Abstract
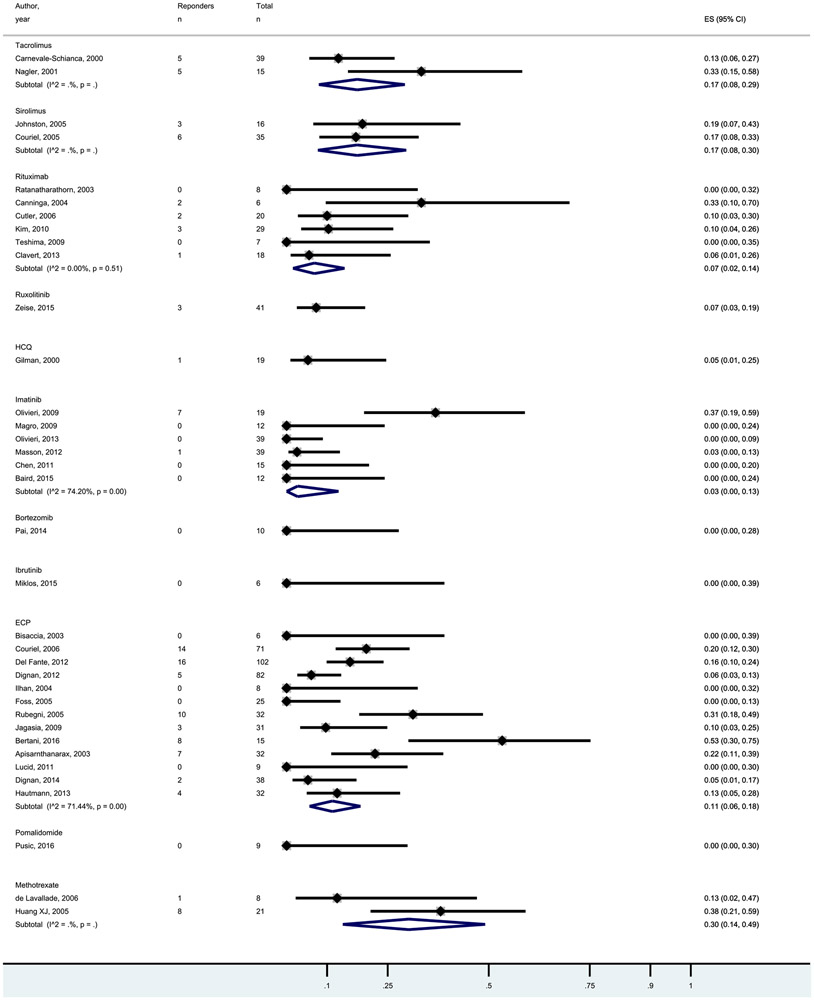
Given the increasing incidence of chronic graft-versus-host disease (cGVHD) and its rapidly escalating costs due to many lines of drug treatments, we aimed to perform a meta-analysis to assess the comparative effectiveness of various treatment options. Using these results, we then conducted a cost-effectiveness analysis for the frequently utilized agents in steroid-refractory cGVHD. We searched for studies examining tacrolimus, sirolimus, rituximab, ruxolitinib, hydroxychloroquine, imatinib, bortezomib, ibrutinib, extracorporeal photopheresis, pomalidomide, and methotrexate. Studies with a median follow-up period shorter than 6 months and enrolling fewer than 5 patients were excluded. Meta-analysis for overall and organ system-specific GVHD response (overall response [ORR], complete response [CR], and partial response [PR]) was conducted for each intervention. Cost per CR and cost per CR + PR were calculated as the quotient of the 6-month direct treatment cost by CR and CR + PR. Forty-one studies involving 1047 patients were included. CR rates ranged from 7% to 30% with rituximab and methotrexate, respectively, and ORR ranged from 30% to 85% with tacrolimus and ruxolitinib, respectively. Cost per CR ranged from US$1,187,657 with ruxolitinib to US$680 with methotrexate. Cost per ORR ranged from US$453 for methotrexate to US$242,236 for ibrutinib. The most cost-effective strategy was methotrexate for all of the organ systems. Pomalidomide was found to be the least cost-effective treatment for eye, gastrointestinal, fascia/joint, skin, and oral GVHD, and imatinib was found to be the least cost-effective treatment for liver and extracorporeal photopheresis for lung GVHD. We observed huge cost-effectiveness differences among available agents. Attention to economic issues when treating cGVHD is important to recommend how treatments should be sequenced, knowing that many patients will cycle through available agents.
Keywords: Cost, GVHD, Allogeneic, Transplant, Survival
INTRODUCTION
Hematopoietic cell transplantation (HCT) is an established procedure for many acquired and congenital disorders of the hematopoietic and the immune system. More than 25,000 allogeneic HCTs are carried out annually worldwide and are increasing each year [1,2]. However, HCT is an expensive procedure and HCT-related costs become increasingly important because of the widespread application of this treatment. According to an Agency for Healthcare Research and Quality report, HCT had the most rapidly growing expenditure among medical procedures between 2004 and 2007, with an increase of 84.9% and a total of US$1.3 billion spent on HCT in 2007 [3]. Most of the studies looking at cost drivers of HCT have focused on early post-transplant costs of HCT [4-9]. The economic impact of long-term costs and chronic graft-versus-host disease (cGVHD) treatments is not clear [10,11].
cGVHD remains the most serious complication affecting long-term survivors of allogeneic HCT [12,13]. Increasing HCT numbers, use of unrelated donors, peripheral blood stem cell utilization, and the inclusion of older recipients utilizing nonmyeloablative regimens have led to an increased incidence of cGVHD [14]. Currently, the therapeutic mainstay for cGVHD is steroids; however, it fails to produce complete response in approximately one-half of the patients and there is no established consensus on the treatment of steroid-refractory cGVHD (SR-cGVHD) patients [15-17]. The available options vary substantially in both cost and effectiveness [18].
Several advances in transplantation techniques and supportive care practices over the last 2 decades have significantly improved survival of patients undergoing HCT [19]. However, along with several other factors (eg, increase in peripheral blood stem cells for HCT), this improved early survival has also contributed to an increase in the incidence of cGVHD. More than 40,000 cGVHD patients are estimated from the last 5 years of trends reported in the registries to require treatment over the next decade [20].
The increasing numbers of patients with cGVHD, and the increasing use of marketed and off-label therapies for them will cause a significant increase in the expenditures of both the private insurers and the Centers for Medicare and Medicaid Services in the future. To delineate the current landscape of cGVHD drug therapy, we aimed to perform a cost-effectiveness analysis (CEA) for the frequently utilized agents in adult patients with SR-cGVHD; before pharmacoeconomic analysis, we performed a comprehensive meta-analysis of the response rates for each drug/extracorporeal photopheresis (ECP) treatment and also for each organ system separately.
MATERIALS AND METHODS
Literature Search Methods
A comprehensive search of several databases for studies published from January 2000 to May 2016 was conducted by an expert medical librarian. The databases searched were: Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, and Scopus. The searched MeSH terms were graft vs host disease augmented by text words such as chronic, second line, steroid refractory, treatment. The search terms were then translated to the preferred terms of the other databases. This terminology was utilized for management with each one of the following therapies: tacrolimus, sirolimus, rituximab, ruxolitinib, hydroxychloroquine, imatinib, bortezomib, ibrutinib, ECP, pomalidomide, and methotrexate. This study was conducted before the approval of ibrutinib by the U.S. Food and Drug Administration for treatment of SR-cGVHD.
Eligibility Criteria
We included prospective and retrospective studies examining tacrolimus, sirolimus, rituximab, ruxolitinib, hydroxychloroquine, imatinib, bortezomib, ibrutinib, ECP, pomalidomide, and low-dose methotrexate as a sole therapy for SR-cGVHD, which was defined as any disease that failed to respond to previous immunosuppressive therapy with steroids at a dose of ≥.5 mg/kg/day for at least 4 weeks or inability to taper it with or without additional immunosuppressive drugs. There were insufficient publications to analyze mycophenolate mofetil, mesenchymal stem cells azathioprine, daclizumab, basiliximab, thalidomide, anti-TNF antibodies, daclizumab, total nodal irradiation, everolimus, and cyclophosphamide. We excluded studies evaluating any of the aforementioned drugs in combination with another drug when introduced concurrently. Review articles and meta-analysis were excluded. We excluded studies enrolling fewer than 5 patients or with <6 months of follow-up, publications in languages other than English, and those that included any pediatric patients unless they had a separate data analysis for the adult patients in the study.
Study Selection
Two reviewers (S.H. and F.F.Y.) independently considered the potential eligibility of each of the abstracts and titles from the retrieved citations and requested full text versions for these potentially eligible studies. Working separately, reviewers assessed the full text of reports to confirm eligibility.
Data Collection and Extraction
Data were extracted using a predefined data extraction form, including general information about the included studies, participants, and outcomes (all measured outcomes, including overall cGVHD and organ system–specific cGVHD responses). The outcomes measured were complete response (CR), partial response (PR), and overall response (ORR) for overall cGVHD and organ-specific cGVHD as defined in the reports. ORR was defined as the sum of CR and PR. Of note, the response rates were rarely based on formal National Institutes of Health consensus criteria and were usually physician-reported responses [21,22]. The response rate represented the cumulative incidence of the outcome variable in the meta-analysis.
Risk of Bias Assessment
We conducted a quality assessment of each study. Randomized controlled trials were assessed using the Cochrane risk of bias tool [23]. The Newcastle Ottawa Scale [24] was used to assess the methodological quality of nonrandomized studies.
Statistical Methods and Analysis
Meta-analysis
For each study, we estimated the response rates (cumulative incidence) and the associated 95% confidence interval. Response rates were pooled across studies using the random effects model. Meta-analysis was conducted using the STATA statistical software package release 14 (StataCorp, College Station, TX).
Cost-effectiveness analysis
Drug prices were obtained from uptodate.com, representing the average wholesale price. Regarding ECP, cost per session was obtained from the manufacturer’s (Therakos, Mallinckrodt plc, Staines-upon-Thames, United Kingdom) internal data as used previously [25].
Treatment protocols for each drug were determined based on the published reports and if different doses were used in different trials for the same drug, then the standard doses (based on consensus of ≥2 practicing hematologists) for a specific drug were utilized for input for analysis. Direct medical costs of each drug for 6 months of treatment were calculated (Table 1). We did not include the direct nonmedical and indirect costs. Cost per CR and cost per CR + PR were calculated as the quotient of the 6-month cost by CR and CR + PR of each drug respectively (Table 2). Costs per CR + PR for each of the organ system were calculated as the quotient of the 6-month cost by CR + PR of each organ system for each of the drug (Table 3).
Table 1.
Treatment Protocols and Costs for 6 Months of Treatment (United States Only)
| Active Ingredient | Brand Names | Dose/Form | Price | Treatment Protocol | 6-Month Drug Cost* |
|---|---|---|---|---|---|
| Tacrolimus | Tacrolimus | 0.5 mg (100 capsules) | $222 | .12 mg/kg/d, 6 mo | $6815 |
| 1 mg (100 capsules) | $445 | ||||
| 5 mg (100 capsules) | $2229 | ||||
| Sirolimus | Sirolimus | 2 mg (100 tablets) | $3149 | 6 mg loading, 2 mg/d, 6 mo | $5731 |
| Rituximab | Rituxan | 100 mg/10 mL (10 mL) | $1042 | 375 mg/m2/wk, 4 wk | $29,184 |
| 500 mg/50 mL (50 mL) | $5212 | ||||
| Ruxolitinib | Jakafi | 5 mg (60 tablets) | $13,856 | 10–20 mg twice daily, 6 mo | $83,136 |
| 10 mg (60 tablets) | $13,856 | ||||
| HCQ | 200 mg (100 tablets) | $408 | 800 mg/d, 6 mo | $2938 | |
| Imatinib | Gleevec | 100 mg (90 tablets) | $10,112 | 100 mg/d, 6 mo | $20,224 |
| Bortezomib | Velcade | 3.5 mg (1 vial) | $1923 | .2 mg/m2/week, 6 months | $46,152 |
| Ibrutinib | Imbruvica | 140 mg (90 capsules) | $13,323 | 420 mg/d, 6 mo | $79,938 |
| ECP | $1348 | † | $41,788 | ||
| Pomalidomide | Pomalyst | 1 mg (21 capsules) | $17,430 | 1–4 mg/d, 21 of 28 d per course, 6 courses | $104,580 |
| 2 mg (21 capsules) | $17,430 | ||||
| 3 mg (21 capsules) | $17,430 | ||||
| 4 mg (21 capsules) | $17,430 | ||||
| Methotrexate | Methotrexate Sodium injection | 25 mg/mL (2 mL) | $8.5 | 7.5 mg/m2/wk, 6 mo | $204 |
HCQ indicates hydroxychloroquine.
Cost calculation based on 170 cm height and 70 kg weight and only includes direct costs calculated per described protocol.
Three times during week 1, and then twice weekly on consecutive days during weeks 2–12. Responding patients in the ECP group could continue 2 ECP treatments every 4 weeks until week 24.
Table 2.
Response Rates and Costs Per Response Types
| Active ingredient | Brand Name | CR Rate (95% CI) | Cost per CR | CR + PR Rate (95% CI) | Cost per CR + PR* |
|---|---|---|---|---|---|
| Tacrolimus | Tacrolimus | .17 (.08–.29) | $40,088 | .30 (.16–.44) | $22,717 |
| Sirolimus | Sirolimus | .17 (.08–.29) | $33,712 | .77 (.57–.92) | $7443 |
| Rituximab | Rituxan | .07 (.02–.14) | $416,914 | .62 (.53–.71) | $47,071 |
| Ruxolitinib | Jakafi | .07 (.003–.19) | $1,187,657 | .85 (.72–.93) | $97,807 |
| HCQ | .05 (.01–.25) | $58,760 | .32 (.15–.53) | $9181 | |
| Imatinib | Gleevec | .03 (.00–.13) | $674,133 | .46 (.32–.62) | $43,965 |
| Bortezomib | Velcade | .00 (.00–.28) | .50 (.24–.76) | $92,304 | |
| Ibrutinib | Imbruvıca | .00 (.00–.39) | .33 (.10–.70) | $242,236 | |
| ECP | .11 (.06–.18) | $379,891 | .62 (.54–.69) | $67,400 | |
| Pomalidomide | Pomalyst | .00 (.00–.30) | .78 (.45–.94) | $134,077 | |
| Methotrexate | Methotrexate Sodium injection | .30 (.14–.49) | $680 | .45 (.26–.64) | $453 |
CI indicates confidence interval.
Cost calculation based on 170 cm height and 70 kg weight and only includes direct costs calculated per described protocol.
Table 3.
Organ System–Specific Response Rates
| Active Ingredient | Eye Rate (95% CI) Cost per CR + PR |
GI Rate (95% CI) Cost per CR + PR |
Fasciae/Joint Rate (95% CI) Cost per CR + PR |
Liver Rate (95% CI) Cost per CR + PR |
Skin Rate (95% CI) Cost per CR + PR |
Lung Rate (95% CI) Cost /perCR + PR |
Mouth Rate (95% CI) Cost per CR + PR |
|---|---|---|---|---|---|---|---|
| Tacrolimus | NA | NA | NA | NA | NA | NA | NA |
| Sirolimus | .59 (.33–83) $9714 | .57 (.10–98) $10,054 | .33 (.06–79) $17,367 | .50 (.23–78) $11,462 | .64 (.48–78) $8955 | NA | .62 (.39–83) $9244 |
| Rituximab | .28 (.09–52) $104,229 | .33 (.01–78) $88,436 | .77 (.47–98) $37,901 | .44 (.19–70) $66,327 | .61 (.51–71) $47,843 | NA | .52 (.22–81) $56,123 |
| Ruxolitinib | NA | NA | NA | NA | NA | NA | NA |
| HCQ | .00 (.00–43) | .00 (.00–49) | .00 (.00–66) | .18 (.05–48) $16,322 | .25 (.09–53) $11,752 | NA | .22 (.06–55) $13,355 |
| Imatinib | .58 (.33–81) $34,869 | .72 (.47–92) $28,089 | .55 (.30–79) $36,771 | .30 (.00–74) $67,413 | .56 (.29–81) $36,114 | .33 (.14–61) $ 61,285 | .30 (.07–58) $67,413 |
| Bortezomib | .67 (.30–90) $68,884 | .50 (.09–91) $92,304 | .50 (.09–91) $92,304 | .00 (.00–79) | .50 (022–78) $92,304 | NA | .56 (.27–81) $82,414 |
| Ibrutinib | NA | NA | NA | NA | NA | NA | NA |
| ECP | .65 (.44–84) $64,289 | .68 (.20, 1.00) $61,453 | .52 (.31–72) $80,362 | .63 (.34–87) $66,330 | .65 (.51–78) $64,289 | .67 (.35–94) $ 62,370 | .69 (.51–84) $60,562 |
| Pomalidomide | .33 (.10–70) $316,909 | .50 (.15–85) $209,160 | .17 (.03–56) $615,176 | .00 (.00–79) | .63 (.31–86) $166,000 | NA | .29 (.08–64) $360,621 |
| Methotrexate | .56 (.11, 0,97) $364 | .80 (.38–96) $255 | .33 (.10–70) $618 | .67 (.40–90) $304 | .78 (.30, 1.00) $262 | NA | .40 (.06–79) $510 |
NA indicates not available; GI, gastrointestinal.
Cost calculation based on 170 cm height and 70 kg weight and only includes direct costs calculated per described protocol.
RESULTS
Selected Studies
The initial search of electronic databases based on MeSH terms identified studies for tacrolimus (189), sirolimus (82), rituximab (122), ruxolitinib (3), hydroxychloroquine (65), imatinib (78), bortezomib (6), ibrutinib (1), ECP (63), pomalidomide (3), and methotrexate (98). Following the review of these, based on the selection criteria, we included studies of tacrolimus (2) [26,27], sirolimus (3) [28-30], rituximab (7) [31-37], ruxolitinib (1) [38], hydroxychloroquine (1) [39], imatinib (6) [40-45], bortezomib (1) [46], ibrutinib (1) [47], ECP (15) [48-62], pomalidomide (1) [63], and methotrexate (3) [64-66] (eTable 1 in the Supplement). Among these, only 1 randomized controlled trial was identified [52]. The quality appraisal for this clinical trial using the Cochrane Collaboration’s tool indicated a low risk of bias considering adequate methodology described for sequence generation, allocation concealment, and for selected outcome reporting.
Pooled Overall cGVHD Responses
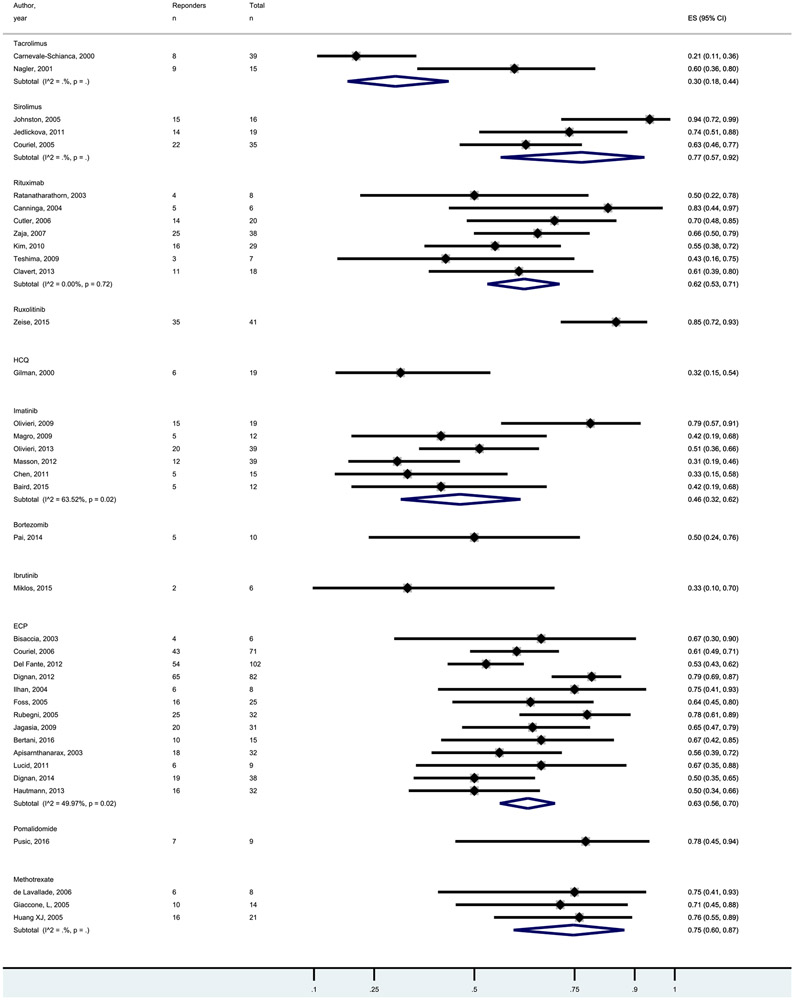
Pooled CR and ORR for the studied agents are shown in Figures 1 and 2. In general, CR rates were low with a median of 7% and range of 0% to 30%. ORR rates were higher with a median of 50% and range of 30% to 85%. Response rates were not statistically significant between the agents, supporting presentation of results as cost per response.
Figure 1.
Forest plot of the CR rates following treatments for SR-cGVHD.
Figure 2.
Forest plot of the ORR rates following treatments for SR-cGVHD.
Cost per CR and ORR
Cost per CR and cost per ORR are shown in Table 2 and ranged from US$453 per ORR for methotrexate to US$242,236 per ORR for ibrutinib. The median was US$47,071 per CR + PR. As rates of CR and ORR were fairly similar between agents, cost-effectiveness differences were largely driven by the costs of the agents/treatments.
Pooled Organ System–Specific Responses
Organ system–specific responses were reported for sirolimus, rituximab, hydroxychloroquine, imatinib, bortezomib, ECP, pomalidomide, and methotrexate.
More than half of the patients experienced an ocular ORR with sirolimus, imatinib, bortezomib, ECP, and methotrexate. The highest response rates are reported with bortezomib and ECP, with 67% and 65%, respectively. Hydroxychloroquine was not effective with 0% reported ORR for ocular cGVHD (eFigure 1 in the Supplement).
Gastrointestinal GVHD ORR are shown in eFigure 2 in the supplement and ranged from 0% and 80%, with a median of 50%. Methotrexate is reported as the most effective agent while hydroxychloroquine was not effective.
The best responses to fascia and joint GVHD are observed with rituximab followed by imatinib, ECP, and bortezomib. The ORR was <50% in patients treated with hydroxychloroquine, pomalidomide, sirolimus, and methotrexate (eFigure 3 in the Supplement).
For liver GVHD, more than half of the patients treated with sirolimus, ECP, and methotrexate responded. The highest reported response 67% was with methotrexate. Bortezomib and pomalidomide were not effective with reported response of 0%. (eFigure 4 in the Supplement).
Skin GVHD ORR are shown in eFigure 5 in the supplement and ranged from 25% and 78%. Methotrexate is reported as the most effective agent while hydroxychloroquine was not found to be effective.
The median ORR for oral GVHD is 46% with the highest responses seen with ECP (eFigure 6 in the Supplement). Less than half of the patients treated with hydroxychloroquine, pomalidomide, imatinib, and methotrexate had a response.
For lung GVHD, the information was not available for majority of treatments. The observed pooled ORR was 33% for imatinib, and 67% for ECP (eFigure 7 in the Supplement).
Cost per Organ System Responses
The CEA per organ responses are summarized in Table 3. The most cost-effective strategy was methotrexate for all of the organs systems. On the other hand, pomalidomide was found to be the least cost-effective treatment for eye, gastrointestinal, fascia/joint, skin, and oral GVHD and imatinib was found to be the least cost-effective treatment for liver and ECP for lung GVHD.
DISCUSSION
The national cost of cancer care in 2010 was estimated to be US$124.57 billion, and this cost in 2020 is estimated to rise to US$157.77 billion, representing a 27% increase from 2010. Taken together, leukemia and lymphoma are the third most expensive cancers in women and second most expensive in men from the perspective of management costs [67]. The costs of HCT within the first 100 days or 1 year are quite high, and key cost drivers in management of these diseases. To date, the vast majority of HCT cost-identification studies have focused on early post-transplant costs of HCT (typically the first few months of HCT) so the economic impact of late complications as well as cGVHD remains unclear [6-10,68].
We calculated 6-month direct drug costs for cGVHD in adult patients for the most frequently studied therapies. Enormous differences were observed between various treatments in the CEA with ruxolitinib associated with a cost per CR of US$1,187,657 and methotrexate with a cost per CR of US$680.Furthermore, for organ system–specific responses, a clear signal of cost effectiveness of a particular drug was observed (eg, pomalidomide was found to be the least cost-effective treatment for eye, gastrointestinal, fascia/joint, skin, and oral cGVHD) (Table 3).
There have been some economic evaluations for GVHD reported in the literature, but there are no reported economic evaluations comparing different drugs in SR-cGVHD. Crespo et al. [69] conducted an excellent pharmacoeconomic evaluation of selected cGVHD treatments. They assessed the cost effectiveness of ECP, rituximab, and imatinib in patients with cGVHD using local cost data (consumer price index; Spain) for the aforementioned agents [69]. Unlike our results, they found ECP to be more cost effective than imatinib and rituximab. The main differences are that they used microsimulation techniques for 1000 hypothetical patients and report the cost effectiveness at a 5-year time horizon, whereas we utilized the base case of 6 months of treatment with effectiveness measured by variables including CR and ORR. Another difference is the cost of the reviewed treatments. In another study Jones et al. [20] evaluated the cost burden of cGVHD by the summation of direct and indirect costs from prior studies. They estimated the total 10-year cGVHD cost burden as US$30.2 billion [20].
Our analysis was limited to direct drug costs for cGVHD and did not include costs necessary to administer a drug. Apart from the cost of medication, the direct costs in various pharmacoeconomic evaluations generally include the costs for the medical services including hospital services, physician and nurse services, medical supplies and laboratory monitoring tests, infusion unit costs (where applicable), and concomitant medications. Direct nonmedical costs were also excluded such as parking, tolls, childcare, and other costs related to receipt of treatment. We also did not include indirect nonmedical costs. These costs include years of labor lost attributable to the disease or its treatment as well. In this study, we did not include the direct nonmedical and indirect costs because an accurate measurement of these costs is limited due to the absence of data in published reports. A large randomized trial comparing cord blood to haploidentical HCT is currently ongoing (BMT-CTN 1101 CEA), which has a formal cost-effectiveness substudy built into the protocol to evaluate the most important determinants of both direct and indirect costs. This study is also restricted to 6-month treatment cost. As well known, cGVHD requires systemic immunosuppressive treatment for a median duration of 2 to 3 years depending on the site of the involvement. However, most of the studies have a short follow-up and did not include the proportions of patients still receiving treatment.
Another limitation of our study is the rapidly evolving literature on the effectiveness of agents for SR-cGVHD and our strict inclusion criteria. Based on our inclusion criteria, at the time of the electronic search, only 1 study existed for 4 drugs—pomalidomide, ruxolitinib, ibrutinib, and bortezomib. As new studies are published, the response rates based on meta-analysis for these agents may change, which will likely result in a change of CEA for each treatment. As an example, a recent study exploring the role of ibrutinib in SR-cGVHD demonstrated an ORR of 67% (28 of 42 patients) [70]. Based on this finding, the U.S. Food and Drug Administration approved ibrutinib for the treatment of adult patients with cGVHD after failure of 1 or more lines of systemic therapy. This is the first Food and Drug Administration–approved therapy for the treatment of cGVHD.
Organ-specific cost rates should be interpreted with caution, especially for some fibrotic manifestations such as contractures, bronchiolitis obliterans, and sicca syndrome, where responses are difficult to achieve. Also it should be kept in mind that, there is a proportion of patients that remain with some deficits but off immunosuppression which also implicates “financial CR.”
In conclusion, this CEA illustrates significant variability in costs associated with various treatment modalities for SR-cGVHD, accompanied by heterogeneous effectiveness results. The lack of clinical trials directly comparing these agents and heterogeneity of trial designs and study populations limit firm conclusions about incremental cost effectiveness. Unfortunately, the majority of the clinical trials being conducted for SR-cGVHD in the current decade are single-arm studies, which makes this pharmacoeconomic analysis important to compare the currently available agents with respect to both their effectiveness and costs for use in the clinic. As patients with cGVHD are treated with several different agents over the course of their disease, attention to economic issues when treating cGVHD can help guide how treatments should be sequenced, knowing that many patients will cycle through the currently available agents. When third-party payers reimburse expensive off-label drugs for cGVHD without sufficient evidence of safety and efficacy, they could be hampering the development of high quality data from randomized clinical trials that could help ensure wise use of resources. Enrollment of patients in clinical trials is encouraged to help advance the field and allow evidence-based treatment decisions.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Patricia J. Erwin, MLS, for conducting professional electronic search for the meta-analysis. Financial disclosure: The authors have nothing to disclose.
Footnotes
Conflict of interest statement: None of the authors declare any relevant conflicts of interest. S.K.H. has received honorarium from Mallinckrodt for educational symposium at the EBMT 2017 meeting.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2018.03.008.
REFERENCES
- 1.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51(6):786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2010;87–105. [PubMed] [Google Scholar]
- 3.Stranges E, Russo A, Friedman B. Statistical Brief #82: Procedures with the Most Rapidly Increasing Hospital Costs, 2004-2007 [Internet]. Available at http://www.hcup-us.ahrq.gov/reports/statbriefs/sb82.jsp. Accessed September 4, 2017.
- 4.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15(5):564–573. [DOI] [PubMed] [Google Scholar]
- 5.Saito AM, Zahrieh D, Cutler C, et al. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone Marrow Transplant. 2007;40(3):209–217. [DOI] [PubMed] [Google Scholar]
- 6.Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Klar N, Weeks JC, Antin JH. Predicting costs of stem-cell transplantation. J Clin Oncol. 2000;18(1):64–71. [DOI] [PubMed] [Google Scholar]
- 8.Svahn BM, Alvin O, Ringden O, Gardulf A, Remberger M. Costs of allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82(2):147–153. [DOI] [PubMed] [Google Scholar]
- 9.Mishra V, Vaaler S, Brinch L. A prospective cost evaluation related to allogeneic haemopoietic stem cell transplantation including pretransplant procedures, transplantation and 1 year follow-up procedures. Bone Marrow Transplant. 2001;28(12):1111–1116. [DOI] [PubMed] [Google Scholar]
- 10.Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera N, Emmert A, Storer BE, Sandmaier BM, Alyea EP, Lee SJ. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. Oncologist. 2014;19(6):639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. [DOI] [PubMed] [Google Scholar]
- 13.Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28(2):121–129. [DOI] [PubMed] [Google Scholar]
- 15.Dignan FL, Amrolia P, Clark A, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012;158(1):46–61. [DOI] [PubMed] [Google Scholar]
- 16.Inamoto Y, Flowers ME. Treatment of chronic graft-versus-host disease in 2011. Curr Opin Hematol. 2011;18(6):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garnett C, Apperley JF, Pavlu J. Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol. 2013;4(6):366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ. New approaches for preventing and treating chronic graft-versus-host disease. Blood. 2005;105(11):4200–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nivison-Smith I, Simpson JM, Dodds AJ, Ma DD, Szer J, Bradstock KF. Relative survival of long-term hematopoietic cell transplant recipients approaches general population rates. Biol Blood Marrow Transplant. 2009;15(10):1323–1330. [DOI] [PubMed] [Google Scholar]
- 20.Jonesn CA, Fernandez L, Mesa OA, Weimersheimer P, Peters C. Burden of cost in chronic graft versus host disease following hematopoietic stem cell transplantation: predictions for the next decade. Value Health. 2015;18(7):A842. [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 22.Jagasia MH, Greinix HT, Arora M, et al. National nstitutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401, e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, Higgins JP, Sterne J, Tugwell P, Reeves BC. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Synth Methods. 2013;4(1):63–77. [DOI] [PubMed] [Google Scholar]
- 25.de Waure C, Capri S, Veneziano MA, et al. Extracorporeal photopheresis for second-line treatment of chronic graft-versus-host diseases: results from a health technology assessment in italy. Value Health. 2015;18(4):457–466. [DOI] [PubMed] [Google Scholar]
- 26.Carnevale-Schianca F, Martin P, Sullivan K, et al. Changing from cyclosporine to tacrolimus as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6(6):613–620. [DOI] [PubMed] [Google Scholar]
- 27.Nagler A, Menachem Y, Ilan Y. Amelioration of steroid-resistant chronic graft-versus-host-mediated liver disease via tacrolimus treatment. J Hematother Stem Cell Res. 2001;10(3):411–417. [DOI] [PubMed] [Google Scholar]
- 28.Johnston LJ, Brown J, Shizuru JA, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(1):47–55. [DOI] [PubMed] [Google Scholar]
- 29.Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M. Therapy of sclerodermatous chronic graft-versus-host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant. 2011;17(5):657–663. [DOI] [PubMed] [Google Scholar]
- 30.Couriel DR, Saliba R, Escalon MP, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005;130(3):409–417. [DOI] [PubMed] [Google Scholar]
- 31.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9(8):505–511. [DOI] [PubMed] [Google Scholar]
- 32.Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, Sanders CJ, van den Tweel JG, Verdonck LF. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood. 2004;104(8):2603–2606. [DOI] [PubMed] [Google Scholar]
- 33.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(3):273–277. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Lee JW, Jung CW, et al. Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study. Haematologica. 2010;95(11):1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teshima T, Nagafuji K, Henzan H, et al. Rituximab for the treatment of corticosteroid-refractory chronic graft-versus-host disease. Int J Hematol. 2009;90(2):253–260. [DOI] [PubMed] [Google Scholar]
- 37.Clavert A, Chevallier P, Guillaume T, et al. Safety and efficacy of rituximab in steroid-refractory chronic GVHD. Bone Marrow Transplant. 2013;48(5):734–736. [DOI] [PubMed] [Google Scholar]
- 38.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilman AL, Chan KW, Mogul A, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6(3A):327–334. [DOI] [PubMed] [Google Scholar]
- 40.Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–718. [DOI] [PubMed] [Google Scholar]
- 41.Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114(3):719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivieri A, Cimminiello M, Corradini P, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood. 2013;122(25):4111–4118. [DOI] [PubMed] [Google Scholar]
- 43.de Masson A, Bouaziz JD, Peffault de Latour R, et al. Limited efficacy and tolerance of imatinib mesylate in steroid-refractory sclerodermatous chronic GVHD. Blood. 2012;120(25):5089–5090. [DOI] [PubMed] [Google Scholar]
- 44.Chen GL, Arai S, Flowers ME, et al. A phase 1 study of imatinib for corticosteroid-dependent/refractory chronic graft-versus-host disease: response does not correlate with anti-PDGFRA antibodies. Blood. 2011;118(15):4070–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baird K, Comis LE, Joe GO, et al. Imatinib mesylate for the treatment of steroid-refractory sclerotic-type cutaneous chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(6):1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pai CC, Chen M, Mirsoian A, et al. Treatment of chronic graft-versus-host disease with bortezomib. Blood. 2014;124(10):1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miklos DB, Arora M, Cutler CS, et al. A multicentral open label phase 1b/2 study of ibrutinib in steroid dependent or refractory chronic graft versus host disease. Chicago, IL: ASCO; 2015. 2015 May 29-June 2. [Google Scholar]
- 48.Bisaccia E, Palangio M, Gonzalez J, Adler KR, Rowley SD, Goldberg SL. Treating refractory chronic graft-versus-host disease with extracorporeal photochemotherapy. Bone Marrow Transplant. 2003;31(4): 291–294. [DOI] [PubMed] [Google Scholar]
- 49.Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107(8):3074–3080. [DOI] [PubMed] [Google Scholar]
- 50.Del Fante C, Scudeller L, Viarengo G, Bernasconi P, Perotti C. Response and survival of patients with chronic graft-versus-host disease treated by extracorporeal photochemotherapy: a retrospective study according to classical and National Institutes of Health classifications. Transfusion. 2012;52(9):2007–2015. [DOI] [PubMed] [Google Scholar]
- 51.Dignan FL, Greenblatt D, Cox M, et al. Efficacy of bimonthly extracorporeal photopheresis in refractory chronic mucocutaneous GVHD. Bone Marrow Transplant. 2012;47(6):824–830. [DOI] [PubMed] [Google Scholar]
- 52.Greinix HT, van Besien K, Elmaagacli AH, et al. Progressive improvement in cutaneous and extracutaneous chronic graft-versus-host disease after a 24-week course of extracorporeal photopheresis–results of a crossover randomized study. Biol Blood Marrow Transplant. 2011;17(12):1775–1782. [DOI] [PubMed] [Google Scholar]
- 53.Ilhan O, Arat M, Arslan O, et al. Extracorporeal photoimmunotherapy for the treatment of steroid refractory progressive chronic graft-versus-host disease. Transfus Apher Sci. 2004;30(3):185–187. [DOI] [PubMed] [Google Scholar]
- 54.Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35(12):1187–1193. [DOI] [PubMed] [Google Scholar]
- 55.Rubegni P, Cuccia A, Sbano P, et al. Role of extracorporeal photochemotherapy in patients with refractory chronic graft-versus-host disease. Br J Haematol. 2005;130(2):271–275. [DOI] [PubMed] [Google Scholar]
- 56.Jagasia MH, Savani BN, Stricklin G, et al. Classic and overlap chronic graft-versus-host disease (cGVHD) is associated with superior outcome after extracorporeal photopheresis (ECP). Biol Blood Marrow Transplant. 2009;15(10):1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertani G, Santoleri L, Ferri U, et al. Response of steroid-refractory chronic graft-versus-host disease to extracorporeal photopheresis correlates with the dose of CD3+ lymphocytes harvested during early treatment cycles. Transfusion. 2016;56(2):505–510. [DOI] [PubMed] [Google Scholar]
- 58.Apisarnthanarax N, Donato M, Korbling M, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31(6):459–465. [DOI] [PubMed] [Google Scholar]
- 59.Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant. 2011;46(3):426–429. [DOI] [PubMed] [Google Scholar]
- 60.Dignan FL, Aguilar S, Scarisbrick JJ, et al. Impact of extracorporeal photopheresis on skin scores and quality of life in patients with steroid-refractory chronic GVHD. Bone Marrow Transplant. 2014;49(5):704–708. [DOI] [PubMed] [Google Scholar]
- 61.Seaton ED, Szydlo RM, Kanfer E, Apperley JF, Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood. 2003;102(4):1217–1223. [DOI] [PubMed] [Google Scholar]
- 62.Hautmann AH, Wolff D, Hahn J, et al. Extracorporeal photopheresis in 62 patients with acute and chronic GVHD: results of treatment with the COBE Spectra System. Bone Marrow Transplant. 2013;48(3):439–445. [DOI] [PubMed] [Google Scholar]
- 63.Pusic I, Rettig MP, DiPersio JF, et al. Phase-1/-2 study of pomalidomide in chronic GvHD. Bone Marrow Transplant. 2016;51(4):612–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Lavallade H, Mohty M, Faucher C, Furst S, El-Cheikh J, Blaise D. Low-dose methotrexate as salvage therapy for refractory graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Haematologica. 2006;91(10):1438–1440. [PubMed] [Google Scholar]
- 65.Giaccone L, Martin P, Carpenter P, et al. Safety and potential efficacy of low-dose methotrexate for treatment of chronic graft-versus-host disease. Bone Marrow Transplant. 2005;36(4):337–341. [DOI] [PubMed] [Google Scholar]
- 66.Huang XJ, Jiang Q, Chen H, et al. Low-dose methotrexate for the treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36(4):343–348. [DOI] [PubMed] [Google Scholar]
- 67.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngamkiatphaisan S, Sriratanaban J, Kamolratanakul P, Intragumtornchai T, Noppakun N, Jongudomsuk P. Cost analysis of hematopoietic stem cell transplantation in adult patients with acute myeloid leukemia at King Chulalongkorn Memorial Hospital. J Med Assoc Thai. 2007;90(12):2565–2573. [PubMed] [Google Scholar]
- 69.Crespo C, Perez-Simon JA, Rodriguez JM, Sierra J, Brosa M. Development of a population-based cost-effectiveness model of chronic graft-versus-host disease in Spain. Clin Ther. 2012;34(8):1774–1787. [DOI] [PubMed] [Google Scholar]
- 70.Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.