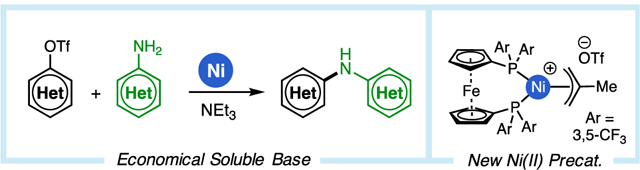
Abstract
Palladium-catalyzed amination reactions using soluble organic bases have provided a solution to the many issues associated with heterogeneous reaction conditions. Still, homogeneous C–N cross-coupling approaches cannot yet employ bases as weak and economical as trialkylamines. Furthermore, organic base-mediated methods have not been developed for Ni(0/II) catalysis, despite some advantages of such systems over analogous Pd-based catalysts. We designed a new air-stable and easily prepared Ni(II) precatalyst bearing an electron-deficient bidentate phosphine ligand that enables the cross-coupling of aryl triflates with aryl amines using triethylamine (TEA) as base. The method is tolerant of sterically-congested coupling partners, as well as those bearing base-and nucleophile-sensitive functional groups. With the aid of density functional theory (DFT) calculations, we determined that the electron-deficient auxiliary ligands decrease both the pKa of the Ni-bound amine and the barrier to reductive elimination from the resultant Ni(II)–amido complex. Moreover, we determined that precluding Lewis acid-base complexation between the Ni catalyst and the base, due to steric factors, is important for avoiding catalyst inhibition.
Graphical Abstract
INTRODUCTION
The development of metal-catalyzed carbon–nitrogen (C–N) bond-forming reactions has had a transformative impact on the synthesis of pharmaceuticals, agrochemicals, organic materials and fine chemicals.1 Catalysts based on palladium and copper have been broadly employed to facilitate the cross-coupling of aryl (pseudo)halides with amine nucleophiles, but these reactions have traditionally required the addition of inorganic bases.2 In recent years, however, there has been increased interest in the use of soluble organic bases in place of commonly used inorganic reagents for cross-coupling reactions generally.3 These single-phase reactions are easily transferrable to high-throughput reaction screening settings, continuous flow chemistry, and microfluidic screening platforms.4 Moreover, the use of weak organic bases avoids functional group incompatibility issues associated with nucleophilic alkoxide and metal amide bases, especially in combination with amines.5 Previously, several phosphazene,6 guanidine,6 amidine,7 and alkyl amine8 bases have been shown to facilitate Pd- and Cu- catalyzed9 C–N bond formation. The weakest among these, alkyl amine bases stand out as an attractive class of reagents, particularly since their steric properties, nucleophilicity, and basicity can be precisely tuned.10 Furthermore, many trialkylamine reagents, including triethylamine (TEA), are produced on large scale directly from alcohols and ammonia,11 making them as inexpensive as common organic solvents.
Previously, our research group demonstrated that a bulky, electron-deficient Pd catalyst can facilitate C–N bond formation in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).12 Mechanistic investigations13 and in-depth reaction optimization studies14 suggested that other organic bases, including TEA and diisopropylethylamine (DIPEA, Hünig’s base) could facilitate the cross-coupling of aryl triflates and anilines, albeit with slower reaction rates. We considered whether an electron-deficient catalyst based on nickel might allow for these very mild and inexpensive trialkylamine bases to be used more effectively in C–N cross-coupling.
The use of Ni was of particular interest to us because the use of weak, soluble organic bases in Ni-catalyzed aryl amination has not yet been systematically explored. Since the first reports of Ni-catalyzed amination,15 the transformation has been significantly improved in terms of scope and efficiency through rational ligand design,16 the development of photocatalytic variants,17 and using electrochemistry.18 While these efforts have greatly expanded the number and type of electrophiles19 and nucleophiles20 that can be cross-coupled under practical conditions, the majority of Ni-catalyzed methods remain predominantly reliant on inorganic bases such as metal tert-butoxides and phosphates to facilitate C–N formation (Figure 1A). Many useful solutions that are compatible with organic bases take advantage of energy input through either photo- or electrocatalysis. These protocols are primarily limited to the coupling of strongly coordinating nucleophiles such as aliphatic amines (Figure 1, B).17,18 Providing a complementary approach, we herein describe the rational discovery of a Ni (pre)catalyst capable of effecting arylation of weakly binding aniline nucleophiles using a trialkylamine base.
Figure 1.
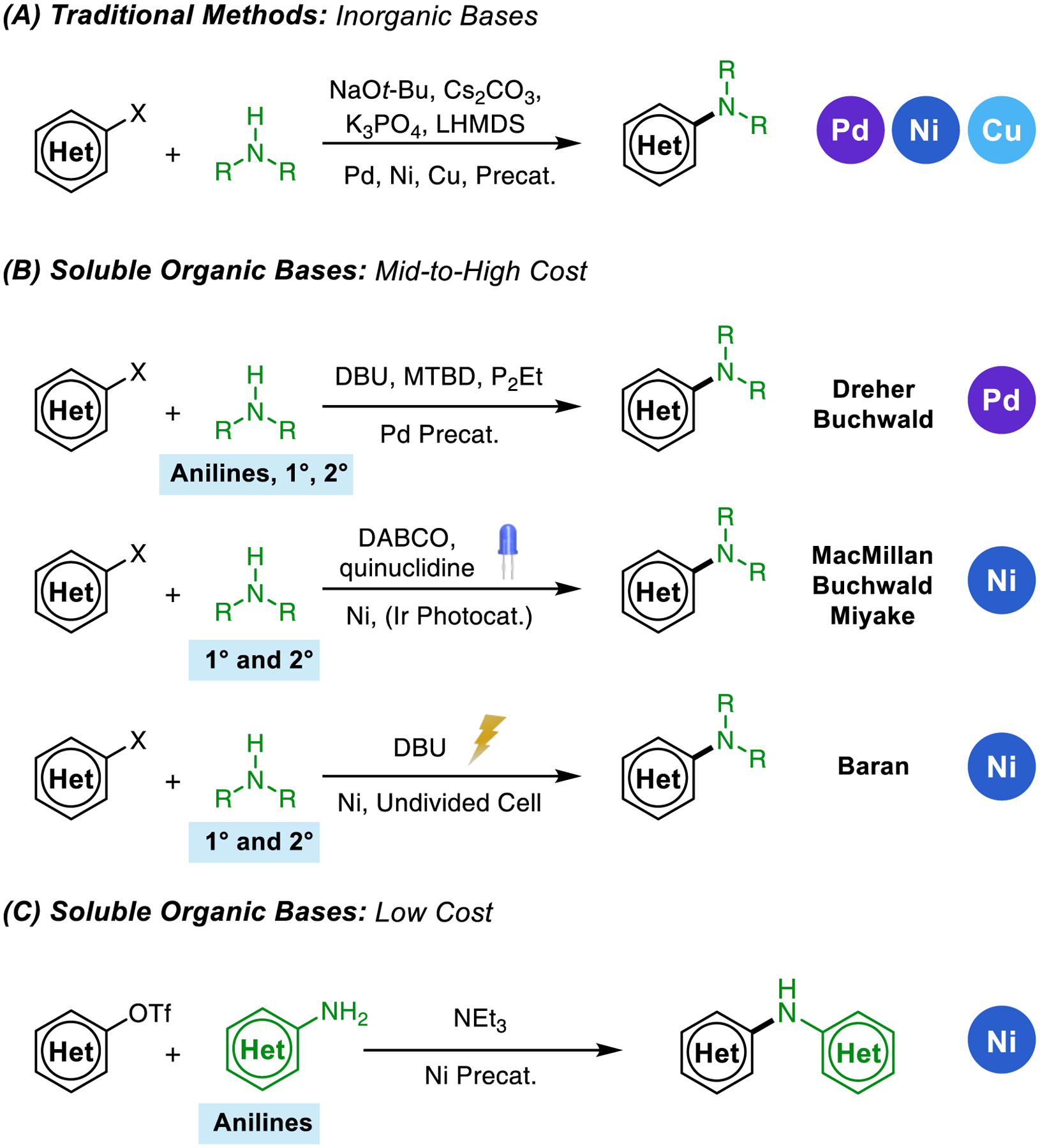
(A) Inorganic bases used in traditional Pd-, Ni-, and Cu-catalyzed C–N cross-coupling methods. (B) Amidine, guanidine, and phosphazene bases used in Pd-catalyzed amination and Ni-catalyzed photo- or electrocatalysis. (C) Nickel-catalyzed C–N cross coupling of aryl triflates and amines facilitated by triethylamine.
RESULTS AND DISCUSSION
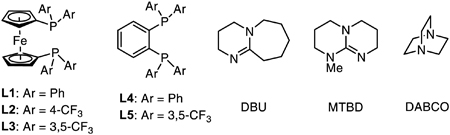
Our studies began with an evaluation of commercially available bidentate phosphine ligands and organic bases in a model transformation, the Ni-catalyzed cross-coupling of phenyl triflate (1) and aniline. Selected results from these studies are summarized in Table 1 (see Supporting Information for further experimental details). When we used Ni(COD)2 (4 mol%) and 1,1′-bis(diphenylphosphino)ferrocene (L1, DPPF) as precatalysts and triethylamine (TEA) as base, a 6% yield of the desired product was observed, with unreacted 1 making up the remainder of the mass balance. As in our previous work on Pd-catalyzed amination, we predicted that a more electron-deficient metal center would better facilitate the deprotonation of an amine-bound Ni complex by a base as weak as TEA.12a Accordingly, we prepared several DPPF derivatives bearing electron-withdrawing trifluoromethyl (−CF3) substituents on the P-aryl groups.21 Indeed, use of the fourfold trifluoromethylated ligand L2 ([CF3]4-DPPF) resulted in 32% yield of the desired product. The yield was further increased to 94% by employing the further trifluoromethylated ligand L3 ([CF3]8-DPPF). A Ni-based catalyst bearing this ligand had previously been shown to facilitate the cross-coupling of aryl chlorides with indoles and primary aliphatic amines using NaOt-Bu as the base.22
Table 1.
Comparison of ligands and bases in the Ni-catalyzed cross-coupling of phenyl triflate (1) and aniline.
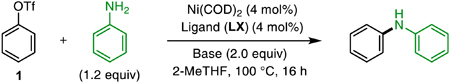 | ||
|---|---|---|
| Ligand | Base | Yield (%) |
| L1, DPPF | NEt3 | 6 |
| L2, [CF3]4-DPPF | NEt3 | 32 |
| L3, [CF3]8-DPPF | NEt3 | 94 |
| L4, DPPBz | NEt3 | Trace |
| L5, [CF3]8-DPPBz | NEt3 | 23 |
| L3, [CF3]8-DPPF | i-Pr2NEt | 87 |
| L3, [CF3]8-DPPF | DABCO | 17 |
| L3, [CF3]8-DPPF | DBU | Trace |
| L3, [CF3]8-DPPF | MTBD | Trace |
 | ||
GC yields were determined relative to hexamethylbenzene internal standard and are reported as a single run. Reaction conditions: phenyl triflate (0.20 mmol), aniline (0.24 mmol), base (0.40 mmol), Ni(COD)2 (0.016 mmol, 4 mol% Ni), ligand (0.016 mmol, 4 mol%), and 2-MeTHF (0.40 mL, 0.50 M). 2-MeTHF = 2-methyltetrahydrofuran.
The ferrocene backbone was also found to be important to the success of these reactions: other ligands containing similar trifluoromethylated aryl groups, such a 1,2-bis(diphenylphosphino)benzene (DPPBz) derivative (L5, [CF3]8-DPPBz) were less effective in promoting the C–N coupling reaction. TEA, besides being advantageous in terms of cost and mildness, was also uniquely efficacious as a base. Several stronger bases that had been reported to facilitate Pd-catalyzed amination reactions, including DBU and 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD), were essentially unable to promote our Ni-catalyzed transformation. Other alkylamine bases, such as 1,4-diazabicyclo[2.2.2]octane (DABCO) and DIPEA, could be used instead of TEA, but with lower reaction yields.
Although Ni(COD)2 is a convenient source of Ni(0) for reaction discovery and mechanistic studies, the complex is highly sensitive to air and moisture, generally requiring the use of an inert atmosphere glovebox to handle.23 To alleviate the associated operational complications, we aimed to develop an air-stable Ni precatalyst bearing L3, the most effective ligand.24 Our initial efforts focused on the use of σ-aryl “oxidative addition” (OA) complexes of aryl bromides and chlorides.25 However, OA complexes bearing L3 and various aryl groups,26 including o-tolyl and mesityl, were unable to facilitate the reaction, even when activated with reducing additives including phenylboronic acid and activated olefins. We hypothesized that the presence of strongly associating halide anions inhibits C–N coupling by outcompeting aniline for binding to Ni (see below for further mechanistic discussion).27 Predicated on this lack of reactivity, we sought to prepare OA complexes bearing non-coordinating triflate anions.28 However, due to the propensity of coordinatively unsaturated Ni(II) complexes to undergo bimetallic decomposition pathways, our attempts to isolate Ni(II) σ-aryl complexes bearing triflate anions were not successful. Based on the work of Nolan29 and Hazari,30 we hypothesized that the introduction of an η3-allyl group would saturate the Ni coordination sphere without introducing new strongly-coordinating ligands such as halides. Combining a commercially available methallyl nickel chloride dimer with L3 in the presence of THF led to the formation of L3-Ni(Cl)(η3-methallyl).31 This complex was not purified, but immediately treated with trimethylsilyl triflate (TMS–OTf), upon which a methallyl nickel triflate complex was rapidly formed.32 The structure of this complex (P1) was unambiguously characterized using X-ray diffraction (Figure 2). Under optimized reaction conditions, this precatalyst (P1) facilitated the C–N coupling reaction and provided the desired product in 98% yield in 2 h. Analysis of the crude reaction mixture (GC/MS) showed that N-methallyl aniline was formed during the reaction, consistent with activation of P1 through outer-sphere nucleophilic attack by aniline at the methallyl ligand.33
Figure 2.
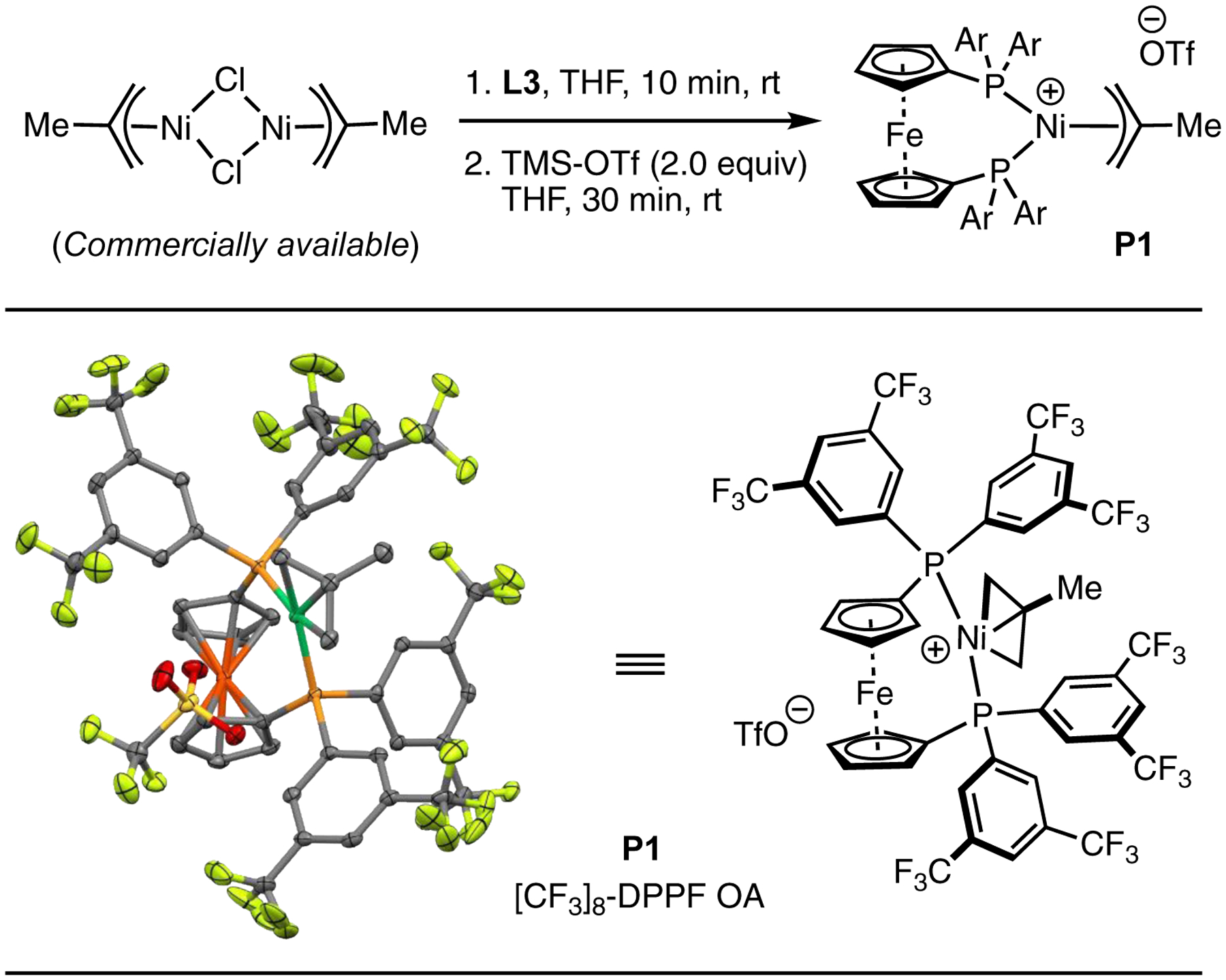
Synthesis and crystal structure of an L3-bound methallyl triflate–nickel oxidative addition complex. Thermal ellipsoids are shown at 50% probability. Hydrogen atoms are omitted for clarity.
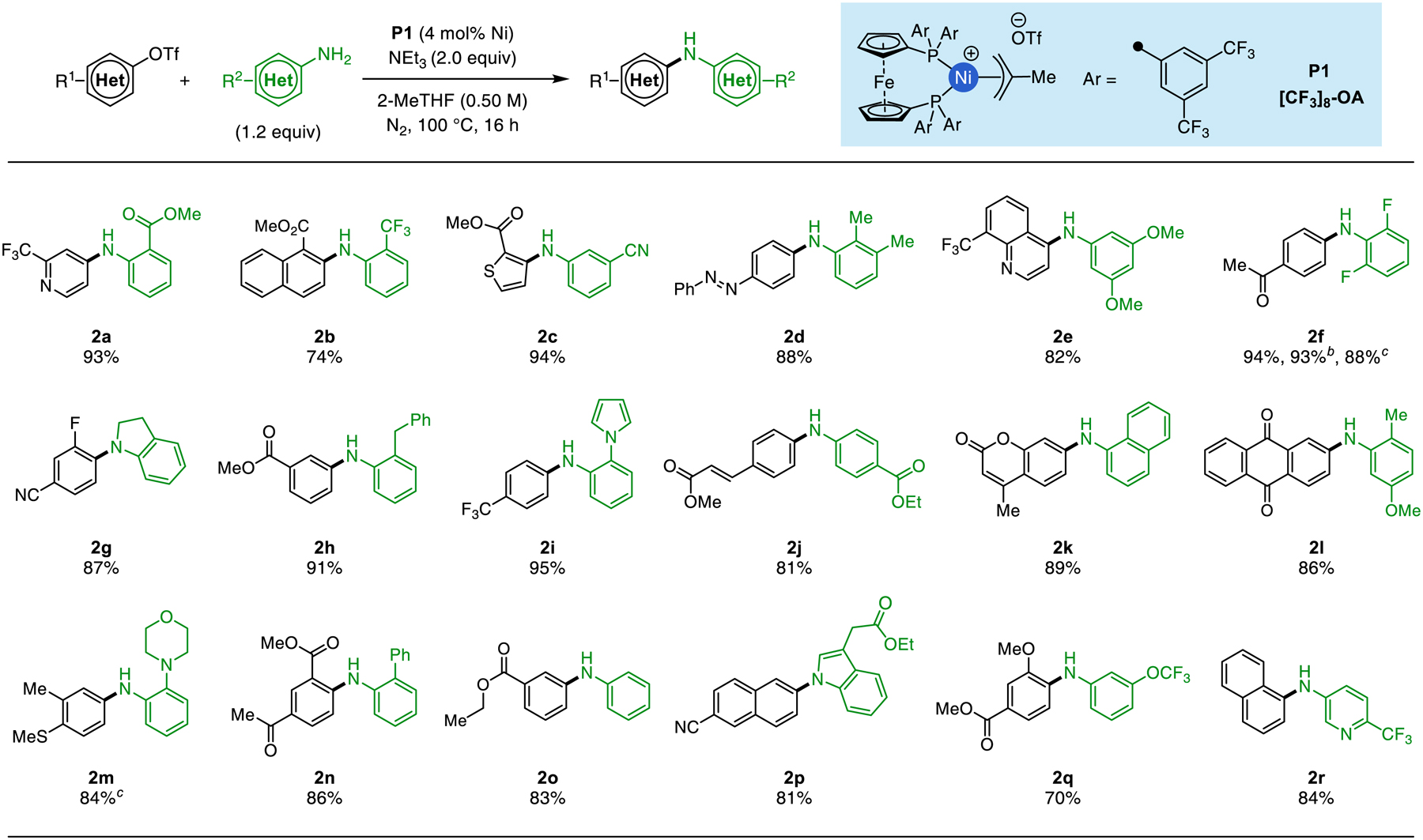
Using this new precatalyst, we explored the scope of the cross-coupling reaction by testing a variety of aryl triflate electrophiles and amine nucleophiles. In contrast to some Pd-catalyzed amination procedures, in particular those facilitated by soluble organic bases, this methodology is tolerant of sterically encumbered coupling partners. Specifically, aryl triflates and anilines bearing bulky ortho-substituents, such as trifluoromethyl (2b), benzyl (2h), morpholino (2m) and phenyl (2n), underwent coupling in high yields. In contrast to traditional Ni-catalyzed amination protocols that work well for strongly coordinating alkylamine nucleophiles, the current method is especially effective for weakly coordinating anilines, including those bearing cyano (2c), trifluoromethoxy (2q), and carbonyl substituents (2a, 2j). Additionally, secondary aryl amines, including indoline (2g) and a 3-substituted indole (2p) could be arylated in high yields.22 We note, however, that aliphatic amines do not react under these conditions, likely due to their decreased acidity compared to anilines.34 Coupling partners containing heterocycles, including pyridines (2a, 2r), a quinoline (2e), a thiophene (2c), and a pyrrole (2i) were tolerated well. Several electrophilic functional groups, including methyl esters (2a-c, 2h, 2n) and nitriles (2c, 2g, 2p), remained intact under the mildly basic reaction conditions. An α,ß-unsaturated ester (2j), and a coumarin derivative (2k) could be cross-coupled under these reaction conditions, despite their potential to react with anilines in metal-catalyzed aza-Michael reactions.35 Moreover, substrates bearing redox-sensitive functional groups, including an anthraquinone (2l) are tolerated.36 Finally, because reproducibility issues associated with stirring rate can occur in amination protocols featuring inorganic bases37 or electric potentials,18b we wished to demonstrate that this method is not dependent on mixing efficiency. To show this, we prepared 2f without using a stir bar or external agitation. The desired product was obtained in 93% yield, which is in line with that obtained when magnetic stirring was used. This result suggests that these single-phase reactions are less prone to reproducibility issues when varied stirring techniques are used.
The proposed catalytic cycle of this Ni-catalyzed amination reaction is summarized in Figure 3.20b,38 First, activation of P1 via nucleophilic attack of the aniline at the methallyl group provides a L3-supported Ni(0) catalyst. Next, Ni undergoes oxidative insertion into the aryl triflate. Then, the amine binds to the Lewis acidic Ni(II) metal center, acidifying its hydrogens for deprotonation by TEA. Finally, reductive elimination from resultant Ni(II)–amido complex affords the desired product and regenerates the Ni(0) catalyst. Although this proposed mechanism is directly analogous to that of other Ni- and Pd- catalyzed C–N cross-coupling reactions,13 it was important to determine how the highly fluorinated ligand L3 might affect the elementary steps, and importantly, how it is able to facilitate the catalytic transformation using such a weak base (TEA).
Figure 3.
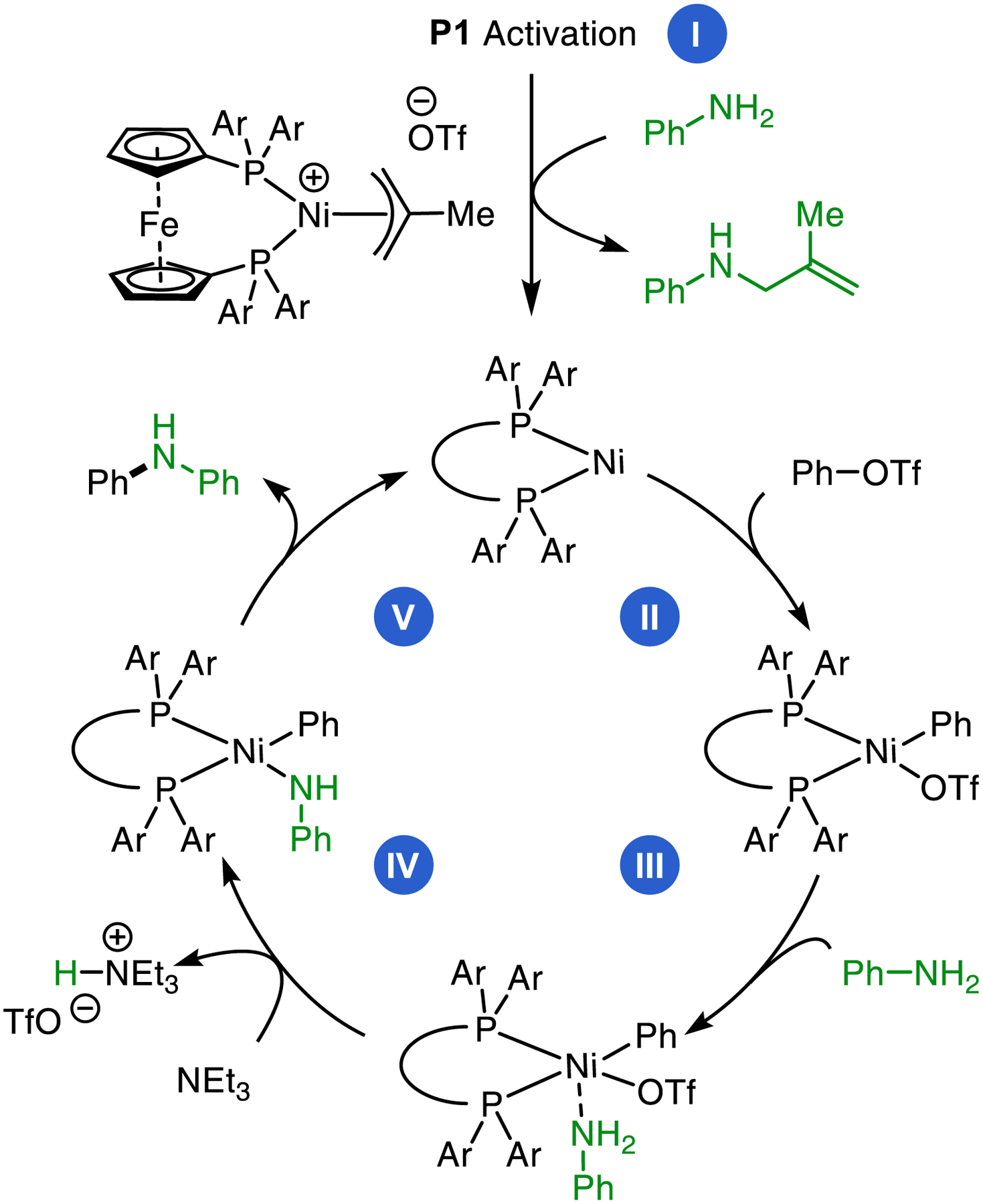
The proposed catalytic cycle for the nickel-catalyzed cross-coupling of aryl halides with anilines: I, activation of P1; II, oxidative addition of an aryl triflate; III, aniline binding to OA complex; IV, deprotonation of an amine-bound OA complex; V, reductive elimination of an amido complex.
Using density functional theory (DFT) calculations we obtained a model of the catalytic mechanism using phenyl triflate (1) and aniline as substrates. The energy profile of this mechanism is illustrated in Figure 4. The binding of phenyl triflate to L3-Ni(0) (complex I) was found to be exergonic by 10.1 kcal/mol (II). From this π-complex, oxidative addition through an SNAr-type mechanism39 was predicted to be extremely rapid, with a barrier of only 9.0 kcal/mol (II-TS), and thermodynamically favorable, releasing 26.5 kcal/mol of free energy (III). In the ground state of the resultant Ni(II) complex III, the triflate anion was bound to Ni, although its dissociation appeared possible under the reaction conditions (+16.9 kcal/mol, IV). Regardless, the displacement of the triflate ligand by aniline is only slightly endergonic (+7.5 kcal/mol, V). Interestingly, deprotonation of this cationic Ni–aniline complex by TEA was predicted to be slightly favorable in free energy (−0.7 kcal/mol, VI). Reductive elimination from this amido complex through a three-membered transition state (+16.4 kcal/mol, VI-TS) would then provide the diphenylamine product.
Figure 4.
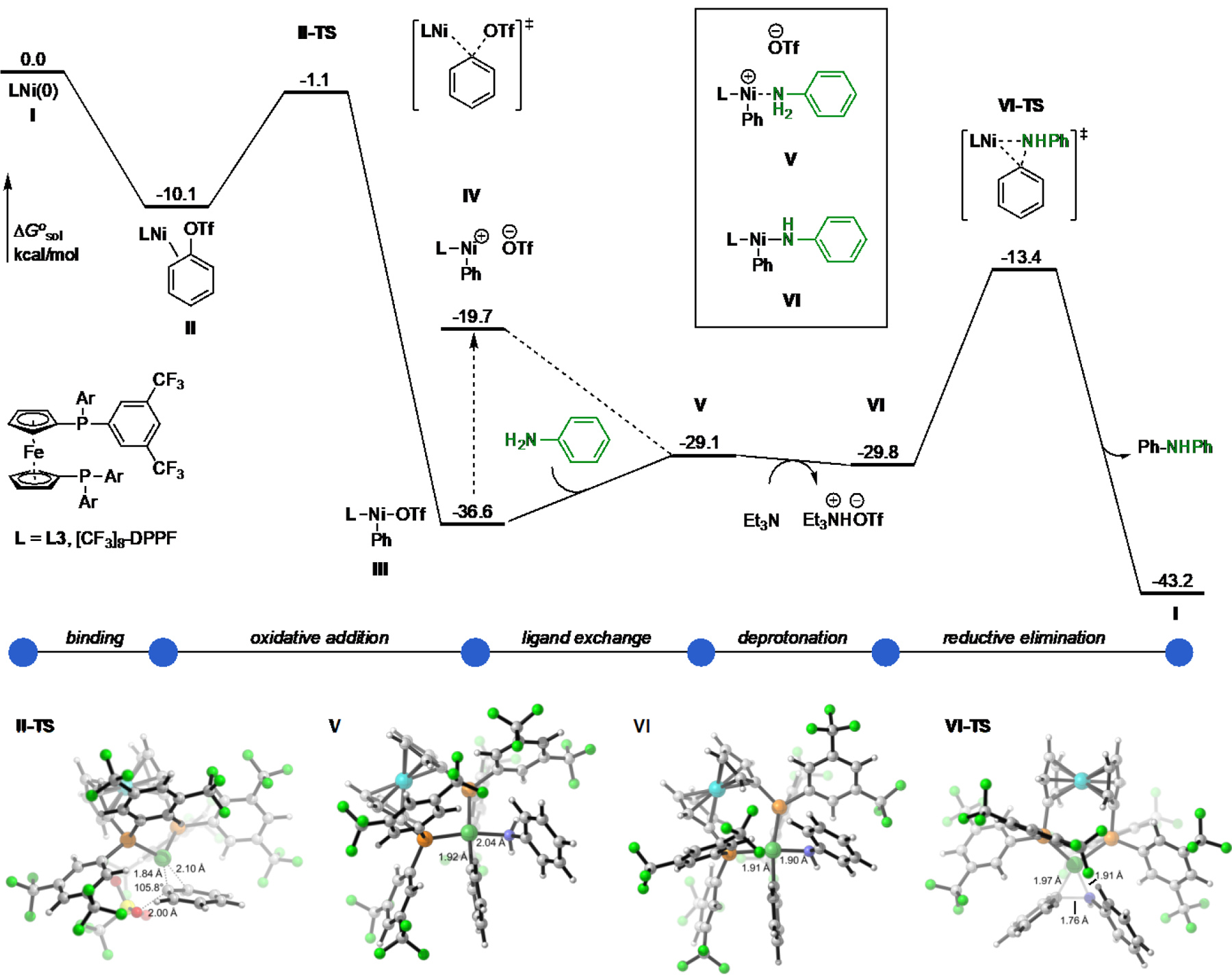
Computed energy profiles for the Ni-catalyzed cross-coupling of 1 and aniline. Gibbs free energy values calculated with M06/6–311+G(d,p)-SDD(Ni,Fe)//B3LYP/6–31G(d)-SDD(Ni,Fe).
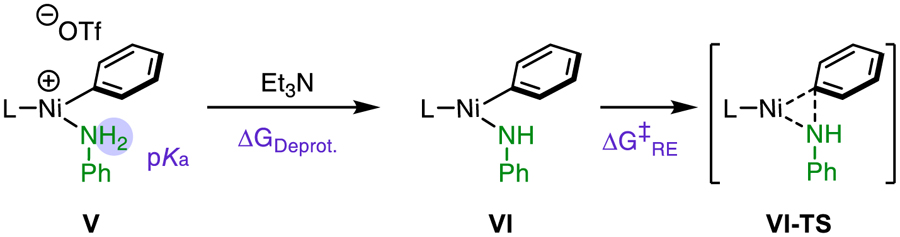
Considering our original hypothesis that the electron-deficiency of the ligand had a favorable influence on the thermodynamics of the deprotonation step, we more closely examined the effect of varying the phosphine ligand on this process. Table 3 shows the pKa of several amine-bound Ni(II) complexes analogous to Vas well as triethylammonium triflate and aniline for comparison purposes. The free energy change associated with the proton transfer step can be calculated on the basis of pKa differences. The deprotonation of weakly acidic aniline (pKa = 28) by triethylamine (pKaH+ = 12.5)40 is thermodynamically highly disfavored. However, association of the aniline to cationic Ni(II) results in dramatic acidification, to the extent of roughly 13 pKa units in THF when the ligand is DPPF (L1). With the addition of electron-withdrawing groups on the ligand, the amine is further acidified. Indeed, in the complex with L3, the aniline is sufficiently activated that it is predicted to be more acidic (pKa = 12.2) than triethylammonium triflate. Thus, deprotonation by triethylamine is in this case slightly thermodynamically favorable.
Table 3.
Ligand effects on deprotonation and reductive elimination. a
 | |||||
|---|---|---|---|---|---|
| Ligand for V | pKa (THF) | ΔGDeprot. (kcal/mol) | ΔG‡RE (kcal/mol) | ΔG‡RE + ΔGDeprot. | Yield (%) |
| L1 | 15.3 | 6.4 | 17.2 | 23.6 | 6% |
| L2 | 13.4 | 2.0 | 16.7 | 18.7 | 32% |
| L3 | 12.2 | −0.6 | 16.4 | 15.8 | 94% |
| L5 | 13.0 | 1.3 | 17.9 | 19.2 | 23% |
| PhNH2 | 28.5 | ||||
| Et3N•HOTf | 12.5 (experimental, see ref. 40) | ||||
 | |||||
Gibbs free energy values and acidities calculated with M06/6–311+G(d,p)-SDD(Ni,Fe)//B3LYP/6–31G(d)-SDD(Ni,Fe), except for the pKa of Et3NHOTf, used as a reference, which was obtained from the literature.40
We also found that the barrier to reductive elimination is also somewhat affected by the electronic properties of the phosphine ligand: as the number of trifluoromethyl substituents on the catalyst increase, the reductive elimination is increasingly facile. For comparison, we also evaluated an analogue derived of L3 from DPPBz (L4). With the L4-ligated catalyst, the free energy of deprotonation and barrier to reductive elimination were both higher (+1.9 kcal/mol and +1.5 kcal/mol, respectively) than from the L3-bound complexes. Thus, not only the identity of the P-aryl groups, but the backbone structure of the chelating ligand significantly influences these steps. The combined barrier from deprotonation–reductive elimination sequence is also shown in Table 3. The net activation energies are qualitatively consistent with the experimentally determined yields using these catalysts.
Finally, our model also explained the superior performance of triethylamine compared to other organic bases, even those that were significantly stronger bases. Previously, in experimental13a and theoretical13b mechanistic studies of Pd-catalyzed amination using DBU, we found that off-cycle binding of the base to Pd could have an inhibitory effect. We investigated the relative binding ability of TEA, DBU, and aniline to the cationic intermediate IV (Figure 5). As a reference, we had found earlier that the binding of triflate to IV is exergonic by 16.9 kcal/mol. Due presumably to steric interactions, the binding of TEA to IV is significantly disfavored (ΔG° = +7.8 kcal/mol), in a manner similar to well-known “frustrated” Lewis acid-base pairs.41 Accordingly, aniline can outcompete the base for binding (ΔG° = −9.4 kcal/mol for aniline binding to IV), and the productive reaction can take place. In contrast, when DBU is present, we found that it tightly coordinates to IV (ΔG° = −20.8 kcal/mol), sequestering Ni in this off-cycle resting state and thus increasing the overall activation energy for cross-coupling. We believe that this effect explains the unique effectiveness of TEA compared to stronger, more nucleophilic organic bases.
Figure 5.
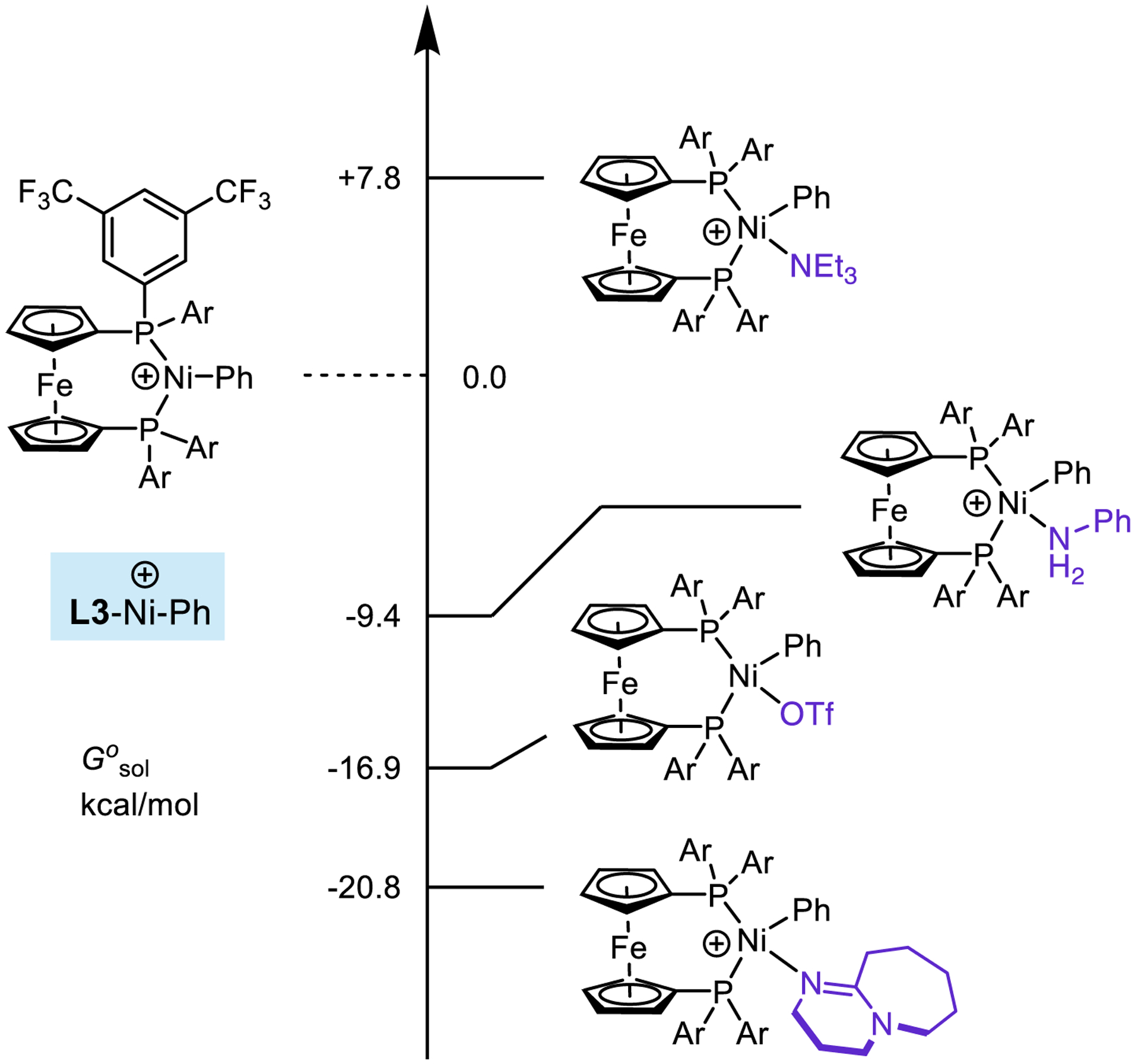
Relative binding energies of triethylamine, triflate anion, and DBU to an L3-supported, cationic nickel complex. Gibbs free energy values calculated with M06/6–311+G(d,p)-SDD(Ni,Fe)//B3LYP/6–31G(d)-SDD(Ni,Fe).
CONCLUSION
In summary, we have developed a novel Ni(II) precatalyst bearing an electron-deficient DPPF-derived ligand (L3) that is able to facilitate the cross-coupling of aryl triflates with primary anilines, as well as indolines and indoles. The precatalyst is a rare example of a bench-stable cationic Ni(II) triflate complex and represents a new class of halide-free Ni precatalysts that might have general applicability to reactions currently requiring Ni(COD)2. Using DFT calculations, relationships between ligand structure and the energetics of the key deprotonation and reductive elimination steps were elucidated. Moreover, we determined that the unique effectiveness of alkylamine bases can be attributed to their steric bulk, which prevents unwanted binding to cationic Ni intermediates. We anticipate that these mechanistic insights can assist in the development of new Ni-catalyzed cross-coupling methodologies that employ soluble organic bases.
Supplementary Material
Table 2.
Amination of Aryl Triflates using P1a
|
Isolated yields are reported as the average of two runs. Unless noted, standard reaction conditions: aryl triflate (1.0 mmol), aryl amine (1.2 mmol), triethylamine (2.0 mmol), P1(0.04 mmol, 4% Ni), 2-MeTHF (2.0 mL, 0.5 M), 100 °C for 16 h.
Single reaction performed without stirring.
5.0 mmol scale reaction using 2.0% Ni.
1.5 equiv of aryl amine was used.
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institutes of Health (R35GM122483) and the NSF Graduate Research Fellowship Program under Grant No. 1122374 (J.M.D.). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIH or NSF. The authors thank Dr. Frieda Zhang for her assistance in elucidating the substrate scope of this methodology and Jacob Rodriguez for assistance with mass spectroscopy experiments. We acknowledge Dr. James Bour for helpful discussions throughout the duration of this study and Dr. Peter Müller for crystallographic analysis. We also thank Dr. Scott McCann, Dr. Alexander Schuppe, and Dr. Christine Nguyen for their assistance in the preparation of this manuscript.
Footnotes
Supporting Information
Experimental procedures; computational, spectral, and reaction optimization data; X-ray data.
REFERENCES
- (1).(a) Ruiz-Castillo P; Buchwald SL Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev 2016, 116, 12564–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Magano J; Dunetz JR Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev 2011, 111, 2177–2250. [DOI] [PubMed] [Google Scholar]
- (2).Beletskaya IP; Cheprakov AV The Complementary Competitors: Palladium and Copper in C–N Cross-Coupling Reactions. Organometallics 2012, 31, 7753–7808. [Google Scholar]
- (3).For recent examples, see:; (a) (b) Xu J; Liu RY; Yeung CS; Buchwald SL Monophosphine Ligands Promote Pd-Catalyzed C–S Cross-Coupling Reactions at Room Temperature with Soluble Bases. ACS Catalysis 2019, 9, 6461–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Laffoon SD; Chan VS Fickes MG; Kotechki B; Ickes AR Henle J; Napolitano JG; Franczyk TS; Dunn TB; Barnes DM; Haight AR; Henry RF Shekhar S Pd-Catalyzed Cross-Coupling Reactions Promoted by Biaryl Phosphorinane Ligands. ACS Catal. 2019, 9, 11691–11708. [Google Scholar]; (c) For a recent example of a reaction featuring a fully soluble trimethylsilanolate base, see:; Delaney CP; Kassel VM; Denmark SE Potassium Trimethylsilanolate Enables Rapid, Homogeneous Suzuki-Miyaura Cross-Coupling of Boronic Esters. ACS Catal. 2020, 10, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Brewer AC; Hoffman PC; Martinelli JR; Kobierski ME; Mullane N; Robbins D Development and Scale-Up of a Continuous Aerobic Oxidative Chan-Lam Coupling. Org. Process Res. Dev 2019, 23, 1484–1498. [Google Scholar]; (b) Kashani SK; Sullivan RJ; Andersen M; Newman SG Overcoming Solid Handling Issues in Continuous Flow Substitution Reactions Through Ionic Liquid Formation. Green Chem. 2018, 20, 1748–1753. [Google Scholar]
- (5).For an example of this, see:; Sperry JB; Price Wiglesworth KE; Edmonds I; Fiore P; Boyles DC; Damon DB; Dorow RL; Piatnitski Chekler EL; Langille J; Coe JW Kiloscale Buchwald–Hartwig Amination: Optimized Coupling of Base-Sensitive 6-Bromoisoquinoline-1-carbonitrile with (S)-3-Amino-2-methylpropan-1-ol. Org. Process Res. Dev 2014, 18, 1752–1758. [Google Scholar]
- (6).(a) Buitrago Santanilla A; Christensen M; Campeau L-C; Davies IW; Dreher SD P2Et Phosphazene: A Mild, Functional Group Tolerant Base for Soluble, Room Temperature Pd-Catalyzed C–N, C–O, and C–C Cross-Coupling Reactions. Org. Lett 2015, 17, 3370–3373. [DOI] [PubMed] [Google Scholar]; (b) Buitrago Santanilla A; Regalado EL; Pereira T; Shevlin M; Bateman K; Campeau L-C; Schneeweis J; Berritt S; Shi Z-C; Nantermet P; Liu Y; Helmy R; Welch CJ; Vachal P; Davies IW; Cernak T; Dreher SD Nanomole-Scale High-Throughput Chemistry for the Synthesis of Complex Molecules. Science 2015, 347, 49–53. [DOI] [PubMed] [Google Scholar]; (c) Ahneman DT; Estrada JG; Lin S; Dreher SD; Doyle AG Predicting Reaction Performance in C–N Cross-Coupling Using Machine Learning. Science 2018, 360, 186–190. [DOI] [PubMed] [Google Scholar]; (d) Gesmundo NJ; Sauvagnat B; Curran PJ; Richards MP; Andrews CL; Dandliker PJ; Cernak T Nanoscale Synthesis and Affinity Ranking. Nature 2018, 557, 228–232. [DOI] [PubMed] [Google Scholar]; (e) Uehling MR; King RP; Krska SW; Cernak T Buchwald SL Pharmaceutical Diversification via Palladium Oxidative Addition Complexes. Science 2019, 363, 405–408. [DOI] [PubMed] [Google Scholar]
- (7).(a) Tundel RE; Anderson KW; Buchwald SL Expedited Palladium-Catalyzed Amination of Aryl Nonaflates through the Use of Microwave-Irradiation and Soluble Organic Amine Bases. J. Org. Chem 2006, 71, 430–433. [DOI] [PubMed] [Google Scholar]; (b) Beutner GL Coombs JR; Green RA; Inankur B; Lin D; Qiu J;Roberts F; Simmons EM; Wisniewski SR Palladium-Catalyzed Amidation and Amination of (Hetero)aryl Chlorides under Homogeneous Conditions Enabled by a Soluble DBU/NaTFA Dual-Base System. Org. Process Res. Dev 2019, 23, 1529–1537. [Google Scholar]; (c) Kashani SK; Jessiman JE; Newman S Exploring Homogeneous Conditions for Mild Buchwald-Hartwig Amination in Batch and Flow. Preprint: 10.26434/chemrxiv.10094048.v1. [DOI] [Google Scholar]
- (8).Murthy Bandaru SS; Bhilare S; Chrysochos N; Gayakhe V; Trentin I; Schulzke C; Kapdi AR Pd/PTABS: Catalyst for Room Temperature Amination of Heteroarenes. Org. Lett 2018, 20, 473–476. [DOI] [PubMed] [Google Scholar]
- (9).(a) Liu L; Frohn M; Xi N; Dominguez C; Hungate R; Reider PJ A Soluble Base for the Copper-Catalyzed Imidazole N-Arylations with Aryl Halides. J. Org. Chem 2005, 70, 10135–10138. [DOI] [PubMed] [Google Scholar]; (b) Sung S; Sale D; Braddock DC; Armstrong A Brennan C; Davies RP Mechanstic Studies on the Copper-Catalyzed N-Arylation of Alkylamines Promoted by Soluble Ionic Bases. ACS Catal. 2016, 6, 3965–3974. [Google Scholar]; (c) Lo QA; Sale D; Braddock DC; Davies RP Mechanistic and Performance Studies on the Ligand-Promoted Ullmann Amination Reaction. ACS Catal. 2018, 8, 101–109. [Google Scholar]
- (10).Morgenthaler M; Schweizer E; Hoffmann-Röder A; Benini F; Martin RE; Jaeschke G; Wagner B; Fischer H; Bendels S; Zimmerli D; Schneider J; Diederich F; Kansy F; Müller K Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem 2007, 2, 1100–1115. [DOI] [PubMed] [Google Scholar]
- (11).Roose P; Eller K; Henkes E; Rossbacher Höke, H. Ullmann’s Encyclopedia of Industrial Chemistry. In Amines, Aliphatic, Wiley, Hoboken, 2015. [Google Scholar]
- (12).(a) Dennis JM; White NA; Liu RY; Buchwald SL Breaking the Base Barrier: An Electron-Deficient Palladium Catalyst Enables the Use of a Common Soluble Base in C–N Coupling. J. Am. Chem. Soc 2018, 140, 4721–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) For additional applications of this catalyst system, see:; Engl PS; Häring AP; Berger F; Berger G; Pérez-Bitrián A; Ritter T C–N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc 2019, 141, 13346–13351. [DOI] [PubMed] [Google Scholar]
- (13).(a) Dennis JM; White NA; Liu RY; Buchwald SL Pd-Catalyzed C–N Coupling Reactions Facilitated by Organic Bases: Mechanistic Investigation Leads to Enhanced Reactivity in the Arylation of Weakly Binding Amines. ACS Catal. 2019, 9, 3822–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]; For computational mechanistic studies, see:; (b) Kim S-T; Pudasaini B; Baik M-H Mechanism of Palladium-Catalyzed C–N Coupling with 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) as a Base. ACS Catal. 2019, 9, 6851–6856. [Google Scholar]; (c) Sunesson Y; Limé E; Nilsson Lill SO; Meadows RE; Norrby P-O Role of the Base in Buchwald–Hartwig Amination. J. Org. Chem 2014, 79, 11961–11969. [DOI] [PubMed] [Google Scholar]
- (14).Baumgartner LM; Dennis JM; White NA; Buchwald SL; Jensen KF Use of a Droplet Platform To Optimize Pd-Catalyzed C–N Coupling Reactions Promoted by Organic Bases. Org. Process Res. Dev 2019, 23, 1594–1601. [Google Scholar]
- (15).(a) Christau HJ; Desmurs JR Arylation of Hard Heteroatomic Nucleophiles Using Bromoarenes Substrates and Cu, Ni, Pd-Catalysts. Ind. Chem. Libr 1995, 7, 240. [Google Scholar]; For the first general method, see:; (b) Wolfe JP; Buchwald SL Nickel-Catalyzed Amination of Aryl Chlorides. J. Am. Chem. Soc 1997, 119, 6054–6058. [Google Scholar]; (b) For a detailed history of this topic, see:; Marín M; Rama RJ; Nicasio MC Ni-Catalyzed Amination Reactions: An Overview. Chem. Rec 2016, 16, 1819–1832. [DOI] [PubMed] [Google Scholar]
- (16).For recent examples, see:; (a) Lavoie CM; Tassone JP; Ferguson MJ; Zhou Y; Johnson ER; Stradiotto M Probing the Influence of PAd-DalPhos Ancillary Ligand Structure on Nickel-Catalyzed Ammonia Cross-Coupling. Organometallics, 2018, 37, 4015–4023. [Google Scholar]; (b) McGuire RT; Paffile JFJ; Zhou Y; Stradiotto M Nickel-Catalyzed C-N Cross-Coupling of Ammonia, (Hetero)anilines, and Indoles with Activated (Hetero)aryl Chlorides Enabled by Ligand Design. ACS Catal., 2019, 9, 9292–9297. [Google Scholar]; For a thorough review on this topic in regard to bisphosphines, see:; (c) Lavoie CM; Stradiotto M Bisphosphines: A Prominent Ancillary Ligand Class for Application in Nickel-Catalyzed C–N Cross-Coupling. ACS Catal. 2018, 8, 7228–7250. [Google Scholar]
- (17).(a) Corcoran EB; Pirnot MT; Lin S; Dreher SD; DiRocco DA; Davies IW; Buchwald SL; MacMillan DWC Aryl Amination Using Ligand-Free Ni(II) Salts and Photoredox Catalysis. Science 2016, 353, 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kudisch M; Lim C-H; Thordarson P; Miyake GM Energy Transfer to Ni-Amine Complexes in Dual Catalytic, Light-Driven C–N Cross-Coupling reactions. J. Am. Chem. Soc 2019, 141, 19479–19486. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lim C-H; Kudisch M; Liu B Miyake GM C–N Cross-Coupling via Photoexcitation of Nickel-Amine Complexes. J. Am. Chem. Soc 2018, 140, 7667–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Park BY; Pirnot MT; Buchwald SL Visible Light-Mediated (Hetero)aryl Amination Using Ni(II) Salts and Photoredox Catalysis in Flow: A Synthesis of Tetracaine. J. Org. Chem 2020, 10.1021/acs.joc.9b03107. [DOI] [PubMed] [Google Scholar]
- (18).(a) Li C; Kawamata Y; Nakamura H; Vantourout JC; Liu Z; Hou Q; Bao D; Starr JT; Chen J; Yan M; Baran PS Electrochemically Enabled, Nickel-Catalyzed Amination. Angew. Chem. Int. Ed 2017, 56, 13088–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kawamata Y; Vantourout JC; Hickey DP; Bai P; Chen L; Hou Q; Qiao W; Barman K; Edwards MA; Garrido-Castro AF; deGruyter JN; Nakamura H; Knouse K; Qin C; Clay KJ; Bao D; Li C; Starr JT; Garcia-Irizarry C; Sach N; White HS; Neurock M; Minteer SD; Baran PS Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications. J. Am. Chem. Soc 2019, 141, 6392–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).(a) Wiensch EM; Montgomery J Nickel-Catalyzed Amination of Silyloxyarenes through C–O Bond Activation. Angew. Chem. Int. Ed 2018, 57, 11045–11049. [DOI] [PubMed] [Google Scholar]; (b) Harada T; Ueda Y; Iwai T; Sawamura M Nickel-Catalyzed Amination of Aryl Fluoride with Primary Amines. Chem. Commun 2018, 54, 1718–1721. [DOI] [PubMed] [Google Scholar]
- (20).(a) Borzenko A; Rotta-Loria NL; MacQueen PM; Lavoie CM McDonald, R.; Stradiotto, M. Nickel-Catalyzed Monoarylation of Ammonia. Angew. Chem. Int. Ed, 2015, 54, 3773–3777. [DOI] [PubMed] [Google Scholar]; (b) Ge S; Green RA; Hartwig JF Controlling First-Row Catalysts: Amination of Aryl and Heteroaryl Chlorides and Bromides with Primary Aliphatic Amines Catalyzed by a BINAP-Ligated Single-Component Ni(0) Complex. J. Am. Chem. Soc 2014, 136, 1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Green RA; Hartwig JF Nickel-Catalyzed Amination of Aryl Chlorides with Ammonia or Ammonium Salts. Angew. Chem. Int. Ed 2015, 54, 3768–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).For discussion on the preparation and use of DPPF derivatives in Ni-catalyzed amination reactions, see:; McGuire RT; Clark JSK Gatien AV; Shen MY; Ferguson MJ; Stradiotto M Bulky 1,1’-Ferrocenyl Ligands Featuring Diazaphospholene or Dioxaphosphepine Donor Fragments: Catalytic Screening in Nickel-Catalyzed C-N Cross-Coupling. Eur. J. Inorg. Chem 2019, 4112–4116. [Google Scholar]
- (22).Clark JSK; Voth CN; Ferguson MJ; Stradiotto M Evaluating 1,1’-Bisphosphinoferrocene Ancillary Ligand Variants in the Nickel-Catalyzed C–N Cross-Coupling of (Hetero)aryl Chlorides. Organometallics 2017, 36, 679–686. [Google Scholar]
- (23).Nattmann L; Saeb R; Nöthling N; Cornella J An Air-Stable Binary Ni(0)–Olefin Catalyst. Nat. Catal 2020, 3, 6–13. [Google Scholar]
- (24).For an overview of Ni precatalysts, see:; Hazari N; Melvin PR; Mohadjer Beromi M Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat. Rev. Chem 2017, 1, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).For applications of these precatalysts, see:; Standley EA; Jamison TF Simplifying Nickel(0) Catalysis: An Air-Stable Nickel Precatalyst for the Internally Selective Benzylation of Terminal Alkenes. J. Am. Chem. Soc 2013, 135, 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).For a discussion on the effect of the aryl group on the reactivity of DPPF-ligated σ-aryl precatalysts, see:; Mohadjer Beromi M; Banerjee G; Brudvig GW; Charboneau DJ; Hazari N; Lant HMC; Mercado BQ Modifications to the Aryl Group of DPPF-Ligated Ni σ-Aryl Precatalysts: Impact on Speciation and Catalytic Activity in Suzuki-Miyaura Coupling Reactions. Organometallics 2018, 37, 3943–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).The addition of halide scavengers, including AgOTf and AgBF4, resulted in one turnover of the catalyst followed by catalyst death and the formation of Ni black.
- (28).For an example of Pd precatalysts bearing triflate anions, see:; DeAngelis AJ, Gildner PG, Chow R; Colacot TJ Generating Active “L-Pd(0)” Via Neutral or Cationic π-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphosphines: Synthetic, Mechanistic, and Structure–Activity Studies in Challenging Cross-Coupling reactions. J. Org. Chem 2015, 80, 6794–6813. [DOI] [PubMed] [Google Scholar]
- (29).Martin AR; Nelson DJ; Meiries S; Slawin AMZ; Nolan SP Efficient C–N and C–S Bond Formation Using the Highly Active [Ni(allyl)Cl(IPr*OMe)] Precatalyst. Eur. J. Org. Chem 2014, 3127–3131. [Google Scholar]
- (30).Beromi MM; Banerjee G; Brudvig GW; Hazari N Mercado, B. Q. Nickel(I) Aryl Species: Synthesis, Properties, and Catalytic Activity. ACS Catal. 2018, 8, 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).These catalysts have been shown to be active in other Ni-catalyzed cross-coupling methodologies. See:; Ge S; Hartwig JF Highly Reactive, Single-Component Nickel Catalyst Precursor for Suzuki– Miyuara Cross-Coupling of Heteroaryl Boronic Acids with Heteroaryl Halides. Angew. Chem. Int. Ed 2012, 51, 12837–12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Weber JM; Longstreet AR; Jamison TF Bench-Stable Nickel Precatalysts with Heck-type Activation. Organometallics 2018, 37, 2716–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).For another example of this reactivity, see:; Pawlas J; Nakao Y; Kawatsura M; Hartwig JF A General Nickel-Catalzyed Hydroaminatino of 1,3-Dienes by Alkylamines: Catalyst Selection, Scope, and Mechanism. J. Am. Chem. Soc 2002, 124, 3669–3669. [DOI] [PubMed] [Google Scholar]
- (34).Preliminary results have shown that stronger organic bases, including phosphazene base tert-butylimino-tri(pyrrolidino)phosphorane (BTTP), can facilitate the cross-coupling of phenyl triflate and benzyl amine in the presence of P1.
- (35).For examples of the addition of aniline derivatives to α,ß-unsaturated electrophiles, see:; (a) Kim S; Kang S; Kim G; Lee Y Copper-Catalyzed Aza-Michael Addition of Aromatic Amines or Aromatic Aza-Heterocycles to α,ß-Unsaturated Olefins. J. Org. Chem 2016, 81, 4048–4057. [DOI] [PubMed] [Google Scholar]; (b) Fei X-D; Zhou Z; Li W; Zhu Y-M; Shen J-K Buchwald-Hartwig Coupling/Michael Addition Reactions: One-Pot Synthesis of 1,2-Disubstituted 4-Quinolones from Chalcones and Primary Amines. Eur. J. Org. Chem 2012, 3001–3008. [Google Scholar]
- (36).Bachman JE; Curtiss LA; Assary RS Investigation of the Redox Chemistry of Anthraquinone Derivatives Using Density Functional Theory. J. Phys. Chem. A 2014, 118, 8852–8860. [DOI] [PubMed] [Google Scholar]
- (37).(a) Meyers C; Maes BUW; Loones KTJ; Bal G; Lemière GLF; Dommisse RA Study of a New Rate Increasing “Base Effect” in the Palladium-Catalyzed Amination of Aryl Iodides. J. Org. Chem 2004, 69, 6010–6017. [DOI] [PubMed] [Google Scholar]; (b) Kuethe JT; Childers KG; Humphrey GR; Journet M; Peng Z A Rapid, Large-Scale Synthesis of a Potent Cholecystokinin (CCK) 1R Receptor Agonist. Org. Process Res. Dev 2008, 12, 1201–1208. [Google Scholar]
- (38).(a) Uthayopas C; Surawatanawong P Aryl C–O Oxidative Addition of Phenol Derivatives to Nickel Supported by an N-Heterocyclic Carbene via a Ni0 Five-Centered Complex. Dalton Trans. 2019, 48, 7817–7827. [DOI] [PubMed] [Google Scholar]; (b) Rull SG; Funes-Ardoiz I; Maya C; Maseras F; Fructos MR; Belderrain TR; Nicasio MC Elucidating the Mechanism of Aryl Aminations Mediated by NHC-Supported Nickel Complexes: Evidence for a Nonradical Ni(0)/Ni(II) Pathway. ACS Catal. 2018, 8, 3733–3742. [Google Scholar]; For a discussion on catalytically-active Ni(I) and Ni(II) species, see:; (c) Lavoie CM; McDonald R; Johnson ER; Stradiotto M Bisphosphine-Ligated Nickel Pre-Catalysts in C(sp2)–N Cross-Couplings of Aryl Chlorides: A Comparison of Nickel(I) and Nickel(II). Adv. Synth. Catal 2017, 359, 2972–2980. [Google Scholar]
- (39).Bajo S; Laidlaw G; Kennedy AR; Sproules S; Nelson DJ Oxidative Addition of Aryl Electrophiles to a Prototypical Nickel(0) Complex: Mechanism and Structure/Reactivity Relationships. Organometallics 2017, 36, 1662–1672. [Google Scholar]
- (40).Rodima T; Kaljurand I; Pihl A; Maemets V; Leito I; Koppel IA Acid–Base Equilibria in Nonpolar Media. 2.1 Self-Consistent Basicity Scale in THF Solution Ranging from 2-Methoxypyridine to EtP1(pyrr) Phosphazene. J. Org. Chem 2002, 67, 1873–1881. [DOI] [PubMed] [Google Scholar]
- (41).For some recent reviews, see:; (a) Stephan DW The Broadening Reach of Frustrated Lewis Pair Chemistry. Science 2016, 354, aaf7229. [DOI] [PubMed] [Google Scholar]; (b) Stephan DW Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res 2015, 48, 306–316. [DOI] [PubMed] [Google Scholar]; (c) Stephan DW; Erker G Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem. Int. Ed 2015, 54, 6400–6441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


