Abstract
Background:
The reticular thalamus gates thalamocortical information flow via finely tuned inhibition of thalamocortical cells in the mediodorsal thalamus. Brain imaging studies in humans show that the psychedelic lysergic acid diethylamide (LSD) modulates activity and connectivity within the cortico-striato-thalamo-cortical (CSTC) circuit, altering consciousness. However, the electrophysiological effects of LSD on the neurons in these brain areas remain elusive.
Methods:
We employed in vivo extracellular single-unit recordings in anesthetized adult male mice to investigate the dose–response effects of cumulative LSD doses (5–160 µg/kg, intraperitoneal) upon reticular thalamus GABAergic neurons, thalamocortical relay neurons of the mediodorsal thalamus, and pyramidal neurons of the infralimbic prefrontal cortex.
Results:
LSD decreased spontaneous firing and burst-firing activity in 50% of the recorded reticular thalamus neurons in a dose–response fashion starting at 10 µg/kg. Another population of neurons (50%) increased firing and burst-firing activity starting at 40 µg/kg. This modulation was accompanied by an increase in firing and burst-firing activity of thalamocortical neurons in the mediodorsal thalamus. On the contrary, LSD excited infralimbic prefrontal cortex pyramidal neurons only at the highest dose tested (160 µg/kg). The dopamine D2 receptor (D2) antagonist haloperidol administered after LSD increased burst-firing activity in the reticular thalamus neurons inhibited by LSD, decreased firing and burst-firing activity in the mediodorsal thalamus, and showed a trend towards further increasing the firing activity of neurons of the infralimbic prefrontal cortex.
Conclusion:
LSD modulates firing and burst-firing activity of reticular thalamus neurons and disinhibits mediodorsal thalamus relay neurons at least partially in a D2-mediated fashion. These effects of LSD on thalamocortical gating could explain its consciousness-altering effects in humans.
Keywords: LSD, reticular thalamus, thalamocortical, corticothalamic, psychedelic
Introduction
The cortico-striato-thalamo-cortical (CSTC) circuit represents one of the potential neural circuits of consciousness, by taking part in the generation of arousal, attention, and emotions via gating thalamocortical information flow (Alkire et al., 2008; Crick, 1984; Geyer and Vollenweider, 2008; Ward, 2011). Several psychiatric disorders, including schizophrenia (SCZ), obsessive–compulsive disorder (OCD) and neurodevelopmental disorders such as autism spectrum disorder (ASD), present dysfunctions of one or more nodes of the CSTC circuit (Ferrarelli and Tononi, 2011; Krol et al., 2018; Zikopoulos and Barbas, 2012), and are underlined by thalamocortical dysrhythmia (Schulman et al., 2011). In this work, we focused on the reticular thalamus, a thin sheet of GABAergic neurons which surrounds the thalamus and keeps thalamocortical relay cells under tight inhibitory control, selectively releasing them from inhibition to integrate and process incoming stimuli (McAlonan et al., 2000; Pinault, 2004).
Human imaging studies have shown that psychedelic compounds, including the semi-synthetic ergosterol lysergic acid diethylamide (LSD), profoundly alter CSTC circuit information flow. Specifically, LSD modulates cortico-striato-thalamic connectivity (Muller et al., 2017; Preller et al., 2019; Vlisides et al., 2017), eliciting a state of increased brain entropy (Carhart-Harris et al., 2014). The psychedelic LSD produces an alteration of consciousness, increased sensory processing, pseudo-hallucinations, thinking expansion, and depersonalization (reviewed in De Gregorio et al., 2016a), thus representing an ideal pharmacological tool to understand the neuronal mechanism of altered states of consciousness as well as psychosis.
A paucity of studies has so far investigated the effects of LSD on the CSTC circuit in animal models, an endeavor which could help shed light on the neural mechanisms underlying the profound changes in network activity and connectivity observed in human imaging studies in response to psychedelic compounds (Vollenweider and Preller, 2020), and which might shed light on potential novel treatments for thalamocortical dysrhythmia and altered thalamic gating in psychiatric disorders such as SCZ and ASD (Orekhova et al., 2008; Schulman et al., 2011; Tregellas et al., 2007). In the present study, performing a series of extracellular in vivo electrophysiological experiments, we investigated whether acute cumulative doses of LSD affect the spontaneous neuronal activity of GABAergic neurons of the reticular thalamus (primary outcome measure), thalamocortical relay neurons of the mediodorsal nucleus of the thalamus, and pyramidal neurons of the infralimbic prefrontal cortex (IL-PFC) (secondary outcome measures). In addition, given that dopamine antagonists can be used for treating LSD-induced psychosis, and given that they modulate reticular thalamus burst-firing and spindle activity, normalizing thalamocortical neurotransmission in SCZ patients (Ferrarelli and Tononi, 2011), we assessed whether the dopamine 2 receptor (D2) antagonist haloperidol administered following cumulative doses of LSD reverses the effects of the drug in the brain regions of interest (secondary outcome measure).
Materials and methods
All procedures were approved by the McGill University Ethics Committee (Protocol 5764) and are in line with the Canadian Institute of Health Research for Animal Care and Scientific Use, and the Animal Care Committee of McGill University. All efforts were made to minimize animal suffering.
Animals
Adult male C57/BL6 mice (Charles Rivers, Saint-Constant, Quebec, Canada) (N = 30) specific pathogen-free, aged 60–70 days were housed under standard laboratory conditions with a 12 h light–dark cycle (lights off at 19:00 h) with ad libitum access to food and water, enriched with nesting material. Animals were housed in a maximum number of five per cage. All experiments were performed between 09:00 h and 17:00 h to avoid concerns regarding the light–dark phase switch.
Drugs
LSD (Sigma-Aldrich, London, UK), was dissolved in a 0.9% NaCl vehicle (VEH) solution and injected intraperitoneally (i.p.). Cumulative doses of 5, 10, 20, 40, 80, and 160 µg/kg were administered acutely in dose–response experiments. The doses were chosen based on our previous work in which we found a D2 involvement at high doses (De Gregorio et al., 2016b). The D2 receptor antagonist haloperidol (Sigma-Aldrich, Oakville, ON, Canada) was dissolved in a solution of 0.039% of 2-hydroxypropyl-γ-cyclodextrin in 0.9% NaCl (De Gregorio et al., 2016b).
In vivo electrophysiology
Mice were anesthetized with urethane (1.2 g/kg, 10 mL/kg injection volume, i.p.), and placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Deep anesthesia was confirmed regularly by the absence of whisker movement and absence of response to toe pinch. To maintain an appropriate depth on the anesthesia plane, supplemental doses of urethane (10% of the initial dose, i.p.) were administered as needed. Recordings were performed using single-barreled glass micropipettes pulled from 2-mm Stoelting (Wood Dale, IL, USA) capillary glass on a Narashige (Tokyo, Japan) PE-21 pipette puller. For recordings of reticular thalamus GABAergic neurons, the micropipettes were filled with 2% pontamine sky blue dye dissolved in 2M sodium acetate at final pH of 7.5 (Ochoa-Sanchez et al., 2011). For recordings of thalamocortical relay neurons of the mediodorsal thalamus, and for recordings of pyramidal IL-PFC neurons, the micropipettes were filled with 2% Pontamine sky blue dye dissolved in 2M sodium chloride (De Gregorio et al., 2016b). The micropipette tips were broken down to diameters of 1–3 μm to reach an electrode impedance of 1–3 MΩ. Single-unit activity was recorded as action potentials captured by a software window discriminator, amplified by an AC Differential MDA-3 amplifier (BAK Electronics, Inc., FL, USA), post-amplified and band-pass filtered by a Realistic 10 band frequency equalizer, digitized by a CED 1401 interface system (Cambridge Electronic Design, Cambridge, UK), processed online, and analysed off-line using Spike2 software version 5.20 for Windows. Upon recordings termination, and following anesthetic overdose, Pontamine sky blue dye was iontophoretically injected by passing a constant positive current of 25 μA for 10 min through the recording pipette to mark the recording site. Subsequently, mice were decapitated, and their brains removed and stored at −80°C. Localization of the recording site was performed by cutting 20 μm-thick brain sections using a microtome (Leica CM 3050 S), until the blue dot was recognizable to the naked eye. Anatomical localization of recording electrode placement was confirmed with a microscope (Olympus U-TVO.5 × C-3). Recordings outside the areas of interest (n = 3) were excluded for all subsequent analyses. One neuron per animal was tested. For each neuron/treatment, the burst-firing parameters analyzed were (a) the number of bursts in 100 s, (b) the % of burst-firing, (c) the number of spikes per burst, (d) the mean intraburst frequency, and (e) the burst length. Once a stable neuron was found, baseline firing rate was recorded for at least 5 min, and VEH, followed by LSD at increasing cumulative doses (5–160 µg/kg), was injected i.p. at 5-min intervals. The D2 receptor antagonist haloperidol (150 µg/kg, i.p.) was administered 10 min after the highest dose of LSD, and the activity of the neuron was recorded for additional 10 min.
Recording of reticular thalamus GABAergic neurons
In vivo single-unit extracellular recordings of GABAergic reticular thalamus neurons were performed following our and others’ previous work (Ochoa-Sanchez et al., 2011; Steriade et al., 1991). Briefly, the electrode was advanced slowly into the rostral reticular thalamus, guided by coordinates from the Franklin and Paxinos mouse brain atlas : −0.5 to −0.8 mm anteroposterior (AP) relative to Bregma, 0.8–1.2 mm mediolateral relative to the midline, 2.7–4 mm dorsoventral, relative to the dura mater (Franklin, 2008). These coordinates were chosen to target the rostral part of the reticular thalamus (Lee et al., 2019), which represents the limbic part of the reticular thalamus. This specific region receives projections from the striatum and medial prefrontal cortex (mPFC) (Cornwall et al., 1990; Zikopoulos and Barbas, 2006), projects to the rostral mediodorsal thalamus (Groenewegen, 1988), and in turn receives axon collaterals from the same thalamocortical nuclei that it inhibits (Pinault and Deschênes, 1998).
Reticular thalamus neurons were recognized by a low range frequency (0.5–4 Hz), and a faster firing rate (7–14 Hz) (Ochoa-Sanchez et al., 2011; Steriade et al., 1991), an action potential duration of 2 ms and an amplitude of 0.4 mV (Ochoa-Sanchez et al., 2011). Moreover, reticular thalamus neurons were recognized by their long burst (50 ms) and accelerando-decelerando burst-firing pattern (Domich et al., 1986; Fuentealba et al., 2004). The burst of reticular thalamus cells consisted of a discharge of at least four spikes (Contreras et al., 1993), with an onset defined by a maximum interspike interval 20 ms and a preburst and postburst interval 100 ms (Domich et al., 1986). The longest interval allowed within a burst was 70 ms to include the tonic tail, which is merely the end of a spike barrage (Domich et al., 1986).
Recording of mediodorsal thalamus relay neurons
In vivo single-unit extracellular recordings of thalamocortical relay neurons of the mediodorsal thalamus were performed as previously described (Copeland et al., 2015) with modifications. Briefly, the electrode was advanced slowly into the mediodorsal thalamus, guided by coordinates from the Franklin and Paxinos mouse brain atlas (Franklin, 2008); −1.3 to −1.8 mm AP relative to Bregma, 0.1–0.5 mm mediolateral relative to the midline, 2.7–3.5 mm dorsoventral relative to the dura mater.
This area corresponds to the rostral portion of the mediodorsal thalamus, which receives GABAergic inhibitory input from the rostral reticular thalamus and sends excitatory input to the mPFC (Groenewegen, 1988; Krettek and Price, 1977). Thalamocortical relay cells were identified based on their firing rate of 0.5–4 Hz (Huh and Cho, 2013; Santana et al., 2011; Sittig and Davidowa, 2001), long action potentials lasting 2 ms (Santana et al., 2011), and their firing pattern, mostly characterized by bursting activity during sleep and urethane anesthesia (Huh and Cho, 2013), which switches to tonic firing every few hundred milliseconds to a few seconds (Ramcharan et al., 2000; Wang et al., 2006). Bursts were identified with the following parameters: two spikes with minimum and maximum interspike interval of 4 ms and 100 ms, respectively (Copeland et al., 2015; Huh and Cho, 2013; Ramcharan et al., 2000; Wang et al., 2006).
Recording of IL-PFC pyramidal neurons
In vivo single-unit extracellular recordings of pyramidal neurons of the IL-PFC were performed according to our protocols (Bambico et al., 2010, 2013). Briefly, the electrode was lowered into the infralimbic region of the prefrontal cortex (+1.5–2.0 mm AP relative to Bregma; 2.5–3.5 mm ventral to the dura mater; 0.25 mm from the midline, within layer 5–6 of the cortex). Pyramidal neurons were identified based on their physiological firing rates (0.5–10 Hz in the IL-PFC) (Ashby et al., 1994; Bambico et al., 2010; El Mansari and Blier, 1997). Neurons were also identified based on their large amplitude (0.5–1.2 mV), long duration (0.8–1.2 ms), and positive single-action potential patterns alternating with complex spike discharges (Bambico et al., 2010, 2013; Barthó et al., 2004; Gobbi and Janiri, 2006). Bursts were identified with the following parameters: three consecutive spikes with minimum and maximum interspike interval of 20 ms and 45 ms (Parsegian et al., 2011).
Statistical analysis
Repeated measures one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparison post-hoc test was performed using GraphPad Prism Version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA). For dose–response experiments, only the last 100 s of each dose-bin were considered for analyses concerning cell firing frequency, burst-firing activity, and burst-firing parameters. Two-way ANOVA followed by Bonferroni multiple comparison post-hoc test was used to compare the basal and post-LSD electrophysiological characteristics of the two subpopulations of reticular thalamus neurons observed. For comparisons in which two-way ANOVA was employed, specific mention appears in brackets in the results section. We considered a neuron as increased when the final firing rate was more than +15% compared with baseline and decreased when the final firing rate was less than −15% compared with baseline. This threshold was based on the observation that the neurons recorded did not exhibit firing rate changes greater than 15% in response to VEH, therefore ruling out the possibility that the changes observed would result from the effect of the manipulation due to the injection and/or VEH injection rather than the active drug. However, all statistical analyses were performed comparing the firing frequency changes to the firing frequency observed after the administration of vehicle to account for these differences. The statistical significance threshold was set at p < 0.05. Detailed statistical tests are reported in Supplementary Table S1.
Results
LSD modulates firing and burst-firing activity of reticular thalamus GABAergic neurons
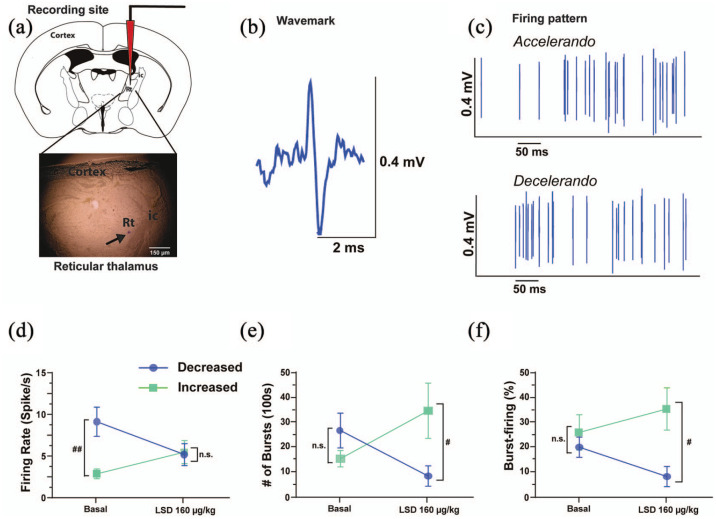
We recorded the acute response of reticular thalamus GABAergic neurons (N = 14; Figure 1(a)–(c)) and found that, according to our criteria, 50% (n = 7) of the neurons were inhibited by LSD whereas the other 50% (n = 7) was excited (two-way ANOVA interaction, F1, 24 = 6.068, p = 0.0213; Figure 1(d)).
Figure 1.
In vivo single unit extracellular electrophysiological recordings of reticular thalamus GABAergic neurons: (a) representative photomicrograph of the recording site in the reticular thalamus. The black arrow indicates the site of the electrode recording labeled with pontamine sky blue dye. (b) The typical wavemark of GABAergic reticular thalamus neurons, and (c) their classical accelerando–decelerando firing pattern. Cumulative doses of LSD (5–160 μg//kg, i.p.) modulate in a dual fashion the spontaneous (d) cell firing frequency, (e) the number of bursts in 100 s, and (f) the percentage of burst-firing in one subpopulation of neurons that is inhibited following LSD administration (50%, n = 7, blue lines) and another subpopulation of neurons that is excited by LSD administration (50%, n = 7, green lines). Two-way analysis of variance followed by Bonferroni post-hoc test for significant interaction.
#p > 0.05; ##p < 0.01 compared with the other subpopulation at the same timepoint.
ic: internal capsule; LSD: lysergic acid diethylamide; ms: milliseconds; mV: millivolts; Rt: reticular thalamus; μg: micrograms
Neurons that were inhibited by LSD had significantly higher basal firing rate than those excited by LSD (9.6 ± 1.8 spikes/s and 3.3 ± 0.6 spikes/s, respectively, p = 0.0056; Figure 1(d)). Contrastingly, no statistically significant differences were observed between these two populations concerning the basal number of bursts observed in 100 s (26.3 ± 7.1 and 14.9 ± 3.3, respectively, two-way ANOVA interaction, F1, 23 = 6.627, p = 0.0170, post-hoc test under basal conditions p = 0.5447; Figure 1(e)) or the % of basal burst-firing (26.1% ± 7.2% and 20.1% ± 4.1%, respectively, two-way ANOVA interaction, F1,23 = 2.759, p = 0.1103; Figure 1(f)). Despite their different spontaneous cell-firing frequency, the firing rates of the two subpopulations observed became almost identical after LSD at the highest doses tested (160 µg/kg) (5.6 ± 1.3 spikes/s and 5.9 ± 1.4 spikes/s, respectively; Figure 1(d)). On the contrary, the number of bursts observed in 100 s (decreasing cells from 20.1 ± 4.1 to 8.1 ± 4.1 bursts in 100 s, increasing cells from 14.9 ± 3.3 to 34.3 ± 11.2 bursts in 100 s) became statistically different at the highest doses tested (post-hoc test p = 0.0406; Figure 1(e)). Similarly, the percentage of burst-firing became statistically different at the highest doses tested (decreasing cells from 20.1% ± 4.1% to 8.1% ± 4.1%, increasing cells from 26.1% ± 7.2% to 35.8% ± 8.7%, post-hoc test p = 0.0145; Figure 1(f)).
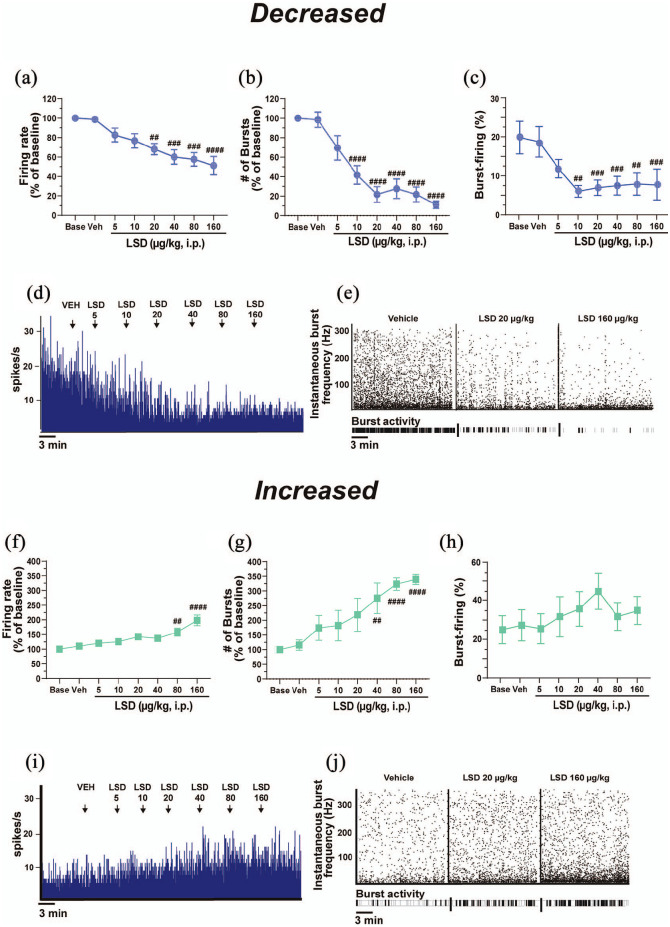
In the decreasing neurons, LSD produced a dose-dependent decrease of cell firing frequency (F7, 42 = 9.677, p < 0.0001; Figure 2(a) and (d)) and the number of bursts in 100 s (F7, 41 = 20.94, p < 0.0001; Figure 2(b) and (e)). In this subpopulation, the decrease in firing frequency was statistically significant starting from the dose of 20 µg/kg (−31.8% ± 5.7% compared with VEH, post-hoc test p = 0.0051, which equates to 120 µg for a 75 kg person, or a “full” dose, which can elicit psychedelic effects including auditory and visual hallucinations) (De Gregorio et al., 2016a, 2018; Nair and Jacob, 2016), up to the dose of 160 µg/kg (−48.2% ± 9.5% compared with VEH, post-hoc test p < 0.0001; Figure 2(a) and (d)). However, LSD did not impact at these doses the coefficient of variation (F7, 36 = 1.645, p = 0.1543), meaning that reticular thalamus GABAergic neurons do not lose their intrinsic discharge features in response to LSD. Concerning the number of bursts in 100 s, the decrease reached statistical significance at the dose of 10 µg/kg (−57.6% ± 9.6% compared with VEH, post-hoc test p < 0.0001), up to the dose of 160 µg/kg (−88.6% ± 3.6% compared with VEH, post-hoc test p < 0.0001; Figure 2(b) and (e)). Cumulative doses of LSD also decreased the percentage of burst-firing in this subpopulation of neurons (F7, 39 = 6.887, p < 0.0001; Figure 2(c)). Post-hoc analyses unveiled that the percentage of spikes in burst was significantly decreased at the dose of 10 µg/kg (−61.7% ± 11.1%, post-hoc test compared with VEH p = 0.0244) and continued to be statistically decreased up to the dose of 160 µg/kg (post-hoc test compared with VEH p < 0.0071; Figure 2(c)). No differences were observed in this population of neurons regarding the number of spikes per burst (F7, 44 = 0.9073, p = 0.5097), the mean intraburst frequency (F7, 1 = 0.7273, p = 0.6505) or the burst length (F7, 40 = 1.125, p = 0.3667).
Figure 2.
LSD modulates reticular thalamus firing and burst-firing activity in a dual dose–response fashion. Two subpopulations of reticular thalamus GABAergic neurons are differentially affected by LSD administration. Cumulative doses of LSD (5–160 μg/kg, i.p.) decrease the spontaneous (a) cell firing frequency, (b) number of bursts in 100 s, and (c) percentage of burst-firing in a subpopulation (50%, n = 7) of reticular thalamus neurons in a dose–response manner. (d) A typical integrated histogram of spontaneous firing rate of a reticular thalamus GABAergic neuron from the subpopulation of neurons decreased following the i.p. injection of cumulative doses of LSD. (e) The instantaneous bursting activity. On the contrary, LSD increases the spontaneous (f) cell firing frequency and (g) the number of bursts in 100 s in another subpopulation (50%, n = 7) of reticular thalamus neurons, as well as (h) the percentage of burst-firing. (i) A typical integrated histogram of the spontaneous firing rate of a reticular thalamus GABAergic neuron from the subpopulation of neurons increased following the i.p. injection of cumulative doses of LSD. (j) The instantaneous burst activity. Repeated measures one-way analysis of variance followed by Bonferroni post-hoc test.
##p < 0.01; ###p < 0.001; ####p < 0.0001 compared with VEH.
i.p.: intraperitoneal; LSD: lysergic acid diethylamide; ms: milliseconds; mV: millivolts; VEH: vehicle; μg: micrograms
In the neurons that were excited by LSD (seven out of 14), cumulative doses of LSD produced a dose-dependent increase of cell firing frequency (F7, 42 = 11.70, p < 0.0001; Figure 2(f) and (i)) and of the number of bursts in 100 s (F7, 35 = 10.86, p < 0.0001; Figure 2(g) and (j)). The increase in firing activity reached statistical significance at the dose of 80 µg/kg (+49.8% ± 14.3% compared with VEH, post-hoc test p = 0.0066; Figure 2(f)), up to the dose of 160 µg/kg (+90.9% ± 17.2%, post-hoc test p < 0.0001; Figure 2(f)). However, the coefficient of variation (F7, 40 = 0.8047, p = 0.5883) was not affected by LSD, suggesting that neurons maintained their intrinsic discharge features. Similarly, the increase in the number of bursts in 100 s was statistically significant starting from the dose of 40 µg/kg (+175.4% ± 51.8%, p = 0.0015; Figure 2(g)), up to the dose of 160 µg/kg (+239.6% ± 17.8% compared with VEH, post-hoc test compared with VEH p < 0.0001, post-hoc test compared with LSD 40 µg/kg, p = 0.0094; Figure 2(g)). In this subpopulation, LSD produced an increase in the percentage of burst-firing (F7, 35 = 2.506, p = 0.0338, post-hoc test compared with VEH p > 0.05; post-hoc test of LSD 40 µg/kg compared with VEH p = 0.0511; Figure 2(h)) and in the number of spikes per burst (+25.2% ± 7.5%, F7, 42 = 4,775, p = 0.0006) only at the dose of 160 µg/kg (post-hoc test compared with VEH p = 0.0266). No differences were observed regarding the mean intraburst frequency (F7, 40 = 0.9994, p = 0.4460) or the burst length (F7, 41 = 1.757, p = 0.1225).
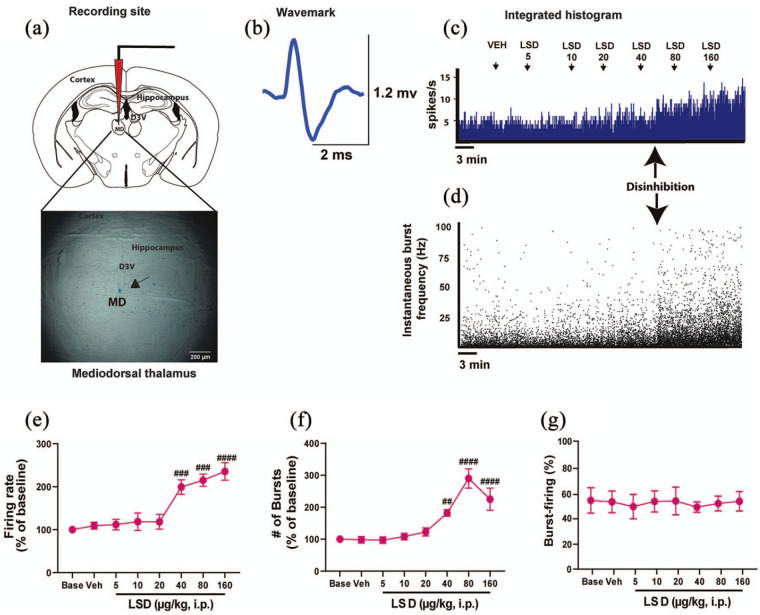
LSD disinhibits thalamocortical relay neurons of the mediodorsal thalamus
As mentioned before, reticular thalamus GABAergic neurons send inhibitory projections to only the mediodorsal, and no other extra-thalamic area, with the main function of keeping relay neurons in the mediodorsal and other thalamic nuclei under inhibitory control, and releasing them from inhibition as needed, thereby gating the thalamocortical flow of information. Therefore, given that we observed a profound modulatory effect of LSD on reticular thalamus GABAergic neurons, we subsequently recorded the acute response of thalamocortical relay neurons of the mediodorsal thalamus to cumulative doses of LSD (n = 5) (Figure 3(a)–(d)). LSD produced an increase of mediodorsal thalamocortical neurons firing frequency (F7, 26 = 14.19, p < 0.0001; Figure 3(c) and (e)) and the number of bursts in 100 s (F7, 25 = 21.06, p < 0.0001; Figure 3(d) and (f)) in all neurons tested. The increased firing rate was statistically significant at the dose of 40 µg/kg (+99.3% ± 17%, post-hoc test compared with VEH p = 0.0007, which equates to 3.3 µg/kg in humans, or 240 µg for a 75 kg person, (Nair and Jacob, 2016), up to the dose of 160 µg/kg (+135.5% ± 20.3%, post-hoc test compared with VEH p < 0.0001; Figure 3(e)). No effect of LSD was observed on the coefficient of variation (F7, 25 = 2.063, p = 0.0863), suggesting that the intrinsic discharge features of these neurons were not altered by the drug. The increase in the number of bursts in 100 s was statistically significant starting from the dose of 40 µg/kg (+81.9% ± 10.5%, post-hoc test compared with VEH p = 0.0049) up to the dose of 160 µg/kg (+124.8% ± 34.7, post-hoc test compared with VEH p < 0.0001; Figure 3(f)). No effects of LSD were observed on the mean intraburst frequency (F7, 25 = 0.6601, p = 0.7030), the percentage of burst-firing (F7, 28 = 0.2128, p = 0.9795) (Figure 3(g)) or the burst length (F7, 28 = 0.6482, p = 0.7114).
Figure 3.
LSD disinhibits thalamocortical relay neurons in the mediodorsal thalamus: (a) a representative photomicrograph of the recording site in the mediodorsal thalamus. The black arrow indicates the site of the electrode recording labeled with pontamine sky blue dye. (b) The typical wavemark of a thalamocortical relay neuron in the mediodorsal thalamus. (c) A typical integrated histogram of spontaneous firing rate of a thalamocortical relay neuron in the mediodorsal thalamus showing disinhibition at the dose of 40 μg//kg. (d) The instantaneous bursting activity, in which the disinhibition is also visible at the dose of 40 μg//kg. Cumulative doses of LSD (5–160 μg//kg, i.p., n = 5) increase the spontaneous (e) cell firing frequency and the (f) the number of bursts in 100 s of thalamocortical neurons of the mediodorsal thalamus. No changes were observed concerning (g) the percentage of burst-firing. Repeated measures one-way analysis of variance followed by Bonferroni post-hoc test. ##p < 0.01; ###p < 0.001; ####p < 0.0001 compared with VEH.
i.p.: intraperitoneal; LSD: lysergic acid diethylamide; MD: mediodorsal thalamic nucleus; ms: milliseconds; mV: millivolts; VEH: vehicle; μg: micrograms
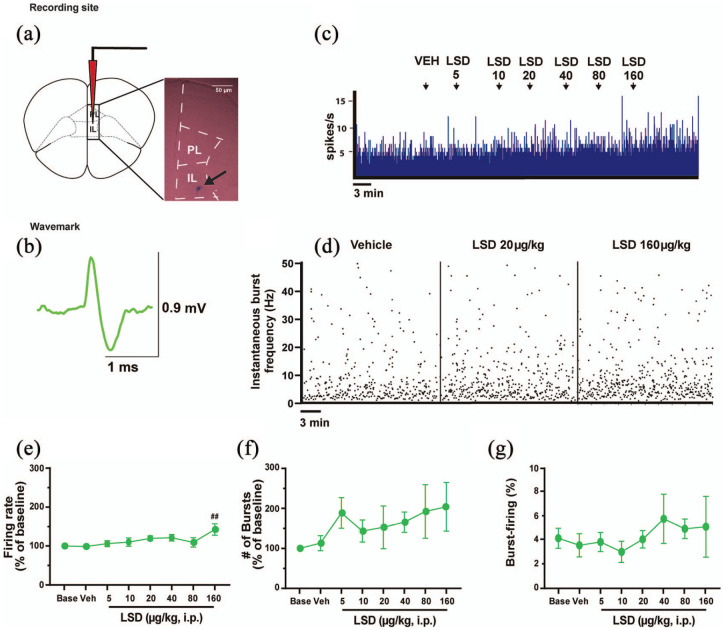
LSD increases the spontaneous firing activity of IL-PFC pyramidal neurons
When reticular thalamus GABAergic neurons discharge in bursts in physiological condition, the mPFC is inhibited (Halassa et al., 2011; Steriade, 2005; Vantomme et al., 2019). Thus, we next investigated the response of IL-PFC pyramidal neurons (n = 6) to cumulative doses of LSD which were shown to affect reticular thalamus and mediodorsal thalamus neurons (Figure 4(a)–(d)). Out of six neurons recorded, five were excited according to our criteria, whilst one was not affected by LSD (<15% variation at the maximum tested dose compared with VEH). In IL-PFC pyramidal neurons, LSD increased cell firing frequency at the highest dose tested of 160 µg/kg (F7, 35 = 2.624, p = 0.0274, post-hoc test compared with VEH p = 0.0088; Figure 4(e)) but did not affect the number of bursts in 100 s (F7, 35 = 1.299, p = 0.2797; Figure 4(f)), the percentage of burst-firing (F7, 32 = 0.4787, p = 0.8428; Figure 4(g)), the number of spikes per burst (F7, 31 = 0.9020, p = 0.51174), or the burst length (F7, 31 = 1.411, p = 0.2364).
Figure 4.
LSD increases the spontaneous firing activity of IL-PFC pyramidal neurons only at the dose of 160 μg/kg. (a) A representative photomicrograph of the recording site in the IL-PFC. The black arrow indicates the site of the electrode recording labeled with pontamine sky blue dye. (b) The typical wavemark of IL-PFC pyramidal neurons. (c) A typical integrated histogram of the spontaneous firing rate of an IL-PFC pyramidal neuron which is excited by LSD at the highest doses tested. (d) The instantaneous burst frequency. Cumulative doses of LSD (5–160 μg//kg, i.p., n = 6) increase (e) cell firing frequency but not (f) the number of bursts in 100 s in IL-PFC pyramidal neurons. Similarly, (g) the percentage of burst-firing is not affected by LSD. Repeated measures one-way analysis of variance followed by Bonferroni post-hoc test.
##p < 0.01 compared with VEH.
IL-PFC: infralimbic prefrontal cortex; PL: prelimbic prefrontal cortex; i.p.: intraperitoneal; LSD: lysergic acid diethylamide; ms: milliseconds; mV: millivolts; VEH: vehicle; μg: micrograms
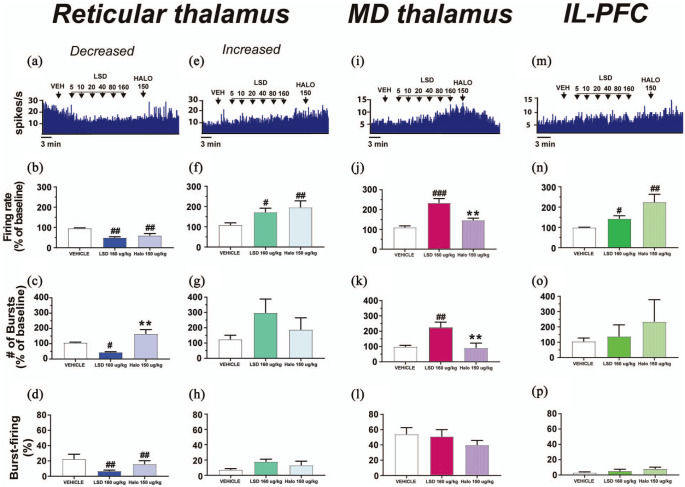
The D2 antagonist haloperidol administered after LSD increases the burst activity of reticular thalamus GABAergic neurons inhibited by LSD and decreases the firing rate of thalamocortical neurons in the mediodorsal thalamus
Aside from its well-documented 5-HT2A receptor affinity, LSD shows affinity for D2 receptors, especially at high doses (De Gregorio et al., 2016b; Marona-Lewicka et al., 2005; Passie et al., 2008; Pieri et al., 1974). Therefore, we decided to assess whether the D2 antagonist haloperidol administered following cumulative doses of LSD could reverse the modulatory effects of the drug on neurons of the brain regions under investigation.
In the reticular thalamus, when the D2 antagonist haloperidol was administered following cumulative doses of LSD, the cell firing frequency of neurons that were inhibited by LSD did not change (F2, 8 = 16.74, p = 0.0014, post-hoc test compared with LSD 160 µg/kg, p = 0.4613; Figure 5(a) and (b)). However, in this subpopulation of reticular thalamus GABAergic neurons, the D2 antagonist haloperidol reversed the decrease elicited by LSD in the number of bursts in 100 s (F2, 6 = 16.15, p = 0.0038, post-hoc test compared with LSD 160 µg/kg p = 0.0026; Figure 5(a) and (c)), suggesting that the D2 antagonism with haloperidol increased the burst-firing activity of these neurons to levels comparable to baseline. No effects of haloperidol after LSD (p = 0.1667) were found concerning the percentage of burst-firing (post-hoc test compared with LSD 160 µg/k p = 0.5316; Figure 5(d)). No effects of LSD and LSD plus haloperidol were observed on the number of spikes per burst (F2, 7 = 0.3008, p = 0.7493) and the burst length (F2, 7 = 0.9402, p = 0.9034).
Figure 5.
The D2 receptor antagonist haloperidol increases burst activity in those reticular thalamus GABAergic neurons inhibited by LSD and rescues the disinhibition of mediodorsal thalamocortical relay neurons. (a) A typical integrated histogram of the spontaneous firing rate of a reticular thalamus GABAergic neuron inhibited by LSD, with subsequent administration of the D2 receptor antagonist haloperidol. Haloperidol administered after cumulative doses of LSD does not restore (b) the cell firing frequency, but it does restore (c) the number of bursts in 100 s in those reticular thalamus GABAergic neurons inhibited by LSD. In this subpopulation of neurons haloperidol does not affect (d) the percentage of burst-firing. (e) A typical integrated histogram of the spontaneous firing rate of a reticular thalamus GABAergic neuron excited by LSD, with subsequent administration of haloperidol. Haloperidol administered after LSD further increases (f) the spontaneous cell firing frequency of the reticular thalamus neurons excited by LSD, but it does not affect (g) the number of bursts in 100 s, or (h) the percentage of burst-firing. (i) A typical integrated histogram of the spontaneous firing rate of thalamocortical relay neurons of the mediodorsal thalamus disinhibited by LSD, with subsequent administration of haloperidol. Haloperidol administered following LSD rescues mediodorsal thalamus thalamocortical neurons disinhibition via decreasing (j) cell firing frequency and (k) the number of bursts in 100 s, without affecting (l) the percentage of burst-firing. (m) A typical integrated histogram of the spontaneous firing rate of an IL-PFC pyramidal neuron excited by LSD, with subsequent administration of haloperidol. Haloperidol administered after repeated cumulative doses of LSD does not change (n) the cell firing frequency, (o) the number of bursts in 100 s, or (p) the percentage of burst-firing in IL-PFC pyramidal neurons. Repeated measures one-way analysis of variance followed by Bonferroni post-hoc test.
#p < 0.05; ##p < 0.01; ###p < 0.001 compared with VEH; **p < 0.01 compared with LSD 160 μg/kg.
Halo: haloperidol; IL-PFC: infralimbic prefrontal cortex; i.p.: intraperitoneal; LSD: lysergic acid diethylamide; MD: mediodorsal thalamus; ms: milliseconds; mV: millivolts; VEH: vehicle; μg: micrograms
When the D2 antagonist haloperidol was administered after LSD no differences were observed concerning the spontaneous firing frequency in those reticular thalamus GABAergic neurons that were excited by the drug (F2, 6 = 10.61, p = 0.0107, post-hoc test compared with LSD 160 µg/kg p = 0.5453; Figure 5(e) and (f)), the number of bursts in 100 s (F2, 6 = 1.443, p = 0.3079, post-hoc test compared with LSD 160 µg/kg p = 0.6555; Figure 5(g)), the % of burst-firing (F2, 6 = 3.384, p = 0.1038; Figure 5(h)), or the burst length (F2, 6 = 2.412, p = 0.1703). In this subpopulation of reticular thalamus GABAergic neurons, administration of the D2 antagonist haloperidol reversed the LSD-induced increase in the number of spikes per burst (F2, 6 = 5.550, p = 0.0432, post-hoc test compared with VEH p = 0.3062, post-hoc test compared with LSD 160 µg/kg p = 0.0316).
In the mediodorsal thalamus, the D2 antagonist haloperidol administered following cumulative doses of LSD rescued the LSD-induced disinhibition of thalamocortical relay cells, restoring to basal levels the cell firing frequency (F2, 10 = 19.82, p = 0.0003, post-hoc test compared with VEH p = 0.1944, post-hoc test compared with LSD 160 µg/kg p = 0.0002; Figure 5(i) and (j)) and the number of bursts in 100 s (F2, 6 = 13.40, p = 0.0061, post-hoc test compared with VEH p > 0.9999, post-hoc test compared with LSD 160 µg/kg p = 0.0088; Figure 5(k)). No differences in the percentage of spikes in burst (F2, 6 = 1.088, p = 0.3951; Figure 5(l)), or the burst length (F2, 6 = 1.854, p = 0.2361) were observed in these neurons following administration of the D2 antagonist haloperidol.
When haloperidol was administered following cumulative doses of LSD, we observed a trend towards a further increase in cell firing frequency of pyramidal IL-PFC neurons compared with LSD 160 µg/kg, which did not reach statistical significance (F2, 10 = 8.304, p = 0.0075, post-hoc test compared with LSD 160 µg/kg p = 0.0512; Figure 5(m) and (n)). In these neurons, we did not observe effects of haloperidol on the number of bursts in 100 s (F2, 14 = 0.5581, p = 0.5845; Figure 4(o)) or the percentage of burst-firing (F2, 10 = 1.937, p = 0.1945; Figure 5(p)). Figure 6 summarizes the effects of LSD and haloperidol injected after LSD in the different neuronal subpopulations here investigated.
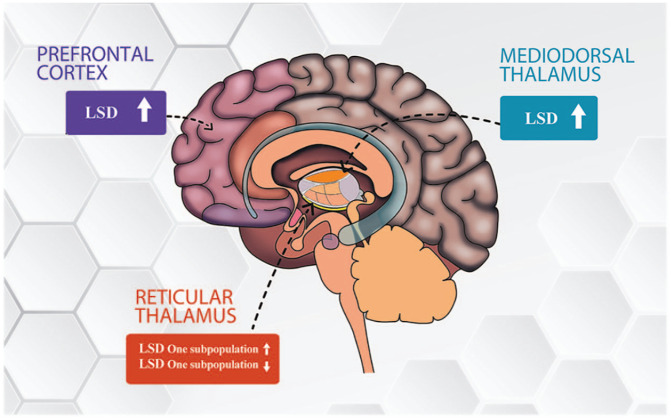
Figure 6.
Effects of LSD in the brain areas investigated in this study. We observed that LSD modulates in a dual fashion GABAergic neurons of the reticular thalamus, inhibiting one subpopulation starting from the lower doses tested, and exciting another starting from the higher doses tested. Moreover, LSD disinhibits thalamocortical relay neurons of the mediodorsal thalamus starting at intermediate doses. Lastly, LSD excites spontaneously firing pyramidal neurons of the infralimbic prefrontal cortex only at the highest dose tested.
LSD: lysergic acid diethylamide
Discussion
In this study, we performed a series of extracellular in vivo single unit electrophysiological recordings of GABAergic neurons of the reticular thalamus, thalamocortical relay neurons of the mediodorsal thalamus, and pyramidal neurons of the infralimbic prefrontal cortex to investigate whether cumulative doses of LSD affect the spontaneous firing and burst-firing activity of neurons in these brain areas and whether the D2 antagonist haloperidol administered after LSD blocks the effects.
Reticular thalamus
We found that LSD has a modulatory effect on GABAergic neurons of the reticular thalamus. These effects were accompanied by a disinhibition of thalamocortical relay neurons of the mediodorsal thalamus and an increase in firing frequency in IL-PFC pyramidal neurons only at high doses. The D2 antagonist haloperidol administered following LSD restored burst-firing activity in those reticular thalamus neurons that were inhibited by LSD and reversed the disinhibition of thalamocortical relay neurons of the mediodorsal thalamus to baseline levels, while further increasing the cell firing frequency of pyramidal neurons of the IL-PFC. Even if bioequivalent pharmacokinetics studies have not been performed to compare LSD in mice and humans, based on the Food and Drug Administration equivalent doses mice–humans (Nair and Jacob, 2016) and use of LSD in humans, low doses of LSD (⩽10 µg/kg) may corresponds to micro-doses in humans (i.e. 10–70 µg; see also Bershad et al. (2019)), while high doses in mice (⩾40 µg/kg) may correspond to psychedelic doses in humans (200–250 µg; see also De Gregorio et al.(2016a)).
It is possible that the two opposite responses observed in the reticular thalamus neurons arose from the observation of heterogeneous populations of GABAergic neurons previously reported to be present in the reticular thalamus (Clemente-Perez et al., 2017; Contreras et al., 1993; Lee et al., 2007; Pinault, 2004). For example, using intracellular recordings combined with electroencephalogram (EEG), two populations of reticular thalamus neurons were correlated to the generation of EEG, delta oscillations (for neurons discharging 0.5–4 Hz) and to spindle activity (for neurons discharging at 7–14 Hz) (Steriade et al., 1991). Other groups using different techniques have also characterized different populations in the reticular thalamus, including a group of neurons responding with inhibition to glutamatergic input (Cox and Sherman, 1999). Interestingly, two subpopulations with distinct intrinsic membrane excitability have been characterized by Clemente-Perez et al. (2017) in the mouse reticular thalamus, which are somatostatin (SOM+) and parvalbumin (PV+) GABAergic neurons, two biomarkers which can also be used to differentiate subpopulations of reticular thalamus GABAergic neurons in humans. While SOM+ neurons are linked to limbic circuitries, and project to higher order relay nuclei, PV+ are rhythmogenic and seem to project mostly to first-order relay nuclei (Clemente-Perez et al., 2017). However, more studies are necessary to better characterize these different populations of reticular thalamus neurons, correlating them with their physiological functions.
The marked effect of LSD on the reticular thalamus could be related to the 5-HT-modulating effects of LSD (De Gregorio et al., 2016b, Inserra et al., 2021) and the strong presence of 5-HT receptors in this region. Indeed, GABAergic neurons of the reticular thalamus express pre- and postsynaptic 5-HT2A and 5-HT1A receptors (Aznar et al., 2003; Goitia et al., 2016; Pompeiano et al., 1992; Rodriguez et al., 2011) and receive serotonergic projections from the DRN, the brain areas in which most of the serotonergic neurons are located (RodrÍGuez et al., 2011). The reticular thalamus receives also norepinephrinergic projections from the locus coeruleus (Asanuma, 1992), which together with the 5-HT/ dorsal raphe nucleus (DRN) input keeps the reticular thalamus in a slightly depolarized state. Such depolarization is necessary for the generation of T-type Ca2+ channel-mediated bursts, which are involved in the generation and propagation of thalamocortical spindles (Fuentealba and Steriade, 2005; Morrison and Foote, 1986). However, if monoaminergic input is removed, reticular thalamus neurons are more hyperpolarized, leading to T-type Ca2+ channel de-inactivation, which blocks burst activity (Bosch-Bouju et al., 2013; Fernandez et al., 2018). Given that LSD decreases 5-HT firing in the DRN (Aghajanian and Vandermaelen, 1982; De Gregorio et al., 2016b; Nair and Jacob, 2016), the LSD-inhibited neurons of the reticular thalamus may be neurons receiving direct monoaminergic input from the DRN and locus coeruleus.
Electrophysiological studies have pointed out that reticular thalamus neurons are affected by a number of consciousness-altering drugs, such as the dissociative anesthetics ketamine (Anderson et al., 2017; Mahdavi et al., 2020) and phencyclidine (PCP) (Troyano-Rodriguez et al., 2014) and the psychostimulants cocaine and caffeine (Goitia et al., 2016). For example, ketamine decreases burst-firing activity in the reticular thalamus, shifting the firing mode from burst to tonic, thus eliciting an arousal-like effect (Mahdavi et al., 2020). Similarly, PCP decreases local field potential in the reticular thalamus (Troyano-Rodriguez et al., 2014). Consistent with the decrease of a subpopulation of reticular thalamus neurons, which should decrease the inhibitory input from the reticular thalamus to specific thalamic nuclei (Pinault, 2004), we observed a disinhibition of thalamocortical relay neurons in the mediodorsal nucleus of the thalamus.
Mediodorsal thalamus
This study is one of the first electrophysiological investigations examining the effects of LSD in the mediodorsal thalamus. Interestingly, the mediodorsal thalamus is low in 5-HT2A (Pompeiano et al., 1994), but rich in D2 in humans and rodents (Boyson et al., 1986; Khan et al., 1998; Rieck et al., 2004; Young and Wilcox, 1991). Consequently the disinhibition observed only at high doses of LSD could be D2-mediated given that in our laboratory we previously demonstrated that LSD at doses >40 µg/kg activates dopaminergic (DA) neurons of the ventral tegmental area (VTA) through a D2 receptor mechanism (De Gregorio et al., 2016b). Similarly, PCP increases mediodorsal thalamus neuronal firing, while eliciting c-fos expression in neurons which express the vesicular glutamate transporter 1 in both mediodorsal thalamus and the cortex (Santana et al., 2011). Moreover, in line with the current findings, previous studies reported that 2,5-Dimethoxy-4-iodoamphetamine (DOI) disinhibits thalamocortical neurons (Puig et al., 2003). The disinhibition of thalamocortical relay neurons in the mediodorsal thalamus by LSD, which was reversed by the D2 antagonist haloperidol, might be at least partially responsible for the increased functional thalamocortical connectivity observed in human brain imaging studies (Preller et al., 2019).
Infralimbic prefrontal cortex
The IL-PFC is rich in postsynaptic 5-HT2A receptors, which are thought to play a major role in the effects of psychedelics (López-Giménez and González-Maeso, 2018). In line with the excitation of IL-PFC neurons observed in this study, LSD and DOI were previously reported to elevate prefrontal-limbic glutamate release via stimulating postsynaptic 5-HT2A on pyramidal cells in the PFC (Aghajanian and Marek, 1999; Muschamp et al., 2004). Accordingly, a previous study reported that DOI activates 5-HT2A thalamocortical heteroreceptors, leading to glutamate release in superficial layer V to deep III of the mouse PFC, and an AMPA-dependent expression of c-fos (Scruggs et al., 2000). Accordingly, LSD potentiates AMPA and 5-HT2A- mediated neurotransmission in the mPFC via an increased phosphorylation of the mammlian target of rapamycin 1 (mTORC1) (De Gregorio et al., 2021). On the other hand, PCP has a double effect in mPFC neuronal activity and delta-range cortical synchrony, and this effect is reversed by the D2 antagonist haloperidol and the mixed dopamine-serotonin receptor antagonist clozapine (Kargieman et al., 2007).
In human brain imaging studies, LSD and psilocybin administration seems to elicit a dual effect: on the one hand, an integration of sensory and somato-motor brain networks, on the other hand a disintegration of associative brain regions, including the default mode network (DMN), which is mainly represented by the mPFC and posterior cingulate cortex (PCC), the latter being a brain area recently reported to be involved in dissociation (Carhart-Harris et al., 2016; Kaelen et al., 2016; Lebedev et al., 2015; Preller et al., 2018, 2020; Vesuna et al., 2020). The net outcome seems to be an increased capacity of processing sensory information, possibly due to decreased reticular thalamus gating, and a decreased integration capacity due to disruption of associative networks, such as the DMN. Accordingly, in the present study, while we observed a modulatory action of LSD in the reticular thalamus and mediodorsal thalamus at doses ranging from 10 µg/kg to 40 µg/kg, we observed an excitation of IL-PFC pyramidal neurons only at the highest dose tested of 160 µg/kg. This strengthens the notion of a de-coupling between the effects of psychedelics on the CSTC circuit and the DMN.
This study should be considered in light of some limitations. First, it was performed in anesthetized mice; therefore our findings might not completely translate to the waking state; although urethane anesthesia partially depresses thalamocortical excitability (Huh and Cho, 2013), it was previously reported that the electrophysiological signature of hypnotic drugs on sleep and reticular thalamus neurons are still appreciable under urethane anesthesia (Ochoa-Sanchez et al., 2011; Pagliardini et al., 2013). Second, due to the limited sample size we were not able to perform predictive analyses such as K-cluster analysis, to predict the response of the two subpopulations of reticular thalamus neurons based on their spontaneous electrophysiological characteristics. Third, we did not assess the effects of the D2 antagonist haloperidol per se on the neuronal populations observed and did not assess the effects of 5-HT2A receptor antagonists administered post-LSD. Fourth, we did not perform antero- or retrograde tracing studies to identify the topographical connection of the neurons recorded.
Despite these limitations, this study represents the first attempt to understand the modulation of LSD on thalamocortical gating and expands our understanding of the neurobiological mechanism of action of LSD. The present findings suggest that LSD might disrupt the physiological functioning of the reticular thalamus, thereby allowing unrestrained information flow within the CSTC circuit. Thus, LSD represents a pharmacological tool to better understand the neural mechanisms of consciousness in humans and to further explore the role of the reticular thalamus in physiological and pathological thalamic gating. In particular, the knowledge generated could be exploited for the understanding and treatment of psychiatric disorders characterized by altered CSTC circuit function and thalamic gating and connectivity, including OCD and SCZ, and neurodevelopmental disorders such as ASD.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_0269881121991569 for Lysergic acid diethylamide differentially modulates the reticular thalamus, mediodorsal thalamus, and infralimbic prefrontal cortex: An in vivo electrophysiology study in male mice by Antonio Inserra, Danilo De Gregorio, Tamim Rezai, Martha Graciela Lopez-Canul, Stefano Comai and Gabriella Gobbi in Journal of Psychopharmacology
Acknowledgments
We thank Dr. Franco Recchia and Photo Grafic (www.photo-grafic.it) for help with the preparation of the figures.
Footnotes
Author contribution: GG, SC, and AI designed the experiments. AI performed the experiments, wrote and edited the manuscript. TR, MGLC helped performing some of the experiments. AI, DDG, and SC analysed the results of the experiments. AI, DDG, GG, and SC wrote and edited the manuscript. All authors approved the final version of this manuscript.
Availability of data and material: Data is available upon request to the authors.
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.G. is a consultant for Diamond Therapeutics Inc. The other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: All experimental procedures were approved by the McGill University Ethics Committee (Protocol # 5764) and are in line with the Canadian Institute of Health Research for Animal Care and Scientific Use, and the Animal Care Committee of McGill University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Quebec Network for Suicide, Mood Disorders, and Related Disorders. (RQSHA grant #268065) and by the Canadian Institute of Health Research (CIHR grant #PJT-173556). DDG received a fellowship from the Canadian Institutes of Health Research (CIHR) and by the Fonds pour la recherche en Santé du Québec (FRQS). MLC received a fellowship from the Ferring family to the Faculty of Medicine of McGill University. G.G. and D.D.G. are consultant at Diamond Therapeutics Inc, Toronto, ON, Canada. G.G. and D.D.G. are inventors of a provisional patent regarding the use of LSD.
ORCID iDs: Antonio Inserra  https://orcid.org/0000-0002-7261-5659
https://orcid.org/0000-0002-7261-5659
Gabriella Gobbi  https://orcid.org/0000-0003-4124-9825
https://orcid.org/0000-0003-4124-9825
Supplemental material: Supplemental material for this article is available online.
References
- Aghajanian GK, Marek GJ. (1999) Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Research 825: 161–171. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Vandermaelen CP. (1982) Intracellular recordings from serotonergic dorsal raphe neurons: Pacemaker potentials and the effect of LSD. Brain Research 238: 463–469. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. (2008) Consciousness and anesthesia. Science 322: 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PM, Jones NC, O’Brien TJ, et al. (2017) The N-methyl D-aspartate glutamate receptor antagonist ketamine disrupts the functional state of the corticothalamic pathway. Cerebral Cortex 27: 3172–3185. [DOI] [PubMed] [Google Scholar]
- Asanuma C. (1992) Noradrenergic innervation of the thalamic reticular nucleus: A light and electron microscopic immunohistochemical study in rats. Journal of Comparative Neurology 319: 299–311. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Wang RY. (1994) Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: An lontophoretic study. Synapse 17: 173–181. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, et al. (2003) The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Research 959: 58–67. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Cassano T, Dominguez-Lopez S, et al. (2010) Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 35: 2083–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Lacoste B, Hattan PR, et al. (2013) Father absence in the monogamous California mouse impairs social behavior and modifies dopamine and glutamate synapses in the medial prefrontal cortex. Cerebral Cortex 25: 1163–1175. [DOI] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, et al. (2004) Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. Journal of Neurophysiology 92: 600–608. [DOI] [PubMed] [Google Scholar]
- Bershad AK, Schepers ST, Bremmer MP, et al. (2019) Acute subjective and behavioral effects of microdoses of lysergic acid diethylamide in healthy human volunteers. Biological Psychiatry 86: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bouju C, Hyland BI, Parr-Brownlie LC. (2013) Motor thalamus integration of cortical, cerebellar and basal ganglia information: Implications for normal and parkinsonian conditions. Frontiers in Computational Neuroscience 7: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. (1986) Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. Journal of Neuroscience 6: 3177–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, et al. (2014) The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Frontiers in Human Neuroscience 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. (2016) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proceedings of the National Academy of Sciences of the United States of America 113: 4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Perez A, Makinson SR, Higashikubo B, et al. (2017) Distinct thalamic reticular cell types differentially modulate normal and pathological cortical rhythms. Cell Reports 19: 2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Curró Dossi R, Steriade M. (1993) Electrophysiological properties of cat reticular thalamic neurones in vivo. The Journal of Physiology 470: 273–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Neale SA, Salt TE. (2015) Neuronal activity patterns in the mediodorsal thalamus and related cognitive circuits are modulated by metabotropic glutamate receptors. Neuropharmacology 92: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. (1990) Projections to the rostral reticular thalamic nucleus in the rat. Experimental Brain Research 80: 157–171. [DOI] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. (1999) Glutamate inhibits thalamic reticular neurons. The Journal of Neuroscience 19: 6694–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. (1984) Function of the thalamic reticular complex: The searchlight hypothesis. Proceedings of the National Academy of Sciences of the United States of America 81: 4586–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio D, Comai S, Posa L, et al. (2016. a) d-Lysergic acid diethylamide (LSD) as a model of psychosis: Mechanism of action and pharmacology. International Journal of Molecular Sciences 17: 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio D, Enns JP, Nunez NA, et al. (2018) d-Lysergic acid diethylamide, psilocybin, and other classic hallucinogens: Mechanism of action and potential therapeutic applications in mood disorders. Progress in Brain Research 242: 69–96. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Posa L, Ochoa-Sanchez R, et al. (2016. b) The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Pharmacological Research 113: 81–91. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Popic J, Enns JP, et al. (2021) Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proceeding of the National Academy of Sciences of the United States of America 118: e2020705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domich L, Oakson G, Steriade M. (1986) Thalamic burst patterns in the naturally sleeping cat: A comparison between cortically projecting and reticularis neurones. The Journal of Physiology 379: 429–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansari M, Blier P. (1997) In vivo electrophysiological characterization of 5-HT receptors in the guinea pig head of caudate nucleus and orbitofrontal cortex. Neuropharmacology 36: 577–588. [DOI] [PubMed] [Google Scholar]
- Fernandez LM, Vantomme G, Osorio-Forero A, et al. (2018) Thalamic reticular control of local sleep in mouse sensory cortex. Elife 7: e39111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G. (2011) The thalamic reticular nucleus and schizophrenia. Schizophrenia Bulletin 37: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin P. (2008) The Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams, 3rd ed. Academic Press. [Google Scholar]
- Fuentealba P, Steriade M. (2005) The reticular nucleus revisited: Intrinsic and network properties of a thalamic pacemaker. Progress in Neurobiology 75: 125–141. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Timofeev I, Steriade M. (2004) Prolonged hyperpolarizing potentials precede spindle oscillations in the thalamic reticular nucleus. Proceedings of the National Academy of Sciences of the United States of America 101: 9816–9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. (2008) Serotonin research: Contributions to understanding psychoses. Trends in Pharmacological Sciences 29: 445–453. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Janiri L. (2006) Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacology (Berlin) 185: 255–262. [DOI] [PubMed] [Google Scholar]
- Goitia B, Rivero-Echeto MC, Weisstaub NV, et al. (2016) Modulation of GABA release from the thalamic reticular nucleus by cocaine and caffeine: Role of serotonin receptors. Journal of Neurochemistry 136: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ. (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24: 379–431. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, et al. (2011) Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nature Neuroscience 14: 1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh Y, Cho J. (2013) Urethane anesthesia depresses activities of thalamocortical neurons and alters its response to nociception in terms of dual firing modes. Frontiers in Behavioral Neuroscience 7: 141–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inserra A, De Gregorio G, Gobbi G. (2021) Psychedelics in psychiatry: Neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacological Reviews 73: 202–277. [DOI] [PubMed] [Google Scholar]
- Kaelen M, Roseman L, Kahan J, et al. (2016) LSD modulates music-induced imagery via changes in parahippocampal connectivity. European Neuropsychopharmacology 26: 1099–1109. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, et al. (2007) Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proceedings of the National Academy of Sciences of the United States of America 104: 14843–14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutiérrez A, Martín R, et al. (1998) Differential regional and cellular distribution of dopamine D2-like receptors: An immunocytochemical study of subtype-specific antibodies in rat and human brain. Journal of Comparative Neurology 402: 353–371. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. Journal of Comparative Neurology 171: 157–191. [DOI] [PubMed] [Google Scholar]
- Krol A, Wimmer RD, Halassa MM, et al. (2018) Thalamic reticular dysfunction as a circuit endophenotype in neurodevelopmental disorders. Neuron 98: 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Lovden M, Rosenthal G, et al. (2015) Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Human Brain Mapping 36: 3137–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Govindaiah G, Cox CL. (2007) Heterogeneity of firing properties among rat thalamic reticular nucleus neurons. The Journal of Physiology 582: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Latchoumane CV, Park J, et al. (2019) The rostroventral part of the thalamic reticular nucleus modulates fear extinction. Nature Communications 10: 4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Giménez JF, González-Maeso J. (2018) Hallucinogens and serotonin 5-HT(2A) receptor-mediated signaling pathways. Current Topics in Behavioral Neurosciences 36: 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ, Bowman EM. (2000) Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. Journal of Neuroscience 20: 8897–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi A, Qin Y, Aubry AS, et al. (2020) A single psychotomimetic dose of ketamine decreases thalamocortical spindles and delta oscillations in the sedated rat. Schizophrenia Research 222: 362–374. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE. (2005) Distinct temporal phases in the behavioral pharmacology of LSD: Dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berlin) 180: 427–435. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL. (1986) Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. Journal of Comparative Neurology 243: 117–138. [DOI] [PubMed] [Google Scholar]
- Müller F, Lenz C, Dolder P, et al. (2017) Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatrica Scandinavica 136: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, et al. (2004) Lysergic acid diethylamide and [−]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Research 1023: 134–140. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S. (2016) A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Sanchez R, Comai S, Lacoste B, et al. (2011) Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. Journal of Neuroscience 31: 18439–18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, et al. (2008) Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters 434: 218–223. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Gosgnach S, Dickson CT. (2013) Spontaneous sleep-like brain state alternations and breathing characteristics in urethane anesthetized mice. PLoS One 8: e70411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Jr, Lavin A, et al. (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biological Psychiatry 69: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passie T, Halpern JH, Stichtenoth DO, et al. (2008) The pharmacology of lysergic acid diethylamide: A review. CNS Neuroscience & Therapeutics 14: 295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri L, Pieri M, Haefely W. (1974) LSD as an agonist of dopamine receptors in the striatum. Nature 252: 586–588. [DOI] [PubMed] [Google Scholar]
- Pinault D. (2004) The thalamic reticular nucleus: Structure, function and concept. Brain Research Reviews 46: 1–31. [DOI] [PubMed] [Google Scholar]
- Pinault D, Deschênes M. (1998) Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: A three-dimensional, graphic, and morphometric analysis. Journal of Comparative Neurology 391: 180–203. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios J, Mengod G. (1992) Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: Correlation with receptor binding. The Journal of Neuroscience 12: 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Molecular Brain Research 23: 163–178. [DOI] [PubMed] [Google Scholar]
- Preller KH, Burt JB, Ji JL, et al. (2018) Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife 7: e35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Duerler P, Burt JB, et al. (2020) Psilocybin induces time-dependent changes in global functional connectivity. Biological Psychiatry 88: 197–207. [DOI] [PubMed] [Google Scholar]
- Preller KH, Razi A, Zeidman P, et al. (2019) Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proceedings of the National Academy of Sciences of the United States of America 116: 2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Celada P, Díaz-Mataix L, et al. (2003) In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: Relationship to thalamocortical afferents. Cerebral Cortex 13: 870–882. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. (2000) Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Visual Neuroscience 17: 55–62. [DOI] [PubMed] [Google Scholar]
- Rieck RW, Ansari M, Whetsell WO, et al. (2004) Distribution of dopamine D2-like receptors in the human thalamus: Autoradiographic and PET studies. Neuropsychopharmacology 29: 362–372. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Noristani HN, Hoover WB, et al. (2011) Serotonergic projections and serotonin receptor expression in the reticular nucleus of the thalamus in the rat. Synapse 65: 919–928. [DOI] [PubMed] [Google Scholar]
- Santana N, Troyano-Rodriguez E, Mengod G, et al. (2011) Activation of thalamocortical networks by the N-methyl-D-aspartate receptor antagonist phencyclidine: Reversal by clozapine. Biological Psychiatry 69: 918–927. [DOI] [PubMed] [Google Scholar]
- Schulman J, Cancro R, Lowe S, et al. (2011) Imaging of thalamocortical dysrhythmia in neuropsychiatry. Frontiers in Human Neuroscience 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, et al. (2000) DOI-induced activation of the cortex: Dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. The Journal of Neuroscience 20: 8846–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig N, Davidowa H. (2001) Histamine reduces firing and bursting of anterior and intralaminar thalamic neurons and activates striatal cells in anesthetized rats. Behavioural Brain Research 124: 137–143. [DOI] [PubMed] [Google Scholar]
- Steriade M. (2005) Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends in Neurosciences 28: 317–324. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi R, Nunez A. (1991) Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: Cortically induced synchronization and brainstem cholinergic suppression. The Journal of Neuroscience 11: 3200–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, et al. (2007) Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophrenia Research 92: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyano-Rodriguez E, Llado-Pelfort L, Santana N, et al. (2014) Phencyclidine inhibits the activity of thalamic reticular gamma-aminobutyric acidergic neurons in rat brain. Biological Psychiatry 76: 937–945. [DOI] [PubMed] [Google Scholar]
- Vantomme G, Osorio-Forero A, Luthi A, et al. (2019) Regulation of local sleep by the thalamic reticular nucleus. Frontiers in Neuroscience 13: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna S, Kauvar IV, Richman E, et al. (2020) Deep posteromedial cortical rhythm in dissociation. Nature 586: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Preller KH. (2020) Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nature Reviews Neuroscience 21: 611–624. [DOI] [PubMed] [Google Scholar]
- Vlisides PE, Bel-Bahar T, Lee U, et al. (2017) Neurophysiologic Correlates of Ketamine Sedation and Anesthesia: A High-density Electroencephalography Study in Healthy Volunteers. Anesthesiology 127: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jones HE, Andolina IM, et al. (2006) Functional alignment of feedback effects from visual cortex to thalamus. Nature Neuroscience 9: 1330–1336. [DOI] [PubMed] [Google Scholar]
- Ward LM. (2011) The thalamic dynamic core theory of conscious experience. Consciousness and Cognition 20: 464–486. [DOI] [PubMed] [Google Scholar]
- Young KA, Wilcox RE. (1991) Characterization of D2 receptors and dopamine levels in the thalamus of the rat. Life Sciences 48: 1845–1852. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. (2006) Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. The Journal of Neuroscience 26: 7348–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. (2012) Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. Journal of Neuroscience 32: 5338–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_0269881121991569 for Lysergic acid diethylamide differentially modulates the reticular thalamus, mediodorsal thalamus, and infralimbic prefrontal cortex: An in vivo electrophysiology study in male mice by Antonio Inserra, Danilo De Gregorio, Tamim Rezai, Martha Graciela Lopez-Canul, Stefano Comai and Gabriella Gobbi in Journal of Psychopharmacology