Abstract
SARS coronavirus 1 (SARS-CoV-1) causes a respiratory infection that can lead to acute respiratory distress characterized by inflammation and high levels of cytokines in the lung tissue. In this study we constructed a herpes simplex virus 1 replication-defective mutant vector expressing SARS-CoV-1 spike protein as a potential vaccine vector and to probe the effects of spike protein on host cells. The spike protein expressed from this vector is functional in that it localizes to the surface of infected cells and induces fusion of ACE2-expressing cells. In immunized mice, the recombinant vector induced antibodies that bind to spike protein in an ELISA assay and that show neutralizing activity. The spike protein expressed from this vector can induce the expression of cytokines in an ACE2-independent, MyD88-dependent process. These results argue that the SARS-CoV-1 spike protein intrinsically activates signaling pathways that induce cytokines and contribute directly to the inflammatory process of SARS.
Keywords: Herpes simplex virus, SARS coronavirus, Cytokines
1. Introduction
Severe acute respiratory syndrome (SARS) coronavirus 1 (SARS-CoV-1) causes acute inflammation and cytokine storm in the respiratory tract, leading to acute respiratory distress (Drosten et al., 2003; Ksiazek et al., 2003; Peiris et al., 2003). The SARS-CoV genome encodes 4 structural proteins, S (spike), M (membrane), E (envelope protein), and N (nucleoprotein), and a series of non-structural and accessory proteins (Perlman and Masters, 2020). The spike (S) is the major surface protein responsible for binding to the host cell receptor and promoting entry of the virus. S protein is the target of neutralizing antibodies against the virus.
SARS-CoV-1 causes cytopathic effects in infected cells (Ksiazek et al., 2003; Peiris et al., 2003), and this could contribute to viral pathogenesis, but the mechanisms of the full pathogenic process including the cytokine storm remain to be explained fully. SARS-CoV-1 is known to inhibit type 1 interferon signaling by blocking IRF-3 and NF-κB signaling (Devaraj et al., 2007; Frieman et al., 2009) by the action of several proteins (Perlman and Masters, 2020), but the mechanism(s) of induction of pro-inflammatory cytokines have not been defined. Two studies have found weak induction of IL-6 and TNF-ɑ by soluble fragments of SARS spike protein (Wang et al., 2007) or induction of IL-8 by baculovirus expressing SARS spike protein (Chang et al., 2004), although high concentrations of soluble protein (e.g., 1–20 μg/ml) were needed for the effects observed.
Several viral recombinant vectors using modified vaccinia Ankara virus, parainfluenza virus, and adenovirus have been constructed that express the SARS spike protein as potential vaccines for SARS (Taylor, 2006). We have constructed herpes simplex virus 1 (HSV-1) replication-defective mutant viruses (Murphy et al., 2000; Watanabe et al., 2007) that serve as vaccine vectors for simian immunodeficiency virus (SIV) in non-human primates (Murphy et al., 2000), human immunodeficiency virus (HIV) in humanized mice (Claiborne et al., 2019), and West Nile virus in mice (Taylor et al., 2016). We specifically utilized the HSV-1 d106 recombinant virus that has deletions in the two copies of the ICP4 (R S 1) gene, and deletions in the ICP22/ICP47 promoter sequence (Samaniego et al., 1998) as a vaccine vector (Liu et al., 2009; Watanabe et al., 2007).
Viral recombinant vectors can also serve as vectors for expression of microbial proteins to define the effects of the expressed protein on host cells under reduced containment conditions. In this study we constructed an HSV-1 d106 replication-defective mutant virus that expresses the SARS-CoV-1 spike protein, evaluated its immunogenicity in mice, and studied the effect of spike protein expression on cytokine expression in host cells.
2. Results
2.1. Comparison of the vector potential of HSV-1 d106-GFP with Ad5-GFP
To assess the vector potential of HSV-1 d106 virus, we compared expression of green fluorescent protein (GFP) from identical expression cassettes (CMV-eGFP) contained in the HSV-1 d106 virus (Fig. 1 A) or an adenovirus 5 recombinant virus, Ad5-GFP, under conditions where there were equal numbers of viral genomes of the two viruses in infected cells. To define conditions where equal numbers of GFP sequences were in the infected cells, we infected human foreskin fibroblast (HFF) cells with varying amounts of the two viruses using different multiplicities of infection (MOI) and measured GFP gene sequences in the infected cells by PCR. We determined that at MOI = 1.25 for d106 and MOI = 0.125 for Ad5-GFP that similar GFP DNA copy numbers were observed in infected cells (not shown). We therefore infected sets of cells with the two viruses under these conditions and measured GFP expression by immunoblotting (Fig. 2 A) or flow cytometry (Fig. 2B). We observed higher expression of GFP protein in d106 infected cells as compared with Ad5-GFP virus (Fig. 2A). Furthermore, when we quantified fluorescent fluorescence using flow cytometry, we observed that GFP fluorescence was 10-fold higher with d106 as compared with Ad5-GFP (Fig. 2B). We concluded that d106 is a very efficient vector for expression of a transgene.
Fig. 1.
Map of genome of HSV-1 d106 virus (A) and the HSV-1 d106-SARS-CoV-1S virus (B). (A) Map of d106 viral genome. Four IE genes, ICP27, ICP4, ICP22, and ICP47, contain deletions and are not expressed in d106 virus, and a GFP expression cassette is inserted in the ICP27 gene deletion site. ICP0 is the sole IE gene product expressed. B. Map of the d106-SARS-CoV-1S virus. GFP was replaced with a SARS-CoV-1 spike gene to generate d106-SARS-CoV-1S. UL: unique long region, US: unique short region, purple boxes: terminal and internal repeats in UL, Gray boxes; terminal and internal repeats in US. Arrowheads: the direction of transcription of individual IE genes.
Fig. 2.
Expression of GFP protein from recombinant viruses.
Human foreskin fibroblasts (HFF) were infected with d106-GFP (MOI = 1.25) or Ad5-GFP (MOI = 0.125) which gave similar intracellular GFP sequence copy numbers in preliminary experiments. Panel A. One day post transduction, cells were lysed with SDS sample buffer, and GFP levels were analyzed by SDS PAGE and immunoblotting. Panel B. One day post transduction, single cell suspensions were generated by enzymatic dissociation, and GFP expression was analyzed by flow cytometry.
2.2. Construction and testing of an HSV-1 vector expressing SARS coronavirus 1 spike protein
We constructed an HSV-1 d106 recombinant vector, d106-SARS-CoV-1-S (Fig. 1B), expressing SARS CoV-1 spike protein by homologous recombination as described previously (Taylor et al., 2016). SARS spike protein coding sequences from the SARS-SOptimized plasmid (Li et al., 2003), generously provided by Michael Farzan, were inserted into the CMV expression vector pCIΔAflII (Murphy et al., 2000), and the expression cassette was cloned into the transfer plasmid pPs27pd1 plasmid (Rice et al., 1989), which contains the HSV genomic sequences surrounding the U L 54 (ICP27) gene to generate the pd27SARS-S plasmid. The pd27SARS-S plasmid was linearized and co-transfected into E11 complementing cells with infectious HSV-1 d106 viral DNA. Progeny viruses were harvested, and potential recombinants were screened for by the formation of non-fluorescent plaques by visualization with an inverted fluorescence microscope. Recombinants were confirmed by the detection of S protein in cell lysates by Western blot analysis. The resulting d106-SARS-CoV-1S recombinant virus was triple-plaque purified, and stocks were grown in the complementing E11 cells.
To monitor expression of S protein, we infected human foreskin fibroblast (HFF) cells with d106-SARS-CoV-1S virus or mock-infected, prepared cell lysates and extracellular supernatant, and detected S protein by immunoblotting with the monoclonal antibody SW111 that we described previously (Petit et al., 2005). In d106-SARS-CoV-1S virus-infected cells, we detected a major protein with apparent molecular weight of 180,000, similar to that in SARS virus-infected cells (Babcock et al., 2004) but none in the cell supernatant (Fig. 3 , panel A). Immunofluorescence (IF) studies showed the S protein expressed by the viral vector was localized to the surface of infected cells as shown by IF using staining of unfixed or fixed, unpermeabilized cells (Fig. 3, panels B and C, respectively). IF analysis of permeabilized cells showed there was also accumulation of S protein in the peri-nuclear region, similar to the distribution of the endoplasmic reticulum (Fig. 3, panel C).
Fig. 3.
Expression and localization of SARS-CoV-1 S protein. Panel A: expression of SARS-CoV-1 S protein in infected cells. Human foreskin fibroblast (HFF) cells (1 × 105) were infected with d106-SARS-CoV-1S virus at a MOI of 10 PFU per cell or mock-infected. The cells were harvested by scraping at 24 hpi and lysed in 100 μl RIPA buffer. Proteins from 10 μl of 100 μl cell lysate or 30 μl of 1.5 ml cell culture supernatant were resolved in a 4–12% polyacrylamide gradient gel, and spike protein was detected by immunoblotting using the mouse anti-SARS S protein hybridoma SW111 as the primary antibody. Panels B–D: localization of S protein in infected cells. HFF cells were infected with d106-SARS-CoV-1S virus at an MOI of 3. At 20 hpi, the cells were stained with SW111 antibody specific for spike. Spike protein expression on the cell surface was detected in unfixed (panel B) or fixed cells (panel C) without permeabilization. Total cellular spike protein was detected in fixed and permeabilized cells (panel D). The nuclei were stained with DAPI.
To test if the S protein expressed on the surface of d106-SARS-CoV-1S virus-infected cells was functional, we infected ACE2-293T cells that express the SARS virus receptor, ACE2 (Fig. 4 A). We observed that the infected cells formed syncytia (Fig. 4B), indicating that the S protein expressed by d106-SARS-CoV-1S virus was functional and at least part was localized on the surface of infected cells.
Fig. 4.
Cell-cell fusion induced by d106-SARS-CoV-1S infection. (A) ACE2 expression was detected by immunoblotting. HEK 293T and 293T/ACE2 cells were harvested and cell lysates (lanes: 1: 1.5 × 105, 0.5: 7.5 × 104, 0.25: 3.75 × 104 cells) were loaded to SDS-PAGE gel. ACE2 and GAPDH were detected their specific antibodies. (B) Cell-cell fusion was induced by d106-SARS-CoV-1S infection. HEK 293T/ACE2 cells were infected with d106 or d106-SARS-CoV-1S (3 × 106 PFU/well), and cell-cell fusion was detected at 20 hpi. GFP was detected as a control for d106 infection.
2.3. Antibody responses in immunized mice
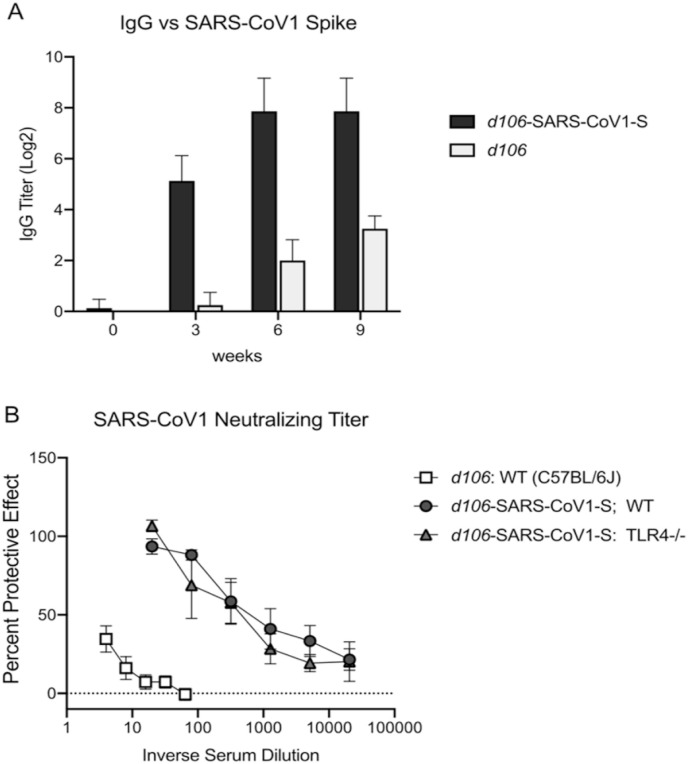
To define the immunogenicity of the recombinant vector, we immunized Balb/C mice with d106-SARS-CoV-1S or d106 virus subcutaneously in the rear flank at 0 and 3 weeks and obtained sera for testing at 3, 6, and 9 weeks. We performed an ELISA test using baculovirus-expressed S protein (Petit et al., 2005), as described previously and found that mice immunized with d106-SARS-CoV-1 virus had spike protein binding antibodies (Fig. 5 A). Sera from C57BL/6 mice immunized with d106-SARS-CoV-1 developed SARS-CoV-1 neutralizing activity (protection from CPE). With a single dose of d106-SARS-CoV-1, titers of 1:40–1:80 were achieved (data not shown). Following a second dose of d106-SARS-CoV-1, neutralizing titers increased to ≥1:640 for wild type C57BL/6 mice (Fig. 5B). Neutralizing titers in TLR4−/− mice were indistinguishable from titers in WT mice. (Fig. 5B). In contrast, the d106 vector alone induced minimal SARS-CoV-1 neutralizing activity at all time points (titer <1:4).
Fig. 5.
Ab titers and virus neutralization of SARS-CoV-1 in sera of mice immunized with d106-SARS-CoV-1S or control d106 virus.
(A) Total IgG anti-spike Ab levels. C57BL/6J mice were immunized twice with d106-SARS-CoV-1S vector or d106 vector alone. Sera were collected 3, 6 and 9 weeks later and anti-Spike IgG Ab levels were measured by ELISA. (B) Neutralizing Ab titers. WT C57BL/6 and TLR4−/− mice were immunized and boosted with d106-SARS-CoV-1S vector or d106 vector alone or were mock treated. Sera were collected 4 weeks after the boost. Neutralizing titers of anti-SARS-CoV-1 Ab measured by in CPE protection assay and are expressed as the inverse titer conferring 50% protection from SARS-CoV-1 infection induced CPE. Neutralizing titers: WT C57BL/6 d106 vector alone, <4; WT d106-SARS-CoV-1S, ≥640; TLR4−/− d106-SARS-CoV-1S, ≥640.
2.4. Induction of innate responses
We defined the effect of SARS S protein on cells by infecting various murine WT cells and cells defective for specific innate signaling proteins with the d106-SARS-CoV-1S virus or the empty vector, d106 virus. We found that the d106-SARS-CoV-1S virus induced expression of higher levels of several cytokines than the d106 virus itself when used in challenge studies of human and mouse cells. High levels of MCP-1, IL-6 and RANTES protein secretion were induced by d106-SARS-CoV-1S from human PBMC (Fig. 6 A and B) and from murine embryonic fibroblasts (Fig. 7 A and B). Cytokine induction by d106-SARS-CoV-1S was dose-dependent (Fig. 7A and B). The cytokine responses to SARS-S were reduced in MyD88-deficient compared to WT cells (Fig. 7A and B) as were cytokine responses to the MyD88-dependent TLR2 ligand Pam3CSK4 (Fig. 7C and D).
Fig. 6.
Induction of innate cytokines in PBMC by infection with d106-SARS-CoV-1S virus.
PBMCs were plated at 106 per well and infected with d106-SARS-CoV-1S vector or d106 vector alone at MOI 1 and 10. Controls included challenge with medium alone, LPS (TLR4 ligand, 10 ng/ml), Pam2CSK4 (TLR2 ligand, 100 ng/ml), Pam3CSK4 (TLR2 ligand, 100 ng/ml), or IL-1β (10 ng/ml). Supernatants were collected 18 h later and (A) MCP-1 and (B) IL-6 levels were measured by ELISA.
Fig. 7.
d106-SARS-CoV-1S virus induced cytokines in MEFs is MyD88-dependent
C57BL/6 WT and MyD88−/− MEFs (106 per well) were infected with d106-SARS-CoV-1S vector or d106 vector alone at varying MOI. (A) MCP-1 and (B) RANTES cytokine levels in culture supernatants were measured 18 h later by ELISA. (C, D) Controls include challenge with medium alone and Pam3CSK4, a MyD88-dependent TLR2 agonist, and measurement of (C) MCP-1 and (D) RANTES levels.
3. Discussion
In these studies, we constructed an HSV-1 replication-defective recombinant vector strain expressing SARS spike protein, tested its immunogenicity, and used it to probe the effects of spike protein on host cells. The spike protein expressed from this vector is functional in that it localizes to the surface of infected cells and induces fusion of ACE2-expressing cells. In immunized mice, the recombinant vector induced antibodies that bind to spike protein in an ELISA assay and show neutralizing activity. The spike protein expressed from this vector can induce the expression of cytokines in an ACE2-independent, MyD88-dependent process. These results argue that the SARS spike protein intrinsically activates signaling pathways that induce cytokines and could contribute directly to the inflammatory process of SARS.
3.1. Efficiency of HSV-1 d106 as a vector
We compared the HSV-1 d106 vector for expression of GFP protein with an Ad5 recombinant virus expressing the same GFP expression cassette under infection conditions where both vectors were delivering similar amounts of GFP DNA sequences into the host cells. We observed that HSV-1 d106 expressed more viral protein as detected on an immunoblot and 10-fold more fluorescence than Ad-GFP under these conditions. Thus, d106 virus is a very efficient vector for expression of a transgene in normal human fibroblasts. This is likely due, at least in part, to the expression of the HSV-1 ICP0 immediate-early protein, which promotes gene expression from the d106 genome (Samaniego et al., 1998) by promoting the degradation of a number of host restriction factors (Everett et al., 2008; Orzalli et al., 2012) and de-silencing the viral genome (Cliffe and Knipe, 2008; Lee et al., 2016). In contrast, the Ad5 vector is deleted for E1A, which in part serves the same purpose of combatting the host cell epigenetic silencing of the viral genome. Further studies are needed to determine the mechanisms that define the relative efficiencies of gene expression by these vaccine vectors.
3.2. Expression of SARS spike protein
The S protein expressed by the d106 vector is localized in part on the infected cell surface and is functional as assayed for its ability to promote fusion of 293T cells expressing the SARS receptor, ACE2. Mice immunized with d106-SARS-CoV-1 produced binding and neutralizing antibodies. The d106 vector has been used to immunize mice and nonhuman primates and has produced neutralizing antibody responses for West Nile virus (Taylor et al., 2016), CD8+ T cells and antibody responses for simian immunodeficiency virus (SIV) and HIV in mice (Watanabe et al., 2009), antibody, T cell and protective immunity against SIV (Kaur et al., 2007), and protective immunity against HIV in humanized mice (Claiborne et al., 2019). The vector is safe in non-human primates (Kaur et al., 2007), and a similar HSV-2 replication-defective recombinant virus has been produced as a clinical product, tested, and found to be safe and immunogenic in a phase I trial for a genital herpes vaccine (Dropulic et al., 2019). Therefore, HSV-1 d106 could be developed as a vaccine vector platform for SARS-CoV-2 and other infectious diseases.
Infection of PBMCs, fibroblasts and macrophages (not shown) with d106-SARS-CoV-1S virus led to an induction of expression of several cytokines, including MCP-1, RANTES, and IL-6, relative to the d106 GFP vector. This argues that the spike protein can induce these cytokines, likely independently, but possibly in conjunction with an HSV virion protein. Induction of the cytokines requires MyD88, suggesting that Toll-like receptors or other innate pathways are involved in the cytokine induction. We observed a partial dependence on TLR2 or TLR4, two innate receptors that signal via MyD88, in macrophages challenged with d106-SARS-CoV-1S (not shown), suggesting that multiple pathways may be contributing to the induction of inflammation. In contrast to inflammatory cytokine production, we noted that TLR4 was not required for Ab responses to S protein. These results argue that S protein is part of the mechanism of induction of inflammatory cytokines by SARS virus. This is ACE2-independent because it is observed in murine cells.
A previous study using transfection of lung epithelial and fibroblast cells expressing SARS spike protein showed activation of the IL-8 gene promoter (Chang et al., 2004). Soluble S protein, transfection of plasmid encoding S protein, or infection with a baculovirus vector expressing S protein induced IL-8 expression (Chang et al., 2004). Their results argued for AP-1 induction and not NF-κB and a dependence on ACE2 for the induction. Another study using soluble S protein incubation with murine RAW264.7 macrophages showed induction of IL-6 and TNF (Wang et al., 2007). This induction involved IKB degradation and NF-κB signaling. Therefore, different systems have yielded different mechanisms and different cytokine activation, but in total, the results argue that SARS spike protein may contribute to or even be a major contributor to the cytokine storm observed in SARS.
Induction of cytokines could be due, at least in part, to over-expression or even normal levels of spike protein by the d106 vector and activation of the unfolded protein response (UPR) as observed previously (Chan et al., 2006). The UPR has been associated with cytokine induction (reviewed by Smith, 2018). SARS CoV-1 viral infection has been shown to activate UPR (Chan et al., 2006) and this has been speculated to increase viral assembly. Further studies should define the relationships between these observations.
The implications of these results are two-fold. Approaches that block the signaling induced by the SARS spike protein could reduce the inflammation and immune pathology. Furthermore, the results point to the possibility that immunization with a vector expressing SARS protein or with SARS protein itself might lead to local inflammatory cytokine production.
4. Materials and methods
4.1. Cells and viruses
HSV-1 d106 virus (Samaniego et al., 1998) was kindly provided by Neal DeLuca, University of Pittsburgh. Infectious full-length HSV-1 d106 viral DNA was purified from infected E11 cell lysates by sodium iodide gradient centrifugation (Walboomers and Schegget, 1976), as described (Knipe et al., 1979).
An adenovirus 5 recombinant expressing GFP (Ad5CMV GFP) was obtained from the Viral Vector Core at the University of Iowa (VVC–U of Iowa-4 Ad5CMVeGFP).
4.2. Antibodies
Anti-ACE2 antibody (R&D, Cat#: AF933, 1:1000) and Anti-GAPDH ([6C5], Mouse monoclonal, abcam, Cat. #: ab8245, 1:5000) were used in this study.
4.3. Cell-cell fusion assay
Human embryonic kidney 293T/ACE2 cells (kind gift from Michael Farzan) (5 × 105) were plated in 6-well plates. On the next day, the cells were infected with d106-SARS-CoV-1S virus (3 × 106 PFU/well), and cell-cell fusion was assessed at 20 h post infection (hpi). Images were acquired using a Nikon TE200 microscope.
4.4. SDS-PAGE and immunoblotting
Immunoblotting was performed as described previously (Oh et al., 2014). Briefly, cells were lysed in 1x RIPA buffer (10 mM Tris-Cl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl) with protease inhibitor (Complete Protease Inhibitor Cocktail, Millipore Sigma). The proteins were resolved in NuPAGE 4–12% Bis-Tris Gels (Life Technologies) and transferred to a Nitrocellulose Membrane (Bio-Rad, #1620112). The membranes were then incubated in Odyssey Blocking Buffer (LI-COR) for 1 h at room temperature and incubated with antibodies specific for ACE2 (1:1000, polyclonal goat, R&D, Cat#: AF933) or GAPDH (1:10,000, mAb, Abcam, Cat#: ab8245). The membranes were incubated with secondary antibodies, IRDye 680RD or IRDye 800 (LI-COR), for 45 min at room temperature. Near-infrared fluorescence was detected using Odyssey (LI-COR) and imaged using ImageStudio V4 (LI-COR).
4.5. Immunofluorescence
HFF cells (4–8x104) were seeded on glass coverslips in a 24-well plate. On the next day, the cells were infected with d106-SARS-CoV-1S virus at an MOI of 3 for 20 h. To detect spike protein expression on the cell surface, unfixed or fixed, unpermeabilized cells were used. Fixation of cells was with 3.7% paraformaldehyde in PBS for 10 min, and permeabilization was performed using 0.1% Triton X-100 in PBS for 10 min, both as indicated. Permeabilized cells were blocked in 10% normal goat serum (Jackson ImmunoResearch) + human IgG (0.16%) in DMEM for 1 h at room temperature. Cells were incubated with monoclonal antibody SW111 specific for spike protein (Petit et al., 2005) for 1 h at room temperature. Secondary antibodies conjugated to the dye Alexa 594 were incubated with the cells for 1 h at room temperature. Nuclei were stained with 4,6-diamidino-2- phenylindole (DAPI; Life Technologies), and ProLong Gold Antifade mounting reagent (Life Technologies) was used to mount coverslips. Wide-field images of cells were acquired on a Zeiss Axioplan 2 microscope and the Zeiss AxioVision 4.8 image acquisition software.
4.6. Flow cytometry
HFF cells in 6-well trays were infected with HSV d106, Ad5-GFP or mock infected in 500 μL medium and incubated at 37 °C. Two h later, the inoculum was aspirated, and cells were washed 4 times with warm PBS to remove free virus. Fresh culture medium was added and the cells were incubated at 37 °C. At 24 hpi, infected and mock infected cells were washed with PBS and dissociated into single cell suspensions using 1 ml of AccuMax solution (Innovative Cell Technologies, Cat# AM105). Suspended cells were run on a FACScalibur flow cytometer retrofitted with optics and fluidics from Cytek (Fremont, CA). All samples were acquired and analyzed with FlowJo (FlowJo, Ashland OR). Debris was gated out by selecting the cell population by forward scatter (FSC) versus vs side scatter (SSC) plot, and singlets were selected on an FSC-H vs FSC-A plot. The GFP-expressing population was determined by plotting the mock vs infected single cells using the FITC-A (GFP channel in our cytometer). Using the mock infected cells as GFP negative control, a GFP positive gate was determined and applied to all samples. The GFP positive population followed a normal distribution and the mean fluorescence intensity (MFI) was determined for the GFP populations.
4.7. Cytokine assays
WT, TLR4−/−, and MyD88−/− mice were bred in the University of Massachusetts Medical School Animal Facility. MEFs were isolated as described previously (Kurt-Jones et al., 2004). MEFs were plated at 5 × 105 per well in 24-well plates and infected with d106 viruses at varying MOI or challenged with TLR ligands (LPS (TLR4 ligand, Sigma E. coli 011:B4), 10 ng/ml; Pam2CSK4 and Malp2 lipopeptides (TLR2 ligands, EMC Microcollections), 100 ng/ml), or cultured in medium alone. Culture supernatants were collected 18 h later, and cytokine levels were measured by ELISA (OptEIA, BD Pharmingen).
Peripheral blood mononuclear cells (PBMC) were prepared from heparinized blood of healthy donors. PBMC were plated at 106 per well in 24-well plates and infected with d106 viruses at varying MOI or challenged with TLR ligands or cultured in medium alone. Culture supernatants were collected 18 h later, and cytokine levels were measured by ELISA (Quantitkine, R&D Systems).
4.8. Animal studies
For immunization studies, 8wk old C57BL/6J WT or TLR4−/− mice were infected ip with 105 pfu d106 or d106-SARS (primary immunization). Four weeks later, a cohort of mice were boosted with a second ip infection of 105 pfu d106 viruses (secondary immunization). Sera were collected at 4 wks (post-primary immunization) and 7 wks (i.e., 3 wks post-secondary boosting). Sera were tested for SARS spike protein binding by ELISA and for SARS neutralization activity in CPE reduction assays.
4.9. Neutralization assays (CPE reduction)
The neutralizing activity of serum was measured as described previously (Greenough et al., 2005). Vero E6 cells were seeded at 5000 cells/well, in 96-well microtiter plates, on assay day −1 in a volume of 100 μL. On assay day 0, two-fold serum dilutions were preincubated for 1 h with 100 TCID50 of virus stock (Urbani strain; generously provided by Larry Anderson [Centers for Disease Control and Prevention, Atlanta]; low passage in Vero E6 cells). These mixtures of virus and serum dilutions then were added to cells in duplicate. One additional set of serum dilutions without virus was included as a control to detect toxicity. Virus stock was back-titrated in each assay, to ensure that the inoculum was 30–300 TCID50/well. Presence or absence of cytopathic effect (CPE) after 72–96 h of incubation was determined by microscopy. The dilution of serum that completely prevented CPE in 50% of the wells was calculated by means of the Reed-Muench formula. After microscopic visualization of CPE, the medium was replaced by PBS, CellTiter96 reagent (Promega) was added, and plates were incubated for 2–4 h until gradations of color between uninfected and infected controls were easily distinguished visually. CellTiter96 is metabolized to a soluble, colored product, the concentration of which is proportional to the number of viable cells in the culture. Absorbance is reduced in wells with significant CPE. To inactivate virus, 10% SDS was added, and the absorbance (optical density measured at 490 nm) was read by use of a universal plate reader (EL 800; Bio-Tek Instruments). Percent protective effect was calculated as follows: 100 (observed CPE-maximum CPE)/(minimum CPE-maximum CPE), where “maximum CPE” refers to absorbance in control wells with virus and no serum and “minimum CPE” refers to absorbance in control wells with no virus and no serum.
CRediT authorship contribution statement
Evelyn A. Kurt-Jones: Conceptualization, Investigation, Writing – original draft, Supervision. Timothy E. Dudek: Investigation. Daisuke Watanabe: Investigation. Leisa Mandell: Investigation. Jenny Che: Investigation. Shenghua Zhou: Investigation. LuCheng Cao: Investigation. Thomas Greenough: Investigation, Writing – original draft, Supervision. Gregory J. Babcock: Investigation. Fernando Diaz: Investigation. Hyung Suk Oh: Investigation, Writing – original draft. Changhong Zhou: Investigation. Robert W. Finberg: Conceptualization, Supervision, Writing – original draft, Funding acquisition. David M. Knipe: Conceptualization, Supervision, Writing – original draft, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by grants from the New England Regional Center for Excellence in Biodefense and Emerging Infectious Diseases (DMK and RWF) and by a grant from the Massachusetts Consortium for Pathogen Readiness (DMK).
References
- Babcock G.J., Esshaki D.J., Thomas W.D., Jr., Ambrosino D.M. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J. Virol. 2004;78:4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.P., Siu K.L., Chin K.T., Yuen K.Y., Zheng B., Jin D.Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80:9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.J., Liu C.Y., Chiang B.L., Chao Y.C., Chen C.C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol. 2004;173:7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Claiborne D.T., Dudek T.E., Maldini C.R., Power K.A., Ghebremichael M., Seung E., Mellors E.F., Vrbanac V.D., Krupp K., Bisesi A., Tager A.M., Knipe D.M., Boutwell C.L., Allen T.M. Immunization of BLT humanized mice redirects T cell responses to gag and reduces acute HIV-1 viremia. J. Virol. 2019;93 doi: 10.1128/JVI.00814-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A.R., Knipe D.M. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J. Virol. 2008;82:12030–12038. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S.G., Wang N., Chen Z., Chen Z., Tseng M., Barretto N., Lin R., Peters C.J., Tseng C.T., Baker S.C., Li K. Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2007;282:32208–32221. doi: 10.1074/jbc.M704870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropulic L.K., Oestreich M.C., Pietz H.L., Laing K.J., Hunsberger S., Lumbard K., Garabedian D., Turk S.P., Chen A., Hornung R.L., Seshadri C., Smith M.T., Hosken N.A., Phogat S., Chang L.J., Koelle D.M., Wang K., Cohen J.I. A randomized, double-blinded, placebo-controlled, phase 1 study of a replication-defective herpes simplex virus (HSV) type 2 vaccine, HSV529, in adults with or without HSV infection. J. Infect. Dis. 2019;220:990–1000. doi: 10.1093/infdis/jiz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Everett R.D., Parada C., Gripon P., Sirma H., Orr A. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 2008;82:2661–2672. doi: 10.1128/JVI.02308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M., Ratia K., Johnston R.E., Mesecar A.D., Baric R.S. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J. Virol. 2009;83:6689–6705. doi: 10.1128/JVI.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough T.C., Babcock G.J., Roberts A., Hernandez H.J., Thomas W.D., Jr., Coccia J.A., Graziano R.F., Srinivasan M., Lowy I., Finberg R.W., Subbarao K., Vogel L., Somasundaran M., Luzuriaga K., Sullivan J.L., Ambrosino D.M. Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J. Infect. Dis. 2005;191:507–514. doi: 10.1086/427242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A., Sanford H.B., Garry D., Lang S., Klumpp S.A., Watanabe D., Bronson R.T., Lifson J.D., Rosati M., Pavlakis G.N., Felber B.K., Knipe D.M., Desrosiers R.C. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology. 2007;357:199–214. doi: 10.1016/j.virol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D.M., Ruyechan W.T., Roizman B. Molecular genetics of herpes simplex virus. III. Fine mapping of a genetic locus determining resistance to phosphonoacetate by two methods of marker transfer. J. Virol. 1979;29:698–704. doi: 10.1128/jvi.29.2.698-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., Group S.W. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E.A., Sandor F., Ortiz Y., Bowen G.N., Counter S.L., Wang T.C., Finberg R.W. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J. Endotoxin Res. 2004;10:419–424. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Raja P., Knipe D.M. Herpesviral ICP0 protein promotes two waves of heterochromatin removal on an early viral promoter during lytic infection. mBio. 2016;7:e02007–e02015. doi: 10.1128/mBio.02007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lin L., Wang H., Yin J., Ren Y., Zhao Z., Wen J., Zhou C., Zhang X., Li X., Wang J., Zhou Z., Liu J., Shao J., Lei T., Fang J., Xu N., Liu S. The epitope study on the SARS-CoV nucleocapsid protein. Dev. Reprod. Biol. 2003;1:198–206. doi: 10.1016/S1672-0229(03)01025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Broberg E., Watanabe D., Dudek T., Deluca N., Knipe D.M. Genetic engineering of a modified herpes simplex virus 1 vaccine vector. Vaccine. 2009;27:2760–2767. doi: 10.1016/j.vaccine.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C.G., Lucas W.T., Means R.E., Czajak S., Hale C.L., Lifson J.D., Kaur A., Johnson R.P., Knipe D.M., Desrosiers R.C. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 2000;74:7745–7754. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H.S., Bryant K.F., Nieland T.J., Mazumder A., Bagul M., Bathe M., Root D.E., Knipe D.M. A targeted RNA interference screen reveals novel epigenetic factors that regulate herpesviral gene expression. mBio. 2014;5 doi: 10.1128/mBio.01086-13. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzalli M.H., DeLuca N.A., Knipe D.M. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y., Group H.U.S.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Masters P.S. In: seventh ed. Knipe D.M., Howley P.M., Whelan S.P.J., editors. vol. 1. Wolters Kluwer; Philadelphia: 2020. Coronaviridae; pp. 410–448. (Fields Virology). [Google Scholar]
- Petit C.M., Melancon J.M., Chouljenko V.N., Colgrove R., Farzan M., Knipe D.M., Kousoulas K.G. Genetic analysis of the SARS-coronavirus spike glycoprotein functional domains involved in cell-surface expression and cell-to-cell fusion. Virology. 2005;341:215–230. doi: 10.1016/j.virol.2005.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S.A., Su L.S., Knipe D.M. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J. Virol. 1989;63:3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego L.A., Neiderhiser L., DeLuca N.A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A. Regulation of cytokine production by the unfolded protein response; implications for infection and autoimmunity. Front. Immunol. 2018;9:422. doi: 10.3389/fimmu.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.R. Obstacles and advances in SARS vaccine development. Vaccine. 2006;24:863–871. doi: 10.1016/j.vaccine.2005.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T.J., Diaz F., Colgrove R.C., Bernard K.A., DeLuca N.A., Whelan S.P.J., Knipe D.M. Production of immunogenic West Nile virus-like particles using a herpes simplex virus 1 recombinant vector. Virology. 2016;496:186–193. doi: 10.1016/j.virol.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J.M., Schegget J.T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976;74:256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., Kong L., Fang X., Zheng H., Wu Z., She Y. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128:1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D., Brockman M.A., Ndung'u T., Mathews L., Lucas W.T., Murphy C.G., Felber B.K., Pavlakis G.N., Deluca N.A., Knipe D.M. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357:186–198. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Sekine H., Uruma T., Nagasaki S., Tsunoda T., Machida Y., Kobayashi K., Igarashi H. Increase of atypical lymphocytes expressing CD4+/CD45RO+ in an infectious mononucleosis-like syndrome associated with hepatitis A virus infection. J. Infect. Chemother. 2009;15:187–190. doi: 10.1007/s10156-009-0677-9. [DOI] [PubMed] [Google Scholar]