This cross-sectional study investigates demographic data reporting and demographic representation by sex, age, and race/ethnicity in premarketing and postmarketing studies of novel cancer therapeutics.
Key Points
Question
Are the demographic characteristics of patients enrolled in premarketing and postmarketing studies used by the US Food and Drug Administration (FDA) to evaluate the safety and efficacy of novel cancer therapeutics clearly reported and representative of the US cancer population?
Findings
In this cross-sectional study of 77 premarketing studies and 56 postmarketing studies for FDA-approved cancer therapeutics, there was inconsistent reporting of demographic data and underrepresentation of older adults and Black patients in premarketing and postmarketing studies of cancer therapeutics.
Meaning
Premarketing and postmarketing studies used by the FDA to evaluate novel cancer therapeutics may not adequately represent the US cancer population.
Abstract
Importance
Adequate representation of demographic subgroups in premarketing and postmarketing clinical studies is necessary for understanding the safety and efficacy associated with novel cancer therapeutics.
Objective
To characterize and compare the reporting of demographic data and the representation of individuals by sex, age, and race in premarketing and postmarketing studies used by the Food and Drug Administration (FDA) to evaluate novel cancer therapeutics.
Design, Setting, and Participants
In this cross-sectional study, premarketing and postmarketing studies for novel cancer therapeutics approved by the FDA from 2012 through 2016 were identified. Study demographic information was abstracted from publicly available sources, and US cancer population demographic data was abstracted from US Cancer Statistics. Analyses were conducted from February 25 through September 21, 2020.
Main Outcomes and Measures
The percentages of trials reporting sex, age, and race/ethnicity were calculated, and participation to prevalence ratios (PPRs) were calculated by dividing the percentage of study participants in each demographic group by the percentage of the US cancer population in each group. PPRs were constructed for premarketing and postmarketing studies and by cancer type. Underrepresentation was defined as PPR less than 0.8.
Results
From 2012 through 2016, the FDA approved 45 cancer therapeutics. The study sample included 77 premarketing studies and 56 postmarketing studies. Postmarketing studies, compared with premarketing studies, were less likely to report patient sex (42 studies reporting [75.0%] vs 77 studies reporting [100%]; P < .001) and race (27 studies reporting [48.2%] vs 62 studies reporting [80.5%]; P < .001). Women were adequately represented in premarketing studies (mean [SD] PPR, 0.91; 95% CI, 0.90-0.91) and postmarketing studies (mean PPR, 1.00; 95% CI, 1.00-1.01). Although older adults and Black patients were underrepresented in premarketing studies (older adults: mean PPR, 0.73; 95% CI, 0.72-0.74; Black patients: mean PPR, 0.32; 95% CI, 0.31-0.32), these groups continued to be underrepresented in postmarketing studies (older adults: mean PPR, 0.75; 95% CI, 0.75-0.76; Black patients: mean PPR, 0.21; 95% CI, 0.21-0.21).
Conclusions and Relevance
This study found that older adults and Black patients were underrepresented in postmarketing studies of novel cancer therapeutics to a similar degree that they were underrepresented in premarketing studies. These findings suggest that postmarketing studies are not associated with improvements to gaps in demographic representation present at the time of FDA approval.
Introduction
In the United States, the Food and Drug Administration (FDA) is responsible for the approval of novel therapeutics through the evaluation of clinical trials.1 Clinical trials are done to ensure that a therapeutic is safe and efficacious in the population expected to use the therapeutic. Therefore, clinical trial participants should be representative of the patient population.2 However, there are well-documented concerns that women, older adults, and racial/ethnic minority groups are underrepresented in premarketing studies for cancer therapeutics.3,4,5,6 Additionally, some evidence suggests that race/ethnicity data are underreported in cancer clinical trials.3
Accordingly, the FDA has had increased focus on improving demographic representation in clinical trials.7,8 The FDA presented an action plan in 2014 to improve demographic subgroup analysis in the evaluation of new therapeutics, with specific guidelines for improving the quality of demographic data collected in postmarketing surveillance systems.9
Postmarketing studies with diverse patient populations are especially important for understanding the safety and efficacy profile of cancer therapeutics, given that many cancer therapeutics are approved for marketing at earlier stages with less robust evidence through expedited approval pathways; for example, 76% of cancer therapeutics received priority review over that last 3 decades.10,11,12 Because postmarketing studies are meant to address any uncertainties about safety and efficacy at the time of approval and address the well-known underrepresentation of demographic subgroups in premarketing studies, adequate demographic representation in postmarketing studies is particularly important. The FDA has asserted that a more diverse patient population in postmarketing studies will “provide valuable information to better inform providers and the FDA about the safe and effective use of new therapeutics.”13
There are some concerns with postmarketing requirements (PMRs) and postmarketing commitments (PMCs), including that many are not associated with improvements to gaps in the foundation of clinical evidence present at the time of FDA approval.14,15,16 However, to our knowledge, there is no evidence evaluating whether postmarketing studies for cancer therapeutics are associated with improved representation of women, older adults, or racial/ethnic minority groups, compared with premarketing studies. To address these knowledge gaps, we characterized and compared the quality of demographic data reporting and demographic representation of patients enrolled in premarketing studies vs postmarketing studies evaluating novel cancer therapeutics approved by the FDA between 2012 and 2016.
Methods
The Yale Human Investigations Committee office determined that this cross-sectional study was not subject to institutional review board approval or informed consent because the study was conducted using only publicly available study reports and manuscripts. In this study, we identified all novel therapeutics approved by the FDA for an oncologic indication from 2012 through 2016. Using FDA medical review documents, we identified premarketing and postmarketing studies for these therapeutics. Using medical review documents, ClinicalTrials.gov, journal publications, and sponsors’ websites, we analyzed reporting of patient demographic data and compared the demographic composition of these studies with the demographic composition of the 2012 to 2016 US cancer population by indication.
Study Sample
Using the yearly published Center for Drug Evaluation and Research New Molecular Entity Drug and Original Biologic Approvals list, 2 investigators (TV and JJS) identified all novel drugs and biologics approved by the FDA for oncologic indications from 2012 through 2016.17 To ensure that there was enough time for the completion and reporting of results of postmarketing studies, cancer therapeutics approved after 2016 were not included in our sample. Using medical review documents in the Drugs@FDA database,18 we determined whether therapeutics were approved through expedited pathways (ie, priority review, accelerated approval, breakthrough designation, and fast track) or designated as orphan drugs, as well as each therapeutic’s indication according to cancer type. From the Review Strategy section of the medical review documents, we identified all clinical studies that provided some evidence regarding the efficacy of the therapeutic. Two investigators (TV and DS) classified studies as pivotal if they were explicitly described as pivotal or if the studies provided evidence of efficacy or safety that was essential to FDA approval, as noted in the medical review documents. All other trials were classified as nonpivotal. Two investigators (JJS and JDW) identified all PMRs and PMCs outlined in the original approval letters available in the Drugs@FDA database.14,15,16
Among this sample of premarketing and postmarketing studies, we excluded studies that evaluated only the therapeutics’ safety, pharmacokinetics and pharmacodynamics, bioavailability, dose escalation, or drug interactions (eTable 1 in the Supplement). Additionally, Phase 1 studies, extension studies, expanded access studies, and studies that included patients with a different indication or only healthy patients were excluded. All PMRs and PMCs that were nonclinical studies, extension studies, recruiting patients as of July 2020, aggregates of unspecified trials, or for trials listed in more than 1 PMR or PMC were excluded (eTable 1 in the Supplement). Trials that were designated as both premarketing studies (pivotal or nonpivotal) and postmarketing studies (PMR or PMC) were included in our sample only as postmarketing studies.
Demographic Characteristics of Study Patients
To determine the demographic characteristics of patients enrolled in our sample of premarketing studies, we abstracted the number of participants who were women, were older adults (defined as ages 65 years or older), and belonged to racial/ethnic minority groups from medical review documents in the Drugs@FDA database. If demographic information was not available in these documents, we abstracted data from ClinicalTrials.gov, indexed publications from the clinical trial entry on ClinicalTrials.gov, or clinical trials synopses on the study sponsor’s website, in this order. Demographic information in the FDA’s medical review documents, even if available for only a portion of the study sample, superseded demographic information of the entire trial in ClinicalTrials.gov.
For postmarketing studies, we abstracted demographic information from ClinicalTrials.gov, indexed publications of the clinical trial entry on ClinicalTrials.gov, or clinical trials synopses on the study sponsor’s website. Given that many PMRs and PMCs did not have results reported on ClinicalTrials.gov, we also searched the Scopus database for publications (Elsevier) using a previously described approach.14,15,19
Data on the demographic distribution of patients diagnosed with cancer in the United States was abstracted from the 2012 to 2016 US Cancer Statistics data set, which includes cancer registry data from the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) data set.20 This data set includes demographic data for the proportion of US cancer patients who identify as women; older adults; and White, Black, or Asian, by cancer type. Analyses were conducted from February 25 through September 21, 2020.
Statistical Analysis
We calculated the percentage of premarketing and postmarketing studies that reported the proportion of patients who were women and older adults and the proportion by race/ethnicity. To assess whether there was a significant difference in demographic data reporting between premarketing and postmarketing studies, we calculated a χ2 test of independence. Results were considered statistically significant at a P value of < .05. All tests were 2-tailed. Data analyses were conducted from February 25 through September 21, 2020, using Excel version 16.16.27 (Microsoft Corporation).
We aggregated demographic data by cancer type and calculated the proportion of patients who were women and older adults and the proportion by race (ie, White, Black, or Asian). To evaluate the demographic composition of patients in trials relative to the disease population, we constructed the participation to prevalence ratio (PPR), as developed by Poon et al.21 The PPR was calculated by dividing the percentage of a particular population among the study participants (eg, percentage of women among participants in lung cancer trials) by the percentage of the particular population in the disease population (eg, percentage of women among individuals in the US with lung cancer) by cancer type. A PPR from 0.8 to 1.2 indicates adequate representation; a PPR less than 0.8 indicates underrepresentation, and a PPR greater than 1.2 indicates overrepresentation.21 Within each indication, we compared the PPR of premarketing and postmarketing studies by sex, age, and race. We constructed CIs using the CI formula for sample proportions using the total number of study participants for each indication as the denominator.22 Only studies with complete race data were included in the calculation of PPR by race
We were unable to calculate the PPR for basal cell carcinoma or for Asian patients with neuroblastoma because the data were unavailable in US Cancer Statistics. Age data for the population diagnosed with soft tissue sarcoma were taken from SEER.23 We did not calculate the PPR for older adults with neuroblastoma because the incidence of neuroblastoma among older adults is very low.24
Results
Among 45 therapeutics approved by the FDA for an oncologic indication from 2012 through 2016, 31 were drugs and 14 were biologics (Table); 24 therapeutics (53.3%) received accelerated approval, and 35 therapeutics (77.8%) were granted orphan drug status. The therapeutics in our sample were approved for 16 broad indications (eTable 2 in the Supplement). Multiple myeloma and leukemia were the most common indications (Table). We identified 77 premarketing studies (including 42 pivotal studies [54.5%] and 35 nonpivotal studies [45.5%]) and 56 postmarketing studies (described in 45 PMRs [80.4%] and 11 PMCs [19.6%]) for the therapeutics in our sample.
Table. Characteristics of Oncologic Therapeutic Approvals.
| Characteristic | No. (%) (N = 45) |
|---|---|
| Type | |
| Drug | 31 (68.9) |
| Biologic | 14 (31.1) |
| Year | |
| 2012 | 11 (24.4) |
| 2013 | 8 (17.8) |
| 2014 | 8 (17.8) |
| 2015 | 14 (31.1) |
| 2016 | 4 (8.9) |
| Approval pathway | |
| Priority review | 34 (75.6) |
| Accelerated approval | 24 (53.3) |
| Fast track | 24 (53.3) |
| Breakthrough | 18 (40.0) |
| Orphan drug status | 35 (77.8) |
| Indication | |
| Multiple myeloma | 6 (13.3) |
| Leukemia | 6 (13.3) |
| Melanoma | 5 (11.1) |
| Lung cancer | 5 (11.1) |
| Breast cancer | 3 (6.7) |
| Colorectal cancer | 3 (6.7) |
| Non-Hodgkin lymphoma | 3 (6.7) |
| Ovarian cancer | 2 (4.4) |
| Thyroid cancer | 2 (4.4) |
| Basal cell carcinoma | 2 (4.4) |
| Prostate cancer | 2 (4.4) |
| Soft tissue sarcoma (including liposarcoma) | 2 (4.4) |
| Othera | 4 (8.8) |
The indications for the 4 therapeutics are urothelial cancer, neuroblastoma, gastric cancer, and renal cell carcinoma.
Availability of Demographic Data
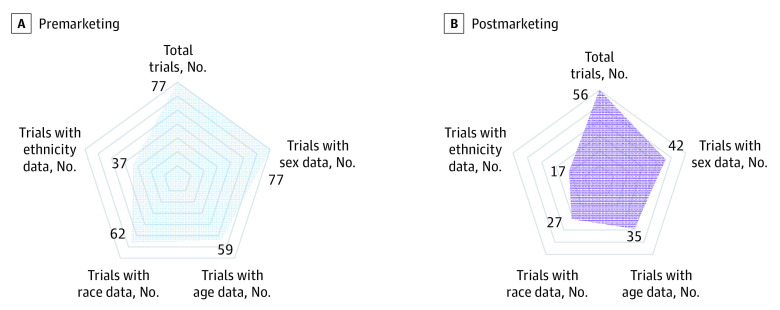
Postmarketing studies, compared with premarketing studies, were less likely to report on patient sex (42 studies reporting [75.0%] vs 77 studies reporting [100%]; P < .001) and race (27 studies reporting [48.2%] vs 62 studies reporting [80.5%]; P < .001) (Figure 1). There was no significant difference between postmarketing and premarketing studies in reporting of the proportion of older adults (35 studies [62.5%] vs 59 studies [76.6%]; P = .08) or the reporting of ethnicity (17 studies [30.4%] vs 37 studies [48.1%]; P = .07). We found that while all premarketing studies reported some demographic data, only 42 postmarketing studies [75.0%] reported some demographic data (eTable 3 in the Supplement).
Figure 1. Availability of Demographic Data.
Demographic Representation in Premarketing and Postmarketing Studies
Sex
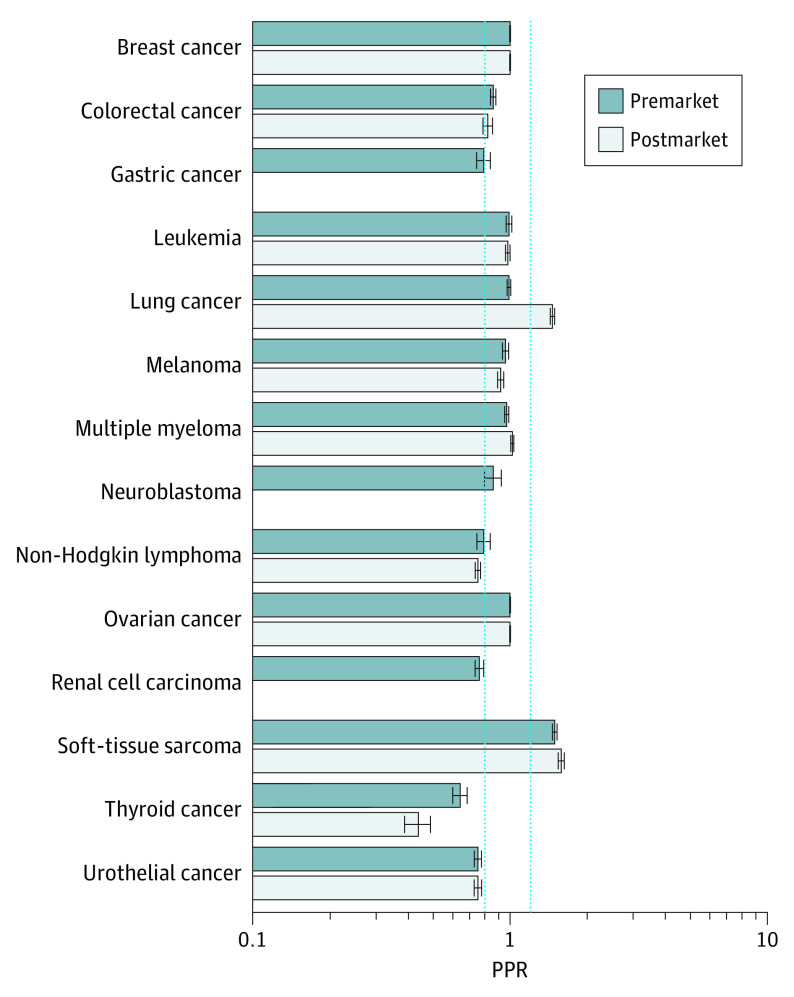
Women were adequately represented in premarketing studies (mean PPR, 0.91; 95% CI, 0.90-0.91) and postmarketing studies (mean PPR, 1.00; 95% CI, 1.00-1.01) across all therapeutics (Figure 2). Of 3 indications in which women were underrepresented in premarketing studies (ie, PPR < 0.8) (renal cell carcinoma: 0.76; 95% CI, 0.73-0.79; thyroid cancer: 0.64; 95% CI, 0.60-0.68; and urothelial cancer: 0.75; 95% CI, 0.72-0.77), women continued to be underrepresented in postmarketing studies for all indications except renal cell carcinoma, for which there were no postmarketing studies (thyroid cancer: 0.44; 95% CI, 0.39-0.49 and urothelial cancer: 0.75; 95% CI, 0.73-0.79) (Figure 2). Study-specific PPRs for women by cancer type can be found in eFigure 1 in the Supplement.
Figure 2. Participation to Prevalence Ratio (PPR) for Women.
The PPR is calculated by dividing the proportion of study patients who are women by the proportion of US cancer patients who are women, by cancer type. A PPR from 0.8 to 1.2 indicates adequate representation, a PPR less than 0.8 indicates underrepresentation, and a PPR greater than 1.2 indicates overrepresentation. Dotted blue lines indicate these boundaries; error bars, CIs. The number of trials included in the PPR by cancer type can be found in eTable 3 in the Supplement. Data for sex were missing for 14 postmarketing studies.
Age
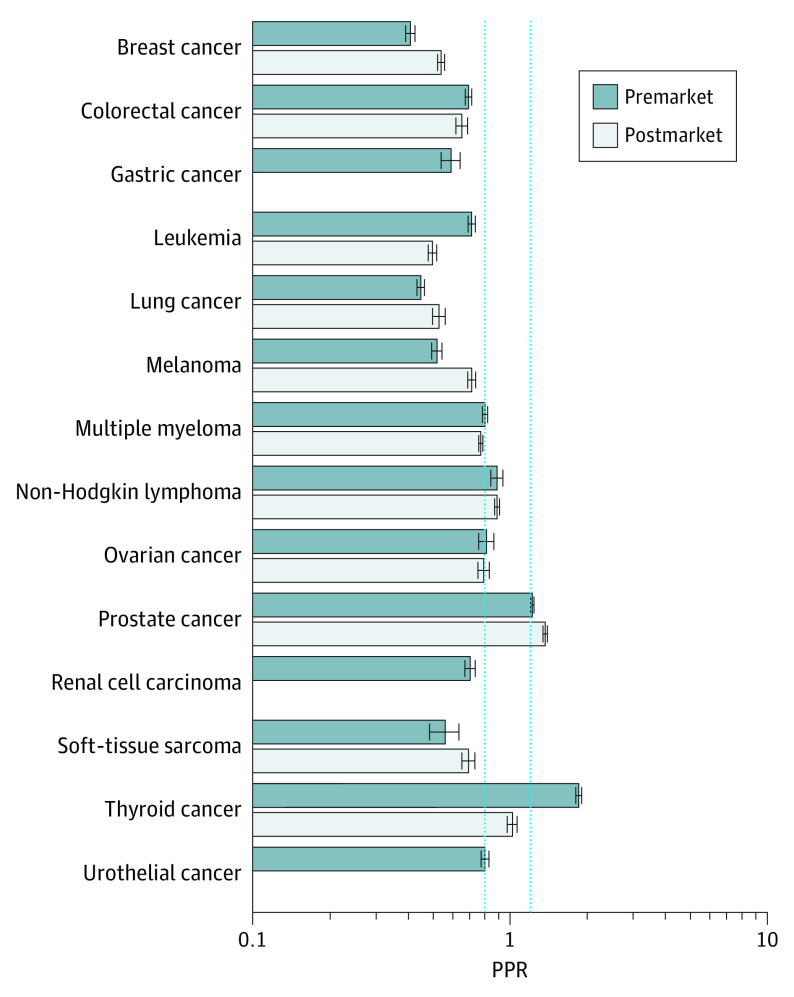
Older adults were underrepresented in premarketing studies (mean PPR, 0.73; 95% CI, 0.72-0.74) and postmarketing studies (mean PPR, 0.75; 95% CI, 0.75-0.76). There were 8 indications in which older adults were underrepresented in premarketing studies according to PPR: breast cancer: 0.41 (95% CI, 0.49-0.43); colorectal cancer: 0.69 (95% CI, 0.67-0.71); gastric cancer: 0.59 (95% CI, 0.54-0.64); leukemia: 0.71 (95% CI, 0.69-0.74); lung cancer: 0.45 (95% CI, 0.43-0.46); melanoma: 0.52 (95% CI, 0.49-0.54); renal cell carcinoma: 0.70 (95% CI, 0.66-0.73); and soft tissue sarcoma: 0.56 (95% CI, 0.49-0.63). Among these, 6 indications had postmarketing studies that reported patient age (Figure 3). These 6 indications continued to have underrepresentation of older adults in postmarketing studies according to PPR: breast cancer: 0.54 (95% CI, 0.52-0.55); colorectal cancer: 0.65 (95% CI, 0.62-0.69); leukemia: 0.45 (95% CI, 0.48-0.52); lung cancer: 0.53 (95% CI, 0.50-0.56); melanoma: 0.71 (95% CI, 0.68-0.73); and soft tissue sarcoma: 0.69 (95% CI, 0.65-0.73). Study-specific PPRs for older adults by cancer type can be found in eFigure 2 in the Supplement.
Figure 3. Participation to Prevalence Ratio (PPR) for Older Adults (Aged ≥65 Years).
The PPR is calculated by dividing the proportion of study patients who are older adults by the proportion of US cancer patients who are older adults, by cancer type. A PPR from 0.8 to 1.2 indicates adequate representation, a PPR less than 0.8 indicates underrepresentation, and a PPR greater than 1.2 indicates overrepresentation. Dotted blue lines indicate these boundaries; error bars, CIs. The number of trials included in the PPR by cancer type can be found in eTable 3 in the Supplement. Data on older adults were missing for 18 premarketing studies and 21 postmarketing studies.
The absolute difference in the proportion of older adults in our sample of studies and the proportion in the US cancer population by indication ranged from 156 of 560 individuals (39.8%) vs 52 243 of 243 109 individuals (21.5%), for a difference of 18.3 percentage points, for thyroid cancer to 1287 of 3947 patients (30.9%) vs 762 725 of 1 104 350 individuals (69.1%), for a difference of −38.2 percentage points, for lung cancer in premarketing studies. It ranged from 690 of 858 individuals (80.4%) vs 555 830 of 946 936 individuals (58.7%) , for a difference of 21.7 percentage points, for prostate cancer to 364 of 1114 individuals (36.6%) vs 762 725 of 1 104 350 individuals (69.1%), for a difference of −32.5 percentage points, for lung cancer in postmarketing studies (eTable 4 in the Supplement).
Race
White patients were adequately represented in premarketing studies (mean PPR, 0.95; 95% CI, 0.94-0.85) and postmarketing studies (mean PPR, 0.92; 95% CI, 0.91-0.93) (Figure 4). Black patients were underrepresented in premarketing studies (mean PPR, 0.32; 95% CI, 0.31-0.32) and postmarketing studies (mean PPR, 0.21; 95% CI, 0.20-0.22). Among 15 indications that had race data, Black patients were underrepresented in premarketing studies for 14 indications according to PPR: breast cancer 0.32 (95% CI, 0.31-0.33); colorectal cancer: 0.19 (95% CI, 0.19-0.20); gastric cancer: 0.17 (95% CI, 0.15-0.19); leukemia: 0.55 (95% CI, 0.53-0.56); lung cancer: 0.11 (95% CI, 0.10-0.11); melanoma: 0.63 (95% CI, 0.63-0.63); multiple myeloma: 0.31 (95% CI, 0.30-0.32); neuroblastoma: 0.59 (95% CI, 0.55-0.62); non-Hodgkin lymphoma: 0.58 (95% CI, 0.56-0.61); ovarian cancer: 0.27 (95% CI, 0.26-0.29); prostate cancer: 0.17 (95% CI, 0.16-0.17); renal cell carcinoma: 0.05 (95% CI, 0.04-0.05); thyroid cancer: 0.23 (95% CI, 0.22-0.24); and urothelial cancer 0.23 (95% CI, 0.21-0.24) (Figure 4). Of these 14 indications, 10 indications had postmarketing studies that reported patient race. All 10 indications continued to have underrepresentation of Black patients in postmarketing studies according to PPR: breast cancer: 0.24 (95% CI, 0.23-0.25); colorectal cancer: 0.15 (95% CI, 0.14-0.16); leukemia: 0.42 (95% CI, 0.41-0.43); lung cancer: 0.07 (95% CI, 0.07-0.08); melanoma: 0; multiple myeloma: 0.12 (95% CI, 0.11-0.12); non-Hodgkin lymphoma: 0.31 (95% CI, 0.30-0.32); ovarian cancer: 0.13 (95% CI, 0.12-0.14); prostate cancer: 0.20 (95% CI, 0.19-0.22); soft tissue sarcoma: 0.23 (95% CI, 0.22-0.25); urothelial cancer: 0.06 (95% CI, 0.06-0.07).
Figure 4. Participation to Prevalence Ratio (PPR) by Race.
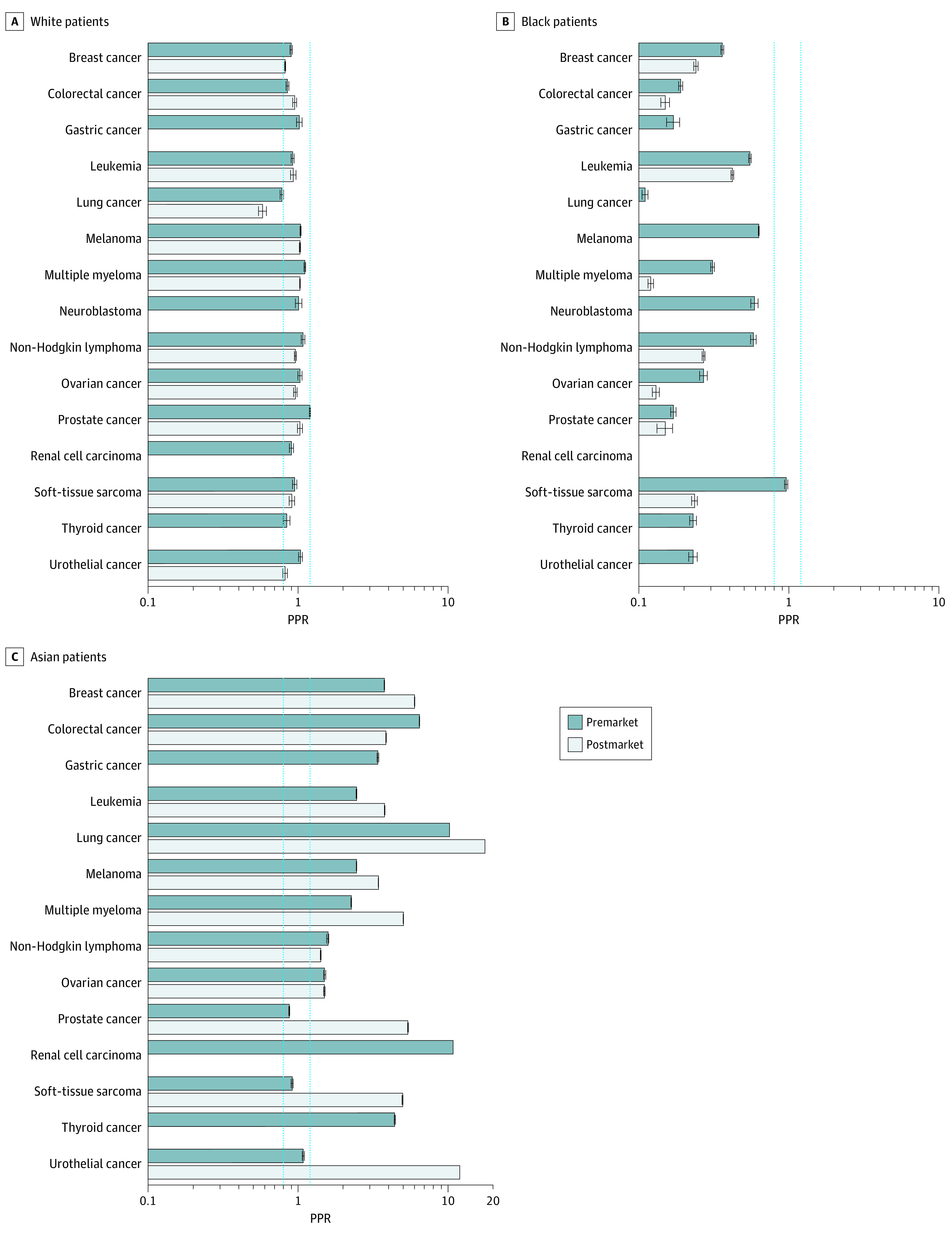
The PPR is calculated by dividing the proportion of study patients who are White, Black, or Asian by the proportion of US cancer patients who are White, Black, or Asian, by cancer type. A PPR from 0.8 to 1.2 indicates adequate representation, a PPR less than 0.8 indicates underrepresentation, and a PPR greater than 1.2 indicates overrepresentation. Dotted blue lines indicate these boundaries; error bars, CIs. The number of trials included in the PPR by cancer type can be found in eTable 3 in the Supplement. Data on race were missing for 15 premarketing studies and 29 postmarketing studies.
The absolute difference in the proportion of Black individuals in our sample of studies and the proportion in the US cancer population by indication ranged from 2 of 662 individuals (0.30%) vs 1875 of 391 393 individuals (0.48%), for a difference of −0.18 percentage points, for melanoma to 164 of 2573 individuals (6.4%) vs 26 077 of 127 402 individuals (20.5%), for a difference of −14.1 percentage points, for multiple myeloma in premarketing studies. It ranged from 0 of 945 individuals (0%) vs 1875 of 371 542 individuals (0.48%), for a difference of −0.48 percentage points, for melanoma to 58 of 2278 individuals (2.6%) vs 26 077 of 95 670 individuals (20.8%), for a difference of −18.2 percentage points for multiple myeloma in postmarketing studies (eTable 4 in the Supplement).
Asian patients were overrepresented in premarketing studies (mean PPR, 3.99; 95% CI, 3.99-4.00) and postmarketing studies (mean PPR, 4.26; 95% CI, 4.26-4.27). In premarketing studies, Asian patients were overrepresented for 11 indications according to PPR: breast cancer: 3.27 (95% CI, 3.26-3.29); colorectal cancer: 6.40 (95% CI, 6.38-6.42); gastric cancer: 3.39 (95% CI, 3.35-3.44); leukemia: 2.44 (95% CI, 2.43-2.46); lung cancer: 10.16 (95% CI, 10.14-10.18); melanoma: 2.44 (95% CI, 2.43-2.44); multiple myeloma: 2.25 (95% CI, 2.24-2.25); non-Hodgkin lymphoma: 1.57 (95% CI, 1.54-1.59); ovarian cancer: 1.50 (95% CI, 1.48-1.53); renal cell carcinoma: 10.72 (95% CI, 10.69-10.74); and thyroid cancer: 4.39 (95% CI, 4.35-4.43) (Figure 4). Of these 11 indications, 8 indications had postmarketing studies that reported patient race. Asian patients were overrepresented in postmarketing studies for all 8 indications according to PPR: breast cancer: 5.95 (95% CI, 5.94-5.96); colorectal cancer: 3.84 (95% CI, 3.83-3.86); leukemia: 3.76 (95% CI, 3.75-3.78); lung cancer: 17.47 (95% CI, 17.43-17.51); melanoma: 3.41 (95% CI, 3.41-3.42); multiple myeloma: 5.00 (95% CI, 4.99-5.01); non-Hodgkin lymphoma: 1.41 (95% CI, 1.41-1.51); ovarian cancer 1.49 (95% CI, 1.48-1.51); prostate cancer: 5.37 (95% CI, 5.33-5.40); soft tissue sarcoma: 4.94 (95% CI, 4.90-4.97); and urothelial cancer: 10.01 (95% CI, 9.98-10.04). Study-specific PPRs by race and by cancer type can be found in eFigure 3 in the Supplement.
Discussion
In this cross-sectional study of 45 cancer therapeutics, we found that postmarketing studies were less likely to report patient sex and race than premarketing studies. We found little evidence that postmarketing studies were associated with improvements to poor demographic representation in premarketing studies. Older adults and Black patients were consistently underrepresented in premarketing and postmarketing studies. Inadequate demographic representation in premarketing and postmarketing studies calls into question the generalizability of cancer clinical trial results to clinical practice as well as the FDA’s ability to encourage or enforce demographic representation in clinical studies.
Older adults were underrepresented in premarketing and postmarketing studies for most cancer indications, suggesting that postmarketing studies are not associated with improvements to the representation of older adults at the time of approval. Given that cancer incidence increases with age, representation of older adults is particularly important.25 There may also be differences in drug response and toxic effects by age.26 Additionally, older adults are more likely to have comorbidities and use medications concomitantly, both of which can be associated with therapeutics’ safety and efficacy outcomes.27 In fact, comorbid conditions and concomitant medications are often exclusion criteria in cancer clinical trials.28,29,30 While the FDA has provided guidelines for increasing enrollment of older adults, our findings suggest that there are further opportunities to improve representation of older adults in cancer clinical trials.31
Our study suggests that Black patients are significantly underrepresented in premarketing and postmarketing studies for cancer therapeutics. Prior work suggests that Black patients are underrepresented in industry-sponsored and federally sponsored cancer clinical trials, although there is some evidence suggesting that representation is improving in federally sponsored trials.3,5,6,32,33 It is worth noting that all trials in our sample were industry-sponsored. Barriers to representation of Black patients in cancer clinical trials include structural barriers (eg, transportation, child care, and access to health care) and a disproportionate burden of comorbidities.34,35,36,37,38,39,40 Furthermore, there is a well-documented and justified mistrust of research institutions among Black patients stemming from a long history of exploitation of Black patients by medical research.41 Structural racism in the US health care system continues to reinforce this mistrust.42 Studies of interpersonal discrimination have found that research and clinical professionals view Black patients as less likely to enroll in clinical research and, therefore, clinicians spend less time discussing clinical trials with Black patients.43,44,45 It is particularly problematic that Black patients are underrepresented in postmarketing studies, given that postmarketing studies evaluate therapeutics for which there is already preliminary evidence suggesting effectiveness. It is worth noting that representation of racial/ethnic minority groups in clinical trials is important not because there is a biological basis for race but because understanding the safety and efficacy of novel therapeutics requires diverse patient populations.
We found that women were adequately represented in premarketing and postmarketing studies. Our findings support current literature that suggests that women are adequately represented in cancer clinical trials.5,6,46 Interestingly, Asian patients were overrepresented in premarketing and postmarketing studies. This may be because several studies in our sample were done in Asian countries. Although our findings do mirror current evidence that Asian patients are adequately represented or overrepresented in clinical trials, there is evidence of barriers to clinical trial participation for Asian patients.3,47,48
The FDA published several guidelines from 2014 to 2020 for improving reporting and participation of women, older patients, and racial and ethnic minority groups in clinical research.8,9,49 However, our findings suggest that translating these guidelines into practice remains a challenge. Several strategies have been proposed to increase the inclusion of older adults and Black patients in cancer clinical trials. In February 2020, the FDA and the American Association of Cancer Research made recommendations to improve Black patient representation in multiple myeloma clinical trials, including designing protocols with explicit targets for representation of racial/ethnic minority groups and specific plans for how these targets will be met.50 Another strategy is to create incentives for manufacturers to test therapeutics in older adults by providing manufacturers with 6-month patent extensions.51,52 This would be similar to the pediatric market exclusivity incentive, which has been associated with increased research among pediatric patients.53
Given that exclusion criteria based on comorbidities or concomitant medication use can disproportionately exclude older adults and Black patients, these criteria should be included only when necessary.54,55 Addressing logistical barriers of clinical trial participation, as well as developing partnerships with community organizations, may also increase participation.2,56,57 Ultimately, the FDA should require demographic representation in premarketing and postmarketing studies that approximates the population expected to use the therapeutic by investing in adequate recruitment a priori to ensure that ensuring demographic representation does not risk delaying study completion for new drugs.
Limitations
This study has a number of limitations. First, several studies were missing demographic data, which undermined our ability to evaluate demographic representation across all trials in our sample. For instance, we were unable to calculate the PPR for Latinx patients. This speaks to the inconsistency of race/ethnicity subgroup reporting in cancer clinical trials, as reported in other studies.3,58 Second, we did not exclude trials conducted outside of the United States, and therefore, not all trial populations in this study demographically represent the US population. This is particularly true for the PPR for Asian patients, given that several premarketing studies were done in Japan. We justify their inclusion in this analysis because these studies were submitted by sponsors to support approval of the therapeutic in the United States. Third, our study did not represent clinical studies for recently approved therapeutics. We selected therapeutics approved from 2012 through 2016 to allow for adequate completion and results reported from postmarketing studies. Fourth, our analysis of demographic data reporting in premarketing and postmarketing studies had a relatively small sample size. We found that there was no significant difference between premarketing and postmarketing studies in reporting of age or ethnicity, despite consistent trends that postmarketing studies reported less demographic data than premarketing studies. Fifth, our study did not consider any genetic factors that may contribute to the racial composition of trial participants. For example, research suggests that the EGFR mutation may be more prevalent in people of East Asian ancestry, which may be associated with the disproportionately high representation of Asian individuals in some lung cancer studies.59,60
Conclusions
In this study, we characterized the representation of individuals by sex, age, and race in 77 premarketing studies and 56 postmarketing studies for 45 novel cancer therapeutics approved by the FDA from 2012 through 2016. We found that postmarketing studies were more likely to underreport demographic data about patient sex and race compared with premarketing studies. Additionally, demographic representation in postmarketing studies was not different from that of premarketing studies, in which older adults and Black patients were significantly underrepresented. Ultimately, these findings suggest that the FDA must do more to improve the transparency of clinical trial demographic data and to ensure that women, older adults, and racial/ethnic minority groups are adequately represented in premarketing and postmarketing studies of novel cancer therapeutics.
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Novel Therapeutics With Oncologic Approvals by Cancer Type (2012-2016)
eTable 3. Availability of Demographic Data by Cancer Type
eTable 4. Absolute Difference in Proportion of Women and Older Adults and Proportion by Race Between Study Sample and Patient Population by Cancer Type
eFigure 1. Participation to Prevalence Ratio (PPR) for Women by Cancer Type
eFigure 2. Participation to Prevalence Ratio (PPR) for Older Adults by Cancer Type
eFigure 3. Participation to Prevalence Ratio (PPR) for Race by Cancer Type
References
- 1.Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-377. doi: 10.1001/jama.2013.282034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol. 2019;44(5):148-172. doi: 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870-e191870. doi: 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen A, Wright H, Itana H, et al. Representation of Women and Minorities in Clinical Trials for New Molecular Entities and Original Therapeutic Biologics Approved by FDA CDER from 2013 to 2015. J Womens Health (Larchmt). 2018;27(4):418-429. doi: 10.1089/jwh.2016.6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downing NS, Shah ND, Neiman JH, Aminawung JA, Krumholz HM, Ross JS. Participation of the elderly, women, and minorities in pivotal trials supporting 2011-2013 U.S. Food and Drug Administration approvals. Trials. 2016;17:199. doi: 10.1186/s13063-016-1322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. Evaluation and reporting of age-, race- and ethnicity-specific data in medical device clinical studies. Accessed July 23, 2020. https://www.fda.gov/media/98686/download
- 8.Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research . Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs: guidance for industry. US Food and Drug Administration. Accessed November 9, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial
- 9.US Food and Drug Administration. FDA Action Plan to Enhance Collection and Availability of Demographic subgroup data. Accessed July 23, 2020. https://www.fda.gov/media/89307/download
- 10.Batta A, Kalra BS, Khirasaria R. Trends in FDA drug approvals over last 2 decades: an observational study. J Family Med Prim Care. 2020;9(1):105-114. doi: 10.4103/jfmpc.jfmpc_578_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesselheim AS, Wang B, Franklin JM, Darrow JJ. Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study. BMJ. 2015;351:h4633. doi: 10.1136/bmj.h4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilal T, Gonzalez-Velez M, Prasad V. Limitations in clinical trials leading to anticancer drug approvals by the US Food and Drug Administration. JAMA Intern Med. 2020;180(8):1108-1115. doi: 10.1001/jamainternmed.2020.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA in brief: FDA affirms commitment to postmarket study requirements; warns company for failure to submit final postmarket study report. US Food and Drug Administration. Accessed October 26, 2020. https://www.fda.gov/news-events/fda-brief/fda-brief-fda-affirms-commitment-postmarket-study-requirements-warns-company-failure-submit-final
- 14.Wallach JD, Egilman AC, Dhruva SS, et al. Postmarket studies required by the US Food and Drug Administration for new drugs and biologics approved between 2009 and 2012: cross sectional analysis. BMJ. 2018;361:k2031. doi: 10.1136/bmj.k2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallach JD, Luxkaranayagam AT, Dhruva SS, Miller JE, Ross JS. Postmarketing commitments for novel drugs and biologics approved by the US Food and Drug Administration: a cross-sectional analysis. BMC Med. 2019;17(1):117. doi: 10.1186/s12916-019-1344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skydel JJ, Zhang AD, Dhruva SS, Ross JS, Wallach JD. U.S.. Food and Drug Administration utilization of postmarketing requirements and postmarketing commitments, 2009-2018. medRxiv. Published online September 2, 2020:2020.08.31.20184184. doi: 10.1101/2020.08.31.20184184 [DOI]
- 17.Compilation of CDER new molecular entity (NME) drug and new biologic approvals. US Food and Drug Administration. Accessed July 15, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/compilation-cder-new-molecular-entity-nme-drug-and-new-biologic-approvals
- 18.Drugs@FDA: FDA-approved drugs. US Food and Drug Administration. Accessed March 26, 2021. https://www.accessdata.fda.gov/scripts/cder/daf/
- 19.Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637. doi: 10.1136/bmj.i637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States cancer statistics. US Centers for Disease Control and Prevention. Accessed July 15, 2020. https://www.cdc.gov/cancer/uscs/index.htm
- 21.Poon R, Khanijow K, Umarjee S, et al. Participation of women and sex analyses in late-phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007-2009. J Womens Health (Larchmt). 2013;22(7):604-616. doi: 10.1089/jwh.2012.3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane DM. Proportion. OnlineStatBook.com. Accessed August 30, 2020. https://onlinestatbook.com/2/estimation/proportion_ci.html
- 23.Centers for Disease Control and Prevention . Cancer stat facts: soft tissue including heart cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed July 21, 2020. https://seer.cancer.gov/statfacts/html/soft.html
- 24.Rogowitz E, Babiker HM, Kanaan M, Millius RA, Ringenberg QS, Bishop M. Neuroblastoma of the elderly, an oncologist’s nightmare: case presentation, literature review and SEER database analysis. Exp Hematol Oncol. 2014;3:20. doi: 10.1186/2162-3619-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute . Age and cancer risk. Accessed February 19, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
- 26.FDA in brief: FDA encourages inclusion of older adult patients in cancer clinical trials. US Food and Drug Administration. Accessed July 21, 2020. https://www.fda.gov/news-events/fda-brief/fda-brief-fda-encourages-inclusion-older-adult-patients-cancer-clinical-trials
- 27.Turner JP, Shakib S, Singhal N, et al. Prevalence and factors associated with polypharmacy in older people with cancer. Support Care Cancer. 2014;22(7):1727-1734. doi: 10.1007/s00520-014-2171-x [DOI] [PubMed] [Google Scholar]
- 28.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. doi: 10.5888/pcd10.120137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockett J, Sauma S, Radziszewska B, Bernard MA. Adequacy of inclusion of older adults in NIH-funded phase III clinical trials. J Am Geriatr Soc. 2019;67(2):218-222. doi: 10.1111/jgs.15786 [DOI] [PubMed] [Google Scholar]
- 30.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One. 2012;7(8):e41601. doi: 10.1371/journal.pone.0041601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oncology Center of Excellence, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Inclusion of older adults in cancer clinical trials. US Food and Drug Administration. Accessed October 6, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/inclusion-older-adults-cancer-clinical-trials
- 32.Khan MS, Shahid I, Siddiqi TJ, et al. Ten-Year trends in enrollment of women and minorities in pivotal trials supporting recent US Food and Drug Administration approval of novel cardiometabolic drugs. J Am Heart Assoc. 2020;9(11):e015594. doi: 10.1161/JAHA.119.015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger JM, Hershman DL, Osarogiagbon RU, et al. Representativeness of Black Patients in Cancer Clinical Trials Sponsored by the National Cancer Institute Compared With Pharmaceutical Companies. JNCI Cancer Spectr. 2020;4(4):a034. doi: 10.1093/jncics/pkaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivers D, August EM, Sehovic I, Lee Green B, Quinn GP. A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemp Clin Trials. 2013;35(2):13-32. doi: 10.1016/j.cct.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 35.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: study design barriers. J Clin Oncol. 2004;22(4):730-734. doi: 10.1200/JCO.2004.03.160 [DOI] [PubMed] [Google Scholar]
- 36.Avis-Williams A, Khoury A, Lisovicz N, Graham-Kresge S. Knowledge, attitudes, and practices of underserved women in the rural South toward breast cancer prevention and detection. Fam Community Health. 2009;32(3):238-246. doi: 10.1097/FCH.0b013e3181ab3bbb [DOI] [PubMed] [Google Scholar]
- 37.Brown M, Moyer A. Predictors of awareness of clinical trials and feelings about the use of medical information for research in a nationally representative US sample. Ethn Health. 2010;15(3):223-236. doi: 10.1080/13557851003624281 [DOI] [PubMed] [Google Scholar]
- 38.Evans KR, Lewis MJ, Hudson SV. The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J Cancer Educ. 2012;27(2):299-305. doi: 10.1007/s13187-011-0300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gooden KM, Carter-Edwards L, Hoyo C, et al. Perceptions of participation in an observational epidemiologic study of cancer among African Americans. Ethn Dis. 2005;15(1):68-75. [PubMed] [Google Scholar]
- 40.Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health. 2011;101(12):2217-2222. doi: 10.2105/AJPH.2011.300279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harriet A. Washington. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present. Doubleday; 2006. [Google Scholar]
- 42.Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21(3):879-897. doi: 10.1353/hpu.0.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eggly S, Barton E, Winckles A, Penner LA, Albrecht TL. A disparity of words: racial differences in oncologist-patient communication about clinical trials. Health Expect. 2015;18(5):1316-1326. doi: 10.1111/hex.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinsky PF, Ford M, Gamito E, et al. Enrollment of racial and ethnic minorities in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Med Assoc. 2008;100(3):291-298. doi: 10.1016/S0027-9684(15)31241-4 [DOI] [PubMed] [Google Scholar]
- 45.Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126(9):1958-1968. doi: 10.1002/cncr.32755 [DOI] [PubMed] [Google Scholar]
- 46.Eshera N, Itana H, Zhang L, Soon G, Fadiran EO. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA From 2010 to 2012. Am J Ther. 2015;22(6):435-455. doi: 10.1097/MJT.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 47.Alexander GA, Chu KC, Ho RCS. Representation of Asian Americans in clinical cancer trials. Ann Epidemiol. 2000;10(8)(suppl):S61-S67. doi: 10.1016/S1047-2797(00)00198-8 [DOI] [PubMed] [Google Scholar]
- 48.Paterniti DA, Chen MS Jr, Chiechi C, et al. Asian Americans and cancer clinical trials: a mixed-methods approach to understanding awareness and experience. Cancer. 2005;104(12)(suppl):3015-3024. doi: 10.1002/cncr.21522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Center for Drug Evaluation and Research, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Office of the Commissioner Office of Minority Health and Health Equity, Office of the Commissioner Office of Women's Health . Collection of race and ethnicity data in clinical trials. US Food and Drug Administration. Accessed August 27, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials
- 50.FDA-AACR workshop to examine under-representation of African Americans in multiple myeloma clinical trials; February 13, 2020; Washington, DC. Accessed January 30, 2021. https://www.aacr.org/professionals/policy-and-advocacy/regulatory-science-and-policy/events/multiple-myeloma-clinical-trials/ [Google Scholar]
- 51.Hurria A, Levit LA, Dale W, et al. ; American Society of Clinical Oncology . Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33(32):3826-3833. doi: 10.1200/JCO.2015.63.0319 [DOI] [PubMed] [Google Scholar]
- 52.Levit L, Balogh E, Nass S, Ganz P. Delivering high-quality cancer care: charting a new course for a system in crisis. Institute of Medicine of the National Academies. Accessed October 14, 2020. https://commed.vcu.edu/Chronic_Disease/Cancers/2014/CancerCare2013_IOM.pdf [PubMed]
- 53.Kesselheim AS. An empirical review of major legislation affecting drug development: past experiences, effects, and unintended consequences. Milbank Q. 2011;89(3):450-502. doi: 10.1111/j.1468-0009.2011.00636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duma N, Kothadia SM, Azam TU, et al. Characterization of comorbidities limiting the recruitment of patients in early phase clinical trials. Oncologist. 2019;24(1):96-102. doi: 10.1634/theoncologist.2017-0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unger JM, Hershman DL, Fleury ME, Vaidya R. Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol. 2019;5(3):326-333. doi: 10.1001/jamaoncol.2018.5953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otado J, Kwagyan J, Edwards D, Ukaegbu A, Rockcliffe F, Osafo N. Culturally competent strategies for recruitment and retention of African American populations into clinical trials. Clin Transl Sci. 2015;8(5):460-466. doi: 10.1111/cts.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327-337. doi: 10.1177/107327481602300404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu P, Ross JS, Ioannidis JP, Dhruva SS, Vasiliou V, Wallach JD. Prevalence and significance of race and ethnicity subgroup analyses in Cochrane intervention reviews. Clin Trials. 2020;17(2):231-234. doi: 10.1177/1740774519887148 [DOI] [PubMed] [Google Scholar]
- 59.Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology—mainland China subset analysis of the PIONEER study. PLoS One. 2015;10(11):e0143515. doi: 10.1371/journal.pone.0143515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892-2911. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Novel Therapeutics With Oncologic Approvals by Cancer Type (2012-2016)
eTable 3. Availability of Demographic Data by Cancer Type
eTable 4. Absolute Difference in Proportion of Women and Older Adults and Proportion by Race Between Study Sample and Patient Population by Cancer Type
eFigure 1. Participation to Prevalence Ratio (PPR) for Women by Cancer Type
eFigure 2. Participation to Prevalence Ratio (PPR) for Older Adults by Cancer Type
eFigure 3. Participation to Prevalence Ratio (PPR) for Race by Cancer Type