Abstract
Aim:
To characterize lipid profiles in women with different gestational diabetes mellitus (GDM) physiologic subtypes.
Methods:
We measured seven lipid markers (total cholesterol, LDL, HDL, triglycerides, non-esterified fatty acids (NEFA), ApoA, ApoB) in fasting plasma collected in a prospective cohort of 805 pregnant women during second trimester. We estimated insulin sensitivity and secretion using oral glucose tolerance test-based validated indices. We categorized GDM physiologic subtypes by insulin sensitivity and secretion defects defined as values below the 25th percentile among women with normal glucose tolerance (NGT), as previously established. We compared lipid markers across NGT and GDM subtypes. We explored associations between lipid markers and newborn anthropometry in the overall group and stratified by glucose tolerance status.
Results:
Among 805 women, 67 (8.3%) developed GDM. Women with GDM had higher body mass index (BMI; 29.3 vs. 26.6 kg/m2), while ethnicity (97.3% vs. 97.0% European ancestry) and age (28 vs. 29 years) were similar. In comparison to women with NGT, women with GDM characterized by a predominant insulin sensitivity defect had significantly higher triglycerides (2.20 vs. 1.82, P = 0.002), lower HDL (1.64 vs. 1.90, P = 0.01) and higher NEFA (0.34 vs. 0.24, P < 0.0001). GDM women with a predominant insulin secretion defect differed from women with NGT with respect to NEFA (0.32 vs. 0.24, P = 0.003) while other lipid markers were similar. These associations remained significant after adjusting for maternal age and BMI. Greater maternal levels of NEFA were associated with higher birth weight z-scores in women with an insulin secretion defect (BMI-adjusted r = 0.58, P = 0.01). We did not find significant associations between other lipid markers and newborn anthropometry in other groups.
Conclusion:
Women with GDM have distinct lipid profiles based on their GDM physiologic subtype which may not be apparent when investigating GDM as a single group.
Keywords: Gestational diabetes, Lipids, Non-esterified fatty acids, Insulin sensitivity, Insulin secretion
1. Introduction
Pregnancy markedly alters lipid metabolism. The first trimester is characterized by increased lipogenesis; later in pregnancy there is a shift to a more lipolytic state, in accordance with fetal growth requirements [1,2]. Previous studies have suggested that pregnancies with gestational diabetes mellitus (GDM) are characterized by more severe hyperlipidemia as compared to pregnancies in which normal glucose tolerance (NGT) is maintained, but reports are inconsistent regarding which lipids are elevated [3–6]. A possible explanation is that GDM is a heterogeneous condition. We previously demonstrated that GDM can be subdivided into three physiologic subtypes [7]. We also observed that women with an insulin-sensitivity defect had higher complication rates at delivery suggesting other fuels may contribute to macrosomia and related birth complications [7]. However, it is unknown if lipids differ among GDM physiologic subtypes. Thus, the purpose of this study was to characterize gestational lipid profiles among three physiologic GDM subtypes and compare them to NGT women.
2. Methods
2.1. Study Details
This study used data from the Genetics of Glucose regulation in Gestation and Growth (Gen3G) cohort, a prospective cohort study of maternal-child pairs followed from the first trimester through delivery, previously described [8]. The ethics committee at Centre Hospitalier Universitaire de Sherbrooke (CHUS) approved the study and all participants provided written informed consent.
2.2. Gestational Diabetes Mellitus Status
After excluding women with pre-existing diabetes at enrollment, we classified women as having GDM if their second trimester 75 g oral glucose tolerance test (OGTT) based on international criteria [9]. We used plasma glucose and insulin values obtained at 0, 60 and 120 min during OGTT to calculate insulin sensitivity [10] (Matsuda index) and secretion indices [11,12] (Stumvoll first-phase estimate). We defined women as having insulin-sensitivity defect if their Matsuda insulin sensitivity index was below the 25th percentile of the Matsuda index distribution (GDM-Sensitivity); we defined women as having insulin-secretion defect if their Stumvoll insulin secretion was below the 25th percentile of the Stumvoll estimate distribution (GDM-Secretion) (both distributions determined among NGT women) as previously described [7]. We categorized women with both defects as GDM-mixed.
2.3. Measurements
We obtained ≥8-hour fasting samples during the second trimester visit for the following biomarkers: total cholesterol (TC), high-density lipoprotein-cholesterol (HDL), low-density lipoprotein-cholesterol (LDL), Apolipoprotein A and B (Apo A and B), triglycerides, non-esterified fatty acids (NEFA), fasting glucose, HbA1c and insulin using methods described elsewhere [8]. The CHUS biomedical laboratory measured fasting TC, HDL, and triglycerides using a colorimetric method (Johnson & Johnson Clinical Diagnostics) and calculated LDL using Friedewald’s equation. We measured NEFA levels [minimum level of detection of 0.0014 mM; intra-assay coefficients of variation (CV) 0.8%] using a colorimetric assay (Wako Chemicals). We excluded three NGT women whose LDL >7.0 mmol/L as this was suggestive of familial hypercholesterolemia.
2.4. Statistical Analysis
We presented participants’ characteristics as medians (interquartile range) for continuous measures and frequencies for categorical values. We used the rank-sum test to compare the GDM group overall with the NGT group. We used Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables to compare differences across groups. When P values from Kruskal-Wallis or Fisher exact tests were <0.05, we did pairwise comparisons between groups using Dunn’s tests [13] or Fisher exact tests with Bonferroni correction of P values. We used linear regression to control for maternal BMI and age, transforming non-normally distributed dependent variables with either the natural-log or square-root transformations to achieve normality prior to regression. We assessed the relationships between NEFA and insulin sensitivity, secretion and oral disposition indices (controlling for second trimester maternal BMI) and all lipids and birth weight using pairwise and partial correlation analyses (controlling for BMI or gestational weight gain). We considered a two-tailed P < 0.05 statistically significant. We performed analyses using SAS version 9.4 (Cary, North Carolina) and Stata version 15 (Stata Corp LP, College Station, Texas).
3. Results
We included 805 women in our current analyses, 67 (8.3%) diagnosed with GDM (34 GDM-Sensitivity, 20 GDM-Secretion and 12 GDM-Mixed) and 739 who maintained normoglycemia. GDM-Sensitivity women had a significantly higher BMI as compared to NGT women [7]. There was a higher frequency of previous GDM or macrosomia among GDM-Secretion vs. NGT women (Table 1). Additional subtype differences in characteristics have been reported previously [7].
Table 1.
Characteristics and lipid values of women with NGT or GDM, by physiological subtype.
| GDM-Secretion Median (IQR)/n (%) | Pb | GDM-Mixed Median (IQR)/n (%) | Pb | GDM-Sensitivity Median (IQR)/n (%) | Pb | NGT Median (IQR)/n (%) | Pa | All GDM Median (IQR) | Pc | |
|---|---|---|---|---|---|---|---|---|---|---|
| nd | 20 (29.9% of GDM) | – | 12 (17.9% of GDM) | – | 34 (50.7% of GDM) | – | 739 (91.8% of total n) | – | 67 (8.3% of total n) | – |
| Age (years) | 30 (28–32) | – | 31 (23–33) | – | 28 (25–35) | – | 28 (25–31) | 0.16 | 29 (26–33) | 0.05 |
| % European descent | 18 (90.0%) | – | 12 (100.0%) | – | 34 (100.0%) | – | 714 (97.1%) | 0.22 | 65 (97.0%) | 0.95 |
| % Nulliparous | 5 (25.0%) | – | 4 (33.3%) | – | 17 (50.0%) | – | 382 (51.8%) | 0.07 | 27 (40.3%) | 0.07 |
| % Patient history of GDM or macrosomia | 8 (40.0%) | 0.001 | 3 (25.0%) | 0.23 | 5 (14.7%) | 0.61 | 62 (8.4%) | 0.0001 | 16 (23.9%) | <0.0001 |
| First-trimester visit | ||||||||||
| Gestational age (weeks) | 8.8 (7.5–10.8) | – | 10.2 (8.2–12.1) | – | 9.4 (7.2–11.6) | – | 9.2 (8.1–11.4) | 0.85 | 9.3 (7.4–12.0) | 0.89 |
| BMI (kg/m2) | 21.9 (20.7–26.0) | 0.15 | 25.3 (21.3–29.8) | 0.98 | 30.1 (26.9–37.7) | <0.0001 | 23.9 (21.5–27.5) | <0.0001 | 27.0 (22.0–32.4) | 0.002 |
| Second-trimester visit | ||||||||||
| Gestational age (weeks) | 26.4 (25.9–27.7) | – | 26.2 (25.3–27.8) | – | 26.3 (25.6–26.6) | – | 26.2 (25.6–27.1) | 0.65 | 26.3 (25.6–34.5) | 0.91 |
| BMI (kg/m2) | 25.0 (23.7–28.3) | 0.21 | 28.7 (24.6–32.8) | 0.65 | 32.3 (28.9–40.9) | <0.0001 | 26.6 (24.2–30.1) | <0.0001 | 29.3 (25.6–34.5) | 0.0004 |
| Insulin sensitivity (Matsuda) | 7.4 (6.2–8.5) | >0.99 | 5.4 (4.7–5.5) | 0.0003 | 2.9 (2.3–4.0) | <0.0001 | 7.9 (5.8–11.2) | <0.0001 | 4.6 (2.9–6.2) | <0.0001 |
| Insulin secretion (Stumvoll) | 594 (501–739) | <0.0001 | 748 (694–870) | <0.0001 | 1364 (1063–1677) | 0.0003 | 1121 (934–1286) | <0.0001 | 969 (676–1368) | 0.004 |
| DIo | 4491 (3947–5318) | <0.0001 | 4082 (3824–4374) | <0.0001 | 4038 (3655–4613) | <0.0001 | 8557 (6797–12,004) | <0.0001 | 4144 (3760–4850) | <0.0001 |
| Lipids | ||||||||||
| Fasting NEFA (nmol/L) | 0.32 (0.27–0.38) | 0.01 | 0.32 (0.23–0.36) | 0.17 | 0.34 (0.28–0.39) | <0.0001 | 0.24(0.18–0.31) | <0.0001 | 0.33 (0.26–0.38) | <0.0001 |
| Triglycerides (mmol/L) | 1.75 (1.33–1.85) | 0.54 | 2.04 (1.71–2.66) | 0.18 | 2.20 (1.73–2.78) | 0.004 | 1.82 (1.48–2.24) | 0.001 | 1.97 (1.58–2.61) | 0.02 |
| HDL cholesterol (mmol/L) | 2.20 (1.79–2.30) | 0.29 | 1.92 (1.46–2.33) | >0.99 | 1.64 (1.42–2.03) | 0.01 | 1.90 (1.63–2.18) | 0.01 | 1.89 (1.46–2.22) | 0.28 |
| LDL cholesterol (mmol/L) | 3.26 (2.92–3.52) | 0.29 | 4.39 (3.31–5.43) | 0.07 | 2.99 (2.18–3.81) | 0.06 | 3.41 (2.83–4.03) | 0.01 | 3.24 (2.67–3.82) | 0.19 |
| Total cholesterol (mmol/L) | 6.10 (5.55–6.47) | >0.99 | 7.15 (6.52–7.88) | 0.01 | 5.98 (5.01–6.61) | 0.28 | 6.18 (5.51–6.92) | 0.01 | 6.23 (5.34–6.96) | 0.68 |
| Apolipoprotein A (mmol/L) | 2.21 (2.08–2.36) | – | 2.16 (2.03–2.35) | – | 2.08 (1.84–2.20) | – | 2.11 (1.93–2.30) | 0.22 | 2.17 (1.91–2.24) | 0.74 |
| Apolipoprotein B (mmol/L) | 1.10 (1.08–1.25) | – | 1.52 (1.10–1.60) | – | 1.19 (0.96–1.38) | – | 1.18 (1.03–1.38) | 0.18 | 1.14 (1.08–1.38) | 0.98 |
NGT, normal glucose tolerance; GDM, gestational diabetes mellitus; IQR, interquartile range; GCT, glucose challenge test; DIo, oral disposition index; NEFA, non-esterified fatty acid.
Differences across groups were compared using the Kruskal-Wallis test for continuous variables and the Fisher exact test for categorical variables.
When the P values from the Kruskal-Wallis test or Fisher exact test were <0.05, pairwise comparisons were made between the NGT group and each of the GDM subtypes using Dunn’s test or Fisher exact test. P values were adjusted using the Bonferroni method and are presented in the third, fifth and seventh columns.
Differences between the All GDM group and the NGT group were compared using the rank sum test with P values given in the eleventh column.
Missing data on second-trimester LDL (n = 34) and second-trimester HDL (n = 28); all other variables had data missing for ≤5 participants. There were a total of 67 participants with GDM, however one participant could not be categorized into a subtype leaving a total of 66 available for subtype analyses.
GDM women had higher median triglycerides and NEFA vs. NGT women: 1.97 vs. 1.82 (P = 0.02) and 0.33 vs. 0.24 (P < 0.0001), respectively (Table 1). Regarding GDM subtypes, we noted differences in NEFA, triglycerides, HDL, LDL and TC across groups (Table 1 and Fig. 1). Compared to NGT women, GDM-Sensitivity women had lower HDL (1.64 vs. 1.90, P = 0.01) and higher triglycerides (2.20 vs. 1.82, P = 0.002) and NEFA (0.34 vs. 0.24, P < 0.0001). GDM-Sensitivity women also had lower HDL and higher triglycerides than GDM-Secretion women (Fig. 1). These differences remained statistically significant after maternal BMI and age adjustment. In contrast, we did not find significant differences in most lipid markers in GDM-Secretion compared to NGT women except for NEFA (0.32 vs. 0.24, P = 0.003). NEFA levels in GDM-Mixed women were similar to other subtypes (Fig. 1) and demonstrated higher levels but not statistically different from NGT women. GDM-Mixed women had higher TC compared to NGT, and higher TC and LDL compared to GDM-Sensitivity and GDM-secretion (Fig. 1); these associations were maintained after adjustments for maternal age and BMI.
Fig. 1.
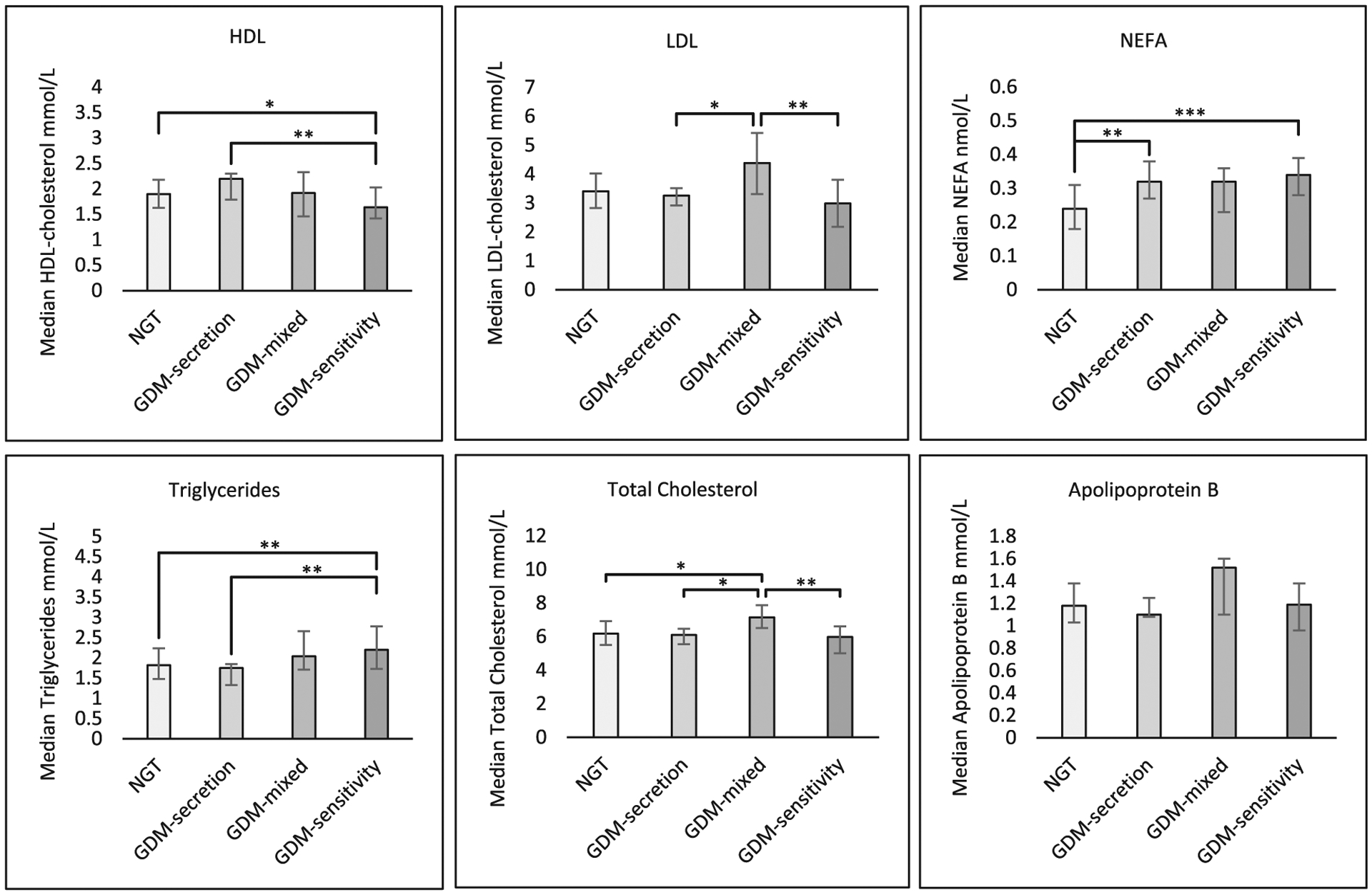
Median and interquartile range for lipids, by physiological subtype. Pairwise comparisons between groups with statistically significant differences shown by brackets. *P-value < 0.05; **P-value < 0.01; ***P-value < 0.0001; GDM: gestational diabetes mellitus; NGT: normal glucose tolerance; HDL: high-density lipoprotein-cholesterol; LDL: low-density lipoprotein cholesterol; NEFA: non-esterified fatty acid.
To further our understanding of the pathophysiologic role of NEFA in pregnancy, we evaluated the relationship between NEFA and the oral disposition index (DIo). After controlling for BMI, we observed a weak negative partial correlation in the NGT group (r = −0.14, P = 0.001). We did not find significant correlations between NEFA and DIo in overall GDM or in any GDM subtype. We did not find significant associations between NEFA and insulin sensitivity and secretion indices in women with NGT or GDM.
Finally, we explored links between lipids and newborn anthropometry: we did not find significant relationships between triglycerides, HDL, LDL, TC and birth weight by glycemic status (adjusted for maternal BMI or gestational weight gain). However, we found that greater maternal NEFA levels were associated with higher birth weight z-scores in GDM-Secretion subtype (adjusted for BMI or gestational weight gain, r = 0.58, P = 0.01).
4. Discussion
We found that the lipid profile differs between GDM and NGT women and demonstrated heterogeneity in lipid profiles between GDM physiologic subtypes. Women with GDM-Sensitivity defect had higher triglycerides and lower HDL when compared with NGT or GDM Secretion women. Conversely, the lipid profiles of women with GDM-Secretion defect did not differ significantly from those of NGT women, apart from NEFA which were elevated in all GDM subtypes. TC and LDL were significantly higher in the GDM-Mixed subtype compared to the GDM-Secretion and GDM-Sensitivity subtypes.
By investigating GDM subtypes, we demonstrated striking differences in lipid profiles that were not apparent when investigating GDM as one group, with most prominent differences for women with GDM-Sensitivity defect. Prior literature suggests that NEFA and triglycerides, after crossing the placenta, are released into fetal circulation and may serve as fetal fuel in addition to glucose [14,15]. We expected both to be higher in GDM-Sensitivity, since we observed higher birth weight in this subtype [7]. However, our finding of elevated NEFA across all GDM physiologic subtypes indicates other factors could be influencing birth weight. Yet we did not observe any significant relationship between birth weight and triglycerides, HDL, LDL and TC, thus other lipids should be investigated.
Study strengths include using validated indices to estimate insulin sensitivity and secretion, and previously characterized GDM physiologic subtypes allowing us to observe distinct lipid profiles. Study limitations include most participants were of European origin, limiting generalizability, and small sample sizes contributing to limited power and lack of statistically significant differences. We did not assess glycemia at the end of pregnancy, but all GDM women were treated at the same multidisciplinary clinic and proportion of insulin treatment did not differ across GDM subtypes [7].
5. Conclusions
Our results demonstrate that GDM physiologic subtypes differ in their gestational lipid profiles, underscoring the presence of physiologic heterogeneity in GDM. Future studies should investigate small lipid fractions and metabolites in GDM physiologic subtypes and their role in fetal overgrowth.
Acknowledgements
The authors are thankful to all Gen3G participants. The authors acknowledge the CHUS Blood sampling in pregnancy clinic, the assistance of clinical nurses and research assistants, and the CHUS biomedical laboratory.
Funding
Gen3G was supported by FRQS #20697 (M.F.H.); CIHR #MOP 115071 (M.F.H.), and Diabète Québec grants (P.P. and L.B.). M.F.H. is supported by American Diabetes Association (1-15-ACE-26); C.E.P. is supported by NIDDK (K23DK113218) and the Robert Wood Johnson Foundation (74256).
Abbreviations:
- GDM
gestational diabetes mellitus
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- TC
total cholesterol
- HDL
high-density lipoprotein-cholesterol
- LDL
low-density lipoprotein-cholesterol
- Apo A and B
apolipoprotein A and B
- NEFA
non-esterified fatty acid
- DIo
oral disposition index
Footnotes
Declarations of Interest
None.
References
- [1].Herrera E, Amusquivar E, López-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res 2006;65(Suppl. 3):S59–64. [DOI] [PubMed] [Google Scholar]
- [2].Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000;71(Suppl. 5):S1256–61. [DOI] [PubMed] [Google Scholar]
- [3].Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 2015;122:643–51. [DOI] [PubMed] [Google Scholar]
- [4].Di Cianni G, Seghieri G, Lencioni C, Cuccuru I, Anichini R, De Bellis A, et al. Normal glucose tolerance and gestational diabetes mellitus: what is in between? Diabetes Care 2007;30(7):1783–8. [DOI] [PubMed] [Google Scholar]
- [5].Couch SC, Philipson EH, Bendel RB, Pujda LM, Milvae RA, Lammi-Keefe CJ. Elevated lipoprotein lipids and gestational hormones in women with diet-treated gestational diabetes mellitus compared to healthy pregnant controls. J Diabetes Complications 1998;12(1):1–9. [DOI] [PubMed] [Google Scholar]
- [6].Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31(9):1858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Powe CE, Allard C, Battista MC, Doyon M, Bouchard L, Ecker JL, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 2016;39(6):1052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guillemette L, Allard C, Lacroix M, Patenaude J, Battista MC, Doyon M, et al. Genetics of Glucose regulation in Gestation and Growth (Gen3G): a prospective prebirth cohort of mother-child pairs in Sherbrooke, Canada. BMJ Open 2016;6 (2):e010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. , for the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22:1462–70. [DOI] [PubMed] [Google Scholar]
- [11].Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001;24:796–7. [DOI] [PubMed] [Google Scholar]
- [12].Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- [13].Dinno A Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J 2015;15:292–300. [Google Scholar]
- [14].Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Invest 2016;26(2):109–27. [DOI] [PubMed] [Google Scholar]
- [15].Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34 (10):2198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]


