Abstract
Antiphospholipid syndrome (APS) with cerebral venous sinus thrombosis (CVST) is a relatively rare phenomenon, and this observational study aimed to investigate the clinical characteristics of APS patients complicated with CVST. We retrospectively investigated the clinical characteristics of CVST events in APS and compared differential characteristics and associated factors between APS patients with and without CVST. Twenty-one CVST patients with APS were enrolled including 14 females (9.4%) and 7 males (5.8%). The median age and disease duration at onset of CVST was 33 years (IQR 28-48) old and 1.3 months (IQR 0.7-4), respectively. Among APS patients with CVST, 12 (57.1%) cases presented with neurologic symptoms of CVST as the initial manifestation. Onset of CVST was mainly chronic (52.4%). Headache (90.5%) was the most common neurological symptom. The common locations of CVST were transverse sinus (76.2%) and superior sagittal sinus (57.1%), with more frequently (76.2%) dual or multiple sinuses involved. All patients with CVST were treated with anticoagulant, and 5 (23.8%) patients received endovascular therapy. Sixteen (84.2%) patients had good outcomes and 3 (15.8%) patients died at last follow-up. There were no significant differences (P > 0.05) between two groups in the analysis of related APS indicators. There were no significant differences (P > 0.05) between two groups in the analysis of related APS indicators. Although APS complicated with CVST is rare and predominately chronic developed. The evaluation of CVST should be performed for APS patients with intracranial hypertension syndrome. The routine screening of antiphospholipid antibodies (aPLs) is highly recommended in unexplained CVST patients. Most CVST patients with APS will have a good prognosis after treatment, and endovascular therapy is an alternative treatment.
Keywords: antiphospholipid syndrome, cerebral venous sinus thrombosis, magnetic resonance venography, anticoagulation, endovascular therapy
Introduction
Antiphospholipid syndrome (APS), also known as “Hughes Syndrome,” is a systemic non-inflammatory autoimmune disease. APS is characterized by recurrent arterial and/or venous thrombosis, recurrent miscarriages, recurrent fetal losses, and thrombocytopenia,1,2 accompanied by the presence of antiphospholipid antibodies (aPLs). This syndrome can occur alone (primary APS) or may be associated with other existing connective diseases, such as systemic lupus erythematosus (SLE), Sjogren’s syndrome, certain infections, etc.3,4 Estimates have indicated that the incidence of APS is around 5 new cases per 100,000 individuals per year and the prevalence around 40-50 cases per 100,000 persons.5
Thrombotic events are one of the prominent clinical features of APS, which may occur in virtually any blood vessel. Venous thromboembolism (VTE), particularly deep vein thrombosis (usually in the legs), is the most frequent manifestation of APS, with a prevalence of 31.7%-38.9% of VTE.6,7 However, cerebral venous sinus thrombosis (CVST), as a venous thrombus, is a relatively rare phenomenon of APS. The etiology of CVST is complicated. Contraceptive use, pregnancy, puerperium, infectious diseases, prothrombotic states, malignancy, and autoimmune inflammatory diseases were risk factors of CVST. Hypercoagulable state is the main pathologic mechanism of venous thrombosis. Clinically, because of protean symptoms, there is difficult to make a confirmed diagnose early and is potentially fatal to the APS patients due to delayed diagnose. More insights in CVST and APS will be essential to improve diagnosis and prognosis of disease.
To date, the pathogenic mechanisms of CVST behind APS are complex and not fully elucidated, which could be related to the presence of aPLs by interfering different pathways, immune-mediated damage, and complement activation. Recurrent thrombosis is an important characteristic in patients with APS despite adequate anticoagulation. Although the current treatment guidelines for APS emphasize the importance of early diagnosis and recommend aggressive therapy to prevent recurrence of thrombosis.8,9 However, the optimal duration and intensity of anticoagulation therapy of CVST in patients with APS remains controversial.10,11 Besides, the diagnosis and treatment of recurrent thrombosis in APS patients requires further clarification.
Currently, due to the low incidence, few studies have conducted a detailed study of CVST in APS patients, and the available data concerning clinical characteristics, imaging manifestations, treatment and prognosis of CVST in APS patients are limited. Therefore, the purpose of this observational study is to explore clinical features, treatment, outcome and risk factors for CVST in APS patients via a retrospective data. This study may help practitioners to increase the awareness so the correct diagnosis and effective therapy can be performed to improve patient prognosis.
Materials and Methods
Patients
Patients with APS identified using ICD-10 coding system from the medical records from November 2009 to October 2019 at Xuanwu Hospital were enrolled. APS was diagnosed on the basis of the criteria proposed in 2006 in Sydney.12 In the APS cohort, 21 patients with CVST were diagnosed by an experienced neurologist, and confirmed according to typical clinical features, imaging characteristics (magnetic resonance imaging (MRI) combined with magnetic resonance venography (MRV), computed tomography venography (CTV), or cerebral digital subtraction angiography (DSA)) following established diagnostic criteria.13 Two of 21 APS patients with CVST were secondary to SLE, who were diagnosed based on the 2012 Systemic Lupus International Collaborating Clinics classification criteria.14 One of 21 APS patients with CVST was secondary to Behçet’s Disease, who was diagnosed based on the new International Criteria for Behçet’s Disease.15 Simultaneously, for each CVST case, age-, gender-matched APS patients without CVST (at 1:3 ratio) were randomly selected as control.
This study was approved by the Research Ethics Committee of Xuanwu hospital, Capital Medical University. Due to the retrospective design of this study based on a review of medical records, the requirement for written informed consent was waived. The patient’s records/information was anonymized and de-identified prior to analysis.
Data Collection
We retrospectively reviewed the medical records system and collected the following data with a case report form, including demographic characteristics, clinical symptoms and signs, lumbar puncture results, neurologic imaging (MRI, CTV, MRV, DSA), laboratory examinations, treatment, and prognosis.
Symptom onset was categorized as acute (< 48 hour from symptom onset to admission), subacute (48 hour to 1 month from symptom onset to admission), or chronic (more than 1 month from symptom onset to admission).13
Follow-up visits were performed from at 3 months after discharge to the end of date collection by direct interview in the outpatient department or telephone interview of the patient or a relative. The functional outcome was evaluated according to the modified Rankin scale (mRS) as complete recovery (mRS = 0-1); partial recovery, independent (mRS = 2); dependent (mRS = 3-5); and death (mRS = 6). Favorable and unfavorable outcomes were defined as mRS of 0-2 and > 2 (3-6), respectively. In addition, clinical data and laboratory examination results of APS patients with CVST and without CVST were compared to analyze the risk factors of CVST.
Statistical Analysis
All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, USA). Continuous quantitative variables of non-normal distribution were presented as median (interquartile range, IQR) and analyzed with a Mann-Whitney U test. Categorical variables were reported as counts and percentages and analyzed by Chi-square test or Fisher’s exact test, depending on the sample size. A 2-sided P value < 0.05 was considered to be statistically significant.
Results
Baseline Characteristics
In this study, 269 of the patients with APS were identified and 21 (21/269, 7.8%) APS patients were diagnosed with CVST, including 14 females (14/149, 9.4%) and 7 males (7/120, 5.8%). The median age at onset of CVST was 33 years (IQR 28-48). And the median duration of CVST was 1.3 months (IQR 0.7-4). Of the 21 patients with CVST, 18 were primary APS and 3 were secondary APS, including 2 cases of SLE and 1 case of BD. Among all APS patients, the incidence of CVST was 9.4% in females and 5.8% in males, and no significant difference between male and female patients was observed (P = 0.279).
Clinical Features
Clinical characteristics of the 21 patients are shown in Tables 1 and 2. The most forms of the clinical course were chronic (11/21, 52.4%), followed by subacute (9/21, 42.9%) and acute (1/21, 4.8%). Among APS patients with CVST, 12 (57.1%) cases presented with neurologic symptoms of CVST as the initial manifestation, and the first symptom of CVST included headache (18/21, 85.7%), dizziness (1/21, 4.8%), hemiplegia (1/21, 4.8%), and visual (1/21, 4.8%). The most common complaints were headache (19/21, 90.5%), followed by visual loss (10/21, 47.6%), nausea/vomiting (8/21, 38.1%), diplopia (6/21, 28.6%), dizziness (5/21, 23.8%), hemiplegia (4/21, 19.0%), conscious disturbance (3/21, 14.3%), earache (2/21, 9.5%), and neck pain (2/21, 9.5%). A few other symptoms included ophthalmodynia, dysarthria, limb numbness, seizure, tinnitus, and memory decline. In addition, 11 of 21 (52.4%) patients with CVST were detected with papilledema.
Table 1.
Neurologic Features of the APS Patients With CVST (n = 21).
| Parameter | Value |
|---|---|
| Age at CVST onset, years (IQR) | 33 (28-48) |
| CVST as initial feature | 12 (57.1) |
| Onset patterns | |
| Acute | 1 (4.8) |
| Subacute | 9 (42.9) |
| Chronic | 11 (52.4) |
| Symptoms | |
| Headache | 19 (90.5) |
| Visual loss | 10 (47.6) |
| Nausea/vomiting | 8 (38.1) |
| Diplopia | 6 (28.6) |
| Dizziness | 5 (23.8) |
| Hemiplegia | 4 (19.0) |
| Conscious disturbance | 3 (14.3) |
| Earache | 2 (9.5) |
| Neck pain | 2 (9.5) |
| Ophthalmodynia | 1 (4.8) |
| Dysarthria | 1 (4.8) |
| Seizure | 1 (4.8) |
| Limb numbness | 1 (4.8) |
| Tinnitus | 1 (4.8) |
| Memory decline | 1 (4.8) |
| Papilledema | 11 (52.4) |
| Cerebrospinal fluid examination | |
| Intracranial hypertension | 19 (90.5) |
| CSF WBC count, n (%) | |
| Raise | 3 (14.3) |
| Normal | 18 (85.7) |
| Number of sinuses involved | |
| Single sinus | 5 (23.8) |
| Dual or multiple sinuses | 16 (76.2) |
| Occluded sinus/vein | |
| Transverse sinus | 16 (76.2) |
| Superior sagittal sinus | 12 (57.1) |
| Sigmoid sinus | 11 (52.4) |
| Internal jugular vein | 8 (38.1) |
| Confluence of sinuses | 4 (19.0) |
| Straight sinus | 3 (14.3) |
| Galen’s vein | 1 (4.8) |
| Inferior sagittal sinus | 1 (4.8) |
| Cortical veins | 1 (4.8) |
| Cerebral parenchymal lesions | |
| No lesion | 11 (52.4) |
| Cerebral infarction | 9 (42.9) |
| Cerebral venous infarction | 6 (28.6) |
| Intracerebral hemorrhage | 3 (14.3) |
| Hemorrhagic venous infarction | 2 (9.5) |
| mRS at admission (n = 21) (IQR) | 1 (1-3) |
| mRS = 0-1 | 14 (66.6) |
| mRS = 2 | 1 (4.8) |
| mRS = 3-5 | 4 (19.0) |
| Death = 6 | 2 (9.5) |
| mRS at last follow-up (n = 19) (IQR) | 0 (0-1) |
| mRS = 0-1 | 15 (78.9) |
| mRS = 2 | 1 (5.3) |
| mRS = 3-5 | 0 |
| Death = 6 | 3 (15.8) |
Table 2.
Clinical Characteristics of APS Patients With CVST.
| No | Gender/age (years) | Onset | Initial features | Major clinical features | LAC | Cerebral parenchymal lesions | Site of CVST (CTV/MRV/DSA) | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/28 | Chronic | Headache | Headache | – | None | LTS, SiS, IJ | ACT | Good |
| 2 | F/41 | Subacute | Headache | Headache, nausea/vomiting | + | None | SSS | ACT | Good |
| 3 | M/19 | Subacute | Headache | Headache, nausea/vomiting | – | Left parietal CVI | LTS, SSS, CoS, Cortical veins | ACT | Good |
| 4 | M/25 | Chronic | Headache | Headache, visual loss | – | None | BTS, SSS, SiS, | ACT, EMT | Good |
| 5 | M/33 | Chronic | Headache | Headache | – | None | RTS | ACT, APT | Good |
| 6 | F/50 | Chronic | Headache | Headache, visual loss, dizziness | – | BSI; CSI | LTS | ACT, CS | Good |
| 7 | F/33 | Chronic | Headache | Headache, earache, neck pain | + | None | RTS, SSS, SS, SiS, CoS | ACT, APT, CS, HCQ, MTX | Good |
| 8 | F/35 | Subacute | Dizziness | Visual loss, nausea/vomiting, dizziness | + | None | BTS | ACT, APT, CS | Good |
| 9 | F/51 | Subacute | Headache | Headache, visual loss, nausea/vomiting, diplopia, right earache | – | LFH; LTH | LTS, SSS, SiS, SS, CoS, IJ | ACT, EMT | Good |
| 10 | M/51 | Chronic | Visual loss | Visual loss, left hemiplegia, dysarthria | – | Left frontal, temporal, and parietal CVI and HVI | BTS, SSS, SiS, CoS | ACT, APT | Death |
| 11 | M/42 | Acute | Hemiplegia | Headache, hemiplegia, conscious disturbance | – | Right frontal, left frontal, temporal, parietal, basal nucleus, callosum, and mesencephalon CVI | BTS, SSS | ACT | Good |
| 12 | F/28 | Subacute | Headache | Headache, nausea/vomiting, hemiplegia, conscious disturbance, seizure | – | Right thalamic CVI and HVI | SS, ISS, Galen’s vein | ACT, LET, EMT, CS, HCQ | Death |
| 13 | F/46 | Chronic | Headache | Headache, diplopia, left hemiplegia | – | Right parietal CVI | RTS, SSS, BSiS | ACT, LET, EMT | Good |
| 14 | F/21 | Subacute | Headache | Headache, nausea/vomiting, dizziness, conscious disturbance | + | RBNI | RTS, SSS, RSiS, RIJ | ACT, APT, CS, HCQ, AZA | Good |
| 15 | F/50 | Subacute | Headache | Headache, diplopia, dizziness | – | None | LIJ, SiS | ACT | Good |
| 16 | M/11 | Chronic | Headache | Headache, visual loss, nausea/vomiting, diplopia | – | None | TS, RIJ, SiS, SSS | ACT, HCQ, ONSF | Good |
| 17 | F/33 | Subacute | Headache | Headache, nausea/vomiting, diplopia, dizziness, neck pain | – | None | TS, LIJ, LSiS | ACT, APT, CS, STENT | Good |
| 18 | F/38 | Chronic | Headache | Headache, visual loss, ophthalmodynia, limb numbness | – | None | SSS | ACT, CS | Good |
| 19 | F/33 | Chronic | Headache | Headache, visual loss, tinnitus, memory decline | + | LSFI | LIJ | ACT, CS, HCQ | Good |
| 20 | F/30 | Subacute | Headache | Headache, visual loss, diplopia | + | None | LTS, SiS, BIJ | ACT, APT, CS, ONSF | Good |
| 21 | M/70 | Chronic | Headache | Headache, visual loss | – | Right occipital CVI | BTS, SSS | ACT, CS, HCQ | Death |
Laboratory Examinations
The results of laboratory investigations are listed in Tables 2 and 3. Laboratory analysis of APS patients with CVST showed that 8 cases (8/18, 44.4%) were antinuclear antibodies (ANAs) positive, 7 cases (7/21, 33.3%) were D-dimer positive, 10 cases (10/21, 47.6%) were anti-cardiolipin (aCL) positive, and 15 cases (15/17, 88.2%) were anti-β2-glycoprotein-I (anti-β2GP-1) positive. Lupus anticoagulant (LAC) was tested in 6 (28.6%) patients, with all positive. Erythrocyte sedimentation rate (ESR) and homocysteine were higher than normal in 13 patients (13/21, 61.9%) and 5 patients (5/21, 23.8%), respectively. Hemoglobin and platelet (PLT) were lower than normal in 5 patients (5/21, 23.8%) and 6 patients (6/21, 28.6%), respectively. The international normalized ratio, thrombophilia factor (antithrombin III, protein S, and protein C) tests were within the normal ranges.
Table 3.
Clinical Comparison of APS Patients With and Without CVST.
| Parameters | APS with CVST (n = 21) | APS without CVST (n = 63) | P value |
|---|---|---|---|
| Gender (F/M) | 14/7 | 42/21 | 1.000 |
| Age, median (IQR) years | 33 (28-48) | 39 (28-50) | 0.521 |
| Disease duration, median (IQR) months | 1.3 (0.7-4) | 2 (0.5-12.2) | 0.616 |
| Secondary APS | 3 (14.3) | 9 (14.3) | 1.000 |
| Other thrombosis | 5 (23.8) | 30 (47.6) | 0.055 |
| HGB decreased | 5 (23.8) | 14 (22.2) | 1.000 |
| PLT decreased | 6 (28.6) | 12 (19.0) | 0.539 |
| D-dimer positive | 7 (33.3) | 23/59 (39.0) | 0.648 |
| ESR increased | 13 (61.9) | 27 (42.9) | 0.130 |
| ANAs positive | 8/18 (44.4) | 20/61 (32.8) | 0.364 |
| aCL positive | 10 (47.6) | 43 (68.3) | 0.090 |
| anti-β2GP-1 positive | 15/17 (88.2) | 53 (84.1) | 0.969 |
Cerebral Spinal Fluid Analysis
Lumbar puncture confirmed elevated intracranial pressure in 19 of 21 patients (90.5%). The cerebrospinal fluid (CSF) pleocytosis were showed only in 3 patients (14.3%), and elevated protein of CSF was detected in 5 cases (23.8%). The chloride and glucose level of CSF and etiological examination were all unremarkable.
Neuroimaging Findings
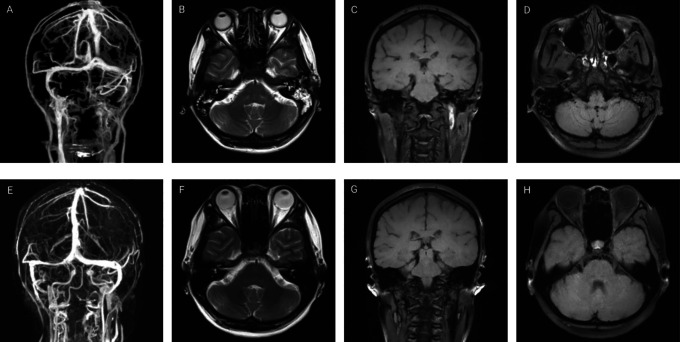
In present study, CVST was diagnosed by MRV or CTV in all patients (Tables 1 and 2). Multiple sinuses were involved in 16 (76.2%) patients and single sinus in 5 (23.8%) patients. Moreover, 10 patients underwent magnetic resonance black-blood thrombus imaging (MRBTI) to confirmed CSVT. The most frequently involved venous sinuses was the transverse sinus in 76.2% (16/21), followed by superior sagittal sinus in 57.1% (12/21), sigmoid sinus in 52.4% (11/21), internal jugular vein in 38.1% (8/21), confluence of sinuses in 19.0% (4/21), straight sinus in 14.3% (3/21). In addition, occlusions of Galen’s vein (1/21, 4.8%), inferior sagittal sinus (1/21, 4.8%), and cortical veins (1/21, 4.8%) were also noted. Parenchymal lesions with abnormal MRI findings were confirmed in 10 (47.6%) patients, which mainly included cerebral venous infarction in 6 (28.6%), intracerebral hemorrhage in 3 (14.3%), and hemorrhagic venous infarction in 2 patients (9.5%). Neuroimaging detection of one representative patient is presented in Figures 1 and 2.
Figure 1.
Neuroimaging of a 33-year-old female with subacute cerebral sinus venous thrombosis. (A) Computed tomography venography demonstrates the absence of flow in the left partial transverse sinus, sigmoid sinuses and internal jugular vein. (B) Axial T2-weighted sequence shows absence of flow-void in the left transverse sinus. (C) Coronal magnetic resonance black-blood thrombus imaging (MRBTI) shows hyperintense signal in the superior segment of left internal jugular vein. (D) Axial MRBTI shows an isointense signal inside the left sigmoid sinuses. Three-dimensional reconstruction magnetic resonance venography (E), axial T2-weighted sequence (F), coronal MRBTI (G) and axial MRBTI (H) at 5 months follow-up demonstrate an almost complete recanalization of the left transverse sinus, sigmoid sinuses and internal jugular vein.
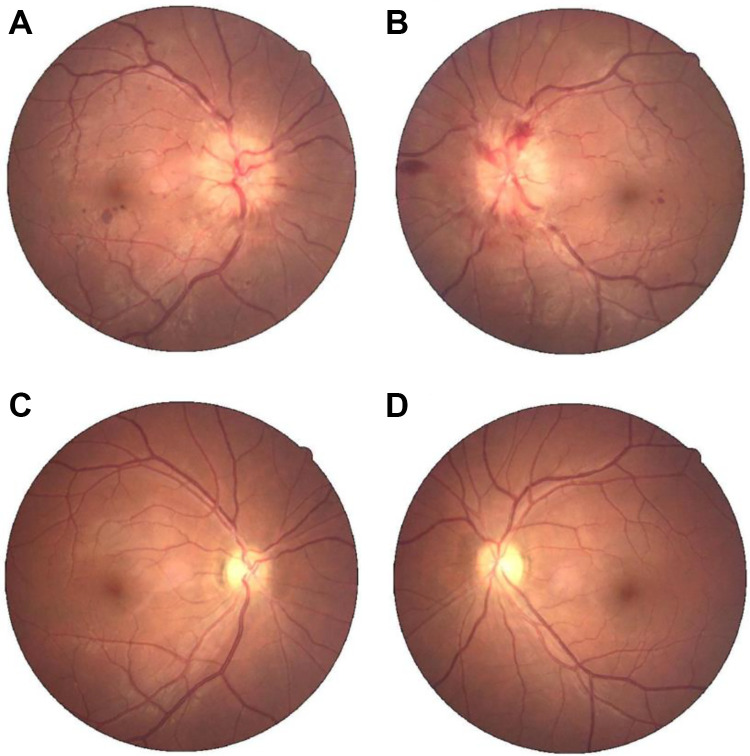
Figure 2.
Colored fundus photograph of a 33-year-old female with subacute cerebral sinus venous thrombosis. A-B Initial colored fundus photograph shows florid papilledema both eyes at admission. C-D Repeated colored fundus photograph 5 months later shows recovery after endovascular stenting of internal jugular vein and other treatment.
Treatment and Outcome
All patients were treated with low-molecular-weight heparin (LMWH, 90 U/kg each time, subcutaneously, twice daily), then followed with long-term oral anticoagulation including warfarin in 17 (81.0%) or rivaroxaban in 4 (19.0%) patients. Meanwhile, 7 (33.3%) patients were treated with antiplatelet drugs. Furthermore, some patients received endovascular mechanical thrombectomy (4/21, 19.0%), local endovascular thrombolysis (2/21, 9.5%), and internal jugular veins stenting (1/21, 4.8%). Additional treatments included corticosteroid (10/21, 47.6%), hydroxychloroquine (6/21, 28.6%), azathioprine (1/21, 4.8%), and methotrexate (1/21, 4.8%). In addition, some patients received symptomatic treatment, such as reducing intracranial hypertension, and antiepileptic therapy according to the actual complaints. After a median 39 months follow-up (range 3-121), 18 patients achieved clinically improved without recurrence, and 3 patients died of cerebral herniation (Tables 1 and 2). The median mRS was 1 (IQR 1-3) at admission and 0 (IQR 0-1) at last follow-up respectively. Sixteen patients (84.2%) had good outcomes, and 3 patients (15.8%) had poor outcomes at last follow-up. Two patients were lost to follow-up after discharge (Table 1).
Comparison With APS Cases Without CVST
Table 3 shows a comparison of the characteristics between the APS with CVST and APS without CVST groups. Sixty-three APS patients without CVST served as control with 42 females (66.7%) and 21 males (33.3%).
The median age and disease duration of APS patients without CVST was 39 years (IQR 28-50) and 2 months (IQR 0.5-12.2), respectively. Among them, 54 were primary and 9 were secondary APS. As showed in Table 3, there were no significant differences (P > 0.05) between two groups in the analysis of related indicators such as duration of disease, other thrombosis laboratory markers including ESR, ANAs, aCL, anti-β2GP1, PLT etc.
Discussion
CVST is an uncommon and severe cerebrovascular disease, usually accounts for 10-20% of stroke in young individuals.16 Early and accurate diagnosis of CVST is still a great challenge in clinical practice because of varying and non-specific clinical manifestations, which include a wide range of symptoms such as headache, focal neurological deficits, seizures, and altered mental status.17 As a systemic autoimmune disease, APS can cause cerebral venous and arterial thrombosis, which can be the initial presenting symptom or occur in the disease course.1 In present study, we presented detailed clinical characteristics, radiology findings, laboratory results, treatments, and outcomes of CVST in 21 patients with APS.
CVST is rare complication in patients with APS. A retrospective cohort study reported that the prevalence of CVST in APS was 0.7%,7 while the incidence of CVST at 7.8% in our study was higher than that in previous literature. This difference might be due to the following factors: differences of ethnic, geographical distribution and advances within the imaging techniques during recent decades. In addition, most study objects with CVST in APS were enrolled from a neurology center and were usually referred to our center from other cities in the country.
In this study, younger individuals were prone to CVST, mainly female. Different from the common onset form of CVST (chronic only in 7% of patients),18 52% of our APS patients with CVST presented with chronic onset and only one patient with acute onset. It is not known whether the pathogenesis of APS-related CVST is different from those with other causes, then induced different onset pattern.19 The CVST patients with APS have a wide spectrum of clinical presentations. Similar to previous reports on CVST,18 headache was the most common presenting symptoms at (90.5%) of all our cases, which was also the primary reason for visiting the doctors in this study. The headache of CVST usually presents as a refractory and diffuse headache, and distribute to anterior/posterior bilateral head,20 frequently accompanied by epileptic seizure, focal deficits or intracranial hypertension syndrome.21 Therefore, the diagnosis of CVST should be considered in APS patients with idiopathic intracranial hypertension, who complaining of severe unexplained headache, or young patients presenting with stroke symptoms, or the overlapping clinical presentation of these conditions.16,17 Moreover, other manifestation included tinnitus, memory decline, dysarthria, and ophthalmodynia were unusual but helpful for diagnosis in CVST patients with APS. So, more recognition of CVST will be essential to early diagnosis.
More than half of our patients (57.1%) were admitted to the department of neurology with neurologic symptoms as initial presentation, and definite diagnosis of APS with CVST was yielded after detailed neurological examination. The etiology of CVST is complicated, which usually include infectious and non-infectious causes. Any alteration in one of the composition of blood, blood flow stasis or vascular endothelium can attribute to CVST.13 The International Study on Cerebral Venous Thrombosis (ISCVT) study indicated that 34.1% of 624 enrolled patients had a prothrombotic conditions (including antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibody, etc.). Moreover, hematologic disorders and systemic diseases account for 19.2% of the patients.18 CVST is a complication of APS, mainly owing to the prothrombotic states and impaired clot dissolution. APS is responsible for approximately 6-17% of patients with CVST.22 Therefore, when search for the causes of CVST, we should take the prothrombotic conditions, hematologic disorders, and systemic diseases into account, especially the screening of APS.
So far, the pathogenesis of CVST in APS remains largely unclear. Studies suggested that CVST is associated with the production of aPLs which targets at cerebral venous and platelet in APS.23–26 APLs, namely LAC, aCL, anti-β2-GP1, are a heterogeneous group of auto-antibodies directed against phospholipid binding proteins. The involvement of aPLs in clinically significant normal procoagulant and anticoagulant reactions and on certain cells altering the expression and secretion of various molecules are the basis for possible mechanisms. Previous studies indicated that aPLs could induce a prothrombotic state through: (a) potentiating platelet aggregation and adhesiveness through upregulation of the thromboxane A223; (b) enhancing surface expression of tissue factor expression on endothelial cells and activate the extrinsic coagulation pathway27; and (c) activation of the classical complement pathway and interference with endogenous anticoagulant mechanisms.28
To date, the conclusion about the triple positivity (LAC-positive, aCL-positive, and anti-β2GP-1 positive) have the highest risk for venous thrombosis29 are inconsistent in previous studies. Moreover, LAC is regarded as the strongest risk factor for thrombosis as demonstrated in many studies.30 However, in one retrospective study, an unexpectedly high prevalence of aCL-positive (53%) patients with CVST was found but none was found to have LAC.31 The presence of aCL appears to predispose patients to develop CVST at a relatively younger age and to have more extensive superficial and deep cerebral venous system involvement.32 The positive rate of LAC, aCL, and anti-β2GP1 were 100%, 47.6%, and 88.2% in our CVST with APS, respectively. However, we did not find that aCL and anti-β2GP-1 were specific predictive factors for CVST in APS. This could be related to the limited number of cases, and the detection index was not comprehensive in the same blood sample. So, clinical trials with larger sample size should be designed to make further demonstration in the future.
In our research, thrombocytopenia was found in nearly a third of CVST patients, however, which was no difference with control groups (19.0%). Thrombocytopenia occurs in at least 29.6% of individuals with APS and is most marked at times of thrombosis formation.6 Moreover, thrombocytopenia might be associated with other systemic manifestations of APS, such as obstetrical morbidity, venous and/or arterial thrombosis, myocardial infarction and valve vegetations.33 Cautious assessment is required for combined antiplatelet therapy in APS-induced CVST patients with thrombocytopenia, since thrombocytopenia increases the risk of bleeding from antiplatelet and anticoagulant therapy.
Diagnostic imaging has played an increasing role in the recognition, diagnosis, and management of CVST. As shown in this study, 28.6% CVST patients presented with cerebral venous infarction as the main manifestation of parenchymal lesions, followed by intracerebral hemorrhage (14.3%). Previous publications22 showed that parenchymal lesions occurred in 60% of patients with CVST, and two-thirds of them have a hemorrhagic component. All these cerebral parenchymal lesions of CVST can be better visualized and depicted on MRI. As similar to most of the previous literatures,34,35 transverse sinus and superior sagittal sinus were the most frequently involved sites of CVST, and more than 3-quarter of the patients were affected by multiple sinuses. The location of the CVST is frequently associated with the predisposing causes. Cavernous sinus thrombosis was more common in patients with infection-associated CVST, whereas superior sagittal sinus and transverse sinus thrombosis were more common in the non-infectious CVST.36 This information suggests that the risk factors may be non-inflammatory diseases when the thrombus occurs in the superior sagittal sinus, transverse sinus or straight sinus. These also could explain why CVST associated APS often involved transverse sinus and superior sagittal sinus. Currently, MRI is the reference standard imaging technique for diagnosis of CVST. Maximum accuracy is obtained with the combination of classic MRI sequences, which are able to show the thrombus and parenchymal lesions, together with MRV, which can show reduction or absence of flow.37 Theoretically, DSA was the most accurate technique, due to its invasiveness and radiation, is only reserved for patients with an inconclusive CTV and MRV or undergoing endovascular therapeutic.38 In our study, there were 2 patients to be free of thrombus by DSA, However, the presence of thrombus was confirmed eventually through clinical symptoms and other examinations (especially MRBTI), which presumably demonstrated that DSA also has some limitations in diagnose of CVST. Moreover, thrombus was verified in 3 cases (case 7, case 13, case 15) of our CVST patients with inconclusive CTV or MRV by undergoing MRBTI. The MRBTI, as an emerging technique, can be used as a promising first-line diagnostic imaging tool of achieving accurate detection of thrombus in the cerebral venous system, which allows selective visualization of thrombus by effectively suppressing blood signal.39
The recurrence rate of thrombotic events in the APS patients is high with 7.5 per 100 patient-years in the first 5 years despite anticoagulation.40 Therefore, for CVST patients with APS, the recommended initial therapy is unfractionated or LMWH, followed by indefinite long-term anticoagulant therapy with a vitamin K antagonist (VKA) such as warfarin (target INR, 2 to 3).11,13 Followed these principles, in addition to initiating with LMWH, all our patients received warfarin or rivaroxaban as long-term oral anticoagulation. So far, little is known about the efficacy and safety of new oral anticoagulants (NOACs) in APS patients with venous thrombosis. Some research data support the fact that low-risk aPLs patients with sole venous thrombosis and without vascular risk factors could profit from the use of NOACs. Besides, compared with VKA, NOACs are an appealing alternative without the necessity of INR monitoring. For APS, some patients with CVST were treated with hydroxychloroquine which can serve as a potential add-on treatment for APS patients with recurrent venous thrombosis despite adequate dose of anticoagulant therapy.11 Furthermore, anticoagulation is not the only option for CVST. In this study, 5 CVST patients received endovascular therapy due to no improvement or clinical deterioration despite anticoagulation, or with severe neurological deficits. And the symptoms of 5 patients improved with no recurrence during follow-up. This outcome provided a further evidence that endovascular therapy may be safe and effective in APS patients with CVST with ineffective anticoagulant therapy or clinical deterioration. Endovascular mechanical thrombectomy and thrombolysis have arisen as viable options for medically refractory CVST.41,42 In addition, optic nerve sheath fenestration (ONSF) was performed in 2 patients with vision improved. The intracranial hypertension secondary to CVST can progress rapidly with fulminant papilledema and rapid visual loss. For these patients, ONSF may be a safe and effective alternative to protect visual function.43 After antithrombotic therapy, the majority of CVST cases with APS showed good response to antithrombotic therapy without recurrence, and 84.2% achieved favorable prognosis with mRS of 0-2 with active treatment. Unfortunately, 3 patients (15.8%) died of cerebral herniation due to acute hemorrhagic lesion, higher than the previous study (4.39%).44 This may be explained by the fact that this study was conducted in a national referral center, and there are more critical CVST patients from other hospital with relatively inadequate treatment. Decompressive craniectomy should be considered when selecting therapy for patients with progressive herniation.45
There were several limitations in our study. First, because of a single-center study, the data were obtained from a relatively small sample size which may not be able to represent whole CVST patients with APS. Second, since this study was an observational, retrospective study, the incomplete laboratory examination and limited evaluation index limit to draw a definitive conclusion.
Despite the limitations of retrospective study, present study also has certain enlightening significance to the further research. More than 20% CVST patients have better functional outcomes after endovascular therapy, so further prospective studies can be carried out to provide more options for severe CVST patients with APS. Furthermore, more clinical trials should be designed to search the prediction factors of CVST in APS patients and provide more reliable clinical conclusions.
Conclusion
In conclusion, CVST is rare and severe complication of APS. Clinical manifestation is extremely varied due to different venous sinus of involvements. For better prognosis, prompt evaluation and effective management is required for a well recognition of this disease. The routine screening of aPLs is recommended in unexplained CVST patients. On the other hand, for APS patients with headache, visual loss, and nausea/vomiting, evaluation of CVST should be performed as soon as possible. Most CVST patients with APS will eventually have a good long-term prognosis after LMWH overlapping indefinite OACs treatment. Endovascular therapy for CVST is a feasible option on the condition of failed anticoagulation therapy or contraindication.
Acknowledgments
We thank physicians, patients, and family members who provided clinical information.
Authors’ Note: Our institution does not require ethical approval for reporting individual cases or case series.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the National Key Research and Development Program of China (No. 2016YFC1300600, 2017-2020), the National Key Research and Development Program of China, Precision Medicine Program (No. 2016YFC0901004).
ORCID iD: Xiaoqin Huang  https://orcid.org/0000-0001-6148-3577
https://orcid.org/0000-0001-6148-3577
References
- 1. Schreiber K, Sciascia S, de Groot PG, et al. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:18005. [DOI] [PubMed] [Google Scholar]
- 2. Hughes GR. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br Med J (Clin Res Ed). 1983;287(6399):1088–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amiral J, Peyrafitte M, Dunois C. Anti-phospholipid syndrome: current opinion on mechanisms involved, laboratory characterization and diagnostic aspects. Transfus Apher Sci. 2017;56(4):612–625. [DOI] [PubMed] [Google Scholar]
- 4. Tyagi S, Ahuja A. Antiphospholipid antibody syndrome-diagnostic issues of lupus anticoagulants. J Hematol Thromboembolic Dis. 2016;4(253):2. [Google Scholar]
- 5. Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48-49:20–25. [DOI] [PubMed] [Google Scholar]
- 6. Cervera R, Serrano R, Pons-Estel GJ. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74(6):1011–1018. [DOI] [PubMed] [Google Scholar]
- 7. Cervera R, Piette J, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46(4):1019–1027. [DOI] [PubMed] [Google Scholar]
- 8. Uthman I, Noureldine MHA, Ruiz-Irastorza G. Management of antiphospholipid syndrome. Ann Rheum Dis. 2019;78:155–161. [DOI] [PubMed] [Google Scholar]
- 9. Nalli C, Andreoli L, Casu C, Tincani A. Management of recurrent thrombosis in antiphospholipid syndrome. Curr Rheumatol Rep. 2014;16(3):405. [DOI] [PubMed] [Google Scholar]
- 10. Les I, Ruiz-Irastorza G, Khamashta MA. Intensity and duration of anticoagulation therapy in antiphospholipid syndrome. Semin Thromb Hemost. 2012;38(4):339–347. [DOI] [PubMed] [Google Scholar]
- 11. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018; 378:2010–2021. [DOI] [PubMed] [Google Scholar]
- 12. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. [DOI] [PubMed] [Google Scholar]
- 13. Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192. [DOI] [PubMed] [Google Scholar]
- 14. Petri M, Orbai A, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davatchi F, Assaad-Khalil S, Calamia KT. The International Criteria for Behcet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–347. [DOI] [PubMed] [Google Scholar]
- 16. Dmytriw AA, Song JSA, Yu E. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology. 2018;60(7):669–685. [DOI] [PubMed] [Google Scholar]
- 17. Linn J, Ertl-Wagner B, Seelos KC, et al. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J Neuroradiol. 2007;28(5):946–952. [PMC free article] [PubMed] [Google Scholar]
- 18. Ferro JM, Canhao P, Stam J, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–670. [DOI] [PubMed] [Google Scholar]
- 19. Shlebak A. Antiphospholipid syndrome presenting as cerebral venous sinus thrombosis: a case series and a review. J Clin Pathol. 2016;69(4):337–343. [DOI] [PubMed] [Google Scholar]
- 20. Timóteo Â, Inácio N, Machado S. Headache as the sole presentation of cerebral venous thrombosis: a prospective study. J Headache Pain. 2012;13(6):487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Amorim LCD, Maia FM, Rodrigues CEM. Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus. 2017;26(5):529–536. [DOI] [PubMed] [Google Scholar]
- 22. Silvis SM, de Sousa DA, Ferro JM. Cerebral venous thrombosis. Nat Rev Neur 2017;13:555–565. [DOI] [PubMed] [Google Scholar]
- 23. Muscal E, Brey RL. Neurologic manifestations of the antiphospholipid syndrome: integrating molecular and clinical lessons. Curr Rheumatol Rep. 2008;10(1):67–73. [DOI] [PubMed] [Google Scholar]
- 24. Graf J. Central nervous system manifestations of antiphospholipid syndrome. Rheum Dis Clin North Am. 2017;43(4):547–560. [DOI] [PubMed] [Google Scholar]
- 25. Zhu D, Fu J, Zhang Y, et al. Neurological antiphospholipid syndrome: clinical, neuroimaging, and pathological characteristics. J Neurol Sci. 2014;346(1-2):138–144. [DOI] [PubMed] [Google Scholar]
- 26. Du VX, Kelchtermans H, de Groot PG. From antibody to clinical phenotype, the black box of the antiphospholipid syndrome: pathogenic mechanisms of the antiphospholipid syndrome. Thromb Res. 2013;132(3):319–326. [DOI] [PubMed] [Google Scholar]
- 27. Fleetwood T, Cantello R, Comi C. Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy. Front Neurol. 2018;9:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27(9):1404–1414. [DOI] [PubMed] [Google Scholar]
- 29. Pengo V, Banzato A, Bison E, Denas G, Padayattil Jose S, Ruffatti A. Antiphospholipid syndrome: critical analysis of the diagnostic path. Lupus. 2010;19(4):428–431. [DOI] [PubMed] [Google Scholar]
- 30. Devreese KMJ. Antiphospholipid antibodies: evaluation of the thrombotic risk. Thromb Res. 2012;130(Suppl 1):S37–S40. [DOI] [PubMed] [Google Scholar]
- 31. Bousser MG, Chiras J, Bories J. Cerebral venous thrombosis—a review of 38 cases. Stroke. 1985;16(2):199–213. [DOI] [PubMed] [Google Scholar]
- 32. Carhuapom JR, Mitsias P, Levine SR. Cerebral venous thrombosis and anticardiolipin antibodies. Stroke. 1997;28(12):2363–2369. [DOI] [PubMed] [Google Scholar]
- 33. Krause I, Blank M, Fraser A, et al. The association of thrombocytopenia with systemic manifestations in the antiphospholipid syndrome. Immunobiology. 2005;210(10):749–754. [DOI] [PubMed] [Google Scholar]
- 34. Gunes HN, Cokal BG, Guler SK, et al. Clinical associations, biological risk factors and outcomes of cerebral venous sinus thrombosis. J Int Med Res. 2016;44(6):1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ozcan A, Canpolat M, Doganay S. Cerebral sinus venous thrombosis and prothrombotic risk factors in children: a single-center experience from Turkey. J Pediatr Hematol Oncol. 2018;40(6):e369–e372. [DOI] [PubMed] [Google Scholar]
- 36. Korathanakhun P, Petpichetchian W, Sathirapanya P, Geater SL. Cerebral venous thrombosis: comparing characteristics of infective and non-infective aetiologies: a 12-year retrospective study. Postgrad Med J. 2015;91(1082):670–674. [DOI] [PubMed] [Google Scholar]
- 37. Weimar C. Diagnosis and treatment of cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep. 2014;14(1):417. [DOI] [PubMed] [Google Scholar]
- 38. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018;16(10):1918–1931. [DOI] [PubMed] [Google Scholar]
- 39. Yang Q, Duan J, Fan Z. Early detection and quantification of cerebral venous thrombosis by magnetic resonance black-blood thrombus imaging. Stroke. 2016; 47: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bazzan M, Vaccarino A, Stella S, et al. Patients with antiphosholipid syndrome and thrombotic recurrences: a real world observation (the Piedmont cohort study). Lupus. 2016;25(5):479–485. [DOI] [PubMed] [Google Scholar]
- 41. Salottolo K, Wagner J, Frei DF, et al. Epidemiology, endovascular treatment, and prognosis of cerebral venous thrombosis: US center study of 152 Patients. J Am Heart Assoc. 2017;6(6):e5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siddiqui FM, Dandapat S, Banerjee C, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. 2015;46(5):1263–1268. [DOI] [PubMed] [Google Scholar]
- 43. Chen H, Zhang Q, Tan S, Fu H, Farris BK, Yang Z. Update on the application of optic nerve sheath fenestration. Restor Neurol Neurosci. 2017;35(3):275–286. [DOI] [PubMed] [Google Scholar]
- 44. Haghighi AB, Edgell RC, Cruz-Flores S, et al. Mortality of cerebral venous–sinus thrombosis in a large national sample. Stroke. 2012;43(1):262–264. [DOI] [PubMed] [Google Scholar]
- 45. Canhao P, Ferro JM, Lindgren AG, et al. Causes and predictors of death in cerebral venous thrombosis. Stroke. 2005;36(8):1720–1725. [DOI] [PubMed] [Google Scholar]