Abstract
Ozone (O3) exposure has been reported to contribute to various cutaneous inflammatory conditions, such as eczema, psoriasis, rush etc. via a redox-inflammatory pathway. O3 is too reactive to penetrate cutaneous tissue; it interacts with lipids present in the outermost layer of skin, resulting in formation of oxidized molecules and hydrogen peroxide (H2O2). Interestingly, several inflammatory skin pathologies demonstrate altered levels of antimicrobial peptides (AMPs). These small, cationic peptides are found in various cells, including keratinocytes, eccrine gland cells, and seboctyes. Classically, AMPs function as antimicrobial agents. Recent studies indicate that AMPs also play roles in inflammation, angiogenesis, and wound healing. Since altered levels of AMPs have been detected in pollution-associated skin pathologies, we hypothesized that exposure to O3 could affect the levels of AMPs in the skin. We examined levels of AMPs using qRT-PCR, Western blotting, and immunofluorescence in vitro (human keratinocytes), ex vivo (human skin explants), and in vivo (human volunteer subjects exposed to O3) and observed increased levels of all the measured AMPs upon O3 exposure. In addition, in vitro studies have confirmed the redox regulation of AMPs in keratinocytes. This novel finding suggests that targeting AMPs could be a possible defensive strategy to combat pollution-associated skin conditions.
Keywords: Skin, LL37, ROS, Antimicrobial peptides, Pollution
Abbreviations: AMPs, antimicrobial peptides; hBDs, human beta defensins, ozone (O3); CAMP, cathelicidin antimicrobial peptide; hCAP18, human cationic bacterial protein of 18 kDa; WHO, World Health Organization; NOx, nitrogen oxides; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; AP-1, activator protein-1; H2O2, hydrogen peroxide; 4HNE, 4-hydroxy-nonenal; CFU, colony forming unit; VDR, vitamin D receptor; AhR, aryl hydrocarbon receptor; ROS, reactive oxygen species; COX2, cyclo-oxygenase 2; UV, ultraviolet; PM, particulate matter; TLR, toll-like receptor; PUFAs, polyunsaturated fatty acids
Graphical abstract
Highlights
-
•
AMPs (hBDs1-3, CAMP) increase in O3 exposed human skin by a redox mechanism.
-
•
Transcriptional upregulation of AMPs in response to O3 exposure is due to an altered redox status.
-
•
Pollution increase AMPs could be the connection between pollution exposure and the development/exacerbation of inflammatory skin conditions.
1. Introduction
Tropospheric ozone (O3) is formed at the ground level through chemical reactions between nitrogen oxides (NOx) and volatile organic compounds, when pollutants are emitted by cars, industrial boilers, refineries, and chemical plants in the presence of sunlight [1]. Exposure to O3 has been associated with negative effects on respiratory, cardiovascular, and neurological functions [1]. However, only recently has the noxious effects of O3 exposure on cutaneous tissues been studied. O3 exposure has been correlated with premature skin aging, contact dermatitis, atopic dermatitis, and psoriasis [[2], [3], [4], [5], [6]]. O3 interacts with lipids present in the outermost layer of skin and initiates formation of bioactive products, such as aldehydes and free radicals, causing cutaneous redox imbalance (Valacchi et al., 2002). Oxidative stress induced by O3 exposure is believed to contribute to the development and/or exacerbation of inflammatory skin disorders, but the mechanisms remain unclear. In this study, we have identified a potential role for AMPs as mediators of O3-associated oxinflammation [7].
AMPs are key effectors in innate immune responses and protect from pathogens by disrupting pathogen membrane integrity [8]; they are divided into different groups, depending on their secondary structures. Defensins are small (~4 kDa), cationic peptides, characterized by three intramolecular disulfide bonds that stabilize two or more β-sheets, and are further classified as either beta or alpha, depending on arrangement of disulfide bonds [8]. Alpha defensins are primarily expressed by neutrophils and Paneth cells. Beta defensins are expressed in epithelial cells. Types of beta defensins found in human skin include human beta defensin 1 (hBD1), hBD2, and hBD3. Cutaneous hBD1 is constitutively expressed [9]; transcription of hBD2 and hBD3 is induced in response to infection, inflammation, or injury [[10], [11], [12], [13], [14]]. Cathelicidins are amphipathic, alpha-helical AMPs that contain a conserved N-terminal cathelin domain and a variable C-terminal antimicrobial domain [8,10]. Although 30 members have been found in mammalians, humans only express one cathelicidin (CAMP gene). The precursor is called human cationic bacterial protein of 18 kDa (hCAP18), and the mature antibacterial peptide, generated through extracellular proteolytic cleavage, is named LL37 [15]. In skin, transcription of CAMP is induced in response to infection, inflammation, and injury [[16], [17], [18]]. We believe that oxidative stress, a consequence of O3 exposure [19], can regulate AMP transcription, due to regulation of AMP-associated redox sensitive transcription factors (NF-κB and AP-1) [12,20,21].
Classically, AMPs function as antimicrobial agents. Recent research has indicated that AMPs can also regulate activation/chemotaxis of immune cells, stimulate angiogenesis, and induce production of proinflammatory cytokines [[22], [23], [24], [25], [26], [27], [28], [29]]. Furthermore, the concentrations required for antibacterial activity of hBD3 are ~100–1000 fold excess, compared to its immunomodulatory activity [30]. Interestingly, increased levels of AMPs are detected in active lesions of inflammatory skin diseases, such as psoriasis and atopic dermatitis [17,[31], [32], [33], [34]], although this finding is still controversial for atopic dermatitis [33] both hBD2 and hBD3 have been originally isolated from psoriatic patients [11,35]; Korting et al., 2012). Higher genomic copy numbers of hBD3 also correlate with an increased risk in developing psoriasis [36], and self-DNA and LL37 complexes, as well as self-RNA and LL37 complexes, have been detected in psoriatic lesions, contributing to immune cell activation [37,38].
Since exposure to pollutants as well as altered AMPs levels are associated with the development/progression of inflammatory skin conditions, we hypothesized that exposure to the pollutant O3 could affect cutaneous AMPs levels through a redox mechanism. In the present work we examined levels of hBD1, hBD2, hBD3, and CAMP in vitro using human keratinocytes, ex vivo using human skin biopsies, and in vivo in human volunteer subjects exposed to O3. We observed increased levels of these AMPs in response to O3 in all models analyzed, likely due to redox regulation of AMPs.
This novel finding brings new insights on the possible role of pollutants in redox regulation of AMPs and how O3 could affect the development/progression of inflammatory skin conditions, suggesting eventual new therapeutic anti-pollution strategies. This study could contribute to explain the increase of AMPs levels in inflammatory skin disorders (like psoriasis), creating a currently unexplored niche in pollution-induced skin pathologies.
2. Results
2.1. Ozone (O3) increases AMPs levels in human keratinocytes
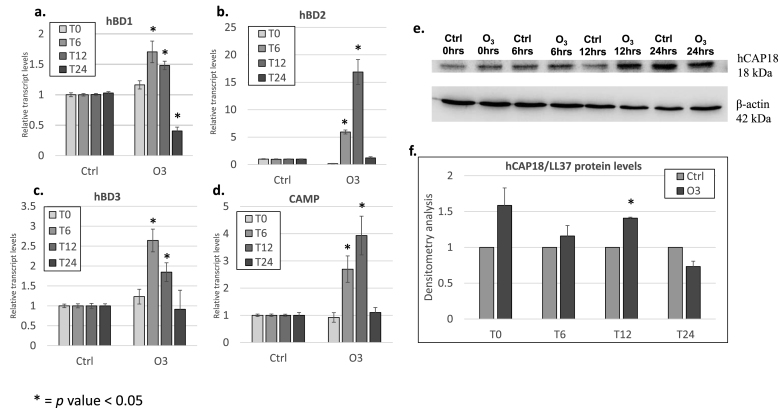
Since both, O3 exposure and increased AMPs levels are independently associated with cutaneous inflammatory conditions, we want to evaluate whether O3 exposure could modulate AMPs expression. Therefore, we determined the levels of various cutaneous AMPs including hBD1, hBD2, hBD3, and CAMP (LL37) in keratinoctyes exposed to 0.2 ppm of O3 for 30 min. We observed a similar trend for hBD1 and hBD3 with the highest response at 6 h post-exposure and a decline at the following time points (12 and 24 h) (Fig. 1a, and c); while the transcript levels of hBD2 and CAMP in O3 exposed cells increased 6 h post-exposure and reach the highest expression 12 h post-exposure (Fig. 1b and d). As expected we did not observe a large induction for the constitutively expressed hBD1 mRNA [9]. On the other hand, the response for hBD2, hBD3, and CAMP were very robust, confirming their susceptibility to different challenges (inflammation, injury, etc) [[10], [11], [12], [13], [14],[16], [17], [18]]. We also assessed hCAP18 (LL37 precursor) protein levels (Fig. 1e) and observed a clear induction of hCAP18 by O3 12 h post-exposure (Fig. 1e and f). These data confirm the hypothesis that O3 exposure is able to affect cutaneous AMPs expression and levels.
Fig. 1.
AMPs are increased in keratinocytes exposed to ozone in vitro. qRT-PCR analysis of transcript levels of hBD1 (a), hBD2 (b), hBD3 (c), and CAMP (d) in keratinocytes exposed to 0.2 ppm of O3 directly after exposure (T0), 6 h (T6), 12 h (T12), and 24 h (T24) post-exposure. (e) Representative immunoblot for hCAP18 levels in keratinocytes at different time points (T0, T6, T12, and T24), quantification is depicted in panel (f). Results from three independent experiments and three replicates. * = p-value<0.05. Error bars are defined as mean±SEM.
2.2. O3 exposure increased AMPs protein levels in human skin biopsies
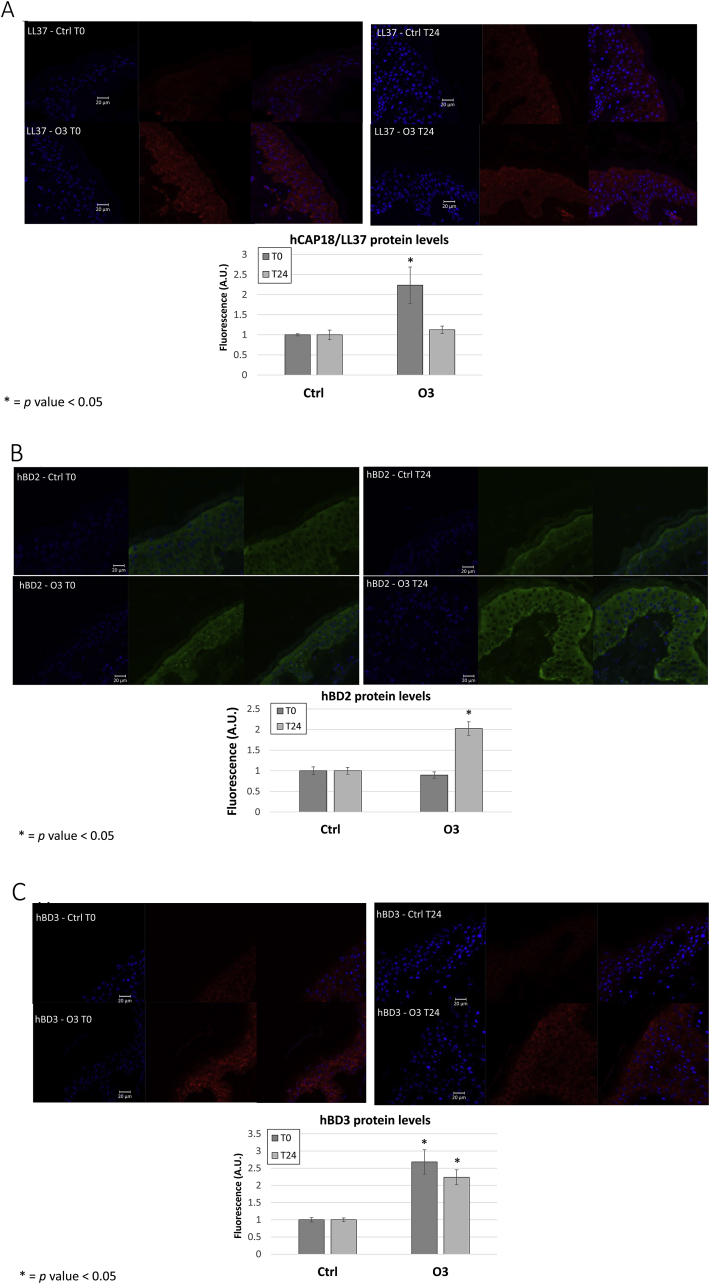
Considering the limitation of the 2D cell culture models, we want to confirm our data in ex vivo human skin biopsies, which incorporate all skin appendages such as hair follicles and sweat glands (produce AMPs). As depicted in Fig. 2, after O3 exposure (T0) there was a clear and significant increase of hCAP18/LL37 staining throughout exposed epithelia (1.25 fold) (Fig. 2A) and this increment was lost 24 h post-exposure. As depicted in Fig. 2B, a clear increase in hBD2 (2 fold) was noticed after 24hr post exposure. In addition, we were able to also detect a significant increase in hBD3 levels immediately after O3 exposure (T0) (1.6 fold) (Fig. 2C) and 24 h post-exposure (1.2 fold). These data confirm the results observed in 2D keratinocytes model.
Fig. 2.
Exposure of human biopsies to ozone increases AMP protein levels in the skin. Ex vivo human abdominoplasty biopsies from three different donors were exposed to 0.4 ppm O3.Sections were collected directly after exposure (T0) and 24 h (T24) post exposure and embedded in paraffin. Immunofluorescence was performed analyzing protein levels of AMPs hCAP18/LL37 (A) hBD2 (B) and hBD3 (C). Images quantification was performed using ImageJ. Graphs depicted are the results of the quantification of three independent experiments. The images reported in this figure were chosen as being most representative of the quantification. Red staining indicates either hCAP18/LL37 or hBD3, green staining indicates hBD2. Blue staining represents DAPI. * = p-value<0.05. Error bars are defined as mean±SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Redox regulation of cutaneous AMPs
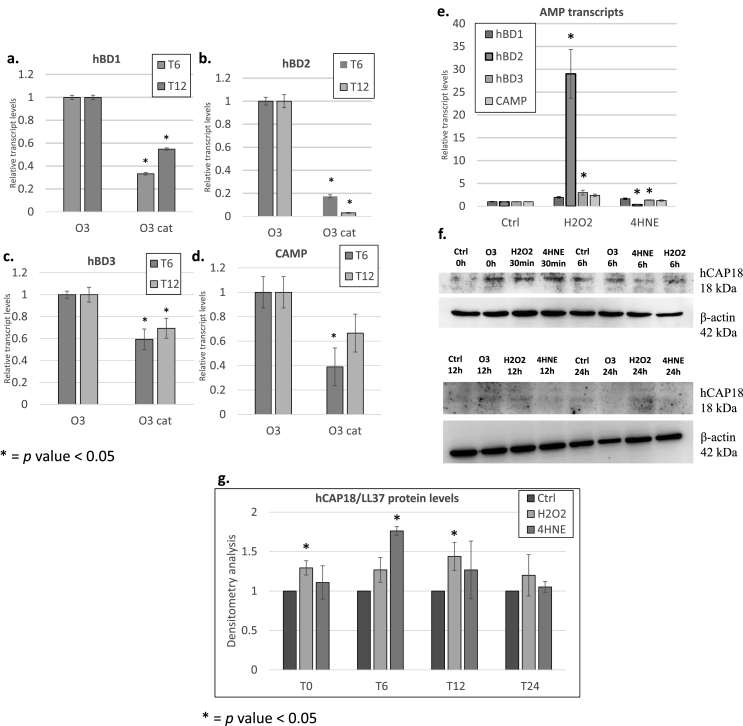
As mentioned before, O3 is not able to penetrate the skin as it reacts instantaneously with the polyunsaturated fatty acids (PUFAs) present in the stratum corneoum, producing several byproducts, including hydrogen peroxide (H2O2) and aldehydes, such as 4-hydroxynonenal (4HNE) [39]. To determine whether O3-induced H2O2 is responsible for increased AMPs transcription, we pre-treated keratinocytes with 800 U/ml of catalase (cat) for 1.5 h then exposed to 0.2 ppm of O3 for 30 min and collected samples 6 and 12 h post-exposure. As depicted in Fig. 3, pretreatment with cat prevented O3-induced hBD1, hBD2, hBD3, and CAMP transcripts (Fig. 3a–d).
Fig. 3.
Ozone-induced signaling mediator H2O2regulates AMP levels in keratinocytes. Transcript levels of hBD1 (a), hBD2 (b), hBD3 (c), and CAMP (d) were assessed in keratinocytes pretreated with 20 μg/mL of catalase (cat) then exposed to O3 at different time points. AMPs transcript levels in keratinocytes treated with 50 μM H2O2 for 6 h (e). Protein levels of hCAP18 in keratinocytes after 30 min, 6 h (T6), 12 h (T12), and 24 h (T24) of treatment with either H2O2 or 4HNE (f). Quantification is depicted in panel (g). *p-value<0.05, compared to control. Error bars = mean ± SEM.
As a proof of concept, we exposed human keratinocytes to H2O2 to understand whether this molecule could be a possible messenger of O3 affects AMPs transcript levels. As shown in Fig. 3e, H2O2 significantly increased transcript levels of all tested AMPs after 6 h with hBD2 the most upregulated (over 20-fold) (Fig. 3e). In addition, we observed a striking increase in protein levels of hCAP18 immediately after exposure to O3 and after 30 min of treatment with H2O2 and 4HNE (the other main bio-product formed by O3 interaction with the epidermis fatty acids) (Fig. 3f and g). We observed no clear differences between hCAP18 levels in keratinocytes treated with either 4HNE or H2O2 after 6 h suggesting that both molecules are able to affect hCAP18 levels. Of note is that the effect of H2O2 was evident up to 12–24hrs post exposure (Fig. 3f). These data suggest that AMPs are increased in response to O3 in skin due to generation of mediators, such as H2O2 and 4HNE.
2.4. Exposure of human volunteers to O3 increases AMPs levels in skin
To further confirm our data, we determine the levels of AMPs after O3 exposure in human volunteers. We exposed forearms of subjects to O3 for 3 h a day for 5 consecutive days (Allendale Institutional Review Board; 7015-090-104/106-002; August 10, 2015) [40]. We examined levels of hCAP18/LL37, hBD2 and hBD3 in 3 mm punch biopsies taken from both unexposed (control) and exposed lateral forearms and observed increases in both LL37 (Fig. 4A), hBD2 (Fig. 4B) and hBD3 (Fig. 4C) positive staining throughout the epithelia of exposed forearms. As depicted in Fig. 4 the increase was statistically significant for all the AMPs analyzed, indicating that O3-induced their upregulation also in vivo and substantiating the in vitro and ex vivo findings (Fig. 1, Fig. 2, Fig. 3).
Fig. 4.
Exposure of human volunteer subjects to ozone increases AMP protein levels in the skin. Forearms of human volunteer subjects were exposed to 0.8 ppm O3 for 3 h a day for 5 consecutive days. We examined protein levels of hCAP18/LL37 (a, c) and hBD3 (b, d) using immunofluorescence in 3 mm punch biopsies taken from the aforementioned subjects from both unexposed (control) and exposed lateral forearms. Images were quantified using ImageJ. Data shown in (c–d) are the results of averages of multiple images from at least three different subjects. Images depicted in Fig. 5 were chosen as being the most representative of results after quantification. * = p-value<0.05. Error bars are defined as mean±SEM.
3. Discussion
O3 exposure has been associated with the development/exacerbation of inflammatory skin conditions, such as atopic dermatitis, acne, and psoriasis [[2], [3], [4],77]. Inflammatory skin conditions are also associated with altered AMPs levels, indeed multiple AMPs were originally isolated from human skin pathologies [11,35]. Since O3 exposure as well as increased levels of AMPs are associated with cutaneous inflammation [1,4,17,[31], [32], [33], [34]], we hypothesized that cutaneous AMPs upregulation due to O3 exposure could be linked to the development/exacerbation of inflammatory skin conditions.
To test this, we used a variety of skin models, ranging from in vitro cell culture, ex vivo human skin explants, and human volunteer subjects. We utilized in vitro cell culture mainly for the mechanistic aspects of our study and ex vivo human skin explants, since this model incorporates skin appendages such as hair follicles and sweat glands (both apparatus involved in AMPs production) to better evaluate cutaneous responses. In addition, human volunteer subjects were also exposed to O3 and AMPs were evaluated in vivo. At this stage we have avoided the use of murine models because the classes of AMPs encoded in mice differs from humans [8,10], the transcriptional regulation of CAMP is different in mice vs. humans, due to presence of a primate-specific retrotransposable element [41,42] and the mechanisms involved in epigenetic regulation also differ between mice and humans [43]. Importantly, in all the skin models analyzed, in vitro, ex vivo, and in vivo, we observed that O3 exposure increased levels of the cutaneous AMPs: hBD1, hBD2, hBD3, and CAMP, and this to our knowledge has never been demonstrated before.
Interestingly, we observed that transcript levels of hBD1 (constitutively produced by all epithelia) is increased in response to O3 exposure. Schroeder et al. demonstrated that reduction of disulfide bonds of hBD1 increases its antimicrobial activity against pathogenic and commensal bacteria and fungi [44]. Raschig et al. showed that the ability of hBD1 form bacteria-entrapping nets depends on reduction of its disulfide bonds [45], which can be mediated by the thioredoxin system [44,45]. In our study the transcript levels of hBD1 increased in keratinocytes exposed to O3, although not evident as other cutaneous AMPs and this was expected considering that hBD1 is the constitutive form. Due to lack of a commercially available antibodies, we could not investigate whether O3 exposure altered redox-dependent antimicrobial activity of hBD1, although it is likely that pollutant exposure affects hBD1 activity, as O3 induces oxidative stress and hBD1 has been shown to be redox sensitive [46].
We also observed increased transcript levels of hBD2, hBD3, and CAMP, in response to O3 exposure in keratinocytes and that hBD2 transcript levels are increased to a much higher level than either CAMP or hBD3, in response to both O3 and H2O2. Transcription of these AMPs is regulated by different signaling pathways. CAMP transcription is regulated by vitamin D, due to presence of a vitamin D response element in the promoter. This promoter is also responsive to vitamin D receptor (VDR)-independent transactivation by AP-1 and NF-κB [42,47,48]. In addition, Steubesand et al. demonstrated that infection with Candida albicans increases hBD2, but not hBD3, transcript levels in an NF-κB-dependent manner in epithelial cells [21]. Further studies by Tsutsumi-Ishii [49] confirmed the susceptibility of hBD2 to NFkB regulation. This could explain the evident increase of hBD2 mRNA in response to H2O2 which has been demonstrated for more than 20 years a very specific and strong NFkB activator via IkB alpha phosphorylation [50]. In silico analyses indicate the hBD2 promoter has multiple potential NF-κB and AP-1 binding sites which is in agreement with several others studies [12,20]. The hBD3 promoter is covered in potential AP-1 binding sites and has three potential AhR binding sites (data not shown). Rademacher et al. demonstrated that blocking AhR signaling decreases Staphylococcus epidermidis-induced increases in hBD3 transcription in skin [51]. Future studies include investigating whether we can detect NF-κB, AP-1, and AhR binding to the hBD2, CAMP, and hBD3 promoters, in response to O3 exposure in skin, since these transcription factors have been shown to be all modulated by O3 [1,52,53]
Based on our results, H2O2 alone is likely not fully responsible for O3-mediated increases in hBD3 and CAMP transcription. It is possible that lipid-derived peroxides or other lipid-derived aldehydes such as 4HNE, affect cutaneous AMPs levels. In skin, O3 exposure induces production of H2O2, as well as lipid peroxides and aldehydes, through interaction with PUFAs [54]. In addition to direct stimulation and/or modifications by lipid peroxides or aldehydes, these products could potentially alter AMPs levels indirectly through further increasing reactive oxygen species (ROS) levels. It has also been demonstrated that O3 exposure increases levels of cyclo-oxygenase 2 (COX2) [55], which is also involved in ROS production. The idea that AMPs regulation is at least partially redox modulated is confirmed by the catalase experiments, suggesting that H2O2 can be one trigger for AMPs transcription.
In vivo O3 exposure could also regulate AMPs levels by affecting the brain-skin axis. In this axis, stress induces production of hormones, neurotransmitters, and secreted factors that can regulate proinflammatory pathways in skin through regulation of NF-κB as well as lamellar body secretion (encapsulates AMPs), resulting in alterations in AMPs mRNA and/or protein levels [[56], [57], [58]].
Whether other pollutants affect AMPs levels has been sparsely investigated. For instance, Ultraviolet Radiation (UV) induces expression of AMPs (hBD1, hBD2, CAMP) in human keratinocytes, which could potentially alter the skin microbiome [[59], [60], [61]]. Exposure to particulate matter (PM) decreases the ability of respiratory epithelial cells to increase AMPs levels in response to infection [62,63]. Vargas Buonfiglio et al. demonstrated that ambient PM (coal fly ash) binds to cationic AMPs, decreasing levels of active AMPs [64]. Whether PM decreases AMPs activity in skin is still unknown.
Activity of many AMPs is regulated by cleavage of signal sequences at N-terminal, which holds the COOH-terminal AMP domain in an inactive form. In skin, hCAP18 is cleaved by kallikrein-related serine proteases to LL37 [8]. LL37 can then be further cleaved into RK-31, KS-30, and K20, which exhibit different antimicrobial and immunomodulatory activities than LL37 [15]. Whether O3 exposure regulates AMPs cleavage in skin is unknown. Another mechanism by which O3 exposure could regulate AMPs activity is through inducing post-translational modifications, such as protein oxidation. Furthermore, O3 exposure, which depletes cellular lipid content, could affect packaging of AMPs into lamellar bodies [8,65]. Disturbance of AMPs levels in skin could alter composition of the skin microbiome [30] and make the skin more susceptible to develop inflammatory conditions.
Independent of antimicrobial activities, AMPs can regulate chemotaxis, activation, and differentiation of immune cells, modulate cellular toll-like receptor (TLR) responses, inflammasome activation, stimulate angiogenesis, and regulate the expression/activity of proinflammatory cytokines/chemokines [8,10,22,23,[27], [28], [29],66,67]. In inflammatory skin conditions, including psoriasis, atopic dermatitis, and rosacea, altered levels of AMPs have been detected, as well as self-RNA/LL37 and self-DNA/LL37 complexes (in psoriasis), which can regulate activity of immune cells involved in these conditions [17,[31], [32], [33], [34],37,38]. The consequences of AMPs induction in response to pollutants could result in development/exacerbation of inflammatory skin disorders, due to the ability of AMPs to regulate adaptive immune responses. Our lab has previously demonstrated that O3 exposure can activate cutaneous inflammasome [68] which become even more relevant considering that LL37 can stimulate skin inflammasome activation [66].
However, AMPs can also act as anti-inflammatory agents, depending on context [69]. In fact, AMPs have recently been utilized as cosmeceuticals to improve the structure and function of aged skin [[70], [71], [72], [73], [74], [75], [76]]. It is currently unclear whether increased levels of AMPs in response to pollution exposure inhibits or promotes inflammation in skin. Their increase upon pollution exposure could be also interpretated as a non-specific response to unknown xenobiotic leading to an altered skin homeostasis.
Despite the fact that AMPs have the function to protect our body, several studies have suggested that these molecules can contribute to the pathogenesis of various skin conditions such as psoriasis, atopic dermatitis, rosacea, acne vulgaris, systemic lupus erythematosus and systemic sclerosis. Thus, AMPs induction and activation can also be considered a double-edged tool where from one side can promote cutaneous immunity and from the other side trigger the pathogenesis of some skin disorders [71]. Therefore, at this stage is hard to understand whether cutaneous AMPs upregulation in response to O3 is a “good” or a “bad” event; it is for sure a response to an oxinflammatory insult that leads to an alteration of skin homeostasis. Further studies need to be performed to better understand whether this induction is a transient response to an acute challenge or can lead to prolonged tissue responses. It is also possible that the induction of AMPs by pollution could affect cutaneous microbiome and therefore affect skin homeostais.
The further step will be to evaluate whether the AMPs secreted are fully functioning as anti-inflammatory molecules. Our attempts using e-coli (see supplementary Figure 1), showed that infected cells exposed to O3 increase their proliferation, suggesting their use in cosmeceuticals as a new possible technology against pollution-induced skin damage although, in a long run the eventual development of bacterial resistance should also be taken in consideration.
4. Materials and methods
4.1. Cell culture
Human keratinocytes (HaCaTs) were cultured, as previously described [72] (see Supplemental Methods). 1 × 106 (RNA) or 9 × 105 (protein) keratinocytes were seeded onto 60 mm or 6-well plates and exposed the following day. For catalase experiments, keratinocytes were pre-treated with 20 μg/mL of catalase (C4963, Sigma, St. Louis, MO) for 1.5 h. For exposure, keratinocytes were exposed for 30 min at 0.2 ppm of O3, as previously described [73]. For 4HNE and H2O2 experiments, keratinocytes were treated with either 50 μM concentration of H2O2 (H1009-100 ML, Sigma, St. Louis, MO) dissolved in water or 20 μM concentration of 4HNE (CAS 75899-68-2, Santa Cruz, Dallas, TX) dissolved in ethanol. Protein and RNA were collected at the indicated time points using protein lysis buffer (Supplemental Methods) or TRIzol (Ambion) [74].
4.2. Ozone generator
O2 was used to generate O3 via electrical corona arc discharge and combined with ambient air to flow into a plexiglass box (ECO3 model CUV-01, Torino, Italy Model 306 Ozone Calibration Source, 2B Technologies, Ozone Solution), as previously described [40,73]. Concentration of O3 in the chamber was adjusted, depending on the model used, and continuously monitored by an O3 detector.
4.3. Preparation of ex vivo human biopsies
Healthy human skin was obtained from elective abdominoplasties from 3 different donors. 12 mm punch biopsies were taken from the human skin. Next, subcutaneous fat was trimmed. Biopsies were rinsed with phosphate-buffered saline (PBS, Gibco, NY, USA) containing 1% antibiotics/antimycotics using sterile technique and then immediately transferred to 6-well dishes and cultured in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, NY, USA) at 37 °C in 5% CO2 humidified atmosphere. Experiments were performed the following day, as previously described by Ref. [68].
4.4. Ex vivo O3 exposure
Biopsies from donor skins were placed in a plexiglass box connected to the ozone generator and exposed to 0.4 ppm O3 for 4 h [68] N = 3. Samples were collected directly after exposure and 24 h post-exposure in 10% neutral buffered formalin and then dehydrated using alcohol gradients, followed by immersion in xylene for paraffin embedding.
4.5. Immunofluorescence
Immunofluorescence analysis was performed using 4 μm thick paraffin-embedded sections of ex vivo human biopsies or in vivo biopsies from human subjects, as previously described [40]. Briefly, sections were deparaffinized in xylene and rehydrated using alcohol gradients. Antigen retrieval was achieved using heat-based epitope retrieval with 10 mM sodium citrate buffer (AP-9003-500, Thermo, Waltham, MA) (pH 6.0) with 0.05% Tween 20 at a sub-boiling temperature in pressure cooker (Instant Pot Duo using the steam setting – Kanata, ON, Canada) for 15 min. After cooling for 30 min, the sections were washed 2 times for 5 min in PBS, blocked with 5% BSA in PBS at RT for 45 min, and incubated overnight at 4 °C with primary antibodies for hCAP18/LL37 (sc-166770, Santa Cruz, Dallas, TX) or hBD2 (ab63982 ABCAM Cambridge MA), hBD3 (sc-59495, Santa Cruz, Dallas, TX) at 1:50 dilutions in 2% BSA in PBS. The next day, sections were washed 3 times in PBS for 5 min, followed by a 1 h incubation with fluorochrome-conjugated secondary antibodies (Alexa Fluor 568 cat. A11004, Invitrogen, Waltham, MA) at 1:1000 dilutions in 2% BSA in PBS at RT, and then washed with PBS 3 times for 5 min. Nuclei were stained with DAPI (cat. 1874814, Invitrogen, Waltham, MA) for 1–2 min in PBS at RT, and sections were then washed 3 times for 5 min with PBS and then with water. The sections were mounted onto glass slides using PermaFluor aqueous mounting medium (TA-006-FM, Thermo, Waltham, MA) and imaged via epifluorescence on a Zeiss LSM10 confocal microscope equipped at 40X magnification. Quantification of raw images was performed using ImageJ.
4.6. Immunoblotting
Total protein lysates were extracted in ice-cold RIPA buffer (cat. AAJ62524AD, Alfa Aesar, Tewksbury, MA) with 1% protease (cat. 78430, Thermo, Waltham, MA) and 1% phosphatase (cat. 1862495, Thermo, Waltham, MA) inhibitor cocktails. Lysates were centrifuged at 13,000 rpm for 15 min at 4 °C. Protein content in supernatants was measured using the Quick Start Bradford Protein Assay Kit (cat. 5000201, Bio-Rad, Hercules, CA). Equivalent amounts of proteins were ran on 15% SDS-PAGE gels, electro-transferred onto nitrocellulose membranes, and blocked in TBS containing 0.1% Tween 20 and 5% not-fat milk (cat. 1706404XTU, BioRad, Hercules, CA). Membranes were incubated with 1:1000 dilution of hCAP18/LL37 (cat. sc-166770, Santa Cruz, Dallas, TX or cat. ab80895, Abcam, Cambridge, UK) or beta actin (cat. A3854, Sigma, 1:50,000 dilution) antibodies in 2% milk TBST. The membranes were then incubated with 1:10,000 dilutions of horseradish peroxidase-conjugated secondary antibodies (cat. 170–6516, BioRad, Hercules, CA) in 2% milk TBST for 1 h at RT, and the bound antibodies were detected in a chemiluminescent reaction (ECL, BioRad, Hercules, CA). Chemiluminescence was detected on ChemiDoc imager (BioRad, Hercules, CA), and the signal was quantified using Image J. For quantification analysis, levels of hCAP18 were first normalized to beta actin then to the control sample for each time point for each experiment (n = 3). Next, we compiled densitometric values of blots from individual experiments together to generate the graph depicted in Fig. 1e.
4.7. RNA extraction and quantitative real time PCR
RNA samples were extracted using TRIzol (Ambion), and RNA was isolated using phenol-chloroform extraction using a modified version of the protocol described by Ref. [74]. Reverse transcription-PCR was performed using iScript cDNA Synthesis Kit (cat. 1708841, BioRad, Hercules, CA), according to the manufacturer's instructions. To investigate the mRNA expression of AMPs, quantitative real-time PCR was performed using SsoAdvanced Universal SYBR Green Supermix (cat. 1725271, Biorad, Hercules, CA), according to the manufacturer's protocol on a Roche LightCycler 480 machine. Quantitative relative gene expression was calculated using the 2 −ΔΔCt method, using the housekeeping gene glyceraldehyde-3-phosphate (GAPDH) to normalize, and control samples as internal calibrators. The primers used are listed in Table 1.
Table 1.
Primers used in the study.
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | TCGGAGTCAACGGATTTGGT | TTCCCGTTCTCAGCCTTGAC |
| hBD1 | TTGTCTGAGATGGCCTCAGGTGGTAAC | ATACTTCAAAAGCAATTTTCCTTTAT |
| hBD2 | GCATTGCACCCAATACCAGT | CCAAAAACACCTGGAAGAGGCA |
| hBD3 | TATCTTCTGTTTGCTTTGCTCTTCC | CGCCTCTGACTCTGCAATAA |
| CAMP | CGGTGTATGGGGACAGTGAC | TGGGTACAAGATTCCGCAAA |
4.8. Human subjects’ exposure
Human subjects consisted of Caucasian male and female (60% male and 40% female) human volunteers aged 18–55, free of systemic or dermatological disorders (N = 15). Written consent was obtained from the subjects. Subject forearms were exposed to 0.8 ppm O3 for 3 h a day for 5 consecutive days. 3 mm punch biopsies were collected immediately after the last exposure (IRB approval - Allendale Institutional Review Board; 7015-090-104/106-002; August 10, 2015), as described by Ref. [40]. We did not observe sex-specific differences in upregulation of inflammatory markers in response to exposure.
4.9. Antibacterial experiments
E. coli strain DH5α (gifted from Hsieh lab at Plants for Human Health Institute (PHHI)) was used for these experiments. Colonies were seeded from frozen stocks and grown overnight at 37 °C in Luria-Bertani (LB) broth with slight agitation. Before antibacterial experiments, the bacterial cells were washed with PBS, and optical density at 600 nm was measured. For these experiments, bacteria were used that had exhibited absorbance of 0.4 at OD600 (in exponential growth phase). Number of CFU to use for infection was calculated using the following formula: OD600 = 4 × 108 CFU/ml of E. coli [75]. The day prior to exposure, human keratinocytes (HaCaTs) were washed three times with PBS and cultured in DMEM with 10% FBS and no antibiotics. 100,000 cells were seeded in 24-well plates, and, the following day, cells were exposed to 0.2 ppm of O3 for 30 min. Directly after exposure, cells were infected with 1 × 105 CFU of bacteria and incubated for 6 h in 5% CO2 humidified incubator at 37 °C. Microscopy images were taken on a Zeiss LSM 710 microscope 6 h post-infection. Aliquots were taken from culture media and serially diluted in LB broth and then plated on LB agar plates. Colonies were counted after overnight incubation at 37 °C. Representative experiment is depicted in Supplementary Figure 1. Quantification was based on counts from multiple serial dilutions that were averaged together to produce graph shown in Fig. 5.
4.10. Statistical analysis
Welch's unequal variance t-test was used to test results for significance. Statistical significance was considered at p < 0.05. Data are expressed as mean ± SEM.
Data availability
No large datasets were generated or analyzed in this study; however, all data will be shared by open request to the corresponding author.
Declaration of competing interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101952.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fuks K.B., Woodby B., Valacchi G. Skin damage by tropospheric ozone. Hautarzt. 2019;70(3):163–168. doi: 10.1007/s00105-018-4319-y. [DOI] [PubMed] [Google Scholar]
- 2.Fuks K.B., Huls A., Sugiri D., Altug H., Vierkotter A., Abramson M.J. Tropospheric ozone and skin aging: results from two German cohort studies. Environ. Int. 2019;124:139–144. doi: 10.1016/j.envint.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Krutmann J., Moyal D., Liu W., Kandahari S., Lee G.S., Nopadon N. Pollution and acne: is there a link? Clin. Cosmet. Invest. Dermatol. 2017;10:199–204. doi: 10.2147/CCID.S131323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu F., Yan S., Wu M., Li F., Xu X., Song W. Ambient ozone pollution as a risk factor for skin disorders. Br. J. Dermatol. 2011;165(1):224–225. doi: 10.1111/j.1365-2133.2011.10349.x. [DOI] [PubMed] [Google Scholar]
- 5.Valacchi G., Porada E., Rowe B.H. Ambient ozone and bacterium Streptococcus: a link between cellulitis and pharyngitis. Int. J. Occup. Med. Environ. Health. 2015;28(4):771–774. doi: 10.13075/ijomeh.1896.00267. [DOI] [PubMed] [Google Scholar]
- 6.Kousha T., Valacchi G. The air quality health index and emergency department visits for urticaria in Windsor. Canada J Toxicol Environ Health A. 2015;78(8):524–533. doi: 10.1080/15287394.2014.991053. [DOI] [PubMed] [Google Scholar]
- 7.Valacchi G., Virgili F., Cervellati C., Pecorelli A. OxInflammation: from subclinical condition to pathological biomarker. Front. Physiol. 2018;9:858. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L.J., Gallo R.L. Antimicrobial peptides. Curr. Biol. 2016;26(1):R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Valore E.V., Park C.H., Quayle A.J., Wiles K.R., McCray P.B., Jr., Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Invest. 1998;101(8):1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cederlund A., Gudmundsson G.H., Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278(20):3942–3951. doi: 10.1111/j.1742-4658.2011.08302.x. [DOI] [PubMed] [Google Scholar]
- 11.Harder J., Bartels J., Christophers E., Schroder J.M. A peptide antibiotic from human skin. Nature. 1997;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 12.Harder J., Meyer-Hoffert U., Teran L.M., Schwichtenberg L., Bartels J., Maune S. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. 2000;22(6):714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 13.Menzies B.E., Kenoyer A. Staphylococcus aureus infection of epidermal keratinocytes promotes expression of innate antimicrobial peptides. Infect. Immun. 2005;73(8):5241–5244. doi: 10.1128/IAI.73.8.5241-5244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neil D.A., Porter E.M., Elewaut D., Anderson G.M., Eckmann L., Ganz T. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 1999;163(12):6718–6724. [PubMed] [Google Scholar]
- 15.Yamasaki K., Schauber J., Coda A., Lin H., Dorschner R.A., Schechter N.M. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb. J. 2006;20(12):2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 16.Dorschner R.A., Pestonjamasp V.K., Tamakuwala S., Ohtake T., Rudisill J., Nizet V. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 2001;117(1):91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 17.Frohm M., Agerberth B., Ahangari G., Stahle-Backdahl M., Liden S., Wigzell H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997;272(24):15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 18.Gallo R.L., Murakami M., Ohtake T., Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002;110(6):823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 19.Valacchi G., Fortino V., Bocci V. The dual action of ozone on the skin. Br. J. Dermatol. 2005;153(6):1096–1100. doi: 10.1111/j.1365-2133.2005.06939.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu L., Wang L., Jia H.P., Zhao C., Heng H.H., Schutte B.C. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222(2):237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 21.Steubesand N., Kiehne K., Brunke G., Pahl R., Reiss K., Herzig K.H. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 2009;10:36. doi: 10.1186/1471-2172-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Y., Chen Q., Schmidt A.P., Anderson G.M., Wang J.M., Wooters J. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192(7):1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koczulla R., von Degenfeld G., Kupatt C., Krotz F., Zahler S., Gloe T. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Invest. 2003;111(11):1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niyonsaba F., Iwabuchi K., Someya A., Hirata M., Matsuda H., Ogawa H. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106(1):20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niyonsaba F., Ogawa H., Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111(3):273–281. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niyonsaba F., Someya A., Hirata M., Ogawa H., Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur. J. Immunol. 2001;31(4):1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Niyonsaba F., Ushio H., Nagaoka I., Okumura K., Ogawa H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 2005;175(3):1776–1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 28.van der Does A.M., Beekhuizen H., Ravensbergen B., Vos T., Ottenhoff T.H., van Dissel J.T. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 2010;185(3):1442–1449. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 29.Yang D., Chertov O., Bykovskaia S.N., Chen Q., Buffo M.J., Shogan J. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 30.Dhople V., Krukemeyer A., Ramamoorthy A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta. 2006;1758(9):1499–1512. doi: 10.1016/j.bbamem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Gambichler T., Skrygan M., Tomi N.S., Othlinghaus N., Brockmeyer N.H., Altmeyer P. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin. Int. Arch. Allergy Immunol. 2008;147(1):17–24. doi: 10.1159/000128582. [DOI] [PubMed] [Google Scholar]
- 32.Harder J., Dressel S., Wittersheim M., Cordes J., Meyer-Hoffert U., Mrowietz U. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J. Invest. Dermatol. 2010;130(5):1355–1364. doi: 10.1038/jid.2009.432. [DOI] [PubMed] [Google Scholar]
- 33.Ong P.Y., Ohtake T., Brandt C., Strickland I., Boguniewicz M., Ganz T. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 34.Peric M., Koglin S., Dombrowski Y., Gross K., Bradac E., Buchau A. Vitamin D analogs differentially control antimicrobial peptide/"alarmin" expression in psoriasis. PloS One. 2009;4(7) doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harder J., Bartels J., Christophers E., Schroder J.M. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276(8):5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 36.Hollox E.J., Huffmeier U., Zeeuwen P.L., Palla R., Lascorz J., Rodijk-Olthuis D. Psoriasis is associated with increased beta-defensin genomic copy number. Nat. Genet. 2008;40(1):23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganguly D., Chamilos G., Lande R., Gregorio J., Meller S., Facchinetti V. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206(9):1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lande R., Gregorio J., Facchinetti V., Chatterjee B., Wang Y.H., Homey B. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 39.Pecorelli A., Woodby B., Prieux R., Valacchi G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. Biofactors. 2019 Jul;45(4):536–547. doi: 10.1002/biof.1513. Epub 2019 May 14. [DOI] [PubMed] [Google Scholar]
- 40.Valacchi G., Pecorelli A., Belmonte G., Pambianchi E., Cervellati F., Lynch S. Protective effects of topical vitamin C compound mixtures against ozone-induced damage in human skin. J. Invest. Dermatol. 2017;137(6):1373–1375. doi: 10.1016/j.jid.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 41.Campbell Y., Fantacone M.L., Gombart A.F. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur. J. Nutr. 2012;51(8):899–907. doi: 10.1007/s00394-012-0415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gombart A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna C.W., Demond H., Kelsey G. Epigenetic regulation in development: is the mouse a good model for the human? Hum. Reprod. Update. 2018;24(5):556–576. doi: 10.1093/humupd/dmy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeder B.O., Wu Z., Nuding S., Groscurth S., Marcinowski M., Beisner J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469(7330):419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 45.Raschig J., Mailander-Sanchez D., Berscheid A., Berger J., Stromstedt A.A., Courth L.F. Ubiquitously expressed Human Beta Defensin 1 (hBD1) forms bacteria-entrapping nets in a redox dependent mode of action. PLoS Pathog. 2017;13(3) doi: 10.1371/journal.ppat.1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puri P., Nandar S.K., Kathuria S., Ramesh V. Effects of air pollution on the skin: a review. Indian J. Dermatol. Venereol. Leprol. 2017;83(4):415–423. doi: 10.4103/0378-6323.199579. [DOI] [PubMed] [Google Scholar]
- 47.Chakraborty K., Maity P.C., Sil A.K., Takeda Y., Das S. cAMP stringently regulates human cathelicidin antimicrobial peptide expression in the mucosal epithelial cells by activating cAMP-response element-binding protein, AP-1, and inducible cAMP early repressor. J. Biol. Chem. 2009;284(33):21810–21827. doi: 10.1074/jbc.M109.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park K., Elias P.M., Oda Y., Mackenzie D., Mauro T., Holleran W.M. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J. Biol. Chem. 2011;286(39):34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsutsumi-Ishii Y., Nagaoka I. NF-κB-mediated transcriptional regulation of human β-defensin-2 gene following lipopolysaccharide stimulation. J. Leukoc. Biol. 2002;71:154–162. [PubMed] [Google Scholar]
- 50.Takada Y., Mukhopadhyay A., Kundu G.C., Mahabeleshwar G.H., Singh S., Aggarwal B.B. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003;278(26):24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 51.Rademacher F., Simanski M., Hesse B., Dombrowsky G., Vent N., Glaser R. Staphylococcus epidermidis activates aryl hydrocarbon receptor signaling in human keratinocytes: implications for cutaneous defense. J Innate Immun. 2019;11(2):125–135. doi: 10.1159/000492162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sticozzi C., Pecorelli A., Lim Y., Maioli E., Pagnin E., Davis P.A. Modulation of skin oxidative stress and inflammatory markers by environmental stressors. Differences between young and old. J. Dermatol. Sci. 2012;65(3):226–228. doi: 10.1016/j.jdermsci.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Valacchi G., Pagnin E., Okamoto T., Corbacho A.M., Olano E., Davis P.A. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem. Biophys. Res. Commun. 2003;305(3):741–746. doi: 10.1016/s0006-291x(03)00812-x. [DOI] [PubMed] [Google Scholar]
- 54.McDaniel D., Farris P., Valacchi G. Atmospheric skin aging-Contributors and inhibitors. J. Cosmet. Dermatol. 2018;17(2):124–137. doi: 10.1111/jocd.12518. [DOI] [PubMed] [Google Scholar]
- 55.Valacchi G., Pagnin E., Corbacho A.M., Olano E., Davis P.A., Packer L. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic. Biol. Med. 2004;36(5):673–681. doi: 10.1016/j.freeradbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Aberg K.M., Radek K.A., Choi E.H., Kim D.K., Demerjian M., Hupe M. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J. Clin. Invest. 2007;117(11):3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paus R., Theoharides T.C., Arck P.C. Neuroimmunoendocrine circuitry of the 'brain-skin connection. Trends Immunol. 2006;27(1):32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J. Clin. Invest. 2007;117(11):3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J.E., Kim B.J., Jeong M.S., Seo S.J., Kim M.N., Hong C.K. Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells, and inflammatory skin diseases. J. Kor. Med. Sci. 2005;20(4):649–654. doi: 10.3346/jkms.2005.20.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patra V., Laoubi L., Nicolas J.F., Vocanson M., Wolf P. A perspective on the interplay of ultraviolet-radiation, skin microbiome and skin resident memory TCRalphabeta+ cells. Front. Med. 2018;5:166. doi: 10.3389/fmed.2018.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo S.J., Ahn S.W., Hong C.K., Ro B.I. Expressions of beta-defensins in human keratinocyte cell lines. J. Dermatol. Sci. 2001;27(3):183–191. doi: 10.1016/s0923-1811(01)00135-9. [DOI] [PubMed] [Google Scholar]
- 62.Chen X., Liu J., Zhou J., Wang J., Chen C., Song Y. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir. Res. 2018;19(1):5. doi: 10.1186/s12931-017-0700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivas-Santiago C.E., Sarkar S., Cantarella Pt, Osornio-Vargas A., Quintana-Belmares R., Meng Q. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect. Immun. 2015;83(6):2507–2517. doi: 10.1128/IAI.03018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vargas Buonfiglio L.G., Mudunkotuwa I.A., Abou Alaiwa M.H., Vanegas Calderon O.G., Borcherding J.A., Gerke A.K. Effects of coal fly ash particulate matter on the antimicrobial activity of airway surface liquid. Environ. Health Perspect. 2017;125(7) doi: 10.1289/EHP876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braff M.H., Di Nardo A., Gallo R.L. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Invest. Dermatol. 2005;124(2):394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 66.Kahlenberg J.M., Carmona-Rivera C., Smith C.K., Kaplan M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013;190(3):1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai Y., Adhikarakunnathu S., Bhardwaj K., Ranjith-Kumar C.T., Wen Y., Jordan J.L. LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PloS One. 2011;6(10) doi: 10.1371/journal.pone.0026632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrara F., Pambianchi E., Pecorelli A., Woodby B., Messano N., Therrien J.P. Redox regulation of cutaneous inflammasome by ozone exposure. Free Radic. Biol. Med. 2019 May 20;152:561–570. doi: 10.1016/j.freeradbiomed.2019.11.031. Epub 2019 Nov 26. [DOI] [PubMed] [Google Scholar]
- 69.Semple F., Dorin J.R. beta-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4(4):337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taub A., Bucay V., Keller G., Williams J., Mehregan D. Multi-center, double-blind, vehicle-controlled clinical trial of an alpha and beta defensin-containing anti-aging skin care regimen with clinical, histopathologic, immunohistochemical, photographic, and ultrasound evaluation. J. Drugs Dermatol. JDD. 2018;17(4):426–441. [PubMed] [Google Scholar]
- 71.Niyonsaba F., Kiatsurayanon C., Chieosilapatham P., Ogawa H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017;26:989–998. doi: 10.1111/exd.13314. [DOI] [PubMed] [Google Scholar]
- 72.Crivellari I., Sticozzi C., Belmonte G., Muresan X.M., Cervellati F., Pecorelli A. SRB1 as a new redox target of cigarette smoke in human sebocytes. Free Radic. Biol. Med. 2017;102:47–56. doi: 10.1016/j.freeradbiomed.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Benedusi M., Frigato E., Beltramello M., Bertolucci C., Valacchi G. Circadian clock as possible protective mechanism to pollution induced keratinocytes damage. Mech. Ageing Dev. 2018;172:13–20. doi: 10.1016/j.mad.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Toni L.S., Garcia A.M., Jeffrey D.A., Jiang X., Stauffer B.L., Miyamoto S.D. Optimization of phenol-chloroform RNA extraction. Methods (Duluth) 2018;5:599–608. doi: 10.1016/j.mex.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krasnodembskaya A., Song Y., Fang X., Gupta N., Serikov V., Lee J.-W. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cell. 2010;28(12):2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Korting H.C., Schollmann C., Stauss-Grabo M., Schafer-kotring M. Antimicrobial peptides and skin: a paradigm of translational medicine. Skin Pharmacol. Physiol. 2021;25:323–334. doi: 10.1159/000341990. [DOI] [PubMed] [Google Scholar]
- 77.Valacchi G., Sticozzi C., Pecorelli A., Cervellati F., Cervellati C., Maioli E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012;1271:75–81. doi: 10.1111/j.1749-6632.2012.06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large datasets were generated or analyzed in this study; however, all data will be shared by open request to the corresponding author.