Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the global COVID-19 pandemic. Rapidly-spreading SARS-CoV-2 variants may jeopardize newly-introduced antibody and vaccine countermeasures. Here, using monoclonal antibodies (mAbs), animal immune sera, human convalescent sera, and human sera from recipients of the BNT162b2 mRNA vaccine, we report the impact on antibody neutralization of a panel of authentic SARS-CoV-2 variants including a B.1.1.7 isolate, chimeric strains with South African or Brazilian spike genes, and isogenic recombinant viral variants. Many highly neutralizing mAbs engaging the receptor binding domain (RBD) or N-terminal domain (NTD), and most convalescent sera and mRNA vaccine-induced immune sera showed reduced inhibitory activity against viruses containing an E484K spike mutation. As antibodies binding to spike RBD and NTD demonstrate diminished neutralization potency in vitro against some emerging variants, updated mAb cocktails targeting highly conserved regions, enhancement of mAb potency, or adjustments to the spike sequences of vaccines may be needed to prevent loss of protection in vivo.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the global COVID-19 pandemic infecting more than 111 million people and causing 2.4 million deaths. Clinical disease in humans ranges from asymptomatic infection to pneumonia, severe respiratory compromise, multi-organ failure, and systemic inflammatory syndromes. The rapid expansion and prolonged nature of the COVID-19 pandemic and its accompanying morbidity, mortality, and destabilizing socioeconomic effects have made the development of SARS-CoV-2 therapeutics and vaccines an urgent global health priority1. Indeed, the emergency use authorization and rapid deployment of antibody-based countermeasures including monoclonal antibodies, immune plasma therapy, and mRNA, inactivated, and viral-vectored vaccines has provided hope for curtailing disease and ending the pandemic.
The spike protein of the SARS-CoV-2 virion binds the cell-surface receptor angiotensin-converting enzyme 2 (ACE2) to promote entry into human cells2. Because the spike protein is critical for viral entry, it has been targeted for vaccine development and therapeutic antibody interventions. SARS-CoV-2 S proteins are cleaved to yield S1 and S2 fragments. The S1 protein includes the N-terminal (NTD) and receptor binding (RBD) domains, whereas the S2 protein promotes membrane fusion. The RBD is recognized by many potently neutralizing monoclonal antibodies3–7, protein-based inhibitors8, and serum antibodies9.
The current suite of antibody therapeutics and vaccines was designed with a spike protein based on strains circulating during the early phases of the pandemic in 2020. More recently, variants with enhanced transmissibility have emerged in the United Kingdom (B.1.1.7), South Africa (B.1.351), Brazil (B.1.1.248) and elsewhere with multiple substitutions in the spike protein including in the NTD and the receptor binding motif (RBM) of the RBD. Preliminary studies with pseudoviruses suggest that neutralization by some antibodies and immune sera may be diminished against variants expressing mutations in the spike gene10–13. Given these concerns, here, we evaluated the neutralizing activity in cell culture of monoclonal and serum-derived polyclonal antibodies against a panel of authentic, infectious SARS-CoV-2 variants including a B.1.1.7 isolate, chimeric Washington strains with a South African (Wash SA-B.1.351) or Brazilian (Wash BR-B.1.1.248) spike gene, and isogenic recombinant variants with designed mutations or deletions at positions 69–70, 417, 484, 501, 614, and/or 681 of the spike protein. Our data show moderate to substantially diminished neutralizing potency of antibodies and sera against chimeric SARS-CoV-2 strains or isogenic variants containing a mutation at position 484.
RESULTS
To evaluate the effects of SARS-CoV-2 strain variation on antibody neutralization, we obtained or generated a panel of authentic infectious SARS-CoV-2 strains with sequence variations in the spike gene (Fig 1a–c). A B.1.1.7 isolate had signature changes in the spike gene14 including the 69–70 and 144–145 deletions, and N501Y, A570D, D614G, and P681H substitutions. We created a chimeric, fully-infectious SARS-CoV-2 with a South African spike gene (Wash SA-B.1.351; D80A, 242–244 deletion, R246I, K417N, E484K, N501Y, D614G, and A701V) and a panel of isogenic spike mutants (D614G, K417N/D614G, E484K/D614G, N501Y/D614G, P681H/D614G, del69–70/N501Y/D614G, E484K/N501Y/D614G, and K417N/E484K/N501Y/D614G) in the Washington strain background (2019n-CoV/USA_WA1/2020 [WA1/2020]). Recombinant viruses and B.1.1.7 were propagated in Vero-TMPRSS2 and Vero-hACE2-TMPRSS2 cells expressing transmembrane protease serine 2 (TMPRSS2) and human angiotensin converting enzyme 2 (hACE2) to prevent the development of adventitious mutations in the spike, especially at or near the furin cleavage site, which accumulate rapidly in Vero E6 cells15 and can impact entry pathways and virulence16. All viruses were used at low passage (p0 or p1) and deep sequenced to confirm the presence of expected mutations and an absence of cell type-dependent adaptations (Supplementary Table S1).
Figure 1. Neutralization of SARS-CoV-2 viral variants by mAbs.
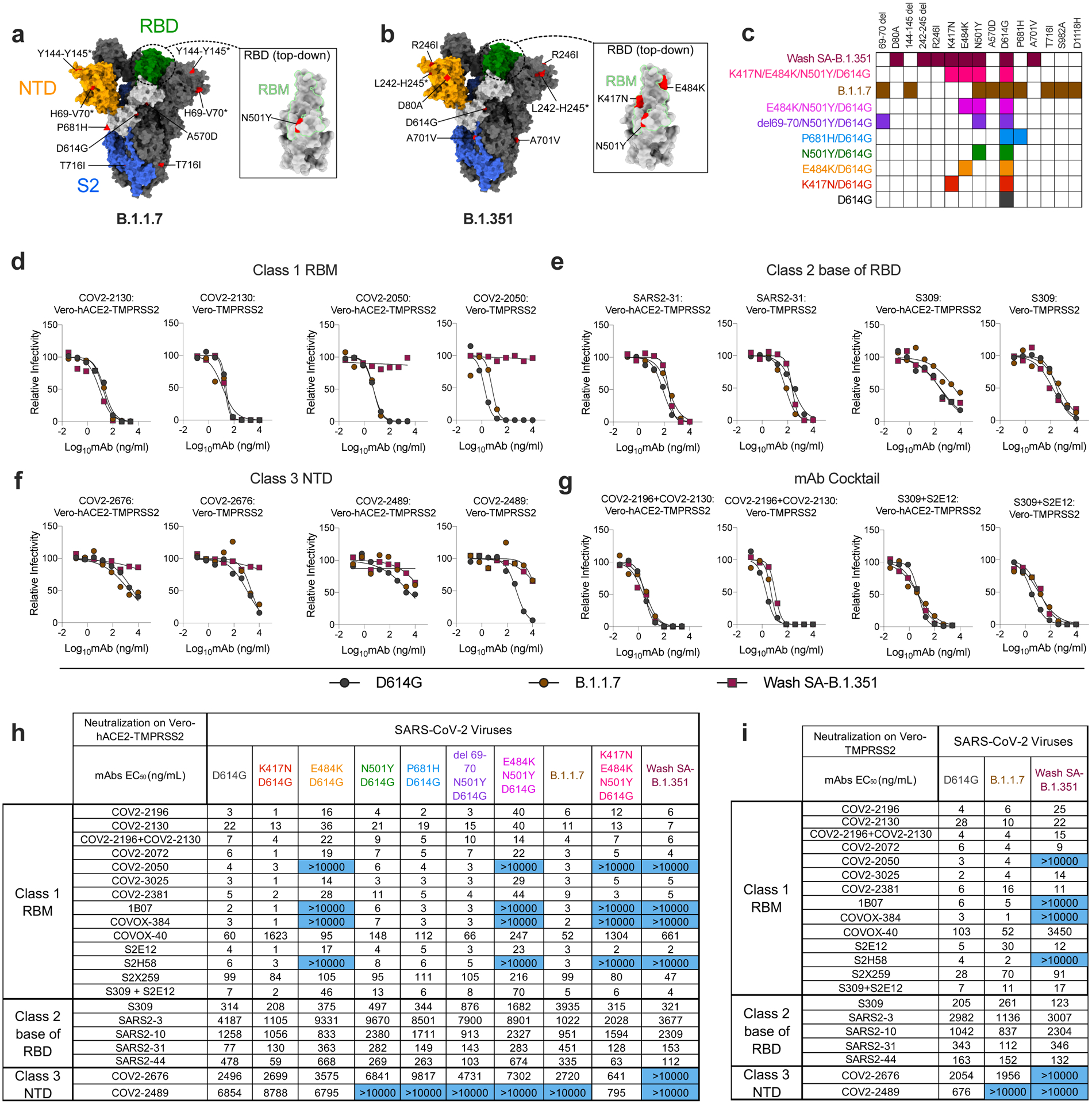
a-b, SARS-CoV-2 spike trimer. One protomer is highlighted, showing the NTD in orange, RBD in green, and S2 portion of the molecule in blue, with N- and C-termini annotated. a, Substitutions in the B.1.1.7 variant (69–70 deletion, 144–145 deletion, A570D, D614G, P681H, and T716I) are shaded in red. Red triangle depicts approximate location of P681H, which was not included in the model. Inset shows a top-down view of the RBD showing the location of the N501Y mutation contextualized with the receptor-binding motif (RBM). b, Substitutions in the Wash-SA B.1.135 variant (242–244 deletion, D80A, R246I, D614G, and A701V) are shaded in red. The red diamond denotes approximate location of D80A, which is buried in this view. Inset shows top-down view of the RBD with Wash SA-B.1.351 substitutions K417N, E484K, and N501Y shaded red and contextualized with the receptor binding motif. For all panels, structures depicting spike protein were modeled using PDB: 7C2L. Structures depicting RBD were modeled using PDB: 6W41. All analyses and figures were generated with UCSF ChimeraX47. c, Viruses with indicated spike mutations. d-f, Neutralization curves in (left panels) Vero-hACE2-TMPRSS2 cells or (right panels) Vero-TMPRSS2 cells comparing the sensitivity of SARS-CoV-2 strains with class 1 (d, COV2–2130 and COV-2150), class 2 (e, SARS2–31 and S309), and class 3 (f, COV2–2676 and COV2–2489) mAbs and indicated viruses. Also shown are the neutralization curves for antibody cocktails (g, COV2–2196 + COV2–2130 and S309 + S2E12). One representative experiment of two performed in technical duplicate is shown. h-i, Summary of EC50 values (ng/ml) of neutralization of SARS-CoV-2 viruses propagated on the indicated cells and performed in Vero-hACE2-TMPRSS2 (h) or Vero-TMPRSS2 (i) cells. Blue shading of cells shows virtually complete loss of neutralizing activity: EC50 > 10,000 ng/mL.
We tested our panel of viruses for antibody-mediated neutralization in Vero-hACE2-TMPRSS2 cells and then repeated some experiments with Vero-TMPRSS2 cells to evaluate for effects of hACE2 over-expression on neutralization17. We performed high-throughput focus reduction neutralization tests (FRNTs)18 using a panel of neutralizing mAbs recognizing distinct and overlapping epitopes in the RBD including some having potential use in humans. Class 1 antibodies (e.g., COV2–2196, COV2–2072, COV2–2050, COV2–2381, COV2–2130, COVOX-384, COVOX-40, 1B07, S2E12, S2H58, and S2X259) are potently neutralizing, block soluble hACE2 binding, and bind multiple proximal sites in the receptor binding motif (RBM) of the RBD as determined by structural or escape mutation analyses (Extended Data Fig 1a)5,7,19,20; class 2 neutralizing antibodies (e.g., S309, SARS2–3, SARS2–10, SARS2–31, SARS2–44) often cross-react with SARS-CoV, bind the base of the RBD (Extended Data Fig 1b), and variably block hACE2 binding (3 and L. VanBlargan and M. Diamond, unpublished results); and class 3 neutralizing mAbs (e.g., COV2–2676 and COV2–2489) recognize the N-terminal domain (NTD) (Extended Data Fig 1c)21.
Initially, we performed neutralization tests with WA1/2020 D614G and the two Vero cell types (Fig 1d–i and Extended Data Fig 2). With the D614G strain, neutralization by the majority of class 1 and class 2 mAbs was similar in Vero-hACE2-TMPRSS2 and Vero-TMPRSS2 cells. However, NTD-reactive mAbs showed greater potency (up to 13-fold) and more complete neutralization on Vero-TMPRSS2 than Vero-hACE2-TMPRSS2 cells (Fig 1f). Given that the expression of hACE2 on recipient Vero cells can impact the neutralizing activity of mAbs binding outside of the RBD, we performed subsequent studies with both cell types.
We next assessed the impact of spike protein mutations on mAb neutralization in Vero-hACE2-TMPRSS2 cells (Fig 1h) and Vero-TMPRSS2 cells (Fig 1i). We observed the following patterns with the variant viruses: (a) The P681H mutation (in the C-terminal region of S1) and the 69–70 deletion (in the NTD) had marginal effects on neutralization potency of the RBM and RBD mAbs we evaluated. It was difficult to assess the impact of the P681H and other mutations on the NTD mAbs, since these mAbs neutralized the D614G virus poorly at baseline in Vero-hACE2-TMPRSS2 cells; (b) The K417N mutation resulted in ~27-fold reduction in neutralization by mAb COVOX-40 but did not negatively affect other mAbs in our panel. If anything, several class 1 mAbs and also SARS2–44 showed slightly improved inhibitory activity (P = 0.002, two-tailed Wilcoxon matched-pairs signed rank test) with this mutation; (c) Mutation at N501Y reduced the neutralizing activity of COVOX-40, SARS2–31, and SARS2–10 slightly but did not alter the potency of other mAbs substantively; this result is consistent with data showing that human convalescent sera efficiently neutralize viruses with N501Y substitutions22–24; (d) The E484K mutation negatively impacted the potency of several class 1 antibodies. Compared to the D614G virus, mAbs COV2–2196, COV2–3025, COV2–2381 and S2E12 showed 4- to 5-fold reduced activity against the E484K virus, and COV2–2050, 1B07, COVOX-384, and S2H58 lost virtually all neutralizing potential; (e) The combination of E484K and N501Y mutations, which is present in the circulating South African B.1.351 and Brazilian B.1.1.248 strains, showed even greater effects (6- to 13-fold reductions) on the activity of class 1 mAbs COV2–2196, COV2–3025, COV2–2381, and S2E12 mAbs; (f) When we tested class 1 mAbs for inhibition of the Wash SA-B.1.351 virus containing the full South African spike sequence, as expected, several mAbs (COV2–2050, 1B07, COVOX-384, and S2H58) lost activity in both Vero-hACE2-TMPRSS2 and Vero-TMPRSS2 cells. However, the reductions in neutralizing potential by other class 1 mAbs (COV2–2196, COV2–3025, COV2–2381, and S2E12) seen against the E484K/N501Y virus were absent with Wash SA-B.1.351, which contains additional mutations. The K417N substitution, which is located at the edge of the RBM (Fig 1b) and enhances neutralization by some class 1 mAbs, may compensate for the negative effects on inhibition of the E484K/N501Y mutations. In comparison, we observed a distinct neutralization pattern with Wash SA-B.1.351 for class 2 and 3 mAbs. Because some mAbs neutralized poorly in Vero-hACE2-TMPRSS2 cells, we performed parallel experiments in Vero-TMPRSS2 cells. Class 2 mAbs binding the base of the RBD showed no substantive loss in potency against the Wash SA-B.1.351. However, the two NTD mAbs in class 3 (COV2–2676 and COV2–2489) lost neutralizing activity against Wash SA-B.1.351 in Vero-hACE2-TMPRSS2 and Vero-TMPRSS2 cells, consistent with recent data with other NTD mAbs and pseudoviruses13; (g) None of the class 1 mAbs lost neutralizing activity against the B.1.1.7 virus on Vero-hACE2-TMPRSS2 cells. Nonetheless, we observed some small reductions in potency (2.5- to 6-fold) with mAbs COV2–2381, S2E12, and S2X259 on Vero-TMPRSS2 cells, although they remained highly neutralizing. In comparison, we observed diminished (6- to 13-fold) neutralizing activity of some class 2 mAbs (SARS2–31 and S309) against the B.1.1.7 strain in Vero-hACE2-TMPRSS2 but not Vero-TMPRSS2 cells. The reduced potency of S309 mAb against B.1.1.7 strain in Vero-hACE2-TMPRSS2 cells contrasts with data showing it binds avidly to the B.1.1.7 spike protein on the surface of cells and potently neutralizes a vesicular stomatitis virus (VSV) pseudotyped with B.1.1.7 spike protein in Vero E6 cells or a WA1/2020 virus derived in Vero CCL81 cells and tested on Vero-hACE2-TMPRSS2 or Vero-TMPRSS2 cells (Extended Data Fig 3a–c). Finally, one of the NTD class 3 mAbs (COV2–2489) lost inhibitory activity against the B.1.1.7 strain in both cell types, possibly due to the deletions present in the NTD (69–70 and 144–145)21.
Several academic and industry groups have developed mAb cocktails to overcome possible emergence of resistance during therapy7,25. We tested two mAb combinations that have potential use in humans (COV2–2196 + COV2–2130 [Vanderbilt University Medical Center; with engineered derivatives being tested in clinical trials by AstraZeneca] and S309 + S2E12 [Vir Biotechnology] for their inhibitory activity against the SARS-CoV-2 variant viruses (Fig 1g–i). The COV2–2196 + COV2–2130 combination generally retained inhibitory activity (< 4-fold reduction) against all strains. Although the S309 + S2E12 combination showed reduced (~10-fold) potency against the E484K/N501Y/D614G strain, it performed effectively against the Wash SA-B.1.351 virus, again suggesting that additional mutations in natural variants (e.g., K417N) enable some antibodies to function better against viruses containing E484K and N501Y mutations.
We next assessed how spike protein mutations impacted the neutralizing activity of polyclonal sera obtained from individuals (n = 19) approximately 1 month after mild SARS-CoV-2 infection26. Based on experiments with the mAbs, we used Vero-hACE2-TMPRSS2 cells and focused our testing on WA1/2020 D614G, B.1.1.7, Wash SA-B.1.351, and WA1/2020 D614G with mutations at K417N, E484K/N501Y, or K417N/E484K/N501Y (Fig 2 and Extended Data Fig 4). When compared to the WA1/2020 D614G virus, we observed the following: (a) differences in neutralization were not observed with the B.1.1.7 strain (Fig 2a); (b) a small increase (1.5-fold, P < 0.05) in neutralization was detected with the K417N virus (Fig 2b), similar to that seen with some mAbs (Fig 1h); (c) serum neutralization titers were lower against E484K/N501Y (5-fold, P < 0.0001), K417N/E484K/N501Y (3.5-fold, P < 0.0001), and Wash SA-B.1.351 (4.6-fold, P < 0.0001) viruses (Fig 2c–e), all of which contain the E484K mutation. A heatmap analysis showed that most individuals lost neutralizing activity against all three viruses containing the E484K and N501Y mutations (Fig 2f). Given these results with viruses encoding E484K mutations, we performed separate studies with human convalescent serum (n =10) and a chimeric SARS-CoV-2 WA1/2020 strain encoding a Brazilian variant spike gene (Wash BR-B.1.1.248; L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I, and V1176F [Extended Data Fig 5a]). As expected, several class 1 (RBM-binding) and class 3 (NTD-binding) mAbs showed reduced neutralizing activity against Wash BR-B.1.1.248 (Extended Data Fig 5b–c). Nonetheless, we observed a smaller yet significant decrease (2.5-fold, P < 0.01) in neutralization potency of convalescent serum against Wash BR-B.1.1.248 (Fig 2g–h and Extended Data Fig 4).
Figure 2. Neutralization of SARS-CoV-2 viral variants by convalescent human serum in Vero-hACE2 TMPRSS2 cells.
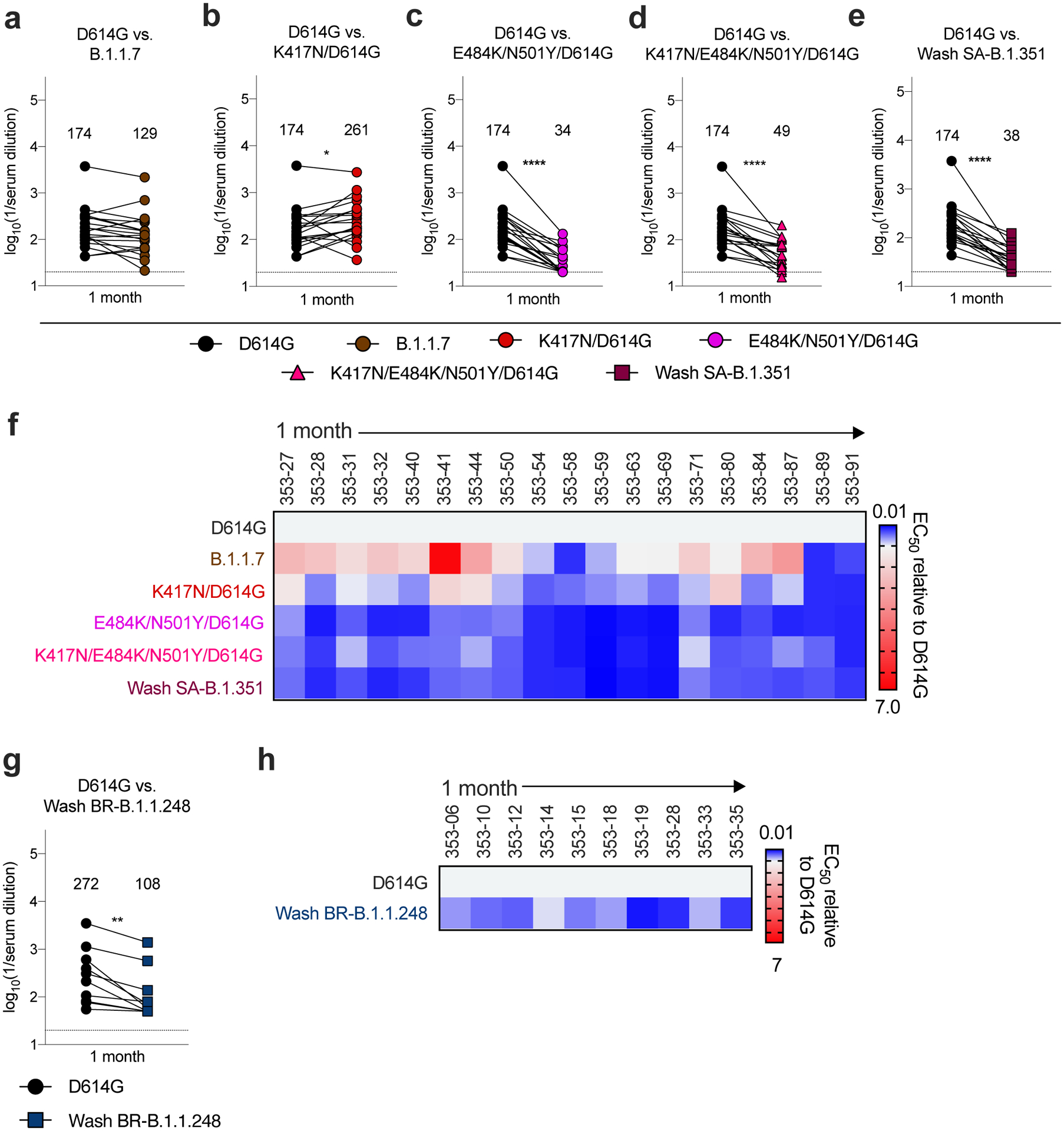
a-e, Paired analysis of neutralizing activity by convalescent human sera (n = 19) obtained approximately 1 month after mild SARS-CoV-2 infection against WA1/2020 D614G and variant viruses in Vero-hACE2-TMPRSS2 cells: (a) B.1.1.7, (b) K417N/D614G, (c) E484K/N501Y/D614G, (d) K417N/E484K/N501Y/D614G, or (e) Wash SA-B.1.351. g. Paired analysis of neutralizing activity by a separate convalescent human sera cohort (n = 10) obtained approximately 1 month after mild SARS-CoV-2 infection against WA1/2020 D614G and Wash BR-B.1.1.248 in Vero-hACE2-TMPRSS2 cells. a-e and g. Results are from one experiment performed in duplicate. Geometric mean neutralization titers (GMT) are shown above each graph. Dotted line represents the limit of detection of the assay. Two-tailed Wilcoxon matched-pairs signed rank test: D614G vs. B.1.1.7, P = 0.0546; D614G vs. K417N/D614G, P = 0.0361; D614G vs. E484K/N501Y/D614G, P < 0.0001; D614G vs. K417N/E484K/N501Y/D614G, P < 0.0001; D614G vs. Wash SA-B.1.351, P < 0.0001; D614G vs. Wash BR-B.1.1.248, P = 0.0020. f, h Heat maps of the relative neutralizing activity of sera from individual convalescent subjects against indicated SARS-CoV-2 viruses compared to recombinant WA1/2020 D614G. Blue, reduction; red, increase.
Given that viruses containing changes at positions 484 and 501 escape neutralization by serum from convalescent humans, we next examined the effects of vaccine-induced antibody responses. Initially, we interrogated sera from mice (n = 10), hamsters (n = 8), and non-human primates (NHP [rhesus macaques], n = 6) obtained one month after immunization with ChAd-SARS-CoV-2, a chimpanzee adenoviral vectored vaccine encoding for a prefusion stabilized form of the spike protein27–29. Using Vero-hACE2-TMPRSS2 cells, we assessed serum neutralization of WA1/2020 D614G, B.1.1.7, Wash SA-B.1.351, and recombinant WA1/2020 D614G viruses with mutations at K417N, E484K/N501Y, or K417N/E484K/N501Y (Extended Data Fig 6). For serum samples from mice, when comparing the GMTs of neutralization to the WA1/2020 D614G strain, we observed a slight increase (1.9-fold, P < 0.05) with K417N (Fig 3b), decreases with E484K/N501Y (9-fold, P < 0.001; Fig 3c), K417N/E484K/N501Y (5-fold, P < 0.01; Fig 3d), and Wash SA-B.1.351 (5-fold, P < 0.01; Fig 3e), yet no significant differences with B.1.1.7. (Fig 3a). In a heatmap plot (Fig 3p), 9 of the 10 mouse sera show a loss of neutralizing activity against multiple viruses containing the E484K mutation. In hamsters, the results were similar. We observed a marked decrease (10- to 12-fold, P < 0.01) in serum neutralization of E484K/N501Y, K417N/E484K/N501Y, and Wash SA-B.1.351 (Fig 3h–j). Statistically significant differences in neutralization were not observed with K417N and B.1.1.7 viruses (Fig 3f, g). This pattern was reflected at the individual sample level (Fig 3q). In NHPs, we also observed a substantial decrease (9- to 11-fold, P < 0.05) in serum neutralization of E484K/N501Y, K417N/E484K/N501Y, and Wash SA-B.1.351 (Fig 3m–o). In comparison, with B.1.1.7 (Fig 3k) or K417N (Fig 3l) viruses, we detected no or small (1.5-fold increase, P < 0.05) significant differences in neutralization, respectively. The heatmap analysis showed that all NHP sera consistently exhibited reduced neutralizing activity against viruses containing the E484K mutation (Fig 3r).
Figure 3. Resistance of SARS-CoV-2 viral variants to neutralization by vaccine-induced serum derived from mice, hamsters, and NHPs.
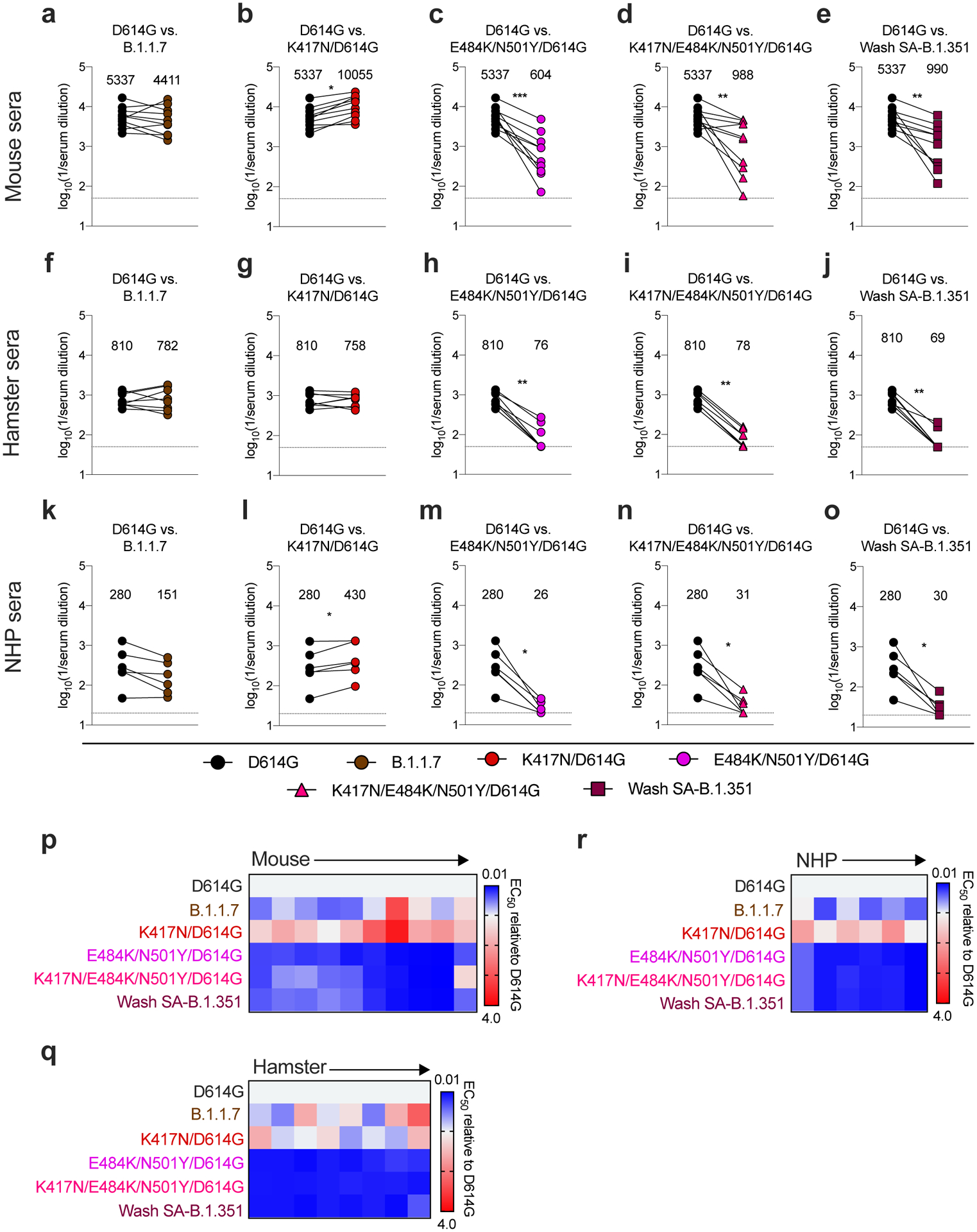
Paired analysis of neutralizing activity by sera from mice (a-e, n = 10), hamsters (f-j, n = 8), and NHPs (k-o, n = 6) obtained ~30 days after a single intranasal immunization with an adenoviral vectored SARS-CoV-2 vaccine (ChAd-SARS-CoV-2-S27). Neutralization data on Vero-hACE2-TMPRSS2 cells is displayed as WA1/2020 D614G versus the variant viruses: (a, f, k) B.1.1.7, (b, g, l) K417N/D614G, (c, h, m) E484K/N501Y/D614G, (d, i, n) K417N/E484K/N501Y/D614G, or (e, j, o) Wash SA-B.1.351. Results are from one experiment performed in duplicate, with some exceptions due to limited sample. GMT values are shown above each graph. Dotted line represents the limit of detection of the assay. Two-tailed Wilcoxon matched-pairs signed rank test: Mouse sera: D614G vs. B.1.1.7, P = 0.323; D614G vs. K417N/D614G, P = 0.0020; D614G vs. E484K/N501Y/D614G, P = 0.0020; D614G vs. K417N/E484K/N501Y/D614G, P = 0.0039; D614G vs. Wash SA-B.1.351, P = 0.0020. Hamster sera: D614G vs. B.1.1.7, P = 0.9453; D614G vs. K417N/D614G, P > 0.9999; D614G vs. E484K/N501Y/D614G, P = 0.0078; D614G vs. K417N/E484K/N501Y/D614G, P = 0.0078; D614G vs. Wash SA-B.1.351, P = 0.0078. NHP sera: D614G vs. B.1.1.7, P = 0.0625; D614G vs. K417N/D614G, P = 0.0312; D614G vs. E484K/N501Y/D614G, P = 0.0312; D614G vs. K417N/E484K/N501Y/D614G, P = 0.0312; D614G vs. Wash SA-B.1.351, P = 0.0312. p-r, Heat maps of the relative neutralizing activity of sera from individual mice (p), hamsters (q), and NHPs (r) against indicated SARS-CoV-2 viruses compared to WA1/2020 D614G. Blue, reduction; red, increase.
Because samples from human immunization trials with ChAd-SARS-CoV-2 are not yet available, we interrogated sera from individuals who received the Pfizer-BioNTech (BNT162b2) vaccine, a lipid nanoparticle encapsulated-mRNA that encodes a similar membrane-bound, prefusion stabilized form of the full-length SARS-CoV-2 spike protein30. We tested sera (Extended Data Fig 7 and 8) for neutralization of our panel of SARS-CoV-2 variants (Fig 4a–f). Compared to the WA1/2020 D614G variant, we observed moderate reductions in neutralizing activity (GMTs) of B.1.1.7 (2-fold, P < 0.01; Fig 4a) and E484K/N501Y (4-fold, P < 0.0001; Fig 4c) and larger decreases in activity against Wash SA-B.1.351 (10-fold, P < 0.0001; Fig 4d), with all subjects showing substantially reduced potency (Fig 4f), results that agree with pseudovirus studies13. Analogous to the results with human convalescent sera (Fig 2g), we observed a smaller decrease (2.2-fold, P < 0.01) in neutralization potency of serum from vaccine recipients against the Wash BR-B.1.1.248 virus (Fig 4e). Significant differences in neutralizing activity were not detected with K417N/D614G (Fig 4b).
Figure 4. Resistance of SARS-CoV-2 viral variants to neutralization by human serum from Pfizer-BioNTech BNT162b2 mRNA vaccinated individuals in Vero-hACE2-TMPRSS2 cells.
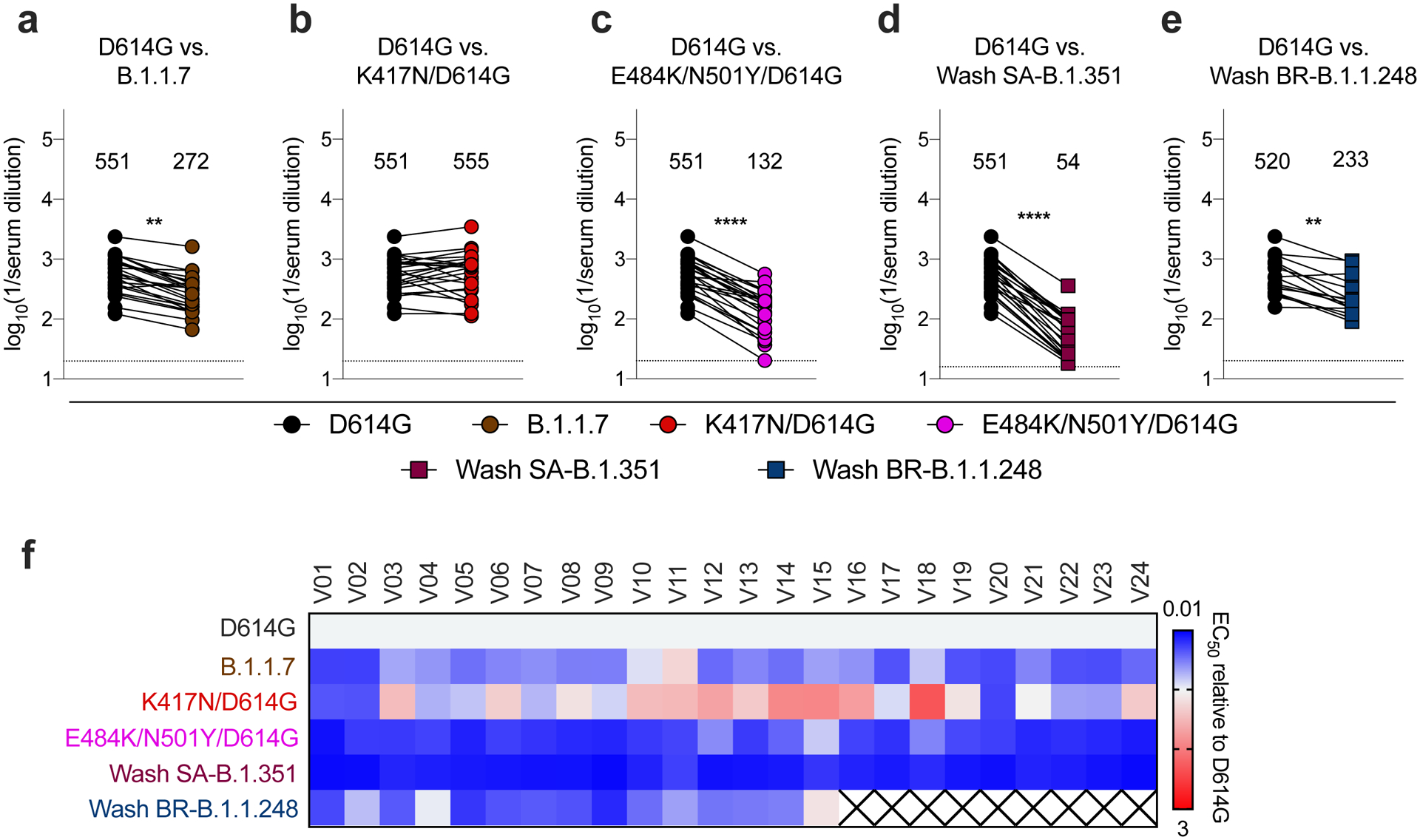
Paired analysis of neutralizing activity by sera from humans (n = 24) obtained after boosting with the BNT162b2 mRNA vaccine. Neutralization data on Vero-hACE2-TMPRSS2 cells is displayed with WA1/2020 D614G versus the variant viruses: (a) B.1.1.7, (b) K417N/D614G, (c) E484K/N501Y/D614G, (d) Wash SA-B.1.351, or (e) Wash BR-B.1.1.248 (n = 15). Results are from one experiment performed in duplicate. GMT values are shown above each graph. Dotted line represents the limit of detection of the assay. Two-tailed Wilcoxon matched-pairs signed rank test: D614G vs. B.1.1.7, P < 0.0001; D614G vs. K417N/D614G, P = 0.6231; D614G vs. E484K/N501Y/D614G, P < 0.0001; D614G vs. K417N/E484K/N501Y/D614G, P = 0.8774; D614G vs. Wash SA-B.1.351, P < 0.0001; D614G vs. Wash BR-B.1.1.248, P = 0.0020. f, Heat map of the relative neutralizing activity of sera from vaccinated individuals against indicated SARS-CoV-2 viruses compared to WA1/2020 D614G. Blue, reduction; red, increase. An X indicates sera was not evaluated.
Because of the differences in neutralization seen with some mAbs on Vero-hACE2-TMPRSS2 and Vero-TMPRSS2 cells (Fig 1h–i), we also evaluated the impact of hACE2 receptor expression on neutralizing activity of serum samples from convalescent adults (Fig 5a–d) and from BNT162b2 mRNA-vaccinated individuals (Fig 5e–h). Given the limited remaining serum quantities, we performed neutralization experiments on Vero-TMPRSS2 cells with WA1/2020 D614G, B.1.1.7, and Wash SA-B.1.351, and Wash BR-B.1.1.248 viruses. These experiments (Extended Data Fig 9) revealed the following: (a) Convalescent and vaccine sera showed small yet significant reductions (1.7- to 2.5- fold, P < 0.01) in neutralizing activity of B.1.1.7 compared to the WA1/2020 D614G virus (Fig 5a, e). (b) Sera from both convalescent and vaccinated individuals showed a marked 6- to 9-fold reduction (P < 0.01) in neutralizing potency against the Wash SA-B.1.351 virus (Fig 5b, f); (c) we again observed a smaller decrease (1.7- to 4.5-fold, P < 0.01) in neutralization potency of serum against Wash BR-B.1.1.248, (Fig 5c, g). The results were similar in magnitude between Vero-hACE2-TMPRSS2 and Vero-TMPRSS2 cells (see also Fig 4a, d, e) and suggest that cellular expression of hACE2 does not markedly impact neutralization outcome of polyclonal antibodies in these assays.
Figure 5. Resistance of SARS-CoV-2 viral variants to neutralization by human serum from convalescent and vaccinated individuals in Vero-TMPRSS2 cells.
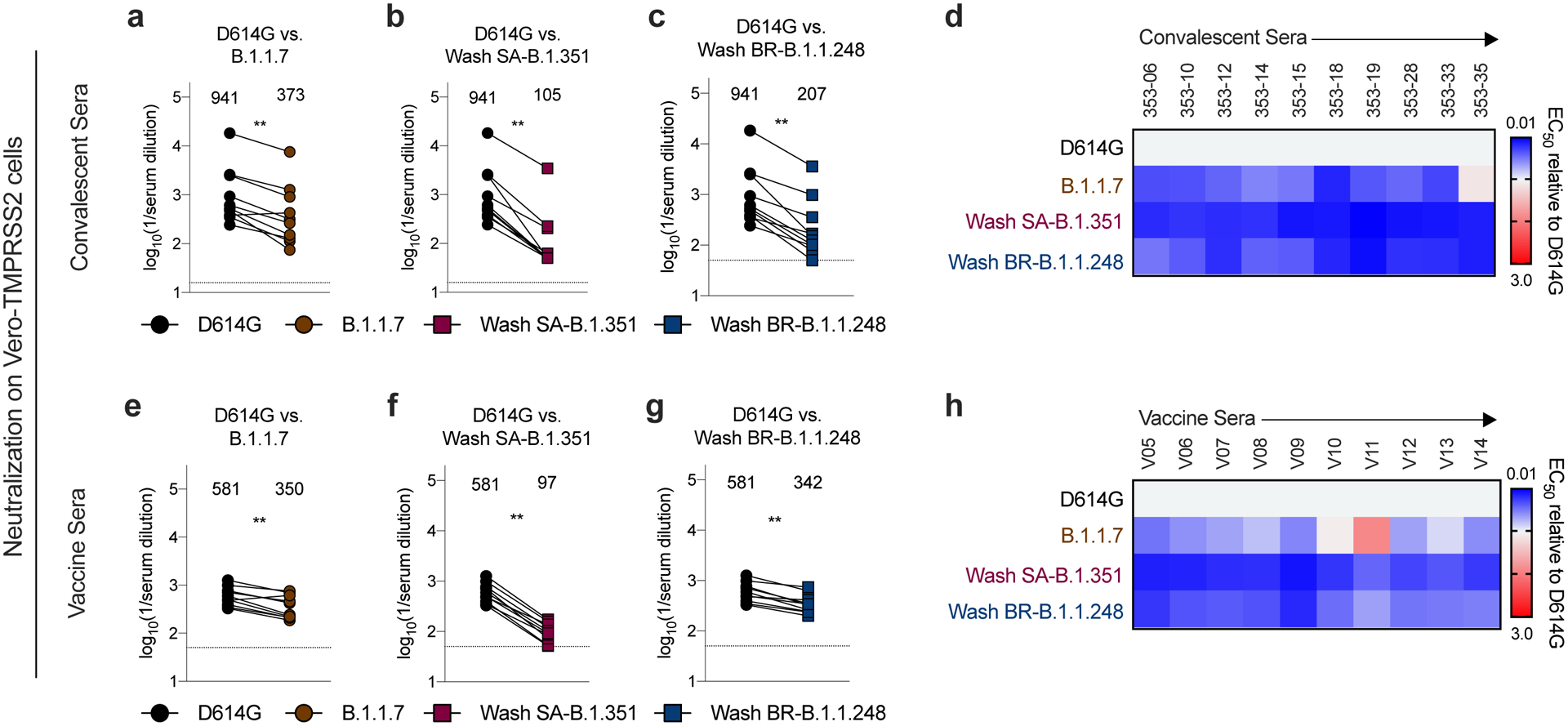
Sera from individuals who had been infected with SARS-CoV-2 (a-c; n = 10, ~1-month post-infection) or vaccinated with the Pfizer-BioNTech mRNA vaccine (d-f; n = 10) were tested for neutralization of the indicated SARS-CoV-2 strains (WA1/2020 D614G (a, b, c, e, f, g), B.1.1.7 (a, e), Wash SA-B.1.351 (b, f), or Wash BR-B.1.1.248 (c, g) using a FRNT in Vero-TMPRSS2 cells. Results are from one experiment performed in duplicate, with some exceptions due to limited sample. GMT values are shown above each graph. Dotted line represents the limit of detection of the assay. Two-tailed Wilcoxon matched-pairs signed rank test: Convalescent sera: D614G vs. B.1.1.7, P = 0.0039; D614G vs. Wash SA-B.1.351, P = 0.0020; D614G vs. Wash BR-B.1.1.248, P = 0.0020. Vaccine sera: D614G vs. B.1.1.7, P = 0.0039; D614G vs. Wash SA-B.1.351, P = 0.0020; D614G vs. Wash BR-B.1.1.248, P = 0.0020. d, h, Heat maps of the relative neutralizing activity of sera from convalescent (d) or vaccinated (h) individuals against indicated SARS-CoV-2 viruses compared to WA1/2020. Blue, reduction; red, increase.
DISCUSSION
Our in vitro experiments using a B.1.1.7 isolate and engineered variants in the backbone of the WA1/2020 strain establish that mutations in the spike can impact the potency of antibody neutralization. Some neutralizing mAbs targeting the base of the RBD or NTD showed reduced activity against the B.1.1.7 isolate, whereas others targeting the RBM or NTD failed to inhibit infection of Wash SA-B.1.351, Wash BR-B.1.1.248, or variants containing the E484K mutation. These finding are potentially important because the RBM has functional plasticity31,32, and additional mutations in this region that occur as the pandemic evolves could further impact the efficacy of mAb therapies or vaccines. Our results establishing the E484K substitution as a vulnerability for multiple neutralizing mAbs are consistent with deep mutational scanning or VSV-SARS-CoV-2-based neutralization escape screening campaigns31,33,34. However, several other highly neutralizing mAbs (e.g., COV2–2196, COV2–2381, COV2–3025, and S2E12) showed intact or only mildly diminished inhibitory activity against the suite of variant viruses we tested, possibly because they bind the RBM at sites other than the E484K residue (Table 1). Moreover, cocktails of mAbs binding different epitopes of the spike protein overcame virus resistance to individual mAbs. Alternative approaches to addressing the diminished mAb neutralization activity by variant SARS-CoV-2 lineages include targeting of conserved regions of the spike and identifying clonal mAb variants with greater potency, such that a given dose of mAb can protect against a range of variants despite some decrease in neutralization activity.
Table 1.
Monoclonal antibodies used in this study
| MAb | Region | Species | EC50 values (ng/mL)a | block ACE2 binding | Contact residues within RBMb | Binding site residues, aa position | Functionally important residuesc | Other mapping data | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D614G | E484K/D614G | ||||||||||
| CLASS 1 | COV2–2196 | RBD - RBM | human | 3 | 16 | yes | yes | 455–456, 475–479, 484–489, 493 | F486A, N487A (LOB) | Not competitive with COV2–2130 | 5,19,58 |
| COV2–2130 | RBD - RBM | human | 22 | 36 | yes | yes | 345–346, 439–441, 443–447, 449–450, 452, 484, 490, 492–494 | K444A, G447R (LOB); and R346I, K444R, K444E (NE) | Not competitive with COV2–2196 | 5,19,58 | |
| COV2–2072 | RBD - RBM | human | 6 | 19 | yes | yes | N/A | N/A | COV2–2196 competitive | 5,19 | |
| COV2–2050 | RBD - RBM | human | 4 | >10,000 | yes | yes | N/A | E484K (LOB and NE) | COV2–2196 and COV2–2130 competitive | 5,19,31 | |
| COV2–3025 | RBD - RBM | human | 3 | 14 | yes | yes | N/A | N/A | COV2–2196 competitive | 5,19 | |
| COV2–2381 | RBD - RBM | human | 5 | 28 | yes | yes | N/A | N/A | COV2–2196 competitive | 5,19 | |
| 1B07 | RBD - RBM | mouse-human chimera | 2 | >10,000 | yes | yes | N/A | E484A/D/G/K, F486Y (NE) | N/A | 20,33 | |
| S2E12 | RBD - RBM | human | 4 | 17 | yes | yes | 455 to 458, 473 to 493 | G476S, F486A (LOB) | N/A | 7, Starr, Corti et al. unpublished | |
| COVOX-384 | RBD - RBM | human | 3 | >10,000 | yes | yes | 455–456, 456, 482–486 | N/A | N/A | 57 | |
| COVOX-40 | RBD - RBM | human | 60 | 95 | yes | yes | 417, 409, 505 | N/A | N/A | 57 | |
| S2H58 | RBD-RBM | human | 6 | >10,000 | yes | yes | N/A | E484K, F490L, S494P (LOB) | Starr, Corti et al. unpublished | ||
| S2X259 | RBD | human | 99 | 105 | yes | no | G504D (LOB) | 56 | |||
| CLASS 2 | S309 | RBD- BASE | human | 314 | 375 | no | no | 333–335,337, 339–341, 343, 346,354, 356–361, 440–441, 444,509 | N/A | N/A | 3 |
| SARS2–3 | RBD- BASE | mouse | 4187 | 9331 | no | no | N/A | N/A | CR3022 competitive | VanBlargan and Diamond, unpublished | |
| SARS2–10 | RBD- BASE | mouse | 1258 | 833 | yes | no | N/A | N/A | CR3022 competitive | VanBlargan and Diamond, unpublished | |
| SARS2–31 | RBD- BASE | mouse | 130 | 282 | yes | no | N/A | K378E/Q, R408K, G504D (NE) | CR3022 competitive | VanBlargan and Diamond, unpublished | |
| SARS2–44 | RBD- BASE | mouse | 59 | 269 | yes | no | N/A | N/A | CR3022 competitive | VanBlargan and Diamond, unpublished | |
| CLASS 3 | COV2–2676 | NTD | human | 2496 | 3575 | no | no | N/A | Y144A, N164A (LOB) and F140S (NE) | COV2–2489 competitive | 5,19,21 |
| COV2–2489 | NTD | human | 6854 | 6795 | no | no | N/A | G142A, Y144A, F157A, N164A (LOB); and G142D, R158S (NE) | COV2–2676 competitive | 5,19,21 | |
Neutralization potency determined by FRNT assay in Vero-hACE2-TMPRSS2 cells from Figure 1 with D614G and E484K/D614G isolates.
Identified as engaging residues within the RBM site by direct binding ELISA or by epitope mapping using loss-of binding assays.
LOB, loss-of-binding to spike protein, and NE, neutralization escape mutants assessed with SARS-CoV-2 WA1/2020.
N/A, data not available
Our studies with human sera from convalescent subjects and recipients of the BNT162b2 mRNA vaccine, and animal sera after immunization with a vaccine encoding a similar spike gene, demonstrate a lower potency of neutralization against E484K and N501Y-containing viruses (note: we did not perform studies with the single-mutation viruses, due to limited serum availability). This observation is unexpected given that antibody responses in animals and humans are polyclonal and in theory, should overcome resistance associated with individual mutations and loss of activity of particular B cell clones.
Our analyses agree with some studies showing substantial or complete escape against spike proteins corresponding to the South African lineage (B.1.351 or 501Y.V2) by antibodies in convalescent or vaccine-immune plasma using lentiviral-based pseudotype neutralization assays10,11,23. Moreover, they are consistent with studies showing loss of neutralization potency of human convalescent serum against VSV-SARS-CoV-2 chimeric virus variants containing the E484K mutation35 and selection of escape E484K mutants under serial passage of convalescent COVID-19 plasma36. Indeed, similar findings with authentic SARS-CoV-2 viruses encoding E484K mutations were recently reported37. One unique trend we noticed was that convalescent and vaccine-induced immune sera neutralized infection of the chimeric SARS-CoV-2 strains encoding the Brazilian spike (B.1.1.248) better than the South African spike (B.1.351) even though both viruses encoded E484 and N501 mutations. While follow-up corroborating studies are warranted, this result could be due to the distinct set of mutations and/or deletions in the NTD region or enhanced neutralization of B.1.1.248 by anti-RBD antibodies that bind outside of the RBM (see Extended Data Fig 5c). Overall, our findings may have therapeutic implications, as immune plasma derived from individuals infected early during the pandemic might fail to protect patients infected with more recent isolates containing the E484K mutation.
Limitations of the study.
These studies focused exclusively on the impact of sequence changes in the spike protein on antibody neutralization in cell culture. Despite observing differences in serum neutralizing activity against authentic SARS-CoV-2 variant viruses, it remains unclear how this finding translates into effects on protection in the context of secondary infection or infection after vaccination with platforms using historical spike gene sequences. Although serum neutralizing titers are an anticipated correlate of protection38, this measurement does not account for Fc effector functions; Fcγ receptor or complement protein engagement by non-, weakly-, or strongly- neutralizing antibodies that bind the SARS-CoV-2 spike protein on the surface of infected cells could confer substantial protection39–41. Also, the role of memory T or B cells in protection against variant viruses is unknown and could prevent severe infection even in the setting of compromised serum antibody responses42–44.
Moreover, the field still does not know whether Vero or other cell-based neutralization assays predict antibody-mediated protection. Indeed, primary cells targeted by SARS-CoV-2 in vivo can express unique sets of attachment and entry factors45, which could impact receptor and entry blockade by specific antibodies. We observed that the cell line used can affect the potency of antibody neutralization against different SARS-CoV-2 variants. Such results may impact the congruity of data across laboratories and interpretation of effects of viral variants on vaccine efficacy. As an example, recent studies with Vero E6 cell-derived SARS-CoV-2 with spike proteins containing some (E484K, N501Y, and D614G) or all of the South African mutations showed smaller 1.2 to 2.7-fold decreases in neutralization potency by BNT162b2 mRNA vaccine-elicited human immune sera22,46. When we compared neutralization of deep-sequenced confirmed p0 (Vero E6 cell-produced) and p1 (Vero-hACE2-TMPRSS2 cell-produced) K417N/E484K/N501Y/D614G viruses by immune serum from vaccinated animals or humans, or naturally infected humans in the same recipient Vero-hACE2-TMPRSS2 cells, the viruses produced in Vero E6 cells were neutralized more efficiently (2 to 3-fold, P < 0.05) than those propagated in Vero-hACE2-TMPRSS2 cells (Extended Data Fig 10). We speculate that TMPRSS2 might modify the spike protein of authentic SARS-CoV-2 in the producer cell such that some classes of antibodies no longer efficiently block infection of the recipient cell.
While our analysis of neutralizing antibody responses with authentic infectious SARS-CoV-2 variants on Vero-hACE2-TMPRSS2 and Vero-TMPRSS2 cells suggests that adjustments to some therapeutic antibody cocktails or existing spike sequences in vaccines might be necessary, corroborating in vivo studies are needed. Sequential infection and/or vaccination/infection studies in animals and analysis of vaccine efficacy in the setting of new variant infections ultimately will determine the impact of emerging SARS-CoV-2 lineages, especially those containing E484K mutations.
METHODS
Cells.
Vero E6 (CRL-1586, American Type Culture Collection (ATCC)), Vero-TMPRSS248 (gift of S. Ding, Washington University), and Vero-hACE2-TMPRSS2 (gift of A. Creanga and B. Graham, NIH) cells were cultured at 37°C in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES pH 7.3, 1 mM sodium pyruvate, 1× non-essential amino acids, and 100 U/ml of penicillin–streptomycin. Vero-TMPRSS2 cell cultures were supplemented with 5 μg/mL of blasticidin. TMPRSS2 expression was confirmed using an anti-V5 antibody (Thermo Fisher 2F11F7) or anti-TMPRSS2 mAb (Abnova, Clone 2F4) and APC-conjugated goat anti-mouse IgG (BioLegend, 405308). Vero-hACE2-TMPRSS2 cell cultures were supplemented with 10 μg/ml of puromycin.
Viruses.
The 2019n-CoV/USA_WA1/2020 isolate of SARS-CoV-2 was obtained from the US Centers for Disease Control (CDC). The B.1.1.7 isolate was obtained from an infected individual. Individual point mutations in the spike gene (D614G, K417N/D614G, E484K/D614G, N501Y/D614G, P681H/D614G, del69–70/N501Y/D614G, and E484K/N501Y/D614G) were introduced into an infectious cDNA clone of the 2019n-CoV/USA_WA1/2020 (WA1/2020) strain as described previously49. Nucleotide substitutions were introduced into a subclone puc57-CoV-2-F5–7 containing the spike gene of the SARS-CoV-2 wild-type infectious clone50. The South African (B.1.351) and Brazilian (B.1.1.248) variant spike genes were produced synthetically by Gibson assembly. The full-length infectious cDNA clones of the variant SARS-CoV-2 viruses were assembled by in vitro ligation of seven contiguous cDNA fragments following the previously described protocol50. In vitro transcription was then performed to synthesize full-length genomic RNA. To recover the mutant viruses, the RNA transcripts were electroporated into Vero E6 cells. The viruses from the supernatant of cells were collected 40-h later and served as p0 stocks51. All viruses were passaged once in Vero-hACE2-TMPRSS2 or Vero-TMPRSS2 cells and subjected to deep sequencing after RNA extraction to confirm the introduction and stability of substitutions (Supplementary Table 1). Viral RNA from cell culture supernatants was used to generate next generation sequencing libraries using either the Illumina TruSeq Stranded Total RNA Library Prep with Ribo-Zero kit or the Illumina Stranded Total RNA Prep, Ligation with Ribo-Zero Plus kit per the manufacturer’s protocol. The final indexed libraries were quantified using Agilent’s Bioanalyzer and pooled at an equal molar concentration. Illumina’s NextSeq sequencer was used to generate paired end 150 base pair reads. Raw sequencing data was processed using fastp52 v.0.20.1 (https://github.com/OpenGene/fastp) to trim adapters and filter out sequence with < Q30. Alignment to the SARS-CoV-2 reference genome (MN908947.3) was performed using BWA53 v0.7.17-r1188 (http://bio-bwa.sourceforge.net). DeepVariant54 v1.1.0 (https://github.com/google/deepvariant) was used to call variants with an allele frequency >= 50%. Variants were annotated using SNPEff55 5.0c (https://sourceforge.net/projects/snpeff/). All virus preparation and experiments were performed in an approved Biosafety level 3 (BSL-3) facility.
Monoclonal antibodies.
The human mAbs studied in this paper (COV2–2196, COV2–2072, COV2–2050, COV2–2381, COV2–2130, COVOX-384, COVOX-40, S309, S2E12, S2H58, S2X333, VIR-7381, and S2X259) were isolated from blood samples from individuals in North America or Europe with previous laboratory-confirmed symptomatic SARS-CoV or SARS-CoV-2 infection. The original clinical studies to obtain specimens after written informed consent were previously described3,5,7,56,57 and approved by the Institutional Review Board of Vanderbilt University Medical Center, the Institutional Review Board of the University of Washington, the Research Ethics Board of the University of Toronto, and the Canton Ticino Ethics Committee (Switzerland). Chimeric mAb 1B07 with a murine Fv and human Fc (human IgG1) were isolated from C57BL/6 mice immunized with recombinant spike and RBD proteins and described previously20. Murine mAbs were generated in BALB/c or C57BL/6 mice immunized with recombinant spike and RBD proteins and described previously33,35.
Human immune sera.
Multiple sources of human serum samples were used in this study: Convalescent serum samples were obtained from a cohort recruited from the St. Louis metropolitan area who experienced mild SARS-CoV-2 infection. None of those patients required intubation, and the study was approved by Washington University School of Medicine Institutional Review Board (202003186 (WU353)). The serum samples from individuals immunized with the Pfizer-BioNTech (BNT162b2) mRNA vaccine were obtained prior to primary immunization or one week after boosting from young adults, and the studies were approved by Washington University School of Medicine Institutional Review Board (202012081 (WU368) and 202012084 (COVaRiPAD))
Mouse, hamster, and NHP immune sera.
The mouse, hamster, and NHP immune sera were obtained one month after intranasal immunization with ChAd-SARS-CoV-2, a chimpanzee adenoviral vectored vaccine encoding for a prefusion stabilized form of the spike protein. Details of the immunization protocol and functional analyses have been described elsewhere27–29.
Focus reduction neutralization test.
Serial dilutions of mAbs (starting at 10 μg/ml dilution) or serum were incubated with 102 focus-forming units (FFU) of different strains or variants of SARS-CoV-2 for 1 h at 37°C. Antibody-virus complexes were added to Vero-hACE2-TMPRSS2 or Vero-TMPRSS2 cell monolayers in 96-well plates and incubated at 37°C for 1 h. Subsequently, cells were overlaid with 1% (w/v) methylcellulose in MEM supplemented with 2% FBS. Plates were harvested 24 h later by removing overlays and fixed with 4% PFA in PBS for 20 min at room temperature. Plates were washed and sequentially incubated with an oligoclonal pool of SARS2–2, SARS2–11, SARS2–16, SARS2–31, SARS2–38, SARS2–57, and SARS2–71 anti-S antibodies and HRP-conjugated goat anti-mouse IgG (Sigma, 12–349) in PBS supplemented with 0.1% saponin and 0.1% bovine serum albumin. SARS-CoV-2-infected cell foci were visualized using TrueBlue peroxidase substrate (KPL) and quantitated on an ImmunoSpot microanalyzer (Cellular Technologies).
ELISA.
Assays were performed in 96-well plates (MaxiSorp; Thermo) coated with 100 μL of recombinant spike or RBD protein20 in PBS, and plates were incubated at 4°C overnight. Plates were then blocked with 10% FBS and 0.05% Tween20 in PBS. Serum were serially diluted in blocking buffer and added to the plates. Plates were incubated for 90 min at room temperature and then washed 3 times with 0.05% Tween-20 in PBS. Goat anti-human IgG-HRP (Jackson ImmunoResearch, 115-035-003; 1:2,500) was diluted in blocking buffer before adding to wells and incubating for 60 min at room temperature. Plates were washed 3 times with 0.05% Tween-20 in PBS, and then washed 3 times with PBS before the addition of peroxidase substrate (SigmaFAST o-Phenylenediamine dihydrochloride, Sigma-Aldrich). Reactions were stopped by the addition of 1 M HCl. Optical density measurements were taken at 490 nm. The half-maximal binding dilution for each serum or plasma sample was calculated using nonlinear regression. The limit of detection was defined as 1:30.
Transient expression of recombinant SARS-CoV-2 spike proteins and flow cytometry.
The full-length S gene of SARS-CoV-2 strain (SARS-CoV-2-S) isolate BetaCoV/Wuhan-Hu-1/2019 (accession number MN908947) carrying D614G was codon-optimized for expression in hamster cells and cloned into the pcDNA3 expression vector. Amino acid substitutions for B.1.1.7, P.1 (Brazilian lineage: L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, H655Y, T1027I, and V1167F), and B.1.351 variants were introduced by overlap extension PCR. Briefly, DNA fragments with overlap sequences were amplified by PCR (step 1). Mutations were introduced by amplification with primers with similar melting points (Tm). Deletion of the C-terminal 21 amino acids was introduced to increase surface expression of the recombinant spike. Next, three contiguous overlapping fragments were fused by a first overlap PCR (step 2) using the utmost external primers of each set, resulting in three larger fragments with overlapping sequences. A final overlap PCR (step 3) was performed on the three large fragments using the utmost external primers to amplify the S gene and the flanking sequences including the restriction sites KpnI and NotI. This fragment was digested and cloned into the expression plasmid phCMV1. For all PCR reactions, the Q5 Hot Start High fidelity DNA polymerase was used (New England Biolabs), according to the manufacturer’s instructions and adapting the elongation time to the size of the amplicon. After each PCR step, the amplified regions were separated on agarose gel and purified using Illustra GFX™ PCR DNA and Gel Band Purification Kit (Merck KGaA).
Expi-CHO cells (ThermoFisher, A29127) were transiently transfected with SARS-CoV-2-S expression vectors using Expifectamine CHO Enhancer. Two days later, cells were collected for immunostaining with mAbs. Binding of mAbs to transfected cells was analyzed by flow-cytometry using a ZE5 Cell Analyzer (Biorard) and FlowJo software (v9, TreeStar). Positive binding was defined by differential staining of CoV-S-transfectants versus mock-transfectants.
SARS-CoV-2 pseudotyped virus production.
293T/17 cells (ATCC CRL-11268) were seeded in 10-cm dishes for 80% next day confluency. The next day, cells were transfected with the plasmid pcDNA3.1(+)-spike-D19 (encoding the SARS-CoV-2 spike protein) or pcDNA3.1(+)-spike-D19 variants using the transfection reagent TransIT-Lenti according to the manufacturer’s instructions. One day post-transfection, cells were infected with VSV-luc(VSV-G) at an MOI of 3. The cell supernatant containing SARS-CoV-2 pseudotyped virus was collected at day 2 post-transfection, centrifuged at 1,000 × g for 5 min to remove cellular debris, aliquoted and frozen at −80°C. The SARS-CoV-2 pseudotyped virus preparation was quantified using Vero E6 cells seeded at 20,000 cells/well in clear bottom black 96 well plates the previous day. Cells were inoculated with 1:10 dilution series of pseudotyped virus in 50 μL DMEM for 1 h at 37°C. An additional 50 μL of DMEM was added, cells were incubated overnight at 37°C. Luciferase activity was quantified with Bio-Glo reagent by adding 100 μL of Bio-Glo (diluted 1:1 in PBS), incubated at room temperature for 5 min, and relative light units (RLU) were read on an EnSight or EnVision plate reader.
Neutralization of SARS-CoV-2 pseudotyped virus.
Vero E6 cells were seeded into clear bottom black-walled 96-well plates at 20,000 cells/well in 100 μL medium and cultured overnight at 37°C. Twenty-four hours later, 1:3 8-point serial dilutions of mAb were prepared in medium, with each dilution tested in duplicate on each plate (range: 10 μg/mL to 4 ng/mL final concentration). Pseudovirus was diluted 1:25 in medium and added 1:1 to 110 μL of each antibody dilution. Pseudovirus:antibody mixtures were incubated for 1 h at 37°C. Media was removed from the Vero E6 cells and 50 μL of pseudovirus:antibody mixtures were added to the cells. One hour post-infection, 100 μL of medium was added to wells containing pseudovirus:antibody mixtures and incubated for 17 h at 37°C. Media then was removed and 100 μL of Bio-Glo reagent (diluted 1:1 in DPBS) was added to each well. The plate was shaken on a plate shaker at 300 RPM at room temperature for 20 min, and RLUs were read on an EnSight or EnVision plate reader.
Data availability.
All data supporting the findings of this study are available within the paper and are available from the corresponding author upon request. Deep sequencing datasets of viral stocks are available at NCBI BioProject PRJNA698378 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA698378).
Statistical analysis.
All statistical tests were performed as described in the indicated figure legends. Non-linear regression curve fitting was performed to calculate EC50 values. Statistical significance was calculated using a non-parametric two-tailed Wilcoxon matched-pairs signed rank test and Prism 8.0. The number of independent experiments used are indicated in the relevant Figure legends.
Extended Data
Extended Data Fig. 1. MAb-spike structures.
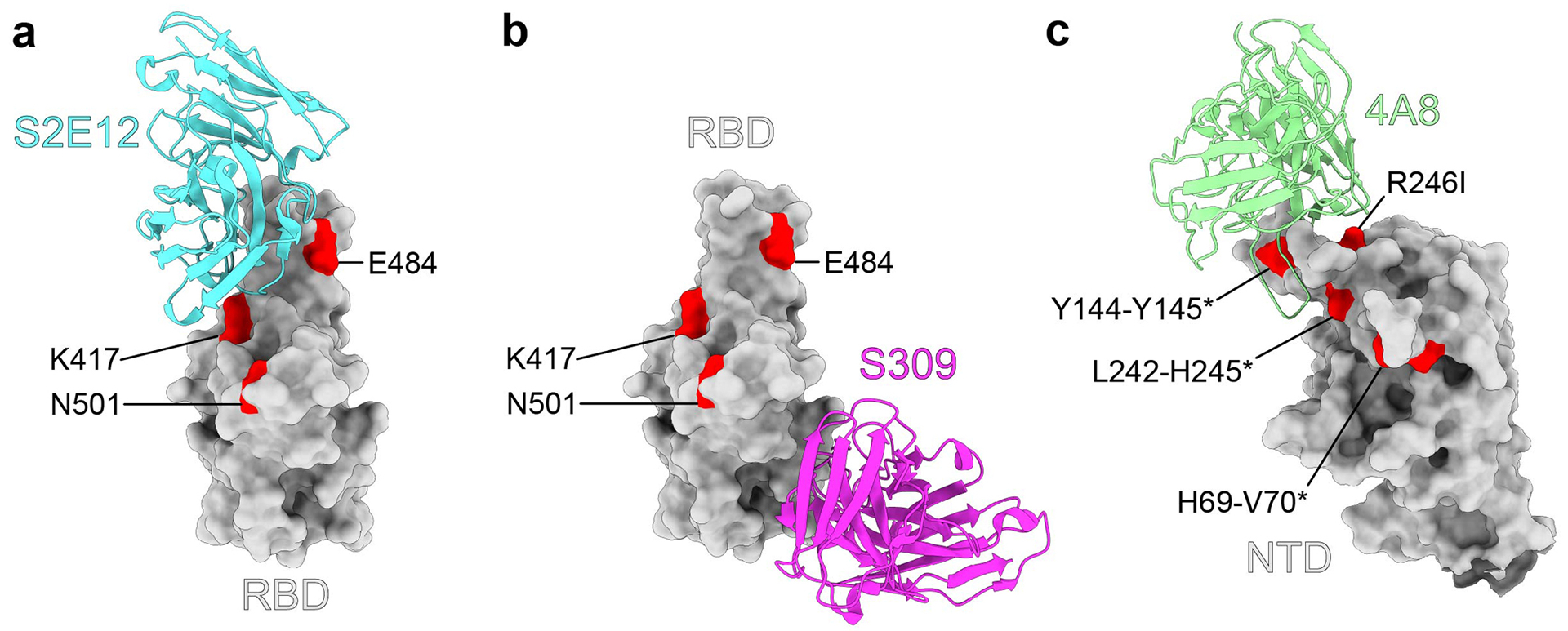
Structures of the SARS-CoV-2 RBD in complex with a representative neutralizing antibody from (a) class 1 (S2E12, PDB: 7K45), or (b) class 2 (S309, PDB: 6WPS). c, Structure of the SARS-CoV-2 spike N-terminal domain (NTD) in complex with a representative class 3 neutralizing antibody (4A8, PDB: 7C2L). All structural analysis and figures were generated with UCSF ChimeraX47.
Extended Data Fig. 2. Neutralization curves with mAbs and variant SARS-CoV-2 strains.

Anti-SARS-CoV-2 human mAbs were tested for neutralization of infection of the indicated viral variants and isolates using a FRNT on Vero-hACE2-TMPRSS2 or Vero-TMPRSS2 cells. One representative experiment of two performed in duplicate is shown.
Extended Data Fig. 3. Binding and neutralizing activity of mAbs to SARS-CoV-2 variants.
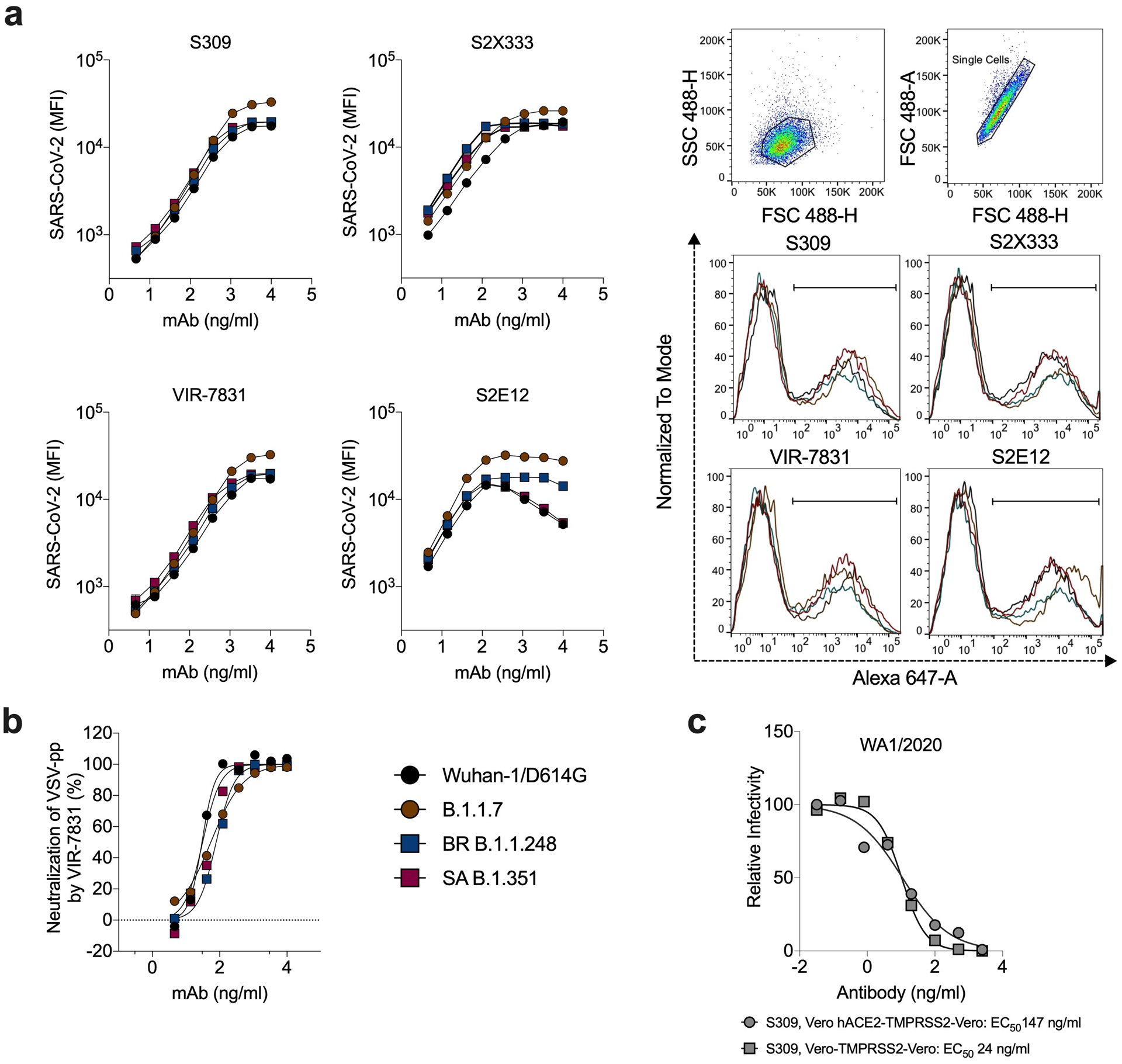
a, Binding of mAbs S2E12 (class 1, RBM), S309 (class 2, RBD base), VIR-7381 (class 2, RBD-base), and S2X333 (class 3, NTD) to SARS-CoV-2 spike proteins from the indicated strains when expressed on the surface of ExpiCHO cells (symbols, mean of duplicates from one experiment). Gating strategy and binding of mAbs S2E12, S309, VIR-7831, and S2X333 (at 370 ng/mL) to SARS-CoV-2 spike from indicated variants when expressed on the surface of ExpiCHO cells. Shown on histograms is the gating strategy of the population of positive cells (percentage ranging between 37 and 46%) used to calculate MFI. b, Neutralization of VSV-SARS-CoV-2 pseudotyped viruses (with indicated spike proteins) on Vero E6 cells. Mean ± standard deviation of sextuplicates is shown for all pseudoviruses, except for SARS-CoV-2 WT (mean of triplicates). WT, Wuhan-1 + D614G. c, Serial dilutions of S309 mAb were mixed with Vero CCL81 cell-derived WA1/2020 and added to Vero-hACE2-TMPRSS2 or Vero-TMPRSS2 cells for evaluation of neutralizing activity by FRNT. One representative experiment of two is shown. The EC50 values are provided in the legend in ng/mL.
Extended Data Fig. 4. Neutralization curves with convalescent human sera from longitudinal cohort and variant SARS-CoV-2 strains.

Serum from individuals (n = 29) who had been infected with SARS-CoV-2 (samples obtained at ~1-month post-infection) were tested for neutralization of the indicated viral variants and isolates in Vero-hACE2-TMPRSS2 cells using a FRNT. One experiment performed in duplicate is shown.
Extended Data Fig. 5. Neutralization curves with mAbs and Wash BR-B.1.1.248 virus.

a, Surface representation of SARS-CoV-2 spike showing the NTD in orange, RBD in green, and S2 portion of the molecule in blue, with N- and C-termini annotated. Substitutions seen in the B.1.1.248 Brazilian variant (L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I, and V1176F) are shaded red. Red hexagon depicts approximate location of R190S, which is obscured in this view. Red star indicates approximate location of T1027I, which is obscured in this view. V1176F is not shown, as it exists beyond the C-terminus of this model, which ends at residue D1146. Inset shows top-down view of the RBD with B.1.1.248 RBD substitutions (K417T/E484K/N501Y) shaded red and contextualized with the receptor binding motif. Spike was modelled using PDB: 7C2L; RBD was modelled using PDB: 6W41. All structural analyses and figures were generated with UCSF ChimeraX47. b, Selected anti-SARS-CoV-2 human mAbs (selected ones used are indicated) were tested for neutralization of the indicated Wash BR-B.1.1.248 using a FRNT on Vero-hACE2-TMPRSS2 cells (top) or on Vero -TMPRSS2 cells (bottom). One representative experiment of two performed in duplicate is shown. c, Summary of EC50 values (ng/ml) of neutralization of Wash BR-B.1.1.248 performed in Vero-hACE2-TMPRSS2 cells (top) or on Vero-TMPRSS2 cells (bottom). Data are an average of two experiments, each performed in duplicate. Blue shading of cells shows virtually complete loss of neutralizing activity: EC50 > 10,000 ng/mL.
Extended Data Fig. 6. Neutralization curves with animal sera from ChAd-CoV-2 vaccinated animals and variant SARS-CoV-2 strains.

Serum samples were collected from mice (n = 10), hamsters (n = 8), or rhesus macaques (NHP, n = 6) ~30 days after a single intranasal immunization with ChAd-SARS-CoV-2-S. Sera were tested for neutralization of infection of the indicated viral variants and isolates in Vero-hACE2-TMPRSS2 cells using a FRNT. One experiment performed in duplicate is shown.
Extended Data Fig. 7. S and RBD binding activity of human sera from individuals vaccinated with BNT162b2 mRNA vaccine.

Individuals were vaccinated and boosted with the Pfizer-BioNTech mRNA vaccine. At seven days after boosting, sera were collected and tested for binding to S or RBD proteins (WA1/2020 strain) by ELISA. One experiment performed in duplicate is shown.
Extended Data Fig. 8. Neutralization curves in Vero-hACE2-TMPRSS2 cells with human sera from subjects vaccinated with the BNT162b2 mRNA vaccine and variant SARS-CoV-2 strains.
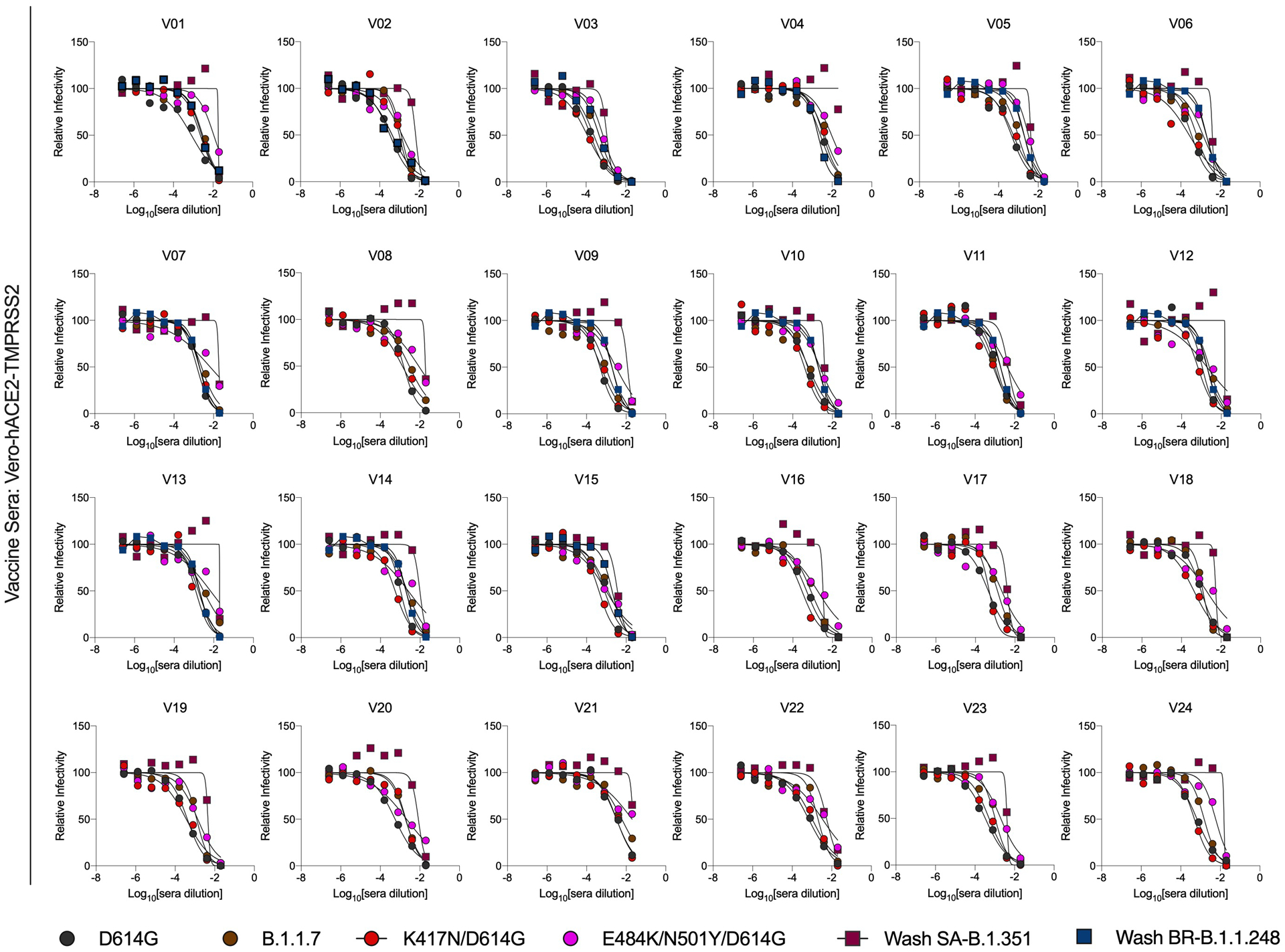
Individuals were vaccinated and boosted with the Pfizer-BioNTech mRNA vaccine. Sera were collected and tested for neutralization of infection of the indicated viral variants and isolates using a FRNT and Vero-hACE2-TMPRSS2 cells. One experiment performed in duplicate is shown.
Extended Data Fig. 9. Neutralization curves of variant SARS-CoV-2 strains in Vero-TMPRSS2 cells with human sera from convalescent subjects or those vaccinated with the BNT162b2 mRNA vaccine.
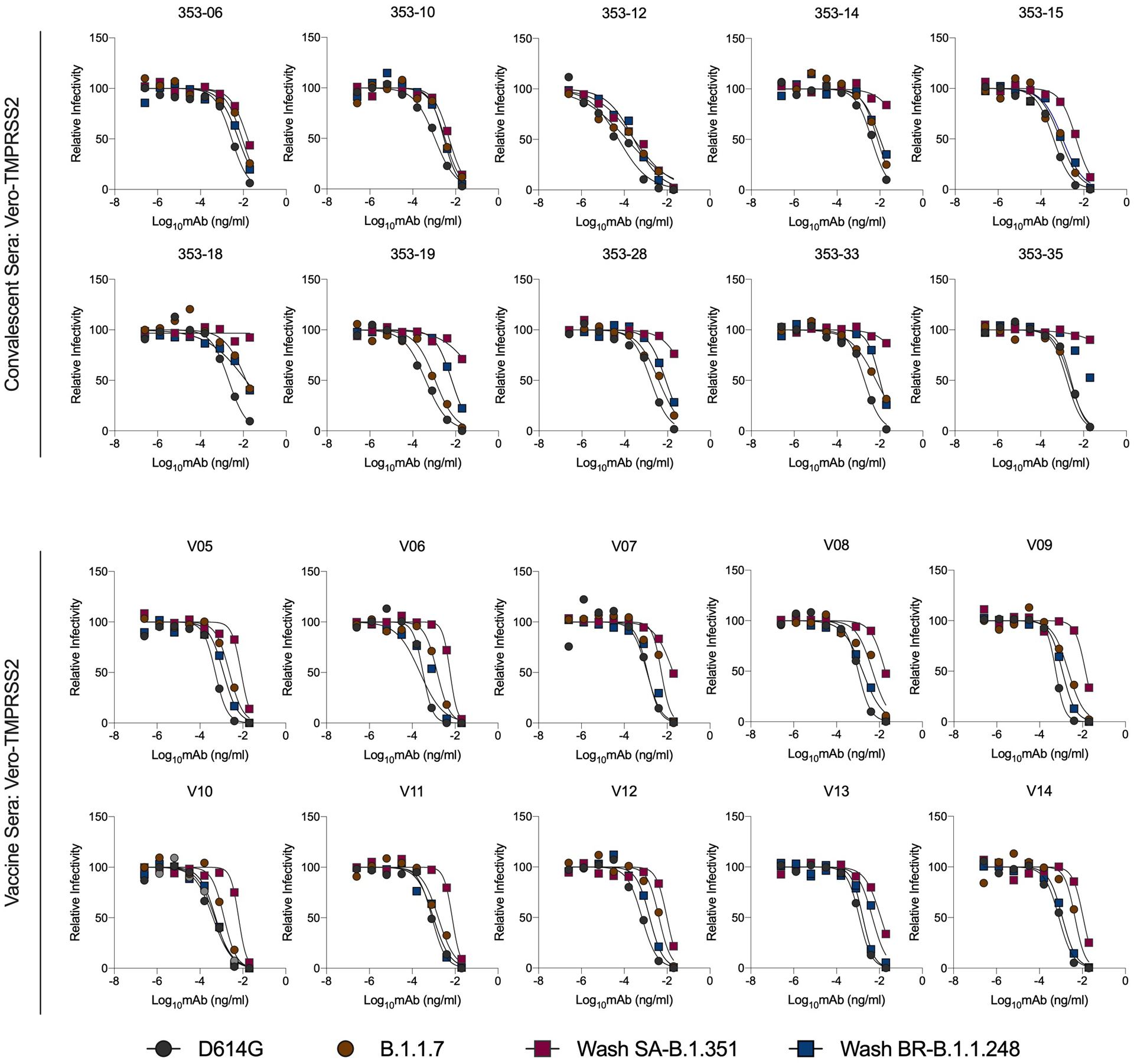
Serum from individuals (n = 10) who had been infected with SARS-CoV-2 (~ 1-month time point) or vaccinated with the Pfizer-BioNTech mRNA vaccine (n = 10) were tested for neutralization of the indicated SARS-CoV-2 strains (D614G, B.1.1.7, Wash SA-B.1.351, Wash BR-B.1.248) using a FRNT and Vero-TMPRSS2 cells. One experiment performed in duplicate is shown, with some exceptions due to limited sample.
Extended Data Fig. 10. Differential serum neutralization of SARS-CoV-2 produced in Vero E6 and Vero-hACE2-TMPRSS2 cells.
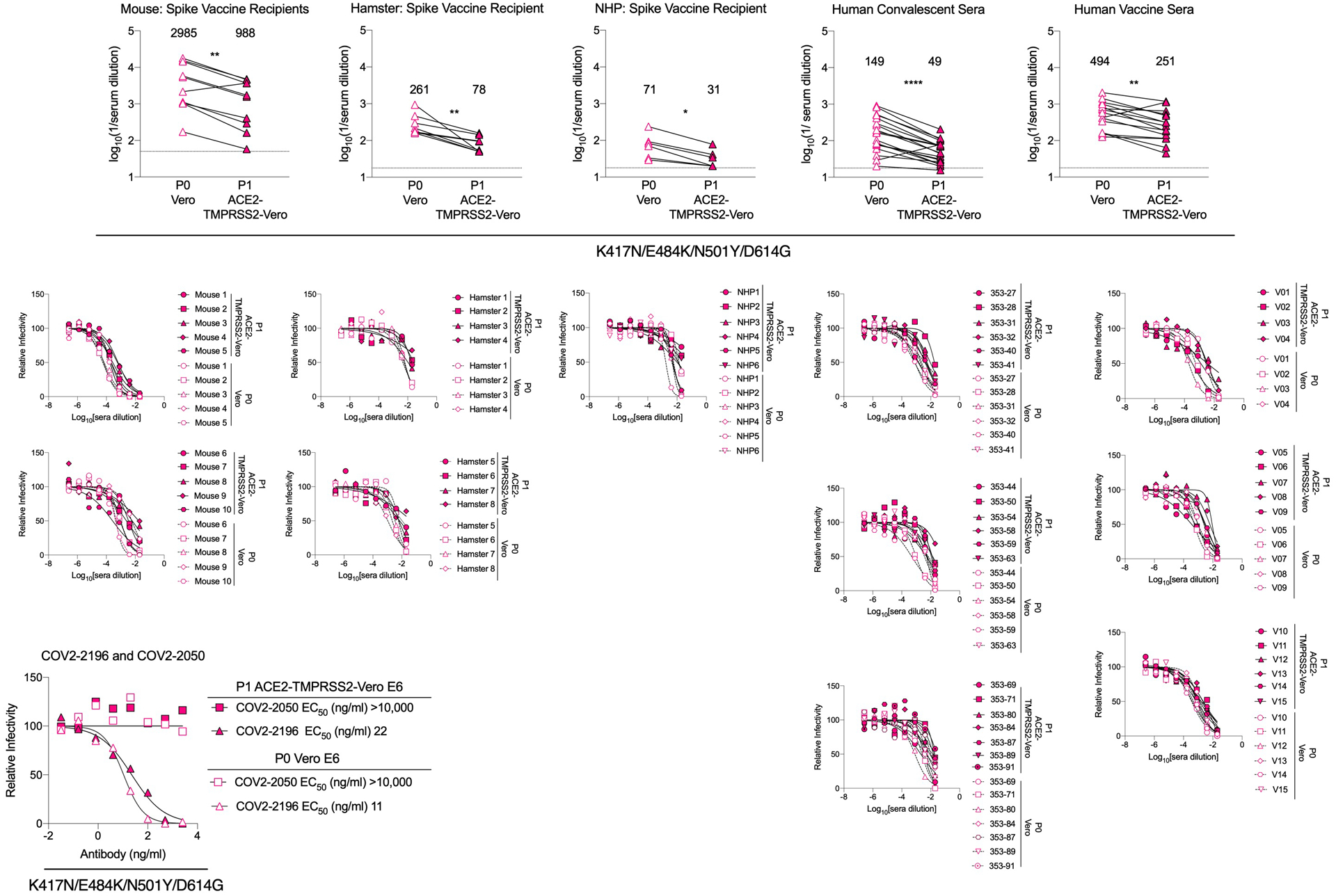
((Top panels) Immune or vaccine-derived sera from mice, hamsters, NHP, or humans (see Figures 2, 3, and 4) were incubated with deep-sequenced confirmed p0 (Vero cell-produced) or p1 (Vero-hACE2-TMPRSS2 cell-produced) versions of K417N/E484K/N501Y/D614G virus and then subjected to a FRNT in Vero-hACE2-TMPRSS2 recipient cells. EC50 values were calculated from one experiment performed in duplicate. GMT values are shown above each graph. Dotted line represents the limit of detection of the assay. Two-tailed Wilcoxon matched-pairs signed rank test: Mouse vaccine sera, P = 0.0039; Hamster vaccine sera, P = 0.0078; NHP vaccine sera, P = 0.0312; Human convalescent sera, P = 0.0001; Human vaccine sera, P = 0.0026. (Middle panels) Serum neutralization curves with K417N/E484K/N501Y/D614G virus (p0, generated in Vero E6 cells; p1, generated in Vero-hACE2-TMPRSS2 cells) using a FRNT and Vero-hACE2-TMPRSS2 cells. One experiment performed in duplicate is shown. (Bottom left panel) Neutralization curves and EC50 values with COV2–2050 and COV2–2196 mAbs using the p0 (Vero cell-produced) or p1 (Vero-hACE2-TMPRSS2 cell-produced) viruses and recipient Vero-hACE2-TMPRSS2 cells.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by contracts and grants from NIH (75N93019C00062, 75N93019C00051, 75N93019C00074, HHSN272201400006C, HHSN272201400008C, R01 AI157155, U01 AI151810, R01 AI142759, R01 AI134907, R01 AI145617, UL1 TR001439, P30 AR073752, U01AI141990), and the Defense Advanced Research Project Agency (HR001117S0019), the Dolly Parton COVID-19 Research Fund at Vanderbilt University, Fast Grants (Mercatus Center, George Mason University), and the Future Insight Prize (Merck KGaA; to J.E.C). J.B.C. is supported by a Helen Hay Whitney Foundation postdoctoral fellowship, E.S.W. is supported by F30 AI152327, and J.S.T. is supported by 5T32CA009547. P-Y.S. is supported by awards from the Sealy & Smith Foundation, the Kleberg Foundation, the John S. Dunn Foundation, the Amon G. Carter Foundation, the Gilson Longenbaugh Foundation, and the Summerfield Robert Foundation. P.D. is supported by a Junior Faculty Development Award from the American College of Gastroenterology. T.K.T. is funded by the EPA Cephalosporin Early Career and Teaching Fellowship and Townsend Jeantet Charitable Trust (charity number 1011770). We thank Rachel Nargi and Robert Carnahan for assistance with mAb generation and purification, Gavin Screaton for providing COVOX--0 and COVOX-384, Lisa Purcell for critical comments on experiments and the manuscript, and Adam Bailey, Adrian Creanga and Barney Graham for the Vero-hACE2-TMPRSS2 cells, and the Laboratory of Virology, Division of Intramural Research, NIAID, NIH for the NHP immune sera.
COMPETING FINANCIAL INTERESTS
M.S.D. is a consultant for Inbios, Vir Biotechnology, NGM Biopharmaceuticals, and Carnival Corporation, and on the Scientific Advisory Boards of Moderna and Immunome. The Diamond laboratory has received funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions. J.E.C. has served as a consultant for Eli Lilly and Luna Biologics, is a member of the Scientific Advisory Boards of CompuVax and Meissa Vaccines and is Founder of IDBiologics. The Crowe laboratory at Vanderbilt University Medical Center has received sponsored research agreements from AstraZeneca and IDBiologics. Vanderbilt University (J.E.C.) and Washington University (A.H.E., A.C.M.B., M.S.D., and D.H.F.) have applied for patents related to antibodies described in this paper. The Ellebedy laboratory has received funding support in sponsored research agreements from AbbVie and Emergent BioSolutions. The Boon laboratory has received funding support in sponsored research agreements from AI Therapeutics, GreenLight Biosciences Inc., AbbVie Inc., and Nano targeting & Therapy Biopharma Inc. The Shi laboratory has received sponsored research agreements from Pfizer, Gilead, Merck, and IGM Sciences Inc. D.P., D.C., and H.W.V. are employees of Vir Biotechnology and may hold equity in Vir Biotechnology. H.W.V. is a founder of Casma Therapeutics and PierianDx. P.D. has served as an advisory board member for Janssen, Pfizer, Prometheus Biosciences, and Arena Pharmaceuticals and has received funding support in sponsored research agreements from Takeda Pharmaceuticals.
REFERENCES
- 1.Sempowski GD, Saunders KO, Acharya P, Wiehe KJ & Haynes BF Pandemic Preparedness: Developing Vaccines and Therapeutic Antibodies For COVID-19. Cell 181, 1458–1463 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letko M, Marzi A & Munster V Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology 5, 562–569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto D, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182, 73–84 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zost SJ, et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat Med 26, 1422–1427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes CO, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tortorici MA, et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science 370, 950–957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao L, et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 370, 426–431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathe JA, et al. SARS-CoV-2 Serologic Assays in Control and Unknown Populations Demonstrate the Necessity of Virus Neutralization Testing. J Infect Dis (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wibmer CK, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv (2021). [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tada T, et al. Neutralization of viruses with European, South African, and United States SARS-CoV-2 variant spike proteins by convalescent sera and BNT162b2 mRNA vaccine-elicited antibodies. bioRxiv (2021). [Google Scholar]
- 13.Wang P, et al. Increased Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7 to Antibody Neutralization. bioRxiv (2021). [Google Scholar]
- 14.Leung K, Shum MH, Leung GM, Lam TT & Wu JT Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill 26(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klimstra WB, et al. SARS-CoV-2 growth, furin-cleavage-site adaptation and neutralization using serum from acutely infected hospitalized COVID-19 patients. J Gen Virol 101, 1156–1169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson BA, et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappazzo CG, et al. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Case JB, et al. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host and Microbe 28, 475–485 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zost SJ, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 584, 443–449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsoussi WB, et al. A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J Immunol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suryadevara N, et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. bioRxiv (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie X, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nature Medicine, In press (2021). [DOI] [PubMed] [Google Scholar]
- 23.Wu K, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv (2021). [Google Scholar]
- 24.Rathnasinghe R, et al. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post-vaccination human sera. medRxiv : the preprint server for health sciences (2021). [Google Scholar]
- 25.Baum A, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellebedy A, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Research square (2020). [DOI] [PubMed] [Google Scholar]
- 27.Hassan AO, et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 183, 169–184.e113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bricker TL, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. bioRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan AO, et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. bioRxiv (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greaney AJ, et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain that Escape Antibody Recognition. Cell Host Microbe 29, 44–57.e49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccoli L, et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 183, 1024–1042.e1021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. bioRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisblum Y, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife 9(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreano E, et al. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. bioRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jangra S, et al. The E484K mutation in the SARS-CoV-2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post-vaccination sera. medRxiv : the preprint server for health sciences (2021). [Google Scholar]
- 38.Kim JH, Marks F & Clemens JD Looking beyond COVID-19 vaccine phase 3 trials. Nat Med (2021). [DOI] [PubMed] [Google Scholar]
- 39.Schäfer A, et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med 218(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler ES, et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions and monocytes for optimal therapeutic protection. bioRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zohar T, et al. Compromised Humoral Functional Evolution Tracks with SARS-CoV-2 Mortality. Cell 183, 1508–1519.e1512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dan JM, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipsitch M, Grad YH, Sette A & Crotty S Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat Rev Immunol 20, 709–713 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sette A & Crotty S Adaptive immunity to SARS-CoV-2 and COVID-19. Cell (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey AL & Diamond MS A Crisp(r) New Perspective on SARS-CoV-2 Biology. Cell 184, 15–17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, et al. Neutralizing Activity of BNT162b2-Elicited Serum - Preliminary Report. N Engl J Med (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goddard TD, et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zang R, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 5(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plante JA, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie X, et al. An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe 27, 841–848.e843 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie X, et al. Engineering SARS-CoV-2 using a reverse genetic system. Nat Protoc (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S, Zhou Y, Chen Y & Gu J fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H & Durbin R Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poplin R, et al. A universal SNP and small-indel variant caller using deep neural networks. Nat Biotechnol 36, 983–987 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCallum M, et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. bioRxiv (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dejnirattisai W, et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong J, et al. Genetic and structural basis for recognition of SARS-CoV-2 spike protein by a two-antibody cocktail. bioRxiv (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and are available from the corresponding author upon request. Deep sequencing datasets of viral stocks are available at NCBI BioProject PRJNA698378 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA698378).


