Abstract
Although much is known about the biochemical regulation of glycolytic enzymes, less is understood about how they are organized inside cells. We systematically examine the dynamic subcellular localization of glycolytic protein phosphofructokinase-1/PFK-1.1 in Caenorhabditis elegans. We determine that endogenous PFK-1.1 localizes to subcellular compartments in vivo. In neurons, PFK-1.1 forms phase-separated condensates near synapses in response to energy stress from transient hypoxia. Restoring animals to normoxic conditions results in cytosolic dispersion of PFK-1.1. PFK-1.1 condensates exhibit liquid-like properties, including spheroid shapes due to surface tension, fluidity due to deformations, and fast internal molecular rearrangements. Heterologous self-association domain cryptochrome 2 promotes formation of PFK-1.1 condensates and recruitment of aldolase/ALDO-1. PFK-1.1 condensates do not correspond to stress granules and might represent novel metabolic subcompartments. Our studies indicate that glycolytic protein PFK-1.1 can dynamically form condensates in vivo.
Significance
Our study demonstrates that glycolytic proteins can dynamically relocalize in vivo into liquid-like compartments. The study provides a biophysical characterization of the properties of these subcellular compartments in vivo. These biophysical properties might be conserved to organize local metabolism in cells.
Introduction
Cells are internally organized through compartmentalization. Cellular compartments can delineate areas and locally concentrate molecules of a pathway (1). Although most of our cell biological understanding of compartmentalization is derived from the concept of membrane-bound organelles, an increasing body of work has revealed the existence of membraneless organelles—structures such as the nucleolus, germ granules, and Cajal bodies, which lack physical barriers like lipid membranes but can still self-organize into biomolecular condensates (2,3). An important question in cell biology has become which cytoplasmic processes are organized into these membraneless organelles.
Glycolysis is a metabolic pathway that consists of 10 enzymatic steps. Unlike oxidative phosphorylation, which is housed within the membrane-bound mitochondrion, glycolytic enzymes are soluble proteins in the cytosol for most eukaryotic cells. Early biochemical studies using rat muscle and brain extracts demonstrated that glycolytic proteins interchange between soluble and particulate states depending on different metabolites or hypoxic conditions, suggestive of transient interactions that could result in the subcellular organization of glycolysis (4, 5, 6, 7, 8, 9). Studies in red blood cells, which lack mitochondria, suggest that glycolytic proteins compartmentalize near the plasma membrane and that their subcellular organization is important for the regulation of cellular cation pumps (10, 11, 12). Immunohistochemical studies on isolated Drosophila flight muscle revealed a distinct pattern of colocalization of glycolytic protein isoforms to muscle sarcomeres (13) and vertebrate endothelial cells display enrichment of glycolytic proteins to lamellipodia and filopodia, where mitochondria are mostly absent (14). Together, these studies and others suggest that glycolytic proteins could be compartmentalized in specific tissues (15,16). Yet, the notion of a subcellular organization for glycolytic proteins has remained controversial, largely because of the lack of studies examining the dynamic distribution of these enzymes in vivo (17,18).
We recently reported that in Caenorhabditis elegans neurons, glycolytic proteins dynamically relocalize near synapses upon energy stress induced by transient hypoxia (19). Glycolytic enzymes were also shown to colocalize into subcellular compartments termed “G-bodies” in yeast (20) and in mammalian tissue culture cells (16). A human isoform of phosphofructokinase (PFK) was shown to assemble into tetramers that oligomerize into higher-ordered filamentous structures in vitro and to dynamically compartmentalize in tissue culture cells in response to specific metabolites (21). Together, these studies indicate that in living cells, glycolytic proteins are not simply diffusely distributed in the cytosol, and suggest the existence of regulatory mechanisms that organize glycolytic proteins in vivo. The purported organization of glycolytic proteins in vivo could have important consequences for the subcellular regulation of this metabolic pathway (15,22,23) and its enzymatic activity (24). How proteins of the glycolytic pathway are subcellularly organized and how their localization is regulated are key questions that remain to be answered.
To examine these questions, we developed a hybrid microfluidic-hydrogel device for use with C. elegans to systematically examine and quantify the dynamic subcellular localization of glycolytic proteins in vivo. We focused our study on the rate-limiting enzyme of glycolysis, phosphofructokinase-1/PFK-1.1, and performed high-resolution in vivo imaging of its subcellular localization while precisely and dynamically controlling oxygen levels to transiently inhibit oxidative phosphorylation and induce acute energy stress. Using this system, we observed that in cells in which PFK-1.1 is diffusely localized in the cytosol, such as neurons, the enzyme can dynamically relocalize into biomolecular condensates in response to transient energy stress. Upon return to normoxic conditions, PFK-1.1 dispersed in the cytosol. We further determined that PFK-1.1 condensates exhibit liquid-like properties and that their molecular dynamics, including its viscosity and biophysical properties, change with prolonged hypoxic conditions. We observe that PFK-1.1 and ALDO-1 genetically interact to recruit each other into condensates, indicative of a self-association event that drives a feed-forward loop to recruit glycolytic proteins into condensates. Together, our studies demonstrate that the glycolytic proteins such as PFK-1.1 and ALDO-1 can dynamically compartmentalize in vivo in response to acute energy stress as phase-separated condensates.
Materials and methods
C. elegans strains and transgenic lines
C. elegans were maintained at 20°C using OP50 Escherichia coli as a food source as previously described (25). CRY2 expression clone carrying transgenic lines were kept separately in the dark in the same 20°C incubator. C. elegans N2 Bristol strain was used as the wild-type. The following mutant strains were received from Shohei Mitani (Tokyo Women’s Medical University, Tokyo, Japan): aldo-1(tm5782) and pfk-1.1(tm5741). The other strain used in this study is pfk-1.1(ola72). See Supporting materials and methods for details.
Hybrid microfluidic-hydrogel device setup and calibration
A reusable microfluidic polydimethylsiloxane (PDMS) device was fabricated to deliver gases through a channel adjacent to immobilized animals, following protocols as previously described (26). A 50 μm, oxygen-permeable PDMS membrane was permanently bonded using air plasma to create an enclosed arena for gas flow, and the opposite side of the device was permanently bonded to a glass slide for structural integrity. Animals were kept stationary during high-resolution imaging and exposure to shifting gas concentrations at the membrane surface by hydrogel immobilization (27). The gas device was carefully centered and lowered on top of the hydrogel assembly and clamped into a P2 series holder (Warner Instruments, Hamden, CT) to complete the closed system. Lastly, microfluidic tubing and components were connected to a nitrogen tank set to ∼1–10 psi using a low-pressure regulator. To generate hypoxic conditions in the hydrogel, nitrogen flow was delivered continuously through the device assembly and confirmed by observing bubbles in a waste beaker filled with water connected with tubing to the device outlet. To generate normoxic conditions in the hydrogel, the nitrogen tank was turned off and the inlet tubing was immediately disconnected at the tank. Note that in addition to the 0% oxygen condition, we also repeated the hypoxic experiments using an 8% oxygen condition in the same setup and obtained similar results (data not shown). C. elegans can survive anoxic condition for a whole day (28), and for our hypoxic experiments, which ranged from minutes to an hour, the animals were rescued and shown to be viable post-transient hypoxia. For complete details on the hybrid microfluidic-hydrogel device setup and calibration, see Supporting materials and methods.
Fluorescence recovery after photobleaching of PFK-1.1
For fluorescence recovery after photobleaching (FRAP), a 60 CFI Plan Apo VC (Nikon, Tokyo, Japan), numerical aperture 1.4, oil-immersion objective on an UltraView Vox spinning-disk confocal microscope (PerkinElmer Life and Analytical Sciences, Waltham, MA) and Volocity FRAP Plugin were used. To calibrate the FRAP Plugin, steps laid out in the Volocity User Manual (September 2011 edition), “Calibrating the Photokinesis Accessory,” were followed.
For a full FRAP, z-stack images covering the entire volume of the neurite were acquired. A small, circular region, approximately the size of the PFK-1.1 punctum, was used to bleach the entire PFK-1.1 punctum. Recovery images were acquired every 20 s. In ImageJ, acquired images were max projected. Images that passed the following three criteria were used for the analysis: 1) the fluorescence of the PFK-1.1 punctum was not saturated before photobleaching, 2) the punctum was photobleached to at least 80%, and 3) the neurite did not go out of focus during the image acquisition. For the fluorescence recovery calculations, a small region of interest (ROI) was drawn around the bleached punctum to measure its mean fluorescence. The mean fluorescence was then multiplied by the area of the ROI for total fluorescence. The same ROI was used to calculate a background fluorescence level. Background fluorescence was then subtracted from the total fluorescence for each time point. This calculated value was then normalized to the range of prebleach value as 1 and postbleach value as 0 and plotted against time. In comparing the fluorescence recovery at different time points, p-values were calculated using the Mann-Whitney U-test.
For a partial FRAP, a small circular ROI was made and used for photobleaching: 17 (w, 2.05 μm) × 14 (h, 1.69 μm) × 1 (d, 1 μm) dimension. For consistency, the same dimension was kept constant for all FRAP experiments. This ROI was placed just slightly overlapping with the PFK-1.1::EGFP punctum to cause partial bleaching but not full bleaching of the entire punctum. Single-plane images were acquired at maximum speed, resulting in ∼0.1 s intervals for postbleached images. Partial FRAP time-lapse images were analyzed in two ways: 1) kymograph to visualize the fluorescence recovery and 2) to individually calculate the fluorescence change in the bleached and unbleached regions. For calculating the fluorescence change, a small ROI was drawn within the bleached and unbleached regions, and fluorescence was measured throughout time. Kymograph analysis was also performed using ImageJ.
Viscosity estimation from partial FRAP
To understand the diffusion dynamics of PFK-1.1, we used partial photobleaching of PFK-1.1::EGFP in soluble state and in a condensate form. Because PFK-1.1 condensates were slightly bigger in the neuronal cell bodies, partial photobleaching and measurement of fluorescence recovery were conducted on PFK-1.1 found in the neuronal cell bodies. For the normoxia condition, diffuse PFK-1.1 were photobleached and recovery measured. For transient hypoxia condition, PFK-1.1 condensates were induced with nitrogen gas for 10–20 min using the hybrid microfluidic-hydrogel device and then partially photobleached. From there, fluorescence recovery curves were obtained using the FRAP analysis plugin developed by Robert Haase of the Scientific Computing Facility at Max Planck Institute of Molecular Cell Biology and Genetics. In summary, the measured signal was corrected using a reference point, and all values were normalized to the corrected signal at photobleaching as 0 for both the fluorescence recovery and time. The plotted FRAP data were then fitted to an exponential equation, y = 1 − ae−bt − c. Here, t is time in seconds; y is the normalized fluorescence recovery fraction; and a, b, and c are variables, where a and b are positive values and a + c is close to . From this exponential curve fitting, half time (τ, in seconds) was calculated and related to the diffusion coefficient (D) via a diffusion equation in a two-dimensional space, r2 = 4Dτ. Using the fluorescence recovery, viscosity was approximated as previously described (29). Briefly, the Stokes-Einstein relationship was used, where kB is the Boltzmann’s constant, T is the temperature, α is the radius of the PFK-1.1 particle (which we roughly estimated as 5 nm based on Webb et al. (30)), and η is the viscosity. In comparing the calculated viscosity values, p-value was calculated using the Mann-Whitney U test.
Aspect ratio calculations and size measurement of PFK-1.1
The aspect ratio was calculated with the max projected images. After thresholding the images to obtain an outline for each punctum, the “Analyze Particles” function in ImageJ was used to calculate aspect ratio values for each punctum and for all time points. Considering the resolution limit of a spinning-disk confocal (∼300 nm), any structure with a diameter less than 500 nm and an area smaller than 0.2 μm2 was excluded from the analyses. PFK-1.1 condensate size or area was measured after thresholding the images and using the “Analyze Particles” function.
PFK-1.1 rate of formation, dispersion, and initiation time calculations
Using the acquired time-lapse videos of PFK-1.1 cluster formation and dispersion with the hybrid microfluidic-hydrogel device, change in the max fluorescence of individual clusters were tracked over time using ImageJ. From plotting the max fluorescence change over time and performing linear regression, the region of where max fluorescence increases (for the formation) or decreases (for the dispersion) linearly was identified using the following parameters: minimum slope of at least 5 (fluorescence intensity/s) and minimum length of half the time of the total treatment time. Any punctum with a starting fluorescence or pixel value of 1.2 × 105 AU was excluded from the calculations because the max fluorescence did not further peak after this value or quickly reached a saturation point. MATLAB (The MathWorks, Natick, MA) was used to conduct linear regression with the criteria as mentioned above. Rate was defined as the slope of the fitted line, and the initiation time was defined as the time for the first data point of the fitted line. The p-value comparing the rates of PFK-11. cluster formation and dispersion was calculated using the Mann-Whitney U-test.
Results
PFK-1.1 localizes to specific subcellular compartments in vivo
To better understand the subcellular localization of glycolytic proteins in living animals, we generated transgenic C. elegans strains expressing functional fluorophore-tagged PFK-1.1 (PFK-1.1::EGFP) under its own promoter (Fig. S1 A). Expression of PFK-1.1::EGFP rescues synaptic vesicle cycle defects observed in pfk-1.1 loss-of-function alleles, indicating that tagged PFK-1.1 is both functional and able to recapitulate the endogenous expression pattern of the gene ((19) and data not shown). In vivo examination of the PFK-1.1 expression pattern revealed, as expected, broad expression of PFK-1.1 in multiple tissue types, including muscles and neurons (Figs. 1 A and S1, B and C). In most examined tissues, there were high levels of cytoplasmic PFK-1.1 and no pronounced subcellular organization.
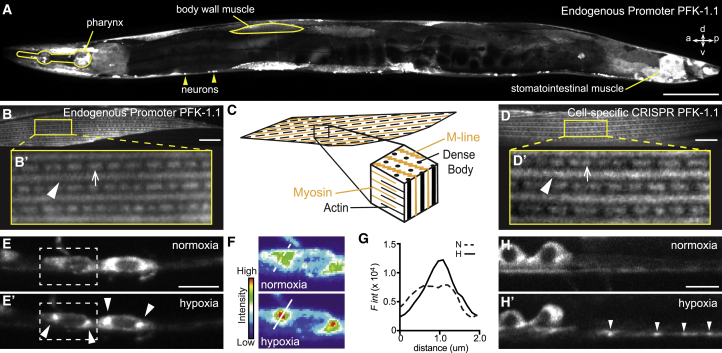
Figure 1.
Subcellular localization of PFK-1.1 in neurons and muscles of C. elegans. (A) Localization of PFK-1.1::EGFP expressed under its own promoter in C. elegans, with specific tissues labeled. For orientation: anterior (a), posterior (p), dorsal (d), and ventral (v). Scale bars, 100 μm. (B and B′) C. elegans body wall muscle showing the localization of PFK-1.1 in M-lines (arrowhead) and dense bodies (arrow). PFK-1.1 was expressed under its own promoter. Scale bars, 10 μm. (C) A schematic showing the myofibrils of the C. elegans body wall muscle. M-line and dense bodies act as anchors for myosin and actin filament, respectively. (D–H′) The mig-13 promoter was used in conditional CRISPR lines to tag the endogenous PFK-1.1 with GFP in a subset of tissues. (D and D′) Endogenous PFK-1.1 also localizes to M-lines (arrowhead) and dense bodies (arrow) in the body wall muscle. Scale bars, 10 μm. (E and E′) PFK-1.1 clusters form in the neuronal cell bodies (arrowheads) over time under hypoxia. Here, elapsed time of hypoxia was 55 min. (F) Pseudocolor based on fluorescence intensity of the dashed box in (E) for the two conditions. (G) Quantification of fluorescence intensity along the lines shown in (F) for each condition: N (normoxia) and H (hypoxia). (H and H′) PFK-1.1 clusters can be seen in the neurites (arrowheads) after hypoxia. Here, elapsed time of hypoxia was 10 min.
In some tissues, PFK-1.1 displayed subcellular organization. In muscles, PFK-1.1 localized into a striking pattern of longitudinal bands with alternating interdigitated foci (Fig. 1, B and B′), consistent with the protein being specifically enriched at two subcellular compartments: 1) M-lines within the A band of muscle sarcomeres and 2) dense body structures (functionally analogous to vertebrate Z-disks) (Fig. 1 C). M-lines and dense bodies function as anchors for thick myosin fibers and thin actin fibers, respectively, and their organization within muscle cells is necessary for filament cross-bridge formation and muscle contractions (31, 32, 33, 34). Consistent with our observations, immunohistological studies in Drosophila have also shown that glycolytic proteins are enriched at M-lines and Z-disks in flight muscles (13).
To better examine the in vivo subcellular localization of endogenous PFK-1.1 in single cells, we generated conditional transgenic lines via CRISPR-Cas strategies (35, 36, 37). In brief, we used CRISPR-Cas to introduce two flippase recombinase target (FRT) sites that flank a transcriptional stop motif followed by a GFP sequence to the C-terminus of the endogenous pfk-1.1 locus (Fig. S1 D). Introduction of these FRT sites did not result in detectable mutant phenotypes, including the synaptic vesicle phenotype observed for the PFK-1.1 loss-of-function alleles (data not shown), suggesting that the genomic modifications do not alter PFK-1.1 expression or function. Using cell-specific promoters, we then drove the expression of flippase to excise the FRT-flanking transcriptional stop motif, allowing for the expression of the endogenous GFP-tagged PFK-1.1 in single cells (see Supporting materials and methods). Flippase expression using mig-13 and unc-47 promoters resulted in the expression of PFK-1.1::GFP in subsets of tissues and with a low signal because of endogenous labeling. Consistent with the localization pattern of the overexpressed PFK-1.1::EGFP (Fig. 1 B), we also observed the localization of PFK-1.1 at M-lines and dense bodies in the muscles of the cell-specific CRISPR lines (Fig. 1, D and D′).
We then examined the subcellular localization of PFK-1.1 in neurons. Expression of PFK-1.1:EGFP in single neurons revealed that although PFK-1.1 is not equally distributed throughout the neurite, it is, for the most part, diffusely localized in the cytosol (Fig. S1 E). Previously, we observed that under conditions known to cause energy stress, such as transient hypoxia, PFK-1.1 dynamically relocalizes to subcellular compartments (19). These former studies were done by overexpressing PFK-1.1 from cell-specific promoters in single neurons. Consistent with these observations, an examination of the conditional CRISPR lines revealed that upon transient hypoxia, endogenous PFK-1.1 relocalizes from a diffuse cytosolic pattern to subcellular clusters both in the neuronal soma and in neurites (Figs. 1, E–H′ and S1 F).
Our findings demonstrate that in vivo and in metazoans, PFK-1.1 localizes to specific subcellular compartments. They also reveal that the localization pattern of PFK-1.1 varies between tissues. Importantly, our studies demonstrate that even in cells that have no obvious subcellular organization of PFK-1.1, such as the neurons, the localization of endogenous PFK-1.1 can dynamically change in response to cues within the cellular environment.
PFK-1.1 dynamically relocalizes to distinct subcellular compartments in response to transient hypoxia
Transient hypoxia inhibits oxidative phosphorylation at the mitochondria and induces cellular energy stress. To characterize the dynamic responses of PFK-1.1 to controlled changes in oxygen concentration, we developed a hybrid microfluidic-hydrogel device that enables precise regulation of oxygen concentration in the animal’s surrounding environment during high-resolution and long-term imaging of PFK-1.1 localization (Fig. 2, A–C; (26)). Calibration of our device enabled us to make precise and rapid switches between steady-state hypoxic (0% O2) and normoxic (21% O2) conditions within 1 min (Fig. S2, A–D). Animals under sustained steady-state normoxic conditions (21% O2) did not show relocalization of PFK-1.1 proteins in neurons (Fig. S3 A), indicating that variables such as air flow or mechanical forces during mounting to the device do not affect the localization of PFK-1.1. Induction of transient hypoxia, however, resulted in robust relocalization of PFK-1.1 in neurons from a diffuse state in the cytoplasm into distinct clusters near synapses (Fig. 2 D). The increased signal of PFK-1.1 near synapses was not the result of an increase in total protein levels at the neurite because the total fluorescence in the neurite did not increase upon hypoxic stimuli (Fig. S2 E).
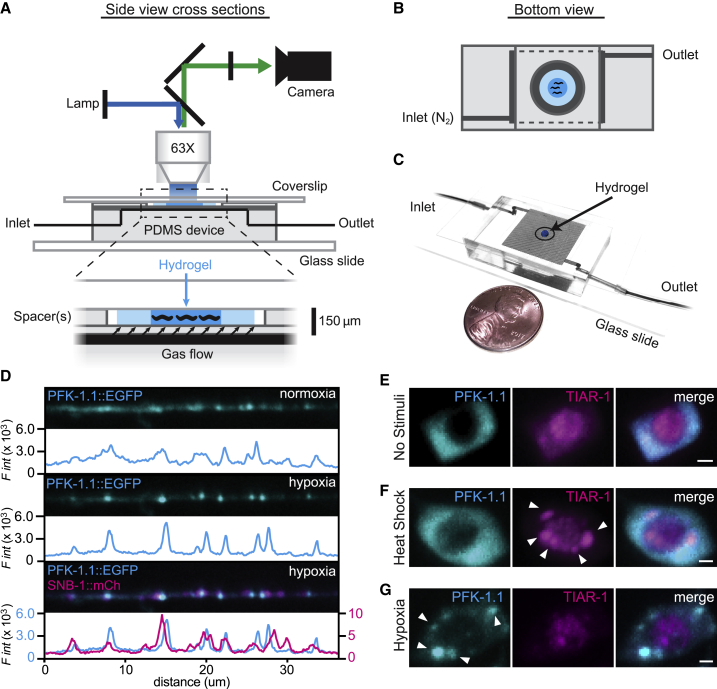
Figure 2.
Hybrid microfluidic-hydrogel device enables high-resolution imaging of PFK-1.1 localization and clustering dynamics during transient hypoxia. (A) A schematic of the hybrid microfluidic-hydrogel device used to induce transient hypoxic conditions in C. elegans while imaging PFK-1.1 continuously and at high resolution. Zoom-in (below) shows micron-scale device geometries for animal immobilization, gas delivery, and imaging. (B) A bottom view schematic of the device shows geometry of inlet and outlet channels connected to the gas arena (dark gray area). Animals are immobilized in hydrogel (dark blue) surrounded by buffer (light blue) and sealed by a circular, hole-punched PDMS spacer. Oxygen conditions experienced by the animals are controlled by inlet gas flow. (C) A picture of the microfluidic device, size comparable to a penny, with inlet and outlet tubing connected. (D) PFK-1.1::EGFP (cyan) in GABAergic neuron under normoxia (top panels) and after 10 min of transient hypoxia (middle panels). Coexpression with synaptobrevin-1/SNB-1::mCh (magenta, lower panel) shows that PFK-1.1 relocalized into clusters near synaptic sites upon transient hypoxia. Corresponding fluorescence intensity for each image is shown immediately below the corresponding image, with colors matching traced protein distribution in the image. (E) PFK-1.1::mRuby-3 (cyan) and TIAR-1::EGFP (magenta) in the cell body of neurons with no stimuli (normoxic conditions and 22°C). (F) Heat shock (37°C for 1 h) leads to formation of stress granules, as observed with TIAR-1 aggregates (arrowheads). PFK-1.1 does not form clusters under this condition. (G) Under transient hypoxic conditions (25–45 min), PFK-1.1 forms clusters in the cell body (arrowheads) that do not colocalize with TIAR-1. All scale bars, 1 μm.
Cellular stress is known to cause the aggregation of cytoplasmic RNAs and proteins into stress granules (38). To examine whether PFK-1.1 in neurons was relocalizing into stress granules, we simultaneously imaged PFK-1.1 and the stress granule protein, TIAR-1, under heat shock conditions known to cause stress granule formation (39,40). Consistent with previous studies, TIAR-1 in neurons was enriched in the soma and could be seen in both the cytoplasm and nuclei (Fig. 2 E). Heat shock by incubating worms at 37°C for 1 h resulted in the aggregation of TIAR-1 in both the cytoplasm and nuclei, as expected. However, PFK-1.1 did not form clusters under conditions that caused TIAR-1 aggregation (Fig. 2 F). We then examined whether stress granules are formed upon transient hypoxic conditions known to induce PFK-1.1 clustering. TIAR-1 has been shown to aggregate under several hours of prolonged hypoxic conditions, but not under transient hypoxia (41,42). Consistent with those reports, we observed that although transient hypoxia (less than 20 min) caused PFK-1.1 clustering, the same hypoxic conditions did not cause TIAR-1 clustering (Fig. 2 G). Lack of colocalization was also observed between PFK-1.1 and stress granule markers TDP-43 and G3BP1 (data not shown). Our findings demonstrate that TIAR-1 and PFK-1.1 cluster under different cellular conditions and suggest that PFK-1.1 clusters correspond to a new subcellular compartment distinct from stress granules.
PFK-1.1 reversibly organizes into biomolecular condensates
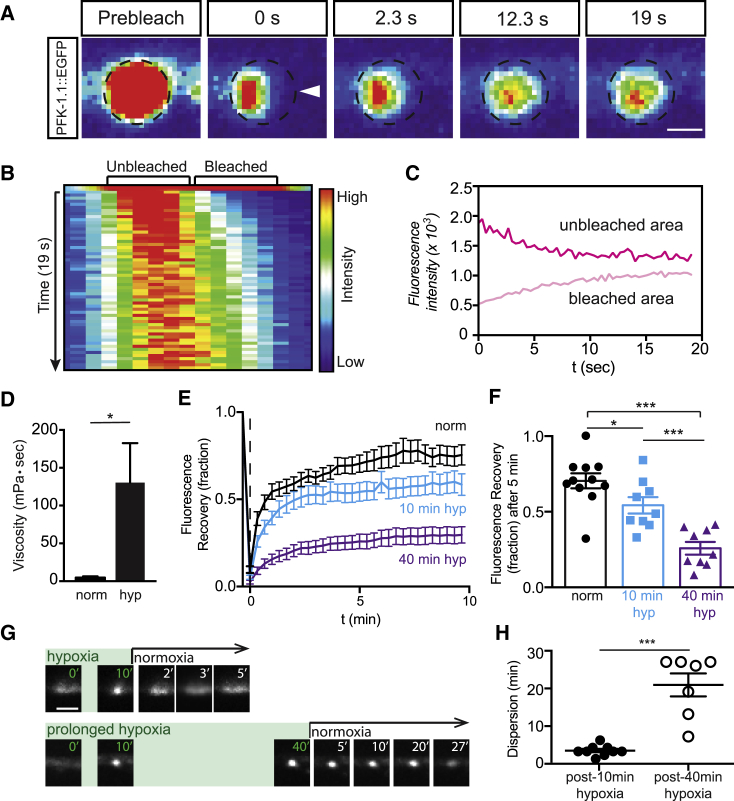
To examine the dynamics of PFK-1.1 clusters, we performed high-resolution time-lapse imaging of PFK-1.1 in single neurons of living animals upon transient hypoxia exposure. We focused our analyses in 3 μm neurite regions that displayed PFK-1.1 enrichment during normoxia. We observed that induction of transient hypoxia resulted in a redistribution of the PFK-1.1 protein within these regions, with PFK-1.1 concentrating into 0.5–1 μm compartments (Fig. 3, A and B). We also observed a concomitant decrease of PFK-1.1 levels in regions adjacent to the emerging compartments (Fig. 3 C). A kymograph of PFK-1.1 in neurites showed that the local formation of PFK-1.1 clusters, which remained relatively immobile, was due to a redistribution of diffuse PFK-1.1 proteins to concentrated compartments (Fig. 3 D). Together, our results indicate that PFK-1.1 puncta emerge through the local condensation of PFK-1.1 material into concentrated clusters, which we now refer as condensates (2,43).
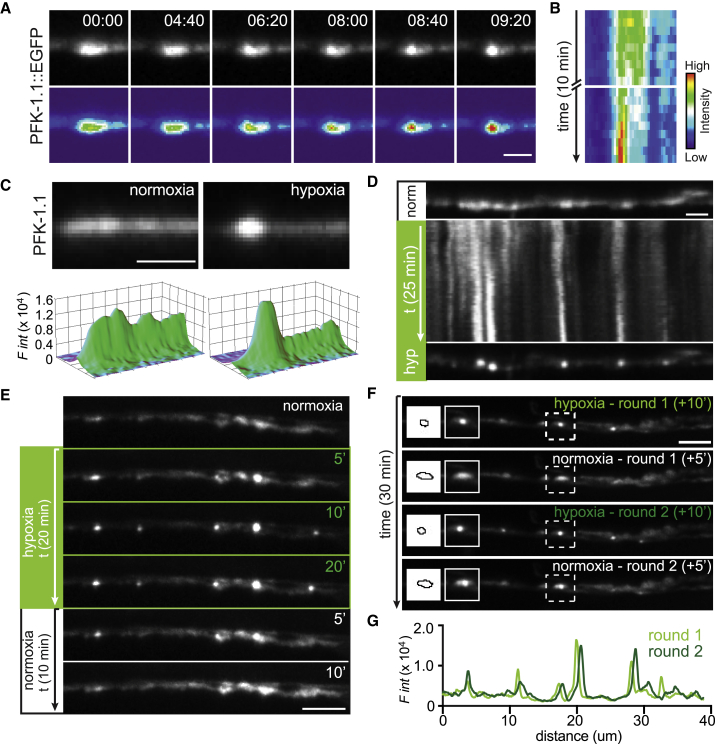
Figure 3.
PFK-1.1 reversibly organizes into biomolecular condensates. (A) Upon minutes of transient hypoxia, PFK-1.1 relocalizes from a dispersed localization pattern in the cytosol to a concentrated punctum. Time elapsed is indicated for each image in minutes:seconds. Bottom images have been pseudocolored based on fluorescence intensity. Scale bars, 2 μm. (B) A kymograph of PFK-1.1 localization shown in (A) over 10 min of transient hypoxia. 0–3 and 7–10 min are shown. (C) Localization of PFK-1.1 in a neurite during normoxia (left panel) and after 18 min of transient hypoxia (right panel). Corresponding three-dimensional surface rendering projections of fluorescence intensity in panels below. Scale bars, 1 μm. (D) Kymograph showing PFK-1.1 localization change over time, starting from normoxic condition (norm, top panel) to 25 min of transient hypoxia (hyp) (kymograph and lower panel). Scale bars, 2 μm. (E) Localization of PFK-1.1 through a normoxia-hypoxia-normoxia cycle at different time points (as indicated by minutes in upper right hand of images). PFK-1.1 condensates induced by transient hypoxic condition (green numbers, in minutes) can be seen dispersing away when the condition is returned to normoxia (white numbers, in minutes). See also Video S1. Scale bars, 5 μm. (F) PFK-1.1 localization under two cycles of transient hypoxia (rounds 1 and 2). PFK-1.1 forms condensates during 10 min of transient hypoxia and becomes diffuse during 5 min of normoxia. Dashed box in main panel corresponds to zoomed in image on the left (along with the outline of the corresponding morphology of the condensate). See also Video S2. Scale bars, 5 μm. (G) Line scan of first round (light green) and second round (dark green) of PFK-1.1 condensate localization in the neurite shown in (F) under transient hypoxic conditions. Note that during repeated cycles of normoxia and transient hypoxia, PFK-1.1 condensates reappear at similar subcellular locations.
We observed that condensates of PFK-1.1 were visible within 5 min of transient hypoxia. The persistence of hypoxic treatment resulted in the emergence of additional condensates, resulting in the almost complete relocalization of all visible PFK-1.1::EGFP into clusters (Fig. 3 E). Interestingly, restoring animals to normoxic conditions resulted in the dispersion of PFK-1.1 in the cytosol in both the neurite and cell body (Figs. 3 E and S3 B; Video S1). Thus, we asked whether PFK-1.1 proteins were capable of repeated cycles of condensation and dispersion in neurons. We observed that PFK-1.1 could undergo multiple cycles of condensation, displaying similar dynamics in each cycle (Fig. 3 F; Video S2). Importantly, during the two rounds of transient hypoxia and normoxia, we observed that PFK-1.1 condensates reappeared in similar locations (Figs. 3 G and S3 C). The repeated formation of PFK-1.1 clusters at the same location in the neurite occurred even after PFK-1.1 proteins completely diffused back to their initial state (Fig. S3 D). The reappearance of PFK-1.1 clusters at the same sites is consistent with our observations that PFK-1.1 clusters form preferentially (but not exclusively) near synapses (Fig. 2 D; (19)). The observation may suggest the existence of synaptic nucleating factors or the existence of subcellular conditions at the synapse that instructs local assembly of PFK-1.1 upon transient hypoxia.
PFK-1.1 condensates exhibit liquid-like behaviors
Biomolecular condensates, including the nucleolus, germ granules, prion proteins, and postsynaptic proteins, have been shown to exhibit liquid-like characteristics (29,44, 45, 46, 47). To examine whether PFK-1.1 condensates transiently induced by hypoxic treatment also exhibit liquid-like properties, we tested for the three key features that define liquid-like compartments: 1) liquid-like compartments are spherical because of surface tension and deformed because of cellular geometry constraints, 2) they can fuse and relax into a single spherical droplet, and 3) they exhibit fast internal molecular rearrangements (48). We systematically tested the PFK-1.1 clusters for their biophysical characteristics.
Liquid-like compartments are spherical because of surface tension and deformed because of cellular geometry constraints
Our qualitative observations on the emerging clusters revealed that most of the PFK-1.1 condensates were circular (and, because there was no obvious z axis asymmetry, likely spherical). To examine the shape of the PFK-1.1 condensates and to establish a quantitative criterion that defines the emergence of the clusters during condensation of PFK-1.1, we computed the aspect ratio, a ratio between the major and minor axes of the emerging clusters, over time (Figs. 4 A and S4, A and B; (44,49)). Only the condensates with sizes above the diffraction limit were quantified (see Materials and methods). Aspect ratio calculations of hypoxia-induced PFK-1.1 clusters in neurites revealed a distribution with a mean aspect ratio of 1.32 ± 0.04 (mean ± SEM; N = 38) (Fig. 4 B). A perfect sphere has an aspect ratio of 1, so our data suggest that PFK-1.1 clusters were similar, on average, to elongated spheroids.
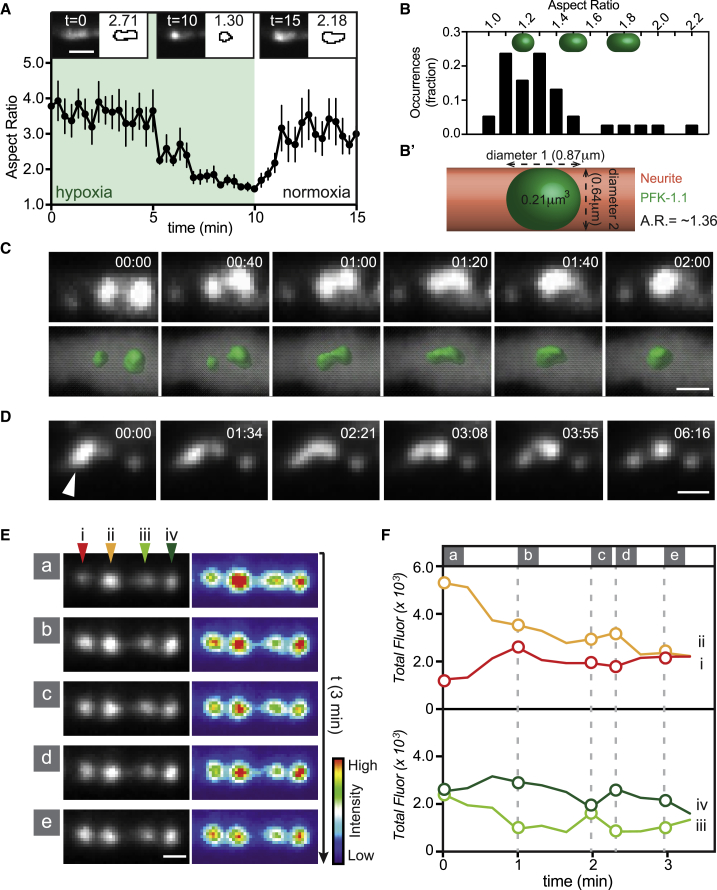
Figure 4.
PFK-1.1 condensates exhibit liquid-like behaviors. (A) Reversibility of PFK-1.1 localization as quantified by the aspect ratio of a population of PFK-1.1 condensates through one cycle of hypoxia-normoxia (N = 8 condensates). Mean (black circles) and SEM values are shown for each time point. Images on top and corresponding outlines are representative morphologies of condensates: from left to right, the beginning of hypoxia, after 10 min of transient hypoxia, and after 5 min of recovery in normoxia. Scale bars, 2 μm. (B) A histogram of the fraction of occurrence of the PFK-1.1 condensate aspect ratio after 40 min of transient hypoxia (N = 38 condensates). A perfect sphere has an aspect ratio of 1, and PFK-1.1 condensates have an average aspect ratio of 1.3. Representative spherocylindrical shapes for different aspect ratios (1.2, 1.5, and 1.8) are shown above. (B′) Schematic and calculation of aspect ratio for a theoretical spherical PFK-1.1 condensate with a volume of 0.21 μm3 that fits into a neurite with a diameter of 0.64 μm. Corresponding predicted aspect ratio (A.R.) is 1.36. (C) Time-lapse images of adjacent PFK-1.1 condensates under transient hypoxic conditions fusing and relaxing into a spheroid (above) and their corresponding three-dimensional surfaced rendered images (below). Elapsed time from the beginning of the imaging session is shown for each image in minutes:seconds. Here, 00:00 is 08:40 into the hypoxic treatment. See also Video S3. Scale bars, 1 μm. (D) A fluid-like movement recorded for a PFK-1.1 condensate (arrowhead) during transient hypoxia. Elapsed time from the beginning of the imaging session is indicated for each image in minutes:seconds. Scale bars, 1 μm. To see all frames recorded, refer to Fig. S5D. (E) Four adjacent PFK-1.1 condensates (i–iv) during transient hypoxia displayed exchange of PFK-1.1::EGFP in the span of 3 min. The first image (a) is after approximately 6 min into hypoxic treatment. See also Video S4. Scale bars, 1 μm. (F) Quantification of the fluorescence of the PFK-1.1 condensates in (E), with graphs pseudocolored to corresponding arrowheads in (i)–(iv) and time points (a)–(e) in (E).
Because we qualitatively also observed a relationship between the size of the puncta and the extent of the elongated spheroids (Fig. S4 C), we hypothesized that neurite space constraints could affect the shape of the larger (liquid) PFK-1.1 condensates. Liquids are capable of deformation, and liquid droplets confined within a cylindrical-like space such as a neurite would be expected to elongate into capsule-shaped spherocylinders. To test whether the larger PFK-1.1 condensates were constrained capsule-shaped spherocylinders, we first calculated the average diameter of the neurites by measuring cytoplasmic mCherry expressed in GABAergic neurons, and by examining electron micrographs of GABAergic neurites (50). From these calculations, we determined the average diameter of the examined neurites to be 0.64 ± 0.06 μm (mean ± SEM; N = 20) (Fig. S4 D). Empirical measurement of the area of the PFK-1.1 condensates (see Supporting materials and methods) revealed that these structures had an average area of 0.43 ± 0.04 μm2 (mean ± SEM; N = 38) (Fig. S4 E). Assuming radial symmetry of the neurite, the average condensate volume would be 0.21 ± 0.03 μm3, which is larger than the maximum sphere fitting within the neurite diameter, 0.14 ± 0.04 μm3. A hypothetical spherocylinder with a volume of 0.21 μm3 and radial diameter of 0.64 μm would have a length of 0.87 μm and an aspect ratio of 1.36 (Fig. 4 B′), a value similar to the one we empirically calculated, which was 1.32. Of note, the spherocylinder represents an optimal geometry for the condensate in the context of the constraints of the neurite because this shape minimizes surface area as compared to several smaller spherical droplets of equal volume. Thus, our findings suggest that PFK-1.1 condensates have liquid-like properties that result in spherocylindrical morphologies because of constraints by two physical forces: the surface tension of the condensate and the dimensions of the neurite.
Liquid-like structures can fuse and relax into a single spherical droplet
Phase-separated compartments display stereotypical fluid-like behaviors that include fusion. We reasoned that if PFK-1.1 condensates displayed liquid-like characteristics, condensates that happened to nucleate in close proximity (within one micron from each other) would fuse. Indeed, PFK-1.1 condensates that came into physical contact fused into new, larger clusters (Figs. 4 C and S5, A and B; Videos S3 and S4). Quantification of the total area of the two PFK-1.1 condensates before fusion was similar to the total area of the single PFK-1.1 condensate postfusion (Fig. S5 C). The new clusters relaxed into spheroid shapes (within the geometrical constraints of the neurite) upon fusion, consistent with a thermodynamically favored response of a liquid compartment to surface tension (29). Moreover, whereas most PFK-1.1 particles in vivo retained spheroid shapes upon formation, some particles would occasionally become deformed and even shear, presumably because of cytoplasmic flux or transport of organelles disrupting the PFK-1.1 condensates in the tightly packed spaces of the neurite (Figs. 4 D and S5 D). Shearing of droplets might represent an interaction between the phase-separated condensates and the cellular medium, which could occasionally provide an energetic cost that favors the formation of smaller droplets (51, 52, 53). Importantly, deformation of the condensates resulted in transient fluid-like movements and eventual relaxation back into spheroid structures.
Quantification of the levels of fluorescence in nearby condensates revealed that growth of a condensate happened at the expense of adjacent condensates in a process that was reminiscent of Ostwald ripening (Fig. 4 E). But unlike Ostwald ripening, which is a thermodynamically spontaneous process in which smaller droplets dissolve into energetically favored larger droplets (54), some PFK-1.1 condensates in vivo did not result in larger droplets. Instead, the exchange of material reached a dynamic equilibrium between adjacent condensates as they approached similar fluorescence levels (Fig. 4, E and F and Video S5). The observed in vivo dynamics between adjacent condensates could be because of active cellular processes that drive the formation of two similar condensates (55,56), or because of other unidentified phenomena. Importantly, the exchange of material between adjacent clusters is consistent with liquid-like behaviors of the PFK-1.1 condensates.
Liquid compartments exhibit fast internal molecular rearrangements
To further probe the molecular dynamics of PFK-1.1 condensates, we performed FRAP experiments. We observed that upon partial FRAP of the PFK-1.1 condensate, fluorescence in the bleached area recovered within seconds of postbleaching (Figs. 5, A and B and S5, E and E′). Consistent with an internal rearrangement resulting in fluorescence recovery, we observed that fluorescence in the bleached region increased at the expense of the unbleached areas (Fig. 5 C). The observed molecular dynamics for PFK-1.1 within condensates suggest that PFK-1.1 remains in liquid phase droplets capable of undergoing internal dynamic rearrangements (29).
Figure 5.
PFK-1.1 compartments exhibit fast internal molecular rearrangements that harden with time. (A) FRAP of a PFK-1.1::EGFP condensate after 10–20 min of transient hypoxia. PFK-1.1::EGFP condensate initial shape is outlined with dashed circle, and partial area bleached is highlighted with arrowhead in second panel. Scale bars, 1 μm. (B) A kymograph of fluorescence distribution across the bleached punctum in (A). (C) Change in fluorescence values for the unbleached region (dark pink line) and the bleached region (light pink line) of the condensate in (A) over time (seconds). (D) Viscosity estimates of PFK-1.1 proteins under normoxia (labeled “norm”) and 10–20 min of transient hypoxia (labeled “hyp”). (N = 4 neurons for each conditions). (E and F) FRAP of cytosolic PFK-1.1 after normoxia (black line, labeled “norm”), PFK-1.1 condensate after 10 min of transient hypoxia (blue line, labeled “10 min hyp”), and PFK-1.1 condensate after 40 min of transient hypoxia (purple line, labeled “40 min hyp”) and corresponding fraction of fluorescence recovery 5 min postbleaching calculated in (F). In all experiments, PFK-1.1 was photobleached to a minimum of 80% of its original fluorescence value. Note how recovery dynamics decrease with increased exposure to transient hypoxia, suggesting that PFK-1.1 condensates harden with time. (G) Comparison of PFK-1.1 condensate reversal in normoxia after 10 min (top row) and 40 min (bottom row) of hypoxia (marked by green). Time (in minutes) for each image is indicated on the top right corner, and corresponding images displaying dispersion due to normoxia are shown for the two conditions examined (10 min of hypoxia in the top row, and 40 min of hypoxia in the bottom row). Scale bars, 2 μm. (H) Quantification of the time it takes for PFK-1.1 condensates to disperse upon return to normoxia when condensates were formed under 10 min of hypoxia (black circles; N = 9) or 40 min of hypoxia (open circles; N = 7). Error bars denote SEM. ∗p < 0.05. ∗∗p < 0.01. ∗∗∗p < 0.001 between indicated groups.
PFK-1.1 condensates harden with time
To test whether transient hypoxia was inducing a change in the molecular dynamics of PFK-1.1 resulting in liquid-liquid phase separation, we measured the half-time recovery and lateral diffusion of PFK-1.1 in its soluble state during normoxia versus its condensate state during hypoxia by partially photobleaching the condensates (29). Using FRAP, we determined that the average half-time recovery in the photobleached regions for diffusely localized PFK-1.1 was 0.6 s, whereas in the condensate, it was 3.8 s (data not shown). Our findings indicate a substantial change in the diffusion dynamic of PFK-1.1 between normoxia and hypoxia and are consistent with changes in molecular dynamics during condensation.
To approximate the fluidity of PFK-1.1 under the two states, we used the Stokes-Einstein equation (see Materials and methods) to estimate the viscosity of PFK-1.1. Although the accuracy of the viscosity calculations is limited by the small size of the puncta, we note that, in the soluble state, PFK-1.1 had an estimated viscosity of 4.8 ± 1.7 mPa ⋅ s (mean ± SEM; N = 4), which is ∼5 times more viscous than water. This value is comparable to the reported viscosity (2.0–3.0 mPa ⋅ s) of the cytoplasm (57), consistent with PFK-1.1 being largely diffusely localized in the cytosol under normoxic conditions. Upon transient hypoxic conditions, however, the calculated viscosity for PFK-1.1 was 130.5 ± 52.1 mPa ⋅ s (mean ± SEM; N = 4) (Fig. 5 D). Our findings suggest that PFK-1.1 increases two orders of magnitude in viscosity after it forms condensates. Importantly, our findings indicate that the biophysical properties of PFK-1.1 change upon transient hypoxia and suggest that these changes could contribute to PFK-1.1 relocalizing into phase-separated condensates.
Changes in the viscoelastic properties of molecular condensates contribute to liquid-liquid phase separation. Yet, it has also been documented that changes in the material phase of the condensates can contribute to their maturation into more “gel-like” or “solid-like” states that eventually result in the (pathological) aggregation of proteins (45,58). We reasoned that if transient hypoxia was inducing a change in the molecular dynamics of PFK-1.1, one would expect that conditions of prolonged hypoxia would further alter the molecular dynamics and the biophysical properties of the condensates. To test whether prolonged hypoxia alters the PFK-1.1 dynamics, we compared the fluorescence recovery of PFK-1.1 condensates that were fully photobleached after 10 min (newly formed condensates) and 40 min (“mature” condensates) of hypoxic treatment. We observed that the recovery dynamics of mature PFK-1.1 condensates were significantly slower than those of newly formed condensates (Figs. 5, E and F and Video S6. PFK-1.1 Condensates Do Not All Fuse, But Do Have Fluid-Like Movements, Document S2. Article plus supporting material). The slower recovery dynamics due to prolonged hypoxia are consistent with an increase in the immobile fraction, which could be from changes in the state of the liquid condensates into solid or gel-like states. Also supportive of a change in PFK-1.1 condensate into solid or gel-like state, we observed that PFK-1.1 condensates formed during 10 min of hypoxia required an average of 3.2 ± 0.5 min (± SEM; N = 9) in normoxia before dispersion was observed, whereas PFK-1.1 condensates formed and maintained during 40 min of hypoxia required an average of 20.9 ± 3.1 min (± SEM; N = 7)) for dispersion (Fig. 5, G and H). Our findings are consistent with the increased viscosity observed for newly formed PFK-1.1 condensates (Fig. 5 D) and suggest that changes in the viscoelastic properties of PFK-1.1 contribute to the biophysical properties of the condensates, including transitions into liquid-like or gel-like states.
In the course of our studies, we also imaged mature condensates that happened to be adjacent to one another. These condensates sometimes came into physical contact with each other, just as we had previously documented for the fusion events occurring in newly formed condensates (Fig. 4 C). Interestingly, however, and unlike the newly formed condensates, some of these mature condensates deformed and “bounced” off each other upon contact (Fig. S6 B; Video S6). Our observations of these dynamics suggest that the viscoelastic properties of PFK-1.1 condensates change upon persistent hypoxia.
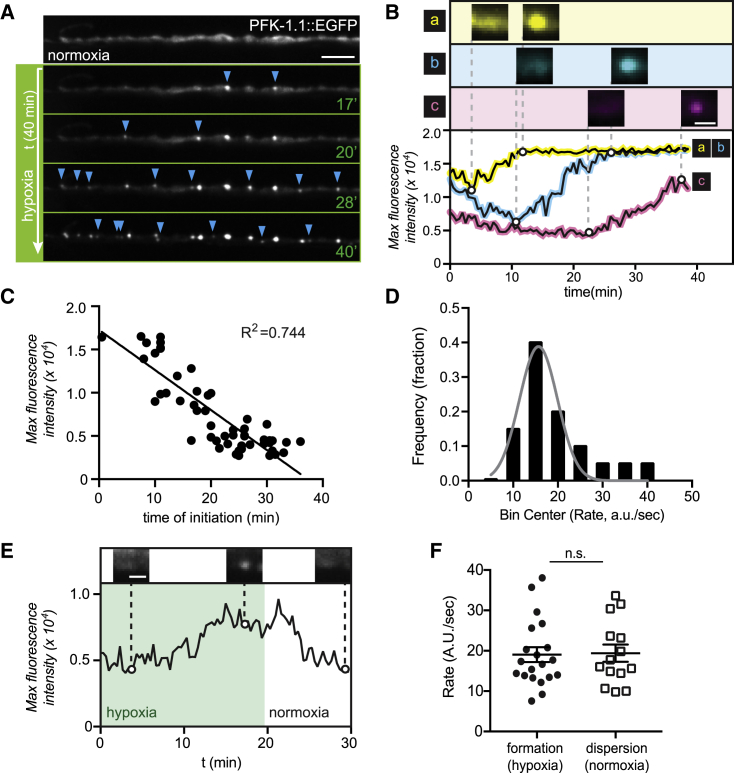
Local concentration of PFK-1.1 correlates with the initiation time of condensates
Physical principles of phase transitions influence the assembly of membraneless organelles such as p-granules and the nucleolus (59). A key biophysical aspect that influence the dynamics of membraneless organelle assembly is protein concentration (43,48,60). Because we observed a nonhomogeneous distribution of PFK-1.1 proteins throughout the neurite even during normoxia (Fig. S1 E), we hypothesized that PFK-1.1 concentration may influence cluster formation at subcellular regions. To test this hypothesis, we monitored the formation of PFK-1.1 condensates for specific subcellular regions that had varying concentrations of PFK-1.1 under conditions of persistent hypoxia (see Materials and methods). From the lengthened hypoxic treatment, we observed a continuous and asynchronous formation of PFK-1.1 condensates throughout the neurite, with PFK-1.1 condensates forming at different time points in different cellular regions (Fig. 6, A and B; Video S7). Comparisons of the local concentration of PFK-1.1 proteins in specific subcellular regions of the neurite, and the time at which the PFK-1.1 condensates initiate in those regions, revealed an inverse correlation between these two variables (Fig. 6 C). Our findings are consistent with initial PFK-1.1 concentrations affecting the time of initiation of the condensates, with a higher level of PFK-1.1 proteins corresponding to an earlier appearance of PFK-1.1 condensates.
Figure 6.
Local concentration of PFK-1.1 drives initiation of condensates. (A) PFK-1.1 dynamics through persistent hypoxia. Blue arrowheads mark newly formed PFK-1.1 condensates at each time point (green numbers indicate minutes into hypoxic treatment), indicating asynchronous formation of PFK-1.1 condensates throughout the neurite. Scale bars, 5 μm. See also Video S7. (B) Three separate regions in neurites, where PFK-1.1 condensates appear, are shown (a–c). The corresponding maximum fluorescence value over time for the three regions is shown below, pseudocolored to correspond to the region specified above (also labeled with a–c). PFK-1.1 condensate formation occurred with a linear increase in maximum fluorescence intensity through time in response to transient hypoxia. Scale bars, 1 μm. (C) The maximum fluorescence intensity of PFK-1.1::EGFP just before PFK-1.1 condensates are observed was plotted and compared with the time when PFK-1.1 condensates first appear (time of initiation). Note how the time of initiation negatively correlates with the maximum fluorescence intensity (equivalent to the amount of PFK-1.1 in a specific region of the neurite where PFK-1.1 condensates appear). (D) Rates for PFK-1.1 condensate formation were calculated for 20 condensates as maximum fluorescence intensity change over time, and their distribution was plotted. The rates are constant, regardless of initial concentration, and a normal (Gaussian) distribution (N = 20 condensates). (E) Maximum fluorescence intensity of a PFK-1.1 condensate calculated over time (minutes) during transient hypoxia (shaded green) and subsequent return to normoxic conditions. Representative images of PFK-1.1 condensates are shown for given time points above the graph. (F) Rates of formation and dispersion of PFK-1.1 condensates under transient hypoxia (green circles, left) and normoxia (white squares, right), respectively. Note how the rates of formation and dispersion are both constant, normally distributed (compare with D) and not significantly (specified as n.s. in graph) different. Error bars denote SEM.
Interestingly, we also observed that once PFK-1.1 condensates appeared and regardless of where or when they appeared in the neurite, they underwent similar linear rates of increase in protein fluorescence. The constant increase of fluorescence did not vary based on the starting concentration and instead displayed a normal distribution around an average constant rate (Fig. 6 D). Furthermore, calculations of the rates of PFK-1.1 dispersion upon return to normoxic conditions also showed a normal distribution around an average rate that was similar to the average rate of condensation (Fig. 6, E and F).
Together, we found two properties regarding the local emergence of PFK-1.1 condensates across subcellular regions: 1) concentration, which correlates with the timing of initiation of PFK-1.1 condensates, and 2) rates of PFK-1.1 condensate growth (during transient hypoxia) and dispersion (during normoxia), which share a similar normal distribution regardless of initial concentration at subcellular locations. Thus, the asynchronous formation of PFK-1.1 condensates throughout the neurite could result from the initial uneven distribution of PFK-1.1 in neurites, which in turn determines the local concentration and the initiation time of PFK-1.1 condensates.
PFK-1.1 and aldolase are interdependent for the formation of glycolytic condensate
Multivalent interactions between proteins or proteins and RNAs play important roles in driving condensates into liquid-like protein droplets (2). Multivalent interactions can be mediated via folded protein domains, or intrinsically disordered regions with multiple interacting motifs (48,61, 62, 63, 64, 65, 66, 67). These interactions leading to phase separation can be recapitulated in vivo and in vitro by engineering self-association domains (63,68,69), underscoring the importance of multivalent interactions in the formation of condensates. PFK-1.1 does not have predicted intrinsically disordered but is a protein with dihedral symmetry capable of forming higher-order oligomers via multivalent interactions (21,70, 71, 72).
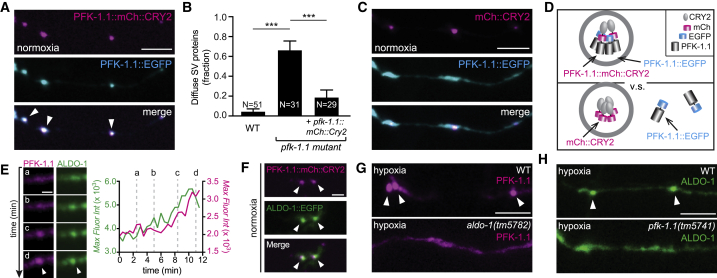
To test whether multivalent interactions could induce the formation of PFK-1.1 condensates, we tagged PFK-1.1 with cryptochrome 2 (CRY2), a class of flavoproteins from Arabidopsis thaliana that are capable of mediating oligomerization (73). Optogenetic control of CRY2 has been used to drive phase separation of intrinsically disordered proteins (69). The extent of CRY2-induced oligomerization depends on the protein that the CRY2 is fused to, and it has been observed that tetrameric proteins highly enhance the homo-oligomerization of CRY2 (74,75). We observed that CRY2-tagged PFK-1.1 formed condensates even in the absence of any light stimulation and under normoxic conditions (Figs. 7, A and F and S7 A). We also note that the PFK-1.1 condensates promoted by CRY2 were functional and capable of rescuing the synaptic vesicle phenotype in pfk-1.1(ola72) mutant animals (Fig. 7 B). Interestingly, the CRY2-tagged PFK-1.1 condensates localized to the synapse (Fig. S7 A), suggesting the existence of signals or scaffolds that spatially instruct the site of phase separation, even when promoted by CRY2. These results indicate that PFK-1.1 self-association via CRY2 is sufficient to drive its condensation even in the absence of stimuli, and result in functional condensates capable of sustaining the synaptic vesicle cycle at synapses.
Figure 7.
PFK-1.1 and aldolase condensate formation. (A) Under normoxia, PFK-1.1::mCh::CRY2 (magenta, top panel) is sufficient to cause PFK-1.1::EGFP (cyan, middle panel) to form condensates. Merged image (bottom panel) shows colocalization of the CRY2-tagged (magenta) and non-CRY2-tagged (cyan) PFK-1.1 condensates (white arrowheads). (B) In transient hypoxic conditions, the fraction of animals displaying a diffuse pattern of RAB-3 in AIY neurons in the wild-type, the loss-of-function allele pfk-1.1(ola72) (labeled as pfk-1.1 mutant), and AIY cell-specific expression of the pfk-1.1::mCh::CRY2 array in pfk-1.1(ola72) animals. Note that the expression of pfk-1.1::mCh::CRY2 rescues the synaptic vesicle phenotype in pfk-1.1(ola72) mutant animals, indicating that pfk-1.1::mCh::CRY2 is functional. (C) Under the same normoxic condition as in (A), when coexpressed with mCh::CRY2 (magenta, top panel), PFK-1.1::EGFP (cyan, middle panel) remains diffuse. A merged image of the two is shown in the bottom panel. (D) A schematic of the localization of PFK-1.1::EGFP in the presence of PFK-1.1::mCh::CRY2 (top panel) or control mCh::CRY2. (E) PFK-1.1 and ALDO-1 dynamically form clusters in response to a hypoxic condition. Left: formation of PFK-1.1::mRuby-3 (magenta) and ALDO-1::EGFP (green) condensate (arrowheads) through times (a)–(d). Right: change in the max fluorescence intensity of the PFK-1.1 (magenta) and ALDO-1 (green) over time. The corresponding four images of (a)–(d) on the left are indicated with dotted gray lines on the graph. Scale bars, 1 μm. (F) PFK-1.1::EGFP condensates in wild-type (upper image) and aldo-1(tm5782) mutant animals (lower image) after 20 min of hypoxia. Arrowheads point at condensates. Note the absence of PFK-1.1 condensates in aldo-1(tm5782) mutant animals. (G) Same as (F), but examining ALDO-1::EGFP in pfk-1.1 (tm5741) mutant animals. Note the absence of ALDO-1 condensates in pfk-1.1(tm5741) mutant animals. (A), (B), (G), (H) scales, 5 μm. (H) ALDO-1 (green) coexpressed with PFK-1.1::mCh::CRY2 (magenta) in normoxic condition (as in A). Note CRY2-promoted condensates of PFK-1.1 recruit ALDO-1. Colocalization of the PFK-1.1::CRY2 and ALDO-1 condensates is marked with arrowheads. Scale bars, 2 μm. N = number of animals. Error bars denote SEM. ∗p < 0.05. ∗∗p < 0.01. ∗∗∗p < 0.001 between indicated group.
We then asked whether the PFK-1.1::mCherry::CRY2 ectopic condensates were sufficient to drive condensation of non-CRY2-tagged glycolytic proteins. We examined this by simultaneously expressing PFK-1.1::mCherry::CRY2 and PFK-1.1::EGFP in single neurons. As a control, we expressed mCherry::CRY2 along with the examined glycolytic protein. We observed that in neurons expressing mCherry::CRY2 and PFK-1.1::EGFP, PFK-.1.1 remained diffusely localized in the cytoplasm under normoxia, as expected (Fig. 7, C and D). However, in neurons coexpressing PFK-1.1::mCherry::CRY2 and PFK-1.1::EGFP, ectopic PFK-1.1::mCherry::CRY2 condensates recruited PFK-1.1::EGFP even under normoxia (Fig. 7, A and D).
Is recruitment of PFK-1.1 to condensates necessary for the recruitment of other glycolytic proteins? We used the microfluidic setup to simultaneously examine PFK-1.1 and glycolytic protein aldolase/ALDO-1. Aldolase, like PFK-1.,1, is diffusely localized in the cytosol under normoxic conditions. Upon transient hypoxia, we observed that the two glycolytic proteins condense at the same sites and with similar recruitment kinetics (Fig. 7 E; Video S8).
We then examined whether recruitment of PFK-1.1 to condensates depends on ALDO-1 by observing PFK-1.1 localization in aldo-1 loss-of-function mutants. Interestingly, PFK-1.1 condensate formation was suppressed in aldo-1(tm5782) mutant animals, suggesting a requirement for ALDO-1 in PFK-1 recruitment (Figs. 7 F and S7, B and C). Similarly, ALDO-1 condensate formation was suppressed in pfk-1.1(tm5741) loss-of-function mutants, indicating a requirement for PFK-1.1 in the recruitment of ALDO-1 (Figs. 7 G and S7, B and C). Together, our findings indicate that PFK-1.1 and ALDO-1 genetically interact to recruit each other into condensates.
Is PFK-1.1 condensation sufficient to recruit other glycolytic proteins, like ALDO-1, into the condensates? We expressed PFK-1.1::mCherry::CRY2 and ALDO-1:GFP and observed that even under normoxic conditions, when PFK-1.1 condensates are facilitated by CRY-2, ALDO-1 is recruited into the condensates (Figs. 7 H and S7 C). Our findings suggest that PFK-1.1 self-association, promoted by the introduction of the CRY2 domain, is capable of recruiting other glycolytic proteins. Together, our findings suggest that PFK-1.1 self-association drives a feed-forward loop that results in the recruitment of other glycolytic proteins and that there is a mutual dependency between glycolytic enzymes for glycolytic protein condensate formation.
Discussion
PFK-1.1 localizes in subcellular compartments in vivo. Although widely regarded as cytosolic in nature, glycolytic enzymes like PFK have long been observed, in both biochemical and immunohistological studies, to self-associate into complexes and be enriched at subcellular compartments in specific cell types (4, 5, 6, 7, 8, 9, 10, 11, 12, 13,76). These observations led to the coining of the term “ambiquitous,” used to refer to the ability of glycolytic proteins to exist both in soluble and particulate or membrane-bound states (9). Biochemical studies indicated that the state of the glycolytic proteins depended on the metabolic state (and identity) of the tissue from which they were harvested (Wilson (8,9)). The ambiquitous properties of glycolytic proteins, however, remained controversial because the observed biochemical associations were weak, transient, and dependent on the presence of particular metabolites (17,77,78) and because of the lack of supporting in vivo evidence (18). In our study and through a systematic examination of endogenous PFK-1.1 via the use of a hybrid microfluidic-hydrogel device, we conclusively determine that PFK-1.1 indeed displays distinct patterns of subcellular localization in specific tissues in vivo. Our findings are consistent with the hypothesis of the ambiquitous nature of glycolytic enzymes (6,7,9), and with more recent cell biological studies that also observed compartmentalization of glycolytic proteins in response to specific stimuli (14,16,20,79, 80, 81).
Our findings on the biophysical properties of PFK-1.1 during transient hypoxia, the fluid-like movements of the condensates, and the correlation of the concentration with the time of emergence indicate that PFK-1.1 clusters represent metastable liquid condensates. The resulting spheroid condensate shapes suggest an interplay between the confinement of the condensate in the neurite’s cellular geometry and the surface tension of the condensate. The biophysical properties of PFK-1.1 are different from those observed for other metabolic enzymes with more static assemblies, such as filamentous structures and crystalline-like assemblies (82). Interestingly, “aged” PFK-1.1 condensates slow down their dynamics, including nonfusing properties, decreased FRAP recovery, and time of dispersion upon normoxia. These findings suggest that changes in the metabolic state of the cell induced by hypoxia result in consistent (and predictable) changes in PFK-1.1 biophysical properties. Our findings also suggest that thermodynamic forces drive phase separation during PFK-1.1 condensation. We note, however, that we also observed in vivo nonequilibrium properties to PFK-1.1 localization that are likely the result of the interplay between cell biological regulatory mechanisms and phase-separated condensates (what have been called “active emulsions” (56)). For example, we observed the exchange of material resulting, not in Ostwald ripening as would be predicted from thermodynamic equilibrium, but instead in similarly sized adjacent condensates. We also observed that the localization of the PFK-1.1 condensates occurred near synapses, where PFK-1.1 was previously shown to be required for sustaining the synaptic vesicle cycle (19). In the muscle, we observed PFK-1.1 is enriched at M-lines and dense body structures, functionally important sites of ATP consumption and muscle contraction (83). Although we have not directly examined the functional implications of phase separation in this study, a recent in vitro study showed that compartmentalization of glycolytic proteins into liquid droplets accelerates the glycolytic reactions (24). Together with our in vivo findings, these observations are consistent with a local “on-demand” assembly of PFK-1.1 condensates, similar to what has been described for other reaction-controlled assemblies of liquid compartments (43,55). Importantly, our findings suggest that thermodynamic driving forces and regulatory biological mechanisms control the ad hoc formation of PFK-1.1 liquid condensates at specific subcellular compartments.
Multivalent interactions in PFK-1.1 are likely important for its condensation. Liquid-liquid phase separation can be induced via regulated multivalent protein-protein and protein-RNA interactions (2,48,61, 62, 63, 64, 65, 66, 67). For example, in yeast, cellular redox states regulate the phase separation of ataxin-2, which in turn regulates TORC1 activity in response to the activity state of the mitochondria. Phase separation of ataxin-2 in yeast is regulated via a low complexity domain, composed of a stretch of methionine residues, which mediate protein-RNA interactions (84). Also in yeast, RNA promotes phase separation of glycolytic enzymes during prolongued hypoxia (15,20,85,86). Although we do not find RNA-interacting domains, intrinsically disordered regions, or stretches of methionine residues in PFK-1.1, PFK exists as a homotetramer capable of self-associating into higher-order structures (21). Similar to other self-associating proteins such as hemoglobin, PFK possesses unique geometrical symmetries that result in multivalent interactions that could contribute to phase separation (70, 71, 72). Consistent with this model, engineering a CRY2 self-association domain promoted formation of PFK-1.1 condensates. Importantly, induction of PFK-1.1 condensates was sufficient to drive the localization of PFK-1.1 lacking the self-association CRY2 domain and glycolytic protein aldolase/ALDO-1, suggesting that nucleation of PFK-1.1 condensates drive a feed-forward reaction that results in the compartmentalization of glycolytic proteins into condensates. PFK tetramerization and self-association can be regulated by its substrate, by metabolites (including AMP and ATP), and by phosphorylation of its regulatory domain (87). ATP can also act like a biological hydrotrope to solubilize proteins (88). We hypothesize that hypoxic conditions that block the oxidative phosphorylation pathway may result in local changes in concentration of metabolites (such as AMP and ATP), which regulate PFK-1.1 oligomerization states and the local formation of condensates. We note recurring formation of PFK-1.1 condensates at the same location in the neurite. This observation may be reflective of an underlying biology that underpins local metabolite changes or needs. Together, our data are consistent with a model whereby PFK-1.1 oligomerization in response to local metabolites might lead to multivalent interactions that drive its self-association into condensates.
PFK-1.1 condensates might represent a novel glycolytic compartment. The idea that glycolytic proteins compartmentalize into a glycolytic metabolon was first proposed after classical biochemical studies could not explain, based on known biochemical principles, the observed cellular rates of glycolysis (76,89). This led to the hypothesis that higher organizing principles, such as subcellular compartmentalization, must influence the observed flux for the glycolytic metabolic reaction in cells (90,91). Consistent with this, in vitro biochemical studies and modeling have demonstrated that the interaction between PFK and ALDO increases their enzymatic activities (92,93) and that higher-order oligomerization of PFK is associated with an increase in its activity (94). We had previously reported that PFK-1.1 dynamically colocalizes into clusters with other glycolytic proteins, such as aldolase (ALDO) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (19). In yeast, prolonged (24-h) hypoxia also results in PFK phase separating with other glycolytic proteins into membraneless subcompartments, termed G-bodies (20,95). In our study, we demonstrate that promoting PFK-1.1 condensates through the use of CRY2 results in recruitment of aldolase to the PFK-1.1 condensates. Observations of glycolytic compartments in multiple different species and cell types suggest that although the regulation, composition, and formation kinetics of the glycolytic condensates may differ, compartmentalization of glycolytic enzymes may be a conserved feature of metabolic organization. We hypothesize that the compartmentalization described here represents a mechanism that changes enzymatic parameters of the kinase to suit changing metabolic needs in the cell. The subcellular organization and biophysical dynamics observed for PFK-1.1 might extend to other glycolytic proteins, and PFK-1.1 condensates might represent the hypothesized “glycolytic metabolon” (4,6) or a novel membraneless organelle that could regulate glycolysis.
Compartmentalization is a common way for cells to internally organize their reactions and cellular processes (1,22,96,97). Membraneless compartments in cells can serve multiple purposes, from inhibiting enzymatic activity by sequestering molecules to enhancing activity through mass action effects (2,48,97, 98, 99, 100). Although metabolic reactions by cytosolic proteins are generally thought of as distributed in cells, emerging evidence suggests that they could be compartmentalized in response to cellular states (15,23,101,102). For example, systematic studies on ∼800 metabolic enzymes in yeast identified widespread reorganization of proteins involved in intermediary metabolism upon nutrient starvation (103). Enzymes involved in purine biogenesis are compartmentalized to enhance or inhibit their activity (104,105). Metabolic enzymes such as asparagine synthetase paralogs, CTP synthase, and glucokinase polymerize in response to cellular metabolic changes (106, 107, 108). We now show that PFK-1.1 dynamically compartmentalizes into condensates. The principles we uncover here on the subcellular organization of PFK-1.1 could represent important principles underlying the subcellular regulation of glycolysis and conserved concepts on how metabolic processes are dynamically organized to modulate subcellular states.
Author contributions
S.J., Z.X., R.C.L., L.M.J., A.P., D.R.A., A.A.H., and D.A.C.-R. designed research. S.J., Z.X., R.C.L., I.J.G., M.S., S.P., and H.S.K. performed research. S.J., Z.X., and R.C.L. analyzed data. S.J., Z.X., R.C.L., D.R.A., A.A.H., and D.A.C.-R. wrote the manuscript.
Acknowledgments
We thank Emmanuel Levy and Sucharita Dey (Weizmann Institute), Ceciel Jegers (Hyman lab), Robert Haase and Simon Alberti (MPI-CBG), Ann Cowan (UConn), Leslie Loew (UConn), John Kim and Mindy Clark (Johns Hopkins), Michael Rosen (UT Southwestern), Amy Gladfelter (UNC Chapel Hill), Joseph Hoffman (Yale University), Richard Goodman (Vollum Institute), and members of the Colón-Ramos lab for insightful discussions on the work and advice on the project. We thank the Research Center for Minority Institutions program, the Marine Biological Laboratories, and the Instituto de Neurobiología de la Universidad de Puerto Rico for providing meeting and brainstorming platforms.
Support for S.J. was provided by T32-GM007223. A.A.H., A.P., and L.M.J. were supported by a direct grant from the Max Planck Society. D.R.A. and R.C.L. were supported by National Science Foundation EF1724026 and CBET1605679. Research in the D.A.C.-R. lab was supported by National Institutes of Health R01NS076558 and DP1NS111778 and by an HHMI Scholar Award. The collaboration between D.A.C.-R. lab and A.A.H. is supported by an HFSP Award.
Editor: Anne Kenworthy.
Footnotes
Avinash Patel’s present address is Dewpoint Therapeutics GmbH, Dresden, Germany.
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2020.08.002.
Supporting Citations
References (109,110) appear in the Supporting Material.
Supporting material
References
- 1.Walter H., Brooks D.E. Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett. 1995;361:135–139. doi: 10.1016/0014-5793(95)00159-7. [DOI] [PubMed] [Google Scholar]
- 2.Banani S.F., Lee H.O., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitrea D.M., Kriwacki R.W. Phase separation in biology; functional organization of a higher order. Cell Commun. Signal. 2016;14:1. doi: 10.1186/s12964-015-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke F.M., Masters C.J. On the association of glycolytic components in skeletal muscle extracts. Biochim. Biophys. Acta. 1974;358:193–207. doi: 10.1016/0005-2744(74)90270-8. [DOI] [PubMed] [Google Scholar]
- 5.Knull H.R. Association of glycolytic enzymes with particulate fractions from nerve endings. Biochim. Biophys. Acta. 1978;522:1–9. doi: 10.1016/0005-2744(78)90316-9. [DOI] [PubMed] [Google Scholar]
- 6.Kurganov B.I., Sugrobova N.P., Mil’man L.S. Supramolecular organization of glycolytic enzymes. J. Theor. Biol. 1985;116:509–526. doi: 10.1016/s0022-5193(85)80086-2. [DOI] [PubMed] [Google Scholar]
- 7.Masters C. Interactions between glycolytic enzymes and components of the cytomatrix. J. Cell Biol. 1984;99:222s–225s. doi: 10.1083/jcb.99.1.222s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson J.E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J. Biol. Chem. 1968;243:3640–3647. [PubMed] [Google Scholar]
- 9.Wilson J.E. Ambiquitous enzymes - variation in intracellular-distribution as a regulatory mechanism. Trends Biochem. Sci. 1978;3:124–125. [Google Scholar]
- 10.Chu H., Puchulu-Campanella E., Hoffman J.F. Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proc. Natl. Acad. Sci. USA. 2012;109:12794–12799. doi: 10.1073/pnas.1209014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green D.E., Murer E., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch. Biochem. Biophys. 1965;112:635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- 12.Mercer R.W., Dunham P.B. Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J. Gen. Physiol. 1981;78:547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan D.T., MacIntyre R., Ramizel L. Analysis of glycolytic enzyme co-localization in Drosophila flight muscle. J. Exp. Biol. 2003;206:2031–2038. doi: 10.1242/jeb.00367. [DOI] [PubMed] [Google Scholar]
- 14.De Bock K., Georgiadou M., Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Kastritis P.L., Gavin A.-C. Enzymatic complexes across scales. Essays Biochem. 2018;62:501–514. doi: 10.1042/EBC20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohnhorst C.L., Kyoung M., An S. Identification of a multienzyme complex for glucose metabolism in living cells. J. Biol. Chem. 2017;292:9191–9203. doi: 10.1074/jbc.M117.783050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks S.P., Storey K.B. Where is the glycolytic complex? A critical evaluation of present data from muscle tissue. FEBS Lett. 1991;278:135–138. doi: 10.1016/0014-5793(91)80101-8. [DOI] [PubMed] [Google Scholar]
- 18.Menard L., Maughan D., Vigoreaux J. The structural and functional coordination of glycolytic enzymes in muscle: evidence of a metabolon? Biology (Basel) 2014;3:623–644. doi: 10.3390/biology3030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S., Nelson J.C., Colón-Ramos D.A. Glycolytic enzymes localize to synapses under energy stress to support synaptic function. Neuron. 2016;90:278–291. doi: 10.1016/j.neuron.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin M., Fuller G.G., Kim J.K. Glycolytic enzymes coalesce in G bodies under hypoxic stress. Cell Rep. 2017;20:895–908. doi: 10.1016/j.celrep.2017.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb B.A., Dosey A.M., Barber D.L. The glycolytic enzyme phosphofructokinase-1 assembles into filaments. J. Cell Biol. 2017;216:2305–2313. doi: 10.1083/jcb.201701084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wombacher H. Molecular compartmentation by enzyme cluster formation. A view over current investigations. Mol. Cell. Biochem. 1983;56:155–164. doi: 10.1007/BF00227216. [DOI] [PubMed] [Google Scholar]
- 23.Zecchin A., Stapor P.C., Carmeliet P. Metabolic pathway compartmentalization: an underappreciated opportunity? Curr. Opin. Biotechnol. 2015;34:73–81. doi: 10.1016/j.copbio.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Ura T., Tomita S., Shiraki K. Dynamic formation of liquid droplets triggered by sequential enzymatic reactions. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11930403.v1. [DOI] [PubMed] [Google Scholar]
- 25.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagoy R.C., Albrecht D.R. Microfluidic devices for behavioral analysis, microscopy, and neuronal imaging in Caenorhabditis elegans. Methods Mol. Biol. 2015;1327:159–179. doi: 10.1007/978-1-4939-2842-2_12. [DOI] [PubMed] [Google Scholar]
- 27.Burnett K., Edsinger E., Albrecht D.R. Rapid and gentle hydrogel encapsulation of living organisms enables long-term microscopy over multiple hours. Commun. Biol. 2018;1:73. doi: 10.1038/s42003-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell-Coffman J.A. Hypoxia signaling and resistance in C. elegans. Trends Endocrinol. Metab. 2010;21:435–440. doi: 10.1016/j.tem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Brangwynne C.P., Eckmann C.R., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 30.Webb B.A., Forouhar F., Barber D.L. Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations. Nature. 2015;523:111–114. doi: 10.1038/nature14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis G.R., Waterston R.H. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J. Cell Biol. 1985;101:1532–1549. doi: 10.1083/jcb.101.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gieseler K., Qadota H., Benian G.M. Development, structure, and maintenance of C. elegans body wall muscle. WormBook. 2017;2017:1–59. doi: 10.1895/wormbook.1.81.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moerman D.G., Williams B.D. Sarcomere assembly in C. elegans muscle. WormBook. 2006:1–16. doi: 10.1895/wormbook.1.81.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qadota H., Benian G.M. Molecular structure of sarcomere-to-membrane attachment at M-Lines in C. elegans muscle. J. Biomed. Biotechnol. 2010;2010:864749. doi: 10.1155/2010/864749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickinson D.J., Pani A.M., Goldstein B. Streamlined genome engineering with a self-excising drug selection cassette. Genetics. 2015;200:1035–1049. doi: 10.1534/genetics.115.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flavell S.W., Pokala N., Bargmann C.I. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154:1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz M.L., Jorgensen E.M. SapTrap, a toolkit for high-throughput CRISPR/Cas9 gene modification in Caenorhabditis elegans. Genetics. 2016;202:1277–1288. doi: 10.1534/genetics.115.184275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Protter D.S.W., Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huelgas-Morales G., Silva-García C.G., Navarro R.E. The stress granule RNA-binding protein TIAR-1 protects female germ cells from heat shock in Caenorhabditis elegans. G3 (Bethesda) 2016;6:1031–1047. doi: 10.1534/g3.115.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Yang P., Zhang H. A genome-wide RNAi screen identifies genes regulating the formation of P bodies in C. elegans and their functions in NMD and RNAi. Protein Cell. 2011;2:918–939. doi: 10.1007/s13238-011-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardner L.B. Hypoxic inhibition of nonsense-mediated RNA decay regulates gene expression and the integrated stress response. Mol. Cell. Biol. 2008;28:3729–3741. doi: 10.1128/MCB.02284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottschald O.R., Malec V., Hänze J. TIAR and TIA-1 mRNA-binding proteins co-aggregate under conditions of rapid oxygen decline and extreme hypoxia and suppress the HIF-1α pathway. J. Mol. Cell Biol. 2010;2:345–356. doi: 10.1093/jmcb/mjq032. [DOI] [PubMed] [Google Scholar]
- 43.Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 44.Brangwynne C.P., Mitchison T.J., Hyman A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franzmann T.M., Jahnel M., Alberti S. Phase separation of a yeast prion protein promotes cellular fitness. Science. 2018;359:eaao5654. doi: 10.1126/science.aao5654. [DOI] [PubMed] [Google Scholar]
- 46.Patel A., Lee H.O., Alberti S. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 47.Zeng M., Shang Y., Zhang M. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell. 2016;166:1163–1175.e12. doi: 10.1016/j.cell.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alberti S., Gladfelter A., Mittag T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell. 2019;176:419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gopal P.P., Nirschl J.J., Holzbaur E.L.F. Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proc. Natl. Acad. Sci. USA. 2017;114:E2466–E2475. doi: 10.1073/pnas.1614462114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xuan Z., Manning L., Kurshan P.T. Clarinet (CLA-1), a novel active zone protein required for synaptic vesicle clustering and release. eLife. 2017;6:e29276. doi: 10.7554/eLife.29276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Style R.W., Sai T., Dufresne E.R. Liquid-liquid phase separation in an elastic network. Phys. Rev. X. 2018;8:011028. [Google Scholar]
- 52.Rosowski K.A., Sai T., Dufresne E.R. Elastic ripening and inhibition of liquid-liquid phase separation. Nat. Phys. 2020;16:422–425. doi: 10.1038/s41567-019-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zwicker D., Seyboldt R., Jülicher F. Growth and division of active droplets provides a model for protocells. Nat. Phys. 2017;13:408–413. [Google Scholar]
- 54.Voorhees P.W. Ostwald ripening of two-phase mixtures. Annu. Rev. Mater. Sci. 1992;22:197–215. [Google Scholar]
- 55.Berry J., Brangwynne C.P., Haataja M. Physical principles of intracellular organization via active and passive phase transitions. Rep. Prog. Phys. 2018;81:046601. doi: 10.1088/1361-6633/aaa61e. [DOI] [PubMed] [Google Scholar]
- 56.Weber C.A., Zwicker D., Lee C.F. Physics of active emulsions. Rep. Prog. Phys. 2019;82:064601. doi: 10.1088/1361-6633/ab052b. [DOI] [PubMed] [Google Scholar]
- 57.Mastro A.M., Babich M.A., Keith A.D. Diffusion of a small molecule in the cytoplasm of mammalian cells. Proc. Natl. Acad. Sci. USA. 1984;81:3414–3418. doi: 10.1073/pnas.81.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feric M., Vaidya N., Brangwynne C.P. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hyman A.A., Weber C.A., Jülicher F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- 60.Banani S.F., Rice A.M., Rosen M.K. Compositional control of phase-separated cellular bodies. Cell. 2016;166:651–663. doi: 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elbaum-Garfinkle S., Kim Y., Brangwynne C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA. 2015;112:7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langdon E.M., Qiu Y., Gladfelter A.S. mRNA structure determines specificity of a polyQ-driven phase separation. Science. 2018;360:922–927. doi: 10.1126/science.aar7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li P., Banjade S., Rosen M.K. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nott T.J., Petsalaki E., Baldwin A.J. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith J., Calidas D., Seydoux G. Spatial patterning of P granules by RNA-induced phase separation of the intrinsically-disordered protein MEG-3. eLife. 2016;5:e21337. doi: 10.7554/eLife.21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J., Choi J.-M., Hyman A.A. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 2018;174:688–699.e16. doi: 10.1016/j.cell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H., Elbaum-Garfinkle S., Gladfelter A.S. RNA controls PolyQ protein phase transitions. Mol. Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bracha D., Walls M.T., Brangwynne C.P. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell. 2018;175:1467–1480.e13. doi: 10.1016/j.cell.2018.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin Y., Berry J., Brangwynne C.P. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell. 2017;168:159–171.e14. doi: 10.1016/j.cell.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Q., Vekilov P.G., Hirsch R.E. Liquid-liquid phase separation in hemoglobins: distinct aggregation mechanisms of the beta6 mutants. Biophys. J. 2004;86:1702–1712. doi: 10.1016/S0006-3495(04)74239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galkin O., Chen K., Vekilov P.G. Liquid-liquid separation in solutions of normal and sickle cell hemoglobin. Proc. Natl. Acad. Sci. USA. 2002;99:8479–8483. doi: 10.1073/pnas.122055299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Seisdedos H., Empereur-Mot C., Levy E.D. Proteins evolve on the edge of supramolecular self-assembly. Nature. 2017;548:244–247. doi: 10.1038/nature23320. [DOI] [PubMed] [Google Scholar]
- 73.Bugaj L.J., Choksi A.T., Schaffer D.V. Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods. 2013;10:249–252. doi: 10.1038/nmeth.2360. [DOI] [PubMed] [Google Scholar]
- 74.Che D.L., Duan L., Cui B. The dual characteristics of light-induced cryptochrome 2, homo-oligomerization and heterodimerization, for optogenetic manipulation in mammalian cells. ACS Synth. Biol. 2015;4:1124–1135. doi: 10.1021/acssynbio.5b00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park H., Kim N.Y., Heo W.D. Optogenetic protein clustering through fluorescent protein tagging and extension of CRY2. Nat. Commun. 2017;8:30. doi: 10.1038/s41467-017-00060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moses V. Mechanism for the intracellular compartmentation of newly synthesized proteins. In: Srere P.A., Estabrook R.W., editors. Microenvironments and Metabolic Compartmentation. Academic Press; 1978. pp. 169–185. [Google Scholar]
- 77.Vas M., Batke J. Evidence for absence of an interaction between purified 3-phosphoglycerate kinase and glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta. 1981;660:193–198. doi: 10.1016/0005-2744(81)90159-5. [DOI] [PubMed] [Google Scholar]
- 78.Weber J.P., Bernhard S.A. Transfer of 1,3-diphosphoglycerate between glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase via an enzyme-substrate-enzyme complex. Biochemistry. 1982;21:4189–4194. doi: 10.1021/bi00260a042. [DOI] [PubMed] [Google Scholar]
- 79.Araiza-Olivera D., Chiquete-Felix N., Uribe-Carvajal S. A glycolytic metabolon in Saccharomyces cerevisiae is stabilized by F-actin. FEBS J. 2013;280:3887–3905. doi: 10.1111/febs.12387. [DOI] [PubMed] [Google Scholar]
- 80.Graham J.W.A., Williams T.C.R., Sweetlove L.J. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell. 2007;19:3723–3738. doi: 10.1105/tpc.107.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miura N., Shinohara M., Ueda M. Spatial reorganization of Saccharomyces cerevisiae enolase to alter carbon metabolism under hypoxia. Eukaryot. Cell. 2013;12:1106–1119. doi: 10.1128/EC.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petrovska I., Nüske E., Alberti S. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife. 2014;3:e02409. doi: 10.7554/eLife.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wojtas K., Slepecky N., Sullivan D. Flight muscle function in Drosophila requires colocalization of glycolytic enzymes. Mol. Biol. Cell. 1997;8:1665–1675. doi: 10.1091/mbc.8.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kato M., Yang Y.-S., Tu B.P. Redox state controls phase separation of the yeast ataxin-2 protein via reversible oxidation of its methionine-rich low-complexity domain. Cell. 2019;177:711–721.e8. doi: 10.1016/j.cell.2019.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castello A., Fischer B., Hentze M.W. Comprehensive identification of RNA-binding domains in human cells. Mol. Cell. 2016;63:696–710. doi: 10.1016/j.molcel.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mazurek S., Hugo F., Eigenbrodt E. Studies on associations of glycolytic and glutaminolytic enzymes in MCF-7 cells: role of P36. J. Cell. Physiol. 1996;167:238–250. doi: 10.1002/(SICI)1097-4652(199605)167:2<238::AID-JCP7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 87.Sola-Penna M., Da Silva D., Zancan P. Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life. 2010;62:791–796. doi: 10.1002/iub.393. [DOI] [PubMed] [Google Scholar]
- 88.Patel A., Malinovska L., Hyman A.A. ATP as a biological hydrotrope. Science. 2017;356:753–756. doi: 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- 89.Sies H. Academic Press; Cambridge, MA: 1982. Metabolic Compartmentation. [Google Scholar]
- 90.Cori C.F. Problems of cellular biochemistry. In: Green D.E., editor. Currents in Biochemical Research. Interscience; 1956. pp. 198–214. [Google Scholar]
- 91.Srere P.A., Mosbach K. Metabolic compartmentation: symbiotic, organellar, multienzymic, and microenvironmental. Annu. Rev. Microbiol. 1974;28:61–83. doi: 10.1146/annurev.mi.28.100174.000425. [DOI] [PubMed] [Google Scholar]
- 92.Marcondes M.C., Sola-Penna M., Zancan P. Muscle-type 6-phosphofructo-1-kinase and aldolase associate conferring catalytic advantages for both enzymes. IUBMB Life. 2011;63:435–445. doi: 10.1002/iub.464. [DOI] [PubMed] [Google Scholar]
- 93.Raïs B., Ortega F., Cascante M. Quantitative characterization of homo- and heteroassociations of muscle phosphofructokinase with aldolase. Biochim. Biophys. Acta. 2000;1479:303–314. doi: 10.1016/s0167-4838(00)00047-9. [DOI] [PubMed] [Google Scholar]
- 94.Su J.Y., Storey K.B. Fish muscle phosphofructokinase - influences of protein-concentration on enzyme-kinetic behavior. Int. J. Biochem. Cell Biol. 1995;27:1277–1283. [Google Scholar]
- 95.Fuller G.G., Han T., Kim J.K. RNA promotes phase separation of glycolysis enzymes into yeast G bodies in hypoxia. eLife. 2020;9:e48480. doi: 10.7554/eLife.48480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alberti S. Phase separation in biology. Curr. Biol. 2017;27:R1097–R1102. doi: 10.1016/j.cub.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 97.Schmitt D.L., An S. Spatial organization of metabolic enzyme complexes in cells. Biochemistry. 2017;56:3184–3196. doi: 10.1021/acs.biochem.7b00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Case L.B., Zhang X., Rosen M.K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science. 2019;363:1093–1097. doi: 10.1126/science.aau6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee C., Zhang H., Gladfelter A.S. Protein aggregation behavior regulates cyclin transcript localization and cell-cycle control. Dev. Cell. 2013;25:572–584. doi: 10.1016/j.devcel.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su X., Ditlev J.A., Vale R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 2016;352:595–599. doi: 10.1126/science.aad9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Connell J.D., Zhao A., Marcotte E.M. Dynamic reorganization of metabolic enzymes into intracellular bodies. Annu. Rev. Cell Dev. Biol. 2012;28:89–111. doi: 10.1146/annurev-cellbio-101011-155841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prouteau M., Loewith R. Regulation of cellular metabolism through phase separation of enzymes. Biomolecules. 2018;8:160. doi: 10.3390/biom8040160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Narayanaswamy R., Levy M., Marcotte E.M. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc. Natl. Acad. Sci. USA. 2009;106:10147–10152. doi: 10.1073/pnas.0812771106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An S., Kumar R., Benkovic S.J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 105.Pedley A.M., Benkovic S.J. A new view into the regulation of purine metabolism: the purinosome. Trends Biochem. Sci. 2017;42:141–154. doi: 10.1016/j.tibs.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noree C., Monfort E., Wilhelm J.E. Common regulatory control of CTP synthase enzyme activity and filament formation. Mol. Biol. Cell. 2014;25:2282–2290. doi: 10.1091/mbc.E14-04-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Noree C., Sirinonthanawech N., Wilhelm J.E. Saccharomyces cerevisiae ASN1 and ASN2 are asparagine synthetase paralogs that have diverged in their ability to polymerize in response to nutrient stress. Sci. Rep. 2019;9:278. doi: 10.1038/s41598-018-36719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stoddard P.R., Lynch E.M., Garner E.C. Independent evolution of polymerization in the Actin ATPase clan regulates hexokinase activity. bioRxiv. 2019 doi: 10.1101/686915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mello C., Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- 110.Thévenaz P., Ruttimann U.E., Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.