Abstract
In this study, we investigated the therapeutic efficacy of VERU-111 in vitro and in vivo model systems of cervical cancer. VERU-111 treatment inhibited cell proliferation and, clonogenic potential, induce accumulation of p53 and down regulated the expression of HPV E6/E7 expression in cervical cancer cells. In addition, VERU-111 treatment also decreased the expression of phosphorylation of Jak2 (TyR1007/1008) and STAT3 at Tyr705 and Ser727. VERU-111 treatment arrested cell cycle in the G2/M phase and modulated cell cycle regulatory proteins (cyclin B1, p21 p34cdc2 and pcdk1). Moreover, VERU-111 treatment induced apoptosis and modulated the expression of Bid, Bcl-xl, Survivin, Bax, Bcl2 and cleavage in PARP. In functional assays, VERU-111 markedly reduced the tumorigenic, migratory, and invasive potential of cervical cancer cells via modulations of MMPs. VERU-111 treatment also showed significant (P<0.05) inhibition of orthotopic xenograft tumor growth in athymic nude mice. Taken together, our results demonstrate the potential anti-cancer efficacy of VERU-111 in in vitro and in vivo. VERU-111 can be explored as a potent therapeutic agent for the treatment of cervical cancer.
1. Introduction
Cervical cancer is the world fourth largest cause of cancer-related death. The American Cancer Society estimates that there will be 4,250 deaths, and 13,170 new cases of cervical cancer in the US in the year 2019 [1]. High-risk human papillomaviruses (HPV) plays a central role in the development of 99.5% of cervical cancers [2]. HPV acts through infecting the genital mucosa, and is integrated into the host genome, leading to the overexpression of E6 and E7 oncoproteins, then immortalizating the host cells by disrupting p53 and pRb functions, respectively [3]. Oncoprotein E6 binds to p53 and targets it for ubiquitin-mediated degradation [4]. Activation of p53 further induces downstream target the gene involved in cell cycle arrest, apoptosis, or attempts to repair the damaged DNA [5]. Cyclin-dependent kinase (cdk) inhibitor p21cip1/waf1 and Bax mediate the cytotoxic and apoptotic effect of p53 respectively [6]. Thus, activation of the p53 function represents a viable option for the effective therapeutic targeting and management of cervical cancer [7]. Reactivation of p53 is regulated either via inhibition of viral oncoproteins expression and function or in prevention of proteasomal degradation of p53 [8]. To activate p53 in cervical cancer, several different strategies like small molecule compounds, direct anti-E6 approaches, gamma-irradiation, certain cytotoxic drugs, and ribozyme techniques have been adopted [4]. It has been reported that STAT3 bind with the HPV16 LCR and regulate the abnormal E6 and E7 expression, binding to p53, pRb and degrading HPV16 [9]. In addition, various non-coding RNAs that modulate expression of oncogenic and tumor-suppressive genes and micro-RNAs (miRNA) play a major role in cervical carcinogenesis development [10]. This can be a novel approach in the treatment of cervical cancer to target these oncogenic signaling pathways and miRNAs. Chemotherapy is currently one of the most common methods of treating advanced metastatic cervical cancer. But the clinical application of this approach often presents serious challenges due to the of chemoresistance and toxic side effects. A new non-toxic modality for preventing and treating cervical cancer therefore urgently needs to be developed.
VERU-111 was designed and synthesized based on previously reported ABI-I and ABI-II analogues known as ABI-231 [11–13]. The novel VERU-111 (2-aryl-4-benzoyl-imidazole) scaffold exerts its potent anti-proliferative effects by interacting with the colchicine-binding site in tubulin. VERU-111 was found to be active on panels of melanoma, prostate and pancreatic cancer cell lines at the nanomolar concentration and strongly suppresses melanoma tumors in vivo [14, 15].
In this study, we evaluated the activities of VERU-111 to inhibit cervical cancer in vitro and in vivo and investigate its underlying molecular mechanisms of action. Mechanistically, we report that VERU-111 suppresses the expression of HPV E6 and E7 oncoproteins and restoration of p53 levels. This which further leads to sequential reactivation of p53-dependent tumor suppressor activity by downstream modulation of proteins involved in cell proliferation, cell cycle progression and apoptosis.
2. Material and methods
2.1. Cell culture, growth conditions and treatment
The human cervical cancer cells (CaSki, HeLa and SiHa) that were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) were cytogenetically tested and authenticated. Cervical cancer cells, CaSki were cultured in an RPMI-1640 and SiHa and HeLa in DMEM medium, supplemented with 10% fetal bovine serum, and 1% antibiotic and antimycotic solution (Thermo Fisher Scientific, Waltham, MA, USA). Cervical cancer cells were maintained in an incubator at 37°C, 5% CO2, and a humidified atmosphere.
2.2. Antibodies
Antibodies against p21 (cat. # 2947), p53 (cat. # 2524), PCNA (cat. # 13110), (JAK2 (cat. # 3230), pJAK2 (cat. # 3771), STAT3 (cat. #9139), pstat3Tyr (705) (cat. # 9145), pstat3 ser (727) (cat # 9134), (Bax (cat # 2772), Bcl2 (cat # 2872), PARP (cat. #9532), pcdk1 (cat # 9111), Cyclin B1 (cat # 4138), Bid (cat # 2002), Bim (cat # 2819), Bcl-xl (cat #2764), Survivin (cat # 2808) and GAPDH (cat # 5174), were obtained from Cell Signaling Technology, Inc (Danvers, MA, USA). P34cdc2 (cat# 8395), HPV E6 (cat # SC-480) and HPV E7 (cat. # SC-698) antibodies were obtained from Santa Cruz Biotechnology, Dallas, TX, USA.
2.3. Cell viability and Colony forming assay
The effect of VERU-111 on cell viability was determined by the 3-(4,5-dimethylthiazole- 2-yl)-2, 5-biphenyl tetrazolium bromide (MTT) assay as previously reported [16]. To investigate the effect of VERU −111 on the clonogenic potentials of cervical cancer cells, the colony formations assay was performed [16–18].
2.4. Transient transfection
CaSki, cells were transiently transfected with STAT3 siRNA (100 nM), p53 siRNA (50 nM) or control siRNA (50 nM) using Lipofectamine 2000 according to the manufacturer’s protocol [19].
2.5. RNA preparations and Real time PCR
RNA was extracted from cell lines and tumor xenograft by using TRIzol (Invitrogen). The qRT-PCR was performed as previously described [16]. The primers sequences are listed in supplementary Table. S1.
2.6. Western blotting
Western blot analysis was performed as previously reported [16, 20].
2.7. p53 transactivation activity
p53 transactivation assay, ELISA kit (Active Motif, Carlsbad, CA, USA) was used for the p53 transactivation assays. The cervical cancer cells were treated with VERU-111 and nuclear extract (Nuclear and Cytosolic Extraction Kit, Pierce, Rockford, IL, USA) was isolated according to the manufacturer’s instructions.
2.8. Cell migration and invasion
The scratch and migration assay was performed using a cell migration 96 well, pore size, 8 μm; Corning plate [21]. We also investigated the effect of VERU-111 on cellular motility by an agarose bead-based cell motility assay as described earlier [21]. The invasion assay was performed by using a cell invasion kit (BD Biocoat™ Matrigel Invasion Chambers; BD Biosciences, San Jose, CA, USA) as describe previously [22–24].
2.9. Real time cell proliferation and migration by xCELLigence assays
To further confirm the effect of VERU-111 on migration, invasion, and proliferation of cervical cancer cells, real-time proliferation, migration and invasion assays were performed using the xCELLigence system as described [25, 26].
2.10. Cell cycle and apoptosis analysis
For cell cycle analysis, cells were exposed to different concentrations of VERU-111 at (0– 50 nM) for 24 h. Samples were analyzed with Accuri C6 (BD Biosciences) flow cytometer in the FL2 channel as described [25]. Further, the Annexin V-7AAD apoptosis kit (BD Biosciences, San Diego, CA,USA) was utilized to determine VERU-111’s ability to induce apoptosis in cervical cancer cells as described [25]. To analyze the effect of VERU-111 on mitochondrial membrane potential (ΔΨM), Tetramethyl rhodamine ethyl ester (TMRE) (Invitrogen) stain method was employed as described [27].
2.11. Orthotopic xenograft study
Six-week-old female athymic nude mice were used in accordance to protocol reviewed and approved by the UTHSC Institutional Animal Care and Use Committee (UTHSC-IACUC). Briefly, CaSki cells (4 × 106) were dispersed in 100 μL PBS (1X) and 100 μ L Matrigel (BD Biosciences) and injected directly into the cervix of each mouse without any surgery. When tumor volume reached ~200 mm3, tumor-bearing mice were randomly divided into two groups (n=6 per group). VERU-111 (50 μg/mice) and the vehicle control (1X PBS) were injected intratumorally three times per week for three weeks. The weight of mice as well as tumors volume – was measured every week starting from the day when they were administered. When tumor volume, of the control mice reached 1000 mm3, the mice were sacrificed, and tumors were excised and used for tissue sectioning, histopathology RNA isolations and lysate preparations.
2.12. Immunofluorescence and immunohistochemistry analysis
Immunofluorescence and immunohistochemistry analysis were performed as describe previously [25].
2.13. Statistical analysis
Statistical analysis was performed with Graph Pad Prism 5 by using a Student’s t- test.
3. Results
3.1. VERU-111 exhibits antiproliferative activity and clonogenic potential of cervical cancer cells
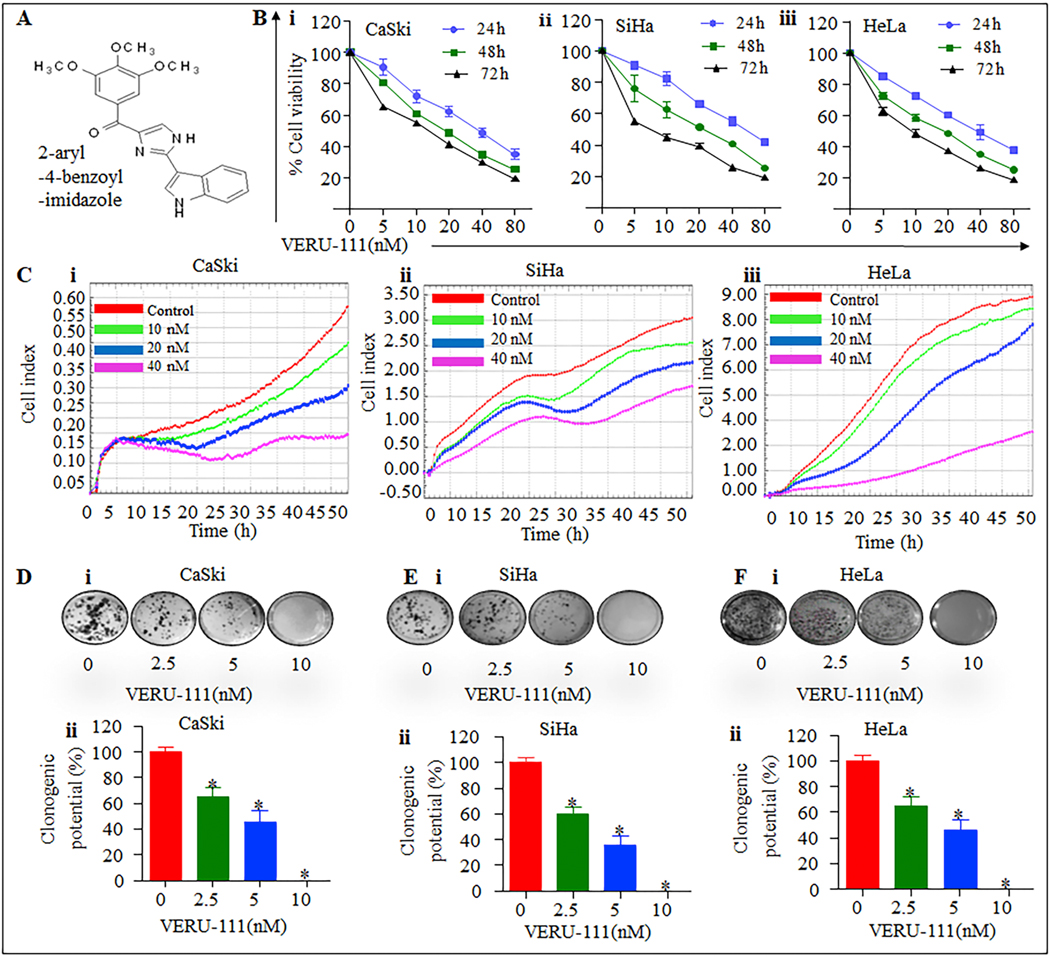
HPV infections are associated with a majority of cervical cancer cases [28]. We investigated the cytotoxic effect of VERU −111 (Figure. 1A) on cervical cancers cell lines, CaSki, HeLa, SiHa and at various concentrations (5–80 nM). VERU-111 inhibited the growth of all three cervical cell lines in a dose dependent manner (Figure;1Bi-iii). This finding was confirmed by using the xCELLigence real time cell proliferation system (Figure. 1Ci–iii). For clonogenic potential, VERU-111 significantly (p<0.05) inhibited the number of cells formed in all three cervical cancers cell lines compared with respective controls (Figure.1 D–F). These findings suggest that VERU-111 treatment inhibits the proliferation and clonogenic potential of cervical cancer cells.
Figure 1. VERU-111 inhibits the growth cervical cancer cells.
A. Structure of VERU-111 B. Effect of VERU-111 on cell viability of CaSki (i), SiHa (ii) and HeLa (iii) cells. Briefly, cells (5000) were seeded in 96-well plates and after overnight incubation; cells were treated with the indicated concentrations of VERU-111 for 24, 48 and 72 h. Cell viability was assessed by MTT assay. The line graph represents the percentage of viable cells compared to the untreated-treated group cells. The concentration value is the mean ± SE of triplicate wells of each group. C. Effect of VERU-111 on cervical cancer cells proliferation by xCELLigence Real Time Cell Analyzer (RTCA). Briefly, cervical cancer cells (5,000 cells/well) were seeded in an E-plate (xCELLigence) following the xCELLigence Real Time Cell Analyzer (RTCA). After 24 h, VERU-111 was added and the experiment was allowed to run for 50 h. The Average baseline cell index for VERU- 111 treated CaSki (i), SiHa (ii) and HeLa (iii) cells were compared to the untreated control group. D. Effect of VERU-111 on clonogenic potential of cervical cancer cells. Representative colony images of control and VERU-111treated CaSki (Di), SiHa (Ei) and HeLa (Fi) cervical cancer cells. Bar graphs indicating quantification of colony formation in CaSki (Dii), SiHa (Eii) and HeLa (Fii) cervical cancer cells. Asterisk (*) denotes the significant value p<0.05.
3.2. VERU-111 induces activation of p53 in cervical cancer cells
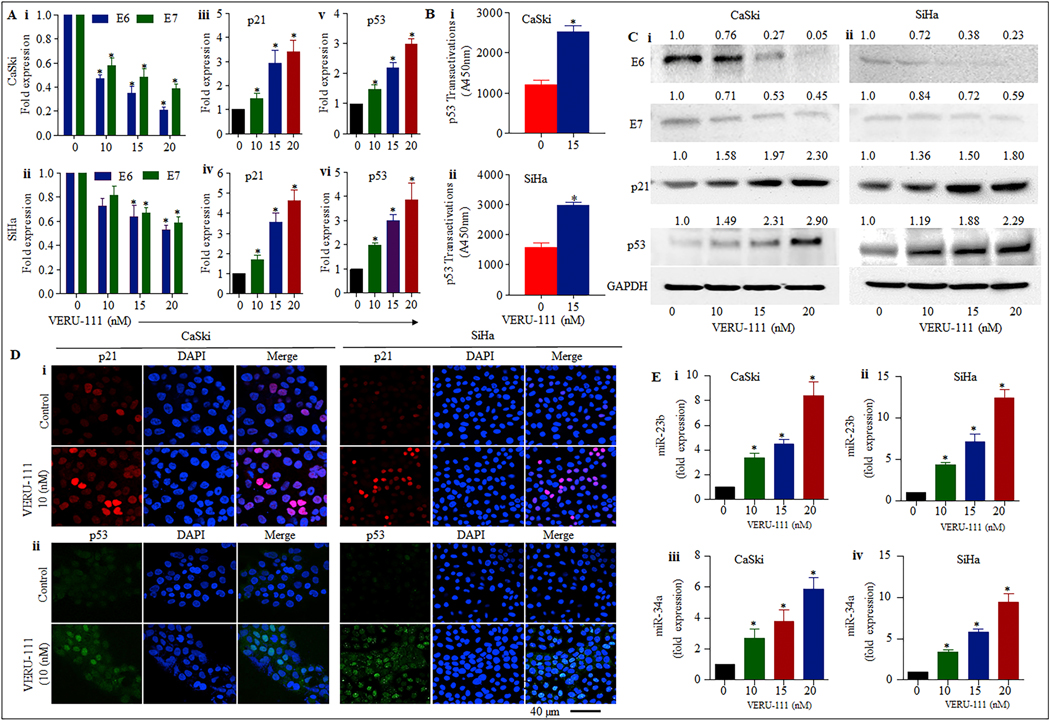
The effect of VERU-111 on HPV E6 and E7 oncogenes, p21 and p53 in CaSki and SiHa cells was investigated by qPCR, western blot analysis and confocal microscopy. Our results indicate a significant downregulation of both HPV16 E6 and E7 transcripts by VERU-111 in a dose dependent manner (Figure. 2Ai–ii) by qPCR and western blot analysis (Figure. 2Ci–ii). Moreover, the p53 dependent apoptotic pathway is are mediated through its downstream target of p21 [29]. Therefore, we investigated the effect of VERU- 111 on p21. Interestingly, the expression of p21 levels was significantly increased in both the mRNA (Figure 2Aiii-iv) and the protein level (Figure. 2Ci–ii) indicating the involvement of p53 dependent apoptosis in these cells. In addition, VERU-111 significantly induces the expression of p53 in a dose dependent manner on mRNA levels (Figure 2Av–vi); and western blot analysis (Figure.2Ci–ii) confirmed functional restorations in p53. Moreover, the increased p53 transactivation following VERU-111 for 24h cervical cancer cells, confirmed that the p53 had been functionally restored (Figure. 2Bi–ii). Further, confocal microscopic analysis again made evident that VERU-111 led to a functional restoration of p53 and p21 as confirmed through the increased nuclear translocation of p53 and p21 in cervical cancer cells (Figure 2Di–ii). The above results confirmed that VERU-111 repressed both transcription and translation of E6 and E7 oncogenes, thereby leading to the restoration of the vital tumor suppressor pathways.
Figure 2: Effect of VERU-111 on expression of p53, miR-23b and miR-34a in cervical cancer cells.
A. Effect of VERU-111 on the expression of HPV oncogenes E6 and E7 (i-ii), p21 (iii-iv) and p53 (v-vi) in cervical cancer cells was analyzed through real time PCR expression analysis where GAPDH RNA was used for normalization (*p < 0.05). Data presented as mean ± SD and are representative of three independent experiments. B. Increased level of p53 was measured by p53 transactivation assay in CaSki and SiHa cells treated with VERU-111 for 24h in comparison to controls. C. Effect of VERU-111 on expression of E6, E7, p53 and p21 proteins in cervical cancer was detected by western blot analysis and GAPDH was used as loading control. The representative data of three independent experiments is presented. D. Expression and localization p21 (red) and p53 (green) in cervical cancer cells treated with VERU-111 (10 nM) for 18h was determined by immunofluorescence and confocal microscopy. Images were captured under 40x magnification and are representative images of three independent experiments. E. Increase in the expression of 23b and miR-34a in cervical cancer cells treated with VERU-111 for 24 h in comparison to non- treated controls. (*p < 0.05) was determined through real time PCR. Data presented as mean ± SD and are representative of three independent experiments.
3.3. VERU-111 treatment induces the expression of 23b and miR-34a in cervical cancer cells
Modulations of MicroRNAs are involved in cervical cancer development, progression and metastasis. We investigated the effect of VERU-111 on the expression of miR-23b and miR-34a by qRT-PCR in cervical cancer cells. Our results illustrated an 8- and 14-fold induction of miR-23b expression in VERU-111 treated (20 nM) in CaSki and SiHa cells, respectively as compared to the untreated control (Figure.2Ei–ii). MiR-34a is also significantly upregulated by VERU-111 in dose dependent compared to untreated cells (Figure. 2Eiii–iv). These results suggest that VERU-111 has the potential to induce the expression of tumor suppressor miRNAs in cervical cancer cells.
3.4. VERU-111 inhibits JAK2/ STAT3 signalling pathways
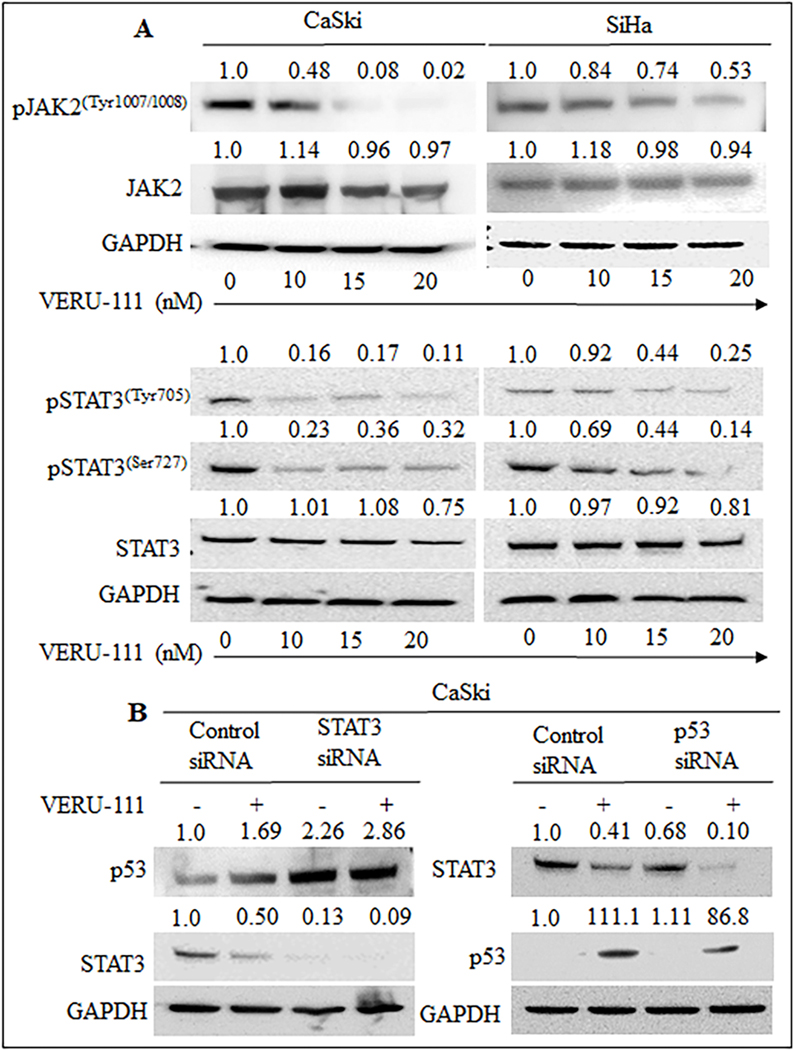
The JAK2 kinases phosphorylate STAT3 (Signal transducers and activators of transcription 3) is known to contribute to oncogenesis in cervical cancer [30]. Thus, we investigated the effect of VERU-111 on JAK2/STAT3 and regulations of p53 in cervical cancer cells. Interestingly, VERU-111 down regulated the expression of p-JAK2 dose dependently in cervical cancer cell lines as determine by western blot analysis (Figure. 3A). On the other hand, VERU-111 decreased the levels of pSTAT (Ser727) and pSTAT (Tyr705) in a dose-dependent manner (Figure. 3A) as well as total STAT3 at a higher concentration (Figure. 3A). VERU-111 treatment also restored the expression of p53 levels in part due to STAT3 silencing, suggesting STAT3 inactivation as one of the mechanisms of action. VERU-111 resulted in lower levels of STAT3 when p53 was knocked down. This result suggests that VERU-111 induced p53, which further caused decreases in STAT3 levels (Figure. 3B). These results suggest that VERU-111 regulated STAT3 expression and induced p53.
Figure 3: Effect of VERU-111 on JAK2/ STAT3 signaling pathway.
(A) Briefly, cervical cancer cells were treated with VERU-111 at indicated concentration for 24 h and subjected to western blot analysis to detect protein levels (B) CaSki cells were transfected with control siRNA, siSTAT3 or sip53, followed by treatment with VERU-111 at 20 nM for 24 h. Cell lysates were used for western blot analysis with STAT3, p53 and GAPDH antibodies.
3.5. VERU-111 arrests cell cycle at G2/M phase
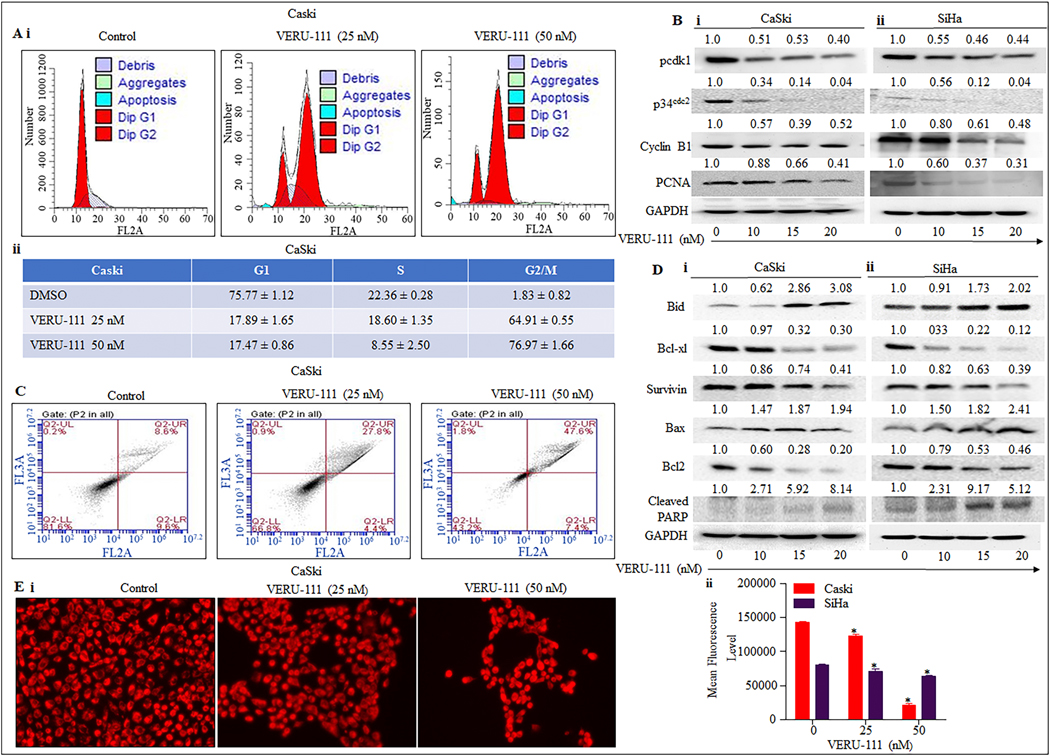
Excessive cell cycle progression is a major problem with cancerous cells, so drugs that can block cell cycle progression are highly desirable. CaSki and SiHa cells were stained with Propidium Iodide, and flow cytometer results revealed that VERU-111 arrested cell cycle progression of CaSki (Figure. 4Ai–ii) and SiHa (Supplementary Figure. S1Ai–ii) cells at G2/M phase in dose dependent manner in contrast to the vehicle control. Our western blot analysis demonstrated that a decrease in cyclin B1 levels, suggests a possible late G2 or M- phase arrest in cervical cancer cells (Figure. 4Bi–ii). Since VERU-111 induced G2/M phase arrest, we were interested into determining its effect on p34cdc2, a key regulator of G2/M transition and its interaction with cyclin B1. Interestingly, our data illustrated substantial dose-dependent reeducation in both p34cdc2 and phosphor- Cdk1 levels (Figure. 4Bi–ii) confirming G2/M arrest by VERU-111. Studies have shown that p21cip1/waf1 downstream target of p53 directly bind to PCNA and inhibit cell cycle progression [31]. VERU-111 treatment upregulated p21cip1/waf1 and downregulated PCNA levels (Fig. 3B). Thus, our results suggest G2/M arrest and modulation of p53-mediated cell cycle regulators by VERU-111 in cervical cancer cells.
Figure 4. Effect of VERU-111 on cell cycle progression and apoptosis in cervical cancer cells.
A. VERU-111 arrests CaSki cells cycle in G2/M phase as determined by flow Cytometry. Histogram (Ai) and table (Aii) represent the cell-cycle distribution in CaSki cells. B. Effect of VERU-111 on protein levels of cell cycle regulatory proteins (pcdk1, p34cdc2 CyclinB1and PCNA in both CaSki (Bi), and SiHa (Bii) cells. Briefly, cervical cancer cells were treated with the indicated concentrations of VERU-111 for 24 h; total cell lysates were prepared and subjected to Western blot analysis. Equal loading of protein in each lane was determined by probing the blots with GAPDH antibody. C. VERU-111 treatment induces apoptosis in cervical cancer cells. CaSki cells were treated with the indicated concentration of VERU-111 for 24 h and processed for apoptosis analysis using the Annexin V-7AAD Apoptosis Kit. Representative FL3-A and FL2-A plots showing dose-dependent increase of apoptosis in CaSki cells. D. VERU-111 treatment regulates apoptosis regulatory protein in cervical cancer cells. Briefly, 70% confluent cervical cancer cells were treated with VERU-111 at different concentrations for 24 h and whole cell lysates was prepared and subjected to Western blot analysis of apoptosis regulatory proteins (Bid, Bcl-xL, Survivin, Bax, Bcl2, and PARP cleavage) in both CaSki (Di), and SiHa (Dii). E. Effect of VERU-111 on mitochondrial membrane potential (ΔΨM) as determined by TMRE staining. Representative fluorescence images showing dose-dependent effect of VERU-111 on TMRE staining in CaSki cells (Ei). Bar graph indicating dose dependent inhibition of mitochondrial membrane potential (ΔΨM) in VERU-111 -treated CaSki cells as determined by flow cytometry (Ei). Asterisk (*) denotes the significant value P < 0.05.
3.6. VERU-111 induces apoptosis in cervical cancer cells
To determine the type of cell death induced by VERU-111, Annexin V-7AAD-based flow cytometry - (Annexin V early apoptotic cells;)-7AAD (stains dead cells) was used for detection of the percent apoptotic or necrotic cells. VERU-111 increased the percentage of apoptotic cells in both early and late apoptotic phases in CaSki (Figure. 4C) and SiHa (Supplementary Figure. S1B) in a dose dependent manner indicating apoptotic cell death. Furthermore, we investigated the effect of VERU-111 on modulations of apoptotic proteins by western blot analysis. Our results demonstrated that VERU-111 treatment markedly increased the protein levels of the pro-apoptotic Bid, whereas it decreased the protein levels of anti-apoptotic Bcl-xl and Survivin in CaSki and SiHa compared with the control group (Figure. 4Di–ii). Collectively, our results suggest that VERU-111 significantly inhibits proliferation through apoptotic cell death in cervical cancer cells.
Several studies have reported that Bcl-2 maintained the mitochondrial integrity, while Bax destroyed mitochondrial integrity and caused loss of mitochondrial membrane potential (ΔΨM) [32]. The susceptibility to apoptosis, and thus, the resulting life or death of a cell was dependent on the ratio between Bcl-2 and Bax [32]. The depolarization of mitochondrial membrane is considered a crucial cellular event of the intrinsic apoptotic pathway. We also determined VERU-111’s impact on the mitochondrial membrane potential of both cervical cancer cell lines. Cervical cancer cells were treated with VERU-111 at concentrations 25, 50 for 24 h, and we used both flow cytometer and fluorescence microscope studies with a Tetramethylrhodamine ethyl ester (TMRE) stain to examine mitochondrial membrane potential. Our results revealed that VERU-111 significantly decreased the mitochondrial membrane potential of both CaSki (Figure. 4Ei–ii) and SiHa cells (Supplementary Figure. S1C). VERU-111 treatment modulates p53-dependent apoptotic markers such as Bcl2 and Bax. As expected, PARP cleavage increased in a dose-dependent manner in both cell lines (Figure.4Di–ii). Taken together; these results showed that VERU-111 was able to induce apoptosis by altering the regulation of apoptotic genes, particularly through the upregulation of Bax and downregulation of Bcl-2.
3.7. VERU-111 inhibits cell migration, invasion, and modulate MMPs expression in cervical cancer
The effect of VERU-111 on cell migration was examined by wound healing assay at several concentrations (2.5–5 nM) for 24 h. We found the wound gaps in the 2.5 and 5 nM VERU-111 treated groups were significantly wider than those in the untreated group (Figure.5A i–ii). The cell migration was further analyzed by Transwell assay corning plate. Similar to the results of the wound healing assay, VERU-111effectively inhibited cell migration in a dose-dependent manner (Figure. 5C i–iii). Agarose bead assay experiments showed a similar effect (Figure. 5B i–ii). These findings were confirmed by using the xCELLigence real time cell migration system in cervical cancer cells (Figure. 5D i–ii) further verifying the effects on cellular motility.
Figure 5. Effect of VERU-111 on cell invasion, migration, and MMPs markers in cervical cancer cells.
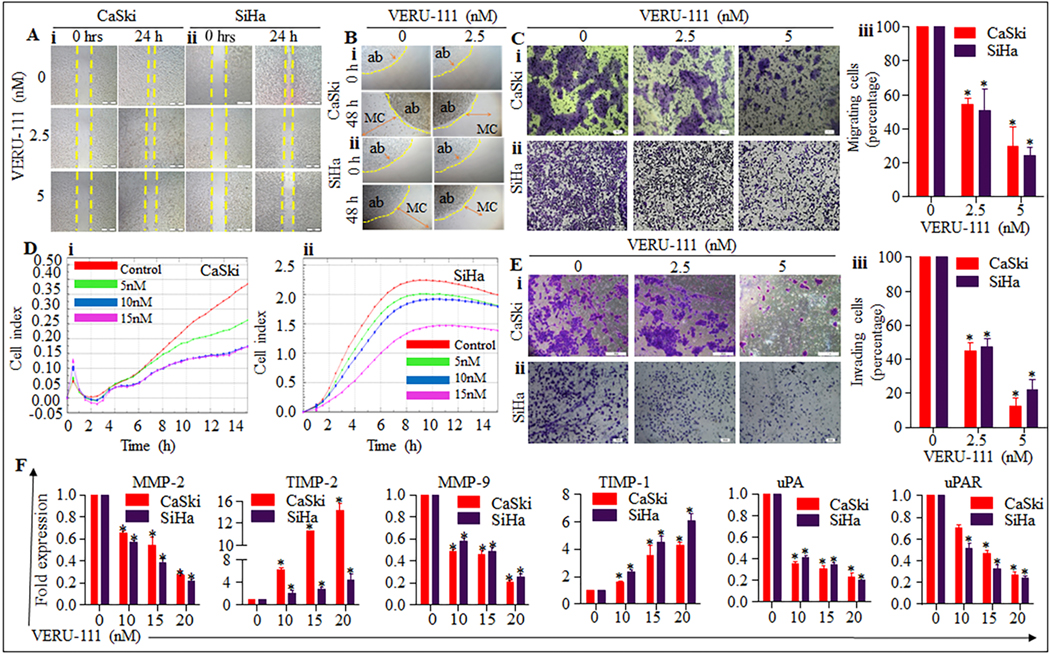
A, B. Effect of VERU-111 on cell motility of cervical cancer cells as determined by scratch wound and agarose bead assays. (A) Representative images of migratory cervical cancer cells in control and treated groups at 0, 24 h as determined by scratch wound assay. (B) Representative images of migratory cells in control and VERU-111 treated groups at 0 and 24 h as determined by agarose bead assays. AB denotes agarose bead. C, D Effect of VERU-111 on cell migration of cervical cancer cells as determined by Boyden chamber and xCELLigence assays. C. Representative images (20 X original magnification) showing inhibition of cervical cancer cells migration by Boyden chamber assay. D. Effect of VERU-111 on real-time cell migration as determined by xCELLigence assay. Briefly, cervical cancer cells (7× 104) were seeded in migration plate and VERU-111 treatment (2.5–5 nM) was given after 15 h and plate was allowed to incubate at 37ºC and 5% CO2 for real-time migration assay up to 48 h. Results indicate significant decrease in migratory potential of VERU-111 treated cervical cancer cells compared with control. E. Effect of VERU-111 on invasion of cervical cancer cells as determined by Boyden chamber. Representative photographs (20x original magnification) of invaded cells of control and VERU-111 treated cervical cancer cells as determined by Boyden Chamber Kit. F. Effect of VERU-111 on expression on MMPs in cervical cancer cells as determined by qRT-PCR. Briefly, 70% confluent cervical cancer cells were treated with VERU-111 (5–20 mM) for 24 h. RNA was isolated and cDNA was prepared and subjected to qRT-PCR for MMPs analysis. Asterisk (*) denotes the significant value P < 0.05.
Our cell invasion assays demonstrate that VERU-111 reduces the invaded cell in a dose dependent manner in cervical cancer cells (Figure. 5E i–ii). Compared to the control group, VERU-111 suppressed invasion by 55.3 %, 87.66% in CaSki and 52.63%, and 77.66 % in SiHa at 2.5 and 5 nM, respectively.
Matrix metalloproteinase (MMPs) such as MMP-2 and MMP-9 have been reported to play a critical role in cancer cell migration and invasion by facilitating the degradation of the extracellular matrix (ECM) and basement membrane BM [33]. Interestingly, our data illustrated that the mRNA level of MMP-2, MMP-9, UPA and uPAR were significantly (p< 0.05) reduced and those of TIMP-1 and TIMP-2 were significantly (p< 0.05) elevated at dose dependent manner in CaSki and SiHa cervical cancer cell (Figure. 5F). These data show that VERU-111 most likely inhibits cervical cancer cell migration, invasion and metastasis by downregulating the levels of uPA, uPAR, MMP-2, and MMP-9 proteolytic enzymes, and by upregulating TIMP-1 and TIMP-2.
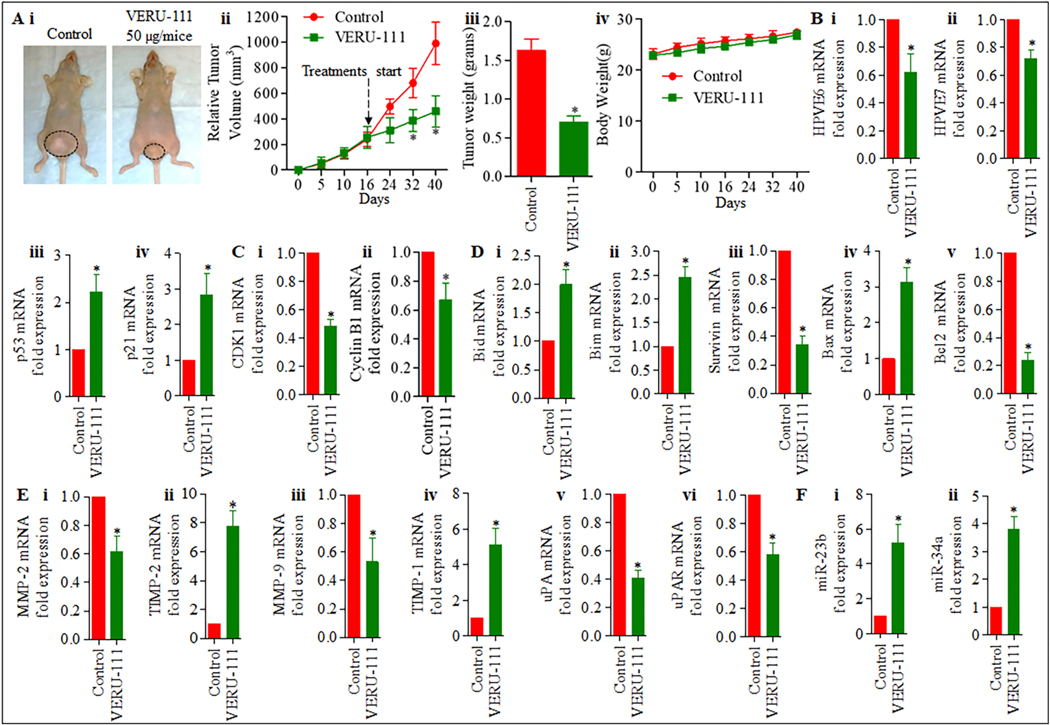
3.8. VERU-111 inhibits cervical cancer cell-derived orthotopic xenograft tumors in athymic nude mice
To determine an effective dose, we treated the mice with VERU-111 (50 μg/mice, three times a week) intratumorally in orthotopic xenograft. Our results demonstrate that intra-tumoral administration of VERU-111 significantly inhibited CaSki cell-derived orthotopic xenograft tumors in athymic nude mice compared to an untreated control (Figure. 6Ai–iv). VERU-111 administration significantly (p < 0.05) reduced both tumor volume (Figure. 6Ai - ii) and weight (Figure. 6Aiii) compared to control groups. During experiments, no signs of toxicity were present as reflected by unaltered body weights compared with the control group (Figure.6Aiv). No mortality occurred during the study period. We observed that the mRNA expression of E6 and E7 (Figure. 6Bi–ii.) was down-regulated, and that p21 and p53 (Figure. 6Biii–iv) are upregulated in VERU-111 treated tumors. Furthermore, the mRNA level expression of cell cycle regulatory markers (CDK1 and cyclin B1) was significantly reduced in VERU-111 treated orthotopic xenograft tissue (Figure. 6Ci–ii.). VERU-111 increase the expression of pro-apoptotic markers Bid and Bim (Figure. 6Di–ii) and modulates the expression of anti-apoptotic Bax, Bcl2, and Survivin on the mRNA level in orthotopic xenograft tissue compared with the untreated group (Figure. 6Diii–v) which correlates with our cell culture studies. We also investigated the effect of VERU-111 on modulation of MMPs and their inhibitors in xenograft tumors. Our qRT-PCR result demonstrates that expression of MMP2, MMP9, uPA, and uPAR is significantly downregulated and TIMP2, TIMP1 markedly upregulated in VERU-111 treated groups compared to the control group (Figure. 6Ei–vi). Interestingly, the expression of miR-23b and miR-34a was significantly restored in VERU-111 treated xenograft tumors as determined by qRT-PCR (Figure.6Fi–ii.). We also measured PCNA, E6, E7, p21, p53, MMP-2, MMP-9, STAT3, pSTAT3ser727, and pSTAT3tyr705 protein expression in xenograft tumor tissues by IHC. Compared to the control, the expression of PCNA, E6, E7 MMP-2, MMP-9, STAT3, pSTAT3ser727, and pSTAT3tyr705 significantly decreased, while the expression of p53 and p21 markedly increased in the treatment group (Figure. 7i–ii), which correlates with the in vitro assessment.
Figure 6. VERU-111 inhibits cervical tumor growth in xenograft mouse model.
A, Effect of VERU-111 on CaSki cells derived xenograft tumors in female athymic nude mice. In brief, 12 mice were used in this experiment and were divided into two groups. A total of 4× 106 CaSki cells were injected directly into the cervix of each mouse without any surgery. VERU-111 was administered (50 μg, intra-tumorally three times per week for three weeks) and control group mice received PBS as vehicle control. Representative mouse picture of control and VERU-111 -treated tumor bearing mouse (i). Average tumor volume of each group of mice at different days (ii). Bar graph representing tumor weight of each group of mice (iii). Body weights compared with the control group (iv). B. Effect of VERU-111 on expression of E6 (i), E7 (ii), p53 (iii), and p21 (iv) in treated or control excised tumors as determined by qRT-PCR analysis. C. Effect of VERU-111 on mRNA expression of cell cycle regulatory markers (i-ii). D. Pro-apoptotic markers (i-ii), anti-apoptotic markers (iii-v), and E. MMPs and their inhibitors (i-vi) in orthotopic xenograft tumor treated with VERU-111 as determined by qRT-PCR analysis. F. Effect of VERU-111 on the expression of miR-23b and miR-34a (i-ii) in orthotopic xenograft tumor treated with VERU-111 as determined by qRT-PCR analysis. Value in graph represents mean ± SE of six mice in each group. Asterisk (*) denotes the significant value P < 0.05.
Figure 7. VERU-111 regulate the expression of, PCNA, E6, E7, p21, p53, MMP-2, MMP-9, STAT3, pSTAT3ser, pSTAT3tyr in excised tumors in xenograft mouse model.
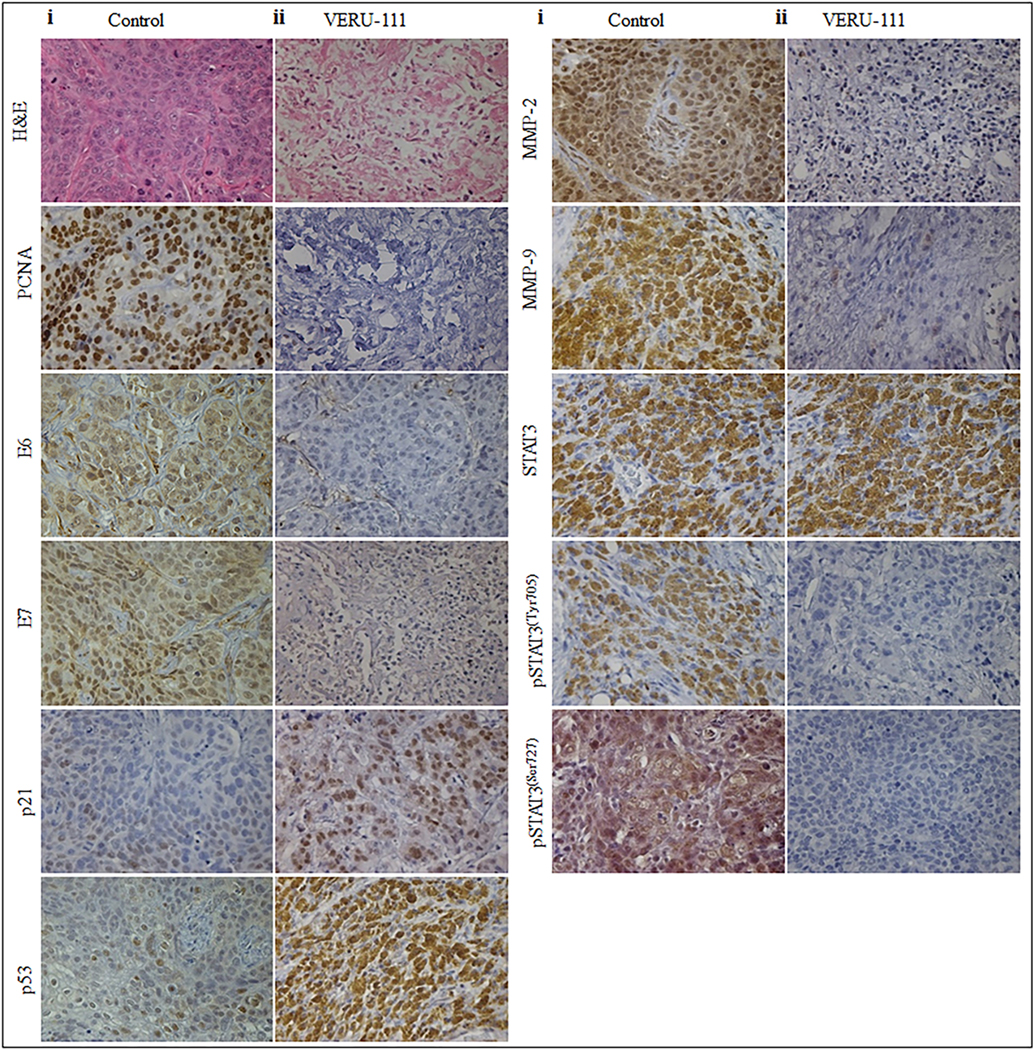
Effect of VERU-111 on the expressions of, PCNA, E6, E7, p21, p53, MMP-2, MMP-9, STAT3, STAT3ser, pSTAT3tyr in excised tumors of control (i) and VERU-111 (ii) treated mice as determined by IHC analysis. All IHC analyses were captured at 20X magnification.
Discussion
In current study, we tested the anticancer activity of novel compound VERU-111 and explored the possible associated mechanism of action in cervical cancer. During In vitro treatment, VERU-111 significantly inhibited growth and cell proliferation in three cervical cancer cells with slightly different IC50 values. The sensitivity of VERU-111 is different in all three cell lines depending on several factors such as growth rate, HPV type, copy number, and basal level of cellular tumor-suppressor protein p53. It is well documented that HPV oncoproteins E6 and E7 are often crucial for host cell immortalization and transformation by targeting tumor suppressor p53 and pRb [34, 35]. Therefore, it is hypothesized that the agent, which suppresses oncogenes E6, and E7 must upregulate the expression of cellular tumor-suppressor proteins. Our findings suggest that VERU-111 represses HPV E6 and E7 and increases expression of p53 and its effector molecules p21cip1/waf1, indicating a possible p53-mediated apoptosis in these cells.
The degradation of p53 was found to decrease the expression of miR-23b, which leads to an increase in expression of urokinase-type plasminogen activator (uPA), thus, inducing migration of human cervical carcinoma [36]. Our qRT-PCR results indicated an 8- and 14-fold increase in miR-23b expression in VERU-111 treated (20nM) CaSki and SiHa cells, respectively, as compared to the untreated control. Several studies have previously reported that miR-34a is a direct transcriptional target of p53. The binding of p53 to a consensus triggers transactivation of the miR-34a expression-binding site present in the miR-34a promoter region [37, 38]. MiR-34a expression is downregulated in most cervical cancer tissues with oncogenic HPV infection by destabilizations of p53. Thus, we investigated the effect of VERU-111 on expression of miR-34a in cervical cancer cells. VERU-111 significantly (p < 0.05) induced the expressions of miR-34a. This suggests that VERU-111 has the potential to induce the expression of tumor suppressor miRNAs in cervical cancer cells.
Janus kinase 2 (JAK2), is a non-receptor tyrosine kinase involved in many signaling pathways that modulate cell apoptosis, autophagy and proliferation [39]. The JAK2 phosphorylates STAT3, and activated STAT3 plays a major role in tumor progression by promoting cell proliferation, angiogenesis and prevention of apoptosis induced by chemotherapeutic agents [40]. On the other hand, previous studies have demonstrated that the binding of activated STAT3 to the HPV16 LCR regulates the aberrant expression of E6 and E7. These further controls the cellular p53 and pRb, which leads to their degradation during the natural history of HPV16 infection [9]. The increased level of STAT3 is also associated with an increased expression of Bcl-xL, Survivin and mcl-1, which have shown a strong correlation with pSTAT3 levels in cervical lesion [41]. In our results, VERU-111 not only decreased the expression of pJAK2 at Tyr1007/1008 and STAT3 phosphorylation at Tyr705 and Ser727 residues; it also decreased the expression of total STAT3 levels (at higher concentrations). Interestingly, the induced expression of p53 by VERU-111 also suggests that this agent must partly be responsible for lowering STAT3 levels. Thus, our findings suggest that VERU-111 inhibited STAT3 levels both directly as well as indirectly, via the restored p53 expression in CaSki cells.
Abnormal regulation of cell cycle progression is one of the hallmarks of cancer cells [42]. Our results show that VERU-111 arrests the cell cycle at the G2/M phase and modulates cell cycle regulators such as cyclin B1, p21cip1/waf1 and p34cdc2. VERU-111 decreased p34cdc2 and pCdk1, further leading to decreased cyclin B1- p34cdc2 complex formation, in turn causing cell cycle arrest at the G2/M phase. Several studies have demonstrated that p21cip1/waf1, a universal inhibitor of cdk, is known to inhibit p34cdc2 [43]. Therefore, decreased expression of p34cdc2 could also be due to an increase in p21cip1/waf1 as caused by VERU-111. Upon activation, p21cip1/waf1 binds to PCNA and results in growth arrest by preventing DNA replication [44]. VERU-111 treated cervical cancer cells clearly demonstrated a decrease in PCNA levels and an increased interaction between p21cip1/waf1 and PCNA. Therefore, we presume that p21cip1/waf1, partly due to interaction with PCNA, may have a proapoptotic effect[45, 46].
A number of small molecules are known to cause DNA damage and activate the p53 pathway to induce growth arrest and apoptosis [47]. It has been previously reported that this effect is due to direct activation of the proapoptotic protein Bax in p53-mediated apoptosis [48]. Our western blot results confirm that VERU-111-induced cell death in cervical cancer cells and was accompanied by an upregulation of Bax and downregulation of the antiapoptotic protein Bcl2. An increased ratio of Bax: Bcl2 was found to increase the cells’ susceptibility to undergo apoptosis and activate the release of cytochrome c from mitochondria into the cytosol [49]. Subsequently, cytochrome c cleaves procaspase-3 into active caspase 3, which in turn leads to PARP cleavage. Our study clearly demonstrates the restoration of p53 levels by VERU-111, resulting in the modulation of the Bax/Bcl2 ratio and caspase-3 mediated apoptosis in cervical cancer cells.
Cervical cancer cells collectively proliferate and invade the stroma, forming groups, nests and tubular architecture. Cell migration is a key characteristic of cancer cells. Metastasis is the main reason for treatment failure in cancer [50]. In cervical cancer with poor prognosis, proteolytic enzymes such as Matrix MMP-2, MMP-9 and uPA play a very important role in ECM and BM degradation [33]. Moreover, ECM degradation promotes cell invasion and migration [51]. Our results demonstrate that VERU-111 not only reduced the invasion and the migration of cervical cancer cells, but also significantly inhibited the expression of MMP-2, MMP-9, uPA and uPAR., Interestingly, treatment of VERU-111 significantly increased the expression of TIMP-1 and TIMP-2, which indicated that VERU-111 inhibited the invasion and migration of cervical cancer cells through downregulation of the expression of MMP-2, MMP-9 and uPA.
Based on the high antiproliferative activity of VERU-111 against cervical cancer cells in vitro, we tested its effective dose against cervical cancer cell-derived orthotopic xenograft tumors in athymic nude mice. In our in vivo findings, downregulation of HPV16 E6 and E7 oncoproteins and an increase in p53 levels in the VERU-111 treated animals were consistent with cell culture findings. Our results demonstrate that VERU-111 has a strong and significant in vivo antiproliferative activity against cervical cancer growth, in a p53-dependent manner.
In summary, we have the shown potential anti-cancer effects of VERU-111 against cervical cancer cells and in vivo. VERU-111 efficiently represses HPV E6 and E7 oncogenes, resulting in sequential reactivation of p53 dependent tumor suppressor activity leading to cervical cancer tumor growth. Our findings provide a strong basis for further exploration of VERU-111 as a therapeutic drug against cervical cancer in monotherapy or adjuvant to standard chemotherapeutic agents, to treat primary/ persistent/ recurrent cervical cancer patients.
Supplementary Material
Effect of VERU-111 on cell cycle arrest and apoptosis in SiHa cells A. VERU-111 arrests SiHa cell cycle in G2/M phase as determined by flow Cytometry. Histogram (Ai) and table (Aii) represent the cell-cycle distribution in SiHa cells. B. VERU-111 treatment induces apoptosis in SiHa cells. Briefly, SiHa cells were treated with indicated concentration of VERU-111 for 24 h and processed for apoptosis analysis using Annexin V-7AAD Apoptosis Kit. Representative FL3-A and FL2-A plots showing dose-dependent increase of apoptosis in SiHa cells. C. Effect of VERU-111 on mitochondrial membrane potential (ΔΨM) as determined by TMRE staining. Representative fluorescence images showing dose-dependent effect of VERU-111 on TMRE staining in SiHa cells.
List of qRT- PCR Primers
Figure 8.
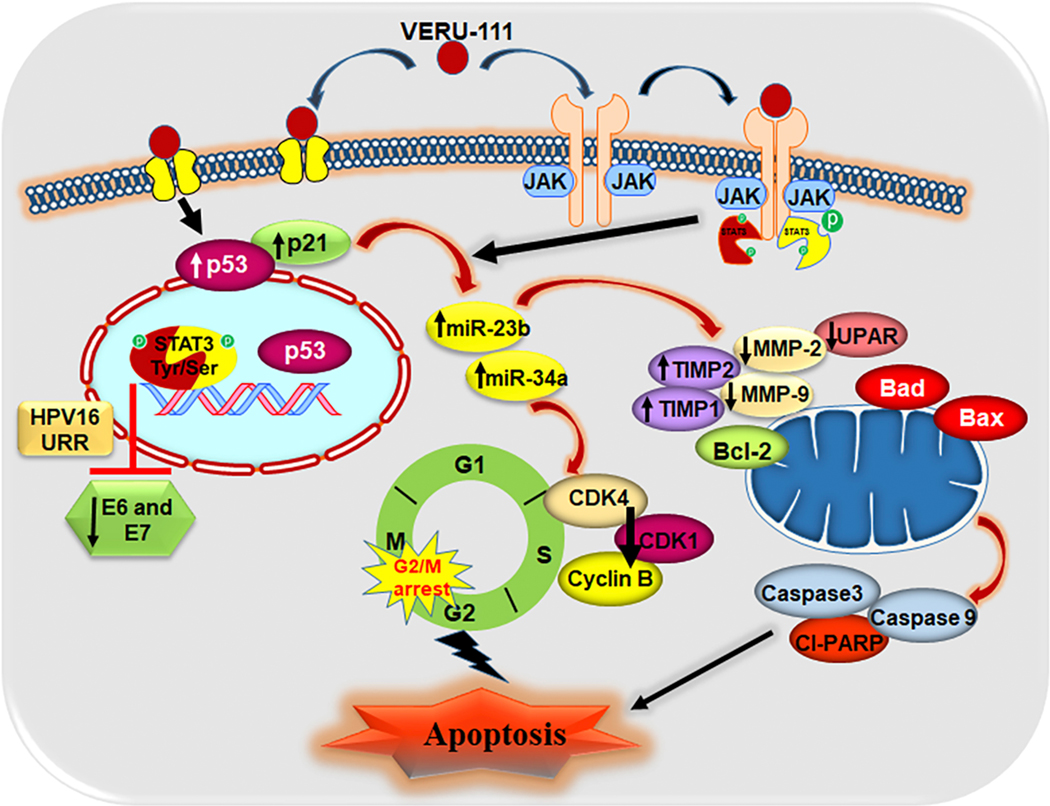
Schematic presentation of possible mechanism of VERU-111 in CxCa
Highlights.
HPV E6 and HPV E7 oncoproteins play important roles in tumorigenesis and cervical cancer progression.
VERU-111 concomitantly downregulates HPV E6 and E7 expression via the activations of p53.
VERU-111 decreased the expression of phosphorylation of Jak2 / STAT3 and display p53-mediated apoptosis.
VERU-111 exhibits potent anti-tumor activity in in vitro and in vivo orthotopic xenograft mouse model of cervical cancer.
Acknowledgments
Funding
This work was supported by NIH/NC1 grants (R01 CA210192, R01 CA206069 and R01 CA204552) to SCC, R01 CA148706 to WL and DDM, 1S10OD010678 and RR026377 to WL
Footnotes
Supplementary Material
Supplementary data are available at Cancer letter online.
Conflicts of Interest
The authors declare that they have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J Clin, 69 (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Scheffner M, Munger K, Byrne JC, Howley PM, The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines, Proc Natl Acad Sci U S A, 88 (1991) 5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Munger K, Howley PM, Human papillomavirus immortalization and transformation functions, Virus Res, 89 (2002) 213–228. [DOI] [PubMed] [Google Scholar]
- [4].Tan S, de Vries EG, van der Zee AG, de Jong S, Anticancer drugs aimed at E6 and E7 activity in HPV-positive cervical cancer, Curr Cancer Drug Targets, 12 (2012) 170–184. [DOI] [PubMed] [Google Scholar]
- [5].Vousden KH, Lu X, Live or let die: the cell’s response to p53, Nat Rev Cancer, 2 (2002) 594–604. [DOI] [PubMed] [Google Scholar]
- [6].Ravizza R, Gariboldi MB, Passarelli L, Monti E, Role of the p53/p21 system in the response of human colon carcinoma cells to Doxorubicin, BMC Cancer, 4 (2004) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Horner SM, DeFilippis RA, Manuelidis L, DiMaio D, Repression of the human papillomavirus E6 gene initiates p53-dependent, telomerase-independent senescence and apoptosis in HeLa cervical carcinoma cells, J Virol, 78 (2004) 4063–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koivusalo R, Mialon A, Pitkanen H, Westermarck J, Hietanen S, Activation of p53 in cervical cancer cells by human papillomavirus E6 RNA interference is transient, but can be sustained by inhibiting endogenous nuclear export-dependent p53 antagonists, Cancer Res, 66 (2006) 11817–11824. [DOI] [PubMed] [Google Scholar]
- [9].Shukla S, Mahata S, Shishodia G, Pandey A, Tyagi A, Vishnoi K, Basir SF, Das BC, Bharti AC, Functional regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis, PLoS One, 8 (2013) e67849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM, Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth, PLoS One, 3 (2008) e2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen J, Ahn S, Wang J, Lu Y, Dalton JT, Miller DD, Li W, Discovery of novel 2-aryl-4-benzoyl-imidazole (ABI-III) analogues targeting tubulin polymerization as antiproliferative agents, J Med Chem, 55 (2012) 7285–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen J, Wang Z, Li CM, Lu Y, Vaddady PK, Meibohm B, Dalton JT, Miller DD, Li W, Discovery of novel 2-aryl-4-benzoyl-imidazoles targeting the colchicines binding site in tubulin as potential anticancer agents, J Med Chem, 53 (2010) 7414–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen J, Li CM, Wang J, Ahn S, Wang Z, Lu Y, Dalton JT, Miller DD, Li W, Synthesis and antiproliferative activity of novel 2-aryl-4-benzoyl-imidazole derivatives targeting tubulin polymerization, Bioorg Med Chem, 19 (2011) 4782–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li CM, Chen J, Lu Y, Narayanan R, Parke DN, Li W, Ahn S, Miller DD, Dalton JT, Pharmacokinetic optimization of 4-substituted methoxybenzoyl-aryl-thiazole and 2-aryl-4benzoyl-imidazole for improving oral bioavailability, Drug Metab Dispos, 39 (2011) 1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kashyap VK, Wang Q, Setua S, Nagesh PKB, Chauhan N, Kumari S, Chowdhury P, Miller DD, Yallapu MM, Li W, Jaggi M, Hafeez BB, Chauhan SC, Therapeutic efficacy of a novel betaIII/betaIV-tubulin inhibitor (VERU-111) in pancreatic cancer, J Exp Clin Cancer Res, 38 (2019) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zaman MS, Chauhan N, Yallapu MM, Gara RK, Maher DM, Kumari S, Sikander M, Khan S, Zafar N, Jaggi M, Chauhan SC, Curcumin Nanoformulation for Cervical Cancer Treatment, Sci Rep, 6 (2016) 20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nagesh PKB, Chowdhury P, Hatami E, Boya VKN, Kashyap VK, Khan S, Hafeez BB, Chauhan SC, Jaggi M, Yallapu MM, miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy, Cancers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chowdhury P, Nagesh PKB, Khan S, Hafeez BB, Chauhan SC, Jaggi M, Yallapu MM, Development of polyvinylpyrrolidone/paclitaxel self-assemblies for breast cancer, Acta Pharm Sin B, 8 (2018) 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Munagala R, Kausar H, Munjal C, Gupta RC, Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells, Carcinogenesis, 32 (2011) 1697–1705. [DOI] [PubMed] [Google Scholar]
- [20].Nagesh PKB, Chowdhury P, Hatami E, Kumari S, Kashyap VK, Tripathi MK, Wagh S, Meibohm B, Chauhan SC, Jaggi M, Yallapu MM, Cross-Linked Polyphenol-Based Drug Nano-Self-Assemblies Engineered to Blockade Prostate Cancer Senescence, ACS Appl Mater Interfaces, 11 (2019) 38537–38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sikander M, Malik S, Chauhan N, Khan P, Kumari S, Kashyap VK, Khan S, Ganju A, Halaweish FT, Yallapu MM, Jaggi M, Chauhan SC, Cucurbitacin D Reprograms Glucose Metabolic Network in Prostate Cancer, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumari S, Khan S, Gupta SC, Kashyap VK, Yallapu MM, Chauhan SC, Jaggi M, MUC13 contributes to rewiring of glucose metabolism in pancreatic cancer, Oncogenesis, 7 (2018) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chowdhury P, Nagesh PKB, Hatami E, Wagh S, Dan N, Tripathi MK, Khan S, Hafeez BB, Meibohm B, Chauhan SC, Jaggi M, Yallapu MM, Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells, J Colloid Interface Sci, 535 (2019) 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Parekh A, Das S, Parida S, Das CK, Dutta D, Mallick SK, Wu PH, Kumar BNP, Bharti R, Dey G, Banerjee K, Rajput S, Bharadwaj D, Pal I, Dey KK, Rajesh Y, Jena BC, Biswas A, Banik P, Pradhan AK, Das SK, Das AK, Dhara S, Fisher PB, Wirtz D, Mills GB, Mandal M, Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo, Oncogene, 37 (2018) 4546–4561. [DOI] [PubMed] [Google Scholar]
- [25].Hafeez BB, Ganju A, Sikander M, Kashyap VK, Hafeez ZB, Chauhan N, Malik S, Massey AE, Tripathi MK, Halaweish FT, Zafar N, Singh MM, Yallapu MM, Chauhan SC, Jaggi M, Ormeloxifene Suppresses Prostate Tumor Growth and Metastatic Phenotypes via Inhibition of Oncogenic beta-catenin Signaling and EMT Progression, Mol Cancer Ther, 16 (2017) 2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Massey AE, Sikander M, Chauhan N, Kumari S, Setua S, Shetty AB, Mandil H, Kashyap VK, Khan S, Jaggi M, Yallapu MM, Hafeez BB, Chauhan SC, Next-generation paclitaxel-nanoparticle formulation for pancreatic cancer treatment, Nanomedicine, (2019) 102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nagesh PKB, Hatami E, Chowdhury P, Kashyap VK, Khan S, Hafeez BB, Chauhan SC, Jaggi M, Yallapu MM, Tannic Acid Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Prostate Cancer, Cancers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karuri AR, Kashyap VK, Yallapu MM, Zafar N, Kedia SK, Jaggi M, Chauhan SC, Disparity in rates of HPV infection and cervical cancer in underserved US populations, Front Biosci (Schol Ed), 9 (2017) 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Benson EK, Mungamuri SK, Attie O, Kracikova M, Sachidanandam R, Manfredi JJ, Aaronson SA, p53-dependent gene repression through p21 is mediated by recruitment of E2F4 repression complexes, Oncogene, 33 (2014) 3959–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Menet CJ, Rompaey LV, Geney R, Advances in the discovery of selective JAK inhibitors, Prog Med Chem, 52 (2013) 153–223. [DOI] [PubMed] [Google Scholar]
- [31].Cayrol C, Knibiehler M, Ducommun B, p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells, Oncogene, 16 (1998) 311–320. [DOI] [PubMed] [Google Scholar]
- [32].Sharpe JC, Arnoult D, Youle RJ, Control of mitochondrial permeability by Bcl-2 family members, Biochim Biophys Acta, 1644 (2004) 107–113. [DOI] [PubMed] [Google Scholar]
- [33].Solovsmall u CNI, Timoshenko OS, Gureeva TA, Kugaevskaya EV, [Matrix metalloproteinases and their endogenous regulators in squamous cervical carcinoma (review of the own data)], Biomed Khim, 61 (2015) 694–704. [DOI] [PubMed] [Google Scholar]
- [34].Zuna RE, Allen RA, Moore WE, Mattu R, Dunn ST, Comparison of human papillomavirus genotypes in high-grade squamous intraepithelial lesions and invasive cervical carcinoma: evidence for differences in biologic potential of precursor lesions, Mod Pathol, 17 (2004) 1314–1322. [DOI] [PubMed] [Google Scholar]
- [35].Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N, Human papillomavirus is a necessary cause of invasive cervical cancer worldwide, J Pathol, 189 (1999) 12–19. [DOI] [PubMed] [Google Scholar]
- [36].Duffy MJ, Maguire TM, McDermott EW, O’Higgins N, Urokinase plasminogen activator: a prognostic marker in multiple types of cancer, J Surg Oncol, 71 (1999) 130–135. [DOI] [PubMed] [Google Scholar]
- [37].Appleman LJ, Uyeki J, Frey AB, Mouse embryo fibroblasts transformed by activated ras or dominant-negative p53 express cross-reactive tumor rejection antigens, Int J Cancer, 61 (1995) 887–894. [DOI] [PubMed] [Google Scholar]
- [38].Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M, Transcriptional activation of miR-34a contributes to p53-mediated apoptosis, Mol Cell, 26 (2007) 731–743. [DOI] [PubMed] [Google Scholar]
- [39].Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA, Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells, Cancer Res, 67 (2007) 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dutta P, Sabri N, Li J, Li WX, Role of STAT3 in lung cancer, JAKSTAT, 3 (2014) e999503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA, Cheng G, Hall BM, Lin J, Stat3 activation in human endometrial and cervical cancers, Br J Cancer, 96 (2007) 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell, 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [43].He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R, Khokhar AR, Kuang J, Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities, Oncogene, 24 (2005) 2929–2943. [DOI] [PubMed] [Google Scholar]
- [44].Waga S, Hannon GJ, Beach D, Stillman B, The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA, Nature, 369 (1994) 574–578. [DOI] [PubMed] [Google Scholar]
- [45].Jaiswal AS, Bloom LB, Narayan S, Long-patch base excision repair of apurinic/apyrimidinic site DNA is decreased in mouse embryonic fibroblast cell lines treated with plumbagin: involvement of cyclin-dependent kinase inhibitor p21Waf-1/Cip-1, Oncogene, 21 (2002) 5912–5922. [DOI] [PubMed] [Google Scholar]
- [46].Maga G, Blanca G, Shevelev I, Frouin I, Ramadan K, Spadari S, Villani G, Hubscher U, The human DNA polymerase lambda interacts with PCNA through a domain important for DNA primer binding and the interaction is inhibited by p21/WAF1/CIP1, FASEB J, 18 (2004) 1743–1745. [DOI] [PubMed] [Google Scholar]
- [47].Yu Q, Restoring p53-mediated apoptosis in cancer cells: new opportunities for cancer therapy, Drug Resist Updat, 9 (2006) 19–25. [DOI] [PubMed] [Google Scholar]
- [48].Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR, Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis, Science, 303 (2004) 1010–1014. [DOI] [PubMed] [Google Scholar]
- [49].Katiyar SK, Roy AM, Baliga MS, Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation, Mol Cancer Ther, 4 (2005) 207–216. [PubMed] [Google Scholar]
- [50].Sun K, Tang XH, Xie YK, Paclitaxel combined with harmine inhibits the migration and invasion of gastric cancer cells through downregulation of cyclooxygenase-2 expression, Oncol Lett, 10 (2015) 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G, Matrix metalloproteinases and cancer - roles in threat and therapy, Asian Pac J Cancer Prev, 15 (2014) 1085–1091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of VERU-111 on cell cycle arrest and apoptosis in SiHa cells A. VERU-111 arrests SiHa cell cycle in G2/M phase as determined by flow Cytometry. Histogram (Ai) and table (Aii) represent the cell-cycle distribution in SiHa cells. B. VERU-111 treatment induces apoptosis in SiHa cells. Briefly, SiHa cells were treated with indicated concentration of VERU-111 for 24 h and processed for apoptosis analysis using Annexin V-7AAD Apoptosis Kit. Representative FL3-A and FL2-A plots showing dose-dependent increase of apoptosis in SiHa cells. C. Effect of VERU-111 on mitochondrial membrane potential (ΔΨM) as determined by TMRE staining. Representative fluorescence images showing dose-dependent effect of VERU-111 on TMRE staining in SiHa cells.
List of qRT- PCR Primers