Abstract
Maintaining adherence to treatment for tuberculosis (TB) is essential if the disease is to be eliminated. As part of formative research to develop an intervention to improve adherence, we documented the lived experiences of adults receiving anti-TB treatment (ATT) in three UK cities and examined how personal, social, and structural circumstances interacted to impact on individuals’ adherence to treatment. Using a topic guide that explored social circumstances and experiences of TB care, we conducted in-depth interviews with 18 adults (six women) who were being or had been treated for TB (patients) and four adults (all women) who were caring for a friend, relative, or partner being treated for TB (caregivers). We analysed transcripts using an adapted framework method that classified factors affecting adherence as personal, social, structural, health systems, or treatment-related. Eleven of 18 patients were born outside the UK (in South, Central, and East Asia, and Eastern and Southern Africa); among the seven who were UK-born, four were Black, Asian, or Minority Ethnic and three were White British. TB and its treatment were often disruptive: in addition to debilitating symptoms and side effects of ATT, participants faced job insecurity, unstable housing, stigma, social isolation, worsening mental health, and damaged relationships. Those who had a strong support network, stable employment, a routine that could easily be adapted, a trusting relationship with their TB team, and clear understanding of the need for treatment reported finding it easier to adhere to ATT. Changes in circumstances sometimes had dramatic effects on an individual’s ability to take ATT; participants described how the impact of certain acute events (e.g., the onset of side effects or fatigue, episodes of stigmatisation, loss of income) were amplified by their timing or through their interaction with other elements of the individual’s life. We suggest that the dynamic and fluctuating nature of these factors necessitates comprehensive and regular review of needs and potential problems, conducted before and during ATT; this, coupled with supportive measures that consider (and seek to mitigate) the influence of social and structural factors, may help improve adherence.
Keywords: Compliance, Medication, Outcomes, Determinants, Person-centred care, Elimination
1. Introduction
Elimination of tuberculosis (TB) is a realistic prospect in low TB burden countries such as the United Kingdom (UK), where incidence has declined from 15 per 100,000 population in 2011 to 8 per 100,000 in 2019 [1]. Treating TB disease currently requires at least six months of daily therapy [2], [3]; non-adherence to anti-TB therapy (ATT) worsens individual outcomes [4], increases the risk of drug-resistant TB (DR-TB) [5], [6], and may prolong infectiousness [7]. Optimising outcomes and maintaining adherence to treatment are essential if the World Health Organization (WHO) ‘pre-elimination’ target of annual incidence <1 per 100,000 is to be reached [8], [9].
TB in the UK is predominantly present in people who are migrants [10], socioeconomically disadvantaged, or both [11], [12]: 74% of people who developed TB in England in 2019 were born outside of the UK [13]. Many individuals diagnosed with TB in the UK also have so-called ‘social risk factors’ (SRFs: drug or alcohol misuse, homelessness, or current or previous imprisonment). In 2019, 35% and 23% of people with TB who were born in the UK and outside the UK, respectively, had at least one SRF [13].
Formal adherence support for ATT in the UK is based on a standardised risk assessment conducted by a specialist TB nurse (usually the individual’s case manager) at treatment initiation. Individuals considered at high risk of non-adherence are offered enhanced case management, which can include directly observed therapy (DOT), video-observed therapy (VOT), and other forms of practical treatment support, such as a weekly dosette box [14]. Enhanced case management is offered most often to people with SRFs, though individuals without recognised SRFs are also at risk of non-adherence: a study of nearly 13,000 people treated for TB in London found that although the odds of non-adherence were highest in those with at least one SRF, they were also high in some migrant communities, people who had previously had TB, those with pulmonary disease, and those who were aged 16–24 years (versus those aged 25–34 years) [15]. Yet the evidence around risk of non-adherence is inconclusive: a recent systematic review found that adherence to treatment for DR-TB was no different in migrants and non-migrants (n = 8 studies, all in high-income countries) [16]. Therefore, to focus supportive resources more efficiently on those with the greatest need (i.e., those most likely to have difficulty taking treatment), screening methods are required that categorise risk of non-adherence by considering a wider range of criteria than simply ‘static’ demographics.
“Non-adherence” is a term that encompasses a spectrum of behaviours at various points throughout the patient’s journey and care pathway [17]. The WHO’s call for “care and support, [that is] sensitive and responsive to patients’ educational, emotional and material needs” [18] reflects the increasing prominence of the discussion around patient-centred care for TB [19], [20], [21], yet the most widely-used form of ATT adherence support, DOT, has been criticised by patients as being inflexible and paternalistic; critics suggest that it seeks to ‘enforce’ treatment-taking, does not promote patient self-management, and focuses on pill-taking as the defining feature of treatment adherence [22], [23], [24], [25]. Evidence-based, supportive approaches are needed that can be adapted for use in all individuals starting ATT.
Globally, numerous qualitative studies have explored adherence to ATT: a 2007 systematic review by Munro et al. synthesised qualitative data from 44 studies and classified the key determinants of adherence into four categories: 1) personal factors, including knowledge and beliefs; 2) structural factors, including poverty, gender, and law; 3) social factors, including family, community, and stigma; and 4) health service-related factors, including the organisation of care and side effects [26]. However, assessing these dimensions as distinct, though useful for analysis, risks over-simplification, as adherence behaviour is not constant [27] and relationships between different dimensions can be dynamic and complex [28].
Despite these studies, there are few data available to help shape evidence-based, supportive approaches for use across the highly diverse populations encountered in high-income, low incidence settings [29], [30]. The small number of qualitative studies reporting on the experiences of individuals with TB in the UK have focused on sub-populations living in extreme situations or within specific communities [31], [32], [33], [34].
Using an adaptation of the Munro framework that includes greater consideration of the dynamic relationships across different levels and types of determinants of adherence [29], we conducted in-depth interviews with a diverse group of adults receiving (and caring for those on) ATT in three cities in the UK. In this analysis, we examine how individuals’ experiences of the TB care trajectory, including their engagement with the health system and their social networks, influenced how they felt about treatment and their ability to take ATT. We use these data to map the relationships between and across factors that enabled and impeded adherence to ATT and discuss the implications for assessing risk of non-adherence and intervening to improve adherence.
2. Methods
Interviews were conducted from April to October 2019 as part of formative qualitative research for a study seeking to develop, pilot, and evaluate an intervention to support patients taking ATT (“Intervening with a Manualised Package to AChieve treatment adherence in people with Tuberculosis” [IMPACT]) [35]. Data from qualitative interviews were used to shape the form and content of the intervention; all interviews were conducted prior to the implementation of any part of the intervention.
2.1. Sites of data collection
Formative research was based in four National Health Service (NHS) Trusts: two in London and one each in Edinburgh and Southampton; recruitment and interviews took place at eight physical sites across the three cities. London overall has the highest TB incidence in the country, at 18.6 notifications per 100,000 in 2019; [13] the two London Trusts involved in the study employed had slightly different modes of care, with, for example, a dedicated social care team in one Trust, but not the other. Edinburgh (incidence 4.9 per 100,000 in 2018) [36] and Southampton (three-year average incidence 11.5 per 100,000) [13] have fewer TB patients than London and smaller dedicated services to deal with them. In both Trusts, people with TB are co-managed by the Respiratory and Infectious Diseases teams, with two or three specialist TB nurses responsible for case management.
2.2. Sampling and recruitment of participants
Adults (aged ≥18 years) who were taking or had taken ATT (patients) and adults who were caring for or had cared for a friend, relative, or partner on ATT (caregivers) were asked to participate; formal inclusion and exclusion criteria applied to patients and caregivers are described in Supplementary table 1. A purposive sampling approach was adopted; it was attempted, throughout, to represent as fully as possible the wide range of perspectives and backgrounds within the patient populations at each site. Further details of recruitment and consent procedures are provided in Appendix 1.
2.3. Data collection
Interviews were conducted in confidential settings in hospitals or community health centres, often in consultation rooms that also contained a workstation, examination couch, and sink. All interviews were conducted in person, by one or two interviewers (one/two of ASK, KK, ASKJ, and MD), in English, and with the help of a topic guide (Appendix 1) that covered the following topics: pathways to TB care; treatment adherence issues; knowledge and perceptions of TB and treatment; adherence to ATT (experiences, enablers, and barriers, and incidence of and reasons for non-adherence); social support; and structural and health systems issues (interactions with and potential obstacles to care). During the interviewing process, the lead interviewer (ASK) confirmed with each participant what was being said through short summarisation at various points in the interview.
2.4. Data management and analysis
Interviews were audio recorded (using an Olympus DS-9500 [Olympus Corporation, Tokyo, Japan]), securely encrypted, and transferred to a password-protected computer. Hand-written notes were scanned and password protected, and the originals destroyed. Transcripts of interviews were used for analysis after being checked for comprehensiveness and completeness.
Analysis was undertaken by ASK and KK (both of whom cross-checked transcripts, cross-checked themes identified, and piloted the coding system) in a stepwise manner, using an adapted framework analysis method. First, transcripts were read several times; narrative profiles of each patient were constructed that included accounts of how patients presented themselves and their social networks; their TB diagnosis and early experiences of care; and their experiences of being ‘on treatment’. Attention was paid to the unique trajectories of individuals and how demographic features such as gender, age, ethnicity, and social status impacted on pathways to and experiences of care, and the effects of TB and ATT on their lives. Second, using a conceptual framework of determinants of adherence derived from the systematic review by Munro et al. [26] and a previously described scoping review [29], information was categorised from the profiles into a matrix to compare and contrast data on the five key themes (personal, social, structural, health systems, and treatment-related; Appendix 1) until theme saturation was reached. Third, the summarised information allowed for the creation of separate files for each of the five thematic areas; retrieved data segments were supplemented with information from the original transcripts and reviewed through a constant comparison method to ensure that content and meaning of the themes were consistently applied [37].
To illustrate the ways in which various factors interacted with one another and the mechanisms by which they might influence adherence behaviour, relationships between determinants and their potential effects on an individual’s ability to take ATT were mapped based on explicit descriptions by interviewees or through inference by the authors after analysis of all interviews. Determinants were categorised as ‘distal’ when their influence on adherence behaviour was indirect or mediated through intervening factors, or ‘proximal’ when they exerted a more direct effect on adherence behaviour. Data were collated and analysed using NVivo (v12, QSR International, Doncaster, Australia) and relationships were visually depicted using Vensim (Ventana Systems UK; https://www.ventanasystems.co.uk/) and InkScape (https://inkscape.org/).
2.5. Ethical considerations
This study received approval from the Camberwell St Giles Ethics Committee (REC reference 18/LO/1818). Written informed consent was obtained from each individual prior to data collection (further details in Appendix 1). Pseudonyms are used in the manuscript to humanise accounts and quotes; no information that could potentially identify a participant has been included.
3. Results
3.1. Participant characteristics
Interviews were conducted with 18 patients and four caregivers. Participants varied widely in age, ethnicity, migration status, level of education, profession, knowledge of TB, social support networks, disease type and severity, experiences of treatment, adherence behaviour, and health literacy (Supplementary table 2).
Ages ranged from 20 to 65 years, but most participants were aged 20–35 years. The two youngest individuals lived with their parents and siblings; others in their 20s and 30s had young children or lived alone. Four of the six individuals aged 50 years or older had additional medical problems, including cancer and depression. Two-thirds of patients were male; all four caregivers were female and had cared for a (male) partner. Three patients were White British; four were born in the UK to Black and Asian families; and the remaining 11 were migrants, originating from South, Central, and East Asia, and Eastern and Southern Africa.
TB disease severity varied widely and was not necessarily linked to age or comorbidities. One of the youngest participants had disseminated, drug-resistant TB. Two participants had recurrent TB disease, needing two courses of ATT in close succession, and another had re-started treatment several times (due to non-adherence) and was waiting for surgery to address complications of TB. Other participants presented with only neck swelling or experienced mild symptoms. Participants were at various stages of the treatment journey at the time of interview: all had received at least three to four months of treatment and four individuals had completed treatment a year or more prior to interview.
Six participants reported high levels of alcohol use, incarceration, emergency migration, or homelessness; these individuals had usually been identified as ‘high risk’ for non-adherence soon after diagnosis and had received some form of treatment support. Some patients who lived alone had parents available for support, but others depended on employers, friends, and, in one case, a landlord. Three participants did not report any strong social ties, and described interactions limited to colleagues or casual acquaintances.
3.2. Lived experiences of illness and the care pathway
3.2.1. Symptom onset and entry into care
I coughed for probably, like, one, two weeks. …I did some research online, it says it might be, you know, even something like lung cancer. And I was so scared. I was so scared. I was so scared
Zhen, a student in his 20s who lived alone and made several trips to his GP and to the Emergency Department before he was diagnosed with TB.
Experiences early in the treatment journey had lasting effects on individuals’ attitude to their illness and engagement with care. Care-seeking behaviour and the time taken to enter the TB care pathway were influenced by disease severity and could be triggered by a dramatic event or acute deterioration in wellbeing, but were also affected by the individual’s priorities around their own health, which were sometimes displaced by alcohol, insecure housing, or financial commitments; their sense of security (i.e., freedom to seek help); and their previous experiences of health care.
…I started coughing blood. But I didn't take any notice. I said, '[inaudible], like, leave it'. And my sister she's a nurse, she noticed that I'm losing weight, I'm not eating. She called the ambulance. I got to go to [hospital], and they found I had TB.
Yousuf, a patient in his 30s who consumed large amounts of alcohol and had been unwell for some time before he sought care; he had persistent problems with adherence and developed DR-TB.
The time between symptom onset and entry into care was often filled with anxiety, and participants described feeling frightened and vulnerable. Delays sometimes occurred within the health system, through early misdiagnosis or extended investigation. Some described losing trust in the system if a health care provider (HCP) set expectations that were later not met.
Yeah, so I didn't take it [the medication] - so that hospital, so eventually I moved out, so I wasn't there no more. And then I had to sign up in a new hospital, then start the whole, sort of, treatment again. (…) So I was there for about six months without taking no meds and knowing I've got TB
Eunice, a patient in her 20s who had spent time in prison and was living in social housing; she described a tenuous relationship with her TB team.
The way an individual responded to diagnosis was shaped by their knowledge and beliefs about TB. These, in turn, were often tied to age, culture, class, country of origin, and previous knowledge of the disease. For example, some UK-born participants thought of TB as something of ‘the past’ or associated it with ‘foreigners’, whereas participants from high TB burden countries were more likely to consider it a disease of ‘the poor’ or of marginalised communities.
…no one actually in my family, my relatives have ever been diagnosed with TB. So I was quite surprised when I was first diagnosed. It's like, back in [South Asian country], the number of reported cases are really high but then usually people who suffer from malnutrition, or belonging to, probably, not so well-to-do families, especially the nutrition part, would get affected, and that's how the illness was probably triggered.
Rita, a patient in her 30s who moved to the UK to pursue her career.
3.2.2. Time on treatment
3.2.2.1. Relationships with immediate family
By the time they started ATT, many individuals had depleted physical and emotional resources, having been through weeks of illness and investigation. For those whose lives were supported by ‘fragile’ infrastructure (tenuous relationships, insecure housing, or irregular employment), TB and its treatment were sometimes seen as additional burdens (“a hell crisis”) to be shouldered by both the patient and their family.
TB medication being started last year in November I think - that was a horrible experience. So he has to leave his job because he couldn't cope, like you know he can't sleep. Every time he is feeling hungry, so he wants to eat something, he is getting up from that, so even I couldn't sleep sometimes. I went upstairs to sleep because I had to sleep - I can't break my sleep - I will get headache because I need to wake up early morning to drop my children.
Jaya speaking about her husband Arif, who resigned from his job a short time after starting ATT, making her the family’s main breadwinner.
The existence of at least one stable, close relationship was important in allowing the patient to continue to function, often by relieving them of some of their normal responsibilities.
She’s been a rock, to be honest. She's… I could just share everything, you know what I mean - we don't hide anything from each other. If I come to any consultants or consultations, she comes with me. I prefer that because, as I say, I've got a bad memory, and she remembers everything.
Alastair, a patient in his 50s, talking about his wife, Aileen
I always put on a face to Alastair and my family, and I don't think they realised how depressed I was after the cancer thing. But I know exactly - that's the - you know the worst thing, it's probably that I know how he's feeling, you know? (…) So that's helped him as well. I think if he hadn’t been through that, I don't think he'd be coping.
Aileen, Alastair’s wife, speaking about her previous illness and how it helped with her husband’s TB care
In contrast, participants whose families were overseas felt the absence of a local support network acutely, and often felt conflicted about how much they could divulge to or ask from family who were far away.
My folks back home didn't even know that I was diagnosed because I didn't want to upset them. (…) Both of my parents are pretty old, I didn't want to stress them out.
Rita, who lived alone
In some cases, the stability provided by relationships worked to counter the ‘chaos’ introduced by drugs or alcohol, or the disruption caused by multiple hospital visits. However, TB could also put strain on existing relationships (e.g., through stigma or changes in power relations), in some cases leading to lasting changes in their character.
The first week I stayed at the hospital, my mum was like, ‘Don’t come home – you’re full of TB, I don’t want you to come home. I don’t want to take care of you.’
Janella, a patient in her 20s who became tearful recounting this experience.
3.2.2.2. Wider interactions
Relationships beyond the immediate family were also important to many participants; for those without partners, social groups sometimes formed their primary support network, with friends stepping in to help with cooking, shopping, and other day-to-day tasks.
They said to me, 'For the first two weeks, try to have as little contact with the general public.’ (…) So I just phoned up one of my mates and said, 'Get us this from the shop, or whatever, and I'll sort out the money when you come up'. They'd do that.
Michael, a patient in his 50s who lived alone but had a circle of close, supportive friends.
Interactions with others could also prove difficult, and many patients experienced discrimination. Several participants did not disclose their diagnosis to their employers, and one even resigned without giving TB as a reason, hoping that this would boost his chances of re-employment once he recovered. Episodes of discrimination and rejection, even if fleeting, sometimes had substantial negative effects on individual’s confidence and sense of identity.
He offered me a job… He said, 'If you wanna come down, you can stay in the caravan on the holiday park and do a bit'. I said, 'I can't. Got TB'. (…) He said, 'You know, no one will touch you - if this gets out, that you've got it, then most firms… because you're public liability'. That's his attitude and mentality. If you're working with other people, then they'll all just, 'Oh, what happens if they catch it?'
Michael describing a conversation with a previous employer that made him feel suicidal.
3.2.2.3. Health care professionals
Relationships with HCPs were frequently cited as important to patients and caregivers, and the person with whom patients had the most contact was usually their TB case manager (a nurse) or DOT care worker. Trust in these relationships was strengthened by clear, easily accessible two-way communication (e.g., a phone number to use out of hours); early demonstrations of confidence and competence; a willingness to adapt, including flexibility in communication style and acknowledgment of difficult life circumstances; a sense of ‘completeness’ of the information received; and collaborative decision-making.
I think the nurses done really, really well here. They are on the ball and they get to know you and they know their patients and if someone is going to mess around with the tablets. They know who would be committed and who wouldn’t, as they do get to know you on that level, which is - I can’t fault the team here at all. (…) I’ve still got their numbers. They text, say ‘How are you?’
Naomi, a patient in her 50s who spoke to us a year after completing treatment.
Yeah, because couple of times I was running out the medicine and then I had to call her; when I called her she made sure that she got the medicine ready for me to come and pick it up. Basically what I think - they know what they're doing. That's it.
Aalok, a patient in his 40s, describing his confidence in his TB nurses.
Interactions with the health service could be challenging for participants with serious comorbidities, who sometimes received conflicting advice from different specialties, leading to a disjointed experience and mistrust. Previous experience of illness sometimes proved useful, providing pre-established medication routines or familiarity with the health system. However, for those who were unfamiliar with the system (usually migrants with no family support), obtaining adequate support could be an all-encompassing activity, requiring weeks of correspondence, multiple visits to relevant authorities, and, in some cases, legal action. These individuals often had to rely on assistance from members of their TB team.
I don't blame the doctor or medical system - they are so kind. But I don't understand why they make problem with sickness person. Make hard, difficult, complicated. Like, I don't have energy to do that. I run to - they said, “Go to [institution]”. I run to them, they say, “We don't do this, we don't do that.” (…) Sometimes, when I pop in, they see my face - not English - they said, “Oh, we don't do this, we don't that.” And they never ask, sit down, explain. And I went around, they say, “Go there!” I went there, they say, “Go this!”
Suchin, a patient in her 60s who lived alone and received support from the TB social care team to fight her eviction in court
3.2.2.4. Routines
An established routine or the ability to quickly adopt a new routine was described by some participants as ‘the secret’ of adherence. This was more difficult for people with relatively unstructured lives, in uncertain housing situations, or with serious alcohol or drug habits. Some participants found the externally imposed routine in hospital to be useful in building a treatment-taking habit. Though many found hospital isolating and alienating, it was seen by others as a respite from daily life, allowing time for reflection and rest.
It wasn’t terrible but it gives you a lot of time to think (…) You think you are going to lose your life, to be honest, so it’s given me a bigger incentive to do more - but I was at my lowest point.
Naomi, describing the two months she spent in hospital at the start of her treatment.
Even in those who managed to establish robust routines, the number of pills and associated side effects could make it difficult to maintain regularity. Many individuals developed ‘adjunct’ systems to help keep track of the different tablets, such as keeping a written log, organising them within a cupboard or box, or setting reminders.
I think when I was first taking it, I didn't really fully understand, and some of my questions weren’t answered properly. So, I had like a sheet with all the medicines that I'm supposed to have - I think that was kind of confusing. So what I did when I first got home, was I write it down myself, just to make it easy to understand.
Imran, a patient in his early 20s who lived with his parents and siblings
Side effects could be debilitating, and for some participants occurred daily for several months. Patients often had to rearrange their lives around periods when they knew they would be “out of it”.
“It’s so depressing. You're having to sit indoors for about four hours before - you're waiting for the person to come round with the tablets, and then, you know, you can't go out. Cos I don't want to be walking on the high street and vomit coming up - people will look at you, and next thing you know they get the police on you. So I'm like, 'No, I gotta wait until I feel right'. It's soul-destroying.”
Michael, speaking about the effects of treatment, side effects, and DOT on his daily routine and mental state
3.3. Dynamic interactions and turning points in adherence behaviour
Only a few of the processes, relationships, and events discussed above, particularly those with a more direct effect on adherence, fit cleanly into one of the ‘personal’, ‘social’, ‘structural’, ‘health systems’, or ‘treatment-related’ categories, and often the combined effects of two or more factors (from different spheres of an individual’s life) were cited as influencing adherence-related behaviour. The dynamic nature of these interactions was illustrated most clearly when participants described changes in circumstances that occurred while they were taking ATT, and how ‘ripples’ from these events led to sometimes profound changes in other aspects of their lives, including their ability to maintain a medication habit. Some changes were more predictable, such as a latent alcohol habit that became more prominent a few months into treatment, or employment that was already unstable but ended after starting treatment. Others were less predictable, such as the loss of a foundational relationship (due to TB-related or other reasons) or, most commonly, fluctuations and deteriorations in mental health. Several patients described feeling profoundly anxious and depressed, sometimes suicidal, and some described dramatic changes in their personality or a loss of identity after starting ATT.
I was just so afraid that my heart will get something wrong. And [nurse] said, 'Do you have anxiety'? Now I started to think about it – probably, because of all those kind of issues, I might have some level of anxiety.
Zhen, who described persistent anxiety about his health several months after completing ATT
Changes could occur slowly over the course of illness, for example, a progressive increase in the amount of alcohol consumed or a gradual reduction in mental wellbeing, or could be triggered by specific events or phenomena, such as severe side effects (e.g., leading to a sudden deterioration in function or hospital admission), pain, fatigue (such that someone who was previously able to work had to stop), social or physical isolation, episodes of stigmatisation or rejection, eviction, a loss of income, or setbacks in treatment. For example, one participant experienced worsening fatigue and side effects that meant he could no longer work; unemployment then created financial pressure for his family and led to the re-emergence of a previous alcohol habit, which strained relationships with his wife and children and worsened his previous mild cognitive impairment, culminating in him stopping ATT entirely. Reduced mental wellbeing, in particular, often put a strain on existing relationships, which in turn led to worsening anxiety, isolation, and difficulties maintaining a treatment-taking routine.
When I take the medication, four, five months, my behaviour also is changing… Other people are saying, 'What kind of behaviour are you doing?' …I asked one of the colleagues, 'What happened? I don't know what I am doing.' He told me, ‘Your head is going crazy.'
Gopal, a patient in his 30s who described a progressive loss of identity and confidence over several months of treatment, to where he was reluctant to venture out of the house after dark.
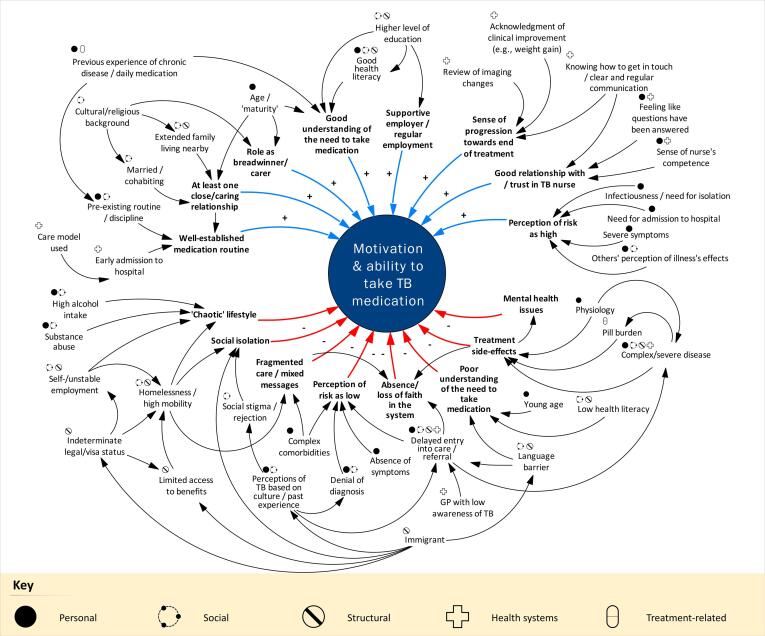
Using data from all individuals interviewed, Fig. 1 illustrates some of the key factors identified and maps their interactions and the mechanisms through which they might affect adherence. Sixteen ‘proximal’ characteristics (eight ‘positive’ [top of the figure] and eight ‘negative’ [bottom of the figure]) were identified that had a more direct effect on an individual’s ability to take treatment: positive characteristics included an ability to create and maintain a medication routine, the existence of at least one caring or close relationship, and regular employment and/or a supportive employer; and negative characteristics included a ‘chaotic’ lifestyle, fragmented care, and severe treatment side effects.
Fig. 1.
Mechanisms* by which personal, social, structural, health systems, and treatment-related factors† influenced individuals’ motivation and ability to take anti-TB treatment, based on interviews with 18 patients and four caregivers. ‘Positive’ influences are depicted in the top half of the figure (blue arrows, ‘+’ notation) and ‘negative’ influences in the bottom half (red arrows,’-‘ notation)*Only key mechanisms have been included for clarity. Additional relationships are undoubtedly present but not represented.†Although for some composite factors both the ‘positive’ and ‘negative’ are described explicitly (e.g., good understanding and poor understanding), the same holds true for most of the elements included in the figure. For example, the absence of a medication routine was likely to make it more difficult to take treatment, and more integrated (i.e., less fragmented) care was likely to make it easier to take treatment. GP: general practitioner; TB: tuberculosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In three UK cities, we conducted in-depth interviews with 22 people who had taken or cared for someone taking ATT. TB and being ‘on treatment’ were often experienced as disruptive: in addition to debilitating symptoms, side effects, and stigma, many participants also had to balance income loss or reduction, unstable housing, social isolation, worsening mental health, and damaged relationships. Those who had a strong support network, stable employment, an adaptable routine, a trusting relationship with their TB team, and clearly understood the need for treatment reported being more easily able to adhere to ATT, whereas those with a ‘chaotic’ lifestyle, who were socially isolated, experienced severe side effects, or faced structural barriers (such as language or recourse to public funds) reported finding it more difficult to adhere.
Based on these data, we frame adherence as a complex, dynamic phenomenon that spans a longer timeframe than conventionally examined. Rather than focusing on static determinants or conceptualising adherence as the act of taking or not taking pills as prescribed, we suggest the adoption of a relational view (Arakelyan et al., in preparation) that sees an individual’s life in the context of its complex social and structural connections; recognises that health-related behaviour is intertwined with other aspects, such as identity, community, and relationships; and allows for the examination of how medicine-taking patterns reflect the temporal and socio-spatial fluctuations inherent to many individuals’ experience of having TB and receiving care. In exploring the mechanisms that may facilitate or impede adherence, we demonstrate the extensive potential for non-adherence, even in people with so-called ordinary lives.
Experiences of social exclusion, the detrimental effects of reduced social support and stigma, and the importance of routine were important themes in our data and were also highlighted by a 2015 study conducted among mostly homeless individuals in London [32]. Though our participants had fewer ‘established’ risk factors for non-adherence than those in the 2015 study, many of the same issues were encountered by people with more ‘ordinary’ lives, suggesting that these factors occur along a spectrum and defy simplistic binary assessment (as does non-adherence itself) [17]. Two studies among African migrants in Sheffield (Somali adults, 2013) and London (African adults, 2015) discussed experiences that were shared by many of our participants, including frustration with the diagnostic process in primary care, difficulties in maintaining relationships while receiving treatment, the positive effect of a supportive social network on adherence, and the toll on mental health over the course of treatment [33], [34]. Similar themes were also identified by a recent critical synthesis of barriers to TB care among migrants, which placed ‘relationships’ at the centre of a theoretical model of experiences relating to adherence, with ‘cumulative vulnerability’ (the compounding of difficulties faced by migrants across multiple systems), ‘acculturation’ (balancing one’s identity with relationships with society), and ‘interpretations of illness’ (individual understanding of the disease and the experience) exerting important effects on those relationships [38].
4.1. Assessment and intervention
In the absence of robust routine data on adherence [39], there remains a need for consistent, widespread use of contextually modifiable, nuanced methods to assess for the likelihood of non-adherence in every person starting treatment for TB, as well as socially and culturally sensitive, sustainable approaches to support them through treatment [40]. The current TB adherence landscape in the UK is encouraging: the need to ensure a “high treatment completion rate” is part of the national TB strategy [41]; most NHS Trusts follow the case management model, where one specialist TB nurse coordinates an individual’s care from diagnosis to discharge [14]; and adherence policy (across conditions) is based loosely around the ‘Perceptions and Practicalities’ (PAPA) framework, which considers individual motivation and ability, as well as a number of intrinsic and extrinsic factors, to specify the ‘minimum ingredients’ of adherence support, targeted to the needs of the patient [42]. Although DOT remains the mainstay of adherence support in most Trusts, VOT [43] is increasingly available, as are medication aids (e.g., dosette boxes) and a range of ‘softer’ measures, such as occasional home visits or additional phone contact. However, our data suggest that despite the emphasis placed by the health system on medicine-taking, for many people it remains only one aspect of being ‘on treatment’ and may not be prioritised by those trying to balance numerous other issues. Some of the major challenges faced by our participants (unstable housing and income, restricted access to common resources, and social exclusion and isolation) are likely to affect a high proportion of those with TB in the UK, given the disproportionate burden of disease in people who are migrants, socially deprived, or both [13]. Although some TB services (e.g., North Central London) have a dedicated ‘social care’ team to help respond to these issues [44], most do not, leading to disparities in the care available to patients in different parts of the country.
The need for including social support in TB care has been discussed for some time, particularly in lower income settings [45], but there is limited evidence available around the use of supportive interventions in higher income settings upon which to base policy. A systematic review of psycho-emotional and socio-economic support interventions for TB (which found an association between the use of these interventions and better treatment outcomes) included only six studies from high income settings published between 1990 and 2015 [46], and in a scoping review we found that 70% of the included TB adherence interventions targeted only one aspect of adherence, most often a personal or health systems component [29]. This is partially reflective of the fragmented disciplinary assumptions about when, why, and how people take medicines, resulting in the view that knowledge, beliefs, and practices around medicine-taking form the crux of adherence (premised on a definition that centres on maintaining behaviour that meets HCP recommendations) [47], [48], and that this is the domain most amenable to intervention. This is not restricted to TB: a meta-analysis of theories underpinning adherence interventions (n = 124 studies across a range of conditions) found that 65% were based around motivation, cognition, or beliefs [49], and others have suggested that interventions that do not consider wider influences on adherence or that target only one aspect of behaviour are likely to be variably effective [50].
In considering approaches to intervention, it may help to conceptualise an individual’s life as a complex system [28], [51], [52], [53], with the person at the centre and their actions framed in the context of the multiple, relationships with their environment and the people around them. This would also allow us to describe the introduction of disease and care into that system in energetic terms: complex systems require energy to maintain their organisation or ‘order’ [54], and an approach that demands the taking of tablets at all costs (i.e., the imposition of ‘rigid order’) [55] will likely require the input of a large amount of energy from the patient, their family, and/or the health system. This is particularly the case in patients whose lives are already chaotic, or who have less robust support structures. A supportive approach that looks more broadly at the ‘system’ (i.e., the patient, their relationships, their environment), harnesses existing strengths, identifies vulnerabilities, and focuses on improving capability, instead of concentrating interventions primarily on the act of medicine-taking, is likely to be more efficient and therefore easier to scale and sustain.
In addition, if we consider the system to be ‘adaptive’ (that each element within the system has the potential to change or evolve based on its interactions), every contact between patient and HCP then has the potential to be an ‘intervention’. Thus the act of risk assessment, if conducted in a way that engages the patient in their own care, could itself reduce the risk of non-adherence by improving the individual’s awareness of their vulnerabilities and enabling collaborative strategies to be developed that can improve treatment-taking ability. Critically, our data suggest that assessments should be repeated at regular intervals throughout treatment to account for changes in circumstance or capability.
It is also important to state that many experienced clinicians, particularly specialist TB nurses, already work in this patient-centred way, though this often requires them to go beyond what the health service expects (or supports) them to do. If the universal standard of care for people with TB is to be truly “sensitive and responsive” [18] to their needs, we cannot rely on the compassion of individual HCPs, but must adopt person-centredness throughout the health system [56].
4.2. Limitations and strengths
This study has limitations. Interviews were conducted in health care facilities by a medical doctor, which may have influenced the information volunteered by participants, though this was not explicitly discussed with participants, the interviewer was not involved in routine care provision, and one other non-clinician was present for most interviews. Despite reassurances of anonymity, participants who were still receiving care may have moderated their criticisms of the health service out of fear of repercussions. Individuals with DR-TB are likely under-represented in our data, though many of the issues we discuss are likely to be relevant to them, given the longer and more arduous treatment regimens. Interviews were conducted in English, and some migrant experiences will not have been captured; however, many of our participants had limited English and several described challenges around language and communication. Qualitative data were not linked to empirical estimates of adherence in any of the patients interviewed and the designation of factors as ‘key determinants’ as well as the relationships between factors depended on self-report by participants and interpretation by authors. Our study also has strengths: participants were recruited from four NHS Trusts in three cities, reflecting some of the variation in care models and resources available in different parts of the country; and participants were not recruited from any particular risk group, and their common experiences may be closer to those of the ‘average’ person treated for TB in the UK.
5. Conclusions
‘Taking tablets’ is only one aspect of treatment for TB. Supportive measures that are truly patient-centred will need to be grounded in a deep, contextual understanding of patients’ experiences of, views towards, and behaviours associated with TB and its care. Improving adherence among all persons treated for TB, as part of efforts towards elimination in low TB incidence countries, will require supportive approaches that consider not only an individual’s beliefs about TB and ATT, but also social and structural circumstances and changes in them over time, and look to improve capability, rather than enforce medicine-taking. Policy and guidelines need to acknowledge the need for regular assessment during treatment if we are to reduce non-adherence to ATT.
Funding
This work was supported by the National Institute for Health Research (NIHR) Health Technology Assessment Programme, UK grant number 16/88/06.
CRediT authorship contribution statement
Aaron S. Karat: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Annie S.K. Jones: Investigation, Writing - review & editing. Ibrahim Abubakar: Conceptualization, Funding acquisition, Writing - review & editing. Colin N.J. Campbell: Conceptualization, Funding acquisition, Writing - review & editing. Amy L. Clarke: Investigation, Writing - review & editing. Caroline S. Clarke: Conceptualization, Funding acquisition, Writing - review & editing. Marcia Darvell: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Adam T. Hill: Conceptualization, Funding acquisition, Writing - review & editing. Robert Horne: Conceptualization, Funding acquisition, Writing - review & editing. Heinke Kunst: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - review & editing. Mike Mandelbaum: Conceptualization, Funding acquisition, Writing - review & editing. Ben G. Marshall: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - review & editing. Ceri McSparron: Investigation, Writing - review & editing. Ananna Rahman: Investigation, Writing - review & editing. Helen R. Stagg: Conceptualization, Funding acquisition, Methodology, Writing - review & editing. Jacqui White: Investigation, Writing - review & editing. Marc C.I. Lipman: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing - review & editing. Karina Kielmann: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
ASKJ, IA, ALC, CSC, ATH, HK, MM, BGM, CM, AR, and JW have no competing interests to declare. ASK reports grants from the National Institute for Health Research (UK), during the conduct of the study; personal fees from The Aurum Institute (South Africa), The Center for Health Policies and Studies (Republic of Moldova), Edanz Group (Japan), Vital Strategies (Singapore), University of Cape Town (South Africa), the Bill & Melinda Gates Foundation (USA), and Bloomberg Philanthropies (USA) outside the submitted work; non-financial support from Kyoto University (Japan) and the Africa Health Research Institute (South Africa) outside the submitted work. CNJC reports personal fees from Public Health England outside the submitted work. HRS reports grants from Medical Research Council (UK) and the National Institute for Health Research (UK) during the conduct of the study; other from Korean CDC and Johnson and Johnson and other from Latvian Society Against Tuberculosis outside the submitted work; and HRS is a core group member of the World Health Organization’s European Tuberculosis Research Initiative and co-chair of UK Academics and Professionals Against Tuberculosis. KK, MCIL, and MD report grants from the National Institute for Health Research (NIHR) during the conduct of the study. RH is supported by the National Institute for Health Research (NIHR, Collaboration for Leadership in Applied Health Research and Care, North Thames at Bart's Health NHS Trust and Asthma UK (AUKCAR). Speaker engagements with honoraria with the following companies: Abbvie, Amgen, Astellas, AstraZeneca, Biogen, Erasmus, Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp Dohme, Novartis, Pfizer, Roche, Shire Pharmaceuticals, and TEVA. RH is founding director of a UCL-Business spin-out company (Spoonful of Sugar Ltd) providing consultancy on treatment engagement and patient support programmes to healthcare policy makers, providers, and industry.
Acknowledgments
Acknowledgments
Our thanks to all participants for giving us their time and sharing their experiences with us. We are also grateful to NHS staff who were instrumental in facilitating interviews: Clare Campbell, Francisca Nwoguh, and Maddy Wickers. Thanks also to the wider IMPACT team (Ahmed Ali, Helen Clegg, Andrew Copas, Sharda Lavingia, Viren Lavingia, Elisha Pickett, Mylah Ramirez, Mairi Redman, Alison Rodger, Alistair Story, Nicole Vidal, Elizabeth Walker, and Fatima Wurie) and the members of the project oversight group (Steve Bradley, Martin Dedicoat, Amy McConville, Anita Roche, and Alice Sitch) for their many inputs.
Ethical statement
This study received approval from the Camberwell St Giles Ethics Committee (REC reference 18/LO/1818). Written informed consent was obtained from each individual prior to data collection. Pseudonyms are used to refer to participants; care has been taken to disclose no information that could reveal participants’ identities.
Disclaimer
The views expressed are those of the authors and not necessarily those of the National Health Service, UK, the National Institute for Health Research, or the Department of Health and Social Care.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2021.100233.
Contributor Information
Aaron S. Karat, Email: akarat@qmu.ac.uk.
Karina Kielmann, Email: kkielmann@qmu.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. The Global Health Observatory: United Kingdom of Great Britain and Northern Ireland. 2021. https://www.who.int/data/gho/data/countries/country-details/GHO/united-kingdom-of-great-britain-and-northern-ireland?countryProfileId=9ecfbce7-f44a-4660-97d6-44b6a5d54887 (accessed 2021 Jan 7).
- 2.World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care: 2017 update. 2017. https://www.who.int/tb/publications/2017/dstb_guidance_2017/en/ (accessed 2020 May 28).
- 3.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. 2019. https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ (accessed 2020 Jun 4). [PubMed]
- 4.Alipanah N, Jarlsberg L, Miller C, et al. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. Murray M, editor. PLOS Med. 2018;15(7):e1002595. [DOI] [PMC free article] [PubMed]
- 5.Pradipta I.S., Forsman L.D., Bruchfeld J., Hak E., Alffenaar J.-W. Risk factors of multidrug-resistant tuberculosis: A global systematic review and meta-analysis. J Infect. 2018;77(6):469–478. doi: 10.1016/j.jinf.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global tuberculosis report 2019. 2019. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed 2019 Dec 1).
- 7.World Health Organization. Guiding principles to reduce tuberculosis transmission in the WHO European region. 2018. http://www.euro.who.int/__data/assets/pdf_file/0008/377954/ic-principles-eng.pdf?ua=1 (accessed 2020 Jun 6).
- 8.World Health Organization. Towards tuberculosis elimination: an action framework for low-incidence countries. 2015. https://apps.who.int/iris/bitstream/handle/10665/132231/9789241507707_eng;jsessionid=6446646661CD15F1B2E4F8A09F4F59CD?sequence=1 (accessed 2020 Jun 6).
- 9.Adam B.U., Cosford P., Anderson S.R., Abubakar I. Sustaining tuberculosis decline in the UK. The Lancet. 2017;389(10075):1176–1177. doi: 10.1016/S0140-6736(17)30755-9. [DOI] [PubMed] [Google Scholar]
- 10.Story A., Murad S., Roberts W., Verheyen M., Hayward A.C. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. 2007;62(8):667–671. doi: 10.1136/thx.2006.065409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England. Public Health Outcomes Framework: Health Equity Report. Focus on ethnicity. 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733093/PHOF_Health_Equity_Report.pdf (accessed 2020 May 28).
- 12.Public Health England. Health profile for England 2019. 2019. https://www.gov.uk/government/publications/health-profile-for-england-2019 (accessed 2020 May 28).
- 13.Public Health England. Tuberculosis in England: 2020 report. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/934954/TB_Annual_Report_2020.pdf (accessed 2020 Dec 1).
- 14.Royal College of Nursing. A Case Management Tool for TB Prevention, Care and Control in the UK. 2019. https://www.rcn.org.uk/professional-development/publications/pub-006194 (accessed 2020 Dec 1).
- 15.Anderson C., Anderson S.R., Maguire H., Hayward A.C., Story A. Tuberculosis in London: the convergence of clinical and social complexity. Eur Respir J. 2016;48(4):1233–1236. doi: 10.1183/13993003.00749-2016. [DOI] [PubMed] [Google Scholar]
- 16.Nellums L.B., Rustage K., Hargreaves S., Friedland J.S. Multidrug-resistant tuberculosis treatment adherence in migrants: a systematic review and meta-analysis. BMC Med. 2018;16(1) doi: 10.1186/s12916-017-1001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagg HR, Flook M, Martinecz A. All non-adherence is equal, but is some more equal than others? Adherence to treatment for tuberculosis disease in the digital era. ERJ Open Res. 2020 Nov;26(4):00315–02020. doi: 10.1183/23120541.00315-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. The End TB strategy: Global strategy and targets for tuberculosis prevention, care and control after 2015. 2015. http://www.who.int/tb/post2015_strategy/en/ (accessed 2016 May 5).
- 19.Jaramillo J., Yadav R., Herrera R. Why every word counts: towards patient- and people-centered tuberculosis care. Int J Tuberc Lung Dis. 2019;23(5):547–551. doi: 10.5588/ijtld.18.0490. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO Regional Office for Europe: A people-centred model of TB care. Blueprint for EECA countries. https://www.euro.who.int/__data/assets/pdf_file/0004/342373/TB_Content_WHO_PRO_eng_final.pdf (accessed 20Sep 15).
- 21.O'Donnell M.R., Daftary A., Frick M., Hirsch-Moverman Y., Amico K.R., Senthilingam M., Wolf A., Metcalfe J.Z., Isaakidis P., Davis J.L., Zelnick J.R., Brust J.C.M., Naidu N., Garretson M., Bangsberg D.R., Padayatchi N., Friedland G. Re-inventing adherence: toward a patient-centered model of care for drug-resistant tuberculosis and HIV. Int J Tuberc Lung Dis. 2016;20(4):430–434. doi: 10.5588/ijtld.15.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan M, Walley J, Witter S, Shah S, Javeed S. Tuberculosis patient adherence to direct observation: results of a social study in Pakistan. Health Policy Plan. 2005;20(6):354–65. [DOI] [PubMed]
- 23.Sagbakken M, Bjune GA, Frich JC. Humiliation or care? A qualitative study of patients’ and health professionals’ experiences with tuberculosis treatment in Norway: humiliation or care? Scand J Caring Sci. 2012;26(2):313–23. [DOI] [PubMed]
- 24.Fiseha D., Demissie M. Assessment of Directly Observed Therapy (DOT) following tuberculosis regimen change in Addis Ababa, Ethiopia: a qualitative study. BMC Infect Dis. 2015;15(1) doi: 10.1186/s12879-015-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bojorquez I., Salazar I., Garfein R.S., Cerecer P., Rodwell T.C. Surveillance or support: The experience of direct observation during tuberculosis treatment. Global Public Health. 2018;13(7):804–818. doi: 10.1080/17441692.2016.1240823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. Rylko-Bauer B, editor. PLoS Med. 2007;4(7):e238. [DOI] [PMC free article] [PubMed]
- 27.Stagg H.R., Lewis J.J., Liu X., Huan S., Jiang S., Chin D.P., Fielding K.L. Temporal factors and missed doses of tuberculosis treatment. A causal associations approach to analyses of digital adherence data. Annals ATS. 2020;17(4):438–449. doi: 10.1513/AnnalsATS.201905-394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zekovic M., Krajnovic D., Marinkovic V., Tasic L. The complexity of adherence: a review of its scope and determinants. Acta Medica Median. 2016;55(1):51–58. [Google Scholar]
- 29.Kielmann K, Vidal N, Karat AS, Stagg HR, Lipman M. Supporting adherence to treatment for tuberculosis (TB): a relational view. In: The 50th Union World Conference on Lung Health (30 Oct-02 Nov 2019; Hyderabad, India) Abstract PS-21-731-01. 2019.
- 30.Pujol-Cruells A., Vilaplana C. Specific interventions for implementing a patient-centered approach to TB care in low-incidence cities. Front Med. 2019;6:273. doi: 10.3389/fmed.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig G.M., Joly L.M., Zumla A. ‘Complex’ but coping: experience of symptoms of tuberculosis and health care seeking behaviours – a qualitative interview study of urban risk groups, London, UK. BMC Public Health. 2014;14(1):618. doi: 10.1186/1471-2458-14-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig G.M., Zumla A. The social context of tuberculosis treatment in urban risk groups in the United Kingdom: a qualitative interview study. Int J Infectious Dis. 2015;32:105–110. doi: 10.1016/j.ijid.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Gerrish K., Naisby A., Ismail M. Experiences of the diagnosis and management of tuberculosis: a focused ethnography of Somali patients and healthcare professionals in the UK. J Adv Nurs. 2013;69(10):2285–2294. doi: 10.1111/jan.12112. [DOI] [PubMed] [Google Scholar]
- 34.Nnoaham K.E., Pool R., Bothamley G., Grant A.D. Perceptions and experiences of tuberculosis among African patients attending a tuberculosis clinic in London. Int J Tuberc Lung Dis. 2006;10(9):1013–1017. [PubMed] [Google Scholar]
- 35.Stagg H.R., Abubakar I., Campbell C.N. IMPACT study on intervening with a manualised package to achieve treatment adherence in people with tuberculosis: protocol paper for a mixed- methods study, including a pilot randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-032760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Health Protection Scotland. Enhanced Surveillance of Mycobacterial Infections in Scotland: 2019 Tuberculosis Annual Report for Scotland. 2019. https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2887/documents/1_tb-annual-report-2019.pdf (accessed 2020 May 28).
- 37.Glaser B.G. The constant comparative method of qualitative analysis. Soc Probl. 1965;12(4):436–445. [Google Scholar]
- 38.Lin S., Melendez-Torres G.J. Critical interpretive synthesis of barriers and facilitators to TB treatment in immigrant populations. Trop Med Int Health. 2017;22(10):1206–1222. doi: 10.1111/tmi.12938. [DOI] [PubMed] [Google Scholar]
- 39.Vernon A, Fielding K, Savic R, Dodd L, Nahid P. The importance of adherence in tuberculosis treatment clinical trials and its relevance in explanatory and pragmatic trials. PLOS Med. 2019;16(12):e1002884. [DOI] [PMC free article] [PubMed]
- 40.Garner P. Promoting adherence to tuberculosis treatment. Bull World Health Organ. 2007;85(5):404–406. doi: 10.2471/BLT.06.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Public Health England. Collaborative Tuberculosis Strategy for England 2015 to 2020. 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/403231/Collaborative_TB_Strategy_for_England_2015_2020_.pdf (accessed 2020 Apr 15).
- 42.Horne R., Cooper V., Wileman V., Chan A. Supporting adherence to medicines for long-term conditions: a perceptions and practicalities approach based on an extended common-sense model. Eur Psychol. 2019;24(1):82–96. [Google Scholar]
- 43.Story A., Aldridge R.W., Smith C.M., Garber E., Hall J., Ferenando G., Possas L., Hemming S., Wurie F., Luchenski S., Abubakar I., McHugh T.D., White P.J., Watson J.M., Lipman M., Garfein R., Hayward A.C. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019;393(10177):1216–1224. doi: 10.1016/S0140-6736(18)32993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izzard A., Wilders S., Smith C. Improved treatment completion for tuberculosis patients: the case for a dedicated social care team. J Infect. 2020;82(3):e1–e3. doi: 10.1016/j.jinf.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Farmer P., Robin S., Ramilus S.L., Kim J.Y. Tuberculosis, poverty, and ‘compliance’: lessons from rural Haiti. Semin Respir Infect. 1991;6(4):254–260. [PubMed] [Google Scholar]
- 46.van Hoorn R, Jaramillo E, Collins D, Gebhard A, van den Hof S. The effects of psycho-emotional and socio-economic support for tuberculosis patients on treatment adherence and treatment outcomes – a systematic review and meta-analysis. PLOS ONE. 2016;11(4):e0154095. [DOI] [PMC free article] [PubMed]
- 47.Stagg H.R., Lewis J.J., Liu X. How do tuberculosis patients really take their treatment? a detailed quantitative approach. [Abstract PS04-443-25] Int J Tuberc Lung Dis. 2018;22(11) S171. [Google Scholar]
- 48.World Health Organization. Adherence to long-term therapies: evidence for action. 2003. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (accessed 2020 May 29).
- 49.Conn V.S., Enriquez M., Ruppar T.M., Chan K.C. Meta-analyses of theory use in medication adherence intervention research. Am J Hlth Behav. 2016;40(2):155–171. doi: 10.5993/AJHB.40.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munro S., Lewin S., Swart T., Volmink J. A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health. 2007;7(1) doi: 10.1186/1471-2458-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaffee M.W., McNeill M.M. A model of nursing as a complex adaptive system. Nurs Outlook. 2007;55(5):232–241.e3. doi: 10.1016/j.outlook.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Lauffenburger J.C., Choudhry N.K. A call for a systems-thinking approach to medication adherence: stop blaming the patient. JAMA Intern Med. 2018;178(7):950. doi: 10.1001/jamainternmed.2018.0790. [DOI] [PubMed] [Google Scholar]
- 53.Berben L., Dobbels F., Engberg S., Hill M.N., De Geest S. An ecological perspective on medication adherence. West J Nurs Res. 2012;34(5):635–653. doi: 10.1177/0193945911434518. [DOI] [PubMed] [Google Scholar]
- 54.The Health Foundation. Complex adaptive systems. 2010. https://www.health.org.uk/sites/default/files/ComplexAdaptiveSystems.pdf (accessed 2020 Sep 18).
- 55.Rihani S. Complex systems theory and development practice. Understanding Non-Linear Realities. Zed Books; London: 2002. The whole case in a nutshell; pp. 6–14. [Google Scholar]
- 56.Sheikh K., Ranson M.K., Gilson L. Explorations on people centredness in health systems. Health Policy and Planning. 2014;29(suppl 2):ii1–ii5. doi: 10.1093/heapol/czu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.