Abstract
Adult tissue stem cells mediate organ homeostasis and regeneration, and thus are continually making decisions about whether to remain quiescent, proliferate, or differentiate into mature cell types. These decisions often integrate external cues, such as energy balance and nutritional status of the organism. Metabolic substrates and byproducts that regulate epigenetic and signaling pathways are now appreciated to have instructive, rather than bystander roles in regulating cell fate decisions. In this review, we highlight recent literature focused on how metabolites and dietary manipulations can impact cell fate decisions, with a focus on regulation of adult tissue stem cells.
Keywords: adult stem cells, differentiation, metabolism, diet, metabolomics
Adult Tissue-Derived Stem Cells: Roles in Homeostasis and Regeneration
Cell fate decisions are essential for development and lie at the core of multicellular organismal biology. Embryonic development is initiated by pluripotent stem cells that become progressively fate restricted and differentiate into all mature cell types in the body. Postnatal organs contain populations of persistent tissue or adult stem cells (ASCs, see Glossary) that are capable of both self-renewal and differentiation to mature cell types specifically in their tissue of origin [1]. ASCs can be multipotent or unipotent, and typically transition through a progenitor stage followed by terminal differentiation. These specialized tissue-resident stem cells and progenitors play crucial roles in both homeostasis and regeneration in response to injury. To date, ASC/progenitor cells have been described in the majority of mammalian tissues [2]. Rapidly cycling tissues rely heavily on the homeostatic action of ASCs. For example, a population of intestinal stem cells supports the renewal of the lining of the small intestine every 4–5 days, and hair follicle stem cells (HFSCs) undergo iterative rounds of quiescence and activation during the hair cycle. Other tissues have basally low levels of turnover and mainly rely on tissue stem cells in the face of injury, such as skeletal muscle stem cells that are activated in the context of muscle damage to expand and differentiate into mature myocytes. Decades of research have focused on the innate developmental and transcriptional pathways that control the balance between self-renewal and differentiation in embryonic and adult stem cells. However, it is now becoming clear that metabolic state can also greatly influence cell fate transitions. ASCs are constantly tasked with making decisions about whether to remain quiescent, proliferate, or differentiate into mature cell types, and specific metabolic fuels are utilized depending on where stem cells lie along the continuum of differentiation. The metabolic requirements of pluripotent stem cells have been elegantly discussed elsewhere [3–6], and thus this review will focus on metabolism and cell fate choices in ASCs.
Metabolic Communication with Epigenetics and Signaling Pathways
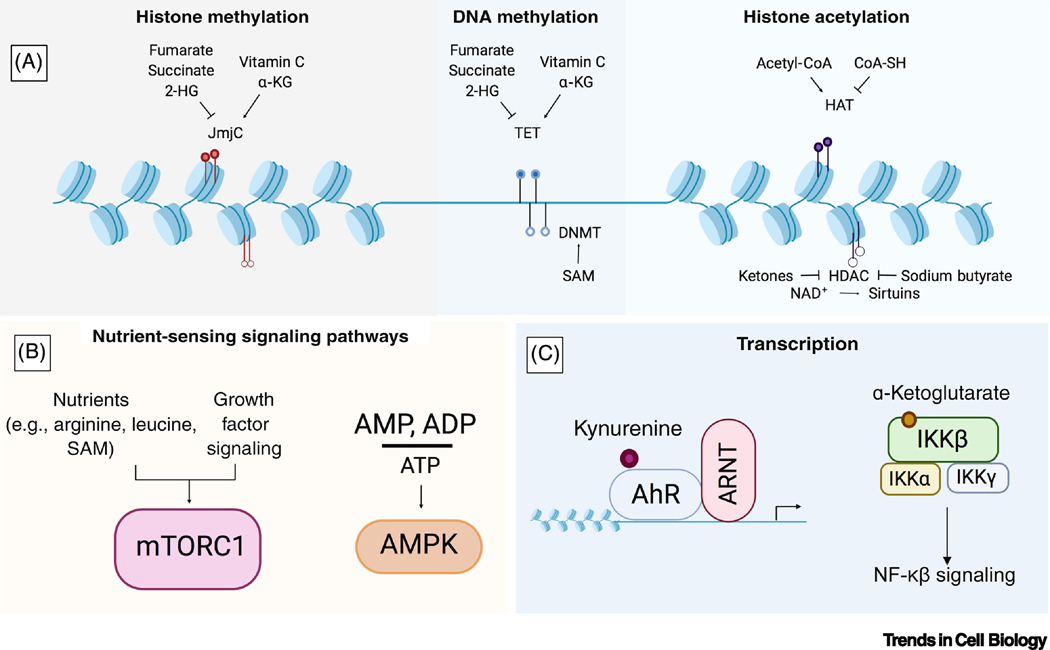
Metabolism encompasses interactions between diet, the microbiome, and cellular enzymatic processes that generate the chemical pathways necessary to sustain life. How are specific metabolites in our cells able to communicate with genetic and developmental pathways? Importantly, both endogenous metabolites and nutrients that are primarily derived from the diet can directly influence epigenetic enzymes [7]. Epigenetic modifications to DNA and histone proteins alter cell fate by controlling chromatin accessibility and downstream gene expression patterns. The enzymes that catalyze these modifications are broadly grouped into the epigenetic “writers” that deposit modifications, and “erasers” that facilitate removal [8]. The majority of substrates and co-factors for chromatin-modifying enzymes are derived from metabolic pathways involving the tricarboxylic acid (TCA) cycle, methionine cycle, folate cycle, glycolysis, β-oxidation, and the hexosamine pathway [9]. These complex and interconnected networks generate intermediates that co-activate epigenetic enzymes and/or serve as direct substrates for modifications, including acetyl-CoA, alpha ketoglutarate (α-KG), succinate, fumarate, S-adenosyl methionine (SAM), UDP-GlcNAc, ketone bodies, lactate, and the reducing equivalents NADH and FADH2 [10, 11]. In addition, diet-derived nutrients such as ascorbate (Vitamin C) and sodium butyrate also regulate activity of chromatin and DNA modifying proteins. Because the physiological concentrations of most chromatin substrates are in the range of enzymatic Km values, the activity of epigenetic writers and erasers is indeed sensitive to metabolic context, providing a direct link between metabolism and gene expression [12] (Figure 1A).
Figure 1. Metabolic Effectors of Chromatin, Signaling Pathways, and Transcription Factors.
(A) Many substrates and co-factors for chromatin-modifying enzymes are derived from metabolic pathways involving the TCA cycle, methionine cycle, folate cycle, glycolysis, β-oxidation, and the hexosamine pathway. These metabolites can serve as activators or inhibitors of epigenetic writers such as Jumonji C (JmjC) domain-containing proteins, DNA methyltransferases (DNMTs), and histone acetyltransferases (HATs), Ten-eleven translocase DNA demethylases (TETs), and histone deacetylases (HDACs). (B) Metabolites can influence nutrient sensing signaling pathways. The kinase complex mTORC1 can only be activated by growth factor-induced signaling when the amino acids arginine and leucine, as well as the co-factor S-adenosyl methionine (SAM), are sensed inside the cell. In addition, energy balance communicated through the cellular AMP/ADP to ATP ratio can be sensed by AMPK. (C) Transcription factors can be directly regulated by metabolites; for example, the tryptophan metabolite kynurenine is an endogenous agonist for the aryl hydrocarbon receptor, and α-KG binds to and activates IKKβ and initiates NF-κβ signaling.
Importantly, this link is bidirectional, with epigenetic changes also able to directly influence metabolism by altering expression of metabolic genes. There are several emerging examples of this phenomenon in the cancer metabolism field. In the setting of oncogenic NRAS mutations, loss of the histone methyltransferase EZH2 leads to aberrant activation of branched-chain amino acid metabolism and malignant transformation in leukemia-initiating cells [13]. Increased expression of EZH2 can also be oncogenic and lead to metabolic reprogramming: in hepatocellular and clear cell renal cell carcinoma, EZH2 represses expression of the enzyme fructose-1,6,-bisphosphatase, thus altering glucose metabolism and promoting tumor growth [14]. A number of congenital disorders caused by aberrant epigenetic regulatory mechanisms also feature hallmarks of metabolic dysfunction. For example, mutations in in the DNA methylation binding protein MECP2 cause Rett syndrome, an X-linked neurodegenerative disorder that is also characterized by dysregulated lipid metabolism [15].
In addition to the connection between metabolism and epigenetic pathways, nutrients can also impact cellular state by modulating signaling pathway activity. One clear example is through the mechanistic target of rapamycin (mTOR) signaling pathway, and in particular mTOR complex 1 (mTORC1), which regulates cell growth only when both nutrients and growth factors are present. Depletion of specific nutrients, including arginine, leucine, and SAM, prevents growth factor-induced mTORC1 activation by blocking Rag GTPase-mediated mTORC1 recruitment to the lysosome where it can be activated by the Rheb GTPase [16]. Another way nutrients are sensed to impact cellular state is through AMP-activated protein kinase (AMPK), which under low cellular ATP levels, phosphorylates substrates to restore the energy balance of the cell and in the process regulates cell growth and autophagy [17] (Figure 1B).
Metabolic Regulation of Tissue Stem Cell Fate and Function
ASCs typically reside deep within resident tissues in the niche: hematopoietic stem cells (HSCs) in the trabecular bone marrow, HFSCs in the follicular bulge, gut stem cells at the base of intestinal crypts, and muscle stem cells outside the sarcolemma alongside the muscle basement membrane [18]. The tissue niche has a large influence on the function of resident ASCs; for example, HFSCs are quiescent > 90% of the time within the niche but will rapidly divide if transferred outside the niche to ex vivo culture [19, 20]. The tissue niche is also capable of influencing ASC metabolism. Much of the work on tissue stem cell metabolism has focused on central carbon metabolism, that is, the generation of metabolic building blocks via glycolysis, oxidative phosphorylation, or the pentose phosphate pathway. It is generally thought that the quiescent stem cell state is characterized by an inherently glycolytic metabolism, followed by a transition to favor mitochondrial oxidative phosphorylation during commitment and differentiation [21–24]. However, mounting evidence suggests that metabolism during quiescence, activation, and differentiation likely varies between tissues, integrating signaling cues and metabolic inputs from the niche and organism as a whole.
Historically it has been difficult to study metabolism in adult stem cells because these populations are rare, lack robust/selective markers for isolation, and activate different metabolic pathways depending on differentiation status. Recent technological advances have increased sensitivity in small cell populations and improved analysis tools. Metabolomics provides snapshots into cellular pathways by looking at pool size or flux of metabolic substrates and products (metabolites) through different pathways, with newly expanded untargeted metabolomics platforms facilitating identification of unknown species [25, 26]. Coupled with transcriptomic and proteomic analysis, our insight into how metabolism affects cell fate (and vice versa) is advancing rapidly. Here we discuss several recent examples across a number of mammalian tissues (Figure 2).
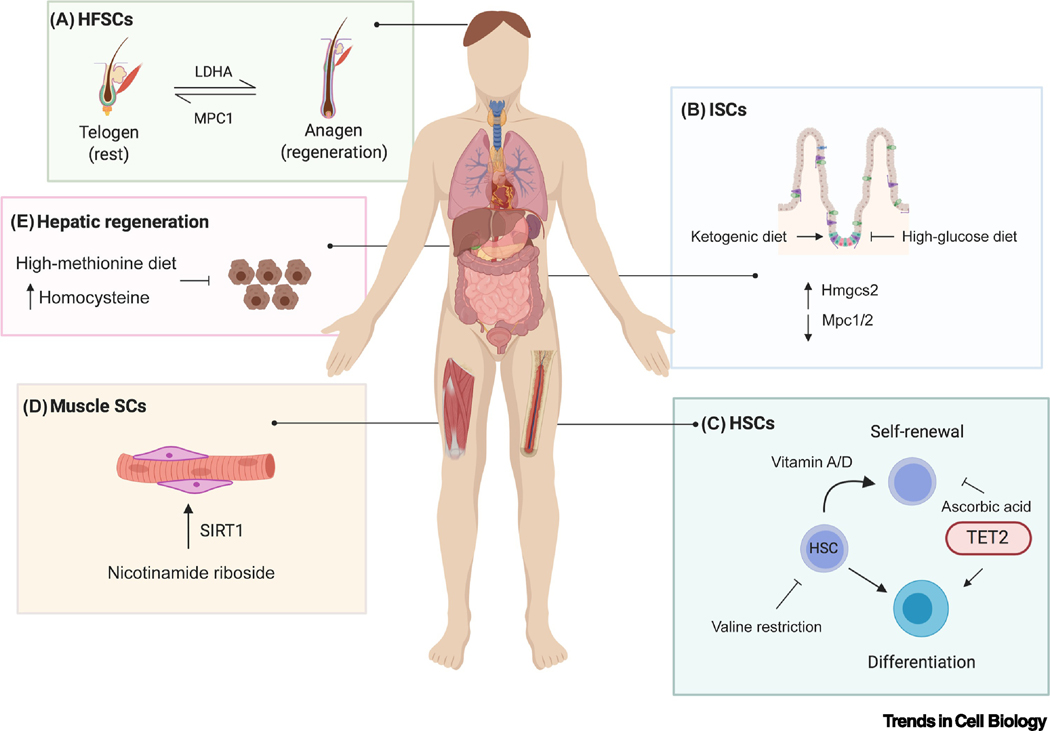
Figure 2. Nutrient Regulation of Adult Tissue Stem Cells.
Dietary manipulations and metabolites can affect tissue stem cell fate decisions, as highlighted in the small intestine (intestinal stem cells, ISCs), hematopoietic system (hematopoietic stem cells, HSCs), liver, muscle (muscle stem cells/satellite cells, SCs), and hair follicles (hair follicle stem cells, HFSCs). (A) In HFSCs, MPC1 (mitochondrial pyruvate carrier 1) and LDHA (lactate dehydrogenase regulate the balance between telogen and anagen during the hair cycle. (B) In ISCs, Hmgcs2 (3-hydroxy-3-methylglutaryl-CoA synthase) is highly expressed, whereas Mpc1/2 are expressed at low levels. Manipulating fuel sources with a ketogenic or high glucose diet regulates the balance of ISC self-renewal. (C) HSC self-renewal and differentiation can be regulated by manipulating the levels of vitamins, C, A, or D. HSC self-renewal is also impaired upon valine restriction. (D) Providing aged mice with the NAD+ precursor nicotinamide riboside is able to enhance muscle stem cell numbers and function in a SIRT1-dependent manner. (E) A high methionine diet, which increases plasma levels of homocysteine, impairs liver regeneration following partial hepatectomy. We note that these dietary manipulations have all been performed thus far in mice; the human image is for illustrative purposes only.
Intestine
The small intestine, comprised of the duodenum, jejunum, and ileum, is the most rapidly self-renewing organ in mammals. Interestingly, the small intestine displays region-specific metabolic programs, with higher levels of fatty acid oxidation occurring in the upper small intestine and declining distally towards the ileum [27]. High rates of intestinal self-renewal are enabled by a population of LGR5+ intestinal stem cells (ISCs) at the base of intestinal crypts [28]. ISCs give rise to more restricted progenitors cells that then undergo several rounds of cell division followed by differentiation into absorptive or secretory epithelial cells as they move upwards towards intestinal villi. Cell types within the intestine can communicate through metabolic signals, with differentiated Paneth cells secreting lactate to support intestinal stem cell function [21]. The balance between stem and differentiated cell fate can also be affected by cell-intrinsic changes in central carbon metabolism. The mitochondrial pyruvate carrier (MPC), comprised of MPC1 and MPC2 subunits, is required for pyruvate oxidation across species by enabling pyruvate entry into the mitochondria [29, 30]. Interestingly, MPC expression is low in intestinal stem cells and increases during differentiation. Genetic deletion of the MPC1 subunit or MPC inhibition in intestinal organoids skews cell metabolism towards glycolysis and increases ISC proliferation. Conversely, overexpression of MPC1/MPC2 reduced LGR5+ positivity in vitro [31].
A recent study demonstrated that expression of the enzyme Hmgcs2, which regulates the rate-limiting step in ketone body synthesis, is enriched in LGR5+ ISCs. Loss of Hmgcs2 impairs ISC regeneration and promotes promiscuous differentiation to the Paneth cell lineage [32]. Mechanistically, the ketone body β-hydroxybutyrate inhibits class I histone deacetylases to enhance transcriptional activation of Notch signaling and maintain stem cell self-renewal. Mice fed a ketogenic diet for 4–6 weeks showed enhanced numbers of intestinal stem cells and progenitor cells. Conversely, supplementation with 13% glucose in the drinking water dampens intestinal ketogenesis, reduces ISC numbers, and enhances differentiation.
In addition, the intestine constantly encounters diet-derived nutrients and is thus uniquely primed to respond to nutritional cues [33]. For example, studies performed in intestinal organoids from patient-derived normal and tumor organoids demonstrated that vitamin D levels can shift the equilibrium between stem and differentiated cell fates [34]. Intestinal stem cell activity, including proliferation and differentiation rates, is also affected by large deviations in nutrient availability as occurs in the context of high-fat diet or fasting [35, 36]. Taken together, it is clear that adult stem cells in the intestine are constantly integrating metabolic cues from their environment, which has broad implications for intestinal regeneration and disease therapies.
Hematopoietic System
HSCs are another well-characterized type of somatic stem cell. These cells predominantly reside in the bone marrow, but are also found in the peripheral blood supply, as well as in umbilical cord blood at birth. HSCs generate all cell types of the myeloid and lymphoid lineages, and aberrant HSC expansion results in hematological malignancies. Recently, vitamin C was shown to play a key role in regulating HSC expansion. In addition to its role as an antioxidant, vitamin C is a co-factor for α-ketoglutarate dependent dioxygenases, including the TET family of DNA demethylating enzymes [37]. Heterozygous Tet2 loss-of-function mutations are a common feature of hematological malignancies such as acute myeloid leukemia [37]. Mice that are unable to generate endogenous ascorbate show significantly higher HSC frequency in the bone marrow and spleen. However, vitamin C supplementation is able to restore normal HSC expansion and suppress leukemogenesis, in part through TET2 [26]. In addition, a separate study found that ascorbate supplementation can enhance residual wildtype TET activity in Tet2-deficient HSCs, thus preventing colony expansion in mutant cultures and leading to upregulated expression of myeloid lineage-specific genes [38]. Together, these results indicate that adequate vitamin C intake should be noted in leukemic patients with Tet2 mutations.
Vitamins A and D can also regulate HSC fate. Vitamin A is oxidized to retinoic acid (RA), and genes involved in RA metabolism are transcriptionally upregulated in HSCs compared to more restricted progenitor populations [39]. Adult mice maintained on a vitamin A deficient diet for 14–17 weeks had significantly decreased HSC frequency in the bone marrow and show decreased self-renewal capacity [40]. Supplementation with the active vitamin D metabolite 1,25(OH)D3 was found to increase HSC production in adult zebrafish kidney marrow, as well as promote ex vivo HSC proliferation in human umbilical cord blood [41]. In the future it will be of interest to determine if combinatorial studies manipulating vitamin A, C, and D intake can restrict or promote HSC expansion in the setting of normal development and/or blood disorders.
Successful bone marrow transplants require that the host HSC niche is first erased through irradiation or chemotherapy. Dietary valine restriction in vivo reduces HSC numbers; importantly, valine starvation permits HSC transplant without irradiation into immunocompromised mice [42]. Altering the balance of the branched chain amino acids valine, leucine, and isoleucine is even more effective, highlighting the potential for non-invasive dietary methods to facilitate bone marrow transplants [43].
Skeletal Muscle
Muscle stem cells, termed satellite cells, are responsible for maintaining adult muscle mass and repair following injury. Several recent studies have started to unravel how innate metabolism changes during differentiation from satellite cells to mature myocytes [44]. For example, single-cell mapping of histone acetylation marks showed that acetylation levels tend to be low in quiescent cells, increase during activation and proliferation, and fall again at the onset of differentiation. Differentiation is marked by increased glucose utilization for mitochondrial respiration, thus limiting availability of acetyl-CoA and acetylation marks on stem/cell-cycle related genes which facilitates maturation [45].
A separate study found that freshly isolated quiescent muscle stem cells highly express fatty acid oxidation enzymes/transporters; however, as they exit quiescence and enter the cell cycle for proliferation, a metabolic transition occurs to favor glycolysis [46]. Mechanistically, the histone deacetylase SIRT1 is a target of enhanced glycolysis. SIRT1 represses expression of mature skeletal muscle-specific genes, as well as genes involved in mitochondrial biogenesis. Enhanced glycolysis depletes NAD+, an essential metabolic co-factor of SIRT1. This in turn reduces SIRT1 activity, promoting downstream activation of these mature muscle-specific genes and concomitant differentiation [47]. Supplementing aged mice with the NAD+ precursor nicotinamide riboside in the diet is able to enhance muscle stem cell numbers and function in a SIRT1-dependent manner [48]. Interestingly, this study also noted that dietary nicotinamide riboside rejuvenated neural and melanocyte stem cells, suggesting a common mechanism may exist in multiple ASC populations.
Liver
The liver is a remarkable organ, capable of regeneration following partial hepatectomy in just one week. This is accomplished primarily through quiescent hepatocytes that re-enter the cell cycle and proliferate to restore tissue mass to its original size [49]. Liver resection is a common surgical procedure and thus it would be valuable to determine if any dietary changes could influence post-operative outcomes. For example, glucose supplementation impairs liver regeneration, while caloric restriction enhances hepatocellular proliferation following resection [50]. In addition, methionine metabolism, which occurs predominately in the liver, has an impact on regeneration dynamics [51]. Mice with genetic deletion of MAT1A, the enzyme responsible for catalyzing the formation of SAM from methionine, have elevated blood methionine levels and show impaired regeneration after partial hepatectomy [52]. Further, supplementing mice with a 2% methionine diet for 3 months increases plasma levels of the metabolite homocysteine, which induces oxidative stress and significantly impairs hepatocyte replication after liver resection [53]. Interestingly, there is evidence to suggest folic acid supplementation may reduce superoxide anion production and thus limit the adverse effects of increased homocysteine levels in the liver [54].
In the setting of chronic injury or disease that exhausts the proliferative capacity of hepatocytes, facultative adult stem cells in the liver known as hepatic progenitors or “oval cells” appear in the Canals of Hering near the biliary ducts. These cells are thought to be bipotent and able to differentiate into both hepatocytes and biliary epithelial cells [55, 56]. While some dietary manipulations can efficiently induce the oval cell response in mice, there is still relatively little known about how nutrients can influence the activation of facultative progenitors in the adult liver [57]. It will be of future interest to determine if certain metabolites can stimulate activation the oval cell response in vivo and/or promote differentiation towards the hepatic versus biliary lineage.
Hair Follicles
Recent work demonstrated that HFSCs display changes in their metabolic state as they transition from quiescence to activation, including an increase in lactate dehydrogenase activity. Altering metabolism in HFSCs by genetically or pharmacologically manipulating pyruvate fate in HFSCs alters differentiation decisions, with lactate dehydrogenase deletion blocking HFSC activation and Mpc1 deletion promoting HFSC activation and entry into the hair cycle. Interestingly, topical administration of the MPC inhibitor UK-5099 also promotes HFSC activation and hair growth [24]. Given that many companies currently market dietary supplements to promote hair growth, it will be of great scientific and public interest to mechanistically dissect how metabolites can regulate activation of HFSCs in vivo.
Tissue Stem cells and Tumors: Lessons from Cancer Metabolism Studies
Stem cells and cancer cells share common features: both cell types are able to re-enter the cell cycle and are wired to support proliferation through similar anabolic programs [3]. In certain contexts, tissue stem cells act as tumor initiating cells, with recent evidence demonstrating that changes in metabolism can alter the propensity of a tissue stem cell to initiate cancer [58]. For example, genetic loss of Mpc1 in intestinal stem cells increases glycolytic activity and expression of stem cell markers, which is sufficient to promote intestinal tumor formation in vivo [59]. However, loss of Mpc1 in HFSCs is not sufficient to promote tumor formation in vivo and has no effect on tumor initiation or aggressiveness in a DMBA-TPA chemically-induced squamous cell carcinoma model [60]. These studies suggest tissue-specific differences in ASC metabolism-directed contributions to cancer initiation, highlighting the need for future studies to parse out the role of tissue stem cell metabolism in instructing fate decisions in tissue-specific contexts.
Given established connections between ASC metabolism and cancer, the tissue stem cell field will benefit greatly from ongoing studies in the cancer metabolism field that explore dietary strategies as a cancer therapeutic [61]. As discussed above, vitamin C supplementation can inhibit expansion of the hematopoietic stem cell compartment and suppress leukemogenesis [26, 38]. Further, high-dose intraperitoneal injection of vitamin C restricts KRAS and BRAF mutated colorectal cancer cells by depleting intracellular glutathione levels and inducing ROS, and a recent study demonstrated that intravenous vitamin C administration can similarly reduce hepatocellular carcinoma xenograft growth in animals by inducing oxidative stress [62, 63]. Conversely, dietary supplementation with vitamin E or B vitamins (folic acid/vitamin B12) correlates with an increased incidence of prostate and lung cancer respectively [64, 65].
Altering the amino acid composition in the diet can also have a vast impact on tumor growth by limiting access to nutrients in rapidly proliferating cells. A low serine/glycine diet can reduce tumor growth and extend survival in genetic models of colon cancer and lymphoma [66]. Histidine catabolism consumes intracellular tetrahydrofolate, a key intermediate in nucleotide synthesis; thus, histidine supplementation enhances sensitivity of cancer cells to the common chemotherapeutic methotrexate [67]. Reducing asparagine availability through genetic, pharmacologic, or dietary means limits progression of breast cancer metastasis, and reduced arginine levels underlie impaired tumor growth in the setting of defective autophagy [68, 69]. In addition, dietary methionine restriction, which has been demonstrated to be achievable in humans, remodels one-carbon metabolism and has therapeutic benefits in patient-derived xenograft models of colorectal cancer and sarcoma [70] (Figure 3). As the connection between metabolism, diet, and cancer progression is strengthened, it is possible that personalized nutrition strategies may be incorporated into treatment plans. For example, the efficacy of PI3K inhibitors used to target tumor growth was found to increase when combined with a ketogenic diet in mice [71]. Therefore, it will be prudent for the stem cell field to explore dietary strategies employed in the cancer field for potential effects on ASC populations.
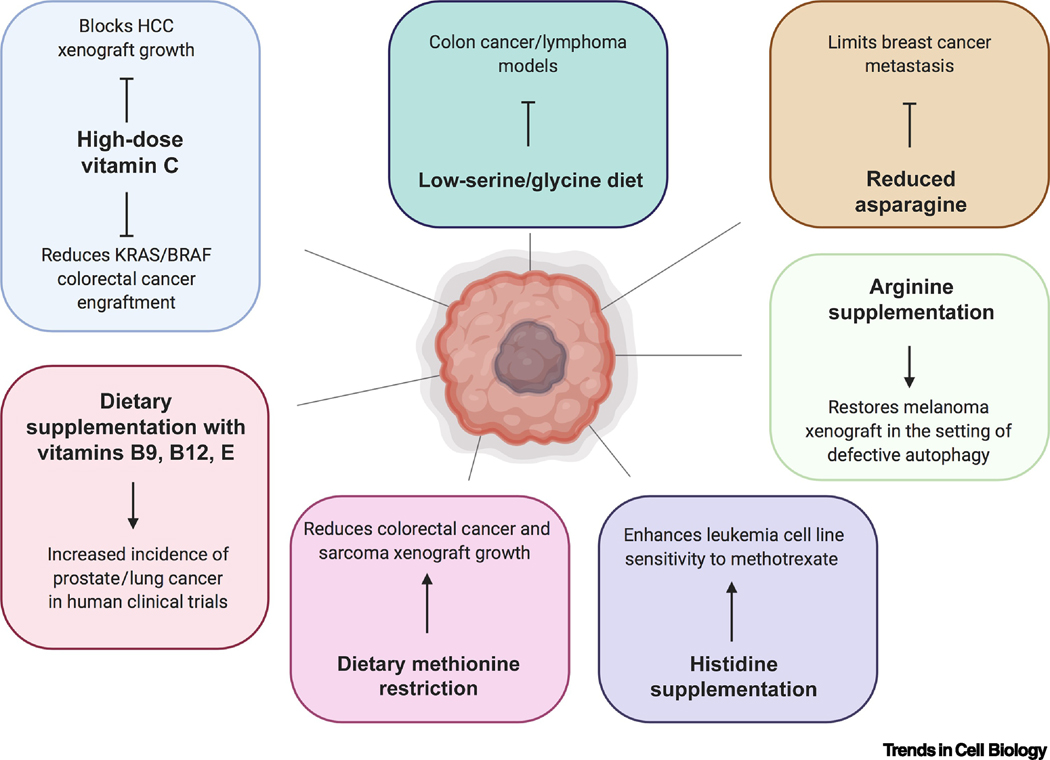
Figure 3. Metabolite and Dietary Manipulations Affecting Cancer Cell Growth.
Nutrients such as vitamins and amino acids can rewire tumor metabolism and alter cancer growth in genetic and xenograft models of cancer in mice. In addition, some large-scale clinical trials have observed that certain nutrients are associated with higher incidence of certain cancers in humans.
Concluding Remarks and Future Perspectives
Defining metabolic regulation of the ASC populations described above has been facilitated by the presence of well-defined markers, temporal control of stem cell activation, robust injury response, and/or the presence of in vitro models. However, we note that tissue stem cells have been described in almost all adult organs including the pancreas, brain, lung, adipose, stomach, and others [2]. Future research defining dietary and metabolic control of cell fate decisions in these tissues will be of broad significance in the fields of metabolism and regenerative medicine (see Outstanding Questions). Metabolic pathways and chromatin modifications are intimately linked and thus many changes in metabolism influence epigenetic changes and alter gene expression. As our knowledge of the intersection between metabolism and cell fate change expands, it will be important to remain open to other mechanisms that may be caused by metabolic shifts. For example, signaling pathways including mTORC, AMPK, MAPK and others are all sensitive to changes in nutrient levels. In addition, there are emerging examples of transcription factors being directly regulated by metabolites [72, 73] (Figure 1C). It is possible that the transcriptional machinery itself may also be responsive to nutrients; for example RNA polymerase II is modified by O-GlcNAc, a metabolite derived from the hexosamine biosynthesis pathway [74]. Taken together, it is likely that epigenetic/signaling pathways and transcription are concomitantly affected by changing nutrient levels.
Outstanding Questions.
What tissues are susceptible to metabolite-induced cell fate decisions? Are there differences in the propensity for tissues to regulate fate decisions through metabolic cues; for example, do tissues that directly encounter diet-derived nutrients like the intestine employ different mechanisms to modulate responses?
How do dietary manipulations being explored in the cancer metabolism field, such as amino acid restriction, affect various adult stem cell populations in the setting of homeostasis and/or response to injury?
What mechanisms beyond changes in epigenetic marks and nutrient sensing signaling pathways are involved in the cellular response to altered metabolite availability, and how are multiple inputs from the same metabolite coordinated? Do common metabolic pathways or enzymes have recurring roles across multiple tissues?
How will new technologies advance our understanding of in vivo metabolism, particularly in tissue stem cells that heavily rely on their native signaling niche to direct cell fate decisions?
How do changes in metabolite availability alter developmental decisions during gestation?
Do dietary choices that manipulate stem cell fate decisions factor into different individuals’ propensity to acquire disease? Can dietary manipulations be leveraged to target diseases such as cancer, or conditions exacerbated by impaired regenerative responses?
In addition, a focus of the stem cell metabolism literature has centered on central carbon metabolism and the balance between glycolysis versus oxidative phosphorylation in regulating cell fate. We note that manipulating common central pathways can have effects on tissue stem cells in multiple populations. For example, altering pyruvate fate though loss- or gain-of-function of the mitochondrial pyruvate carrier alters stem cell fate in both the intestine and hair follicles. It is likely that roles for metabolites in other pathways will increasingly come to light as research advances and the field begins to tackle the technical challenges that accompany metabolic studies in rare cell populations. Metabolic enzymes catalyze reactions on the timescale of seconds to minutes, creating technical limitations for current methods aimed at unraveling metabolic pathways. Fluorescence-activated cell sorting is a common analysis technique that isolates specific cell types from a heterogenous population. However, there are several drawbacks to using this technique for mapping metabolism, namely the extended amount of time required to sort cells (on the timescale of hours) and the mechanical strain imposed during sorting, which can activate stress-related pathways and cause marked changes in cellular metabolism [75, 76]. Thus, new methods will be necessary to facilitate rapid isolation of intact adult stem cells from their tissue of origin.
In addition, the field should focus on developing improved techniques to investigate metabolic transitions in vivo. 2D and organoid culture methods facilitate studies that cannot reasonably be performed in vivo; however, culture conditions expose cells to supraphysiologic levels of many metabolites. A recent study developed physiologic medium, which mimics the polar metabolite profile of human plasma, and observed widespread changes in cellular metabolism relative to cells cultured in traditional medium [77]. Importantly, recent in vivo studies using isotope tracing in both humans and mice have revealed critical differences in the usage of major carbon sources by normal and cancer cells in vivo compared to cell culture [78]. For example, cultured cancer cells typically consume glucose and secrete lactate, whereas in vivo human lung tumors predominantly consume lactate to fuel the TCA cycle [79, 80]. Transitioning to the in vivo setting will reveal additional differences in metabolism under physiological conditions, and will be particularly important in tissue stem cells that heavily rely on the appropriate signaling niche to direct cell fate decisions [81]. Overall, cell culture and organoid systems are invaluable for screening purposes and detailed mechanistic studies, but caution should be taken in interpreting metabolic profiling studies performed in vitro. As discussed above, in vivo metabolic imaging and tracer analyses are established non-invasive techniques that can maintain structural and anatomical tissue integrity [82]. Newer mass spectrometry imaging technologies detect multiple metabolites in situ by direct ionization and thus hold great promise for metabolic mapping in endogenous tissue stem cells [83, 84].
Emphasizing the connection between diet and development will be important in maternal-fetal medicine by expanding our mechanistic knowledge of how specific nutrients can affect developmental pathways [85]. A recent study demonstrated that glucose inhibits maturation of human embryonic stem cells to cardiomyocytes by promoting nucleotide biosynthesis via the pentose phosphate pathway. Interestingly, maternal hyperglycemia during pregnancy is associated with increased incidence of congenital heart disease [86], and a diabetic mouse model showed reduced maturation of fetal cardiomyocytes in vivo, thus providing a mechanistic link between altered metabolism and cardiac development [87]. Studies manipulating maternal diet have already revealed metabolic effects on development. For example, low choline diet during embryonic days 11–17 of gestation reduces cortical neural progenitor cells in fetal brains [88], and maternal ascorbic acid is required for germline demethylation and proper germ cell development in female mice [89]. Thus, it will be of great interest to determine how specific nutrients and supplements can affect developmental processes to complement studies in adult stem cells.
A current focus in the metabolism field is investigating how specific metabolites and nutrients impact disease progression and treatment. It is now becoming clear that diet-regulated nutrients can also regulate normal homeostatic processes by altering adult stem cell fate decisions, which may factor into currently unexplained variability in the susceptibility of different populations to acquire age-related diseases such as neurodegenerative disorders or cancer. Looking to the future, the implications for understanding how diet influences cellular transitions is immense and will guide precision-based nutrition to improve both general health and therapeutic strategies across a broad range of diseases.
Highlights.
Adult stem cells, which are capable of both self-renewal and differentiation to mature cell types, are responsible for maintaining homeostasis and responding to injury stimuli in a tissue-specific manner.
Metabolites derived from glycolysis, oxidative phosphorylation, and other metabolic pathways can influence adult stem cell fate decisions by regulating gene expression and/or signaling pathways.
Adult stem cells residing in their niche have innate metabolic programs that typically vary between the quiescent, activated and differentiated state.
Manipulating metabolite availability (including the prevalence of compounds derived from the diet such as vitamins, ketone bodies, and amino acids) can change adult stem cell fate decisions.
Stem cells and cancer cells share common metabolic features, with cancer cell fate also influenced by nutrient availability.
Acknowledgments:
We respectfully note that we were unable to comprehensively cite many worthy contributions to the field due to space limitations. We thank our colleagues in the Christofk lab for helpful comments and discussion. S.N.S is supported by a postdoctoral training grant from the Eli and Edythe Broad Stem Cell Research Center at UCLA. H.R.C. is supported by a Research Scholar Grant (RSG-16–111-01-MPC) from the American Cancer Society, NIH/NCI R01 CA215185, NIH/NIAMS R01 AR070245, and the UCLA Jonsson Comprehensive Cancer Center and Eli and Edythe Broad Center for Regenerative Medicine Ablon Scholars Program.
Glossary
- Activation:
the process by which stem cells receive signals from the surroundings to re-enter the cell cycle and proliferate to respond to injury or replenish lost cells
- Adult stem cells (ASCs):
an undifferentiated cell present amongst differentiated cells in a tissue or organ. ASCs can self-renew or differentiate into a specialized cell type in the resident tissue
- Differentiation:
the progressive transformation of a less specialized cell to a more specialized cell type, involving iterative alterations in local gene expression, cell morphology, signaling pathways, and metabolic milieu
- Progenitor:
early descendants of stem cells that can differentiate to form one or more cell types but are not capable of indefinite self-renewal
- Quiescence:
a reversible state in which the cell remains dormant but is capable of re-entering the cell cycle and proliferating in response to stimulation
- Niche:
the specialized microenvironment in which stem cells reside, consisting of multiple cell types and a niche-specific extracellular matrix. The niche provides morphogens, structural signals, and metabolic instructions to stem cells to maintain the balance between stemness and differentiation
- Metabolite:
the products or intermediates of cellular metabolism. Metabolites are typically small molecules (sugars, amino acids, vitamins, etc.) that play key roles in biochemical, signaling, gene regulation, and other pathways
- Metabolomics:
a technique used to measure the compendium of metabolites present in cells, tissues, fluids, or other biological samples in a high-throughput manner using mass spectrometry-based methods
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avgustinova A. and Benitah SA (2016) Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol 17 (10), 643–58. [DOI] [PubMed] [Google Scholar]
- 2.Perez LM et al. (2018) Unhealthy Stem Cells: When Health Conditions Upset Stem Cell Properties. Cell Physiol Biochem 46 (5), 1999–2016. [DOI] [PubMed] [Google Scholar]
- 3.Intlekofer AM and Finley LWS (2019) Metabolic signatures of cancer cells and stem cells. Nature Metabolism 1 (2), 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryall JG et al. (2015) Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 17 (6), 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shyh-Chang N. and Ng HH (2017) The metabolic programming of stem cells. Genes Dev 31 (4), 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey A. et al. (2019) Interplay between Metabolites and the Epigenome in Regulating Embryonic and Adult Stem Cell Potency and Maintenance. Stem Cell Reports 13 (4), 573–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaelin WG Jr. and McKnight SL (2013) Influence of metabolism on epigenetics and disease. Cell 153 (1), 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrowsmith CH et al. (2012) Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov 11 (5), 384–400. [DOI] [PubMed] [Google Scholar]
- 9.Kinnaird A. et al. (2016) Metabolic control of epigenetics in cancer. Nat Rev Cancer 16 (11), 694–707. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D. et al. (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574 (7779), 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabari BR et al. (2016) Metabolic regulation of gene expression through histone acylations. Nature Reviews Molecular Cell Biology 18, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid MA et al. (2017) The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat Cell Biol 19 (11), 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Z. et al. (2019) Loss of EZH2 Reprograms BCAA Metabolism to Drive Leukemic Transformation. Cancer Discovery 9 (9), 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao K. et al. (2020) A Feedback Circuitry between Polycomb Signaling and Fructose-1, 6-Bisphosphatase Enables Hepatic and Renal Tumorigenesis. Cancer Research 80 (4), 675. [DOI] [PubMed] [Google Scholar]
- 15.Buchovecky CM et al. (2013) A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat Genet 45 (9), 1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfson RL and Sabatini DM (2017) The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metabolism 26 (2), 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia D. and Shaw RJ (2017) AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Molecular Cell 66 (6), 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraro F. et al. (2010) Adult stem cels and their niches. Advances in experimental medicine and biology 695, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanpain C. et al. (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118 (5), 635–48. [DOI] [PubMed] [Google Scholar]
- 20.Chacón-Martínez CA et al. (2017) Hair follicle stem cell cultures reveal self-organizing plasticity of stem cells and their progeny. The EMBO Journal 36 (2), 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Colman MJ et al. (2017) Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424. [DOI] [PubMed] [Google Scholar]
- 22.Snoeck HW (2017) Mitochondrial regulation of hematopoietic stem cells. Curr Opin Cell Biol 49, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X. et al. (2016) Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 5, e13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores A. et al. (2017) Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol 19 (9), 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinschen MM et al. (2019) Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol 20 (6), 353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agathocleous M. et al. (2017) Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549 (7673), 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stine RR et al. (2019) PRDM16 Maintains Homeostasis of the Intestinal Epithelium by Controlling Region-Specific Metabolism. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T. et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459 (7244), 262–5. [DOI] [PubMed] [Google Scholar]
- 29.Bricker DK et al. (2012) A Mitochondrial Pyruvate Carrier Required for Pyruvate Uptake in Yeast, Drosophila, and Humans. Science 337 (6090), 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herzig S. et al. (2012) Identification and Functional Expression of the Mitochondrial Pyruvate Carrier. Science 337 (6090), 93. [DOI] [PubMed] [Google Scholar]
- 31.Schell JC et al. (2017) Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol 19 (9), 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng CW et al. (2019) Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell 178 (5), 1115–1131 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso S. and Yilmaz ÖH. (2018) Nutritional Regulation of Intestinal Stem Cells. Annual Review of Nutrition 38 (1), 273–301. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Barral A. et al. (2019) Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyaz S. et al. (2016) High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531 (7592), 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mihaylova MM et al. (2018) Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 22 (5), 769–778 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimmino L. et al. (2018) Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol 28 (9), 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cimmino L. et al. (2017) Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 170 (6), 1079–1095 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabezas-Wallscheid N. et al. (2014) Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell 15 (4), 507–522. [DOI] [PubMed] [Google Scholar]
- 40.Cabezas-Wallscheid N. et al. (2017) Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 169 (5), 807–823 e19. [DOI] [PubMed] [Google Scholar]
- 41.Cortes M. et al. (2016) Developmental Vitamin D Availability Impacts Hematopoietic Stem Cell Production. Cell Rep 17 (2), 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taya Y. et al. (2016) Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science, aag3145. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson AC et al. (2018) Branched-chain amino acid depletion conditions bone marrow for hematopoietic stem cell transplantation avoiding amino acid imbalance-associated toxicity. Exp Hematol 63, 12–16 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryall JG and Lynch GS (2018) The molecular signature of muscle stem cells is driven by nutrient availability and innate cell metabolism. Curr Opin Clin Nutr Metab Care 21 (4), 240–245. [DOI] [PubMed] [Google Scholar]
- 45.Yucel N. et al. (2019) Glucose Metabolism Drives Histone Acetylation Landscape Transitions that Dictate Muscle Stem Cell Function. Cell Rep 27 (13), 3939–3955 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado L. et al. (2017) In Situ Fixation Redefines Quiescence and Early Activation of Skeletal Muscle Stem Cells. Cell Rep 21 (7), 1982–1993. [DOI] [PubMed] [Google Scholar]
- 47.Ryall James G. et al. (2015) The NAD+-Dependent SIRT1 Deacetylase Translates a Metabolic Switch into Regulatory Epigenetics in Skeletal Muscle Stem Cells. Cell Stem Cell 16 (2), 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H. et al. (2016) NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352 (6292), 1436. [DOI] [PubMed] [Google Scholar]
- 49.Post Y. and Clevers H. (2019) Defining Adult Stem Cell Function at Its Simplest: The Ability to Replace Lost Cells through Mitosis. Cell Stem Cell 25 (2), 174–183. [DOI] [PubMed] [Google Scholar]
- 50.Huang J. and Rudnick DA (2014) Elucidating the metabolic regulation of liver regeneration. Am J Pathol 184 (2), 309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudnick DA and Huang J. (2015) Metabolic Regulation of Liver Regeneration. In Liver Regeneration, pp. 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L. et al. (2004) Impaired liver regeneration in mice lacking methionine adenosyltransferase 1A. The FASEB Journal 18 (7), 914–916. [DOI] [PubMed] [Google Scholar]
- 53.Liu W-H. et al. (2010) Hepatocyte Proliferation During Liver Regeneration Is Impaired in Mice with Methionine Diet-Induced Hyperhomocysteinemia. The American Journal of Pathology 177 (5), 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo CW et al. (2006) Hyperhomocysteinemia induces liver injury in rat: Protective effect of folic acid supplementation. Biochim Biophys Acta 1762 (7), 656–65. [DOI] [PubMed] [Google Scholar]
- 55.Huch M. et al. (2013) In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494 (7436), 247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huch M. et al. (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160 (1–2), 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bria A. et al. (2017) Hepatic progenitor cell activation in liver repair. Liver Research 1 (2), 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mihaylova Maria M. et al. (2014) Dietary and Metabolic Control of Stem Cell Function in Physiology and Cancer. Cell Stem Cell 14 (3), 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bensard CL. et al. (2020) Regulation of Tumor Initiation by the Mitochondrial Pyruvate Carrier. Cell Metab 31 (2), 284–300 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flores A. et al. (2019) Increased lactate dehydrogenase activity is dispensable in squamous carcinoma cells of origin. Nat Commun 10 (1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanarek N. et al. (2020) Dietary modifications for enhanced cancer therapy. Nature 579 (7800), 507–517. [DOI] [PubMed] [Google Scholar]
- 62.Lv H. et al. (2018) Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis Oncol 2 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yun J. et al. (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350 (6266), 1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klein EA et al. (2011) Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306 (14), 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ebbing M. et al. (2009) Cancer Incidence and Mortality After Treatment With Folic Acid and Vitamin B12. JAMA 302 (19), 2119–2126. [DOI] [PubMed] [Google Scholar]
- 66.Maddocks ODK et al. (2017) Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544 (7650), 372–376. [DOI] [PubMed] [Google Scholar]
- 67.Kanarek N. et al. (2018) Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 559 (7715), 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knott SRV et al. (2018) Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554 (7692), 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poillet-Perez L. et al. (2018) Autophagy maintains tumour growth through circulating arginine. Nature 563 (7732), 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X. et al. (2019) Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572 (7769), 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hopkins BD et al. (2018) Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560 (7719), 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mezrich JD et al. (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185 (6), 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X. et al. (2019) alpha-Ketoglutarate-Activated NF-kappaB Signaling Promotes Compensatory Glucose Uptake and Brain Tumor Development. Mol Cell 76 (1), 148–162 e7. [DOI] [PubMed] [Google Scholar]
- 74.Lewis BA et al. (2016) Human RNA Polymerase II Promoter Recruitment in Vitro Is Regulated by O-Linked N-Acetylglucosaminyltransferase (OGT). J Biol Chem 291 (27), 14056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Llufrio EM et al. (2018) Sorting cells alters their redox state and cellular metabolome. Redox Biol 16, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binek A. et al. (2019) Flow Cytometry Has a Significant Impact on the Cellular Metabolome. J Proteome Res 18 (1), 169–181. [DOI] [PubMed] [Google Scholar]
- 77.Cantor JR et al. (2017) Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell 169 (2), 258–272 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hui S. et al. (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551 (7678), 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hensley CT et al. (2016) Metabolic Heterogeneity in Human Lung Tumors. Cell 164 (4), 681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faubert B. et al. (2017) Lactate Metabolism in Human Lung Tumors. Cell 171 (2), 358–371 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Redondo PA et al. (2017) Elements of the niche for adult stem cell expansion. Journal of tissue engineering 8, 2041731417725464–2041731417725464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Gialleonardo V. et al. (2016) The Potential of Metabolic Imaging. Seminars in nuclear medicine 46 (1), 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miura D. et al. (2012) In situ metabolomic mass spectrometry imaging: recent advances and difficulties. J Proteomics 75 (16), 5052–5060. [DOI] [PubMed] [Google Scholar]
- 84.Buck A. et al. (2017) In Situ Metabolomics in Cancer by Mass Spectrometry Imaging. Adv Cancer Res 134, 117–132. [DOI] [PubMed] [Google Scholar]
- 85.Hambidge KM and Krebs NF (2018) Strategies for optimizing maternal nutrition to promote infant development. Reprod Health 15 (Suppl 1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simeone RM et al. (2015) Diabetes and congenital heart defects: a systematic review, meta-analysis, and modeling project. Am J Prev Med 48 (2), 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakano H. et al. (2017) Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife 6, e29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y. et al. (2016) Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J 30 (4), 1566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiTroia SP et al. (2019) Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature 573 (7773), 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]