Abstract
Background
Neural tube defects are common congenital anomalies that result from early malformation in the development of the spinal cord and brain. It is related to substantial mortality, morbidity, disability, and psychological and economic costs. The aim of this review is to determine the pooled birth prevalence of neural tube defects and associated risk factors in Africa.
Methods
The first outcome of this review was the pooled birth prevalence of the neural tube defects and the second outcome was the pooled measure of association between neural tube defects and associated risk factors in Africa. We systematically searched PubMed, PubMed Central, Joanna Briggs Institute, Google Scopus, Cochrane Library, African Journals Online, Web of Science, Science Direct, Google Scholar, and Medline databases. The heterogeneity of studies was assessed using the Cochrane Q test statistic, I2 test statistic, and, visually, using Forest and Galbraith’s plots. A random-effect model was applied to get the pooled birth prevalence of neural tube defects. Subgroup, sensitivity, meta-regression, time-trend, and meta-cumulative analyses were undertaken. The fixed-effect model was used to analyze the association between neural tube defects and associated risk factors.
Results
Forty-three studies with a total of 6086,384 participants were included in this systematic review and meta-analysis. The pooled birth prevalence of the neural tube defects was 21.42 (95% CI (Confidence Interval): 19.29, 23.56) per 10,000 births. A high pooled birth prevalence of neural tube defects was detected in Algeria 75 (95% CI: 64.98, 85.02), Ethiopia 61.43 (95% CI: 46.70, 76.16), Eritrea 39 (95% CI: 32.88, 45.12), and Nigeria 32.77 (95% CI: 21.94, 43.59) per 10,000 births. The prevalence of neural tube defects has increased over time. Taking folic acid during early pregnancy, consanguineous marriage, male sex, and substance abuse during pregnancy were assessed and none of them was significant.
Conclusions
The pooled birth prevalence of neural tube defects in Africa was found to be high. The risk factors evaluated were not found significant.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-021-02653-9.
Keywords: Africa, Neural tube defects, Systematic review and meta-analysis
Background
Neural tube defects are common congenital anomalies that result from early malformation in the development of the brain and spinal cord [1–8]. It is the main cause of fetal loss and disabilities in neonates and it is considered a significant public health problem [3, 9–17]. The defects occur around 28th day after conception due to the failure of neurulation or alterations in the morphogenesis or histogenesis of the nervous tissue [1–3, 8].
Because of its complicated embryologic history, abnormal development of the spinal cord and brain is common [1]. Anencephaly, encephalocele, and spina bifida are the main types of neural tube defects [18–23]. The defects are correlated with substantial mortality, morbidity, disability, and psychological and economic costs [24]. Patients with these defects mostly have problems related to neurogenic bladder, orthopedic complications, kidney involvement, and hydrocephalus [25]. Patients with neural tube defects face lifelong physical problems that need lifetime medical care that add a significant burden to the affected patients and their families [25–27]. The challenges of parents begin with high distress at the time of diagnosis with defects during pregnancy and face either the grief of a termination/stillbirth or financial and emotional challenges of caring for a child with defects [25]. The lifetime direct medical costs and indirect costs for affected patients, parents, families, and at the national level are found very significant [25]. Prevention ensures that this multi-factorial burden does not have to happen at all [25, 28], and more literature is needed to fill the gaps. Worldwide, neural tube defects are among the top five most serious birth defects [13]. There are more than 400,000 births born affected by neural tube defects each year, causing around 88,000 deaths [9, 13, 29]. More than 10 % of newborns’ mortality happened due to the malformation of the spinal cord and brain [9]. In Africa, the most common birth defects are neural tube defects. It affects approximately 1–3/1000 births annually [18, 30–32]. In addition to its burden, stigmatization towards neural tube defects by the community has been documented elsewhere in Africa [13, 33, 34], affecting the quality of life of caring families with social, economic, and emotional distress.
The factors causing neural tube defects are genetic, nutritional, environmental, or a combination of these [1, 12, 13, 18, 35]. Epidemiologic studies have revealed that folic acid supplements and/or a vitamin-B taken before conception and continued for at least 3 months during pregnancy reduce the occurrence of neural tube defects [1, 23, 29, 36, 37]. Folic acid/folate intake can be increased either through consumption of a folic acid-containing supplement or consumption of staple foods fortified with folic acid in addition to a diet high in natural food of folate [13, 21]. Importantly, the folic acid fortification was revealed to significantly decrease the prevalence of the defects in countries around the globe [1, 37]. The prevalence of folic acid supplementation in Africa varies widely and showed folate deficiencies. Nevertheless, it is still difficult to conclude on the extent of folate deficiencies in Africa due to the limited amount of data available [38]. In addition, neural tube defects are influenced by certain drugs (e.g., valproic acid, if given during 4th-week development as the neural folds are fusing), prenatal factors (e.g., maternal infection or thyroid disorder, Rh factor incompatibility, and some hereditary conditions), presence of chronic disease during pregnancy, and substance use during pregnancy [1, 2, 12, 14, 18, 25, 39].
The aim of this systematic review and meta-analysis is to determine the pooled birth prevalence of neural tube defects and to identify the pooled measure of association between the neural tube defects and associated risk factors in Africa.
Methods
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) statements were adapted to report the present review of meta-analysis [40] (Supplementary file 1). The international prospective register of a systematic review (PROSPERO) registered (CRD registration number is CRD42020169443) this review (https://www.crd.york.ac.uk/).
Review outcomes
The first outcome of this review was the pooled birth prevalence of neural tube defects. The second outcome was the pooled measure of association between neural tube defects and associated risk factors in Africa. Birth prevalence of neural tube defects is defined as the number of neural tube defect cases of live births and/or stillbirths at birth from the total number of births (live births and/or stillbirths) during the study period.
Study eligibility criteria
The inclusion criteria for this review were published and unpublished studies in any period (the study period was not restricted for inclusion), and study designs that report the birth prevalence (live births and/or stillbirths) and/or associated risk factors of neural tube defects in Africa. Case reports, anonymous reports, editorials, and conferences were excluded. The study was excluded if the total number of cases as well as the total number of births were under-reported for the prevalence objective.
Searching strategies and information sources
PubMed, PubMed Central, Google Scopus, Medline, Cochrane Library, JBI Library, Web of Science, Science Direct, Popline, CINAHL, African Journals Online, UCSF, WHO, and Embase databases were systematically searched up to April 18, 2020, for relevant studies. Grey literature and other sources were retrieved using Google and advanced Google Scholar searches. Reference lists, bibliographies, of identified studies were navigated for additional studies. The corresponding authors were contacted for missing important data. The primary search was performed in an advanced PubMed database, using Medical Subject Heading [MeSH] terms, (Supplementary file 2). Besides, the search in other databases was performed using the mentioned core search terms interchangeably (neural tube defects, newborns/live births/stillbirths, and Africa).
Study selection
After retrieving all studies from the databases, we exported citations to the bibliographic software, Endnote Version 7 Software, to remove the duplicate studies. Then, the reviewers screened studies based on the abstract and title for possible inclusion. Two reviewers (MO and AT) independently considered the criteria (pre-determined selection criteria) to select studies. The first two authors, independent of each other, selected all articles. Studies were deeply reviewed entirely in order to identify the final included article.
Methodological quality
We used the Joanna Briggs Institute (JBI) quality appraisal scale to assess the risk of bias in each study [41]. Essentially, two reviewers (MO and MS) independently assessed the quality of each study. Disagreements raised between reviewers were solved based on discussions or by taking the average score of the two reviewers. The JBI quality appraisal scales were adapted for the cohort studies, cross-sectional studies, case-control studies, and for the studies reporting the prevalence data (Supplementary file 3). The study was considered low risk if the study scored five and above points in all quality assessment items.
Data extraction
After including the eligible studies, three reviewers (AT, MS, and MO) extracted all essential data independently using a standardized, pre-specified, data abstraction format. The pre-specified format minimized the reviewers’ conflict of interest in the data extraction process but for any discrepancy of interests raised, the discussion was used to solve raised issues. If necessary, the main author of the study was communicated.
The data extraction format included first author, study country, publication year, sample size, study duration, study design, prevalence period, study setting, birth outcome, the birth prevalence of neural tube defects, and associated risk factors (adjusted odds ratio with a confidence interval of the variables were taken based on available literature). Prevalence reports of all studies in the different denominators have been converted into per 10, 000 births to maintain uniformity. Then, we have used per 10, 000 prevalence estimates for reporting the findings of this review. The assessed factors were folic acid supplementation during early pregnancy, consanguineous marriage, male newborn, and substance abuse during pregnancy.
Meta-analyses
The data analyses were conducted using STATA Version 14 Statistical Software. The data were extracted in Microsoft Excel and it was exported into STATA 14 Software for further analyses. For all studies, the median value, interquartile range, and the minimum and maximum values of neural tube defects were calculated.
The heterogeneity between the studies was assessed using visual and statistical techniques. Visually, the Galbraith plot and Forest plot were used to assessing the presence of heterogeneity. The Q test and I-Squared (I2) test statistics were considered to examine the variations. The heterogeneity was declared as low, moderate, or high when the I2 test statistic result became 25 %, 50 %, and 75 %, respectively [42]. This review displayed that there was significant heterogeneity among studies (P-value < 0.001). Thus, we adopted the random effect model to get the birth prevalence of neural tube defects [43]. However, the analysis demonstrated that there was a non-significant heterogeneity in estimating an association, and the fixed-effect model was adopted to analyze the association between neural tube defects and factors [44].
Given the variations in estimating the pooled birth prevalence, we conducted a subgroup analysis based on identified covariates to reduce the heterogeneity. Random effect meta-regression analyses were accounted for to determine the source of heterogeneity. We performed the sensitivity analyses to evaluate the influence of the study on the overall pooled estimates (the outputs of influence/sensitivity analyses were displayed graphically as well). We performed the time-trend analysis in order to visualize the random variations in the time sequence. A meta-cumulative analysis was done to display the pattern of effects and to show the significance of cumulative effect over the publication years.
The publication bias was checked using Egger’s regression test (and Begg’s test) statistics [45, 46] and we declared the presence of significant publication bias if a P-value became less than 0.05. Egger’s plot and the funnel plot were also considered. The trim and fill analyses were considered to mitigate the publication bias.
Results
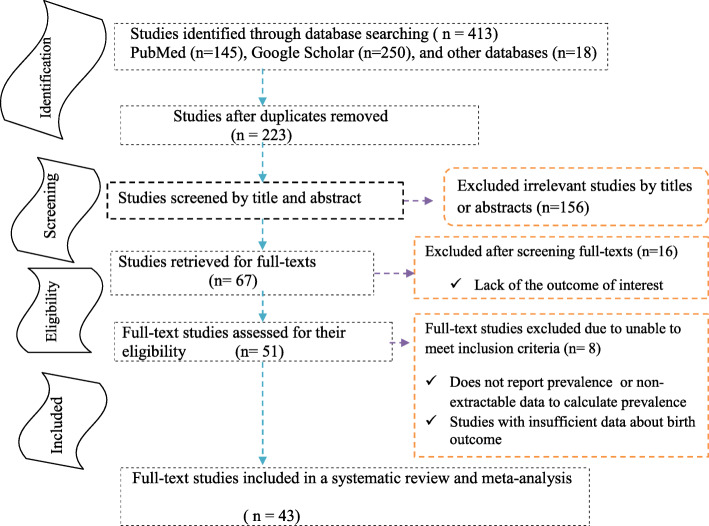
The comprehensive search of databases yielded 413 studies about neural tube defects and associated risk factors in Africa. Of 223 unduplicated studies, we excluded 156 after reviewing the titles and abstracts. The remaining sixty-seven studies were screened and sixteen were excluded because of the outcome interests. Thus, fifty-one studies were assessed for eligibility, and 43 studies, were fulfilled the criteria, were included in this systematic review and meta-analysis (Fig. 1). All included original studies were cross-sectional (29), case-control (5), and prospective cohort (9) study designs [2–23, 47–68]. Of these studies, we used thirty-six for prevalence estimates and all these were cross-sectional and prospective study designs [2, 3, 5–17, 47–55, 57–68]. The total number of participants included was 6086, 384. Ten studies had been conducted in Ethiopia [2, 5, 9–11, 17–20, 64], five in Tunisia [3, 8, 21–23], eight in Nigeria [6, 12, 15, 16, 50, 51, 65, 66], two in Algeria [4, 7], six in South Africa [55, 59, 60, 63, 67, 68], two in Sudan [48, 62], and two in Ghana [49, 57]. Study was conducted in Kenya [13], Eritrea [14], Libya [52], Egypt [53], Cameron [54], Malawi [58], Tanzania [61], and Democratic Republic (DR) of Congo [47] (Table 1). The period prevalence, birth outcome, study setting, and study quality were presented in Table 2. In addition to studies explained in Table 2, studies done by Atlaw et al. [18], Berihu et al. [19], Aynalem et al. [20], Nasri et al. [21], Bourouba et al. [4], Kitova et al. [22], and Nasri et al. [23] were declared low risk.
Fig. 1.
Study selection flow diagram, a figure adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) group statement
Table 1.
The characteristics of the studies included in the systematic review and meta-analysis, 2020
| First author | Year | Country | Study design | Sample size | Duration/months | Prevalence per 10, 000 births |
|---|---|---|---|---|---|---|
| Gedefaw et al. [2] | 2018 | Ethiopia | Cross-sectional | 8677 | 7 | 63 |
| Nasri et al. [3] | 2014 | Tunisia | Cross-sectional | 3,803,889 | 240 | 2 |
| Adane et al. [5] | 2018 | Ethiopia | Cross-sectional | 19,650 | 36 | 52 |
| Anyanwu et al. [6] | 2015 | Nigeria | Cross-sectional | 1456 | 9 | 27 |
| Houchar et al. [7] | 2008 | Algeria | Cross-sectional | 28,500 | 36 | 75 |
| Berihu et al. [9] | 2018 | Ethiopia | Cross-sectional | 14,903 | 9 | 131 |
| Taye et al. [10] | 2019 | Ethiopia | Cross-sectional | 76,201 | 6 | 40 |
| Abebe et al. [11] | 2020 | Ethiopia | Cross-sectional | 45,951 | 60 | 41 |
| Nnadi et al. [12] | 2016 | Nigeria | Prospective | 10,163 | 36 | 22 |
| Githuku et al. [13] | 2014 | Kenya | Cross-sectional | 6041 | 72 | 3 |
| Estifanos etal [14]. | 2017 | Eritrea | Cross-sectional | 39,803 | 24 | 39 |
| Toma et al. [15] | 2018 | Nigeria | Cross-sectional | 1046 | 35 | 250 |
| Audu et al. [16] | 2004 | Nigeria | Cross-sectional | 2250 | 48 | 80 |
| Legesse et al. [17] | 2019 | Ethiopia | Prospective | 956 | 7 | 63 |
| Nasri et al. [8] | 2015 | Tunisia | Cross-sectional | 764,431 | 48 | 2 |
| Atlaw et al. [18] | 2019 | Ethiopia | Case-control | 462 | 6 | – |
| Berihu et al. [19] | 2019 | Ethiopia | Case-control | 617 | 9 | – |
| Aynalem etal [20]. | 2018 | Ethiopia | Case-control | 180 | 7 | – |
| Nasri et al. [21] | 2015 | Tunisia | Case-control | 150 | 7 | – |
| Bourouba et al. [4] | 2018 | Algeria | Case-control | 130 | 12 | – |
| Kitova et al. [22] | 2013 | Tunisia | Prospective | 150 | 36 | – |
| Nasri et al. [23] | 2016 | Tunisia | Prospective | 132 | 9 | – |
| Ahuka et al. [47] | 2006 | DR Congo | Cross-sectional | 8824 | 96 | 10 |
| Oumer et al. [48] | 2016 | Sudan | Cross-sectional | 36,785 | 12 | 28 |
| Alhassan et al. [49] | 2017 | Ghana | Cross-sectional | 35,426 | 48 | 16 |
| Alrede et al. [50] | 1992 | Nigeria | Prospective | 5, 977 | 36 | 70 |
| Ekanem et al. [51] | 2008 | Nigeria | Cross-sectional | 127,929 | 276 | 5 |
| Singh et al. [52] | 2000 | Libya | Prospective | 15, 938 | 12 | 8 |
| Mohammed etal [53]. | 2011 | Egypt | Cross-sectional | 5000 | 7 | 16 |
| Njamnshi et al. [54] | 2008 | Cameron | Cross-sectional | 52,710 | 120 | 19 |
| Sayed et al. [55] | 2008 | South Africa | Prospective | 53,000 | 9 | 10 |
| Masamati et al. [58] | 2000 | Malawi | Cross-sectional | 25,562 | 24 | 6 |
| Venter et al. [59] | 1995 | South Africa | Prospective | 10,380 | 40 | 36 |
| Buccimazzaetal [60]. | 1994 | South Africa | Cross-sectional | 516,252 | 240 | 12 |
| Kinasha et al. [61] | 2003 | Tanzania | Cross-sectional | 34,000 | 24 | 30 |
| Elsheikh et al. [62] | 2009 | Sudan | Prospective | 18,378 | 12 | 35 |
| Krzesinski etal [63]. | 2019 | South Africa | Cross-sectional | 93,609 | 72 | 7 |
| Anyebuno et al. [57] | 1993 | Ghana | Cross-sectional | 19,094 | 24 | 12 |
| Adetiloye et al. [66] | 1993 | Nigeria | Cross-sectional | 23, 438 | 120 | 5 |
| Sorri et al. [64] | 2015 | Ethiopia | Cross-sectional | 28, 961 | 36 | 54 |
| Ugwo et al. [65] | 2007 | Nigeria | Cross-sectional | 2, 891 | 48 | 128 |
| Cornell et al. [67] | 1983 | South Africa | Cross-sectional | 116, 859 | 60 | 9 |
| Kromberg et al. [68] | 1982 | South Africa | Cross-sectional | 29, 633 | – | 8 |
Table 2.
The study period, setting, birth outcome, and quality of included studies in the systematic review and meta-analysis, 2020
| First author | Birth outcome | Prevalence period | Study setting | Study quality |
|---|---|---|---|---|
| Gedefaw et al. [2] | LB + SB | 2016 | Institution-based | Low risk |
| Nasri et al. [3] | LB + SB | 1991–2011 | Institution-based | Low risk |
| Adane et al. [5] | LB + SB | 2015–2017 | Institution-based | Low risk |
| Anyanwu et al. [6] | LB | 2013 | Institution-based | Low risk |
| Houchar et al. [7] | LB + SB | 2004–2006 | Institution-based | Low risk |
| Berihu et al. [9] | LB + SB | 2016–2017 | Institution-based | Low risk |
| Taye et al. [10] | LB | 2015 | Institution-based | Low risk |
| Abebe et al. [11] | LB + SB | 2011–2015 | Institution-based | Low risk |
| Nnadi et al. [12] | LB + SB | 2011–2013 | Institution-based | Low risk |
| Githuku et al. [13] | LB | 2005–2010 | Institution-based | Low risk |
| Estifanos et al. [14] | LB + SB | 2007–2011 | Institution-based | Low risk |
| Toma et al. [15] | LB + SB | 2013–2016 | Institution-based | Low risk |
| Audu et al. [16] | LB | 2000–2003 | Institution-based | Low risk |
| Legesse et al. [17] | LB + SB | 2018–2019 | Institution-based | Low risk |
| Nasri et al. [8] | LB + SB | 2008–2011 | Institution-based | Low risk |
| Ahuka et al. [47] | LB | 1993–2001 | Institution-based | Low risk |
| Oumer et al. [48] | LB + SB | 2014–2015 | Institution-based | Low risk |
| Alhassan et al. [49] | LB + SB | 2010–2014 | Institution-based | Low risk |
| Alrede et al. [50] | LB + SB | 1987–1990 | Institution-based | Low risk |
| Ekanem et al. [51] | LB + SB | 1980–2003 | Institution-based | Low risk |
| Singh et al. [52] | LB + SB | 1995–1996 | Institution-based | Low risk |
| Mohammed et al. [53] | LB | 2007 | Institution-based | Low risk |
| Njamnshi et al. [54] | LB + SB | 1997–2006 | Institution-based | Low risk |
| Sayed et al. [55] | LB + SB | 2004–2005 | Institution-based | Low risk |
| Masamati et al. [58] | LB + SB | 1998–1999 | Institution-based | Low risk |
| Venter et al. [59] | LB | 1989–1992 | Institution-based | Low risk |
| Buccimazza etal [60]. | LB + SB | 1973–1992 | Institution-based | Low risk |
| Kinasha et al. [61] | LB | 2000–2002 | Institution-based | Low risk |
| Elsheikh et al. [62] | LB + SB | 2003–2004 | Institution-based | Low risk |
| Krzesinski et al. [63] | LB + SB | 2003–2013 | Institution-based | Low risk |
| Anyebuno et al. [57] | LB + SB | 1991–1992 | Institution-based | High risk |
| Adetiloye et al. [66] | LB + SB | 1982–1992 | Institution-based | High risk |
| Sorri et al. [64] | LB + SB | 2009–2012 | Institution-based | – |
| Ugwo et al. [65] | LB + SB | 2002–2005 | Institution-based | – |
| Cornell et al. [67] | LB + SB | 1975–1980 | Institution-based | – |
| Kromberg et al. [68] | LB + SB | – | Institution-based | – |
Key: LB Live births, SB Stillbirths
The pooled birth prevalence of neural tube defects in the present meta-analysis was 21.42 (95% CI: 19.29, 23.56) per 10,000 births. A forest plot showed that there was significant heterogeneity across the studies (P-value < 0.001, I2 = 98.5%). Therefore, a random-effect model was applied to pool the overall prevalence [2, 3, 5–17, 47–55, 57–68] (Fig. 2). For thirty-six studies, the median value of neural tube defects was 24.5 and the inter-quartile range was between 8.5 and 53 per 10, 000 births. The minimum and maximum values were 2 and 250 per 10, 000 births.
Fig. 2.
Forest plot showing the pooled prevalence of neural tube defects in Africa, 2020
Subgroup analyses based on the period prevalence, region/country, the birth outcome, and design was performed. The highest and the lowest prevalence rate was found in Algeria (75.0, 95% CI: 64.98, 85.02) and in Tunisia (2.0, 95% CI: 1.87, 2.13, per 10,000 births) (Supplementary file 4). Based on the birth outcome (I2 = 98.5%), the prevalence for live births only was 26.85 (95% CI: 13.43, 40.27) and for both live birth and stillbirths was 19.76 (95% CI: 17.49, 22.03) per 10,000 births. Concerning the study designs (I2 = 98.5%), the prevalence for the cross-sectional (21.01, 95% CI: 18.74, 23.27) was lower than the prospective cohort study designs (28.35, 95% CI: 17.53, 39.17, per 10, 000 births). Based on the period prevalence (I2 = 98.5%), the burden of neural tube defects for the period after 2010 was 49.55 (95% CI: 36.50, 62.61), 2001–2010 was 29.48 (95% CI: 22.10, 36.87), 1991–2011 was 2.0 (95% CI: 1.86, 2.14), 1991–2000 was 12.42 (95% CI: 6.46, 18.38), 1980–2003 was 5.0 (95% CI: 3.77, 6.22), and before 1990 was 10.65 (95% CI: 6.52, 14.77) per 10,000 births.
Sample size (P-value = 0.78), year of publication (P-value = 0.37), duration of the study in months (P-value = 0.74), study quality score (P-value = 0.69), study country (P-value = 0.03), study design (P-value = 0.84), birth outcome (P-value = 0.63), and period prevalence (P-value = 0.47) were analyzed for the source of heterogeneity and only study country was found statistically significant.
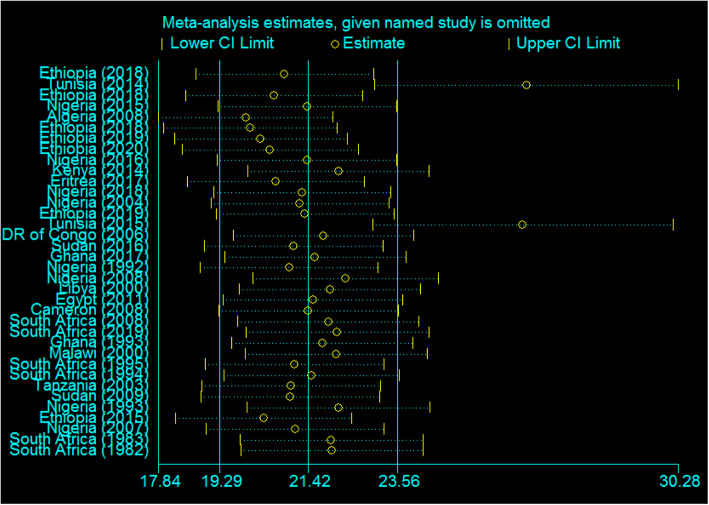
In the current systematic review and meta-analysis, except for two Tunisian studies (years 2014 and 2015), the influence of studies on the overall estimates was uniform (Fig. 3). Meta-influence estimates were analyzed by removing one article at a time and the uniform influence was displayed and the prevalence after removing only the 2014 Tunisia study was 26.64 (95% CI: 23.0, 30.28), and after removing only the 2015 Tunisia study was 26.56 (95% CI: 22.96, 30.16) (Fig. 3). If both studies are omitted together, the prevalence was 28.24 (95% CI: 24.22, 32.27) with uniform influence. Even if the whole analysis was repeated after omitting the two studies, the heterogeneity across studies was not decreased (97.5%, only 1% reduction). We looked at the effect of low-quality studies on the overall estimates by limiting those studies included in a meta-analysis. The meta-analysis estimate was found by including studies that only scored greater than or equal to five, high-quality studies; therefore, its pooled estimate was 22.31 per 10,000 births.
Fig. 3.
Sensitivity analysis showed the influence of each individual study in overall estimates in Africa, 2020
The relationship between the burden of neural tube defects and the study publication years from 1982 (8.0) to 2020 (41 per 10,000 births) was visualized using the time trend analyses. Besides, the pattern of effects on the time, from the year 1982 (8.0) to the year 2020 (21.42 per 10,000 births), was displayed using the meta-cumulative analyses and the cumulative effects of all studies were significant (Fig. 4).
Fig. 4.
Meta-cumulative analysis showing cumulative effect of neural tube defects in relation to time in Africa, 2020
In estimating the birth prevalence, a significant publication bias was identified by Egger’s tests (P-value < 0.001) and its plot (Fig. 5). We conducted the trim and fill meta-analyses to adjust this bias. We analyzed fifty-five studies (19 articles were filled in the 36 studies) in the fill meta-analyses. As a result, the birth prevalence of neural tube defects using the random-effect model was 5.14 (95% CI: 2.90, 7.38) per 10,000 births. This adjusted estimate suggested a lower risk of bias than the original analysis. However, publication bias is still significant after fill and trim analyses have been done.
Fig. 5.
Egger’s publication bias plot, 2020
In this meta-analysis, folic acid supplementation during early pregnancy, consanguineous marriage, male newborn, and substance abuse during pregnancy (smoking, alcohol, especially) were the variables analyzed for association with neural tube defects. In estimating the association of all factors, there was no statistically significant publication bias among studies. Similarly, the Galbraith plot visualized that there was no heterogeneity among the studies. The summary of studies (odds ratio, confidence interval, etc.) included in the meta-analyses for an association was explained in Table 3.
Table 3.
Summary of studies included in the meta-analysis for association with neural tube defects, 2020
| First author | Year | Country | Associated factors | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Folic acid | UCI | LCI | ||||
| Gedefaw et al. [2] | 2018 | Ethiopia | 0.47 | 0.95 | 0.23 | |
| Bourouba et al. [4] | 2018 | Algeria | 0.24 | 1.15 | 0.03 | |
| Anyanwu et al. [6] | 2015 | Nigeria | 0.36 | 19.27 | 0.03 | |
| Atlaw et al. [18] | 2019 | Ethiopia | 0.095 | 0.29 | 0.001 | |
| Berihu et al. [19] | 2019 | Ethiopia | 0.48 | 1.04 | 0.2 | |
| Nasri et al. [8, 21] | 2015 | Tunisia | 1.19 | 2.44 | 0.58 | |
| Nasri et al. [23] | 2016 | Tunisia | 0.15 | 0.44 | 0.04 | |
| Pooled/net odds ratio | 0.51 | 2.29 | 0.11 | |||
| Consanguineous marriage | ||||||
| Atlaw et al. [18] | 2019 | Ethiopia | 5.54 | 20.9 | 1.47 | |
| Nasri et al. [8, 21] | 2015 | Tunisia | 2.09 | 6.1 | 0.76 | |
| Kitova et al. [22] | 2013 | Tunisia | 2.46 | 6.37 | 0.95 | |
| Nasri et al. [23] | 2016 | Tunisia | 2.59 | 11.9 | 0.69 | |
| Nasri et al. [8, 21] | 2015 | Tunisia | 1.27 | 4.59 | 0.35 | |
| Pooled/net odds ratio | 2.41 | 18.47 | 0.31 | |||
| Male newborn | ||||||
| Gedefaw et al. [2] | 2018 | Ethiopia | 0.56 | 0.94 | 0.33 | |
| Nasri et al. [3] | 2014 | Tunisia | 0.68 | 0.79 | 0.59 | |
| Anyanwu et al. [6] | 2015 | Nigeria | 0.92 | 12.77 | 0.07 | |
| Houchar et al. [7] | 2008 | Algeria | 0.7 | 0.92 | 0.52 | |
| Atlaw et al. [18] | 2019 | Ethiopia | 0.72 | 1.37 | 0.38 | |
| Aynalem et al. [20] | 2018 | Ethiopia | 0.58 | 1.14 | 0.3 | |
| Nasri et al. [8, 21] | 2015 | Tunisia | 0.49 | 1.27 | 0.19 | |
| Pooled/net odds ratio | 0.67 | 1.06 | 0.42 | |||
| Substance abuse during pregnancy | ||||||
| Atlaw et al. [18] | 2019 | Ethiopia | 11.08 | 62.7 | 1.96 | |
| Berihu et al. [19] | 2019 | Ethiopia | 10.3 | 88.5 | 1.19 | |
| Aynalem et al. [20] | 2018 | Ethiopia | 0.56 | 1.5 | 0.21 | |
| Pooled/net odds ratio | 1.52 | 33.28 | 0.07 | |||
Key: The different numbers of articles in different analysis/variables is due to a lack of similarity in studies reporting the risk factors
Taking folic acid during early pregnancy (Pooled OR (Odds Ratio) = 0.51, 95% CI: 0.11, 2.29), consanguineous marriage (Pooled OR = 2.41, 95% CI: 0.31, 18.47), male sex (Pooled OR = 0.67, 95% CI: 0.42, 1.06), and substance abuse during pregnancy (Pooled OR = 1.52, 95% CI: 0.07, 33.28) were assessed and none of them was statistically significant (Supplementary file 4).
Discussion
The present systematic review and meta-analysis were conducted to assess the pooled birth prevalence of neural tube defects and to identify the risk factors associated with the occurrence of neural tube defects. This review revealed the pooled birth prevalence in Africa and it evaluated the risk factors (folic acid uptake, consanguineous marriage, male newborn, and substance abuse during pregnancy) for association with neural tube defects. The hidden burden of neural tube defects is very high in Africa. The primary data research and systematic review/meta-analysis that show this burden are scarce. However, the effects of the defects are related to substantial mortality, disability, and psychological costs and it is an important public health problem [24, 69–73].
The pooled birth prevalence of the neural tube defects in the present meta-analysis was found 21.42 per 10,000 births with a range of 19.29–23.56. Different prevalence rates have been reported by the review conducted in Indian [74], Latin America [75], and worldwide [24]. Variation in estimates was also observed in reviews reported elsewhere [69, 70, 74, 75]. The prevalence of the defect remains high in less-developed countries of Africa, Latin America, Asia, and the Far East [1, 71–73]. The variation in estimates may be due to the difference in countries’ health policy, income levels, and the institution of folic acid fortification [24, 72]. The findings have stressed the need for more surveillance efforts, particularly in low-income countries [69]. In the current review, a relatively high-pooled birth prevalence of neural tube defects was detected in Algeria, Ethiopia, Eritrea, and Nigeria. Of all, the highest and lowest rates were detected in Algeria (75) and Tunisia (2), respectively. The magnitude of the defect among African countries showed geographic variations as other previous reviews have shown in various regions of the world [24, 69–75]. Thus, the variation detected across studies in estimating the pooled prevalence of neural tube defects was due to differences in study countries, period prevalence, study design, and birth outcome. The variation of estimates across countries may be also due to the difference in the folic acid supplementation/fortification, prenatal care/antenatal screening, and countries’ health policy.
The increment of prevalence over time may be due to a change in detection methods, an increment of the practices in documenting and reporting cases, an increase of the demands for fetal pathological examinations over these years, or a real increase in disease. Besides, it may be due to an increment of practice changes that could lead to increased detections, for instance, nowadays more children are born in hospitals and more women are became tested/screened.
Taking folic acid during early pregnancy had a non-significant association with the incidence of neural tube defects. However, this finding is not supported by different previous literature [24, 25, 29, 32]. Although folic acid has been revealed to decrease the risk of neural tube defects in previous studies [36, 37, 39, 76], the potential of folic acid to decrease the occurrence of the defect has not been yet examined in most African countries and preventable neural tube defects continue to occur [25]. Furthermore, the utilization is affected by the persistence of socioeconomic and educational issues in the consumption of folic acid, ethnic disparities, and the existence of age-based variation of supplement use [25]. Despite there are folate supplements, there is a low utilization, it is difficult to attain the recommended daily intake of folate for different reasons (relatively poor availability of folate in natural foods, easy destruction during cooking, for instance) [77]. May be the lack of significance is due to the inclusion of a small number of studies (and may be these are low folic acid utilized countries, non-mandatory folic acid users) in the analyses.
Strength and limitations of the review
The present systematic review and meta-analysis gave cumulative and up-to-date evidence on neural tube defects and associated risk factors in Africa. The review finding is estimated from the pooled estimate of forty-three studies in Africa and it provides valuable information to the policymakers, and this should be the ultimate contribution of this review to the field.
The findings of the current review should be interpreted based on some limitations. The estimate did not consider the terminated pregnancies of the defect and this may reduce the pooled prevalence estimates. Moreover, the presence of significant variation across countries may underestimate the overall burden of neural tube defects in Africa. Underestimation of the burden of neural tube defects should be considered due to the missing of many stillbirths and home births that are delivered in the community setting. Furthermore, the variability of the sample size in the included studies might influence the pooled birth prevalence estimates. The risk factors are harder to assess given the limitations on that data. All studies in this review were institution-based studies. Although moderate publication bias was detected in prevalence estimates, we adjusted the bias using the trim and fill analysis.
Conclusions
The pooled birth prevalence of neural tube defects in Africa was found high. A high-pooled prevalence of neural tube defects was detected in Algeria, Ethiopia, Eritrea, and Nigeria. The risk factors evaluated were not found significant.
We would like to inform policymakers that the pooled birth prevalence estimates are may be underestimated due to different mentioned factors and the pooled estimate should not impact policy decisions on prevention efforts negatively in Africa where policymakers may feel that this is not a big problem to prioritize the prevention funds. Strong prevention and control measures should be the priority. Moreover, limited available data on neural tube defects inform the need for additional primary, wide scope research that would improve the true burden of the defects and facilitate preventive policies on preventive factors in Africa.
Supplementary Information
Additional file 1: Supplementary file 1. PRISMA reporting checklist
Additional file 2: Supplementary file 2. PubMed Searching methods
Additional file 3: Supplementary file 3. JBI critical appraisal checklists for all designs
Additional file 4: Supplementary file 4. Additional Table and Figures
Acknowledgments
Not applicable.
Abbreviations
- CI
Confidence Interval
- DR
Democratic Republic
- I2
I-Squared
- JBI
Joanna Briggs Institute
- MeSH
Medical Subject Heading
- OR
Odds Ratio
- PROSPERO
Prospective Register of a Systematic Review
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Authors’ contributions
MO, AT, and MS participated in the conceptualization of the review protocol, formal analysis, methodology or study design, writing-original draft, interpretation, writing-review and editing, and approving the final draft. MO, MS, and AT: Quality assessment, data extraction, and literature review. All authors read and approved the manuscript.
Funding
No funding sources for this review.
Availability of data and materials
The data sets used and/or analyzed during the current systematic review and meta-analysis are included in the review and available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moore KL, Persaud TVN. The developing human. Clinically oriented Embryology. 8th edition Chapter. Congenital anomalies of the brain and spinal cord. Philadelphia: Saunders; 2008.
- 2.Gedefaw A, Teklu S, Tilahun BT. Magnitude of Neural Tube Defects and Associated Risk Factors at Three Teaching Hospitals in Addis Ababa, Ethiopia. London: Hindawi, BioMed Research International; 2018. p. 10. Article ID 4829023. 10.1155/2018/4829023. [DOI] [PMC free article] [PubMed]
- 3.Nasri K, et al. Epidemiology of neural tube defect subtypes in Tunisia, 1991–2011. Pathol Res Pract. 2014. 10.1016/jprp201406027. [DOI] [PubMed]
- 4.Bourouba R, Houcher B, Akar N. Risk factors of neural tube defects: a reality of Batna region in Algeria. Egypt J Med Hum Genet. 2018;19:225–229. doi: 10.1016/jejmhg201710003. [DOI] [Google Scholar]
- 5.Adane F, Seyoum G. Prevalence and associated factors of birth defects among newborns at referral hospitals in Northwest Ethiopia. Ethiop J Health Dev. 2018;32(3):157–62.
- 6.Anyanwu LC, Danborno B, Hamman WO. The prevalence of neural tube defects in live born neonates in Kano, North-Western Nigeria. Sub-Saharan Afr J Med. 2015;2(3):105–109. doi: 10.4103/2384-5147.164417. [DOI] [Google Scholar]
- 7.Houcher B, Bourouba R, Djabi F, Houcher Z. The prevalence of neural tube defects in Sétif University maternity hospital, Algeria-3 years review (2004-2006) Pteridines. 2008;19:12–18. doi: 10.1515/pteridines.2008.19.1.12. [DOI] [Google Scholar]
- 8.Nasri K, et al. An increase in spina bifida cases in Tunisia, 2008–2011. Pathol Res Pract. 2015. 10.1016/jprp201412011. [DOI] [PubMed]
- 9.Berihu BA, Welderufael AL, Berhe Y, Magana T, Mulugeta A, Asfaw S, et al. High burden of neural tube defects in Tigray, Northern Ethiopia: Hospital-based study. PLoS One. 2018;13(11):e0206212. doi: 10.1371/journalpone0206212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taye M, Afework M, Fantaye W, Diro E, Worku A. Congenital anomalies prevalence in Addis Ababa and the Amhara region, Ethiopia: A descriptive cross-sectional study. BMC Pediatr. 2019;19:234. [DOI] [PMC free article] [PubMed]
- 11.Abebe S, Gebru G, Amenu D, Dube L. Prevalence and patterns of birth defects among newborns in southwestern Ethiopia: retrospective study. 2020. [Google Scholar]
- 12.Nnadi DC, Singh S. The prevalence of neural tube defects in north-West Nigeria. Saudi J Health Sci. 2016;5(1):6–10. doi: 10.4103/2278-0521.182858. [DOI] [Google Scholar]
- 13.Githuku JN, Azofeifa A, Valencia D, et al. Assessing the prevalence of spina bifida and encephalocele in a Kenyan hospital from 2005–2010: implications for a neural tube defects surveillance system. Pan Afr Med J. 2014;18:60. doi: 10.11604/pamj201418604070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estifanos D, Adgoy ET, Sereke D, Zekarias B, Marzolf S, Tedla K. The prevalence, trend, and associated demographic factors of neural tube defects at Orotta National Referral Maternity Hospital, Asmara: retrospective record review study. Sci J Public Health. 2017;5(6):452. doi: 10.11648/j.sjph.20170506.17. [DOI] [Google Scholar]
- 15.Toma BO, Shilong DJ, Shwe DD, Bot GM, Diala UM, Ofakunrin AO, Prince A, Binitie PO. The prevalence and pattern of central nervous system anomalies in a neonatal unit in a tertiary hospital in Jos, north-Central Nigeria. J Med Trop. 2018;20(1):63–67. doi: 10.4103/jomt.jomt_10_18. [DOI] [Google Scholar]
- 16.Audu L, Shehu BB, Thom-Manuel, Mairami AB. Open Neural tube defect at the National Hospital, Abuja: Analysis of clinical patterns and neonatal outcome. Niger J Pediatr. 2004;31:131. [Google Scholar]
- 17.Legesse A, Zawidneh D, Gorfu Y. Assessment of prevalence, types and associated risk factors of neural tube defects in pregnant women visiting health centers in Addis Ababa. 2019. [Google Scholar]
- 18.Atlaw D, Worku A, Taye M, Woldeyehonis D, Muche M. Neural Tube Defect and Associated Factors in Bale Zone Hospitals, Southeast Ethiopia. J Pregnancy Child Health. 2019;6(3):412.
- 19.Berihu BA, et al. Maternal risk factors associated with neural tube defects in Tigray regional state of Ethiopia. Brain Dev. 2019;41:11–18. doi: 10.1016/jbraindev201807013. [DOI] [PubMed] [Google Scholar]
- 20.Aynalem F, et al. Determinants of neural tube defect among children, at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. A case control study. 2018. [Google Scholar]
- 21.Nasri K, et al. Association of Maternal Homocysteine and VitaminsStatus with the Risk of Neural Tube Defects in Tunisia: A Case–Control Study. Birth Defects Res (Part A) 2015;103:1011–1020. doi: 10.1002/bdra.23418. [DOI] [PubMed] [Google Scholar]
- 22.Kitova TT, Karaslavova EG, Masmoudi A, Gaigi SS. Maternal factors and associated anomalies in NTD fetuses from Tunisia. Cent Eur J Med. 2013;8(6):707–712. doi: 10.2478/s11536-013-0238-6. [DOI] [Google Scholar]
- 23.Nasri K, et al. Maternal 25-hydroxyvitamin D level and the occurrence of neural tube defects in Tunisia. Int J Gynecol Obstet. 2016. 10.1016/jijgo201601014. [DOI] [PubMed]
- 24.Blencowe H, Kancherla V, Moorthie S, Darlison MW, Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann N Y Acad Sci. 2018;1414(1):31–46. doi: 10.1111/nyas.13548. [DOI] [PubMed] [Google Scholar]
- 25.Yi Y, Lindemann M, Colligs A, Snowball C. Economic burden of neural tube defects and impact of prevention with folic acid: A literature review. Eur J Pediatr. 2011;170(11):1391–1400. doi: 10.1007/s00431-00011-01492-00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zurmohle UM, Homann T, Schroeter C, Rothgerber H, Hommel G, Ermert J. Psychosocial adjustment of children with spina bifida. J Child Neurol. 1998;13(2):64–70. doi: 10.1177/088307389801300204. [DOI] [PubMed] [Google Scholar]
- 27.Date I, Yagyu Y, Asari S, Ohmoto T. Long-term outcome in surgically treated spina bifida cystica. Surg Neurol. 1993;40(6):471–475. doi: 10.1016/0090-3019(93)90049-7. [DOI] [PubMed] [Google Scholar]
- 28.Postma MJ, Londeman J, Veenstra M, De Walle HEK, De Jong van den Berg LTW. Cost-effectiveness of periconceptional supplementation of folic acid. Pharm World Sci. 2002;24(1):8–11. doi: 10.1023/A:1014848928212. [DOI] [PubMed] [Google Scholar]
- 29.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LYC, Gindler J, Hong SX, Hao L, Gunter E, Correa A. Prevention of neural-tube defects with folic acid in China. N Engl J Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 30.Shaer C, Chadduck W, Donahue D, Allen J, Gonzales E, Mulhauser L. Answering your questions about spina bifida. Washington, DC: Children’s National Medical Center; 1995. [Google Scholar]
- 31.Bulatao RA, Stephens PW. Global estimates and projections of mortality by cause, 1970-2015. World Bank Publications; 1992. [Google Scholar]
- 32.Locksmith G, Duff P. Preventing neural tube defects: the importance of periconceptional folic acid supplements. Obstet Gynecol. 1998;91(6):1027–1034. doi: 10.1016/s0029-7844(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 33.Veer TV, Meester H, Poenaru D, Kogei A, Augenstein K, Bransford R. Quality of life for families with spina bifida in Kenya. Trop Dr. 2008;38(3):160–162. doi: 10.1258/td.2007.070053. [DOI] [PubMed] [Google Scholar]
- 34.Munyi N, Poenaru D, Bransford R, Albright L. Encephalocele-a single institution African experience. East Afr Med J. 2009;86(2):51–54. doi: 10.4314/eamj.v86i2.46931. [DOI] [PubMed] [Google Scholar]
- 35.Greene ND, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18(R2):R113–R129. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council vitamin study. Lancet. 1991;338(8760):131–137. doi: 10.1016/0140-6736(91)90133-A. [DOI] [PubMed] [Google Scholar]
- 37.Berry BJ, Bailey L, Mulinare J, Bower C. Folic acid working G: fortification of flour with folic acid. Food Nutr Bull. 2010;31(1 Suppl):S22–S35. doi: 10.1177/15648265100311S103. [DOI] [PubMed] [Google Scholar]
- 38.Bationo F, Songré-Ouattara LT, Hama-Ba F, Baye K, Hemery YM, Parkouda C, et al. Folate Status of Women and Children in Africa –Current Situation and Improvement Strategies. Food Rev Int. 2019. 10.1080/87559129.87552019.81608558.
- 39.American Academy Of Pediatrics Folic acid for the prevention of neural tube defects. Pediatrics. 1999;104:325. doi: 10.1542/peds.104.2.325. [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journalpmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, et al., editors. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; 2017. [Google Scholar]
- 42.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557. doi: 10.1136/bmj3277414557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 44.Borenstein M, Hedges LV, Higgins J, Rothstein HR. A basic introduction to fixed effect and random effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm12. [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bio Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. 10.2307/2533446. [PubMed]
- 47.Ahuka OL, Toko RM, Omanga FU, Tshimpanga BJ. Congenital malformations in the north-eastern Democratic Republic of Congo during civil war. East Afr Med J. 2006;8:95–99. doi: 10.4314/eamj.v83i2.9395. [DOI] [PubMed] [Google Scholar]
- 48.Omer IM, Abdullah OM, Mohammed IN, Abbasher LA. Research: Prevalence of neural tube defects Khartoum, Sudan August 2014–July 2015. BMC Res Notes. 2016;9:495. doi: 10.1186/s13104-016-2298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alhassan A, Adam A, Nangkuu D. Prevalence of neural tube defect and hydrocephalus in Northern Ghana. J Med Biomed Sci. 2017;6(1):18–23. doi: 10.4314/jmbsv6i13. [DOI] [Google Scholar]
- 50.Airede KI. Neural tube defects in the middle belt of Nigeria. J Trop Pediatr. 1992;38:27–30. doi: 10.1093/tropej/38.1.27. [DOI] [PubMed] [Google Scholar]
- 51.Ekanem TB, Okon DE, Akpantah AO, Mesembe OE, Eluwa MA, Ekong MB. Prevalence of congenital malformations in Cross River and Akwa Ibom states of Nigeria from 1980–2003. Congenit Anom. 2008;48:167–170. doi: 10.1111/j17414520200800204x. [DOI] [PubMed] [Google Scholar]
- 52.Singh R, Al-Sudani O. Major congenital anomalies at birth in Benghazi, Libyan Arab Jamahiriya, 1995. East Mediterr Health J. 2000;6:65–75. [PubMed] [Google Scholar]
- 53.Mohammed YA, Shawky RM, Soliman AA, Ahmed MM. Chromosomal study in newborn infants with congenital anomalies in Assiut University hospital: cross-sectional study. Egypt J Med Hum Genet. 2011;12(1):79–90. doi: 10.1016/j.ejmhg.2011.02.003. [DOI] [Google Scholar]
- 54.Njamnshi AK, Djientcheu VDP, Lekoubou A, Guemse M, Obama MT, Mbu R, et al. Neural tube defects are rare among black Americans but not in sub-Saharan black Africans: The case of Yaounde-Cameroon. J Neurol Sci. 2008;270:13–17. doi: 10.1016/j.jns.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res Part A Clin Mol Teratol. 2008;82(4):211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- 56.Houcher B, Akar N, Begag S, Egin Y. Neural tube defects in Algeria. London: INTECH Open Access Publisher; 2012.
- 57.Anyebuno M, Amofa G, Peprah S, Affram A. Neural tube defects at Korle Bu Teaching Hospital, Accra, Ghana. East Afr Med J. 1993;70:572–574. [PubMed] [Google Scholar]
- 58.Msamati BC, Igbigbi PS, Chisi JE. The incidence of cleft lip, cleft palate, hydrocephalus and spina bifida at Queen Elizabeth Central Hospital, Blantyre, Malawi. Cent Afr J Med. 2000;46:292–296. doi: 10.4314/cajm.v46i11.8572. [DOI] [PubMed] [Google Scholar]
- 59.Venter PA, Christianson AL, Hutamo CM, Makhura MP, Gericke GS. Congenital anomalies in rural black South African neonates-a silent epidemic? S Afr Med J. 1995;85:15–20. [PubMed] [Google Scholar]
- 60.Buccimazza SS, Molteno CD, Dunne TT, Viwoen D. Prevalence of neural tube defects in Cape Town, South Africa. Teratology. 1994;50:194–9. [DOI] [PubMed]
- 61.Kinasha AD, Manji KP. The incidence and pattern of neural tube defects in Dar Es Salaam, Tanzania. Eur J Pediatr Surg Suppl. 2002;12:S25–52. [PubMed]
- 62.Elsheikh GEA, Ibrahim SA. Neural tube defects in Omdurman maternity hospital, Sudan. Khatoum Med J. 2009;2:185–190. [Google Scholar]
- 63.Krzesinski EL, Geerts L, Urban MF. Neural tube defect diagnosis and outcomes at a tertiary south African hospital with intensive case ascertainment. S Afr Med J. 2019;109(9):698–703. doi: 10.7196/SAMJ.2019.v7109i7199.1386. [DOI] [PubMed] [Google Scholar]
- 64.Sorri G, Mesfin E. Patterns of neural tube defects at two teaching hospitals in Addis Ababa, Ethiopia. A three years retrospective study. Ethiop Med J. 2015;53(3):119–126. [PubMed] [Google Scholar]
- 65.Ugwu RO, Eneh AU, Oruamabo RS. Neural tube defects in a university teaching hospital in southern Nigeria: trends and outcome. Niger J Med. 2007;16(4):368–371. doi: 10.4314/njm.v4316i4314.37340. [DOI] [PubMed] [Google Scholar]
- 66.Adetiloyea VA, Dareb FO, Oyelami OA. A ten-year review of encephalocele in a teaching hospital. Int J Gynecol Obstet. 1993;41(3):241–249. doi: 10.1016/0020-7292(93)90550-G. [DOI] [PubMed] [Google Scholar]
- 67.Cornell J, Nelson MM, Beighton P. Neural tube defects in the Cape Town area, 1975-1980. S Afr Med J. 1983;64(3):83–84. [PubMed] [Google Scholar]
- 68.Kromberg JK, Jenkins T. Common birth defects in south African blacks. S Afr Med J. 1982;62(17):599–602. [PubMed] [Google Scholar]
- 69.Zaganjor I, Sekkarie A, Tsang BL, Williams J, Razzaghi H, Mulinare J, et al. Describing the Prevalence of Neural Tube Defects Worldwide: A Systematic Literature Review. PLoS One. 2016;11(4):e0151586. doi: 10.1371/journalpone0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Annie L, Dora P, Simrita S. Estimating the burden of neural tube defects in low– and middle–income countries. J Glob Health. 2014;4(1):010402. [DOI] [PMC free article] [PubMed]
- 71.Salih MA, Murshid WR, Seidahmed MZ. Epidemiology, prenatal management, and prevention of neural tube defects. Review article. Saudi Med J. 2014;35(Suppl 1):S15–S28. [PMC free article] [PubMed]
- 72.Sadler TW. Langman’s Medical Embryology. 11th Edition. Chapter: Clinical correlates in central nervous system Embryology. 2010.
- 73.Sadler TW. Chapter: Clinical correlates in central nervous system Embryology. In: Langman’s Medical Embryology. 12th ed. Philadelphia: Wolters Kluwer. Lippincotte Williams and Wilkins; 2011.
- 74.Bhide P, Gurdeep S, Sagoo GS, Moorthie S, Kar A. Systematic Review of Birth Prevalence of Neural Tube Defects in India. Birth Defects Res (Part A) 2013;97:437–443. doi: 10.1002/bdra.23153. [DOI] [PubMed] [Google Scholar]
- 75.Rosenthal J, Casas J, Taren D, Alverson CJ, Flores A, Frias J. Neural tube defects in Latin America and the impact of fortification: a literature review. Public Health Nutr. 2013;17(3):537–550. doi: 10.1017/S1368980013000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolff T, Witkop CT, Miller T, Syed S. Folic acid supplementation for the prevention of neural tube defects: an update of the evidence for the US preventive services task force. Ann Intern Med. 2009;150(9):632–639. doi: 10.7326/0003-4819-150-9-200905050-00010. [DOI] [PubMed] [Google Scholar]
- 77.Ami N, Bernstein M, Boucher F, Rieder M, Parker L. Canadian Paediatric society, drug therapy and hazardous substances committee: Folate and neural tube defects: the role of supplements and food fortification. Paediatr Child Health. 2016;21(3):145–154. doi: 10.1093/pch/21.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary file 1. PRISMA reporting checklist
Additional file 2: Supplementary file 2. PubMed Searching methods
Additional file 3: Supplementary file 3. JBI critical appraisal checklists for all designs
Additional file 4: Supplementary file 4. Additional Table and Figures
Data Availability Statement
The data sets used and/or analyzed during the current systematic review and meta-analysis are included in the review and available from the corresponding author on reasonable request.