Abstract
Lipid miscibility phase separation has long been considered to be a central element of cell membrane organization. More recently, protein condensation phase transitions, into three-dimensional droplets or in two-dimensional lattices on membrane surfaces, have emerged as another important organizational principle within cells. Here, we reconstitute the linker for activation of T cells (LAT):growth-factor-receptor-bound protein 2 (Grb2):son of sevenless (SOS) protein condensation on the surface of giant unilamellar vesicles capable of undergoing lipid phase separations. Our results indicate that the assembly of the protein condensate on the membrane surface can drive lipid phase separation. This phase transition occurs isothermally and is governed by tyrosine phosphorylation on LAT. Furthermore, we observe that the induced lipid phase separation drives localization of the SOS substrate, K-Ras, into the LAT:Grb2:SOS protein condensate.
Significance
Protein condensation phase transitions are emerging as important organizing principles in cells. One such condensate plays a key role in T cell receptor signaling. Immediately after receptor activation, multivalent phosphorylation of the adaptor protein linker for activation of T cells (LAT) at the plasma membrane leads to a networked assembly of a number of signaling proteins into a two-dimensional condensate on the membrane surface. In this study, we demonstrate that LAT condensates in reconstituted vesicles are sufficient to drive lipid phase separation. This lipid reorganization drives another key downstream signaling molecule, Ras, into the LAT condensates. These results show that the LAT condensation phase transition, which is actively controlled by phosphorylation reactions, extends its influence to control lipid phase separation in the underlying membrane.
Introduction
In 1973, shortly after the classic fluid mosaic description of cell membranes was published (1), a series of articles from Harden McConnell’s lab described discovery of lateral phase separation in the lipids of cell membranes (2, 3, 4, 5, 6). Contemporary work from Sackmann and colleagues confirmed an intriguing heterogeneity in the organization of lipids in the fluid membrane (7). This phenomenon later developed into the lipid raft model of cell membranes, as articulated by Simons and Ikonen (8, 9, 10). The field of lipid rafts has since both flourished and attracted great controversy (11, 12, 13, 14). Although lipid miscibility phase transitions are readily and spectacularly visualized in purified lipid membranes (Fig. 1 A; (15, 16, 17)), their unambiguous detection in living cells proved much more challenging (18, 19, 20, 21). Reports of definitive observation are sparse (22), suggesting more complex behavior may prevail in most circumstances. There is evidence that cell membranes are poised near a miscibility phase transition (23), which naturally leads one to speculate that this may be actively controlled by the cell. However, a longstanding criticism of the lipid raft model questions how lipid phase separation could be controlled with the specificity required for biological functions, whereas the underlying interactions between lipids and cholesterol that enable the phase transition are rather nonspecific (24). Clearly proteins must play a commanding role controlling lipid phase separation in the physiological setting, but we have very limited mechanistic understanding of how this is actually achieved in specific cases (25).
Figure 1.
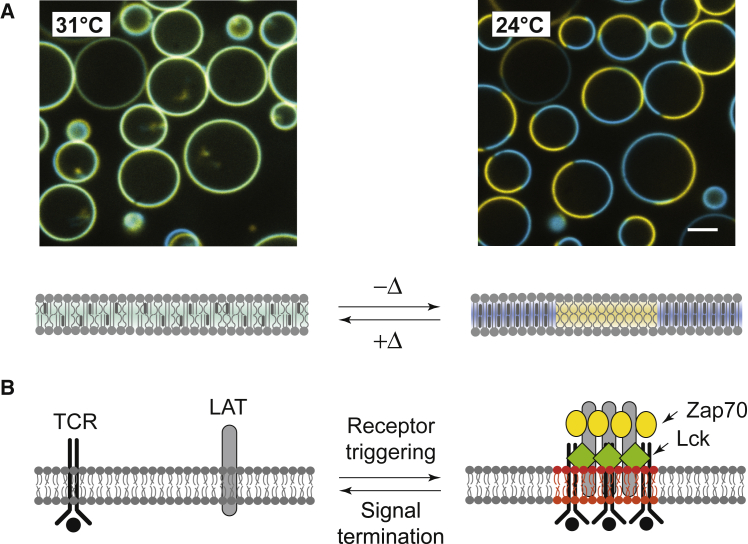
(A) Representative giant unilamellar vesicles (GUVs) showing temperature-dependent liquid-liquid phase separation. At 31°C, which is above the transition temperature Tmisc of 29°C, the distribution of lipids is homogeneous across the membrane for 100% of the sample of ∼100 vesicles. Below Tmisc at 24°C (right), lipids compartmentalize into macroscopic domains for 99% of the sample of ∼100 vesicles: the Ld domain (TR-DHPE; yellow) is enriched with unsaturated lipids, and the Lo with saturated lipids (OG-DHPE; blue). All GUV experiments are performed in buffer with matching osmolarity (50 mM Tris and 150 mM NaCl (pH 7.4)). Typical vesicle concentrations were ∼0.2 mg/mL. Scale bars, 5 μm. The ensemble average temperature-dependent phase separation (n ∼100 vesicles) is shown in Fig. 4 (right panel, empty circles). (B) In lipid raft theory, clusters of signaling proteins, such as the TCRs, are “carried” on ordered lipid domains to facilitate signal transduction.
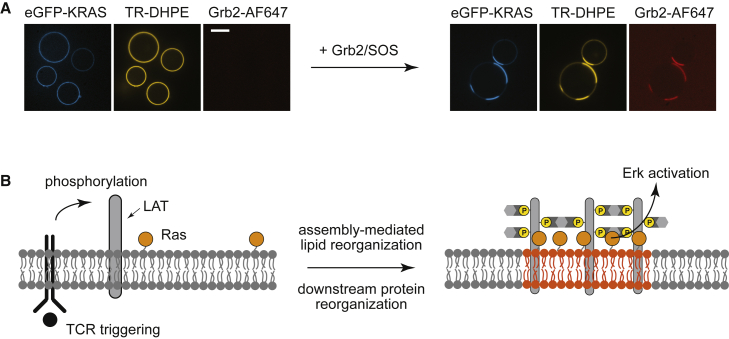
One prominent example that captures this debate is the T cell receptor (TCR) signaling system (Fig. 1 B). TCR and a number of downstream proteins, including linker for activation of T cells (LAT), phospholipase C γ 1, and the Ras activator son of sevenless (SOS), form clusters on the membrane (26, 27, 28, 29, 30, 31, 32). Earlier studies using detergent-resistant membrane extraction have suggested that these molecules reside on lipid rafts (33, 34, 35). However, subsequent studies have failed to conclusively establish lipid rafts as the driving force for TCR-induced signaling clusters (36, 37, 38). Furthermore, it remains unclear how signaling activity—in the case of TCR, the receptor activation and tyrosine phosphorylation of downstream proteins including LAT—could trigger the lipid phase separation. This disconnect is further underscored by the fact that at physiological ligand densities (39), individual TCR are capable of triggering the entire signaling pathway without ever forming clusters themselves (40, 41, 42, 43, 44).
Modular binding interactions among proteins present another type of mediated molecular assembly process in cells (45,46). With sufficient multivalency, these interactions can lead to protein condensation phase transitions into three-dimensional droplets (47), sometimes called membraneless organelles, or two-dimensional assemblies on the membrane surface (48, 49, 50, 51). Similar biomolecular condensates can also incorporate nucleic acids and play a role in transcription regulation (52,53).
It has recently been discovered that LAT can participate in a protein condensation phase transition in reconstituted membranes (48,49,54,55). LAT is a transmembrane scaffold protein that becomes phosphorylated at multiple tyrosines upon TCR activation. Three of the phosphotyrosines on LAT are canonical docking sites for the SH2 domain of growth-factor-receptor-bound protein 2 (Grb2), a cytosolic adaptor protein (56). Grb2 additionally has SH3 domains, which bind to the proline-rich domain of SOS, a guanine nucleotide exchange factor that activates Ras (57). A single SOS can associate with at least two Grb2 molecules, and these multivalent interactions result in an extended two-dimensional network assembly of LAT:Grb2:SOS on the membrane in a phosphorylation-dependent manner (58,59). This complex has been shown to play an important role in T cell signaling (60,61). The LAT:Grb2:SOS protein condensation phase transition is reversible, and because it is governed by tyrosine phosphorylation, it is directly under the control of competing kinase and phosphatase reactions in the TCR signaling system.
Here, we reconstitute the LAT:Grb2:SOS protein condensate from purified proteins on giant unilamellar vesicle (GUV) membranes that can undergo lipid phase separation. The LAT condensation induces lipid phase separation in GUVs, even above the miscibility temperature of the lipid composition. We additionally observe that protein condensation-induced lipid phase separation further directs spatial organization of the downstream membrane-bound molecule, K-Ras. These results illustrate how a protein condensation phase transition, which is directly under control of a specific signaling system, can drive miscibility phase transitions in the underlying lipid membrane.
Results
LAT:Grb2:SOS condensation on vesicles drives lipid phase separation
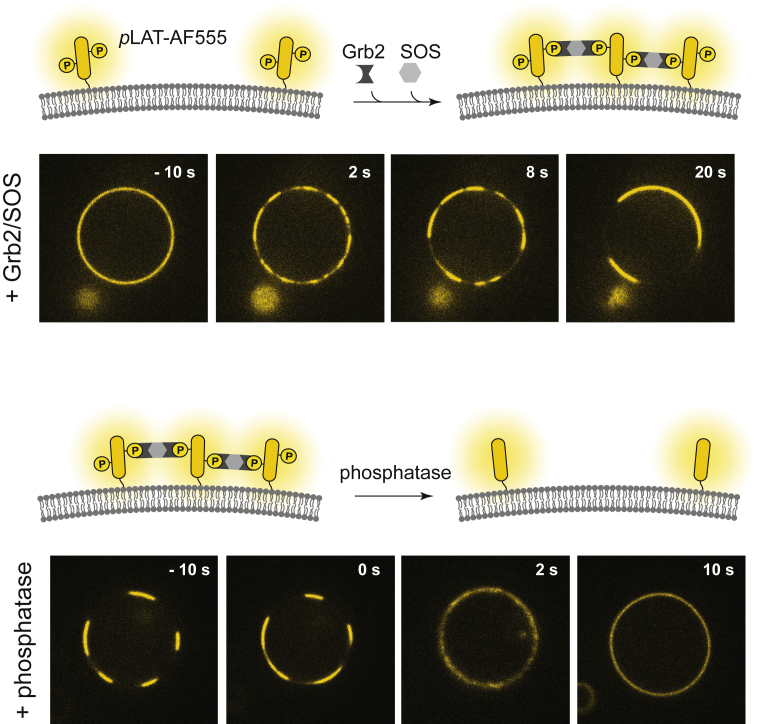
Phosphotyrosine-mediated LAT:Grb2:SOS condensates were reconstituted in GUVs with lipid composition that can undergo lipid phase separation. The cytoplasmic domain of LAT was purified with an N-terminal His6 tag and labeled with Alexa Fluor 555 (AF555) at Cys146 via maleimide-thiol chemistry. LAT was phosphorylated by the kinase domain of Hck in solution. Then, phosphorylated LAT (pLAT) was linked to the membrane by the binding of the His6 tag to the Ni-nitrilotriacetic-acid lipids in the membrane. This membrane-linked pLAT exhibits free lateral diffusion and remains monomeric before any assembly (48,49). The addition of full-length Grb2 and the proline-rich domains of SOS leads to the networked condensation of LAT:Grb2:SOS on the membrane surface of GUVs, as shown in Fig. 2 (top row). Here, the condensates are visualized as concentrated regions of pLAT-AF555 fluorescence on GUVs by confocal microscopy. This condensate is mediated by tyrosine phosphorylation on LAT and is reversible (Fig. 2, bottom row). The rapid (<10 s) dispersion of the condensed structure upon phosphatase (YopH) addition indicates that the individual Grb2:pLAT bonds must be highly dynamic and offer little protection from solution phosphatases. Incidentally, the membrane-linked phosphatase CD45 has been reported to be excluded from LAT condensates, possibly providing some degree of positive feedback with respect to this phosphatase (48). These basic features of LAT condensates on GUVs are similar to LAT condensates on supported membranes (48,49,55); detailed characterizations on supported membranes show that LAT condensates form simultaneously across the membrane and coalesce into larger domains over time. The faster diffusivity in GUVs led to larger domains that simplify the comparison with the lipid organization.
Figure 2.
The LAT:Grb2:SOS protein condensate was reconstituted on GUVs. His-tagged pLAT is associated with the vesicles by chelating to Ni-nitrilotriacetic-acid lipids. The introduction of a 1.2-μM, full-length Grb2 and 0.8 μM proline-rich domain of SOS results in extended networks of LAT condensate that is visualized by the AF555 fluorescence in the confocal microscopy (top). The LAT:Grb2:SOS assembly can be reversed by dephosphorylation of LAT by phosphatase (5 μM YopH) (bottom). Under the experimental conditions used, LAT showed condensation on most (>95%) of the vesicles and reversal by phosphatase (two independent experiments, n ∼50 vesicles). Scale bars, 5 μm. To see this figure in color, go online.
We next examined how the lipid phase transition behavior of GUVs is perturbed by the LAT condensate. GUVs that are composed of a ternary mixture of saturated lipids, unsaturated lipids, and sterols (in a roughly 1:1:1 ratio) exhibit temperature-dependent miscibility phase separation. Below the miscibility transition temperature (Tmisc), the vesicles separate into coexisting liquid-ordered (Lo) and liquid-disordered (Ld) regions (16,62). As a crude guideline, the Lo region is rich in saturated phosphatidylcholine lipids such as dipalmitoylphosphatidylcholine (DPPC), whereas Ld is rich in unsaturated phospholipids such as dioleoylphosphatidylcholine (DOPC) (63). For our experiments, GUVs composed of 29.2% DOPC, 33.2% DPPC, 33.3% cholesterol, 4% Ni-DOGS, 0.1% Texas-Red (TR)-N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (DHPE), and 0.2% Oregon-Green (OG)-DHPE were used. This composition is an approximation of the well-characterized equimolar mixture of DOPC, DPPC, and cholesterol, and the observed Tmisc is also close to the reported value, 29°C (62). Even though it is not critical into which lipid phase LAT partitions in our experiments, the full-length protein has been shown to partition into clusters without lipid raft makers (glycosylphosphatidylinositol (GPI) anchors) in live cells (38)—suggesting that LAT does not partition into the Lo-like phase in cells. In our experiments, because the Ni-DOGS chain is unsaturated (18:1-18:1), Ni-chelated pLAT is expected to partition into the Ld region. This is confirmed by its colocalization with TR-DHPE, which is a well-established reporter of the Ld phase (16). On the other hand, TR and OG fluorescence exclude each other upon phase separation, indicating that OG-DHPE partitions into the Lo phase (Fig. 1 A).
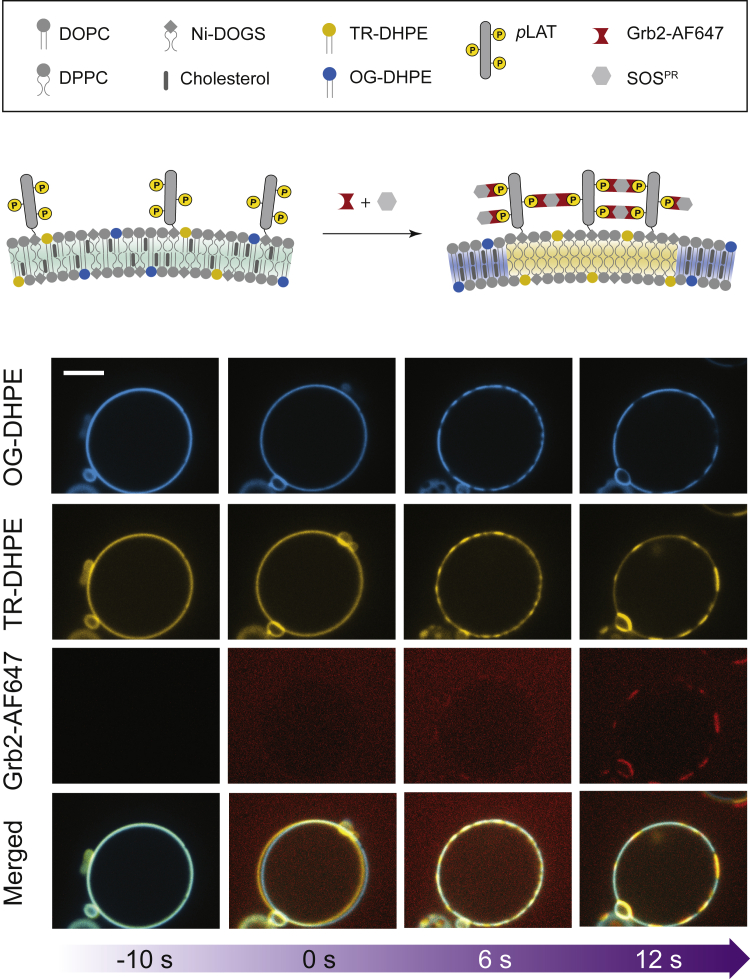
First, we examined whether LAT condensation could induce phase transitions in initially uniform vesicles near the Tmisc. The experiment is shown in Fig. 3. In the imaging chamber maintained at 31°C, the pLAT-associated vesicle membranes exhibit a homogeneous distribution of fluorescent markers (TR-DHPE and OG-DHPE), as expected because this temperature is slightly above the Tmisc of 29°C. The addition of Grb2-AF647 and SOS triggers a rapid LAT:Grb2:SOS condensation on the membrane surface, which is readily visualized by the appearance of concentrated regions of 647-nm fluorescence, tracking Grb2. This is accompanied by a clear partitioning of TR-DHPE (yellow) and OG-DHPE (blue), indicating a miscibility phase transition within the lipids has also occurred, although under isothermal conditions here. The LAT condensate is coincident with the Lo region marked by TR-DHPE, whereas the Ld region, visualized by OG-DHPE, is excluded from LAT.
Figure 3.
An example of a video showing the LAT:Grb2:SOS condensate-induced lipid phase separation on GUVs. Starting with a temperature (31°C) above its Tmisc (29°C), the lipids are initially spatially homogeneous. As the proteins assembled (visualized by doping unlabeled Grb2 with 1% Grb2-AF647), the lipids undergo liquid-liquid phase transition. OG-DHPE and TR-DHPE mark the Lo and Ld regions, respectively. All vesicles that were capable of phase separation transitioned within 30 s. The ensemble average data for the temperature-dependent phase separation (n ∼100 vesicles) are shown in Fig. 4 (right panel, red circles). Scale bars, 5 μm. To see this figure in color, go online.
LAT condensation increases the apparent miscibility temperature of lipid phase separation
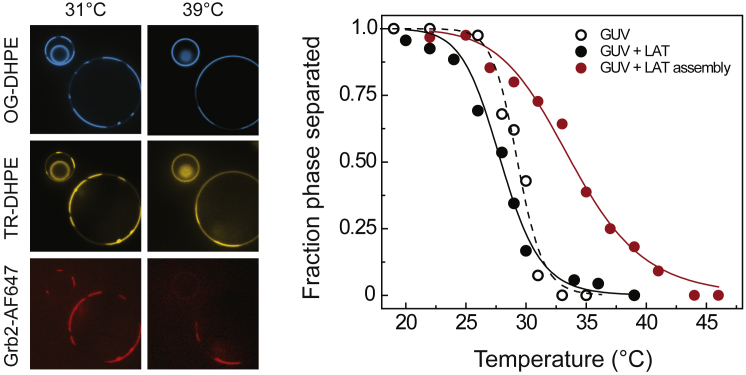
The presence of LAT condensation alters the temperature-dependent phase separation behavior of the vesicles in equilibrium (Fig. 4). In this experiment, the temperature of the imaging chamber was increased gradually at a rate of ∼1°C/min and held at each temperature data point for 2 min. Then, multichannel confocal images of a population (n ∼ 100) of vesicles were obtained. The chamber was cooled back to 20°C at the same rate. The number of phase-separated vesicles were counted at each temperature point. All observations were the same regardless of the direction of the temperature ramp, indicating that all processes, including protein assembly and lipid phase separation, are reversible. Bare GUVs (empty black circles) show a Tmisc of 29°C. With pLAT associated with the vesicles (solid black circles), Tmisc is shifted slightly but remains essentially the same at 28°C. When the LAT condensate is formed by the addition of Grb2 and SOS, however, the apparent Tmisc is increased to 34°C (red solid circles). This is consistent with the previous experiment in which the lipid phase separation is driven by the protein condensate at a temperature at which it would otherwise be homogeneous.
Figure 4.
(Left) At 31°C, the GUVs associated with LAT:Grb2:SOS clusters are phase separated. Note that the smallest vesicle, invaginated within a larger vesicle, is inaccessible to the proteins and remains homogeneous. At 39°C, Grb2 has dissociated from the smaller vesicle, which became homogeneous. For the larger vesicle on which the protein condensate remains, the lipid of the phase separation also remains. Scale bars, 2 μm. (Right) The miscibility transition temperatures (Tmiscs) were measured for bare GUVs (Tmisc = 29.3 ± 0.5°C), GUVs with LAT (Tmisc = 27.8 ± 0.4°C), and GUVs with the LAT condensate (Tmisc = 33.9 ± 0.5°C) by counting the fraction of phase-separated vesicles as a function of temperature then fitting them to the logistic function. Although the difference in Tmisc between bare GUVs and LAT-associated GUVs are minimal, it is increased significantly in the presence of the protein assembly. The data primarily reflect temperature-dependent LAT:Grb2:SOS interactions rather than GUV phase separation because the protein assembly becomes unstable at high temperatures and dissociates from the vesicles. However, hypothetically, stable LAT:Grb2:SOS interactions would further increase the apparent Tmisc. The GUV counts are shown in Table S1. To see this figure in color, go online.
The apparent ΔTmisc of 5°C in the presence of the protein condensate is not actually a shift in the lipid Tmisc. Rather, the protein condensate itself becomes unstable at higher temperatures, and Grb2 and SOS are released from the vesicle surfaces. This can be seen in Fig. 4, bottom right: at 39°C, the Grb2-AF647 fluorescence is not redistributed on the membranes but rather reduced overall because it was lost to the solution. The fluorescence signal is recovered when the temperature is lowered, indicating that the protein condensation is also a reversible, temperature-dependent process. As long as the protein condensate is present, vesicles remained phase separated with the Ld region templating the protein condensate (Fig. 4). This suggests that the actual ΔTmisc is greater than the apparent value of 5°C and probably lies outside the experimentally accessible temperature range in which both the GUV phase separation and LAT:Grb2:SOS condensate can be observed.
LAT-condensation-induced lipid phase separation drives K-Ras into the condensates
The ability for condensates to induce lipid phase separation raises a potential mechanism to spatially reorganize downstream membrane-bound proteins. K-Ras is a small GTPase and a substrate of SOS, and an SOS-catalyzed nucleotide exchange from its GDP-bound state to its GTP-bound state triggers downstream signal activation. The various Ras isoforms serve as hubs for signaling pathways, such as phosphoinositide 3-kinases (PI3K) and mitogen-activated protein kinase (MAPK), and Ras misregulation is among the most common causes of cancer (64,65). Native K-Ras is localized to the membrane by a farnesyl lipid modification as well as electrostatic interactions between its positively charged region and anionic phospholipids in cellular membranes (66). Therefore, the organization of lipids is expected to play an important role in determining the location of K-Ras. Previous studies have shown that K-Ras partitions to the Ld region on GUVs largely because of the highly branched farnesyl anchor (67). Therefore, we anticipated that K-Ras may similarly be directed by the lipid phase separation induced by the LAT:Grb2:SOS condensate. To examine this, enhanced green fluorescent protein (eGFP)-labeled full-length K-Ras with its native membrane anchor, including both the farnesylation and methylation of the terminal cysteine (68,69) (20 nM final concentration), was introduced into GUVs of similar composition as in the previous experiments but with 10% anionic 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS) lipids (final composition: 19.3% DOPC, 33.3% DPPC, 33.3% cholesterol, 10% DOPS, 4% Ni-DOGS, and 0.1% TR-DHPE). The negatively charged lipids are necessary for the stable association of K-Ras to the membrane (69, 70, 71). The bottom panel of Fig. 5 shows that before the introduction of Grb2 and SOS, eGFP-K-Ras (blue) as well as TR-DHPE (yellow) are initially distributed homogeneously on the vesicles. After Grb2 (red) and SOS are added, the lipid membrane becomes phase separated as the protein assemblies form on its surface. K-Ras, LAT:Grb2:SOS, and TR are observed to partition together in the Ld region. The enrichment of K-Ras within the condensates was ∼5-fold compared with molecules outside of the condensates (the apparent partition coefficient K = ∼ 4.7 ± 0.4, where ± denotes SEM). Coupling of the lipid miscibility phase separation to the LAT:Grb2:SOS protein condensation localizes K-Ras with the condensate. Because K-Ras does not colocalize with the protein condensate on supported lipid bilayers that are incapable of phase transitions (Fig. S1), its partitioning on GUVs is likely to be driven by its anchor participating in the lipid phase transition. This phase transition and subsequent protein colocalization between SOS and K-Ras occurs isothermally and under the control of tyrosine phosphorylation reactions.
Figure 5.
(A) The LAT:Grb2:SOS condensate on GUVs results in the segregation of K-Ras into the Ld region with the condensate, suggesting that spatial organization mediated by protein assemblies can propagate downstream of the signaling pathway via lipids. K-Ras templated the Ld region marked by TR on >95% of the vesicles (two independent experiments, n ∼ 30 vesicles) with the apparent partition coefficient (where ± denotes SEM). The temperature was 22°C, at which all of the vesicles were phase separated. Scale bars, 5 μm. (B) This lipid phase separation induced by protein organization may underlie lipid rafts seen in TCR clusters. To see this figure in color, go online.
Discussion
In summary, we have reconstituted the T cell signaling condensate, LAT:Grb2:SOS, on vesicles capable of undergoing liquid-liquid miscibility phase transitions. We observed that the formation of protein condensate can drive the lipid phase transition under isothermal conditions, redistributing lipids in a signal-dependent manner. Furthermore, we have shown that K-Ras, which does not directly participate in the LAT:Grb2:SOS condensation, nonetheless colocalizes with the condensate through its sensitivity to the lipid environment. Lipid phase separation can also be induced by actin polymerization (72,73) and lipid cross-linking by cholera toxin (74). Unique to the observations reported here, however, is that the LAT:Grb2:SOS protein condensation occurs immediately downstream of TCR activation, and as a direct result of ZAP70 kinase activation on triggered TCRs (28,75). ZAP70 is an Syk family kinase that exhibits a distinctive substrate specificity that is orthogonal to that of other kinases in the TCR signaling system, and strongly favors phosphorylation of the specific tyrosine residues on LAT that are involved in the LAT condensate (76). In this way, the LAT condensation phase transition is selectively controlled by TCR signaling.
The native LAT protein has been reported to exhibit a similar preference for the Ld lipid phase as the lipid-linked LAT in our experiments (38). However, in light of the significant number of other membrane-associated and transmembrane proteins in the cellular context of the LAT signaling condensate (59), we would refrain from extrapolating these results to predict specific details of the lipid phase associated with LAT in the natural physiological setting. The important point is that LAT condensation perturbs the underlying lipids and is capable of inducing lipid phase separation now under the control of TCR signaling (Fig. 5). The LAT:Grb2:SOS protein condensate is not unique. Other two-dimensional condensates have been discovered with their own signaling specificities (50,51), and more are likely to emerge (e.g., with EGFR, which shares multivalent Grb2 and SOS interactions much like LAT). Such protein condensates on the membrane may play a broad role directly connecting receptor signaling activity with membrane lipid phase structure.
From a more physical perspective, a distinctive feature of the coupled protein-membrane system is that it exhibits phase transitions isothermally and under the control of competitive kinase-phosphatase reactions. At a single temperature and composition, the molecular interactions themselves change (as a function of LAT phosphorylation), and the phase state of the system follows. This differs from typical observations of lipid miscibility phase transitions in which the molecular properties of the lipids are fixed, and other control parameters such as temperature potentiate the phase transition (16,62,77,78). This control over LAT condensation through tyrosine phosphorylation not only enables the specific connection with cellular signaling systems, it also opens the door to various nonequilibrium chemical phenomena.
An example for such nonequilibrium phenomena can be found in a competitive lipid kinase-phosphatase reaction, which is similar to the tyrosine kinase-phosphatase competition governing LAT phosphorylation. The lipid kinase-phosphatase system has recently been observed to exhibit scale sensitivity in which the final outcome of the reaction depends on the size of the reaction system (e.g., a corralled lipid membrane in micron scales) (79). In this case, under identical concentrations of lipid kinases and phosphatases in solution, the membrane reaction system reaches a PIP2-dominated or PIP1-dominated (lipid kinase and phosphatase products, respectively) state based on size and degree of confinement by the corralled membranes. Even partially confined membrane features, such as filopodia, are sufficient to flip the reaction outcome, and more elaborate pattern formations occur under different geometric restrictions. As with all kinase-phosphatase competitive cycles, this example is a dissipative process that continuously consumes ATP. The system is intrinsically out of equilibrium, and the mechanism of this reaction scale sensitivity is rooted in nonequilibrium aspects of the kinetic system. The tyrosine kinase-phosphatase reactions upstream of the LAT condensate are qualitatively similar to the lipid kinase-phosphatase system mentioned above, albeit with even more complex feedback and regulatory couplings (80,81). In the case of the LAT condensate, functionally critical properties, such as nucleation threshold, size distribution, and growth-dispersion characteristics, are likely to be set by the kinase-phosphatase reactions controlling LAT phosphorylation. The LAT condensates, as well as any lipid phase structure they cause, will thus reflect the chemical states of the signaling system—including those arising from nonequilibrium processes—rather than equilibrium phase separation. At this time, very little is known about physical characteristics of LAT condensates in living cells, leaving a wealth of opportunities for detailed studies of these systems. From a functional perspective, one may speculate that lipid miscibility phase separation in living cells is inextricably coupled to numerous specific protein assemblies and signaling processes, many of which are only beginning to gain visibility.
Author contributions
J.K.C., W.Y.C.H., and J.T.G. conceived the research. J.K.C., W.Y.C.H., C.B.C., and L.M.N. performed the experiments. J.K.C., W.Y.C.H., C.B.C., R.D.V., A.N.P., and J.T.G. analyzed the data and interpreted the results. J.K.C., W.Y.C.H., and J.T.G. wrote the manuscript. All authors commented on the manuscript.
Acknowledgments
We thank John-Paul Denson, William K. Gillette, and Andrew G. Stephen (National Cancer Institute RAS Initiative, Frederick National Laboratory for Cancer Research) for the eGFP-K-Ras construct. We thank Young Kwang Lee, Hiu Yue Monatrice Lam, Shalini Low-Nam, and Emily C. Laubscher for assistance with the pilot experiments.
This work was supported by the Novo Nordisk Foundation Challenge Program under the Center for Geometrically Engineered Cellular Systems. Additional support was provided by National Institutes of Health, National Cancer Institute, Physical Sciences in Oncology Network project 1-U01CA202241 and by National Institutes of Health grant P01 A1091580.
Editor: Rohit Pappu.
Footnotes
Jean K. Chung and William Y. C. Huang contributed equally to this work.
Jean K. Chung’s present address is Department of Chemistry, Colorado State University, Fort Collins, Colorado.
William Y. C. Huang’s present address is Department of Chemical and Systems Biology, Stanford University, Stanford, California.
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2020.09.017.
Supporting material
References
- 1.Singer S.J., Nicolson G.L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 2.Shimshick E.J., McConnell H.M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973;12:2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- 3.Brûlet P., McConnell H.M. Kinetics of phase equilibrium in a binary mixture of phospholipids. J. Am. Chem. Soc. 1976;98:1314–1318. doi: 10.1021/ja00422a003. [DOI] [PubMed] [Google Scholar]
- 4.Hong-wei S., McConnell H. Phase separations in phospholipd membranes. Biochemistry. 1975;14:847–854. doi: 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- 5.Luna E.J., McConnell H.M. Lateral phase separations in binary mixtures of phospholipids having different charges and different crystalline structures. Biochim. Biophys. Acta. 1977;470:303–316. doi: 10.1016/0005-2736(77)90108-0. [DOI] [PubMed] [Google Scholar]
- 6.Luna E.J., McConnell H.M. Multiple phase equilibria in binary mixtures of phospholipids. Biochim. Biophys. Acta. 1978;509:462–473. doi: 10.1016/0005-2736(78)90240-7. [DOI] [PubMed] [Google Scholar]
- 7.Stier A., Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim. Biophys. Acta. 1973;311:400–408. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- 8.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 9.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 10.Simons K., Gerl M.J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 11.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 12.Nichols B. Cell biology: without a raft. Nature. 2005;436:638–639. doi: 10.1038/436638a. [DOI] [PubMed] [Google Scholar]
- 13.Hancock J.F. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levental I., Levental K.R., Heberle F.A. Lipid rafts: controversies resolved, mysteries remain. Trends Cell Biol. 2020;30:341–353. doi: 10.1016/j.tcb.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgart T., Hess S.T., Webb W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 16.Veatch S.L., Keller S.L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 2002;89:268101. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- 17.Oglęcka K., Rangamani P., Parikh A.N. Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials. eLife. 2014;3:e03695. doi: 10.7554/eLife.03695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie M. Mysteries of the cell. Do lipid rafts exist? Science. 2011;334:1046–1047. doi: 10.1126/science.334.6059.1046-b. [DOI] [PubMed] [Google Scholar]
- 19.Sevcsik E., Brameshuber M., Schütz G.J. GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane. Nat. Commun. 2015;6:6969. doi: 10.1038/ncomms7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I.H., Saha S., Groves J.T. Live cell plasma membranes do not exhibit a miscibility phase transition over a wide range of temperatures. J. Phys. Chem. B. 2015;119:4450–4459. doi: 10.1021/jp512839q. [DOI] [PubMed] [Google Scholar]
- 21.Saha S., Lee I.H., Mayor S. Diffusion of GPI-anchored proteins is influenced by the activity of dynamic cortical actin. Mol. Biol. Cell. 2015;26:4033–4045. doi: 10.1091/mbc.E15-06-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rayermann S.P., Rayermann G.E., Keller S.L. Hallmarks of reversible separation of living, unperturbed cell membranes into two liquid phases. Biophys. J. 2017;113:2425–2432. doi: 10.1016/j.bpj.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veatch S.L., Cicuta P., Baird B. Critical fluctuations in plasma membrane vesicles. ACS Chem. Biol. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 24.Rao M., Mayor S. Active organization of membrane constituents in living cells. Curr. Opin. Cell Biol. 2014;29:126–132. doi: 10.1016/j.ceb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Stone M.B., Shelby S.A., Veatch S.L. Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. eLife. 2017;6:e19891. doi: 10.7554/eLife.19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monks C.R., Freiberg B.A., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 27.Dustin M.L., Cooper J.A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 28.Yokosuka T., Sakata-Sogawa K., Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 29.Rapp M., Granseth E., von Heijne G. Identification and evolution of dual-topology membrane proteins. Nat. Struct. Mol. Biol. 2006;13:112–116. doi: 10.1038/nsmb1057. [DOI] [PubMed] [Google Scholar]
- 30.Lillemeier B.F., Mörtelmaier M.A., Davis M.M. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dustin M.L., Groves J.T. Receptor signaling clusters in the immune synapse. Annu. Rev. Biophys. 2012;41:543–556. doi: 10.1146/annurev-biophys-042910-155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi J., Balagopalan L., Samelson L.E. TCR microclusters form spatially segregated domains and sequentially assemble in calcium-dependent kinetic steps. Nat. Commun. 2019;10:277. doi: 10.1038/s41467-018-08064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerný J., Stockinger H., Horejsí V. Noncovalent associations of T lymphocyte surface proteins. Eur. J. Immunol. 1996;26:2335–2343. doi: 10.1002/eji.1830261010. [DOI] [PubMed] [Google Scholar]
- 34.Janes P.W., Ley S.C., Kabouridis P.S. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 35.Drevot P., Langlet C., He H.T. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 2002;21:1899–1908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglass A.D., Vale R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glebov O.O., Nichols B.J. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat. Cell Biol. 2004;6:238–243. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- 38.Bunnell S.C., Hong D.I., Samelson L.E. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell A.W., Croft N.P., Tscharke D.C. Immunology by numbers: quantitation of antigen presentation completes the quantitative milieu of systems immunology! Curr. Opin. Immunol. 2016;40:88–95. doi: 10.1016/j.coi.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Lin J.J., O’Donoghue G.P., Groves J.T. Membrane association transforms an inert anti-TCRβ Fab’ ligand into a potent T cell receptor agonist. Biophys. J. 2020;118:2879–2893. doi: 10.1016/j.bpj.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brameshuber M., Kellner F., Huppa J.B. Monomeric TCRs drive T cell antigen recognition. Nat. Immunol. 2018;19:487–496. doi: 10.1038/s41590-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossboth B., Arnold A.M., Schütz G.J. TCRs are randomly distributed on the plasma membrane of resting antigen-experienced T cells. Nat. Immunol. 2018;19:821–827. doi: 10.1038/s41590-018-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Donoghue G.P., Pielak R.M., Groves J.T. Direct single molecule measurement of TCR triggering by agonist pMHC in living primary T cells. eLife. 2013;2:e00778. doi: 10.7554/eLife.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J.J.Y., Low-Nam S.T., Groves J.T. Mapping the stochastic sequence of individual ligand-receptor binding events to cellular activation: T cells act on the rare events. Sci. Signal. 2019;12:eaat8715. doi: 10.1126/scisignal.aat8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 46.Banani S.F., Lee H.O., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P., Banjade S., Rosen M.K. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su X., Ditlev J.A., Vale R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 2016;352:595–599. doi: 10.1126/science.aad9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang W.Y., Yan Q., Groves J.T. Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. Proc. Natl. Acad. Sci. USA. 2016;113:8218–8223. doi: 10.1073/pnas.1602602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Case L.B., Ditlev J.A., Rosen M.K. Regulation of transmembrane signaling by phase separation. Annu. Rev. Biophys. 2019;48:465–494. doi: 10.1146/annurev-biophys-052118-115534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banjade S., Rosen M.K. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife. 2014;3:e04123. doi: 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brangwynne C.P., Eckmann C.R., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 53.Hnisz D., Shrinivas K., Sharp P.A. A phase separation model for transcriptional control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang W.Y.C., Alvarez S., Groves J.T. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science. 2019;363:1098–1103. doi: 10.1126/science.aau5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang W.Y.C., Chiang H.K., Groves J.T. Dynamic scaling analysis of molecular motion within the LAT:Grb2:SOS protein network on membranes. Biophys. J. 2017;113:1807–1813. doi: 10.1016/j.bpj.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houtman J.C., Higashimoto Y., Samelson L.E. Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry. 2004;43:4170–4178. doi: 10.1021/bi0357311. [DOI] [PubMed] [Google Scholar]
- 57.Houtman J.C., Yamaguchi H., Samelson L.E. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat. Struct. Mol. Biol. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 58.Kortum R.L., Balagopalan L., Samelson L.E. The ability of Sos1 to oligomerize the adaptor protein LAT is separable from its guanine nucleotide exchange activity in vivo. Sci. Signal. 2013;6:ra99. doi: 10.1126/scisignal.2004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balagopalan L., Kortum R.L., Samelson L.E. The linker for activation of T cells (LAT) signaling hub: from signaling complexes to microclusters. J. Biol. Chem. 2015;290:26422–26429. doi: 10.1074/jbc.R115.665869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu M., Janssen E., Zhang W. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J. Immunol. 2003;170:325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 61.Lin J., Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J. Biol. Chem. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 62.Veatch S.L., Keller S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veatch S.L., Polozov I.V., Keller S.L. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellis C.A., Clark G. The importance of being K-Ras. Cell. Signal. 2000;12:425–434. doi: 10.1016/s0898-6568(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 65.Friday B.B., Adjei A.A. K-ras as a target for cancer therapy. Biochim. Biophys. Acta. 2005;1756:127–144. doi: 10.1016/j.bbcan.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weise K., Kapoor S., Winter R. Membrane-mediated induction and sorting of K-Ras microdomain signaling platforms. J. Am. Chem. Soc. 2011;133:880–887. doi: 10.1021/ja107532q. [DOI] [PubMed] [Google Scholar]
- 68.Gillette W.K., Esposito D., Stephen A.G. Farnesylated and methylated KRAS4b: high yield production of protein suitable for biophysical studies of prenylated protein-lipid interactions. Sci. Rep. 2015;5:15916. doi: 10.1038/srep15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung J.K., Lee Y.K., Groves J.T. K-Ras4B remains monomeric on membranes over a wide range of surface densities and lipid compositions. Biophys. J. 2018;114:137–145. doi: 10.1016/j.bpj.2017.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hancock J.F., Paterson H., Marshall C.J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 71.Cadwallader K.A., Paterson H., Hancock J.F. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol. Cell. Biol. 1994;14:4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu A.P., Fletcher D.A. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys. J. 2006;91:4064–4070. doi: 10.1529/biophysj.106.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honigmann A., Sadeghi S., Vink R. A lipid bound actin meshwork organizes liquid phase separation in model membranes. eLife. 2014;3:e01671. doi: 10.7554/eLife.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammond A.T., Heberle F.A., Feigenson G.W. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W., Sloan-Lancaster J., Samelson L.E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 76.Shah N.H., Wang Q., Kuriyan J. An electrostatic selection mechanism controls sequential kinase signaling downstream of the T cell receptor. eLife. 2016;5:e20105. doi: 10.7554/eLife.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaizuka Y., Groves J.T. Structure and dynamics of supported intermembrane junctions. Biophys. J. 2004;86:905–912. doi: 10.1016/S0006-3495(04)74166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parthasarathy R., Yu C.H., Groves J.T. Curvature-modulated phase separation in lipid bilayer membranes. Langmuir. 2006;22:5095–5099. doi: 10.1021/la060390o. [DOI] [PubMed] [Google Scholar]
- 79.Hansen S.D., Huang W.Y.C., Groves J.T. Stochastic geometry sensing and polarization in a lipid kinase-phosphatase competitive reaction. Proc. Natl. Acad. Sci. USA. 2019;116:15013–15022. doi: 10.1073/pnas.1901744116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Courtney A.H., Lo W.L., Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem. Sci. 2018;43:108–123. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abraham R.T., Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.