Abstract
Introduction:
Night work requires inversion of the natural, diurnal human activity-rest cycle and is associated with decreased alertness and some measures of performance, reduced safety, adverse health effects, and chronic disruption of the melatonin cycle that has been associated with increased risk for several major diseases. Previous studies show that red light exposures at night can promote alertness and improve performance while not negatively affecting melatonin secretion.
Method:
This ongoing crossover, mixed (within- and between-subjects) design field study is testing the efficacy and acceptance of red light delivered to day-shift and night-shift workers using personal light glasses while they are at work. Each participant experienced three lighting interventions at the eyes: red light (50 lx, 630 nm, the treatment intervention), blue light (50 lx, 460 nm, the positive control intervention), and dim white light (10 lx, 3000 K, the placebo control). During the interventions, participants underwent visual performance testing, submitted salivary melatonin and cortisol samples, and provided subjective reports of sleepiness, sleep disturbance, and general health over the 20-week protocol. Due to the ongoing nature of the study, only the performance and subjective reports are presented here.
Results:
Preliminary results indicate that response times were improved by the red and blue interventions, but not accuracy and hit rates. Blue light was associated with improvements to self-reported sleep disturbances compared to dim light.
Conclusions:
These field results partially support our laboratory results that showed a positive effect of red light for promoting alertness and certain performance outcomes during the day and at night.
Practical Applications:
Red light may be used to improve response times in shift workers. Continued research will elucidate the lighting interventions’ effects on melatonin and objective sleep measures (actigraphy).
Keywords: alertness, circadian stimulus, health, healthcare workers, red light, shift work, sleep, visual performance testing
1. Introduction
Over 16% of all full-time wage and salary workers in the United States follow schedules that fall outside conventional daytime (07:00–18:00) work hours, with over 10 million Americans working evening, night, or rotating shifts that involve nights (U.S. Bureau of Labor Statistics, 2019). Because it requires inversion of the natural, diurnal human activity-rest cycle, night work is associated with decreased alertness and some measures of performance, reduced safety, and adverse health effects (Ganesan et al., 2019; Rajaratnam, Howard, & Grunstein, 2013). Working at night, especially when part of a rotating shift schedule, can also disrupt circadian system functions such as the melatonin cycle. Circadian disruption has been associated with increased risk for metabolic syndrome, diabetes, cardiovascular disease, and cancer (Khan, Duan, Yao, & Hou, 2018; Potter et al., 2016). There are indications that the cancer risk is potentially mediated through the suppression of melatonin by exposure to light at night (LAN), with several studies concluding that women performing night-shift work had elevated risk for breast cancer, specifically (Davis, Mirick, & Stevens, 2001; Hansen, 2001a, 2001b; Schernhammer, Kroenke, Laden, & Hankinson, 2006; Schernhammer et al., 2001; Tynes, Hannevik, Andersen, Vistnes, & Haldorsen, 1996).
Nurses and patient care associates who work evening and night shifts are also at greater risk for work-related injuries (Trinkoff, Le, Geiger-Brown, & J., 2007), and that risk appears to increase when working consecutive 12-h shifts compared to shifts that are preceded by time off (Hopcia, Dennerlein, Hashimoto, Orechia, & Sorensen, 2012). A recent study of 1,744 newly licensed registered nurses found that those working overtime experienced a 32% increased risk for needle-stick injuries, and nurses working night shifts experienced a more modest (but still significant) 16% increased risk (Stimpfel, Brewer, & Kovner, 2015). Alertness is therefore especially important during evening and night work to minimize risk for accidents and promote healthcare workers’ safety, health, and well-being.
Light can serve as an important countermeasure to somnolence at night, as it is capable of eliciting an acute alerting response in humans shortly after the light exposure occurs, similar to the effect of a cup of coffee. This response has been confirmed by laboratory studies documenting elevated core body temperature, increased EEG brain activity, improved scores in certain types of performance testing, subjective and objective reports of improved alertness, and subjective reports of improved health and well-being in response to bright light at all times of day and night (Badia, Myers, Boecker, Culpepper, & Harsh, 1991; Lowden, Akerstedt, & Wibom, 2004; Okamoto, Rea, & Figueiro, 2014; Sahin & Figueiro, 2013; Viola, James, Schlangen, & Dijk, 2008). Acute exposure to high levels of bright light (typically 100–10,000 lx at the eyes) at night has been shown to promote alertness, but because the human circadian system is maximally sensitive to short-wavelength light (as measured by acute melatonin suppression) (Brainard et al., 2001; Rea, Figueiro, Bullough, & Bierman, 2005; Thapan, Arendt, & Skene, 2001), much lower levels of 470-nm (blue) light are also effective (Souman, Tinga, Te Pas, van Ee, & Vlaskamp, 2018). It is well known, however, that nighttime exposure to bright light (>2500 lx) (Lewy, Wehr, Goodwin, & Newsome, 1980) or blue light (as low as 40 lx) (Figueiro & Rea, 2010) can suppress the body’s secretion of the hormone melatonin, thereby increasing the aforementioned health risks. Light exposures can also disrupt other key circadian processes such as the sleep/wake cycle by delaying bedtimes and wake times, feeding a vicious cycle that further exacerbates problems with health, mood, and alertness.
It has become clear, on the other hand, that light’s alerting effects are not solely linked to its ability to suppress the hormone melatonin, which is secreted by the pineal gland in darkness and signals the body that it is time for sleep. Studies have shown that light exposures can elicit alertness during the daytime, when melatonin levels are low (Phipps-Nelson, Redman, Dijk, & Rajaratnam, 2003; Vandewalle et al., 2006) (Sahin, Wood, Plitnick, & Figueiro, 2014). Consistently, research has also indicated that light’s alerting effect extends to longer wavelength light (e.g., saturated red light, peak wavelength [λmax] at 630 nm) that exerts minimal influence on melatonin levels and the circadian system (Figueiro, Bierman, Plitnick, & Rea, 2009; Papamichael, Skene, & Revell, 2012). These findings have potentially significant practical applications for healthcare personnel who work through the night, in that red light could help them maintain alertness without suppressing melatonin or shifting the timing of their circadian rhythms.
This ongoing crossover, mixed design (within- and between-subjects) design field study seeks to test the efficacy and acceptance of a carefully specified and administered red light exposure, delivered via personal light glasses while participants are working in healthcare settings. Our overall hypothesis is that both the red and blue lighting interventions will reduce sleepiness and improve performance outcomes compared to the control condition, but that only blue light will affect melatonin levels in night-shift workers. Because we hypothesized that no significant differences would exist between the red and blue lighting interventions, no post hoc comparisons between these two conditions were performed. This contribution presents only the visual performance testing and subjective-scale results for those who have completed baseline and intervention weeks for at least one of the three lighting conditions employed.
2. Materials and Methods
2.1. Participants
The 70 participants (mean age 39.2 years [SD = 11.5], 69 females) in this ongoing study have been recruited from four hospitals in Albany, NY; Cooperstown, NY; Schenectady, NY; South Bend, IN; and Syracuse, NY. Forty-three participants are day-shift workers and 27 are night-shift workers. The data reported here are for all participants who have completed at least 4 weeks (2 weeks baseline and 2 weeks intervention) of the study’s 20-week protocol. For inclusion in the study, evening/night-shift participants must have been working at least three shifts per week on evenings or nights (8-h or 12-h shifts starting at 19:00 or later) for 6 months prior to the study. Day shift (control) participants must not have worked shifts that began after 19:00 for 6 months prior to the study. Participants were excluded from the study if they had cardiovascular disease or diabetes, were taking beta blocker medication or melatonin supplements, or were pregnant or lactating. No other exclusion criteria were applied.
This study conforms to the Code of Federal Regulations (CFR) document Protection of Human Subjects, 45 CFR 46 (2018) and international ethical standards (Portaluppi, Smolensky, & Touitou, 2010). The protocol was reviewed, approved, and monitored by Rensselaer Polytechnic Institute’s Institutional Review Board. Informed written consent was obtained from all study participants.
2.2. Experimental conditions and lighting apparatus
Each participant was provided with a single pair of light glasses that was capable of delivering the study’s three lighting interventions. The custom-built device is composed of a USB-rechargeable lithium-ion battery power supply, a microcontroller to adjust the intensity of the white and RGB (red, green, blue) light-emitting diode (LED) integrated driver chips, four mini-LEDs (two per eye), an on/off switch, and a push-button lighting intervention selector, all enclosed in a 3-D-printed housing and mounted on a lightweight, lensless safety eyeglasses frame. Polycarbonate translucent filter material covers the LEDs to minimize discomfort glare and any risk for blue-light hazard. The device was designed to fit over existing eyeglasses, if necessary. While at work during the intervention periods only, the participants wore the light glasses for a 30-min interval in the first, middle, and final hours of their shifts (see Procedure).
The light glasses delivered red light (the treatment intervention), blue light (the positive control intervention), and dim white light (the placebo control intervention) to the participants’ eyes. The red-light treatment intervention was designed to promote alertness by delivering a high level of light (50 lx at the eyes) while providing minimal circadian stimulus (CS), thereby avoiding melatonin suppression and significant effects on the participants’ circadian systems. The blue-light positive control was also designed to promote alertness by delivering a high level of light (50 lx at the eyes) while providing maximal CS. The blue light was also expected to suppress melatonin in night-shift workers and advance sleep in day-shift workers. The dim white-light placebo control was designed to provide a minimal alerting stimulus and minimal CS by delivering a low level of light (10 lx at the eyes).
The lighting interventions were specified in terms of spectrum and light level (i.e., photopic illuminance) to provide targeted amounts of circadian light (CLA) (Rea, Figueiro, Bierman, & Bullough, 2010) and circadian stimulus (CS) to the participants’ eyes (Rea & Figueiro, 2018; Rea, Figueiro, Bierman, & Hamner, 2012; Rea et al., 2005). Briefly, CLA is irradiance weighted by the spectral sensitivity of the retinal phototransduction mechanisms stimulating the response of the biological clock, based on nocturnal melatonin suppression. CS is a transformation of CLA into a relative scale from approximately 0.1 (≈10%), the threshold for circadian system activation, to approximately 0.7 (≈70%), response saturation, and is equivalent to nocturnal melatonin suppression in percent after a 1-h exposure to light.
The specifications for the interventions are shown along with their order of administration (by participant group) in Table 1, and their relative spectral power distributions are shown in Fig. 1. The devices were calibrated using a spectrometer (Oriel Instaspec IV, Oriel Instruments, Stratford, CT, US).
Table 1.
Specifications and order of the three lighting interventions administered to the study’s three participant groups.
| Lighting intervention | λmax (nm) | FWHM (nm) | Illuminance at the eyes (lx) | CS | Intervention order, by participant group | ||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |||||
| Red (treatment) | 630 | 24 | 50 | <0.01 | 2nd | 1st | 3rd |
| Blue (positive control) | 460 | 20 | 50 | 0.60 | 1st | 3rd | 2nd |
| Dim white, 3000 K (placebo control) | — | — | 10 | 0.01 | 3rd | 2nd | 1st |
λmax = peak wavelength, FWHM = full width at half maximum, CS = circadian stimulus.
Fig. 1.
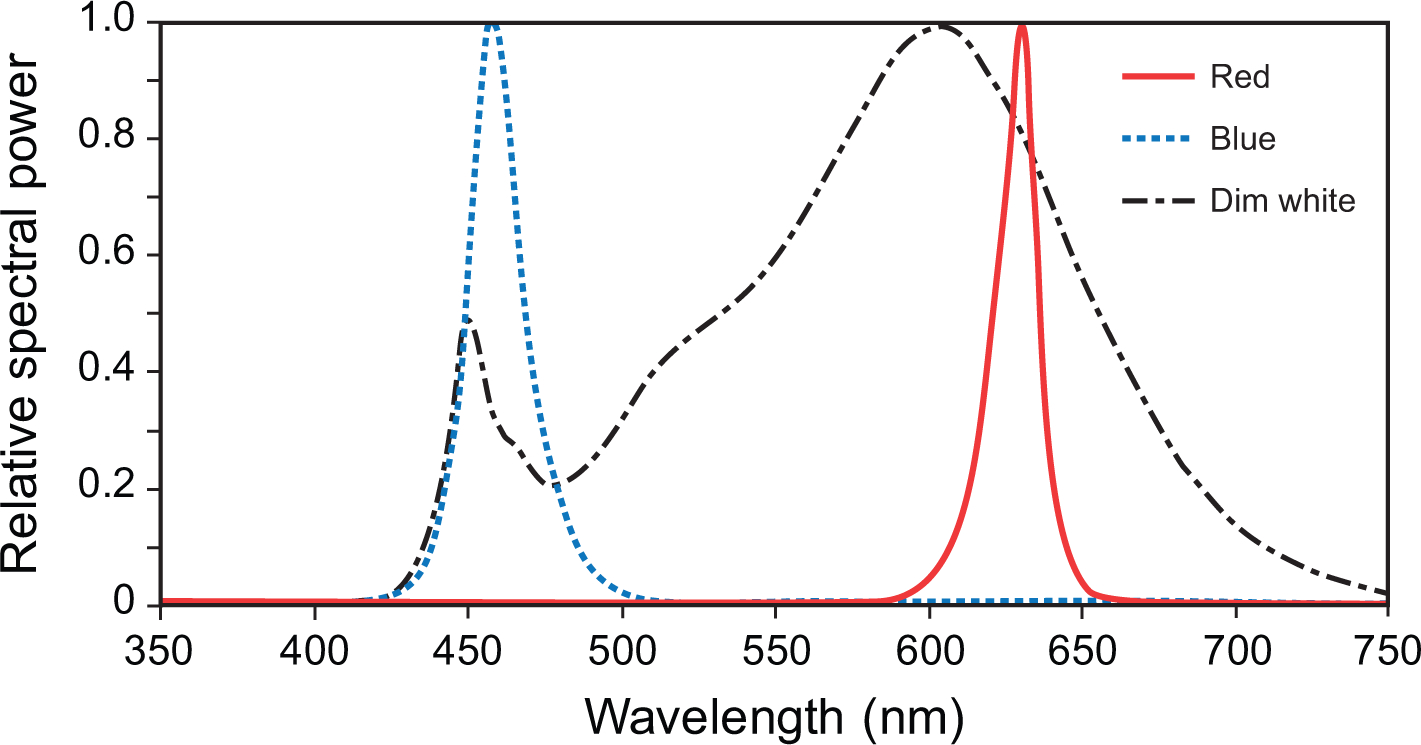
The spectral power distributions of the red (λmax = 630 nm), blue (λmax = 460 nm), and dim white (3000 K) lighting interventions used in the study.
2.3. Outcomes
2.3.1. Visual performance testing
Visual performance was assessed via psychomotor vigilance test (PVT), 1-back, and go/no-go (GNG) tasks that were presented to the participants on digital displays connected to personal computers equipped with keyboards. The PVT task presented a black circle on a white field for 50 milliseconds (ms) every 2–10 s over a period of 3 min. Participants were instructed to press the computer keyboard’s spacebar when the black circle appeared on the display. The 1-back task presented one of seven uppercase letters (A, O, D, G, J, X, M, T), in black on a white field, for a duration of 500 ms every 4 s over a period of 3 min. Participants were instructed to press the computer keyboard’s “A” key if the current letter matched the previous letter and press the “L” key if the current letter did not match. The GNG task presented one of two colored circles (red or green) on a white field for a duration of 250 ms every 2–10 s over a period of 3 min. Participants were instructed to press the computer keyboard’s spacebar only when the green circle appeared, which occurred in 70% of the trials.
The PVT task outcomes include response time (in seconds) and hit rate (i.e., the number of correct responses divided by the number of trials). Outcomes for the 1-back task include response time (for all correct responses, in seconds), accuracy rate, number of correct matches, number of correct no-matches (i.e., the number of times participants correctly responded with “no-match” when the current and previous trials were not the same), correct match response time (in seconds), and correct no-match response time (in seconds). Outcomes for the GNG task include response time (in seconds), hit rate (the number of correct responses to “go” trials divided by the total number of trials), and false positive rate (the number of incorrect responses to “go” trials divided by the total number of trials).
2.3.2. Subjective scales
Sleepiness while at work was assessed using the Karolinska Sleepiness Scale (KSS) (Åkerstedt & Gillberg, 1990), wherein participants report their feelings of sleepiness on a scale ranging from 1 to 9, where 1 = “very alert,” 5 = “neither alert nor sleepy,” and 9 = “very sleepy, fighting sleep, an effort to remain awake.”
Participants rated their sleep quality using the Pittsburgh Sleep Quality Index (PSQI), a well-validated 19-item questionnaire that assesses general sleep quality over the past month (Buysse et al., 1991). In the PSQI, 19 individual items generate seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. These seven component scores are summed to obtain a global score.
Sleep disturbance was measured using the eight-item PROMIS Sleep Disturbance Short Form (PROMIS-SD), which assesses the pure domain of sleep disturbance in individuals aged 18 years and older (Cella et al., 2010). Participants rate each PROMIS-SD item on a 5-point scale (1 = “never,” 2 = “rarely,” 3 = “sometimes,” 4 = “often,” 5 = “always”), producing scores ranging from 8 to 40, with higher scores indicating greater severity of sleep disturbance. The raw scores for all eight items are summed to obtain a global score.
Participants provided self-reports of physical and mental health using the PROMIS Global Health (PROMIS-GH), version 1.0/1.1, instrument (Hays, Bjorner, Revicki, Spritzer, & Cella, 2009), which is composed of 10 global health domains. For the purposes of the present study, global scores for physical health comprise the sum of four domains (physical health and function, pain, and fatigue) and global scores for mental health comprise the sum of three domains (mental health, social satisfaction, and emotional problems). The domains generally rate responses on a five-point scale, with higher scores indicating superior outcomes. The PROMIS-GH instrument utilizes T-score distributions of participant responses that are standardized so that a value of 50 represents the mean for the US general population (SD = 10). Thus, a T-score value of 60 is one standard deviation better (healthier) than the general population.
2.4. Procedure
Prior to the experiment, the project manager pre-screened participants to ensure that they passed the study’s exclusion criteria. A training session was held prior to the study’s first baseline data collection period to instruct participants on wearing the devices, collecting biomarker samples, and executing the performance tasks (to avoid learning effects).
In this study’s crossover, mixed design, the participants were randomly assigned to three groups that received the lighting interventions in counterbalanced order (see Table 1). Each group experienced three 2-week lighting intervention periods (i.e., weeks 2–4, 10–12, and 18–20) that exposed them to either the blue, red, or dim white light. Each intervention period was preceded by a 2-week baseline assessment period and separated by a 4-week washout period. Participants wore the energized light glasses for 30 min while working during the first (0–1.0 h), middle (5.5–6.5 h), and final (11–12 h) hours of every shift during the intervention weeks only (Fig. 2).
Fig. 2.
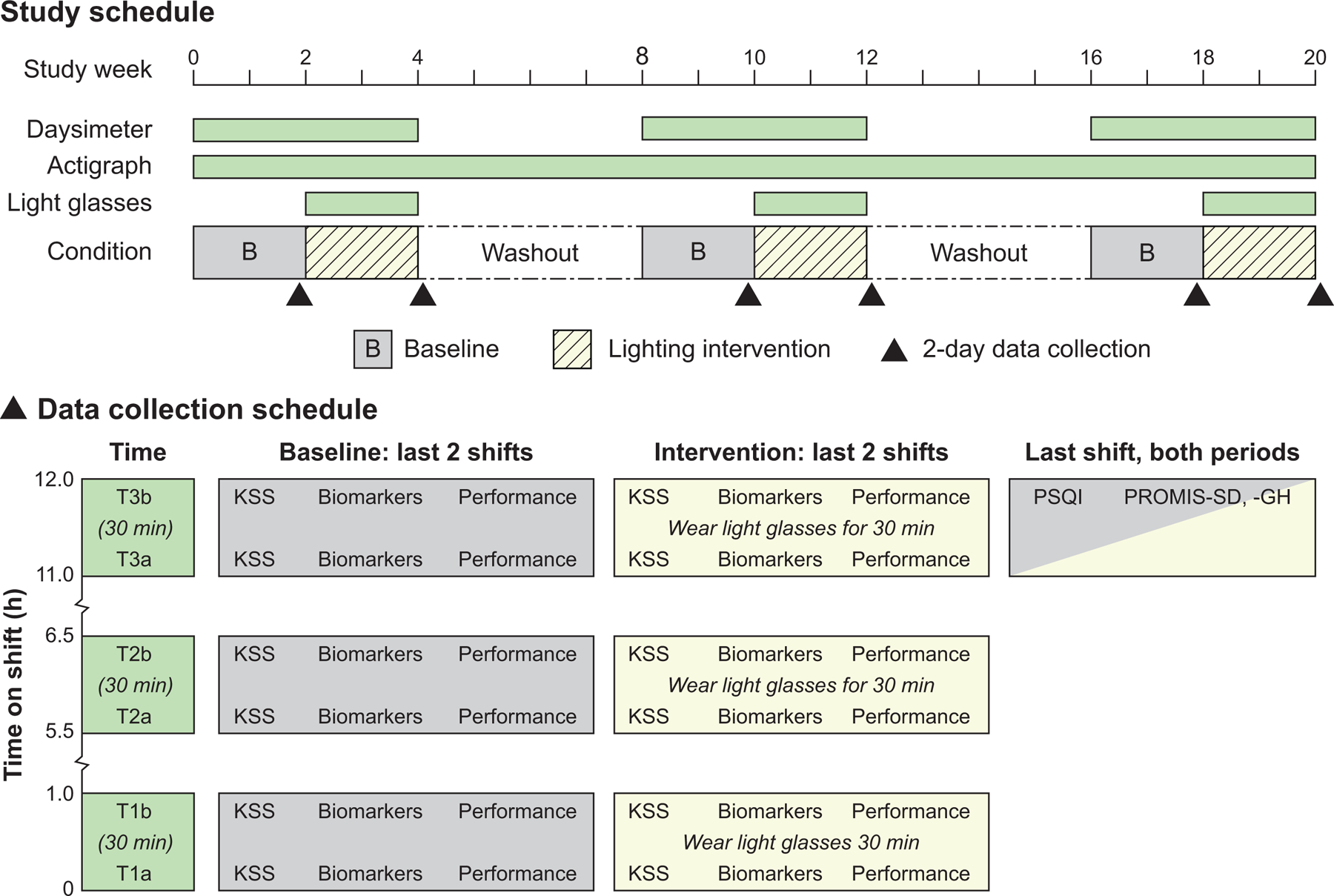
The experimental protocol. The KSS questionnaire, biomarkers, and performance data were collected six times throughout the 20-week protocol on the last two shifts of the baseline assessment and lighting intervention periods. Six series of data were collected per shift, twice during each of the shift’s first (T1), middle (T2), and final (T3) hours, with the first series (T1a, T2a, T3a) separated from the second (T1b, T2b, T3b) by 30-min intervals within each hour. The PSQI, PROMIS-SD, and PROMIS-GH questionnaires were filed during the final hour of the baseline and intervention periods.
On the last two shifts of each baseline assessment and intervention period, the participants completed two KSS questionnaires, collected two biomarker samples, and underwent two visual performance test batteries during the shift’s first (T1), middle (T2), and final (T3) hours. The first series of data were collected at times T1a, T2a, and T3a, and the second series of data were collected 30 min later at corresponding times T1b, T2b, and T3b. During the intervention, upon completion of the first performance test battery, participants energized the light glasses and wore them for the 30 min interval, just as they had done at the same times on all other intervention shifts. The light glasses were not energized during the performance testing to avoid any reduction in visibility of the digital displays. During baseline data collection, participants simply remained in the existing facility lighting until beginning the second series of data collection 30 min later (see Fig. 2).
2.5. Data analyses
Data for all outcomes were analyzed using IBM SPSS Statistics (version 26.0, IBM Inc., Armonk, NY). For each participant and each experimental condition, the performance and KSS data collected at T1, T2 and T3 were normalized to those collected at T1a, T2a and T3a. Next, the intervention data were normalized to the baseline for each participant and each light condition. Variables shift type (day/night), light color (blue/red/white), and time (T1/T2/T3) were entered as fixed factors and participant was entered as a random factor. Autoregressive covariance structure was used for the repeated factors. Time was not entered as a fixed factor in the linear mixed-effects models (LMEMs) for the sleep quality/disturbance (i.e., PSQI and PROMIS-SD) and physical/mental health (PROMIS-GH) outcomes. These questionnaire data were also not normalized to baseline, so a condition factor (baseline/intervention) was entered as fixed factor in the analyses. The numbers of participants included in the analyses for all outcomes, by total and shift, are provided in Supplementary Table 1.
Two-tailed unpaired Student’s t-tests were used to evaluate the differences between any variables in all outcome measures that reached statistical significance in the LMEMs. When the LMEMs identified significant main effects of light color or interactions involving light color, pairwise comparisons were made between the red vs. dim white and blue vs. dim white interventions only. This strategy followed our a priori hypothesis that no significant differences would exist between the red and blue lighting interventions.
For all outcomes in the LMEM and post hoc analyses, the criterion for statistical significance was p <0.05. Only those outcomes that reached statistical significance are reported in Results.
3. Results
3.1. Visual performance testing
3.1.1. Psychomotor vigilance test (PVT) task
3.1.1.1. Response time
The LMEM for PVT response time revealed a significant interaction between shift type and time (F2,208 = 4.49, p = 0.012). Response times were significantly lower (i.e., faster) at T2 (i.e., the middle of both shifts) during the night shift compared to the day shift (Supplementary Table 2). Within shifts, response times were significantly lower at T3 compared to T2 during the day shift, and at T2 compared to T1 during the night shift (Supplementary Table 3).
3.1.1.2. Hit rate
The LMEM for PVT hit rate revealed a significant main effect of light color (F2,140 = 3.25, p = 0.042) in which hit rates were significantly lower during the red intervention compared to the dim white intervention (Fig. 3 and Supplementary Table 4).
Fig. 3.
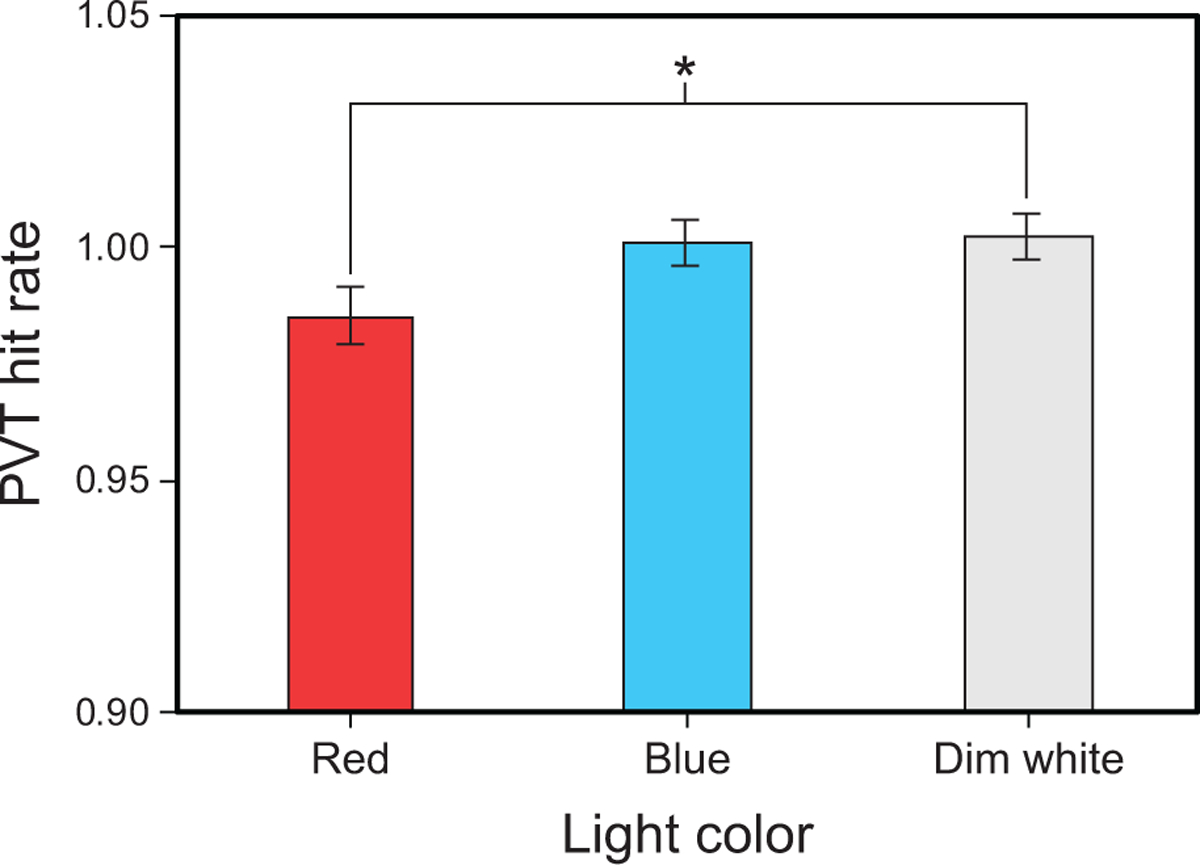
Results for PVT hit rate by light color. The error bars represent SEM; * represents p <0.05.
3.1.2. 1-back task
3.1.2.1. Response time
The LMEM for 1-back response time revealed a significant three-way interaction between shift type, light color, and time (F4,222 = 2.42, p = 0.049). For the day shift, post hoc comparisons showed that response times were significantly faster (i.e., lower values) during the red intervention at T3 (i.e., end of shift) compared to the same intervention at T1 and the dim white light placebo control at T3 (Fig. 4 and Supplementary Tables 5 and 6). Day-shift response times at T3 were also significantly faster during the blue intervention compared to the dim white intervention (Supplementary Table 7).
Fig. 4.
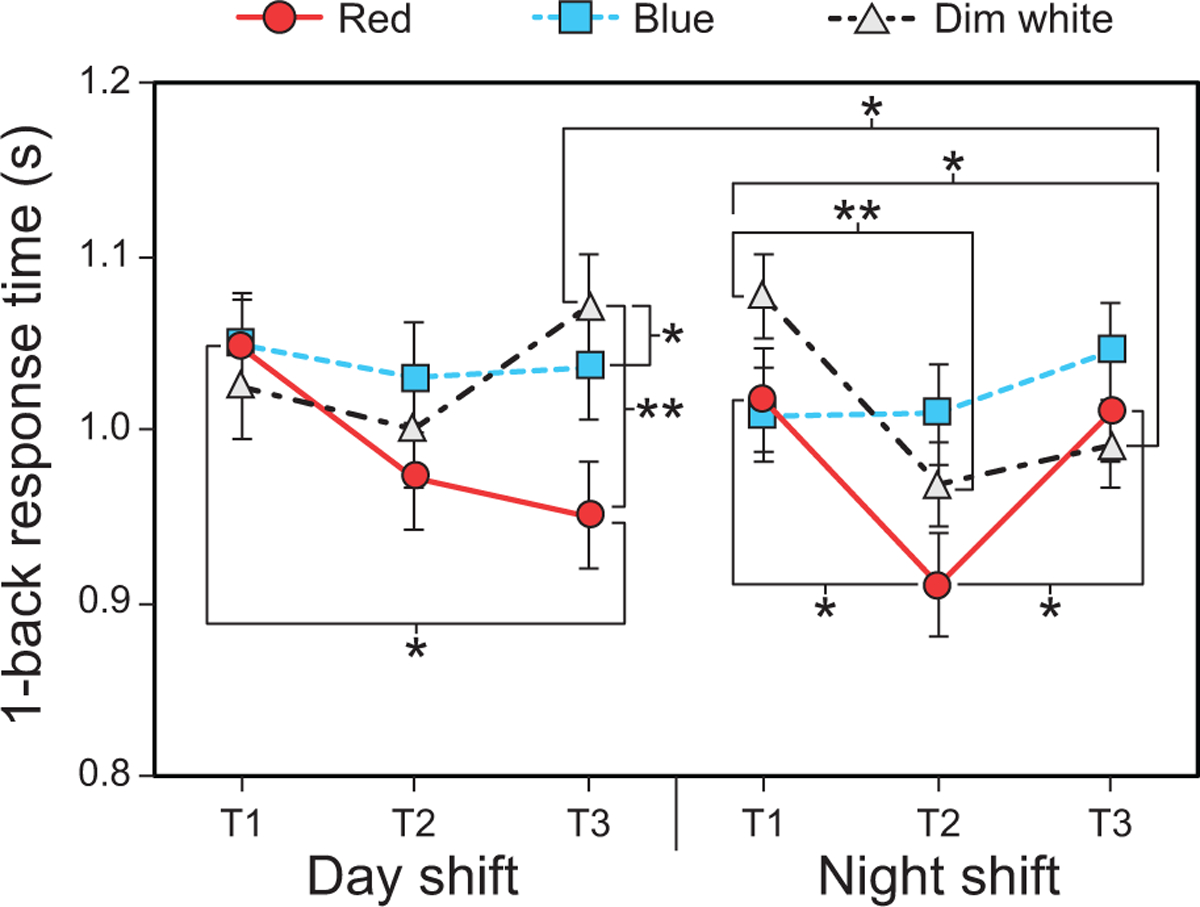
Results for 1-back response time by shift type, time, and light color. The error bars represent SEM; * represents p <0.05, ** represents p <0.01.
For the night shift, post hoc comparisons showed that 1-back response times were significantly faster during the red intervention at T2 (i.e., middle of shift) compared to T1 and T3. Response times were also significantly faster during the dim white intervention at T2 and T3 compared to T1 (i.e., beginning of shift) (Fig. 4 and Supplementary Table 5).
Finally, response times during the dim white intervention were significantly faster at T3 (i.e., end of shift) on the night shift compared to same time during the day shift (Supplementary Table 7).
3.1.2.2. Accuracy rate
The LMEM for 1-back accuracy rate revealed significant light color by shift type (F2,120 = 6.27, p = 0.003) and time by shift type (F2,200 = 3.40, p = 0.035) interactions. For the day shift, post hoc comparisons showed that accuracy rates were significantly lower during the red intervention compared to the dim white intervention (Fig. 5 and Supplementary Table 8). Night-shift accuracy rates were also significantly lower during the blue and dim white interventions compared to day shift (Fig. 5 and Supplementary Table 9). While night-shift 1-back accuracy rates remained essentially flat through time, day-shift accuracy rates were significantly higher at T2 compared to T1 and T3 (Supplementary Table 3).
Fig. 5.
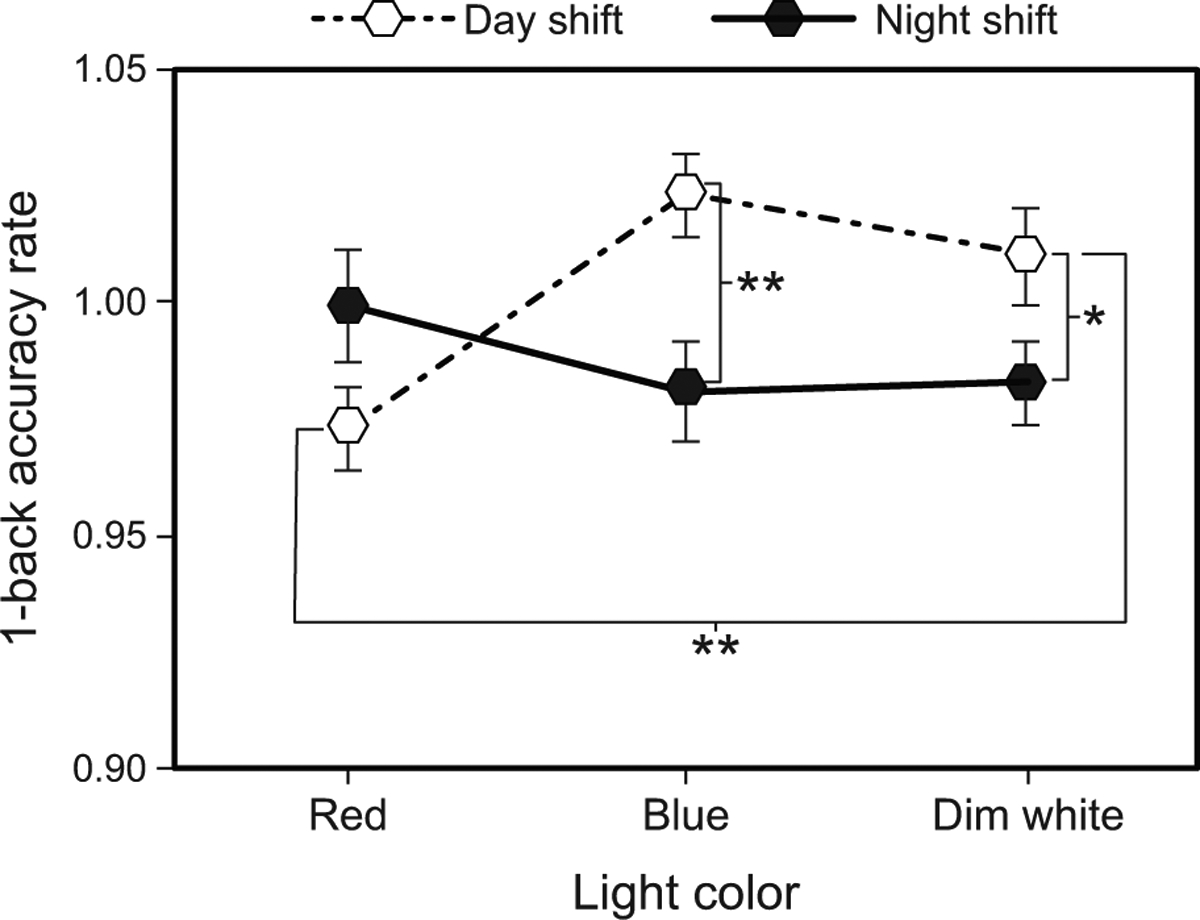
Results for 1-back accuracy rate by light color and shift type. The error bars represent SEM; * represents p <0.05, ** represents p <0.01.
3.1.2.3. Number of correct no-matches
The LMEM for 1-back number of correct no-matches revealed a significant main effect of time (F2,198 = 3.84, p = 0.023) and a significant interaction between shift type and light color (F2,110 = 3.29, p = 0.041). Post hoc comparisons showed that the number of correct no-matches was significantly greater at T2 compared to T1 across all interventions and shift types combined (Supplementary Table 10). For the night shift, the number of correct no-matches was also significantly greater during the dim white intervention compared to the blue intervention (Fig. 6 and Supplementary Table 8).
Fig. 6.
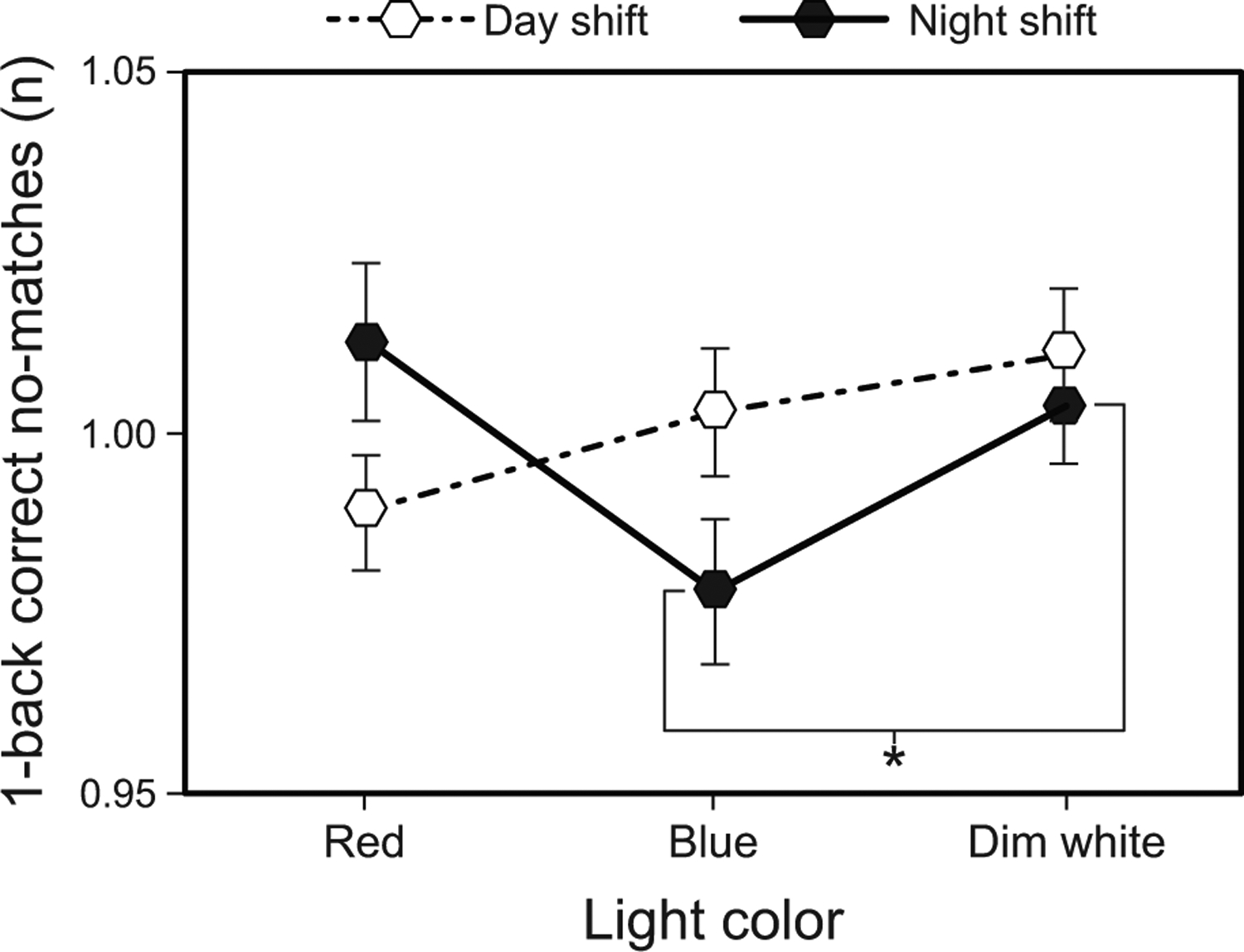
Results for 1-back number of correct no-matches by light color and shift type. The error bars represent SEM; * represents p <0.05.
3.1.2.4. Correct match response time
The LMEM for 1-back correct match response time revealed a significant three-way interaction between shift type, light color, and time (F4,203 = 2.74, p = 0.03). With respect to shift type, the post hoc comparisons for the night shift showed that 1-back correct match response times were significantly slower during the blue intervention at T1 and T3 compared to T2 (Fig. 7A and Supplementary Table 5). Also for the night shift, response times during the dim white intervention were significantly faster at T2 and T3 compared to T1 (Fig. 7A and Supplementary Table 5).
Fig. 7.
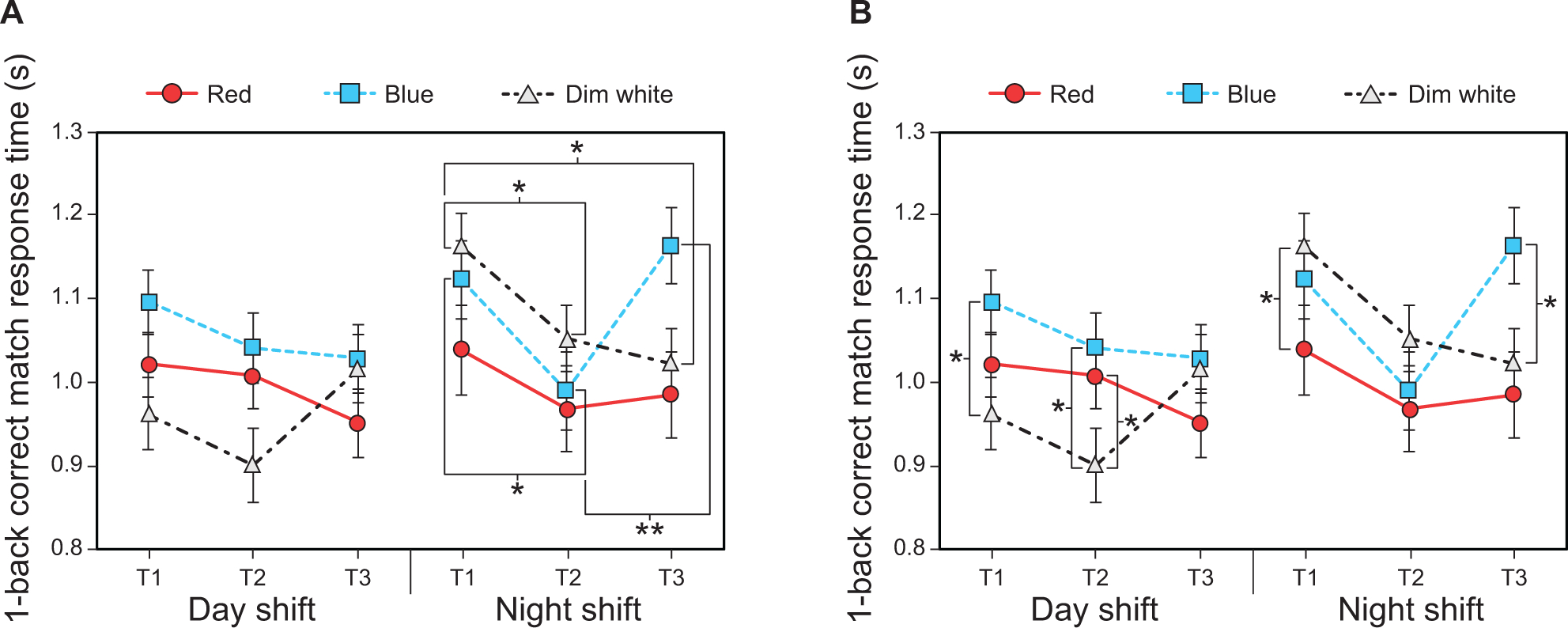
Results for 1-back correct match response time: (A) time comparisons by light color, time, and shift type, and (B) light color comparisons by time and shift type. The error bars represent SEM; * represents p <0.05, ** represents p <0.01.
With respect to time, the post hoc comparisons showed that response times for the day shift were significantly faster during the dim white intervention compared to the blue intervention at T1, and significantly faster than both the red and blue interventions at T2 (Fig. 7B and Supplementary Table 6). For the night shift, response times were significantly slower during the dim white intervention compared to the red intervention at T1 and faster compared to the blue intervention at T3 (Fig. 7B and Supplementary Table 6).
3.1.2.5. Correct no-match response time
The LMEM for correct no-match response time revealed a significant three-way interaction between shift type, light color, and time (F4,203 = 2.74, p = 0.03). For the day shift, post hoc comparisons showed that response times were significantly faster during the red intervention at T3 (i.e., end of shift) compared to T1 (Fig. 8 and Supplementary Table 5). Response times were also significantly faster during the red intervention compared to the dim white intervention at T3. For the night shift, post hoc comparisons showed that response times were significantly faster during the dim white intervention at T2 and T3 compared to T1 (Fig. 8 and Supplementary Table 5).
Fig. 8.
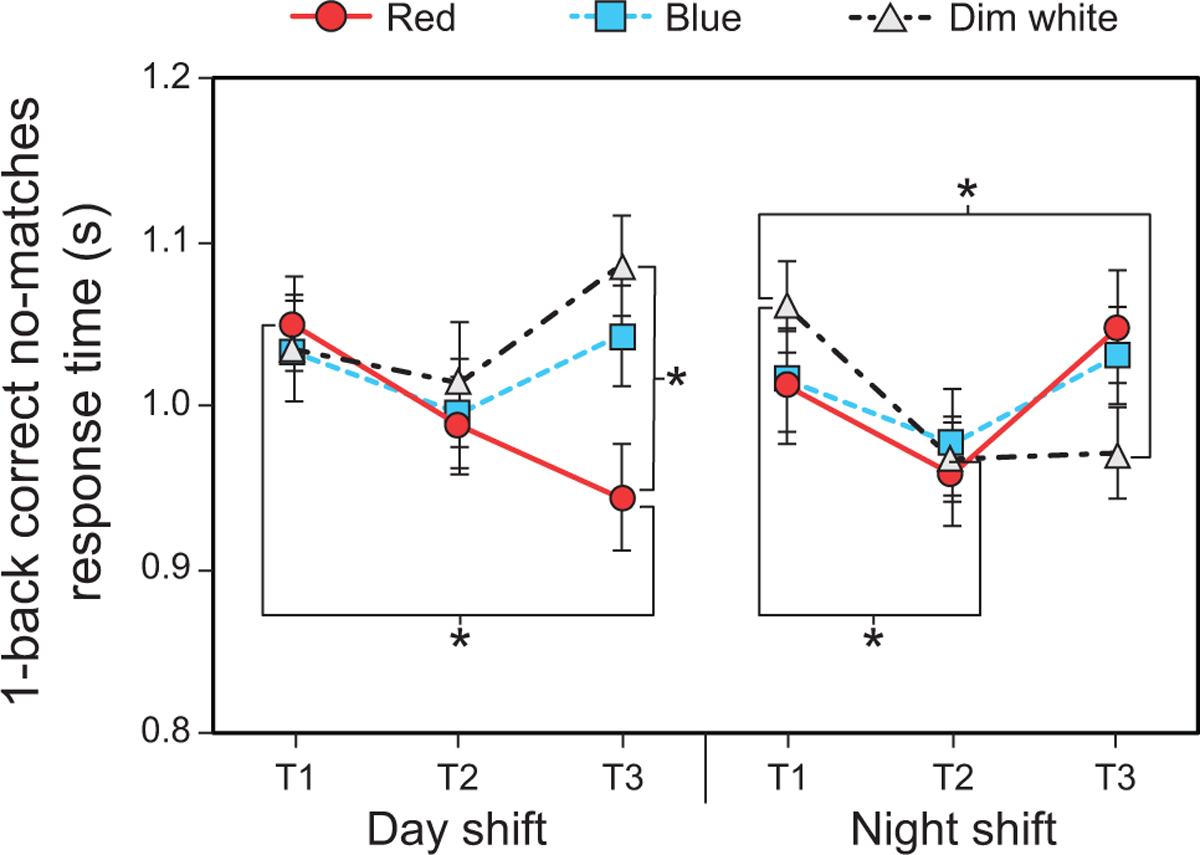
Results for 1-back correct no-match response time by shift type, time, and light color. The error bars represent SEM; * represents p <0.05.
3.1.3. GNG Task
3.1.3.1. False positive rate
The LMEM for GNG false positive revealed a significant main effect of shift type (F1, 98 = 5.48, p = 0.021). Post hoc comparisons showed that the false positive rate was greater during the day shift compared to the night shift (Supplementary Table 11).
3.1.3.2. Response time
The LMEM for GNG response time revealed a significant light color by shift type interaction (F1, 142 = 5.78, p = 0.004). For the day shift, post hoc comparisons showed that response times were significantly faster during the blue intervention compared to the dim white intervention (Fig. 9 and Supplementary Table 8). For the night shift, response times were close to being statistically significantly faster during the blue intervention compared to the dim white intervention (Fig. 9 and Supplementary Table 8). Finally, day-shift response times during the dim white intervention were significantly faster than those for the night shift (Fig. 9 and Supplementary Table 9).
Fig. 9.
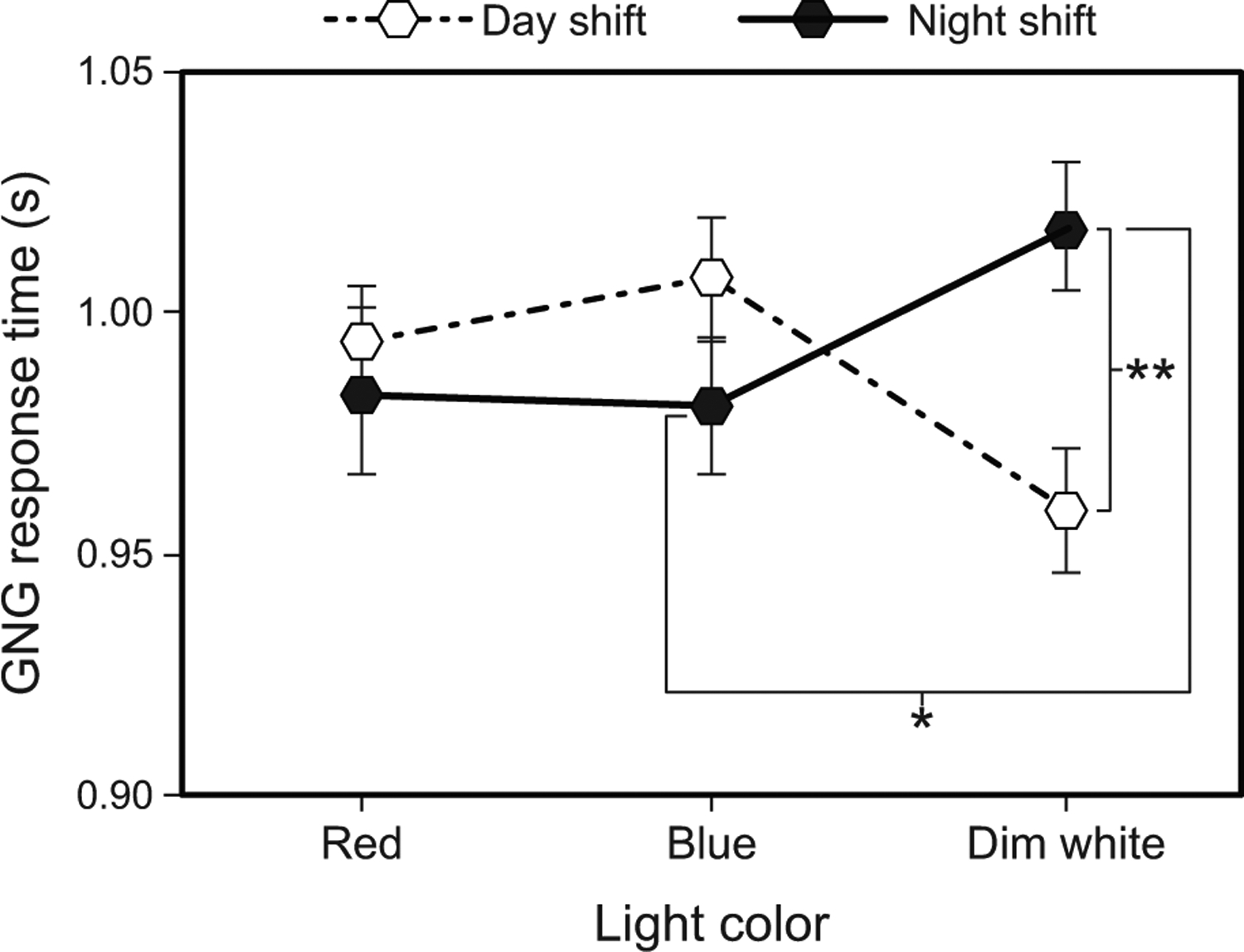
Results for GNG response time by light color and shift type. The error bars represent SEM; * represents p <0.05, ** represents p <0.01.
3.2. Subjective scales
The LMEMs for subjective sleep quality (PSQI) as well as physical and mental health (PROMIS-GH) did not reveal statistically significant main effects or interactions.
3.2.1. Subjective sleepiness (KSS)
The LMEM for KSS revealed a significant interaction between time and light color (F4,288 = 3.02, p = 0.018). Post hoc comparisons showed that normalized KSS scores were significantly higher (i.e., indicating greater sleepiness) during the blue intervention compared dim white interventions at T1 (Fig. 10 and Supplementary Table 12). During the blue intervention, KSS scores were significantly lower at T2 compared to T1 (Fig. 10 and Supplementary Table 13).
Fig. 10.
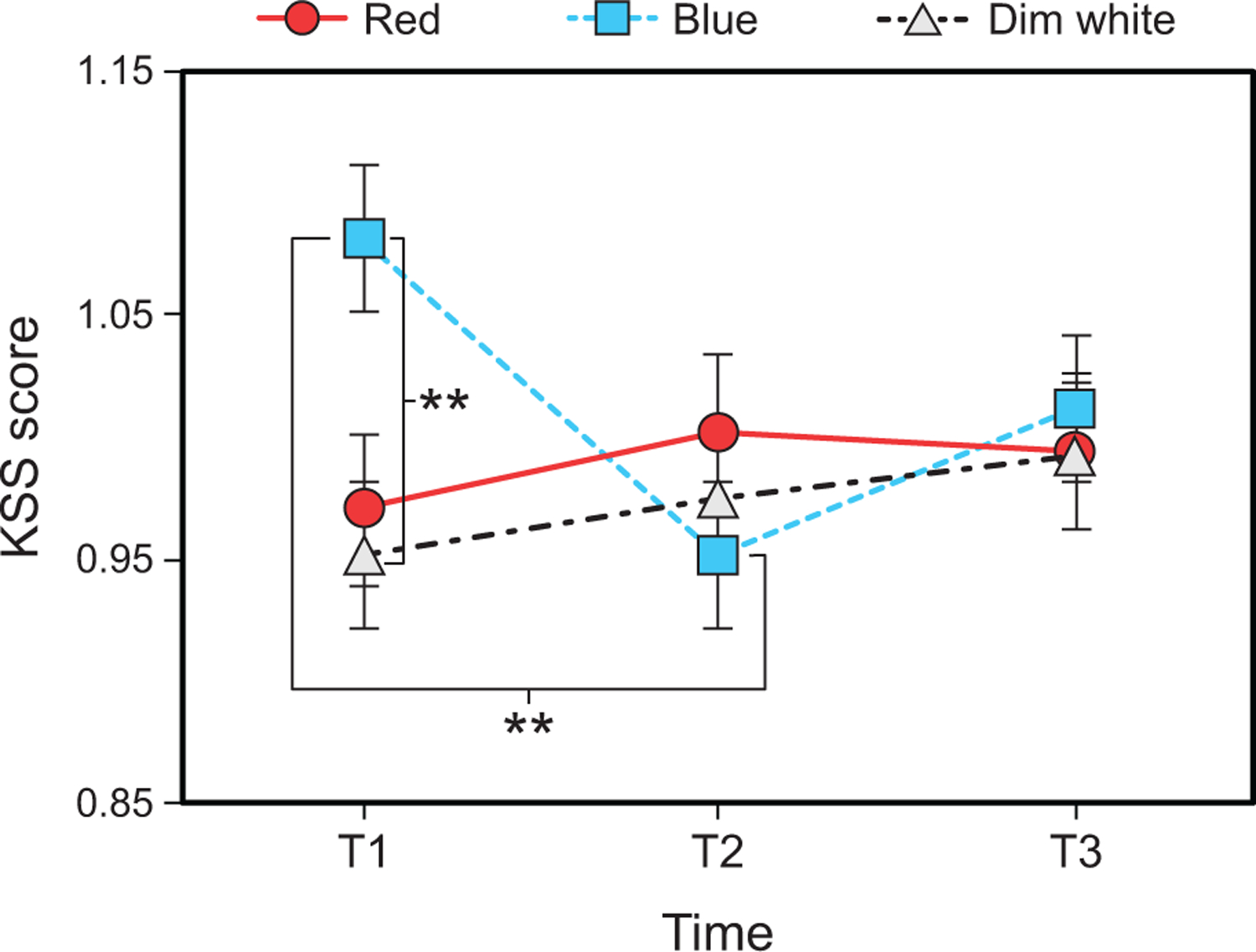
Results for KSS score by time and light color. The error bars represent SEM; ** represents p <0.01.
3.2.2. Sleep disturbance (PROMIS-SD)
The LMEM for PROMIS-SD revealed a significant main effect of light color (F2,198 = 3.117, p = 0.046). Post hoc comparisons showed that PROMIS-SD scores were significantly higher (i.e., worse) in the dim white intervention compared to the blue intervention (Fig. 11 and Supplementary Table 14).
Fig. 11.
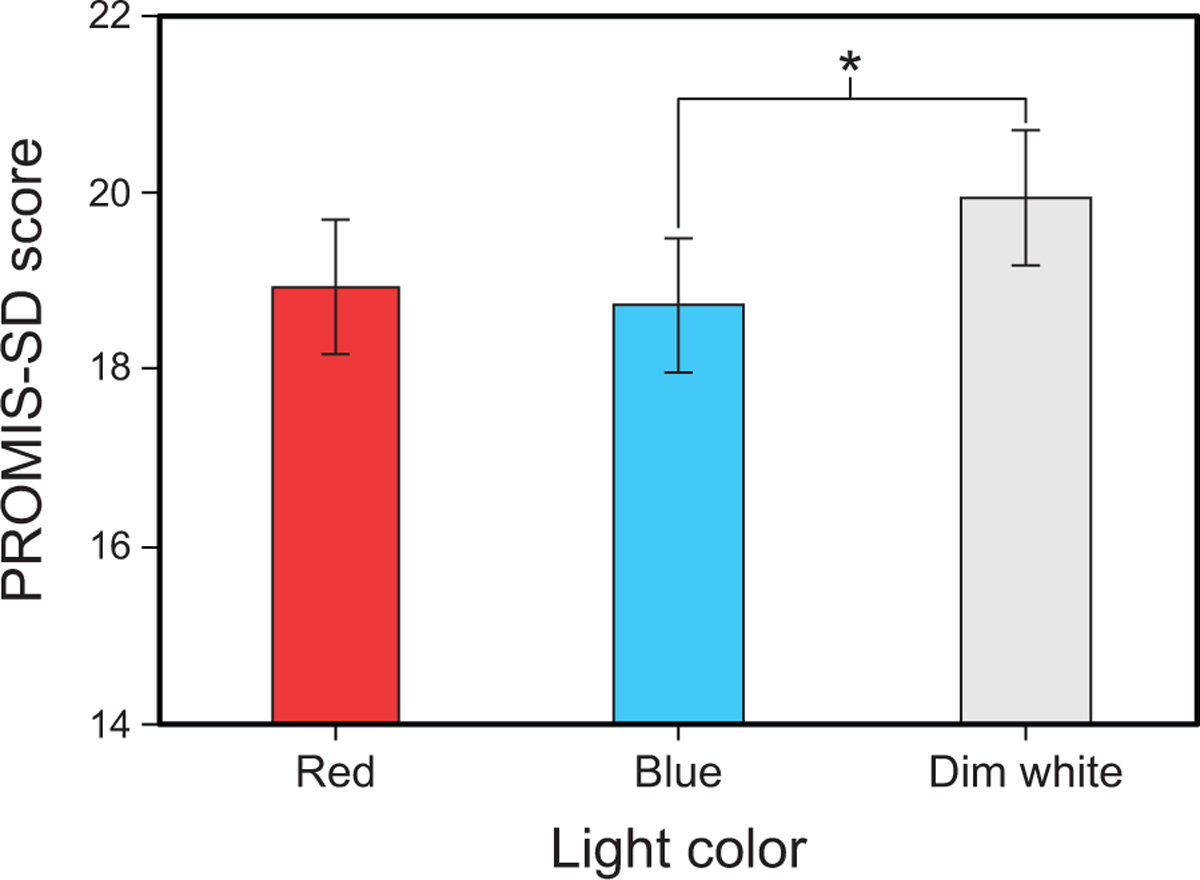
Results for PROMIS-SD score by light color. The error bars represent SEM; * represents p <0.05.
4. Discussion
In this ongoing study investigating the impact of light on visual performance and subjective responses in day- and night-shift workers, preliminary data show that day-shift workers were slower to respond in the middle of the afternoon (T2) on the PVT task while night-shift workers were faster to respond in the middle of the night. The overall hit rate in the PVT task was, however, significantly lower during the red intervention compared to the dim white placebo control intervention.
In tasks that carry a greater cognitive demand than the PVT task (1-back and GNG tasks), the red and blue interventions improved some performance outcomes compared to the dim white intervention, as shown by the reduced response times in the 1-back task (i.e., for the overall response time, correct match response time, and correct no-match response time outcomes). Response times after the red intervention improved during both day and night shifts, while response times in the blue intervention improved only at the end (T3) of the day shift. These results are consistent with our laboratory studies showing that participants’ responses tend to be faster after exposure to red light, during both night and day. These results also corroborate studies showing that short-wavelength (blue) light is not needed to increase alertness and maintain performance in certain tasks, as reviewed in Souman et al. (2018). Nevertheless, it should be emphasized that, overall, the results in the field are less robust than those shown under laboratory conditions (Figueiro, Sahin, Wood, & Plitnick, 2016; Okamoto et al., 2014; Sahin & Figueiro, 2013).
The impact of the lighting interventions on accuracy and hit rates, however, did not show the expected results. In fact, despite being faster to respond, in some cases, participants were less accurate in their responses after the blue and red interventions compared to the dim white intervention. These results suggest that light may have a stronger effect on the sympathetic system (i.e., fight or flight response) than on cognition and memory. Improvements in cognitive performance after both nighttime and daytime light exposures can be task dependent, and such improvements have not always been demonstrated in various laboratory and field studies (Correa, Barba, & Padilla, 2016; Segal, Sletten, Flynn-Evans, Lockley, & Rajaratnam, 2016; Young et al., 2015).
In terms of the study’s subjective scales, subjective sleepiness (KSS) was surprisingly flat over the course of both shifts. The only significant difference was after the blue intervention at T1, when participants reported greater sleepiness compared to the other two interventions. This result could have been due to factors other than the lighting intervention, but there is no way to determine that from the available data.
Another interesting result was that exposure to blue light reduced subjective sleep disturbance (PROMIS-SD) scores (indicating better sleep) compared to exposure to dim white light. The red light also reduced self-reports of sleep disturbance compared to the dim white placebo control, but the difference did not reach statistical significance. Blue light exposure at night was expected to delay sleep in night-shift workers and advance sleep in day-shift workers, possibly improving sleep only in those receiving blue light during the day, but the shift type by light color interaction was not significant.
The fact that the present research is a field study can be counted as both a noteworthy limitation and a strength. By their very nature, field studies can introduce uncontrollable extraneous variables to the data collection. For example, we encountered many instances where performance tests were interrupted or not completed, the timing of data collection varied from night to night, distractions may have affected performance of tests, and other work or personal related issues may have influenced participants’ subjective responses. Despite these limitations, however, these results are nonetheless promising because they provide field data that are consistent with carefully performed laboratory studies showing that red light increases objective and subjective alertness and improves certain types of short-term performance. New data that are currently being collected will provide us with more information about the positive effects of light on day- and night-shift workers.
5. Conclusions
The significance of these results is that shift workers, especially night-shift workers, can possibly use red light to help them counteract their natural tendency to fall asleep without affecting their melatonin levels during the shift or delaying or disturbing their sleep after the shift is over. It is well known that bright light (>500 lx at the eye) or blue light (as low as 20 lx at the eye) can promote nighttime alertness but will also suppress melatonin and may delay sleep onset after the shift. Therefore, solutions like the ones presented in this study may have important implications for workers’ safety and well-being.
Supplementary Material
Acknowledgements
The study was funded by NIOSH (Grant #R01OH01668). The authors would like to thank Barbara Plitnick, Sharon Lesage, Charles Roohan, Greg Ward, Danuel Carr, and Levent Sahin for their technical assistance. The authors also wish to acknowledge the study’s participating nurses and hospitals, and offer specific thanks to the following individuals who helped make the study possible: Ghafar Kurdieh, Bassett Medical Center, Cooperstown, NY; Dr. Leslyn Williamson and Emily Spinner, Ellis Hospital and Bellvue Woman’s Center, Schenectady, NY; Mashelle Monhaut, Memorial Hospital, South Bend, IN; and Karen Klingman, Upstate University Hospital, Syracuse, NY.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- Åkerstedt T, & Gillberg M (1990). Subjective and objective sleepiness in the active individual. International Journal of Neuroscience, 52(1–2), 29–37. doi: 10.3109/00207459008994241 [DOI] [PubMed] [Google Scholar]
- Badia P, Myers B, Boecker M, Culpepper J, & Harsh JR (1991). Bright light effects on body temperature, alertness, EEG and behavior. Physiology and Behavior, 50(3), 583–588. doi: 10.1016/0031-9384(91)90549-4 [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, & Rollag MD (2001). Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. Journal of Neuroscience, 21(16), 6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, & Kupfer DJ (1991). Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep, 14(4), 331–338. doi: 10.1093/sleep/14.4.331 [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, … Choi S (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa Á, Barba A, & Padilla F (2016). Light effects on behavioural performance depend on the individual state of vigilance. PLoS One, 11(11), e0164945–e0164945. doi: 10.1371/journal.pone.0164945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Mirick DK, & Stevens RG (2001). Night shift work, light at night, and risk of breast cancer. Journal of the National Cancer Institute, 93(20), 1557–1562. doi: 10.1093/jnci/93.20.1557 [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Bierman A, Plitnick B, & Rea MS (2009). Preliminary evidence that both blue and red light can induce alertness at night. BMC Neuroscience, 10, 105. doi: 10.1186/1471-2202-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro MG, & Rea MS (2010). The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. International Journal of Endocrinology, 2010, 829351. doi: 10.1155/2010/829351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro MG, Sahin L, Wood B, & Plitnick B (2016). Light at night and measures of alertness and performance: Implications for shift workers. Biological Research for Nursing, 18(1), 90–100. doi: 10.1177/1099800415572873 [DOI] [PubMed] [Google Scholar]
- Ganesan S, Magee M, Stone JE, Mulhall MD, Collins A, Howard ME, … Sletten TL (2019). The Impact of shift work on sleep, alertness and performance in healthcare workers. Scientific Reports, 9(1), 4635. doi: 10.1038/s41598-019-40914-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J (2001a). Increased breast cancer risk among women who work predominantly at night. Epidemiology, 12(1), 74–77. doi: 10.1097/00001648-200101000-00013 [DOI] [PubMed] [Google Scholar]
- Hansen J (2001b). Light at night, shiftwork, and breast cancer risk. Journal of the National Cancer Institute, 93(20), 1513–1515. doi: 10.1093/jnci/93.20.1513 [DOI] [PubMed] [Google Scholar]
- Hays RD, Bjorner JB, Revicki DA, Spritzer KL, & Cella D (2009). Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research, 18(7), 873–880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopcia K, Dennerlein JT, Hashimoto D, Orechia T, & Sorensen G (2012). Occupational injuries for consecutive and cumulative shifts among hospital registered nurses and patient care associates: A case-control study. Workplace Health & Safety, 60(10), 437–444. doi: 10.1177/216507991206001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Duan P, Yao L, & Hou H (2018). Shiftwork-mediated disruptions of circadian rhythms and sleep homeostasis cause serious health problems. International Journal of Genomics, 2018, 8576890. doi: 10.1155/2018/8576890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, & Newsome DA (1980). Light suppresses melatonin secretion in humans. Science, 210(4475), 1267–1269. doi: 10.1126/science.7434030 [DOI] [PubMed] [Google Scholar]
- Lowden A, Akerstedt T, & Wibom R (2004). Suppression of sleepiness and melatonin by bright light exposure during breaks in night work. Journal of Sleep Research, 13(1), 37–43. doi: 10.1046/j.1365-2869.2003.00381.x [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Rea MS, & Figueiro MG (2014). Temporal dynamics of EEG activity during short- and long-wavelength light exposures in the early morning. BMC Research Notes, 7, 113. doi: 10.1186/1756-0500-7-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichael C, Skene DJ, & Revell VL (2012). Human nonvisual responses to simultaneous presentation of blue and red monochromatic light. Journal of Biological Rhythms, 27(1), 70–78. doi: 10.1177/0748730411431447 [DOI] [PubMed] [Google Scholar]
- Phipps-Nelson J, Redman JR, Dijk DJ, & Rajaratnam SM (2003). Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep, 26(6), 695–700. doi: 10.1093/sleep/26.6.695 [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, & Touitou Y (2010). Ethics and methods for biological rhythm research on animals and human beings. Chronobiology International, 27(9–10), 1911–1929. doi: 10.3109/07420528.2010.516381 [DOI] [PubMed] [Google Scholar]
- Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, & Hardie LJ (2016). Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocrine Reviews, 37(6), 584–608. doi: 10.1210/er.2016-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam SMW, Howard ME, & Grunstein RR (2013). Sleep loss and circadian disruption in shift work: health burden and management. The Medical Journal of Australia, 199(8), S11–S15. doi: 10.5694/mja13.10561 [DOI] [PubMed] [Google Scholar]
- Rea MS, & Figueiro MG (2018). Light as a circadian stimulus for architectural lighting. Lighting Research & Technology, 50(4), 497–510. doi: 10.1177/1477153516682368 [DOI] [Google Scholar]
- Rea MS, Figueiro MG, Bierman A, & Bullough JD (2010). Circadian light. Journal of Circadian Rhythms, 8(1), 2. doi: 10.1186/1740-3391-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Bierman A, & Hamner R (2012). Modelling the spectral sensitivity of the human circadian system. Lighting Research & Technology, 44(4), 386–396. doi: 10.1177/1477153511430474 [DOI] [Google Scholar]
- Rea MS, Figueiro MG, Bullough JD, & Bierman A (2005). A model of phototransduction by the human circadian system. Brain Research Reviews, 50(2), 213–228. doi: 10.1016/j.brainresrev.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Sahin L, & Figueiro MG (2013). Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiology and Behavior, 116–117, 1–7. doi: 10.1016/j.physbeh.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Sahin L, Wood B, Plitnick B, & Figueiro MG (2014). Daytime light exposure: Effects on biomarkers, measures of alertness, and performance. Behav Brain Res, 274, 176–185. doi: 10.1016/j.bbr.2014.08.017 [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Laden F, & Hankinson SE (2006). Night work and risk of breast cancer. Epidemiology, 17(1), 108–111. doi: 10.1097/01.ede.0000190539.03500.c1 [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, & Colditz GA (2001). Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. Journal of the National Cancer Institute, 93(20), 1563–1568. doi: 10.1093/jnci/93.20.1563 [DOI] [PubMed] [Google Scholar]
- Segal AY, Sletten TL, Flynn-Evans EE, Lockley SW, & Rajaratnam SM (2016). Daytime exposure to short- and medium-wavelength light did not improve alertness and neurobehavioral performance. Journal of Biological Rhythms, 31(5), 470–482. doi: 10.1177/0748730416659953 [DOI] [PubMed] [Google Scholar]
- Souman JL, Tinga AM, Te Pas SF, van Ee R, & Vlaskamp BNS (2018). Acute alerting effects of light: A systematic literature review. Behav Brain Res, 337, 228–239. doi: 10.1016/j.bbr.2017.09.016 [DOI] [PubMed] [Google Scholar]
- Stimpfel AW, Brewer CS, & Kovner CT (2015). Scheduling and shift work characteristics associated with risk for occupational injury in newly licensed registered nurses: An observational study. International Journal of Nursing Studies, 52(11), 1686–1693. doi: 10.1016/j.ijnurstu.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, & Skene DJ (2001). An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. The Journal of Physiology, 535, 261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkoff AM, Le R, Geiger-Brown J, & J. L (2007). Work schedule, needle use, and needlestick injuries among registered nurses. Infection Control and Hospital Epidemiology, 28(2), 156–164. doi: 10.1086/510785 [DOI] [PubMed] [Google Scholar]
- Tynes T, Hannevik M, Andersen A, Vistnes A, & Haldorsen T (1996). Incidence of breast cancer in Norwegian female radio and telegraph operators. Cancer Causes and Control, 7(2), 197–204. doi: 10.1007/BF00051295 [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of Labor Statistics. (2019). Table 7. Workers by shift usually worked and selected characteristics, averages for the period 2017–2018. Retrieved from https://www.bls.gov/news.release/flex2.t07.htm
- Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, … Maquet P (2006). Daytime light exposure dynamically enhances brain responses. Current Biology, 16(16), 1616–1621. doi: 10.1016/j.cub.2006.06.031 [DOI] [PubMed] [Google Scholar]
- Viola AU, James LM, Schlangen LJ, & Dijk DJ (2008). Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scandinavian Journal of Work, Environment & Health, 34(4), 297–306. doi: 10.5271/sjweh.1268 [DOI] [PubMed] [Google Scholar]
- Young CR, Jones GE, Figueiro MG, Soutière SE, Keller MW, Richardson AM, … Rea MS (2015). At-sea trial of 24-h-based submarine watchstanding schedules with high and low correlated color temperature light source. Journal of Biological Rhythms, 30(2), 144–154. doi: 10.1177/0748730415575432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


