Summary
Splenic Ly6Chigh monocytes are innate immune cells involved in the regulation of central nervous system-related diseases. Recent studies have reported the shaping of peripheral immune responses by the gut microbiome via mostly unexplored pathways. In this study, we report that a 4-day antibiotic treatment eliminates certain families of the Bacteroidetes, Firmicutes, Tenericutes, and Actinobacteria phyla in the gut and reduces the levels of multiple pattern recognition receptor (PRR) ligands in the serum. Reduction of PRR ligands was associated with reduced numbers and perturbed function of splenic Ly6Chigh monocytes, which acquired an immature phenotype producing decreased levels of inflammatory cytokines and exhibiting increased phagocytic and anti-microbial abilities. Addition of PRR ligands in antibiotic-treated mice restored the number and functions of splenic Ly6Chigh monocytes. Our data identify circulating PRR ligands as critical regulators of the splenic Ly6Chigh monocyte behavior and suggest possible intervention pathways to manipulate this crucial immune cell subset.
Subject areas: Immunology, Microbiology, Microbiome
Graphical abstract

Highlights
-
•
A 4-day antibiotic treatment eliminates certain bacterial families in the gut
-
•
Gut dysbiosis is followed by reduced levels of PRR ligands in the circulation
-
•
Reduction of PRR ligands relates to perturbation of splenic Ly6Chigh monocytes
-
•
Addition of PRR ligands restores splenic Ly6Chigh monocyte numbers and function
Immunology; Microbiology; Microbiome
Introduction
The gut microbiome has recently emerged as a key regulator of the peripheral innate immunity as well as myelopoiesis in the bone marrow (BM) (Belkaid and Harrison, 2017). At steady state, gut microbiome-derived products and metabolites cross the intestinal barrier and enter the bloodstream affecting the differentiation potential of BM precursor cells (Khosravi et al., 2014; Balmer et al., 2014) as well as the function of innate immune cells in peripheral tissues (Gorjifard and Goldszmid, 2016). Microbial peptidoglycan fragments have been reported to be present in the sera and BM of naive mice regulating the anti-microbial ability of BM-derived neutrophils (Clarke et al., 2010). Also, the bacteria-derived lipodipeptide, lipid 654, a Toll-like receptor (TLR) 2 ligand, is present in the serum of healthy individuals, but its levels are significantly decreased in patients with multiple sclerosis (Farrokhi et al., 2013), whereas its administration at low levels in mice attenuates experimental autoimmune encephalomyelitis (EAE) (Anstadt et al., 2016). Recently, the muramyl dipeptide (MDP), a NOD2 ligand, was found to be ubiquitously present in the sera of healthy humans, mice, and monkeys and was reported to participate in the immunoregulation of autoimmune arthritis and EAE (Huang et al., 2019). Another major group of microbial metabolites regulating the peripheral innate immune responses are short-chain fatty acids (SCFAs), products of fermentation of indigestible dietary fibers in the cecum and colon (Kim, 2018). The aforementioned findings demonstrate that in the absence of infection, there is a continuous communication between the gut microbiota and peripheral innate cells mediated by circulating microbial signals. Perturbation of this cross talk affects peripheral autoimmune processes and disease development.
Among peripheral innate immune cells, splenic-resident Ly6Chigh/low monocytes play a prominent immunoregulatory role under inflammatory conditions as they are mobilized and rapidly recruited to injured tissues (Guilliams et al., 2018). During the onset of EAE, splenic Ly6Chigh monocytes acquire an immunosuppressive function (Zhu et al., 2007), which they maintain within the central nervous system (CNS) as they express Nos2 and Arg1, hallmark genes of myeloid-derived suppressor cells (Zhu et al., 2007; Giladi et al., 2020). Their abundance has recently been related to a milder EAE course (Melero-Jerez et al., 2020). Splenic Ly6Chigh monocytes are also involved in the regulation of amyotrophic lateral sclerosis (ALS) because they acquire an M1 signature before disease onset and treatment with an anti-Ly6C monoclonal antibody leads to reduced monocyte recruitment to the spinal cord, diminished neuronal loss, and extended survival in mice (Butovsky et al., 2012). Furthermore, splenic Ly6Chigh monocytes regulate the acute and post-acute phases of spinal cord injury since prevention of early monocyte infiltration has been associated with improved disease recovery (Blomster et al., 2013). These studies highlight the regulatory function of splenic monocytes during the development of CNS-related diseases and demonstrate the need to further understand their biology.
In this study, we investigated the premise that the gut microbiome generates and secretes pattern recognition receptor (PRR) ligands in the circulation that regulate splenic Ly6Chigh monocyte homeostasis and function at steady state. We performed a 4-day antibiotic treatment to perturb the gut microbiome and simultaneously avoid impairment of hematopoiesis in the BM (Josefsdottir et al., 2017) that is known to affect myeloid cell populations in the spleen (Khosravi et al., 2014). We profiled immediate changes in the levels of several circulating PRR ligands and in the number and function of splenic-resident Ly6Chigh monocytes. Our findings (1) considerably expand the number of known PRR ligands present in the circulation at steady state and (2) demonstrate the role of PRR ligands in modulating the splenic Ly6Chigh monocyte immune profile during steady state.
Results
Families of the Bacteroidetes, Firmicutes, Tenericutes, and Actinobacteria phyla are the most susceptible taxa to our antibiotic treatment
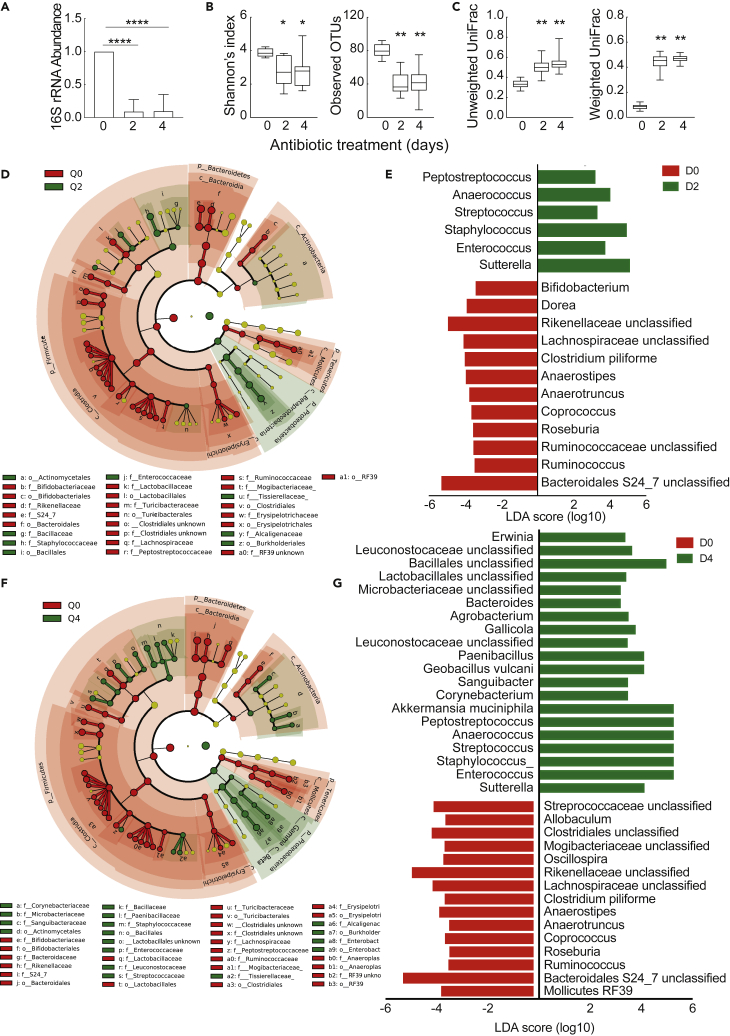
To investigate whether the gut microbiome has a regulatory role on the splenic monocyte homeostasis and function, we initiated our study by treating C57BL/6 mice with a broad-spectrum antibiotic cocktail for 4 days to disturb the gut microbiome. A significant decrease in bacterial abundance was detected in fecal samples 2 and 4 days post antibiotic administration (Figure 1A). Bacterial composition was significantly changed at the levels of alpha (measured by Shannon's index and observed operational taxonomic units) and beta (measured by unweighted and weighted UniFrac analysis) diversity 2 and 4 days post antibiotic administration (Figures 1B and 1C, and S1). Taxonomic analysis using the linear discriminant analysis effect size (LEfSe) algorithm showed a significant decrease in the relative abundance of Bacteroidetes, Firmicutes, Tenericutes, and Actinobacteria with a concomitant increase in Proteobacteria in the antibiotic-treated group (Figures 1D and 1F). Among the most affected taxa were members of the S24_7 and Rikenellaceae families of the Bacteroidetes phylum because their relative abundance was shifted from 40.71% to 1.1% and 0.49% and from 20.6% to 2.58% and 1.14%, respectively, during treatment (Figures 1E and 1G and Tables S1 and S2). Within the Firmicutes phylum, several members of the Clostridiaceae, Lachnospiraceae, Ruminococcaceae, and Mogibacteriaceae families underwent a significant decrease, whereas a modest reduction was detected for Mollicutes RF39, Tenericutes phylum, and Bifidobacterium, Actinobacteria phylum, in the treated group. Overall, our data describe significant quantitative and qualitative changes in the homeostasis of the gut microbiota after 2 and 4 days of antibiotic treatment and identify certain families of the Bacteroidetes, Firmicutes, Tenericutes, and Actinobacteria phyla as the most susceptible bacterial taxa to our treatment.
Figure 1.
Families of the Bacteroidetes, Firmicutes, Tenericutes, and Actinobacteria phyla are the most susceptible taxa to our antibiotic treatment
(A) Relative expression of 16S rRNA in fecal samples of mice drinking water with or without antibiotics for 2 and 4 days. Quantification was performed using qPCR analysis. Relative expression was normalized to milligrams of fecal sample. Data represent mean ± SD, n = 8.
(B) Shannon's entropy index and observed OTUs were calculated for fecal microbial communities to compare differences in alpha-diversity between samples from antibiotic-treated and untreated animals for the indicated time period. Statistical significance was calculated using the Kruskal-Wallis pairwise test.
(C) Beta-diversity values were calculated using the unweighted and weighted UniFrac distance, to examine differences in overall microbial communities. Statistical significance was calculated using the PERMANOVA pair wise test.
(D) Taxonomic cladogram obtained from LEfSe analysis of 16S sequences from fecal samples of antibiotic-treated and untreated animals. Red color indicates the bacterial taxa enriched in untreated animals (Q0), whereas green color indicates bacterial taxa enriched in mice treated with antibiotics for 2 days (Q2).
(E) Linear discriminant analysis (LDA) scores of bacterial genera enriched in untreated mice shown in red as negative values; in treated mice for 2 days shown in green as positive values.
(F) Taxonomic cladogram comparing fecal bacterial composition in untreated (red) and treated animals for 4 days (green).
(G) LDA scores of bacterial genera enriched in untreated mice (Q0) shown in red as negative values; in treated mice for 4 days (Q4) shown in green as positive values.
Data represent mean ± SD, n = 8 mice per group. p values are shown as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Decreased levels of PRR ligands in the circulation and perturbation of the splenic monocyte homeostasis upon dysbiosis of the gut microbiota
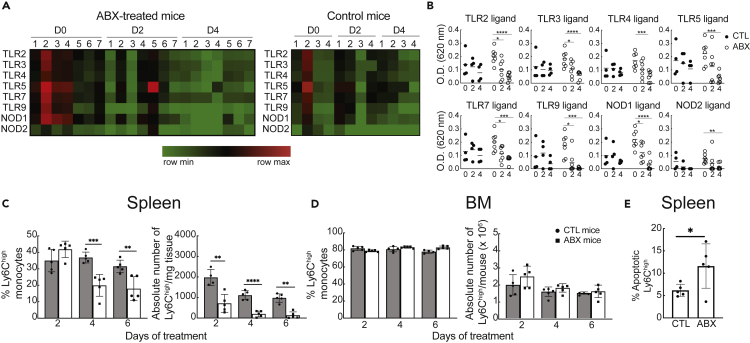
We next addressed whether dysbiosis of the gut microbiota affected the levels of microbial products in the circulation of antibiotic-treated animals. Mice were administered drinking water with (n = 7) or without (n = 4) antibiotics, and sera were collected before (day 0) and on days 2 and 4 of treatment. Detection of PRR ligands in the sera of individual mice was tested using the HEK-Blue PRR reporter cell lines. Ligands for TLR2, 3, 4, 5, 7, and 9 and NOD1 and 2 were constitutively present at low levels on day 0 in all mice from both groups (Figures 2A and 2B). By day 4, the levels of all PRR ligands were significantly decreased only in mice receiving antibiotic treatment, suggesting (1) that the gut microbiome is the source producing and secreting PRR ligands into the circulation at steady state and (2) that a brief exposure to antibiotics was sufficient to mediate this reduction in PRR ligand levels.
Figure 2.
Decreased levels of PRR ligands in the circulation and perturbation of the splenic monocyte homeostasis upon dysbiosis of the gut flora
(A and B) Levels of PRR ligands in the d0, d2, and d4 sera of individual mice treated with (n = 7) or without (n = 4) antibiotics using reporter HEK Blue cells lines for detection as described in transparent methods. In the heatmap, each column represents the serum response of an individual mouse against the PRR ligand listed in each row. Each cell line was incubated with serum in duplicate wells. Data are representative of four independent experiments. Statistical significance was assessed by paired Student's t test.
(C and D) Frequency and absolute number of Ly6Chigh monocytes in the (C) spleen and (D) BM of mice treated with or without antibiotics for the indicated time period. Flow cytometric analysis was gated on live LIN−CD11b+ cells as described in transparent methods. Absolute number of Ly6Chigh monocytes was normalized to milligrams of tissue for spleen, whereas total BM cells recovered from each mouse are shown for BM. Data show the mean ± SEM, and they are representative of three independent experiments (n = 6 per group).
(E) Ex vivo detection of apoptosis in splenic Ly6Chigh monocytes from mice treated with or without antibiotics for 3 days. Splenocyte suspensions were incubated with FAM-FLIVO, and 1 h later, cells were washed twice and fixed and analyzed by flow cytometry.
Data show the frequency of apoptotic cells gated on Ly6Chigh monocytes (mean ± SEM, n = 5 mice per group). Statistical significance was assessed by Student's t test. p values are shown as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
To examine whether the reduction in the levels of PRR ligands would correlate with changes in the splenic monocyte homeostasis, we analyzed the frequency and absolute numbers of Ly6Chigh monocytes in the spleens of mice treated with or without antibiotics for 2, 4, and 6 days. Both the frequency and absolute numbers of splenic Ly6Chigh monocytes were significantly decreased in the experimental group after 4 and 6 days of antibiotic treatment compared with the untreated mice (Figure 2C). Similar observations were not detected in the BM of the same animals (Figure 2D) suggesting that the short-term antibiotic treatment did not impair hematopoiesis in the BM, known to affect the numbers of splenic monocytes (Khosravi et al., 2014). Of note, the frequency and absolute numbers of Ly6Chigh cells were not significantly altered in antibiotic-treated versus untreated germ-free (GF) mice (Figure S2) suggesting that perturbation of the monocyte homeostasis is microbiome-mediated rather than a direct toxic effect of the antibiotic cocktail on immune cells. To test whether the antibiotic treatment induced increased cell death of splenic monocytes, we performed an apoptosis assay on Ly6Chigh monocytes ex vivo because apoptotic cells do not accumulate at steady state in vivo (Poon et al., 2014). Following antibiotic administration for 3 days, the apoptotic rate was significantly higher in the splenic Ly6Chigh monocytes from the antibiotic-treated group compared with the control group (Figure 2E). Cell death was not related to bacterial translocation into peripheral organs because we did not detect bacterial growth in mesenteric lymph node, liver, and splenic cell suspensions of mice treated with antibiotics under aerobic and anaerobic conditions (data not shown). Splenic monocytes from antibiotic-treated mice neither underwent increased differentiation to dendritic cells/macrophages nor received signals promoting their immediate exit from the tissue during treatment, as previously shown in angiotensin II-infused mice (Swirski et al., 2009), using an intrasplenic monocyte transfer approach (Figure S3). Taken together, our data demonstrate that decreased levels of PRR ligands in the circulation of antibiotic-treated mice correlate with perturbation of the splenic monocyte homeostasis due to an increased rate of apoptosis in this cell population.
Phenotypic and functional changes in splenic Ly6Chigh monocytes after depletion of the gut microbiota
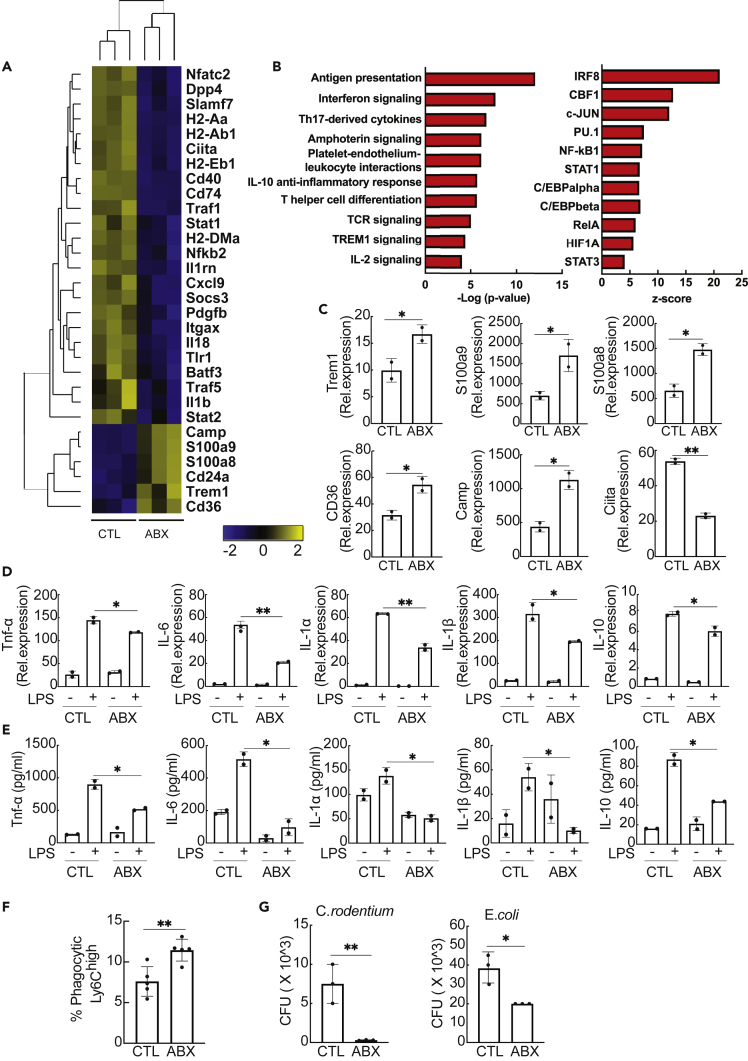
Next, we examined the phenotype of splenic monocytes after a 3-day treatment with antibiotics using the Nanostring nCounter platform. RNA profiling of Ly6Chigh monocytes revealed that genes involved in antigen presentation (Ciita, H2-Aa, H2-Ab1, Cd74, Cd40, H2-DMa) as well as SOCS3, Stat1, Traf1, and Nkb2 were significantly downregulated following antibiotic treatment (Figure 3A). Significant upregulation was detected for Trem1, a triggering receptor; Cd36, a phagocytic receptor; Camp, cathelicidin anti-microbial peptide (del Fresno et al., 2009); and S100a8 and S100a9, endogenous alarmins that can induce hyporesponsiveness (Austermann et al., 2014). Gene Enrichment Analysis predicted the top 10 biological pathways associated with the differentially expressed (DE) genes such as antigen presentation, interferon and amphoterin signaling, as well as the top 10 putative transcriptional factors regulating their expression (Figure 3B). Changes in expression of Trem1, CD36, S100a8, S100a9, Camp, and Ciita were validated by qPCR (Figure 3C) and were not detected in Ly6Chigh monocytes from antibiotic-treated GF mice (Figure S2). The aforementioned data suggest that splenic Ly6Chigh monocytes acquire an immature phenotype marked by decreased expression of antigen-presenting molecules and increased expression of genes involved in phagocytosis and anti-microbial defense at the RNA level following antibiotic treatment.
Figure 3.
Phenotypic and functional changes in splenic Ly6Chigh monocytes after depletion of the gut microbiota
(A) Heatmap depicting hierarchical clustering of significantly upregulated (yellow) and downregulated (blue) genes in splenic Ly6Chigh monocytes from untreated and treated animals for 3 days as calculated by the nSolver software.
(B) The 10 most significant biological networks and 10 top upstream transcriptional regulators of the DE genes of Ly6Chigh monocytes as predicted by the MetaCore software.
(C) Validation of changes in gene expression of Trem-1, S100a8, S100a9, Cd36, Camp, and Ciita by qPCR. Gene expression is presented relative to Gapdh. Data show mean ± SEM, and they are representative of two experiments (n = 10 for antibiotic-treated group, n = 5 for control group).
(D) Quantitative PCR analysis of the expression of Tnf-α, IL-6, IL-1α, IL-1β, and IL-10 in splenic Ly6Chigh monocytes from antibiotic-treated and untreated mice after ex vivo stimulation with or without LPS. Gene expression has been normalized to Gapdh. Data show mean ± SD, and they are representative of two experiments (n = 10 for antibiotic-treated group, n = 5 for control group).
(E) Detection of Tnf-α, IL-6, IL-1α, IL-1β, and IL-10 in the culture supernatant of splenic Ly6Chigh monocytes from antibiotic-treated and untreated mice after ex vivo stimulation with or without LPS. Data show mean ± SD, and they are representative of two experiments (n = 10 for antibiotic-treated group, n = 5 for control group).
(F) Percentage of splenic Ly6Chigh monocytes from antibiotic-treated and untreated mice containing fluorescently labeled beads, 24 h after intra-splenic bead injection (n = 7 per group). Data show mean ± SD, and they are representative of two experiments.
(G) Gentamicin protection assay on splenic Ly6Chigh monocytes from antibiotic-treated and untreated mice with C. rodentium and E. coli. Values (mean ± SD) show absolute colony-forming unit (CFU) counts after overnight incubation of cell lysates at 37°C and are representative of three experiments (n = 10 for antibiotic-treated group, n = 5 for control group).
Statistical significance was assessed by Student's t test for pairwise comparisons. p values are shown as ∗p < 0.05, ∗∗p < 0.01.
At the functional level, splenic Ly6Chigh monocytes from antibiotic-treated mice produced significantly lower levels of TNF-α, IL-6, IL-1α, IL-1β, and IL-10 after lipopolysaccharide (LPS) stimulation compared with control monocytes both at the RNA and protein levels (Figures 3D and 3E). This difference was not due to impaired ex vivo survival of splenic monocytes from antibiotic-treated mice, as previously reported (Hergott et al., 2016) (Figure S4). Also, increased phagocytic ability was detected in Ly6Chigh monocytes from antibiotic-treated mice compared with control mice after intrasplenic injection of fluorescently labeled microspheres (Figure 3F). Finally, Ly6Chigh monocytes had a higher anti-microbial capacity toward E. coli, K12 strain, and Citrobacter rodentium compared with the control group in a gentamicin protection assay (Figure 3G). Overall, these results demonstrate that splenic Ly6Chigh monocytes acquire an immature phenotype with decreased expression of antigen-presenting molecules accompanied by decreased production of inflammatory cytokines and increased phagocytic and anti-microbial abilities following gut dysbiosis.
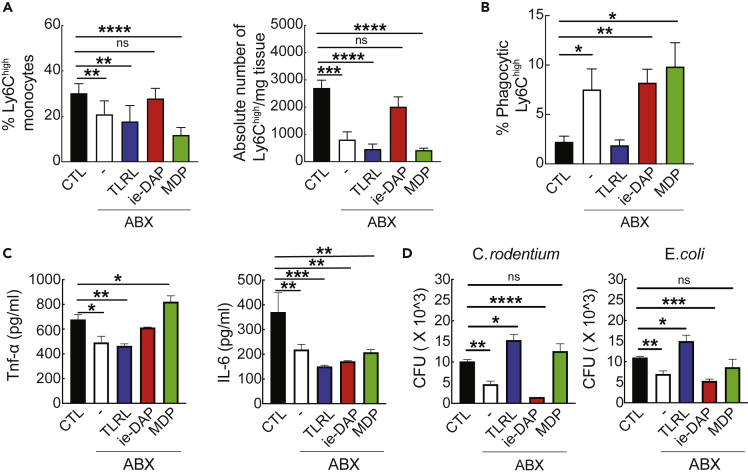
Addition of PRR ligands restores splenic Ly6Chigh monocyte numbers and functions in antibiotic-treated mice
Last, we investigated whether addition of certain PRR ligands in the circulation would restore splenic monocyte homeostasis and function in antibiotic-treated mice. As described in transparent methods, after a 3-day treatment with antibiotics, mice were injected intraperitoneally (i.p.) with low levels of (1) a TLR ligand (TLRL) cocktail, (2) ie-DAP (a NOD1 ligand), or (3) MDP (a NOD2 ligand). Injection of ie-DAP, but not of TLRL cocktail or MDP, in antibiotic-treated mice increased the frequency and the absolute numbers of splenic Ly6Chigh cells (Figure 4A). At the functional level, the phagocytic ability of splenic Ly6Chigh cells from antibiotic-treated mice was restored to levels similar to the untreated group when mice were injected with the TLRL cocktail but not with ie-DAP or MDP (Figure 4B). In terms of cytokines, we monitored the production of TNF-α and IL-6 in ex vivo-obtained Ly6Chigh monocytes after LPS stimulation. In antibiotic-treated mice, ie-DAP and MDP, but not TLRL cocktail, produced TNF-α at similar or higher levels compared with untreated mice (Figure 4C). Interestingly, injection of any of the PRR ligands restored the synthesis of IL-6 in cells from the treated group (Figure 4C). Regarding the antimicrobial capacity of splenic Ly6Chigh monocytes, addition of TLRL cocktail and MDP in antibiotic-treated mice brought back to control levels the ability of C. rodentium and E. coli to survive intracellularly (Figure 4D). Taken together, our data suggest that addition of certain PRR ligands in the circulation of antibiotic-treated mice restores the numbers and normal function of splenic Ly6Chigh monocytes. These findings highlight that (1) perturbation of the splenic Ly6Chigh numbers and function in antibiotic-treated mice is caused by the absence of certain circulating PRR ligands and (2) circulating PRR ligands have diverse and pleiotropic effects on the immune functions of splenic Ly6Chigh monocytes.
Figure 4.
Addition of PRR ligands restores splenic Ly6Chigh monocyte number and functions in antibiotic-treated mice
(A) Percentage and absolute number of splenic Ly6Chigh monocytes from untreated and antibiotic-treated mice injected i.p. with either ie-DAP, MDP, or TLRL or PBS. Data show mean ± SD and are representative of two experiments (n = 6 per group).
(B) Percentage of splenic Ly6Chigh monocytes from untreated and antibiotic-treated mice injected i.p. with either ie-DAP, MDP, or TLRL or PBS containing fluorescently labeled beads. Flow cytometric analysis was assessed 24 h after intra-splenic bead injection (n = 6 per group). Data show mean ± SD, and they are representative of three experiments.
(C) Presence of TNF-α and IL-6 in the culture supernatant of LPS-stimulated splenic Ly6Chigh monocytes from untreated and antibiotic-treated mice injected i.p. with either ie-DAP, MDP, or TLRL or PBS. Data (mean ± SD) are representative of two experiments (n = 6 per group).
(D) Gentamicin protection assay on splenic Ly6Chigh monocytes from untreated and antibiotic-treated mice injected i.p. with either ie-DAP, MDP, or TLRL or PBS with C. rodentium and E. coli. Absolute CFU counts (mean ± SD) after overnight incubation of cell lysates at 37°C are representative of two experiments (n = 6 per group).
Statistical significance was assessed by one-way ANOVA test for pairwise comparisons. p values are shown as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Discussion
In this study, we examined whether circulating PRR ligands produced constitutively by the gut microbiome regulate the splenic Ly6Chigh monocyte homeostasis and function during steady state. Our study focused on splenic Ly6Chigh monocytes as these immune cells play a regulatory role in the pathogenesis of many diseases such as EAE (Zhu et al., 2007; Melero-Jerez et al., 2020), ALS (Butovsky et al., 2012), stroke (Seifert and Offner, 2018; Pennypacker and Offner, 2015), atherosclerosis (Robbins et al., 2012, Swirski et al., 2016), ischemic myocardial injury (Leuschner et al., 2012; Swirski et al., 2009), lung ischemia-reperfusion injury (Hsiao et al., 2018), colitis (Griseri et al., 2012) and several tumor models (Cortez-Retamozo et al., 2012; Richards et al., 2013; Wu et al., 2018). We induced dysbiosis of the gut microbiome by administering a broad-spectrum antibiotic cocktail for a very short time period, compared with other studies (Kennedy et al., 2018), to identify the direct role of circulating PRR ligands on the maintenance of monocytes. Our findings delineate a new level of regulation by the microbiome in addition to the regulation of hematopoiesis, previously described in experiments with a 2- to 5-week exposure to antibiotics (Josefsdottir et al., 2017; Khosravi et al., 2014). Similar observations have been reported for neutrophils where the gut microbiome directly affects their function in blood in addition to their production in the BM (Zhang and Frenette, 2019). We conducted our experiments using female mice as the innate immune responses are generally stronger in females than in males (Klein and Flanagan, 2016) and the intestinal microbiota appears consistent throughout the estrous cycle (Wallace et al., 2018).
In our study, we identified the S24_7 and Rikenellaceae families (Bacteroidetes phylum); Clostridiaceae, Lachnospiraceae, Ruminococcaceae, and Mogibacteriaceae families (Firmicutes phylum); Mollicutes RF39 (Tenericutes phylum); and Bifidobacterium (Actinobacteria phylum) as the most susceptible taxa to our antibiotic treatment. This finding, in combination with the parallel reduction of PRR ligands in the circulation of treated animals, demonstrates that these bacterial families may be among the primary sources of PRR ligand production within the gut during steady state. Conversely, our data suggest that all the bacterial taxa that underwent significant expansion during the treatment, including Sutterella, Staphylococcus, and Akkermansia muciniphila, may not contribute significantly to the production of circulating PRR ligands. The simultaneous perturbation of the gut microbiota homeostasis and reduction of PRR ligands from the circulation of antibiotic-treated animals further supports the premise that the gut microbiome constitutively produces and secretes bacterial-derived products in the circulation, as previously shown in antibiotic, streptozotoxin-treated mice (Thaiss et al., 2018). To our knowledge, our data report for the first time the simultaneous presence of TLR2, 3, 4, 5, 7, and 9 and NOD1 and NOD2 ligands at low levels in the sera of naive animals, supporting previous studies that have detected NOD1 (Clarke et al., 2010), NOD2 (Huang et al., 2019) and TLR2 (Farrokhi et al., 2013) ligands in the circulation of naive mice.
Reduction of PRR ligands from the circulation of antibiotic-treated mice was associated with perturbation of splenic but not BM-derived Ly6Chigh monocytes. This observation could be explained either by a differential regulation of spleen versus BM-derived monocytes by the gut microbiome or by a prompt replenishment of monocytes by progenitors within the BM of treated animals. Our data propose that the homeostasis of splenic Ly6Chigh monocytes does not depend alone on BM hematopoiesis effects as suggested earlier (Khosravi et al., 2014) but that it can also be impacted directly by signals from commensal bacteria. This apparent discrepancy could be due to the short-term antibiotic treatment we performed (4 days) versus the long-term treatment (4–5 weeks) performed by Khosravi et al. Disruption of the monocyte homeostasis is microbiome-mediated because we did not observe similar changes in the numbers of splenic Ly6Chigh monocytes in antibiotic-treated GF animals. Perturbation was also related to increased apoptotic rate within the spleen further supporting that deprivation of gut microbiota-derived signals has an immediate impact on the homeostasis of splenic monocytes. We did not detect increased differentiation to DC/macrophages or increased trafficking of monocytes from the spleen to the bloodstream or to the gut of antibiotic-treated mice in response to increased bacterial cell death.
Splenic Ly6Chigh monocytes underwent transcriptional and functional changes after 3 days of antibiotic administration. Molecules involved in antigen presentation pathways (Ciita, H2-Aa, H2-Ab1, Cd74, Cd40, H2-DMa) as well as Stat1, Traf1, and Nkb2 were significantly downregulated, whereas Trem1, a proinflammatory factor; CD36, a scavenger receptor; and Camp, an antimicrobial peptide, were significantly upregulated. Functionally, Ly6Chigh monocytes from antibiotic-treated mice produced lower levels of proinflammatory cytokines and they had acquired increased phagocytic and antimicrobial abilities, hallmarks of endotoxin-tolerant monocytes (del Fresno et al., 2009). It could be that in the absence of circulating tonic signals, S100a8 and S100a9, two calcium-binding proteins, act as endogenous alarmins inducing a state of hyporesponsiveness to monocytes, similar to exogenous LPS (Austermann et al., 2014).
As dysbiosis of the gut microbiome can destabilize the whole metabolome signature in the circulation (Vernocchi et al., 2016), we addressed whether perturbation of the splenic Ly6Chigh monocyte numbers and function in antibiotic-treated mice is specifically regulated by the levels of circulating PRR ligands. We report that the NOD1 signaling pathway regulates the lifespan of splenic Ly6Chigh monocytes confirming findings from a previous study (Hergott et al., 2016). The presence of TLRL increased the phagocytic properties of Ly6Chigh cells, as has previously been described in cell lines in vitro (Doyle et al., 2004). Further studies need to address which TLRL, individually or in combination, has this regulatory role. Interestingly, our observation that the antimicrobial capacity of Ly6Chigh monocytes is regulated by TLRL and MDP is in agreement with previous studies suggesting enhancement of the antimicrobial capacity of phagocytes by TLRs (Brightbill et al., 1999) and MDP (O'Reilly and Zak, 1992). In terms of cytokine production, the levels of TNF-α were restored after injection of ie-DAP and MDP in LPS-stimulated Ly6Chigh monocytes, whereas any PRR ligand affected the production of IL-6. Our data demonstrate that individual circulating PRR ligands have pleiotropic effects on the immune functions of splenic Ly6Chigh monocytes. Further studies will be performed to dissect the underlying mechanisms of these complex interactions. Also, in our study, we have not addressed the impact of the gut dysbiosis on the levels of other microbial metabolites, such as SCFAs, β-glucans, and bile acids, known to affect the biology of monocytes (Fiorucci et al., 2018; van de Wouw et al., 2019).
Taken together, our findings suggest that the ecology of the intestinal microbiota can control the homeostasis and function of splenic Ly6Chigh monocytes during steady state via regulation of the levels of circulating PRR ligands. Identification of the PRR ligand-monocyte crosstalk provides an opportunity to develop intervention strategies to modulate splenic monocytes, an important immune cell subset in the pathogenesis of cardiovascular and CNS-related diseases.
Limitations of the study
In this study we demonstrate that circulating PRR ligands produced by the gut microbiome regulate the number and immune functions of splenic Ly6Chigh monocytes. Whether circulating PRR ligands act directly upon splenic Ly6Chigh monocytes or indirectly via other immune cells in vivo can be further investigated in future studies.
Resource availability
Lead contact
Howard L. Weiner, Ann Romney Center for Neurologic Diseases, Brigham and Women's Hospital, Harvard Medical School, Building for Transformative Medicine, 60 Fenwood Road, 10002G, Boston, MA, 02115-6128, hweiner@rics.bwh.harvard.edu.
Materials availability
This study did not generate new unique reagents.
Data and code availability
The raw data from the 16S rRNA sequencing library and the Nanostring data have been uploaded in Mendeley (https://doi.org/10.17632/tjdwjnzskr.1).
All datasets supporting the current study are available from the corresponding author on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Vladimir Yeliseyev for technical assistance with GF mouse experiments, Joseph Rone for technical assistance with bacterial cultures, Kit Fuhrman and Clement David for assistance with analysis of Nanostring data, Biopolymers Facility at Harvard Medical School for sequencing data, and Deneen E. Kozoriz and Rajesh K. Krishnan for sorting. The research was supported by NIH grant RO1# NS087226 to H.W.
Author contributions
Conceptualization, P.K. and H.L.W.; investigation, P.K., A.S., and V.W.; methodology, P.K., S.L., L.M.C., M.F., R.R., and D.G.; resources: S.L.; formal analysis, P.K., L.M.C., and V.W.; writing – original draft, P.K. and H.L.W.; writing – review & editing, P.K. and H.L.W.; funding acquisition, H.L.W.; supervision, H.L.W.
Declaration of interests
The authors declare no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102356.
Supplemental information
References
- Anstadt E.J., Fujiwara M., Wasko N., Nichols F., Clark R.B. TLR tolerance as a treatment for central nervous system. Autoimmun. J. Immunol. 2016;197:2110–2118. doi: 10.4049/jimmunol.1600876. [DOI] [PubMed] [Google Scholar]
- Austermann J., Friesenhagen J., Fassl S.K., Petersen B., Ortkras T., Burgmann J., Barczyk-Kahlert K., Faist E., Zedler S., Pirr S. Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions. Cell Rep. 2014;9:2112–2123. doi: 10.1016/j.celrep.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Balmer M.L., Schürch C.M., Saito Y., Geuking M.B., Cuenca M., Kovtonyuk L.V., McCoy K.D., Hapfelmeier S., Ochsenbein A.F. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J. Immunol. 2014;193:5273–5283. doi: 10.4049/jimmunol.1400762. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Harrison O.J. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomster L.V., Brennan F.H., Lao H.W., Harle D.W., Harvey A.R., Ruitenberg M.J. Mobilisation of the splenic monocyte reservoir and peripheral CX₃CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 2013;247:226–240. doi: 10.1016/j.expneurol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Brightbill H.D., Libraty D.H., Krutzik S.R., Yang R.B., Belisle J.T., Bleharski J.R., Maitland M., Norgard M.V., Plevy S.E., Smale S.T. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Butovsky O., Siddiqui S., Gabriely G., Lanser A.J., Ben Dake, Murugaiyan G., Doykan C.E., Wu P.M., Gali R.R., Iyer L.K. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke T.B., Davis K.M., Lysenko E.S., Zhou A.Y., Yu Y., Weiser J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez-Retamozo V., Etzrodt M., Newton A., Rauch P.J., Chudnovskiy A., Berger C., Ryan R.J.H., Iwamoto Y., Marinelli B., Gorbatov R. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Fresno C., Garcia-Rio F., Gomez-Pina V., Soares-Schanoski A., Fernandez-Ruiz I., Jurado T., Kajiji T., Shu C., Marin E., Gutierrez del Arroyo A. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 2009;183:2194. doi: 10.4049/jimmunol.0803350. [DOI] [PubMed] [Google Scholar]
- Doyle S.E., O'Connell R.M., Miranda G.A., Vaidya S.A., Chow E.K., Liu P.T., Suzuki S., Suzuki N., Modlin R.L., Yeh W.-C. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhi V., Nemati R., Nichols F.C., Yao X., Anstadt E., Fujiwara M., Grady J., Wakefield D., Castro W., Donaldson J. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin. Transl. Immunol. 2013;2:e8. doi: 10.1038/cti.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S., Biagioli M., Zampella A., Distrutti E. Bile acids Activated receptors regulate innate immunity. Front. Immunol. 2018;9:1853. doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi A., Wagner L.K., Li H., Dörr D., Medaglia C., Paul F., Shemer A., Jung S., Yona S., Mack M. Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol. 2020;21:525–534. doi: 10.1038/s41590-020-0661-1. [DOI] [PubMed] [Google Scholar]
- Gorjifard S., Goldszmid R.S. Microbiota--myeloid cell crosstalk beyond the gut. J. Leukoc. Biol. 2016;100:865–879. doi: 10.1189/jlb.3RI0516-222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griseri T., McKenzie B.S., Schiering C., Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37:1116–1129. doi: 10.1016/j.immuni.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., Mildner A., Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49:595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Hergott C.B., Roche A.M., Tamashiro E., Clarke T.B., Bailey A.G., Laughlin A., Bushman F.D., Weiser J.N. Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood. 2016;127:2460–2471. doi: 10.1182/blood-2015-10-675173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H.-M., Fernandez R., Tanaka S., Li W., Spahn J.H., Chiu S., Akbarpour M., Ruiz-Perez D., Wu Q., Turam C. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1β. J. Clin. Invest. 2018;128:2833–2847. doi: 10.1172/JCI98436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Wang J., Xu X., Wang H., Qiao Y., Chu W.C., Xu S., Chai L., Cottier F., Pavelka N. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat. Microbiol. 2019;4:766–773. doi: 10.1038/s41564-019-0381-1. [DOI] [PubMed] [Google Scholar]
- Josefsdottir K.S., Baldridge M.T., Kadmon C.S., King K.Y. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129:729–739. doi: 10.1182/blood-2016-03-708594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E.A., King K.Y., Baldridge M.T. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front. Physiol. 2018;9:1534. doi: 10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi A., Yáñez A., Price J.G., Chow A., Merad M., Goodridge H.S., Mazmanian S.K. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H. Immune regulation by microbiome metabolites. Immunology. 2018;154:220–229. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Leuschner F., Rauch P.J., Ueno T., Gorbatov R., Marinelli B., Lee W.W., Dutta P., Wei Y., Robbins C., Iwamoto Y. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Jerez C., Alonso-Gómez A., Moñivas E., Lebrón-Galán R., Machín-Díaz I., de Castro F., Clemente D. The proportion of myeloid-derived suppressor cells in the spleen is related to the severity of the clinical course and tissue damage extent in a murine model of multiple sclerosis. Neurobiol. Dis. 2020;140:104869. doi: 10.1016/j.nbd.2020.104869. [DOI] [PubMed] [Google Scholar]
- O'Reilly T., Zak O. Enhancement of the effectiveness of antimicrobial therapy by muramyl peptide immunomodulators. Clin. Infect. Dis. 1992;14:1100–1109. doi: 10.1093/clinids/14.5.1100. [DOI] [PubMed] [Google Scholar]
- Pennypacker K.R., Offner H. The role of the spleen in ischemic stroke. J. Cereb. Blood Flow Metab. 2015;35:186–187. doi: 10.1038/jcbfm.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon I.K.H., Lucas C.D., Rossi A.G., Ravichandran K.S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D.M., Hettinger J., Feuerer M. Monocytes and macrophages in cancer: development and functions. Cancer Microenviron. 2013;6:179–191. doi: 10.1007/s12307-012-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins C.S., Chudnovskiy A., Raunch P.J., Figueiredo J.-L., Iwamoto Y., Gorbatov R., Etzrodt M., Weber G.F., Ueno T., Rooijen N.V. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H.A., Offner H. The splenic response to stroke: from rodents to stroke subjects. J. Neuroinflamm. 2018;15:195. doi: 10.1186/s12974-018-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.-L., Kohler R.H., Chudnovskiy A., Waterman P. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F.K., Nahrendorf M., Libby P. Mechanisms of Myeloid Cell Modulation of Atherosclerosis. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.MCHD-0026-2015. [DOI] [PubMed] [Google Scholar]
- Thaiss C.A., Levy M., Grosheva I., Zheng D., Soffer E., Blacher E., Braverman S., Tengeler A.C., Barak O., Elazar M. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- van de Wouw M., Boehme M., Dinan T.G., Cryan J.F. Monocyte mobilisation, microbiota & mental illness. Brain Behav. Immun. 2019;81:74–91. doi: 10.1016/j.bbi.2019.07.019. [DOI] [PubMed] [Google Scholar]
- Vernocchi P., Del Chierico F., Putignani L. Gut microbiota profiling: metabolomics based approach to unravel compounds affecting human health. Front. Microbiol. 2016;7:1144. doi: 10.3389/fmicb.2016.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J.G., Potts R.H., Szamosi J.C., Surette M.G., Sloboda D.M. The murine female intestinal microbiota does not shift throughout the estrous cycle. PLoS One. 2018;13:e0200729. doi: 10.1371/journal.pone.0200729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Ning H., Liu M., Lin J., Luo S., Zhu W., Xu J., Wu W.-C., Liang J., Shao C.-K. Spleen mediates a distinct hematopoietic progenitor response supporting tumor-promoting myelopoiesis. J. Clin. Invest. 2018;128:3425–3438. doi: 10.1172/JCI97973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Frenette P.S. Cross talk between neutrophils and the microbiota. Blood. 2019;133:2168–2177. doi: 10.1182/blood-2018-11-844555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Bando Y., Xiao S., Yang K., Anderson A.C., Kuchroo V.K., Khoury S.J. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data from the 16S rRNA sequencing library and the Nanostring data have been uploaded in Mendeley (https://doi.org/10.17632/tjdwjnzskr.1).
All datasets supporting the current study are available from the corresponding author on request.