Abstract
FBXO17 is a newly studied F-box protein associated with high-grade glioma. However, its exact role in glioma remains unclear. In the present study, we aimed to investigate the role of FBXO17 in glioma both in vitro and in vivo and explore the underlying mechanism. Our results showed that FBXO17 mRNA and protein levels were upregulated in glioma cells including U87, U251, SHG44, and U-118-MG cells as compared to the HA1800 cells. Downregulation of FBXO17 significantly suppressed the cellular behaviors of glioma cells including cell proliferation, migration, and invasion. In addition, FBXO17 knockdown induced E-cadherin expression and inhibited N-cadherin and vimentin expression at mRNA and protein levels in glioma cells. In contrast, overexpression of FBXO17 promoted cell proliferation, migration, invasion and EMT process. Furthermore, FBXO17 regulated the Akt/GSK-3β/snail signaling pathway in glioma cells with significant changes in the expression levels of p-Akt, p-GSK-3β and snail. Additionally, inhibition of Akt by LY294002 reversed the effects of FBXO17 overexpression on cellular behaviors of glioma cells. Finally, in vivo mouse xenograft assay proved that downregulation of FBXO17 suppresses the tumorigenesis of glioma. In conclusion, these findings demonstrated that FBXO17 acted as a promotor of glioma development via modulating Akt/GSK-3β/snail signaling pathway.
Keywords: FBXO17, glioma, invasion, EMT, Akt/GSK-3β/snail pathway
Introduction
Glioma is the most prevalent primary tumor of the brain and spinal cord and accounts for over 80% of malignant brain tumors1. Glioblastoma (GBM) is the most common and most malignant type of glioma with highest incidence among those 75-84-years old2. Previous studies have revealed the association between characteristic genetic alterations, epigenetic profiles, and different types of gliomas. The specific molecular characteristics can be used to refine glioma classification, to improve prediction of patient outcomes, and to guide individualized treatment3.
F-box proteins are the substrate-recognition subunits of SKP1-cullin 1-F-box protein (SCF) E3 ligase complexes, which have pivotal roles in the ubiquitylation by the ubiquitin proteasome system (UPS)4,5. Due to the multiple functions of UPS, F-box proteins have been found to be involved in various cellular processes such as cell proliferation, cell cycle progression, transcription and apoptosis through ubiquitylation and subsequent degradation of target proteins6. Notably, increasing evidence has demonstrated that dysregulation of F-box proteins may lead to human malignancies and mediate therapy resistance7,8. Given that a substantial number of F-box proteins have tumor-suppressive or oncogenic roles, targeting F-box proteins have shown promising therapeutic potential in the cancer treatment.
The PI3K/AKT signaling pathway is a well-known pathway in the regulation of tumorigenesis, and is significantly activated in glioma9. The activation of Akt increases the nuclear expression and transcriptional activity of Snail by inhibitory phosphorylation of GSK-3β, thereby triggering cell migration and epithelial-to-mesenchymal (EMT)10. Previous studies have showed that AKT/GSK-3β/Snail signaling pathway may induce EMT to induce tumorigenesis11,12. Thus, identification of key regulators implicated in modulating Akt/GSK-3β/Snail pathway in gliomagenesis is advantageous for exploring novel approaches for its therapeutic targeting.
FBXO17 is a poorly studied F-box protein that has been shown to play a role in tumor development and progression. FBXO17 expression is associated with pathological grade and the overall survival rate of hepatocellular carcinoma (HCC) patients and promotes malignant progression of HCC13. FBXO17 is significantly associated with patients’ survival in esophageal squamous cell carcinoma (ESCC)14. FBXO17 modulates the tumorigenesis of lung cancer through promoting cell proliferation15. Moreover, high-grade glioma (HGG) patients with elevated FBXO17 expression have a significantly shorter overall survival (OS), implying that FBXO17 has a potential as a stratification factor for clinical decision-making in HGG16. However, the biological role of FBXO17 in glioma and the mechanism has not been studied.
The current study was aimed to investigate the role of FBXO17 in the regulation of glioma cell proliferation, migration, invasion and EMT process and the underlying mechanisms.
Materials and Methods
Cell Culture
Normal human astrocyte cell line HA1800 and human glioma cell lines (U87, U251, SHG44 and U-118-MG) were purchased from Cell Bank of Chinese academy of science (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco Laboratories), penicillin (100 U/mL; Gibco Laboratories) and streptomycin (100 µg/mL; Gibco Laboratories) and maintained in a 5% CO2 atmosphere at 37°C.
Cell Transfection
To knock down FBXO17 in glioma cells, cells were infected with lentivirus-mediated vector containing small hair RNAs (shRNA) targeting FBXO17, shRNA1-FBXO17 or shRNA2-FBXO17 (Hanbio Biotechnology Co., Ltd (Shanghai, China). The cells infected with shRNA-NC were served as control cells.
To overexpress FBXO17 in U251 and SHG44 cells, cells were transfected with pcDNA3.1 or pcDNA3.1-FBXO17 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After 48 h post transfection, the qRT-PCR and western blot was performed to detect the mRNA and protein levels of FBXO17.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the cell lines using RNAiso Plus (Takara Biotechnology, Dalian, China) and then reversed transcribed into complementary DNA (cDNA) using a PrimeScript RT Master Mix (Takara). Following cDNA synthesis, mRNA expression levels of FBXO17, E-cadherin, N-cadherin, and vimentin were assessed using the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA, USA). The GAPDH transcript was used as the reference gene. The qPCR protocols were: denaturation at 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 30 seconds and extension at 72°C for 60 seconds; and a final step of 72°C for 10 minutes. Relative gene expressions were calculated using the 2-ΔΔCq method. The primer sequences for the genes were as follows: FBXO17, 5′-TGG GGA AGA TTG GGA AAG GC-3′ (forward) and 5′-TCG CCC ATT GGC TAC ATC TC-3′ (reverse); E-cadherin, 5′-GTG TCA TCC AAC GGG AAT GC-3′ (forward) and 5′-TGG CGG CAT TGT AGG TGT TC-3′ (reverse); N-cadherin, 5′-CTT GCC AGA AAA CTC CAG GG-3′ (forward) and 5′-TGT GCC CTC AAA TGA AAC CG-3′ (reverse); vimentin, 5′-ATG ACC GCT TCG CCA ACT AC-3′ (forward) and 5′-CGG GCT TTG TCG TTG GTT AG-3′ (reverse); GAPDH, 5′-TGA CAA CTT TGG TAT CGT GGA AGG-3′ (forward) and 5′-AGG CAG GGA TGA TGT TCT GGA GAG-3′ (reverse).
Western Blot
Glioma cells were collected for the extraction of total protein using lysis buffer (Tiangen Biotech, Beijing, China), followed by protein concentration determination using the BCA method (Tiangen Biotech). Subsequently, the protein samples were subjected to 100°C heating for 5 min for denaturation and then separated by 10% SDS-PAGE electrophoresis. Then the proteins were transferred to nitrocellulose (PVDF) membranes and blocked using 5% non-fat dry milk for 1 hour. The membranes were then incubated with appropriate dilutions of primary antibodies against FBXO17, E-cadherin, N-cadherin, vimentin, p-Akt, Akt, p-GSK-3β, GSK-3β, snail, and GAPDH (Abcam, Cambridge, MA, USA) overnight at 4°C. The membranes were added with suitable secondary antibodies (Abcam) at room temperature for 1 h. The ECL kit (Thermo Fisher Scientific, Waltham, MA, USA) was utilized for the visualization of bands, and then the Image J software (National Institutes of Health, NIH, Bethesda, MD, USA) was used for the determination of gray value.
Cell Proliferation Assay
Transfected U251 and SHG44 cells were cultured for 48 h and then subjected to the CCK-8 assay. In general, 10 µL of CCK-8 reagent (Beyotime) were added to each well and cells were continually cultured for 2 h. The absorbance at 450 nm was measured using a microplate reader (Bio-Tek Instruments, Winooski, VT, UST).
Migration and Invasion Assays
After transfection for 48 h, the media of U251 and SHG44 cells were replaced with FBS-free media and added into the upper chambers coated with or without Matrigel. Complete medium (500 µl) was added into the lower chambers. After incubation for 24 h, cells were fixed in 4% paraformaldehyde for 30 min and stained in Giemsa buffer for 30 min. Cells in five fields were counted using a microscope.
Tumor Growth in a Mouse Xenograft Model
For in vivo xenograft assay, female BALB/c nude mice (6 weeks old) were purchased from the Medical Laboratory Animal Center of Xi’an Jiaotong University (Xi’an, China). The mice were housed in a specific pathogen-free animal facility with free access to food and water, and randomly divided into two groups (n = 6 per group). Each mouse was subcutaneously injected with approximately 1 × 107 lentivirus-infected U251 cells. After injection, tumor size and volume were monitored every week. The mice were euthanized 4 weeks after implantation, and then tumors were collected for examination. Tumor volume was calculated as follows: Volume = length × (width)2/2. The animal experiments were approved by the Animal Experimental Ethical Committee of the First Affiliated Hospital of Xi’an Jiaotong University.
Statistical Analysis
All of the experiments were repeated in triplicate and the data were analyzed by SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Difference was determined with Student’s t-test or one-way analysis variance (ANOVA) followed by Tukey’s post hoc test, where appropriate. P < 0.05 was considered to be statistically significant.
Results
FBXO17 is Present at Higher Expression Levels in Human Glioma Tissues and Cell Lines
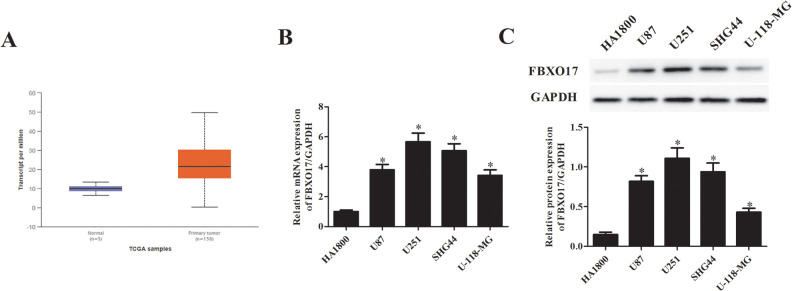
To determine the involvement of FBXO17 in glioma, we compared the expression levels of FBXO17 in glioma and normal tissues using the ENCORI Pan-Cancer Analysis Platform. We observed that FBXO17 expression was significantly higher in glioma tissues than that in normal tissues (Fig. 1A). In addition, we used qRT-PCR to detect the mRNA level of FBXO17 in normal human astrocyte cell line HA1800 and human glioma cell lines (U87, U251, SHG44, and U-118-MG). As shown in Fig. 1B, the mRNA levels of FBXO17 were markedly increased in human glioma cell lines including U87, U251, SHG44, and U-118-MG cells. Similarly, western blot analysis demonstrated that the protein levels of FBXO17 were also upregulated in the four glioma cell lines (Fig. 1C). The U251 and SHG44 cells exhibited higher expression levels of FBXO17 than other glioma cell lines. Thus, U251 and SHG44 cells were selected for the following experiments.
Figure 1.
FBXO17 is present at higher expression levels in human glioma tissues and cell lines. (A) The expression of FBXO17 in glioma was analyzed with ENCORI Pan-Cancer Analysis Platform. (B, C) The mRNA and protein levels of FBXO17 in normal human astrocyte cell line HA1800 and human glioma cell lines (U87, U251, SHG44 and U-118-MG) were detected using qRT-PCR and western blot, respectively. *P < 0.05 vs HA1800 cells.
Downregulation of FBXO17 Suppresses Glioma Cell Proliferation
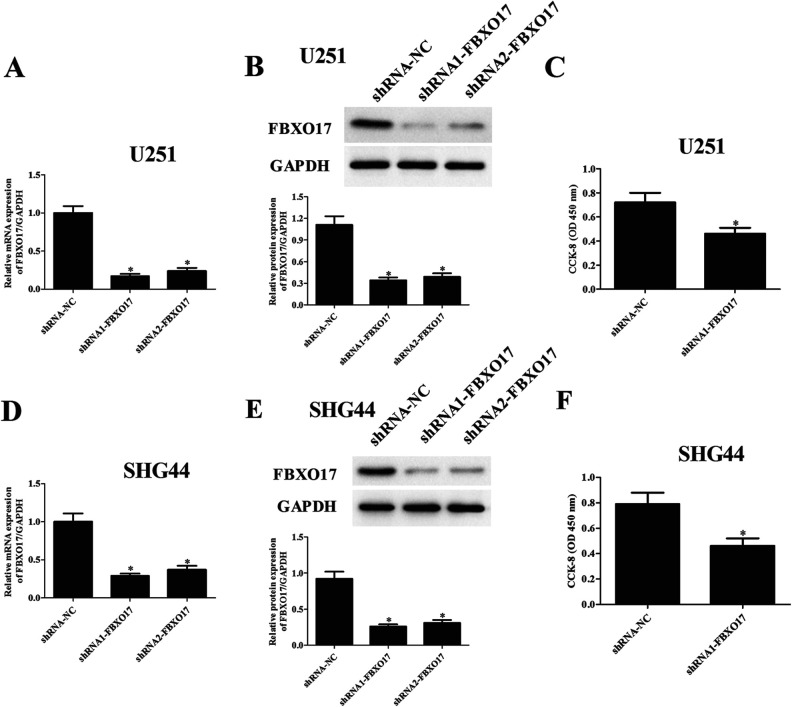
Considering FBXO17 might be involved in the development of glioma, we further constructed FBXO17-silencing U251 and SHG44 cells through infection with shRNA1-FBXO17 or shRNA2-FBXO17. Transfection efficiency was assessed by fluorescence imaging after transfection in U251 and SHG44 cells (Supplemental Fig. 1). The data of fluorescence-activated cell sorting (FACS) assay demonstrated that the positive cell percentage of glioma cells transfected with shRNA1/2-FBXO17 and shRNA-NC lentivirus was >80% in U251 and SHG44 cells (Supplemental Fig. 2). In addition, compared with the shRNA-NC group, the infection efficiency of shRNA1-FBXO17 was higher than that of shRNA2-FBXO17 in U251 (Fig. 2A, B) and SHG44 cells (Fig. 2D, E). For the following experiments, shRNA1-FBXO17 infected U251 and SHG44 cells were used to evaluate the role of FBXO17 in glioma. We next used the CCK-8 assay to assess the proliferation of U251 and SHG44 cells. The results demonstrated that cell proliferation of U251 and SHG44 cells were significantly decreased after infection with shRNA1-FBXO17 (Fig. 2C, F).
Figure 2.
Downregulation of FBXO17 suppresses glioma cell proliferation. We further constructed FBXO17-silencing U251 and SHG44 cells through infection with shRNA1-FBXO17 or shRNA2-FBXO17. U251 and SHG44 cells infected with shRNA-NC served as controls. (A, D) Comparison of mRNA levels of FBXO17 in U251 and SHG44 cells with different infection. (B, E) Comparison of protein levels of FBXO17 in U251 and SHG44 cells with different infections. (C, F) CCK-8 assay was performed to evaluate the proliferation of U251 and SHG44 cells with different infection. *P < 0.05 vs shRNA-NC group.
Downregulation of FBXO17 Suppresses Glioma Cell Migration and Invasion
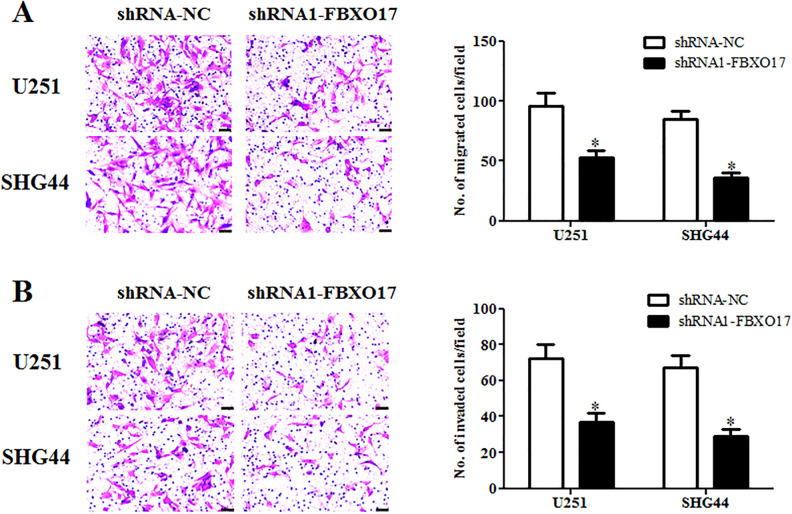
To further explore the role of FBXO17 in regulating cell migration and invasion, the transwell assays were performed. As indicated in Fig. 3A, cell migration of U87 and U-118-MG cells were significantly decreased in shRNA1-FBXO17 infection group as compared to shRNA-NC infection group. As expected, shRNA1-FBXO17 infection also caused a significant decrease in cell invasion in both U251 and SHG44 cells (Fig. 3B).
Figure 3.
Downregulation of FBXO17 suppresses glioma cell migration and invasion. The transwell assays were performed to examine the cell migration and invasion of U251 and SHG44 cells after different infections. (A) Comparison of cell migration of U251 and SHG44 cells with different infection. (B) Comparison of cell invasion of U251 and SHG44 cells with different infection. *P < 0.05 vs shRNA-NC group.
Downregulation of FBXO17 Suppresses the EMT Process in Glioma Cells
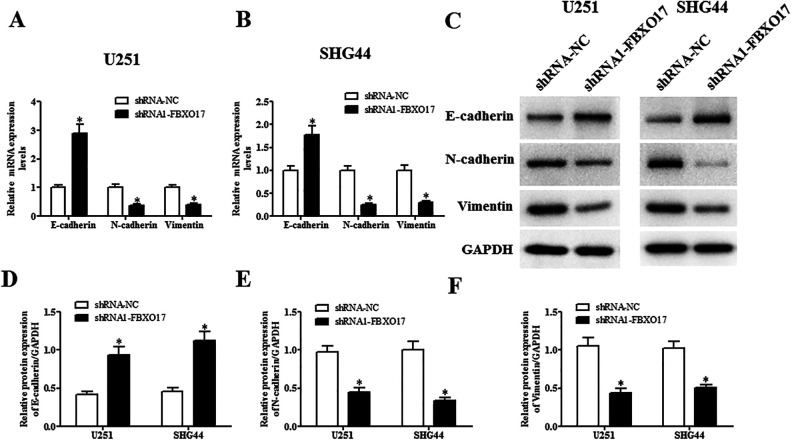
Next, we evaluated the changes in the expression levels of EMT markers including E-cadherin, N-cadherin and vimentin. Results in qRT-PCR showed that the mRNA level of E-cadherin was increased, while the mRNA levels of N-cadherin and vimentin were decreased in shRNA1-FBXO17-infected U251 and SHG44 cells (Fig. 4A, B). In consistent with qRT-PCR, western blot analysis proved that shRNA1-FBXO17 also greatly induced E-cadherin expression and inhibited N-cadherin and vimentin expression at protein levels in U251 and SHG44 cells, respectively (Fig. 4C–F).
Figure 4.
Downregulation of FBXO17 suppresses the EMT process in glioma cells. U251 and SHG44 cells were infected with shRNA-NC or shRNA1-FBXO17 for 48 h. The EMT process was assessed by detecting the expression levels of EMT markers including E-cadherin, N-cadherin, and vimentin using qRT-PCR and western blot, respectively. (A, B) Comparison of the mRNA levels of E-cadherin, N-cadherin, and vimentin in U251 and SHG44 cells. (C–F) Comparison of the protein levels of E-cadherin, N-cadherin and vimentin in U251 and SHG44 cells. *P < 0.05 vs shRNA-NC group.
Upregulation of FBXO17 Promotes Cell Proliferation, Migration, Invasion and EMT
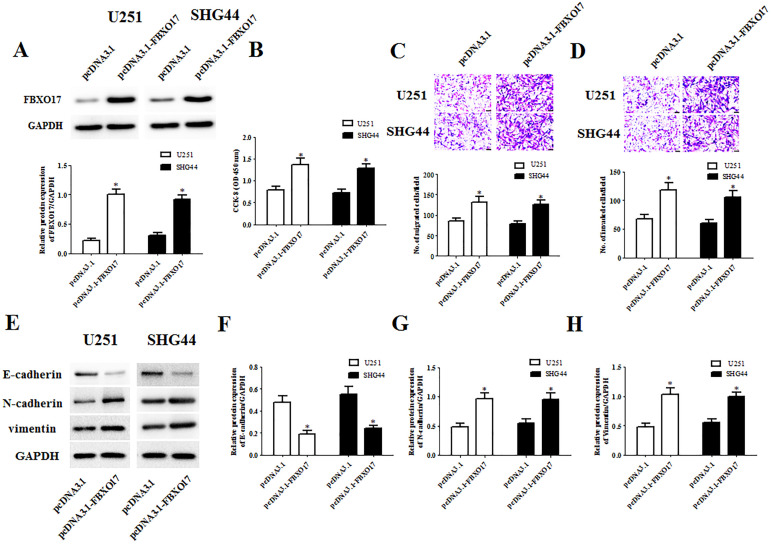
Subsequently, we also evaluated the effects of FBXO17 overexpression on the cellular behaviors of glioma cells. As indicated in Fig. 5A, the protein expression levels of FBXO17 in U251 and SHG44 cells were significantly increased by transfection with pcDNA3.1-FBXO17. The data of CCK-8 assay showed that compared to the pcDNA3.1 group, the cell proliferation in pcDNA3.1-FBXO17 group was markedly increased (Fig. 5B). Transwell assays proved that cell migration and invasion of U251 and SHG44 cells were significantly induced by pcDNA3.1-FBXO17 transfection (Fig. 5C, D). Similarly, we found that FBXO17 overexpression also greatly promoted the proliferation, migration and invasion of U-118-MG cells (Supplemental Fig. 3A–D). Moreover, a significant decrease in E-cadherin protein expression and obvious increases in N-cadherin and vimentin protein expressions were observed in pcDNA3.1-FBXO17 transfected U87 and U-118-MG cells (Fig. 5E–H).
Figure 5.
Upregulation of FBXO17 promotes the proliferation, migration, invasion, and EMT in glioma cells. We next constructed FBXO17-overexpressing U251 and SHG44 cells through infection with pcDNA3.1-FBXO17. U251 and SHG44 cells infected with pcDNA3.1 served as controls. (A) Comparison of protein levels of FBXO17 in U251 and SHG44 cells with different transfections. (B) Comparison of cell proliferation of U251 and SHG44 cells with different transfections. (C, D) Comparison of cell migration and invasion of U251 and SHG44 cells with different transfections. (E–H) Comparison of the protein levels of E-cadherin, N-cadherin, and vimentin. *P < 0.05 vs pcDNA3.1 group.
Regulation of Akt/GSK-3β/Snail Signaling Pathway by FBXO17 in Glioma Cells
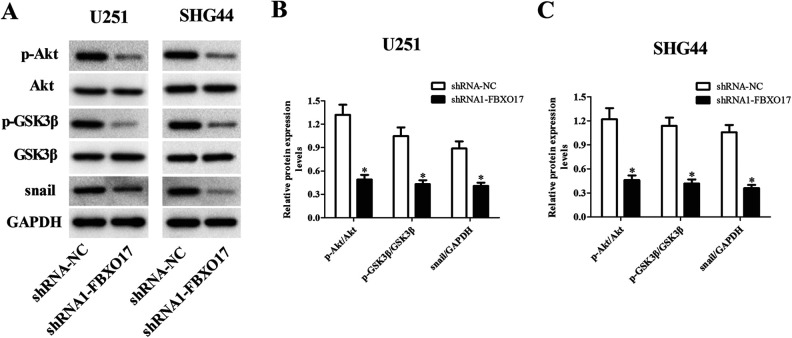
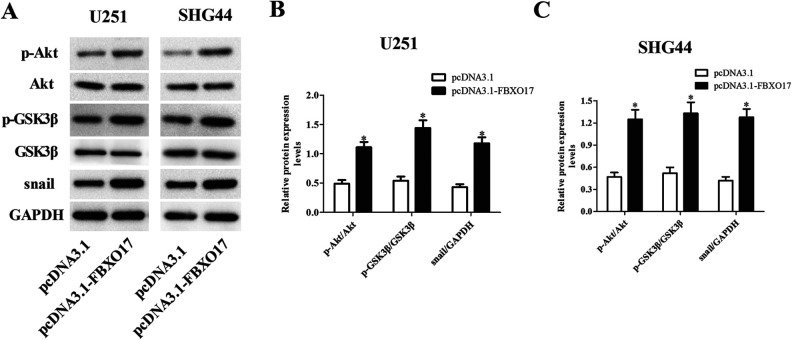
FBXO17 has been reported as an important regulator of Akt pathway15. GSK-3β and snail are the downstream molecules of Akt pathway. Therefore, we hypothesized that there might be a relationship between FBXO17 and this pathway. We investigated the action of FBXO17 on Akt/GSK-3β/snail signaling pathway in glioma cells. As shown in Fig. 6A–C, downregulation of FBXO17 significantly suppressed the Akt/GSK-3β/snail signaling pathway with deceased expression levels of p-Akt, p-GSK-3β and snail in both U251 and SHG44 cells. Besides, the expression levels of p-Akt, p-GSK-3β and snail were markedly increased in pcDNA3.1-FBXO17 transfected U251 and SHG44 cells (Fig. 7A–C).
Figure 6.
Knockdown of FBXO17 inhibits the activation of Akt/GSK-3β/snail pathway in glioma cells. U251 and SHG44 cells were infected with shRNA-NC or shRNA1-FBXO17 for 48 h. (A) The expression levels of p-Akt, Akt, p-GSK-3β, GSK-3β and snail were detected using western blot in U251 and SHG44 cells. (B) Quantification analysis of p-Akt/Akt, p-GSK-3β/GSK-3β and snail/GAPDH in U251 cells. (C) Quantification analysis of p-Akt/Akt, p-GSK-3β/GSK-3β, and snail/GAPDH in SHG44 cells. *P < 0.05 vs shRNA-NC group.
Figure 7.
Overexpression of FBXO17 promotes the activation of Akt/GSK-3β/snail pathway in glioma cells. U251 and SHG44 cells were infected with pcDNA3.1 or pcDNA3.1-FBXO17 for 48 h. (A) The expression levels of p-Akt, Akt, p-GSK-3β, GSK-3β and snail were detected using western blot in U251 and SHG44 cells. (B) Quantification analysis of p-Akt/Akt, p-GSK-3β/GSK-3β, and snail/GAPDH in U251 cells. (C) Quantification analysis of p-Akt/Akt, p-GSK-3β/GSK-3β, and snail/GAPDH in SHG44 cells. *P < 0.05 vs pcDNA3.1 group.
LY294002 Reversed the Effects of FBXO17 Overexpression on Cellular Behaviors of Glioma Cells
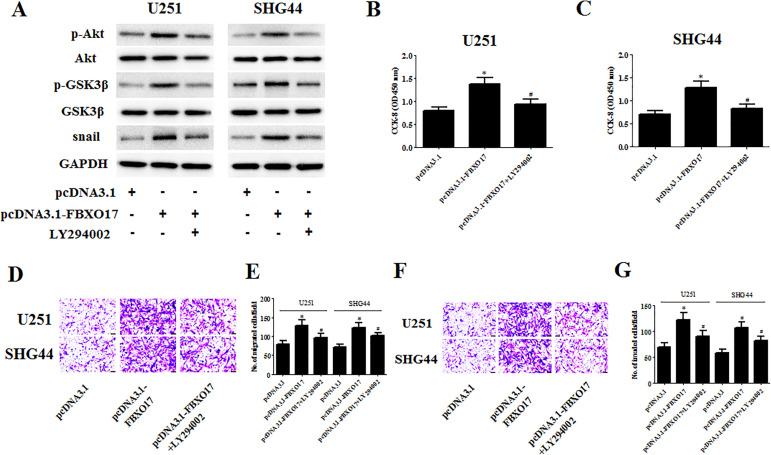
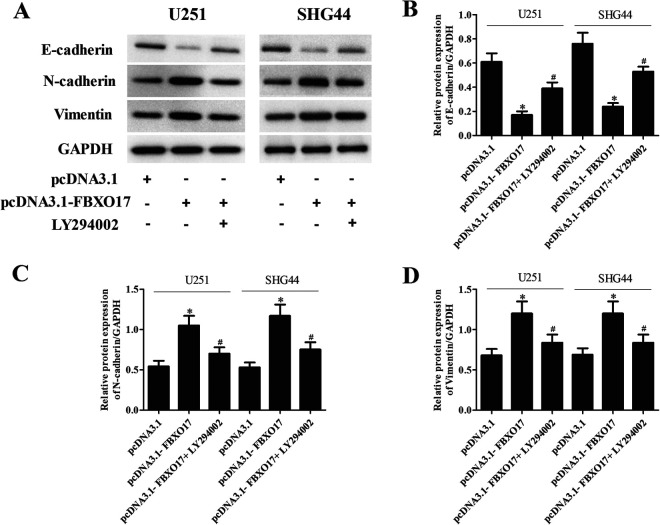
To verify that Akt/GSK-3β/snail signaling contributes to the modulation of the FBXO17-mediated effect in glioma cells, we examined the effect of LY294002 on the FBXO17-induced effect. Treatment with LY294002 greatly decreased the expression levels of p-Akt, p-GSK-3β, and snail in pcDNA3.1-FBXO17 transfected U251 and SHG44 cells (Fig. 8A). In addition, the increased cell proliferation, migration, and invasion caused by FBXO17 overexpression were reversed by LY294002 treatment in U251 and SHG44 cells, respectively (Fig. 8B–G). Moreover, the decreased E-cadherin expression and increased N-cadherin and vimentin expression in pcDNA3.1-FBXO17 transfected U251 cells were also mitigated by LY294002 treatment (Fig. 9A–D).
Figure 8.
LY294002 reversed the effects of FBXO17 overexpression on glioma cell proliferation, migration, and invasion. U251 and SHG44 cells were transfected with pcDNA3.1-FBXO17 in the presence of LY294002 (5 µM). (A) The expression levels of p-Akt, Akt, p-GSK-3β, GSK-3β, and snail were detected using western blot in U251 and SHG44 cells. (B, C) CCK-8 assay was performed to evaluate the proliferation of U251 and SHG44 cells. (D–G) Transwell assays were performed to examine the migration and invasion of U251 and SHG44 cells. *P < 0.05 vs pcDNA3.1 group, #P < 0.05 vs pcDNA3.1-FBXO17 group.
Figure 9.
LY294002 reversed the effects of FBXO17 overexpression on EMT process in glioma cells. U251 and SHG44 cells were transfected with pcDNA3.1-FBXO17 in the presence of LY294002 (5 µM). (A) The EMT process was assessed by detecting the expression levels of E-cadherin, N-cadherin and vimentin using western blot in U251 and SHG44 cells. (B–D) Quantification analysis of E-cadherin, N-cadherin and vimentin. *P < 0.05 vs pcDNA3.1 group, #P < 0.05 vs pcDNA3.1-FBXO17 group.
Downregulation of FBXO17 Suppresses Tumorigenesis of Glioma
To test the tumor-suppressing efficiency of FBXO17 knockdown in vivo, we established a xenograft model in nude mice. As indicated in Fig. 10A, tumor growth in FBXO17 knockdown group was significantly lower than that in control group. The tumor volume was greatly decreased in nude mice injected with shRNA1-FBXO17 infected U87 cells (Fig. 10B). Moreover, tumor weight was also significantly reduced in shRNA1-FBXO17 group compared with those in shRNA-NC group (Fig. 10C). In the tissues from shRNA1-FBXO17-tumors, the levels of p-Akt, p-GSK-3β and snail were significantly decreased (Fig. 10D).
Figure 10.
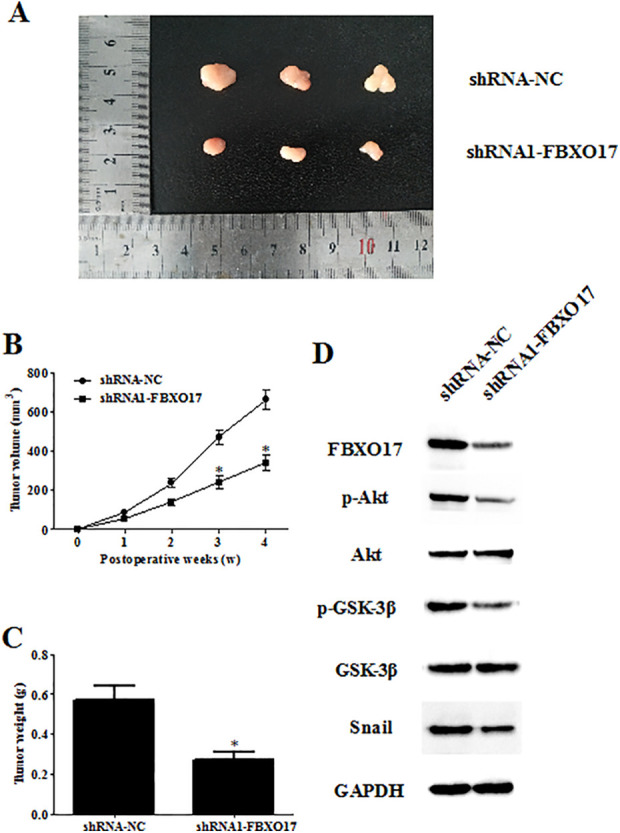
Downregulation of FBXO17 suppresses tumorigenesis of glioma. A xenograft model in nude mice was established to test the tumor-suppressing efficiencyof FBXO17 knockdown in vivo. Tumor growth was monitored every week. (A) Tumor growth was suppressed in FBXO17 knockdown group. (B, C) The mice were euthanized 4 weeks after implantation, and then tumors were collected for examination of tumor volume and weight. (D) Western blot detection of the expression levels of p-Akt, Akt, p-GSK-3β, GSK-3β, and snail in tumor tissues. *P < 0.05 vs shRNA-NC group.
Discussion
In the current study, we investigated the role of FBXO17 in glioma in vitro and explored the involvement of potential signaling pathway. We found that FBXO17 exerted an oncogenic role in glioma through regulation of glioma cell proliferation, migration, invasion, and EMT process. In vivo mouse xenograft assay proved that downregulation of FBXO17 suppresses tumorigenesis of glioma. Furthermore, the Akt/GSK-3β/snail signaling pathway was involved in the effects of FBXO17.
Researchers have found that FBXO17 is associated with the development of several types of cancers via regulating cellular processes such as cell survival, proliferation, apoptosis, migration, and invasion. FBXO17 is highly expressed in some lung cancer cell lines and regulates the cell proliferation and survival15. Inhibition of FBXO17 suppresses cell proliferation and metastasis ability and induces apoptosis of HCC cell line, while overexpression of FBXO17 markedly increases cell proliferation and metastasis ability and decreases cell apoptosis13. In the present study, we found that FBXO17 exhibited higher expression levels in human glioma cell lines. Knockdown of FBXO17 suppressed the proliferation, migration, invasion and EMT process in glioma cells. In contrast, upregulation of FBXO17 promoted cell proliferation, migration, invasion and EMT. These findings indicated that FBXO17 exerted an oncogenic role in glioma.
Deregulation of many signaling pathways has been found to be involved in the pathogenesis of glioma17–19. One of these pathways is PI3K/Akt pathway, which is intensively studied and widely described so far20. This pathway is often overactivated in glioma and regulates many biological processes such as cell metabolism, growth, and survival17,21. It is perhaps not surprising that inhibitors of PI3K/Akt may improve the outcomes in glioma. Previous study has demonstrated that FBXO17 causes Akt activation in lung cancer cells, while FBXO17 knockdown reduces Akt Ser 473 phosphorylation, and thereby modulates tumorigenesis15.
In recent years, more and more attention has been paid to the role of GSK-3β, a target of Akt, in the regulation of cancer development22–24. It is well established that activated Akt translocates to the various subcellular compartments and causes phosphorylation of several targets, including GSK-3β25. A growing body of evidence indicates that Akt/GSK-3β signaling is implicated in glioma26–28. Moreover, FBXO17 was found to recognize and mediate the polyubiquitination of GSK-3β in murine lung epithelial cells29. Both endogenous and ectopically expressed FBXO17 associate with GSK-3β, and its overexpression leads to decreased protein levels of GSK-3β29. Besides, silencing FBXO17 functions as oncogene in HCC through upregulating GSK-3β level and downregulating the expression of proteins in Wnt/β-catenin pathway such as c-Myc, MMP-9, and MMP-213.
Snail is a zinc-finger transcription factor that induces EMT, accompanied by increased cell migration and metastasis in cancer cells30,31. Additionally, GSK-3β may cause nuclear exportation and cytoplasmic translocation of Snail and lead to phosphorylation of Snail, while inhibition of GSK-3β results in rescues of snail32–34. Previous studies have revealed that Akt/GSK-3β/snail pathway is widely accepted in modulating EMT35,36, therefore we hypothesized that there might be a relationship between FBXO17 and this pathway. Our results found that FBXO17 silencing resulted in downregulation of p-Akt, p-GSK-3β and snail in glioma cells; on the contrary, FBXO17 overexpression upregulated the levels of p-Akt, p-GSK-3β, and snail. These findings suggested that FBXO17 regulated the Akt/GSK-3β/snail pathway in glioma cells. Moreover, inhibition of Akt reversed the effects of FBXO17 overexpression on cellular behaviors of glioma cells. Taken together, the oncogenic role of FBXO17 in glioma might be mediated by Akt/GSK-3β/snail signaling pathway.
In summary, the present study identified that FBXO17 was upregulated in glioma cells and regulated the cellular behaviors of glioma cells including cell proliferation, migration, invasion and EMT process. Moreover, FBXO17 acted as a regulator of glioma carcinogenesis via modulating the Akt/GSK-3β/snail signaling pathway.
Supplemental Material
Supplemental Material, sj-pdf-1-cll-10.1177_09636897211007395 for Overexpression of FBXO17 Promotes the Proliferation, Migration and Invasion of Glioma Cells Through the Akt/GSK-3β/Snail Pathway by Ning Wang, Qian Song, Hai Yu and Gang Bao in Cell Transplantation
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical Approval is not applicable for this article.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Animal Experimental Ethical Committee of the First Affiliated Hospital of Xi’an Jiaotong University (APPROVAL NUMBER: 2020015) approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Funding: This study was supported by the Natural Science Foundation of Shaanxi, China (Program No. 2021JM-264).
ORCID iD: Gang Bao  https://orcid.org/0000-0003-0576-4408
https://orcid.org/0000-0003-0576-4408
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503; quiz 491 p following 516. [DOI] [PubMed] [Google Scholar]
- 2. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat Rev Clin Oncol. 2017;14(7):434–452. [DOI] [PubMed] [Google Scholar]
- 4. Ho MS, Tsai PI, Chien CT. F-box proteins: the key to protein degradation. J Biomed Sci. 2006;13(2):181–191. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Mallampalli RK. Small molecule therapeutics targeting F-box proteins in cancer. Semin Cancer Biol. 2016;36:105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nature Reviews Cancer. 2014;14(4):233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adamovich AI, Toland AE, Parvin JD. F-box protein-mediated resistance to PARP inhibitor therapy. Mol Cell. 2019;73(2):195–196. [DOI] [PubMed] [Google Scholar]
- 8. Diaz VM, de Herreros AG. F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol. 2016;36:71–79. [DOI] [PubMed] [Google Scholar]
- 9. Dasari VR, Kaur K, Velpula KK, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PloS one. 2010;5(4):e10350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6(10):931–940. [DOI] [PubMed] [Google Scholar]
- 11. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, Fan J, Dai Z, Zhou J. CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3beta/Snail signaling. Cancer Lett. 2015;358(2):124–135. [DOI] [PubMed] [Google Scholar]
- 13. Liu FH, Cui YP, He YK, Shu RH. FBXO17 promotes malignant progression of hepatocellular carcinoma by activating wnt/beta-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23(19):8265–8273. [DOI] [PubMed] [Google Scholar]
- 14. Lu T, Chen D, Wang Y, Sun X, Li S, Miao S, Wo Y, Dong Y, Leng X, Du W, Jiao W. Identification of DNA methylation-driven genes in esophageal squamous cell carcinoma: a study based on the cancer genome atlas. Cancer Cell Int. 2019;19:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suber TL, Nikolli I, O’Brien ME, Londino J, Zhao J, Chen K, Mallampalli RK, Zhao Y. FBXO17 promotes cell proliferation through activation of Akt in lung adenocarcinoma cells. Respir Res. 2018;19(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du D, Yuan J, Ma W, Ning J, Weinstein JN, Yuan X, Fuller GN, Liu Y. Clinical significance of FBXO17 gene expression in high-grade glioma. BMC Cancer. 2018;18(1):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta S, Lo Cascio C. Developmentally regulated signaling pathways in glioma invasion. Cell Mol Life Sci. 2018;75(3):385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meel MH, Schaper SA, Kaspers GJL, Hulleman E. Signaling pathways and mesenchymal transition in pediatric high-grade glioma. Cell Mol Life Sci. 2018;75(5):871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan QW, Weiss WA. Targeting the RTK-PI3K-mTOR axis in malignant glioma: overcoming resistance. Curr Top Microbiol Immunol. 2010;347:279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–33450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang K, Chen Z, Gao J, Shi W, Li L, Jiang S, Hu H, Liu Z, Xu D, Wu L. The key roles of gsk-3beta in regulating mitochondrial activity. Cell Physiol Biochem. 2017;44(4):1445–1459. [DOI] [PubMed] [Google Scholar]
- 23. Kisoh K, Hayashi H, Itoh T, Asada M, Arai M, Yuan B, Tanonaka K, Takagi N. Involvement of GSK-3beta phosphorylation through pi3-k/akt in cerebral ischemia-induced neurogenesis in rats. Mol Neurobiol. 2017;54(10):7917–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E, Giampietri C. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin J, Song T, Li C, Mao W. GSK-3beta in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim Biophys Acta Mol Cell Res. 2020;1867(5):118659. [DOI] [PubMed] [Google Scholar]
- 26. Zhu B, Zhang S, Meng N, Zhang H, Yuan S, Zhang J. Long non-coding RNA RNCR3 promotes glioma progression involving the Akt/GSK-3beta pathway. Oncol Lett. 2019;18(6):6315–6322. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Li B, Li X. Overexpression of hsa_circ_0007534 predicts unfavorable prognosis for osteosarcoma and regulates cell growth and apoptosis by affecting AKT/GSK-3beta signaling pathway. Biomed Pharmacother. 2018;107:860–866. [DOI] [PubMed] [Google Scholar]
- 28. Shin SY, Choi BH, Ko J, Kim SH, Kim YS, Lee YH. Clozapine, a neuroleptic agent, inhibits Akt by counteracting Ca2+/calmodulin in PTEN-negative U-87MG human glioblastoma cells. Cell Signal. 2006;18(11):1876–1886. [DOI] [PubMed] [Google Scholar]
- 29. Suber T, Wei J, Jacko AM, Nikolli I, Zhao Y, Zhao J, Mallampalli RK. SCF(FBXO17) E3 ligase modulates inflammation by regulating proteasomal degradation of glycogen synthase kinase-3beta in lung epithelia. J Biol Chem. 2017;292(18):7452–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith BN, Odero-Marah VA. The role of Snail in prostate cancer. Cell Adh Migr. 2012;6(5):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Li J, Zhang G, Da Q, Chen L, Yu S, Zhou Q, Weng Z, Xin Z, Shi L, Ma L, et al. HMGB1-Induced p62 overexpression promotes snail-mediated epithelial-mesenchymal transition in glioblastoma cells via the degradation of GSK-3beta. Theranostics. 2019;9(7):1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Zhou H, Zhu R, Ding F, Li Y, Cao X, Liu Z. SPSB3 targets SNAIL for degradation in GSK-3beta phosphorylation-dependent manner and regulates metastasis. Oncogene. 2018;37(7):768–776. [DOI] [PubMed] [Google Scholar]
- 34. Liu ZC, Chen XH, Song HX, Wang HS, Zhang G, Wang H, Chen DY, Fang R, Liu H, Cai SH, Du J. Snail regulated by PKC/GSK-3beta pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358(2):491–502. [DOI] [PubMed] [Google Scholar]
- 35. Yuan L, Zhou M, Huang D, Wasan HS, Zhang K, Sun L, Huang H, Ma S, Shen M, Ruan S. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial-mesenchymal transition via the AKT/GSK-3beta/Snail signaling pathway. Mol Med Rep. 2019;20(3):2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang L, Zhou Y, Cao XP, Lin JX, Zhang L, Huang ST, Zheng M. KPNA2 promotes migration and invasion in epithelial ovarian cancer cells by inducing epithelial-mesenchymal transition via Akt/GSK-3beta/Snail activation. J Cancer. 2018;9(1):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cll-10.1177_09636897211007395 for Overexpression of FBXO17 Promotes the Proliferation, Migration and Invasion of Glioma Cells Through the Akt/GSK-3β/Snail Pathway by Ning Wang, Qian Song, Hai Yu and Gang Bao in Cell Transplantation