Abstract
Background:
Several national organizations have advocated for inpatient antiretroviral stewardship to prevent the consequences of medication-related errors. This study aimed to evaluate the impact of a stewardship initiative on outcomes in people with HIV (PWH).
Methods:
A pharmacist-led audit and review of adult patients admitted with an ICD-10 code for HIV was implemented to an existing antimicrobial stewardship program. A quasi-experimental, retrospective cohort study was conducted comparing PWH admitted during pre- and post-intervention periods. Rates of antiretroviral therapy (ART)-related errors and infectious diseases (ID) consultation with linkage to care were evaluated through selection of a random sample of patients receiving ART in each period. Length of stay (LOS) and mortality were assessed by analyzing all admissions in the post-intervention period. Clinical outcomes including LOS, 30-day all-cause hospital readmission, and in-hospital and 30-day mortality in the post-intervention group were stratified by patients not on ART, on ART at admission, and started on ART as a result of the intervention.
Results:
A total of 100 patients in the pre-intervention period and 103 patients in the post-intervention period were included to assess ART-related errors and linkage to care. A reduction in errors (70.0 versus 25.7%, p < 0.001) and increased linkage to care (19.0 versus 39.6%, p < 0.01) were demonstrated. Of 389 admissions during the post-intervention period, 30-day mortality rates were similar between PWH on ART at admission and those initiated on ART during admission (5% versus 8%, respectively), but less than those not on ART (21%). A longer LOS was observed in the patients started on ART during admission (5 days if ART started during admission versus 3 days if not started during admission, p < 0.01).
Conclusions:
This interdisciplinary intervention was successful in reducing inpatient ART-related errors and increasing ID consultation with linkage to care among PWH.
Keywords: antiretroviral stewardship, HIV, medication-related errors, transitions of care
Introduction
The Infectious Diseases Society of America (IDSA), HIV Medicine Association (HIVMA), and American Academy of HIV Medicine (AAHIVM) recently released a call to action for incorporation of antiretroviral stewardship into existing stewardship initiatives.1 The complexity of antiretroviral regimens and lack of experience with HIV treatment by inpatient providers may lead to medication-related errors; error rates of up to 86% related to antiretroviral therapy (ART) in hospitalized patients have been reported in the literature.2 Errors in antiretroviral prescribing can lead to drug resistance, treatment failure, and adverse effects if not promptly addressed. Transitions of care between community and inpatient settings magnify the potential for these medication-related antiretroviral errors.2,3
In addition, admission to an inpatient facility presents an opportunity for linkage or reengagement in care, but ART is occasionally held or stopped during hospitalizations without arrangement of HIV follow up. Several factors may result in ART suspension or discontinuation, such as acute changes in renal and/or hepatic function, introduction of new medications resulting in severe drug–drug interactions (DDIs) with ART or concerns for enteric absorption requiring administration via nasogastric tubes.4 ART dramatically reduces HIV-associated morbidity and mortality; however, only 55% of people with HIV (PWH) in the United States (US) have suppressed viral loads, often resulting from failure to link or retain diagnosed patients in care.5 ART stewardship programs should be able to reduce medication errors and use inpatient admissions as an opportunity to engage PWH in care.
Koren et al. suggest that institutions augment their stewardship programs to include an interdisciplinary team comprised of an infectious diseases (ID)-trained pharmacist and physician to manage, maintain, and monitor ART prescribing.1 Upon internal review of baseline data at our institution, inpatient medication-related ART errors were noted in up to 70% of patients. In response, a comprehensive, interdisciplinary intervention aimed at improving outcomes for PWH in our large health-system was implemented. The objectives of this initiative were to decrease medication-related errors during hospitalization, increase the proportion of PWH with linkage to care after hospital discharge, and improve clinical outcomes such as hospital readmission and mortality. Here, we describe our experience and outcomes of an HIV stewardship intervention and provide a framework for implementation for other health-systems aiming to incorporate recommendations from national organizations.
Methods
Intervention
An initiative was implemented targeting stewardship pharmacist-led review of all PWH admitted to Carolinas Medical Center – an 875-bed tertiary care hospital in Charlotte, NC, USA. With a goal to implement review at all 10 inpatient facilities in the system, the initiative was first piloted at the flagship hospital. Key stakeholders from ID physician and pharmacy groups developed a process to identify PWH hospitalized for any reason (HIV-related or not) and ensure optimal use of ART. An alert-based report for ART orders was built into the stewardship clinical decision support platform, Theradoc® (Premier Healthcare Solutions, Charlotte, NC, USA). To capture patients not receiving ART, an office administrator identified PWH via a daily report of International Classification of Diseases, Tenth Edition (ICD-10) diagnosis of HIV (B20-B24, R75, and Z21), which was emailed each morning to the stewardship pharmacists. These reports were reconciled and incorporated into usual antimicrobial stewardship workflow with at least twice weekly review beginning in April 2018. A group of four ID pharmacists rotated through this position monthly. Early in the initiative, interventions were piloted in a small subset of services (e.g., hospitalist, critical care) and eventually expanded to the entire hospital by October 2018. For patients on ART, the ID pharmacist reviewed the medication profile for all admitted PWH twice weekly to ensure that ART was continued appropriately (e.g., correct regimen, formulation, dose, scheduling) and that no DDIs were present, and that opportunistic infection prophylaxis was prescribed when indicated. At Atrium Health, ID physicians round daily with the ID pharmacist to review cases requiring intervention. If PWH were not on ART or if a potential need for an ART switch was identified (e.g., major DDIs, chronic change in renal function, etc.), the ID pharmacist contacted the ID physician on-call to initiate or change ART and link or reengage the patient in care, if needed. All of the approximately 15 ID physicians staffing at the flagship hospital participated in this initiative when on-call. In March 2018, educational materials summarizing the operational workflow were developed, presented, and shared among pharmacists and physicians, both via email and the hospital intranet. Additionally, ID physicians were educated live at the local antibiotic subcommittee meeting. To minimize medication errors, all ART agents were placed on the hospital’s formulary, including combination tablets. Finally, ID physicians were encouraged to contact one of two social workers devoted to HIV linkage to care to discuss the importance of regimen adherence with patients and schedule outpatient follow-up visits, which were usually within 1 month of discharge or sooner.
Study design
A quasi-experimental, retrospective cohort study of all adult (⩾18 years of age) PWH identified via ICD-10 code admitted during pre- and post-intervention periods was conducted. This study was approved by Atrium Health Institutional Review Board (#09-17-04E) and determined to be exempt from full review with a waiver of patient consent. The pre-intervention period was January 2017 through June 2017, and the post-intervention period was October 2018 through March 2019. The post-intervention period was selected to be several months after the pre-intervention period to allow for justification of services to pharmacy and ID leadership with pre-intervention data, process development, provider and pharmacy education, and implementation once hospital-wide support was established. Patients were excluded if receiving ART for non-HIV treatment indications (e.g., hepatitis B, pre-exposure prophylaxis) or if admitted solely to transition to hospice. Readmission was connected to an index admission and was not considered a discrete encounter.
The primary endpoint was the rate of inpatient medication-related ART errors comparing pre- and post-intervention periods. Secondary outcomes included the frequency of formal ID consultation and linkage to care after discharge, and clinical outcomes such as length of stay (LOS), 30-day all-cause hospital readmission, and in-hospital and 30-day mortality. An error was defined as a DDI, omission of a regimen component, inappropriate dose, wrong formulation dispensed, or duplicative therapy that was not corrected during the inpatient encounter. ART DDIs were identified based on HIV guideline recommendations from the Department of Health and Human Services and included inappropriate separation of doses where indicated [e.g., divalent cations with integrase strand transfer inhibitors (INSTIs)], prescription of contraindicated concomitant medication or inappropriate dosing of medication due to an interaction with ART.5 To assess medication-related errors and ID consultation with linkage to care, 100 and 103 patients (respectively) were selected randomly from a pharmacy-generated list of patients receiving ART in both the pre-intervention and post-intervention periods. When assessing clinical outcomes, all patients with an ICD-10 code for HIV in the post-intervention period were included; three subgroups were then compared: patients on ART prior to admission and continued, no ART prior to or during admission, and no ART prior to admission but started during admission as a result of the intervention. Data collection, review, and extraction were performed by ID pharmacists and physicians via a standardized data collection instrument and guidance tool in REDCap™.
Data were collected on receipt of ART at various time points (prior to, during admission, and at discharge), obtainment of ID consultation, linkage to care, medication-related errors during hospitalization, and pertinent laboratory tests including HIV viral load and CD4 T-cell count at baseline. Hospital LOS, readmission, and mortality were assessed by review of the medical record for patients in the post-intervention period.
Statistical analysis
Preliminary univariate analysis was performed between intervention groups in the form of frequency and percentages for categorical variables, and medians with interquartile ranges for continuous variables. Between-group comparisons were analyzed for demographic and primary outcomes. Medication-related ART errors and rate of ID consultation were compared between patients hospitalized during the pre- and post-intervention periods using Pearson’s chi-square and Fisher’s exact tests for association. Clinical outcomes such as LOS and mortality in the overall population were compared between patients on ART prior to admission and continued, those not on ART, and those started on ART as a result of the intervention. For this analysis, the chi-square test or Fisher’s exact test were used to analyze categorical variables and one-way analysis of variance (ANOVA) was used for continuous variables. Additionally, baseline comparisons were made between the pre- and post-intervention data using one-way ANOVAs for continuous variables, and Pearson’s chi-square and Fisher’s exact tests for categorical variables. Statistical tests were found significant at a p value of 0.05 and were conducted using SAS/STAT, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Medication-related errors and linkage to care
The post-intervention frequency of medication-related errors decreased significantly (70.0% versus 25.7%, respectively, p < 0.001) (Table 1). As ART was not actively monitored during the pre-intervention period, only one intervention was made by the stewardship team, compared with 119 made during the post-intervention period. Nearly all (97%) interventions were accepted by the primary provider. More patients received single-tablet, INSTI-based regimens in the post-intervention period (62.1% versus 43.0%, p < 0.01). The most common error types in both periods were DDIs and omission of a component of the regimen, both of which decreased during the post-intervention period (44.0–20.4%, p < 0.01 and 33.0–0.97%, p < 0.01, respectively). Most DDIs identified were with INSTIs and divalent cations that were inappropriately timed (37/65, 55%) and protease inhibitors (PI) or cobicistat with statins (12/65, 18%). The rate of ID consultation increased significantly in the post-intervention period (19.0 versus 39.6%, p < 0.01).
Table 1.
Impact on medication related errors and ID consultation.
| Factor | Pre-intervention (N = 100) | Post-intervention (N = 103) | p value |
|---|---|---|---|
| Age (median, IQR) | 47 (36–59) | 44 (31–54) | 0.43 |
| Male sex [n (%)] | 64 (64.0) | 68 (66.0) | 0.87 |
| Black [n (%)] | 80 (80.0) | 93 (90.3) | 0.45 |
| Single tablet regimen [n (%)] | 43 (43.0) | 64 (62.1) | <0.01 |
| Base agent [n (%)] | |||
| INSTI | 76 (76.0) | 93 (90.3) | <0.01 |
| PI | 28 (28.0) | 14 (13.6) | 0.01 |
| NNRTI | 16 (16.0) | 6 (5.8) | 0.02 |
| Overall medication-related errors | 70 (70.0) | 26 (25.7) | <0.001 |
| Error type [n (%)] | |||
| DDI | 44 (44.0) | 21 (20.4) | <0.01 |
| Component omission | 33 (33.0) | 1 (0.97) | <0.01 |
| Inappropriate dose adjustment | 2 (2.0) | 0 | 0.24 |
| ID consult involvement [n (%)] | 19 (19.0) | 40 (39.6) | <0.01 |
DDI, Drug–drug interaction; ID, infectious diseases; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Clinical outcomes
In the post-implementation period, 389 patient admissions in 275 unique PWH were identified. This cohort was largely Black (80%) and male (61%) with a mean age of 52 years (Table 2). Of 389 patient admissions, 294 (76%) had ART continued at the time of hospitalization. In patients on ART at admission, CD4 count and rate of viral suppression were significantly higher, and inpatient mortality lower compared with those not on ART (Table 2). For the 95 patient admissions in PWH not on ART at the time of hospitalization, 38 patients were started on ART and offered linkage to outpatient HIV care as a result of the stewardship intervention. Inpatient and 30-day mortality rates were similar to those already receiving ART at admission (Figure 1). Patients who started on ART during admission tended to have longer LOS by approximately 1 day [median 3 days (interquartile range, IQR, 1–5) in patients not on ART, 4 days (IQR 2–6) if on ART at admission, and 5 days (IQR 2–10) if started on ART during admission] (Figure 2). No difference was observed between the groups with regard to 30-day readmission (14% in patients not on ART, 15% if on ART at admission, 13% if started on ART during admission, p = 0.98).
Table 2.
Baseline characteristics and impact of ART initiation on clinical outcomes in post-intervention period.
| Factor | No ART started during admission (N = 57) | On ART at admission (N = 294) | ART started during admission (N = 38) | p value |
|---|---|---|---|---|
| Age (median, IQR) | 45 (29–60) | 53 (40–61) | 47 (36–57) | 0.09 |
| Male sex [n (%)] | 40 (70.2) | 169 (57.5) | 27 (71.1) | 0.08 |
| Black [n (%)] | 51 (89.5) | 232 (78.9) | 30 (78.9) | 0.45 |
| CD4 (median, IQR) | 148 (39–419) | 384 (178–677) | 147 (27–306) | <0.01 |
| Viral load < 200 copies/ml [n (%)] | 10 (20.4) | 163 (70.0) | 4 (13.3) | <0.01 |
ART, antiretroviral therapy; IQR, interquartile range.
Figure 1.
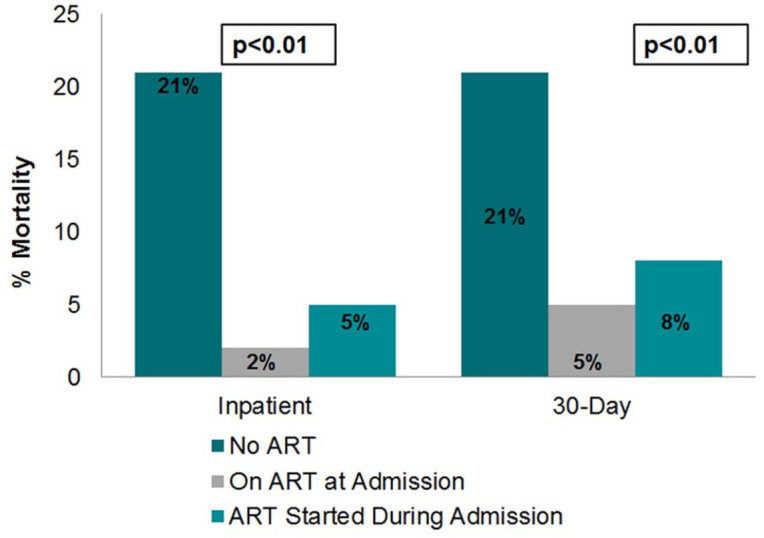
Impact of ART initiation on admitted mortality in the post-intervention period.
ART, antiretroviral therapy.
Figure 2.
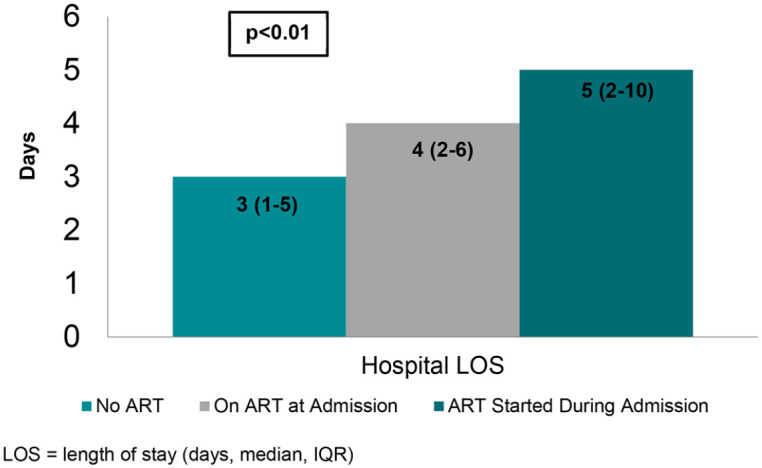
Impact of ART initiation on LOS in post-intervention period.
IQR, interquartile range; LOS, length of stay (days, median, IQR).
Consistent with findings in the investigation of medication-related errors, the most common ART regimens started by ID providers were INSTI-based. In these 38 patients, 14 (37%) were started on bictegravir/emtricitabine/tenofovir alafenamide, 9 (24%) were started on dolutegravir plus emtricitabine/tenofovir disoproxil fumarate, 8 (21%) were started on elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, and 7 (18%) were initiated on alternative regimens. A total of 31 (82%) of these patients were scheduled for follow up within our healthcare system, of whom 20 (64%) were subsequently seen in clinic (others who were scheduled for follow up did not attend their scheduled appointment), and 14 (45%) achieved virologic suppression within 90 days of hospital discharge. The remaining 7 of 38 patients had follow up scheduled at a clinic outside of the system.
We also examined the reasons why the remaining 57 of 85 patients had not started on ART during hospitalization. In 24 (42%) of cases, ART was deferred due to physician-defined reasons such as the perceived inability of the patient to follow up in clinic or limitations due to mental health or substance abuse. Therapy was declined in 15 patients (26%). ART was not initiated in five (9%) of patients because a short LOS resulted in inadequate time for pharmacist intervention, and other reasons for lack of ART initiation were cited in 13 (23%) of patients.
Discussion
We sought to develop a comprehensive program devoted to care of hospitalized PWH. Uniquely, our intervention used ICD-10 code to identify all hospitalized PWH for case review where existing antimicrobial stewardship programs and prior published studies used active orders for ART alone, which may have missed patients who had fallen out of care, had treatment held upon admission, or had a new diagnosis.6–10 In a recent study by Brizzi and colleagues, a more comprehensive program focused on both antiretroviral stewardship and transitions of care also demonstrated a reduction of medication errors, decreased 30-day all-cause readmission rates, and increased linkage to care.11 The transitions of care component involved the pharmacist ensuring that patients had access to medications at time of hospital discharge, which included communication with the outpatient pharmacy, completing prior authorizations, patient assistance program applications, enrollment into the AIDS Drug Assistance Program (ADAP), and contacting high-risk patients via telephone to evaluate understanding of and adherence to medications. A number of these tasks may be challenging in settings with limited resources or in stewardship programs with other significant responsibilities. We employed an interdisciplinary approach with support from ID physicians and pharmacists, but also incorporated essential support from social work and an office administrator. This multifaceted intervention was successful in decreasing the rate of medication-related errors and increasing the rate of ID consultation and linkage to care.
A wide range of identified medication-related errors in PWH have previously been reported in the literature, from 5% to 77%.2 A medication-related error rate of 70% in our pre-intervention group was identified, which is consistent with rates that have been published in previous literature where ID trained pharmacists assessed medication errors.6,7 The implementation of a targeted review of all PWH successfully decreased the frequency of medication-related errors; however, the incidence of ART-related errors persisted in a significant portion of cases (26%) in the post-intervention period. This is in contrast to previous programs, which have been able to decrease medication error frequency to as low as 5% with pharmacist intervention.7,12 Additionally, all ART agents were placed on hospital formulary, in attempt to minimize errors including combination tablets. The high error frequency despite formulary availability and pharmacist intervention seen in this study could be attributed to the heavy workload of the stewardship pharmacist when balancing other antimicrobial stewardship activities in addition to ART stewardship. Time devoted to this process occurred for approximately 2 h twice weekly; therefore, interventions on all errors were not feasible. After reviewing our outcomes with hospital administrators, we obtained funding for a full-time pharmacist dedicated to review of all hospitalized PWH at all facilities in our 10-hospital system. Additional focus will be placed on transitions of care and ensuring that patients have access to ART upon discharge.
We sought to evaluate the clinical impact of inpatient initiation of ART. We utilized the hospital encounter as an opportunity to reengage PWH in care and restart ART in an intervention program modeled after outpatient test and treat or rapid ART initiation programs. In one study of outpatients referred to a rapid ART initiation program with ART started in 1–2 days compared with traditional clinic follow up, significantly higher retention in care and viral suppression were observed in patients referred for rapid ART initiation.13 Early initiation or re-initiation of ART may lead to improved clinical outcomes due to less time off ART and enhanced engagement in care before loss to follow up.14,15 Similarly, we observed better than expected rates of outpatient follow up and virologic suppression at 3 months in inpatients who were initiated on ART with 45% of patients who could be tracked achieving undetectable viremia by 3 months of therapy.
Our results comparing patients with ART withheld, continued, or newly started during admission suggest that inpatient mortality may be improved in patients where ART is reinitiated in the inpatient setting. Mortality in patients initiated on ART while admitted mirrored the rate in PWH admitted already on ART. This finding challenges the common practice of deferring ART initiation until after discharge during clinic follow up. Prior observational studies examining the impact of ART in PWH with acute infections have highlighted the potential for improved outcomes with timely ART treatment due to attenuation of immune dysregulation with acute viral suppression and downregulation of immune activation from HIV.16–20 Ongoing HIV replication drives immune activation, which is increased in PWH not on ART. ART decreases immune activation and helps normalize inflammation.20 The impact of ART on immune regulation and inflammation might explain the improved outcomes observed in all of the hospitalized PWH started on ART compared with those left off therapy.
A longer LOS was observed in the group who started ART during admission. The cause of this is uncertain but it is possible that physicians may have deferred inpatient ART initiation if the patient was soon to be discharged, leading to a bias towards ART initiation in patients with longer LOS. There was no observed difference in 30-day hospital readmission.
This study has several limitations. First, it is possible that the rate of medication-related errors may have decreased during the post-intervention period due to the increased use of single-tablet, INSTI-based regimens rather than the intervention itself. However, DDIs are still common with INSTI-based regimens and a significant decrease in INSTI-related interactions were observed in the post-intervention period. Also, with the availability of multiple single-tablet products with identical components except for the salt formulation of tenofovir (e.g., Stribild® and Genvoya®), the potential for medication-related errors remains. Regarding clinical outcomes and the observed difference in mortality in patients started on ART during inpatient admission, it is possible that this difference was driven by higher severity of illness in the patients not on ART during admission. However, this is unlikely as most patients who were not started on therapy during admission were patients with known compliance issues admitted for psychiatric-related conditions rather than critical illness requiring care in an intensive care unit. Even with our targeted intervention, of the 85 patient admissions in PWH who were not on ART during hospitalization, 57 remained untreated during their inpatient stay largely as a result of physician perceived barriers. This may represent an opportunity to further educate physicians caring for these patients on the benefits of inpatient initiation of ART and develop further programs to engage these PWH in care. We recognized 97% of interventions were accepted, which may be due to the multi-disciplinary collaboration with infectious diseases providers; however, this finding may also warrant further exploration into expanding the scope of practice for inpatient ID pharmacists.
In conclusion, an interdisciplinary intervention targeted at improving comprehensive inpatient management and transitions of care in PWH was successful in reducing medication-related errors, increasing ART prescription rates, and improving linkage to care. In contrast with other published literature, one in four patients still had an error observed in the post-intervention period. This highlights the importance of having ID trained pharmacists that have dedicated time, sufficient to perform ART stewardship on all hospitalized PWH, including those who are not actively on ART by using ICD-10 codes. Initiation of inpatient ART should be strongly considered to improve clinical outcomes. Interdisciplinary collaboration was vital for the program’s success and should be encouraged at other institutions exploring implementation of an inpatient ART review program in response to the call to action from the IDSA, HIVMA, and AAHIVM.
Footnotes
Author contributions: DR and CP conceptualized the project idea and developed the methodology. DR, JM, RJ, KH, BS, and JC conducted the data collection and investigation. MM and NR conducted the formal analysis. All authors contributed to the preparation and editing of the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Danya Roshdy  https://orcid.org/0000-0003-1223-8983
https://orcid.org/0000-0003-1223-8983
Contributor Information
Danya Roshdy, Department of Pharmacy, Carolinas Medical Center, 1000 Blythe Blvd, Charlotte, NC 28203, USA.
Maggie McCarter, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, NC, USA.
Jacqueline Meredith, Department of Pharmacy, Atrium Health, Charlotte, NC, USA.
Rupal Jaffa, Department of Pharmacy, Atrium Health, Charlotte, NC, USA.
Katie Hammer, Department of Pharmacy, Atrium Health, Charlotte, NC, USA.
Barbara Santevecchi, Department of Pharmacotherapy and Translational Research, College of Pharmacy, University of Florida, Gainesville, FL, USA.
Nigel Rozario, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, NC, USA.
Jamie Campbell, Department of Internal Medicine, Atrium Health, Charlotte, NC, USA.
Michael Leonard, Department of Internal Medicine, Atrium Health, Charlotte, NC, USA; Division of Infectious Diseases, Atrium Health, Charlotte, NC, USA.
Christopher Polk, Department of Internal Medicine, Atrium Health, Charlotte, NC, USA; Division of Infectious Diseases, Atrium Health, Charlotte, NC, USA.
References
- 1. Koren DE, Scarsi KK, Farmer EK, et al. A call to action: the role of antiretroviral stewardship in inpatient practice, a joint policy paper of the Infectious Diseases Society of America, HIV Medicine Association, and American Academy of HIV Medicine. Clin Infect Dis 2020; 70: 2241–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li EH, Foisy MM. Antiretroviral and medication errors in hospitalized HIV-positive patients. Ann Pharmacother 2014; 48: 998–1010. [DOI] [PubMed] [Google Scholar]
- 3. Chiampas TD, Kim H, Badowski M. Evaluation of the occurrence and type of antiretroviral and opportunistic infection medication errors within the inpatient setting. Pharm Pract (Granada) 2015; 13: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbier F, Mer M, Szychowiak P, et al. Management of HIV-infected patients in the intensive care unit. Intensive Care Med 2020; 46: 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Washington, DC: HHS, 2020. [Google Scholar]
- 6. Pastakia SD, Corbett AH, Raasch RH, et al. Frequency of HIV-related medication errors and associated risk factors in hospitalized patients. Ann Pharmacother 2008; 42: 491–497. [DOI] [PubMed] [Google Scholar]
- 7. Daniels LM, Raasch RH, Corbett AH. Implementation of targeted interventions to decrease antiretroviral-related errors in hospitalized patients. Am J Heal Pharm 2012; 69: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carcelero E, Tuset M, Martin M, et al. Evaluation of antiretroviral-related errors and interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med 2011; 12: 494–499. [DOI] [PubMed] [Google Scholar]
- 9. Billedo JAS, Berkowitz LB, Cha A. Evaluating the impact of a pharmacist-led antiretroviral stewardship program on reducing drug interactions in HIV-infected patients. J Int Assoc Provid AIDS Care 2016; 15: 84–88. [DOI] [PubMed] [Google Scholar]
- 10. DePuy AM, Samuel R, Mohrien KM, et al. Impact of an antiretroviral stewardship team on the care of patients with human immunodeficiency virus infection admitted to an academic medical center. Open Forum Infect Dis 2019; 6: ofz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brizzi MB, Burgos RM, Chiampas TD, et al. Impact of pharmacist-driven antiretroviral stewardship and transitions of care interventions on persons with human immunodeficiency virus. Open Forum Infect Dis 2020; 7: ofaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corrigan MA, Atkinson KM, Sha BE, et al. Evaluation of pharmacy-implemented medication reconciliation directed at antiretroviral therapy in hospitalized HIV/AIDS patients. Ann Pharmacother 2010; 44: 222–223. [DOI] [PubMed] [Google Scholar]
- 13. Halperin J, Conner K, Butler I, et al. A care continuum of immediate ART for newly diagnosed patients and patients presenting later to care at a federally qualified health center in New Orleans. Open Forum Infect Dis 2019; 6: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koenig SP, Dorvil N, Dévieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med 2017; 14: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016; 13: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwizera A, Nabukenya M, Peter A, et al. Clinical characteristics and short-term outcomes of HIV patients admitted to an African intensive care unit. Crit Care Res Pract 2016; 2016: 2610873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moreira J. The burden of sepsis in critically ill human immunodeficiency virus-infected patients—a brief review. Braz J Infect Dis 2015; 19: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morquin D, Le Moing V, Mura T, et al. Short- and long-term outcomes of HIV-infected patients admitted to the intensive care unit: impact of antiretroviral therapy and immunovirological status. Ann Intensive Care 2012; 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casalino E, Wolff M, Ravaud P, et al. Impact of HAART advent on admission patterns and survival in HIV-infected patients admitted to an intensive care unit. AIDS 2004; 18: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 20. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]


