Introduction
Melanomas of the conjunctiva and eyelid present unique management challenges for the ophthalmologist and ocular oncologist. Surgical excision, a mainstay of treatment, may be disfiguring with variable rates of local recurrence.1 Conjunctival melanomas have a local recurrence rate ranging from 18% to 83% with data largely coming from patients treated with excision with or without cryotherapy.2–4 Cutaneous melanomas of the eyelid skin have a local recurrence rate ranging from 7% to 78% depending on technique and extent of excision.5–7 The rate of regional lymph node metastasis was 41% in a study of conjunctival melanoma patients treated primarily with local excision, the authors compared their finding to rates in cutaneous melanoma of the head and neck which ranged from 14% to 44% or eyelid skin melanoma at 29%.4 Efforts to improve outcomes of these tumors have continually advanced with surgical techniques and adjunctive treatments such as topical therapy, radiation, and systemic chemotherapy.8 Recent advances in immunotherapy, specifically checkpoint inhibitors, has allowed for primary and adjuvant treatment of cutaneous melanomas with medical therapy. These have been successful in the setting of metastatic cutaneous melanoma and other cancers. This review of the literature summarizes the current understanding and use of checkpoint inhibitors, with a particular focus for the ophthalmic surgeon.
Immune checkpoint inhibitors are relatively new therapies, developed with the rationale of stimulating a patient’s own immune system to better respond to malignancies. This strategy progressed from the discovery of specific receptor proteins that promote immune tolerance, that is, inhibit immune responsiveness, which tumors may take advantage of to proliferate unhindered. Monoclonal antibodies were developed to block these cellular checkpoints. Clinically available therapies include ipilimumab, which targets cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), nivolumab, pembrolizumab, and cemiplimab, which target programmed cell death-1 (PD-1), and atezolizumab, avelumab, and durvalumab, which target programmed cell death ligand-1 (PD-L1). Checkpoint inhibitors have demonstrated efficacy in a range of malignancies including non–small cell lung cancer, urothelial cancer, renal cell cancer, squamous cell carcinoma of the head and neck, Merkel cell carcinoma, and metastatic cutaneous melanoma.9
In recent years there has been emerging interest in checkpoint inhibitor therapy for oculoplastic applications, specifically eyelid and conjunctival melanomas.10 However, there is limited clinical experience with the use of these drugs for this indication. Eyelid and conjunctival melanomas were not specifically studied in the trials leading to the development of these therapies, resulting in limited knowledge of their potential benefits and risks. This chapter reviews the available literature regarding checkpoint inhibitors for eyelid and conjunctival melanomas.
Immune Checkpoint Inhibitors
The human immune system has cellular and humoral mediated immunity. The cellular mediated response against tumors is escalated by T-cell recognition of tumor antigens presented by antigen-presenting cells in the activation phase. The activated T-cell multiplies and responds to tumor cells in the effector phase. This response can be de-escalated by multiple immune checkpoints.11 A clinically relevant checkpoint is CTLA-4. This receptor expressed on T-cells binds to CD80 and CD86 on antigen-presenting cells, preventing costimulatory signals that activate T cells.12 A tumor cell may take advantage of the required costimulatory signaling to inhibit the immune response. In a landmark study by Leach et al,13 blockage of CTLA-4 was demonstrated to cause tumor rejection, as well as future immunologic memory. Subsequent clinical trials on CTLA-4 inhibitors included 2 fully humanized antibodies: ipilimumab (IgG1 monoclonal antibody) and tremelimumab (IgG2 monoclonal antibody). In 2010, a phase 3 study of ipilimumab14 led to the Food and Drug Administration (FDA) approval for the treatment of metastatic cutaneous melanoma. This study randomized 676 advanced or metastatic melanoma patients who had progressed on systemic therapy to ipilimumab in combination with gp100 (peptide tumor vaccine), gp100 alone, or ipilimumab alone. Results showed significant improvement in overall survival in the arms with ipilimumab. Ipilimumab also had favorable survival when combined with dacarbazine versus dacarbazine alone (standard of care) for metastatic melanoma15 and as adjuvant therapy.16
More recently, several anti-PD-1 and PD-L1 trials have shown even more promise with further improved outcomes. PD-1 is expressed on T cells and binds to PD-L1, a ligand expressed on tumor cells and macrophages. Increased expression of this ligand on tumor cells leads to increased binding on T cells, which impairs their response to tumors. Available agents include nivolumab, pembrolizumab, and cemiplimab against PD-1, and atezolizumab, avelumab, and durvalumab against PD-L1. In 2014, the FDA approved nivolumab based on a study in which patients with metastatic melanoma without BRAF mutations were treated with either nivolumab or dacarbazine, with results showing that nivolumab resulted in superior overall survival and progression-free survival.17 In addition for patients that progressed on ipilimumab (anti-CTLA-4), subsequent nivolumab (anti-PD-1) was superior to cytotoxic agents such as dacarbazine or paclitaxel plus carboplatin.18,19 Other comparative studies between nivolumab and ipilimumab as adjuvant therapy after resection confirmed the superior recurrence-free survival and lower grade 3 or 4 adverse events with nivolumab (14.4%) versus ipilimumab (45.9%).20 Similarly, pembrolizumab, also an anti-PD-1 agent, was shown to be superior to cytotoxic chemotherapy for ipilimumab-refractory melanoma.21 Pembrolizumab also had superior overall survival even against the CTLA-4 checkpoint inhibitor ipilimumab, with lower rates of adverse events.22 In addition, combination checkpoint blockade using CTLA-4 (ipilimumab) and PD-1 (nivolumab) inhibition is also a first-line treatment option for metastatic melanoma. This regimen was approved based on CheckMate 067, which compared the combination of ipilimumab and nivolumab, nivolumab alone, and ipilimumab alone; 5-year follow-up has shown this combination regimen to have the highest efficacy thus far in metastatic melanoma with over 50% of patients surviving at 5 years. However, there is additive toxicity with this combination, so not all patients may qualify for this therapy.23 These checkpoint inhibitors have fundamentally altered the medical treatment of metastatic melanoma, and have evolved rapidly in the past decade.
Checkpoint Inhibitors for Conjunctival Melanoma
Conjunctival melanoma is a relatively rare melanoma, representing 2% to 5% of all ocular malignancies.24,25 These tumors of melanocytes usually present as pigmented conjunctival lesions, ranging between nodular or flat growth patterns (Fig. 1). Timely diagnosis and management of conjunctival melanoma are critical as it has the potential for local invasion and systemic spread, with high rates of recurrence.26 Early-stage conjunctival melanoma can be managed with complete excision with wide margins, cryotherapy, or brachytherapy. The role of topical chemotherapy in adjuvant disease is debated but is not indicated for primary disease.8,27 In contrast, locally advanced and metastatic conjunctival melanoma may require external beam or proton beam radiation therapy. Orbital exenteration is done for unresectable disease; however, this has not been shown to improve survival.28 Management of metastatic conjunctival melanoma has been limited, primarily utilizing systemic chemotherapy29 without significant success. Interest in finding more efficacious treatments for conjunctival melanoma include efforts to apply literature from other tumors such as cutaneous and mucosal melanoma.
Figure 1.
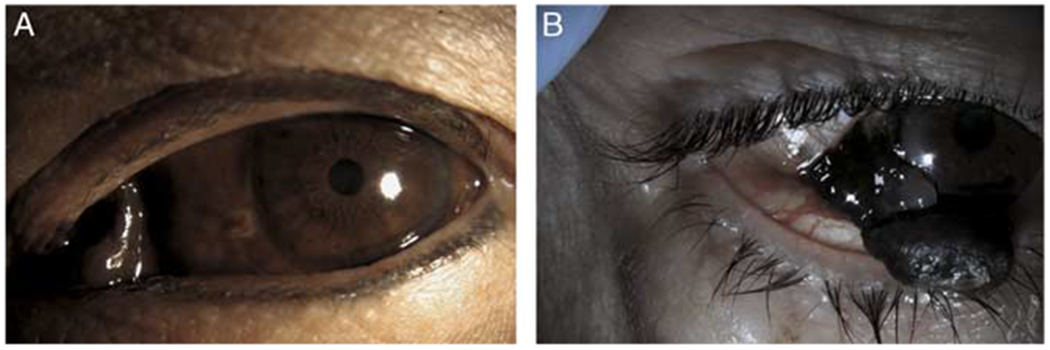
Representative photos of conjunctival melanoma. A, Caruncle involving conjunctival melanoma. A 63-year-old Thai female presented with a rapidly growing pigmented lesion involving the caruncle in the setting of diffuse primary acquired melanosis that had been present for years per the patient. The patient was not primarily surgically resectable but wanted to avoid exenteration and therefore was treated with wide local excision followed by adjunctive cryotherapy to any nodular areas and topical mitomycin C 0.04%. Sentinel lymph node biopsy was deferred and there was no metastatic disease. Her local disease was controlled for 3 years until she was noted to have a local amelanotic recurrence. Head and neck magnetic resonance imaging showed lymph node involvement, which was confirmed on biopsy. She was treated systemically, however succumbed to her disease 4 years after presentation. B, A 65-year-old Hispanic male with rapidly growing pigmented lesion in the setting of primary acquired melanosis. He underwent wide local excision. Pathology was consistent with a conjunctival melanoma. There was no radiologic evidence of locally advanced or metastatic disease.
A degree of molecular similarities between cutaneous and conjunctival melanoma, and the expression of PD-1/PD-L1 in a subset of conjunctival melanomas, suggest the potential relevance of checkpoint inhibition as a treatment option for conjunctival melanoma. Cao and colleagues found PD-1 and PD-L1 expression (cutoff for positivity 5%) in a subset of conjunctival melanoma by immunofluorescence; their study included 27 primary conjunctival melanoma patients, with 5 (19%) having PD-L1 expression in the tumor and 17 (63%) having PD-1 expression in the tumor. The expression of PD-L1 was associated with distant metastases and worse melanoma-related survival, and PD-1 expression was found primarily in the more advanced T2 stage tumors.30 In addition, cutaneous and conjunctival melanomas share several significant mutational similarities including high expression of BRAF, NRAS, numerous copy number variations, and heat shock protein 90 expressions while having low rates of GNA11, p16, and KIT.28,31–37 In contrast, conjunctival melanoma differs from uveal melanoma, which instead has higher GNAQ/GNA11 mutations.38
The molecular similarities of cutaneous and conjunctival melanoma, in contrast to mucosal or uveal melanoma, appear to have some correlation with the clinical response of these tumors to checkpoint inhibitors. The response rate of uveal and mucosal melanomas to checkpoint inhibitors is poor: a study reviewing the uveal melanoma literature for checkpoint inhibitors found 20 papers, with 18 of the reports showing response rates between 0% and 16.7%, with only two outlier reports of 26.6% and 30% response rates.39 A study reviewing the mucosal melanoma literature for checkpoint inhibitors found 5 papers, showing response rates to nivolumab monotherapy of 23.3% and to combination nivolumab and ipilimumab of 37.1%.40 In contrast, cutaneous melanoma had a response rate to nivolumab monotherapy of 40.9% and to combination nivolumab and ipilimumab of 60.4%.40 Overall the literature reveals poor response rates for uveal and mucosal melanoma to checkpoint inhibitor therapy, whereas cutaneous melanoma has notably higher response rates. The molecular similarities of conjunctival melanoma to cutaneous melanoma, in contrast to uveal and mucosal melanoma, may thus predict a higher response rate of conjunctival melanomas to checkpoint inhibitors.
Only in recent years have there been clinical reports of checkpoint inhibitor therapy for conjunctival melanoma (Table 1). Notably, these studies feature small sample sizes, variable design, and no comparative arms. Finger and Pavlick41 published a series of 5 patients with conjunctival melanoma, 3 in which anti-PD-1 agents were used after all other medical options and in place of exenteration, and 2 for metastatic conjunctival melanoma. In their series, all patients had disease response, with moderate systemic adverse reactions. Similarly, Sagiv et al42 published a series of 5 cases of metastatic conjunctival melanoma, 4 of which were treated with nivolumab and 1 with pembrolizumab. They found 4 of the cases had a complete response with no evidence of disease at 1 to 36 months after completion of treatment, and the last patient had stable disease for 6 months. Kini et al43 published a single case of conjunctival melanoma treated with pembrolizumab followed by excision and cryotherapy. There was the resolution of the tumor and no recurrence at 1 year, with no side effects noted. Part of the reasoning for pembrolizumab instead of exenteration, in this case, was that the involved eye was the patient’s seeing eye, underscoring the need for effective globe sparing therapy. Chaves et al44 also published a single case of conjunctival melanoma treated with debulking, brachytherapy, sentinel lymph node biopsy, and finally ipilimumab for adjuvant therapy due to high recurrence risk. Pinto Torres et al46 reported 2 cases of conjunctival melanoma treated initially with excision but metastases with systemic therapy, one of which was treated with pembrolizumab with complete resolution and without significant adverse effects. Notably, the patient on pembrolizumab in Pinto Torres’ study had a history of the human immunodeficiency virus (HIV) with normal CD4 count and undetectable viral load. There were no HIV related complications or reactivation of the virus noted. Other experience on the case report level also supports the successful use of checkpoint inhibitors despite the presence of HIV.47 This has also been demonstrated outside of melanoma.48,49 It is difficult to interpret a response rate of conjunctival melanoma to checkpoint inhibitors from these small case series, as there is likely reporting bias for successful cases. However, the overwhelmingly positive response rates are suggestive of therapeutic potential that warrants ongoing study.
Table 1.
Conjunctival Melanoma Treated by Checkpoint Inhibitor
| References | Tumor Stage | Checkpoint Inhibitor | Resection Prior | Topical Therapy Prior | Radiation Prior | Metastatic Disease | Previous Systemic Therapy | Tumor Response | Follow-up | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Finger et al41 | AJCC-T3bN0M0 | Ipilimumab, then pembrolizumab | Yes | Yes | No | No | Yes | 36 mo since initiation of therapy, 2y NED | Adrenal insufficiency, dermatitis | |
| AJCC-cT3bN0M0, pT4b | Pembrolizumab, then ipilimumab | No | No | No | No | Yes | None | |||
| AJCC-T3bN0M0 | Pembrolizumab, then combination with ipilimumab | Yes | Yes | Brachytherapy | No | No | Yes | > 18 mo | None | |
| M1 | Ipilimumab, then pembrolizumab in combination and alone | Yes | Yes | Regional at site of metastases | Yes | No | Yes | > 24 mo | ||
| M1 | Ipilimumab, Nivolumab | Yes | Yes | Yes | No | Yes | > 36 mo | Grade II hepatotoxicity, grade III colitis, grade II pneumonitis | ||
| Sagiv et al42 | M1 | Nivolumab | Yes | No | No | Yes | No | Yes | 9 mo | |
| M1 | Nivolumab | Yes | Yes | No | Yes | No | Yes | 36 | ||
| M1 | Nivolumab | Yes | No | No | Yes | Interferon | Yes | 7 | Colitis | |
| M1 | Pembrolizumab, then ipilimumab | Yes | No | Yes | Yes | Yes | 2 | Grade IV hepatotoxicity | ||
| M1 | Nivolumab | Yes | No | No | Yes | No | Yes | 1 | Colitis | |
| Kini et al43 | Localized disease | Pembrolizumab | Yes | No | No | No | No | Yes | 12 mo | |
| Chaves et al44 | AJCC-T3bN1M0 | Ipilimumab | Yes | No | Custom iodine-125 device | Yes | No | Yes | Mild fatigue | |
| Ford et al45 | M1 | Nivolumab | Yes | Yes | No | Yes | No | Yes | ~24 mo | Not addressed |
| Pinto Torres et al46 | M1 | Pembrolizumab | Yes | No | No | Yes | No | Yes | 9 mo | None noted |
NED indicates no evidence of disease.
Checkpoint Inhibitors for Eyelid Melanoma
There are no published reports directly studying checkpoint inhibitors for eyelid-specific cutaneous melanoma: searches in PubMed for the terms “eyelid” and: “checkpoint inhibitor,” “ipilimumab,” “nivolumab,” “pembrolizumab,” “atezolizumab” yield no pertinent results.
It may be reasonable to suggest that eyelid melanomas are a specifically localized subset of cutaneous melanomas, and thus the previously reviewed cutaneous melanoma literature with a strong response rate would be relevant towards this subtype. However, certain factors clinically distinguish the presentation (Fig. 2) and management of cutaneous melanoma on the eyelid. For example, earlier diagnosis of melanoma of the eyelid may occur, compared with elsewhere on the body, due to the prominent location. There may also be differences in aggressiveness of initial resection for melanomas of the eyelid compared with elsewhere due to the cosmetic (and functional) sensitivity of the location. These factors may alter the timing and success rate of checkpoint inhibitors as adjuvant therapy for eyelid melanomas.
Figure 2.
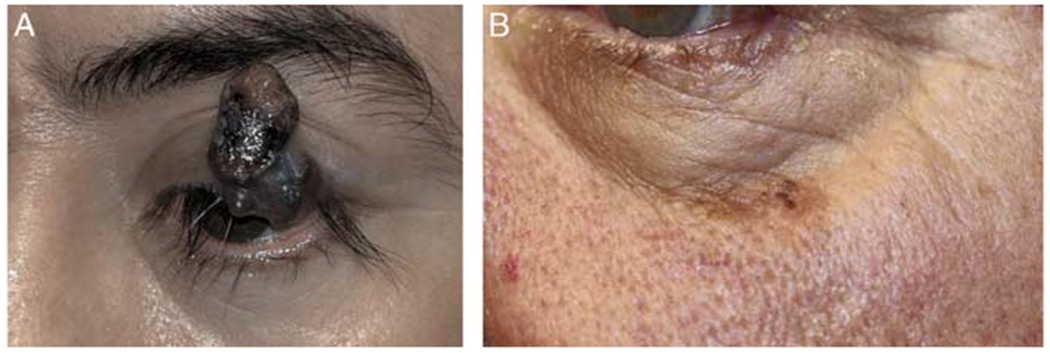
Representative photos of eyelid involving cutaneous melanoma. A, Left upper eyelid melanoma. B, Left lower eyelid/cheek melanoma.
Ophthalmic Complications and Side-effects of Systemic Immunotherapy
Immune checkpoints are a naturally evolved step in the human immune system for modulating the immune response. The therapeutic blockage of these checkpoints with monoclonal antibodies predictably comes with unintended consequences. The adverse effects are exceedingly diverse but largely fall into the categories of autoimmune and inflammatory processes. In a review of 14 clinical trials with 1498 patients receiving ipilimumab, 64% of patients experienced immune therapy-related adverse events of any grade and 17% experienced grade 3 or 4 toxicity (grading of severity of adverse events are 1 to 5: 1 is mild, 2 is moderate, 3 is severe, 4 is life-threatening or disabling, and 5 is death, with specific descriptions of signs and symptoms consisting each grade based on organ system).50 Adverse events can lead to discontinuation of therapy in about 40% of patients.51 Systemic adverse events from checkpoint inhibitors differ in presentation and management from chemotherapies with other mechanisms.9 Affected organ systems can include skin, liver, gastrointestinal tract, respiratory tract, nervous system, and endocrine systems.51–56 Prompt diagnosis and management are important to prevent subsequent sequelae of these adverse effects.
Ocular complications can be diverse including euthyroid Graves’ ophthalmopathy,57 optic neuropathy with disc edema,58 ocular rosacea,59 orbital inflammation,60,61 peripheral ulcerative keratitis,60 and mild to severe panuveitis with or without serous retinal detachment.62–64 There have been several reports of uveitis in association with checkpoint inhibitors, with proposed mechanisms. The eye is an immune-privileged organ.65 Wang et al62 reviewed the evidence for PD-L1 being one of the mechanisms for this immune privilege, which may be compromised by checkpoint inhibitors.66–68 This suggests a basis for the reports of therapy-related uveitis, and they further theorize that uveitis may even be used as a sign of response to checkpoint inhibitor therapy. This is a similar rationale with other immune toxicities as well. The broad presentations of ocular complications from checkpoint inhibitors may warrant comprehensive ophthalmic examinations, although practice patterns vary and there are no consensus guidelines for screening. There is a noted association between ocular inflammation and colitis, so patients with colitis should undergo an ophthalmological examination.69 Many conventional chemotherapeutic agents may also cause a broad range of ophthalmic side effects and also require ophthalmic examinations and treatment.70 Because of the relatively novel nature of checkpoint inhibitors and ongoing refinements of dosing and duration, the ocular and systemic adverse effects will require continued study.
Adverse effects may change in rate or intensity as the dosing of checkpoint inhibitors change. There are limited data demonstrating a dose-dependent effect with the CTLA-4 inhibitor, ipilimumab, but not so for PD-1 or PD-L1 inhibitors.71 The use of checkpoint inhibitors in combination also appears to increase toxicity.23,72 The duration of existing protocols may also change as data from patients with early termination of treatment due to adverse effects still retain treatment effect longer than anticipated.51 These and other changes in the application of checkpoint inhibitors may improve the ratio of benefit to harm.
Conclusions
Immune checkpoint inhibitors, including ipilimumab, nivolumab, and pembrolizumab, represent a promising new tool for the management of conjunctival and eyelid melanomas. Adding immunotherapy using checkpoint inhibitors to the armamentarium for melanoma may allow for the reduction of the morbidity associated with surgery or cytotoxic chemotherapies, while also providing superior outcomes. These novel agents may have a role in primary treatment, adjuvant therapy, or as an alternative option to surgery. Checkpoint inhibitors have also demonstrated promising results for metastatic conjunctival melanoma. However, the overall quality of available literature is still limited, both for potential benefits as well as the varied adverse effects. Patient informed consent should include discussion of the novel nature of these agents especially when used for eyelid and conjunctival melanoma.
There are numerous future opportunities to better understand the role of checkpoint inhibitors in ophthalmology. First, existing case series are small, retrospective, heterogenous, and noncomparative. Future study design on checkpoint inhibitors for ophthalmology will benefit from addressing these aspects, although the low incidence makes it challenging to conduct well-designed prospective studies, and thus, may require collaborative efforts across multiple institutions. In addition, synergistic effects of multiagent therapies have also been seen in cutaneous melanoma,73,74 but this has not been significantly explored yet for conjunctival melanomas. The mutational similarities and differences of conjunctival melanoma (more similar to cutaneous, and dissimilar to mucosal and uveal melanomas) with other ocular melanomas reviewed in this chapter show some correlation with clinical outcomes. This suggests other insights may come from future studies of conjunctival and eyelid melanomas on the molecular level. Finally, the orbit has a high density of delicate and important periocular structures that increases the risk of morbidity with extensive surgery and radiation. There is only one report in the literature specifically exploring checkpoint inhibitors for orbital disease, with a positive outcome.45
In summary, checkpoint inhibitors have limited but encouraging literature on the case report level for conjunctival melanoma to support further study and potential use, while little is known about checkpoint inhibitors for eyelid-specific melanoma. These agents may have a role in the appropriate patient with advanced or metastatic eyelid and conjunctival melanoma. Close collaboration with medical oncologists and other members of a multidisciplinary team is critical for the administration and systemic monitoring of these immunotherapies.
Acknowledgments
Supported by an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness, New York, NY.
Footnotes
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Shields CL, Chien JL, Surakiatchanukul T, et al. Conjunctival tumors: review of clinical features, risks, biomarkers, and outcomes—The 2017 J. Donald M. Gass Lecture. Asia Pac J Ophthalmol (Phila). 2017;6:109–120. [DOI] [PubMed] [Google Scholar]
- 2.De Potter P, Shields CL, Shields JA, et al. Clinical predictive factors for development of recurrence and metastasis in conjunctival melanoma: a review of 68 cases. Br J Ophthalmol. 1993;77:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seregard S, Kock E. Conjunctival malignant melanoma in Sweden 1969-1991. Acta Ophthalmol (Copenh). 2009;70:289–296. [DOI] [PubMed] [Google Scholar]
- 4.Esmaeli B Patterns of regional and distant metastasis in patients with conjunctival melanoma Experience at a cancer center over four decades. Ophthalmology. 2001;108:2101–2105. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri M, Buffam FV, Martinka M, et al. Clinicopathologic features and behavior of cutaneous eyelid melanoma 1 1. Ophthalmology. 2002;109:901–908. [DOI] [PubMed] [Google Scholar]
- 6.Harish V, Bond JS, Scolyer RA, et al. Margins of excision and prognostic factors for cutaneous eyelid melanomas. J Plast Reconstr Aesthet Surg. 2013;66:1066–1073. [DOI] [PubMed] [Google Scholar]
- 7.Andersson AP, Gottlieb J, Drzewiecki KT, et al. Skin melanoma of the head and neck: prognostic factors and recurrence-free survival in 512 patients. Cancer. 2010;69:1153–1156. [DOI] [PubMed] [Google Scholar]
- 8.Vora GK, Demirci H, Marr B, et al. Advances in the management of conjunctival melanoma. Surv Ophthalmol. 2017;62:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Reilly A, Larkin J. Checkpoint inhibitors in advanced melanoma: effect on the field of immunotherapy. Expert Rev Anticancer Ther. 2017;17:647–655. [DOI] [PubMed] [Google Scholar]
- 10.Wierenga APA, Cao J, Luyten GPM, et al. Immune checkpoint inhibitors in uveal and conjunctival melanoma. Int Ophthalmol Clin. 2019;59:53–63. [DOI] [PubMed] [Google Scholar]
- 11.Furue M, Ito T, Wada N, et al. Melanoma and immune checkpoint inhibitors. Curr Oncol Rep. 2018;20:29. [DOI] [PubMed] [Google Scholar]
- 12.Walker LSK. PD-1 and CTLA-4: two checkpoints, one pathway? Sci Immunol. 2017;2:eaan3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maio M, Grob J-J, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 18.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 19.Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 21.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 23.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. [DOI] [PubMed] [Google Scholar]
- 24.McCartney AC. Pathology of ocular melanomas. Br Med Bull. 1995;51:678–693. [DOI] [PubMed] [Google Scholar]
- 25.Isager P, Østerlind A, Engholm G, et al. Uveal and conjunctival malignant melanoma in Denmark, 1943–1997: incidence and validation study. Ophthalmic Epidemiol. 2005;12:223–232. [DOI] [PubMed] [Google Scholar]
- 26.Wong JR, Nanji AA, Galor A, et al. Management of conjunctival malignant melanoma: a review and update. Expert Rev Ophthalmol. 2014;9:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Midena E, Frizziero L, Parrozzani R. Pharmacotherapy and immunotherapy of conjunctival tumors. Asia Pac J Ophthalmol (Phila). 2017;6:121–131. [DOI] [PubMed] [Google Scholar]
- 28.Paridaens AD, Minassian DC, McCartney AC, et al. Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases. Br J Ophthalmol. 1994;78:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dagi Glass LR, Lawrence DP, Jakobiec FA, et al. Conjunctival melanoma responsive to combined systemic BRAF/MEK inhibitors. Ophthal Plast Reconstr Surg. 2017;33:e114–e116. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Brouwer NJ, Richards KE, et al. PD-L1/PD-1 expression and tumor-infiltrating lymphocytes in conjunctival melanoma. Oncotarget. 2017;8:54722–54734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westekemper H, Karimi S, Süsskind D, et al. Expression of HSP 90, PTEN and Bcl-2 in conjunctival melanoma. Br J Ophthalmol. 2011;95:853–858. [DOI] [PubMed] [Google Scholar]
- 32.Pahlitzsch M Conjunctival melanoma and BRAF inhibitor therapy. J Clin Exp Ophthalmol. 2014;5:322. [Google Scholar]
- 33.Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143–3152. [DOI] [PubMed] [Google Scholar]
- 34.Zoroquiain P, Fernandes BF, González S, et al. p16ink4a expression in benign and malignant melanocytic conjunctival lesions. Int J Surg Pathol. 2012;20:240–245. [DOI] [PubMed] [Google Scholar]
- 35.Spendlove HE, Damato BE, Humphreys J, et al. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma Res. 2004;14:449–452. [DOI] [PubMed] [Google Scholar]
- 36.Lake SL, Jmor F, Dopierala J, et al. Multiplex ligation-dependent probe amplification of conjunctival melanoma reveals common BRAF V600E gene mutation and gene copy number changes. Invest Ophthalmol Vis Sci. 2011;52:5598–5604. [DOI] [PubMed] [Google Scholar]
- 37.Karim RZ, Li W, Sanki A, et al. Reduced p16 and increased cyclin D1 and pRb expression are correlated with progression in cutaneous melanocytic tumors. Int J Surg Pathol. 2009;17:361–367. [DOI] [PubMed] [Google Scholar]
- 38.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jindal V Role of immune checkpoint inhibitors and novel immunotherapies in uveal melanoma. Chin Clin Oncol. 2018;7:8. [DOI] [PubMed] [Google Scholar]
- 40.D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer. 2019;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sagiv O, Thakar SD, Kandl TJ, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018;136:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kini A, Fu R, Compton C, et al. Pembrolizumab for recurrent conjunctival melanoma. JAMA Ophthalmol. 2017;135:891. [DOI] [PubMed] [Google Scholar]
- 44.Chaves LJ, Huth B, Augsburger JJ, et al. Eye-sparing treatment for diffuse invasive conjunctival melanoma. Ocul Oncol Pathol. 2018;4:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ford J, Thuro BA, Thakar S, et al. Immune checkpoint inhibitors for treatment of metastatic melanoma of the orbit and ocular adnexa. Ophthal Plast Reconstr Surg. 2017; 33:e82–e85. [DOI] [PubMed] [Google Scholar]
- 46.Pinto-Torres S, Tomás T, Gouveia E, et al. Systemic treatment of metastatic conjunctival melanoma. Case Rep Oncol Med. 2017;2017:4623964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davar D, Wilson M, Pruckner C, et al. PD-1 blockade in advanced melanoma in patients with hepatitis C and/or HIV. Case Rep Oncol Med. 2015;2015:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-a phase 1 study. JAMA Oncol. 2019;5:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tio M, Rai R, Ezeoke OM, et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. 2018;104:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim R, Berman D, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. ASCO Annual Meeting Proceedings; 2011. [Google Scholar]
- 51.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byun DJ, Wolchok JD, Rosenberg LM, et al. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117–122. [DOI] [PubMed] [Google Scholar]
- 56.Wada N, Uchi H, Furue M. Case of remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab in a patient with advanced malignant melanoma. J Dermatol. 2017;44:e196–e197. [DOI] [PubMed] [Google Scholar]
- 57.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh OL, Francis CE. Ipilimumab-associated bilateral optic neuropathy. J Neuroophthalmol. 2015;35:144–147. [DOI] [PubMed] [Google Scholar]
- 59.Brouwer NJ, Haanen JBAG, Jager MJ. Development of ocular rosacea following combined ipilimumab and nivolumab treatment for metastatic malignant skin melanoma. Ocul Oncol Pathol. 2017;3:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papavasileiou E, Prasad S, Freitag SK, et al. Ipilimumab-induced ocular and orbital inflammation—a case series and review of the literature. Ocul Immunol Inflamm. 2016;24: 140–146. [DOI] [PubMed] [Google Scholar]
- 61.Sheldon CA, Kharlip J, Tamhankar MA. Inflammatory orbitopathy associated with ipilimumab. Ophthal Plast Reconstr Surg. 2017;33:S155–S158. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Lam WC, Chen L. Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2019;68:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanno H, Ishida K, Yamada W, et al. Uveitis induced by programmed cell death protein 1 inhibitor therapy with nivolumab in metastatic melanoma patient. J Infect Chemother. 2017;23:774–777. [DOI] [PubMed] [Google Scholar]
- 64.Arai T, Harada K, Usui Y, et al. Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol. 2017;44:975–976. [DOI] [PubMed] [Google Scholar]
- 65.Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep. 2010;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L, Pai V, Levinson R, et al. Constitutive Neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Ocul Immunol Inflamm. 2009;17:47–55. [DOI] [PubMed] [Google Scholar]
- 67.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. [DOI] [PubMed] [Google Scholar]
- 68.Zamani MR, Aslani S, Salmaninejad A, et al. PD-1/PD-L and autoimmunity: a growing relationship. Cell Immunol. 2016;310:27–41. [DOI] [PubMed] [Google Scholar]
- 69.Tarhini A Immune-mediated adverse events associated with ipilimumab CTLA-4 blockade therapy: the underlying mechanisms and clinical management. Scientfica. 2013;2013:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kunkler AL, Binkley EM, Mantopoulos D, et al. Known and novel ocular toxicities of biologics, targeted agents, and traditional chemotherapeutics. Graefes Arch Clin Exp Ophthalmol. 2019;257:1771–1781. [DOI] [PubMed] [Google Scholar]
- 71.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611–622. [DOI] [PubMed] [Google Scholar]
- 72.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


