Abstract
Storing information from incoming stimuli in working memory (WM) is essential for decision-making. The prefrontal cortex (PFC) plays a key role to support this process. Previous studies have characterized different neuronal populations in the PFC for working memory judgements based on whether an originally presented stimulus matches a subsequently presented one (matching-rule decision-making). However, much remains to be understood about this mechanism at the population level of PFC neurons. Here, we hypothesized differences in processing of feature vs. spatial WM within the PFC during a matching-rule decision-making task. To test this hypothesis, the modulation of neural activity within the PFC during two types of decision-making tasks (spatial WM and feature WM) in comparison to a passive fixation task was determined. We discovered that neural population-level activity within the PFC is different for the match vs. non-match condition exclusively in the case of the feature-specific decision-making task. For this task, the non-match condition exhibited a greater firing rate and lower trial-to-trial variability in spike count compared to the feature-match condition. Furthermore, the feature-match condition exhibited lower variability compared to the spatial-match condition. This was accompanied by a faster behavioral response time for the feature-match compared to the spatial-match WM task. We attribute this lower across-trial spiking variability and behavioral response time to a higher task-relevant attentional level in the feature WM compared to the spatial WM task. The findings support our hypothesis for task-specific differences in the processing of feature vs. spatial WM within the PFC. This also confirms the general conclusion that PFC neurons play an important role during the process of matching-rule governed decision-making.
Keywords: Decision-making, Feature-based attention, Trial-to-trial variability, Prefrontal cortex (PFC), Task-specific, Working memory (WM)
Introduction
Our brain is capable of recognizing whether two sequential objects are the same (match) or different (non-match) during a cognitive task like decision-making. This matching-rule decision-making process involves working memory (WM) that intrinsically employs attention for this judgment (Desimone 1996; Downing 2000; Soto et al. 2005). Attention is posited to play a key role in many cognitive functions of the brain (Treue and Trujillo 1999; Treue and Maunsell 1999; Sigala and Logothetis 2002; Williford and Maunsell 2006; Liu and Newsome 2006; Yao et al. 2016; Xue et al. 2017; Backen et al. 2018). It causes various statistical changes in the neural responses. For example, it increases the firing rate (Cohen and Maunsell 2009), neural synchronization, and across-trial correlation (Fries et al. 2001, 2008; Lakatos et al. 2008; Siegel et al. 2008; Bichot et al. 2015). It also decreases the across-trial variability and noise correlation among simultaneous activities of two or more neurons (Mitchell et al. 2007; Cohen and Maunsell 2009; Merrikhi et al. 2017). There are different types of attention relying on the aspect of an object that is attended, most importantly feature and spatial-based attention (Peelen and Mruczek 2008; Greenberg et al. 2010; Heuer et al. 2016). In feature-based attention, a specific feature of an object such as shape is considered, while, in spatial-based attention, the focus of attention is oriented to the location of that object.
The prefrontal cortex (PFC) is considered as an area critical for the allocation of attentional resources of the brain that plays a central role in finding objects with certain features (Bichot et al. 2015). This process of the so-called feature-based attention in PFC is accomplished even earlier than lower brain areas (Katsuki and Constantinidis 2012; Bichot et al. 2015; Sereno and Lehky 2018). PFC, in the top place of brain hierarchy, contributes to various high-level cognitive functions such as working memory, decision-making, and attention (Gregoriou et al. 2009; Noudoost and Moore 2011; Clark and Noudoost 2014; Johnson et al. 2017, 2018b; Sarafyazd and Jazayeri 2019). Several studies have revealed different modulation of PFC neurons depending on the demands (Wilson et al. 1993; Asaad et al. 2000; Johnston and Everling 2006; Warden and Miller 2010; Johnson et al. 2018a). Consequently, it is more likely that decision-making in this area would be modulated in a task-specific manner.
Investigating brain mechanism is usually accomplished through several repetitions (trails) of one stimulus. This allows us to examine across trials response variability in addition to the strength of average response activity. Response variability has been considered as a hallmark in several cognitive functions. A single exposure to a stimulus could cause differences in inter-trial response variability. Previous studies have indicated the decreasing of trial-to-trial response variability by stimulus onset (Churchland et al. 2010; Deco and Hugues 2012; Ponce-Alvarez et al. 2013), attention (Mitchell et al. 2007) and preparation of movement (Churchland et al. 2006). It also has been shown that inter-trial response variability increases during decision-making (Ratcliff et al. 2009; Churchland et al. 2011). Trial-to-trial variability could reflect the information in a population of neurons rather than a single neuron. In other words, estimates across several trials of one stimulus condition may estimate response variability in a short time epoch across a population of neurons. This variation may reflect an aspect of the decision-making process within the PFC over a short time frame.
The literature on matching-rule decision-making has characterized different neuronal populations that respond to match/non-match conditions (Wallis and Miller 2003; Machens et al. 2005; Soltani and Wang 2006; Muhammad et al. 2006; Rawley and Constantinidis 2011; Roth and Rust 2018). One group of neurons exhibits higher firing rate to the match condition and the other group creates higher firing rate to the non-match condition. However, the neural population-level response of this process is not fully understood. In this study, we hypothesized that the matching-rule decision-making process within the PFC is task-specific, as a spatial attention is uniquely different in forming the matching modulation of PFC neurons at the population level. To address this, we analyzed the spiking activity (obtained by electrophysiological recordings from the PFC area of two rhesus monkeys) during performance of a passive and two WM tasks. In the feature/spatial WM task, monkeys were required to attend to the shape feature/spatial location of stimuli (feature and spatial-based attention). No attention was needed for the passive task, which was implemented before the training. Therefore, comparing the two spatial and feature attention/WM tasks could reveal the task dependency of PFC neurons. Likewise, comparing WM tasks requiring attention with a passive non-attentive task would clarify the role of attention in the process.
The elusive PFC matching-rule modulation at the population level could be formed from a compromise between differential neuronal responses, namely match-enhancement and match-suppression neurons. We postulated that feature-based attention, in the feature WM task, has an enhancement mechanism for specific neural representations. Hence, it may be more effective in orienting the PFC population response for matching-rule decision-making. To test this idea, we compared the match vs. non-match conditions at the population level within the three aforementioned tasks. Using neural activity in terms of firing rate, and trial-to-trial spike count variability in terms of Fano factor and across-trial correlation, we evaluated the matching-rule modulation in the PFC. The comparison of matching-rule modulation between spatial and feature WM tasks elucidates the task-specific function of PFC neurons for decision-making. In addition, contrasting these two WM tasks with the passive task explicates the role of attention on it.
Materials and methods
The procedure of surgery, experimental paradigm, and data acquisition are described in (Meyer et al. 2011). Here, we provide further details necessary for understanding the current study.
Statement on animal research
Research with non-human primates represents an indispensable component of neuroscience research (Calapai et al. 2017; Berger et al. 2018). This study was accomplished according to and with commitment to the animal welfare protocols to fulfill the best possible science with the least possible harm to the animals. The main goal of this study was to examine the task-specific differences in processing of feature vs. spatial WM within the PFC for decision-making during a matching-rule task. To this end, we compared the neuronal responses between match vs. non-match conditions. We analyzed the spiking activity responses at the population level in terms of spike firing rate and across-trial spiking variability. All animal procedures of this study were carried out in compliance with the guidelines set forth by the National Institutes of Health as reviewed and approved by the Wake Forest University Animal Care and Use Committee.
Behavioral task
Two monkeys were trained to perform a delayed match-to-sample task. The experimental paradigm consisted of three steps. First, before any training, monkeys were passively presented with a set of two stimuli, each one of which had a duration of 0.5 s and was followed by a delay period duration of 1.5 s. The sample and test stimuli (1st and 2nd stimuli) could be match or non-match in terms of spatial location or shape feature. Animals needed to maintain his gaze on the fixation point throughout the task to get a reward (passive task, Fig. 1a). The shape and location of stimuli were chosen randomly from the feature and spatial sets. The feature set consisted of eight different geometric shapes (circle: ●, diamond: ♦, H letter: H, number sign: #, plus sign: +, square:, triangle: ▲, inverted Y: ⅄). Also, the spatial set included nine possible locations arranged on a 3 × 3 grid (starting from the right, counterclockwise, with 9 at the center location) (Fig. 1b). The passive task was performed in two distinct paradigms. In one paradigm, the shapes of sample and test stimuli remained the same, and their locations were selected randomly from the spatial set. The second paradigm was vice versa; the locations remained the same, and the shapes were selected randomly from the feature set. Subsequently, monkeys were trained to remember the spatial location of the sample stimulus and identify the matching status (matched/non-matched) of the following test stimulus accordingly (spatial WM task, Fig. 1c). Finally, monkeys were trained to identify the matching status of the sample and test stimuli with respect to their shape identity (feature WM task, Fig. 1d). The timeline and procedure of these two WM tasks were similar to the passive task. However, in the two WM tasks, following the second delay, the fixation point disappeared and two target choices (a green and a blue square) were presented on the screen. Monkeys were required to saccade to the green/blue target in the case of match/non-match condition, respectively. Hence, monkeys had to attend to the sample and test stimuli to report the matching status of them correctly and get a drop of juice reward.
Fig. 1.
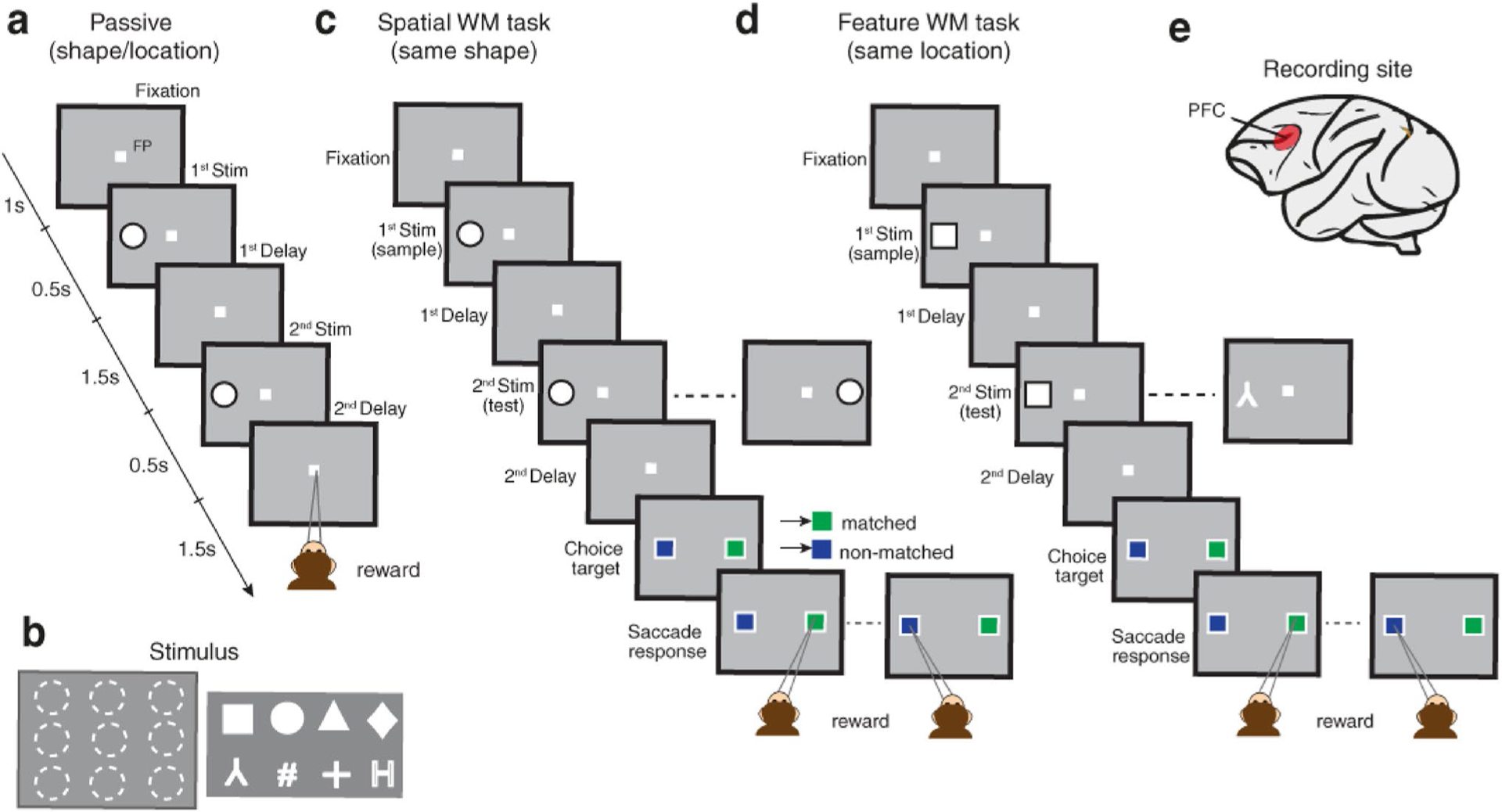
Behavioral passive and WM tasks. a In the passive task, two sequential stimuli were followed by two delay periods. Stimuli could be match or non-match relative to the spatial location or shape feature aspect. Regardless of stimuli properties, animals needed to maintain their gaze on the fixation point to receive a drop of juice reward. b Spatial set consisted of nine locations (left panel) and feature set included of eight different shapes (right panel). c, d In the WM tasks, monkeys were trained for making a decision regarding the match/non-match status between sample and test stimuli. In the spatial WM task, the shape of sample and test stimuli were identical and monkeys should identify the match or non-match status of their location. In contrast, in the feature WM task, the location of stimuli remained the same and monkeys should identify the matching status based on their shapes. The WM tasks included two additional phases in comparison to the passive task; choice target and saccade response epochs. e The prefrontal cortex (red region) as the target recording area
Electrophysiological recording
Single unit activity was recorded from 1365 neurons over the prefrontal cortex (Fig. 1e) of two Rhesus monkeys, while they performed a task (passive/WM). Animals were trained to maintain their gaze on a fixation point (0.2°) displayed on the center of the screen. Throughout the task, the eye positions were monitored via an eye-tracking system (model RK-716; ISCAN- with 240 Hz sampling). Data were recorded from single-electrode [tungsten electrodes of 250 μm diameter, with an impedance of 1 MΩ at 1 kHz (Alpha-Omega Engineering, Nazareth)] inserted in the ventral/dorsal part of PFC. The data were recorded by a modular data acquisition system (APM system, FHC, Bowdoin, ME). Recording signals were online amplified and passed from a bandpass filter in the range of 500–8000 Hz. The spiking activity of each recording site was digitized and used for offline analyses. Spike sorting was performed using an automated cluster analysis method based on the KlustaKwik algorithm (Harris et al. 2000).
Data analysis
To examine the matching-rule discrimination, we compared the neural activities of the match and non-match conditions in a passive and two WM tasks. All our analyses of the recorded data were performed using MATLAB 2016b (The MathWorks, Inc., USA).
To investigate the neural discrimination between two matching conditions, we first computed the spike density function (SDF) during the time-course of analysis from − 0.5 to 4 s following the sample stimulus onset. The action potentials were sorted online, and the SDF was calculated by convolving the individual spike times with a Gaussian kernel with σ = 20. The average SDF of trials in the match/non-match condition was calculated as the neural response. All recording sites of both monkeys were preprocessed separately and the correctly completed trials (hits) pooled together for further analyses. The statistic rank-sum test between two distributions in each particular time point indicated the time-course of variation with significant differences. Furthermore, to show this modulation, the difference between them was calculated based on normalized d-prime between two conditions. The d-prime is defined as follows:
where Resmatch and Resnonmatch are the averaged SDF of the match and non-match trials during the time-course of analysis. The boundary of chance level for distribution of difference was computed based on 1000 times label shuffling and re-calculating the difference between two assumed groups.
Subsequently, we quantified the discrimination strength between matching conditions based on the analysis of variance (ANOVA). To this end, first, the trials of each condition were equalized between three tasks. Then, within each task, the ANOVA was applied in 5 ms non-overlapped windows during − 0.5 and 4 s with respect to the sample stimulus onset. The F-statistic value was considered as a discrimination measure between two categories or conditions. In a similar fashion, we calculated the chance distribution of the discrimination by re-calculating ANOVA for 1000 times with randomly shuffling all the labels. Next, using the t test, we determined the time bins with statistically significant discrimination of matching conditions compared to the chance distribution. To identify the main epoch involved in the matching-rule decision-making, the distribution of F-statistic values was determined during each epoch, separately (fixation, sample stimulus, 1st delay, test stimulus, and 2nd delay). Then, the percentage of points with a significant difference between the two matching conditions were reported as modulation strength of that epoch.
To study the matching-rule decision-making effect on the response variability, we calculated the trial-to-trial spike count variability for each condition (Mitchell et al. 2007). To this end, we computed the Fano factor in each neuron and within individual stimulus conditions using a 100 ms sliding window with 1 ms stepping size. Then, the average of Fano factor values (considered as the response variability) compared between the two matching conditions. The Fano factor (FF) which is the variance relative to the mean of spike counts was calculated as follows:
We also compared the trial-to-trial spike count variability between three target tasks (passive, spatial WM, and feature WM tasks). For this purpose, after calculating the trial-to-trial variability (based on Fano factor), the ANOVA was applied to quantify the difference variability between them.
To investigate the impact of each condition (match or non-match) on the matching modulation strength, we employed another variability measure. For this purpose, across-trial correlation of spike count within the same neuron was calculated in non-overlapping 100 ms windows. Following a normalization step within each neuron (normalized to the maximum value of match and non-match groups), the cross-correlation values were averaged across neurons for the match and non-match conditions, separately. This process was performed for all three desired tasks. The modulation of this measure during the test stimulus with respect to the fixation period declares the effect of matching modulation.
We employed t test, rank-sum, and one-way analysis of variance (ANOVA) tests for statistical comparison between two or more conditions. All statistical analyses were performed using a Bonferroni correction or a false discovery rate (FDR) multiple-comparison correction of p < 0.05 (Benjamini and Hochberg 1995).
Results
Two rhesus monkeys were trained to perform a delayed match-to-sample (DMS) task (Fig. 1, see “Materials and methods”). Spiking activity was recorded from PFC neurons during a passive (before training) and two WM tasks. We examined the matching-rule modulation by calculating the match vs. non-match discrimination at the population level of PFC neurons. To this end, based on single-unit activity (in terms of firing rate) and also trial-to-trial spike count variability (based on Fano factor and across-trial correlation), we compared matching-rule discrimination between the aforementioned tasks.
Matching-rule decision-making modulates the PFC population response in the feature WM task
First, we examined the discrimination between the two matching conditions (match vs. non-match) at the population level of PFC neurons for the passive (spatial/feature passive), spatial WM, and feature WM tasks. To this end, we calculated the average spike density function (SDF) for each matching condition. Figure 2 shows the dynamics of neural activity throughout the passive and WM tasks for the match and non-match trials, separately. Here, we divided the passive task into two spatial passive and feature passive tasks according to their properties (identical location or identical shapes in sample and test stimuli). Our data show a significant increase in the population response of single-unit activity of non-match condition compared to the match condition exclusively in the feature WM task (Fig. 2d t test, Bonferroni correction, p < 0.05). It rises about 250 ms after test stimulus onset and is maintained in the memory epoch (2nd delay). This overall population effect is present, while there are differential population responses in the PFC neurons for match and non-match conditions. Figure S1 shows these different PFC neural responses in two sample recording sites for both spatial WM and feature WM tasks.
Fig. 2.
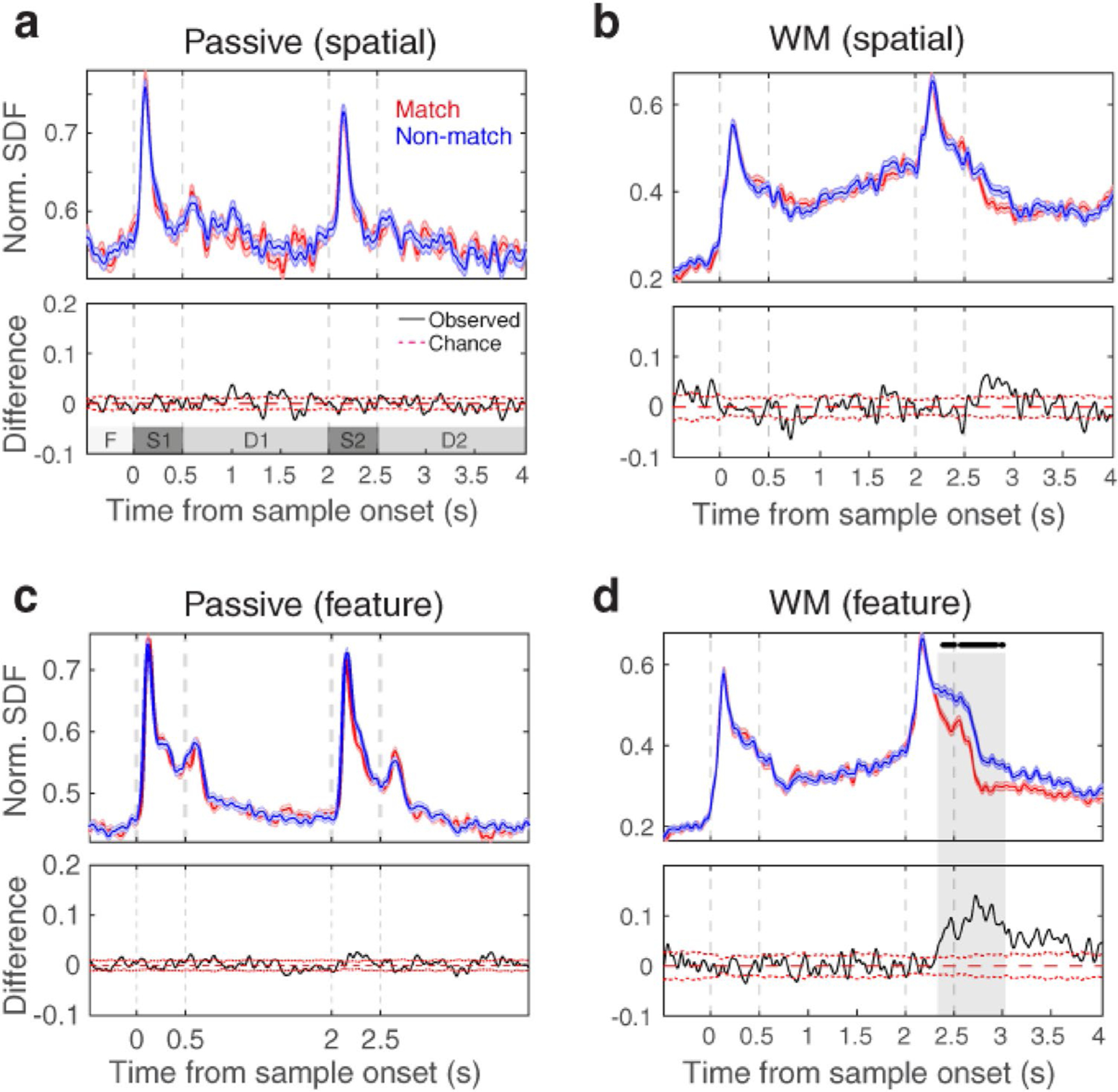
Matching-rule decision-making modulates the PFC neural activity in the feature WM task. The top panels show the average spiking activity (based on SDF) during − 0.5 to 4 s relative to the sample stimulus onset (F fixation, S1 sample stimulus, D1 1st delay, S2 test stimulus, D2 2nd delay). They exhibit the neural responses of the match (red line) and non-match (blue line) conditions within spatial passive (a), spatial WM (b), feature passive (c), and feature WM (d) tasks. Black dots on top of the SDFs indicate the time points with a significant difference between the population response of match and non-match conditions. The differences between average responses of the match and non-match conditions were displayed in the lower panels. Gray dashed lines show the period of sample and test stimuli presentation. Red dashed lines indicate the standard error from the mean (SEM) of the chance distribution
To better show this modulation, the normalized difference between population responses (d-prime) of the match and non-match conditions is calculated and compared with the chance level (Fig. 2, lower panels of each sub-figures). Results show that the d-prime modulation enhanced in the test stimulus period and remained during memory epoch for the feature WM task. Due to similar results of two kind of passive tasks (and no modulation of d-prime for them as expected), we considered the spatial passive task as a non-attentive ‘control’ condition to compare with two WM tasks requiring attention.
Subsequently, to find the proportion of points with a significant difference between the two matching conditions, we quantified the selectivity between match and non-match conditions. Using one-way analysis of variance (ANOVA), we calculated F-statistic values as the discrimination strength between matching conditions in each 5 ms non-overlapped window. Then, we smoothed it with a 40 ms sliding window. The time-course of discrimination between two matching conditions is shown in Fig. 3a and the histogram of F-statistic values is depicted in Fig. 3b. Every five epochs were analyzed separately [i.e., fixation (F), sample stimulus (S1), 1st delay (D1), test stimulus (S2), and 2nd delay (D2)]. The percentage of bins with significant discrimination was denoted by an integer in each panel (Fig. 3b, ANOVA test, p < 0.05). Results show that the matching-rule modulation at the population level occurs dominantly in the feature WM task (39% of bins reach significance in the 2nd stimulus period, and 73% significance in the 2nd delay period). We employed the ANOVA test to obtain both the discrimination strength (F-statistic value) and the significance of this modulation (p value). As a result, we saw the population matching-rule decision modulation arises exclusively in the feature WM task. Notably, computing this discriminability using other measures, namely the area under the receiver operating characteristic (ROC) curve and Shannon entropy that all the neurons convey about the match/non-match conditions, gave similar results (not shown here).
Fig. 3.
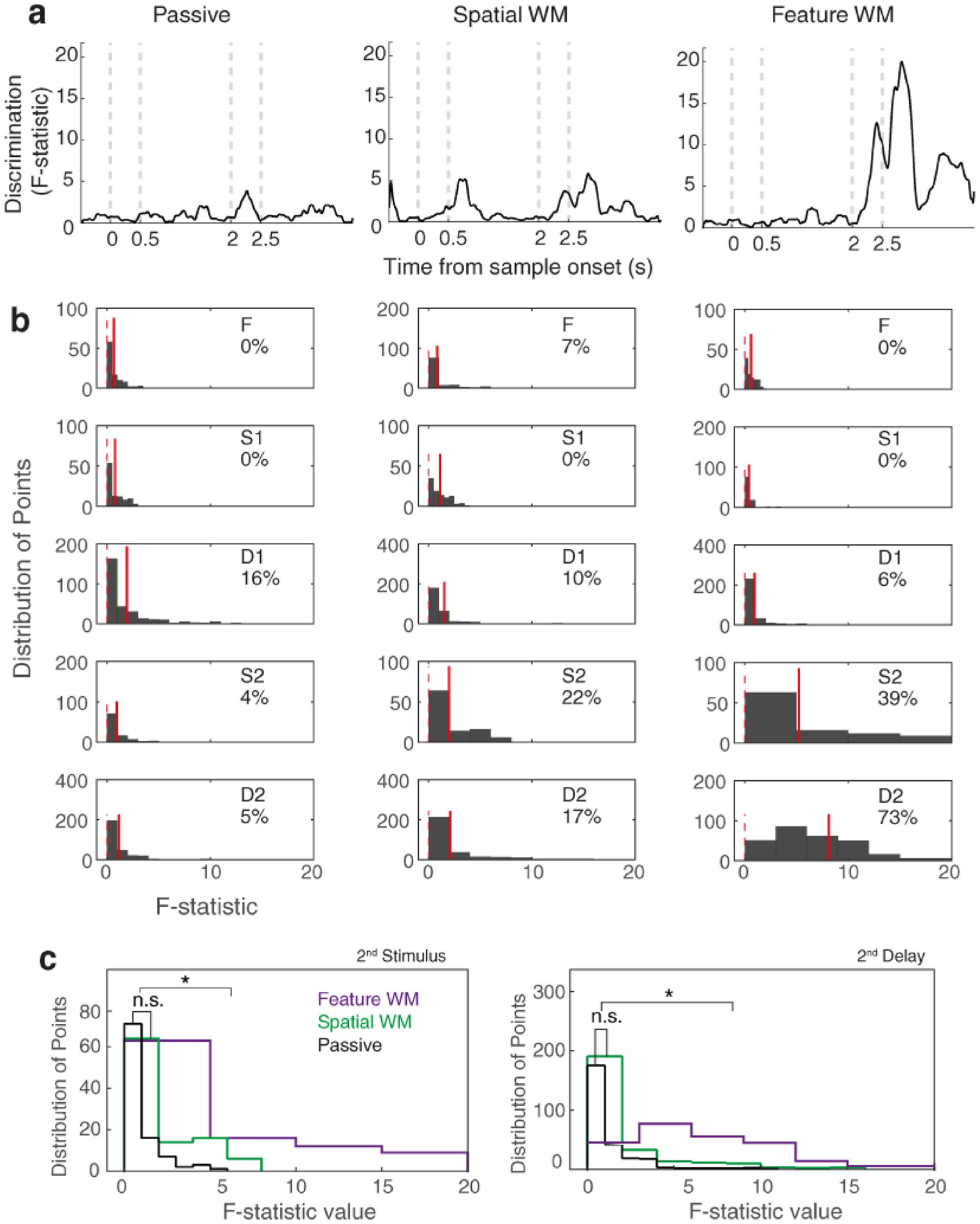
Matching-rule modulation arises during the late test period and its following delay interval. a Time-course of discrimination between firing rate response of match and non-match conditions based on F-statistic values obtained by ANOVA across the population of neurons (left: passive, middle: spatial WM, and right: feature WM). b Histogram of the F-statistic values of each epoch in the passive and WM tasks, separately (F fixation, S1 sample stimulus, D2 1st delay, S2 test stimulus, D2 2nd delay). The integers represent the percentage of points with significant selective responses for matching conditions. Red solid lines show the mean F-statistic in each epoch and indicate the distance of the mean selectivity value from zero (red dashed lines). c Statistical comparisons between the histograms of the three tasks during test stimulus interval (left panel) and its following delay period (right panel). Notation ‘ns’ denotes non-significant effect and *Shows significant effect
We further compared the histograms of F-statistic values between the passive and two WM tasks during the test stimulus period and its corresponding delay epoch (Fig. 3c). The feature WM task with a significant positive distribution of F-statistic value (with the mean value of ~ 9) shows a population selectivity of the matching-rule decision-making (Wilcoxon rank-sum test between passive and feature WM distributions, p < 0.05). This is while the spatial WM task shows no significant selectivity compared to the passive task (with mean values of ~ 1 and ~ 2 for the passive and spatial WM task, respectively).
Matching-rule decision modulates the PFC trial-to-trial response variability in the feature WM task
Given that recent studies have suggested that attention modulates not only mean firing rate but also its variability (Churchland et al. 2010), we further evaluated the trial-to-trial response variability in terms of spiking activity in two matching conditions. To this end, we calculated the Fano factor per each neuron and stimulus condition. Then, the average Fano factor was measured for match and non-match trials separately over the time-course of analysis. Figure 4 shows the time-course of trial-to-trial variability for match and non-match conditions in the three target tasks. Similar to the firing rate, the trial-to-trial spike count variability differs between the matching conditions exclusively in the feature WM task (Fig. 4c; t test, FDR corrected, p < 0.05).
Fig. 4.
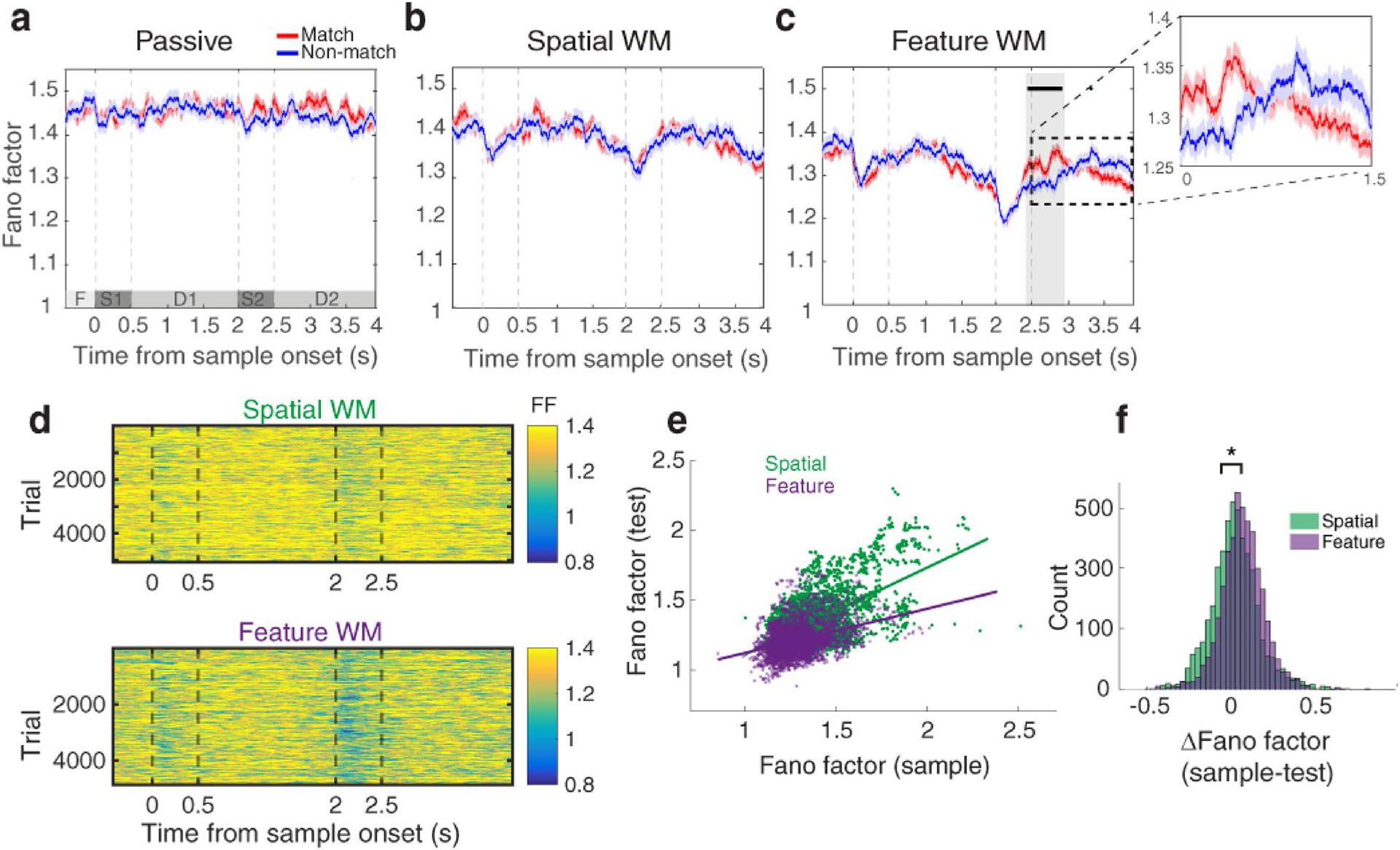
Matching-rule decision-making modulates the PFC spiking variability in the feature WM task. a–c The Fano factor during the time-course of analysis from − 0.5 to 4 s with respect to the sample stimulus onset in the passive (a), spatial WM (b), and feature WM (c) tasks. Each time point shows the middle of the corresponding window that the Fano factor was calculated. Red and blue traces show the averaged Fano factor of the match and non-match conditions, respectively. The black horizontal line indicates the time interval with a significant difference between matching conditions. Dashed lines display stimulus presentation epochs. d The variation of Fano factor across different trials of spatial WM (top panel) and feature WM (bottom panel) tasks. e Scatter plot of Fano factor values of the sample and test stimuli for spatial (green) and feature (purple) WM tasks. f The distribution of Fano factor changes between sample and test stimuli (sample test) for the spatial (green) and feature (purple) WM tasks. *Significant effect
We then evaluated how the Fano factor is changed during the time within each WM tasks. To this end, after calculating the Fano factor across trials in a time resolved fashion (Fig. 4d), we compared scatter plots of Fano factor in the test versus sample period between two WM tasks (Fig. 4e). A significant difference was observed between the sample and test Fano factors in the feature WM task but not the spatial WM task (Fig. 4f; Wilcoxon rank-sum test, FDR corrected, p < 0.05). This could imply a preferential attentional representation for the matching-rule decision in the feature WM task compared to the spatial WM task. Importantly, resembling the same procedure on the firing rate results in no significant difference between the two WM tasks (Fig. S2).
To rule out the possibility of trial-to-trial spike count variability modulation as a simple effect of firing rate difference between two matching conditions, we further recalculated the Fano factor in the trials with a similar firing rate for match and non-match conditions (Fig. S3). In the trials with an almost equal match and non-match firing rate (Fig. S3a), the coefficient of variation shows similar values for both conditions (Fig. S3b). Furthermore, in this case, with a similar coefficient of variation, two matching conditions show different Fano factor modulation (Fig. S3c). This could imply that the Fano factor effect is independent of the firing rate.
Subsequently, to examine which status (match/non-match) modulates this across-trial variability, we estimated the correlation of the mean firing rate across trials in each non-overlapping 100 ms window. Figure S4 shows the average correlation of match (red lines) and non-match (blue lines) conditions during the time-course of analysis. Results show no significant modulation of cross-correlation in the passive task (t test, p > 0.05, Fig. S4a), a weak modulation in the spatial WM task (two windows of 2nd delay; t test, FDR corrected, p < 0.05, Fig. S4b), and a noticeable significant decrease of the match condition in the feature WM task (six windows of 2nd delay; t test, FDR corrected, p < 0.05, Fig. S4c). These findings suggest about the relation between attention and across-trial correlation that appears dominantly in the feature WM task in line with Fano factor results. In other words, it seems that the match condition desynchronized across-trial spiking activity in the WM interval following the test stimulus appearance.
We also compared the mean difference of firing rate, Fano factor, and across-trial correlation between match and non-match conditions in a larger window length (with a 500 ms non-overlapping window) (Fig. S5a–c). All three metrics show a modulation of the population response exclusively in the feature WM task. The firing rate modulation arises in the test stimulus epoch and remains during its following memory epoch (t test, FDR corrected, p < 0.05). The Fano factor variability dissociates between the two matching conditions in the 2nd delay (t test, FDR corrected, p < 0.05). The across trial correlation shows a modulation in the 2nd delay and within similar windows to the trial-to-trial variability (t test, FDR corrected, p < 0.05).
Higher attentional level in the feature WM compared to the spatial WM task
Ultimately, we investigated the plausible reason for matching modulation in the feature WM task but not in the spatial WM task despite the presence of attention in both of them. One hypothesis could be a difference in the attentional level between spatial and feature WM tasks. It has been shown that there is a close relationship between the attentional resource and across-trial spiking variability (Castellanos et al. 2005; Mitchell et al. 2007; Desimone et al. 2011; Falkner et al. 2013; Katsuki and Constantinidis 2014; Moore and Zirnsak 2017; Ni et al. 2018; Lowet et al. 2018). A more recent study shows that attention quenches neural response variability (Cohen and Maunsell 2009; Arazi et al. 2019). Accordingly, we hypothesized the attentional level as a plausible factor to impact on matching-rule modulation at the population level. To evaluate this, we estimated the attentional level in the three target tasks by comparing their mean Fano factor (regardless of the match or non-match status) (Fig. 5a). Results show a lower trial-to-trial spike count variability for two WM tasks (in the context of attention) compared to the passive task. Moreover, we found that the trial-to-trial variability in the feature WM task was less than the spatial WM task. Comparison between the Fano factors of the three tasks revealed a differential across-trial spiking variability, dominantly in the period around the test stimulus (Fig. 5b, ANOVA, Bonferroni correction, p < 0.05). This period is more likely as a prominent epoch that required more attention (compared to other times of the task) for performing a DMS task. We further compared the behavioral reaction times (RT) between two WM tasks (Fig. 5c). As a result, a significantly faster RT was observed in the feature WM task in comparison to the spatial WM task. Taken together, these results may suggest a higher attentional level in the feature WM task compared to the spatial WM task which could be the reason for the matching-rule modulation exclusively in this task.
Fig. 5.
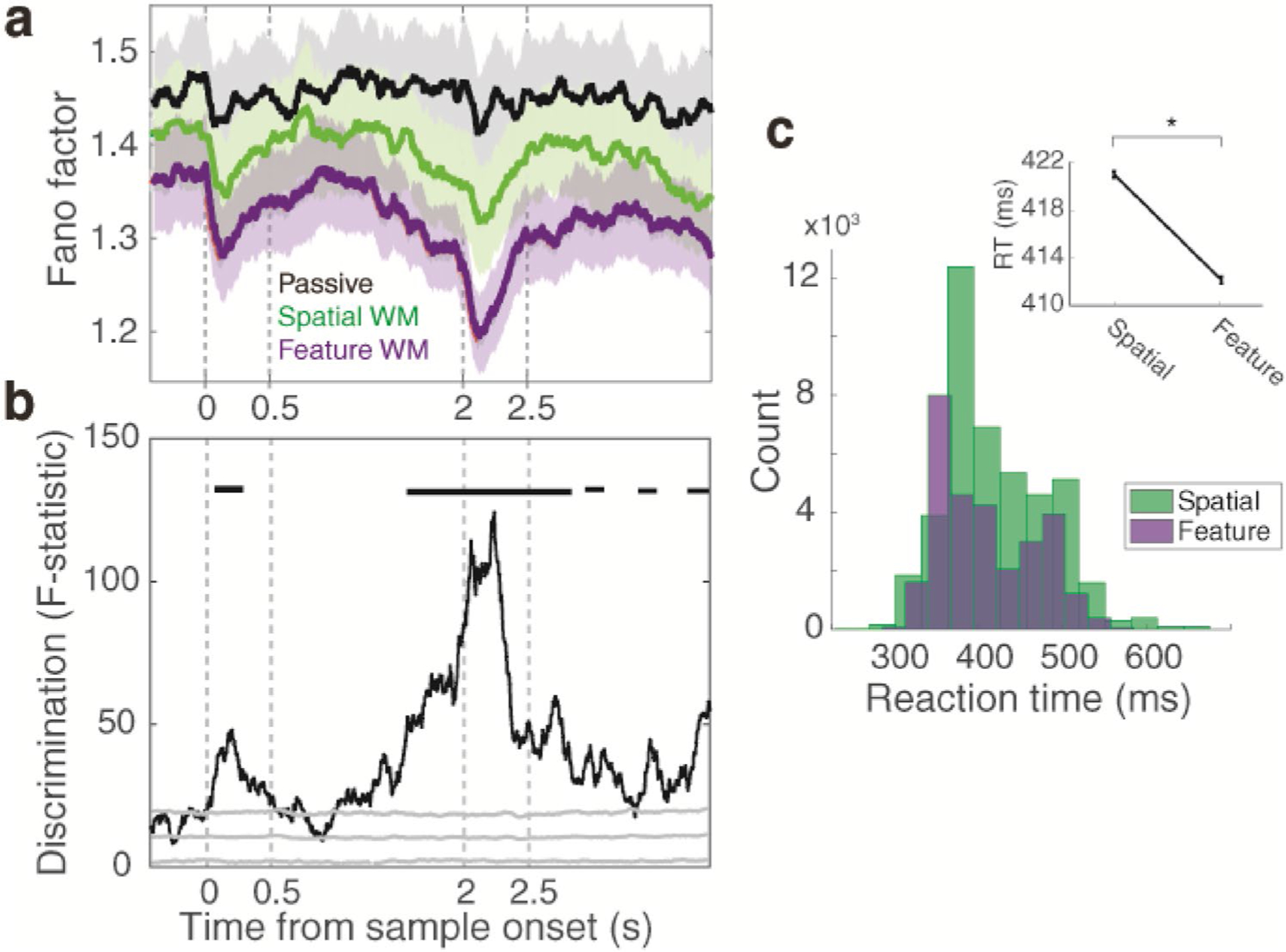
The different discriminability of WM tasks is due to their differential attentional level. a Mean Fano factor throughout the passive (black line), spatial WM (green line), and feature WM (purple line) tasks. Shaded lines display the standard error of the mean (SEM) across all neuron conditions. b Discrimination strength between the Fano factors of the three tasks. The gray solid line denotes the chance level of the null distribution. Black horizontal lines show the time intervals with a significant discrimination effect. c Distribution of reaction times (RT) in two WM tasks. The inset plot shows the significant difference in behavioral reaction times between the two WM tasks
Discussion
We found a matching-rule modulation at the population of PFC neurons in the context of feature-based attention. Previous studies have shown two populations of neurons in the PFC for encoding the matching-rule modulation, match enhancement and match suppression neurons (Rawley and Constantinidis 2011; Engel and Wang 2011; Qi et al. 2012). Indeed, our data identified these differential populations of single-site matching-rule modulation in both WM tasks. These populations reflected the key attentional resources to form the matching-rule modulation. Importantly, population-level analyses revealed a significant matching-rule modulation only in the feature WM task. This highlighted the task dependence modulation of the PFC area, here in the context of decision-making.
Our data showed not only a greater firing rate, but also lower across-trial spiking variability for non-match condition compared to the match condition in the feature WM task. These modulations were exhibited during the late test period and its following delay interval. As expected (Stein et al. 2005; Chang et al. 2012), the variability modulation was independent of firing rate.
Several investigations have revealed that trial-to-trial neural response variability supports several neural (Mitchell et al. 2007; Cohen and Maunsell 2009) or behavioral (Cohen and Newsome 2008; Churchland et al. 2010) conditions. This trial-to-trial variability often conveys a different kind of information compared to spike rate information. For instance, regardless of firing rate changes, decreasing of the across-trial spiking variability may increase the information characterized in cortical circuits (Cohen and Newsome 2008). Generally, it seems that the presence of information may be associated with lower neural response variability.
Previous studies have revealed that the neural response variability is decreased by stimulus onset throughout the cortex and increased again after stimulus offset (Churchland et al. 2010; Deco and Hugues 2012; Ponce-Alvarez et al. 2013). In our data, the increase of the trial-to-trial spike count variability after stimulus offset observed later in the non-match condition compared to the match condition. This could be interpreted as a longer stimulus-induced trial-to-trial variability decreasing in the non-match condition compared to the match condition. Furthermore, based on across-trial correlation analysis, we showed that the matching-rule modulation is more likely related to the non-match condition. This finding could be in line with the mismatch negativity effect of EEG studies (Näätänen et al. 2007; Skrandies and Reuther 2008; Garrido et al. 2009) or stimulus-specific adaptation in electrophysiological studies (Parto Dezfouli and Daliri 2015; Parto Dezfouli et al. 2019; Zarei et al. 2020) which imply to a modulation due to a deviation from a usual or memory record description.
An interesting finding in our study was the population-level matching-rule modulation in the feature WM task, while no significant change was observed in the spatial WM task. This could be explained by two factors: (i) two different attentional mechanisms, feature-based and spatial attention, or (ii) learning of different feature aspects, spatial and non-spatial features.
First, we can identify two types of attention for our WM tasks, feature-based and spatial attention for the feature WM and spatial WM tasks, respectively. Accordingly, two attentional mechanisms could lead to different levels of attention and therefore different modulations in the PFC neurons. Moreover, previous studies have shown distinct attentional networks and engaged areas for orienting attention to the object’s spatial or non-spatial features (Bisley and Goldberg 2003; Chambers et al. 2004; Peelen and Mruczek 2008; Greenberg et al. 2010; Heuer et al. 2016). They have shown that the extrastriate visual cortex and parietal cortex are dominantly engaged with orienting attention to the object’s feature and spatial location, respectively (feature-based/spatial attention) (Chambers et al. 2004; Maunsell and Treue 2006). Accordingly, it is likely that other areas such as parietal cortex may have a greater modulation for spatial location matching-rule modulation. Feature-based attention is posited to play a key role in the enhancement of neural representation and information selectivity (Saenz et al. 2002; Martinez-Trujillo and Treue 2004). The variability modulation only in feature WM task may imply to feature-based attention as a mechanism for neural response variability modulation.
Second, these tasks were run in blocks during recordings; first passive, second spatial WM, and, finally, feature WM task. As we know, there is a positive relationship between learning and attention (Qi and Constantinidis 2012; Leong et al. 2017; Ni et al. 2018). Training and learning to perform a WM task lead the attentional level to increase. Selective attention to a relevant feature dimension leads to focus learning (Leong et al. 2017). It has been shown that learning impacts on several cognitive functions such as selectivity and motion perception (Meyer et al. 2011; Qi et al. 2011; Constantinidis and Klingberg 2016; Liu and Pack 2017; Krause et al. 2017). Furthermore, learning to attend to an isolated feature or to multiple features engages different areas and is supported by two different (ventral and dorsal) visual pathways (Meyers et al. 2018). Importantly, both attention and learning lead to a reduction in the trial-by-trial variation of neural response (Constantinidis and Klingberg 2016; Ni et al. 2018). Here, we found two manners of PFC population responses in the context of learning to attend to spatial location or shape feature. This may imply task-specific PFC modulation in line with the previous reports (Wilson et al. 1993; Asaad et al. 2000; Johnston and Everling 2006; Warden and Miller 2010; Sebastian et al. 2016). In other words, this effect could be explained by different population responses of PFC neurons related to the task context (Asaad et al. 2000; Balaban and Luria 2016; Heuer et al. 2016). It revealed that in the context of various demands, similar stimuli result in different neural responses. Here, we found different modulations for a single mechanism (matching-rule decision-making) in two WM demands.
The comparison of trial-to-trial spike count variability between the passive and two WM tasks showed a lower across-trial variability for the feature WM task compared to the spatial WM and passive tasks. Less neural response variability potentially is related to a greater attentional level (Arazi et al. 2019). Accordingly, three attentional levels could be assumed to our three target tasks: feature WM > spatial WM > passive tasks. This difference in the neural response variability (attentional level) might be due to different attentional mechanisms or various WM demands. It highlights the impact of feature-based attention in cognitive functions. Taken together, our data showed that the matching-rule modulation of PFC at the population level arises in the context of attention and is task-specific. This similarity-based matching-rule modulation in the PFC is related to the top-down memory-guided activity and documents that attention plays a crucial role in matching-rule decision-making.
The current study has exclusively focused on PFC neurons. As mentioned above, this population-level matching modulation in other relative areas such as parietal cortex may have a different pattern of modulation for spatial and feature WM tasks. In other words, we expect other areas with a significant population-level modulation for matching-rule decision-making. However, this matter cannot be addressed based on these data.
In conclusion, our data suggest that attention is necessary for matching-rule modulation in PFC neurons, and that differences in firing rate and trial-to-trial spike count variability between tasks could be due to higher attentional demands of the feature WM task compared to the spatial WM task (in these data). These results highlight the task-specific role of PFC neurons in terms of matching-rule decision-making.
Supplementary Material
Acknowledgements
The project was supported by the Wake Forest University School of Medicine, Department of Neurobiology and Anatomy database. We would like to thank Dr. Shima T. Moein, and Dr. Morteza Saraf for their comments on the manuscript.
Footnotes
Data availability The data that support the current findings are deposited to the Wake Forest University School of Medicine, Department of Neurobiology and Anatomy database. Requests for data should be directed to the Prof. C. Constantinidis: cconstan@wfubmc.edu.
Conflict of interest The authors declare no competing financial interests.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00429-020-02191-7.
References
- Arazi A, Yeshurun Y, Dinstein I (2019) Neural variability is quenched by attention. J Neurosci. 10.1523/JNEUROSCI.0355-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK (2000) Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol 84:451–459. 10.1152/jn.2000.84.1.451 [DOI] [PubMed] [Google Scholar]
- Backen T, Treue S, Martinez-Trujillo JC (2018) Encoding of spatial attention by primate prefrontal cortex neuronal ensembles. eNeuro. 10.1523/ENEURO.0372-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban H, Luria R (2016) Object representations in visual working memory change according to the task context. Cortex 81:1–13. 10.1016/j.cortex.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berger M, Calapai A, Stephan V et al. (2018) Standardized automated training of rhesus monkeys for neuroscience research in their housing environment. J Neurophysiol 119:796–807. 10.1152/jn.00614.2017 [DOI] [PubMed] [Google Scholar]
- Bichot NP, Heard MT, DeGennaro EM, Desimone R (2015) A source for feature-based attention in the prefrontal cortex. Neuron 88:832–844. 10.1016/J.NEURON.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME (2003) Neuronal activity in the lateral intraparietal area and spatial attention. Science 299:81–86. 10.1126/science.1077395 [DOI] [PubMed] [Google Scholar]
- Calapai A, Berger M, Niessing M et al. (2017) A cage-based training, cognitive testing and enrichment system optimized for rhesus macaques in neuroscience research. Behav Res Methods 49:35–45. 10.3758/s13428-016-0707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A et al. (2005) Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry 57:1416–1423. 10.1016/J.BIOPSYCH.2004.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Stokes MG, Mattingley JB (2004) Modality-specific control of strategic spatial attention in parietal cortex. Neuron 44:925–930. 10.1016/j.neuron.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Chang MH, Armstrong KM, Moore T (2012) Dissociation of response variability from firing rate effects in frontal eye field neurons during visual stimulation, working memory, and attention. J Neurosci 32:2204–2216. 10.1523/JNEUROSCI.2967-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV (2006) A central source of movement variability. Neuron 52:1085–1096. 10.1016/j.neuron.2006.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP et al. (2010) Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci 13:369–378. 10.1038/nn.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Chaudhuri R et al. (2011) Variance as a signature of neural computations during decision making. Neuron 69:818–831. 10.1016/j.neuron.2010.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Noudoost B (2014) The role of prefrontal catecholamines in attention and working memory. Front Neural Circuits 8:33. 10.3389/fncir.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR (2009) Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12:1594–1600. 10.1038/nn.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT (2008) Context-dependent changes in functional circuitry in visual area MT. Neuron 60:162–173. 10.1016/j.neuron.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Klingberg T (2016) The neuroscience of working memory capacity and training. Nat Rev Neurosci 17:438–449. 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- Deco G, Hugues E (2012) Neural network mechanisms underlying stimulus driven variability reduction. PLoS Comput Biol 8:e1002395. 10.1371/journal.pcbi.1002395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R (1996) Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A 93:13494. 10.1073/PNAS.93.24.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Albright T, Gross C, Bruce C (2011) Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci 4:2051–2062. 10.1523/jneurosci.6798-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE (2000) Interactions between visual working memory and selective attention. Psychol Sci 11:467–473. 10.1111/1467-9280.00290 [DOI] [PubMed] [Google Scholar]
- Engel TA, Wang X-J (2011) Same or different? A neural circuit mechanism of similarity-based pattern match decision making. J Neurosci 31:6982–6996. 10.1523/JNEUROSCI.6150-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Goldberg ME, Krishna BS (2013) Spatial representation and cognitive modulation of response variability in the lateral intraparietal area priority map. J Neurosci 33:16117–16130. 10.1523/JNEUROSCI.5269-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–1563. 10.1126/science.1055465 [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R (2008) The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci 28:4823–4835. 10.1523/JNEUROSCI.4499-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, Friston KJ (2009) The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol 120:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D et al. (2010) Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci 30:14330–14339. 10.1523/JNEUROSCI.4248-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R (2009) High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324:1207–1210. 10.1126/science.1171402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J et al. (2000) Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84:401–414. 10.1152/jn.2000.84.1.401 [DOI] [PubMed] [Google Scholar]
- Heuer A, Schubö A, Crawford JD (2016) Different cortical mechanisms for spatial vs. feature-based attentional selection in visual working memory. Front Hum Neurosci 10:415. 10.3389/fnhum.2016.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Dewar CD, Solbakk A-KK et al. (2017) Bidirectional frontoparietal oscillatory systems support working memory. Curr Biol 27:1829–1835.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Adams JN, Solbakk A-K et al. (2018a) Dynamic frontotemporal systems process space and time in working memory. PLOS Biol 16:e2004274. 10.1371/journal.pbio.2004274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Tang L, Yin Q et al. (2018b) Direct brain recordings reveal prefrontal cortex dynamics of memory development. Sci Adv 4:3702. 10.1126/sciadv.aat3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Everling S (2006) Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cogn Neurosci 18:749–765. 10.1162/jocn.2006.18.5.749 [DOI] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C (2012) Early involvement of prefrontal cortex in visual bottom-up attention. Nat Neurosci 15:1160–1166. 10.1038/nn.3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C (2014) Bottom-Up and Top-Down Attention. Neurosci 20:509–521. 10.1177/1073858413514136 [DOI] [PubMed] [Google Scholar]
- Krause MR, Zanos TP, Csorba BA et al. (2017) Transcranial direct current stimulation facilitates associative learning and alters functional connectivity in the primate brain. Curr Biol 27:3086–3096.e3. 10.1016/J.CUB.2017.09.020 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD et al. (2008) Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320:110–113. 10.1126/science.1154735 [DOI] [PubMed] [Google Scholar]
- Leong YC, Radulescu A, Daniel R et al. (2017) Dynamic interaction between reinforcement learning and attention in multi-dimensional environments. Neuron 93:451–463. 10.1016/J.NEURON.2016.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Newsome WT (2006) Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J Neurosci 26:7779–7790. 10.1523/JNEUROSCI.5052-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LD, Pack CC (2017) The contribution of area MT to visual motion perception depends on training. Neuron 95:436–446.e3. 10.1016/J.NEURON.2017.06.024 [DOI] [PubMed] [Google Scholar]
- Lowet E, Gomes B, Srinivasan K et al. (2018) Enhanced neural processing by covert attention only during microsaccades directed toward the attended stimulus. Neuron 99:207–214.e3. 10.1016/J.NEURON.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machens CK, Romo R, Brody CD (2005) Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science 307:1121–1124. 10.1126/science.1104171 [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S (2004) Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol 14:744–751. 10.1016/J.CUB.2004.04.028 [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Treue S (2006) Feature-based attention in visual cortex. Trends Neurosci 29:317–322. 10.1016/J.TINS.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Merrikhi Y, Clark K, Albarran E et al. (2017) Spatial working memory alters the efficacy of input to visual cortex. Nat Commun 8:15041. 10.1038/ncomms15041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Qi X-L, Stanford TR, Constantinidis C (2011) Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J Neurosci 31:6266–6276. 10.1523/JNEUROSCI.6798-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EM, Liang A, Katsuki F, Constantinidis C (2018) Differential processing of isolated object and multi-item pop-out displays in LIP and PFC. Cereb Cortex 28:3816–3828. 10.1093/cercor/bhx243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH (2007) Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55:131–141. 10.1016/J.NEURON.2007.06.018 [DOI] [PubMed] [Google Scholar]
- Moore T, Zirnsak M (2017) Neural mechanisms of selective visual attention. Annu Rev Psychol 68:47–72. 10.1146/annurev-psych-122414-033400 [DOI] [PubMed] [Google Scholar]
- Muhammad R, Wallis JD, Miller EK (2006) A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. J Cogn Neurosci 18:974–989. 10.1162/jocn.2006.18.6.974 [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K (2007) The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol 118:2544–2590 [DOI] [PubMed] [Google Scholar]
- Ni AM, Ruff DA, Alberts JJ et al. (2018) Learning and attention reveal a general relationship between population activity and behavior. Science 359:463–465. 10.1126/science.aao0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T (2011) Control of visual cortical signals by prefrontal dopamine. Nature 474:372–375. 10.1038/nature09995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parto Dezfouli M, Daliri MR (2015) The effect of adaptation on the tuning curves of rat auditory cortex. PLoS ONE 10:e0115621. 10.1371/journal.pone.0115621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parto Dezfouli M, Zarei M, Jahed M, Daliri MR (2019) Stimulus-specific adaptation decreases the coupling of spikes to LFP phase. Front Neural Circuits 13:44. 10.3389/fncir.2019.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Mruczek REB (2008) Sources of spatial and feature-based attention in the human brain. J Neurosci 28:9328–9329. 10.1523/JNEUROSCI.3562-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Alvarez A, Thiele A, Albright TD et al. (2013) Stimulus-dependent variability and noise correlations in cortical MT neurons. Proc Natl Acad Sci U S A 110:13162–13167. 10.1073/pnas.1300098110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X-L, Constantinidis C (2012) Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS ONE 7:e41053. 10.1371/journal.pone.0041053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X-L, Meyer T, Stanford TR, Constantinidis C (2011) Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb Cortex 21:2722–2732. 10.1093/cercor/bhr058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X-L, Meyer T, Stanford TR, Constantinidis C (2012) Neural correlates of a decision variable before learning to perform a match/non-match task. J Neurosci 32:6161–6169. 10.1523/JNEUROSCI.6365-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Philiastides MG, Sajda P (2009) Quality of evidence for perceptual decision-making is indexed by trial-to-trial variability of the EEG. Proc Natl Acad Sci U S A 106:6539–6544. 10.1073/pnas.0812589106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C (2011) Effects of task and coordinate frame of attention in area 7a of the primate posterior parietal cortex. J Vis 10:12–12. 10.1167/10.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N, Rust NC (2018) Inferotemporal cortex multiplexes behaviorally-relevant target match signals and visual representations in a manner that minimizes their interference. PLoS ONE 13:e0200528. 10.1371/journal.pone.0200528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM (2002) Global effects of feature-based attention in human visual cortex. Nat Neurosci 5:631–632. 10.1038/nn876 [DOI] [PubMed] [Google Scholar]
- Sarafyazd M, Jazayeri M (2019) Hierarchical reasoning by neural circuits in the frontal cortex. Science 364:eaav8911. 10.1126/SCIENCE.AAV8911 [DOI] [PubMed] [Google Scholar]
- Sebastian A, Jung P, Neuhoff J et al. (2016) Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Struct Funct 221:1635–1651. 10.1007/s00429-015-0994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB, Lehky SR (2018) Attention effects on neural population representations for shape and location are stronger in the ventral than dorsal stream. eNeuro. 10.1523/ENEURO.0371-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R et al. (2008) Neuronal Synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60:709–719. 10.1016/j.neuron.2008.09.010 [DOI] [PubMed] [Google Scholar]
- Sigala N, Logothetis NK (2002) Visual categorization shapes feature selectivity in the primate temporal cortex. Nature 415:318–320. 10.1038/415318a [DOI] [PubMed] [Google Scholar]
- Skrandies W, Reuther N (2008) Match and mismatch of taste, odor, and color is reflected by electrical activity in the human brain. J Psychophysiol 22:175–184. 10.1027/0269-8803.22.4.175 [DOI] [Google Scholar]
- Soltani A, Wang X-J (2006) A biophysically based neural model of matching law behavior: melioration by stochastic synapses. J Neurosci 26:3731–3744. 10.1523/JNEUROSCI.5159-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Heinke D, Humphreys GW, Blanco MJ (2005) Early, involuntary top-down guidance of attention from working memory. J Exp Psychol Hum Percept Perform 31:248–261. 10.1037/0096-1523.31.2.248 [DOI] [PubMed] [Google Scholar]
- Stein RB, Gossen ER, Jones KE (2005) Neuronal variability: noise or part of the signal? Nat Rev Neurosci 6:389–397. 10.1038/nrn1668 [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH (1999) Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci 19:7591–7602. 10.1523/JNEUROSCI.19-17-07591.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Trujillo JCM (1999) Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399:575–579. 10.1038/21176 [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK (2003) From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol 90:1790–1806. 10.1152/jn.00086.2003 [DOI] [PubMed] [Google Scholar]
- Warden MR, Miller EK (2010) Task-dependent changes in short-term memory in the prefrontal cortex. J Neurosci 30:15801–15810. 10.1523/JNEUROSCI.1569-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford T, Maunsell JHR (2006) Effects of spatial attention on contrast response functions in macaque area V4. J Neurophysiol 96:40–54. 10.1152/jn.01207.2005 [DOI] [PubMed] [Google Scholar]
- Wilson FAW, Scalaidhe SP, Goldman-Rakic PS (1993) Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260:1955–1958. 10.1126/science.8316836 [DOI] [PubMed] [Google Scholar]
- Xue C, Kaping D, Ray SB et al. (2017) Spatial attention reduces burstiness in macaque visual cortical area MST. Cereb Cortex 27:83–91. 10.1093/cercor/bhw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Ketkar M, Treue S, Krishna BS (2016) Visual attention is available at a task-relevant location rapidly after a saccade. Elife. 10.7554/eLife.18009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Parto Dezfouli M, Jahed M, Daliri MR (2020) Adaptation modulates spike-phase coupling tuning curve in the rat primary auditory cortex. Front Syst Neurosci 14:55. 10.3389/fnsys.2020.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


