Abstract
Background:
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend triple therapy (TT) for chronic obstructive pulmonary disease (COPD) patients based on severity. TT utilization by severity is infrequently studied in real-world settings and may deviate significantly from current clinical recommendations. This study describes prescribing pathways to TT among patients with COPD in the United States.
Methods:
This study analyzed Geisinger Health System electronic medical records from 1 January 2004 to 30 November 2016. Two retrospective cohorts of COPD patients were included: (1) incident COPD, and (2) incident TT users. COPD treatment patterns, including time to TT, were summarized. Time to TT was estimated using Kaplan–Meier methods. Predictors of the relative hazard for TT among incident COPD patients were estimated using Cox proportional hazards regressions.
Results:
Incident COPD and TT cohorts included 57,141 and 8173 patients, respectively. TT was used by 9.6% of incident COPD patients. In the year before TT, 34.3% of incident TT patients received treatment combinations recommended before TT according to GOLD recommendations, which mainly included: long-acting muscarinic antagonists (LAMAs), long-acting beta agonists (LABAs) + LAMAs, and inhaled corticosteroids + LABAs. Among incident TT patients, median time from COPD diagnosis to TT exceeded 2 years. The hazard for TT over time was associated with lower forced expiratory volume in 1 s values, more frequent exacerbations, current/previous smoking, and comorbid lung conditions such as pulmonary vascular disease, acute respiratory failure, and lung cancer. About 15–20% of the incident TT patients stepped down to a one- or two-drug regimen. Median time to TT discontinuation or step-down were 2 and 9 months, respectively.
Conclusion:
The study has revealed discrepancies in the treatment of COPD patients between GOLD guidelines and actual clinical practices in the United States. Pathways to TT differed from recommended therapy regimes. Further studies are needed to understand barriers to the use of guideline-recommended TTs by healthcare providers.
The reviews of this paper are available via the supplemental material section.
Keywords: chronic obstructive pulmonary disease, GOLD guidelines, prescribing pathways, retrospective cohort study, triple therapy
Background
Chronic obstructive pulmonary disease (COPD) is a slowly developing, progressive respiratory condition that causes breathlessness and predisposes patients to exacerbations and serious illness. It affects 16 million adults in the United States1 with 5-year mortality rates ranging from 40% to 70% by severity.2 COPD prevalence in the overall US population ranges from 10.2% to 20.9%, compared with 9% to 10% in most other countries,3 posing a significant clinical and economic burden.4 Compared with patients with mild disease, patients who experience frequent exacerbations have a faster deterioration in lung function, poorer quality of life, and higher mortality.5
Pharmacologic therapies are available for COPD but do not necessarily slow or prevent disease progression and may only offer symptom relief.4 COPD management relies on inhaled drugs, including inhaled corticosteroids (ICSs), long-acting beta agonists (LABAs), and long-acting muscarinic antagonists (LAMAs).6 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations identify COPD phenotypes based on patient risk and suggest a preferred treatment protocol for each risk category.7 The 2016 GOLD recommended that patients with moderate-to-severe COPD be treated with drug combinations that include LABAs or ICSs, and patients with severe COPD be treated with a combination of LABAs, ICSs, and/or LAMAs, or a combination of all three drug types, known as ‘triple therapy’ (TT).8 Fixed-dose TT combinations were not available prior to 2017.
Previous studies have demonstrated that clinical management and COPD prescribing patterns deviate significantly from recommended practice.9–12 Mannino et al.10 reported that prescribing patterns deviated from GOLD recommendations for about 64% of patients with COPD in the United States. The magnitude of TT use and the pathways thereto are not well understood. To date, there are limited real-world studies that describe patterns of TT prescriptions, characteristics of patients receiving TT, and related outcomes. Therefore, the purpose of this study is to: (1) quantify the proportions of patients receiving TT before, at, and after initial COPD diagnosis; (2) identify pathways to TT among these patients; and (3) assess the time to initiation of TT from initial diagnosis of COPD.
Methods
Study population and data source
This was a noninterventional, retrospective cohort study using the Geisinger Health System (GHS) database.13 The data include electronic medical records from an integrated health system including more than 700 multi-specialty physicians across 55 clinical practice sites that provide adult and pediatric primary and specialty care, three hospitals, and a clinical reference laboratory.14 The GHS service area is limited to the state of Pennsylvania. The captured data include primary and specialty ambulatory care; inpatient stays; laboratory results including spirometry; vital signs; medication orders; procedures; diagnoses; and smoking history.15 It is likely that the prescription records in the database are incomplete because ambulatory prescriptions dispensed by private national pharmacy chains are not always reported back to the clinic system. This study was approved by the GHS Institutional Review Board; given that this study was based exclusively on analyses of a large electronic de-identified healthcare database, informed consent was not required.
The study included two overlapping cohorts of patients with at least one encounter with a diagnosis code indicative of COPD (see e-Appendix 1 for International Classification of Diseases, 9th Revision, Clinical Modification codes) from 1 January 2004 to 30 November 2016. Patients <40 years old as of their first-time COPD diagnosis were excluded. Additional eligibility criteria for the (1) incident COPD cohort and (2) incident TT cohort (i.e. patients with COPD who initiated TT) appear below. Incident COPD cohort was intended to provide insights on complete treatment pathways among these newly diagnosed COPD patients, while incident TT cohort would demonstrate time to discontinuation and step-down from TT within the same study period.
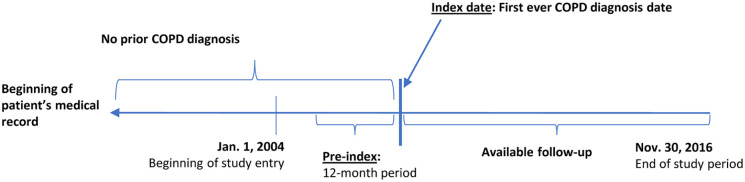
Incident COPD cohort patients had a first-time diagnosis of COPD from 1 January 2005 to 30 November 2016 and had at least 12 months of available information before first COPD diagnosis. Incident COPD cohort patients were followed from first COPD diagnosis until death, loss to follow-up, or the end of the study period, whichever occurred first (Figure 1).
Figure 1.
Schematic selection of incident COPD cohort.
COPD, chronic obstructive pulmonary disease.
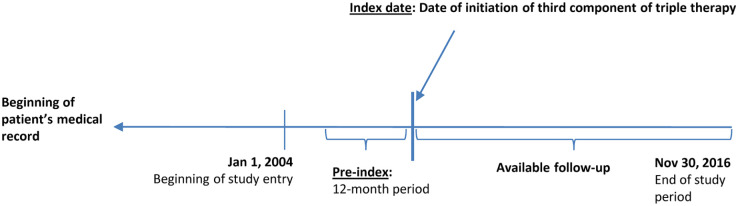
Incident TT cohort patients had ⩾1 courses of TT from 1 January 2005 to 30 November 2016. TT was inferred from ⩾30 consecutive days with overlapping TT medications (LABAs, LAMAs, and ICSs), derived from prescription dates and the recorded days’ supply for each prescription. When LABA, LAMA, and ICS prescriptions overlapped for ⩾30 days but began on different dates, the date of the third prescribed component defined TT initiation. Incident TT cohort patients were also required to have at least 12 months of available information before the start of the first course of TT. Incident TT cohort patients were followed from start of TT until death, loss to follow-up, or the end of the study period, whichever occurred first (Figure 2).
Figure 2.
Schematic selection of triple therapy cohort.
Study measures and outcomes
Multiple algorithms were applied to infer evidence of exacerbations. Event sequences qualifying as an exacerbation are defined in e-Table 1. The earliest date among any qualifying event in the algorithms above was considered the start date of an exacerbation. Exacerbation episodes were defined as lasting 21 days, and any episodes satisfying multiple exacerbation criteria were counted as a single episode. We estimated GOLD 2016 severity8 from the forced expiratory volume in 1 s (FEV1) results and exacerbations from 12 months before until 2 weeks after start of follow-up. Patients with available FEV1 % of predicted values were classified as GOLD 1 (⩾80% predicted), GOLD 2 (⩾50%, <80% predicted), GOLD 3 (⩾30%, <50% predicted), or GOLD 4 (<30% predicted). Exacerbation counts were used to derive GOLD A/B (⩽1, not including hospital admission) or GOLD C/D (⩾2 or inpatient exacerbation). We assigned patients to subgroups based on the following COPD phenotype definitions (e-Table 2): (1) asthma with COPD overlap [ACO (acronym used for brevity only)]; (2) frequent exacerbators; (3) nonfrequent exacerbators; (4) nonexacerbators; or (5) emphysema.
Patient characteristics were assessed during the 12 months prior to follow-up or at the beginning of study follow-up. Demographics included age at start of follow-up, sex, race and ethnicity, and beginning year of follow-up. Clinical characteristics included smoking history; body mass index; Charlson comorbidity index;16 most recent blood eosinophil count before start of follow-up; and the presence of specific respiratory, cardiovascular, metabolic, and other COPD-related comorbidities. We recorded use of the following COPD medications before start of follow-up: LAMAs, LABAs, and ICSs (including specific fixed-dose combinations), short-acting beta agonists (SABAs), short-acting muscarinic antagonists (SAMAs), methylxanthines, and phosphodiesterase-4 inhibitors.
We estimated median time from COPD diagnosis to TT in both cohorts. We measured the COPD medications observed in the 12 months before start of TT in the incident TT cohort and at any time from diagnosis to TT in the incident COPD cohort. Among TT users, step-down (discontinuation of one or two of LABA, LAMA, ICS components) or complete discontinuation (of all three components) required a 60-day gap at any time following initiation of TT. The date of step-down or complete discontinuation was defined as the last date where all three components overlapped. We also measured COPD medications prescribed during the 30 days following step-down, and exacerbations observed in within 60 or 180 days of step-down. TT persistence was calculated from start of TT until step-down, discontinuation, or end of follow-up.
Statistical analyses
We summarized baseline characteristics, COPD severity and phenotypes, and study outcomes with counts and percentages for categorical variables and with means and standard deviations for continuous variables. We constructed Kaplan–Meier estimators for time from start of follow-up to TT and from start of TT to step-down or discontinuation. In the incident COPD cohort, we estimated factors predicting TT initiation with Cox proportional hazards regressions. We fitted full and reduced Cox regressions, with a single step eliminating parameters (certain comorbid conditions) with significance level of p ⩾ 0.05, except for several variables forced to remain in the reduced model (age, sex, smoking, body mass index, and asthma in the year before index date). All analyses were conducted in SAS version 9.4.
Results
We identified 57,141 patients in the incident COPD cohort and 8173 patients in the incident TT cohort. Median [interquartile range (IQR)] follow-up after index date for incident COPD cohort and incident TT cohort were 29 (IQR: 10–63) months and 20 (IQR: 9–45) months, respectively. Duration of available follow-up after initiation of TT in incident COPD cohort was 19 (IQR: 9–42) months.
Baseline characteristics and exposures
The mean age at COPD diagnosis in the incident COPD cohort was 65 years (Table 1). Most patients were white (97.6%), female (50.5%), and either current (33.1%) or former (33.8%) smokers. Hypertension (55.5%), mood disorders (50.7%; includes tobacco use disorder), and hypercholesterolemia and hyperlipidemia (47.4%) were the most common comorbidities. About 38% of patients were nonfrequent or frequent exacerbators, and 44% had ACO. Only 10% had one or more recorded FEV1 measurements between 12 months before and 2 weeks after first diagnosis, so GOLD 1–4 status was unclassified for the remaining 90%. Mild-to-moderate COPD (GOLD A/B), as measured by exacerbation frequency and severity, was observed in 97.1% of patients. Nearly 62% received prescriptions for at least one COPD medication as monotherapy during the baseline period [Table 2(a)]. These included 21.2% receiving SABAs and 10.9% receiving LAMAs. Baseline combination therapy was observed in 7.8% of patients, including 3.5% with SABAs + LABAs + ICSs and 5.6% with LABAs + ICSs. At initial COPD diagnosis, the most frequent new prescriptions were reliever medications in various combinations [Table 2(b)].
Table 1.
Baseline characteristics of incident COPD and incident triple therapy cohort.
| Characteristics | Category | Incident COPD cohort (N = 57,141) | Incident triple therapy cohort (N = 8173) |
|---|---|---|---|
| Age at start of follow-up | Mean (SD) | 65.2 (12.5) years. | 63.8 (11.1) years. |
| Sex, n (%) | Male | 28,262 (49.5) | 4104 (50.2) |
| Ethnicity, n (%) | White | 55,777 (97.6) | 8050 (98.5) |
| African American | 741 (1.3) | 90 (1.1) | |
| Others/unknown | 623 (1.05) | 33 (0.41) | |
| Smoking history, n (%) | Current smokers | 18,900 (33.1) | 2657 (32.5) |
| Ex-smokers | 19,288 (33.8) | 4309 (52.7) | |
| Nonsmokers | 10,358 (18.1) | 855 (10.5) | |
| Missing | 8595 (15.0) | 352 (4.3) | |
| BMI category, n (%) | Underweight <18.5 | 1896 (3.3) | 351 (4.3) |
| Normal 18.5–25 | 14,178 (24.8) | 1943 (23.8) | |
| Overweight >25–30 | 15,105 (26.4) | 2108 (25.8) | |
| Obese >30 | 21,836 (38.2) | 3677 (44.9) | |
| Missing | 4126 (7.2) | 94 (1.2) | |
| Respiratory-related comorbidities, n (%) | Respiratory infection (except pneumonia) | 11,741 (20.6) | 2063 (25.2) |
| Pulmonary vascular disease | 3795 (6.6) | 863 (10.6) | |
| Acute respiratory failure | 3525 (6.2) | 1199 (14.7) | |
| Lung cancer | 1619 (2.8) | 447 (5.5) | |
| Asthma | 13,279 (23.2) | 2985 (36.5) | |
| Cardiovascular-related comorbidities, n (%) | Hypertension | 31,696 (55.5) | 4800 (58.7) |
| Ischemic heart disease | 16,713 (29.3) | 2929 (35.8) | |
| Other comorbidities, n (%) | Hypercholesterolemia/hyperlipidemia | 27,098 (47.4) | 4267 (52.2) |
| Cancer (except lung and thoracic) | 8162 (14.3) | 1472 (18.0) | |
| Diabetes mellitus | 13,871 (24.3) | 2349 (28.7) | |
| Mood disorders | 28,948 (50.7) | 4528 (55.4) | |
| Anemia | 12,045 (21.1) | 1953 (23.9) | |
| Dementia | 644 (1.1) | 49 (0.6) | |
| Alzheimer’s disease | 743 (1.3) | 48 (0.6) | |
| Sinusitis | 1303 (2.3) | 264 (3.2) | |
| Osteoporosis | 5467 (9.6) | 1003 (12.3) | |
| Osteoarthritis | 10,528 (18.4) | 1737 (21.3) | |
| Cholelithiasis (gallstones) | 1826 (3.2) | 321 (3.9) | |
| CCI score | Mean (SD) | 2.3 (2.0) | 2.4 (2.1) |
| COPD phenotype, n (%) | ACO | 25,162 (44.0) | 3048 (37.3) |
| Frequent exacerbators | 4986 (8.7) | 2351 (28.8) | |
| Nonfrequent exacerbators | 16,771 (29.4) | 2641 (32.3) | |
| Nonexacerbators | 35,384 (61.9) | 3181 (38.9) | |
| Emphysema | 8015 (14.0) | 1632 (19.9) | |
| Exacerbations in the 12 months prior or two weeks after index, n (%) | 0 | 34,033 (59.6) | 2150 (26.3) |
| 1 | 17,810 (31.2) | 3182 (38.9) | |
| 2 or more | 5298 (9.2) | 2841 (34.7) | |
| Number of FEV1 records/patient in the 12 months prior and two weeks after index date | 0 | 51,478 (90.1) | 6186 (75.7) |
| 1 | 5232 (9.2) | 1662 (20.3) | |
| 2 or more | 431 (0.8) | 325 (3.9) | |
| GOLD 2016 (A/B/C/D), n (%) | A/B | 55,456 (97.1) | 6835 (83.6) |
| C/D | 1685 (2.9) | 1338 (16.4) |
ACO, asthma with COPD overlap; BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SD, standard deviation.
Table 2a.
Baseline medication use in incident COPD and incident triple therapy cohort prior to start of follow-up.
| Medication categorya | Incident COPD cohort (N = 57,141) (%) | Incident triple therapy cohort (N = 8173) | |
|---|---|---|---|
| Baseline medications prior to start of follow-up | LAMA | 6258 (10.9) | 1471 (18.0) |
| LABA | 197 (0.3) | 152 (1.9) | |
| LAMA + LABA | 23 (0.04) | 22 (0.3) | |
| ICS | 1698 (2.9) | 921 (11.3) | |
| LABA + ICS | 3191 (5.6) | 1930 (23.6) | |
| SABA | 12,125 (21.2) | 4164 (50.9) | |
| SAMA | 869 (1.5) | 425 (5.2) | |
| SABA + SAMA | 8420 (14.7) | 3207 (39.2) | |
| PDE-4 | 24 (0.04) | 64 (0.8) | |
| Methylxanthines | 2713 (4.8) | 912 (11.2) | |
| COPD treatment combinations prior to start of follow-up | LABA + ICS | 3179 (5.6) | — |
| SABA + LAMA + LABA + ICS | 626 (1.1) | — | |
| SAMA + SABA + LAMA + LABA + ICS | 287 (0.5) | — | |
| SABA + LABA + ICS | 2008 (3.5) | — | |
| LABA + LAMA | 925 (1.6) | — | |
The medication categories are not mutually exclusive that is, the sum of total number of patients for all the medication categories could exceed 100%.
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; PDE-4, phosphodiesterase-4; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist.
Table 2b.
Baseline medication use in incident COPD and incident triple therapy cohort at start of follow-up.
| Medication categorya | Incident COPD cohort (N = 57,141) (%) | Incident triple therapy cohort (N = 8173) (%) | |
|---|---|---|---|
| Baseline medications at start of follow-up | LAMA | 1504 (2.6) | 7829 (95.8) |
| LABA | 99 (0.2) | 283 (3.5) | |
| LAMA + LABA | 60 (0.1) | 42 (0.5) | |
| ICS | 614 (1.1) | 617 (7.6) | |
| LABA + ICS | 1885 (3.3) | 7192 (88.0) | |
| SABA | 6594 (11.5) | 4923 (60.2) | |
| SAMA | 380 (0.7) | 164 (2.0) | |
| SABA + SAMA | 4266 (7.5) | 2708 (33.1) | |
| PDE-4 | 23 (0.04) | 93 (1.1) | |
| Methylxanthines | 883 (1.6) | 380 (4.7) | |
| COPD treatment combinations at start of follow-up | LABA + ICS | 1779 (3.1) | — |
| SABA + LAMA + LABA + ICS | 353 (0.6) | — | |
| SAMA + SABA + LAMA + LABA + ICS | 145 (0.3) | — | |
| SABA + LABA + ICS | 1101 (1.9) | — | |
| LABA + LAMA | 491 (0.9) | — | |
| COPD triple therapy combinations at start of follow-up | LABA/LAMA + ICS | 5 (0.01) | 36 (0.4) |
| LAMA + ICS/LABA | 1429 (2.5) | 7865 (96.2) | |
| LAMA + LABA + ICS | 45 (0.1) | 310 (3.8) | |
The medication categories are not mutually exclusive that is, the sum of total number of patients for all the medication categories could exceed 100%.
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; PDE-4, phosphodiesterase-4; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist.
The mean age at COPD diagnosis in the incident TT cohort was about 64 years (Table 1). Most patients were white (98.5%); male (50.2%), and were either current (32.5%) or former (52.7%) smokers. Hypertension (58.7%), mood disorders (55.4%; including tobacco use disorder), and hypercholesterolemia and hyperlipidemia (52.2%) were the most common comorbidities. About 61% of patients were nonfrequent or frequent exacerbators; 37.3% of patients had ACO. One or more FEV1 tests were observed in 24.3% of the cohort, leaving GOLD 1–4 undetermined for 75.7% of patients. Mild-to-moderate COPD (GOLD A/B), measured from frequency and severity of exacerbations, was observed in 83.6% of patients. During the year before TT, 99.2% of patients received a prescription for at least one monotherapy. SABA was most frequently observed (50.9%), followed by LAMA (18.0%) [Table 2(a)]. Overall, 63.1% of patients were using at least one combination therapy during the pre-index period, including SABAs + SAMAs (39.2%) and LABAs + ICSs (23.6%).
Pathways to TT
In the incident COPD cohort, 9.6% initiated TT before the end of the study period, including 1.9% who began TT prior to their COPD diagnosis. In the incident TT cohort, most patients (82.1%) received TT after their COPD diagnosis, while some received TT before (14.2%) or at COPD diagnosis (3.8%).
In the incident COPD cohort, 4407 patients initiated TT at or after COPD diagnosis. Between diagnosis and TT initiation, patients could receive different treatments, both rescue and maintenance therapies. Some patients may receive monotherapies while others may receive combinations of them; 57.9% of patients received at least one monotherapy and 84.8% received at least one combination therapy (Table 3). SABA was the most frequently prescribed monotherapy (46.9%), followed by LAMA (17.6%) and methylxanthines (12.5%). The most frequent maintenance therapy was ICSs + LABAs (28.5%); other most frequently prescribed therapy combinations included SAMAs + SABAs (47.9%). Similarly, among patients stratified by severity as defined by GOLD A/B and C/D categories (e-Table 3), SABA was the most frequently prescribed monotherapy (47.0% and 45.5% in GOLD A/B and C/D cohorts, respectively) while ICSs + LABAs were the most frequently prescribed combination therapy (28.2% and 35.5% in GOLD A/B and C/D cohorts, respectively). SABAs + SAMAs were also commonly prescribed in GOLD A/B (47.7%) and C/D cohorts (55.6%).
Table 3.
Pathways to triple therapy in incident COPD and incident triple therapy cohort.
| Treatments prior to triple therapy initiationa | Category | Subgroup of patients in incident COPD cohort initiating triple therapy (N = 4407) (%) | Total incident triple therapy cohort (N = 8173) (%) |
|---|---|---|---|
| Monotherapy | LAMA | 776 (17.6) | 861 (10.5) |
| LABA | 45 (1.0) | 44 (0.5) | |
| SABA | 2070 (46.9) | 2797 (34.2) | |
| SAMA | 105 (2.4) | 146 (1.8) | |
| ICS | 280 (6.4) | 359 (4.4) | |
| PDE-4 inhibitors | 24 (0.5) | 32 (0.4) | |
| Methylxanthines | 549 (12.5) | 612 (7.5) | |
| Any monotherapy | 2555 (57.9) | 3747 (45.9) | |
| Combination therapy | LABA + ICS | 1257 (28.5) | 1915 (23.4) |
| SABA + LABA + ICS | 849 (19.3) | 1265 (15.5) | |
| SAMA + LABA + ICS | 595 (13.5) | 879 (10.8) | |
| Any other combinations | 290 (6.5) | 355 (4.4) | |
| Any combination therapy | 1362 (30.9) | 2071 (25.3) | |
| Other combination therapy | ICS + SABA | 1145 (25.9) | 1752 (21.4) |
| LAMA + SABA | 663 (15.0) | 786 (9.6) | |
| SAMA + SABA | 2115 (47.9) | 3309 (40.5) | |
| Any other combination therapy | 2373 (53.9) | 3764 (46.1) |
Period observed for incident COPD cohort includes all time between initial diagnosis and TT initiation; for incident TT cohort, period includes 12 months before TT initiation.
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; PDE-4, phosphodiesterase-4; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist.
In the incident TT cohort, 45.9% had at least one prescription for monotherapy in the 12 months before TT initiation, with SABAs being the most prevalent monotherapy (34.2%) followed by LAMAs (10.5%) and methylxanthines (7.5%); 71.4% of patients had at least one prescription for combination therapy, the most commonly prescribed combinations were ICSs + LABAs (23.4%) and SABAs + LABAs + ICSs (15.5%). Other commonly prescribed combinations included SAMAs + SABAs (40.5%) and ICSs + SABAs (21.4%) (Table 3). Among frequent exacerbators, 54.9% received SABAs, 62.1% received SAMAs + SABAs, 32.2% received ICSs + LABAs, 23.3% received ICSs + LABAs + LAMAs in the 12 months prior to TT initiation during this period (e-Table 4).
Time to initiation of TT
Only 9.6% of the incident COPD cohort received TT; median time to TT was not defined in Kaplan–Meier estimates (Figure 3). In the incident TT cohort, the median time to TT initiation from initial COPD diagnosis was 24.1 months (IQR 2.0–61.3 months).
Figure 3.
Kaplan–Meier plots for treatment patterns in incident COPD cohort.
COPD, chronic obstructive pulmonary disease.
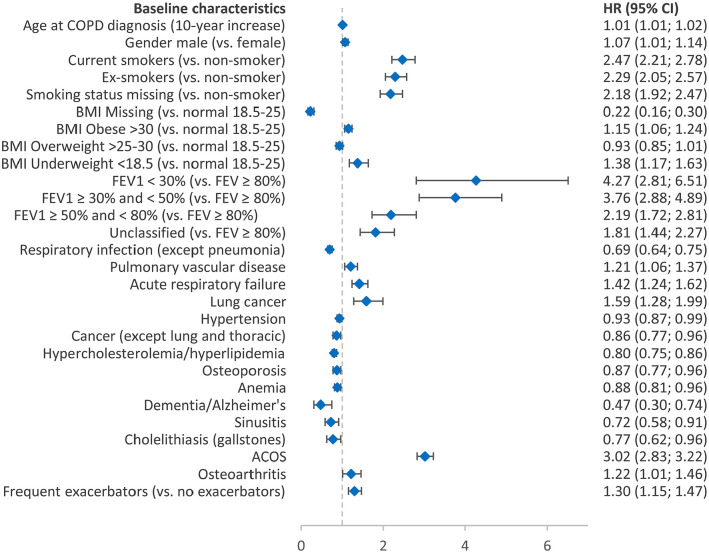
In Cox proportional hazards regressions of the incident COPD cohort, decreasing FEV1 values predicted increased hazards for TT (Figure 4). Those with FEV1 <30% had a hazard four-times greater than that for patients with an FEV1 ⩾80% [hazard ratio (HR) = 4.27; 95% confidence interval (CI): 2.81, 6.51]. ACO predicted a threefold increase in the hazard for TT (HR = 3.02; 95% CI: 2.83, 3.22). Frequent and nonfrequent exacerbators were associated with 30% (HR = 1.30; 95% CI: 1.15, 1.47) and 21% (HR = 1.21; 95% CI: 1.13, 1.30) increased hazards for TT, respectively. Age at COPD diagnosis (HR = 1.01; 95% CI: 1.01, 1.02) and male sex (HR = 1.07; 95% CI: 1.01, 1.14) was also found to be associated with increased hazards of TT.
Figure 4.
Predictors for initiation of triple therapy among the incident COPD cohort.
ACOS, asthma with COPD overlap syndrome; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume.
Comorbidities that increased the hazard of TT included pulmonary vascular disease, acute respiratory failure, osteoarthritis, and lung cancer (HR = 1.21–1.59; all p ⩽ 0.05). Current or ex-smokers had twice the hazard of starting TT compared to nonsmokers (HR = 2.47; 95% CI: 2.21, 2.77 and HR = 2.29; 95% CI: 2.05, 2.57, respectively).
Discontinuation and step-down from TT
Among incident COPD patients who initiated TT, 72.67% of patients discontinued treatment completely, and another 15.7% (n = 866) stepped down to one- or two-drug regimens (Table 4). Choice of step-down therapy included 7.89% to monotherapy, the most common of which was LAMA monotherapy (7.5%), and another 7.3% to combination therapy, the most common combination being LABAs + ICSs (7.2%). Median time to complete discontinuation and to step-down were both 13 months in this subgroup (Figure 3). Exacerbations were observed in 15% of patients within 60 days and 33% of patients within 180 days after discontinuation or step-down.
Table 4.
Pathways to step-down from triple therapy in the COPD incident cohort and the incident triple therapy cohort.
| Category | Incident COPD patients who initiate on triple therapy before the end of follow-up (N = 5499) (%) | Incident triple therapy cohort (N = 8173) (%) | |
|---|---|---|---|
| Overall step-down, n (%) | 866 (15.8) | 1571 (19.2) | |
| Monotherapy | LAMA | 412 (7.5) | 931 (11.4) |
| Any other monotherapy | 82 (1.5) | 191 (2.3) | |
| Any monotherapy | 434 (7.9) | 963 (11.8) | |
| Combination Therapy | LABA + ICS | 393 (7.2) | 539 (6.6) |
| Any other combination therapy | 90 (1.7) | 124 (1.5) | |
| Any combination therapy | 401 (7.3) | 552 (6.8) | |
| Any other combination therapy | 74 (1.4) | 133 (1.6) | |
| Persistent | 637 (11.6) | 905 (11.1) | |
| Complete discontinuation (without step-down) | 3996 (72.7) | 5697 (69.7) | |
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist.
Among incident TT cohort, complete TT discontinuation was observed in nearly 70% of this cohort; another 19% stepped down to one- or two-drug combinations during the follow-up (Table 4). Of 1571 patients who stepped down, 11.8% switched to monotherapy and 7.3% stepped down to other combination therapies. The most frequently prescribed monotherapy was LAMAs (11.4%), and the most frequently prescribed combination was LABAs + ICSs (6.6%). The median time to complete discontinuation and step-down were 2 and 9 months (95% CI: 8–12), respectively (Figure 5). Exacerbations were observed in 15% of patients within 60 days and 36% of patients within 180 days after discontinuation or step-down.
Figure 5.
Kaplan–Meier plots for treatment patterns in incident triple therapy cohort.
COPD, chronic obstructive pulmonary disease.
Discussion
GOLD 2016 treatment guidelines consider TT to be a progression from prior combination therapies for more severe patients, after they have experienced inadequate symptom control or exacerbations. According to these guidelines,8 patients in GOLD A/B categories should start on single bronchodilator (SABAs, SAMAs, LABAs, or LAMAs), while patients in GOLD C/D categories should start with either LAMA monotherapy or one of two specific combination therapies (LAMAs + LABAs or ICSs + LABAs). Our findings indicate there is a significant gap between recommended initial treatments and prescribed treatments. Our analysis also suggested long progressions from first COPD diagnosis to TT, without clear evidence of orderly progression of therapies consistent with GOLD recommendations. Median time from COPD diagnosis to TT exceeded 2 years, although our regression models identified predictable markers of COPD severity (FEV1, exacerbations, male sex, increased age, current or previous smoking, and some comorbid lung conditions) associated with shorter time to TT.
An important limitation of this study is that persistence was underestimated due to missing refills data. Although our data came from an integrated health system with its own pharmacy, ambulatory prescriptions can be dispensed locally by several national pharmacy chains that compete aggressively for patient business, and which do not always communicate refill data back to the clinic system. With incomplete access to refills, more than two-thirds of TT patients appeared to discontinue TT completely, with another 15% to 20% stepping down to a one- or two-drug regimen. Incident TT patients who appeared to discontinue TT did so in a median of 2 months (the earliest possible under study definitions), and the remainder who stepped down did so over a median of 9 months. Exacerbations were observed with some frequency after step-down or complete discontinuation; however, with the incomplete access to refill data, it is difficult to infer whether treatment changes increased exacerbation risk or vice versa.
Studies based on large databases are subjected to possible diagnostic and miscoding bias, including local clinical practices that influence coding. In this study, for example, the high comorbidity rate for mood disorders included diagnoses of tobacco use disorder, which coincided frequently with COPD patients’ prescriptions for nicotine replacement therapy. In addition, patients included in our study were required to have at least one diagnosis code for COPD, which could have increased the risk of misclassification. Further research is needed to understand the impact of different coding algorithms (e.g. identification of patients with at least two diagnosis codes). Likewise, we suspect that spirometry was recorded less frequently in clinic system records than it was performed, which constrained our ability to characterize GOLD severity as measured by FEV1. Lack of availability of fixed-dose TT combinations during the study period could have further impacted on our findings. Given the variations in prescribing of reliever medications such as SABAs and SAMAs, the utilization of such medications may not be accurately reflected in the study. Future studies are needed to take them into account. Given that the data used in this study were from an integrated health system in Pennsylvania, the generalizability of the findings is limited. Given that our study period was from 2004 to 2016, the pathways to TT reported in this study may be outdated. While the open-label TT combinations could have been appropriately captured in our study, the advent of various new fixed-dose combinations approved in the United States since 2016 were not captured. Therefore, further studies are needed to evaluate treatment pathways after 2016.
Despite these limitations, our large cohort-based study provides robust estimates of ‘real-world’ TT utilization and treatments received prior to and after TT initiation. The data captured over a decade give unique insights of treatment patterns, which is critical given the limited evidence available.
Interpretation
TT appears to be an infrequent treatment option among US patients diagnosed with COPD. Results from this study shows that treatments other than TT are more commonly utilized among this patient population, suggesting gaps between usual care and GOLD guidelines. Initial therapies for incident COPD patients frequently differed from GOLD recommendations, and substantial proportions of patients transition from incident diagnosis to TT without exposure to treatment combinations normally recommended before TT. Further studies are needed to understand barriers for adaptation of these guidelines by healthcare providers.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Acknowledgments
We thank J.C. Simeone, (Evidera), D. Lambrelli, (Evidera), and N.K. Leidy, (Evidera).
Footnotes
Author contributions: V. Schabert, S. Shah, C. Cabrera and U. Holmgren contributed to the study design, data analysis and interpretation, and the writing of the manuscript. All the authors approved this version to be published and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by AstraZeneca.
Disclosures: This study was supported by AstraZeneca. U. Holmgren and C. Cabrera are current employees of AstraZeneca. S. Shah is an employee of Evidera and V. Schabert is a former employee of Evidera. All authors helped interpret the results, wrote or provided substantive comments on the manuscript, approved the final version of the manuscript, and agreed to be accountable for its contents. In addition, C. Cabrera conceived the study; C. Cabrera, U. Holmgren, V. Schabert, and S. Shah designed the study; and analyzed the results.
Guarantor statement: The corresponding author, C. Cabrera is the guarantor of the content of the manuscript, including the data and analysis.
Other contributions: Third-party data analysis support was provided by hMetrix, a business management consultancy in Pennsylvania, US and was funded by AstraZeneca.
Notation of prior abstract publication/presentation: None
ORCID iD: Surbhi Shah  https://orcid.org/0000-0001-8351-0265
https://orcid.org/0000-0001-8351-0265
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Vernon Schabert, Independent, Sarajevo, Bosnia and Herzegovina.
Surbhi Shah, Evidera Inc, Waltham, MD, USA.
Ulf Holmgren, AstraZeneca plc, Gothenburg, Sweden.
Claudia Cabrera, BioPharmaceuticals Medical, AstraZeneca, KC6 SE-431 83 Mölndal, Gothenburg, Sweden.
References
- 1. Centers for Disease Control and Prevention. Basics about COPD, https://www.cdc.gov/copd/basics-about.html (2019, accessed 15 January 2020).
- 2. Nishimura K, Tsukino M. Clinical course and prognosis of patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med 2000; 6: 127–132. [DOI] [PubMed] [Google Scholar]
- 3. Ho T, Cusack RP, Chaudhary N, et al. Under- and over-diagnosis of COPD: a global perspective. Breathe (Sheff) 2019; 15: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guarascio AJ, Ray SM, Finch CK, et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013; 5: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George C, Zermansky W, Hurst JR. Frequent exacerbations in chronic obstructive pulmonary disease. BMJ 2011; 342: d1434. [DOI] [PubMed] [Google Scholar]
- 6. Malerba M, Nardin M, Santini G, et al. Single-inhaler triple therapy utilizing the once-daily combination of fluticasone furoate, umeclidinium and vilanterol in the management of COPD: the current evidence base and future prospects. Ther Adv Respir Dis 2018; 12: 1753466618760779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Safka KA, Wald J, Wang H, et al. GOLD stage and treatment in COPD: a 500 patient point prevalence study. Chronic Obstr Pulm Dis 2016; 4: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD, http://goldcopd.org/ (2016, accessed 22 February 2020). [DOI] [PubMed]
- 9. Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J 2008; 15: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mannino DM, Yu TC, Zhou H, et al. Effects of GOLD-adherent prescribing on COPD symptom burden, exacerbations, and health care utilization in a real-world setting. Chronic Obstr Pulm Dis 2015; 2: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lane DC, Stemkowski S, Stanford RH, et al. Initiation of triple therapy with multiple inhalers in chronic obstructive pulmonary disease: an analysis of treatment patterns from a US retrospective database study. J Manag Care Spec Pharm 2018; 24: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis 2017; 12: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geisinger. System-wide electronic health record, https://www.geisinger.edu/research/research-at-geisinger/resources/system-wide-electronic-health-records (2019, accessed 17 January 2020).
- 14. Paulus RA, Davis K, Steele GD. Continuous innovation in health care: implications of the Geisinger experience. Health Aff (Millwood) 2008; 27: 1235–1245. [DOI] [PubMed] [Google Scholar]
- 15. Geisinger. About Geisinger, https://www.geisinger.org/about-geisinger (2019, accessed 17 January 2020).
- 16. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211001018 for Prescribing pathways to triple therapy in patients with chronic obstructive pulmonary disease in the United States by Vernon Schabert, Surbhi Shah, Ulf Holmgren and Claudia Cabrera in Therapeutic Advances in Respiratory Disease