Abstract
PCp-I is a polysaccharide isolated and identified from the Psoralea corylifolia L. by our research group. In this study, the immunomodulatory effects of PCp-I on RAW264.7 cells was evaluated. PCp-I could enhance the level of NO along with up-regulation of iNOS mRNA in RAW264.7 cells. The PCp-I could significantly up-regulate the mRNA expression of TNF-α and IL-6 in RAW264.7 cells, and then the expression of TNF-α, IL-6, ROS and the phagocytic activity were increased. Additionally, PCp-I could significantly up-regulate the phosphorylation level of p65, p38, ERK and JNK proteins, which proved that PCp-I could activate the macrophages by MAPKs and NF-κB signalling pathway and the TLR4 may be one of the receptors of PCp-I regulate the RAW264.7 cells.
Keywords: Psoralea corylifolia L, polysaccharide, immunomodulation, RAW264.7 cells
Introduction
Psoralea corylifolia L. (P. corylifolia), belonging to Leguminosae, was used to cure psoriasis and vitiligo.1 In our previous researches, one polysaccharide (PCp-I) with the average molecular weight of 2.721 × 104 g/mol and irregular porous structure was purified and identified from P. corylifolia. The PCp-I was composed of rhamnose, arabinose, xylose, mannose, glucose and galactose.2 Polysaccharide has a wide range of biological activities, such as immunomodulation,3–5 anti-tumor,6 anti-oxidation7,8 and anti-virus.9 Immunomodulatory activity is recognized as the most important effective biological effects of polysaccharide and some scholars have found that the structure of polysaccharide including the monosaccharide composition, glycosidic-linkage position, molecular weight and branching are closely related to its immunostimulatory activity.10 Contrary to bacterial polysaccharides, plant derived polysaccharides are usually nontoxic and without side effects, so they become potential therapeutic candidates for cancer and immune disorders.11 Inhibiting effect of PCp-I on the A549 lung cancer cells was confirmed in our previous research while the immune activity has not been studied.2 The main targets responding to immune molecules are monocytes, macrophages and neutrophils.11 In this research, the mouse macrophage RAW264.7 was selected to study the immunomodulatory effects of PCp-I.
Due to the large molecular mass, polysaccharide cannot penetrate cell membrane directly, so the first step in the modulation of cellular events is to bind to Pattern Recognition Receptors (PRRs),12 such as Scavenger Receptors (SRs), Complement Receptor (CR) and Toll-Like Receptors (TLRs).10,13,14 Until now, a total of 10 functional TLRs (TLR1-TLR10) in humans and 13 active TLRs in laboratory mice have been discovered.15 PRRs will trigger immune signalling pathways, leading to regulation of relevant gene expression and protein synthesis. In macrophages, Mitogen Activated Protein Kinases (MAPKs) and Nuclear Factor-κB (NF-κB) are main immunoregulate signalling pathways reported in previous researches.16 When they are activated, macrophages can increase the secretion of the immune molecules, including Nitric Oxide (NO) and other pro-inflammatory cytokines, such as Tumour Necrosis Factor-α (TNF-α), interleukin (IL)-1, IL-6, IL-8, IL-12 and Interferon (IFN)-γ.17–19 Especially, NF-κB is recognized as a key regulator of cytokine expression and is closely associated with the generation of ROS and induction of apoptosis in macrophages.20 Besides secretion the immune molecules, macrophages can exert their immunoregulation effects by devouring foreign pathogens and cell fragments.21 In addition, they can process and present the endogenous and exogenous antigens by increasing the expression of Major Histocompatibility Complex (MHC) and Cluster of Differentiation (CD) molecules on the cell surface.22
In this paper, the immunomodulatory effects and corresponding regulation mechanism of P. corylifolia polysaccharide were studied by the mouse macrophage RAW264.7.
Materials and methods
Polysaccharide preparation and reagents
Preparation of PCp-I
PCp-I were isolated and purified by Henan Joint International Research Laboratory of Drug Discovery of Small Molecules, Zhengzhou, China.2 In brief, The P. corylifolia dried powder was soaked with petroleum ether and 75% ethanol to eliminate some fat-soluble substances and the residue was extracted three times with ultra-pure water at 85°C. The crude polysaccharide was precipitate from the water extraction solution by adding ethanol. The Sevage method was used to remove proteins. The DEAE-52 cellulose column and Sephadex G-100 column chromatography were used to refine polysaccharide. ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit was used to detect endotoxin content and it ruled out the contamination of endotoxin in PCp-I used in the present study.
Chemicals and reagents
Dulbecco’s Modified Eagle’s Medium (DMEM) and neutral red were purchased from Solarbio (Beijing, China). Fetal Bovine Serum (FBS) was purchased from Gibco (Grand Island, NY, USA). Nitric oxide kit was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). TNF-α and IL-6 ELISA kit were obtained from Beijing 4A Biotech Co, Ltd (Beijing, China). Primer iNOS, TNF-α, IL-6, TLR4 and MyD88 were purchased from Thermo Fisher scientific (Shanghai, China). Reactive Oxygen Species Assay Kit were purchased from Beyotime Biotechnology (Shanghai, China). PrimeScriptTMRT reagent kit with gDNA Eraser kit and TB Green TM Ex TaqTM II (Tli RNadeH Plus) and Bulk kit were purchased from TaKaRa. Antibody NF-κB p65, phospho-NF-κB p65, p38 MAPK, phospho-p38 MAPK, SAPK/JNK, phospho-SAPK/JNK, p44/p22 MAPK and phospho-p44/p22 MAPK were purchased from Cell Signaling (Beverly, MA, USA). NF-κB inhibitor (PDTC), ERK inhibitor (PD98059), JNK inhibitor (SP600125) and p38 inhibitor (SB203580) Lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St. Louis, MO, USA)
Methods
Cell culture and treatment
RAW264.7 cells were obtained from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, China). The cells were cultured in DMEM medium with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin and incubated in a humidified incubator of 5% CO2 at 37°C. LPS (1 μg/mL) was used as the positive control.
Cell viability assay
RAW264.7 cells were seeded in 96-well plates at a density of 1 × 105 cells/mL and cultured at incubator, and then the cells were treated with 100 μL culture medium or PCp-I at different concentration (25, 50, 100, 200, 400 and 800 μg/mL) for 24 h. The culture medium was then removed and 100 μL/well of the MTT solution (1 mg/mL) was added. After 4 h incubation, the medium was discarded and 100 μL of DMSO was added to each well to solubilize blue-purple crystal. Finally, the absorbance was measured at 490 nm.
Phagocytic activity
Effects of PCp-I on the pinocytosis activity of RAW264.7 cells was measured by the method of neutral red uptake as described by previous articles.23 Briefly, 100 μL cells (1 × 105 cells/well) were seeded in 96-well plates and treated with specified concentrations of PCp-I or LPS (1 μg/mL). After 24 h culturing, the supernatant was replaced with 0.075 % neutral red solution. And then for, cell lysis solution (1% acetic acid/anhydrous alcohol, 1:1/v:v) was added to each well after 4 h incubation of neutral red solution. Finally, the absorbance value was measured at 540 nm.
Measurement of NO, TNF-α and IL-6
RAW264.7 cells were added into 24-well plates (1 × 105 cells/well) and treated with PCp-I for 24 h. The supernatant was collected and the levels of NO and pro-inflammatory cytokines were determined according to the manufacturer’s instructions.
Determination of ROS generation
Intracellular ROS level was measured by detecting the changes of fluorescence intensity resulting from the oxidation of the fluorescent probe DCFH-DA.5 Cells (2 × 105 cells/mL) were added into 6-well plates and incubated with PCp-I (400 μg/mL) or LPS (1 μg/mL) for 24 h. Next, 10 μM DCFH-DA was added into each well and cells were incubated for 30 min at 37°C in dark. Later, the cells were washed three times by the PBS, and the Mean Fluorescence Intensity (MFI) of RAW264.7 cells (1 × 104 cells)were measured by the flow cytometry.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Similar to the above cell processing methods, RAW264.7 cells was treated with LPS or PCp-I (400 μg/mL) with 6 h. Then cells total RNA was isolated by Trizol Reagent and reversed to cDNA through Prime Script TMRT reagent kit with gDNA Eraser Kit. For detection of the mRNA expression, qRT-PCR was performed by TB Green TM Ex TaqTM II (Tli RNadeH Plus), Bulk kit. The data were presented as 2−ΔΔct. GAPDH was assessed as on normalization control and the sequences were listed in Table 1.
Table 1.
Primers for real-time RT-PCR.
| Name | Sequences |
|---|---|
| GAPDH | Forward: 5′ - ACCCCAGCAAGGACACTGAGCAAG-3′ |
| Reverse: 5′ - GGCCCCTCCTGTTATTATGGGGGT -3′ | |
| iNOS | Forward: 5’- GCTCGCTTTGCCACGGACGA -3′ |
| Reverse: 5′- AAGGCAGCGGGCACATGCAA -3′ | |
| TNF-α | Forward: 5′- CCCTCCTGGCCAACGGCATG -3′ |
| Reverse: 5′- TCGGGGCAGCCTTGTCCCTT -3′ | |
| IL-6 | Forward: 5′- AGACAAAGCCAGAGTCCTTCAGAGA -3′ |
| Reverse: 5″- GCCACTCCTTCTGTGACTCCAGC -3′ | |
| TLR4 | Forward: 5′- TGAGCAGTCGTGCTGGTATC -3′ |
| Reverse: 5′- CAGGGCTTTTCTGAGTCGTC -3′ | |
| MyD88 | Forward: 5′-ACTCGCAGTTTGTTGGATG -3′ |
| Reverse: 5′- CACCTGTAAAGGCTTCTCG -3′ |
Western blot
The total proteins were extracted by the weak RIPA Lysis Buffer. The cytoplasmic and nuclear proteins were extracted by nuclear protein kit. The BCA kits were used to measure the protein concentrations. The proteins (30 μg, boiling for 10 min) were separated by 8% SDS-PAGE and then transferred to the polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% skim milk for 2 h and incubated with the phosphorylated or non-phosphorylated antibodies (NF-κB p65, ERK1/2, SAPK/JNK and p38) overnight at 4°C. Subsequently, the membrane with PBST was washed and the membrane with an HRP-conjugated secondary antibody was incubated. Washed again and the proteins signal were visualized with ECL chemiluminescence detection kit. The band gray value was quantitatively analyzed by Image J software.
Statistical analysis
The data were expressed as mean ± SD. All experiments were performed in triplicate and analyzed statistically by SPSS 19.0 software. Statistical significance was calculated by the one-way ANOVA analysis.
Results and discussions
PCp-I increases the proliferation of RAW264.7 cells
MTT assay was used to detect the proliferation of cells. In Figure 1, PCp-I could significantly promote the proliferation of RAW264.7 cells at the concentration of 200–400 μg/mL (P < 0.05) and 800 μg/mL (P < 0.01) compared with the control group. PCp-I polysaccharide could not present inhibiting effect at the concentrations less than 800 μg/mL. The above results also indicated that PCp-I (25–800 μg/mL) did not exhibit any cytotoxic effect to the RAW264.7 cells.
Figure 1.
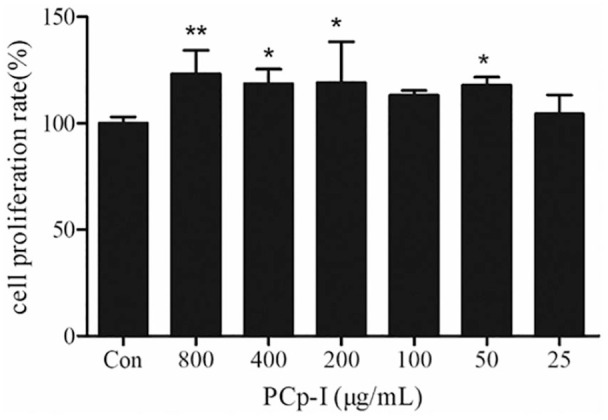
Effects of PCp-I on the proliferation rate of RAW264.7 cells.
Data represent the mean ± SD, n = 3. Compared with control group,**P < 0.01,*P < 0.05.
PCp-I enhances the phagocytic activity of RAW264.7 cells
As a professional scavenger in the body, macrophages play an important role in clearance of foreign matters and pathogens.24 Pathogens inside the macrophages are broken by low pH, hydrolysis and deep offence. Then pathogen-derived compounds would be presented at the cell surface (antigen presentation), allowing the activation of acquired immunity.25,26 In Figure 2, the phagocytosis of the cell treated with LPS (1 μg/mL), as expected, obviously increased nearly twice as many as that in the control group (P < 0.001). Compared with the normal control group, the phagocytic activities of RAW264.7 cells treated with PCp-I from 100–800 μg/mL for 24 h were significantly enhanced (P < 0.001) in a dose-dependent manner. These results suggested that PCp-I treatment could enhance the phagocytic activity of RAW264.7 cell, which was important to the body’s immunomodulatory.
Figure 2.
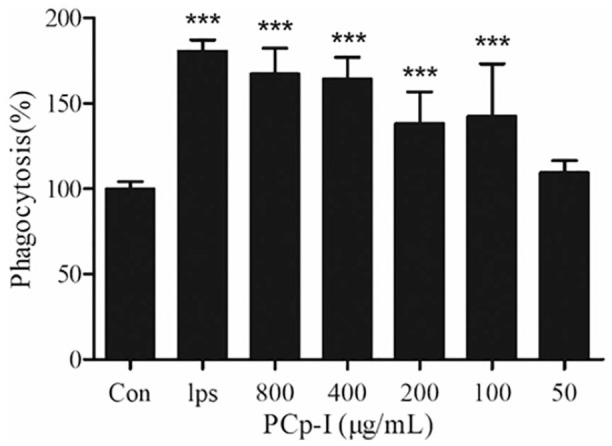
Effects of PCp-I on phagocytic activity of RAW264.7 cells.
Data represent the mean ± SD, n = 3. Compared with the control, ***P < 0.001.
Effects of PCp-I on NO production and iNOS expression
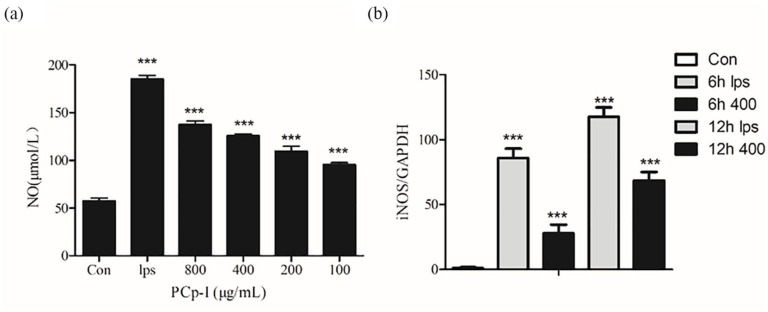
As one of the most important pro-inflammatory mediators in immune system, NO reveals immunity function through killing microorganisms and tumour cells.27,28 Hence, NO can be used to assess the effects of PCp-I on immune regulation. In Figure 3a, the level of NO production in RAW264.7 cells increased significantly after the stimulation from LPS (1 μg/mL) and PCp-I (P < 0.001) compared with the normal control group. Along with an increase of the concentration of PCp-I, the enhancement of the NO secretion became stronger and stronger. In order to further verify the accuracy of the experiment and explore the regulation mode of NO release in RAW264.7 cells, qRT-PCR was used to detect the relative mRNA expression level of iNOS. The results showed that LPS and PCp-I (400 μg/mL) increased the expression levels of iNOS mRNA at 6 and 12 h treatment respectively (Figure 3b).
Figure 3.
Effects of PCp-I on NO production (a) and iNOS expression (b) in Raw264.7 cells.
Data represent mean ± SD, n = 3. Compared with the control, ***P < 0.001.
Effects of PCp-I on the expression of cytokines and mRNA in RAW264.7 cells
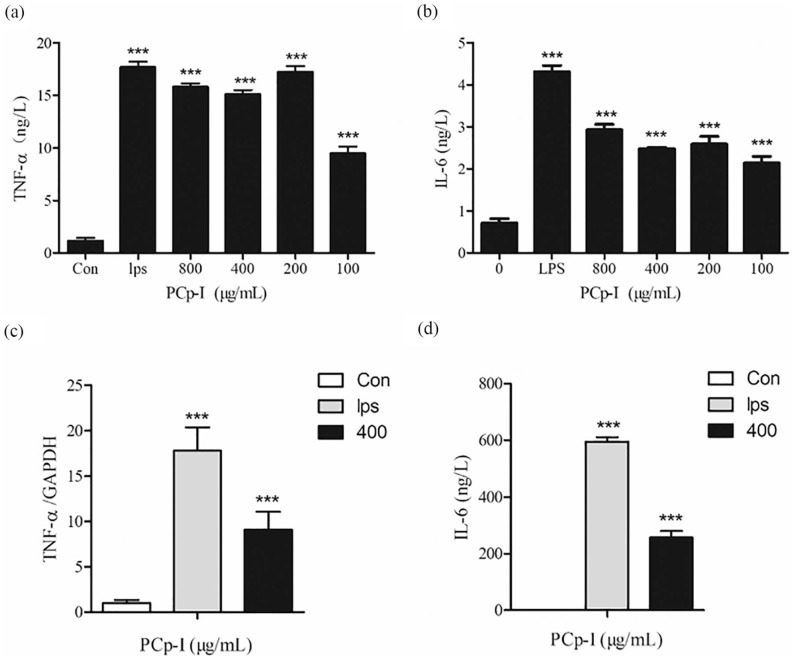
When macrophages are activated, more cytokines will be secreted, including TNF-α, IL-6, IL-1β etc, which all exert immunomodulatory activity. TNF-α increase the production of a series of inflammatory factors and enhance the immune responses of monocytes and macrophages through autocrine manner.29 In addition, as a kind of pivotal cytokines inducing acquired immune response, IL-6 plays a significant role in promoting the differentiation of the T cells and B cells.30,31
In Figure 4a to d, the level of TNF-α and IL-6 and the expression of their corresponding mRNAs were significantly increased after the treatment of LPS (1 μg/mL) (P < 0.001). In Figure 4a to b, PCp-I could obviously enhance the secretion level of TNF-α and IL-6 compared with the control group. Moreover, PCp-I (400 μg/mL) significantly promoted the mRNA expression of TNF-α and IL-6 (P < 0.001) ( Figure 4c and d).
Figure 4.
The effects of PCp-I on the expression of TNF-α (a), IL-6 (b), and TNF-α (c), IL-6 (d) mRNA in Raw264.7 cells.
Data represent mean ± SD, n = 3. Compared with the control, ***P < 0.001.
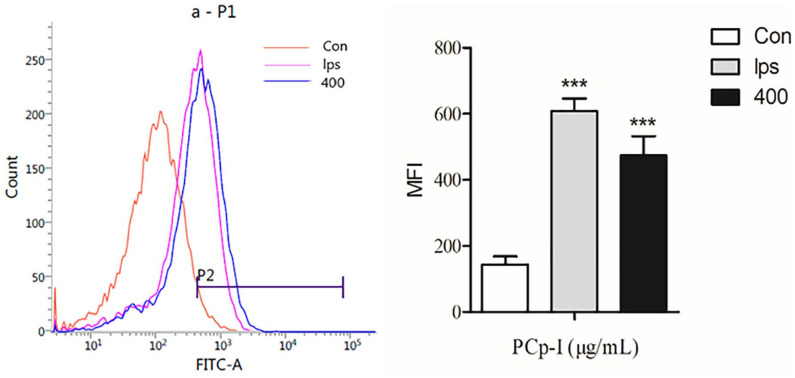
PCp-I induces ROS production of RAW264.7cells
ROS acts as an immunomodulatory signalling molecule in macrophage like other pro-inflammatory cytokines.32 According to an earlier report, NF-κB is a transcription factor that is redox-regulated, with the generation of reactive oxygen species (ROS) in macrophages closely associated with the NF-κB pathway activating.20 NF-κB by many agents can result in the formation increase of intracellular ROS.33–35 Besides, ROS also has a certain promotion effect on cell apoptosis.36 The Figure 5 showed that compared with the control group, the peak shape of the model group shifted to the right significantly, which indicated that PCp-I could enhance the release of ROS. ROS indicated that PCp-I (400 μg/mL) could promote the production of ROS in RAW264.7 cells.
Figure 5.
The effects of PCp-I on ROS in Raw264.7 cells.
Data represent mean ± SD, n = 3. Compared with the control, ***P < 0.001.
PCp-I induces the activation of MAPKs and NF-κB pathways in RAW264.7 cells
In macrophages, NF-κB and MAPKs are main signalling pathways to control immune responses. NF-κB, as a master transcription factor including five members (p50, p52, p65 (RelA), RelB, c-Rel), regulates immune responses and exists mainly in cells as p65 and p50 heterodimers.37,38 In normal cells, NF-κB usually exists in the cytoplasm through combining with the inhibitor of kappa B (I-κB). After activation, different upstream pathways will converge on the inhibitor of nuclear factor kappa B kinase (IKK) complex. Then IKK complex phosphorylates I-κB, leading to the release of NF-κB from the cytoplasm and translocation into the nucleus to activate specific target genes.39,40 MAPK family contains three major kinases including extracellular signal-regulated kinase 1/2 (ERK 1/2), c-jun N-terminal kinase (JNK) and p38.36 AP-1 is a downstream transcription factor of MAPKs and its major forms are Fos/Jun heterodimers which have high affinity with the protein by binding to AP-1 sites. Besides, AP-1 induces gene expression involved in cell differentiation and proliferation.41,42
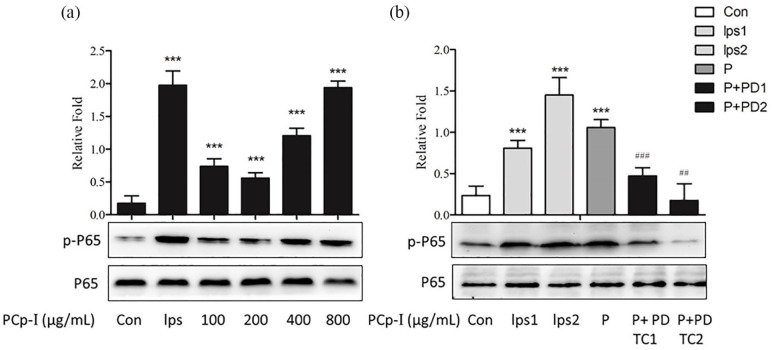
It was clear (shown in Figure 6a) that the expression level of phosphorylated p65 increased significantly by the treatment of PCp-I, which enhanced the proportion of p-p65/p65. Compared with the control group, the concentration of PCp-I with 800 μg/mL could increase the level of NF-κB as high as the positive control group. The results suggested that PCp-I could accelerate the translocation of NF-κB from cytoplasm to nucleus. In order to further determine the role of NF-κB in the activation of RAW264.7 cells evoked by PCp-I, NF-κB specific inhibitors (PDTC) was used. We pretreated the PCp-I drug group with different concentrations of PDTC (10 and 30 μM, respectively). The results were shown in Figure 6b. LPS1 (100 ng/mL) and LPS2 (1 μg/mL) were used as a positive control, we found that the phosphorylated p65 expression level was significantly attenuated through PDTC pretreatment compared with the PCp-I (800 μg/mL) group. All of these results indicated that PCp-I played an immunomodulatory role through activating the NF-κB signalling pathway.
Figure 6.
Effects of PCp-I on the expression of phosphorylation NF-κB P65 protein (a) inRAW264.7 cells and PDTC on protein expression after activation of PCp-I (b).
Data represent mean ± SD, n = 3. In Figure 6a and b, compared with the control, ***P < 0.001. In Figure 6b, compared with PCp-I, ##P < 0.01, ###P < 0.001.
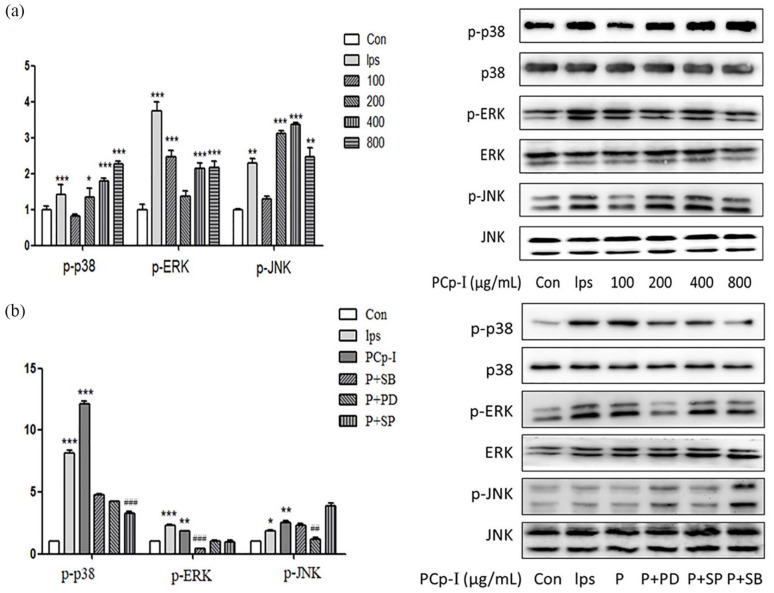
In Figure 7, the expression of p-p38, p-ERK, p-JNK protein were observably higher than the control group. It is clear that in Figure 7a, LPS or PCp-I had no effects on the expression of non-phosphorylated p38, ERK and JNK, while PCp-I enhanced the expression of MAPKs protein specially at the concentration of 100–800 μg/mL. As in the above case, the MAPK signalling pathway inhibitors were used to further verify the activation effects of PCp-I, including PD98059 (ERK inhibitor), SP600125 (SAPK/JNK inhibitor) and SB203580 (p38 inhibitor). In Figure 7b, PD98059 (30 μM), SP600125 (3 μM) and SB203580 (10 μM) pretreatment specifically reduced the expression of corresponding protein phosphorylation, which validated the results of Figure 7a. Morever, the regulatory effects of PCp-I on p38 and ERK is particularly obvious than JNK. The results showed that PCp-I could up-regulate the level of MAPKs phosphorylation in RAW264.7 cells to exhibit the role of regulating immunity.
Figure 7.
Effects of PCp-I on the proteins expression of p38, ERK and JNK in RAW264.7 cells (a) and corresponding inhibitors on proteins expression after activation of PCp-I (b).
Data represent mean ± SD, n = 3. In Figure 7a and b, compared with the control, *P < 0.05, **P < 0.01, ***P < 0.001. In Figure 7b, compared with PCp-I, ##P < 0.01, ###P < 0.001.
Many studies have reported that polysaccharides derived from Angelica dahurica, Sutherlandia frutescens and Ganoderma atrum can activate immunocyte through MAPK phosphorylation and NF-κB translocation.3,43,44 It is consistent with previous studies, our results demonstrated that MAPK and NF-κB signalling pathways played a significant role in RAW264.7 cells activation induced by PCp-I.
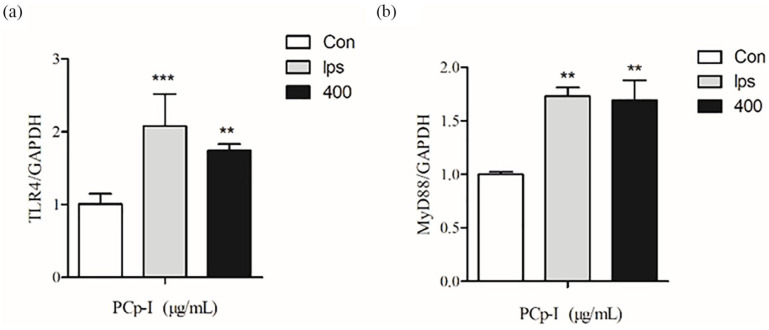
Effect of PCp-I on TLR4 and MyD88 in RAW264.7 cells
All TLRs bind to ligands, recruit the adapter molecule Myeloid differentiation factor 88 (MyD88) to regulate immune responses.45 TLR4 has been identified as an important membrane receptor of polysaccharides. Some polysaccharides from A. gigas Nakai, Cordyceps militaris, and Acanthopanax koreanum have been reported to activate B cells and DCs through TLR4.46–48 The TLR4 bind to ligands, resulting in recruitment of the adapter molecule Myeloid differentiation factor 88 (MyD88), which in turn leads to the activation of NF-κB and MAPK transduction cascades in macrophages.49,50
In Figure 8, RT-PCR analysis showed that PCp-I directly up-regulated the mRNA expression levels of TLR4 and MyD88 in RAW264.7. Similar results concerning about TLR4 expression in macrophages were observed following LPS.51 TLR4 may be one of the important receptors for PCp-I regulate the immune activity of RAW264.7 cells.
Figure 8.
Effects of PCp-I on the expression of TLR4 (a) and MyD88 (b) mRNA.
Data represent mean ± SD, n = 3. compared with the control, **P < 0.01, ***P < 0.001.
Conclusions
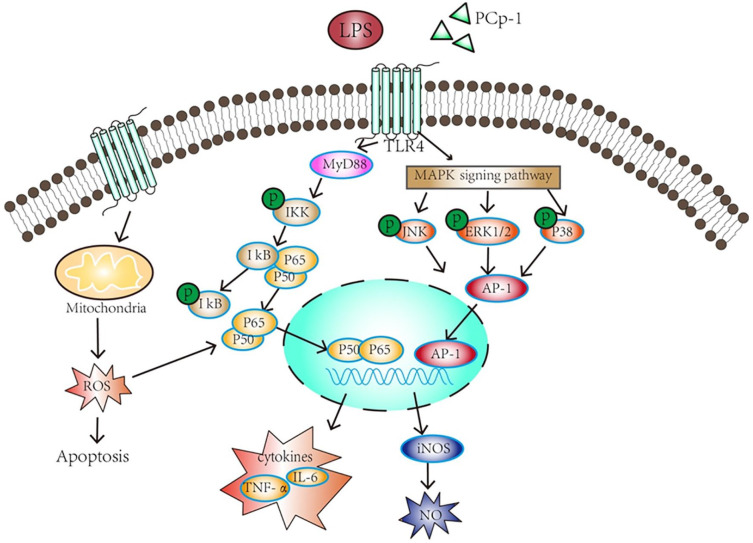
Previous researches have reported that 35 kinds of polysaccharides from 22 different plants could enhance the function of macrophages.24 In our study, we found PCp-I had immunomodulatory effect on RAW264.7 macrophages and specific mechanism. Polysaccharide PCp-I can regulate the activation of NF-κB and MAPK signalling pathway in the RAW264.7 cells. The activation of NF-κB have a closely relationship with the increase of active oxygen release. NF-κB and AP-1 can be transferred into the nuclear to regulate the transcription of genes, which increase the secretion of NO and pro-inflammatory cytokines, including TNF-α and IL-6. In addition, PCp-I may bind to TLR4, induce the recruitment of MyD88 to activate of NF-κB and MAPK signalling pathway. This detailed process is shown in Figure 9. Therefore, PCp-I has potential to exploit as inherent immune response regulators, which has a significance in preventing and treating human immunosuppress diseases. However, the specific membrane receptor, and even the cellular messenger which play an important role in improving the immunity function of PCp-I need to be further studied.
Figure 9.
Signalling pathways involved in macrophage activation by PCp-I.
Footnotes
Author Contributions: Honglin Wang, Xiaoqing Xu and Zhenhua Yin contributed equally to this work.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Key project in Science and Technology Agency of Henan Province (192102110214 and 202102110283).
Ethical approval: Ethical approval was not sought for the present study because the subjects in this research were RAW264.7 cells, one type of mouse macrophage cells.
ORCID iD: Wenyi Kang  https://orcid.org/0000-0002-1822-6249
https://orcid.org/0000-0002-1822-6249
Data availability: The [DATA TYPE] data used to support the findings of this study are included within the article.
References
- 1. Kim YJ, Lim HS, Lee J, et al. (2016) Quantitative analysis of psoralea corylifolia linne and its neuroprotective and anti-neuroinflammatory effects in HT22 hippocampal cells and BV-2 microglia. Molecules 21(8): 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yin Z, Zhang W, Zhang J, et al. (2019) Two novel polysaccharides in psoralea corylifolia L and anti-A549 lung cancer cells activity in vitro. Molecules 24(20): 3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang JY, Wang HL, Zhang HL, et al. (2019) Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway. International Journal of Biological Macromolecules 132: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 4. Xu X, Wu X, Wang Q, et al. (2014) Immunomodulatory effects of alginate oligosaccharides on murine macrophage RAW264.7 cells and their structure-activity relationships. Journal of Agricultural and Food Chemistry 62(14): 3168–3176. [DOI] [PubMed] [Google Scholar]
- 5. Yang F, Li X, Yang Y, et al. (2019) A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264.7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-κB signaling pathways. International Journal of Biological Macromolecules 140: 895–906. [DOI] [PubMed] [Google Scholar]
- 6. Zhao L, Dong Y, Chen G, et al. (2010) Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydrate Polymers 80(3): 783–789. [Google Scholar]
- 7. Wang ZJ, Xie JH, Kan LJ, et al. (2015) Sulfated polysaccharides from Cyclocarya paliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. International Journal of Biological Macromolecules 80: 410–417. [DOI] [PubMed] [Google Scholar]
- 8. You L, Gao Q, Feng M, et al. (2013) Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chemistry 138(4): 2242–2249. [DOI] [PubMed] [Google Scholar]
- 9. Mazumder S, Ghosal PK, Pujol CA, et al. (2002) Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). International Journal of Biological Macromolecules 31(1–3): 87–95. [DOI] [PubMed] [Google Scholar]
- 10. Ferreira SS, Passos CP, Madureira P, et al. (2015) Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydrate Polymers 132: 378–396. [DOI] [PubMed] [Google Scholar]
- 11. Schepetkin IA, Quinn MT. (2006) Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential International Immunophar-macology 6(3): 317–333. [DOI] [PubMed] [Google Scholar]
- 12. Lull C, Wichers HJ, Savelkoul HFJ. (2005) Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators of Inflammation 2: 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song DH, Lee JO. (2012) Sensing of microbial molecular patterns by Toll-like receptors. Immunological Reviews 250(1): 216–229. [DOI] [PubMed] [Google Scholar]
- 14. Canton J, Neculai D, Grinstein S. (2013) Scavenger receptors in homeostasis and immunity. Nature Reviews Immunology 13(9): 621–634. [DOI] [PubMed] [Google Scholar]
- 15. Vijay K. (2018) Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. International Immunopharmacology 59: 391–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu JY, Yang FL, Lu CP, et al. (2008) Polysaccharides from dioscorea batatas induce tumor necrosis factor-α secretion via toll-like receptor 4-mediated protein kinase signaling pathways,” Journal of Agricultural and Food Chemistry 56(21): 9892–9898. [DOI] [PubMed] [Google Scholar]
- 17. Tang C, Sun J, Liu J, et al. (2019) Immune-enhancing effects of polysaccharides from purple sweet potato. International Journal of Biological Macromolecules 123: 923–930. [DOI] [PubMed] [Google Scholar]
- 18. Xie G, Schepetkin IA, Siemsen DW, et al. (2008) Fractionation and characterization of biologically-active polysaccharides from Artemisia tripartita. Phytochemistry 69(6): 1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta PK, Rajan MGR, Kulkarni S. (2017) Activation of murine macrophages by G1-4A, a polysaccharide from Tinospora cordifolia, in TLR4/MyD88 dependent manner. International Immunopharmacology 50: 168–177. [DOI] [PubMed] [Google Scholar]
- 20. Geoffrey G, Sylvie LP, Jacques P. (2006) NF-κB activation by reactive oxygen species: Fifteen years later. Biochemical Pharmacology 72(11): 1493–1505. [DOI] [PubMed] [Google Scholar]
- 21. Lee KY, Jeon YJ. Macrophage activation by polysaccharide isolated from Astragalus membranaceus. International Immunopharmacology 5(7–8): 1225–1233. [DOI] [PubMed] [Google Scholar]
- 22. Zhang XJ, Li Y, Tai GX, et al. (2005) Effects of activin A on the activities of the mouse peritioneal macrophages. Cellular & Molecular Immunology 2(1): 63–67. [PubMed] [Google Scholar]
- 23. Liu X, Xie J, Jia S, et al. (2017) Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. International Journal of Biological Macromolecules 98: 576–581. [DOI] [PubMed] [Google Scholar]
- 24. Henneke P, Golenbock DT. (2004) Phagocytosis, innate immunity, and host-pathogen specificity. The Journal of Experimental Medicine 199(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aderem A. (2003) Phagocytosis and the inflammatory response. The Journal of Infectious Diseases 187: 340–345. [DOI] [PubMed] [Google Scholar]
- 26. Cheng XD, Wu QX, Zhao J, et al. (2019) Immunomodulatory effect of a polysaccharide fraction on RAW 264.7 macrophages extracted from the wild Lactarius deliciosus. International Journal of Biological Macromolecules 128: 732–739. [DOI] [PubMed] [Google Scholar]
- 27. Bosca L, Zeini M, Traves PG, et al. (2005) Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 208(2): 249–258. [DOI] [PubMed] [Google Scholar]
- 28. Michel T, Vanhoutte PM. (2010) Cellular signaling and NO production, Pflugers Archiv. European Journal of Physiology 459(6): 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mosser DM. (2003) The many faces of macrophage activation. Journal of Leukocyte Biology 73(2): 209–212. [DOI] [PubMed] [Google Scholar]
- 30. Neurath MF. (2014) Cytokines in inflammatory bowel disease, nature reviews. Immunology 14(5): 329–342. [DOI] [PubMed] [Google Scholar]
- 31. Scheller J, Chalaris A, Schmidt-Arras D, et al. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta 1813(5): 878–888. [DOI] [PubMed] [Google Scholar]
- 32. Mittal M, Siddiqui MR, Tran K, et al. (2014) Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling 20(7): 1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asehnoune K, Strassheim D, Mitra S, et al. (2004) Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappaB,” Journal of Immunology 172: 2522–2529. [DOI] [PubMed] [Google Scholar]
- 34. Baeuerle PA, Henkel T. (1994) Function and activation of NF-κB in the immune system. Annual Review of Immunoloy 12: 1994. [DOI] [PubMed] [Google Scholar]
- 35. Toledano MB, Leonard WJ. (1991) Modulation of transcription factor NF-κB binding activity by oxidation-reduction in vitro. PNAS 88: 4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang C, Ding R, Sun J, et al. (2019) The impacts of natural polysaccharides on intestinal microbiota and immune responses–A review. Food & Function 10(5): 2290–2312. [DOI] [PubMed] [Google Scholar]
- 37. Sakurai H, Chiba H, Miyoshi H, et al. (1999) IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. The Journal of Biological Chemistry 274(43): 30353–30356. [DOI] [PubMed] [Google Scholar]
- 38. Hayden MS, Ghosh S. (2008) Shared principles in NF-κB signaling. Cell 132(3): 344–362. [DOI] [PubMed] [Google Scholar]
- 39. Natoli G, Chiocca S. (2008) Nuclear ubiquitin ligases, NF-kappaB degradation, and the control of inflammation,” Science Signaling 1(1): pe1. [DOI] [PubMed] [Google Scholar]
- 40. O’Dea E, Hoffmann A. (2010) The regulatory logic of the NF-κB signaling system. Cold Spring Harbor Perspect 2: a000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karin M. (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. The Journal of Biological Chemistry 270(28): 16483–16486. [DOI] [PubMed] [Google Scholar]
- 42. Karin M, Liu ZG, Zandi E. (1997) AP-1 function and regulation. Current Opinion in Cell Biology 9(2): 240–246. [DOI] [PubMed] [Google Scholar]
- 43. Lei W, Browning JD, Jr, Eiche PA, et al. (2015) Immuno-stimulatory activity of a polysaccharide-enriched fraction of Sutherlandia frutescens occurs by the toll-like receptor-4 signaling pathway. Ethnopharmacol 172: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu Q, Nie SP, Wang JQ, et al. (2012) Polysaccharide from Ganoderma atrum induces tumor necrosis factor-α secretion via phosphoinositide 3-kinase/Akt, mitogen-activated protein kinase and nuclear factor-κB signaling pathways in RAW264.7 cells. International Immunopharmacology 14: 362–368. [DOI] [PubMed] [Google Scholar]
- 45. Akira S, Hoshino K. (2003) Myeloid differentiation factor 88-dependent and -independent pathways inToll-like receptor signaling. Journal of Infectious Diseases 187: S356–S363. [DOI] [PubMed] [Google Scholar]
- 46. Kim HS, Kim JY, Kang JS, et al. (2010) Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food and Chemical Toxicology 48: 1926–1933. [DOI] [PubMed] [Google Scholar]
- 47. Kim HS, Kim JY, Kang JS, et al. (2010) Induction of dendritic cell maturation by beta-glucan isolated from Sparassis crispa. International Immunopharmacology 10: 1284–1294. [DOI] [PubMed] [Google Scholar]
- 48. Kim JY, Yoon YD, Ahn JM, et al. (2007) Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. International Immunopharmacology 7: 78–87. [DOI] [PubMed] [Google Scholar]
- 49. Beutler B, Hoebe K, Du X, et al. (2003) How we detect microbes and respond to them: The Toll-like receptors and their transducers. Journal of Leukocyte Biology 74: 479–485. [DOI] [PubMed] [Google Scholar]
- 50. Wei W, Xiao HT, Bao WR, et al. (2016) TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. Ethnopharmacol 179: 243–252. [DOI] [PubMed] [Google Scholar]
- 51. An HZ, Xu HM, Yu YZ, et al. (2002) Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activationof NF-κB, ERK and p38 MAPK signal pathways. Immunology Letters 81: 165–169. [DOI] [PubMed] [Google Scholar]