Abstract
We report a method to prepare α-chiral carboxylic acid derivatives, including those bearing all-carbon quaternary centers, through an enantioselective CuH-catalyzed hydrocarboxylation of allenes with a commercially available fluoroformate. A broad range of heterocycles and functional groups on the allenes were tolerated in this protocol, giving enantioenriched α-quaternary and tertiary carboxylic acid derivatives in good yields with exclusive branched regioselectivity. The synthetic utility of this approach was further demonstrated by derivatization of the products to afford biologically important compounds, including the antiplatelet drug indobufen.
All-carbon quaternary stereocenters, a structural feature that can impart significant chemical and biological impact to a molecule, are critical to many synthetic and medicinal applications.1–4 Consequently, catalytic and enantioselective approaches for constructing all-carbon quaternary centers, especially functionalized stereocenters, are highly desirable.5–8 Carboxylic acids, a chemically versatile functional group, that can bear an α-stereogenic center often serve as useful synthetic intermediates.9–13 More importantly, α-chiral carboxylic acid derivatives themselves constitute an essential class of compounds in pharmaceutical, agrochemical, and natural product arenas (Figure 1A).14–16 Methods for generating enantioenriched α-chiral carboxylic acids have long been sought after.17 Prominent synthetic strategies targeting α-chiral carboxylic acids or esters via asymmetric catalysis include hydrogenation of α,β-unsaturated carboxylic acids,18 carbene-induced C–H insertion with diazoacetates,19–21 enantioselective protonation22,23 or hydrogen atom transfer24 processes, and α-functionalization of carboxylic acid derivatives.25–50 Nonetheless, catalytic access51 to enantioenriched acyclic carboxylic acids or esters featuring an all-carbon α-quaternary stereocenter remains challenging.5,6 In this regard, common synthetic methods include allylic alkylation of geometrically pure alkenes,52–55 often with superstoichiometric organometallic reagents, and α-functionalization of carboxylic acid derivatives,35–44,50 which typically necessitates a β-directing group or electron-withdrawing group (Figure 1B).
Figure 1.
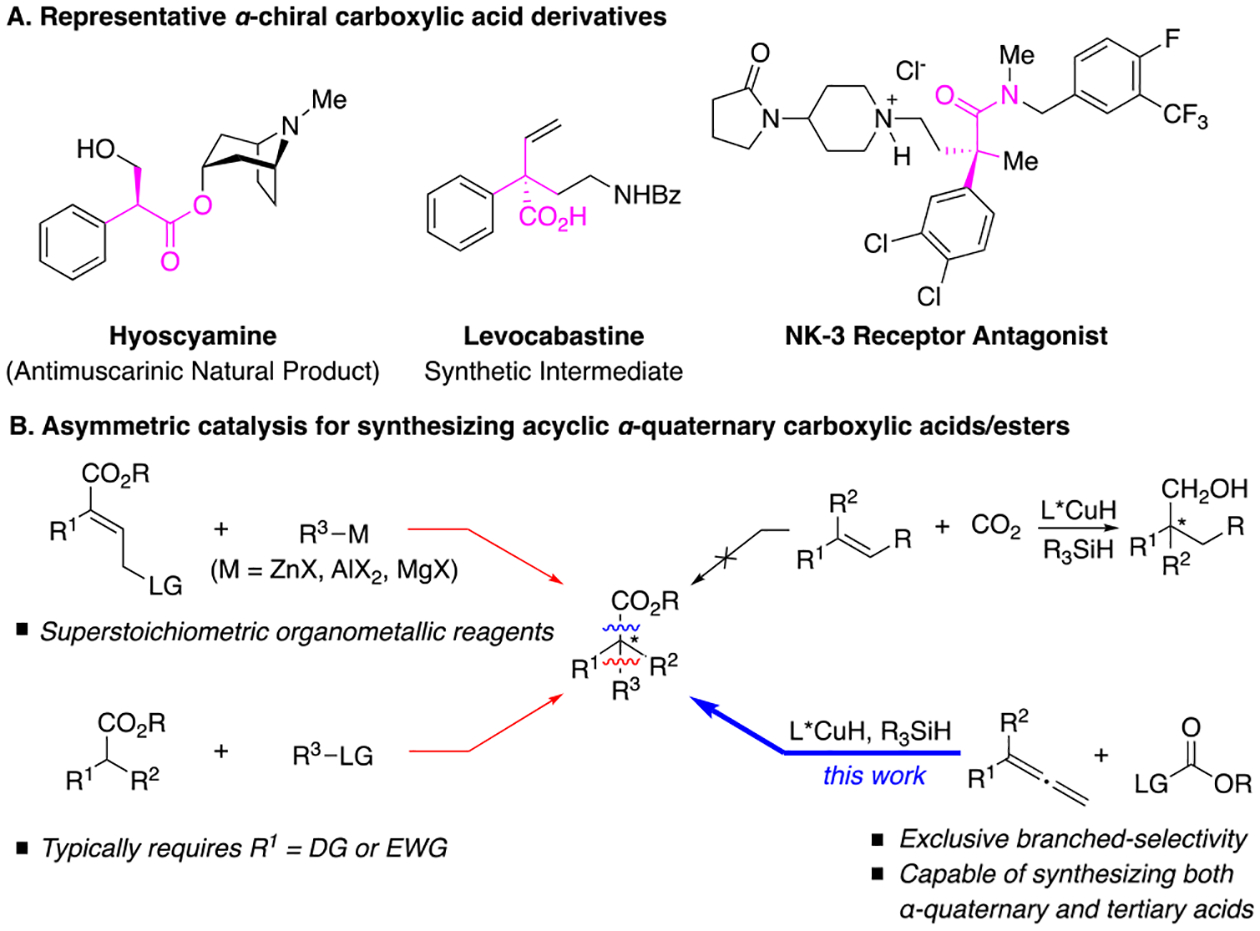
(A) Overview of bioactive α-chiral carboxylic acid derivatives. (B) Previous strategies and our approach to synthesize acyclic α-quaternary carboxylic acid derivatives.
As an alternative, the hydrocarboxylation56–67 of prochiral unsaturated substrates represents a straightforward approach for preparing carboxylic acids. Asymmetric hydrocarboxylation has typically68,69 been achieved through palladium-catalyzed hydroxy- and alkoxycarbonylation processes using CO gas or a carbon monoxide surrogate.70–77 Despite significant advances in this area, the vast majority of the methods can only synthesize α-tertiary acids or esters from vinyl arenes, and a highly enantioselective technique for the assembly of α-quaternary carboxylic acids through a hydrocarboxylation or hydroesterification of unsaturated substrates is still unknown.68
Based upon our research program in copper hydride (CuH)-catalyzed asymmetric hydrofunctionalization of unsaturated substrates,78–91 we sought to develop a hydrocarboxylation method for constructing enantioenriched carboxylic acids, especially α-quaternary acids. Specifically, we envisioned that a chiral organocopper species, generated in situ from the hydrocupration of an unsaturated substrate, could engage a suitable carboxylation reagent to afford enantioenriched carboxylic acids. Previously, when CO2 was used as an electrophile in CuH-catalyzed olefin hydrofunctionalization reactions, the initially formed silylated carboxylic acid intermediates underwent facile reduction and led to the formation of hydroxymethylene products.92–96 To circumvent this reduction pathway, we targeted the CuH-catalyzed hydroesterification, as the products are unreactive under the reaction conditions and can be readily hydrolyzed to give the corresponding carboxylic acids. An ester directly attached to a leaving group is proposed as the electrophile for realizing the hydrocarboxylation process (Figure 1B). In order to obtain α-quaternary esters and acids, we sought to perform a regioselective hydrocarboxylation of allenes as the unsaturated substrate. Herein, we report a highly enantioselective CuH-catalyzed hydrocarboxylation to furnish both α-quaternary and tertiary carboxylic acid derivatives.
We chose 1-phenyl-1-methylallene (1a) as our model substrate since the branched selective hydrocarboxylation of 1-aryl-1-alkylallenes would produce valuable acyclic quaternary α-vinyl-α-aryl carboxylic acids that have been used as intermediates in the preparation of (+)-epilaurene13 and several pharmaceutical ingredients.10,52 We began our investigation with diphenyl carbonate (2a) as the reagent for carboxylate introduction. A series of chiral bisphosphine ligands were evaluated in the hydrocarboxylation of 1a with diphenyl carbonate (Table S1), and the highest level of enantioselectivity was obtained with (R,R)-Ph-BPE (L1). Under these conditions, the ester product was formed in 42% yield (90:10 er) exclusively as the branched isomer (Table 1, entry 1). In addition to the moderate level of enantioselectivity that was observed, the use of 2a appeared to result in a sluggish reaction rate. We next attempted to improve the activity of electrophile by replacing 2a with Boc2O (2b) or methyl chloroformate (2c), which resulted in no desired hydroesterification product being formed (Table 1, entries 2–3). With 2c, we needed an alkoxide base to regenerate LCuH from a LCuCl intermediate,97 and we ascribed the low yield to the incompatibility between the base and methyl chloroformate. Since LCuH regeneration from LCuF complexes can proceed in the absence of a base additives,98 we investigated the use of fluoroformates99,100 as potential carboxylation reagents. When commercially available 1-adamantyl fluoroformate (2d) was employed, product 3 was obtained in 83% yield (Table 1, entry 4). Upon reexamining the suitability of different ligands in reactions with 2d (Table 1, entry 5–6, and Table S2), we found that when (R)-DTBM-SEGPHOS (L2) was used (Table 1, entry 5), the branched product was obtained as a single regioisomer in 92% yield and 99:1 er.
Table 1.
Evaluation of Reaction Conditions for the CuH-Catalyzed Hydrocarboxylation of Allenea
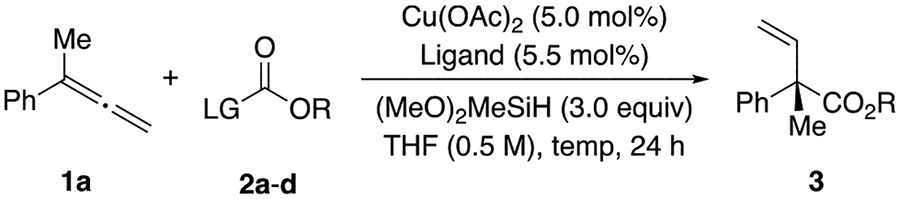 | |||||
|---|---|---|---|---|---|
| entry | ligand | electrophile | temp (°C) | yield (%)b | erc |
| 1 | L1 | 2a | 40 | 42 | 10:90 |
| 2 | L1 | 2b | 40 | <5 | − |
| 3d | L1 | 2c | 25 | <5 | − |
| 4 | L1 | 2d | 25 | 83 | 13:87 |
| 5e | L1 | 2d | 25 | 92 | 99:1 |
| 6f | L1 | 2d | 25 | 77 | 96:4 |
Conditions: 0.10 mmol 2 (1.0 equiv), 1a (2.0 equiv), copper(II) acetate (5.0 mol %), ligand (5.5 mol %), dimethoxy(methyl)silane (3.0 equiv) in THF (0.5 M).
Yield was determined by 1H NMR spectroscopy of the crude reaction mixture, using 1,3,5-trimethoxybenzene as an internal standard.
Enantiomeric ratio was determined by SFC analysis.
Either LiOMe (1.1 equiv) or CsOBz (1.1 equiv) was used as an additive; 1a (1.5 equiv) was used.
1a (1.2 equiv) was used.
1a (1.0 equiv) and 2 (1.2 equiv) were used.
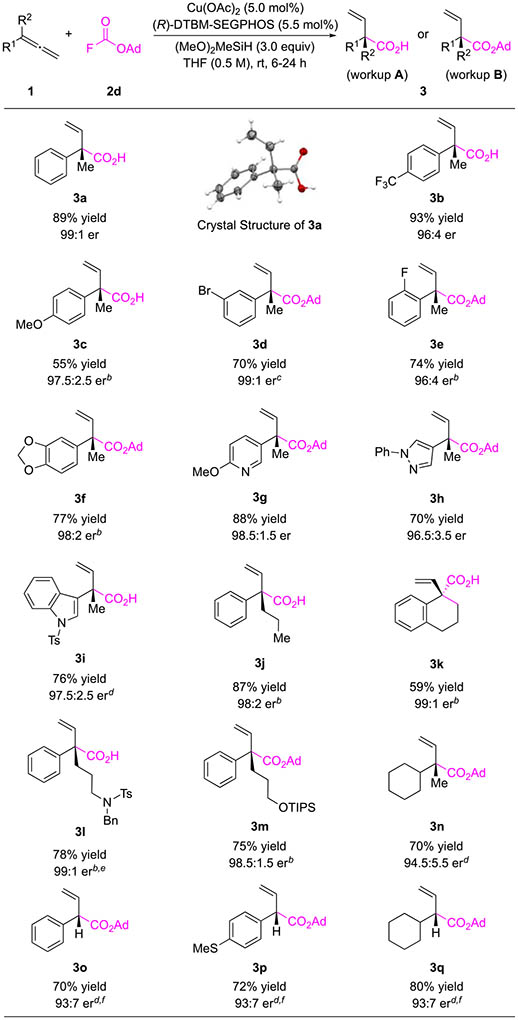
With the optimal reaction conditions identified, we first examined the substrate scope using 1,1-disubstituted allenes (Table 2). We found that a broad range of 1,1-disubstituted allenes in combination with 2d were transformed to the desired products in good yields and with excellent enantioselectivity. Moreover, the ester products could be easily hydrolyzed to carboxylic acids in the presence of trifluoroacetic acid (TFA) in near-quantitative yields. To demonstrate the feasibility of this in situ hydrolysis protocol, half of the ester products in Table 2 were isolated as carboxylic acids (3a–c, 3i–l) without any purification of the intermediate esters.101 1-Aryl-1-alkylallenes bearing an electron-withdrawing (3b) and -donating group (3c) on the arenes were both compatible. Additionally, reactions of arenes substituted with para- (3b, 3c), meta- (3d), and ortho- (3e) groups resulted in the formation of the products in high yields and enantioselectivity. Functional groups such as an acetal (3f), a sulfonamide (3l), and a siloxy group (3m) were also well tolerated. Allenes containing heterocycles, including a pyridine (3g) and pyrazole (3h), were suitable substrates for the hydrocarboxylation reaction. However, when an allene substituted with an indole (3i) was utilized, better results were found if ligand L3 was used in place of L2. We speculate that this is due to the sterically demanding environment of the substrate that requires the use of a less bulky ligand. Allenes containing functionalized primary alkyl groups (3j, 3l–m) as well as an exocyclic allene (3k) were also accommodated in this protocol. Furthermore, 1-cyclohexyl-1-methylallene (3n) was efficiently transformed to the hydroxycarboxylation product when ligand L3 was employed.
Table 2.
Substrate Scope for the CuH-Catalyzed Hydrocarboxylation of Allenesa
|
Conditions: 0.50 mmol 2d (1.0 equiv), 1 (1.2 equiv), copper(II) acetate (5.0 mol %), L2 (5.5 mol %), dimethoxy(methyl)silane (3.0 equiv) in THF (0.5 M); workup A: NH4F/MeOH workup followed by hydrolysis using TFA; workup B: NH4F/MeOH workup; yields refer to average isolated yields of two runs; see the Supporting Information for details.
Reaction was carried out at 40 °C.
Reaction was carried out at 30 °C.
L3 was used as the ligand instead.
1 (1.1 equiv) was used. fReaction was carried out at 0 °C in 1,2-dimethoxyethane (DME, 1.0 mL).
We were also interested in expanding this method toward the synthesis of α-tertiary esters, which under many conditions are difficult to access in high enantioselectivity due to the easily epimerizable stereogenic center. Thus, we next examined the reaction of a monosubstituted allene, phenylallene (1o), under our standard reaction conditions. However, the product ester was formed with a poor level of enantioselectivity, 69.5:30.5 er (Table S4). After reevaluating the reaction parameters, the carboxylation product 3o could be isolated in 70% yield and 93:7 er using L3 as ligand (Table 2). A thioether-containing 1-aryl allene (1p) and cyclohexylallene (1q) were also converted to the corresponding α-tertiary esters in good yields and high enantioselectivity.
To further demonstrate the synthetic utility of our method, we examined the transformation of the hydrocarboxylation products into compounds of interest (Scheme 1). For example, chiral α-tertiary amines are found in a variety of natural products and biologically active compounds, and are difficult to access in an enantioenriched form by standard hydroamination reactions.102–104 By employing a Curtius rearrangement, we were able to convert α-quaternary carboxylic acid 3a to α-tertiary amine 6 in a stereoretentive fashion (Scheme 1a). Additionally, we sought to apply our hydrocarboxylation products to the synthesis of enantioenriched γ-amino acid derivatives, which play an important role as γ-aminobutyric acid transaminase inhibitors and in peptide chemistry.105 By derivatization of the resulting vinyl group in 3d, an α-quaternary γ-amino ester 8 could be accomplished using a CuH-catalyzed hydroamination reaction106 (Scheme 1b). We also utilized the method for the preparation of the pharmaceutical indobufen, a platelet aggregation inhibitor marketed under brand name Ibustrin.107 (S)-Indobufen, previously prepared by the separation of the racemic mixture,108 was found to be far more potent than the (R)-enantiomer in terms of its antiplatelet and anti-inflammatory activities,108–110 and thus an enantioselective synthetic route to (S)-indobufen would be of interest. In our approach, CuH-catalyzed hydrocarboxylation of allene 1r gave ester 3r, which underwent subsequent hydrogenation and hydrolysis to furnish (S)-Indobufen (10) in 76% overall yield and 92:8 er, without the need for any chromatographic purification.
Scheme 1.
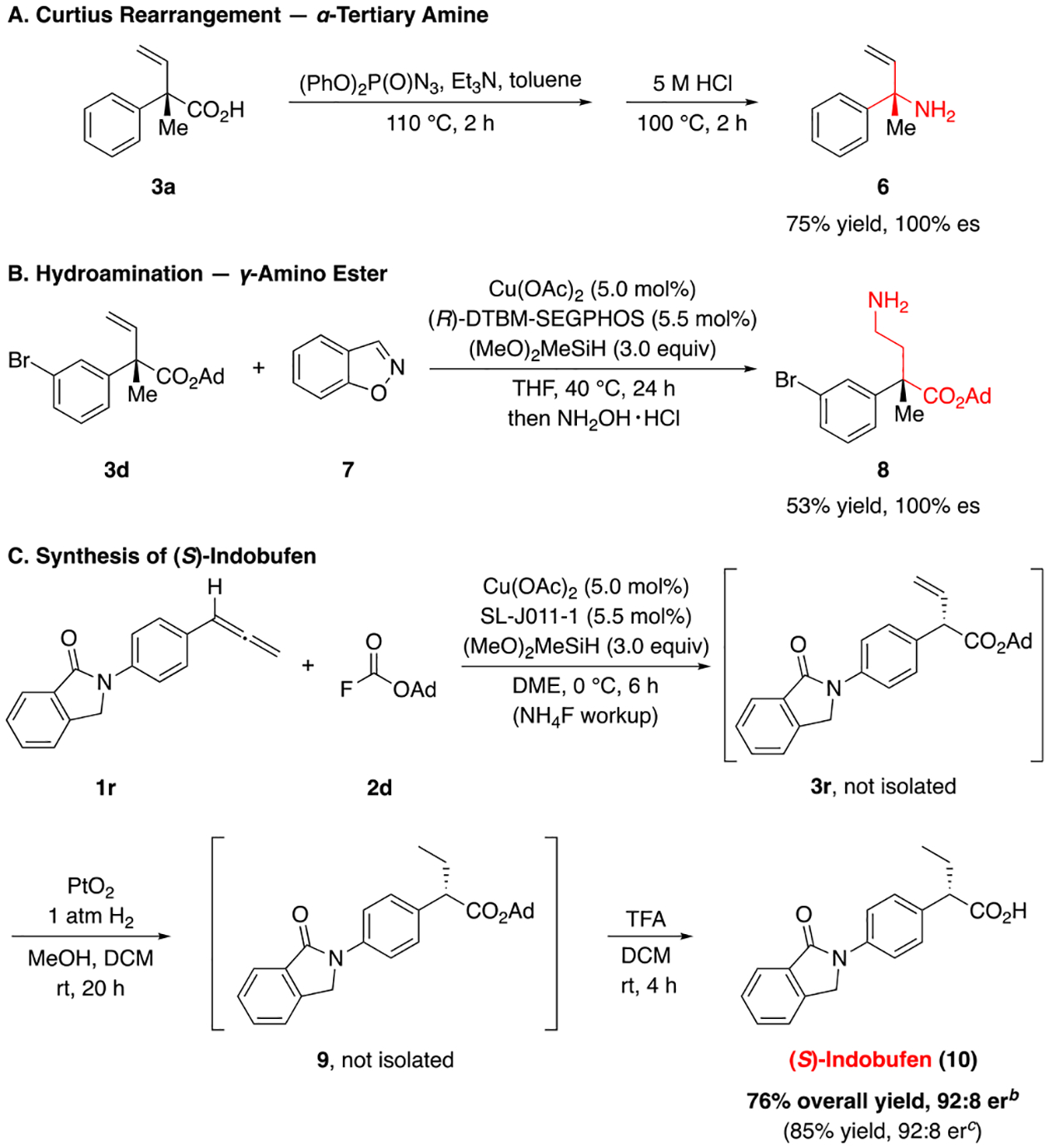
Applications of the CuH-Catalyzed Hydrocarboxylation Reactionsa
aSee the Supporting Information for experimental details. b1r (1.0 equiv) and 2d (1.2 equiv) were used. c2d (1.0 equiv) and 1r (1.2 equiv) were used.
Based on previous DFT calculations on CuH-catalyzed reactions involving allenes,111,112 a plausible mechanism can be proposed for this transformation, as depicted in Figure 2. An allene (1) first undergoes hydrocupration with a CuH catalyst to generate a rapidly equilibrating mixture of allylcopper species (B and C). The less hindered terminal allylic copper (B) reacts preferentially with fluoroformate 2d through an enantio-determining six-membered transition state (D), to form intermediate E. Subsequent collapse of the tetrahedral intermediate by β-fluoride elimination leads to the branched carboxylation product 3 and CuF. A σ-bond metathesis reaction between CuF and the silane regenerates the CuH catalyst. It is worth noting that the presence of the fluorine atom in 2d may lead to unusual energetic preferences in transition state D due to dipole minimization or stereo-electronic effects. Although we can propose a plausible sequence of elementary steps by analogy to related reactions,111,112 at this point we cannot definitively pinpoint stereochemical details of the enantio-determining transition state D and explain the subtle substituent effects on enantioselectivity.
Figure 2.
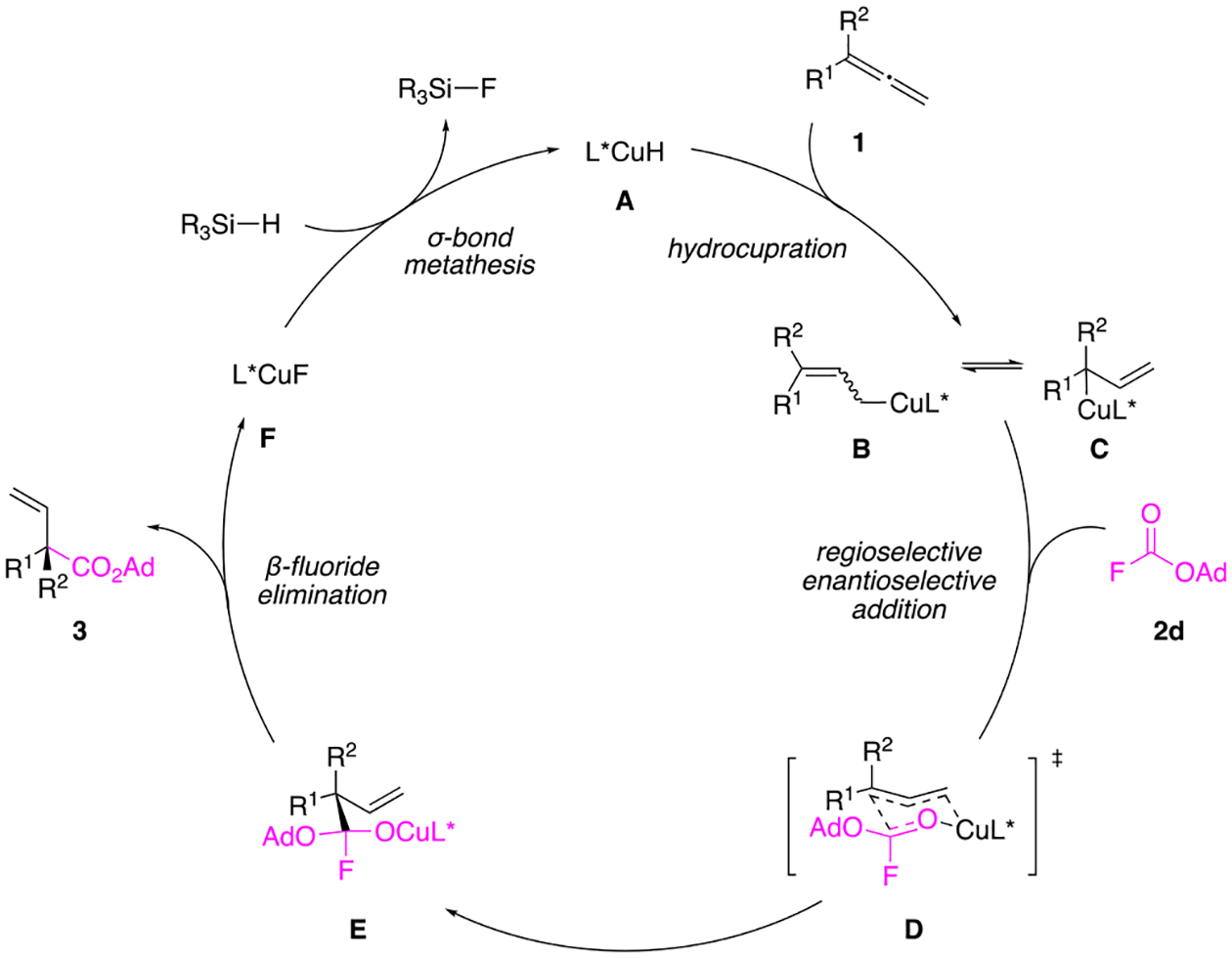
Proposed mechanism for the CuH-catalyzed hydrocarboxylation of allenes.
In conclusion, we have developed a highly enantioselective CuH-catalyzed hydrocarboxylation to synthesize α-chiral carboxylic acids and esters, in particular α-quaternary ones. A commercially available fluoroformate was used as the carboxylation reagent to react with allenes in exclusive branched selectivity. The reaction proceeded under mild conditions and could tolerate a variety of important functional groups and heterocycles. Further derivatization of the carboxylation products provided other pharmaceutically and synthetically useful scaffolds. We anticipate that this carboxylation strategy using a fluoroformate may be extended to the discovery of other types of important asymmetric carboxylation processes.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institutes of Health under award number R35-GM122483. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Solvias AG is acknowledged for a generous gift of SL-J011-1. We thank Drs. Veronika Kottisch, Alexander Schuppe, and Christine Nguyen for their advice on the preparation of this manuscript. We acknowledge Dr. Peter Müller (MIT) for X-ray crystallographic analysis of 3a. We thank the National Institutes of Health for a supplemental grant for the purchase of supercritical fluid chromatography (SFC) equipment (GM058160-17S1).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c01880.
Experimental procedures and spectral data (PDF)
Accession Codes
CCDC 2050451 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.1c01880
The authors declare no competing financial interest.
REFERENCES
- (1).Hu P; Chi HM; DeBacker KC; Gong X; Keim JH; Hsu IT; Snyder SA Quaternary-Centre-Guided Synthesis of Complex Polycyclic Terpenes. Nature 2019, 569, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ling T; Rivas F All-Carbon Quaternary Centers in Natural Products and Medicinal Chemistry: Recent Advances. Tetrahedron 2016, 72, 6729–6777. [Google Scholar]
- (3).Li C; Ragab SS; Liu G; Tang W Enantioselective Formation of Quaternary Carbon Stereocenters in Natural Product Synthesis: A Recent Update. Nat. Prod. Rep 2020, 37, 276–292. [DOI] [PubMed] [Google Scholar]
- (4).Christoffers J; Baro A Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis; Wiley-VCH, 2005. [Google Scholar]
- (5).Feng J; Holmes M; Krische MJ Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev 2017, 117, 12564–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Das JP; Marek I Enantioselective Synthesis of All-Carbon Quaternary Stereogenic Centers in Acyclic Systems. Chem. Commun 2011, 47, 4593–4623. [DOI] [PubMed] [Google Scholar]
- (7).Quasdorf K; Overman LE Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocentres. Nature 2014, 516, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zeng X; Cao Z; Wang Y; Zhou F; Zhou J Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev 2016, 116, 7330–7396. [DOI] [PubMed] [Google Scholar]
- (9).Hoyle J The Synthetic Uses of Carboxylic Acids and Their Derivatives. In The Chemistry of Acid Derivatives; Wiley: 1992. Vol. 2. [Google Scholar]
- (10).Shockley SE; Hethcox JC; Stoltz BM Compositions and Methods for Preparing β,γ-Unsaturated Acids. International Patent WO209243(A1), November. 15, 2018.
- (11).Hanessian S; Jennequin T; Boyer N; Babonneau V; Soma U; Mannoury la Cour C; Millan MJ; De Nanteuil G Design, Synthesis, and Optimization of Balanced Dual NK1/NK3 Receptor Antagonists. ACS Med. Chem. Lett 2014, 5, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Knust H; Nettekoven M; Ratni H; Vifian W; Wu X Piperidine Derivatives as NK3 Receptor Antagonists. International Patent WO033995(A1), March. 19, 2009.
- (13).Fadel A; Canet J-L; Salaün J Asymmetric Construction of Quaternary Carbons from Chiral Malonates: Total Syntheses of (+)-Epilaurene and (−)-Isolaurene. Tetrahedron: Asymmetry 1993, 4, 27–30. [Google Scholar]
- (14).Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals; Lamberth C, Dinges J, Eds.; Wiley-VCH: 2016. [Google Scholar]
- (15).Ramadan M; Goeters S; Watzer B; Krause E; Lohmann K; Bauer R; Hempel B; Imming P Chamazulene Carboxylic Acid and Matricin: A Natural Profen and Its Natural Prodrug, Identified through Similarity to Synthetic Drug Substances. J. Nat. Prod 2006, 69, 1041–1045. [DOI] [PubMed] [Google Scholar]
- (16).Gülcan HO; Ünlü S; Dimoglo A; Şahin Y; Esiringu I; Erçetin T;Öz D; Şahin MF Marginally Designed New Profen Analogues Have the Potential to Inhibit Cyclooxygenase Enzymes. Arch. Pharm 2015, 348, 55–61. [DOI] [PubMed] [Google Scholar]
- (17).Corey EJ; Kürti L Enantioselective Chemical Synthesis: Methods, Logic and Practice; Direct Book Publishing: 2010. [Google Scholar]
- (18).Zhu S; Zhou Q Iridium-Catalyzed Asymmetric Hydrogenation of Unsaturated Carboxylic Acids. Acc. Chem. Res 2017, 50, 988–1001. [DOI] [PubMed] [Google Scholar]
- (19).Zhang XP; Cui X Asymmetric C–H Functionalization by Transition Metal-Catalyzed Carbene Transfer Reactions. In Comprehensive Organic Synthesis, 2nd ed.; Elsevier: 2014; Vol 7, pp 86–120. [Google Scholar]
- (20).Davies HML; Liao K Dirhodium Tetracarboxylates as Catalysts for Selective Intermolecular C–H Functionalization. Nat. Rev. Chem 2019, 3, 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Qiu H; Li M; Jiang L; Lv F; Zan L; Zhai C; Doyle MP; Hu W Highly Enantioselective Trapping of Zwitterionic Intermediates by Imines. Nat. Chem 2012, 4, 733–738. [DOI] [PubMed] [Google Scholar]
- (22).Mohr JT; Hong AY; Stoltz BM Enantioselective Protonation. Nat. Chem 2009, 1, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chen X; Fong JZM; Xu J; Mou C; Lu Y; Yang S; Song B; Chi YR Carbene-Catalyzed Dynamic Kinetic Resolution of Carboxylic Esters. J. Am. Chem. Soc 2016, 138, 7212–7215. [DOI] [PubMed] [Google Scholar]
- (24).Sandoval BA; Meichan AJ; Hyster TK Enantioselective Hydrogen Atom Transfer: Discovery of Catalytic Promiscuity in Flavin-Dependent ‘Ene’-Reductases. J. Am. Chem. Soc 2017, 139, 11313–11316. [DOI] [PubMed] [Google Scholar]
- (25).Cheng Q; Tu H; Zheng C; Qu J; Helmchen G; You S Iridium-Catalyzed Asymmetric Allylic Substitution Reactions. Chem. Rev 2019, 119, 1855–1969. [DOI] [PubMed] [Google Scholar]
- (26).Schwarz KJ; Amos JL; Klein C; Do DT; Snaddon TN Uniting C1-Ammonium Enolates and Transition Metal Electrophiles via Cooperative Catalysis: The Direct Asymmetric α-Allylation of Aryl Acetic Acid Esters. J. Am. Chem. Soc 2016, 138, 5214–5217. [DOI] [PubMed] [Google Scholar]
- (27).Jiang X; Beiger JJ; Hartwig JF Stereodivergent Allylic Substitutions with Aryl Acetic Acid Esters by Synergistic Iridium and Lewis Base Catalysis. J. Am. Chem. Soc 2017, 139, 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kotani S; Yoshiwara Y; Ogasawara M; Sugiura M; Nakajima M Catalytic Enantioselective Aldol Reactions of Unprotected Carboxylic Acids under Phosphine Oxide Catalysis. Angew. Chem., Int. Ed 2018, 57, 15877–15881. [DOI] [PubMed] [Google Scholar]
- (29).Schwarz KJ; Yang C; Fyfe JWB; Snaddon TN Enantioselective α-Benzylation of Acyclic Esters Using π-Extended Electrophiles. Angew. Chem., Int. Ed 2018, 57, 12102–12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Spielvogel DJ; Buchwald SL Nickel-BINAP Catalyzed Enantioselective α-Arylation of α-Substituted γ-Butyrolactones. J. Am. Chem. Soc 2002, 124, 3500–3501. [DOI] [PubMed] [Google Scholar]
- (31).Mermerian AH; Fu GC Catalytic Enantioselective Construction of All-Carbon Quaternary Stereocenters: Synthetic and Mechanistic Studies of the C-Acylation of Silyl Ketene Acetals. J. Am. Chem. Soc 2005, 127, 5604–5607. [DOI] [PubMed] [Google Scholar]
- (32).Huang Z; Liu Z; Zhou J An Enantioselective, Intermolecular α-Arylation of Ester Enolates To Form Tertiary Stereocenters. J. Am. Chem. Soc 2011, 133, 15882–15885. [DOI] [PubMed] [Google Scholar]
- (33).Jette CI; Tong ZJ; Hadt RG; Stoltz BM Copper-Catalyzed Enantioselective Allylic Alkylation with a γ-Butyrolactone-Derived Silyl Ketene Acetal. Angew. Chem., Int. Ed 2020, 59, 2033–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kim B; Kim Y; Lee SY Stereodivergent Carbon–Carbon Bond Formation between Iminium and Enolate Intermediates by Synergistic Organocatalysis. J. Am. Chem. Soc 2021, 143, 73–79. [DOI] [PubMed] [Google Scholar]
- (35).Fujita T; Yamamoto T; Morita Y; Chen H; Shimizu Y; Kanai M Chemo- and Enantioselective Pd/B Hybrid Catalysis for the Construction of Acyclic Quaternary Carbons: Migratory Allylation of O-Allyl Esters to α-C-Allyl Carboxylic Acids. J. Am. Chem. Soc 2018, 140, 5899–5903. [DOI] [PubMed] [Google Scholar]
- (36).He Z; Jiang X; Hartwig JF Stereodivergent Construction of Tertiary Fluorides in Vicinal Stereogenic Pairs by Allylic Substitution with Iridium and Copper Catalysts. J. Am. Chem. Soc 2019, 141, 13066–13073. [DOI] [PubMed] [Google Scholar]
- (37).Zhu Y; Zhang L; Luo S Asymmetric α-Photoalkylation of β-Ketocarbonyls by Primary Amine Catalysis: Facile Access to Acyclic All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc 2014, 136, 14642–14645. [DOI] [PubMed] [Google Scholar]
- (38).Wang D; Zhang L; Luo S Enantioselective Decarboxylative α-Alkynylation of β-Ketocarbonyls via a Catalytic α-Imino Radical Intermediate. Org. Lett 2017, 19, 4924–4927. [DOI] [PubMed] [Google Scholar]
- (39).Wang Y; Chai J; You C; Zhang J; Mi X; Zhang L; Luo S π-Coordinating Chiral Primary Amine/Palladium Synergistic Catalysis for Asymmetric Allylic Alkylation. J. Am. Chem. Soc 2020, 142, 3184–3195. [DOI] [PubMed] [Google Scholar]
- (40).Xie X; Chen Y; Ma D Enantioselective Arylation of 2-Methylacetoacetates Catalyzed by CuI/trans-4-Hydroxy-L-Proline at Low Reaction Temperatures. J. Am. Chem. Soc 2006, 128, 16050–16051. [DOI] [PubMed] [Google Scholar]
- (41).Liu W-B; Reeves CM; Stoltz BM Enantio-, Diastereo-, and Regioselective Iridium-Catalyzed Asymmetric Allylic Alkylation of Acyclic β-Ketoesters. J. Am. Chem. Soc 2013, 135, 17298–17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sawamura M; Sudoh M; Ito Y An Enantioselective Two-Component Catalyst System: Rh–Pd-Catalyzed Allylic Alkylation of Activated Nitriles. J. Am. Chem. Soc 1996, 118, 3309–3310. [Google Scholar]
- (43).Asad SA; Ulicki J; Shevyrev M; Uddin N; Alberch E; Hossain MM First Example of the Intermolecular Palladium-Catalyzed Asymmetric Allylic Alkylation of Hydroxyacrylates: Synthesis of All-Carbon α-Aryl Quaternary Aldehydes. Eur. J. Org. Chem 2014, 2014, 5695–5699. [Google Scholar]
- (44).Hashimoto T; Sakata K; Maruoka K α-Chiral Acetylenes Having an All-Carbon Quaternary Center: Phase Transfer Catalyzed Enantioselective α-Alkylation of α-Alkyl-α-alkynyl Esters. Angew. Chem., Int. Ed 2009, 48, 5014–5017. [DOI] [PubMed] [Google Scholar]
- (45).Dai X; Strotman NA; Fu GC Catalytic Asymmetric Hiyama Cross-Couplings of Racemic α-Bromo Esters. J. Am. Chem. Soc 2008, 130, 3302–3303. [DOI] [PubMed] [Google Scholar]
- (46).Mao J; Liu F; Wang M; Wu L; Zheng B; Liu S; Zhong J; Bian Q; Walsh PJ Cobalt-Bisoxazoline-Catalyzed Asymmetric Kumada Cross-Coupling of Racemic α-Bromo Esters with Aryl Grignard Reagents. J. Am. Chem. Soc 2014, 136, 17662–17668. [DOI] [PubMed] [Google Scholar]
- (47).Jin M; Adak L; Nakamura M Iron-Catalyzed Enantioselective Cross-Coupling Reactions of α-Chloroesters with Aryl Grignard Reagents. J. Am. Chem. Soc 2015, 137, 7128–7134. [DOI] [PubMed] [Google Scholar]
- (48).Guan H; Zhang Q; Walsh PJ; Mao J Nickel/Photoredox-Catalyzed Asymmetric Reductive Cross-Coupling of Racemic α-Chloro Esters with Aryl Iodides. Angew. Chem., Int. Ed 2020, 59, 5172–5177. [DOI] [PubMed] [Google Scholar]
- (49).Wang Z; Yin H; Fu GC Catalytic Enantioconvergent Coupling of Secondary and Tertiary Electrophiles with Olefins. Nature 2018, 563, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wang Z; Yang Z; Fu GC Quaternary Stereocentres via Catalytic Enantioconvergent Nucleophilic Substitution Reactions of Tertiary Alkyl Halides. Nat. Chem 2021, 13, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Yu K; Lu P; Jackson JJ; Nguyen TD; Alvarado J; Stivala CE; Ma Y; Mack KA; Hayton TW; Collum DB; Zakarian A Lithium Enolates in the Enantioselective Construction of Tetrasub-stituted Carbon Centers with Chiral Lithium Amides as Noncovalent Stereodirecting Auxiliaries. J. Am. Chem. Soc 2017, 139, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Murphy KE; Hoveyda AH Catalytic Enantioselective Synthesis of Quaternary All-Carbon Stereogenic Centers. Preparation of α,α′-Disubstituted β,γ-Unsaturated Esters through Cu-Catalyzed Asymmetric Allylic Alkylations. Org. Lett 2005, 7, 1255–1258. [DOI] [PubMed] [Google Scholar]
- (53).Lee Y; Hoveyda AH Lewis Base Activation of Grignard Reagents with N-Heterocyclic Carbenes. Cu-Free Catalytic Enantioselective Additions to γ-Chloro-α,β-Unsaturated Esters. J. Am. Chem. Soc 2006, 128, 15604–15605. [DOI] [PubMed] [Google Scholar]
- (54).Gao F; Lee Y; Mandai K; Hoveyda AH Quaternary Carbon Stereogenic Centers through Copper-Catalyzed Enantioselective Allylic Substitutions with Readily Accessible Aryl- or Heteroaryllitium Reagents and Aluminum Chlorides. Angew. Chem., Int. Ed 2010, 49, 8370–8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Shockley SE; Hethcox JC; Stoltz BM Enantioselective Synthesis of Acyclic α-Quaternary Carboxylic Acid Derivatives through Iridium-Catalyzed Allylic Alkylation. Angew. Chem., Int. Ed 2017, 56, 11545–11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Takaya J; Iwasawa N Hydrocarboxylation of Allenes with CO2 Catalyzed by Silyl Pincer-Type Palladium Complex. J. Am. Chem. Soc 2008, 130, 15254–15255. [DOI] [PubMed] [Google Scholar]
- (57).Williams CM; Johnson JB; Rovis T Nickel-Catalyzed Reductive Carboxylation of Styrenes Using CO2. J. Am. Chem. Soc 2008, 130, 14936–14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Gaydou M; Moragas T; Juliá-Hernández F; Martin R Site-Selective Catalytic Carboxylation of Unsaturated Hydrocarbons with CO2 and Water. J. Am. Chem. Soc 2017, 139, 12161–12164. [DOI] [PubMed] [Google Scholar]
- (59).Seo H; Liu A; Jamison TF Direct β-Selective Hydrocarboxylation of Styrenes with CO2 Enabled by Continuous Flow Photoredox Catalysis. J. Am. Chem. Soc 2017, 139, 13969–13972. [DOI] [PubMed] [Google Scholar]
- (60).Meng Q; Wang S; Huff GS; König B Ligand-Controlled Regioselective Hydrocarboxylation of Styrenes with CO2 by Combining Visible Light and Nickel Catalysis. J. Am. Chem. Soc 2018, 140, 3198–3201. [DOI] [PubMed] [Google Scholar]
- (61).Alkayal A; Tabas V; Montanaro S; Wright IA; Malkov AV; Buckley BR Harnessing Applied Potential: Selective β-Hydrocarboxylation of Substituted Olefins. J. Am. Chem. Soc 2020, 142, 1780–1785. [DOI] [PubMed] [Google Scholar]
- (62).Fujihara T; Xu T; Semba K; Terao J; Tsuji Y Copper-Catalyzed Hydrocarboxylation of Alkynes Using Carbon Dioxide and Hydrosilanes. Angew. Chem., Int. Ed 2011, 50, 523–527. [DOI] [PubMed] [Google Scholar]
- (63).Tani Y; Kuga K; Fujihara T; Terao J; Tsuji Y Copper-Catalyzed C–C Bond-Forming Transformation of CO2 to Alcohol Oxidation Level: Selective Synthesis of Homoallylic Alcohols from Allenes, CO2, and Hydrosilanes. Chem. Commun 2015, 51, 13020–13023. [DOI] [PubMed] [Google Scholar]
- (64).Li H; Dong K; Jiao H; Neumann H; Jackstell R; Beller M The Scope and Mechanism of Palladium-Catalysed Markovnikov Alkoxycarbonylation of Alkenes. Nat. Chem 2016, 8, 1159–1166. [DOI] [PubMed] [Google Scholar]
- (65).Dong K; Fang X; Gülak S; Franke R; Spannenberg A; Neumann H; Jackstell R; Beller M Highly Active and Efficient Catalysts for Alkoxycarbonylation of Alkenes. Nat. Commun 2017, 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Yang J; Liu J; Neumann H; Franke R; Jackstell R; Beller M Direct Synthesis of Adipic Acid Esters via Palladium-Catalyzed Carbonylation of 1,3-Dienes. Science 2019, 366, 1514–1517. [DOI] [PubMed] [Google Scholar]
- (67).Sang R; Kucmierczyk P; Dühren R; Razzaq R; Dong K; Liu J; Franke R; Jackstell R; Beller M Synthesis of Carboxylic Acids by Palladium-Catalyzed Hydroxycarbonylation. Angew. Chem., Int. Ed 2019, 58, 14365–14373. [DOI] [PubMed] [Google Scholar]
- (68).Kawashima S; Aikawa K; Mikami K Rhodium-Catalyzed Hydrocarboxylation of Olefins with Carbon Dioxide. Eur. J. Org. Chem 2016, 2016, 3166–3170. [Google Scholar]
- (69).Yuan Y; Wu F; Schünemann C; Holz J; Kamer PCJ; Wu X Copper-Catalyzed Carbonylative Hydroamidation of Styrenes to Branched Amides. Angew. Chem., Int. Ed 2020, 59, 22441–22445. [DOI] [PubMed] [Google Scholar]
- (70).Godard C; Muñoz BK; Ruiz A; Claver C Pd-Catalysed Asymmetric Mono- and Bis-Alkoxycarbonylation of Vinylarenes. Dalton Trans. 2008, 853. [DOI] [PubMed] [Google Scholar]
- (71).Cometti G; Chiusoli GP Asymmetric Induction in Carbomethoxylation of Vinylaromatics. J. Organomet. Chem 1982, 236, C31–C32. [Google Scholar]
- (72).Alper H; Hamel N Asymmetric Synthesis of Acids by the Palladium-Catalyzed Hydrocarboxylation of Olefins in the Presence of (R)-(−)- or (S)-(+)-1,1′-Binaphthyl-2,2′-diyl Hydrogen Phosphate. J. Am. Chem. Soc 1990, 112, 2803–2804. [Google Scholar]
- (73).Cao P; Zhang X Highly Enantioselective Cyclocarbonylation of Allylic Alcohols Catalyzed by Novel Pd-1,4-bisphosphine Complexes. J. Am. Chem. Soc 1999, 121, 7708–7709. [Google Scholar]
- (74).Konrad TM; Fuentes JA; Slawin AMZ; Clarke ML Highly Enantioselective Hydroxycarbonylation and Alkoxycarbonylation of Alkenes using Dipalladium Complexes as Precatalysts. Angew. Chem., Int. Ed 2010, 49, 9197–9200. [DOI] [PubMed] [Google Scholar]
- (75).Li J; Chang W; Ren W; Dai J; Shi Y Palladium-Catalyzed Highly Regio- and Enantioselective Hydroesterification of Aryl Olefins with Phenyl Formate. Org. Lett 2016, 18, 5456–5459. [DOI] [PubMed] [Google Scholar]
- (76).Li J; Ren W; Dai J; Shi Y Palladium-Catalyzed Regio- and Enantioselective Hydroesterification of Aryl Olefins with CO Gas. Org. Chem. Front 2018, 5, 75–79. [DOI] [PubMed] [Google Scholar]
- (77).Tian D; Xu R; Zhu J; Huang J; Dong W; Claverie J; Tang W Asymmetric Hydroesterification of Diarylmethyl Carbinols. Angew. Chem., Int. Ed 2021, 60, 6305–6309. [DOI] [PubMed] [Google Scholar]
- (78).Pirnot MT; Wang Y; Buchwald SL Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes. Angew. Chem., Int. Ed 2016, 55, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Science 2016, 354, aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Liu RY; Buchwald SL CuH-Catalyzed Olefin Functionalization: From Hydroamination to Carbonyl Addition. Acc. Chem. Res 2020, 53, 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Yang Y; Perry IB; Lu G; Liu P; Buchwald SL Copper-Catalyzed Asymmetric Addition of Olefin-Derived Nucleophiles to Ketones. Science 2016, 353, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Yang Y; Perry IB; Buchwald SL Copper-Catalyzed Enantioselective Addition of Styrene-Derived Nucleophiles to Imines Enabled by Ligand-Controlled Chemoselective Hydrocupration. J. Am. Chem. Soc 2016, 138, 9787–9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Bandar JS; Ascic E; Buchwald SL Enantioselective CuH-Catalyzed Reductive Coupling of Aryl Alkenes and Activated Carboxylic Acids. J. Am. Chem. Soc 2016, 138, 5821–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Yuan Y; Zhang X; Qian H; Ma S Catalytic Enantioselective Allene-Anhydride Approach to β,γ-Unsaturated Enones Bearing an α-All-Carbon-Quaternary Center. Chem. Sci 2020, 11, 9115–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Saxena A; Choi B; Lam HW Enantioselective Copper-Catalyzed Reductive Coupling of Alkenylazaarenes with Ketones. J. Am. Chem. Soc 2012, 134, 8428–8431. [DOI] [PubMed] [Google Scholar]
- (86).Tsai EY; Liu RY; Yang Y; Buchwald SL A Regio- and Enantioselective CuH-Catalyzed Ketone Allylation with Terminal Allenes. J. Am. Chem. Soc 2018, 140, 2007–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Li K; Shao X; Tseng L; Malcolmson SJ 2-Azadienes as Reagents for Preparing Chiral Amines: Synthesis of 1,2-Amino Tertiary Alcohols by Cu-Catalyzed Enantioselective Reductive Couplings with Ketones. J. Am. Chem. Soc 2018, 140, 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Jang WJ; Yun J Copper-Catalyzed Tandem Hydrocupration and Diastereo- and Enantioselective Borylalkyl Addition to Aldehydes. Angew. Chem., Int. Ed 2018, 57, 12116–12120. [DOI] [PubMed] [Google Scholar]
- (89).Shao X; Li K; Malcolmson SJ Enantioselective Synthesis of anti-1,2-Diamines by Cu-Catalyzed Reductive Couplings of Azadienes with Aldimines and Ketimines. J. Am. Chem. Soc 2018, 140, 7083–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Liu RY; Zhou Y; Yang Y; Buchwald SL Enantioselective Allylation Using Allene, a Petroleum Cracking Byproduct. J. Am. Chem. Soc 2019, 141, 2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Tan Y; Tang X; Liu P; Kong D; Chen Y; Tian P; Lin G CuH-Catalyzed Asymmetric Intramolecular Reductive Coupling of Allenes to Enones. Org. Lett 2018, 20, 248–251. [DOI] [PubMed] [Google Scholar]
- (92).Gui Y; Hu N; Chen X; Liao L; Ju T; Ye J; Zhang Z; Li J; Yu D Highly Regio- and Enantioselective Copper-Catalyzed Reductive Hydroxymethylation of Styrenes and 1,3-Dienes with CO2. J. Am. Chem. Soc 2017, 139, 17011–17014. [DOI] [PubMed] [Google Scholar]
- (93).Chen X; Zhu L; Gui Y; Jing K; Jiang Y; Bo Z; Lan Y; Li J; Yu D Highly Selective and Catalytic Generation of Acyclic Quaternary Carbon Stereocenters via Functionalization of 1,3-Dienes with CO2. J. Am. Chem. Soc 2019, 141, 18825–18835. [DOI] [PubMed] [Google Scholar]
- (94).Qiu J; Gao S; Li C; Zhang L; Wang Z; Wang X; Ding K Construction of All-Carbon Chiral Quaternary Centers through CuI-Catalyzed Enantioselective Reductive Hydroxymethylation of 1,1-Disubstituted Allenes with CO2. Chem. - Eur. J 2019, 25, 13874–13878. [DOI] [PubMed] [Google Scholar]
- (95).Li W; Chen L; Lin Z; Man S; Qin X; Lyu Y; Li C; Leng G Theoretical Characterization of Catalytically Active Species in Reductive Hydroxymethylation of Styrene with CO2 over a Bisphosphine-Ligated Copper Complex. Inorg. Chem 2020, 59, 9667–9682. [DOI] [PubMed] [Google Scholar]
- (96).Wang M; Jin X; Wang X; Xia S; Wang Y; Huang S; Li Y; He L; Ma X Copper-Catalyzed and Proton-Directed Selective Hydroxymethylation of Alkynes with CO2. Angew. Chem., Int. Ed 2021, 60, 3984–3988. [DOI] [PubMed] [Google Scholar]
- (97).Appella DH; Moritani Y; Shintani R; Ferreira EM; Buchwald SL Asymmetric Conjugate Reduction of α,β-Unsaturated Esters Using a Chiral Phosphine-Copper Catalyst. J. Am. Chem. Soc 1999, 121, 9473–9474. [Google Scholar]
- (98).Suess AM; Uehling MR; Kaminsky W; Lalic G Mechanism of Copper-Catalyzed Hydroalkylation of Alkynes: An Unexpected Role of Dinuclear Copper Complexes. J. Am. Chem. Soc 2015, 137, 7747–7753. [DOI] [PubMed] [Google Scholar]
- (99).Boreux A; Indukuri K; Gagosz F; Riant O Acyl Fluorides as Efficient Electrophiles for the Copper-Catalyzed Boroacylation of Allenes. ACS Catal. 2017, 7, 8200–8204. [Google Scholar]
- (100).Han J; Zhou W; Zhang P; Wang H; Zhang R; Wu H; Zhang J Design and Synthesis of WJ-Phos, and Application in Cu-Catalyzed Enantioselective Boroacylation of 1,1-Disubstituted Allenes. ACS Catal. 2019, 9, 6890–6895. [Google Scholar]
- (101). The only exception was the hydrolysis to give carboxylic acid 3l, which was carried out after first isolating the corresponding ester in order to facilitate its purification.
- (102).Pascaud X; Honde C; Le Gallou B; Chanoine F; Roman F; Bueno L; Junien JL Effects of Fedotozine on Gastrointestinal Motility in Dogs: Mechanism of Action and Related Pharmocokinetics. J. Pharm. Pharmacol 1990, 42, 546–552. [DOI] [PubMed] [Google Scholar]
- (103).Hager A; Vrielink N; Hager D; Lefranc J; Trauner D Synthetic Approaches Towards Alkaloids Bearing α-Tertiary Amines. Nat. Prod. Rep 2016, 33, 491–522. [DOI] [PubMed] [Google Scholar]
- (104).Bera K; Namboothiri INN Asymmetric Synthesis of Quaternary α-Amino Acids and Their Phosphonate Analogues. Asian J. Org. Chem 2014, 3, 1234–1260. [Google Scholar]
- (105).Ordóñez M; Cativiela C Stereoselective Synthesis of γ-Amino Acids. Tetrahedron: Asymmetry 2007, 18, 3–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Guo S; Yang JC; Buchwald SL A Practical Electrophilic Nitrogen Source for the Synthesis of Chiral Primary Amines by Copper-Catalyzed Hydroamination. J. Am. Chem. Soc 2018, 140, 15976–15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Cattaneo M; Bevilacqua C; Lecchi A; Mannucci PM In vitro and ex vivo Effects of Indobufen on Human Platelet Aggregation, the Release Reaction and Thromboxane B2 Production. Haemostasis 1987, 17, 293–300. [DOI] [PubMed] [Google Scholar]
- (108).Yao Y Right-handed Indobufen and Use for Preparing Medicament. CN101270072(A), September. 24, 2008.
- (109).Cerletti C; Manarini S; Colombo M; Tavani A The (+)-Enantiomer Is Responsible for the Antiplatelet and Anti-Inflammatory Activity of (±)-Indobufen. J. Pharm. Pharmacol 1990, 42, 885–887. [DOI] [PubMed] [Google Scholar]
- (110).Patrignani P; Volpi D; Ferrario R; Romanzini L; Somma MD; Patrono C Effects of Racemic, S- and R-Indobufen on Cyclooxygenase and Lipoxygenase Activities in Human Whole Blood. Eur. J. Pharmacol 1990, 191, 83–88. [DOI] [PubMed] [Google Scholar]
- (111).Ye Y; Kevlishvili I; Feng S; Liu P; Buchwald SL Highly Enantioselective Synthesis of Indazoles with a C3-Quaternary Chiral Center Using CuH Catalysis. J. Am. Chem. Soc 2020, 142, 10550–10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Liu RY; Yang Y; Buchwald SL Regiodivergent and Diastereoselective CuH-Catalyzed Allylation of Imines with Terminal Allenes. Angew. Chem., Int. Ed 2016, 55, 14077–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


