for heat-stable and water-soluble phytochemicals from crude drugs. The percolation method is similar to the maceration as it involves placing the pulverized sample in a closed system and dropping of the solvent gradually from the top towards the bottom (Kaufmann and Christen, 2002; Sticher, 2008). Here, filtration is not required since the percolator devices are equipped with filters that can only allow solvent containing the extract to pass through. The problems of the percolation method are similar to those of maceration method (time-consuming, large solvent volumes) but in addition to the problem of solubility of the polyphenols, sample size, and extraction duration. Moreover, infusion is used for extracting volatile plant sample that can readily dissolve its phytochemicals in an organic solvent. This is simply done by macerating the plant sample using boiled or cold water for a shorter period of time and allowing it to steep in the solvent for a duration of time. Digestion technique is a modified maceration method involving the use of gentle heating provided the temperature does not affect the active phytochemicals in the plant sample. This technique is mostly employed for plant materials that contain polyphenolic compounds or poorly soluble materials. Additionally, serial exhaustive extraction entails the fractionation of crude extracts with solvent of higher polarity from hexane (a non-polar solvent) to butanol (a polar solvent) to achieve the extraction of wider range of phytochemicals. This technique cannot be utilized to extract thermolabile compounds due to the prolong heating (Alara and Abdurahman, 2019).
Regarding soxhlet extraction method (Alara et al., 2018a, Alara et al., 2018b; Luque de Castro and García-Ayuso, 1998), the pulverized samples are placed in timbles (made of cellulose) and positioned in the extraction chamber just over the collecting flask under a reflux condenser. Then, the solvent already added to the heating bottle is heated to produce vapour which will condense under cool running water and drop back into the timbles that hold the sample (Azwanida, 2015). The reflux is maintained severally; finally, the aqueous extract is obtained back from the heating flask. Being a continuous process, soxhlet extraction is advantageous because it requires less time and less solvent compared to percolation and maceration methods (Azwanida, 2015).
However, there is a need to handle the soxhlet extraction process carefully because reports have highlighted the influence of excess heat on the thermolabile polyphenols (Seidel, 2012). Another advantage of the soxhlet extraction method is its convenience (Azwanida, 2015). Despite the differences in these stated methods, they all involve the use of organic solvent at a given feed/liquid ratio. Among the generally utilized solvents for extracting polyphenols are methanol, water, chloroform, n-hexane, ethanol, propanol, ethyl acetate, and acetone (Zhang, 2018). These solvents differ in their polarity; hence, they have different influences on the extraction of phytochemicals. Organic solvents can easily mix; hence, they are considered when the aim is to improve extraction yield as suggested by several studies (Zhang, 2018).
Phytochemicals are mainly extracted using organic solvent and its aqueous formulation; however, there are still doubts regarding the most suitable solvent for polyphenols extraction. For instance, acetone has been proven efficient in polyphenols extraction from lychee flowers compared to methanol, water and ethanol (Liu et al., 2009). However, another study reported water as the better solvent for polyphenols extraction from walnut green husks (Fernández-Agulló et al., 2013). A recent study found aqueous and organic solvent to achieve better extraction efficiencies compared to absolute organic solvents (Metrouh-Amir et al., 2015). Similarly, aqueous methanol had been suggested as an adequate solvent for extracting polyphenols from Phoradendron californicumo oak extracts (Iloki-Assanga et al., 2015). The literature evidenced that there is no solvent generally acceptable as the best for extraction of polyphenols; nevertheless, it is generally believed that solvents of higher polarity often perform best in terms of polyphenols extraction because of high solubility of polyphenols in such solvents. It should be noted that a major influence that detects the solubility of solutes in solvents is the structure of the solute which refers to phenolic compounds in this context.
Because of the effect of several factors including the level of conjugation and presence of multiple hydroxyl groups on the extraction of polyphenols, it is necessary to prior test and adapts the best solvent prior to the main process. Therefore, the determination of the right solvent for the development of a standard method for all forms of polyphenols may be a difficult task even though it seems to conclude that solvent system that allows the maximization of the polyphenols yield without causing significant modification of targets’ chemical nature must be considered good. In this context, the selection of any solvent for extraction purposes must be based on the following factors: Solvent power, solvent polarity, boiling temperature of the solvent, solvents’ reactivity, solvents’ viscosity, solvents’ stability, safety concerns, legislature compatibility for food usages, and potential reusability.
3.2. Unconventional techniques of extraction
Most laboratories opt for conventional extraction methods due to their low cost and ease of use. Furthermore, studies have shown the environmental problems and low efficiency of the conventional methods including maceration, percolation and soxhlet extraction methods due to their requirement of large organic solvent volumes. The conventional extraction process often involves a recovery step which is followed by extract concentration via an evaporation process that is a time-consuming process. Finding the solution to these problems has elicited the development of several methods in the last years. Such methods include supercritical CO2 extraction (SC–CO2), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE), pressurized fluid extraction (PFE), or even a combination of these approaches. As per literature evidence, these novel extraction methods seem to be the methods of choice compared to the conventional methods as they offer numerous advantages which include less solvent volume, higher yields, reduced toxic residues, better process reproducibility, and less extraction time. The following subsections discussed these unconventional extraction techniques.
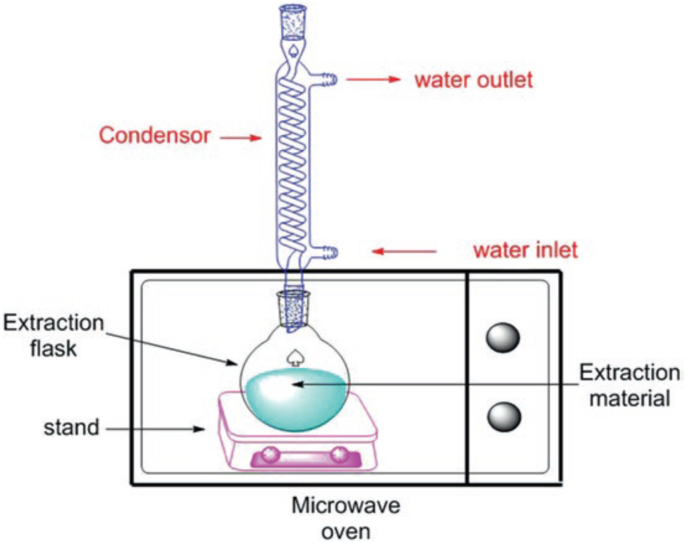
3.2.1. Microwave-assisted extraction (MAE)
Microwave-assisted extraction involves the use of microwave radiation energy to heat-up the solute-solvent mixture (Alara and Abdurahman, 2019b). The pictorial representation of MAE technique is presented in Fig. 5. The generated heat facilitates the solvents’ diffusivity into the sample to improve the diffusion of the target phytochemicals out of the sample (Alara et al., 2018a, Alara et al., 2018b; Kaufmann and Christen, 2002). The diffusion of the solvent through the sample increases the disruption of hydrogen bonds holding the sample, thereby allowing the target compounds to dissolve into the extraction fluid (Shams et al., 2015). The merits of the microwave-assisted extraction are less time-wasting and low solvent volumes (Alara et al., 2018a, Alara et al., 2018b). The MAE has been found useful in the extraction of short-chain polyphenols like phenolic acids and flavonoids; however, it is used sparingly when considering polymeric polyphenols such as anthocyanins and tannins due to the possibility of microwave-assisted extraction destroying polyphenols with several hydroxyl-type substituents and heat-sensitive ones such as anthocyanins. The extraction temperature during MAE is directly a function of the time and power (watts) but relates in an inverse proportion to the solvents’ sample mass and heat capacity (Pinela et al., 2016). Solvent diffusion and extraction kinetics are favoured when using higher temperatures and small sample volumes in microwave-assisted extraction process (Pinela et al., 2016).
Fig. 5.
Pictorial representation of MAE technique.
The MAE system has been used in the extraction of several phytochemicals, including polyphenols, where it seems to provide a good yield of polyphenols in less time and consuming fewer solvents. Nevertheless, the issues to consider when using this technique for polyphenols extraction include the type of material, solvent type and purity, power and time of microwave application, available sample surface area, as well as the operating temperature. The most critical factor is the type of solvent as its effects cut across the whole process, ranging from the solubility of the target components to the process efficiency. Hence, the solvent must be selected with care by considering both its affinity to the target compounds and its microwave energy absorption capability (Zhang et al., 2011). Transparent solvents such as hexane or dichloromethane may not be applicable in MAE due to their inability to heat up under microwave radiation. Other solvents such as methanol, ethanol, or water, with good microwave absorbing capacity can easily heat up, thereby reducing the length of microwave power application time and hence, should be used in the processes (Dudley et al., 2015). Besides, these solvents have no serious influence on thermolabile compounds.
3.2.2. Ultrasound-assisted extraction (UAE) method
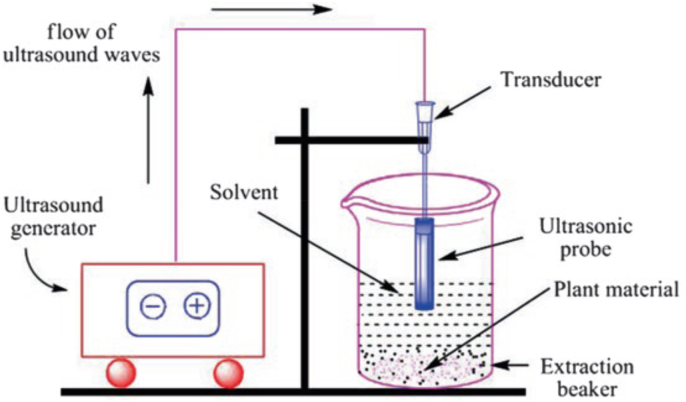
This type of method is an easy extraction technique that utilizes the induced mechanical influence through micro-sized bubbles explosion to give quick tissue disorganization which facilitates the diffusion of phytochemicals from substance into the solvent (Vinatoru, 2001). It is a simple and low-cost method which can be used in both small and large-scale settings (Shirzad et al., 2017). The past few years have witnessed an increased use of UAE for polyphenols extraction from different sources (Dahmoune et al., 2014; Shirzad et al., 2017; Yu et al., 2017). The experimental process generally requires the use of ultrasounds with a frequency range between 20 and 2000 kHz to increase cell wall permeability and produce cavitation. As per various reports, UAE ensures a faster and better extraction of polyphenols with minimized breakdown of compounds in relation to other techniques of extraction (Vinatoru, 2001). The pictorial representation of UAE technique is illustrated in Fig. 6.
Fig. 6.
Pictorial representation of UAE technique.
For instance, ultrasound-assisted extraction had been reported to be more effective in extracting rosmarinic and carnosic acid than that of the conventional extraction techniques (Zu et al., 2012). A recent report presented a maximum polyphenols extraction yield of 13.20 mg/g d. w. from spruce wood bark using UAE method (Ghitescu et al., 2015). Furthermore, the yield of anthocyanin from purple sweet potato was reported to be higher when the UAE was employed (Cai et al., 2016). A common thing among these reports is that the use of ultrasound-assisted extraction increases the rate of compounds solubility into the extraction solvent, thereby reducing the required solvent volume to achieve complete phytochemical recovery. Relying on these reports, this technique seems to be less expensive due to the following reasons: Involvement of lower solvent volume, higher sample volume tested, and requires lower extraction time. It is also agreed that the shorter sonication time and lower temperatures would enhance polyphenols extraction and preservation of the thermolabile compounds. Meanwhile, some reports have outlined that long periods of sonication greater than 40 min at a higher energy level that is above 20 kHz could seriously affect the extracted phytochemicals owing to the decreased rate of diffusion area/rate and increased diffusion distance (Annegowda et al., 2010; Wang et al., 2008). These conditions could facilitate the generation of unintended changes and free radicals in the extracted compounds (Wang et al., 2008).
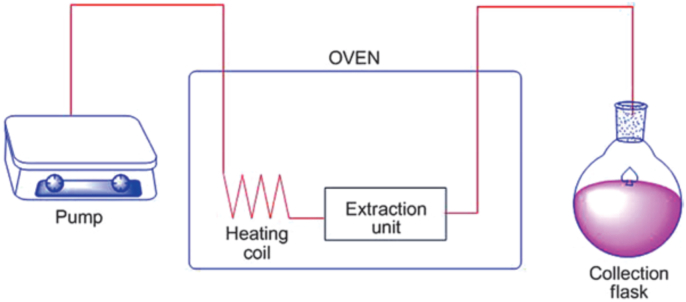
3.2.3. Pressurized liquid extraction (PLE)
The pressurized liquid extraction technique can also be called “accelerated solvent extraction (ASE)” (Fig. 7). It is one of the current techniques developed for extracting phytochemicals. It requires the use of high temperature and pressure (Nieto et al., 2010). High pressure ranging from 3.3 to 20.3 MPa is applied in combination with high temperature; ranging from 40 to 200 °C to facilitate the desorption and solubility molecules into solvents (Zhang et al., 2018). Nieto et al. approved that PLE is an advanced extraction method which ensures rapid extraction process using a few volume of solvents as compared to the conventional methods of extraction (Nieto et al., 2010). Besides, it encourages a better usage of water as extraction solvent such as the so-called subcritical water extraction due to the involvement of high temperature (Teo et al., 2010).
Fig. 7.
Pictorial representation of PLE technique.
At higher temperatures of around 200 °C, there will be a change in the dielectric properties of water, making it act as a standard organic solvent, thereby improving the capacity of the extraction (Plaza and Turner, 2015). Among the reported positives of the PLE as per various researchers include purity of the extracts compared with extracts from the classical methods. This eliminates the need for any purification step especially liquid chromatography mass spectrometry analysis (Sosa-Ferrera et al., 2013). However, the major limitations of PLE are low analytes selectivity during extraction, presence of interferents during the extraction process, high level of extracts dilution especially when using several numbers of cycles, and need for advanced instrumentation which is a costly process. Despite these limitations, pressurized liquid extraction is a common extraction method which had been utilized severally for extracting polyphenols via various sources (Erdogan et al., 2011; Liazid et al., 2014).
In order to improve the efficiency of a typical PLE process, some parameters must be optimized and the most important of these parameters is solvent selection. Even though the properties of any solvent can be modified under the elevated temperature used in PLE, the success of the process still depends on the solvent employed. Several solvents and their mixtures had been utilized for extracting phenolic component through several sources, but the most frequently used solvents are methanol, ethanol and their combination with water in various ratios. The decision on the combination of these solvents must be systematically taken as it has been proven that adequate selection of solvent may affect the level of extraction of several phenolics from the same sample because of the large distinct in the chemical composition of such components.
For instance, phenolic compounds extraction from parsley had been proven to be impossible due to the difficulty of finding a common solvent that will extract all the phenolic compounds in parsley (Maqsood et al., 2014). In fact, the water content of any extraction solvent has an impact on the compounds to be effectively extracted using such a solvent. Water acidification can improve the efficiency of phenolic compounds extraction if the chemical nature of such compounds is considered. This technique has been reportedly used to extract anthocyanin from the grape skin. Having carefully optimized the solvent selection by considering different percentages of acidified water, the complex solvent mixtures consisting of HCL, acetone, methanol, and water at the ratio of 0.1:40:40:20 was found to achieve the total acylated anthocyanins and maximum phenolic compounds recovery (Ju and Howard, 2003).
Similarly, the other parameters including time, temperature, sample packaging inside the extraction cell sample size, and solvent flow rate need to be optimized as they could influence the process. Although pressure as a parameter is believed to facilitate the rupture of the sample matrix and allow a better extraction experience; nevertheless, studies have proven that pressure has no significant influence on the extraction result if its value is highly sufficient in sustaining the solvent at liquid phase throughout the process of extraction.
The pressurized liquid extraction is usually combined with solid-phase extraction in an off-line or in-line mode to achieve better isolation of the target phenolic compounds. Regarding on-line coupling, it implies the positioning of solid phase within the extraction cells together with the targeted extracted sample but demarcated through a dispersing agent. This step ensures a better sample purity prior to chemical analysis. Several studies have employed the off-line and in-line PLE-SPE technique for extracting phenolic compounds (Plaza and Turner, 2015). Other on-line commercial platforms had been reported to be used in quantifying concentration of proanthocyanins in malt (Pritor and Liwei, 2005). This case requires the use of an automated device which will mediate the transfer of extracts from PLE collection bottle to SPE device.
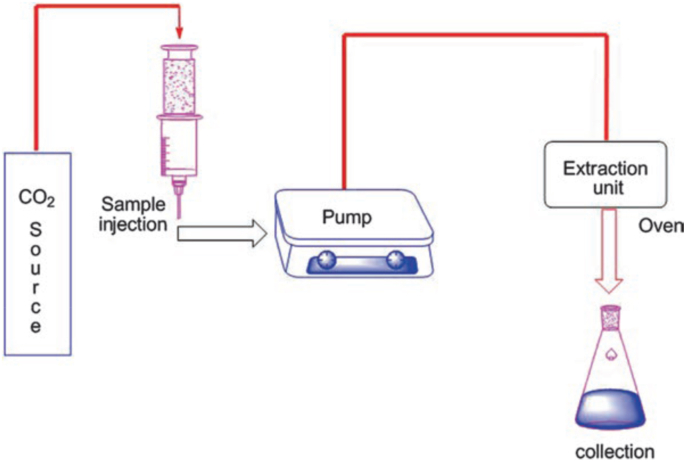
3.2.4. Supercritical CO2 extraction (SC–CO2)
This method requires the use of CO2 as a supercritical fluid (Fig. 8). This fluid is mostly used due to its non-toxicity, non-flammable, cost-effective, and high availability with high purity grade (Morgan, 2013). Under SC-CO2 extraction, different pressure-temperature combinations can be applied (Morgan, 2013), making this technique the method of choice for creating several end products. This possibility of several combinations permits the use of low pressure (7.386 MPa) and temperature (31.60 °C, the critical point of CO2), making SC-CO2 a common method in lab-scale facilities. The combination of low temperatures and pressure ensures the preservation of thermolabile phytochemicals. More advantages of SC-CO2 include: (i) more extraction capacity due to the higher mass transfer between the phases occasioned by decreased viscosity and increased diffusion coefficient of CO2 compared to the solvents; (ii) higher solvent penetration into the samples; (iii) possibility of different pressure-temperature combinations for better extraction conditions adaptability; (iv) recyclability of CO2 as the extraction process ends to ensure no harmful effect to the environment (De Zordi et al., 2014; Morgan, 2013) (Fig. 9).
Fig. 8.
Pictorial representation of SC-CO2 technique.
Fig. 9.
Pictorial representation of EAE technique.
There are limitations to this process, such as those related to the polarity of CO2. As a solvent of low polarity, CO2 is more suitable for the extraction of the non-polar compounds. However, its application can be extended to the other groups of compounds by introducing a small percentage of polar organic solvents into the extraction process to allow the extraction of the polar compounds such as polyphenols. Among the organic solvents suitable for use as co-solvents with CO2 include ethanol and ethyl lactate (Kooi-Yeong et al., 2017).
For instance, the maximum extraction of phenolic compounds from guava seeds had been achieved using supercritical CO2 with ethanol at a temperature of 60 °C and pressure of 100 bar (Tyśkiewicz et al., 2018). The typical percentage of ethanol when used as a co-solvent with CO2 usually ranges from 5 to 15%. Therefore, it is important to carefully optimize this parameter to obtain the best compounds recovery rate. Statistical evidence has shown the percentage and type of co-solvent as the second most vital parameters when extracting polyphenols from pomegranate seeds oil (Zam et al., 2012). The study also used water, ethanol, and hexane as modifiers, but the comparison proved that the co-solvent’s polarity must be reasonably increased to ensure adequate recovery of polyphenols. Therefore, the selection of a suitable co-solvent could be said to depend mainly on the concentration and type of phenolic components contained within the sample; hence, these parameters require careful consideration in relative to the percentage and type of co-solvent for individual process.
Having selected the extraction based on the co-solvent and solvent, the other parameters may require to be optimized, this includes temperature of the process. For example, different pressure (100–400 bar) and temperature (35–55 °C) ranges had been investigated on resveratrol extraction from grapes pomace using 5% ethanol as a modifier (Casas et al., 2010). From the result, a combination of the highest pressure with the lowers temperature achieved the best extraction performance. Thus, it is necessary to use chemometrics and statistics such as the experimental-based approaches for this kind of application. Using these tools will ensure the coverage of a wide range of experimental parameters to establish the best conditions with the least experimental work.
3.2.5. Enzyme-assisted extraction (EAE)
This is a current technique which exploits the capability of the enzymes to breakdown cell wall compartment to ensure the movement of the cytoplasmic content into the extraction fluid such as water (Puri et al., 2012). The enzymes employed during EAE are capable of breaking and weakening cell walls to expose their cellular content to extraction (Gómez-García et al., 2012; Swer et al., 2016). This ensures a better chance of extracting polyphenols from such samples. In fact, most plant phytochemicals are bonded to lignin which is a tough plant cell wall component via hydrogen or hydrophobic bonds, making them conventionally inaccessible (Gómez-García et al., 2012). Therefore, an enzymatic pre-treatment process will be required to facilitate the effective release of such bonded phytochemicals.
Part of these enzymes is utilized to facilitate the extraction of phytochemical components from plant materials include cellulases, hemicellulases, pectinases, and others. They can be utilized to hydrolyze the cell wall lignin to promote extraction efficiency. The positive influence of EAE on polyphenol extraction has been reported severally. A report on the extraction of polyphenols from grape wastes showed a strong performance with respect to polyphenols yield when celluclast®, pectinex®, and novoferm® enzymes were employed as pretreatment enzymes (Gómez-García et al., 2012). Other studies have reported a similar trend by suggesting that EAE ought to be considered an alternative technique for better extraction of carbohydrates-bonded phenolics from wine-making wastes (de Camargo et al., 2016). These studies observed the activity of the enzymes in degrading cell wall components to improve the extraction efficiency of bioactive compounds. Additionally, this method has been identified as an eco-friendly method owing to its use of water as a solvent rather than organic solvents. Besides, it is one of the recent extraction techniques that are receiving much interest due to the recent campaign for the adoption of eco-friendly laboratory techniques.
3.2.6. Combined techniques
Some situations may require a combination of different extraction methods to improve the extraction of phytochemicals. This is usually the case when a single extraction technique is not enough to completely extract the target phytochemicals from the source material. Hence, an integration of different extraction methods might be adequate approach towards extracting such phytochemicals at a higher extraction efficiency.
4. Conclusion
This review has discussed phenolic compounds and different extraction methods employed in extracting them. The conventional extraction techniques widely employed for extracting phenolic compounds from plant materials are soxhlet, percolation and maceration. Even though these techniques are still being used, they suffer some setbacks which include recovery of limited yields, consumption of a higher amount of extraction solvents, longer time of extraction, and enormous accumulation of residues. These had stimulated the emergence of unconventional techniques which include, MAE, UAE, SC-CO2, EAE, and PLE to overcome the drawbacks from conventional techniques. Due to the importance of phenolic compounds to humanity, the demand for new bioactive compounds will continue to encourage the search for innovative extraction techniques to achieve appreciable recovery yields from the plant materials.
CRediT authorship contribution statement
Oluwaseun Ruth Alara: Conceptualization, Methodology, Data curation, Resources, Writing – original draft. Nour Hamid Abdurahman: Visualization, Supervision. Chinonso Ishamel Ukaegbu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alara O.R., Abdurahman N.H. Kinetics studies on effects of extraction techniques on bioactive compounds from Vernonia cinerea leaf. J. Food Sci. Technol. 2019;56:580–588. doi: 10.1007/s13197-018-3512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alara O.R., Abdurahman N.H. GC-MS and FTIR analyses of oils from Hibiscus sabdariffa, Stigma maydis and Chromolaena odorata leaf obtained from Malaysia: potential sources of fatty acids. Chem. Data Collect. 2019;100200 doi: 10.1016/j.cdc.2019.100200. [DOI] [Google Scholar]
- Alara O.R., Abdurahman N.H. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: kinetic modelling and process intensification. Ind. Crop. Prod. 2019;137:528–535. doi: 10.1016/j.indcrop.2019.05.053. [DOI] [Google Scholar]
- Alara O.R., Abdurahman N.H., Ukaegbu C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants. 2018:1–6. doi: 10.1016/j.jarmap.2018.07.003. [DOI] [Google Scholar]
- Alara O.R., Abdurahman N.H., Ukaegbu C.I., Azhari N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018;122:533–544. doi: 10.1016/j.indcrop.2018.06.034. [DOI] [Google Scholar]
- Alfredo A. Phenolic Compounds. 2017. Phenolics in foods: extraction, analysis and measurements; pp. 61–88. [DOI] [Google Scholar]
- Annegowda H.V., Anwar L.N., Mordi M.N., Ramanathan S., Mansor S.M. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacogn. Res. 2010;2:368–373. doi: 10.4103/0974-8490.75457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts I.C., van De Putte B., Hollman P.C. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food Chem. 2000;48:1752–1757. doi: 10.1021/jf000026%2B. [DOI] [PubMed] [Google Scholar]
- Azwanida N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromatic Plants. 2015:3–8. doi: 10.4172/2167-0412.1000196. 04. [DOI] [Google Scholar]
- Bahare S., Abhay P.M., Manisha N., Bilge S., Mehtap K., Mehdi S.-R., Patrick V., Tsouh F., Natália M., Javad S.-R. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018;6:91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Bohlin L. Natural products isolation. Drug Discov. Today. 1998 doi: 10.1016/s1359-6446(98)01266-5. [DOI] [Google Scholar]
- Boyer J., Liu R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Qu Z., Lan Y., Zhao S., Ma X., Wan Q., Jing P., Li P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016;197(Part A):266–272. doi: 10.1016/j.foodchem.2015.10.110. [DOI] [PubMed] [Google Scholar]
- Casas L., Mantell C., Rodríguez M., Martínez de la Ossa E.J., Roldán A., DeOry I., Caro I., Blandino A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010;96:304–308. doi: 10.1016/j.jfoodeng.2009.08.002. [DOI] [Google Scholar]
- Cheynier V. Phenolic compounds: from plants to foods. Phytochemistry Rev. 2012;11:153–177. doi: 10.1007/s11101-012-9242-8. [DOI] [Google Scholar]
- Claudine M., Augustin S., Christine M., Christian R., Liliana J. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:1–9. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćujić N., Šavikin K., Janković T., Pljevljakušić D., Zdunić G., Ibrić S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016;194:135–142. doi: 10.1016/j.foodchem.2015.08.008. [DOI] [PubMed] [Google Scholar]
- D’Archivio M., Filesi C., Di Benedetto R., Gargiulo R., Giovannini C., Masella R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- Dahmoune F., Spigno G., Moussi K., Remini H., Cherbal A., Madani K. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014;61:31–40. doi: 10.1016/j.indcrop.2014.06.035. [DOI] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Camargo A.C., Regitano-d’Arce M.A., Biasoto A.C., Shahidi F. Enzyme-assisted extraction of phenolics from winemaking by-products: antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016;212:395–402. doi: 10.1016/j.foodchem.2016.05.047. [DOI] [PubMed] [Google Scholar]
- De Zordi N., Cortesi A., Kikic I., Moneghini M., Solinas D., Innocenti G., Portolan A., Baratto G., Dall’Acqua S. The supercritical carbon dioxide extraction of polyphenols from propolis: a central composite design approach. J. Supercrit. Fluids. 2014;95:491–498. doi: 10.1016/j.supflu.2014.10.006. [DOI] [Google Scholar]
- Dudley G.B., Richert R., Stiegman A.E. On the existence of and mechanism for microwave specific reaction rate enhancement. Chem. Sci. 2015;6:2144–2152. doi: 10.1039/c4sc03372h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A., Zaccaria M., Polito A., Maiani G., Carcea M. Lignan content in cereals, buckwheat and derived foods. Foods. 2013;2:53–63. doi: 10.3390/foods2010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan S., Ates B., Durmaz G., Yilmaz I., Seckin T. Pressurized liquid extraction of phenolic compounds from Anatolia propolis and their radical scavenging capacities. Food Chem. Toxicol. 2011;49:1592–1597. doi: 10.1016/j.fct.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Fernández-Agulló A., Pereira E., Freire M.S., Valentão P., Andrade P.B., González-Álvarez J., Pereira J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crop. Prod. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- Forni C., Facchiano F., Bartoli M., Pieretti S., Facchiano A., D’Arcangelo D., Norelli S., Valle G., Nisini R., Beninati S., Tabolacci C., Jadeja R.N. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019:1–16. doi: 10.1155/2019/8748253. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu R.-E., Volf I., Carausu C., Bühlmann A.-M., Gilca I.A., Popa V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015;22 doi: 10.1016/j.ultsonch.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Gómez-García R., Martínez-Ávila G.C.G., Aguilar C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. Biotec. 2012;3(2):297–300. doi: 10.1007/s13205-012-0055-7. [DOI] [Google Scholar]
- Herrero M., Plaza M., Cifuentes A., Ibáñez E. Extraction techniques for the determination of phenolic compounds in food. Compr. Sampl. Sample Prep. 2012;4:159–180. doi: 10.1016/B978-0-12-381373-2.00132-0. [DOI] [Google Scholar]
- Hollman P.C.H., van Trijp J.M.P., Buysman M.N.C.P., vd Gaag M.S., Mengelers M.J.B., de Vries M.B., Katan J.H.M. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- Iloki-Assanga S.B., Lewis-Luján L.M., Lara-Espinoza C.L., Gil-Salido A.A., Fernandez-Angulo D., Rubio-Pino J.L., Haines D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes. 2015;8:1–14. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan, Nimal Punyasiri B.P.A., Kottawa-Arachchi J.D., Mahasen A.B.R., Abeysinghe I.S.B., Gunasekare M.T.K., Bandara B.M.R. Genetic variation of flavonols quercetin, myricetin, and kaempferol in the Sri Lankan tea (Camellia sinensis L.) and their health-promoting aspects. Int. J. Food Sci. 2016;2016:6057434. doi: 10.1155/2016/6057434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- José C.V., Ma L.B., Inés C., Justen B.W., Eduardo N. On flavonoid accumulation in different plant parts: variation patterns among individuals and populations in the shore campion (Silene littorea) Front. Plant Sci. 2015;6:939. doi: 10.3389/fpls.2015.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Z.Y., Howard L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003;51:5207–5213. doi: 10.1021/jf0302106. [DOI] [PubMed] [Google Scholar]
- Katz D.L., Doughty K., Ali A. Cocoa and chocolate in human health and disease. Antioxidants Redox Signal. 2011;15:2779–2811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann B., Christen P. Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002;13:105–113. doi: 10.1002/pca.631. [DOI] [PubMed] [Google Scholar]
- Kaur H., Kaur G. A critical appraisal of solubility enhancement techniques of polyphenols. J. Pharm. (Lahore) 2014:1–14. doi: 10.1155/2014/180845. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanbabaee K., van Ree T. Tannins: classification and definition. Nat. Prod. Rep. 2001;18:641–649. doi: 10.1039/B101061L. [DOI] [PubMed] [Google Scholar]
- Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooi-Yeong K., Marie-Odile P., Paul Nicholas S., James R.F. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules. 2017;22:1186. doi: 10.3390/molecules22071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liazid A., Barbero G., Azaroual L., Palma M., Barroso C. Stability of anthocyanins from red grape skins under pressurized liquid extraction and ultrasound-assisted extraction conditions. Molecules. 2014;19:21034. doi: 10.3390/molecules191221034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.-C., Lin J.-T., Wang C.-K., Chen H.-Y., Yang D.-J. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009;114:577–581. doi: 10.1016/j.foodchem.2008.09.088. [DOI] [Google Scholar]
- Luque de Castro M.D., García-Ayuso L.E. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal. Chim. Acta. 1998;369:1–10. doi: 10.1016/S0003-2670(98)00233-5. [DOI] [Google Scholar]
- Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Maqsood S., Benjakul S., Abushelaibi A., Alam A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: a detailed review. Compr. Rev. Food Sci. Food Saf. 2014;13:1125–1140. doi: 10.1111/1541-4337.12106. [DOI] [Google Scholar]
- Martín J., Navas M.J., Jiménez-Moreno A.M., Asuero A.G. Phenolic Compounds - Natural Sources, Importance and Applications. 2017. Anthocyanin pigments: importance, sample preparation and extraction. [DOI] [Google Scholar]
- Metrouh-Amir H., Duarte C.M.M., Maiza F. Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crop. Prod. 2015;67:249–255. doi: 10.1016/j.indcrop.2015.01.049. [DOI] [Google Scholar]
- Morgan E.D. Molecular Sciences and Chemical Engineering. Elsevier; Netherlands: 2013. Natural products|supercritical fluid chromatography. Reference Module in Chemistry. [Google Scholar]
- Naumann H.D., Tedeschi L.O., Zeller W.E., Huntley N.F. The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Rev. Bras. Zootec. 2017;46:929–949. doi: 10.1590/s1806-92902017001200009. [DOI] [Google Scholar]
- Nieto A., Borrull F., Pocurull E., Marcé R.M. Pressurized liquid extraction: a useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trends Anal. Chem. 2010;29:752–764. doi: 10.1016/j.trac.2010.03.014. [DOI] [Google Scholar]
- Olejar K.J., Fedrizzi B., Kilmartin P.A. Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon blanc wine. Food Chem. 2015;183:181–189. doi: 10.1016/j.foodchem.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Pinela J., Prieto M.A., Carvalho A.M., Barreiro M.F., Oliveira M.B.P.P., Barros L., Ferreira I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: a nutraceutical-oriented optimization study. Separ. Purif. Technol. 2016;164:114–124. doi: 10.1016/j.seppur.2016.03.030. [DOI] [Google Scholar]
- Plaza M., Turner C. Pressurized hot water extraction of bioactives. Trends Anal. Chem. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- Potì F., Santi D., Spaggiari G., Zimetti F., Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int. J. Mol. Sci. 2019;20:351. doi: 10.3390/ijms20020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R.L., Liwei G. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry. 2005;66:2264–2280. doi: 10.1016/j.phytochem.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Puri M., Sharma D., Barrow C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012;30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Robards K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A. 2003;1000:657–691. doi: 10.1016/s0021-9673(03)00058-x. [DOI] [PubMed] [Google Scholar]
- Seidel V. Natural Products Isolation, Methods in Molecular Biology. 2012. Initial and bulk extraction of natural products isolation; pp. 27–42. [DOI] [PubMed] [Google Scholar]
- Selvamuthukumaran M., Shi J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017;1:61–81. doi: 10.1093/fqsafe/fyx004. [DOI] [Google Scholar]
- Shah S.R., Ukaegbu C.I., Hamid H.A., Alara O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018 doi: 10.1007/s11694-018-9810-8. 0, 0. [DOI] [Google Scholar]
- Shams K.A., Abdel-azim N.S., Saleh I.A., Hegazy M.F., El-missiry M.M., Hammouda F.M., Bohouth E., Tahrir E. Green technology: economically and environmentally innovative methods for extraction of medicinal & aromatic plants (MAP) in Egypt. J. Chem. Pharmaceut. Res. 2015;7:1050–1074. [Google Scholar]
- Shirzad H., Niknam V., Taheri M., Ebrahimzadeh H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: a nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017;54:2361–2371. doi: 10.1007/s13197-017-2676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Ferrera Z., Mahugo-Santana C., Santana-Rodríguez J.J. Analytical methodologies for the determination of endocrine disrupting compounds in biological and environmental samples. BioMed Res. Int. 2013;23 doi: 10.1155/2013/674838. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher O. Natural product isolation. Nat. Prod. Rep. 2008;25:517–554. doi: 10.1039/B700306B. [DOI] [PubMed] [Google Scholar]
- Stuetz W., Prapamontol T., Hongsibsong S., Biesalski H.K. Polymethoxylated flavones, flavanone glycosides, carotenoids, and antioxidants in different cultivation types of tangerines (Citrus reticulata Blanco cv. Sainampueng) from Northern Thailand. J. Agric. Food Chem. 2010;26:6069–6074. doi: 10.1021/jf904608h. [DOI] [PubMed] [Google Scholar]
- Suwal S., Marciniak A. Technologies for the extraction, separation and purification of polyphenols – a review. Nepal J. Biotechnol. 2018;6:74–91. doi: 10.3126/njb.v6i1.22341. [DOI] [Google Scholar]
- Swer T.L., Chauhan K., Paul P.K., Mukhim C. Evaluation of enzyme treatment conditions on extraction of anthocyanins from Prunus nepalensis L. Int. J. Biol. Macromol. 2016;92:867–871. doi: 10.1016/j.ijbiomac.2016.07.105. [DOI] [PubMed] [Google Scholar]
- Teo C.C., Tan S.N., Yong J.W.H., Hew C.S., Ong E.S. Pressurized hot water extraction (PHWE) J. Chromatogr. A. 2010;1217:2484–2494. doi: 10.1016/j.chroma.2009.12.050. [DOI] [PubMed] [Google Scholar]
- Tiago O., Maicon N., Ivan R.C., Diego N.F., Vinícius J.S., Mauricio F., Alan J.P., Velci Q.S. Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: a review. Afr. J. Agric. Res. 2017;12:71–84. doi: 10.5897/AJAR2016.11677. [DOI] [Google Scholar]
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyśkiewicz K., Konkol M., Rój E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules. 2018;23:2625. doi: 10.3390/molecules23102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D., Rodriguez-Mateos A., Corona G., Oruna-Concha M.J., Spencer J.P.E. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8:303–313. doi: 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Wang L., Li D., Bao C., You J., Wang Z., Shi Y., Zhang H. Ultrasonic extraction and separation of anthraquinones from. Rheum palmatum L. Ultrason. Sonochem. 2008;15:738–746. doi: 10.1016/j.ultsonch.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Wang Q., Ge X., Tian X., Zhang Y., Zhang J., Zhang P. Soy isoflavone: the multipurpose phytochemical (Review) Biomed. Rep. 2013;1:697–701. doi: 10.3892/br.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Li C., Duan Z., Liu B., Duan W., Shang F. Ultrasonic microwave-assisted extraction of polyphenols, flavonoids, triterpenoids, and Vitamin C from Clinacanthus nutans. Czech J. Food Sci. 2017;35:89–94. doi: 10.17221/82/2016-CJFS. [DOI] [Google Scholar]
- Zam W., Bashour G., Abdelwahed W., Khayata W. Effective extraction of polyphenols and proanthocyanidins from Pomegranate’s peel. Int. J. Pharm. Pharmaceut. Sci. 2012;4:675–682. [Google Scholar]
- Zhang H.-F., Yang X.-H., Wang Y. Microwave assisted extraction of secondary metabolites from plants: current status and future directions. Trends Food Sci. Technol. 2011;22:672–688. doi: 10.1016/j.tifs.2011.07.003. [DOI] [Google Scholar]
- Zhang Q., Lin L., Ye W. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu G., Zhang R., Yang L., Ma C., Zu Y., Wang W., Zhao C. Ultrasound-assisted extraction of carnosic acid and rosmarinic acid using ionic liquid solution from Rosmarinus officinalis. Int. J. Mol. Sci. 2012;13:11027. doi: 10.3390/ijms130911027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żwir, Ferenc A., Biziuk M. Solid phase extraction technique – trends, opportunities and applications. Pol. J. Environ. Stud. 2006;15:677–690. [Google Scholar]