Abstract
Purpose
To provide evidence-based recommendations to practicing physicians and others on the management of malignant pleural mesothelioma.
Methods
ASCO convened an Expert Panel of medical oncology, thoracic surgery, radiation oncology, pulmonary, pathology, imaging, and advocacy experts to conduct a literature search, which included systematic reviews, meta-analyses, randomized controlled trials, and prospective and retrospective comparative observational studies published from 1990 through 2017. Outcomes of interest included survival, disease-free or recurrence-free survival, and quality of life. Expert Panel members used available evidence and informal consensus to develop evidence-based guideline recommendations.
Results
The literature search identified 222 relevant studies to inform the evidence base for this guideline.
Recommendations
Evidence-based recommendations were developed for diagnosis, staging, chemotherapy, surgical cytoreduction, radiation therapy, and multimodality therapy in patients with malignant pleural mesothelioma.
INTRODUCTION
The purpose of this guideline is to provide recommendations for the management of patients with malignant pleural mesothelioma (MPM), an aggressive tumor with a poor prognosis. In the United States, about 3,000 new cases are diagnosed each year. The median overall survival of patients with advanced surgically unresectable disease is about 12 months.1 Given the rarity of this malignancy, there have been few large randomized trials, especially for surgical management of this disease. In general, a minority of patients are candidates for surgical resection at time of presentation; thus, the mainstay of treatment is systemic chemotherapy. For patients who are surgical candidates, surgery is performed as part of multimodality therapy involving chemotherapy with or without radiation therapy. The aim of this clinical practice guideline is to outline the management of patients with MPM, including diagnosis, pathologic confirmation, and surgical and medical management.
GUIDELINE QUESTIONS
This clinical practice guideline addresses five overarching clinical questions: (1) What is the optimal approach to obtain an accurate diagnosis of mesothelioma? (2) What initial assessment is recommended before initiating any therapy for mesothelioma? (3) What is the appropriate first- and second-line systemic treatment of patients with mesothelioma? (4) What is the appropriate role of surgical cytoreduction in the management of mesothelioma? (5) When should radiation be recommended for mesothelioma?
METHODS
Guideline Development Process
This systematic review-based guideline product was developed by a multidisciplinary Expert Panel, which included a patient representative and ASCO guidelines staff with health research methodology expertise (Appendix Table A1, online only). The Expert Panel, co-chaired by H.L.K and R.H., met via teleconference and/or webinar and corresponded through e-mail. Based upon the consideration of the evidence, the authors were asked to contribute to the development of the guideline, provide critical review, and finalize the guideline recommendations. Members of the Expert Panel were responsible for reviewing and approving the penultimate version of the guideline, which was then circulated for external review and submitted to Journal of Clinical Oncology for editorial review and consideration for publication. All ASCO guidelines are ultimately reviewed and approved by the Expert Panel and the ASCO Clinical Practice Guideline Committee prior to publication. All funding for the administration of the project was provided by ASCO.
The recommendations were developed by an Expert Panel with multidisciplinary representation using a systematic review (1990 to 2016), which included systematic reviews, meta-analyses, randomized controlled trials (RCTs), prospective and retrospective comparative observational studies, and clinical experience. Articles were selected for inclusion in the systematic review of the evidence based on the following criteria:
Population: Patients diagnosed with MPM.
Interventions that focused on diagnosis, staging, imaging, chemotherapy, radiation, and surgical cytoreduction of patients with MPM.
Study designs included were systematic reviews, meta-analyses, RCTs, and prospective and retrospective comparative observational studies. Some phase II studies were included to address some of the clinical questions for chemotherapy management.
A minimum sample size of 20.
Articles were excluded from the systematic review if they were meeting abstracts not subsequently published in peer-reviewed journals; editorials, commentaries, letters, news articles, case reports, or narrative reviews; or published in a non-English language.
The guideline recommendations are crafted, in part, using the Guidelines Into Decision Support methodology and accompanying BRIDGE-Wiz software.2 In addition, a guideline implementability review is conducted. Based on the implementability review, revisions were made to the draft to clarify recommended actions for clinical practice. Ratings for the type and strength of recommendation, evidence, and potential bias are provided with each recommendation.
Detailed information about the methods used to develop this guideline is available in the Methodology Supplement at www.asco.org/thoracic-cancer-guidelines, including an overview (eg, panel composition, development process, and revision dates), literature search and data extraction, the recommendation development process (Guidelines Into Decision Support and BRIDGE-Wiz), and quality assessment.
The ASCO Expert Panel and guidelines staff will work with co-chairs to keep abreast of any substantive updates to the guideline. Based on formal review of the emerging literature, ASCO will determine the need to update. The Methodology Supplement (available at www.asco.org/thoracic-cancer-guidelines) provides additional information about the Signals approach.
This is the most recent information as of the publication date. Visit the ASCO Guidelines Wiki at www.asco.org/guidelineswiki to submit new evidence.
In some selected cases where evidence is lacking, but there was a high level of agreement among the Expert Panel, informal consensus is used (as noted with the Recommendations).
Guideline Disclaimer
The Clinical Practice Guidelines and other guidance published herein are provided by the American Society of Clinical Oncology, Inc. (ASCO) to assist providers in clinical decision-making. The information herein should not be relied upon as being complete or accurate, nor should it be considered as inclusive of all proper treatments or methods of care or as a statement of the standard of care. With the rapid development of scientific knowledge, new evidence may emerge between the time information is developed and when it is published or read. The information is not continually updated and may not reflect the most recent evidence. The information addresses only the topics specifically identified therein and is not applicable to other interventions, diseases, or stages of diseases. This information does not mandate any particular course of medical care. Further, the information is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. Recommendations reflect high, moderate, or low confidence that the recommendation reflects the net effect of a given course of action. The use of words like “must,” “must not,” “should,” and “should not” indicates that a course of action is recommended or not recommended for either most or many patients, but there is latitude for the treating physician to select other courses of action in individual cases. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. ASCO provides this information on an “as is” basis and makes no warranty, express or implied, regarding the information. ASCO specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. ASCO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information, or for any errors or omissions.
Guideline and Conflicts of Interest
The Expert Panel was assembled in accordance with ASCO’s Conflict of Interest Policy Implementation for Clinical Practice Guidelines (“Policy,” found at http://www.asco.org/rwc). All members of the Expert Panel completed ASCO’s disclosure form, which requires disclosure of financial and other interests, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as a result of promulgation of the guideline. Categories for disclosure include employment; leadership; stock or other ownership; honoraria, consulting or advisory role; speaker’s bureau; research funding; patents, royalties, other intellectual property; expert testimony; travel, accommodations, expenses; and other relationships. In accordance with the Policy, the majority of the members of the Expert Panel did not disclose any relationships constituting a conflict under the Policy.
RESULTS
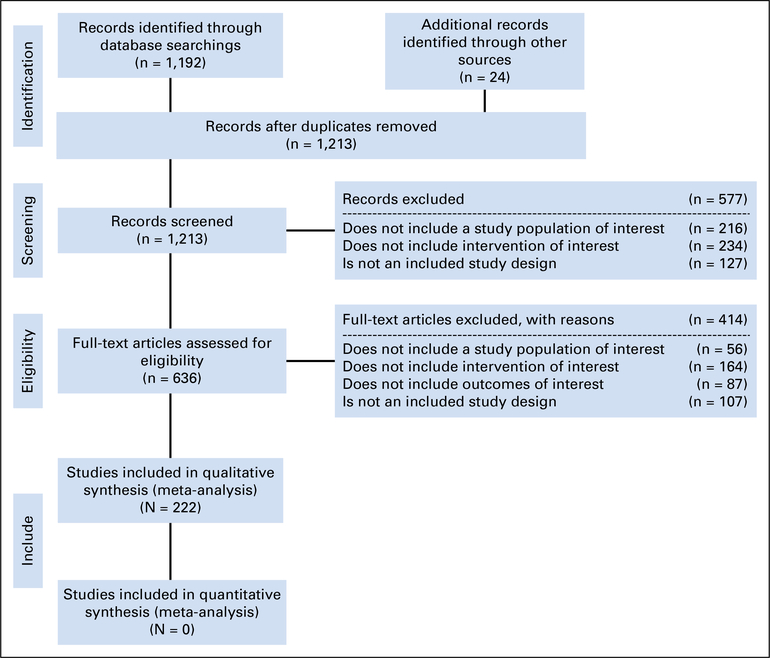
Two hundred twenty-two studies met the eligibility criteria and form the evidentiary basis for the guideline recommendations.1,3–223 The identified trials focused on the diagnosis, staging, chemotherapy treatment, surgical cytoreduction, and radiation therapy treatment of patients with MPM. The primary outcomes reported in studies on therapeutic interventions included overall survival, progression-free survival, response rate, toxicity, quality of life (QoL), and peri- and postoperative complications, while the studies on diagnosis and staging reported primary outcomes on diagnostic accuracy, correlations, and tumor response. Table 1 gives a summary of the study designs of the included studies; details on the study characteristics are included in Data Supplement 1. The systematic review flow diagram is also shown in Figure 1.
Table 1.
Details of Study Design of the Included Studies
| Study Design |
||||||
|---|---|---|---|---|---|---|
| Sections | Systematic Reviews/Meta-Analysis | Randomized Controlled Trial | Prospective Study | Retrospective Study | Prospective Retrospective | Total |
| Diagnosis | 5 | 2 | 15 | 5 | 2 | 29 |
| Staging | 3 | 1 | 14 | 11 | 4 | 33 |
| Chemotherapy | 2 | 10 | 9 23 phase II studies |
5 | 0 | 49 |
| Surgical cytoreduction | 11 | 3 (1 overlap with staging) | 16 1 phase II study |
31 | 1 | 63 |
| Radiation therapy | 6 | 4 | 15 (1 overlap with surgery) 7 phase II studies |
15 | 48 | |
| Total | 27 | 20 | 100 | 67 | 8 | 222 |
Fig 1.
Study flow diagram.
Study Quality Assessment
Study design aspects related to individual study quality, strength of evidence, strength of recommendations, and risk of bias were assessed. Refer to the Methodology Supplement for more information and for definitions of ratings for overall potential risk of bias.
RECOMMENDATIONS
DIAGNOSIS
Clinical Question 1
What is the optimal way to make a diagnosis of pleural mesothelioma? Options include: (a) thoracentesis, (b) core needle biopsy, (c) thoracoscopic biopsy, and (d) open pleural biopsy
Recommendation 1.1.
Clinicians should perform an initial thoracentesis when patients present with symptomatic pleural effusions and send pleural fluid for cytologic examination for initial assessment for possible mesothelioma (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2.
In patients for whom antineoplastic treatment is planned, it is strongly recommended that a thoracoscopic biopsy should be performed. This will: (a) enhance the information available for clinical staging; (b) allow for histologic confirmation of diagnosis; (c) enable more accurate determination of the pathologic subtype of mesothelioma (epithelial, sarcomatoid, biphasic); and (d) make material available for additional studies (eg, molecular profiling) (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 1.2.1.
When performing a thoracoscopic biopsy, the minimal number of incisions (two or fewer) is recommended and should ideally be placed in areas that would be used for subsequent definitive resection to avoid tumor implantation into the chest wall (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 1.3.
In patients with suspected mesothelioma in whom treatment is planned, an open pleural biopsy should be performed if the extent of tumor prevents a thoracoscopic approach. The smallest incision possible is encouraged (generally 6 cm or less is recommended) (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 1.4.
In patients who are not candidates for thoracoscopic biopsy or open pleural biopsy, who also have a nondiagnostic thoracentesis or do not have a pleural effusion, clinicians should perform a core needle biopsy of an accessible lesion (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
The diagnosis of MPM can be quite difficult from a pathologic perspective, as there are several different cell types (epithelioid, sarcomatoid, and mixed [biphasic]), and rarer subtypes (ie, desmoplastic, deciduoid), which can be challenging to distinguish from other primary tumors of the pleura, metastatic malignancy to the pleural surfaces, and benign, inflammatory or fibrotic abnormalities of the pleural space. Given the potential for diagnostic dilemmas, it is critical to have sufficient tissue for immunohistochemical staining utilizing standard antibody panels that aid in distinguishing mesothelioma from carcinoma and sarcoma. Biopsies should be of sufficient depth to be able to confirm the presence of invasion, one of the hallmarks that distinguish malignant mesothelioma from benign mesothelial proliferation.
When a patient with suspected MPM presents with a pleural effusion, the diagnostic work-up should begin with an ultrasound-guided thoracentesis with pleural fluid sent to cytopathology for analysis. Although less than one third of MPM can be diagnosed accurately on pleural fluid cytology,114 thoracentesis is a safe and reliable initial intervention that can also transiently alleviate the common presenting symptoms of dyspnea and chest discomfort. The diagnostic utility of thoracentesis is principally limited to the epithelioid subtype; sarcomatoid and biphasic mesothelioma are rarely detected in pleural fluid specimens.116
More definitive diagnosis necessitates performing thoracoscopy with multiple pleural biopsies, ideally from several different locations throughout the ipsilateral hemithorax. This approach is particularly important in patients for whom further treatment is planned. Biopsies should be of sufficient size and depth to allow for all requisite testing by surgical pathology. The diagnostic yield of thoracoscopy in mesothelioma is > 95%.100,113,114
In patients who present with nodular pleural thickening without a pleural effusion, computed tomography (CT)–guided core biopsy of pleural-based masses is a reasonable diagnostic alternative to more invasive surgical interventions. CT-guided biopsy of pleural nodules under local anesthesia may also be a reasonable option in patients who are poor candidates for thoracoscopy.12,33 Open pleural biopsy, a relatively limited and generally low-risk surgical alternative, can also be considered for these patients, as well as for those without an effusion or a patent pleural space to allow for safe thoracoscopy.
Uncommon variants of MPM may evade diagnostic confirmation even with large thoracoscopic or open pleural biopsies. The classic example is desmoplastic mesothelioma, in which the malignant cells are rare and interspersed within a large volume of densely fibrotic stroma. Sometimes this variant can only be diagnosed either from a large surgical specimen or at autopsy.
Clinical Question 2
Is cytology of pleural fluid as sensitive and specific as histology in making a diagnosis of pleural mesothelioma?
Recommendation 2.0.
Cytologic evaluation of pleural fluid can be an initial screening test for mesothelioma, but it is not a sufficiently sensitive diagnostic test. Whenever definitive histologic diagnosis is needed, biopsies via thoracoscopy or CT guidance offer a better opportunity to reach a definitive diagnosis (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Cytologic examination of pleural fluid is not sufficiently sensitive to make a diagnosis of mesothelioma. This may be attributed to the difficulty of differentiating mesothelioma tumor cells from reactive mesothelial cells, sample preparation, or the extent of disease.26,114 In a study of 75 patients with mesothelioma, 82% of patients with positive pleural fluid cytology had visceral pleural involvement, whereas only 30% of patients with negative pleural fluid cytology had disease involving the visceral pleura.26
Immunohistochemical studies are of limited value to differentiate mesothelioma from benign mesothelial cells.211 Recent studies suggest that the loss of the BRCA1-associated protein (BAP1) and deletion of p16 seen in mesothelioma but not reactive mesothelial cells could be useful adjuncts for cytologic diagnosis of mesothelioma.224,225 Immunohistochemical staining of pleural fluid cytology specimens may help differentiate mesothelioma from adenocarcinoma, however. In a study of 159 malignant pleural effusions, Claudin-4 immunohistochemistry staining was positive in 83 of 84 adenocarcinoma cases and negative in all 64 mesothelioma samples, thereby differentiating adenocarcinoma from mesothelioma with high sensitivity and specificity.158
Clinical Question 3
What panel of immunohistochemistry stains is required to make a diagnosis of mesothelioma?
Recommendation 3.0.
Histologic examination should be supplemented by immunohistochemistry using selected markers expected to be positive in mesothelioma (eg, calretinin, keratins 5/6, and nuclear WT1) as well as markers expected to be negative in mesothelioma (eg, CEA, EPCAM, Claudin 4, TTF-1). These markers should be supplemented with other markers that address the differential diagnosis in that particular situation (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Numerous studies summarized in reviews226,227 suggest immunohistochemical panels to include markers that positively identify mesothelioma. The most important ones are calretinin, keratins 5/6, Wilms tumor protein 1 (WT1), and podoplanin. None is entirely specific for mesothelioma, but together, when interpreted in the context of histologic features, they are all useful. Also recommended are markers expected to be negative in mesothelioma but positive in adenocarcinoma (especially pulmonary adenocarcinoma). Most important are carcinoembryonic antigen (CEA), epithelial cell adhesion molecule (EPCAM, for which two antibodies are commonly used: MOC31, and BerEP4), blood group 8, and Claudin 4. Additional markers typically positive in lung adenocarcinoma and negative in mesothelioma are napsin A and thyroid transcription factor 1 (TTF-1). Positive markers for other tumor types should be used for differential diagnosis of mesothelioma and metastatic carcinomas from various sources, metastatic melanoma, or lymphoma as clinically applicable.226,227 Fluorescent in situ hybridization studies for detection of hetero- or homozygous loss of p16/CDKN2A locus at 9p21202 could be used to support the diagnosis of MPM over a benign process. Loss of BRCA1-associated protein (BAP1) expression is also emerging as an immunohistochemistry marker for MPM and may augur a better prognosis.64
Clinical Question 4
Do the pathologic subtypes of mesothelioma have prognostic significance? What is the optimal way to report histologic composition?
Recommendation 4.1.
Mesothelioma should be reported as epithelial, sarcomatoid, or biphasic, because these subtypes have a clear prognostic significance (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 4.2.
In surgical, thoracoscopic, or open pleural biopsies with sufficient tissue, further subtyping and quantification of epithelial versus sarcomatoid components of mesothelioma may be undertaken (Type of recommendation: informal consensus; Strength of recommendation: moderate).
Literature review and clinical interpretation.
It is insufficient to report the pathologic diagnosis of this disease simply as malignant mesothelioma. The histologic subtype—epithelial, sarcomatoid, or biphasic—should be documented, as it has significant prognostic and therapeutic implications for patients with MPM. Patients with sarcomatoid histology have a much shorter survival than the other subtypes, fail to benefit from surgery, and are less likely to respond to systemic therapy. Biphasic tumors have an intermediate prognosis between epithelial and sarcomatoid.
In the SEER database, the median survival in patients with epithelial, biphasic, and sarcomatoid disease who underwent surgery was 19, 12, and 4 months, respectively (P < .01). Surgery improved survival in patients with epithelioid, but not biphasic or sarcomatoid, histology.79 Thus, surgery is not recommended in patients with sarcomatoid MPM. Emerging evidence also suggests that the percentage of epithelioid differentiation is an independent predictor of survival in patients with biphasic MPM.78 Patients with epithelioid differentiation of 100%, 51% to 99%, and < 50% had median overall survivals of 20.1, 11.8, and 6.62 months, respectively (P < .001) in a 144-patient series.78 A systematic review of 30 MPM trials that reported tumor response rates by histologic subtype documented fewer responses in patients with sarcomatoid histology than in the other subtypes.198
Clinical Question 5
Are there any non–tissue-based biomarkers that can be used to diagnose patients with mesothelioma, to predict outcome, or to monitor tumor response?
Recommendation 5.0.
The non–tissue-based biomarkers that are under evaluation at this time do not have the sensitivity or specificity to predict outcome or monitor tumor response and are therefore not recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
None of the non–tissue-based biomarkers being evaluated at this time for MPM have sufficiently rigorous prospective/blinded validation to recommend their use.
Despite a high-risk, asbestos-exposed population that could be an ideal cohort for the development of diagnostic biomarkers for MPM, the gold standard, soluble mesothelin-related protein (SMRP), has a sensitivity of 95% and a specificity of only 32%. Multiple single-institution cohort studies of serum SMRP have compared levels in patients with MPM to (1) asbestos-exposed non–cancer-bearing controls, (2) healthy controls, (3) non-MPM malignant controls, and (4) controls with inflammatory diseases.203 At a common diagnostic threshold of 2.00 nmol/L, the sensitivities and specificities of SMRP ranged widely (19% to 68% and 88% to 100%, respectively). The utility of SMRP in early diagnosis was evaluated in 217 patients with stage I or II epithelioid and biphasic MPM and 1,612 controls. The resulting area under the receiver operating characteristic curve was 0.77 (95% CI, 0.73 to 0.81). At 95% specificity, SMRP yielded a sensitivity of 32% (95% CI, 26% to 40%).203 In the United States, SMRP measurements are only available through a reference laboratory.
SMRP has been compared with other biomarkers, including osteopontin (OPN) and Fibulin-3 (FBLN3). OPN lacked the specificity for MPM demonstrated by SMRP when nonmesothelioma cohorts were used. Low baseline OPN levels were independently associated with favorable progression-free and overall survival in two studies, while SMRP was not prognostic.97,164 The original publication153 describing FBLN3 reported 100% sensitivity and 94% specificity for stage I or II MPM compared with individuals with asbestos exposure or nonmesotheliomas with pleural effusions; a blinded validation of 48 MPMs and 96 asbestos-exposed controls achieved an area under the curve (AUC) of 0.87. Validation of these data has not been consistent.90 Effusion FBLN3 was an independent significant prognostic factor for survival in patients with MPM153 (hazard ratio [HR], 2.08; P = .017). Patients with MPM with effusion FBLN3 levels below the median survived significantly longer than those above (14.1 v 7.9 months; P = .012). The diagnostic value of FBLN3 for MPM was recently validated89 (sensitivity 93.0%, specificity 90.0%), though a prognostic effect was not observed. The reliability of the FBLN3 enzyme-linked immunosorbent assay is under active investigation and could account for these disparate findings.
Clinical Question 6
Is there a role for tumor genomic sequencing in mesothelioma?
Recommendation 6.0.
While tumor genomic sequencing is currently done on a research basis in mesothelioma and may become clinically applicable in the near future, it is not recommended at this time (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
The current role of genomic sequencing in MPM is limited to research studies. The most comprehensive genomic analysis to date228 describes the exome and transcriptome sequencing of 216 tumor and control specimens of patients with MPM. BAP1, NF2, TP53, SETD2, DDX3X, ULK2, RYR2, CFAP45, SETDB1, and DDX51 were frequently mutated. Many other genes were additionally silenced by copy number changes and chromosomal deletions. Through integrated analyses, alterations in Hippo, mammalian target of rapamycin, histone methylation, RNA helicase, and p53 signaling pathways were identified. Four consensus clusters defined through RNA sequencing correlated with survival and the degree of epithelial-to-mesenchymal transition. The frequencies of mutations of TP53, SETD2, and NF2 were different in the four clusters. BAP1 mutations were present in at least a quarter of each cluster type; these may aid in the diagnosis of MPM and in identifying some familial cases.
STAGING
Clinical Question 1
What are the optimal tests required to stage patients with mesothelioma? (a) CT, (b) positron emission tomography (PET)/CT, (c) magnetic resonance imaging (MRI), (d) mediastinoscopy, (e) thoracoscopy, (f) laparoscopy, and (g) endobronchial ultrasound (EBUS).
Recommendation 1.1.
A CT scan of the chest and upper abdomen with IV contrast is recommended as the initial staging in patients with mesothelioma (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2.
An FDG PET/CT should usually be obtained for initial staging of patients with mesothelioma. This may be omitted in patients who are not being considered for definitive surgical resection (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.3.
If abnormalities that suggest metastatic disease in the abdomen are observed on a chest and upper abdomen CT or on a PET/CT, then consideration should be given to perform a dedicated abdominal (+/− pelvic) CT scan, preferably with IV and oral contrast (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.4.
An MRI (preferably with IV contrast) may be obtained to further assess invasion of the tumor into the diaphragm, chest wall, mediastinum, and other areas (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
Two systematic reviews,208,209 seven prospective cohort studies,104,119,122,124,136,138,152 and five retrospective studies19,31,63,68,157 were identified. One systematic review included 15 studies on PET; another included 14 studies on CT, PET, combination PET/CT and MRI. The prospective studies focused on preoperative CT scan104 [18F]fluorodeoxyglucose (FDG) PET/CT scan,104,136 mediastinoscopy,119 bilateral thoracoscopy,119 laparoscopy,119 MRI,122,124 extended surgical staging,138 and cervical mediastinoscopy.152 The retrospective studies19,31,63,68,157 addressed the potential of both volumetric CT scanning and pleural thickness measurements to determine the T stage of the primary tumor; these remain research questions at present.
CT scan of the chest and upper abdomen with intravenous (IV) contrast is the standard initial imaging study for the clinical staging of MPM. Although CT delineates the overall extent of the primary tumor, it may not precisely define some areas of tumor invasion. The coronal and sagittal CT views are sometimes more helpful than axial cuts in this regard, but interpretation of chest wall or diaphragm invasion can still be problematic. It may also be difficult to distinguish mediastinal adenopathy from adjacent mediastinal pleural tumor on CT, particularly in the subcarinal space. MRI sometimes provides better definition of tumor involvement of the chest wall and diaphragm, but it is not performed in most institutions because it does not routinely add enough information to CT to warrant the additional cost and complexity.
FDG PET/CT scanning identifies metastatic disease not seen on CT in about 10% of patients, and the degree of FDG uptake (as measured by the maximum standardized uptake value) on PET is prognostic of outcome. PET is sometimes used to assess treatment response in patients receiving chemotherapy; however, PET can be problematic to interpret in patients who have received a talc pleurodesis.
Invasive staging techniques can supplement the information obtained from these imaging studies. Mediastinoscopy can confirm the presence of paratracheal and subcarinal lymph node metastases, while EBUS and endoscopic ultrasound also allow access to aortopulmonary window, hilar, and some lower mediastinal and para-esophageal nodes. Unlike primary lung cancers, pleural mesotheliomas often metastasize preferentially to mediastinal rather than hilar lymph nodes, and regional lymph node involvement has consistently been associated with a poor prognosis. Thus, mediastinoscopy, EBUS, and EUS can provide important staging information, but up to half of involved mediastinal lymph nodes are located in areas not accessible with these procedures, including the anterior mediastinum, pericardial fat pad, and peridiaphragmatic and posterior intercostal regions, and may only be diagnosed at exploratory thoracotomy. While some institutions routinely perform mediastinoscopy or EBUS/EUS for staging, others use these procedures selectively, depending on findings from imaging studies and the overall plans for multimodality treatment.
Laparoscopy can clarify whether transdiaphragmatic tumor invasion is present. Bulky tumor in the lower hemithorax often involves and depresses the hemidiaphragm, making it difficult to determine whether T4 or M1 disease is present. Laparoscopy can identify tumor directly extending through the diaphragm (T4) or peritoneal metastases. While some institutions routinely perform staging laparoscopy, most use it selectively to supplement information available from imaging studies.
Recommendation 1.5.
For patients being considered for maximal surgical cytoreduction, a mediastinoscopy and/or endobronchial US should be considered if enlarged and/or PET-avid mediastinal nodes are present (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.6.
In the presence of contralateral pleural abnormalities detected on initial PET/CT or chest CT scan, a contralateral thoracoscopy may be performed to exclude contralateral disease (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 1.7.
In patients with suspicious findings for intra-abdominal disease on imaging and no other contraindications to surgery, it is strongly recommended that a laparoscopy be performed (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
The proper staging of MPM requires a combination of imaging studies (CT/MRI/PET), lymph node sampling (mediastinoscopy, EBUS, EUS), and surgical exploration to determine the extent of involvement of the pleural space. Chest and upper abdomen CT scan with IV contrast is the standard-of-care initial staging modality that allows determination of: involvement of ipsilateral visceral and parietal pleural surfaces; invasion of chest wall, lung parenchyma, and ipsilateral hemidiaphragm; enlargement of mediastinal and/or hilar nodes; presence of metastases in contralateral pleura and/or lung parenchyma; and transdiaphragmatic spread of tumor into the peritoneal cavity. Chest CT is also the basis for monitoring of response to therapy, as the accepted standard of modified Response Evaluation Criteria in Solid Tumors (RECIST) requires calculation of a sum of tumor measurements based upon three separate chest CT scan slices.163
[18F]FDG PET/CT scan can be a valuable adjunct to chest CT scan to help distinguish benign from malignant pleural abnormalities, to assess the likelihood of malignant involvement of mediastinal and hilar lymph nodes, and to detect distant metastases.208,209 Findings on PET/CT scan need to be confirmed by obtaining tissue, especially in surgical candidates. It is important to recognize that the inflammation caused by talc pleurodesis renders subsequent PET images unreliable for the detection of pleural abnormalities.
MRI of the chest with IV contrast, particularly coronal sections, can also serve as an adjunct to chest CT scan in initial staging. MRI is particularly useful for aiding in the determination of chest wall, diaphragmatic, and/or mediastinal invasion/involvement by tumor.122 As with PET/CT, however, findings on MRI should be confirmed with additional interventions (ie, thoracoscopic examination of the pleural space to determine the extent of chest wall invasion), particularly in those patients who are slated to undergo maximal surgical cytoreduction.
Patients who are being assessed for maximal surgical cytoreduction should be considered for minimally invasive staging of mediastinal and hilar nodes. EBUS-guided fine-needle aspiration is more sensitive and specific for determining nodal involvement than standard cervical mediastinoscopy.31 There may also be a role for endoscopic ultrasound for biopsy of subdiaphragmatic and/or paraesophageal lymph nodes. If baseline imaging studies suggest involvement of the contralateral pleural space, this can have significant prognostic and therapeutic implications. For this reason, patients with these findings should undergo a contralateral thoracoscopy with pleural examination and biopsy to confirm the presence of mesothelioma. Similarly, those patients—particularly surgical candidates—who have imaging evidence of transdiaphragmatic invasion and/or involvement of abdominal organs should undergo laparoscopy and biopsy to pathologically confirm intraperitoneal spread of disease.119,152
Clinical Question 2
What are the limitations of the current staging system for surgical and clinical staging of pleural mesothelioma? (a) What are the key discrepancies between clinical and pathologic staging? (b) What are the limitations of staging in predicting prognosis?
Recommendation 2.0.
The current AJCC/UICC staging classification remains difficult to apply to clinical staging with respect to both T and N components and thus may be imprecise in predicting prognosis. Physicians should recognize that in patients with clinical stage I/II disease, upstaging may occur at surgery (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Literature review and clinical interpretation.
One prospective cohort study135 and eight retrospective studies25,30,59,63,66–68,157 were identified. The prospective study assessed resected specimen weight and volume, while six of the retrospective studies25,30,59,66–68 focused on the MPM staging system, proposed adjustments to TNM staging criteria, and supplementary prognostic variables. Two papers63,157 evaluated the potential of CT-based assessment of tumor volume as a means of clinical T staging.
Clinical staging of MPM is challenging because, unlike many solid tumors, the anatomic characteristics of the primary tumor (irregular spread along the pleural surface) do not permit simple uni- or bidimensional measurements on imaging studies. The rarity of this malignancy has also made it difficult to generate data to support a widely accepted staging system. The recent development of a large multinational database through the International Association for the Study of Lung Cancer (IASLC) and the International Mesothelioma Interest Group (IMIG) has generated sufficiently robust analyses of T, N, and M categories in relationship to overall survival to recommend revisions of the staging system for the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging manuals.
Clinical T staging of MPM currently depends on an assessment of the extent and depth of primary tumor involvement in the pleural space. This can be difficult to define accurately on CT or MRI imaging, which explains the frequent discrepancy between clinical and pathologic staging, especially in early-stage disease. Although recent analyses of the IASLC database suggest that this approach is still valid (with some revisions) for the 8th edition of the staging system, there is increasing evidence that measurement of pleural tumor thickness and/or volume may provide an easier and clinically more meaningful assessment of T category, though this approach remains experimental.
Clinical evaluation of nodal involvement (N category) is also problematic because lymph nodes are often difficult to distinguish from the adjacent abnormal pleura on CT, MRI, or PET and because there is no direct correlation between lymph node size and tumor involvement. In addition, the anatomic pattern of metastatic lymphatic disease in MPM differs from lung cancer, with predominant involvement of mediastinal nodes, including those in unusual locations such as the internal mammary, cardiophrenic, and even intercostal regions. Many of these nodes are also outside the reach of staging procedures such as mediastinoscopy or endobronchial and esophageal ultrasound, further reducing the accuracy of clinical staging. Recent analyses of the IASLC database have led to the recommendation to consider all ipsilateral intrathoracic lymph node involvement as N1 disease.
Most MPMs are diagnosed before distant metastases develop, because symptoms such as shortness of breath due to pleural effusion or chest pain prompt evaluation when the tumor is still confined to the chest. Most also tend to remain confined to the ipsilateral hemithorax for much of their clinical course. Although metastases often involve the peritoneum and the contralateral pleura, they can occur in other solid organs. Unlike lung adenocarcinoma, CNS metastases are uncommon, and thus evaluation of M stage is adequately achieved through CT of the chest and upper abdomen and PET imaging; imaging the brain is not required unless the patient has symptoms suggestive of brain metastasis.
Clinical Question 3
What is the optimal approach to radiologic-based tumor measurement and response classification (RECIST 1.1, modified RECIST for mesothelioma, volumetrics)?
Recommendation 3.1.
The optimal approach to mesothelioma measurement requires the expertise of a radiologist to identify measurement sites on CT as per modified RECIST for mesothelioma. This approach requires calculating the sum of up to six measurement sites with at least 1 cm thickness, measured perpendicular to the chest wall or mediastinum, with no more than two sites on each of three CT sections, separated by at least 1 cm axially (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.2.
Assessment of tumor volume by CT scan may enhance clinical staging and provide prognostic information but remains investigational and thus is not recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.3.
It is recommended that tumor response classification be determined based on RECIST criteria from the comparisons of these sums across serial CT scans (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Image-based measurements of mesothelioma are critical for decisions regarding patient enrollment on clinical trials, tumor response assessment, and patient surveillance. The morphology and growth pattern of mesothelioma differ substantively from other solid tumors,112 thus requiring an alternative to the clinically accepted measurement approach of RECIST.213,229 Modified RECIST,163 despite being published as a research study to investigate a known problem in the mesothelioma clinical research community, was quickly adopted as the standard for tumor measurement on the CT scans of patients with MPM.123 The modified RECIST approach requires acquisition of up to six measurements of tumor thickness, each at least 1 cm in extent, measured perpendicularly to the chest wall or mediastinum, with no more than two measurement sites on each of three separate CT sections separated axially by at least 1 cm.163
The acquisition of MPM tumor thickness measurements is conceptually a multistep process, with interobserver variability compounded at each of these steps.230 To mitigate measurement variability, measurements should be performed by a radiologist familiar with modified RECIST and mesothelioma. The same radiologist or radiologists preferably should acquire all measurements from all CT scans for all patients.231 Measurement consistency across the multiple CT scan time points for a patient is important, so measurements should be stored electronically and displayed as annotations superimposed on prior images as a reference for the radiologist acquiring measurements from a current image.154
Modified RECIST did not alter the tumor response classification criteria (the actual numeric values) that separate response categories (partial response, stable disease, and progressive disease); in fact, with the exception of the measurement acquisition approach previously described, modified RECIST implicitly adopted all other aspects of RECIST (and, by extension, the more recent RECIST 1.1).232 Accordingly, the sum of these up-to-six measurements of tumor thickness at each follow-up CT scan are compared with the corresponding sum from all previous scans of the patient to assess tumor response based on the RECIST criteria.
Computer-based extraction of tumor volume from imaging has been investigated65 in the context of staging,63 prognosis,43,133 and response to therapy in MPM,131,132,160,161 but only a modest correlation between CT-based tumor volume and gross tumor specimen volume has been observed.130 While assessment of tumor volume by CT may enhance clinical staging and provide prognostic information, it remains investigational at this time.
CHEMOTHERAPY
Clinical Question 1
In patients with newly diagnosed pleural mesothelioma, is there a role for chemotherapy and does it improve survival and QoL? (a) Who should receive supportive care instead of chemotherapy? (b) Is there a role for additional modalities in these patients?
Recommendation 1.1.
Chemotherapy should be offered to patients with mesothelioma because it improves survival and QoL (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2.
In asymptomatic patients with epithelial histology and minimal pleural disease who are not surgical candidates, a trial of close observation may be offered prior to the initiation of chemotherapy (Type of recommendation: informal consensus; Strength of recommendation: moderate).
Recommendation 1.3.
Selected patients with a poor performance status (PS 2) may be offered single-agent chemotherapy or palliative care alone. Patients with a PS of 3 or greater should receive palliative care (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Literature review and clinical interpretation.
Chemotherapy improves survival and QoL in previously untreated patients with MPM. In the pivotal study by Vogelzang et al,1 the combination of pemetrexed plus cisplatin improved the response rate, progression-free and overall survival compared with cisplatin alone. Using the Lung Cancer Symptom Scale instrument to evaluate QoL, the trial demonstrated statistically significant improvements in dyspnea and pain with combination chemotherapy. A similar study with raltitrexed/cisplatin showed that doublet chemotherapy improved overall survival compared with cisplatin alone. Global health-related QoL (HRQoL) was comparable on both arms (P = .848), and both treatments yielded improvements in dyspnea. Few clinically significant differences between treatment arms were observed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) or Lung Cancer 13.10,17 In the MAPS (Mesothelioma Avastin Cisplatin Pemetrexed Study) trial, the addition of bevacizumab to standard pemetrexed/cisplatin chemotherapy improved progression-free and overall survival. Chemotherapy improved QoL above baseline in both arms.20
The MS01 phase III trial compared active symptom control (ASC) to mitomycin/vinblastine/cisplatin or vinorelbine in 409 previously untreated patients with MPM. Median overall survival was 7.6 months for ASC and 8.5 months for the combined chemotherapy arms, which was not statistically significant (HR, 0.89; P = .29). There were no differences in the QoL subscales of physical functioning, pain, dyspnea, and global health status between arms. Exploratory analyses suggested a survival advantage for vinorelbine compared with ASC alone, which did not reach statistical significance since the study was underpowered (HR, 0.80; P = .08).6
Epithelial MPM can sometimes be quite indolent. In asymptomatic patients with epithelial histology and minimal pleural disease who are not surgical candidates, a trial of close observation may be offered prior to the initiation of chemotherapy. A 43-patient randomized trial compared immediate chemotherapy with mitomycin/vinblastine/cisplatin to chemotherapy at the time of symptomatic progression. Early chemotherapy provided an extended period of symptom control and a trend toward a survival improvement that was not statistically significant.18 The SWAMP (South West Area Mesothelioma and Pemetrexed) trial assessed HRQoL using the EQ-5D, European Organisation for Research and Treatment of Cancer QLQ-C30, and LC-13 in 73 consecutive patients who were fit for first-line pemetrexed/platin chemotherapy; 58 patients received chemotherapy and 15 chose best supportive care (BSC). Patients who received chemotherapy maintained their QoL better than the BSC group (P = .006); the latter experienced a decline in their HRQoL, with worse dyspnea and pain. Patients receiving chemotherapy who had radiographic improvement or a decline in serum mesothelin also had a better HRQoL at 16 weeks.88
It is reasonable to offer selected patients with PS 2 single-agent chemotherapy with pemetrexed,126,129 vinorelbine,6 or gemcitabine.180 Response rates are expected to be quite low. Patients with a PS of 3 or greater should receive palliative care.
Clinical Question 2
What is the best chemotherapy regimen for patients with newly diagnosed pleural mesothelioma who are not candidates for surgery?
Recommendation 2.0.
The recommended first-line chemotherapy for patients with mesothelioma is pemetrexed plus platinum. However, patients should also be offered the option of entering in a clinical trial (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Literature review and clinical interpretation.
Systemic chemotherapy consisting of a platinum plus pemetrexed with folic acid and vitamin B12 supplementation is the recommended first-line systemic therapy for patients with MPM with a good (≤ 2) performance status. The trial that led to US Food and Drug Administration approval of this regimen in MPM was a single-blind, placebo-controlled randomized phase III trial that compared cisplatin (75 mg/m2) with or without pemetrexed (500 mg/m2) in 456 previously untreated patients with MPM. The combination achieved a superior median overall survival (12.1 v 9.3 months; P = .020; HR, 0.77) and progression-free survival (5.7 v 3.7 months; P = .001) and a higher response rate (41.3% v 16.7%; P < .001) when compared with single-agent cisplatin. Vitamin supplementation was instituted after the first 117 patients enrolled, resulting in a significant reduction in toxicity without impairing survival. Toxicity was, of course, greater with the combination, producing grade 3/4 neutropenia, leukopenia, and nausea in 27.9%, 17.7%, and 14.6% of patients, respectively.
A phase III trial that compared the antifolate raltitrexed (80 mg/m2) plus cisplatin (80 mg/m2) to cisplatin alone in 250 patients similarly demonstrated higher response rates (23.6% v 13.6%) and a superior median overall (11.4 v 8.8 months) and 1-year survival (46% v 40%), for the antifolate/platinum combination compared with cisplatin alone. In this study there was no difference in HRQoL between the two arms.10,17
Clinical Question 3
What is the role of adding bevacizumab to the chemotherapy regimen of pemetrexed and cisplatin? Are there patients with mesothelioma who should not get bevacizumab?
Recommendation 3.1.
The addition of bevacizumab to pemetrexed-based chemotherapy improves survival in select patients and therefore may be offered to patients with no contraindications to bevacizumab. The randomized clinical trial demonstrating benefit with bevacizumab used cisplatin/pemetrexed; data with carboplatin/pemetrexed plus bevacizumab are insufficient for a clear recommendation (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: moderate).
Recommendation 3.2.
Bevacizumab is not recommended for patients with PS ≥ 2, substantial cardiovascular comorbidity, uncontrolled hypertension, age > 75, bleeding or clotting risk, or other contraindications to bevacizumab (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
MAPS, an open-label randomized phase III trial in 448 patients with MPM compared standard pemetrexed/cisplatin with or without the addition of bevacizumab, 15 mg/kg every 21 days.20 Eligible patients were age 75 years or younger, with no cardiovascular comorbidity or uncontrolled hypertension, who were not receiving antiaggregant, antivitamin K, low-molecular-weight heparin, or nonsteroidal agents. The three-drug combination produced a longer median overall survival compared with pemetrexed/ cisplatin (18.8 v 16.1 months; P = .0167; HR, 0.77). The superior overall survival in the control arm (which was 12.1 months in the Vogelzang et al1 trial) was attributed in part to the rigorous eligibility criteria for bevacizumab treatment. Progression-free survival was also superior with the triplet (9.2 v 7.3 months; P < .001; HR, 0.61).
As expected, the addition of bevacizumab increased the rate of grade 3/4 toxicity (71% v 62%) especially hypertension (25% v 0%) and thrombosis (6% v 1%); grade 1/2 epistaxis was also more frequent (37.4% v 6.3%). More patients stopped treatment because of toxic effects in the bevacizumab arm than in the control group (24.3% v 6%; P < .001). There was no detriment to QoL with the addition of bevacizumab.
On the basis of these data, it is recommended that the triplet regimen of bevacizumab, pemetrexed, and cisplatin may be offered to patients with no contraindications to bevacizumab. Given the high frequency of cardiovascular comorbidity and hypertension among patients with MPM, however, it is important to carefully select patients who might benefit from the addition of bevacizumab to chemotherapy.
The data for bevacizumab with carboplatin/pemetrexed are insufficient for a clear recommendation. A phase II trial of pemetrexed, carboplatin (AUC 5), plus bevacizumab in 76 previously untreated patients with MPM achieved a partial response rate of 34.2%, with manageable toxicity. The median progression-free and overall survival was 6.9 and 15.3 months, respectively.184 There are no randomized data for this combination.
Clinical Question 4
When should carboplatin be used instead of cisplatin in patients with pleural mesothelioma?
Recommendation 4.0.
In patients who may not be able to tolerate cisplatin, it is recommended that carboplatin may be offered as a substitute for cisplatin (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Carboplatin is generally better tolerated and easier to administer than cisplatin. Although no randomized studies in MPM directly compare carboplatin to cisplatin, data from multiple phase II series and the pemetrexed Expanded Access Program suggest that they are likely equivalent in this disease. In phase II studies,174,176,185 carboplatin (AUC 5) combined with pemetrexed achieved response rates ranging from 19% to 29%, median progression-free survival of 7 to 8 months, and median overall survival of 13 to 14 months,46,185 similar to the pivotal phase III trial of cisplatin and pemetrexed.1 In a retrospective pooled analysis, patients > 70 years of age who were treated with pemetrexed and carboplatin achieved similar outcomes as their younger counterparts, though they experienced more frequent hematologic toxicity.46
Among 1,704 previously untreated patients with MPM in the international Expanded Access Program, comparable response rates (26.3% v 21.7%), time to progression (7 v 6.9 months) and 1-year survival (63.1 v 64%) were reported for treatment with pemetrexed plus cisplatin or carboplatin, respectively. Grade 3/4 neutropenia was greater in patients who received pemetrexed plus carboplatin than pemetrexed plus cisplatin: 36.1% v 23.9%, respectively.127
Based on the available nonrandomized data, substituting carboplatin for cisplatin is an acceptable first-line option for patients with unresectable MPM.
Clinical Question 5
What is the most effective second-line therapy for patients with pleural mesothelioma? Can patients who have previously received pemetrexed be treated again with pemetrexed?
Recommendation 5.1.
Retreatment with pemetrexed-based chemotherapy may be offered in pleural mesothelioma patients who achieved durable (> 6 months) disease control with first-line pemetrexed-based chemotherapy (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 5.2.
Given the very limited activity of second-line chemotherapy in patients with mesothelioma, participation in clinical trials is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 5.3.
In patients for whom clinical trials are not an option, vinorelbine may be offered as second-line therapy (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Literature review and clinical interpretation.
There are few active treatment options for previously treated patients with MPM. A phase III trial in 243 patients who had not received prior pemetrexed demonstrated higher response rates (18.7% v 1.7%; P < .001), superior disease control (59.3% v 19.2%; P < .0001), and longer progression-free survival (3.6 v 1.5 months; P = .0148) in those who received single-agent pemetrexed compared with BSC. This did not translate into an improvement in overall survival, however (8.4 v 9.7 months; P = .74) due to the greater use of subsequent chemotherapy in the BSC arm.7
Retreatment with pemetrexed-based chemotherapy is a reasonable option for patients who achieve durable disease control with first-line pemetrexed-based chemotherapy. A single-center retrospective review reported an overall response rate of 19% and a disease control rate of 48% among 31 patients who achieved disease control with front-line pemetrexed-based chemotherapy for at least 3 months and were then retreated with pemetrexed, alone or with a platinum.184 A multi-institution retrospective analysis of 30 patients documented a 66% disease control rate and decreased pain when patients who had at least 6 months of disease control with front-line pemetrexed/platin were rechallenged with a pemetrexed-based regimen. Time to progression was 5.1 months, and median overall survival was 13.6 months.41 A multicenter retrospective analysis showed that patients with MPM who experienced a time to progression of at least 12 months after first-line therapy had a greater likelihood of disease control with pemetrexed-based rechallenge.28
Vinorelbine is widely used as a second-line therapy in MPM, though there are limited data to support its efficacy. A single-center phase II trial of vinorelbine in 63 patients achieved a response rate of 16% and a median overall survival of 9.6 months. Similarly, a single-center retrospective review in 59 patients reported a 15% response rate and a disease control rate of 49%.233 In contrast, a retrospective review of 60 patients who received either vinorelbine or gemcitabine in the second- or third-line setting documented infrequent responses (none for vinorelbine and 2% for gemcitabine). Median progression-free survival was 1.7 and 1.6 months for vinorelbine and gemcitabine, respectively.60
Given the paucity of active agents in this setting, participation in clinical trials is highly recommended.
Clinical Question 6
What is the optimal duration of front-line chemotherapy for mesothelioma? Is there a role for pemetrexed maintenance therapy in pleural mesothelioma?
Recommendation 6.1.
In select asymptomatic patients with epithelial mesothelioma and a low disease burden who are not surgical candidates, a trial of expectant observation, with close monitoring, may be offered before initiation of systemic therapy (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 6.2.
Front-line pemetrexed-based chemotherapy should be given for four to six cycles. For patients with stable or responding disease, a break from chemotherapy is recommended at that point (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 6.3.
There is insufficient evidence to support the use of maintenance chemotherapy and thus it is not recommended (Type of recommendation: evidence-based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 6.4.
There is insufficient evidence to support the use of pemetrexed maintenance in mesothelioma patients and thus it is not recommended (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: strong).
Literature review and clinical interpretation.
In asymptomatic patients with epithelial histology and minimal pleural disease who are not surgical candidates, a trial of close observation may be considered before the initiation of chemotherapy. A small, 43-patient randomized trial that compared immediate chemotherapy to treatment when symptoms developed demonstrated that early chemotherapy provided a longer period of symptom control and a trend toward superior survival.18 Of the patients randomized to the delayed treatment group, 23% had a performance status deterioration that precluded subsequent chemotherapy. While it is reasonable to delay chemotherapy for patients with low disease burden and few symptoms, such patients should be monitored closely to ensure timely intervention.
In the pivotal study of pemetrexed/cisplatin that led to US Food and Drug Administration approval of this combination, patients received a median of six chemotherapy cycles, with a range of one to 12. The percentage of patients who completed at least four, six, or eight cycles was 71%, 53%, and 5%, respectively.1 Since patients with durable disease control with front-line chemotherapy can respond to retreatment with a pemetrexed-based regimen, a break from chemotherapy after four to six cycles of treatment is recommended.
There is insufficient evidence to support single-agent pemetrexed maintenance in MPM, and thus it is not recommended. A nonrandomized feasibility study in 27 patients demonstrated that maintenance pemetrexed was safe and that responses could be achieved after six cycles of induction chemotherapy. But the heterogeneous patient population (untreated and previously treated), the different induction regimens (pemetrexed/carboplatin or pemetrexed alone), the small number of patients who actually received maintenance therapy (13, only eight of whom had received front-line doublet induction chemotherapy), and the nonrandomized nature of this trial preclude any conclusions about the efficacy of this approach.234 A randomized study of maintenance pemetrexed following induction pemetrexed/platin (Cancer and Leukemia Group B 30901) closed due to poor accrual; preliminary data on this study have not yet been reported.
SURGICAL CYTOREDUCTION
Clinical Question 1
What is the role of surgical cytoreduction in mesothelioma: does it improve survival or QoL? (a) Is surgery for pleural mesothelioma ever curative, and does it prolong survival compared with chemotherapy alone? (b) Is there a role for additional modalities in these patients? (c) Which patient should not be considered for surgical cytoreduction?
Recommendation 1.1.
In selected patients with early stage disease, it is strongly recommended that a maximal surgical cytoreduction should be performed (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Surgical therapy of MPM has evolved since the first description of extrapleural pneumonectomy (EPP) for this disease. The goal, a maximal cytoreduction or a macroscopic complete resection (MCR), is defined as residual tumor after resection of < 1 cm. Surgeons experienced with lung-sparing techniques and EPP continue to debate about which operation does a better job of achieving this goal. Patient selection is key in deciding whether a patient with early disease should have surgery. Patients with less bulky disease measured by CT volumetrics survive longer than those with bulkier disease,19,63,157 including patients with minimal solid disease and those who only have a pleural effusion. These are the ideal candidates for MCR; their median survival can be as long as 48 months.
Nevertheless, it is impossible to predict the biology of an epithelial MPM, even when it presents at an early stage or with minimal bulk. No randomized study comparing surgery for early-stage MPM with favorable prognostic indices to observation or chemotherapy has been performed, and systematic surgical reviews rarely address this issue. An analysis of 14 retrospective studies evaluating EPP, chemotherapy, or palliative surgery reported a 13-month median survival and 37% major morbidity with EPP. In patients with epithelial disease, survival was 19 months with EPP and 7 months for palliative resection.206 Similar studies of patients who could have surgery compared with those who had biopsy only or were unresectable reveal trends toward increasing overall and recurrence-free survival with surgery.32,115 A six-center retrospective analysis reviewed 1,365 consecutive patients with MPM from 1982 to 2012. Median survival for patients who received medical therapy (chemotherapy or BSC), pleurectomy/decortication (P/D), or EPP was 11.7 months, 20.5 months, and 18.8 months, respectively. Patients who underwent resection with adjuvant therapy survived significantly longer than those who received chemotherapy alone (19.8 v 11.7 months; P < .001).24 Age < 70 years, epithelial histology, and receipt of chemotherapy were favorable prognostic factors on multivariate analysis. In patients with all three favorable prognostic factors, median survival was 18.6, 24.6, and 20.9 months for medical therapy, P/D, and EPP, respectively (P = .596). Despite the limits of a retrospective analysis, these data suggest a modest benefit observed with surgery combined with systemic therapy. These data are similar to the most recent edition of the IASLC MPM staging project, which reported a 21-month median survival for patients with pathologic T1 disease.68 Future studies must extend staging supplemental variables with validation of already available clinical prognostic indices (European Organization for Research and Treatment of Cancer trials and Cancer and Leukemia Group B prognostic scoring systems) along with laboratory parameters59 or novel biomarkers164 to define the best surgical candidates.
Recommendation 1.2.
Maximal surgical cytoreduction as a single modality treatment is generally insufficient; additional antineoplastic treatment (chemotherapy and/or radiation therapy) should be administered. It is recommended that this treatment decision should be made with multidisciplinary input involving thoracic surgeons, pulmonologists, medical and radiation oncologists (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
The futility of surgery alone for MPM results from the near impossibility of a complete microscopic resection and the high propensity for local recurrence. Neoadjuvant and adjuvant chemotherapy along with surgery as a multimodality approach evolved rapidly after demonstration of the efficacy of pemetrexed/cisplatin.1 Concurrently, data on extended survival with the use of postoperative adjuvant hemithoracic radiation therapy after EPP181 led to novel delivery approaches after MCR, including intensity-modulated radiation therapy (IMRT).235,236
Systematic reviews have demonstrated a median overall survival of 13 to 23.9 months for patients treated with multimodality therapy.215 The neoadjuvant approach has been hampered by attrition rates for the various treatment stages, so it is important to report outcomes both as intent to treat and in patients receiving all planned therapy. In the larger phase II series of induction chemotherapy followed by surgery, with or without postoperative radiation therapy, the intent-to-treat survival ranged from 14 to 18.4 months, while the survival in those select patients who were able to receive all planned therapies ranged from 20.8 to 59 months.162,190,237 Patients who had a radiographic response to induction chemotherapy also had an improved survival (26.0 v 13.9 months; P = .05).172 In a review of supplementary prognostic variables in 2,141 resected patients from the IASLC staging project, adjuvant therapy was significantly associated with survival in both univariate (HR, 1.7; P < .001) and multivariate analyses (HR, 1.56; P < .001). Whether chemotherapy should be delivered before, during, or after surgery is an unresolved question that is the subject of planned trials. Other unanswered issues being evaluated in ongoing trials include the efficacy of IMRT after pleurectomy and the role of induction radiation therapy before EPP.194,238
Recommendation 1.3.
Patients with transdiaphragmatic disease, multifocal chest wall invasion, or histologically confirmed contralateral mediastinal or supraclavicular lymph node involvement should undergo neoadjuvant treatment before consideration of maximal surgical cytoreduction. Contralateral (N3) or supraclavicular (N3) disease should be a contraindication to maximal surgical cytoreduction (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Clinical staging of MPM is dependent on radiologic findings as well as signs and symptoms of the disease. Diffuse chest wall or transdiaphragmatic involvement represent T4 disease, classically characteristic of a locally advanced, technically unresectable tumor. Suspected T4 disease should be confirmed with sensitive imaging techniques, including MRI. Diffuse chest wall involvement may be physically palpable or seen by PET/CT and is associated with chest wall pain. Suspected transdiaphragmatic extension should be confirmed by staging laparoscopy to avoid unnecessary thoracotomy. The most recent revisions of the IASLC Mesothelioma Staging Project confirm the poor survival for this category; median overall survival was 13.4 and 16.7 months, respectively, for clinical and pathologic stage T4 tumors.68
Surgical intervention after neoadjuvant treatment of T4 disease depends on the response to chemotherapy; rates of completion of all predefined approaches range between 33% and 71%.238 About 25% of patients have radiographic disease progression on chemotherapy, while significant pathologic responses in the resected specimens are rarely observed.238
If studies are suspicious for contralateral mediastinal or supraclavicular lymph node involvement, histologic confirmation must be obtained by EBUS, mediastinoscopy, or direct needle/core biopsy. Clinical evaluation of mediastinal adenopathy is notoriously inaccurate.
The new proposal for revisions of the N descriptors in the forthcoming 8th edition of the TNM classification separates lymph node stations into two categories: N1, metastases in the ipsilateral bronchopulmonary, hilar, or mediastinal (including internal mammary, peridiaphragmatic, pericardial fat pad, or intercostal) lymph nodes; and N2, metastases in the contralateral bronchopulmonary, hilar, or mediastinal lymph nodes or ipsilateral or contralateral supraclavicular lymph nodes.67
With the new classification, the median, 24-month, and 60-month survival of N2 disease is 13.9 months, 27%, and 0%, respectively. This new N2 category needs validation, however, as it only represented 2.3% of the IASLC registry participants. Notably, the poor survival observed with surgery for N2 disease is similar to that reported for pemetrexed/cisplatin chemotherapy without surgery.1 Chemotherapy should be delivered before any consideration of surgery in these patients, since the chances for satisfying MCR criteria are slim. For patients with N2 disease, the brief median survival and the absence of long-term survivors mandates against an initial surgical approach.
Clinical Question 2
Does histology and mediastinal lymph node status affect selection of patients for surgery?
Recommendation 2.1.
Patients with histologically confirmed sarcomatoid mesothelioma should not be offered maximal surgical cytoreduction (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
The importance of obtaining adequate tissue to ascertain the histology is paramount in deciding whether surgery is indicated. In a systematic review of studies with data on prognostic factors in patients who underwent EPP, nonepithelial histology was a significant prognostic factor in 11 of 17 reports; it was a trend in the remaining studies.221 A retrospective analysis of 663 patients treated at three institutions identified nonepithelioid histology as a significant prognostic factor for survival (HR, 1.3; P < .001).106 In a review of 1,183 patients in the SEER database, median survival in patients with epithelial, biphasic, and sarcomatoid disease who underwent surgery was 19, 12, and 4 months, respectively (P < .01). Surgery was associated with improved survival in patients with epithelioid disease, but not in those with biphasic or sarcomatoid histology.79
The percentage of epithelioid differentiation is an independent predictor of survival, which should be carefully considered when recommending surgery for patients with biphasic MPM.78 In a 144-patient series from a single center, patients with epithelioid differentiation of 100%, 51% to 99%, and < 50% had median overall survivals of 20.1, 11.8, and 6.62 months, respectively (P < .001).78
Recommendation 2.2.
Patients with ipsilateral, histologically confirmed mediastinal lymph node involvement should only undergo maximal surgical cytoreduction in the context of multimodality therapy (neoadjuvant or adjuvant chemotherapy). Optimally, these patients should be enrolled in clinical trials (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Lymph node involvement is observed in 35% to 50% of patients with MPM undergoing an MCR239 and is a poor prognostic indicator. A retrospective review of 348 surgical patients reported a median survival of 19 months in those with N0 or N1 disease, compared with 10 months for patients with positive N2, N2/N1, or internal thoracic nodes. Survival was also significantly worse when two or more N2 stations were affected (P < .001).57 In a systematic review of prognostic factors for surgery in MPM, 11 of 14 studies that described nodal involvement found it associated with poor prognosis.221 In a 529-patient retrospective series of EPP for epithelioid MPM, in which N1 disease was defined as within the visceral envelope and N2 as mediastinal, patients with N0, N1, N2, and N3 disease achieved median survivals of 26, 17, 13, and 7 months, respectively. Survival was shorter for patients with concurrent N1/N2 disease (13 months) than those with only N1 or N2 disease (17 and 16 months, respectively).73
The most recent edition of the IASLC Mesothelioma Staging Project recommendations evaluated 2,432 cases, including 851 with pathologic N category information. Survival was significantly inferior in patients with pathologically staged N1 or N2 tumors compared with N0 tumors (HR, 1.51; P < .001). Concurrent N1/N2 involvement portended a worse survival than N2 disease alone. There was no survival difference between N1 and N2 tumors.239 These data form the basis of the recommendation for the 8th edition of the staging system to merge N1 and N2 disease into one N category. N1 disease would refer to all ipsilateral intrathoracic nodal metastases. N3 nodes in the prior system would now be considered N2.
The impact of multimodality therapy on outcomes in patients with ipsilateral nodal disease in MPM is difficult to decipher. The median survival of 10 to 13 months for patients with ipsilateral lymph node involvement who undergo MCR appears no different from outcomes observed with systemic therapy alone. Other contributing factors include histology and T status, response to induction chemotherapy, and whether the patient completes all aspects of multimodality treatment. In a single-center retrospective review of 60 patients who received a variety of neoadjuvant chemotherapies followed by EPP and hemithoracic radiation therapy, the median survival of patients with no involved mediastinal lymph nodes was significantly better if they completed all three treatment modalities (59 v 8 months; P < .001); in patients with pathologic N2 involvement, however, there was no difference in survival (12 v 14 months; P = .9) whether all modalities were completed.162 In contrast, in a 77-patient multicenter phase II trial of neoadjuvant pemetrexed/cisplatin, EPP, and radiation therapy, median overall survival in patients with N1 or N2 disease was 16.6 months, but it was 29.1 months in patients with positive lymph nodes who completed all therapy.172 A single-center retrospective series of 186 patients with MPM who received induction cisplatin with gemcitabine or pemetrexed followed by EPP and radiation therapy reported that response to induction chemotherapy, but not lymph node status, was an independent prognostic factor for survival.133
Recommendations for the treatment of fit individuals with MPM in ipsilateral mediastinal nodes are limited by the lack of prospectively treated patients in randomized trials. Surgery alone is not appropriate in these patients, and a multimodality approach, preferably as part of a clinical trial, should be considered.
Clinical Question 3
What should surgeons consider when deciding the extent of maximal cytoreductive surgery (lung sparing v non–lung sparing)? What are the differences in outcomes (morbidity, QoL, survival) between lung-sparing and non–lung-sparing maximal cytoreductive surgery?
Recommendation 3.0.
Maximal surgical cytoreduction involves either EPP or lung-sparing options (P/D, extended P/D). When offering maximal surgical cytoreduction, lung-sparing options should be the first choice, due to decreased operative and long-term risk. EPP may be offered in highly selected patients when performed in centers of excellence (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
There has been a long-standing controversy regarding the optimal surgery for maximal cytoreduction in MPM. The MARS (Mesothelioma and Radical Surgery) trial was a feasibility study to assess whether patients could accept randomization to chemotherapy or EPP.4 While it was not powered to detect a difference in survival, the poor outcomes observed in this controversial study forced surgeons to consider whether EPP was a gold standard and led to multiple retrospective and prospective series of lung-sparing options.
While the surgical approach to EPP is standardized, the lack of uniform definitions regarding what constitutes a P/D renders comparisons between series quite challenging. A 2011 IASLC-IMIG Consensus Report recommended that P/D should attempt to remove all macroscopic tumor involving the parietal and visceral pleura; when the diaphragm or pericardium is also resected, it should be called an extended P/D.239
In a systematic review of seven studies in 1,145 patients that compared MCR with either EPP or extended P/D,220 perioperative mortality (2.9% v 6.8%; P = .02) and morbidity (27.9% v 62.0%; P < .001) were significantly lower with extended P/D compared with EPP. Survival for extended P/D (13 to 29 months) and EPP (12 to 22 months) was comparable, though the trend favored extended P/D. A meta-analysis of 24 data sets obtained between 1990 and 2014 contained 2,903 patients treated with P/D or EPP. While perioperative mortality was more frequent in patients who underwent EPP compared with P/D (4.5% v 1.7%; P < .05), there was no statistically significant difference in 2-year survival (23.8% v 25%; P = .8).219 In studies where EPP was originally performed by surgeons who later transitioned to P/D MCR, no difference in survival85,240 or improvement in survival with P/D was noted.84,150
Some patients with very bulky disease or disease in the fissure require EPP, as MCR may not otherwise be possible with P/D. Since disease volume is a recognized prognostic factor in MPM, this may confound interpretation of surgical comparisons.19,38,43,63,157 Unfortunately, no studies that compare outcomes from EPP and P/D measured disease volume. Such a study would be able to quantitatively compare the operations in patients with similar disease volumes to see if a lung-sparing approach is equivalent or even superior at a given pathologic stage.
Clinical Question 4
What are the differences in outcome between surgeries with palliative versus curative intent? (a) Which patients are most appropriate for surgery with curative intent? (b) Which patients are most appropriate for procedures with palliative intent?
Recommendation 4.1.1.
A maximal cytoreduction (either lung sparing or non–lung sparing) should only be considered in patients who meet specific preoperative cardiopulmonary functional criteria, have no evidence of extrathoracic disease, and are able to receive multimodality treatment (adjuvant or neoadjuvant) (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Any MCR is associated with perioperative mortality and morbidity. In a review of the Society of Thoracic Surgeons Database, consisting of 225 patients with MPM who underwent EPP or P/D at 48 centers, major morbidity, including acute respiratory distress syndrome, reintubation, unexpected reoperation, and sepsis, was more frequent with EPP (24.2% v 3.8%), as was mortality (10.5% v 3.1%). On multivariate analysis, EPP was an independent predictor of major morbidity or mortality (odds ratio, 6.51; P = .001). These data have been corroborated by others.38,84,220,240 Patients with MPM are usually elderly and therefore must have cardiopulmonary investigations to rule out occult coronary disease, pulmonary hypertension, and respiratory issues.241
MCR is of no value for patients with disease outside the hemithorax, so thorough radiographic242–244 and invasive staging must be performed.31,66,152,245 Multiple studies have documented that the use of combined modality therapy is a prognostic factor,59,71,86,106,155 so patients must be able to tolerate additional therapy either pre- or postoperatively.
Recommendation 4.1.2.
In patients who have a symptomatic pleural effusion, who are PS 2 or greater, or those in whom a maximal cytoreduction cannot be performed (due to disease extent or comorbid conditions), palliative approaches such as a tunneled permanent catheter placement or thoracoscopic exploration with partial resection and/or pleurodesis should be offered. In the latter case, additional biopsy to confirm pathologic diagnosis should be performed during the procedure. If the patient is being evaluated for investigational therapy, material for additional studies (eg, molecular and/or immunologic profiling) should be obtained (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Patients unable to have an MCR may still require palliation for a symptomatic pleural effusion. This can involve video-assisted thoracic surgery (VATS) with or without partial pleurectomy and/or pleurodesis, placement of a tunneled permanent catheter, or talc slurry. The Video-Assisted Surgery or Talc Pleurodesis in Treating Patients With Malignant Mesothelioma (MesoVATs) trial randomly assigned 175 patients with MPM to talc pleurodesis or VATS partial pleurectomy. Overall survival at 1 year was equivalent, but surgical and respiratory complications were significantly more common, and the median hospital stay was longer in patients who underwent VATS pleurectomy.15 Therefore, thoracoscopic talc poudrage without partial pleurectomy or nonthoracoscopic interventions including tunneled pleural catheter drainage or insertion of a talc slurry via a chest tube are preferred in most centers.
Recommendation 4.2.
In patients who have a symptomatic pericardial effusion, percutaneous catheter drainage or pericardial window may be performed (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Literature review and clinical interpretation.
Pericardial effusion is usually a late manifestation of MPM and, if not managed, can impair QoL and prevent the patient from tolerating systemic therapy. Once the diagnosis of a symptomatic pericardial effusion is made, drainage externally via pericardiocentesis with a percutaneous catheter or a pericardial window, either subxyphoid or by VATS, will usually promptly ameliorate symptoms.246–249
Clinical Question 5
Should maximal surgical cytoreduction be combined with chemotherapy and/or radiation? (a) In patients who are candidates for maximal surgical cytoreduction, should chemotherapy be given before or after surgery? (b) What is the optimal duration of neoadjuvant or adjuvant chemotherapy in the multimodality setting?
Recommendation 5.1.
Since surgical cytoreduction is not expected to yield an R0 resection, it is strongly recommended that multimodality therapy with chemotherapy and/or radiation therapy should be administered (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 5.2.
Chemotherapy may be given pre- or postoperatively in the context of multimodality treatment (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 5.3.
Adjuvant radiation therapy may be associated with a decreased risk of local recurrence and may be offered to patients who have undergone maximal cytoreduction. Treatment is complex, and it is recommended that it should be delivered at experienced centers of excellence (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 5.4.
In the context of multimodality treatment, four to six cycles of pemetrexed/platin-based chemotherapy may be administered pre- or postoperatively (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
Generally, the best treatment of a localized solid tumor includes complete surgical extirpation with adequate negative margins with or without additional therapy based on tumor stage. MPM is somewhat unique in that it is technically difficult if not impossible to accomplish complete resection with adequate negative margins (R0), since the pleural cavity extends to generally unresectable structures such as the chest wall, heart, spine, esophagus, airway, and the great vessels. Due to the technical challenges in obtaining an R0 resection, as well as meta-analyses demonstrating that multimodality therapy results in better survival than surgery alone, chemotherapy is recommended as an adjunct either prior to or after surgery.32 In the initial analysis of the IASLC database, for example, patients who received curative-intent surgery alone had a median survival of 11 months, compared with 20 months for those who received multimodality therapy (P < .001).25 Phase 2 trials demonstrate the safety and efficacy of adjuvant or neoadjvuant chemotherapy, though no study compares the two approaches.3,71,108,195,250 These trials commonly delivered about four chemotherapy cycles to minimize delay to surgical therapy and avoid postoperative toxicity.108,110,137,151,250 Adjuvant radiation therapy has been shown in multiple series to improve local control and survival. While preoperative radiation therapy can be associated with lung toxicity, radiation therapy can be given to patients after EPP or P/D as long as it is done by highly experienced centers that ensure that the lung is maximally spared.195
Clinical Question 6
What is the role of peri- or intraoperative intracavitary therapies (chemotherapy, photodynamic therapy)?
Recommendation 6.0.
Intracavitary therapies (chemotherapy or photodynamic therapy) may be administered safely in experienced centers of excellence, preferably in the context of a clinical trial. Their role in improving outcome is indeterminate (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: weak).
Literature review and clinical interpretation.
The fundamental problem with the goal of a complete macroscopic cytoreduction in MPM is that an R0 resection is usually nearly impossible. Due to the proximity of unresectable vital structures in the chest, surgical resection margins will always be very close to remaining tissues. Even after a seemingly complete resection, there is a relatively high rate of local and regional recurrence.75,251 Several intracavitary approaches have been evaluated to try to reduce local recurrence. Hyperthermic intraoperative chemotherapy (HIOC) with cisplatin has demonstrated safety and some efficacy in two phase I or II prospective clinical trials in patients undergoing EPP and P/D immediately after surgery.252,253 A safe maximally tolerated dose and the methodology to reduce associated complications have been established. A direct comparison between surgery with or without HIOC has not been published.62,118,171,252,253 The methodology for intraoperative photodynamic therapy has been similarly evaluated, and its efficacy has been reported.61 Only a limited number of specialized centers have expertise in HIOC and photodynamic therapy, however. Neither approach has been validated in multicenter randomized trials.
Clinical Question 7
What is the optimal management of pleural effusion in patients with mesothelioma? What is the role of pleurodesis versus tunneled pleural catheters in mesothelioma?
Recommendation 7.1.
Tunneled pleural catheters are not recommended in patients who are candidates for maximal surgical cytoreduction, because of the risk of tumor implantation into the chest wall (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 7.2.
In patients who are not candidates for maximal surgical cytoreduction, tunneled pleural catheters or pleurodesis (performed via chest tube or thoracoscopy) may be offered. Multidisciplinary input including surgical consultation with a center of excellence should be sought to optimize management of a pleural effusion and consideration of investigational intracavitary therapies (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
MPM is well known to seed biopsy sites. Thus, it is recommended to minimize the number of ports used during surgical diagnostic biopsies to one or two, placed in the line of future thoracotomy to allow for complete excision at definitive surgery. In addition, one should minimize the use of tunneled catheters in patients with MPM in whom future cytoreduction surgery is planned. The site selected for tunneled catheters is usually inferior in the chest wall to that of a future thoracotomy; the subcutaneous tunnel is a known seeding site for tumor cells.76 Although such seeding can be treated by radiation therapy, these sites can be difficult to resect and eradicate during cytoreduction surgery and may add to postoperative morbidity.
Patients for whom definitive surgery is not an option may be treated with various palliative procedures. These may include VATS decortication and pleurodesis,34,35,37,40,117,125,210 talc pleurodesis,117 bedside instillation of talc,40 placement of indwelling catheters, and intrapleural therapy with fibrinolytic drugs to resolve loculated effusions.37 To minimize morbidity and tumor seeding, these procedures should be performed through minimal incisions; additional biopsies for potential molecular testing can be obtained at the same time. Pleurodesis and pleural drainage can provide substantial symptomatic relief to patients with dyspnea due to lung compression. The incidence of infection is usually < 5% and manageable by antibiotics with or without catheter removal.
RADIATION THERAPY
Clinical Question 1
Should patients receive prophylactic irradiation of intervention tracts (thoracentesis, tunneled pleural catheters, thoracoscopy, and needle biopsy) to prevent tract recurrences?
Recommendation 1.1.
Prophylactic irradiation of intervention tracts should generally not be offered patients to prevent tract recurrences (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: moderate).
Recommendation 1.2.
It is recommended that adjuvant radiation should be offered to patients who have resection of intervention tracts found to be histologically positive (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
Two systematic reviews,204,216 four RCTs,9,11,14,21 and one retrospective study27 were identified. Most used variable radiation therapy doses, some with antiquated techniques (electrons, superficial kVs, etc), and radiation therapy was delivered at various intervals from surgical intervention. The largest retrospective study analyzed 171 patients treated with prophylactic irradiation of intervention tracks (PIT), mostly thoracoscopic procedures. Most patients (84%) received either 21 Gy in three fractions or 20 Gy in four to five fractions. In the PIT group, 13% of the 48 patients developed biopsy tract metastases, compared with 33% of the 123 patients who were not radiated (P = .008). This difference was not statistically significant on multivariate analysis when analyzed as local progression-free survival at the intervention site at 6 or 12 months.
The two systematic reviews included three RCTs.9,11,14 Both concluded that there was neither consensus nor strong justification for PIT, since only one RCT detected a significant difference in intervention site metastases after PIT. In this widely cited 40-patient trial, 21 Gy in three consecutive fractions delivered 10 to 15 days after thoracoscopy reduced intervention site metastases from 40% to 0%.14 An RCT on 58 sites in 43 patients with a much lower dose, 10 Gy in one fraction using 9-MeV electron therapy, reported no significant difference in tract metastases (10% v 7%). A 61-patient RCT compared 21 Gy in three fractions within 21 days after an invasive procedure with BSC. No statistical difference in tract metastases was detected.9 It is important to recognize that most published studies were performed prior to the widespread use of effective chemotherapy. Patients were also not treated with more comprehensive adjuvant radiation therapy techniques delivered to larger parts of the thorax.
Interestingly, despite these data, 75% of United Kingdom survey responders routinely used PIT, and 80% were supportive of a larger RCT to determine its efficacy.204,216 This led to the largest, most rigorously performed multicenter, phase III RCT in 203 patients treated with immediate radiation therapy to 21 Gy in three fractions within 42 days of pleural intervention or deferred radiation therapy at the time of procedure-tract metastases.21 The primary end point was the incidence of tract metastases within 7 cm of the intervention site. No significant difference in tract metastases was identified (9% v 16%; P = .14). There was a suggestion that epithelioid-only histologic subtypes may benefit from PIT, and patients not treated with chemotherapy may have a lower tract recurrence rate with immediate radiation therapy. Further studies in these specific subgroups may be warranted.
Clinical Question 2
What is the role of palliative radiation therapy? What is the optimal radiation dose and fractionation?
Recommendation 2.1.
Radiation therapy should be offered as an effective treatment modality to palliate patients with symptomatic disease (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 2.2.
It is recommended that standard dosing regimens used in other diseases be offered to patients with mesothelioma (8 Gy × one fraction, 4 Gy × five fractions, or 3 Gy × 10 fractions) (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Until recently, the evidence for palliative radiation therapy in MPM was quite limited. A systematic review199 found that the literature consisted mostly of retrospective series and two small single-arm phase II studies that investigated palliative hemithoracic radiation therapy with antiquated techniques. A 111-patient retrospective series from 1994 demonstrated relief of symptoms, principally pain, in over half the patients, with no observed dose-response relationship. The largest retrospective study was in 189 patients treated for a total of 227 courses of radiation therapy.48 Pain, mostly from tumor growing into the chest wall, was the indication for palliative radiation therapy in 77%. While patients were treated with a various radiation therapy regimens, since 1987, sites of symptomatic disease in 91 patients were irradiated to a total dose of 36 Gy in nine fractions, three times weekly. There was a better response rate with radiation therapy doses of 4 Gy or higher per fraction (50% v 39%), with a median time to pain recurrence of 69 days.
The highest-quality data are from the Symptom Study of Radiotherapy in Mesothelioma (SYSTEMS-1), a multicenter single-arm phase II study of 40 patients treated to a total dose of 20 Gy in five fractions.191 All treatments were planned based on CT and PET/CT imaging. Pain was characterized prospectively; 54% of patients presented with neuropathic pain.196 This regimen decreased pain in 47% of patients. No improvement in QoL or other symptoms was detected, possibly due to the short survival after treatment. This study is limited by its relatively small sample size, high attrition rate, poor survival, and variability of radiation field and technique. A follow-up study (SYSTEMS-2) will examine whether a dose-escalated, hypofractionated radiation therapy approach (36 Gy in six fractions) results in clinically significant improvement in pain at 5 weeks when compared with standard palliative radiation therapy (20 Gy in five fractions).
Palliative radiation therapy using standard palliative doses and fractionation can provide significant pain relief in about 50% of patients and should be considered in all patients with MPM with localized disease causing pain or obstructive symptoms.
Clinical Question 3
What is the role of radiation therapy for asymptomatic recurrence? What is the optimal radiation dose and fractionation?
Recommendation 3.0.
Radiation therapy may be offered to patients with localized asymptomatic recurrence. The dosing fractionation is dependent on the site and extent of disease and should be determined by the radiation oncologist in consultation with the patient (Type of recommendation: informal consensus; Strength of recommendation: moderate).
Literature review and clinical interpretation.
The use of radiation therapy for localized asymptomatic recurrences is not clearly described in the literature. However, radiation techniques to treat recurrent MPM are available and can be applied. The difficulty in this setting is the high variability of clinical presentation. The appropriate treatment decision needs to be made in a multidisciplinary context. Options may include close observation, systemic therapies, and local treatment, such as surgical resection and radiation therapy. Prior treatments need to be carefully taken into account.
From a radiation therapy perspective, small, isolated, localized recurrences can potentially be treated with high-dose hypofractionated stereotactic body radiation therapy. This is highly effective and ablative in multiple metastatic histologies, resulting in long-term local control rates of about 90%. Select patients with unresectable pleural disease may be considered for hemithoracic pleural IMRT at centers of excellence with expertise in this approach. Very fit, select patients may be candidates for surgical resection and radiation therapy if feasible.
Radiation therapy should be considered as one of several options to treat asymptomatic recurrences. Dose and fractionation depend on the clinical scenario, prior treatments, currently available treatment options, as well as the patient’s wishes.
Clinical Question 4
What is the role of radiation therapy in patients who get non–lung-sparing cytoreductive surgery? What is the optimal adjuvant radiation approach in this setting?
Recommendation 4.1.
Hemithoracic adjuvant radiation therapy may be offered to patients who undergo non–lung-sparing cytoreductive surgery (EPP), preferably in centers of excellence with experience in this modality for mesothelioma (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 4.2.
Hemithoracic neoadjuvant radiation therapy may be offered to patients who undergo non–lung-sparing cytoreductive surgery. This potentially toxic regimen remains experimental and should only be performed in highly experienced centers within the context of a clinical trial (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Literature review and clinical interpretation.
One systematic review,214 12 prospective cohorts,49,95,96,120,139–142,145,146,148,149,155 six retrospective studies,36,52,54–56,162 and four phase II studies172,181,190,194 were identified. Together, these provide substantial evidence that hemithoracic adjuvant radiation therapy can be safely delivered after EPP. Initially, a conventional combined photon-electron radiation technique targeting the involved hemithorax with anterior-posterior beam arrangements was used.54,181 However, this leads to significant radiation dose inhomogeneities associated with a high risk of local failure.49 Therefore, three-dimensional (3D) conformal radiation therapy (CRT) and IMRT techniques were developed, which provide a higher level of control over the radiation dose distribution and less inhomogeneity.36,145
There was a steep learning curve with the application of more modern techniques, with reports of fatal radiation pneumonitis in the contralateral lung254 due to an increase in the radiation dose to the single remaining lung, which can be higher using 3D-CRT or IMRT if the dose is not tightly controlled.145,146 On multivariate analysis, the volume of lung receiving 20 Gy (V20) > 7% was identified as a significant risk factor for pulmonary-related death.146 With increasing experience, improvement in target coverage, and reduction in radiation therapy doses to the lung, decreased lung toxicity was observed.69,145 Clearly, these complex treatments should be delivered at highly experienced centers. Institutional series with modern radiation techniques have resulted in 2-year locoregional control of 40% to 71% and 2-year overall survival of 18% to 57%.56,139–141,162
Prospective phase II studies on EPP and adjuvant hemithoracic radiation therapy have demonstrated a marked reduction in locoregional recurrence. In a 57-patient single-center trial, locoregional recurrence was only 3.5%; median survival was 33.8 months for stage I/II and 10 months for stage III/IV tumors.181 A multicenter phase II trial of neoadjuvant platinum/ pemetrexed, EPP, and adjuvant hemithoracic radiation therapy in 54 patients achieved a median survival of 29.1 months and a 2-year overall survival of 61.2%.172 Median progression-free survival rates were 48.4% at 1 year and 25.5% at 2 years.
A two-part, multicenter randomized phase II study (Neoadjuvant Chemotherapy and Extrapleural Pneumonectomy of Malignant Pleural Mesothelioma With or Without Hemithoracic Radiotherapy [SAKK 17/04]) tested the feasibility of achieving a complete macroscopic resection with EPP after neoadjuvant chemotherapy (part 1) and the locoregional relapse-free survival after an MCR with or without adjuvant radiation therapy (part 2).190 An MCR was obtained in 64% of 151 patients. In part 2, 54 patients were randomized to adjuvant radiation therapy versus observation. Ninety-three percent of patients received radiation therapy as planned; three different fractionation regimens were allowed. Median locoregional relapse-free survival was 7.6 months in the observation group and 9.4 months with radiation therapy, which was not a statistically significant difference. The authors concluded that their study does not support the use of adjuvant hemithoracic radiation therapy after EPP. However, the trial closed early due to poor accrual and was not adequately powered to meet its primary end point. Quality control of the radiation therapy plans was also insufficient without central plan review for uniformity and protocol adherence.
The Princess Margaret Hospital has pioneered neoadjuvant accelerated hemithoracic IMRT (25 Gy in five fractions with a concomitant boost of 5 Gy) followed by EPP within 1 week after the end of radiation therapy.142,194 In the initial experience, this resulted in a 3-year overall survival of 84% in patients with epithelioid MPM. An expanded report of 62 patients showed a median overall survival of 36 months. Patients with epithelioid MPM had a median overall and disease-free survival of 51 and 47 months, respectively. The rate of complications grade 3 or greater was 39%. Since non–lung-sparing surgical resection must occur after neoadjuvant high-dose hemithoracic radiation therapy to avoid potentially lethal radiation pneumonitis in the irradiated hemithorax, careful patient selection was necessary to guarantee 100% resectability. A retrospective analysis found no significant differences in surgical risk or 90-day mortality between neoadjuvant hemithoracic radiation therapy and induction chemotherapy.52 This high-risk strategy has not been validated by other institutions and should first be established at centers with significant expertise in the multimodality management of MPM before being used by a wider community.
Clinical Question 5
What is the role of radiation therapy in patients who get lung-sparing cytoreductive surgery? What is the optimal radiation approach in this setting?
Recommendation 5.1.
Hemithoracic adjuvant intensity-modulated radiation therapy may be offered to patients who undergo lung-sparing cytoreductive surgery (P/D or extended P/D). This potentially toxic regimen should only be performed in highly experienced centers, preferably in the context of a clinical trial (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 5.2.
Due to the potential for severe pulmonary toxicity, neoadjuvant radiation therapy is not recommended for patients who undergo lung-sparing surgical cytoreductive surgery (Type of recommendation: informal consensus; Strength of recommendation: strong).
Literature review and clinical interpretation.
Two systematic reviews,212,218 four prospective cohort studies,137,143,144,147 six retrospective studies,22,50,53,70,143,255 and one prospective phase II study195 were identified. Adjuvant radiation therapy targeting the ipsilateral pleura after lung-sparing cytoreductive surgery or in unresectable patients with two intact lungs is challenging because of the high radiation sensitivity of normal lung tissue.
With the advent of IMRT, it became technically feasible to target the entire ipsilateral pleura with relative sparing of underlying lung tissue and to escalate the radiation dose to tumoricidal levels. The first report in 36 patients with MPM with two intact lungs showed that hemithoracic adjuvant pleural IMRT could be delivered with a 20% grade 3 or greater pneumonitis risk; one patient had grade 5 pneumonitis.147 The median survival in resectable patients was 26 months. A tomotherapy technique was published with similar toxicity outcomes (17.8% grade ≥ 2 pneumonitis, no fatal respiratory toxicity).144 The volume of lung receiving 5 Gy (V5) of the contralateral lung most strongly correlated with the risk of developing pneumonitis. The radiation dose delivered was slightly higher, with 50 Gy delivered in 25 fractions and a simultaneous boost to 60 Gy for areas of concern for residual disease based on FDG-PET. A matched analysis of P/D, chemotherapy, and IMRT versus EPP, chemotherapy, and IMRT found favorable median overall (28.4 v 14.2 months) and progression-free survival (16.4 v 8.2 months) with trimodality therapy involving P/D compared with EPP.22 A progressive decline in pulmonary function was observed, however.
Local failure rates vary significantly among studies, ranging from 40% to 68% at 2 years.22,53,143 Improved target coverage and improved local control with IMRT was observed in a 55-patient retrospective study comparing 3D-CRT to tomotherapy.50 A systematic review still found a significant risk of local failures in the radiation field, mostly in unresectable patients, emphasizing the importance of a macroscopic complete resection, need for optimization of radiation targeting, and experience with this complex radiation technique.53
A two-institution prospective phase II study assessed the safety of adjuvant hemithoracic intensity-modulated pleural radiation therapy in 27 patients.195 Radiation pneumonitis developed in 29.6% (six grade 2; two grade 3). Median progression-free and overall survival were 12.4 and 23.7 months, respectively. In resectable patients with MPM who received chemotherapy and intensity-modulated pleural radiation therapy, 2-year overall survival was 59%.195 There are no data available on neoadjuvant radiation therapy prior to lung-sparing surgery, and it is not recommended.
Radiation therapy after lung-sparing surgery is challenging due to the risk for radiation pneumonitis, a potentially severe toxicity, especially with an intact ipsilateral lung. Older radiation techniques result in unacceptable toxicity and insufficient local control. Modern techniques, such as IMRT, which can be delivered by linear accelerators or tomotherapy units, are safe and feasible when performed by highly experienced treatment teams, preferably in the context of a clinical trial. There are no randomized data employing these techniques, however. Thus, any advanced radiation therapy techniques should only be used in centers with significant experience in multimodality disease and toxicity management of patients with MPM.
Clinical Question 6
What are the appropriate radiation techniques (electrons, two-dimensional [2D], 3D, IMRT, and protons)?
Recommendation 6.1.
For palliative radiation therapy, electrons, 2D, 3D, and IMRT may be considered appropriate techniques depending on location of the treatment target and organs at risk (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 6.2.
For adjuvant or neoadjuvant hemithoracic radiation therapy, 3D or IMRT may be offered, respecting guidelines of organs at risk. Proton therapy may be considered in centers with significant experience, preferably in the context of a clinical trial (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
Three retrospective studies were identified.36,50,51 The choice of radiation technique depends on the clinical scenario, the goal of radiation therapy, the dose to be delivered, and the relative location of the target area and organs at risk.
For palliative radiation therapy, any technique may be used, but simpler ones such as electrons, 2D or 3D conformal radiation therapy are frequently used, since generally lower doses are delivered and long-term toxicities may be of limited relevance in terminally ill patients.9,21,191 Electrons are particularly useful to deliver effective radiation doses to superficial targets, such as intervention tract failures or chest wall or subcutaneous masses. They may be combined with a bolus to enhance the radiation dose to the skin if the tumor is very superficial. Simple 2D or 3D techniques are appropriate for most palliative settings in which common doses such as 4 Gy × 5 fractions or 3 Gy × 10 fractions are delivered. When prolonged local control is the goal, hypofractionated radiation therapy or stereotactic body radiation therapy using highly conformal 3D, IMRT, or proton techniques may be appropriate. This is also important in the re-irradiation setting and may be the only way to deliver additional adequate radiation doses without exceeding normal tissue constraints.
Most radiation studies have been performed in the adjuvant setting. Conformal techniques such as 3D-CRT or IMRT should be used after EPP, but most studies have shown better dosimetry using IMRT when planned appropriately. Initially, IMRT was associated with excess deaths due to the lack of appreciation of the impact of low radiation doses to the contralateral lung.55 Subsequent studies have demonstrated the safety of IMRT after EPP. IMRT improves target volume coverage over combined photon/electron 3D conformal techniques,36 which is associated with a decrease in local relapse.51 However, IMRT can be associated with an increase in dose to the contralateral mean lung dose and lung volume receiving lower radiation doses, which requires tight control of the contralateral lung dose in an adjuvant radiation therapy plan. When comparing different IMRT delivery techniques, most studies have shown further incremental improvement in target coverage and avoidance of organs at risk by using rotational or arc-based delivery techniques such as tomotherapy and volumetric-modulated arc therapy.256–258
Proton therapy has been explored for adjuvant treatment after EPP. In a theoretical planning study, both IMRT and proton therapy achieved good target coverage and dose homogeneity.259 Proton therapy could further decrease the radiation dose to nearby organs at risk. However, proton therapy plans were more sensitive to intrathoracic density changes, such as air cavities. After lung-sparing surgery, IMRT improves target coverage and local control when compared with 3D-CRT.50 The first study on proton therapy after P/D showed that intensity-modulated proton therapy could successfully be delivered to seven patients, with lower mean doses to organs at risk compared with IMRT.260
The appropriateness of the available radiation technique depends on the clinical scenario, goal of radiation therapy, target dose to be delivered, relative location of the target area, and organs at risk. The experience of a center with a given technique is most relevant with more complex treatment techniques.
Clinical Question 7
What are predictors of radiation toxicity (after lung sparing or non–lung-sparing cytoreductive surgery or after palliative pleurectomy)?
Recommendation 7.0.
It is recommended that standard dosimetric guidelines for organs at risk be used as established predictors of radiation toxicity (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Literature review and clinical interpretation.
There are currently no known predictors of radiation toxicity or response that are specific to MPM. Caution is warranted when using adjuvant radiation after either lung-sparing or non–lung-sparing surgery. Additional studies to determine predictors of radiation pneumonitis in these settings are needed.
The first experience with IMRT after EPP showed that the V5, V20, and mean lung dose (MLD) were associated with the development of pneumonitis.55 There is also an association of MLD and the volume of lung receiving 10 Gy with the development of pneumonitis.235 The lung V20 and MLD are most critical for avoiding fatal pulmonary toxicities; the recommendation is to keep the MLD < 8.5 Gy and the mean lung V20 ≤ 7%.
After lung-sparing P/D and adjuvant tomotherapy, the contralateral lung V5 has been associated with the risk of radiation pneumonitis.144 It appears that the MLD and V5 to V20 of the contralateral lung are most important after EPP. The contralateral lung V5 may also play a role after lung-sparing surgery. In general, limiting radiation doses to organs at risk with these extensive and challenging treatment fields is paramount to minimize risk to the patient. Ongoing studies explore the individual variability in radiation sensitivity of organs at risk beyond the dosimetric parameters of the radiation treatment plan.
FUTURE DIRECTIONS
Although no new drugs have been approved for the treatment of MPM since the approval of pemetrexed plus cisplatin in 2004, there have been significant recent advances in understanding the biology of mesothelioma and identifying new targets for therapy. Ongoing clinical trials suggest promising activity of several new agents in MPM, but they are not sufficiently mature to make treatment recommendations. These include clinical trials of mesothelin-targeted agents as well as antibodies against the immune check-points programmed death 1/programmed death-ligand 1 and cytotoxic T-lymphocyte-associated protein 4. Given the rarity of this disease, large randomized international clinical trials are vital to fully define the role of novel therapeutic drugs for the treatment of patients with MPM.
PATIENT AND CLINICIAN COMMUNICATION
There are two main factors that affect patient communication and their choices in health care decisions. The first is the increasing information asymmetry between physician and patient as our scientific advancement grows. The volume and complexity may seem overwhelming and, because it is couched in technical jargon, nearly impenetrable. Our challenge, as physicians and patient advocates, is to explain these issues in plain language, appearing not condescending yet scientifically sound enough that patients can use these advances in their understanding as well as our understanding as physicians to make informed decisions. We must explain that as our knowledge grows so do their options for care, and, while this may seem complex, we must take each step in their care one at a time and explain how each step may affect the next, clarifying the potential benefits and possible outcomes in terms of absolute, not relative, risk that can be easily understood. We must use informational resources, like Adjuvant or National Health Service Predict, to help patients make these decisions and point out valid Internet sources like the National Cancer Institute site, not commercial ventures.
The second major issue is more difficult, because it reflects the art, not the science, of medicine; it is telling the patient what they need to hear, not what they want to hear. All patients want to hear that they can be cured easily and without complications or personal loss. We must balance the expectations such that it does not take away all hope but does not give the impression of an unrealistic outcome. While explaining the potential consequences of the sequential decision-making process, this decision-making process must include social, financial, and age-related issues and is further complicated by the current trend to include the family, no matter how distant or estranged they may be, in this process.
For recommendations and strategies to optimize patient-clinician communication, see Patient-Clinician Communication: American Society of Clinical Oncology Consensus Guideline.261
HEALTH DISPARITIES
Although ASCO clinical practice guidelines represent expert recommendations on the best practices in disease management to provide the highest level of cancer care, it is important to note that many patients have limited access to medical care. Racial and ethnic disparities in health care contribute significantly to this problem in the United States. Patients with cancer who are members of racial/ethnic minorities suffer disproportionately from comorbidities, experience more substantial obstacles to receiving care, are more likely to be uninsured, and are at greater risk of receiving care of poor quality than other Americans.262–265 Many other patients lack access to care because of their geographic location and distance from appropriate treatment facilities. Awareness of these disparities in access to care should be considered in the context of this clinical practice guideline, and health care providers should strive to deliver the highest level of cancer care to these vulnerable populations.
Disparities in care result in not only delayed diagnosis but also the development of major comorbidities, especially diabetes and hypertension. These compromise treatment decisions because of long-term effects on cardiac and renal function and require coordination of care with the patient’s primary care physician as well as cardiologists and endocrinologists. ASCO has long recognized the critical importance of this by including these issues on certification exams. Our challenge is to explain to the patient the importance of managing these conditions while communicating with our colleagues our treatment decisions and how they will affect renal and cardiac function. Moreover, as the management of cancer becomes a chronic disease, we must emphasize to the patient the importance of managing their chronic conditions, which may be more life threatening than their cancer diagnosis once treated. We then become part of the team that manages the chronic, not just the acute, disease process, realizing that cancer treatment may itself generate other comorbidities that require surveillance. Finally, all of these decisions must reflect the patient’s desire for quality of life, not just quantity.
MULTIPLE CHRONIC CONDITIONS
Creating evidence-based recommendations to inform treatment of patients with additional chronic conditions, a situation in which the patient may have two or more such conditions—referred to as multiple chronic conditions (MCC)—is challenging. Patients with MCC are a complex and heterogeneous population, making it difficult to account for all of the possible permutations to develop specific recommendations for care. In addition, the best available evidence for treating index conditions, such as cancer, is often from clinical trials whose study selection criteria may exclude these patients to avoid potential interaction effects or confounding of results associated with MCC. As a result, the reliability of outcome data from these studies may be limited, thereby creating constraints for expert groups to make recommendations for care in this heterogeneous patient population.
As many patients for whom guideline recommendations apply present with MCC, any treatment plan needs to take into account the complexity and uncertainty created by the presence of MCC and highlight the importance of shared decision making regarding guideline use and implementation. Therefore, in consideration of recommended care for the target index condition, clinicians should review all other chronic conditions present in the patient and take those conditions into account when formulating the treatment and follow-up plan.
In light of the above considerations, practice guidelines should provide information on how to apply the recommendations for patients with MCC, perhaps as a qualifying statement for recommended care. This may mean that some or all of the recommended care options are modified or not applied, as determined by best practice in consideration of any MCC.
GUIDELINE IMPLEMENTATION
ASCO guidelines are developed for implementation across health settings. Barriers to implementation include the need to increase awareness of the guideline recommendations among front-line practitioners, survivors of cancer, and caregivers and also to provide adequate services in the face of limited resources. The guideline Bottom Line Box was designed to facilitate implementation of recommendations. This guideline will be distributed widely through the ASCO Practice Guideline Implementation Network. ASCO guidelines are posted on the ASCO Web site and most often published in Journal of Clinical Oncology and Journal of Oncology Practice.
ASCO believes that cancer clinical trials are vital to inform medical decisions and improve cancer care, and that all patients should have the opportunity to participate
ADDITIONAL RESOURCES
More information, including a Data Supplement with additional evidence tables, a Methodology Supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources, is available at www.asco.org/thoracic-cancer-guidelines and www.asco.org/guidelineswiki. Patient information is available at www.cancer.net. Visit www.asco.org/guidelineswiki to provide comments on the guideline or to submit new evidence.
Supplementary Material
THE BOTTOM LINE.
Guideline Questions
What is the best treatment for patients with malignant pleural mesothelioma?
Target Population
Patients with malignant pleural mesothelioma
Target Audience
Medical, surgical, and radiation oncologists; oncology nurses and physician assistants; pulmonologists; radiologists; pathologists; general practitioners; and patients
Methods
An Expert Panel was convened to develop clinical practice guideline recommendations based on a systematic review of the medical literature.
KEY RECOMMENDATIONS
Diagnosis
Recommendation 1.1:
Clinicians should perform an initial thoracentesis when patients present with symptomatic pleural effusions and send pleural fluid for cytologic examination for initial assessment for possible mesothelioma (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2:
In patients for whom antineoplastic treatment is planned, it is strongly recommended that a thoracoscopic biopsy should be performed. This will: (a) enhance the information available for clinical staging; (b) allow for histologic confirmation of diagnosis; (c) enable more accurate determination of the pathologic subtype of mesothelioma (epithelial, sarcomatoid, biphasic); and (d) make material available for additional studies (eg, molecular profiling) (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 1.2.1:
When performing a thoracoscopic biopsy, the minimal number of incisions (two or fewer) is recommended and should ideally be placed in areas that would be used for subsequent definitive resection to avoid tumor implantation into the chest wall (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 1.3:
In patients with suspected mesothelioma in whom treatment is planned, an open pleural biopsy should be performed if the extent of tumor prevents a thoracoscopic approach. The smallest incision possible is encouraged (generally 6 cm or less is recommended) (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 1.4:
In patients who are not candidates for thoracoscopic biopsy or open pleural biopsy, who also have a nondiagnostic thoracentesis or do not have a pleural effusion, clinicians should perform a core needle biopsy of an accessible lesion (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 2.0:
Cytologic evaluation of pleural fluid can be an initial screening test for mesothelioma, but it is not a sufficiently sensitive diagnostic test. Whenever definitive histologic diagnosis is needed, biopsies via thoracoscopy or CT guidance offer a better opportunity to reach a definitive diagnosis (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.0:
Histologic examination should be supplemented by immunohistochemistry using selected markers expected to be positive in mesothelioma (eg, calretinin, keratins 5/6, and nuclear WT1) as well as markers expected to be negative in mesothelioma (eg, CEA, EPCAM, Claudin 4, TTF-1). These markers should be supplemented with other markers that address the differential diagnosis in that particular situation (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 4.1:
Mesothelioma should be reported as epithelial, sarcomatoid, or biphasic, because these subtypes have a clear prognostic significance (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 4.2:
In surgical, thoracoscopic, or open pleural biopsies with sufficient tissue, further subtyping and quantification of epithelial versus sarcomatoid components of mesothelioma may be undertaken (Type of recommendation: informal consensus; Strength of recommendation: moderate).
Recommendation 5.0:
The non–tissue-based biomarkers that are under evaluation at this time do not have the sensitivity or specificity to predict outcome or monitor tumor response and are therefore not recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 6.0:
While tumor genomic sequencing is currently done on a research basis in mesothelioma and it may become clinically applicable in the near future, it is not recommended at this time (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Staging
Recommendation 1.1:
A CT scan of the chest and upper abdomen with IV contrast is recommended as the initial staging in patients with mesothelioma (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2:
An FDG PET/CT should usually be obtained for initial staging of patients with mesothelioma. This may be omitted in patients who are not being considered for definitive surgical resection (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.3:
If abnormalities that suggest metastatic disease in the abdomen are observed on a chest and upper abdomen CT or on a PET/CT then consideration should be given to perform a dedicated abdominal (+/− pelvic) CT scan, preferably with IV and oral contrast (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.4:
An MRI (preferably with IV contrast) may be obtained to further assess invasion of the tumor into the diaphragm, chest wall, mediastinum, and other areas (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 1.5:
For patients being considered for maximal surgical cytoreduction, a mediastinoscopy and/or endobronchial US should be considered if enlarged and/or PET-avid mediastinal nodes are present (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.6:
In the presence of contralateral pleural abnormalities detected on initial PET/CT or chest CT scan, a contralateral thoracoscopy may be performed to exclude contralateral disease (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 1.7:
In patients with suspicious findings for intra-abdominal disease on imaging and no other contraindications to surgery, it is strongly recommended that a laparoscopy be performed (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 2.1:
The current AJCC/UICC staging classification remains difficult to apply to clinical staging with respect to both T and N components and thus may be imprecise in predicting prognosis. Physicians should recognize that in patients with clinical stage I/II disease, upstaging may occur at surgery (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 3.1:
The optimal approach to mesothelioma measurement requires the expertise of a radiologist to identify measurement sites on CT as per modified RECIST for mesothelioma. This approach requires calculating the sum of up to six measurement sites with at least 1 cm thickness measured perpendicular to the chest wall or mediastinum with no more than two sites on each of three CT sections separated by at least 1 cm axially (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.2:
Assessment of tumor volume by CT scan may enhance clinical staging and provide prognostic information but remains investigational and thus is not recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.3:
It is recommended that tumor response classification be determined based on RECIST criteria from the comparisons of these sums across serial CT scans (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Chemotherapy
Recommendation 1.1:
Chemotherapy should be offered to patients with mesothelioma because it improves survival and quality of life (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2:
In asymptomatic patients with epithelial histology and minimal pleural disease who are not surgical candidates, a trial of close observation may be offered prior to the initiation of chemotherapy (Type of recommendation: informal consensus; Strength of recommendation: moderate).
Recommendation 1.3:
Selected patients with a poor performance status (PS 2) may be offered single-agent chemotherapy or palliative care alone. Patients with a PS of 3 or greater should receive palliative care (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 2.1:
The recommended first-line chemotherapy for patients with mesothelioma is pemetrexed plus platinum. However, patients should also be offered the option of enrolling in a clinical trial (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong)
Recommendation 3.1:
The addition of bevacizumab to pemetrexed-based chemotherapy improves survival in select patients and therefore may be offered to patients with no contraindications to bevacizumab. The randomized clinical trial demonstrating benefit with bevacizumab used cisplatin/pemetrexed; data with carboplatin/pemetrexed plus bevacizumab are insufficient for a clear recommendation (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: moderate)
Recommendation 3.2:
Bevacizumab is not recommended for patients with PS 2, substantial cardiovascular comorbidity, uncontrolled hypertension, age > 75, bleeding or clotting risk, or other contraindications to bevacizumab (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 4.0:
In patients who may not be able to tolerate cisplatin, carboplatin may be offered as a substitute for cisplatin (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 5.1:
Retreatment with pemetrexed-based chemotherapy may be offered in pleural mesothelioma patients who achieved durable (> 6 months) disease control with first-line pemetrexed-based chemotherapy (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 5.2:
Given the very limited activity of second-line chemotherapy in patients with mesothelioma, participation in clinical trials is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 5.3:
In patients for whom clinical trials are not an option, vinorelbine may be offered as second-line therapy (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 6.1:
In asymptomatic patients with epithelial mesothelioma and a low disease burden who are not surgical candidates, a trial of expectant observation may be offered before initiation of systemic therapy (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 6.2:
Front-line pemetrexed-based chemotherapy should be given for four to six cycles. For patients with stable or responding disease, a break from chemotherapy is recommended at that point (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 6.3:
There is insufficient evidence to support the use of pemetrexed maintenance in mesothelioma patients, and thus it is not recommended (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: strong).
Surgical Cytoreduction
Recommendation 1.1:
In selected patients with early-stage disease, it is strongly recommended that a maximal surgical cytoreduction should be performed (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.2:
Maximal surgical cytoreduction as a single modality treatment is generally insufficient; additional antineoplastic treatment (chemotherapy and/or radiation therapy) should be administered. It is recommended that this treatment decision should be made with multidisciplinary input involving thoracic surgeons, pulmonologists, medical and radiation oncologists (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 1.3:
Patients with transdiaphragmatic disease, multifocal chest wall invasion, or histologically confirmed contralateral mediastinal or supraclavicular lymph node involvement should undergo neoadjuvant treatment before consideration of maximal surgical cytoreduction. Contralateral (N3) or supraclavicular (N3) disease should be a contraindication to maximal surgical cytoreduction (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 2.1:
Patients with histologically confirmed sarcomatoid mesothelioma should not be offered maximal surgical cytoreduction (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 2.2:
Patients with ipsilateral histologically confirmed mediastinal lymph node involvement should only undergo maximal surgical cytoreduction in the context of multimodality therapy (neoadjuvant or adjuvant chemotherapy). Optimally, these patients should be enrolled in clinical trials. (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.0:
Maximal surgical cytoreduction involves either extrapleural pneumonectomy (EPP) or lung-sparing options (pleurectomy/decortication [P/D], extended P/D). When offering maximal surgical cytoreduction, lung-sparing options should be the first choice, due to decreased operative and long-term risk. EPP may be offered in highly selected patients when performed in centers of excellence (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 4.1.1:
A maximal cytoreduction (either lung sparing or non–lung sparing) should only be considered in patients who meet specific preoperative cardiopulmonary functional criteria, have no evidence of extrathoracic disease, and are able to receive multimodality treatment (adjuvant or neoadjuvant) (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 4.1.2:
In patients who have a symptomatic pleural effusion, who are PS 2 or greater, or in whom a maximal cytoreduction cannot be performed (due to disease extent or comorbid conditions), palliative approaches such as a tunneled permanent catheter placement or thoracoscopic exploration with partial resection and/or pleurodesis should be offered. In the latter case, additional biopsy to confirm pathologic diagnosis should be performed during the procedure. If the patient is being evaluated for investigational therapy, material for additional studies (eg, molecular and/ or immunologic profiling) should be obtained. (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 4.2:
In patients who have a symptomatic pericardial effusion, percutaneous catheter drainage or pericardial window may be performed (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation 5.1:
Since surgical cytoreduction is not expected to yield an R0 resection, it is strongly recommended that multimodality therapy with chemotherapy and/or radiation therapy should be administered (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 5.2:
Chemotherapy may be given pre- or postoperatively in the context of multimodality treatment (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Recommendation 5.3:
Adjuvant radiation therapy may be associated with a decreased risk of local recurrence and may be offered to patients who have undergone maximal cytoreduction. Treatment is complex, and it is recommended that it should be delivered at experienced centers of excellence (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 5.4:
In the context of multimodality treatment, four to six cycles of pemetrexed/platin-based chemotherapy may be administered pre- or postoperatively (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 6.0:
Intracavitary therapies (chemotherapy or photodynamic therapy) may be administered safely in experienced centers of excellence, preferably in the context of a clinical trial. Their role in improving outcome is indeterminate (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: weak).
Recommendation 7.1:
Tunneled pleural catheters are not recommended in patients who are candidates for maximal surgical cytoreduction, because of the risk of tumor implantation into the chest wall (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 7.2:
In patients who are not candidates for maximal surgical cytoreduction, tunneled pleural catheters or pleurodesis (performed via chest tube or thoracoscopy) may be offered. As noted above, these procedures should be performed using the minimal number and size incisions. Multidisciplinary input including surgical consultation with a center of excellence should be sought to optimize management of a pleural effusion and for consideration of investigational intracavitary therapies (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Radiation Therapy
Recommendation 1.1:
Prophylactic irradiation of intervention tracts should generally not be offered patients to prevent tract recurrences (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: moderate).
Recommendation 1.2:
It is recommended that adjuvant radiation should be offered to patients who have resection of intervention tracts found to be histologically positive (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 2.1:
Radiation therapy should be offered as an effective treatment modality to palliate patients with symptomatic disease (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 2.2:
It is recommended that standard dosing regimens used in other diseases be offered to patients with mesothelioma (8 Gy × one fraction, 4 Gy × five fractions, or 3 Gy × 10 fractions) (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 3.0:
Radiation therapy may be offered to patients with localized asymptomatic recurrence. The dosing fractionation is dependent on the site and extent of disease and should be determined by the radiation oncologist in consultation with the patient (Type of recommendation: informal consensus; Strength of recommendation: moderate).
Recommendation 4.1:
Hemithoracic adjuvant radiation therapy may be offered to patients who undergo non–lung-sparing cytoreductive surgery (EPP), preferably in centers of excellence with experience in this modality for mesothelioma (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 4.2:
Hemithoracic neo-adjuvant radiation therapy may be offered to patients who undergo non–lung-sparing cytoreductive surgery. This potentially toxic regimen remains experimental and should only be performed in highly experienced centers within the context of a clinical trial (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 5.1:
Hemithoracic adjuvant intensity-modulated radiation therapy may be offered to patients who undergo lung-sparing cytoreductive surgery (P/D or EPD). This potentially toxic regimen should only be performed in highly experienced centers, preferably in the context of a clinical trial (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation 5.2:
Due to the potential for severe pulmonary toxicity, neoadjuvant radiation therapy is not recommended for patients who undergo lung-sparing surgical cytoreductive surgery (Type of recommendation: informal consensus; Strength of recommendation: strong).
Recommendation 6.1:
For palliative radiation therapy, electrons, 2D, 3D, and IMRT may be considered appropriate techniques depending on location of the treatment target and organs at risk (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 6.2:
For adjuvant or neoadjuvant hemithoracic radiation therapy, 3D or IMRT may be offered, respecting guidelines of organs at risk. Proton therapy may be considered in centers with significant experience, preferably in the context of a clinical trial (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Recommendation 7.0:
It is recommended that standard dosimetric guidelines for organs at risk be used as established predictors of radiation toxicity (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: strong).
Additional Resources
More information, including a Data Supplement with additional evidence tables, a Methodology Supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources, is available at www.asco.org/thoracic-cancer-guidelines and www.asco.org/guidelineswiki. Patient information is available at www.cancer.net
ASCO believes that cancer clinical trials are vital to inform medical decisions and improve cancer care, and that all patients should have the opportunity to participate.
Related ASCO Guidelines.
Integration of Palliative Care into Standard Oncology Practice266 (http://ascopubs.org/doi/10.1200/JCO.2016.70.1474)
Patient-Clinician Communication261 (http://ascopubs.org/doi/10.1200/JCO.2017.75.2311)
Antiemetics267 (http://ascopubs.org/doi/10.1200/JCO.2017.74.4789)
Management of Chronic Pain in Survivors of Adult Cancers268 (http://ascopubs.org/doi/10.1200/JCO.2016.68.5206)
Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers269 (http://ascopubs.org/doi/10.1200/JCO.2016.70.5400)
Acknowledgment
We thank Minaxi Jhawer, MD, Alex Spira, MD, and the Clinical Practice Guidelines Committee for their thoughtful reviews and insightful comments on this guideline.
Support
M.M. and R.H. received support through the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Hedy L. Kindler
Consulting or Advisory Role: Aduro Biotech, MedImmune, Bayer Health Care Pharmaceuticals Celgene, GlaxoSmithKline, AstraZeneca, Merck, Bristol-Myers Squibb, Boehringer Ingelheim, Ipsen, Erytech Pharma, Five Prime Therapeutics
Research Funding: Aduro Biotech (Inst), AstraZeneca (Inst), Bayer Health Care Pharmaceuticals (Inst), Celgene (Inst), GlaxoSmithKline (Inst), Merck (Inst), MedImmune (Inst), Verastem (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst)
Nofisat Ismaila
No relationship to disclose
Samuel G. Armato III
Consulting or Advisory Role: Aduro Biotech
Patents, Royalties, Other Intellectual Property: I receive royalties and licensing fees related to computer-aided diagnosis technology through my institution.
Raphael Bueno
Honoraria: AstraZeneca, International Symposium Group Oncoclinicas
Consulting or Advisory Role: BioMedical Insights
Research Funding: Verastem (Inst), Genentech (Inst), Myriad Genetics (Inst), Novartis (Inst), Siemens Healthcare Diagnostics (Inst), Castle Biosciences (Inst), Gritstone Oncology (Inst), HTG Molecular Diagnostics (Inst), Exosome Diagnostics (Inst), Genentech (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: Myriad Genetics, AstraZeneca, Roche, Genentech
Other Relationship: Neil Leifer, Esq, Morrison Mahoney, David Weiss, Balick & Balick, Arthur Tuverson, Ferraro Law Firm, Rice Dolan & Kershaw, Satterly & Kelly
Mary Hesdorffer
No relationship to disclose
Thierry Jahan
Research Funding: Aduro Biotech (Inst), Acerta Pharma (Inst), AstraZeneca/MedImmune (Inst), Eli Lilly (Inst), BIND Therapeutics (Inst), Golden Biotechnology (Inst), Verastem (Inst), Boehringer Ingelheim (Inst), Bayer Health Care Pharmaceuticals (Inst), Kadmon (Inst)
Clyde Michael Jones
Honoraria: Novartis, Eli Lilly
Consulting or Advisory Role: Novartis, Alexion Pharmaceuticals (Inst)
Speakers’ Bureau: Novartis, Pfizer, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Co-holder of US patents 4977245 5112948 5286482, and 5292642. These are tumor immunology methods and a protein sequence.
Travel, Accommodations, Expenses: Novartis, Alexion Pharmaceuticals, Eli Lilly
Markku Miettinen
No relationship to disclose
Harvey Pass
Honoraria: Genentech (I), Genomic Health (I)
Consulting or Advisory Role: AstraZeneca
Research Funding: Indi Diagnostics, SomaLogic, Celera, Genentech, Nodality
Patents, Royalties, Other Intellectual Property: Patent pending, use of fibulin for the diagnosis of mesothelioma; patent pending, use of HMGB1 for the diagnosis of mesothelioma, with University of Hawaii; patent pending, use of osteopontin for the diagnosis of mesothelioma, with Wayne State University (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Andreas Rimner
Consulting or Advisory Role: Varian Medical Systems, AstraZeneca, Merck
Research Funding: Varian Medical Systems, Boehringer Ingelheim, Pfizer, AstraZeneca
Valerie Rusch
Research Funding: Genelux (Inst)
Travel, Accommodations, Expenses: da Vinci Surgery
Other Relationship: Bristol-Myers Squibb
Daniel Sterman
Consulting or Advisory Role: Olympus Medical Systems, Ethicon, CSA Medical
Anish Thomas
No relationship to disclose
Raffit Hassan
Research Funding: Aduro Biotech (Inst), Morphotek (Inst), Bayer Health Care Pharmaceuticals (Inst)
Appendix
Table A1.
Malignant Pleural Mesothelioma Guideline Expert Panel Membership
| Name and Designation | Affiliation/Institution | Role/Area of Expertise |
|---|---|---|
| Hedy L. Kindler, co-chair | The University of Chicago, Chicago, IL | Medical oncology |
| Nofisat Ismaila | American Society of Clinical Oncology, Alexandria, VA | Staff/health research methodologist |
| Samuel G. Armato III | The University of Chicago, Chicago, IL | Radiology |
| Raphael Bueno | Harvard Medical School, Boston, MA | Thoracic surgery |
| Mary Hesdorffer | Mesothelioma Applied Research Foundation, Alexandria, VA | Patient representative and nurse practitioner |
| Thierry Jahan | University of California San Francisco, San Francisco, CA | Medical oncology |
| Clyde Michael Jones | Baptist Cancer Center Physicians Foundation, Memphis, TN | PGIN representative, medical oncology and hematology |
| Markku Miettinen | Center for Cancer Research, National Cancer Institute, Bethesda, MD | Pathology |
| Harvey Pass | New York University Langone Medical Center, New York, NY | Thoracic surgery |
| Andreas Rimner | Memorial Sloan Kettering Cancer Center, New York, NY | Radiation oncology |
| Valerie Rusch | Memorial Sloan Kettering Cancer Center, New York, NY | Thoracic surgery |
| Daniel Sterman | New York University Langone Medical Center, New York, NY | Pulmonology |
| Anish Thomas | Center for Cancer Research, National Cancer Institute, Bethesda, MD | Medical oncology |
| Raffit Hassan, co-chair | Center for Cancer Research, National Cancer Institute, Bethesda, MD | Medical oncology |
Abbreviation: PGIN, Practice Guideline Implementation Network.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at jco.org.
Clinical Practice Guideline Committee .
Editor’s note: This American Society of Clinical Oncology Clinical Practice Guideline provides recommendations, with comprehensive review and analyses of the relevant literature for each recommendation. Additional information, including a Data Supplement with additional evidence tables, a Methodology Supplement, slide sets, clinical tools and resources, and links to patient information at www.cancer.net, is available at www.asco.org/thoracic-cancer-guidelines and www.asco.org/guidelineswiki.
ASSOCIATED CONTENT
Contributor Information
Hedy L. Kindler, The University of Chicago, Chicago, IL
Nofisat Ismaila, American Society of Clinical Oncology.
Samuel G. Armato, III, The University of Chicago, Chicago, IL.
Raphael Bueno, Harvard Medical School, Boston, MA.
Mary Hesdorffer, Mesothelioma Applied Research Foundation, Alexandria, VA.
Thierry Jahan, University of California San Francisco, San Francisco, CA.
Clyde Michael Jones, Baptist Cancer Center Physicians Foundation, Memphis, TN.
Markku Miettinen, Center for Cancer Research, National Cancer Institute, Bethesda, MD.
Harvey Pass, New York University Langone Medical Center.
Andreas Rimner, Memorial Sloan Kettering Cancer Center, New York, NY.
Valerie Rusch, Memorial Sloan Kettering Cancer Center, New York, NY.
Daniel Sterman, New York University Langone Medical Center.
Anish Thomas, Center for Cancer Research, National Cancer Institute, Bethesda, MD.
Raffit Hassan, Center for Cancer Research, National Cancer Institute, Bethesda, MD.
REFERENCES
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. : Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21: 2636–2644, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Shiffman RN, Michel G, Rosenfeld RM, et al. : Building better guidelines with BRIDGE-Wiz: Development and evaluation of a software assistant to promote clarity, transparency, and implementability. J Am Med Inform Assoc 19:94–101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krug LM, Kindler HL, Calvert H, et al. : Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol 16:447–456, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Treasure T, Lang-Lazdunski L, Waller D, et al. : Extra-pleural pneumonectomy versus no extrapleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 12:763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metintas M, Ak G, Dundar E, et al. : Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: A randomized, controlled trial. Chest 137: 1362–1368, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Muers MF, Stephens RJ, Fisher P, et al. : Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): A multicentre randomised trial. Lancet 371:1685–1694, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jassem J, Ramlau R, Santoro A, et al. : Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 26:1698–1704, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bottomley A, Coens C, Efficace F, et al. : Symptoms and patient-reported well-being: Do they predict survival in malignant pleural mesothelioma? A prognostic factor analysis of EORTC-NCIC 08983: Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma. J Clin Oncol 25:5770–5776, 2007 [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke N, Garcia JC, Paul J, et al. : A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 84:18–22, 2007 [DOI] [PubMed] [Google Scholar]
- 10.van Meerbeeck JP, Gaafar R, Manegold C, et al. : Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 23:6881–6889, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bydder S, Phillips M, Joseph DJ, et al. : A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer 91:9–10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maskell NA, Gleeson FV, Davies RJ: Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: A randomised controlled trial. Lancet 361: 1326–1330, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Pass HI, Temeck BK, Kranda K, et al. : Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol 4:628–633, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Boutin C, Rey F, Viallat JR: Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 108: 754–758, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Rintoul RC, Ritchie AJ, Edwards JG, et al. : Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (Meso-VATS): An open-label, randomised, controlled trial. Lancet 384:1118–1127, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Kindler HL, Karrison TG, Gandara DR, et al. : Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 30:2509–2515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottomley A, Gaafar R, Manegold C, et al. : Short-term treatment-related symptoms and quality of life: Results from an international randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An EORTC Lung-Cancer Group and National Cancer Institute, Canada, Intergroup Study. J Clin Oncol 24: 1435–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 18.O’Brien ME, Watkins D, Ryan C, et al. : A randomised trial in malignant mesothelioma (M) of early (E) versus delayed (D) chemotherapy in symptomatically stable patients: The MED trial. Ann Oncol 17:270–275, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Pass HI, Temeck BK, Kranda K, et al. : Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 115:310–317, 1998; discussion 317–318 [DOI] [PubMed] [Google Scholar]
- 20.Zalcman G, Mazieres J, Margery J, et al. : Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 387: 1405–1414, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Clive AO, Taylor H, Dobson L, et al. : Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): A multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol 17: 1094–1104, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chance WW, Rice DC, Allen PK, et al. : Hemithoracic intensity modulated radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma: Toxicity, patterns of failure, and a matched survival analysis. Int J Radiat Oncol Biol Phys 91:149–156, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Spaggiari L, Marulli G, Bovolato P, et al. : Extrapleural pneumonectomy for malignant mesothelioma: An Italian multicenter retrospective study. Ann Thorac Surg 97:1859–1865, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Bovolato P, Casadio C, Billè A, et al. : Does surgery improve survival of patients with malignant pleural mesothelioma?: A multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 9:390–396, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Rusch VW, Giroux D, Kennedy C, et al. : Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 7:1631–1639, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Pinelli V, Laroumagne S, Sakr L, et al. : Pleural fluid cytological yield and visceral pleural invasion in patients with epithelioid malignant pleural mesothelioma. J Thorac Oncol 7:595–598, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Froment MA, Fréchette E, Dagnault A: Prophylactic irradiation of intervention sites in malignant pleural mesothelioma. Radiother Oncol 101:307–310, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Zucali PA, Simonelli M, Michetti G, et al. : Second-line chemotherapy in malignant pleural mesothelioma: Results of a retrospective multicenter survey. Lung Cancer 75:360–367, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Kadota K, Suzuki K, Sima CS, et al. : Pleomorphic epithelioid diffuse malignant pleural mesothelioma: A clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 6:896–904, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Richards WG, Godleski JJ, Yeap BY, et al. : Proposed adjustments to pathologic staging of epithelial malignant pleural mesothelioma based on analysis of 354 cases. Cancer 116:1510–1517, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Rice DC, Steliga MA, Stewart J, et al. : Endoscopic ultrasound-guided fine needle aspiration for staging of malignant pleural mesothelioma. Ann Thorac Surg 88:862–868, 2009; discussion 868–869 [DOI] [PubMed] [Google Scholar]
- 32.Flores RM, Zakowski M, Venkatraman E, et al. : Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2:957–965, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Agarwal PP, Seely JM, Matzinger FR, et al. : Pleural mesothelioma: Sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology 241:589–594, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Senyiğit A, Bayram H, Babayiğit C, et al. : Comparison of the effectiveness of some pleural sclerosing agents used for control of effusions in malignant pleural mesothelioma: A review of 117 cases. Respiration 67:623–629, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Horn D, Dequanter D, Lothaire P: Palliative treatment of malignant pleural effusions. Acta Chir Belg 110:32–34, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Krayenbuehl J, Oertel S, Davis JB, et al. : Combined photon and electron three-dimensional conformal versus intensity-modulated radiotherapy with integrated boost for adjuvant treatment of malignant pleural mesothelioma after pleuropneumonectomy. Int J Radiat Oncol Biol Phys 69: 1593–1599, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Thomas R, Piccolo F, Miller D, et al. : Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: A multicenter observational study. Chest 148:746–751, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Lauk O, Hoda MA, de Perrot M, et al. : Extrapleural pneumonectomy after induction chemotherapy: Perioperative outcome in 251 mesothelioma patients from three high-volume institutions. Ann Thorac Surg 98:1748–1754, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Ghanim B, Hoda MA, Klikovits T, et al. : Circulating fibrinogen is a prognostic and predictive biomarker in malignant pleural mesothelioma. Br J Cancer 110:984–990, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fysh ET, Tan SK, Read CA, et al. : Pleurodesis outcome in malignant pleural mesothelioma. Thorax 68:594–596, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Bearz A, Talamini R, Rossoni G, et al. : Rechallenge with pemetrexed in advanced mesothelioma: A multi-institutional experience. BMC Res Notes 5:482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manegold C, Symanowski J, Gatzemeier U, et al. : Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol 16: 923–927, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Gill RR, Richards WG, Yeap BY, et al. : Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: Stratification of survival with CT-derived tumor volume. AJR Am J Roentgenol 198: 359–363, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Oxnard GR, Armato SG III, Kindler HL: Modeling of mesothelioma growth demonstrates weaknesses of current response criteria. Lung Cancer 52: 141–148, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Burt BM, Cameron RB, Mollberg NM, et al. : Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: An analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 148:30–35, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Ceresoli GL, Castagneto B, Zucali PA, et al. : Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: Combined analysis of two phase II trials. Br J Cancer 99:51–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis SR, Tan L, Ball DL: Radiotherapy in the treatment of malignant mesothelioma of the pleura, with special reference to its use in palliation. Australas Radiol 38:212–214, 1994 [DOI] [PubMed] [Google Scholar]
- 48.de Graaf-Strukowska L, van der Zee J, van Putten W, et al. : Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura–a single-institution experience with 189 patients. Int J Radiat Oncol Biol Phys 43:511–516, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Gupta V, Krug LM, Laser B, et al. : Patterns of local and nodal failure in malignant pleural mesothelioma after extrapleural pneumonectomy and photon-electron radiotherapy. J Thorac Oncol 4: 746–750, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Kishan AU, Cameron RB, Wang PC, et al. : Tomotherapy improves local control and changes failure patterns in locally advanced malignant pleural mesothelioma. Pract Radiat Oncol 5:366–373, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Krayenbuehl J, Dimmerling P, Ciernik IF, et al. : Clinical outcome of postoperative highly conformal versus 3D conformal radiotherapy in patients with malignant pleural mesothelioma. Radiat Oncol 9:32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mordant P, McRae K, Cho J, et al. : Impact of induction therapy on postoperative outcome after extrapleural pneumonectomy for malignant pleural mesothelioma: Does induction-accelerated hemithoracic radiation increase the surgical risk? Eur J Cardiothorac Surg 50:433–438, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Rimner A, Spratt DE, Zauderer MG, et al. : Failure patterns after hemithoracic pleural intensity modulated radiation therapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 90: 394–401, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yajnik S, Rosenzweig KE, Mychalczak B, et al. : Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 56:1319–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Allen AM, Den R, Wong JS, et al. : Influence of radiotherapy technique and dose on patterns of failure for mesothelioma patients after extrapleural pneumonectomy. Int J Radiat Oncol Biol Phys 68: 1366–1374, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Gomez DR, Hong DS, Allen PK, et al. : Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol 8:238–245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flores RM, Routledge T, Seshan VE, et al. : The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: Implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 136:605–610, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Flores RM, Riedel E, Donington JS, et al. : Frequency of use and predictors of cancer-directed surgery in the management of malignant pleural mesothelioma in a community-based (Surveillance, Epidemiology, and End Results [SEER]) population. J Thorac Oncol 5:1649–1654, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Pass HI, Giroux D, Kennedy C, et al. : Supplementary prognostic variables for pleural mesothelioma: A report from the IASLC staging committee. J Thorac Oncol 9:856–864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zauderer MG, Kass SL, Woo K, et al. : Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 84:271–274, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedberg JS, Culligan MJ, Mick R, et al. : Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 93:1658–1665, 2012; discussion 1665–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sugarbaker DJ, Gill RR, Yeap BY, et al. : Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 145: 955–963, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Gill RR, Naidich DP, Mitchell A, et al. : North American multicenter volumetric CT study for clinical staging of malignant pleural mesothelioma: Feasibility and logistics of setting up a quantitative imaging study. J Thorac Oncol 11:1335–1344, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Righi L, Duregon E, Vatrano S, et al. : BRCA1-associated protein 1 (BAP1) immunohistochemical expression as a diagnostic tool in malignant pleural mesothelioma classification: A large retrospective study. J Thorac Oncol 11:2006–2017, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Sensakovic WF, Armato SG III, Straus C, et al. : Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys 38: 238–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rusch VW, Chansky K, Kindler HL, et al. : The IASLC Mesothelioma Staging Project: Proposals for the m descriptors and for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Mesothelioma. J Thorac Oncol 11:2112–2119, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Rice D, Chansky K, Nowak A, et al. : The IASLC Mesothelioma Staging Project: Proposals for revisions of the N descriptors in the forthcoming eighth edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 11:2100–2111, 2016 [DOI] [PubMed] [Google Scholar]
- 68.Nowak AK, Chansky K, Rice DC, et al. : The IASLC Mesothelioma Staging Project: Proposals for revisions of the T descriptors in the forthcoming eighth edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 11:2089–2099, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Rice DC, Smythe WR, Liao Z, et al. : Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 69:350–357, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Gupta V, Mychalczak B, Krug L, et al. : Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 63:1045–1052, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Lindenmann J, Matzi V, Neuboeck N, et al. : Multimodal therapy of malignant pleural mesothelioma: Is the replacement of radical surgery imminent? Interact Cardiovasc Thorac Surg 16:237–243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugarbaker DJ, Wolf AS, Chirieac LR, et al. : Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 40:298–303, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Sugarbaker DJ, Richards WG, Bueno R: Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: Novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 260:577–580, 2014; discussion 580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taioli E, Wolf AS, Camacho-Rivera M, et al. : Determinants of survival in malignant pleural mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) study of 14,228 patients. PLoS One 10:e0145039, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baldini EH, Richards WG, Gill RR, et al. : Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 149:1374–1381, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas R, Budgeon CA, Kuok YJ, et al. : Catheter tract metastasis associated with indwelling pleural catheters. Chest 146:557–562, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Bueno R, Reblando J, Glickman J, et al. : Pleural biopsy: A reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg 78:1774–1776, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. : Amount of epithelioid differentiation is a predictor of survival in malignant pleural mesothelioma. Ann Thorac Surg 103:962–966, 2017 [DOI] [PubMed] [Google Scholar]
- 79.Meyerhoff RR, Yang CF, Speicher PJ, et al. : Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 196:23–32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kadota K, Villena-Vargas J, Nitadori J, et al. : Tumoral CD10 expression correlates with aggressive histology and prognosis in patients with malignant pleural mesothelioma. Ann Surg Oncol 22: 3136–3143, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thies S, Friess M, Frischknecht L, et al. : Expression of the stem cell factor nestin in malignant pleural mesothelioma is associated with poor prognosis. PLoS One 10:e0139312, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thapa B, Walkiewicz M, Murone C, et al. : Calretinin but not caveolin-1 correlates with tumour histology and survival in malignant mesothelioma. Pathology 48:660–665, 2016 [DOI] [PubMed] [Google Scholar]
- 83.Lang-Lazdunski L, Bille A, Papa S, et al. : Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg 149:558–565, 2015; discussion 565–566 [DOI] [PubMed] [Google Scholar]
- 84.Infante M, Morenghi E, Bottoni E, et al. : Comorbidity, postoperative morbidity and survival in patients undergoing radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 50: 1077–1082, 2016 [DOI] [PubMed] [Google Scholar]
- 85.Batirel HF, Metintas M, Caglar HB, et al. : Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 151:478–484, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Sharkey AJ, O’Byrne KJ, Nakas A, et al. : How does the timing of chemotherapy affect outcome following radical surgery for malignant pleural mesothelioma? Lung Cancer 100:5–13, 2016 [DOI] [PubMed] [Google Scholar]
- 87.Metintas M, Ak G, Erginel S, et al. : A retrospective analysis of malignant pleural mesothelioma patients treated either with chemotherapy or best supportive care between 1990 and 2005: A single institution experience. Lung Cancer 55:379–387, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Arnold DT, Hooper CE, Morley A, et al. : The effect of chemotherapy on health-related quality of life in mesothelioma: Results from the SWAMP trial. Br J Cancer 112:1183–1189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaya H, Demir M, Taylan M, et al. : Fibulin-3 as a diagnostic biomarker in patients with malignant mesothelioma. Asian Pac J Cancer Prev 16: 1403–1407, 2015 [DOI] [PubMed] [Google Scholar]
- 90.Creaney J, Dick IM, Meniawy TM, et al. : Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 69:895–902, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bayram M, Dongel I, Akbaş A, et al. : Serum biomarkers in patients with mesothelioma and pleural plaques and healthy subjects exposed to naturally occurring asbestos. Lung 192:197–203, 2014 [DOI] [PubMed] [Google Scholar]
- 92.Mollberg NM, Vigneswaran Y, Kindler HL, et al. : Quality of life after radical pleurectomy decortication for malignant pleural mesothelioma. Ann Thorac Surg 94:1086–1092, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Rena O, Casadio C: Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: A harmful procedure. Lung Cancer 77: 151–155, 2012 [DOI] [PubMed] [Google Scholar]
- 94.Nakas A, von Meyenfeldt E, Lau K, et al. : Long-term survival after lung-sparing total pleurectomy for locally advanced (International Mesothelioma Interest Group Stage T3-T4) non-sarcomatoid malignant pleural mesothelioma. Eur J Cardiothorac Surg 41:1031–1036, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Tonoli S, Vitali P, Scotti V, et al. : Adjuvant radiotherapy after extrapleural pneumonectomy for mesothelioma. Prospective analysis of a multi-institutional series. Radiother Oncol 101:311–315, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Giraud P, Sylvestre A, Zefkili S, et al. : Helical tomotherapy for resected malignant pleural mesothelioma: Dosimetric evaluation and toxicity. Radiother Oncol 101:303–306, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Hollevoet K, Nackaerts K, Gosselin R, et al. : Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J Thorac Oncol 6:1930–1937, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Cristaudo A, Bonotti A, Simonini S, et al. : Combined serum mesothelin and plasma osteopontin measurements in malignant pleural mesothelioma. J Thorac Oncol 6:1587–1593, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Creaney J, Yeoman D, Musk AW, et al. : Plasma versus serum levels of osteopontin and mesothelin in patients with malignant mesothelioma–which is best? Lung Cancer 74:55–60, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Galbis JM, Mata M, Guijarro R, et al. : Clinical-therapeutic management of thoracoscopy in pleural effusion: A groundbreaking technique in the twenty-first century. Clin Transl Oncol 13:57–60, 2011 [DOI] [PubMed] [Google Scholar]
- 101.Cristaudo A, Foddis R, Bonotti A, et al. : Comparison between plasma and serum osteopontin levels: Usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int J Biol Markers 25:164–170, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Hollevoet K, Nackaerts K, Thimpont J, et al. : Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am J Respir Crit Care Med 181:620–625, 2010 [DOI] [PubMed] [Google Scholar]
- 103.Grigoriu BD, Chahine B, Vachani A, et al. : Kinetics of soluble mesothelin in patients with malignant pleural mesothelioma during treatment. Am J Respir Crit Care Med 179:950–954, 2009 [DOI] [PubMed] [Google Scholar]
- 104.Sørensen JB, Ravn J, Loft A, et al. : Preoperative staging of mesothelioma by 18F-fluoro-2-deoxy-D-glucose positron emission tomography/ computed tomography fused imaging and mediastinoscopy compared to pathological findings after extrapleural pneumonectomy. Eur J Cardiothorac Surg 34:1090–1096, 2008 [DOI] [PubMed] [Google Scholar]
- 105.Park EK, Sandrini A, Yates DH, et al. : Soluble mesothelin-related protein in an asbestos-exposed population: The dust diseases board cohort study. Am J Respir Crit Care Med 178:832–837, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Flores RM, Pass HI, Seshan VE, et al. : Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 135:620–626, 626.e1–3, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Zucali PA, Ceresoli GL, Garassino I, et al. : Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer 112:1555–1561, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Weder W, Stahel RA, Bernhard J, et al. : Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 18: 1196–1202, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Jänne PA, Wozniak AJ, Belani CP, et al. : Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: Outcomes from a phase IIIB expanded access program. J Thorac Oncol 1:506–512, 2006 [PubMed] [Google Scholar]
- 110.Colaut F, Toniolo L, Vicario G, et al. : Pleurectomy/decortication plus chemotherapy: Outcomes of 40 cases of malignant pleural mesothelioma. Chir Ital 56:781–786, 2004 [PubMed] [Google Scholar]
- 111.Andreopoulou E, Ross PJ, O’Brien ME, et al. : The palliative benefits of MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in patients with malignant mesothelioma. Ann Oncol 15:1406–1412, 2004 [DOI] [PubMed] [Google Scholar]
- 112.van Klaveren RJ, Aerts JG, de Bruin H, et al. : Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer 43:63–69, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Grossebner MW, Arifi AA, Goddard M, et al. : Mesothelioma–VATS biopsy and lung mobilization improves diagnosis and palliation. Eur J Cardiothorac Surg 16:619–623, 1999 [DOI] [PubMed] [Google Scholar]
- 114.Boutin C, Rey F: Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 1: Diagnosis . Cancer 72: 389–393, 1993 [DOI] [PubMed] [Google Scholar]
- 115.Rusch VW, Piantadosi S, Holmes EC: The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J Thorac Cardiovasc Surg 102:1–9, 1991 [PubMed] [Google Scholar]
- 116.Edmondstone WM: Investigation of pleural effusion: Comparison between fibreoptic thoracoscopy, needle biopsy and cytology. Respir Med 84: 23–26, 1990 [DOI] [PubMed] [Google Scholar]
- 117.Lumachi F, Mazza F, Ermani M, et al. : Talc pleurodesis as surgical palliation of patients with malignant pleural effusion. Analysis of factors affecting survival. Anticancer Res 32:5071–5074, 2012 [PubMed] [Google Scholar]
- 118.Ried M, Potzger T, Braune N, et al. : Cytoreductive surgery and hyperthermic intrathoracic chemotherapy perfusion for malignant pleural tumours: Perioperative management and clinical experience. Eur J Cardiothorac Surg 43:801–807, 2013 [DOI] [PubMed] [Google Scholar]
- 119.Alvarez JM, Hasani A, Segal A, et al. : Bilateral thoracoscopy, mediastinoscopy and laparoscopy, in addition to CT, MRI and PET imaging, are essential to correctly stage and treat patients with mesothelioma prior to trimodality therapy. ANZ J Surg 79:734–738, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Holsti LR, Pyrhönen S, Kajanti M, et al. : Altered fractionation of hemithorax irradiation for pleural mesothelioma and failure patterns after treatment. Acta Oncol 36:397–405, 1997 [DOI] [PubMed] [Google Scholar]
- 121.Hasan B, Greillier L, Pallis A, et al. : Progression free survival rate at 9 and 18 weeks predict overall survival in patients with malignant pleural mesothelioma: An individual patient pooled analysis of 10 European Organisation for Research and Treatment of Cancer Lung Cancer Group studies and an independent study validation. Eur J Cancer 50: 2771–2782, 2014 [DOI] [PubMed] [Google Scholar]
- 122.Plathow C, Staab A, Schmaehl A, et al. : Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: Initial results. Invest Radiol 43:737–744, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Plathow C, Klopp M, Thieke C, et al. : Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol 18: 1635–1643, 2008 [DOI] [PubMed] [Google Scholar]
- 124.Heelan RT, Rusch VW, Begg CB, et al. : Staging of malignant pleural mesothelioma: Comparison of CT and MR imaging. AJR Am J Roentgenol 172: 1039–1047, 1999 [DOI] [PubMed] [Google Scholar]
- 125.Fysh ETH, Bielsa S, Budgeon CA, et al. : Predictors of clinical use of pleurodesis and/or indwelling pleural catheter therapy for malignant pleural effusion. Chest 147:1629–1634, 2015 [DOI] [PubMed] [Google Scholar]
- 126.Taylor P, Castagneto B, Dark G, et al. : Single-agent pemetrexed for chemonaïve and pretreated patients with malignant pleural mesothelioma: Results of an International Expanded Access Program. J Thorac Oncol 3:764–771, 2008 [DOI] [PubMed] [Google Scholar]
- 127.Santoro A, O’Brien ME, Stahel RA, et al. : Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: Results of the International Expanded Access Program. J Thorac Oncol 3: 756–763, 2008 [DOI] [PubMed] [Google Scholar]
- 128.Kuribayashi K, Voss S, Nishiuma S, et al. : Safety and effectiveness of pemetrexed in patients with malignant pleural mesothelioma based on all-case drug-registry study. Lung Cancer 75:353–359, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Scagliotti GV, Shin DM, Kindler HL, et al. : Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 21:1556–1561, 2003 [DOI] [PubMed] [Google Scholar]
- 130.Armato SG III, Li P, Husain AN, et al. : Radiologic-pathologic correlation of mesothelioma tumor volume. Lung Cancer 87:278–282, 2015 [DOI] [PubMed] [Google Scholar]
- 131.Frauenfelder T, Tutic M, Weder W, et al. : Volumetry: An alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 38:162–168, 2011 [DOI] [PubMed] [Google Scholar]
- 132.Labby ZE, Armato SG III, Dignam JJ, et al. : Lung volume measurements as a surrogate marker for patient response in malignant pleural mesothelioma. J Thorac Oncol 8:478–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Opitz I, Friess M, Kestenholz P, et al. : A new prognostic score supporting treatment allocation for multimodality therapy for malignant pleural mesothelioma: A review of 12 years’ experience. J Thorac Oncol 10:1634–1641, 2015 [DOI] [PubMed] [Google Scholar]
- 134.Bölükbas S, Eberlein M, Schirren J: Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J Thorac Oncol 7:900–905, 2012 [DOI] [PubMed] [Google Scholar]
- 135.Kircheva DY, Husain AN, Watson S, et al. : Specimen weight and volume: Important predictors of survival in malignant pleural mesothelioma. Eur J Cardiothorac Surg 49:1642–1647, 2016 [DOI] [PubMed] [Google Scholar]
- 136.Klabatsa A, Chicklore S, Barrington SF, et al. : The association of 18F-FDG PET/CT parameters with survival in malignant pleural mesothelioma. Eur J Nucl Med Mol Imaging 41:276–282, 2014 [DOI] [PubMed] [Google Scholar]
- 137.Minatel E, Trovo M, Polesel J, et al. : Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 83:78–82, 2014 [DOI] [PubMed] [Google Scholar]
- 138.Nakas A, Waller D, Lau K, et al. : The new case for cervical mediastinoscopy in selection for radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 42:72–76, 2012; discussion 76 [DOI] [PubMed] [Google Scholar]
- 139.Thieke C, Nicolay NH, Sterzing F, et al. : Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 10:267, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bece A, Tin MM, Martin D, et al. : Hemithoracic radiation therapy after extrapleural pneumonectomy for malignant pleural mesothelioma: Toxicity and outcomes at an Australian institution. J Med Imaging Radiat Oncol 59:355–362, 2015 [DOI] [PubMed] [Google Scholar]
- 141.Bille A, Belcher E, Raubenheimer H, et al. : Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma: Experience of Guy’s and St Thomas’ hospitals. Gen Thorac Cardiovasc Surg 60:289–296, 2012 [DOI] [PubMed] [Google Scholar]
- 142.de Perrot M, Feld R, Leighl NB, et al. : Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 151: 468–473, 2016 [DOI] [PubMed] [Google Scholar]
- 143.Minatel E, Trovo M, Bearz A, et al. : Radical radiation therapy after lung-sparing surgery for malignant pleural mesothelioma: Survival, pattern of failure, and prognostic factors. Int J Radiat Oncol Biol Phys 93:606–613, 2015 [DOI] [PubMed] [Google Scholar]
- 144.Minatel E, Trovo M, Polesel J, et al. : Tomotherapy after pleurectomy/decortication or biopsy for malignant pleural mesothelioma allows the delivery of high dose of radiation in patients with intact lung. J Thorac Oncol 7:1862–1866, 2012 [DOI] [PubMed] [Google Scholar]
- 145.Patel PR, Yoo S, Broadwater G, et al. : Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 83:362–368, 2012 [DOI] [PubMed] [Google Scholar]
- 146.Rice DC, Stevens CW, Correa AM, et al. : Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 84:1685–1692, 2007; discussion 1692–1693 [DOI] [PubMed] [Google Scholar]
- 147.Rosenzweig KE, Zauderer MG, Laser B, et al. : Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 83:1278–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sylvestre A, Mahé MA, Lisbona A, et al. : Mesothelioma at era of helical tomotherapy: Results of two institutions in combining chemotherapy, surgery and radiotherapy. Lung Cancer 74:486–491, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Yan TD, Tin M, Boyer M, et al. : Treatment failure after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Dis 1:23–28, 2009 [PMC free article] [PubMed] [Google Scholar]
- 150.Lang-Lazdunski L, Bille A, Lal R, et al. : Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 7:737–743, 2012 [DOI] [PubMed] [Google Scholar]
- 151.Hasegawa S, Okada M, Tanaka F, et al. : Trimodality strategy for treating malignant pleural mesothelioma: Results of a feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation (Japan Mesothelioma Interest Group 0601 Trial). Int J Clin Oncol, 21:523–530, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rice DC, Erasmus JJ, Stevens CW, et al. : Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 80:1988–1992, 2005; discussion 1992–1993 [DOI] [PubMed] [Google Scholar]
- 153.Pass HI, Goparaju C: Fibulin-3 as a biomarker for pleural mesothelioma. N Engl J Med 368:190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Armato SG III, Ogarek JL, Starkey A, et al. : Variability in mesothelioma tumor response classification. AJR Am J Roentgenol 186:1000–1006, 2006 [DOI] [PubMed] [Google Scholar]
- 155.Yan TD, Boyer M, Tin MM, et al. : Extrapleural pneumonectomy for malignant pleural mesothelioma: Outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 138:619–624, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Friedberg JS, Simone CB II, Culligan MJ, et al. : Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann Thorac Surg 103:912–919, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rusch VW, Gill R, Mitchell A, et al. : A multicenter study of volumetric computed tomography for staging malignant pleural mesothelioma. Ann Thorac Surg 102:1059–1066, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jo VY, Cibas ES, Pinkus GS: Claudin-4 immunohistochemistry is highly effective in distinguishing adenocarcinoma from malignant mesothelioma in effusion cytology. Cancer Cytopathol 122:299–306, 2014 [DOI] [PubMed] [Google Scholar]
- 159.Labby ZE, Armato SG III, Kindler HL, et al. : Optimization of response classification criteria for patients with malignant pleural mesothelioma. J Thorac Oncol 7:1728–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Labby ZE, Nowak AK, Dignam JJ, et al. : Disease volumes as a marker for patient response in malignant pleural mesothelioma. Ann Oncol 24: 999–1005, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu F, Zhao B, Krug LM, et al. : Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol 5:879–884, 2010 [DOI] [PubMed] [Google Scholar]
- 162.de Perrot M, Feld R, Cho BC, et al. : Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 27:1413–1418, 2009 [DOI] [PubMed] [Google Scholar]
- 163.Byrne MJ, Nowak AK: Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257–260, 2004 [DOI] [PubMed] [Google Scholar]
- 164.Pass HI, Goparaju C, Espin-Garcia O, et al. : Plasma biomarker enrichment of clinical prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 11:900–909, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mansfield AS, Roden AC, Peikert T, et al. : B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 9:1036–1040, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arrieta O, López-Macías D, Mendoza-García VO, et al. : A phase II trial of prolonged, continuous infusion of low-dose gemcitabine plus cisplatin in patients with advanced malignant pleural mesothelioma. Cancer Chemother Pharmacol 73:975–982, 2014 [DOI] [PubMed] [Google Scholar]
- 167.Nowak AK, Millward MJ, Creaney J, et al. : A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol 7:1449–1456, 2012 [DOI] [PubMed] [Google Scholar]
- 168.Dowell JE, Dunphy FR, Taub RN, et al. : A multicenter phase II study of cisplatin, pemetrexed, and bevacizumab in patients with advanced malignant mesothelioma. Lung Cancer 77:567–571, 2012 [DOI] [PubMed] [Google Scholar]
- 169.Kovac V, Zwitter M, Rajer M, et al. : A phase II trial of low-dose gemcitabine in a prolonged infusion and cisplatin for malignant pleural mesothelioma. Anticancer Drugs 23:230–238, 2012 [DOI] [PubMed] [Google Scholar]
- 170.Tourkantonis I, Makrilia N, Ralli M, et al. : Phase II study of gemcitabine plus docetaxel as second-line treatment in malignant pleural mesothelioma: A single institution study. Am J Clin Oncol 34:38–42, 2011 [DOI] [PubMed] [Google Scholar]
- 171.Tilleman TR, Richards WG, Zellos L, et al. : Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: A phase II prospective study. J Thorac Cardiovasc Surg 138:405–411, 2009 [DOI] [PubMed] [Google Scholar]
- 172.Krug LM, Pass HI, Rusch VW, et al. : Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 27:3007–3013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hillerdal G, Sorensen JB, Sundström S, et al. : Treatment of malignant pleural mesothelioma with carboplatin, liposomized doxorubicin, and gemcitabine: A phase II study. J Thorac Oncol 3:1325–1331, 2008 [DOI] [PubMed] [Google Scholar]
- 174.Castagneto B, Botta M, Aitini E, et al. : Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 19:370–373, 2008 [DOI] [PubMed] [Google Scholar]
- 175.Kalmadi SR, Rankin C, Kraut MJ, et al. : Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: A phase II study of the Southwest Oncology Group (SWOG 9810). Lung Cancer 60:259–263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ceresoli GL, Zucali PA, Favaretto AG, et al. : Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 24: 1443–1448, 2006 [DOI] [PubMed] [Google Scholar]
- 177.Favaretto AG, Aversa SM, Paccagnella A, et al. : Gemcitabine combined with carboplatin in patients with malignant pleural mesothelioma: A multicentric phase II study. Cancer 97:2791–2797, 2003 [DOI] [PubMed] [Google Scholar]
- 178.Fizazi K, Doubre H, Le Chevalier T, et al. : Combination of raltitrexed and oxaliplatin is an active regimen in malignant mesothelioma: Results of a phase II study. J Clin Oncol 21:349–354, 2003 [DOI] [PubMed] [Google Scholar]
- 179.van Haarst JM, Baas P, Manegold Ch, et al. : Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer 86:342–345, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.van Meerbeeck JP, Baas P, Debruyne C, et al. : A Phase II study of gemcitabine in patients with malignant pleural mesothelioma. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. Cancer 85:2577–2582, 1999 [DOI] [PubMed] [Google Scholar]
- 181.Rusch VW, Rosenzweig K, Venkatraman E, et al. : A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 122: 788–795, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Steele JP, Shamash J, Evans MT, et al. : Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 18:3912–3917, 2000 [DOI] [PubMed] [Google Scholar]
- 183.Byrne MJ, Davidson JA, Musk AW, et al. : Cisplatin and gemcitabine treatment for malignant mesothelioma: A phase II study. J Clin Oncol 17: 25–30, 1999 [DOI] [PubMed] [Google Scholar]
- 184.Ceresoli GL, Zucali PA, Mencoboni M, et al. : Phase II study of pemetrexed and carboplatin plus bevacizumab as first-line therapy in malignant pleural mesothelioma. Br J Cancer 109:552–558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Katirtzoglou N, Gkiozos I, Makrilia N, et al. : Carboplatin plus pemetrexed as first-line treatment of patients with malignant pleural mesothelioma: A phase II study. Clin Lung Cancer 11:30–35, 2010 [DOI] [PubMed] [Google Scholar]
- 186.Jackman DM, Kindler HL, Yeap BY, et al. : Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer 113:808–814, 2008 [DOI] [PubMed] [Google Scholar]
- 187.Jänne PA, Simon GR, Langer CJ, et al. : Phase II trial of pemetrexed and gemcitabine in chemotherapy-naive malignant pleural mesothelioma. J Clin Oncol 26:1465–1471, 2008 [DOI] [PubMed] [Google Scholar]
- 188.Castagneto B, Zai S, Dongiovanni D, et al. : Cisplatin and gemcitabine in malignant pleural mesothelioma: A phase II study. Am J Clin Oncol 28: 223–226, 2005 [DOI] [PubMed] [Google Scholar]
- 189.Nowak AK, Byrne MJ, Williamson R, et al. : A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 87: 491–496, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stahel RA, Riesterer O, Xyrafas A, et al. : Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): A randomised, international, multicentre phase 2 trial. Lancet Oncol 16:1651–1658, 2015 [DOI] [PubMed] [Google Scholar]
- 191.MacLeod N, Chalmers A, O’Rourke N, et al. : Is radiotherapy useful for treating pain in mesothelioma?: A phase II trial. J Thorac Oncol 10:944–950, 2015 [DOI] [PubMed] [Google Scholar]
- 192.Sørensen JB, Frank H, Palshof T: Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma. Br J Cancer 99: 44–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stebbing J, Powles T, McPherson K, et al. : The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 63: 94–97, 2009 [DOI] [PubMed] [Google Scholar]
- 194.Cho BC, Feld R, Leighl N, et al. : A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: The “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol 9:397–402, 2014 [DOI] [PubMed] [Google Scholar]
- 195.Rimner A, Zauderer MG, Gomez DR, et al. : Phase II study of hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) as part of lung-sparing multimodality therapy in patients with malignant pleural mesothelioma. J Clin Oncol 34: 2761–2768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.MacLeod N, Kelly C, Stobo J, et al. : Pain in malignant pleural mesothelioma: A prospective characterization study. Pain Med 17:2119–2126, 2016 [DOI] [PubMed] [Google Scholar]
- 197.Blomberg C, Nilsson J, Holgersson G, et al. : Randomized trials of systemic medically-treated malignant mesothelioma: A systematic review. Anticancer Res 35:2493–2501, 2015 [PubMed] [Google Scholar]
- 198.Mansfield AS, Symanowski JT, Peikert T: Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer 86:133–136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Macleod N, Price A, O’Rourke N, et al. : Radiotherapy for the treatment of pain in malignant pleural mesothelioma: A systematic review. Lung Cancer 83:133–138, 2014 [DOI] [PubMed] [Google Scholar]
- 200.Cao C, Tian DH, Pataky KA, et al. : Systematic review of pleurectomy in the treatment of malignant pleural mesothelioma. Lung Cancer 81:319–327, 2013 [DOI] [PubMed] [Google Scholar]
- 201.Gelvez-Zapata SM, Gaffney D, Scarci M, et al. : What is the survival after surgery for localized malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 16:533–537, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wan C, Shen YC, Liu MQ, et al. : Diagnostic value of fluorescence in situ hybridization assay in malignant mesothelioma: A meta-analysis. Asian Pac J Cancer Prev 13:4745–4749, 2012 [DOI] [PubMed] [Google Scholar]
- 203.Hollevoet K, Reitsma JB, Creaney J, et al. : Serum mesothelin for diagnosing malignant pleural mesothelioma: An individual patient data meta-analysis. J Clin Oncol 30:1541–1549, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nagendran M, Pallis A, Patel K, et al. : Should all patients who have mesothelioma diagnosed by video-assisted thoracoscopic surgery have their intervention sites irradiated? Interact Cardiovasc Thorac Surg 13:66–69, 2011 [DOI] [PubMed] [Google Scholar]
- 205.van der Bij S, Schaake E, Koffijberg H, et al. : Markers for the non-invasive diagnosis of mesothelioma: A systematic review. Br J Cancer 104: 1325–1333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sharif S, Zahid I, Routledge T, et al. : Extrapleural pneumonectomy or supportive care: Treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 12:1040–1045, 2011 [DOI] [PubMed] [Google Scholar]
- 207.Zahid I, Sharif S, Routledge T, et al. : Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 12:812–817, 2011 [DOI] [PubMed] [Google Scholar]
- 208.Sharif S, Zahid I, Routledge T, et al. : Does positron emission tomography offer prognostic information in malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 12:806–811, 2011 [DOI] [PubMed] [Google Scholar]
- 209.Zahid I, Sharif S, Routledge T, et al. : What is the best way to diagnose and stage malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 12: 254–259, 2011 [DOI] [PubMed] [Google Scholar]
- 210.Srivastava V, Dunning J, Au J: Does video-assisted thoracoscopic decortication in advanced malignant mesothelioma improve prognosis? Interact Cardiovasc Thorac Surg 8:454–456, 2009 [DOI] [PubMed] [Google Scholar]
- 211.King J, Thatcher N, Pickering C, et al. : Sensitivity and specificity of immunohistochemical antibodies used to distinguish between benign and malignant pleural disease: A systematic review of published reports. Histopathology 49:561–568, 2006 [DOI] [PubMed] [Google Scholar]
- 212.Chapman E, Berenstein EG, Diéguez M, et al. : Radiotherapy for malignant pleural mesothelioma. Cochrane Database Syst Rev 3:CD003880, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Therasse P, Eisenhauer EA, Verweij J: RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer 42:1031–1039, 2006 [DOI] [PubMed] [Google Scholar]
- 214.Chi A, Liao Z, Nguyen NP, et al. : Intensity-modulated radiotherapy after extrapleural pneumonectomy in the combined-modality treatment of malignant pleural mesothelioma. J Thorac Oncol 6: 1132–1141, 2011 [DOI] [PubMed] [Google Scholar]
- 215.Cao CQ, Yan TD, Bannon PG, et al. : A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 5: 1692–1703, 2010 [DOI] [PubMed] [Google Scholar]
- 216.Lee C, Bayman N, Swindell R, et al. : Prophylactic radiotherapy to intervention sites in mesothelioma: A systematic review and survey of UK practice. Lung Cancer 66:150–156, 2009 [DOI] [PubMed] [Google Scholar]
- 217.Green J, Dundar Y, Dodd S, et al. : Pemetrexed disodium in combination with cisplatin versus other cytotoxic agents or supportive care for the treatment of malignant pleural mesothelioma. Cochrane Database Syst Rev 1:CD005574, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ung YC, Yu E, Falkson C, et al. : The role of radiation therapy in malignant pleural mesothelioma: A systematic review. Radiother Oncol 80:13–18, 2006 [DOI] [PubMed] [Google Scholar]
- 219.Taioli E, Wolf AS, Flores RM: Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 99:472–480, 2015 [DOI] [PubMed] [Google Scholar]
- 220.Cao C, Tian D, Park J, et al. : A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 83: 240–245, 2014 [DOI] [PubMed] [Google Scholar]
- 221.Cao C, Yan TD, Bannon PG, et al. : Summary of prognostic factors and patient selection for extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Ann Surg Oncol 18: 2973–2979, 2011 [DOI] [PubMed] [Google Scholar]
- 222.Teh E, Fiorentino F, Tan C, et al. : A systematic review of lung-sparing extirpative surgery for pleural mesothelioma. J R Soc Med 104:69–80, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Maziak DE, Gagliardi A, Haynes AE, et al. : Surgical management of malignant pleural mesothelioma: A systematic review and evidence summary. Lung Cancer 48:157–169, 2005 [DOI] [PubMed] [Google Scholar]
- 224.Hwang HC, Sheffield BS, Rodriguez S, et al. : Utility of BAP1 immunohistochemistry and p16 (CDKN2A) FISH in the diagnosis of malignant mesothelioma in effusion cytology specimens. Am J Surg Pathol 40:120–126, 2016 [DOI] [PubMed] [Google Scholar]
- 225.Andrici J, Sheen A, Sioson L, et al. : Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Mod Pathol 28:1360–1368, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ordóñez NG: Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: A review and update. Hum Pathol 44:1–19, 2013 [DOI] [PubMed] [Google Scholar]
- 227.Husain AN, Colby T, Ordonez N, et al. : Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 137:647–667, 2013 [DOI] [PubMed] [Google Scholar]
- 228.Bueno R, Stawiski EW, Goldstein LD, et al. : Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 48: 407–416, 2016 [DOI] [PubMed] [Google Scholar]
- 229.Therasse P, Arbuck SG, Eisenhauer EA, et al. : New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216, 2000 [DOI] [PubMed] [Google Scholar]
- 230.Armato SG III, Oxnard GR, MacMahon H, et al. : Measurement of mesothelioma on thoracic CT scans: A comparison of manual and computer-assisted techniques. Med Phys 31:1105–1115, 2004 [DOI] [PubMed] [Google Scholar]
- 231.Tsao AS, Garland L, Redman M, et al. : A practical guide of the Southwest Oncology Group to measure malignant pleural mesothelioma tumors by RECIST and modified RECIST criteria. J Thorac Oncol 6:598–601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247, 2009 [DOI] [PubMed] [Google Scholar]
- 233.Zucali PA, Perrino M, Lorenzi E, et al. : Vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Lung Cancer 84: 265–270, 2014 [DOI] [PubMed] [Google Scholar]
- 234.van den Bogaert DP, Pouw EM, van Wijhe G, et al. : Pemetrexed maintenance therapy in patients with malignant pleural mesothelioma. J Thorac Oncol 1:25–30, 2006 [PubMed] [Google Scholar]
- 235.Kristensen CA, Nøttrup TJ, Berthelsen AK, et al. : Pulmonary toxicity following IMRT after extrapleural pneumonectomy for malignant pleural mesothelioma. Radiother Oncol 92:96–99, 2009 [DOI] [PubMed] [Google Scholar]
- 236.Stevens CW, Wong PF, Rice D, et al. : Treatment planning system evaluation for mesothelioma IMRT. Lung Cancer 49:S75–S81, 2005. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 237.Van Schil PE, Baas P, Gaafar R, et al. : Trimodality therapy for malignant pleural mesothelioma: Results from an EORTC phase II multicentre trial. Eur Respir J 36:1362–1369, 2010 [DOI] [PubMed] [Google Scholar]
- 238.Donahoe L, Cho J, de Perrot M: Novel induction therapies for pleural mesothelioma. Semin Thorac Cardiovasc Surg 26:192–200, 2014 [DOI] [PubMed] [Google Scholar]
- 239.Rice D, Rusch V, Pass H, et al. : Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: A consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 6:1304–1312, 2011 [DOI] [PubMed] [Google Scholar]
- 240.Sharkey AJ, Tenconi S, Nakas A, et al. : The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 49:1632–1641, 2016 [DOI] [PubMed] [Google Scholar]
- 241.Paul S, Neragi-Miandoab S, Jaklitsch MT: Preoperative assessment and therapeutic options for patients with malignant pleural mesothelioma. Thorac Surg Clin 14:505–516, ix, 2004 [DOI] [PubMed] [Google Scholar]
- 242.Schaarschmidt BM, Sawicki LM, Gomez B, et al. : Malignant pleural mesothelioma: Initial experience in integrated (18)F-FDG PET/MR imaging. Clin Imaging 40:956–960, 2016 [DOI] [PubMed] [Google Scholar]
- 243.Truong MT, Viswanathan C, Godoy MB, et al. : Malignant pleural mesothelioma: Role of CT, MRI, and PET/CT in staging evaluation and treatment considerations. Semin Roentgenol 48:323–334, 2013 [DOI] [PubMed] [Google Scholar]
- 244.Krüger S, Pauls S, Mottaghy FM, et al. : Integrated FDG PET-CT imaging improves staging in malignant pleural mesothelioma. Nucl Med (Stuttg) 46:239–243, 2007 [DOI] [PubMed] [Google Scholar]
- 245.Alvarez JM, Ha T, Musk W, et al. : Importance of mediastinoscopy, bilateral thoracoscopy, and laparoscopy in correct staging of malignant mesothelioma before extrapleural pneumonectomy. J Thorac Cardiovasc Surg 130:905–906, 2005 [DOI] [PubMed] [Google Scholar]
- 246.de Ceuninck M, Demedts I, Trenson S: Malignant cardiac tamponade. Acta Cardiol 68:505–507, 2013 [DOI] [PubMed] [Google Scholar]
- 247.Georghiou GP, Stamler A, Sharoni E, et al. : Video-assisted thoracoscopic pericardial window for diagnosis and management of pericardial effusions. Ann Thorac Surg 80:607–610, 2005 [DOI] [PubMed] [Google Scholar]
- 248.McDonald JM, Meyers BF, Guthrie TJ, et al. : Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg 76:811–815, 2003; discussion 816 [DOI] [PubMed] [Google Scholar]
- 249.Jama GM, Scarci M, Bowden J, et al. : Palliative treatment for symptomatic malignant pericardial effusion. Interact Cardiovasc Thorac Surg 19: 1019–1026, 2014 [DOI] [PubMed] [Google Scholar]
- 250.Weder W, Opitz I: Multimodality therapy for malignant pleural mesothelioma. Ann Cardiothorac Surg 1:502–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Baldini EH, DeCamp MM Jr, Katz MS, et al. : Patterns of recurrence and outcome for patients with clinical stage II non-small-cell lung cancer. Am J Clin Oncol 22:8–14, 1999 [DOI] [PubMed] [Google Scholar]
- 252.Zellos L, Richards WG, Capalbo L, et al. : A phase I study of extrapleural pneumonectomy and intracavitary intraoperative hyperthermic cisplatin with amifostine cytoprotection for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 137: 453–458, 2009 [DOI] [PubMed] [Google Scholar]
- 253.Richards WG, Zellos L, Bueno R, et al. : Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 24:1561–1567, 2006 [DOI] [PubMed] [Google Scholar]
- 254.Allen AM, Czerminska M, Jänne PA, et al. : Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 65:640–645, 2006 [DOI] [PubMed] [Google Scholar]
- 255.Hilaris BS, Nori D, Kwong E, et al. : Pleurectomy and intraoperative brachytherapy and postoperative radiation in the treatment of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 10:325–331, 1984 [DOI] [PubMed] [Google Scholar]
- 256.Sterzing F, Sroka-Perez G, Schubert K, et al. : Evaluating target coverage and normal tissue sparing in the adjuvant radiotherapy of malignant pleural mesothelioma: Helical tomotherapy compared with step-and-shoot IMRT. Radiother Oncol 86:251–257, 2008 [DOI] [PubMed] [Google Scholar]
- 257.Scorsetti M, Bignardi M, Clivio A, et al. : Volumetric modulation arc radiotherapy compared with static gantry intensity-modulated radiotherapy for malignant pleural mesothelioma tumor: A feasibility study. Int J Radiat Oncol Biol Phys 77:942–949, 2010 [DOI] [PubMed] [Google Scholar]
- 258.Krayenbuehl J, Riesterer O, Graydon S, et al. : Intensity-modulated radiotherapy and volumetric-modulated arc therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. J Appl Clin Med Phys 14:4130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Krayenbuehl J, Hartmann M, Lomax AJ, et al. : Proton therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. Int J Radiat Oncol Biol Phys 78:628–634, 2010 [DOI] [PubMed] [Google Scholar]
- 260.Pan HY, Jiang S, Sutton J, et al. : Early experience with intensity modulated proton therapy for lung-intact mesothelioma: A case series. Pract Radiat Oncol 5:e345–e353, 2015 [DOI] [PubMed] [Google Scholar]
- 261.Gilligan T, Coyle N, Frankel RM, et al. : Patient-clinician communication: American Society of Clinical Oncology consensus guideline. J Clin Oncol 35: 3618–3632, 2017 [DOI] [PubMed] [Google Scholar]
- 262.American Cancer Society. Cancer Facts & Figures for African Americans 2013–2014. Atlanta, GA, American Cancer Society, 2013 [Google Scholar]
- 263.American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity: Patient-centered care for older adults with multiple chronic conditions: A stepwise approach from the American Geriatrics Society: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc 60: 1957–1968, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.U.S. Cancer Statistics Working Group: United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2014 [Google Scholar]
- 265.Mead H, Cartwright-Smith L, Jones K. et al. : Racial and ethnic disparities in U.S. health care: A chartbook. Washington DC, The Commonwealth Fund, 2008 [Google Scholar]
- 266.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:96–112, 2017 [DOI] [PubMed] [Google Scholar]
- 267.Hesketh PJ, Kris MG, Basch E, et al. : Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35: 3240–3261, 2017 [DOI] [PubMed] [Google Scholar]
- 268.Paice JA, Portenoy R, Lacchetti C, et al. : Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 34:3325–3345, 2016 [DOI] [PubMed] [Google Scholar]
- 269.Armenian SH, Lacchetti C, Barac A, et al. : Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 35:893–911, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.