Abstract
The cytokine receptor subunit γc provides critical signals for T cell survival and differentiation. We investigated the molecular mechanism that controls the cell surface abundance of γc during T cell development in the thymus. We found that the amount of γc was low on CD4+CD8+ double-positive (DP) thymocytes before their positive selection to become mature T cells. The transcription factor RORγt was abundant in immature DP thymocytes, and its loss resulted in an increase in the abundance of surface γc, specifically on preselection DP cells. Rather than directly repressing expression of the gene encoding γc, RORγt acted through the antiapoptotic protein Bcl-xL to reduce the abundance of surface γc, which resulted in decreased cytokine signaling and was associated with inhibition of cell metabolism and mitochondrial biogenesis. Accordingly, overexpression of Bcl-xL in RORγt-deficient thymocytes restored the amount of surface γc to that present on normal preselection DP cells. Together, these data highlight a previously unappreciated role for RORγt and Bcl-xL in limiting γc abundance at the cell surface and reveal a signaling circuit in which survival factors control cytokine signaling by limiting the abundance and surface distribution of a receptor subunit shared by several cytokines.
INTRODUCTION
T cell development in the thymus is driven by the concerted action of T cell receptor (TCR) and cytokine receptor signaling. Whereas TCR signaling is necessary to select and shape a self–major histocompatibility complex (MHC)–restricted T cell repertoire that is not self-reactive (referred to as “self-MHC–restricted”), cytokine signaling is critical for cell survival and differentiation and for the lineage commitment of thymocytes (1-4). Specifically, cytokines of the common γ chain (γc) family are essential for thymopoiesis and also for the generation of various T cell subsets in the thymus, such as CD8+ T cells, Foxp3+ T regulatory cells (Tregs), and natural killer T (NKT) cells (4, 5). The γc family members are defined as cytokines that bind to receptor complexes containing the gc protein as a subunit, and they include interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 (6). In the absence of γc, T cell development is severely impaired, and thymic Foxp3+ Tregs and NKT cells fail to develop (6, 7). The γc subunit stabilizes and increases the binding of the individual cytokines to their cognate cytokine receptors and initiates downstream signaling by activating the tyrosine kinase Janus kinase 3 (8-10). Despite these critical roles, the regulatory mechanism that controls the γc cytokine receptor during T cell development is poorly understood.
Thymic T cell development proceeds along a well-characterized path that can be mapped by the presence or absence of the cell surface molecules CD4 and CD8 (3). The most immature thymocytes display neither CD4 nor CD8 on their surface and are known as double-negative (DN) cells. DN cells produce an immature TCR containing the β chain without the α chain, and activation of this TCR drives their differentiation into CD4+CD8+ double-positive (DP) thymocytes, which are the first cells to have a functional αβ TCR on the cell surface. In parallel to TCR signaling, IL-7 receptor (IL-7R) signaling enhances survival and promotes proliferation during the progression from DN to DP cells. Mice lacking either the IL-7 proprietary IL-7Rα subunit or the shared γc subunit show markedly diminished thymocyte numbers, and thymocytes are arrested at the DN stage of development (11-13). DP cells with self-MHC–restricted TCR specificities are rescued from programmed cell death through a process called “positive selection,” and signaling by intrathymic γc cytokines in positively selected cells imposes lineage fate and induces the expression of genes encoding lineage-specific transcription factors, such as Runx3 and Foxp3 (14-17). Between the proliferative burst at the DN stage and the positive selection of DP cells, γc receptor signaling must be suppressed. Otherwise, the prosurvival effects of intrathymic γc cytokines could result in the survival of cells with inappropriate TCR specificities and, thus, impede the establishment of a functional TCR repertoire (18).
The abundance of γc at the cell surface is markedly reduced on preselection DP thymocytes, and it is reinduced at the cell surface only upon positive selection (7, 16). This reduction in γc abundance on DP cells is specific to γc because other cytokine receptors, such as IL-4Rα and IL-21R, are found in large amounts on DP thymocytes before positive selection (19-22). Thus, the reduction in γc abundance is a developmentally controlled event, but the molecular basis for such transient loss of γc from developing thymocytes is unclear. Here, we focused our attention on a potential role of the transcription factor RORγt in controlling γc because RORγt abundance inversely correlates with surface γc abundance during thymocyte development (23, 24). We found that RORγt deficiency was associated with a marked increase in surface γc proteins on DP thymocytes. Our data suggest that RORγt acted through the antiapoptotic protein Bcl-xL, rather than through direct repression of the gene encoding γc, to reduce the amount of surface γc proteins. Consistently, overexpression of the prosurvival factor Bcl-xL, which is a downstream effector molecule of RORγt (23, 24), reduced γc abundance in the absence of RORγt. Together, these results reveal a causal relationship between RORγt and reduction in the surface abundance of γc in developing thymocytes that is mediated by Bcl-xL.
RESULTS
RORγt reduces γc abundance on DP thymocytes before their positive selection
Compared to DN thymocytes and both CD4 single-positive (SP) and CD8 SP thymocytes, developmentally immature preselection DP cells display markedly lower amounts of surface γc (Fig. 1A) (7, 16). The molecular basis for decreased γc abundance on DP thymocytes, however, is unclear. Among all populations of thymocytes, the transcription factor RORγt is exclusively present in immature DP cells (fig. S1A) (23, 24). Thus, we hypothesized that RORγt might be responsible for reducing γc abundance on DP thymocytes. To test this idea, we quantitated surface γc on thymocytes from wild-type (WT) mice and RORγt-deficient (RORγtKO) mice by flow cytometry. DP cells with low amounts of TCRβ (TCRβlow) are pre–positive selection thymocytes, and SP cells with high amounts of TCRβ (TCRβhi) correspond to post–positive selection cells. Using the relative amount of TCRβ rather than only relying on surface staining for CD4 and CD8 ensured proper segregation of pre- and post-selection thymocytes for analysis.
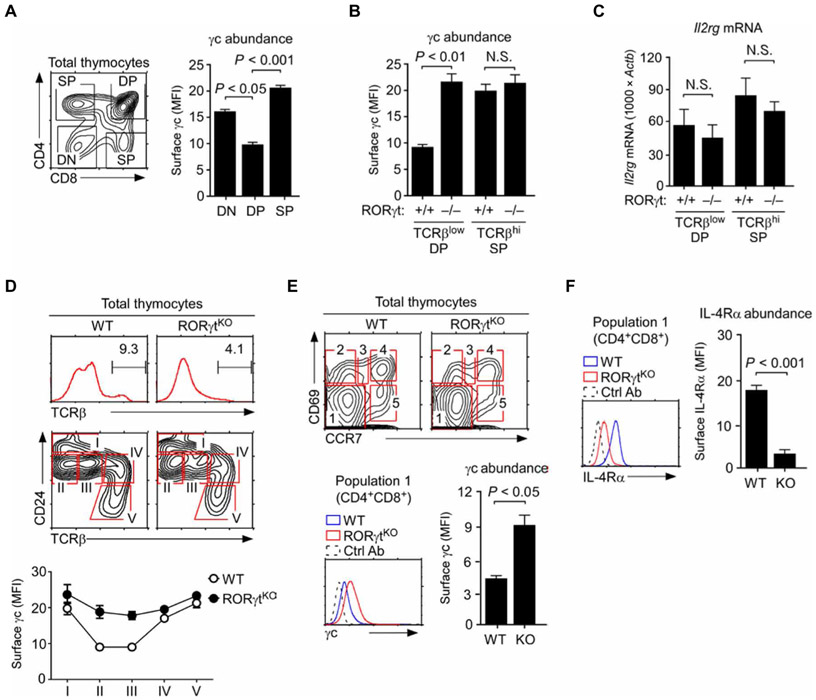
Fig. 1. RORγt deficiency increases the abundance of surface γc on preselection thymocytes.
(A) Surface γc abundance and mean fluorescence intensity (MFI) were assessed in CD4, CD8 DN, DP, and TCRβhi mature CD4 and CD8 SP cells by flow cytometry. Bar graphs show means ± SEM of three independent experiments with a total of six mice. (B) Surface γc abundance (MFI) was determined on TCRβlow preselection DP cells and TCRβhi mature SP cells from WT and RORγtKO mice. Data are means ± SEM of eight independent experiments. (C) Sorted TCRβlow DP and TCRβhi SP cells from WT and RORγtKO thymocytes were assessed for Il2rg mRNA abundance. We performed quantitative real-time polymerase chain reaction (qRT-PCR) analysis with primers specific for the mRNA encoding γc (25), and signals were normalized to that corresponding to Actb. Results show means ± SEM of nine independent experiments. (D) Analysis of the change in surface γc abundance during WT and RORγtKO thymocyte development, as defined by staining for CD24 and TCRβ, followed by flow cytometry. Top and middle: Representative histograms and contour plots. Bottom: Data are means ± SEM of four independent experiments. (E) Surface γc abundance on preselection DP thymocytes as defined by staining for CD69 and CCR7. Top: Representative contour plots. Bottom left: Representative histogram showing γc staining in population 1 cells from the indicated mice. Data are representative of three independent experiments. Bottom right: Data are means ± SEM of three experiments showing the MFI of γc staining on CD4+CD8+ DP cells among population 1 (CD69−CCR7−) cells. Ab, antibody. (F) Flow cytometric analysis of IL-4Rα abundance on the surface of population 1 (CD69−CCR7−) cells from the indicated mice. Left: Representative histograms. Right: Data are means ± SEM of two experiments showing the MFI of IL-4Rα staining on DP cells among population 1 cells. N.S., not significant; KO, knockout.
Here, we found that RORγt was necessary to reduce γc abundance on preselection thymocytes because RORγtKO DP cells showed a substantial increase of surface γc at amounts similar to those observed on mature SP cells of WT mice (Fig. 1B and fig. S1B). Although RORγt is a transcription factor, we found that RORγt did not reduce γc abundance by suppressing the expression of Il2rg mRNA, which encodes γc. Previously, we identified two different species of Il2rg mRNA transcripts that were generated by alternative splicing to produce either a membrane-bound (mγc) or a soluble form (sγc) of γc protein (25). Neither of these γc-encoding transcripts was increased in the absence of RORγt, and the abundance of soluble Il2rg mRNA was rather decreased (Fig. 1C and fig. S1C). These results suggested that RORγt does not suppress Il2rg expression and that RORγt reduces γc surface abundance through posttranscriptional mechanisms.
RORγtKO mice are impaired in thymopoiesis because their thymocytes have a defect in cell survival and are partially blocked in thymocyte maturation (Fig. 1D, top) (23, 24). To assure that the abundance of surface γc was assessed on developmentally comparable thymocyte populations, we first divided WT and RORγtKO thymocytes into five distinct subsets (populations I to V) based on surface staining for CD24 and TCRβ (Fig. 1D and fig. S2A). Note that populations II and III correspond to DP thymocytes (fig. S2A). We confirmed that, in the absence of RORγt, the amount of surface γc was substantially increased in both DP populations (Fig. 1D, bottom). To focus our analysis exclusively on preselection DP thymocytes, we further stained WT and RORγtKO thymocytes for CD69 and CCR7, which are surface molecules induced by TCR signaling (Fig. 1E). Consequently, cells that were negative for both CD69 and CCR7 (CD69−CCR7−; population I) correspond to preselection thymocytes that have not received TCR signals (fig. S2B) (26). Among CD69−CCR7− cells, we gated on CD4+CD8+ DP thymocytes and examined their amount of γc at the surface (Fig. 1E, bottom). We observed a marked increase in surface γc abundance when RORγt was absent (Fig. 1E). This increase was specific to γc because the amount of surface IL-4Rα, which is normally abundant on preselection DP thymocytes (18), was substantially reduced on RORγtKO DP cells (Fig. 1F). This reduction in surface IL-4Rα abundance was associated with markedly decreased expression of Il4ra mRNA, which encodes IL-4Rα (fig. S2C). Thus, RORγt presumably controls the amount of IL-4Rα through transcriptional mechanisms. Together, these results show that the presence of RORγt inversely correlates with the surface abundance of γc proteins and suggest that RORγt limits the amount of γc on thymocytes through a posttranscriptional mechanism.
Enforced RORγt expression fails to reduce γc abundance on mature thymocytes
To directly test whether RORγt could reduce γc abundance, we generated mice expressing a murine RORγt complementary DNA (cDNA) under the control of the proximal Lck promoter (RORγtTg). Transgenic RORγt expression impaired thymocyte development so that the total number of thymocytes was reduced in RORγtTg animals (Fig. 2A). Furthermore, CD4 versus CD8 thymocyte differentiation was altered in RORγtTg mice, such that the mature CD8 SP subpopulation was disproportionally increased among post-selection thymocytes (defined as TCRβhi; Fig. 2A and fig. S3A). The amount of surface γc, however, remained unaffected on RORγtTg thymocytes (Fig. 2B) so that both TCRβlow DP and TCRβhi SP cells displayed the same abundance of γc between WT and RORγtTg mice (Fig. 2B, bottom). Thus, overexpression of RORγt did not further reduce the existing amounts of γc on thymocytes (Fig. 2B, top, and fig. S3, B and C).
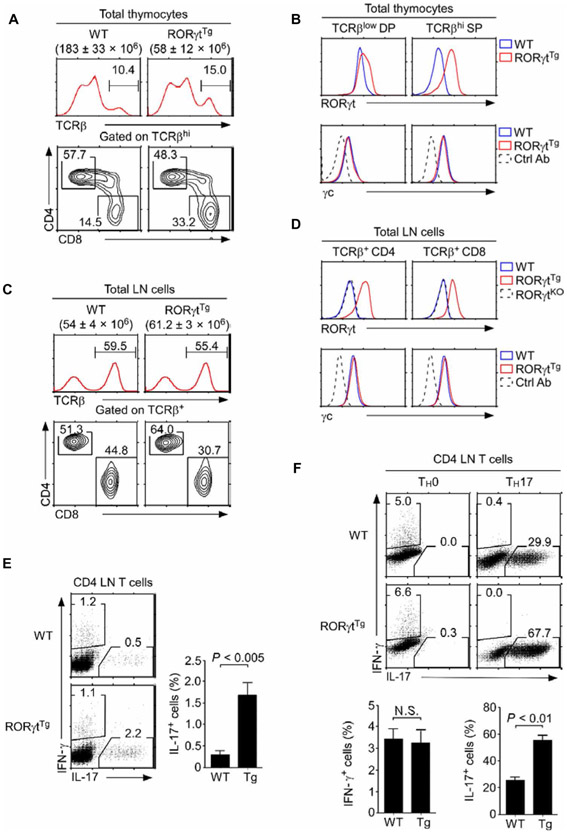
Fig. 2. RORγt overexpression does not reduce γc abundance.
(A) Thymocyte development in RORγtTg mice. Total thymocyte numbers were determined, and CD4, CD8 profiles of TCRβhi cells were assessed for WT and RORγtTg mice by flow cytometry. Cell numbers are means ± SEM, whereas histograms and contour plots are representative of five independent experiments. (B) Intracellular RORγt abundance and surface γc abundance were assessed on TCRβlow preselection DP and TCRβhi mature SP thymocytes of WT and RORγtTg mice by flow cytometry. Histograms are representative of five independent experiments. (C) Total lymph node (LN) cell numbers and CD4, CD8 profiles of TCRβ+ LN cells were determined for WT and RORγtTg mice. Cell numbers are means ± SEM, whereas histograms and contour plots are representative of five independent experiments. (D) Intracellular RORγt and surface γc abundance were assessed on WT, RORγtTg, and RORγtKO LN T cells by flow cytometry. Histograms are representative of five independent experiments. (E) Left: Ex vivo interferon-γ (IFN-γ) and IL-17 production in WT and RORγtTg CD4 LN T cells. Right: Data are means ± SEM of four independent experiments with a total of four mice for each genotype. (F) In vitro differentiation of naïve WT and RORγtTg CD4+ T cells into T helper 17 (TH17) cells. Sorted naïve CD4+ T cells from the indicated mice were cultured for 5 days under TH17-skewing conditions. IFN-γ and IL-17 production were assessed by intracellular staining. Top: Dot plots are representative of two independent experiments. Bottom: Data are means ± SEM of two independent experiments with a total of four mice for each genotype.
In the periphery of RORγtTg mice, we found that RORγt was ectopically expressed in lymph node (LN) T cells. However, the presence of RORγt did not alter the amount of surface γc proteins (Fig. 2, C and D). These results suggest that RORγt alone is insufficient to reduce γc abundance in mature SP thymocytes and peripheral T cells (Fig. 2, B and D). We next confirmed that transgenic RORγt was functionally active. RORγtTg CD4+ T cells showed increased frequencies of IL-17 production ex vivo (Fig. 2E), and they were substantially more effective in generating TH17 cells in vitro than were CD4+ T cells from WT mice (Fig. 2F) (27). Together, these results demonstrate that enforced RORγt expression itself did not suffice to reduce γc abundance on mature thymocytes and T cells.
Thymocytes exhibit an intrinsic requirement for RORγt to control γc abundance
To demonstrate that transgenic RORγt proteins can reduce γc abundance, we introduced the RORγt transgene into germ-line RORγtKO mice to generate RORγtKO/Tg mice. In these mice, RORγt was only expressed in T lineage cells because the transgene is driven by the T lineage–specific proximal Lck promoter (28). Analysis of RORγtKO/Tg thymocytes revealed that T cell–specific RORγt expression restored both thymopoiesis and T cell development in RORγtKO mice (Fig. 3, A and B). In RORγtKO/Tg thymocytes, the percentage of post-selection (TCRβhi) mature thymocytes (Fig. 3A) and the number of total thymocytes (Fig. 3B) were markedly increased and were almost restored to the degree observed in WT mice. Note that RORγt encoded by the transgene was present at similar amounts to that of endogenous RORγt protein in DP cells (Fig. 3C), and transgenic RORγt was sufficient to reduce the abundance of γc in RORγtKO preselection (TCRβlow) DP thymocytes (Fig. 3D). Consequently, the amount of γc on RORytKO/Tg DP cells was comparable to that on WT DP thymocytes. These results indicate a cell-intrinsic role for RORγt in reducing the amount of surface γc on DP cells.
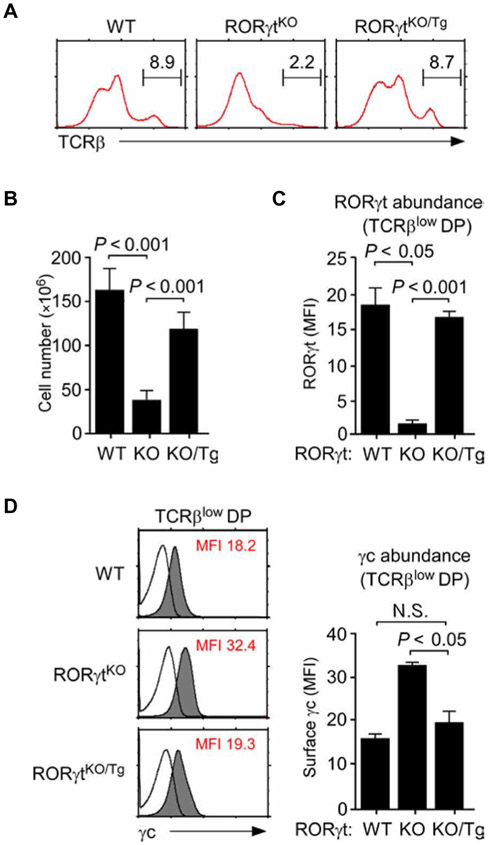
Fig. 3. Transgenic RORγt restores T cell development in RORγtKO mice.
(A and B) Thymocyte development in RORγtKO/Tg mice. Surface TCRβ abundance (A) and total numbers of thymocytes (B) were determined for WT, RORγtKO, and RORγtKO/Tg mice. Data in (A) are representative of three experiments. Data in (B) are means ± SEM of three independent experiments. (C) Quantitation of RORγt protein abundance in TCRβlow preselection DP thymocytes from WT, RORγtKO, and RORγtKO/Tg mice. Data are means ± SEM of four independent experiments. (D) Surface γc abundance on TCRβlow DP cells of WT, RORγtKO, and RORγtKO/Tg mice was assessed by flow cytometry. Left: Histograms are representative of four independent experiments. Right: Data are means ± SEM of four independent experiments with a total of five mice for each genotype.
Bcl-xL acts downstream of RORγt to diminish γc abundance on DP thymocytes
To understand how RORγt could reduce γc abundance, we focused on the downstream effector molecules of RORγt. Immature DP thymocytes are destined to undergo programmed cell death unless they are rescued by positive selection (29). Mcl-1 and Bcl-xL are nonredundant antiapoptotic proteins that maintain DP cell survival (30, 31), and the gene encoding Bcl-xL is a direct target of RORγt (24). As such, RORγt deficiency results in the loss of Bcl-xL and increases apoptosis of preselection DP thymocytes (23, 24). We found that transgenic RORγt markedly increased the abundance of Bcl-xL in RORγtKO/Tg DP thymocytes (fig. S4A) and increased the proportion of TCRβlow preselection DP thymocytes, consistent with their enhanced survival (fig. S4B). Thus, Bcl-xL abundance inversely correlated with the amount of surface γc in preselection thymocytes, suggesting a potential association of the prosurvival effect of RORγt with a decrease in γc abundance. To test whether Bcl-xL was sufficient to reduce γc abundance in immature DP thymocytes, we introduced a Bcl-xL transgene (Bcl-xLTg) into RORγtKO mice to generate RORγtKOBcl-xLTg mice. We found that Bcl-xL overexpression improved the survival of RORγtKO thymocytes (Fig. 4A) and restored the development of preselection thymocytes (Fig. 4B), as indicated by the accumulation of CD69−CCR7− TCR intermediate DP cells, which were reduced in RORγtK° mice (Fig. 4B and fig. S5A).
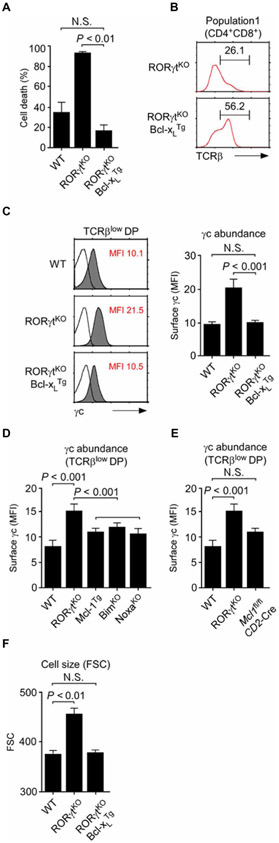
Fig. 4. Transgenic Bcl-xL reduces γc abundance in RORγtKO mice.
(A) Thymocytes of the indicated mice were incubated overnight at 37°C in cell culture medium. Cell viability was determined the next day by assessing propidium iodide (PI) exclusion by flow cytometry. Data are means ± SEM of five independent experiments with a total of five mice for each genotype. (B) TCRβ abundance on CD69−CCR7− (population I) DP thymocytes of RORγtKO and RORγtKOBcl-xLTg mice was assessed by flow cytometry. Histograms are representative of three independent experiments. (C) Surface γc abundance on TCRβlow DP cells of WT, RORγtKO, and RORγtKOBcl-xLTg mice was assessed by flow cytometry. Left: Histograms are representative of five independent experiments. Right: Data are means ± SEM of five independent experiments with a total of eight mice for each genotype. (D) Surface γc abundance on TCRβlow DP thymocytes of the indicated mice was assessed by flow cytometry. Data are means ± SEM of three independent experiments with five WT, four RORγtKO, three Mcl-1Tg four BimKO, and three NoxaKO mice. (E) Surface γc abundance on Mcl-1–deficient TCRβlow DP thymocytes was assessed by gating on human CD4 reporter protein (hCD4)–positive cells in Mcl1fl/flCD2-Cre mice. Data are means ± SEM of three independent experiments with five WT, four RORγtKO, and three Mcl1fl/flCD2-Cre mice. (F) Forward scatter (FSC) signals were determined in thymocytes of the indicated mice as a measure of cell size. Data are means ± SEM of four independent experiments with four mice for each genotype.
To examine whether the increased survival of RORγtKOBcl-xLTg thymocytes involved changes in other pro- or antiapoptotic proteins, we further assessed the mRNA abundance for other prosurvival or proapoptotic proteins in RORγtKO and RORγtKOBcl-xLTg thymocytes. Bcl-2 is found only at very low amounts in immature DP thymocytes (30), and its gene expression was unaffected by the absence or presence of RORγt or the overexpression of Bcl-xL in TCRβlow DP thymocytes (fig. S5B). We also did not find any substantial changes in proapoptotic gene expression when comparing TCRβlow DP thymocytes from WT and RORγtKO mice (fig. S5C). However, transcripts for the antiapoptotic protein Mcl-1 were statistically significantly reduced in RORγtKO cells and then were restored to amounts similar to those in WT cells upon Bcl-xL overexpression (fig. S5B). Thus, these results suggest that Bcl-xL provides antiapoptotic signals both directly and indirectly by positively regulating the expression of another prosurvival gene.
Together with the observation that Bcl-xL can control expression of other molecules, we found that reconstitution of Bcl-xL expression reduced the amount of surface γc on RORγtKO DP thymocytes (Fig. 4C). This Bcl-xL–mediated reduction in γc abundance occurred without suppressing the expression of Il2rg, which encodes γc (fig. S5D). Compared to Il2rg transcripts in RORγtKO DP thymocytes, overexpression of Bcl-xL significantly increased the expression of γc-encoding transcripts in the absence of RORγt (fig. S5D). These results suggest that enforced expression of Bcl-xL was sufficient to replace an RORγt requirement for reducing γc abundance, presumably through posttranscriptional mechanisms.
Bcl-xL is mostly known as an antiapoptotic factor. However, it also plays critical roles in other cellular processes, such as cell metabolism, autophagy, assembly of the NLRP1 inflammasome, and mitochondrial dynamics (32). To discriminate the prosurvival effect of Bcl-xL from its other functions in controlling γc abundance, we investigated whether enforced expression of other antiapoptotic factors or the deletion of proapoptotic molecules could reduce γc abundance. Analysis of γc abundance on preselection DP thymocytes from antiapoptotic Mcl-1 transgenic mice (33) or mice deficient in either of the proapoptotic proteins Bim or Noxa (34, 35) suggested that regulation of γc abundance was independent from the prosurvival effect of Bcl-xL: None of the TCRβlow DP thymocytes from these mice exhibited an increase in γc abundance similar to that observed in the absence of RORγt (Fig. 4D). Moreover, conditional deletion of Mcl1, the gene encoding Mcl-1, at a late stage of T cell development with a CD2-Cre transgene (36) did not increase the amount of surface γc either (Fig. 4E). Using a CD2-Cre transgene that deletes Mcl1 after the DN stage (Mcl1fl/flCD2-Cre) (36), we avoided complications related to the requirement for Mcl-1 in early thymopoiesis (31). Successful deletion of Mcl1 can be monitored on a single-cell basis by assessing the presence of the hCD4 (37). We found no differences in thymocyte populations between cells from WT and Mcl1fl/flCD2-Cre mice (fig. S6A). The abundance of γc between hCD4-positive and hCD4-negative DP thymocytes was also the same (fig. S6B), indicating that the absence of the antiapoptotic protein Mcl-1 did not affect γc abundance. Together, these data suggest that the prosurvival function of Bcl-xL either is not responsible for or is not the only mechanism by which Bcl-xL reduces γc abundance. Therefore, we next examined a role for other cellular changes that were induced by the lack of RORγt and were restored by the Bcl-xLTg. Notably, we found that RORγtKO DP thymocytes were increased in size compared to WT thymocytes and that enforced Bcl-xL expression rescued this phenotype (Fig. 4F). An increase in cell size is an indicator of increased cell metabolism (38), and Bcl-xL reportedly limits bioenergetic and metabolic activity (32). Thus, we hypothesized that a RORγt-controlled Bcl-xL circuit could alter metabolic cues to regulate γc abundance.
Bcl-xL reduces metabolism and mitochondrial dynamics in immature DP thymocytes
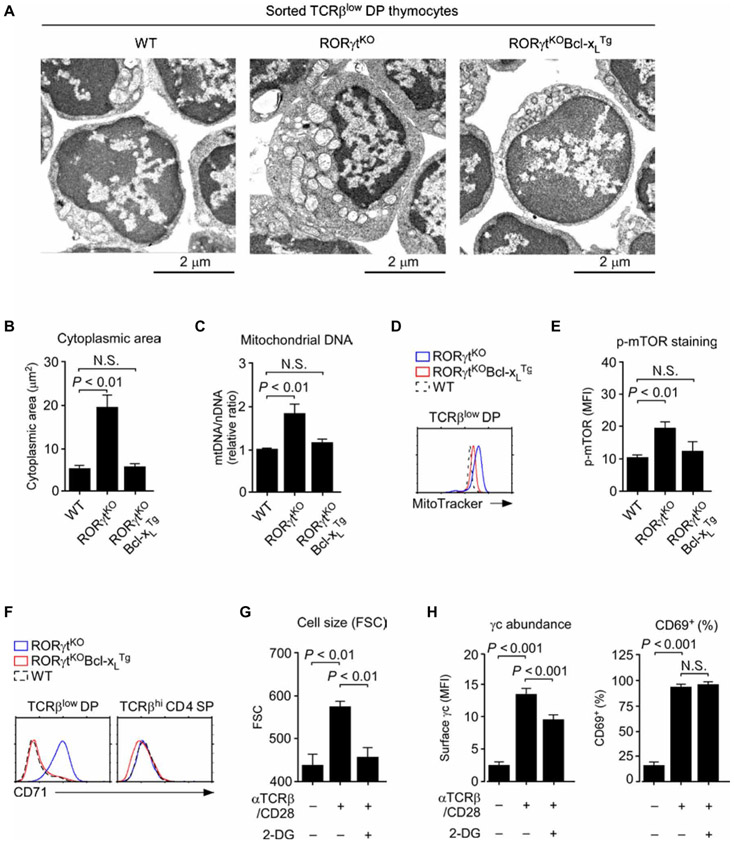
To understand the basis of increased cell size, we used electron microscopy to image TCRβlow DP thymocytes from RORγtKO mice (Fig. 5A). Compared to WT cells, which had a relatively small cytoplasm, RORγtKO cells had substantially increased cytoplasmic areas with abundant mitochondria (Fig. 5, A and B). We assessed the number of mitochondria in two ways. We measured the ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA), which revealed a substantial increase in mitochondrial numbers in preselection DP thymocytes from RORγtKO mice (Fig. 5C). We also quantified mitochondrial mass by MitoTracker Green staining (39), which showed an increase in mitochondrial staining in RORγtKO cells by flow cytometry (Fig. 5D). Enforced expression of Bcl-xL reverted these phenotypes (Fig. 5, A to D), suggesting that the lack of Bcl-xL in RORγtKO thymocytes is the molecular basis for increased cell size and increased mitochondrial content in these cells. Thus, these findings suggest that RORγt deficiency in preselection thymocytes results in increased mitochondrial biogenesis, which would be predicted to enhance mitochondrial and cellular metabolic activity.
Fig. 5. RORγt is required to suppress metabolic activity in immature DP thymocytes.
(A) Electron microscopy of sorted TCRβlow DP thymocytes from the indicated mice. Images are representative of at least five analyses per genotype. (B) Cytoplasmic areas were determined from electron microscopy images of TCRβlow DP thymocytes from the indicated mice using the National Institutes of Health (NIH) ImageJ software. Data are means ± SEM of five to nine cells for each genotype. (C) Mitochondrial content was determined as the ratio of mtDNA to nDNA upon total DNA extraction and PCR analysis of sorted TCRβlow DP thymocytes from the indicated mice. Data are means ± SEM of two independent experiments. (D) Histogram shows representative MitoTracker Green staining of TCRβlow DP thymocytes from the indicated mice. Data are representative of four independent experiments. (E) Analysis of the amount of intracellular phosphorylated mammalian target of rapamycin (p-mTOR) in TCRβlow DP thymocytes from the indicated mice. Data are means ± SEM of four independent experiments. (F) Flow cytometric analysis of cell surface CD71 abundance on TCRβlow DP (left) and mature TCRβhi CD4 SP (right) thymocytes from the indicated mice. Histograms are representative of three independent experiments. (G) FSC signals were used to determine cell size for WT CD4 SP thymocytes stimulated with plate-bound antibodies against TCR and CD28 (αTCRβ/CD28; each at 1 μg/ml) in the presence or absence of 10 mM 2-deoxy-d-glucose (2-DG). Data are means ± SEM of three independent experiments. (H) Cell surface γc (left) and CD69 (right) abundance was determined on WT CD4 SP thymocytes stimulated with plate-bound antibodies against TCR and CD28 (each at 1 μg/ml) in the presence or absence of 10 mM 2-DG. Data are means ± SEM of three independent experiments.
We next assessed phosphorylation of mTOR as an indicator of increased cellular metabolic activity (40). Compared to WT DP thymocytes, RORγt-deficient thymocytes contained significantly increased amounts of phosphorylated mTOR. We found that enforced Bcl-xL expression suppressed this increase (Fig. 5E). Moreover, the surface abundance of CD71, which is a marker for T cell metabolism and activation (41), was also increased in RORγtKO cells compared to that in WT cells but was restored to normal amounts in RORγtKOBcl-xLTg cells (Fig. 5F). Together, these results reveal a role for Bcl-xL as a suppressor of cell metabolism and mitochondrial biogenesis. Thus, we hypothesized that diminished metabolic activity was a mechanism by which Bcl-xL reduced the cell surface abundance of γc in these cells.
To test this hypothesis, we assessed the effect of the metabolic suppressor 2-DG on γc abundance in mature SP thymocytes. T cell activation by antibodies against TCR and CD28 increases metabolic activity and increases γc abundance in mature T cells (25). The addition of 2-DG during TCR/CD28 stimulation suppressed cell metabolism, as indicated by a reduction in cell size (Fig. 5G), and further impaired the increase in cell surface abundance of γc (Fig. 5H). To exclude the possibility that 2-DG would interfere with TCR signaling, we assessed the increased abundance of the cell surface molecule CD69, a marker of T cell activation (42). T cells stimulated in the presence or absence of 2-DG showed a similar increase in CD69 abundance, indicating that 2-DG did not impair T cell activation (Fig. 5H and fig. S7A). Together, these results suggest a mechanism to increase γc abundance that is associated with increased metabolic activity.
Bcl-xL negatively regulates the cell surface abundance of γc
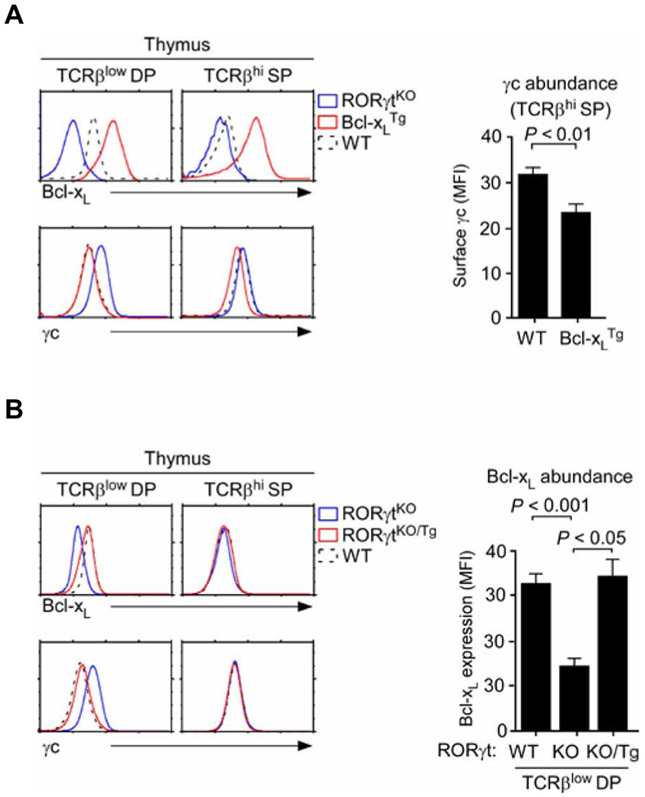
To examine whether Bcl-xL was sufficient to reduce γc abundance, we assessed the relative amount of γc on Bcl-xLTg thymocytes (43). Intracellular staining for Bcl-xL showed an increase in Bcl-xL in both preselection DP and mature SP thymocytes of Bcl-xLTg mice (Fig. 6A, top). Notably, Bcl-xL overexpression was associated with reduced γc abundance even in the absence of RORγt so that mature SP thymocytes of Bcl-xLTg mice had less γc at the cell surface than did WT cells (Fig. 6A, bottom, and fig. S7, B and C). Together, these results place Bcl-xL as an upstream regulator of γc surface abundance in T cells.
Fig. 6. Enforced Bcl-xL expression reduces γc abundance.
(A) Intracellular Bcl-xL and cell surface abundance of γc on thymocytes from RORγtKO, Bcl-xLTg, and WT mice were determined by flow cytometry. Left: Histograms are representative of three independent experiments. Right: Bar graphs are means ± SEM of three independent experiments with a total of three mice for each genotype. (B) Intracellular Bcl-xL and cell surface abundance of γc on thymocytes from RORγtKO, RORγtKO/Tg, and WT mice were determined by flow cytometry. Left: Histograms are representative of three independent experiments. Right: Bar graphs are means ± SEM of three independent experiments with a total of three mice for each genotype.
To understand how overexpression of Bcl-xL (Fig. 6A), but not overexpression of RORγt (Fig. 2B), reduced γc abundance on mature SP thymocytes, we determined the amount of Bcl-xL proteins in RORγtKO/Tg cells. Whereas transgenic RORγt induced an increase in Bcl-xL in TCRβlow DP thymocytes, it did not do so in mature TCRβhi SP thymocytes (Fig. 6B, top and right). Consistent with this lack of an effect on Bcl-xL abundance, the abundance of surface γc on RORγtKO/Tg SP thymocytes was identical to that on WT cells (Fig. 6B, bottom). Thus, because RORγt did not induce an increase in Bcl-xL abundance, it was unable to reduce γc abundance in mature thymocytes. Our results thus suggest that the antiapoptotic protein Bcl-xL is required for RORγt to reduce γc abundance at the surface of developing thymocytes and that this pathway is developmentally limited to preselection DP thymocytes.
DISCUSSION
The molecular mechanisms that regulate the amount of γc proteins on T cells remain poorly understood. Here, we report an unexpected role for the transcription factor RORγt in controlling γc abundance during T cell development. We found that RORγt was necessary to suppress the cell surface expression of γc on immature DP thymocytes and that enforced expression of Bcl-xL was sufficient to replace RORγt in down-regulating the abundance of γc. Because γc signaling increases the expression of genes encoding antiapoptotic factors (6), loss of γc expression results in impaired cell survival. Thus, reducing the abundance of surface γc by RORγt deprives preselection thymocytes of their ability to respond to prosurvival cytokine signals. Consequently, the RORγt-induced decrease in γc abundance is a mechanism that promotes the programmed cell death of preselection thymocytes, unless they are positively selected.
The generation of an immunocompetent TCR repertoire depends on the selective survival of immature thymocytes that have self-MHC–restricted TCR specificities (2, 44). The survival of thymocytes with nonreactive TCR specificities needs to be avoided. Consequently, several mechanisms act in a redundant manner to insulate preselection thymocytes from exposure to indiscriminate prosurvival cytokine signaling. In the mouse thymus, these mechanisms include reductions in the amounts of γc and IL-7Rα proteins (7, 20, 45), an increase in the abundance of suppressor of cytokine signaling 1 (46), and a paucity of IL-7 in the thymic cortex (47, 48). Whereas apoptosis and the removal of nonselected DP thymocytes are essential for thymic selection, it is also important that preselection thymocytes can survive long enough to be tested for their reactivity to self-MHC. In this regard, it is well documented that the premature death of DP thymocytes results in an altered TCR repertoire and impaired T cell development (49). Survival of immature thymocytes depends on Bcl-xL (30), and RORγt is necessary for Bcl-xL production in preselection DP cells (23, 24). Thus, RORγt is a critical factor in T cell development that bridges the survival of immature thymocytes between their entrance into the DP compartment and their exit through positive selection. We consider it not a coincidence that RORγt, which promotes thymocyte survival, also suppresses the surface expression of γc. We propose that RORγt reroutes the survival mechanism from a dependency on extrinsic cytokine signals to a Bcl-xL–mediated, cell-intrinsic survival pathway that is developmentally controlled. According to this scenario, RORγt would suppress surface γc expression to prevent undesired prosurvival cytokine signaling but, at the same time, would compensate for the loss of cytokine signaling by inducing the production of Bcl-xL.
Although RORγt is evidently required to induce Bcl-xL production (23, 24), the mechanistic details of the RORγt-mediated expression of Bcl2l1, which encodes Bcl-xL, remain unclear. Bcl2l1 transcription can be induced and controlled by multiple factors, and at least in DP thymocytes, the transcription factors c-Myb, T cell factor–1 (50), and the enzyme liver kinase B1 are required for its expression (51). Whether RORγt directly regulates Bcl2l1 expression by binding to an RORγt-responsive element in the Bcl2l1 gene or whether it acts indirectly by controlling the expression and action of other factors that induce Bcl2l1 expression has yet to be established (52). The results from our RORγt transgenic T cell study indicate that RORγt alone is not sufficient to increase the abundance of Bcl-xL in mature T cells, although it promoted IL-17 production and the generation of TH17 cells. Thus, in post-selection thymocytes and in mature T cells, Bcl2l1 expression is presumably controlled by distinct mechanisms than in immature thymocytes.
Although RORγt overexpression did not induce Bcl2l1 expression or suppress surface γc expression in mature thymocytes, enforced expression of Bcl2l1 was sufficient for quantitative reduction of γc abundance in the same cells. These results suggest that it is the increase in the abundance of Bcl-xL downstream of RORγt that suppresses γc expression. Moreover, we found that Bcl-xL interfered with γc expression through an unexpected mechanism that involved suppression of metabolic activity and mitochondrial biogenesis. Immature DN thymocytes that undergo β selection receive both activating TCR signals and pro-metabolic Notch signals that drive their differentiation into DP cells (53). Consequently, β-selected thymocytes vigorously proliferate and show highly active metabolism. We found that RORγt expression in DP cells was required to constrain such high metabolic activity and mitochondrial dynamics because RORγt-deficient DP cells displayed features consistent with persistent T cell activation. Therefore, RORγt suppresses metabolism and induces T cell quiescence on freshly selected and proliferating immature thymocytes that enter the DP cell pool. Furthermore, we found that such a role of RORγt could be replaced by Bcl-xL, and our data revealed an underappreciated role of Bcl-xL in suppressing cell bioenergetics and metabolism. Conventionally, Bcl-xL is known as an antiapoptotic protein, which promotes cell survival by protecting the integrity of the mitochondria (54). Consistent with this notion, Bcl-xL is predominantly found in the mitochondrial membrane (55), but it is also present in the endoplasmic reticulum and within the cytosol (56). Outside of the mitochondria, Bcl-xL exerts functions distinct to its role in survival, such as controlling Ca2+ transport, metabolite consumption, and bioenergetics (57). Here, we demonstrated a previously uncharacterized function of Bcl-xL in inhibiting cell metabolism and mitochondrial biogenesis, which suppressed mTOR activation and led to a reduction in cell surface abundance of γc. Thus, these data suggest that increased metabolic activity is the posttranscriptional mechanism that enhances γc surface expression in RORγt-deficient DP thymocytes and that it is metabolic suppression by Bcl-xL that suppresses the increase in γc expression in normal preselection thymocytes.
Although we identified a posttranscriptional mechanism of γc regulation, little is known about the regulatory mechanism of Il2rg mRNA expression. Mapping the Il2rg promoter region suggested a role for GA-binding proteins and the transcription factor Elf-1 in the tissue-specific expression and transcription of γc (58). However, few studies about Il2rg mRNA expression during T cell differentiation have been reported. We previously found a posttranscriptional mechanism that suppresses Il2rg expression upon T cell activation and during T cell development in the thymus (20, 25). We found that Il2rg pre-mRNA transcripts can be alternatively spliced into a new splice isoform that lacks the transmembrane domain and results in the production of sγc at the expense of membrane γc receptors. Thus, alternative splicing into sγc-encoding transcripts is an effective means to reduce the amount of cell surface γc protein. sγc-encoding transcripts are highly enriched in DP thymocytes (20, 25) so that preferential splicing into sγc could potentially serve as a mechanism that suppresses cell surface γc expression on DP cells. On the basis of our observations, we considered a scenario in which RORγt would increase sγc expression and reduce the cell surface abundance of γc; however, we found that lack of RORγt did not affect the abundance of sγc-encoding transcripts in DP thymocytes. Therefore, these results are inconsistent with a role for RORγt in controlling alternative splicing of Il2rg transcripts. Together, our data suggest that the transcription factor RORγt controls the abundance of γc subunits without affecting its gene expression but through regulating the prosurvival factor Bcl-xL, and thus, we document a regulatory circuitry of RORγt, Bcl-xL, and γc expression during T cell development in the thymus.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) mice were purchased from Charles River Laboratories. RORγtKO and Bcl-xLTg mice were previously described (43, 59) and were obtained from the Jackson Laboratory. Bcl-2Tg (60), Mcl-1Tg (33), BimKO (34), and NOXAKO (35) mice were provided by A. Singer [National Cancer Institute (NCI), NIH]. Mcl-1 floxed (Mcl1fl/fl) mice (37) and CD2-Cre transgenic mice (36) were gifts from D. Gray (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) and P. Love (National Institute of Child Health and Human Development, NIH), respectively. The RORγtTg mouse was produced by placing a mouse RORγt-encoding cDNA under the control of the proximal Lck enhancer and promoter and injecting the construct into fertilized B6 oocytes. All animal experiments were approved by the Animal Care and Use Committee of the NCI. Mice were cared for in accordance with NIH guidelines.
Quantitative real-time polymerase chain reaction
TCRβlow DP thymocytes were sorted from thymi of WT, RORγtKO, and RORγtKOBcl-xLTg mice. Total RNA was isolated with a NucleoSpin kit (Clontech) and an RNeasy kit (Qiagen). RNA was reverse-transcribed into cDNA by oligo(dT) priming with the QuantiTect Reverse Transcription kit (Qiagen). qRT-PCR was performed with a QuantStudio 6 Real-Time PCR machine (Applied Biosystems), ABI PRISM 7900HT (Life Technologies), and QuantiTect SYBR Green PCR kits (Qiagen). Primer sequences are as follows: Bcl2, 5′-GGATAACGGAGGCTGGGATGCCT-3′ [forward (F)] and 5′-GGGAAGGCCAGGATTCGA-3′ [reverse (R)]; Bak, 5′-CCGTCCCCTTCTGAACAGC-3′ (F) and 5′-TGTGTCGTAGCGCCGGTT-3′; Bax, 5′-AGGGTTTCATCCAGGATCGA-3′ (F) and 5′-CCACCCGGAAGAAGACCTC-3′ (R); Actb, 5′-GAGAGGGAAATCGTGCGTGA-3′ (F) and 5′-ACATCTGCTGGAAGGTGG-3′ (R); Il4ra, 5 ‘-AAGGAACCCAGGCTGAGCTTC CC-3′ (F) and 5′-AATGATGATGGCCACCAA GGGACT-3 (R); Mcl1, 5′-AGACGGCCTTCCAGGGC-3 (F) and 5′-CCAGTCCCGTTTCGTCCTT-3 (R); membrane Il2rg, 5′-CATGAACCTAGATTCTCCCTGCC-3 (F) and 5′-CCAACCAACAGTACACAAAGATCAG-3′ (R); soluble Il2rg, 5′-CATGAACCTAGATTCTCCCTGCC-3′ (F) and 5′-TGATGGGGGGAATTGGAGIIIIICCTCTAC A-3′ (R).
Flow cytometry
Single-cell suspensions were prepared from the thymus and LNs of mice and then stained with antibodies of the following specificities: CD4 (GK1.5), CD8α (53-6.7), CD62L (MEL-14), CD44 (IM7), CD25 (PC61.5), CD71 (C2), hCD4 (OKT4), CD132 (4G3, TUGm2), CCR7 (4B12), CD69 (H1.2F3), TCRβ (H57-597), RORγt (AFKJS-9), IFN-γ (XMG1.2), and IL-17 (eBio17B7), all from eBioscience; CD24 (M1/69), IL-4Rα (mIL-4R-M1), and p-mTOR (O21-404), all from BD Biosciences; and Bcl-xL (54H6) from Cell Signaling Technology. Cells were analyzed on LSR II, LSRFortessa, or FACSCalibur flow cytometers (BD Biosciences). Dead cells were excluded by forward-light scatter-gating and PI staining. For intracellular cytokine staining, LN T cells were stimulated for 3 hours with phorbol 12-myristate 13-acetate (25 ng/ml) and ionomycin (1 μM) in the presence of brefeldin A (eBioscience). Cells were surface-stained followed by fixation and permeabilization with an IC fixation buffer (eBioscience). RORγt expression was detected using the FoxP3 intracellular staining buffer set according to the manufacturer’s instruction (eBioscience). Intracellular Bcl-2 and Bcl-xL expression was assessed by fixation and permeabilization with the IC Fixation Buffer Kit (eBioscience). For intracellular phosphoprotein staining, cells were fixed using the Foxp3 Transcription Factor Buffer Set (eBioscience) and permeabilized using True-Phos Perm Buffer (BioLegend) according to the manufacturer’s instruction.
mtDNA content analysis
Total DNA from TCRβlow DP thymocytes was extracted using QIAamp DNA Blood Mini kits (Qiagen). The relative expression of the cytochrome c oxidase subunit I (Cox1) gene from the mitochondrial genome and the Ndufv1 gene from the nuclear genome was quantified by qPCR with a QuantiTect SYBR Green PCR kit (Qiagen). The following primers were used for analysis: Cox1, 5′-TGCTAGCCGCAGGCATTAC-3′ (F) and 5′-GGGTGCCCAAAGAATCAGAAC-3′ (R); Ndufv1, 5′-CTTCCCCACTGGCCTCAAG-3′ (F) and 5′-CCAAAACCCAGTGATCCAGC-3′ (R).
Mitochondrial mass analysis
Thymocytes were incubated at a concentration of 2.5 × 106 cells/ml with 1 μM MitoTracker Green dye (Invitrogen) for 20 min at 37°C in serum-free medium. Cells were then washed, stained for additional surface markers, and analyzed by flow cytometry.
Electron microscopy
Transmission electron microscopy analysis was performed by the Electron Microscopy Laboratory of the Frederick National Laboratory for Cancer Research. Briefly, electronically sorted TCRβlow DP thymocytes were fixed with 2% glutaraldehyde and 0.1 M sodium cacodylate. Cell pellets were embedded, sectioned, and carbonized before undergoing imaging on a Hitachi H-7000 Transmission Electron Microscope. Image analysis and quantification were performed with ImageJ software (NIH).
In vitro CD4+ T helper differentiation
Naïve CD4+ T cells were electronically sorted by gating on CD62L+CD44loCD25− cells. Sorted cells were stimulated with plate-bound antibodies against CD3 and CD28 (each at 1 μg/ml) for 5 days, as previously described (61). Cells were cultured for 5 days under nonskewing TH0 conditions (medium alone) or were differentiated into TH17 cells with human TGFβ1 (5 ng/ml; PeproTech), mouse IL-6 (30 ng/ml; BD Biosciences), antibody against mouse IL-4 (10 μg/ml; BD Biosciences), and antibody against mouse IFN-γ (10 μg/ml; BD Biosciences).
Statistical analysis
Data are shown as means ± SEM. Two-tailed Student’s t tests were used to calculate P values for experiments. P values of less than 0.05 were considered to be statistically significant.
Supplementary Material
Fig. S1. Surface abundance of γc on RORγtKO thymocytes.
Fig. S2. Analysis of γc surface abundance on RORγtKO thymocytes.
Fig. S3. Thymocyte development in RORγtTg mice.
Fig. S4. Thymocyte development in RORγtKO and RORγtKO/Tg mice.
Fig. S5. Phenotypic characterization of RORγtKOBcl-xLTg thymocytes.
Fig. S6. Thymocyte development in Mcl1fl/flCD2-Cre mice.
Fig. S7. Surface γc abundance on Bcl-XLTg thymocytes.
Acknowledgments:
We thank A. Singer (NCI, NIH) for critical review of this manuscript and M.-H. Sung (National Institute on Aging, NIH) for verifying that appropriate statistical tests were used to analyze data. We also thank the following investigators for providing experimental mice: P. Love for CD2-Cre transgenes, D. Gray for Mcl1fl/fl mice, and A. Singer for Bcl-2Tg, Mcl-1Tg, BimKO, and NOXAKO mice.
Funding:
This work was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions of this study are available in the paper or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
www.sciencesignaling.org/cgi/content/full/11/545/eaam8939/DC1
REFERENCES AND NOTES
- 1.Gascoigne NRJ, Rybakin V, Acuto O, Brzostek J, TCR signal strength and T cell development. Annu. Rev. Cell Dev. Biol 32, 327–348 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Hogquist KA, Bevan MJ, Positive selection of thymocytes. Annu. Rev. Immunol 13, 93–126 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Singer A, Adoro S, Park J-H, Lineage fate and intense debate: Myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol 8, 788–801 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waickman AT, Park J-Y, Park J-H, The common γ-chain cytokine receptor: Tricks-and-treats for T cells. Cell. Mol. Life Sci 73, 253–269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etzensperger R, Kadakia T, Tai X, Alag A, Guinter TI, Egawa T, Erman B, Singer A, Identification of lineage-specifying cytokines that signal all CD8+-cytotoxic-lineage-fate ‘decisions’ in the thymus. Nat. Immunol 18, 1218–1227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochman Y, Spolski R, Leonard WJ, New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol 9, 480–490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park J-H, Grinberg A, Love P, Feigenbaum L, Erman B, Singer A, Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J. Exp. Med 209, 2263–2276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T, Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266, 1045–1047 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O’Shea JJ, Leonard WJ, Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: Implications for XSCID and XCID. Science 266, 1042–1045 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Lupardus P, LaPorte SL, Garcia KC, Structural biology of shared cytokine receptors. Annu. Rev. Immunol 27, 29–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL, Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89, 1033–1041 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Rodewald H-R, Waskow C, Haller C, Essential requirement for C-KIT and common γ chain in thymocyte development cannot be overruled by enforced expression of Bcl-2. J. Exp. Med 193, 1431–1438 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Freeden-Jeffry U, Solvason N, Howard M, Murray R, The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity 7, 147–154 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY, A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol 6, 1142–1151 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Luckey MA, Kimura MY, Waickman AT, Feigenbaum L, Singer A, Park J-H, The transcription factor ThPOK suppresses Runx3 and imposes CD4+ lineage fate by inducing the SOCS suppressors of cytokine signaling. Nat. Immunol 15, 638–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J-H, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY, Cui Y, Lucas PJ, Gress RE, Kubo M, Hennighausen L, Feigenbaum L, Singer A, Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat. Immunol 11, 257–264 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer DS, Singer A, Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 38, 1116–1128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Park J-H, Doan LL, Erman B, Feigenbaum L, Singer A, Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection. J. Exp. Med 203, 165–175 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luckey MA, Park J-H, γc cytokine signaling: Graduate school in thymic education. Blood 121, 4–6 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Park J-Y, Jo Y, Ko E, Luckey MA, Park YK, Park S-H, Park J-H, Hong C, Soluble γc cytokine receptor suppresses IL-15 signaling and impairs iNKT cell development in the thymus. Sci. Rep 6, 36962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafei M, Rouette A, Brochu S, Vanegas JR, Perreault C, Differential effects of γc cytokines on postselection differentiation of CD8 thymocytes. Blood 121, 107–117 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Yu Q, Erman B, Bhandoola A, Sharrow SO, Singer A, In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J. Exp. Med 197, 475–487 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM, Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. U.S.A 97, 10132–10137 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Unutmaz D, Zou Y-R, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR, Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Hong C, Luckey MA, Ligons DL, Waickman AT, Park JY, Kim GY, Keller HR, Etzensperger R, Tai X, Lazarevic V, Feigenbaum L, Catalfamo M, Walsh STR, Park J-H, Activated T cells secrete an alternatively spliced form of common γ-chain that inhibits cytokine signaling and exacerbates inflammation. Immunity 40, 910–923 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Laethem F, Sarafova SD, Park J-H, Tai X, Pobezinsky L, Guinter TI, Adoro S, Adams A, Sharrow SO, Feigenbaum L, Singer A, Deletion of CD4 and CD8 coreceptors permits generation of αβT cells that recognize antigens independently of the MHC. Immunity 27, 735–750 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Zúñiga LA, Jain R, Haines C, Cua DJ, Th17 cell development: From the cradle to the grave. Immunol. Rev 252, 78–88 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Wildin RS, Garvin AM, Pawar S, Lewis DB, Abraham KM, Forbush KA, Ziegler SF, Allen JM, Perlmutter RM, Developmental regulation of lck gene expression in T lymphocytes. J. Exp. Med 173, 383–393 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surh CD, Sprent J, T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 372, 100–103 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Ma A, Pena JC, Chang B, Margosian E, Davidson L, Alt FW, Thompson CB, Bclx regulates the survival of double-positive thymocytes. Proc. Natl. Acad. Sci. U.S.A 92, 4763–4767 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ, Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671–676 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Michels J, Kepp O, Senovilla L, Lissa D, Castedo M, Kroemer G, Galluzzi L, Functions of BCL-XL at the interface between cell death and metabolism. Int. J. Cell. Biol 2013, 705294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park J-H, Singer A, Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat. Immunol 13, 569–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A, BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A, p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Vacchio MS, Wang L, Bouladoux N, Carpenter AC, Xiong Y, Williams LC, Wohlfert E, Song K-D, Belkaid Y, Love PE, Bosselut R, A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat. Immunol 15, 947–956 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vikstrom I, Carotta S, Lüthje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, Tarlinton DM, Mcl-1 is essential for germinal center formation and B cell memory. Science 330, 1095–1099 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollizzi KN, Waickman AT, Patel CH, Sun IH, Powell JD, Cellular size as a means of tracking mTOR activity and cell fate of CD4+ T cells upon antigen recognition. PLOS ONE 10, e0121710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendergrass W, Wolf N, Poot M, Efficacy of MitoTracker Green™ and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 61, 162–169 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Waickman AT, Powell JD, mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev 249, 43–58 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD, A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J. Immunol 178, 2163–2170 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama WM, Koning F, Kehn PJ, Pereira GM, Stingl G, Coligan JE, Shevach EM, Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J. Immunol 141, 369–376 (1988). [PubMed] [Google Scholar]
- 43.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ, Bcl-XL and Bcl-2 repress a common pathway of cell death. J. Exp. Med 182, 821–828 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K, Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin. Immunol 22, 287–293 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Yu Q, Erman B, Park J-H, Feigenbaum L, Singer A, IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORγt: Impact on thymocyte development. J. Exp. Med 200, 797–803 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong MMW, Cornish AL, Darwiche R, Stanley EG, Purton JF, Godfrey DI, Hilton DJ, Starr R, Alexander WS, Kay TWH, Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity 18, 475–487 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Kim GY, Hong C, Park J-H, Seeing is believing: Illuminating the source of in vivo interleukin-7. Immune Netw. 11, 1–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zamisch M, Moore-Scott B, Su D.-m., Lucas PJ, Manley N, Richie ER, Ontogeny and regulation of IL-7-expressing thymic epithelial cells. J. Immunol 174, 60–67 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He Y-W, Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat. Immunol 3, 469–476 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Yuan J, Crittenden RB, Bender TP, c-Myb promotes the survival of CD4+CD8+ double-positive thymocytes through upregulation of Bcl-xL. J. Immunol 184, 2793–2804 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y, Li H, Liu H, Zheng C, Ji H, Liu X, The serine/threonine kinase LKB1 controls thymocyte survival through regulation of AMPK activation and Bcl-XL expression. Cell Res. 20, 99–108 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Xie H, Huang Z, Wang R, Sun Z, Regulation of thymocyte survival by transcriptional coactivators. Crit. Rev. Immunol 26, 475–486 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Ciofani M, Zúñiga-Pflücker JC, Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat. Immunol 6, 881–888 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Danial NN, Korsmeyer SJ, Cell death: Critical control points. Cell 116, 205–219 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Chen Y.-b., Aon MA, Hsu Y-T, Soane L, Teng X, McCaffery JM, Cheng W-C, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM, Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol 195, 263–276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tagami S, Eguchi Y, Kinoshita M, Takeda M, Tsujimoto Y, A novel protein, RTN-xs, interacts with both Bcl-xL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity. Oncogene 19, 5736–5746 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Williams A, Hayashi T, Wolozny D, Yin B, Su T-C, Betenbaugh MJ, Su T-P, The non-apoptotic action of Bcl-xL: Regulating Ca2+ signaling and bioenergetics at the ER-mitochondrion interface. J. Bioenerg. Biomembr 48, 211–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markiewicz S, Bosselut R, Le Deist F, de Villartay J-P, Hivroz C, Ghysdael J, Fischer A, de Saint Basile G, Tissue-specific activity of the γc chain gene promoter depends upon an Ets binding site and is regulated by GA-binding protein. J. Biol. Chem 271, 14849–14855 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G, In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med 205, 1381–1393 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ, bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67, 879–888 (1991). [DOI] [PubMed] [Google Scholar]
- 61.Waickman AT, Ligons DL, Hwang S, Park J-Y, Lazarevic V, Sato N, Hong C, Park J-H, CD4 effector T cell differentiation is controlled by IL-15 that is expressed and presented in trans. Cytokine 99, 266–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Surface abundance of γc on RORγtKO thymocytes.
Fig. S2. Analysis of γc surface abundance on RORγtKO thymocytes.
Fig. S3. Thymocyte development in RORγtTg mice.
Fig. S4. Thymocyte development in RORγtKO and RORγtKO/Tg mice.
Fig. S5. Phenotypic characterization of RORγtKOBcl-xLTg thymocytes.
Fig. S6. Thymocyte development in Mcl1fl/flCD2-Cre mice.
Fig. S7. Surface γc abundance on Bcl-XLTg thymocytes.