Abstract
Objectives:
Tumour-positive resection margins are a major problem during oral cancer surgery. gGlu-HMRG is a tracer that becomes fluorescent upon activation by gamma-glutamyltranspeptidase (GGT). This study aims to investigate the combination of gGlu-HMRG and a clinical fluorescence imaging system for the detection of tumour-positive resection margins.
Materials and methods:
The preclinical Maestro and clinical Artemis imaging systems were compared in vitro and ex vivo with cultured human head and neck cancer cells (OSC19, GGT-positive; and FaDu, GGT negative) and tumour-bearing nude mice. Subsequently, frozen sections of normal and oral cancer tissues were ex vivo sprayed with gGlu-HMRG to determine the sensitivity and specificity. Finally, resection margins of patients with suspected oral cancer were ex vivo sprayed with gGlu-HMRG to detect tumour-positive resection margins.
Results:
Both systems could be used to detect gGlu-HMRG activation in vitro and ex vivo in GGT positive cancer cells. Sensitivity and specificity of gGlu-HMRG and the Artemis on frozen tissue samples was 80% and 87%, respectively. Seven patients undergoing surgery for suspected oral cancer were included. In three patients fluorescence was observed at the resection margin. Those margins were either tumour-positive or within 1 mm of tumour. The margins of the other patients were clear (≥8 mm).
Conclusion:
This study demonstrates the feasibility to detect tumour-positive resection margins with gGlu-HMRG and a clinical fluorescence imaging system. Applying this technique would enable intraoperative screening of the entire resection margin and allow direct re-resection in case of tumour-positivity.
Keywords: Squamous cell carcinoma, Optical imaging, Fluorescence, Margins of excision, Head and neck neoplasms, Oral cavity
Introduction
Surgery remains the treatment of choice for the curative therapy of squamous cell carcinoma (SCC), including oral cancer. A resection requires wide margins to ensure no residual tumour tissue is left behind after surgery. However, such resections often cause functional loss. In the case of oral cancer, inadequate resection margins (i.e. close and positive margins) are reported in up to 85% of these patients [1,2]. These patients more often develop local recurrences and regional neck metastases, resulting in decreased survival rates [3]. Patients with oral cancer often have leucoplakia, which makes intraoperative discrimination between benign and malignant tissue challenging. Intraoperative frozen section analysis is sometimes used in oral cancer surgery to assess whether the resection margin is free of tumour. However, this method has certain drawbacks. Sampling errors can result in incorrect diagnosis. A pathologist is required for histopathological examination where the subsequent processing of the biopsy would then delay the surgical procedure [4,5]. Ideally, the entire resection surface should be evaluated during the operation, but this is not possible with current strategies.
Recently, several clinical studies with tumour-targeted fluorescent tracers demonstrated the feasibility to visualize tumours, and more importantly their margins, during surgery [6–8]. Cetuximab was conjugated to the near-infrared fluorescent dye IRDye800CW. The combination enabled demarcation of tumours with millimetre-resolution in oral cancer patients [9]. These results are promising, but these tracers have several disadvantages. First, intravenous administration may lead to adverse reactions. Cetuximab, for example, can cause severe infusion reactions and other harmful side effects [10]. Second, antibody-based tracers have long plasma half-lives, and intravenous administration requires relatively high doses to have sufficient tracers reach the tumour. Unbound tracers in the systemic circulation result in non-specific background fluorescence. This reduces the signal-to-background ratio (SBR) and potentially hampers visualization of specific fluorescence. Third, administration of labelled antibodies to patients needs to be applied several days before surgery, which requires an additional visit or earlier admission to the hospital. This requires additional planning and is inconvenient for patients. Fourth, clinical translation of novel fluorescent tracers is costly, time-consuming and requires specific expertise.{11]
All these issues can be solved by topically applying activatable fluorescent tracers instead. This strategy requires a much lower dose and does not suffer from nonspecific fluorescence from unbound tracers. Moreover, spraying activatable tracers on only resected tissue only would not require costly translational research. Furthermore, such an approach would completely diminishes the risk of adverse events.
Recently, γ-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG) was developed for the detection and diagnosis of preclinical cancer [12]. HMRG remains quenched until cleaved in the presence of the enzyme gamma-glutamyltranspeptidase (GGT). After activation HMRG emits fluorescence with a peak at 525 nm. Application of gGlu-HMRG on resected material was investigated to identify tumour-positive resection margins in breast cancer patients [13]. Mizushima et al. [14] showed that fluorescence imaging with gGlu-HMRG could be useful for screening of early-stage head and neck SCC. Shimane et al. demonstrated that gGlu-HMRG has high sensitivity for ex vivo detection of oral SCC [15]. However, to have clinical significance for oral cancer patients during surgery, intraoperative detection of microscopic tumour-positive margins of SCC is required.
The feasibility of this technique was demonstrated with a preclinical fluorescence imaging system [12], which cannot be used in the operating room. To investigate if an intraoperative clinical imaging system such as the Artemis [16] can be used to image the activation of gGlu-HMRG, we compared the sensitivity with the Maestro preclinical imaging system. The aims of this study are outlined as follows: (A) to compare those aforementioned combinations using human head and neck cancer cell lines, (B) to determine the sensitivity and specificity of gGlu-HMRG on ex vivo frozen human oral cancer tissues, and (C) to study the feasibility to detect microscopic tumour-positive resection margins in oral cancer patients.
Materials and methods
Flow cytometry and fluorescence microscopy
The protocol adopted for this study was described previously by Urano et al. [12]. Flow cytometry was performed with the BD LSR-II Flow Cytometer for cell evaluation and subsequent cell measurements were analysed with FlowJo software (FlowJo, LLC, Ashland, OR, USA). Two human SCC cell lines, OSC19 (oral cancer; GGT overexpressing) and FaDu (hypopharyngeal cancer; GGT low expressing), were used. After incubation with gGlu-HMRG, the cells were nuclear stained with Hoechst fluorescent stain (Invitrogen). Images were obtained every 5 min for 1 h after application using the Leica microsystems LAS AF6000 modular systems at 20× magnification. Quantification of the signal over time was assessed using the in-house developed software program, called ‘Stacks’, which operates using the Microsoft Windows operating system. Each measurement was corrected to make translation between separate images possible after which a mask was created based on the last image (t = ’60). Within the mask the average intensity was measured for all images and the standard deviation was calculated as a percentage of the intensity.
Imaging systems
Two imaging systems were compared. The preclinical CRi Maestro imaging system (CRi Inc., Woburn, MA, Perkin Elmer, Waltham, MA, USA) consists of a 300 W xenon-based excitation light source with seven possible excitation and emission filter pairs, covering the complete spectral range from 500 to 950 nm. Every pixel can be analysed by a process called spectral unmixing, in which the signals from the background and fluorophore are separated. The software quantifies each pixel and determines relative concentration of the fluorophore present in that pixel [17,18].
The Artemis fluorescence imaging system [16] (Quest Medical Imaging B.V., The Netherlands) used in this study consisted of a “white” light source, and a light source at 490 nm. Colour video and fluorescence images are simultaneously acquired on separate sensors and displayed in real-time. A pseudo-coloured (lime green) merged image of the colour video and fluorescence images is also displayed. The intensities of the light sources and exposure times could be controlled with the Artemis software.
In vitro imaging of gGlu-HMRG
OSC19 or FaDu cells (2 × 104 cells/well) were seeded into a 12-well plate. The cells were incubated at a timepoint of 48 h after seeding, with gGlu-HMRG at five different concentrations (1, 2, 10, 50, and 100 μM) in PBS for 30 min. PBS without cells, but with similar concentrations of gGlu-HMRG was used as the background signal to calculate a SBR. Images were obtained using the Maestro and Artemis camera system, and analysed by measuring ROIs with the Maestro and ImageJ software (v.1.48, National Institutes of Health, USA), respectively.
In vivo imaging of gGlu-HMRG
The local animal welfare committee of Leiden University Medical Center approved the animal experiments. OSC19 cancer cells (60.000 cells/10 μl medium) were injected into the submucosa of the tip of the tongue of six to eight weeks-old female nude Balb/c mice (Charles River laboratories, France). Health and tumour growth was monitored by weighing and inspecting the mice at least twice a week. Tongue inspections were carried out under isoflurane gas anaesthesia.
One to two weeks after cell injection, when the tumour diameter had reached approximately 5 mm, gGlu-HRMG (100 μl, 50 μM in PBS) was sprayed on the tongue under general anaesthesia (n = 4). A control tongue (n = 1) was sprayed with PBS. The tongues were imaged by the Maestro imaging system 30 min after topical application. Mice were sacrificed and tongues were directly harvested for ex vivo imaging both by Maestro and Artemis. SBRs were calculated with ImageJ.
To confirm signal and tumour overlay, the tongues were cut into cryosections of 10 μm after which they were stained by haematoxylin and eosin (H&E).
Ex vivo evaluation of patient-derived cancer frozen specimen
Frozen tissues of 15 patients with oral cancer were analysed to assess sensitivity and specificity. Normal tissue of the oral cavity of each patient was used as a negative control. Tissues were obtained from the Erasmus Medical Center (Rotterdam, The Netherlands) tissue bank. Cryosections were cut into slices of 10 μm. The cryosections were imaged by fluorescence microscopy (Leica microsystems DM5500 B, Eindhoven, The Netherlands) at 0, 1, 5 and 10 min after application of gGlu-HMRG (50 μl of 50 μM in PBS). Fluorescence intensity (negative, weak, or positive) was scored independently by two authors (MS & HH). Subsequently, each slice was stained using an anti-GGT1 antibody (dilution 1:800; ab55138, Abcam, Cambridge, UK). The staining of epithelium was also scored (negative, weak, or positive) by two authors (LH & HH). Concordance between fluorescence and GGT status was subsequently evaluated.
Ex vivo evaluation of resection margins after oral cancer surgery
Patients with suspected or biopsy-proven oral cancer and scheduled to undergo resection with curative intent were eligible for inclusion. Patients were excluded if they received: (A) radiotherapy, (B) had undergone previous surgery in the oral cavity, or (C) diagnosed with multiple primary oral tumours or any active autoimmune diseases (e.g. Sjögren or lichen planus). The latter category was excluded for their diffuse abnormalities in the oral cavity.
The local medical ethical committee decided no formal approval from patients was required, because the spray was applied ex vivo, the tissue was handled anonymously, the outcomes did not influence treatment, and the surgeon and pathologist were blinded for the study. This pilot study was designed to prevent any potentially harmful change in standard care.
Directly after surgery the edges (margins) of the primary specimen were sprayed with 1 ml of 50 μM gGlu-HRMG per 6 cm2. No intraoperative frozen sections were analyzed with these patients, only the primary tumor resection specimen. Upcoming fluorescent spots were marked with ink. Subsequently, the epithelial side was sprayed, which functioned as a positive control. Fluorescence imaging was performed with the Artemis only. The resection margins were assessed for tumour positivity after embedding into paraffin using standard of care. The tissue was cut into slices of 10 μm and stained for H&E staining and GGT (dilution 1:1600). Spots marked with ink were checked for tumour and GGT status.
Results
Activation and uptake of gGlu-HMRG
Both the oral cancer cell line OSC19 and the hypopharyngeal cancer cell line FaDu, were evaluated for tracer uptake by flow cytometry. OSC19 was found to provide activation of gGlu-HMRG, while the FaDu cell line was identified as almost negative in responding to GGT (Fig. 1A and B). More than 70% of the total intracellular activation of gGlu-HMRG already occurred within 1 min (Fig. 1C), but the signal continued to increase up to 60 min (Fig. 1D). The relatively high standard deviations are explained by the translation between the images at different times and the movement of cells. Activation and internalization of gGlu-HMRG by OSC19 cells was clearly visible with fluorescence microscopy (Fig. 1D).
Fig. 1.
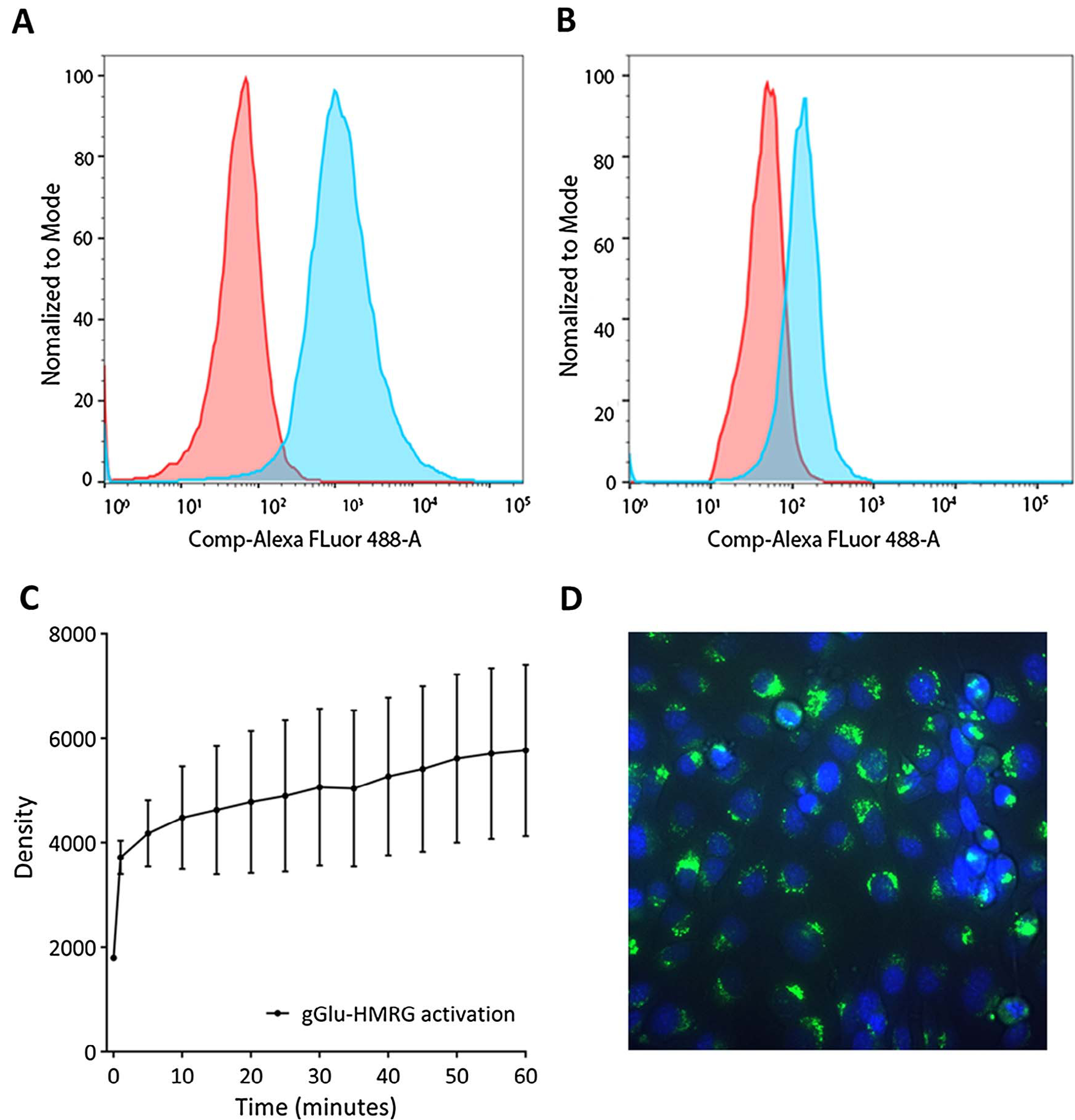
Flow cytometry of the GGT-positive cell line OSC19 (A) and the negative cell line FaDu (B). The majority of the activation of gGlu-HMRG on OSC19 cells occurs within the first ten minutes (C). HMRG is internalized after activation (D; 40 times enlarged).
Maestro vs. Artemis
Tracer activation by OSC19 and FaDu cells was imaged by comparing the performance of the Maestro, allowing spectral unmixing, and the clinical Artemis imaging system. The Maestro, which facilitates spectral unmixing, was capable of generating higher SBRs in OSC19 cells, whilst the Artemis showed already an increase of SBRs at lower concentrations (Fig. 2A and C). The highest SBRs were achieved at an applied concentration of 100 μM performed on the Maestro instrument. However, even when a concentration of 50 μM was applied, the difference in detection between the two systems did not prove to be statically significant (p = .15). The latter concentration was therefore considered optimal and used for further experiments. Moreover, 50 μM resulted in the highest SBR with the Artemis. FaDu showed very weak fluorescence originating from activated gGlu-HMRG with both fluorescence systems (Fig. 2B and D).
Fig. 2.
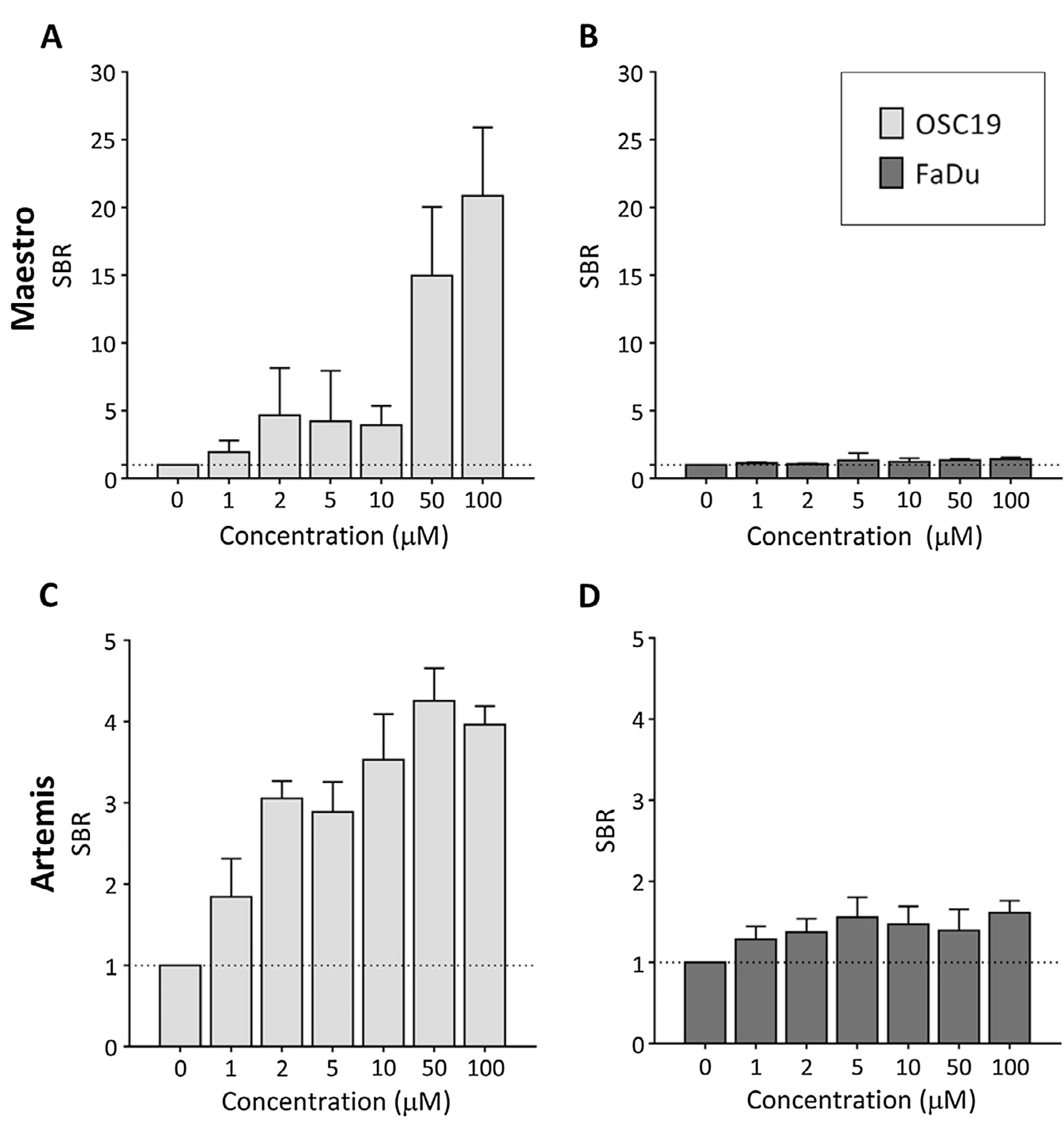
The Maestro was capable of generating high signal-to-background ratios (SBR) in OSC19 cells, especially with 50 μM and 100 μM (A). The Artemis demonstrated already an increase of SBRs at lower concentrations (C). FaDu showed very weak fluorescence originating from activated gGlu-HMRG with both fluorescence systems (B & D).
Fig. 3 demonstrates the visual and numeral differences between the preclinical Maestro and the clinical Artemis system in an orthotopic oral cancer model. SBRs of ex vivo tongues were 22.3 ± 2.7 (control tongue: 1.4) and 4.1 ± 1.2 (control tongue: 1.1) after spraying gGlu-HMRG for the Maestro and Artemis, respectively.
Fig. 3.

Shown are examples of in vivo and ex vivo measurements with the Maestro and colour and fluorescence images made ex vivo with the Artemis. On the right matching signal-to-background ratios of a control (n = 1) and tongues sprayed with gGlu-HMRG (n = 4). Both systems could be used to recognize GGT positive cancer cells of in in vitro and in vivo studies.
In brief, both systems could be used to recognize GGT positive cancer cells of in in vitro and in vivo studies.
Ex vivo evaluation of patient-derived cancer frozen specimen
Fifteen frozen tissue slices with oral cancer were included: 10 with tongue cancer and 5 with cancer of the floor of the mouth (Table 1) (Fig. 4). Fifteen tissue slices without cancer from the same patients were included. Twelve out of 15 malignant lesions showed fluorescence after spraying with gGlu-HMRG (sensitivity 80%). Thirteen normal tissue slices showed either none or only weak fluorescence (specificity 87%). Twelve out of 14 fluorescent lesions (86%) were either weak or positive for GGT. None of the normal tissues stained positive for GGT, while none of the matching malignant lesions were negative for GGT. In five tissue samples some stromal cells showed expression of GGT. However, no fluorescence was observed in these samples and the epithelial cells did not show expression of GGT.
Table 1.
Scoring results.
| Normal | Fluorescence |
Malignant | Fluorescence |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Neg | Weak | Pos | Neg | Weak | Pos | ||||
| GGT | Neg | 6 | 5 | 2 | GGT | Neg | 0 | 0 | 0 |
| Weak | 2 | 0 | 0 | Weak | 0 | 2 | 3 | ||
| Pos | 0 | 0 | 0 | Pos | 1 | 0 | 9 | ||
Fig. 4.
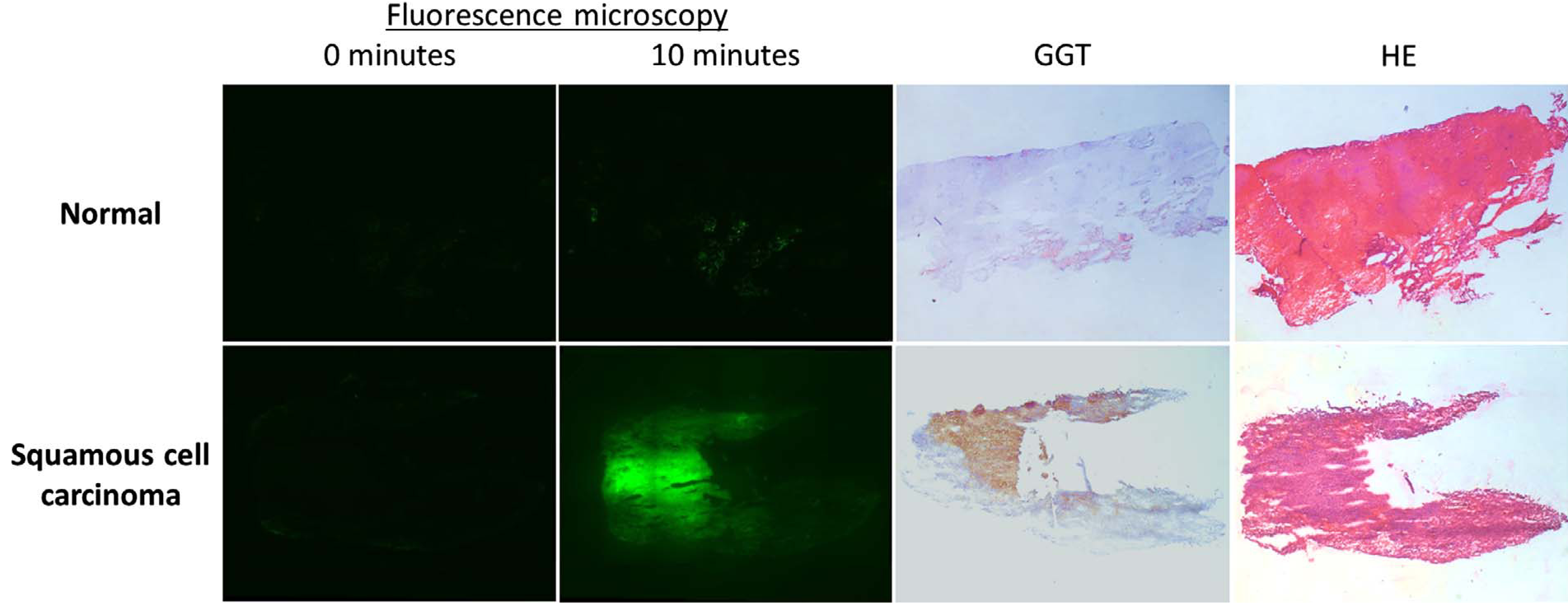
Example of fluorescence microscopy of normal and malignant frozen tissue (2.5 times enlarged). Ten minutes after spraying with gGlu-HMRG, the normal tissue displayed weak fluorescence. Malignant tissue became clearly fluorescent, which correlated with positive GGT staining.
Ex vivo evaluation of resection margins after oral cancer surgery
Seven patients undergoing surgery for suspected oral cancer were included (Table 2). In three patients fluorescence was observed at the resection margin (Fig. 5). In those patients, routine pathological examination demonstrated resection margins that were either tumour-positive or within 1 mm of tumour. The margins of the other four patients were at least 8 mm tumour-free. The epithelial side showed fluorescence in all except one patient. That patient turned out to have no malignancy, but parakeratosis, hyperplasia and a chronic lymphocytic inflammatory infiltrate.
Table 2.
Fluorescence imaging and histopathological evaluation of the primary tumor resection specimen.
| Histopathology |
Fluorescence |
GGT (tumour) | |||
|---|---|---|---|---|---|
| No. | Diagnosis | Margin | RM | ES | |
| 1 | Keratinizing squamous cell carcinoma | Clear (8 mm) | − | + | + |
| 2 | Parakeratosis, hyperplasia | NA | − | − | − |
| 3 | Keratinizing squamous cell carcinoma | Tumour-positive | + | + | + |
| 4 | Keratinizing squamous cell carcinoma | Tumour-positive | + | + + | + |
| 5 | Keratinizing squamous cell carcinoma | Clear (8 mm) | − | + | + |
| 6 | Squamous cell carcinoma | Clear (8 mm) | − | + + | + |
| 7 | Squamous cell carcinoma, well differentiated | Close (4 mm); focal expansion CIS (1 mm) | + | + + | + |
RM: resection margin; ES: epithelial side; NA: not applicable; CIS: carcinoma in situ.
Fig. 5.
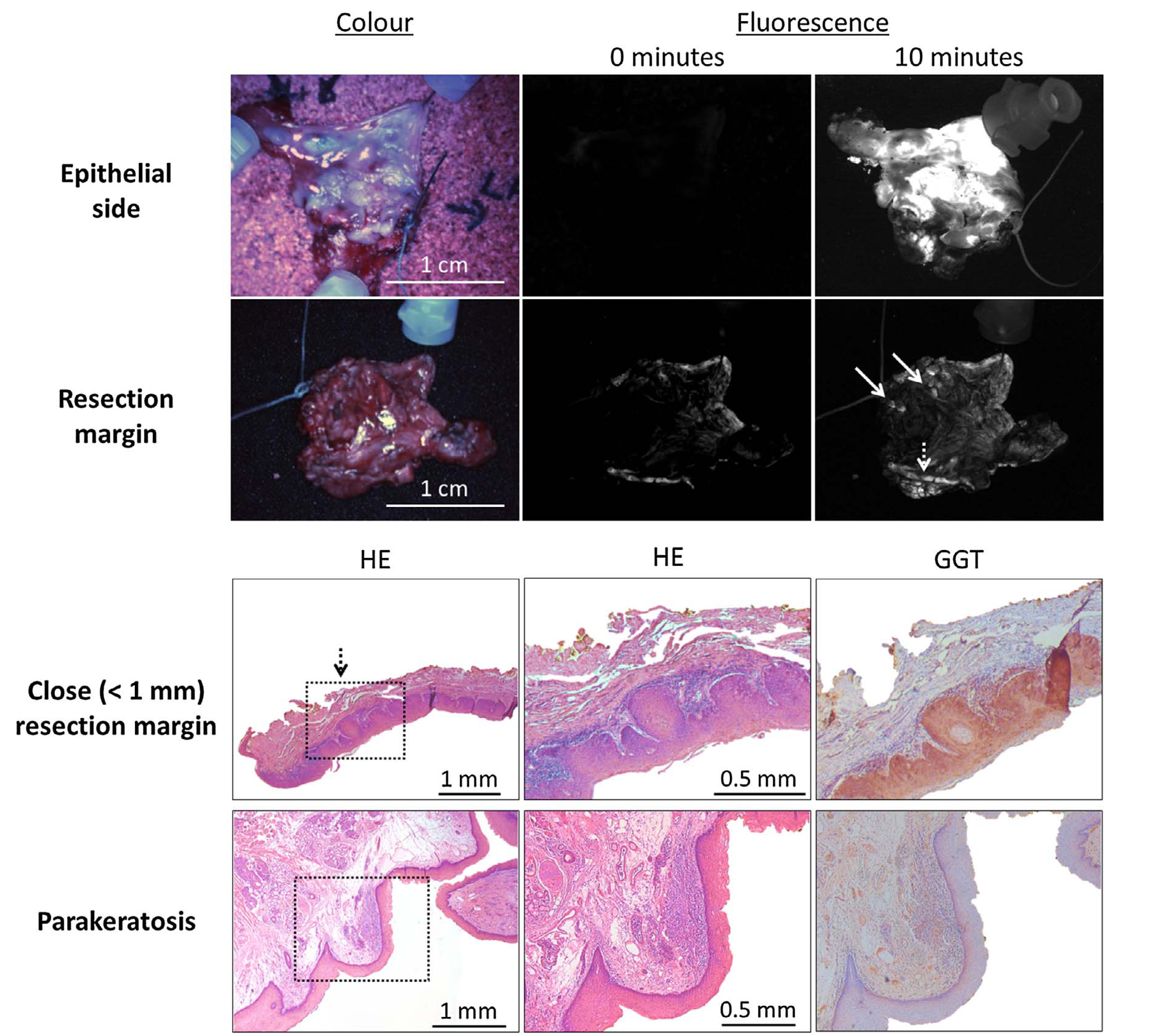
Example of resection margin assessment with gGlu-HMRG and the clinical Artemis imaging system (upper two panels). Ten minutes after spraying on the tissue, several spots became fluorescent in the resection margin (arrows). In one patient with squamous cell carcinoma, focal expansion of a carcinoma in situ < 1 mm from the resection margin was diagnosed with fluorescence imaging (dashed arrow). The epithelial side was used as a positive control.
Following fluorescence imaging, both the epithelial side and resection margin of 16 tissue slices were histopathologically evaluated, resulting in 32 measurements. A total of 11 slices contained oral cancer at the epithelial side and all were fluorescent. Three slices with fluorescent spots at the epithelial side did not contain tumour. Three patients had an inadequate resection margin. Two slices with fluorescent spots contained tumour-positive margins. Four slices were obtained near fluorescent spots in the resection margins of two patients, but no cancer cells could be detected. However, routine histopathological examination in both patients diagnosed a tumour-positive resection margin. Overall, the sensitivity, specificity and accuracy of fluorescence imaging were 100% (13/13), 63% (12/19) and 78% (25/32), respectively.
Discussion
This study demonstrates that fluorescence imaging of resection margins using topically applied activatable tracers can enable detection of microscopic tumour-positive resection margins. Our results pave the way for a larger future study, to include more oral cancer patients, where sensitivity and specificity may be determined. Additionally, the factoring in of recurrence-free and disease-free survival would also help validate this tool, where we could diagnose tumour positive resection margins during surgery. This would permit for the surgeon to directly attempt another resection. Moreover, if the utility of the technique is proven, this could mean a major reduction of costs and time, because intraoperative frozen section analysis will become unnecessary.
Even though assessing the margins of resected tissue with fluorescence imaging could already mean an improvement for surgeons and patients, it would even be better if also the in vivo resection margin could be screened with gGlu-HMRG. This requires certain toxicology studies prior to human use, but topical applications require less extensive and cheaper studies compared to intravenous administered tracers. Systemic uptake will probably be very low, which generally results in favourable safety.
Our work with gGlu-HMRG is not the first activatable tracer to be used clinically for resection. 5-aminolevulinic acid (5-ALA), a nonfluorescent precursor of haemoglobin, is already being used clinically [19,20]. Oral administration of 5-ALA induces accumulation of fluorescent protoporphyrin IX (emission peak at 635 nm) inside malignant tumours. A randomized controlled trial demonstrated that 5-ALA and fluorescence imaging enables a more complete resection and improved progression-free survival in patients with malignant glioma [21]. Even though the diseases and the consequences are not comparable, gGlu-HMRG has the potential to have a similar e?ect on radical resection rates and thereby on surgical outcomes.
In this study no GGT-negative oral tumours were encountered in a total of 21 tumours. Comparably, all ten tumours with oral SCC included in the ex vivo study by Shimane et al. were GGT positive and showed fluorescence 10 min after applying gGlu-HMRG [15]. This is in concordance with literature; most SCCs demonstrate intense GGT overexpression [14,22]. However, in the unlikely case of GGT-negative tumours, applying gGlu-HMRG would result in false-negative results. This can be detected easily by using the epithelial side of the tumour as a positive control, as shown in this study.
The fluorescence signals did not align perfectly in all ex vivo frozen tissues (Fig. 4). There are several explanations. Tissue slices sprayed with gGlu-HMRG stained afterwards negative for GGT. We therefore used consecutive, but not identical, slices. Furthermore, gGlu-HMRG is internalized in vivo after activation. However, this process does not occur ex vivo in frozen tissue, which may cause activated HMRG to spread. Lastly, a GGT1 antibody was used, whereas gGlu-HMRG may be specific for only certain subtypes of GGT. This hypothesis is reinforced by the finding that some stromal cells expressed GGT, but did not show any fluorescence. Nevertheless, fluorescence signals strongly correlated with GGT expression.
Seven tissue slices obtained from the proximity of fluorescent spots did not contain cancer cells. These results were either false-positive, or caused by sampling errors. Some fluorescent spots were very small (see Fig. 5), which made it challenging and sometimes impossible to identify them in a single slice. Unfortunately, the tissue blocks could not completely used, because sufficient tissue needed to be preserved for other research purposes and additional routine histopathological examination.
It was sometimes difficult to discriminate between tissue autofluorescence and fluorescence from activated gGlu-HMRG (see Fig. 5). More research is required to understand the nature of false-positive fluorescence signals. For example, in this study we chose to subjectively discriminate between negative, weak and positive fluorescence (Table 1). The latter two were considered to be positive, hence the sensitivity of 80% and the specificity of 87%. However, if only positive fluorescence would be considered to be positive, the sensitivity would be 93%, with a specificity of 53%. One method to reduce non-specific (auto)fluorescence is to use different fluorophores. For intraoperative imaging, fluorescence with a wavelength around 800 nm is considered optimal [23]. That wavelength is capable of deeper tissue penetration compared to 500 nm [6]. Moreover, tissue autofluorescence is reduced to a minimum, which will increase sensitivity and specificity.
Discrimination between zero measurements and activated fluorescence signals were currently performed by visual inspection. Technology exists that compares spectral differences over time, for example to detect premalignant cervical lesions after spraying acetic acid [24]. This method could allow more accurate detection of gGlu-HMRG activation by tumour-positive resection margins.
Conclusion
In conclusion, this study demonstrates the feasibility of being able to detect microscopic tumour-positive resection margins using an activatable tracer, gGlu-HMRG, in conjunction with a clinical fluorescence imaging system. Applying this technique would allow intraoperative screening of the entire resection margin and facilitate direct re-resection in case of tumour-positivity.
Acknowledgements
We thank Vincent Smit, Antien Mooyaart, Kees Sier and Marieke Prevoo for their assistance and expertise.
Funding
This work was supported by the Dutch Cancer Society (grant UL2010–4732 and UL2012–5561), the European Union’s H2020-MSCA-RISE grant (number 644373-PRISAR) and 734684-CHARMED) and MSCA-ITN-2015-ETN (number 675743-ISPIC).
Footnotes
Conflict of interest statement
None of the authors have possible conflicts of interest or financial ties to disclose.
References
- [1].Smits RW, Koljenovic S, Hardillo JA, Ten Hove I, Meeuwis CA, Sewnaik A, et al. Resection margins in oral cancer surgery: room for improvement. Head Neck 2016;38(Suppl 1):E2197–203. [DOI] [PubMed] [Google Scholar]
- [2].Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol 2005;41:1034–43. [DOI] [PubMed] [Google Scholar]
- [3].Hinni ML, Ferlito A, Brandwein-Gensler MS, Takes RP, Silver CE, Westra WH, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck 2013;35:1362–70. [DOI] [PubMed] [Google Scholar]
- [4].Du E, Ow TJ, Lo YT, Gersten A, Schiff BA, Tassler AB, et al. Refining the utility and role of Frozen section in head and neck squamous cell carcinoma resection. Laryngoscope 2016;126:1768–75. [DOI] [PubMed] [Google Scholar]
- [5].Wenig BM. Intraoperative consultation (IOC) in mucosal lesions of the upper aerodigestive tract. Head Neck Pathol 2008;2:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoogstins CE, Tummers QR, Gaarenstroom KN, de Kroon CD, Trimbos JB, Bosse T, et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res 2016;22:2929–38. [DOI] [PubMed] [Google Scholar]
- [7].Lamberts LE, Koch M, de Jong JS, Adams A, Glatz J, Kranendonk ME, et al. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res 2016. [DOI] [PubMed] [Google Scholar]
- [8].Burggraaf J, Kamerling IM, Gordon PB, Schrier L, de Kam ML, Kales AJ, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med 2015;21:955–61. [DOI] [PubMed] [Google Scholar]
- [9].Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, et al. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res 2015;21:3658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thomas M. Cetuximab: adverse event profile and recommendations for toxicity management. Clin J Oncol Nurs 2005;9:332–8. [DOI] [PubMed] [Google Scholar]
- [11].Keereweer S, Van Driel PB, Snoeks TJ, Kerrebijn JD, Baatenburg de Jong RJ, Vahrmeijer AL, et al. Optical image-guided cancer surgery: challenges and limitations. Clin Cancer Res 2013;19:3745–54. [DOI] [PubMed] [Google Scholar]
- [12].Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, et al. Rapid cancer detection by topically spraying a gamma-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med 2011;3:110ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ueo H, Shinden Y, Tobo T, Gamachi A, Udo M, Komatsu H, et al. Rapid intraoperative visualization of breast lesions with gamma-glutamyl hydroxymethyl rhodamine green. Sci Rep 2015;5:12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mizushima T, Ohnishi S, Shimizu Y, Hatanaka Y, Hatanaka KC, Hosono H, et al. Fluorescent imaging of superficial head and neck squamous cell carcinoma using a gamma-glutamyltranspeptidase-activated targeting agent: a pilot study. BMC Cancer 2016;16:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shimane T, Aizawa H, Koike T, Kamiya M, Urano Y, Kurita H. Oral cancer intraoperative detection by topically spraying a gamma-glutamyl transpeptidase-activated fluorescent probe. Oral Oncol 2016;54:e16–28. [DOI] [PubMed] [Google Scholar]
- [16].van Driel PB, van de Giessen M, Boonstra MC, Snoeks TJ, Keereweer S, Oliveira S, et al. Characterization and evaluation of the artemis camera for fluorescence-guided cancer surgery. Mol Imag Biol 2015;17:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Levenson RM, Lynch DT, Kobayashi H, Backer JM, Backer MV. Multiplexing with multispectral imaging: from mice to microscopy. ILAR J 2008;49:78–88. [DOI] [PubMed] [Google Scholar]
- [18].Mansfield JR, Gossage KW, Hoyt CC, Levenson RM. Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging. J Biomed Opt 2005;10:41207. [DOI] [PubMed] [Google Scholar]
- [19].Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 2000;93:1003–13. [DOI] [PubMed] [Google Scholar]
- [20].Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998;42:518–25. discussion 25–6. [DOI] [PubMed] [Google Scholar]
- [21].Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401. [DOI] [PubMed] [Google Scholar]
- [22].Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol 2006;71:231–8. [DOI] [PubMed] [Google Scholar]
- [23].Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 2013;10:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Louwers J, Zaal A, Kocken M, Ter Harmsel W, Graziosi G, Spruijt J, et al. Dynamic spectral imaging colposcopy: higher sensitivity for detection of premalignant cervical lesions. BJOG 2011;118:309–18. [DOI] [PubMed] [Google Scholar]


