Abstract
Single-agent immune checkpoint inhibitor therapy in advanced non-small cell lung cancer can significantly prolong progression-free and overall survival when compared with cytotoxic chemotherapy. Here, we report a case of newly diagnosed adenocarcinoma of the lung with a solitary brain metastasis and a biopsy confirmed adenocarcinoma in the tail of the pancreas. Cytomorphology and immunohistochemistry suggested the lung and pancreas tumors were distinct primaries. However, molecular analysis of the lung primary and tumor in the pancreas revealed the same mutations of functional significance in PIK3CA, NF1 and TP53, suggesting the tumors were clonal. A total of three cycles of single-agent pembrolizumab, and radiation to the lung and brain administered between cycles 1 and 2, resulted in marked responses in lung, brain and pancreatic tumors. Despite the discontinuation of the pembrolizumab after three cycles due to severe immune-mediated toxicities, the patient has had no progression 11 months after stopping all active treatment. Results of a novel 27-gene immuno-oncology (IO) expression assay revealed strong IO scores for the lung and pancreatic tumors, indicating a favorable tumor immune-microenvironment and possibly explaining the significant response.
Keywords: immune checkpoint inhibitor, lung cancer, pancreas, programmed death ligand 1, tumor immune-microenvironment
Introduction
Adenocarcinoma of the lung can metastasize to any organ but spread to the pancreas, especially as a solitary lesion, is rare. A retrospective study of 2872 patients with non-small cell lung cancer (NSCLC) showed pancreatic metastases in only 17 patients (0.59%).1 Synchronous NSCLC and ductal adenocarcinoma of the pancreas is also very uncommon.2 Patients with locally advanced, non-operable or metastatic NSCLC may be treated with palliative intent systemic therapy and radiation. Immune checkpoint inhibitor (ICI) therapy as a single agent is a first-line treatment option for NSCLC patients whose tumors do not harbor an activating mutation or fusion, especially in tumors with a programmed death ligand 1 (PD-L1) immunohistochemistry (IHC) tumor proportion score (TPS) of ⩾50%.3 However, ICI therapy in pancreatic ductal adenocarcinoma has generally been unsuccessful with the exception of those tumors that are mismatch repair deficient.4,5
In this report, we present a patient with adenocarcinoma of the lung, with a lesion in the brain and in the tail of the pancreas. Biopsy of the pancreatic lesion demonstrated an adenocarcinoma with a different cytomorphology and immunophenotype when compared with the lung. The patient had a significant and sustained partial response in the lung, brain and pancreas lesions after just three cycles of first-line, single-agent pembrolizumab and radiation to the lung and brain. To determine a possible mechanism for the marked response we assessed the lung tumor and the lesion in the pancreas by genomic sequencing and evaluated both tumors with a novel 27-gene immuno-oncology expression assay to study the tumor immune-microenvironment.
Case presentation
A 64-year-old male smoker presented with cough and dyspnea. Computed tomography (CT) scan revealed a right lung mass with mediastinal adenopathy (Figure 1). There was also a 7.4 cm mass in the tail of the pancreas (Figure 1). Magnetic resonance imaging (MRI) of the brain revealed a 1.2 cm mass in the right frontal lobe without significant edema.
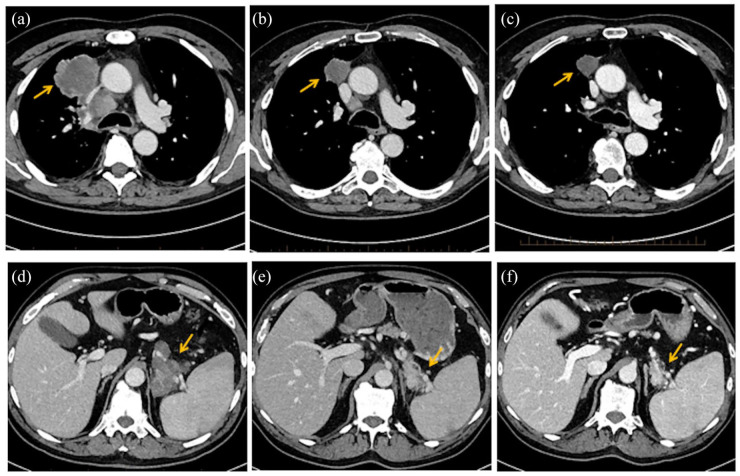
Figure 1.
Radiological evaluation of the patient. (a) Computed tomography scan of the chest before treatment with pembrolizumab and radiation showing the primary lung cancer in the right upper lobe. (b) and (c) Significant regression of the lung tumor and mediastinal adenopathy after three cycles of pembrolizumab and continuing response in the lung tumor 4 months after stopping pembrolizumab. (d) Computed tomography scan of the abdomen prior to treatment with pembrolizumab. (e) and (f) After three cycles of pembrolizumab demonstrating regression of the tumor in the tail of the pancreas and continuing response in the pancreatic tumor 4 months after stopping pembrolizumab.
Fine needle aspiration of the lung mass revealed an adenocarcinoma. IHC showed positivity for thyroid transcription factor-1 (TTF-1), cytokeratin 7 (CK7) and Napsin A, confirming its primary pulmonary origin (Figure 2). The ALK protein was not expressed by IHC, and no EGFR, MET or RAS mutations were detected. Mutations of functional significance were detected in PIK3CA (c.1636C>A), NF1 (c.4836-1G>A) and TP53 (c.809T>C). PD-L1 TPS was determined to be ⩾50% using the 22C3 PharmDx assay (Dako, CA, USA). A core biopsy of the large mass in the tail of the pancreas revealed a different cytomorphology and immunoprofile from the lung biopsy (Figure 2). The IHC results showed TTF-1 to be weakly positive, while Napsin A and CK7 were both negative. These results suggested the lung and pancreatic tumors were not clonal and were synchronous primaries rather than lung cancer metastatic to the pancreas.
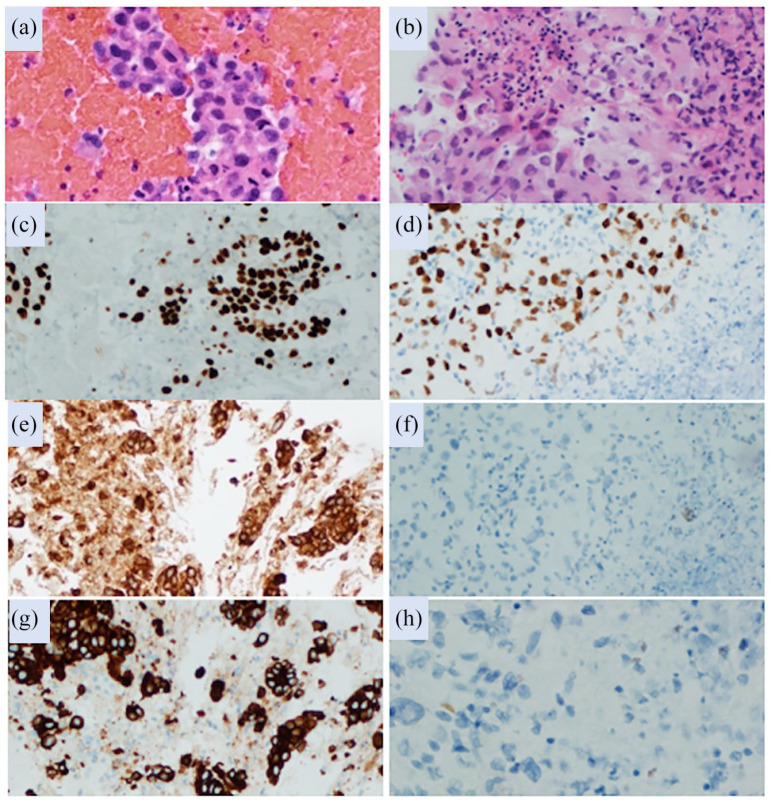
Figure 2.
Histologic and immunohistologic findings. (a) Hematoxylin and eosin stained lung fine needle aspiration cell block preparations composed of cohesive groups of malignant epithelioid cells diagnostic of adenocarcinoma. (b) Core biopsy of the pancreatic mass showing a higher grade pleomorphic tumor composed of more discohesive malignant epithelioid cells with massively enlarged eccentric nucleic, coarse chromatin and large prominent nucleoli. TTF-1 strongly positive staining of lung (c) and weakly positive of the pancreas (d); Napsin A positive staining of the lung (e) and no staining of the pancreas (f); CK7 positive staining of the lung (g) and no staining of the pancreas (h).
The patient declined cytotoxic chemotherapy due to concern regarding possible side effects and the anticipated poor outcome associated with stage IV lung cancer with a possible synchronous cancer in the pancreas. He consented to the use of single-agent pembrolizumab at a dose 2 mg/kg administered intravenously every 3 weeks. One week after the first pembrolizumab infusion the patient presented to the emergency department with focal seizures, mild slurring of speech and weakness. A repeat MRI of the brain demonstrated the enlargement of the right frontal lobe lesion from 1. 2 cm to 2.2 cm with associated focal edema and mild mass effect but no new lesions. The patient was started on dexamethasone 4 mg daily and an antiseizure medication. Palliative radiation to the brain was discussed with the patient and he elected to receive whole brain radiation rather than stereotatic radiotherapy. The patient received 2000 cGy in five fractions and 3000 cGy in 10 fractions to the lung primary and mediastinal adenopathy. He resumed the pembrolizumab 1 week later without incident.
After three cycles of pembrolizumab, the patient developed moderate thrombocytopenia and severe immune-mediated encephalitis that required hospitalization and treatment with high doses of methylprednisolone. A CT scan revealed a marked reduction in the lesions in the chest and pancreas (Figure 1). An MRI of the brain showed almost complete resolution of the metastatic disease and no edema. The patient recovered from his immune-mediated encephalitis but received no further pembrolizumab. Four months after stopping the pembrolizumab, the tumors in the chest and pancreas continued to regress (Figure 1). The patient discontinued corticosteroids after a long taper, but the platelets count remains reduced at between 60 and 90 × 109/L. There is no evidence of progression 11 months after discontinuing pembrolizumab.
In an effort to determine why the patient had such a marked response in the non-radiated tumor in the tail of the pancreas, the pancreas biopsy was tested for PD-L1 expression and subjected to genomic sequencing. The PD-L1 IHC performed on the pancreatic biopsy revealed a TPS of ⩾50%, similar to the PD-L1 TPS of ⩾50% seen on prior testing of the tumor sample from the lung primary at diagnosis. Molecular profiling of the pancreatic tumor revealed the same mutations in PIK3CA, NF1and TP53 as seen in the lung. KRAS was wild type.
The lung and pancreatic tumor biopsies were further analyzed with a novel 27-gene expression assay (Oncocyte, Irvine, CA, USA) to assess the tumour immune-microenvironment.
Methods and materials
Patient consent
A written informed consent was provided from the patient giving permission to study the tumor immune-microenvironment of the lung and pancreatic tumors, and to use the results together with de-identified clinical or imaging data in any subsequent publication.
Twenty-seven-gene immuno-oncology qPCR assay
The 27-gene immune-oncology (IO) assay has previously demonstrated the capability to assess the tumor immune-microenvironment (TIME) to predict response to immunotherapy using data obtained from multiple platforms.6,7 The IO algorithm was derived from a previously established 101-gene triple negative breast cancer-type (TNBCtype) model.8 The algorithm was guided by insights that the IM, M and MSL subtypes of the 101-model would provide information about the TIME.9 A total of 27 genes that most correlated to the IM, M and MSL subtypes of the 101-gene TNBCtype classifier were used to derive the 27-gene IO algorithm. The IO signature 27-gene list is detailed in Nielsen et al.10 Triple negative breast cancer (TNBC) specimens were isolated from datasets obtained by the Gene Expression Omnibus as previously described.8 A threshold for positivity was determined in these specimens and set using area under the curve.
To better address the unmet clinical need of a test capable of fast turnaround time and minimal input requirements, a multiplexed qPCR panel designed to run on the Applied Biosystems QuantStudio (Thermo Fisher Scientific, Waltham, MA, USA) was developed containing the requisite 27 genes. To perform the qPCR panel, RNA is purified from formalin fixed paraffin embedded (FFPE) tissue using the QIAGEN RNeasy FFPE Kit (Qiagen, Germantown, MD, USA), according to the kit protocol. A minimum concentration of 3.57 ng/µL of RNA is required as input using fluorometric quantification, Qubit 2.0. Up to 14 µL of RNA, totaling 50 ng, is used for the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) in a 20 µL volume reaction. Following cDNA synthesis, 2.5 µL of cDNA is added to 7.5 µL of TaqMan PreAmp Master mix (Thermo Fisher Scientific, Waltham, MA, USA) plus a TaqMan PreAmp primer pool for a total volume of 10 µL. The PreAmp reaction is cycled for 14 cycles according to the manufacturer’s recommendations. Following preamplification, a total of 190 µL of TE buffer is added to the 10 µL preamplification product and mixed thoroughly. The properly diluted preamplification product is then used as template for the 27-gene IO qPCR assay, which is spotted in a 384-well plate using the TaqMan Multiplex Master Mix with a 10 µL final volume and run on the QuantStudio 6 according to the manufacturer’s recommendations. Results are exported, quality control metrics are assessed, and data are run through the 27-gene IO algorithm to determine the IO score of each sample (scale −1.0 to 1.0). The IO score positivity cutoff applied to this cohort was determined previously in TNBC and NSCLC and was used for both the lung and the pancreas tumor biopsies.
Results
The 27-gene IO assay gave very high IO score results for both the lung and the pancreas lesions when compared with the threshold established in lung and breast cancer (Table 1). The IO score may also predict intensity of response when the score is considered as a continuous variable, as demonstrated in a previous study where the average IO score of the complete response group was 0.33, indicating the scores observed in this study may be considered strongly positive.7
Table 1.
The IO score results for the lung and pancreas tumor biopsies.
| Run | Lung tumor IO score | Pancreas tumor IO score | Interpretation |
|---|---|---|---|
| 1 | 0.489 | 0.795 | Strongly positive |
| 2 | 0.493 | 0.799 | Strongly positive |
IO, immuno-oncology.
Discussion
Differences in cytomorphology and IHC can help to distinguish synchronous adenocarcinoma of the lung and ductal adenocarcinoma of the pancreas (PDAC) from lung cancer metastasizing to the pancreas. However, poorly differentiated metastatic tumors may have a varied immunophenotype based on differences in protein expression when compared with the primary tumor.11 Therefore, genomic sequencing of the tumors may be required to confirm their sites of origin. The PIK3CA, NF1 and TP53 mutations found in both the lung and pancreatic tumor biopsies strongly suggest that the tumors are clonal rather than synchronous primaries. All three genes have been reported to be mutated in both adenocarcinoma of the lung and PDAC, making it difficult to determine the primary site. NF1 mutations are found more frequently in adenocarcinoma of the lung compared with PDAC and are usually exclusive of KRAS and EGFR mutations, as in this patient.12 One-third of NF1 mutated lung adenocarcinomas will also have a TP53 mutation.12 The absence of a RAS mutation in the pancreatic tumor biopsy sample also strongly argues against the lesion being a primary ductal adenocarcinoma as KRAS is mutated in 94% of PDAC tumors.13
The 27-gene IO assay used in this report was first described in TNBC, then validated in NSCLC and TNBC.6,7 The assay that measures the TIME was based on the immunomodulation signature of a 101-gene TNBC algorithm.8 This algorithm was optimized using genes expressed in both quiescent and immunologically active tumors and may be useful in predicting response to immunotherapies. The 27-gene assay used in this report first demonstrated predictive capabilities in NSCLC patients treated with ICIs, demonstrating a correlation between 1 year progression-free survival and the immune-microenvironment signature of the 27-gene IO assay but not with PD-L1 TPS or TMB.7 Additionally, a three-fold better hazard ratio for IO score positive as compared with IO score negative was observed. Further, the 27-gene IO assay was predictive of response to ICIs and not simply prognostic of outcome. Finally, the 27-gene IO assay showed alignment of the positive IO score with response to durvalumab, along with a better predictive value when compared with current gold standards in TNBC.6
Significant responses to first-line single-agent ICI therapy is well described in the literature, especially in NSCLC patients with elevated PD-L1 expression. The other possible explanation for the responses seen in this patient is that the radiation to the lung primary and brain released tumor associated neoantigens, activating an immune response resulting in the control of the non-irradiated pancreatic tumor, a phenomenon previously described and referred to as the abscopal effect.14,15 There have been a limited number of prospective randomized clinical trials examining the abscopal effect in lung cancer and melanoma patients treated with an ICI with or without radiation to a single site. Unfortunately, the results have not shown clinical benefit.16,17
Our patient’s pancreatic and lung biopsies were performed prior to the initiation of any treatment and each revealed a high IO score (Table 1), suggesting that the TIME was favorable for a response to ICI therapy. The patient received palliative radiation to the lung and brain after the first cycle of pembrolizumab because of concerns of progression but we cannot exclude the possibility that the patient’s symptoms and changes seen on imaging were due to early pseudoprogression and predictive of a response even before radiation was administered. Baseline neutrophil to lymphocyte ratio (NLR) has been inversely associated with immune-related adverse events (irAEs) and poor survival outcomes in cancer patients treated with ICI therapy, especially when the NLR is greater than 3.18,19 The baseline NLR was 1.98 for this patient. Our patient developed severe irAEs requiring the discontinuation of pembrolizumab after only three cycles. Further understanding the TIME may help elucidate characteristics of irAEs associated with ICI therapy and may assist in managing these patients appropriately.
In summary, our report emphasizes that in patients who have adenocarcinoma of the lung with a solitary tumor deposit in the pancreas it is important to use genomic mutational analysis to distinguish between metastatic disease versus a synchronous ductal adenocarcinoma of the pancreas primary. We have also demonstrated that a novel 27-gene immuno-oncology qPCR assay was predictive of a response to single-agent ICI therapy in both the primary and the metastatic tumors.
Acknowledgments
We thank the patient for permitting us to discuss his case and present his imaging.
Footnotes
Conflict of interest statement: DLS, DS, BRV, MH and PKC have no disclosures. TJN, DRH, BLS, FBM, JS and RSS are employees or otherwise affiliated with Oncocyte.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the 27-gene IO assay provided by Oncocyte. The publication costs were provided by the corresponding author.
ORCID iD: David L. Saltman  https://orcid.org/0000-0001-6004-2657
https://orcid.org/0000-0001-6004-2657
Contributor Information
David L. Saltman, BC Cancer, 2410 Lee Avenue, Victoria, BC V8R 6V5, Canada.
Tyler J. Nielsen, Oncocyte Corporation, Irvine, CA, USA
Davide Salina, Department of Laboratory Medicine, Royal Jubilee Hospital, Victoria, BC, Canada.
David R. Hout, Oncocyte Corporation, Irvine, CA, USA
Frank B. McMahon, Oncocyte Corporation, Irvine, CA, USA
Boris R. Valev, BC Cancer, Victoria, BC, Canada
Michael Huk, Department of Medical Imaging, Royal Jubilee Hospital, Victoria, BC, Canada.
Pranil K. Chandra, PathGroup, Nashville, TN, USA
Jeremy Spille, Oncocyte Corporation, Irvine, CA, USA.
Robert S. Seitz, Oncocyte Corporation, Irvine, CA, USA
Brock L. Schweitzer, Oncocyte Corporation, Irvine, CA, USA
References
- 1. Niu FY, Zhou Q, Yang JJ, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer 2016; 16: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puri A, Ma L, Walker GV, et al. Synchronous primary adenocarcinoma of the lung and pancreas: a case series and review of the literature. Lung Cancer Manag 2017; 6: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated analysis of the KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37: 537–546. [DOI] [PubMed] [Google Scholar]
- 4. Henriksen A, Dyhl-Polk A, Chen I, et al. Checkpoint inhibitors in pancreatic cancer. Cancer Treatment Rev 2019; 78: 17–30. [DOI] [PubMed] [Google Scholar]
- 5. Sarantis P, Koustas E, Papadimitropoulou A, et al. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol 2020; 12: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwase T, Pusztai L, Blenman K, et al. Validation of an immunomodulatory gene signature algorithm to predict response to neoadjuvant immunochemotherapy in patients with primary triple-negative breast cancer. J Clin Oncol 2020; 38(Suppl. 15): abstract 3117. [Google Scholar]
- 7. Ranganath H, Jain A, Smith JR, et al. One-year progression-free survival in lung cancer patients treated with immune checkpoint inhibitors is significantly associated with a noval immunomodulatory signature but not PD-L1 staining. J Immunother Cancer 2019; 7(Suppl. 1): P141. [Google Scholar]
- 8. Ring BZ, Hout DR, Morris SW, et al. Generation of an algorithm based on minimal gene sets to clinically subtype triple negative breast cancer patients. BMC Cancer 2016; 16: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lehmann BD, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One 2016; 11: e0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nielsen TJ, Ring BZ, Seitz RS, et al. A novel immuno-oncology alogorithm measuring tumor microenvironment to predict responses to immunotherapies. Heliyon 2021; 7: e06438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olein KA. Pathologic evaluation of unknown primary cancer. Semin Oncol 2009; 36: 8–37. [DOI] [PubMed] [Google Scholar]
- 12. Tlemsani C, Pecuchet N, Gruber A, et al. NF1 mutations identify molecular and clinical subtypes of lung adenocarcinoma. Cancer Med 2019; 8: 4330–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waters A, Der CJ. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect Med 2018; 8: a032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mole R. Whole body irradiation-radiobiology or medicine? Br J Radiol 1953; 26: 234–241. [DOI] [PubMed] [Google Scholar]
- 15. Keam S, Gill S, Ebert MA, et al. Enhancing the efficacy of the immunotherapy using raiotherapy. Clin Transl Immunology 2020; 9: e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotatic body radiation versus pembrolizumab alone on tumor response inpatients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019; 5: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwon ED, Drake GG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that has progressed after docetaxel chemotherapy (CA184-043): a multicentre, radomized, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mezquita L, Auclin E, Ferrara R, et al. Assocation of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 2018; 4: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eggemont AMM, Kicinski M, Blank CV, et al. Association between immune related adverse events and recurrence-free survival among patients randomized to receive pembrolizumab or placebo. JAMA Oncol 2020; 6: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]