Abstract
Background
Soshiho-tang (SST), also known as Xiaochaihu-tang in China and Sho-saiko-to in Japan, is an Oriental herbal formula traditionally used to treat febrile diseases. Recently, several in vitro and in vivo studies have reported the anti-cancer, anti-liver disease, and anti-inflammatory activities of SST. However, there is little evidence of its effects on neurological diseases. We previously reported the inhibitory effects of SST on in vitro acetylcholinesterase (AChE) activation and amyloid-β (Aβ) aggregation, which are crucial hallmarks of Alzheimer's disease (AD). In the present study, we report that SST has preventive effects on memory impairment and neuronal cell changes in an Aβ-induced AD-like mouse model.
Methods
Male mice underwent injection of Aβ aggregates and administered SST (500, 1,000, or 2,000 mg/kg/day) for 20 days. Behavioral tests (passive avoidance task [PAT] and Morris water maze [MWM] test) were conducted. Lastly, brain sections were obtained from sacrificed mice for quantitative analysis.
Results
Intracerebroventricular (ICV) injection of Aβ aggregates significantly decreased the latency time in the PAT and MWM test compared to normal control. In contrast, SST administration markedly reversed the latency caused by Aβ injection. Additionally, our data revealed that SST-mediated improvements in memory impairment are related to its neuroprotective and anti-neuroinflammatory effects. On histological analysis, SST treatment protected neuronal loss and damage as well as microglial activation, and ameliorated amount of Aβ in brain of mouse model of AD.
Conclusion
Our findings suggest that SST may be a promising candidate for the development of novel drugs for AD.
Keywords: Alzheimer's disease, Anti-neuroinflammation, Memory improvement, Neuroprotection, Soshiho-tang
1. Introduction
Soshiho-tang (SST) is a herbal formula firstly introduced in the Sang-Han-Ron in China and also described in the Dong-Eui-Bo-Gam, a traditional Korean medicine book. The herbal composition of SST includes seven medicinal herbs Bupleuri Radix, Scutellariae Radix, Ginseng Radix, Pinelliae Tuber, Glycyrrhizae Radix et Rhizoma, Zingiberis Rhizoma Crudus, and Zizyphi Fructus. SST was traditionally used to control febrile diseases in several Asian countries.1 Recent studies support the notion that SST has potential anti-tumor,2, 3, 4, 5 anti-inflammatory,6, 7, 8 immune regulatory,9,10 and hepatoprotective5,7 activities in preclinical investigations. Most clinical studies using SST have shown its efficacy for liver problems such as hepatic dysfunction,11 chronic liver diseases,12 hepatitis B and C,13,14 and liver cancer.15
In relation to the effects of SST on neuronal diseases, Su et al. suggested the presence of anti-depressant effects in a rodent model.16,17 In addition, Koo et al. reported that SST is effective in improving the symptoms of Parkinson's disease in patients with pontine infarction.18 We recently reported the inhibitory effects of SST extracts on acetylcholinesterase (AChE) activation and amyloid-β (Aβ) aggregation, which are crucial major hallmarks for the pathogenesis of Alzheimer's disease (AD), suggesting the potential of SST as a dual target drug for AD.19 AD is an irreversible dementia caused by genetic and environmental factors.20 According to the information provided at ‘alzheimers.net (www.alzheimers.net)’, around 50 million people worldwide are affected by AD, and the number of patients is increasing continuously. Symptoms of early stage of AD include impairment of memory, thinking, language, and behavior skills, which eventually worsen and leads to death.21,22
However, no effective therapy has been developed for AD, in part due to its complex pathogenesis.23 Therefore, alternative approaches that do not involve targeting single molecule are necessary to overcome the limitations associated with development of AD drugs. Multi-component or multi-drug therapies are new strategies to design multi-target drugs for complex diseases caused by multiple pathophysiological factors. The interaction of multiple components concurrently and synergistically affects multiple targets, and results in better outcome for patients.24 Herbal medicines are characterized by multi-component and multi-target properties.25 Intriguingly, we and others have suggested the possibility of herbal formulas as being anti-AD drug candidates.26, 27, 28, 29 In the present study, we investigated the effects of the herbal medicine SST on memory deficiency and neuronal cell changes using an AD-like animal model to corroborate our previous in vitro data. We established an AD-like mouse model by injecting Aβ aggregates into the brain and conducted behavioral tests to explore memory capacity of the mice. Histological analysis was performed to determine the effects of SST on neuronal loss and inflammation in brain tissues.
2. Methods
2.1. SST and reagents
SST was obtained from Kyungbang Pharm. Co., Ltd. (Incheon, Korea). The specific composition of SST is shown in Table 1. There are five standard compounds of SST, including liquiritin, baicalin, baicalein, and wogonin, which were purchased from Sunny Biotech (Shanghai, China), and glycyrrhizin, which was obtained from ChemFaces Biochemical (Wuhan, China; (purity >98% by high-performance liquid chromatography [HPLC]). HPLC grade solvents acetonitrile and water were obtained from J. T. Baker Chemical (Phillipsburg, NJ, USA), and analytical grade acetic acid (AA) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Table 1.
Composition of SST.
| Latin name | Scientific name | Amount (g) |
|---|---|---|
| Bupleuri Radix | Bupleurum falcatum L. | 2.33 |
| Pinelliae Tuber | Pinellia ternata (Thunb.) Breit. | 1.67 |
| Zingiberis Rhizoma Crudus | Zingiber officinale Roscoe | 1.33 |
| Scutellariae Radix | Scutellaria baicalensis Georgi | 1.00 |
| Zizyphi Fructus | Ziziphus jujuba Mill. | 1.00 |
| Ginseng Radix | Panax ginseng C. A. Meyer | 1.00 |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhiza uralensis Fisch. | 0.67 |
2.2. Apparatus and chromatographic conditions
For the quantitative analysis of the five standard compounds in SST, we used the Waters Alliance e2695 HPLC system (Waters Corp., Milford, MA, USA) equipped with photodiode array (PDA) detector (#2998; Waters Corp.) was used. The data were acquired and processed using Empower software (version 3; Waters Corp). Gemini C18 analytical column (4.6 mm × 250 mm, 5 μm; Phenomenex, Torrance, CA, USA) was used for the chromatographic separation of the five compounds. The column temperature was maintained at 30°C. The mobile phases consisted of two solvents 1.0% (v/v) AA in water (A) and 1.0% (v/v) AA in acetonitrile (B). The gradient conditions were programed as follows: 15-40% B for 20 min, 40-55% B for 20 min, 55-100% B for 5 min, and 100% B for 10 min. The PDA detector recorded ultraviolet (UV) wavelengths ranging from 210 nm to 400 nm for scanning chromatograms. The flow rate and injection volume were 1.0 mL/min and 10 µL, respectively.
2.3. Preparation of sample solutions
SST was dissolved in 80% aqueous methanol to a final concentration of 10 mg/mL and filtered through a syringe filter (0.45-μm pore size). The stock solutions (1.0 mg/mL) of five standard compounds were mixed and diluted with methanol to obtain various concentrations of a standard mixture for quantitative analysis.
2.4. Animals
Male C57BL6 mice (24 ± 2 g) were purchased from the Orient Bio (Seoul, Korea) and acclimated for 1 week prior to the study with a standard rodent diet (2918C, ENVIGO, UK) and water was supplied ad libitum in individual polycarbonate cages. The animals were maintained at a temperature of 23 ± 3°C, with a relative humidity of 55 ± 15%, ventilation frequency of 10-20 times/hr, lighting time of 12 hours (lighted at 8 AM-light offed at 8 PM), and an illumination of 150-300 lux. The experiment was carried out according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee in Nonclinical Research Institute, Chemon Inc. (IACUC Approval No.2019-04-002).
2.5. Intracerebroventricular (ICV) injection of Aβ aggregates and SST administration
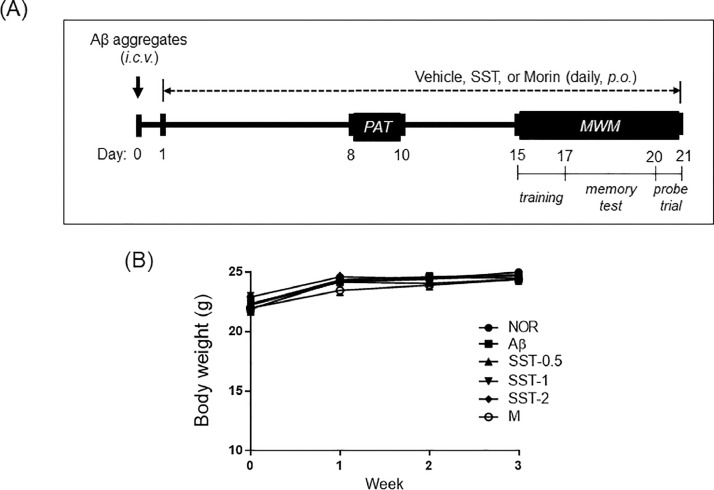
The Aβ1-42 peptides were obtained from Tocris Bioscience (Pittsburgh, PA, USA), dissolved in sterilized 0.1 M phosphate-buffered saline (PBS, pH 7.4) to a concentration of 1 μg/μL, and incubated at -20 °C for 4 weeks to encourage the formation of Aβ aggregates. Mice were anesthetized with mixed zoletil (tiletamine-zolazepam, Verbac, Carros, France) and rompun (Bayer, Leverkusen, Germany) (4:1 v/v) at a dose of 1 mL/kg before the injection of Aβ. The incubated Aβ aggregates were injected at the ICV area using a stereotaxic apparatus (Harvard Apparatus, Holliston, MA, USA), which was fixed at the anterior/posterior (AP) -1.0 mm, mediolateral/lateral +1.0 mm, and dorsal/ventral -2.5 mm. Aggregated Aβ (5 μL) was infused at a rate of 2 μL/min speed. Saline was injected in the same way to the control mice. One day after the Aβ injection, mice were divided into six groups (n = 9 per group), as follows: NOR (normal group), Aβ (Aβ aggregates-injected group), SST -0.5, -1, and -2 (500, 1,000, and 2,000 mg/kg of SST-treated groups into Aβ mice), and M (morin [10 mg/kg]-treated group). SST or morin was orally administered for 20 days from the day after Aβ injection. These animal study procedures were performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animals were housed in the facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International in accordance with the Guide for the Care and Use of Laboratory Animals (Guide, NRC2011).
2.6. Behavioral tests
The passive avoidance task (PAT) was performed using an electronic shock generator with lighted and darkened compartments (Jeungdo Bio & Plant Co. Ltd., Seoul, Korea) and assessed on the 8th to 10th day after Aβ injection. Transfer latency time, representing the time that the mice remained in the lighted compartment within 5 min, was recorded. The Morris water maze (MWM) test was conducted for 7 days between the 15th and 21st day after the injection of Aβ. Mice were put in a pool of four designated release points and allowed to find the platform for 60 sec. After finding the platform, the animals were allowed to rest for 30 sec on the platform. If mice failed to find the platform within 60 sec, the animals were guided to rest on the platform for 30 sec. After all animals finished the first trial, the next trial was started. The release points were randomly chosen every time without overlap. The time taken to find the platform (escape latency) was monitored (training: 2 days, behavioral test: 4 days, and probe trial: 1 day). On the last day (7th day), the platform was removed one hour after SST administration and the probe trial was performed for 60 sec to measure the crossing number on the platform. Experimental mice were administrated SST or morin prior to the behavioral tests and all efforts were made to minimize animal suffering.
2.7. Nissl staining and immunohistochemistry
All mice were sacrificed under anesthesia at the end of the experimental period, and the hippocampal and cortical tissues were immediately isolated on ice and stored at -80°C for further analysis. Three mouse brains from each group were perfused intracardially with PBS (pH 7.4) and fixed in 4% paraformaldehyde. Brain paraffin blocks (5-μm thick) were prepared. The section slides were deparaffinized in xylene and hydrated with a series of graded ethanol. To quench endogenous peroxidase activity, slides were treated with 0.3% hydrogen peroxide in methanol for 25 min and briefly rinsed in PBS. For Nissl staining, slides were immersed in 1% cresyl violet acetate solution, washed with water, and dehydrated through 90% and 100% ethanol for 5 min before mounting in xylene. For antigen retrieval on the brain sections, slides were boiled in citrate buffer (pH 6.0) in a microwave for 15 min. Sections were incubated with horse serum for blocking for 1 hr at 37°C and then incubated overnight at 4°C with primary antibodies anti-neuronal nuclei (NeuN) (Abcam, Cambridge, UK, 1: 250 dilution) and anti-ionized calcium-binding adaptor molecule-1 (Iba-1) (Wako Pure Chemicals, Osaka, Japan. 1:100 dilution), and anti-Aβ (QED Bioscience, San Diego, CA, USA, 1:250 dilution). The slides were incubated with the labeled streptavidin-biotin for 30 min and visualized by diaminobenzidine tetrahydrochloride (DAB, Vector Laboratories, Burlingame, CA, USA). Images were viewed using an Olympus DP71 microscope (Tokyo, Japan) at 400 x magnification. Quantitative analysis of stained cells was performed by blinded investigators using Image J software (Java-based image processing program, NIH, Bethesda, MD, USA).
2.8. Measurement of AChE activity
AChE activity in mouse brain lysates were measured according to the commercial manufacture's protocol using an Acetylcholinesterase Activity Assay Kit (Biomax, Seoul, Korea). The brain homogenates were applied onto the incubated 96-well plates with substrate buffer for 30 min at 37°C and measured for the absorbance of mixture using a microplate reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA) at 570 nm.
2.9. Statistical analysis
All data were expressed as the mean ± SEM and statistical analyses were evaluated via a one-way analysis of variance (ANOVA) followed by an unpaired Student's t-test or Tukey's multiple comparison test. All experiments were performed individually at least three times. GraphPad Prism 8.0 software program (GraphPad Software Inc., San Diego, CA, USA) was used for all analyses. Differences at p < 0.05 were considered statistically significant.
3. Results
3.1. Simultaneous analysis of five standard compounds of SST using HPLC analysis
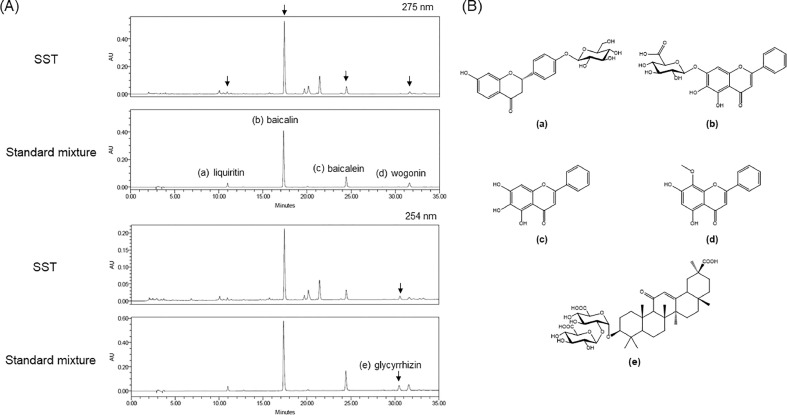
To simultaneously quantify the five standard compounds in SST, the optimized HPLC method was used. The chromatograms showed good separation using mobile phases consisting of 0.1% (v/v) AA in water and 0.1% (v/v) AA in acetonitrile. The UV wavelengths to detect compounds were 275 nm for liquiritin, baicalin, baicalein and wogonin, and 254 nm for glycyrrhizin. The retention times of liquiritin, baicalin, baicalein, wogonin, and glycyrrhizin were 10.95, 17.44, 24.49, 30.64, and 31.66 min, respectively. HPLC chromatograms of standard mixture and SST are presented in Fig. 1A and the chemical structures of the five compounds are shown in Fig. 1B. The calibration curves for the five compounds were calculated by the linear relationships between the peak area (y) and concentration (x, μg/mL) from the following ranges: 1.5625-50 μg/mL for liquiritin and baicalein, 12.5-400 μg/mL for baicalin, 3.125-100 μg/mL for glycyrrhizin, and 0.78125-25 μg/mL for wogonin (Table 2). All correlation coefficient values showed good linearity (r2 ≥ 1.0000). The limit of detection (LOD) for the five compounds ranged from 0.051-0.816 μg/mL and the limit of quantification (LOQ) ranged from 0.156-2.474 μg/mL. The amounts of liquiritin, baicalin, baicalein, wogonin, and glycyrrhizin in SST were 0.671, 6.693, 0.897, 2.419, and 0.332 mg/g, respectively (Table 2).
Fig. 1.
HPLC chromatograms of the ethanol extract of Soshiho-tang (SST) and standard mixture. (A) The five standard compounds liquiritin (a), baicalin (b), baicalein (c), wogonin (d), and glycyrrhizin (e) were separated by a Gemini C18 analytical column at 30°C at 275 or 254 nm. (B) Chemical structures of the standard compounds of SST. Liquiritin (a), baicalin (b), baicalein (c), wogonin (d), glycyrrhizin (e).
Table 2.
Regression equation, linearity, LOD, LOQ, and content of five compounds.
| Compound | Linear range(μg/mL) | Regression equation(y = ax + b)a) | r2 | LODb)(μg/mL) | LOQc)(μg/mL) | Content(mg/g) | |
|---|---|---|---|---|---|---|---|
| Slope (a) | Intercept (b) | ||||||
| Liquiritin | 1.5625 - 50 | 16198 | 1075.6 | 1.0000 | 0.160 | 0.485 | 0.671 ± 0.003 |
| Baicalin | 12.5 - 400 | 63846 | 21716 | 1.0000 | 0.816 | 2.474 | 6.693 ± 0.002 |
| Baicalein | 1.5625 - 50 | 56559 | -9649.7 | 1.0000 | 0.162 | 0.490 | 0.897 ± 0.002 |
| Glycyrrhizin | 3.125 - 100 | 4984.7 | -973.07 | 1.0000 | 0.184 | 0.557 | 2.419 ± 0.005 |
| Wogonin | 0.78125 - 25 | 56066 | 1851 | 1.0000 | 0.051 | 0.156 | 0.332 ± 0.001 |
y=ax+b, y means peak area and x means concentration (μg /mL).
LOD: 3.3 × (standard deviation (SD) of the response / slope of the calibration curve).
LOQ: 10 × (SD of the response / slope of the calibration curve).
3.2. Ameliorating effects of SST on memory impairment in an Aβ-induced mouse model of AD
To investigate whether SST could improve impaired memory, Aβ-induced AD-mice were utilized. As shown in Fig. 2A, a mouse model of AD was established by injecting prepared Aβ aggregates into the ICV regions. From the first day after Aβ injection, the mice were given a daily gavage treatment of vehicle, SST (500, 1,000, or 2,000 mg/kg), or morin (10 mg/kg) for 20 days. No significant difference in body weight was observed among the six groups during the experimental period (Fig. 2B).
Fig. 2.
Establishment of the Aβ-induced AD mouse model. (A) Experimental timeline of Aβ injection into the brain and SST administration. Aβ aggregates were prepared and injected in the intracerebroventricular (ICV) area of male C57BL6 mice with a stereotaxic apparatus. One day after Aβ injection, SST extracts were orally administered at 500, 1,000, or 2,000 mg/kg/day for 20 days. Passive avoidance task (PAT) and Morris water maze (MWM) tests were performed between the 8th-10th day and 15th-21st day, respectively. Morin was used as a positive control of Aβ inhibition. (B) Mean body weight changes of the experimental mice during the three weeks of schedule. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, and M: morin (10 mg/kg)-treated group into Aβ mice.
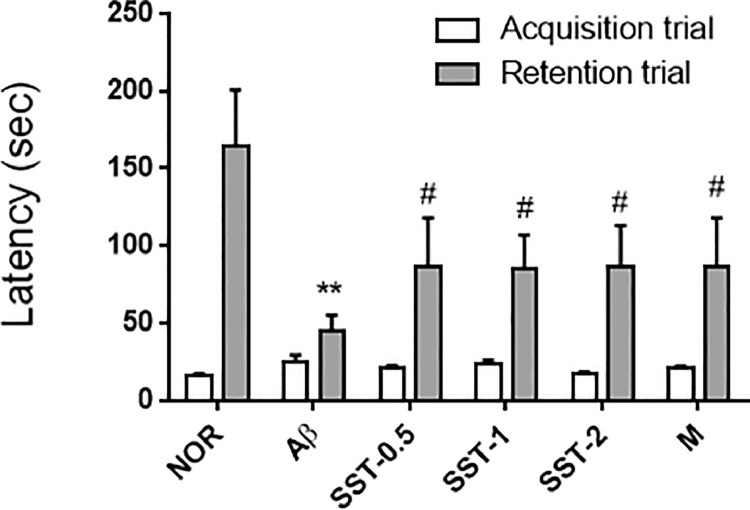
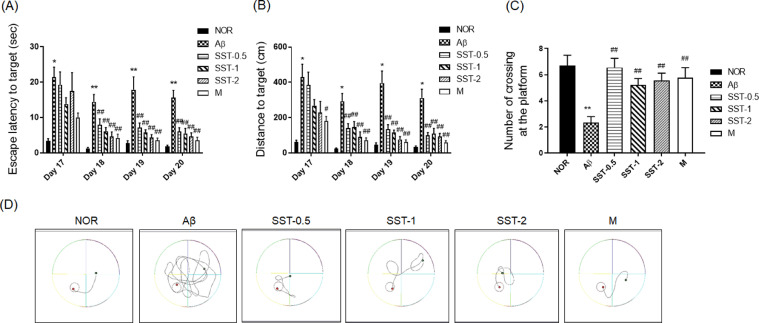
Animal behavioral tests were performed in the middle of the experimental period. Between the 8th and 10th day after Aβ injection, mice underwent the PAT. In the acquisition trial, there was no significant difference between the Aβ and NOR groups, or between the Aβ and SST-0.5, -1, or -2 groups. In the retention trial, the transfer latency was significantly reduced in the Aβ group, whereas Aβ-mediated inhibition was reversed in the SST -0.5, -1, or -2 groups (Fig. 3). The MWM test was carried out from the 15th day for 7 days (trainings for 2 days, memory tests for 6 days, and probe trial for 1 day) after the Aβ injection. On the 17th day after Aβ injection, the latency to find the platform was significantly increased in the Aβ group compared with the NOR group. However, no difference in latency was found between the SST groups and the Aβ group. From the 18th to 20th day, SST -0.5, -1, or -2 groups experienced a significant decrease in the latency time (Fig. 4A). Consistent with results of the latency, a significant decrease in the distance to find the platform was seen in the SST groups compared with the Aβ group from the 18th to 20th day (Fig. 4B). In the probe trial, Aβ group significantly shortened the number of crossings at the platform location compared with the NOR group. In contrast, SST significantly blocked Aβ-mediated suppression of the crossings in the SST groups (Fig. 4C). The tracking patterns in Fig. 4D further confirmed the ameliorating effects of SST on memory loss in the Aβ-induced AD mouse model. Morin, a positive control for Aβ aggregation inhibitor30, also produced a significant inhibition of memory dysfunction.
Fig. 3.
Effects of Soshiho-tang (SST) on memory impairment in an Aβ-induced AD mouse model using passive avoidance task. The passive avoidance task was performed on the 8th to 10th day after Aβ injection with or without SST administration. Transfer latency time was recorded as the time that mice remained in the lighted compartment within a 5 min period. The results are presented as the mean ± SD (n=9/group). ⁎⁎p<0.01 vs NOR group and #p<0.05 vs Aβ group. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, respectively, and M: morin (10 mg/kg)-treated group into Aβ mice.
Fig. 4.
Effects of Soshiho-tang (SST) on memory impairment in an Aβ-induced AD mouse model using the Morris water maze (MWM) test. The MWM test was performed on the 15th to 21st day after the injection of Aβ. (A) The time to reach the platform during training trials (escape latency). (B) The swimming distance traveled to the platform in the training trials. (C) The number of crossings of the platform area in the spatial probe trial. The results are presented as the mean ± SD (n=9/group). *p<0.05 or ⁎⁎p<0.01 vs NOR group, and #p<0.05 or ##p<0.01 vs Aβ group. (D) Representative swimming traces of mice in the spatial probe trial test. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, and M: morin (10 mg/kg)-treated group into Aβ mice.
3.3. Inhibitory effects of SST on neuronal injury and inflammation in an Aβ-induced AD mouse model
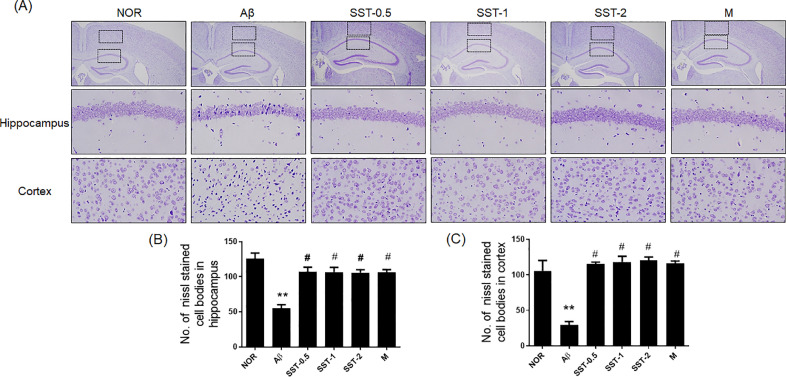
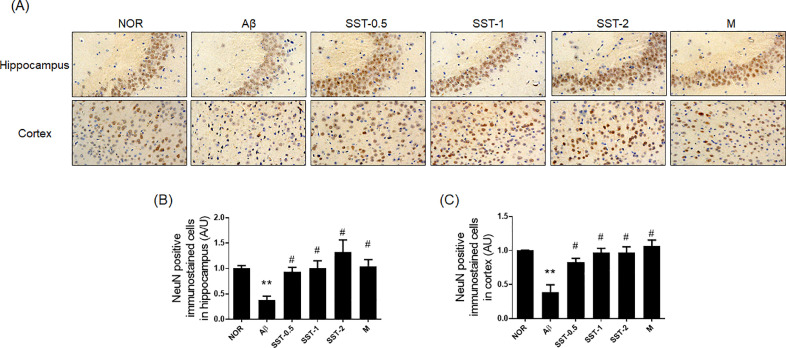
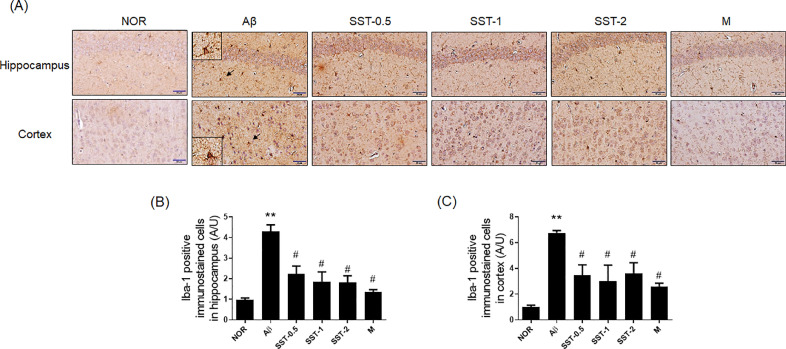
Histological analysis was performed to examine whether the inhibitory effects of SST on memory impairment are associated with neuronal cell changes. The results of Nissl staining displayed that Aβ group significantly reduced the number of surviving neurons (Nissl-stained cell bodies) in the hippocampus and cortex compared to the NOR group. Additionally, neuronal shrinkage or disappearance in the brain tissues were observed in the Aβ group. SST groups significantly attenuated neuronal loss induced by AD relative to that seen in the Aβ group (Fig. 5A-C). Immunohistochemistry with anti-NeuN antibody showed the neuroprotective effects of SST in a mouse model of AD. SST treatment significantly blocked the decrease in the number of NeuN positive cells in the hippocampus and cortex (Fig. 6A-C). We also analyzed the influence of SST on neuroinflammation in the microglia by evaluating the levels of key cellular mediators of neuroinflammation.31 As shown in Fig. 7A-C, the number of Iba-1 positive cells were markedly increased in the Aβ group in the hippocampus and cortex compared with the NOR group. In contrast, the SST groups showed a significant inhibitory activity against microglial activation relative to the Aβ group. Damage of neuronal cells and microglial activation were also restored with morin.
Fig. 5.
Effects of Soshiho-tang (SST) on neuronal loss in the brain tissues of Aβ-induced AD mice. (A) Multiple 5-μm paraffin sections of the hippocampus and cortical regions were prepared from the sacrificed mouse brains. Nissl staining was conducted using cresyl violet solution. Representative photomicrographs are shown at magnification of x400. (B and C) Graphs display the quantitative analysis of Nissl stained cell bodies in hippocampus (B) and cortex (C). The results are presented as the mean ± SD (n=3/group). ⁎⁎p<0.01 vs NOR group and #p<0.05 vs Aβ group. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, respectively, and M: morin (10 mg/kg)-treated group into Aβ mice.
Fig. 6.
Effects of Soshiho-tang (SST) on NeuN in the brain tissues of Aβ-induced AD mice. (A) Multiple 5-μm paraffin sections of hippocampus and cortical regions were prepared from the sacrificed mouse brains. The expression of NeuN, a neuronal marker, was determined by immunohistochemistry at a magnification of x400. (B and C) Graphs display the quantitative analysis of NeuN positive cells in the hippocampus (B) and cortex (C). The staining intensities are reported in arbitrary units (AU). The results are presented as the mean ± SD (n=3/group). ⁎⁎p<0.01 vs NOR group and #p<0.05 vs Aβ group. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, respectively, and M: morin (10 mg/kg)-treated group into Aβ mice.
Fig. 7.
Effects of Soshiho-tang (SST) on microglial activation in brain tissues of Aβ-induced AD mice. (A) Multiple 5-μm paraffin sections of hippocampus and cortical regions were prepared from the sacrificed mouse brains. The expression of Iba-1, a microglial activation marker, was determined by immunohistochemistry at a magnification of x400. (B and C) Graphs display the quantitative analysis of Iba-1 positive cells in the hippocampus (B) and cortex (C). The staining intensities are reported in arbitrary units (AU). The results are presented as the mean ± SD (n=3/group). ⁎⁎p<0.01 vs NOR group and #p<0.05 vs Aβ group. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, respectively, and M: morin (10 mg/kg)-treated group into Aβ mice.
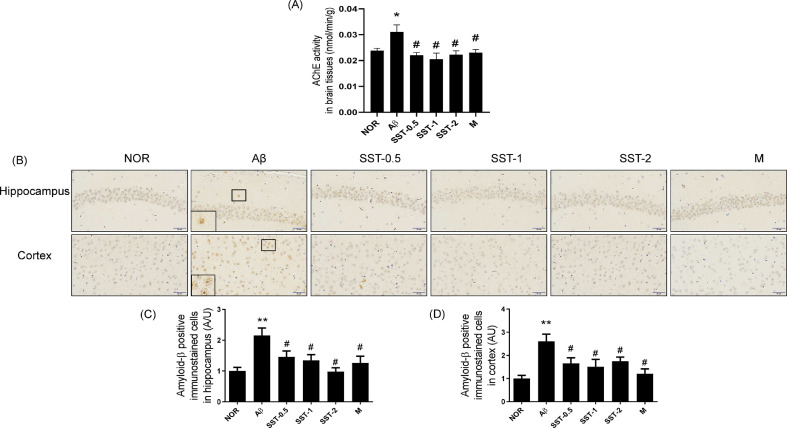
3.4. Preventive effects of SST on the Aβ deposition and AChE activation in an Aβ-induced AD mouse model
Our previous study demonstrated that SST suppressed the AChE activity and enhanced the Aβ disaggregation using in vitro analysis systems.19 In the present study, the inhibitory effects of SST on AChE and Aβ were confirmed using brain tissues of an AD-like mouse model. As shown in Fig. 8A, Aβ injection significantly increased the activity of AChE whereas SST administration significantly reversed the Aβ-mediated AChE inhibition in an AD-like mouse model. We also performed the immunohistochemical analysis using anti-Aβ antibody to examine the effects of SST on Aβ accumulation in neuronal cells. SST treatment into Aβ-injected mice significantly decreased the number of Aβ-positive cells in both hippocampus and cortex areas compared with Aβ-injected AD mice (Fig. 8B-D).
Fig. 8.
Inhibitory effects of Soshiho-tang (SST) on the Aβ deposition and of AChE activity in Aβ-induced AD mice. (A) AChE activity in mouse brain lysates were measured according to the commercial manufacture's protocol using an Acetylcholinesterase Activity Assay Kit (Biomax, Seoul, Korea). (B) Multiple 5-μm paraffin sections of hippocampus and cortical regions were prepared from the sacrificed mouse brains. The expression of Aβ was determined by immunohistochemistry at a magnification of x400. (C and D) Graphs display the quantitative analysis of Aβ-positive cells in the hippocampus (C) and cortex (D). The staining intensities are reported in arbitrary units (AU). The results are presented as the mean ± SD (n=3/group). ⁎⁎p<0.01 vs NOR group and #p<0.05 vs Aβ group. NOR: normal group, Aβ: Aβ-injected group, SST-0.5, -1, or -2: 500, 1,000, or 2,000 mg/kg of SST-treated groups into Aβ mice, respectively, and M: morin (10 mg/kg)-treated group into Aβ mice.
4. Discussion
We here demonstrate that the traditional herbal formula SST inhibits memory impairment and neuronal injury in an AD-like mouse model. We reported the preventive effects of SST on AChE activation and Aβ aggregation in a previous study.19 In the present study, we developed our former study to further confirm the effects of SST on AD-related events using an in vivo model. An AD mouse model was established by the ICV injection of Aβ aggregates. An Aβ-injected AD mouse model has been utilized for AD-related investigations.32, 33, 34 Of note, we recently reported the value of the Aβ-injected AD mice by comparing the differentially gene expression (DEG) pattern with human AD patients.35 Memory problems and cognitive impairment are typical initial symptoms of AD in accordance with the National Institute of Aging (NIA), NIH (https://www.nia.nih.gov/health/what-are-signs-alzheimers-disease). Thus, memory improvement is an important target when developing AD medications, even those targeting the early stage of the disease.
We conducted behavioral tests (PAT and MWM) to examine the effects of SST on memory deficits in a mouse model of AD. The PAT is a fear-aggravated experiment to test memory and learning in rodent models.36 The MWM test aims to evaluate spatial and long term memory in rodents.37 Oral administration dosage of SST for mice were determined by converting from the dose in humans provided by the US FDA guidelines.38 The single administration dose of SST in humans is 9,000 mg/day. In an average human body weight of 60 kg, a single dosage of SST is 150 mg/kg. The equivalent dose for mice was calculated by multiplying the “single dosage in humans” by the “dosage conversion factor to mouse (12.3)”. That is, the calculation equation is as follows: human equivalent dose (HED) to mouse = 150 mg/kg x 12.3 = 1,845 mg/kg. We used three different dosages (500, 1,000, or 2,000 mg/kg) of SST, one above and two below the value of HED in mice. Shin's group previously reported the safety of SST in subacute and subchronic oral toxicity tests.39,40 They demonstrate that the SST treatment did not result in any toxicologically significant changes in mortality, clinical signs, body weights, food and water consumption, ophthalmoscopy, urinalysis, hematological and serum biochemical parameters, gross findings, organ weights, and histopathology. A no-observed-adverse-effect level (NOAEL) of SST was suggested more than 2000 mg/kg. Our results revealed a significant reduction in the transfer latency in AD-like mice. In contrast, Aβ-induced AD mice treated with SST significantly increased the latency compared with Aβ-induced AD mice in both behavioral tests, suggesting the attenuating effects of SST on memory impairment in AD.
The hippocampus and cortex are major brain areas responsible for memory formation. Hence, brain damage in the hippocampus or cortex is considered as a principal cause of memory loss in AD.41 We performed histological Nissl staining using cresyl violet and immunohistochemistry for expression of NeuN, a major neuronal marker42, in hippocampal and cortical tissues of the AD-mice with or without SST treatment. As expected, Aβ injection markedly diminished the number of Nissl stained and NeuN positive cells in the hippocampus and cortex compared with control, indicating neuronal loss and death by Aβ aggregation. In contrast, SST treatment significantly reversed the Aβ-mediated neuronal damage. These results suggest the neuroprotective effects of SST in an in vivo model of AD.
Neuroinflammation plays a central role in the progression of pathological changes in the brains of patients with AD.43 Inflammatory reactions in the brains induce the release of inflammatory cytokines and mediators, which ultimately lead to neuronal cell death. Targeting neuroinflammation in the treatment of AD has been thought to control progression of AD over the past two decades.44 Non-steroidal anti-inflammatory drugs (NSAIDs) and tumor necrosis factor alpha (TNF-α) inhibitors have resulted in improvement in memory and cognition, and suppressed Aβ accumulation in preclinical and clinical studies.45,46 To test the effects of SST on neuroinflammation, immunohistochemical analysis was performed with anti-Iba-1 antibody. Iba-1 is a marker of microglial activation.47 Microglia are the immune cells in the central nervous system which play a major role in the neuroinflammation and are associated with amyloid plaque formation in the brain.48 Our data showed that SST administration significantly decreased the number of Iba-1 expressed cells in the hippocampus and cortex of AD mice compared with Aβ-injected AD mice, indicating the anti-neuroinflammatory activity of SST. Taken together with our previous results, we suggest that SST has multi-target actions in pathogenesis-related factors of AD, such as AChE, Aβ, neuronal damage and neuroinflammation.
Importantly, inflammation is a crucial protective response to neuronal cell and tissue injuries in the neurodegenerative diseases. Damaged neurons cause the sustained activation of glia including microglia and astrocytes that can produce reactive oxygen species (ROS). Excessive generation of toxic ROS induces oxidative stress to neurons, and further promotes neuronal damage and subsequent inflammation.49,50 During the neuroinflammatory process, the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) and inducible nitric oxide synthase (iNOS) is enhanced in microglia, resulting in high levels of superoxide and nitric oxide (NO) can induce the subsequent neuronal damage.51 The activation of signaling pathway by ROS involves the environmental changes in the redox state. Main mechanisms controlled by the redox signaling are activation of the nuclear factor kappa B (NF-κB), and induction of the stress-activated protein kinases c-jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK). In the development of chronic inflammatory and neurodegenerative diseases, NF-κB activation mediates production of the proinflammatory cytokines such as TNFs and interleukines.52,53 In addition, roles of NF-κB in the neuroinflammation promote the constant activation of p38 or JNK by ROS thereby inducing apoptosis related to mitochondrial dysfunction.54,55 We preliminarily examined antioxidant activity of SST by measuring the level of ROS and activation of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) (unpublished data). Our next step is planned to investigate the signaling pathways related to oxidative stress and neuroinflammation to determine the pharmacological mechanisms of SST responsible for the neuroprotective effects in an AD model. Previously, a potential of SST has been reported as anti-inflammatory medication in various diseases such as asthma, atopic dermatitis and liver injury.6,7,8 These reports may support to address the main mechanisms thereby protecting neuronal damages by the SST treatment through activation of the proinflammation-related signaling pathways.
Aβ and acetylchoine (ACh) play pivotal roles in the pathological process of AD. Aβ is a core component of the amyloid plaques observed in the brains of patients with AD.56 Aggregation of Aβ peptides is the consequence of the cleavage of amyloid precursor protein (APP) by β- and γ-secretases, and initiate the pathogenic cascade of AD that results in neuronal death.57 Cholinergic neurodegeneration is one of major pathologic features in the brains of AD patients. AChE is a cholinergic hydrolase found in the central nervous system and terminates termins the synaptic transmission by hydrolyzing acetylcholine into acetate and choline. AChE accelerates Aβ formation and plays a role during amyloid deposition in the brains of AD patients.58 In addition, several studies reported that AChE inhibitors such as donepezil and tacrine protect the neurotoxicity induced by Aβ in both animal and neuronal cell models.59,60 As mentioned above, we previously reported inhibitory effects of SST on the Aβ aggregation and AChE activity in vitro.19 In the present study, we confirmed inhibitory effects of SST on the AChE activation and Aβ aggregation in vivo. Consistent with the results of in vitro assays, the administration of SST extract significantly reduced the AChE activity and the amount of Aβ in the brain of Aβ-induced AD mice. We also observed the protection by SST against the neuronal loss induced by Aβ aggregation using histological analysis. Taken together, our data demonstrate that SST ameliorates the Aβ-mediated neuronal death by inhibiting AChE activity in an AD mouse model, indicating a crucial role of AChE in the neuroprotective action of SST.
Multi-targeting is an attractive property of natural plants composed of multiple components. Herbal formulas are typical multi-target multi-component (MCMT) agents. Several studies provide scientific evidences on the multi-targeting effects of herbal formulas on the pathogenesis of AD using in vitro or in vivo data. For instance, we reported that Bojungikgi-tang possesses positive effects on memory improvement via the inhibition of Aβ aggregation, antioxidation, and neuronal cell death in Aβ-induced AD mice.26 Liu et al. reported that the modified Huang-Lian-Jie-Du decoction ameliorates Aβ synaptotoxicity via the regulation of glutamatergic transmission and the adenosine-related signaling pathway in Aβ-induced AD mice.28 Since donepezil was firstly approved by U.S. FDA as an AD medication in 1996, a number of candidate drugs for AD treatment have been investigated. However, candidates for the treatment of AD had a failure rate of 99% or more. One of the main causes in the drug development failure is that it has focused on amyloid protein removal. According to the Alzheimer's Association's report, there are twenty-nine drugs in phase 3 clinical trials including putative disease-modifying agents (59%) and symptomatic agents (41%).61 Putative disease-modifying agents target the biomolecules Aβ or tau, inflammation/infection/immunity, synaptic plasticity/neuroprotection and so on. Symptomatic agents are cognitive enhancers or behavioral symptom therapies. In comparison to past research, recent studies have expanded to control symptoms of AD rather than focusing on single molecule. Our data reveal the potential of SST as multi-targeting agent for AD to control memory and cognitive impairment, neuronal damage, neuroinflammation, and Aβ. If the multi-targeting potential of SST is proven to be effective and safe in clinical trials, it could be a powerful treatment with the characteristics of both putative disease-modifying agents and symptomatic agents for AD compared to chemical drugs.
Additionally, we carried out the simultaneous quantification of five standard compounds that make up SST to establish the quality control using the HPLC system. SST is composed of seven medicinal herbs Bupleuri Radix, Scutellariae Radix, Ginseng Radix, Pinelliae Tuber, Glycyrrhizae Radix et Rhizoma, Zingiberis Rhizoma Crudus, and Zizyphi Fructus. The standard compounds we used in the HPLC analysis were baicalin, baicalein, and wogonin from Scutellariae Radix, and liquiritin as well as glycyrrhizin from Glycyrrhizae Radix et Rhizoma. The quantitative analysis of the five standard compounds in SST was performed using the established HPLC-PDA method. Consequently, baicalin (6.693 ± 0.002 mg/g) was the most abundant compound in SST. Of the five standard compounds, baicalin, baicalein, and wogonin from Scutellariae Radix have been reported to be effective in treating AD or AD-related memory impairment in vitro and in vivo studies.62, 63, 64, 65, 66 Based on our results, Scutellariae Radix seems to be the main medicinal herb that is most likely to be involved in the protection process of SST.
In conclusion, we demonstrate the potential of SST as a therapeutic drug for patients with AD. Our findings imply that SST improves memory impairments in AD-like mice by protecting neurons and inhibiting inflammation in the hippocampus and cortex. In order to proceed on to the AD drug development stage, well-designed clinical studies will be required as a next step to further verify the efficacy of SST.
Acknowledgments
Author contributions
Conceptualization: S-JJ Methodology: ES and YK. Validation and analysis: ES and YK. Original Draft: ES and S-JJ. Writing - Review & Editing: S-JJ. Supervision: S-JJ.
Conflict of interest
The authors declare no conflict of interest.
Funding
This research was supported by a grant of the Korea Institute of Oriental Medicine (Grant No. KSN2013240).
Ethical statement
All animal experiments in this study were performed according to the ethical standards of the institution and approved by institutional animal care and use committee.
Data availability
The data supporting the findings in this research are provided within the manuscript
References
- 1.Baik YS. A study on the complex efficacy of Sosihotang. J Kor Med Class. 2014;27(2):137. [Google Scholar]
- 2.Kim A, Im M, Ma JY. Sosiho‑tang ameliorates cachexia-related symptoms in mice bearing colon 26 adenocarcinoma by reducing systemic inflammation and muscle loss. Oncol Rep. 2016;35(3):1841–1850. doi: 10.3892/or.2015.4527. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima Y, Kashii T, Tokimitsu Y. Cytotoxic effect of herbal medicine sho-saiko-to on human lung-cancer cell-lines in-vitro. Oncol Rep. 1995;2(1):91–94. doi: 10.3892/or.2.1.91. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Liu L, Zhang Y. The herbal mixture Xiao-chai-hu tang (XCHT) induces apoptosis of human hepatocellular carcinoma Huh7 cells in vitro and in vivo. AJTCAM. 2017;14(3):231–241. doi: 10.21010/ajtcam.v14i3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JK, Kim JH, Shin HK. Therapeutic effects of the oriental herbal medicine Sho-saiko-to on liver cirrhosis and carcinoma. Hepatol Res. 2011;41(9):825–837. doi: 10.1111/j.1872-034X.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeon WY, Shin HK, Shin IS. Soshiho-tang water extract inhibits ovalbumin-induced airway inflammation via the regulation of heme oxygenase-1. BMC Complement Alternat Med. 2015;15:329. doi: 10.1186/s12906-015-0857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin SC, Kim MH, SY Jo. Soshiho-tang protects LPS-induced acute liver injury by attenuating inflammatory response. J Nat Med. 2020 doi: 10.1007/s11418-020-01421-w. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Jo EH, Lee B. Soshiho-Tang, a traditional herbal medicine, alleviates atopic dermatitis symptoms via regulation of inflammatory mediators. Front Pharmacol. 2019;10:742. doi: 10.3389/fphar.2019.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtake N, Yamamoto M, Takeda S. The herbal medicine Sho-saiko-to selectively inhibits CD8+ T-cell proliferation. Eur J Pharmacol. 2005;507(1-3):301–310. doi: 10.1016/j.ejphar.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Kang H, Choi TW, Ahn KS. Upregulation of interferon-gamma and interleukin-4, Th cell-derived cytokines by So-Shi-Ho-Tang (Sho-Saiko-To) occurs at the level of antigen presenting cells, but not CD4 T cells. J Ethnopharmacol. 2009;123(1):6–14. doi: 10.1016/j.jep.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Yaginuma T, Okamura T, Takeuchi T. Preventive effect of traditional herbal medicine, shosaiko-to, on danazol-induced hepatic damage. International journal of gynaecology and obstetrics: the official organ of the international federation of gynaecology and. Obstetrics. 1989;29(4):337–341. doi: 10.1016/0020-7292(89)90359-7. [DOI] [PubMed] [Google Scholar]
- 12.Akase T, Tashiro S, Ishibashi A. Pharmacoepidemiological study of the clinical efficacy of Sho-saiko-to (Xiao-Chai-Hu-Tang) in chronic liver disease patients. J Trad Med. 2001;18:95–106. [Google Scholar]
- 13.Tajiri H, Kozaiwa K, Ozaki Y. Effect of sho-saiko-to(xiao-chai-hu-tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. Am J Chin Med. 1991;19(2):121–129. doi: 10.1142/S0192415X91000193. [DOI] [PubMed] [Google Scholar]
- 14.Deng G, Kurtz RC, Vickers A. A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chai-hu-tang) in chronic hepatitis C patients. J Ethnopharmacol. 2011;136(1):83–87. doi: 10.1016/j.jep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto S, Oka H, Kanno T. [Controlled prospective trial to evaluate Syosakiko-to in preventing hepatocellular carcinoma in patients with cirrhosis of the liver] Gan To Kagaku Ryoho. 1989;16(4 Pt 2-2):1519–1524. [PubMed] [Google Scholar]
- 16.Ma J, Wu CF, Wang F. Neurological mechanism of Xiaochaihutang's antidepressant-like effects to socially isolated adult rats. J Pharm Pharmacol. 2016;68(10):1340–1349. doi: 10.1111/jphp.12616. [DOI] [PubMed] [Google Scholar]
- 17.Su GY, Yang JY, Wang F. Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2014;152(1):217–226. doi: 10.1016/j.jep.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Koo BM, Yang JC, Kim SK. A clinical study about the effects of Soshiho-tang on a case of Parkinson's disease with pontine infarction. j Kor Orient Med. 2007;28(2):34–43. [Google Scholar]
- 19.Lim H-S, Kim YJ, Kim B-Y. Screening of 56 herbal formulas covered by the national health insurance service on dementia-related factors. J Korean Med. 2018;39(3):1–16. [Google Scholar]
- 20.Ballard C, Gauthier S, Corbett A. Alzheimer's disease. Lancet (London, England) 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 21.Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 22.Aging NIo. About Alzheimer's Disease: Symptoms. https://www.webarchiveorg/web/20120115201854/http://www.nianihgov/alzheimers/topics/symptoms 2012.
- 23.Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. Molecular pathogenesis of Alzheimer's disease: an update. Ann Neurosci. 2017;24(1):46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12(1-2):34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Li XJ, Zhang HY. Synergy in natural medicines: implications for drug discovery. Trends Pharmacol Sci. 2008;29(7):331–332. doi: 10.1016/j.tips.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Lim HS, Kim YJ, Sohn E. Bojungikgi-Tang, a traditional herbal formula, exerts neuroprotective effects and ameliorates memory impairments in Alzheimer's disease-like experimental models. Nutrients. 2018;10(12) doi: 10.3390/nu10121952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu X, Wang Q, Wang Z. Danggui-Shaoyao-San: new hope for Alzheimer's disease. Aging Dis. 2016;7(4):502–513. doi: 10.14336/AD.2015.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Du T, Zhang W. Modified Huang-Lian-Jie-Du decoction ameliorates Aβ synaptotoxicity in a murine model of Alzheimer's disease. Oxidative Med Cellular Longevity. 2019;2019 doi: 10.1155/2019/8340192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Guo P, Liu X. Herbal formula Fo Shou San attenuates Alzheimer's disease-related pathologies via the gut-liver-brain axis in APP/PS1 mouse model of Alzheimer's disease. Evidence-based complementary and alternative medicine. eCAM. 2019;2019 doi: 10.1155/2019/8302950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemkul JA, Bevan DR. Morin inhibits the early stages of amyloid β-peptide aggregation by altering tertiary and quaternary interactions to produce "off-pathway" structures. Biochemistry. 2012;51(30):5990–6009. doi: 10.1021/bi300113x. [DOI] [PubMed] [Google Scholar]
- 31.Imai Y, Ibata I, Ito D. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;55:687–700. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 32.Ali T, Kim MJ, Rehman SU. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ(1-42) mouse model of Alzheimer's disease. Mol Neurobiol. 2017;54(8):6490–6506. doi: 10.1007/s12035-016-0136-4. [DOI] [PubMed] [Google Scholar]
- 33.Facchinetti R, Bronzuoli MR, Scuderi C. An animal model of Alzheimer disease based on the intrahippocampal injection of amyloid β-Peptide (1-42) Methods Mol Biol. 2018;1727:343–352. doi: 10.1007/978-1-4939-7571-6_25. [DOI] [PubMed] [Google Scholar]
- 34.Kim HY, Lee DK, Chung BR. Intracerebroventricular injection of amyloid-β peptides in normal mice to acutely induce Alzheimer-like cognitive deficits. J Vis Exp. 2016;(109) doi: 10.3791/53308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BY, Lim HS, Kim Y. Evaluation of animal models by comparison with human late-onset Alzheimer's disease. Mol Neurobiol. 2018;55(12):9234–9250. doi: 10.1007/s12035-018-1036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716(1-2):29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- 37.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 38.USFDA . US Food and Drug Administration; 2005. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Adult Healthy Volunteer. [Google Scholar]
- 39.Lee MY, Seo CS, Shin IS. Evaluation of oral subchronic toxicity of soshiho-tang water extract: the traditional herbal formula in rats. Evidence-Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/590181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin IS, Lee MY, Kim Y. Subacute toxicity and stability of Soshiho-tang, a traditional herbal formula, in Sprague-Dawley rats. BMC Complement. 2012;12:266. doi: 10.1186/1472-6882-12-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jahn H. Memory loss in Alzheimer's disease. Dialog Clin Neurosci. 2013;15(4):445–454. doi: 10.31887/DCNS.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25(10):2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinney JW, Bemiller SM, Murtishaw AS. Inflammation as a central mechanism in Alzheimer's disease. Alzheimer's Dementia (New York, N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ardura-Fabregat A, Boddeke E, Boza-Serrano A. Targeting neuroinflammation to treat Alzheimer's disease. CNS Drugs. 2017;31(12):1057–1082. doi: 10.1007/s40263-017-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim GP, Yang F, Chu T. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging. 2001;22(6):983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 46.Tobinick E, Gross H, Weinberger A. TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study. MedGenMed. 2006;8(2):25. [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed Z, Shaw G, Sharma VP. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55(7):687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 48.Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer's disease. CNS & Neurol Disord Drug Targets. 2010;9(2):156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amor F, Puentes D, Valk Baker P.van der. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discovery. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 51.Brown G. Portland Press Ltd.; 2007. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. [DOI] [PubMed] [Google Scholar]
- 52.Morgan MJ, Liu Z-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signaling. 2005;7(3-4):472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 54.Deng Y, Ren X, Yang L. A JNK-dependent pathway is required for TNFα-induced apoptosis. Cell. 2003;115(1):61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem. 2000;275(34):25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 56.Hamley IW. The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization. Chem Rev. 2012;112(10):5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 57.Chow VW, Mattson MP, Wong PC. An overview of APP processing enzymes and products. NeuroMol Med. 2010;12(1):1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inestrosa NC, Alvarez A, Perez CA. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 59.Fu H, Li W, Lao Y. Bis (7)-tacrine attenuates β amyloid-induced neuronal apoptosis by regulating L-type calcium channels. J Neurochem. 2006;98(5):1400–1410. doi: 10.1111/j.1471-4159.2006.03960.x. [DOI] [PubMed] [Google Scholar]
- 60.Noh MY, Koh SH, Kim SM. Neuroprotective effects of donepezil against A β42-induced neuronal toxicity are mediated through not only enhancing PP 2 A activity but also regulating GSK-3β and n AChR s activity. J Neurochem. 2013;127(4):562–574. doi: 10.1111/jnc.12319. [DOI] [PubMed] [Google Scholar]
- 61.Cummings J, Lee G, Ritter A. Alzheimer's disease drug development pipeline: 2020. Alzheimer's Dementia. 2020;6(1):e12050. doi: 10.1002/trc2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han J, Ji Y, Youn K. Baicalein as a Potential Inhibitor against BACE1 and AChE: mechanistic comprehension through in vitro and computational approaches. Nutrients. 2019;11(11) doi: 10.3390/nu11112694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang DS, Yu YC, Wu CH. Protective effects of Wogonin against Alzheimer's disease by inhibition of amyloidogenic pathway. Evid-Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/3545169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang YK, Jinhua M, Choi BR. Effects of Scutellaria baicalensis on chronic cerebral hypoperfusion-induced memory impairments and chronic lipopolysaccharide infusion-induced memory impairments. J Ethnopharmacol. 2011;137(1):681–689. doi: 10.1016/j.jep.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 65.Jin X, Liu MY, Zhang DF. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci Ther. 2019;25(5):575–590. doi: 10.1111/cns.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang SQ, Obregon D, Ehrhart J. Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model. J Neurosci Res. 2013;91(9):1239–1246. doi: 10.1002/jnr.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings in this research are provided within the manuscript