Abstract
BACKGROUND
The Hu5F9-G4 (hereafter, 5F9) antibody is a macrophage immune checkpoint inhibitor blocking CD47 that induces tumor-cell phagocytosis. 5F9 synergizes with rituximab to eliminate B-cell non-Hodgkin’s lymphoma cells by enhancing macrophage-mediated antibody-dependent cellular phagocytosis. This combination was evaluated clinically.
METHODS
We conducted a phase 1b study involving patients with relapsed or refractory non-Hodgkin’s lymphoma. Patients may have had diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma. 5F9 (at a priming dose of 1 mg per kilogram of body weight, administered intravenously, with weekly maintenance doses of 10 to 30 mg per kilogram) was given with rituximab to determine safety and efficacy and to suggest a phase 2 dose.
RESULTS
A total of 22 patients (15 with DLBCL and 7 with follicular lymphoma) were enrolled. Patients had received a median of 4 (range, 2 to 10) previous therapies, and 95% of the patients had disease that was refractory to rituximab. Adverse events were predominantly of grade 1 or 2. The most common adverse events were anemia and infusion-related reactions. Anemia (an expected on-target effect) was mitigated by the strategy of 5F9 prime and maintenance dosing. Dose-limiting side effects were rare. A selected phase 2 dose of 30 mg of 5F9 per kilogram led to an approximate 100% CD47-receptor occupancy on circulating white and red cells. A total of 50% of the patients had an objective (i.e., complete or partial) response, with 36% having a complete response. The rates of objective response and complete response were 40% and 33%, respectively, among patients with DLBCL and 71% and 43%, respectively, among those with follicular lymphoma. At a median follow-up of 6.2 months among patients with DLBCL and 8.1 months among those with follicular lymphoma, 91% of the responses were ongoing.
CONCLUSIONS
The macrophage checkpoint inhibitor 5F9 combined with rituximab showed promising activity in patients with aggressive and indolent lymphoma. No clinically significant safety events were observed in this initial study. (Funded by Forty Seven and the Leukemia and Lymphoma Society; ClinicalTrials.gov number, NCT02953509.)
Anti-cd20 antibodies such as rituximab are integral components of treatment regimens for virtually all subtypes of B-cell non-Hodgkin’s lymphoma.1 Once these lymphomas become refractory to standard antibody- or chemotherapy-based therapies, the prognosis is poor. The median overall survival among patients with diffuse large B-cell lymphoma (DLBCL) that is refractory to rituximab-containing regimens is approximately 6 months.2 Patients with follicular lymphoma who have progression that occurs less than 2 years after diagnosis or whose disease is refractory to combination regimens with rituximab also have shortened survival.3-5 New therapies are needed to augment the activity of anti-CD20 antibodies, especially in patients with refractory disease.
CD47 is a “do not eat me,” antiphagocytic signal that is overexpressed by virtually all cancers to enable the immune evasion of macrophages and other phagocytes.6 CD47 overexpression is an independent predictor of a poor prognosis in patients with various cancer types, including lymphoma.7 Anti-CD47 antibodies can induce phagocytosis of tumor cells by the blockade of CD47 and its ligand SIRPα.8 In addition, anti-CD47 antibodies induce an antitumor T-cell response by the cross-presentation of tumor antigens by phagocytes to T cells.9,10 Hu5F9-G4 (hereafter, 5F9) is a humanized, IgG4 isotype, CD47-blocking monoclonal antibody.10 In preclinical xenograft models, 5F9 enabled the phagocytic elimination of multiple lymphoma subtypes in addition to many tumor types.7,8,10-14 CD47 is widely expressed on normal cells; however, 5F9 selectively eliminates malignant cells and not normal cells. Phagocytosis is dependent on unmasking prophagocytic “eat me” signals that are expressed only on tumor cells and not on normal cells (with the exception of aging red cells).7
5F9-mediated phagocytosis is augmented through combinations with tumor-targeting antibodies such as rituximab.7 Rituximab induces complement and natural killer (NK) cell–mediated, antibody-dependent, cellular cytotoxic effects by means of its active Fc effector function. In addition, the Fc region of rituximab provides a potent prophagocytic signal for macrophages by stimulating antibody-dependent cellular phagocytosis (Fig. S1A in the Supplementary Appendix, available with the full text of this article at NEJM.org). The combination of rituximab and CD47 blockade generated synergistic and durable antitumor effects in preclinical models of lymphoma.7,10 We tested the combination of 5F9 and rituximab in a phase 1b study involving patients with relapsed or refractory lymphoma.
METHODS
PATIENTS
Eligible patients had CD20-expressing B-cell lymphoma that had relapsed or that was refractory to at least two previous lines of therapy. Patients also had to have an Eastern Cooperative Oncology Group (ECOG)15 performance-status score of to 2 (on a 5-point scale, with higher numbers indicating greater disability), a hemoglobin level of at least 9.5 g per deciliter, an absolute neutrophil count of at least 1000 per cubic millimeter, and a platelet count of at least 50,000 per cubic millimeter. The complete eligibility criteria are provided in the Supplementary Appendix.
STUDY DESIGN
This phase 1b study had three dose-escalation cohorts. We used a 3+3 design, in which a minimum of 3 patients per cohort were enrolled at every dose level, with the safety profile informing the dose escalation for the next cohort.16 Dose-limiting toxic effects were assessed during the first 28 days. All the patients received a priming dose of 5F9 of 1 mg per kilogram of body weight, administered intravenously, followed 1 week later by the receipt of escalating maintenance doses of 10, 20, or 30 mg per kilogram weekly (Fig. S2 in the Supplementary Appendix). In the cohort of patients who received the dose of 30 mg per kilogram, an additional dose of 30 mg per kilogram was administered on day 11.
In all the cohorts, rituximab was administered intravenously at a dose of 375 mg per square meter of body-surface area, weekly in cycle 1 starting in week 2, and then monthly in cycles 2 through 6. 5F9 was administered until disease progression occurred, a lack of clinical benefit was determined, or an unacceptable level of toxic effects occurred.
The primary objectives were to evaluate safety and to determine the recommended phase 2 dose range of 5F9 in combination with rituximab. Secondary objectives were to evaluate efficacy and the pharmacokinetic and immunogenicity profiles of 5F9.
Adverse events were assessed throughout the study and 30 days after the last dose of study drug according to the Common Terminology Criteria for Adverse Events, version 4.03, of the National Cancer Institute.17 We assessed adverse events that occurred or worsened in intensity or frequency after the initiation of treatment (regardless of causality). A treatment-related adverse event was defined as an adverse event as described above that was related to a study drug (5F9, rituximab, or both) as assessed by the investigator. Patients were evaluated for efficacy every 8 weeks with the use of computed tomography and 18F-fluorodeoxyglucose–position-emission tomography. Investigator-assessed efficacy was evaluated according to the Lugano criteria.18 Bone marrow biopsies were required in order to confirm a complete response if disease involvement was present at screening. Rituximab-refractory status was defined as a lack of response to, or progression during, any previous rituximab-containing regimen (monotherapy or combined with chemotherapy) or progression within 6 months after the last rituximab dose.19
Serum levels of 5F9 were measured with the use of a validated enzyme-linked immunosorbent assay. Antidrug antibody levels were measured by a tiered (screening, confirmatory, and titer) approach with the use of a validated electrochemiluminescence assay. CD47-receptor occupancy on target cells was evaluated with the use of a flow-cytometric assay. The antibody tumor penetrance of 5F9 was detected with the use of an antihuman IgG4 antibody that was measured by immunohistochemical testing.
STUDY OVERSIGHT
The study was approved by the institutional review board at each center and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. All the patients provided written informed consent before study entry. The sponsor (Forty Seven) designed the study with several investigators. The investigators and sites collected the data. The sponsor was responsible for data oversight and analysis. The manuscript was written by the authors, with medical writing assistance with copy editing paid for by the sponsor. All the authors vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol (available at NEJM.org).
STATISTICAL ANALYSIS
All the patients who received at least one dose of 5F9 were included in the safety and efficacy analyses. The objective response rate was defined as the proportion of patients who had either a partial response or a complete response according to the Lugano criteria. The time to response was defined as the time from the receipt of the first dose to the date of the first objective response. The duration of response was defined as the time that the first objective response according to protocol-defined assessment was met until the first date of objectively documented progressive disease or the date of last contact.
RESULTS
PATIENTS
From November 2016 through October 2017, a total of 22 patients with relapsed or refractory DLBCL or follicular lymphoma were enrolled across the three dose cohorts (Fig. S2 in the Supplementary Appendix). Data through April 2018 are presented. The characteristics of the patients at baseline are shown in Table 1. The median age of the patients was 59 years (range, 44 to 82), 21 patients (95%) had an ECOG performance-status score of 0 or 1, and 4 (18%) had undergone autologous stem-cell transplantation previously. The median number of previous lines of therapy was 4 (range, 2 to 10), with 21 patients (95%) having tumors that were refractory to previous treatment with rituximab, and 14 patients (64%) having tumors that were refractory to their most recent treatment regimen. Patients who had DLBCL with diverse molecular phenotypes were enrolled (Table S1 in the Supplementary Appendix).
Table 1.
Characteristics of the 22 Patients Who Were Treated.*
| Characteristic | All Patients (N = 22) |
Patients with DLBCL (N = 15) |
Patients with Follicular Lymphoma (N = 7) |
|---|---|---|---|
| Median age (range) — yr | 59 (44–82) | 60 (44–82) | 59 (44–75) |
| Sex — no. (%) | |||
| Male | 12 (55) | 7 (47) | 5 (71) |
| Female | 10 (45) | 8 (53) | 2 (29) |
| Median no. of previous therapies (range) | 4 (2–10) | 4 (2–10) | 4 (2–9) |
| ECOG performance-status score — no. (%)† | |||
| 0 | 7 (32) | 3 (20) | 4 (57) |
| 1 | 14 (64) | 11 (73) | 3 (43) |
| 2 | 1 (5) | 1 (7) | 0 |
| Lugano stage at diagnosis — no. (%)‡ | |||
| I or II | 4 (18) | 3 (20) | 1 (14) |
| III or IV | 15 (68) | 11 (73) | 4 (57) |
| Unknown | 3 (14) | 1 (7) | 2 (29) |
| Disease refractory to previous rituximab regimen — no. (%) | 21 (95) | 14 (93) | 7 (100) |
| Disease refractory to most recent regimen — no. (%) | 14 (64) | 9 (60) | 5 (71) |
| Previous autologous stem-cell transplantation — no. (%) | 4 (18) | 2 (13) | 2 (29) |
| 5F9 maintenance dose level — no. (%) | |||
| 10 mg/kg | 3 (14) | 2 (13) | 1 (14) |
| 20 mg/kg | 6 (27) | 6 (40) | 0 |
| 30 mg/kg | 13 (59) | 7 (47) | 6 (86) |
A total of 15 patients (68%) in this study of Hu5F9-G4 (5F9) had received a diagnosis of diffuse large B-cell lymphoma (DLBCL) and 7 (32%) had received a diagnosis of follicular lymphoma.
Scores for the Eastern Cooperative Oncology Group (ECOG) performance status are assessed on a 5-point scale, with higher numbers indicating greater disability.
A Lugano stage of I indicates disease involving one lymph node or a group of adjacent nodes, II two or more nodal groups on the same side of the diaphragm, III nodes on both sides of the diaphragm or nodes above the diaphragm with spleen involvement, and IV additional noncontiguous extralymphatic involvement.18
The median duration of treatment was 22 weeks (range, 1.7 to 70.7 and ongoing) (Table S2 in the Supplementary Appendix). All 22 patients received 5F9 and rituximab, although 1 patient could not be evaluated for efficacy owing to study-drug discontinuation because of an adverse event (idiopathic thrombocytopenic purpura) at approximately 2 weeks. In the efficacy assessment, this patient was included in the denominator (as a patient who did not have a response). Overall, 3 patients died, all from disease progression; the all-cause mortality was 14%.
SAFETY
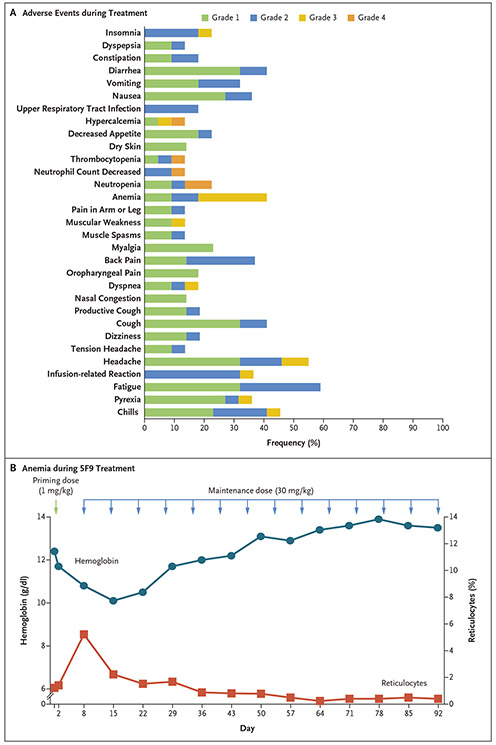
The majority of adverse events during treatment were of grade 1 and 2 (Fig. 1A). The most common treatment-related adverse events were chills (in 9 patients [41%]), headache (in 9 [41%]), anemia (in 9 [41%]), and infusion-related reactions (in 8 [36%]) (Fig. S3A in the Supplementary Appendix). The majority of treatment-related adverse events occurred within the first few weeks (Fig. S3B in the Supplementary Appendix), and no long-term toxic effects were observed. Serious adverse events are shown in Table S3 in the Supplementary Appendix.
Figure 1. Adverse Events Due to Hu5F9-G4 (5F9), Rituximab, or Both and On-Target Anemia Effect of 5F9.
Panel A shows the adverse events that occurred in at least 10% of the patients during treatment. Panel B shows the levels of hemoglobin and reticulocytes over time in a representative patient (with diffuse large B-cell lymphoma) during the study. The priming dose of 1 mg of 5F9 per kilogram of body weight was received on day 1 (green arrow). The patient received maintenance doses of 30 mg of 5F9 per kilogram (blue arrows).
Three dose-limiting toxic effects were observed. In cohort 2, a grade 3 pulmonary embolism was seen. This patient had respiratory symptoms during a 5F9 infusion and was later found to have an occult deep venous thrombosis as a result of vascular compression from lymphoma that was probably the source of the pulmonary embolism. The patient had resolution of the symptoms after receipt of anticoagulation and continued receiving treatment until disease progression several weeks later. This toxic effect led to a cohort expansion to six patients, and no additional dose-limiting toxic effects were observed.
Cohort 3 initially enrolled 6 patients, and no dose-limiting toxic effects were observed. This cohort was expanded to 13 patients in order to collect additional pharmacokinetic and pharmacodynamic data. A total of 2 patients in the expanded cohort had a dose-limiting toxic effect: 1 patient had grade 4 neutropenia, and 1 had grade 3 idiopathic thrombocytopenia purpura. The patient with grade 4 neutropenia had resolution of the event after receipt of granulocyte colony-stimulating factor and continued in the study, with complete resolution of the neutropenia without further growth factor support. The patient with grade 3 idiopathic thrombocytopenic purpura discontinued treatment and received glucocorticoid and intravenous immune globulin treatment, with resolution of the thrombocytopenia.
In cohort 3, the frequency of dose-limiting toxic effects was 15%, which was below the 33% rate that would have exceeded the threshold for the maximum tolerated dose. Thus, no maximum tolerated dose was reached. The 5F9 maintenance dose of 30 mg per kilogram, in combination with rituximab, was established as a recommended phase 2 dose for further study on the basis of the pharmacokinetic data and pharmacodynamic data documenting the saturation of CD47 binding on circulating cells (see below).
Anemia is an expected on-target pharmacodynamic effect of blocking CD47. CD47 blockade can accelerate the elimination of aging red cells and is related to the unmasking of prophagocytic signals on aging red cells (Fig. S1B in the Supplementary Appendix). As red cells age, they lose CD47 expression and gain expression of prophagocytic signals, leading to homeostatic clearance.20 To mitigate this on-target anemia, a priming dose of 1 mg of 5F9 per kilogram was administered in order to eliminate aging red cells selectively while sparing younger red cells, which lack prophagocytic signals10 (Fig. S1B in the Supplementary Appendix). This priming dose led to a predictable and transient mild anemia (given the clearance of old red cells) followed by a compensatory reticulocytosis (generation of new cells) that shifted the overall age of red cells from old to young. Subsequent higher maintenance doses could then be administered, with resolution of the anemia and without recurrence (Fig. 1B, and Fig. S1B in the Supplementary Appendix). This strategy of using priming and maintenance doses substantially mitigated on-target anemia, on the basis of seminal studies of 5F9 in nonhuman primates.10 In support of this observation, the treatment-related anemia that was observed in patients with lymphoma was mainly of grade 1 and 2 and occurred primarily in the first week (Fig. S3A and S3B in the Supplementary Appendix).
Minimal evidence of hemolysis was observed, and there was a mild and transient rise in the indirect bilirubin level that correlated with the initial transient anemia (Fig. S3C in the Supplementary Appendix). A transient decrease in the haptoglobin level below the normal range was observed in 3 of 22 patients (14%) and normalized after the first 2 weeks of treatment. The mean hemoglobin level before treatment was 12.1 g per deciliter. The mean decrease in the hemoglobin level across all 5F9 dose cohorts was 0.9 g per deciliter (maximum decrease, 2.4 g per deciliter). A total of 3 patients underwent red-cell transfusion during the study; 2 patients received only one transfusion (2 units) and the third received four transfusions (8 units). All the transfusions were successfully administered, with the expected increase in the hemoglobin level after the transfusion.
PHARMACOKINETICS, PHARMACODYNAMICS, AND ANTIDRUG ANTIBODIES
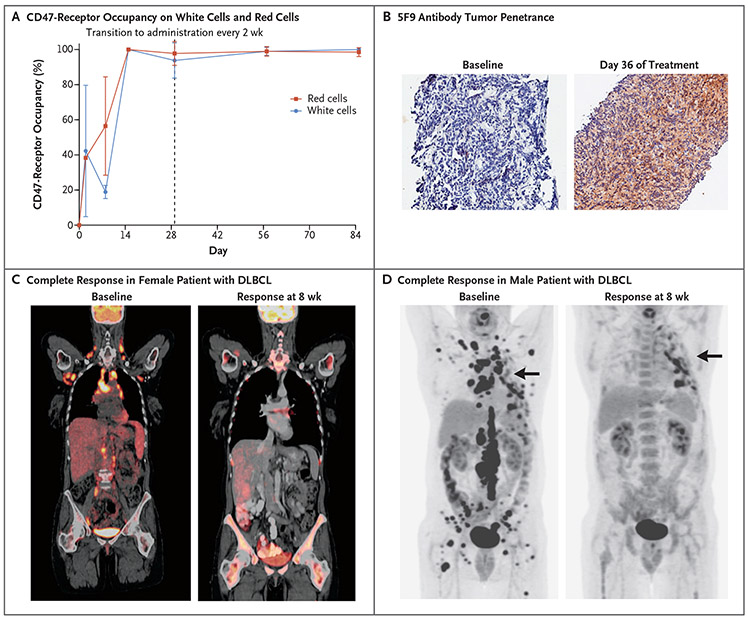
At doses of 10 to 30 mg per kilogram, dose-proportional pharmacokinetic profiles were observed after the fourth maintenance dose, indicating saturation of the CD47 antigen sink (Fig. S4 in the Supplementary Appendix). Once saturation occurred, the apparent terminal half-life of 5F9 was approximately 13 days. Serum samples that were obtained from one patient (5%) at baseline and after the start of 5F9 treatment tested positive for anti-5F9 antibodies. There was no effect on pharmacokinetics in this patient. CD47-receptor occupancy was measured as a pharmacodynamic end point. Given the 5F9 priming dose of 1 mg per kilogram and maintenance doses of 30 mg per kilogram, near 100% CD47-receptor occupancy was observed on circulating red cells and white cells (Fig. 2A). We observed 5F9 antibody tumor penetrance in a patient with DLBCL who was treated with 5F9 (Fig. 2B).
Figure 2. Pharmacodynamic Data on CD47-Receptor Occupancy, Tumor Penetrance, and Responses in Two Patients.
Panel A shows CD47-receptor occupancy on peripheral white cells and red cells. The dashed vertical line at day 29 shows the transition from receipt of the dose weekly to receipt every 2 weeks. Panel B shows 5F9 antibody tumor penetrance in a tumor supraclavicular lymph node in a patient with DLBCL who was treated with 20 mg of 5F9 per kilogram. 5F9 penetrance was measured by immunohistochemical testing with detection by anti-IgG4 staining. Panel C shows positron-emission tomographic–computed tomographic (CT) scans of a 58-year-old woman (Patient 18) with heavily pretreated DLBCL (four previous lines of therapy) who had had rapid disease progression 3 months after undergoing autologous transplantation. The patient had daily fevers before enrollment, which resolved within a few weeks after the initiation of study therapy. The patient had a complete response at 8 weeks, including resolution of bone marrow disease as assessed by means of bone marrow aspirate and biopsy. Panel D shows CT scans of a 56-year-old man (Patient 16) who had primary refractory DLBCL (two previous lines of therapy) with bulky disease. The patient had a complete remission during treatment. Arrows indicate hypermetabolic pleural thickening from previous surgery and not lymphoma.
EFFICACY
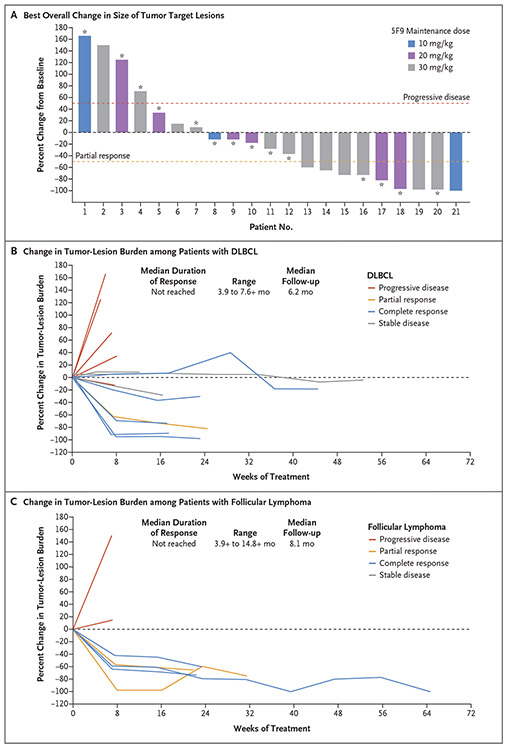
In an intention-to-treat analysis, the objective response rate among all the patients was 50%, with 36% of the patients having a complete response (Table 2 and Fig. 3A). Among patients with DLBCL, the response rate was 40% (6 of 15 patients), with 5 patients (33%) having a complete response. Among patients with follicular lymphoma, the response rate was 71% (5 of 7 patients), with 3 patients (43%) having complete response.
Table 2.
Clinical Responses to Combination Therapy with 5F9 and Rituximab.*
| Response | All Patients (N = 22) |
Patients with DLBCL (N = 15) |
Patients with Follicular Lymphoma (N = 7) |
|---|---|---|---|
| Objective response | 11 (50) | 6 (40) | 5 (71) |
| Complete response | 8 (36) | 5 (33) | 3 (43) |
| Partial response | 3 (14) | 1 (7) | 2 (29) |
| Stable disease | 3 (14) | 3 (20) | 0 |
| Progressive disease | 8 (36) | 6 (40) | 2 (29) |
| Disease control | 14 (64) | 9 (60) | 5 (71) |
Objective response was defined as a complete or partial response. Disease control was defined as a complete response, partial response, or stable disease.
Figure 3. Change in Tumor-Lesion Size and Duration of Responses with 5F9 and Rituximab.
Panel A shows a waterfall plot of the best overall change in the size of tumor target lesions among patients with diffuse large B-cell lymphoma (DLBCL; indicated by an asterisk) or follicular lymphoma, according to the maintenance dose received. Plots of the percentage changes in the tumor burden of target lesions are shown graphically. All the tumor lesions were measured in centimeters. A target lesion size of “too small to measure” was imputed as 0.5 cm2, and “not visible” as 0 cm2. One patient could not be evaluated because of discontinuation of the study before the first protocol-specified response assessment. Thresholds regarding progressive disease and partial response according to the Lugano criteria18 are indicated by dashed lines. Per the Lugano criteria, patients met the criteria for response by either a decrease in the tumor lesion size as assessed by computed tomography (data shown) or a decrease in metabolic activity as assessed by positron-emission tomography (data not shown). Panel B shows spider plots of data from patients with DLBCL, according to response to treatment, and Panel C shows data from patients with follicular lymphoma; the median duration of response and median follow-up are also shown. A plus sign indicates that the response was ongoing at the time of data cutoff. The dashed line at 0 indicates no change from baseline.
The median time to response was 1.7 months (range, 1.6 to 6.6). The median duration of response was not reached among patients with DLBCL or those with follicular lymphoma, at a median follow-up of 6.2 months and 8.1 months, respectively (Fig. 3B and 3C). A total of 10 of 11 patients (91%) with a response had an ongoing response at the time of data cutoff.
One patient with heavily pretreated DLBCL who had had a relapse within 3 months after autologous stem-cell transplantation and who had bulky disease and bone marrow infiltration had a complete response during the study (Fig. 2C). This patient then underwent allogeneic stem-cell transplantation with a matched related donor and continued to have a complete response over a period of 7 months, with the response ongoing at the time of data cutoff.
Two patients with DLBCL had their responses improve over time while they were receiving therapy. One patient had an improvement from stable disease to a complete response at 6 months, and another patient had an improvement from a partial response at 2 months to a complete response at 4 months. Both patients continued to have a complete response at the time of data cutoff. Complete responses were also seen in patients with bulky disease (Fig. 2C and 2D). Responses were also observed across multiple DLBCL subtypes (Table S1 in the Supplementary Appendix).
DISCUSSION
In this phase 1b study, combination therapy with 5F9 plus rituximab was associated with mainly low-grade toxic effects and produced responses in half the patients with relapsed or refractory aggressive and indolent lymphomas. Chemoimmunotherapy is the standard approach for the treatment of B-cell lymphoma but may not be appropriate in patients with relapsed or refractory disease or in patients who cannot receive chemotherapy. Recent biologic insights regarding immune evasion by lymphomas have enabled the development of multiple promising immunotherapeutic strategies.
5F9 is an anti-CD47 antibody that inhibits a key macrophage checkpoint and facilitates macrophage destruction of lymphoma cells. When the drug is administered with a tumor-targeting antibody such as rituximab, a synergistic enhancement of “eat me” signals promotes disease elimination. The safety profile of 5F9 plus rituximab showed no unacceptable side effects, and the maximum tolerated dose was not reached. Most of the adverse events occurred within the first few weeks of treatment, and no late toxic effects were observed (Fig. S3B in the Supplementary Appendix). On-target anemia from CD47 blockade was of low grade and was mitigated considerably because of a priming and maintenance dose regimen of 5F9 owing to the selective clearance of aging red cells, which induces a mild and transient anemia that resolves (Fig. 1B, and Fig. S1B in the Supplementary Appendix). Unlike with T-cell checkpoint inhibitors, minimal immune-mediated adverse events were observed with this treatment. Substantial antitumor activity was observed without the use of chemotherapy in heavily pretreated patients who had rituximab-refractory DLBCL or follicular lymphoma, with complete response rates of 33% and 43%, respectively. The median duration of response was not reached at more than 6 to 8 months of follow-up; longer follow-up is needed. In addition, responses were observed in patients with various DLBCL subtypes, including transformed DLBCL, cell-of-origin subtypes, and double-hit lymphoma (Table S1 in the Supplementary Appendix).
The mechanism of antitumor synergy with 5F9 and rituximab therapy depends largely on macrophage-mediated tumor killing through the blockade of the antiphagocytic CD47 signal by 5F9, combined with rituximab-mediated Fc activation of antibody-dependent cellular phagocytosis.7 This macrophage-mediated mechanism may explain the synergy that has been observed in rituximab-refractory disease in humans. Although a reduction in NK cell–mediated, antibody-dependent, cellular cytotoxic effects is a mechanism of rituximab resistance,21 these clinical data suggest that 5F9 can restore rituximab sensitivity by means of macrophage-mediated, antibody-dependent, cellular phagocytosis. This conclusion is supported by two lines of evidence. First, preclinical data have shown the efficacy of 5F9 plus rituximab therapy in patients with rituximab-resistant disease (Fig. S5 in the Supplementary Appendix). Large-cell lymphoma cells that were resistant to rituximab-dependent phagocytosis were resensitized when rituximab was combined with 5F9. Second, 95% of the patients in our study had rituximab-refractory disease, a fact that minimizes the possibility that rituximab alone induced these responses. Moreover, rituximab monotherapy is associated with a marginal response rate (less than 10 to 15%) in the context of salvage therapy.22 These data suggest that 5F9 plus rituximab can induce antitumor activity in patients with rituximab-resistant disease.
Recently, chemotherapy-free regimens, including monoclonal antibodies, antibody-drug conjugates, and immunotherapies, have shown encouraging activity in patients with lymphoma.23-25 Although T-cell immunotherapies — notably, inhibitors of programmed death 1 (PD-1) and its ligand (PD-L1) — have shown robust activity in patients with solid tumors and those with Hodgkin’s lymphoma, the benefit in patients with non-Hodgkin’s lymphoma has been modest.26 The macrophage-mediated activity of 5F9 plus rituximab may serve as an effective new immunotherapy for stimulating the innate immune system. The ability to induce early responses that remain durable may depend on the ability of 5F9 to induce tumor shrinkage by means of macrophage phagocytosis, followed by activation of the adaptive immune system by tumor antigen cross-presentation from macrophages to T cells.9 The safety profile, ease of administration, and brisk responses that were seen with 5F9 plus rituximab therapy set the stage for larger and longer studies that involve the treatment of patients who have rapidly progressive lymphoma and coexisting conditions.
In conclusion, the new macrophage-activating, anti-CD47 antibody 5F9 in combination with rituximab therapy appeared to be safe and induced durable complete responses in patients with relapsed or refractory DLBCL or follicular lymphoma. Further investigation is ongoing in a phase 2 trial (ClinicalTrials.gov number, NCT02953509).
Supplementary Material
Footnotes
Supported by Forty Seven and the Leukemia and Lymphoma Society.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and families who participated; Wei Dong, Teresa Liu, and Mingqiang Huang for programming; and Karen L. Fink for medical writing assistance with an earlier version of the manuscript.
Contributor Information
Ranjana Advani, Stanford University, Stanford, California
Ian Flinn, Sarah Cannon Research Institute–Tennessee Oncology, Nashville
Leslie Popplewell, City of Hope, Duarte, California
Andres Forero, University of Alabama at Birmingham, Birmingham
Nancy L. Bartlett, Washington University in St. Louis, St. Louis
Nilanjan Ghosh, Levine Cancer Institute–Atrium Health, Charlotte, NC
Justin Kline, University of Chicago, Chicago
Mark Roschewski, National Cancer Institute, Rockville, MD
Ann LaCasce, Dana–Farber Cancer Institute, Boston
Graham P. Collins, University of Oxford, Oxford, United Kingdom
Thu Tran, Stanford University, Stanford, California
Judith Lynn, Forty Seven, Menlo Park, California
James Y. Chen, Forty Seven, Menlo Park, California
Jens-Peter Volkmer, Forty Seven, Menlo Park, California
Balaji Agoram, Forty Seven, Menlo Park, California
Jie Huang, Forty Seven, Menlo Park, California
Ravindra Majeti, Forty Seven, Menlo Park, California
Irving L. Weissman, Stanford University, Stanford, Forty Seven, Menlo Park, California
Chris H. Takimoto, Forty Seven, Menlo Park, California
Mark P. Chao, Forty Seven, Menlo Park, California
Sonali M. Smith, University of Chicago, Chicago
REFERENCES
- 1.Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs 2010;70:1445–76. [DOI] [PubMed] [Google Scholar]
- 2.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol 2015;33:2516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solal-Céligny P, Leconte P, Bardet A, Hernandez J, Troussard X. A retrospective study on the management of patients with rituximab refractory follicular lymphoma. Br J Haematol 2018;180:217–23. [DOI] [PubMed] [Google Scholar]
- 5.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010;142:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng D, Volkmer JP, Willingham SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013;110:11103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Wang L, Zhao F, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 2015;10(9):e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao MP, Alizadeh AA, Tang C, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 2011;71:1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gholamin S, Mitra SS, Feroze AH, et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 2017;9(381):pii:eaaf2968. [DOI] [PubMed] [Google Scholar]
- 14.Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011;118:4890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- 16.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009;101:708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), v4.03 June 14, 2010. (https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol 2016;17:1081–93. [DOI] [PubMed] [Google Scholar]
- 20.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000;288:2051–4. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RP, Lindorfer MA. Antigenic modulation and rituximab resistance. Semin Hematol 2010;47:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czuczman MS, Trněný M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 2017;23:4127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017;377:1331–44. [DOI] [PubMed] [Google Scholar]
- 24.Palanca-Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 2015;16:704–15. [DOI] [PubMed] [Google Scholar]
- 25.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes A, Ansell S, Fowler N, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol 2017;14:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.